User login
Human trafficking: How ObGyns can—and should—be helping survivors

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20

In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).

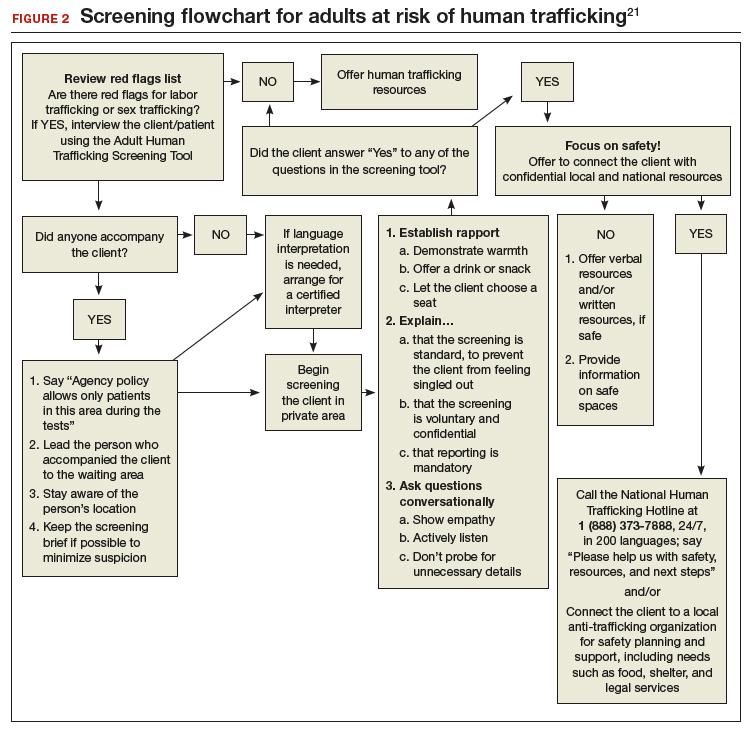
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20

In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).


Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20

In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).


Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
IN THIS ARTICLE
- Clues to raise suspicion
- Medical consequences of trafficking
- Screening algorithm
Alcohol: An unfortunate teratogen
Medical students learn early in their education that alcohol is a teratogen. Despite this widespread knowledge, many obstetricians counsel patients about the safety of low doses of alcohol in pregnancy.1 Indeed, the Royal College of Obstetricians and Gynaecologists’ position on this is, “while the safest approach may be to avoid any alcohol during pregnancy, it remains the case that there is no evidence of harm from low levels of alcohol consumption, defined as no more than one or two units of alcohol once or twice a week.”2
Like many providers, I was aware of this controversy, but it became truly personal when a beloved family member was diagnosed with fetal alcohol syndrome (FAS). In this paper, I will review some of the controversy regarding alcohol in pregnancy, highlight findings from the literature, provide tools for prevention, and identify new developments regarding this devastating, preventable condition.
Charlie
To know my nephew Charlie is to fall in love with my nephew Charlie. One of the happiest moments of my life was when I learned my brother and sister-in-law had adopted twins from Kazakhstan. When my little niece and nephew started their new life in the United States, certain medical issues seemed to merit additional attention. Although both were very small for their age and required significant nutritional support, Charlie seemed to be a bit more rambunctious and required additional supervision.
The children were fortunate enough to have incredibly loving, dedicated parents, who have access to exceptional medical care as residents of Philadelphia, Pennsylvania. After extensive testing, it became clear what was causing Charlie’s developmental delay; his pediatric team made the diagnosis of FAS. My brother and sister-in-law became incredibly well-read about this challenging disorder, and threw themselves into national advocacy work to help prevent this unnecessary tragedy.
Recent data point to teratogenicity, but media confuse the issue
Some recent media coverage3 of celebrities who apparently drank while pregnant was in response to an article in the Journal of Epidemiology and Community Health.4 The authors of this study concluded that, “at age 5 years, cohort members born to mothers who drank up to one to two drinks per week or per occasion during pregnancy were not at increased risk of clinically relevant behavioral difficulties or cognitive deficits, compared with children of mothers in the not-in-pregnancy group.”
This is certainly not the first occasion the popular press has covered a published study that seems to indicate no ill effects of alcohol use in pregnancy. A 2008 report by Kelly and colleagues,5 and its subsequent media coverage, prompted the Fetal Alcohol Spectrum Disorders Study Group to state that the panel of experts was “alarmed” by recent newspaper reports suggesting that light drinking during pregnancy may be beneficial for an unborn child.6 They noted misleading and irresponsible media reports of the findings, which suggested that 3-year-old children whose mothers drank “lightly” during pregnancy were not at risk for certain behavioral problems.
What the study authors proceeded to note, however (that the media did not mention), was that the light drinkers in their study had socioeconomic advantages, compared with nondrinkers.5 (Advantaged economic status is established to be beneficial for childhood development.) They also noted that the study involved preschool-aged children, stating “Generally the adverse effects of light drinking during pregnancy are subtle and may go undetected in young children. However, other group studies of more moderate or ‘social’ drinking levels during pregnancy have shown an adverse impact on multiple aspects of development through adolescence and young adulthood, even when important environmental factors are taken into account.” A sentence I thought was most compelling in their statement was, “It is an inconvenient fact of life that alcohol is a teratogen.” Now, this fact is well supported in the literature.7
There are animal studies regarding the use of “low-dose” or “moderate” alcohol in pregnancy that demonstrate adverse behavioral outcomes with exposure to even small doses of alcohol.8,9 It is an American tragedy that, according to the Centers for Disease Control and Prevention (CDC), rates of FAS in this country range from 0.2 to 2.0 cases per 1,000 live births. Indeed, the rates of fetal alcohol spectrum disorders (FASD) might be at least three times this rate.10 As is the case with other disorders, there are health disparities regarding the prevalence of this condition as well.11
FAS: A long history of preventable disease
1973: Identified. FAS was first described in a 1973 Lancet report, “Pattern of malformation in offspring of chronic alcoholic mothers.”12
1996: Call for prevention. In 1995, the US Surgeon General issued a statement regarding alcohol use in pregnancy, noting, “We do not know what, if any, amount of alcohol is safe.”13 In 1996, the Institute of Medicine released a paper calling FAS and FASD “completely preventable birth defects and neurodevelopmental abnormalities.”14
2000: The troubling effects gathered. The American Academy of Pediatrics (AAP) published a monograph on FAS in 2000, defining it as a constellation of physical, behavioral, and cognitive abnormalities.15
These features classically define FAS:
- dysmorphic facial features
- prenatal and postnatal growth abnormalities
- mental retardation.
Approximately 80% of children with this condition have:
- microcephaly
- behavioral abnormalities.
As many as 50% of affected children also exhibit:
- poor coordination
- hypotonia
- attention-deficit hyperactivity disorder
- decreased adipose tissue
- identifiable facial anomalies (such as maxillary hypoplasia, cleft palate, and micrognathia).
Also common:
- cardiac defects
- hemangiomas
- eye or ear abnormalities.
The AAP further noted that data current to the time (and still true today) did not support the concept of a safe level of alcohol consumption by pregnant women below which no damage to a fetus will occur.15
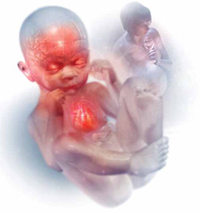
Alcohol intake during pregnancy puts the fetus at risk for cognitive and neuropsychological impairment and physical abnormalities, including dysmorphic facial features (such as micrognathia), restricted prenatal growth, cardiac defects, and eye and ear abnormalities. There is no threshold dose of alcohol that is safe during pregnancy, according to the American College of Obstetricians and Gynecologists.
Despite the knowledge we’ve gained, FAS persists
According to a 2006–2010 CDC analysis involving more than 345,000 women of reproductive age from all 50 states, 7.6% of pregnant women reported alcohol use and 1.4% (or 1 in 71) reported binge drinking (defined, respectively, as at least one alcoholic drink and four or more alcoholic drinks on one occasion in the past 30 days).16 The highest prevalence of obstetric alcohol consumption occurs in women who are:
- aged 35 to 44 years
- white
- college graduates
- employed.
The problem may be bigger than reported. The incidences of alcohol and binge drinking found in the CDC report include women’s self-report—but women drink alcohol without knowing they’re pregnant. Only 40% of women realize they’re pregnant at 4 weeks of gestation, a critical time for organogenesis, and approximately half of all births are unplanned.9
When my brother and sister-in law adopted my beautiful niece and nephew, they were very aware of the risk for conditions like FAS. In an evaluation of 71 children adopted from Eastern Europe at 5 years of age, FAS was diagnosed in 30% of children and “partial FAS” in another 9%.17 Birth defects attributed to alcohol were present in 11% of the children.
Are women’s health providers up to date on FAS education?
In recognition of alcohol’s potentially life-altering consequences for the developing fetus, the American College of Obstetricians and Gynecologists (ACOG) produced an FASD prevention tool kit in 2006 and published a 2011 committee opinion on at-risk drinking and alcohol dependence and their implications for obstetrics and gynecology.18,19 Both guidelines direct clinicians to advise patients to abstain from alcohol during pregnancy.
Results from a 2010 survey of 800 ACOG fellows revealed that only 78% of obstetricians advised abstinence from alcohol during pregnancy. Fifty-eight percent of respondents did not use a validated screening tool for alcohol use in their pregnant patients, and only 72% felt prepared to screen for risky or hazardous drinking.19 (Most were unaware of the ACOG tool kit, which had been published several years earlier.)
In a survey of pediatricians, obstetricians, and family physicians, clinicians said that about 67% of their patients asked about alcohol use in pregnancy, with about 2% of those patients specifically mentioning FAS. About 41% of these same physicians erroneously placed the threshold for FAS at one to three drinks per day,20 when in fact there is no threshold of drinking that has been proven to be safe.
A survey of 1,000 actively practicing ACOG fellows revealed that, while 97% of obstetricians routinely asked their patients about alcohol use, only 20% of providers reported to their patients that abstinence was safest, and 4% of providers didn’t believe that consumption of eight or more drinks weekly posed fetal risk.21
How can we educate our patients about the dangers of alcohol in pregnancy?
Fetal death. A recent Danish study of 79,216 pregnant women revealed that 45% had consumed some alcohol during pregnancy. Two percent reported at least four drinks per week, and 25% admitted to binge drinking during pregnancy. Term infants born to women in the latter two groups had increased neonatal mortality, with hazard ratios of 3.56 (95% confidence interval [CI], 1.15–8.43) and 2.69 (95% CI, 1.27–5.69), respectively.22
Decreased cognitive status. A study by Willford and colleagues evaluated the relationship between prenatal alcohol exposure and cognitive status of 1,360 10-year-old children.23 The authors utilized the Stanford-Binet Intelligence Test, including the composite scores and verbal, abstract/visual, quantitative, and short-term memory scores. After controlling for other variables, among African American offspring they found that, for each additional drink, the average composite score decreased by 1.9 points. This difference was more striking for second-trimester use, and was significant even for one drink daily versus abstention from alcohol.
Impaired neuropsychological development. Another study evaluating light to moderate amounts of prenatal alcohol exposure in 10- and 11-year-old children found significantly worse scores regarding a number of neuropsychological developmental assessments.24
No threshold dose of causation. Results of a 2012 prospective study in California, with data collected on 992 subjects from 1978 until 2005, revealed that many physical FAS features, including microcephaly, smooth philtrum, and thin vermillion border; reduced birth length; and reduced birth weight, were associated with alcohol exposure at specific gestational ages, and were dose-related.25 This paper didn’t reveal any evidence of a threshold dose of causation.
Neurobehavioral outcomes of FAS are not always considered
Another recent study that the media recently highlighted as finding “no association between low or moderate prenatal alcohol exposure and birth defects” was by O’Leary and colleagues.26 Like other similarly limited studies, this one involved only children younger than 6 years and didn’t assess any of the important neurobehavioral outcomes of FAS.
FAS encompasses much more than visible birth defects. As the aforementioned ACOG tool kit stated, “For every child born with FAS, many more children are born with neurobehavioral deficits caused by alcohol exposure but without the physical characteristics of FAS.”
The costs of FAS are felt with dollars, too
The financial cost to our nation is extraordinary. In 1991, Abel and Sokol estimated the incremental annual cost of treating FAS at nearly $75 million, with about three-quarters of that cost associated with FAS cases involving mental retardation.27
A 2002 assessment estimated the lifetime cost for each individual with FAS (adjusting for the change in the cost of medical care services, lost productivity, and inflation) at $2 million. This figure consists of $1.6 million for medical treatment, special education, and residential care for persons with mental retardation, and $0.4 million for productivity losses.28
Where human studies fall short, animal studies can help elucidate causation
Unquestionably, there are flaws in the existing literature on the causation of FAS. Many studies rely on self-reporting by pregnant women, and underreporting in these cases is a real concern. There often are other confounders potentially negatively affecting fetal development, making it difficult to differentiate causation. The animal studies that don’t share these limitations do suggest a causal relationship between antenatal alcohol exposure and poor obstetric outcomes, however.29 These studies suggest mechanisms such as altered gene expression, oxidative stress, and apoptosis (programmed cell death).30
Warren, Hewitt, and Thomas describe how intrauterine alcohol exposure interferes with the function of L1CAM, the L1 cell-adhesion molecule.31 They noted that just one drink could interfere with the ability of L1CAM to mediate cell adhesion and axonal growth. Prenatal alcohol exposure is also thought to contribute to interference in neurotransmitter and N-methyl-D-aspartate receptor coupling, which may have potential therapeutic implications.32
Considerations in FAS identification and treatment
There is a potential to identify alcohol exposure in the womb. The majority of ingested alcohol is eventually converted to carbon dioxide and water in both maternal and fetal circulations, which has hampered the identification of biomarkers for clinical use in FAS. Fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, may prove to be such markers.33 FAEEs have been measured in a variety of tissues, including blood and meconium. FAEEs can be measured in both neonatal and maternal hair samples.
A study evaluating the utility of such testing in 324 at-risk pregnancies revealed 90% sensitivity and 90% specificity for identifying “excessive drinking” using a cutoff of 0.5 ng/mg.34
Research shows potential therapeutic approaches during pregnancy. While the use of biomarkers has the potential to assist with the identification of at-risk newborns, it merely identifies past alcohol use; it doesn’t necessarily permit identification and prevention of the known negative pediatric sequelae. Preliminary animal studies reveal the potential benefit of neuroprotective peptides to prevent brain damage in alcohol-exposed mice.35 Further research is ongoing.
Treatment: The earlier the better
Early diagnosis and a positive environment improve outcomes. It is well established that early intervention improves outcomes. One comprehensive review of 415 patients with FAS noted troubling outcomes in general for adolescents and adults.36 Over their life spans, the prevalence of such outcomes was:
- 61% for disrupted school experiences
- 60% for trouble with the law
- 50% for confinement (in detention, jail, prison, or a psychiatric or alcohol/drug inpatient setting)
- 49% for inappropriate sexual behaviors on repeated occasions
- 35% for alcohol/drug problems.
The odds of escaping these adverse life outcomes are increased up to fourfold when the individual receives a FAS or FASD diagnosis at an earlier age and is reared in a stable environment.36
Barrier to treatment: A mother’s guilt. One of the challenges I’ve learned from my sister-in-law is the stigma mothers face when they bring their child in for services once the diagnosis of FAS is suspected. While adoptive mothers obviously can’t be held accountable for the intrauterine environment to which a fetus is exposed, the same can’t be said of biologic mothers. Therefore, there is a real risk that a mother who is unwilling or unable to face the potentially devastating news that her baby’s issues might be related to choices she made during pregnancy, might not bring her child in for necessary assessment and treatment. Therefore, prevention is a key proponent of treatment.
Prevent FAS: Provide contraception, screen for alcohol use, intervene
While ObGyns aren’t likely to diagnose many children with FAS, we are in an excellent position to try to prevent this tragedy through our counseling of reproductive-aged women. I suspect that most obstetricians spend a considerable amount of time discussing much less frequent obstetric sequelae, such as listeriosis, in the prenatal care setting. Validated alcohol screening tools take moments to administer, and once patients who might have alcohol problems are identified, either a serious discussion about contraception or an honest discussion of FAS may be appropriate. There have been a number of screening tools developed.
The CAGE screen is frequently taught in medical schools, but it isn’t as sensitive for women or minorities.19
The T-ACE (Tolerance, Annoyed, Cut Down, Eye-opener) tool involves four questions that take less than 1 minute to administer (FIGURE 1).39
TWEAK is another potential tool identified by Russell and colleagues (Tolerance, Worry, Eye opener, Amnesia, and Cut down in drinking).39 Other methods utilized include an AUDIT screen and a CRAFFT screen.40 Regardless of which tool is utilized, screening is not time-consuming and is better than merely inquiring about alcohol consumption in general.

FIGURE 1 T-ACE validated alcohol screening tool
Source: American College of Obstetricians and Gynecologists. At risk drinking and illicit drug use: Ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–1460.
When alcohol use is found, intervene
Once patients with at-risk behavior are identified, obstetric staff should offer brief interventions to influence problem drinking. Miller and Sanchez summarized the key elements that were most successful in these programs with the acronym FRAMES: Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy (FIGURE 2).41 This approach has been formally evaluated in the CDC’s multisite pilot study entitled Project CHOICES.42
In this motivational intervention, sexually active, fertile women of reproductive age underwent up to four motivational counseling sessions and one visit to a provider. At 6 months, 69% of women reduced their risk for an alcohol-exposed pregnancy—although the women who drank the least amount had the greatest benefit, primarily by choosing effective contraception, but also by reducing alcohol intake.

FIGURE 2 FRAMES model to deliver brief interventions
Source: American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. Washington, DC: ACOG; 2006.
A single, brief intervention is effective in already-pregnant women. Chang and colleagues conducted a randomized trial of a single-session brief intervention given to pregnant women with positive T-ACE screens and their partners (FIGURE 3).43 Either the study nurse or physician participated in the intervention, and each single session took 25 minutes on average. The pregnant women with the highest level of alcohol use reduced their drinking the most, and this effect was even larger when their partners participated. Other studies of brief interventions showed similar benefits.44,45
Another study evaluating a brief intervention involving training of health-care providers to improve screening rates revealed improved detection and therapy among at-risk patients.46

FIGURE 3 Single session, 25-minute intervention for patients and their partners
Source: Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991–998.
FAS prevention begins with routine counseling and contraception
Although FAS is often thought of in relation to obstetric populations, appointments for preconception counseling or routine health maintenance among women of reproductive age are an essential tool in FAS prevention. As previously mentioned, since approximately half of all pregnancies in this country are unplanned, long-acting reversible contraception is widely available to facilitate improved family planning.
Other contraceptive options also should be discussed. ACOG has teamed up with the CDC to develop a phone app for providers to use at the patient’s bedside to assist with identification and treatment of women at risk for alcohol use during pregnancy.47
The stakes are high, it’s time to step up
As obstetricians, we are powerless to prevent many conditions—such as vasa previa, acute fatty liver of pregnancy, and amniotic band syndrome. FAS is 100% preventable.
There aren’t that many proven teratogens in our profession, and there are none that involve behavior that is more socially acceptable than alcohol consumption. It is time for our profession to encourage women to appreciate how small a percentage of one’s life is spent pregnant, how many more years there are to enjoy an occasional cocktail, and how very high the stakes are during this important period of their lives. Oh, how I wish someone had been able to communicate all of this to sweet Charlie’s biologic mother. I am so grateful he’s getting the exceptional care he’s getting and very optimistic regarding his future. I only hope others in his situation are given the same opportunities.
Prenatal counseling
Louise Wilkins-Haug, MD, PhD (January 2008)
Prevention of fetal alcohol syndrome requires routine screening of all women of reproductive age
We want to hear from you! Tell us what you think.
1. Baram M. Moms-to-be get mixed messages on drinking. ABC News. http://abcnews.go.com/Health/story?id=2654849&page=1#.UM9l-RyeARY. Published November 15 2006. Accessed December 14, 2012.
2. Royal College of Obstetricians and Gynaecologists. Alcohol consumption and the outcomes of pregnancy (RCOG Statement No. 5). London UK: Royal College of Obstetricians and Gynaecologists. January 3, 2006.
3. Pearson C. Alcohol during pregnancy: How dangerous is it really? The Huffington Post. http://www.huffingtonpost.com/2011/04/06/alcohol-during-pregnancy_n_845103.html. Published April 6 2011. Updated September 16, 2011. Accessed December 14, 2012.
4. Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66(1):41-48.Epub Oct 5, 2010.
5. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129-140.Epub Oct 30, 2008.
6. Zhou F. Fetal Alcohol Spectrum Disorders Study Group (FASDSG). Research Society on Alcoholism. http://rsoa.org/fas.html. Updated September 9 2010. Accessed December 14, 2012.
7. Kelly S, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143-149.
8. Vaglenova J, Petkov V. Fetal alcohol effects in rats exposed pre-and postnatally to a low dose of ethanol. Alcohol Clin Exp Res. 1998;22(3):697-703.
9. Schneider M, Moore C, Kraemer G. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25(9):1383-1392.
10. Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders. Data and statistics in the United States. http://www.cdc.gov/ncbddd/fasd/data.html. Updated August 16 2012. Accessed December 14, 2012.
11. Egeland G, Perham-Hestere KA, Gessner BD, Ingle D, Berner JE, Middaugh J. Fetal alcohol syndrome in Alaska 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Pub Health. 1998;88(5):781-786.
12. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267-1271.
13. Institute of Medicine. Fetal alcohol syndrome: diagnosis epidemiology, prevention, and treatment (1996). http://www.come-over.to/FAS/IOMsummary.htm. Accessed December 14, 2012.
14. Committee of Substance Abuse and Committee on Children with Disabilities. American Academy of Pediatrics. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358-361.
15. US Department of Health & Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. http://www.surgeongeneral.gov/news/2005/02/sg02222005.html. Published February 21 2005. Accessed December 13, 2012.
16. Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age–United States 2006–2010. MMWR. 2012;61(28):534-538.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6128a4.htm?s_cid=mm6128a4_w. Accessed December 17, 2012.
17. Landgren M, Svensson L, Stromland K, Gronlund M. Prenatal alcohol exposure and neurodvelopmental disorders in children adopted from Eastern Europe. Pediatrics. 2010;125(5):e1178-1185.doi:10.1542/peds.2009-0712.
18. American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. http://www.acog.org/~/media/Departments/Tobacco%20Alcohol%20and%20Substance%20Abuse/FASDToolKit.pdf?dmc=1&ts=20121217T1504384811. Published 2006. Accessed December 14 2012.
19. Anderson B, Dang E, Floyd R, Sokol R, Mahoney J, Schulkin J. Knowledge opinions, and practice patterns of obstetrician-gynecologist regarding their patients’ use of alcohol. J Addiction Med. 2010;4(2):114-121.
20. Abel EL, Kruger M. What do physicians know and say about fetal alcohol syndrome: a survey of obstetricians pediatricians, and family medicine physicians. Alcohol Clin Exp Res. 1998;22(9):1951-1954.
21. Diekman S, Floyd R, Decoufle P, Schulkin J, Ebrahim S, Sokol R. A survey of obstetrician-gynecologists on their patients’ alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756-763.
22. Strandberg-Larsen K, Gronboek M, Andersen A, Andersen P, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884-891.
23. Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30(6):1051-1059.
24. Richardson G, Ryan C, Willford J, Day N, Goldschmidt. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
25. Feldman H, Jones K, Lindsay S, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36(4):670-676.
26. O’Leary C, Nassar N, Kurinczuk J, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4):e843-850.doi:10.1542/peds.2010-0256.
27. Abel E, Sokol R. A revised conservative estimate of the incidence of FAS and its economic its impact. Alcohol Clin Exp Res. 1991;15(3):514-524.
28. Lupton C. The financial impact of fetal alcohol syndrome. Fetal Alcohol Spectrum Disorders Center for Excellence. www.fasdcenter.samhsa.gov/publications/cost.cfm. Accessed December 14 2012.
29. Bailey B, Sokol R. Prenatal alcohol exposure and miscarriage stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86-91.
30. Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75(5):393-403.
31. Warren K, Hewitt B, Thomas J. Fetal alcohol spectrum disorders: research challenges and opportunities. Alcohol Res Health. 2011;34(1):4-15.
32. Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Chamess ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133(2):381-390.
33. Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl estsers in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82(7):487-493.
34. Kulaga V, Pragst F, Fulga N, Koren G. Hair análisis of fatty acid esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261-266.
35. Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Rev. 2006;52(1):107-118.
36. Streissguth A, Bookstein F, Barr H, Sampson P, O’malley K, Young J. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228-238.
37. 19. Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Committee Opinion No. 496: At-risk drinking and alcohol dependence: Obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383-388.
38. Sokol R, Martier S, Ager J. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160(4):863-868.
39. Chan A, Pristach E, Weite J, Russell M. Use of the TWEAK test in screening for alcoholism/ heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17(6):1188-1192.
40. Floyd R, O’Connor M, Bertrand J, Sokol R. Reducing adverse outcomes from prenatal alcohol exposure: a clinical plan of action. Alcohol Clin Exp Res. 2006;30(8):1271-1275.
41. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard GS Nathan PE, eds. Alcohol use and misuse by young adults. Notre Dame, IN: University of Notre Dame Press; 1994:55–81.
42. Center for Disease Control and Prevention. Motivational intervention to reduce alcohol-exposed pregnancies—Florida Texas, and Virginia, 1997–2001. MMWR. 2003;52(19):441-444.
43. Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991-998.
44. Manwell L, Fleming M, Mundt M, Stauffacher E, Barry K. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24(10):1517-1524.
45. O’Connor M, Whaley S. Brief intervention for alcohol use by pregnant women. Am J Pub Health. 2007;97(2):252-258.
46. Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol Drugs. 2011;72(4):530-535.
47. American College of Obstetricians and Gynecologists. At-risk alcohol use screening and intervention. http://198.87.1.43/womenalcohol/index.html. Published 2011. Accessed December 16 2012.
Medical students learn early in their education that alcohol is a teratogen. Despite this widespread knowledge, many obstetricians counsel patients about the safety of low doses of alcohol in pregnancy.1 Indeed, the Royal College of Obstetricians and Gynaecologists’ position on this is, “while the safest approach may be to avoid any alcohol during pregnancy, it remains the case that there is no evidence of harm from low levels of alcohol consumption, defined as no more than one or two units of alcohol once or twice a week.”2
Like many providers, I was aware of this controversy, but it became truly personal when a beloved family member was diagnosed with fetal alcohol syndrome (FAS). In this paper, I will review some of the controversy regarding alcohol in pregnancy, highlight findings from the literature, provide tools for prevention, and identify new developments regarding this devastating, preventable condition.
Charlie
To know my nephew Charlie is to fall in love with my nephew Charlie. One of the happiest moments of my life was when I learned my brother and sister-in-law had adopted twins from Kazakhstan. When my little niece and nephew started their new life in the United States, certain medical issues seemed to merit additional attention. Although both were very small for their age and required significant nutritional support, Charlie seemed to be a bit more rambunctious and required additional supervision.
The children were fortunate enough to have incredibly loving, dedicated parents, who have access to exceptional medical care as residents of Philadelphia, Pennsylvania. After extensive testing, it became clear what was causing Charlie’s developmental delay; his pediatric team made the diagnosis of FAS. My brother and sister-in-law became incredibly well-read about this challenging disorder, and threw themselves into national advocacy work to help prevent this unnecessary tragedy.
Recent data point to teratogenicity, but media confuse the issue
Some recent media coverage3 of celebrities who apparently drank while pregnant was in response to an article in the Journal of Epidemiology and Community Health.4 The authors of this study concluded that, “at age 5 years, cohort members born to mothers who drank up to one to two drinks per week or per occasion during pregnancy were not at increased risk of clinically relevant behavioral difficulties or cognitive deficits, compared with children of mothers in the not-in-pregnancy group.”
This is certainly not the first occasion the popular press has covered a published study that seems to indicate no ill effects of alcohol use in pregnancy. A 2008 report by Kelly and colleagues,5 and its subsequent media coverage, prompted the Fetal Alcohol Spectrum Disorders Study Group to state that the panel of experts was “alarmed” by recent newspaper reports suggesting that light drinking during pregnancy may be beneficial for an unborn child.6 They noted misleading and irresponsible media reports of the findings, which suggested that 3-year-old children whose mothers drank “lightly” during pregnancy were not at risk for certain behavioral problems.
What the study authors proceeded to note, however (that the media did not mention), was that the light drinkers in their study had socioeconomic advantages, compared with nondrinkers.5 (Advantaged economic status is established to be beneficial for childhood development.) They also noted that the study involved preschool-aged children, stating “Generally the adverse effects of light drinking during pregnancy are subtle and may go undetected in young children. However, other group studies of more moderate or ‘social’ drinking levels during pregnancy have shown an adverse impact on multiple aspects of development through adolescence and young adulthood, even when important environmental factors are taken into account.” A sentence I thought was most compelling in their statement was, “It is an inconvenient fact of life that alcohol is a teratogen.” Now, this fact is well supported in the literature.7
There are animal studies regarding the use of “low-dose” or “moderate” alcohol in pregnancy that demonstrate adverse behavioral outcomes with exposure to even small doses of alcohol.8,9 It is an American tragedy that, according to the Centers for Disease Control and Prevention (CDC), rates of FAS in this country range from 0.2 to 2.0 cases per 1,000 live births. Indeed, the rates of fetal alcohol spectrum disorders (FASD) might be at least three times this rate.10 As is the case with other disorders, there are health disparities regarding the prevalence of this condition as well.11
FAS: A long history of preventable disease
1973: Identified. FAS was first described in a 1973 Lancet report, “Pattern of malformation in offspring of chronic alcoholic mothers.”12
1996: Call for prevention. In 1995, the US Surgeon General issued a statement regarding alcohol use in pregnancy, noting, “We do not know what, if any, amount of alcohol is safe.”13 In 1996, the Institute of Medicine released a paper calling FAS and FASD “completely preventable birth defects and neurodevelopmental abnormalities.”14
2000: The troubling effects gathered. The American Academy of Pediatrics (AAP) published a monograph on FAS in 2000, defining it as a constellation of physical, behavioral, and cognitive abnormalities.15
These features classically define FAS:
- dysmorphic facial features
- prenatal and postnatal growth abnormalities
- mental retardation.
Approximately 80% of children with this condition have:
- microcephaly
- behavioral abnormalities.
As many as 50% of affected children also exhibit:
- poor coordination
- hypotonia
- attention-deficit hyperactivity disorder
- decreased adipose tissue
- identifiable facial anomalies (such as maxillary hypoplasia, cleft palate, and micrognathia).
Also common:
- cardiac defects
- hemangiomas
- eye or ear abnormalities.
The AAP further noted that data current to the time (and still true today) did not support the concept of a safe level of alcohol consumption by pregnant women below which no damage to a fetus will occur.15

Alcohol intake during pregnancy puts the fetus at risk for cognitive and neuropsychological impairment and physical abnormalities, including dysmorphic facial features (such as micrognathia), restricted prenatal growth, cardiac defects, and eye and ear abnormalities. There is no threshold dose of alcohol that is safe during pregnancy, according to the American College of Obstetricians and Gynecologists.
Despite the knowledge we’ve gained, FAS persists
According to a 2006–2010 CDC analysis involving more than 345,000 women of reproductive age from all 50 states, 7.6% of pregnant women reported alcohol use and 1.4% (or 1 in 71) reported binge drinking (defined, respectively, as at least one alcoholic drink and four or more alcoholic drinks on one occasion in the past 30 days).16 The highest prevalence of obstetric alcohol consumption occurs in women who are:
- aged 35 to 44 years
- white
- college graduates
- employed.
The problem may be bigger than reported. The incidences of alcohol and binge drinking found in the CDC report include women’s self-report—but women drink alcohol without knowing they’re pregnant. Only 40% of women realize they’re pregnant at 4 weeks of gestation, a critical time for organogenesis, and approximately half of all births are unplanned.9
When my brother and sister-in law adopted my beautiful niece and nephew, they were very aware of the risk for conditions like FAS. In an evaluation of 71 children adopted from Eastern Europe at 5 years of age, FAS was diagnosed in 30% of children and “partial FAS” in another 9%.17 Birth defects attributed to alcohol were present in 11% of the children.
Are women’s health providers up to date on FAS education?
In recognition of alcohol’s potentially life-altering consequences for the developing fetus, the American College of Obstetricians and Gynecologists (ACOG) produced an FASD prevention tool kit in 2006 and published a 2011 committee opinion on at-risk drinking and alcohol dependence and their implications for obstetrics and gynecology.18,19 Both guidelines direct clinicians to advise patients to abstain from alcohol during pregnancy.
Results from a 2010 survey of 800 ACOG fellows revealed that only 78% of obstetricians advised abstinence from alcohol during pregnancy. Fifty-eight percent of respondents did not use a validated screening tool for alcohol use in their pregnant patients, and only 72% felt prepared to screen for risky or hazardous drinking.19 (Most were unaware of the ACOG tool kit, which had been published several years earlier.)
In a survey of pediatricians, obstetricians, and family physicians, clinicians said that about 67% of their patients asked about alcohol use in pregnancy, with about 2% of those patients specifically mentioning FAS. About 41% of these same physicians erroneously placed the threshold for FAS at one to three drinks per day,20 when in fact there is no threshold of drinking that has been proven to be safe.
A survey of 1,000 actively practicing ACOG fellows revealed that, while 97% of obstetricians routinely asked their patients about alcohol use, only 20% of providers reported to their patients that abstinence was safest, and 4% of providers didn’t believe that consumption of eight or more drinks weekly posed fetal risk.21
How can we educate our patients about the dangers of alcohol in pregnancy?
Fetal death. A recent Danish study of 79,216 pregnant women revealed that 45% had consumed some alcohol during pregnancy. Two percent reported at least four drinks per week, and 25% admitted to binge drinking during pregnancy. Term infants born to women in the latter two groups had increased neonatal mortality, with hazard ratios of 3.56 (95% confidence interval [CI], 1.15–8.43) and 2.69 (95% CI, 1.27–5.69), respectively.22
Decreased cognitive status. A study by Willford and colleagues evaluated the relationship between prenatal alcohol exposure and cognitive status of 1,360 10-year-old children.23 The authors utilized the Stanford-Binet Intelligence Test, including the composite scores and verbal, abstract/visual, quantitative, and short-term memory scores. After controlling for other variables, among African American offspring they found that, for each additional drink, the average composite score decreased by 1.9 points. This difference was more striking for second-trimester use, and was significant even for one drink daily versus abstention from alcohol.
Impaired neuropsychological development. Another study evaluating light to moderate amounts of prenatal alcohol exposure in 10- and 11-year-old children found significantly worse scores regarding a number of neuropsychological developmental assessments.24
No threshold dose of causation. Results of a 2012 prospective study in California, with data collected on 992 subjects from 1978 until 2005, revealed that many physical FAS features, including microcephaly, smooth philtrum, and thin vermillion border; reduced birth length; and reduced birth weight, were associated with alcohol exposure at specific gestational ages, and were dose-related.25 This paper didn’t reveal any evidence of a threshold dose of causation.
Neurobehavioral outcomes of FAS are not always considered
Another recent study that the media recently highlighted as finding “no association between low or moderate prenatal alcohol exposure and birth defects” was by O’Leary and colleagues.26 Like other similarly limited studies, this one involved only children younger than 6 years and didn’t assess any of the important neurobehavioral outcomes of FAS.
FAS encompasses much more than visible birth defects. As the aforementioned ACOG tool kit stated, “For every child born with FAS, many more children are born with neurobehavioral deficits caused by alcohol exposure but without the physical characteristics of FAS.”
The costs of FAS are felt with dollars, too
The financial cost to our nation is extraordinary. In 1991, Abel and Sokol estimated the incremental annual cost of treating FAS at nearly $75 million, with about three-quarters of that cost associated with FAS cases involving mental retardation.27
A 2002 assessment estimated the lifetime cost for each individual with FAS (adjusting for the change in the cost of medical care services, lost productivity, and inflation) at $2 million. This figure consists of $1.6 million for medical treatment, special education, and residential care for persons with mental retardation, and $0.4 million for productivity losses.28
Where human studies fall short, animal studies can help elucidate causation
Unquestionably, there are flaws in the existing literature on the causation of FAS. Many studies rely on self-reporting by pregnant women, and underreporting in these cases is a real concern. There often are other confounders potentially negatively affecting fetal development, making it difficult to differentiate causation. The animal studies that don’t share these limitations do suggest a causal relationship between antenatal alcohol exposure and poor obstetric outcomes, however.29 These studies suggest mechanisms such as altered gene expression, oxidative stress, and apoptosis (programmed cell death).30
Warren, Hewitt, and Thomas describe how intrauterine alcohol exposure interferes with the function of L1CAM, the L1 cell-adhesion molecule.31 They noted that just one drink could interfere with the ability of L1CAM to mediate cell adhesion and axonal growth. Prenatal alcohol exposure is also thought to contribute to interference in neurotransmitter and N-methyl-D-aspartate receptor coupling, which may have potential therapeutic implications.32
Considerations in FAS identification and treatment
There is a potential to identify alcohol exposure in the womb. The majority of ingested alcohol is eventually converted to carbon dioxide and water in both maternal and fetal circulations, which has hampered the identification of biomarkers for clinical use in FAS. Fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, may prove to be such markers.33 FAEEs have been measured in a variety of tissues, including blood and meconium. FAEEs can be measured in both neonatal and maternal hair samples.
A study evaluating the utility of such testing in 324 at-risk pregnancies revealed 90% sensitivity and 90% specificity for identifying “excessive drinking” using a cutoff of 0.5 ng/mg.34
Research shows potential therapeutic approaches during pregnancy. While the use of biomarkers has the potential to assist with the identification of at-risk newborns, it merely identifies past alcohol use; it doesn’t necessarily permit identification and prevention of the known negative pediatric sequelae. Preliminary animal studies reveal the potential benefit of neuroprotective peptides to prevent brain damage in alcohol-exposed mice.35 Further research is ongoing.
Treatment: The earlier the better
Early diagnosis and a positive environment improve outcomes. It is well established that early intervention improves outcomes. One comprehensive review of 415 patients with FAS noted troubling outcomes in general for adolescents and adults.36 Over their life spans, the prevalence of such outcomes was:
- 61% for disrupted school experiences
- 60% for trouble with the law
- 50% for confinement (in detention, jail, prison, or a psychiatric or alcohol/drug inpatient setting)
- 49% for inappropriate sexual behaviors on repeated occasions
- 35% for alcohol/drug problems.
The odds of escaping these adverse life outcomes are increased up to fourfold when the individual receives a FAS or FASD diagnosis at an earlier age and is reared in a stable environment.36
Barrier to treatment: A mother’s guilt. One of the challenges I’ve learned from my sister-in-law is the stigma mothers face when they bring their child in for services once the diagnosis of FAS is suspected. While adoptive mothers obviously can’t be held accountable for the intrauterine environment to which a fetus is exposed, the same can’t be said of biologic mothers. Therefore, there is a real risk that a mother who is unwilling or unable to face the potentially devastating news that her baby’s issues might be related to choices she made during pregnancy, might not bring her child in for necessary assessment and treatment. Therefore, prevention is a key proponent of treatment.
Prevent FAS: Provide contraception, screen for alcohol use, intervene
While ObGyns aren’t likely to diagnose many children with FAS, we are in an excellent position to try to prevent this tragedy through our counseling of reproductive-aged women. I suspect that most obstetricians spend a considerable amount of time discussing much less frequent obstetric sequelae, such as listeriosis, in the prenatal care setting. Validated alcohol screening tools take moments to administer, and once patients who might have alcohol problems are identified, either a serious discussion about contraception or an honest discussion of FAS may be appropriate. There have been a number of screening tools developed.
The CAGE screen is frequently taught in medical schools, but it isn’t as sensitive for women or minorities.19
The T-ACE (Tolerance, Annoyed, Cut Down, Eye-opener) tool involves four questions that take less than 1 minute to administer (FIGURE 1).39
TWEAK is another potential tool identified by Russell and colleagues (Tolerance, Worry, Eye opener, Amnesia, and Cut down in drinking).39 Other methods utilized include an AUDIT screen and a CRAFFT screen.40 Regardless of which tool is utilized, screening is not time-consuming and is better than merely inquiring about alcohol consumption in general.

FIGURE 1 T-ACE validated alcohol screening tool
Source: American College of Obstetricians and Gynecologists. At risk drinking and illicit drug use: Ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–1460.
When alcohol use is found, intervene
Once patients with at-risk behavior are identified, obstetric staff should offer brief interventions to influence problem drinking. Miller and Sanchez summarized the key elements that were most successful in these programs with the acronym FRAMES: Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy (FIGURE 2).41 This approach has been formally evaluated in the CDC’s multisite pilot study entitled Project CHOICES.42
In this motivational intervention, sexually active, fertile women of reproductive age underwent up to four motivational counseling sessions and one visit to a provider. At 6 months, 69% of women reduced their risk for an alcohol-exposed pregnancy—although the women who drank the least amount had the greatest benefit, primarily by choosing effective contraception, but also by reducing alcohol intake.

FIGURE 2 FRAMES model to deliver brief interventions
Source: American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. Washington, DC: ACOG; 2006.
A single, brief intervention is effective in already-pregnant women. Chang and colleagues conducted a randomized trial of a single-session brief intervention given to pregnant women with positive T-ACE screens and their partners (FIGURE 3).43 Either the study nurse or physician participated in the intervention, and each single session took 25 minutes on average. The pregnant women with the highest level of alcohol use reduced their drinking the most, and this effect was even larger when their partners participated. Other studies of brief interventions showed similar benefits.44,45
Another study evaluating a brief intervention involving training of health-care providers to improve screening rates revealed improved detection and therapy among at-risk patients.46

FIGURE 3 Single session, 25-minute intervention for patients and their partners
Source: Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991–998.
FAS prevention begins with routine counseling and contraception
Although FAS is often thought of in relation to obstetric populations, appointments for preconception counseling or routine health maintenance among women of reproductive age are an essential tool in FAS prevention. As previously mentioned, since approximately half of all pregnancies in this country are unplanned, long-acting reversible contraception is widely available to facilitate improved family planning.
Other contraceptive options also should be discussed. ACOG has teamed up with the CDC to develop a phone app for providers to use at the patient’s bedside to assist with identification and treatment of women at risk for alcohol use during pregnancy.47
The stakes are high, it’s time to step up
As obstetricians, we are powerless to prevent many conditions—such as vasa previa, acute fatty liver of pregnancy, and amniotic band syndrome. FAS is 100% preventable.
There aren’t that many proven teratogens in our profession, and there are none that involve behavior that is more socially acceptable than alcohol consumption. It is time for our profession to encourage women to appreciate how small a percentage of one’s life is spent pregnant, how many more years there are to enjoy an occasional cocktail, and how very high the stakes are during this important period of their lives. Oh, how I wish someone had been able to communicate all of this to sweet Charlie’s biologic mother. I am so grateful he’s getting the exceptional care he’s getting and very optimistic regarding his future. I only hope others in his situation are given the same opportunities.
Prenatal counseling
Louise Wilkins-Haug, MD, PhD (January 2008)
Prevention of fetal alcohol syndrome requires routine screening of all women of reproductive age
We want to hear from you! Tell us what you think.
Medical students learn early in their education that alcohol is a teratogen. Despite this widespread knowledge, many obstetricians counsel patients about the safety of low doses of alcohol in pregnancy.1 Indeed, the Royal College of Obstetricians and Gynaecologists’ position on this is, “while the safest approach may be to avoid any alcohol during pregnancy, it remains the case that there is no evidence of harm from low levels of alcohol consumption, defined as no more than one or two units of alcohol once or twice a week.”2
Like many providers, I was aware of this controversy, but it became truly personal when a beloved family member was diagnosed with fetal alcohol syndrome (FAS). In this paper, I will review some of the controversy regarding alcohol in pregnancy, highlight findings from the literature, provide tools for prevention, and identify new developments regarding this devastating, preventable condition.
Charlie
To know my nephew Charlie is to fall in love with my nephew Charlie. One of the happiest moments of my life was when I learned my brother and sister-in-law had adopted twins from Kazakhstan. When my little niece and nephew started their new life in the United States, certain medical issues seemed to merit additional attention. Although both were very small for their age and required significant nutritional support, Charlie seemed to be a bit more rambunctious and required additional supervision.
The children were fortunate enough to have incredibly loving, dedicated parents, who have access to exceptional medical care as residents of Philadelphia, Pennsylvania. After extensive testing, it became clear what was causing Charlie’s developmental delay; his pediatric team made the diagnosis of FAS. My brother and sister-in-law became incredibly well-read about this challenging disorder, and threw themselves into national advocacy work to help prevent this unnecessary tragedy.
Recent data point to teratogenicity, but media confuse the issue
Some recent media coverage3 of celebrities who apparently drank while pregnant was in response to an article in the Journal of Epidemiology and Community Health.4 The authors of this study concluded that, “at age 5 years, cohort members born to mothers who drank up to one to two drinks per week or per occasion during pregnancy were not at increased risk of clinically relevant behavioral difficulties or cognitive deficits, compared with children of mothers in the not-in-pregnancy group.”
This is certainly not the first occasion the popular press has covered a published study that seems to indicate no ill effects of alcohol use in pregnancy. A 2008 report by Kelly and colleagues,5 and its subsequent media coverage, prompted the Fetal Alcohol Spectrum Disorders Study Group to state that the panel of experts was “alarmed” by recent newspaper reports suggesting that light drinking during pregnancy may be beneficial for an unborn child.6 They noted misleading and irresponsible media reports of the findings, which suggested that 3-year-old children whose mothers drank “lightly” during pregnancy were not at risk for certain behavioral problems.
What the study authors proceeded to note, however (that the media did not mention), was that the light drinkers in their study had socioeconomic advantages, compared with nondrinkers.5 (Advantaged economic status is established to be beneficial for childhood development.) They also noted that the study involved preschool-aged children, stating “Generally the adverse effects of light drinking during pregnancy are subtle and may go undetected in young children. However, other group studies of more moderate or ‘social’ drinking levels during pregnancy have shown an adverse impact on multiple aspects of development through adolescence and young adulthood, even when important environmental factors are taken into account.” A sentence I thought was most compelling in their statement was, “It is an inconvenient fact of life that alcohol is a teratogen.” Now, this fact is well supported in the literature.7
There are animal studies regarding the use of “low-dose” or “moderate” alcohol in pregnancy that demonstrate adverse behavioral outcomes with exposure to even small doses of alcohol.8,9 It is an American tragedy that, according to the Centers for Disease Control and Prevention (CDC), rates of FAS in this country range from 0.2 to 2.0 cases per 1,000 live births. Indeed, the rates of fetal alcohol spectrum disorders (FASD) might be at least three times this rate.10 As is the case with other disorders, there are health disparities regarding the prevalence of this condition as well.11
FAS: A long history of preventable disease
1973: Identified. FAS was first described in a 1973 Lancet report, “Pattern of malformation in offspring of chronic alcoholic mothers.”12
1996: Call for prevention. In 1995, the US Surgeon General issued a statement regarding alcohol use in pregnancy, noting, “We do not know what, if any, amount of alcohol is safe.”13 In 1996, the Institute of Medicine released a paper calling FAS and FASD “completely preventable birth defects and neurodevelopmental abnormalities.”14
2000: The troubling effects gathered. The American Academy of Pediatrics (AAP) published a monograph on FAS in 2000, defining it as a constellation of physical, behavioral, and cognitive abnormalities.15
These features classically define FAS:
- dysmorphic facial features
- prenatal and postnatal growth abnormalities
- mental retardation.
Approximately 80% of children with this condition have:
- microcephaly
- behavioral abnormalities.
As many as 50% of affected children also exhibit:
- poor coordination
- hypotonia
- attention-deficit hyperactivity disorder
- decreased adipose tissue
- identifiable facial anomalies (such as maxillary hypoplasia, cleft palate, and micrognathia).
Also common:
- cardiac defects
- hemangiomas
- eye or ear abnormalities.
The AAP further noted that data current to the time (and still true today) did not support the concept of a safe level of alcohol consumption by pregnant women below which no damage to a fetus will occur.15

Alcohol intake during pregnancy puts the fetus at risk for cognitive and neuropsychological impairment and physical abnormalities, including dysmorphic facial features (such as micrognathia), restricted prenatal growth, cardiac defects, and eye and ear abnormalities. There is no threshold dose of alcohol that is safe during pregnancy, according to the American College of Obstetricians and Gynecologists.
Despite the knowledge we’ve gained, FAS persists
According to a 2006–2010 CDC analysis involving more than 345,000 women of reproductive age from all 50 states, 7.6% of pregnant women reported alcohol use and 1.4% (or 1 in 71) reported binge drinking (defined, respectively, as at least one alcoholic drink and four or more alcoholic drinks on one occasion in the past 30 days).16 The highest prevalence of obstetric alcohol consumption occurs in women who are:
- aged 35 to 44 years
- white
- college graduates
- employed.
The problem may be bigger than reported. The incidences of alcohol and binge drinking found in the CDC report include women’s self-report—but women drink alcohol without knowing they’re pregnant. Only 40% of women realize they’re pregnant at 4 weeks of gestation, a critical time for organogenesis, and approximately half of all births are unplanned.9
When my brother and sister-in law adopted my beautiful niece and nephew, they were very aware of the risk for conditions like FAS. In an evaluation of 71 children adopted from Eastern Europe at 5 years of age, FAS was diagnosed in 30% of children and “partial FAS” in another 9%.17 Birth defects attributed to alcohol were present in 11% of the children.
Are women’s health providers up to date on FAS education?
In recognition of alcohol’s potentially life-altering consequences for the developing fetus, the American College of Obstetricians and Gynecologists (ACOG) produced an FASD prevention tool kit in 2006 and published a 2011 committee opinion on at-risk drinking and alcohol dependence and their implications for obstetrics and gynecology.18,19 Both guidelines direct clinicians to advise patients to abstain from alcohol during pregnancy.
Results from a 2010 survey of 800 ACOG fellows revealed that only 78% of obstetricians advised abstinence from alcohol during pregnancy. Fifty-eight percent of respondents did not use a validated screening tool for alcohol use in their pregnant patients, and only 72% felt prepared to screen for risky or hazardous drinking.19 (Most were unaware of the ACOG tool kit, which had been published several years earlier.)
In a survey of pediatricians, obstetricians, and family physicians, clinicians said that about 67% of their patients asked about alcohol use in pregnancy, with about 2% of those patients specifically mentioning FAS. About 41% of these same physicians erroneously placed the threshold for FAS at one to three drinks per day,20 when in fact there is no threshold of drinking that has been proven to be safe.
A survey of 1,000 actively practicing ACOG fellows revealed that, while 97% of obstetricians routinely asked their patients about alcohol use, only 20% of providers reported to their patients that abstinence was safest, and 4% of providers didn’t believe that consumption of eight or more drinks weekly posed fetal risk.21
How can we educate our patients about the dangers of alcohol in pregnancy?
Fetal death. A recent Danish study of 79,216 pregnant women revealed that 45% had consumed some alcohol during pregnancy. Two percent reported at least four drinks per week, and 25% admitted to binge drinking during pregnancy. Term infants born to women in the latter two groups had increased neonatal mortality, with hazard ratios of 3.56 (95% confidence interval [CI], 1.15–8.43) and 2.69 (95% CI, 1.27–5.69), respectively.22
Decreased cognitive status. A study by Willford and colleagues evaluated the relationship between prenatal alcohol exposure and cognitive status of 1,360 10-year-old children.23 The authors utilized the Stanford-Binet Intelligence Test, including the composite scores and verbal, abstract/visual, quantitative, and short-term memory scores. After controlling for other variables, among African American offspring they found that, for each additional drink, the average composite score decreased by 1.9 points. This difference was more striking for second-trimester use, and was significant even for one drink daily versus abstention from alcohol.
Impaired neuropsychological development. Another study evaluating light to moderate amounts of prenatal alcohol exposure in 10- and 11-year-old children found significantly worse scores regarding a number of neuropsychological developmental assessments.24
No threshold dose of causation. Results of a 2012 prospective study in California, with data collected on 992 subjects from 1978 until 2005, revealed that many physical FAS features, including microcephaly, smooth philtrum, and thin vermillion border; reduced birth length; and reduced birth weight, were associated with alcohol exposure at specific gestational ages, and were dose-related.25 This paper didn’t reveal any evidence of a threshold dose of causation.
Neurobehavioral outcomes of FAS are not always considered
Another recent study that the media recently highlighted as finding “no association between low or moderate prenatal alcohol exposure and birth defects” was by O’Leary and colleagues.26 Like other similarly limited studies, this one involved only children younger than 6 years and didn’t assess any of the important neurobehavioral outcomes of FAS.
FAS encompasses much more than visible birth defects. As the aforementioned ACOG tool kit stated, “For every child born with FAS, many more children are born with neurobehavioral deficits caused by alcohol exposure but without the physical characteristics of FAS.”
The costs of FAS are felt with dollars, too
The financial cost to our nation is extraordinary. In 1991, Abel and Sokol estimated the incremental annual cost of treating FAS at nearly $75 million, with about three-quarters of that cost associated with FAS cases involving mental retardation.27
A 2002 assessment estimated the lifetime cost for each individual with FAS (adjusting for the change in the cost of medical care services, lost productivity, and inflation) at $2 million. This figure consists of $1.6 million for medical treatment, special education, and residential care for persons with mental retardation, and $0.4 million for productivity losses.28
Where human studies fall short, animal studies can help elucidate causation
Unquestionably, there are flaws in the existing literature on the causation of FAS. Many studies rely on self-reporting by pregnant women, and underreporting in these cases is a real concern. There often are other confounders potentially negatively affecting fetal development, making it difficult to differentiate causation. The animal studies that don’t share these limitations do suggest a causal relationship between antenatal alcohol exposure and poor obstetric outcomes, however.29 These studies suggest mechanisms such as altered gene expression, oxidative stress, and apoptosis (programmed cell death).30
Warren, Hewitt, and Thomas describe how intrauterine alcohol exposure interferes with the function of L1CAM, the L1 cell-adhesion molecule.31 They noted that just one drink could interfere with the ability of L1CAM to mediate cell adhesion and axonal growth. Prenatal alcohol exposure is also thought to contribute to interference in neurotransmitter and N-methyl-D-aspartate receptor coupling, which may have potential therapeutic implications.32
Considerations in FAS identification and treatment
There is a potential to identify alcohol exposure in the womb. The majority of ingested alcohol is eventually converted to carbon dioxide and water in both maternal and fetal circulations, which has hampered the identification of biomarkers for clinical use in FAS. Fatty acid ethyl esters (FAEEs), nonoxidative metabolites of ethanol, may prove to be such markers.33 FAEEs have been measured in a variety of tissues, including blood and meconium. FAEEs can be measured in both neonatal and maternal hair samples.
A study evaluating the utility of such testing in 324 at-risk pregnancies revealed 90% sensitivity and 90% specificity for identifying “excessive drinking” using a cutoff of 0.5 ng/mg.34
Research shows potential therapeutic approaches during pregnancy. While the use of biomarkers has the potential to assist with the identification of at-risk newborns, it merely identifies past alcohol use; it doesn’t necessarily permit identification and prevention of the known negative pediatric sequelae. Preliminary animal studies reveal the potential benefit of neuroprotective peptides to prevent brain damage in alcohol-exposed mice.35 Further research is ongoing.
Treatment: The earlier the better
Early diagnosis and a positive environment improve outcomes. It is well established that early intervention improves outcomes. One comprehensive review of 415 patients with FAS noted troubling outcomes in general for adolescents and adults.36 Over their life spans, the prevalence of such outcomes was:
- 61% for disrupted school experiences
- 60% for trouble with the law
- 50% for confinement (in detention, jail, prison, or a psychiatric or alcohol/drug inpatient setting)
- 49% for inappropriate sexual behaviors on repeated occasions
- 35% for alcohol/drug problems.
The odds of escaping these adverse life outcomes are increased up to fourfold when the individual receives a FAS or FASD diagnosis at an earlier age and is reared in a stable environment.36
Barrier to treatment: A mother’s guilt. One of the challenges I’ve learned from my sister-in-law is the stigma mothers face when they bring their child in for services once the diagnosis of FAS is suspected. While adoptive mothers obviously can’t be held accountable for the intrauterine environment to which a fetus is exposed, the same can’t be said of biologic mothers. Therefore, there is a real risk that a mother who is unwilling or unable to face the potentially devastating news that her baby’s issues might be related to choices she made during pregnancy, might not bring her child in for necessary assessment and treatment. Therefore, prevention is a key proponent of treatment.
Prevent FAS: Provide contraception, screen for alcohol use, intervene
While ObGyns aren’t likely to diagnose many children with FAS, we are in an excellent position to try to prevent this tragedy through our counseling of reproductive-aged women. I suspect that most obstetricians spend a considerable amount of time discussing much less frequent obstetric sequelae, such as listeriosis, in the prenatal care setting. Validated alcohol screening tools take moments to administer, and once patients who might have alcohol problems are identified, either a serious discussion about contraception or an honest discussion of FAS may be appropriate. There have been a number of screening tools developed.
The CAGE screen is frequently taught in medical schools, but it isn’t as sensitive for women or minorities.19
The T-ACE (Tolerance, Annoyed, Cut Down, Eye-opener) tool involves four questions that take less than 1 minute to administer (FIGURE 1).39
TWEAK is another potential tool identified by Russell and colleagues (Tolerance, Worry, Eye opener, Amnesia, and Cut down in drinking).39 Other methods utilized include an AUDIT screen and a CRAFFT screen.40 Regardless of which tool is utilized, screening is not time-consuming and is better than merely inquiring about alcohol consumption in general.

FIGURE 1 T-ACE validated alcohol screening tool
Source: American College of Obstetricians and Gynecologists. At risk drinking and illicit drug use: Ethical issues in obstetric and gynecologic practice. Obstet Gynecol. 2008;112(6):1449–1460.
When alcohol use is found, intervene
Once patients with at-risk behavior are identified, obstetric staff should offer brief interventions to influence problem drinking. Miller and Sanchez summarized the key elements that were most successful in these programs with the acronym FRAMES: Feedback, Responsibility, Advice, Menu, Empathy, Self-efficacy (FIGURE 2).41 This approach has been formally evaluated in the CDC’s multisite pilot study entitled Project CHOICES.42
In this motivational intervention, sexually active, fertile women of reproductive age underwent up to four motivational counseling sessions and one visit to a provider. At 6 months, 69% of women reduced their risk for an alcohol-exposed pregnancy—although the women who drank the least amount had the greatest benefit, primarily by choosing effective contraception, but also by reducing alcohol intake.

FIGURE 2 FRAMES model to deliver brief interventions
Source: American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. Washington, DC: ACOG; 2006.
A single, brief intervention is effective in already-pregnant women. Chang and colleagues conducted a randomized trial of a single-session brief intervention given to pregnant women with positive T-ACE screens and their partners (FIGURE 3).43 Either the study nurse or physician participated in the intervention, and each single session took 25 minutes on average. The pregnant women with the highest level of alcohol use reduced their drinking the most, and this effect was even larger when their partners participated. Other studies of brief interventions showed similar benefits.44,45
Another study evaluating a brief intervention involving training of health-care providers to improve screening rates revealed improved detection and therapy among at-risk patients.46

FIGURE 3 Single session, 25-minute intervention for patients and their partners
Source: Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991–998.
FAS prevention begins with routine counseling and contraception
Although FAS is often thought of in relation to obstetric populations, appointments for preconception counseling or routine health maintenance among women of reproductive age are an essential tool in FAS prevention. As previously mentioned, since approximately half of all pregnancies in this country are unplanned, long-acting reversible contraception is widely available to facilitate improved family planning.
Other contraceptive options also should be discussed. ACOG has teamed up with the CDC to develop a phone app for providers to use at the patient’s bedside to assist with identification and treatment of women at risk for alcohol use during pregnancy.47
The stakes are high, it’s time to step up
As obstetricians, we are powerless to prevent many conditions—such as vasa previa, acute fatty liver of pregnancy, and amniotic band syndrome. FAS is 100% preventable.
There aren’t that many proven teratogens in our profession, and there are none that involve behavior that is more socially acceptable than alcohol consumption. It is time for our profession to encourage women to appreciate how small a percentage of one’s life is spent pregnant, how many more years there are to enjoy an occasional cocktail, and how very high the stakes are during this important period of their lives. Oh, how I wish someone had been able to communicate all of this to sweet Charlie’s biologic mother. I am so grateful he’s getting the exceptional care he’s getting and very optimistic regarding his future. I only hope others in his situation are given the same opportunities.
Prenatal counseling
Louise Wilkins-Haug, MD, PhD (January 2008)
Prevention of fetal alcohol syndrome requires routine screening of all women of reproductive age
We want to hear from you! Tell us what you think.
1. Baram M. Moms-to-be get mixed messages on drinking. ABC News. http://abcnews.go.com/Health/story?id=2654849&page=1#.UM9l-RyeARY. Published November 15 2006. Accessed December 14, 2012.
2. Royal College of Obstetricians and Gynaecologists. Alcohol consumption and the outcomes of pregnancy (RCOG Statement No. 5). London UK: Royal College of Obstetricians and Gynaecologists. January 3, 2006.
3. Pearson C. Alcohol during pregnancy: How dangerous is it really? The Huffington Post. http://www.huffingtonpost.com/2011/04/06/alcohol-during-pregnancy_n_845103.html. Published April 6 2011. Updated September 16, 2011. Accessed December 14, 2012.
4. Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66(1):41-48.Epub Oct 5, 2010.
5. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129-140.Epub Oct 30, 2008.
6. Zhou F. Fetal Alcohol Spectrum Disorders Study Group (FASDSG). Research Society on Alcoholism. http://rsoa.org/fas.html. Updated September 9 2010. Accessed December 14, 2012.
7. Kelly S, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143-149.
8. Vaglenova J, Petkov V. Fetal alcohol effects in rats exposed pre-and postnatally to a low dose of ethanol. Alcohol Clin Exp Res. 1998;22(3):697-703.
9. Schneider M, Moore C, Kraemer G. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25(9):1383-1392.
10. Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders. Data and statistics in the United States. http://www.cdc.gov/ncbddd/fasd/data.html. Updated August 16 2012. Accessed December 14, 2012.
11. Egeland G, Perham-Hestere KA, Gessner BD, Ingle D, Berner JE, Middaugh J. Fetal alcohol syndrome in Alaska 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Pub Health. 1998;88(5):781-786.
12. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267-1271.
13. Institute of Medicine. Fetal alcohol syndrome: diagnosis epidemiology, prevention, and treatment (1996). http://www.come-over.to/FAS/IOMsummary.htm. Accessed December 14, 2012.
14. Committee of Substance Abuse and Committee on Children with Disabilities. American Academy of Pediatrics. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358-361.
15. US Department of Health & Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. http://www.surgeongeneral.gov/news/2005/02/sg02222005.html. Published February 21 2005. Accessed December 13, 2012.
16. Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age–United States 2006–2010. MMWR. 2012;61(28):534-538.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6128a4.htm?s_cid=mm6128a4_w. Accessed December 17, 2012.
17. Landgren M, Svensson L, Stromland K, Gronlund M. Prenatal alcohol exposure and neurodvelopmental disorders in children adopted from Eastern Europe. Pediatrics. 2010;125(5):e1178-1185.doi:10.1542/peds.2009-0712.
18. American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. http://www.acog.org/~/media/Departments/Tobacco%20Alcohol%20and%20Substance%20Abuse/FASDToolKit.pdf?dmc=1&ts=20121217T1504384811. Published 2006. Accessed December 14 2012.
19. Anderson B, Dang E, Floyd R, Sokol R, Mahoney J, Schulkin J. Knowledge opinions, and practice patterns of obstetrician-gynecologist regarding their patients’ use of alcohol. J Addiction Med. 2010;4(2):114-121.
20. Abel EL, Kruger M. What do physicians know and say about fetal alcohol syndrome: a survey of obstetricians pediatricians, and family medicine physicians. Alcohol Clin Exp Res. 1998;22(9):1951-1954.
21. Diekman S, Floyd R, Decoufle P, Schulkin J, Ebrahim S, Sokol R. A survey of obstetrician-gynecologists on their patients’ alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756-763.
22. Strandberg-Larsen K, Gronboek M, Andersen A, Andersen P, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884-891.
23. Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30(6):1051-1059.
24. Richardson G, Ryan C, Willford J, Day N, Goldschmidt. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
25. Feldman H, Jones K, Lindsay S, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36(4):670-676.
26. O’Leary C, Nassar N, Kurinczuk J, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4):e843-850.doi:10.1542/peds.2010-0256.
27. Abel E, Sokol R. A revised conservative estimate of the incidence of FAS and its economic its impact. Alcohol Clin Exp Res. 1991;15(3):514-524.
28. Lupton C. The financial impact of fetal alcohol syndrome. Fetal Alcohol Spectrum Disorders Center for Excellence. www.fasdcenter.samhsa.gov/publications/cost.cfm. Accessed December 14 2012.
29. Bailey B, Sokol R. Prenatal alcohol exposure and miscarriage stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86-91.
30. Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75(5):393-403.
31. Warren K, Hewitt B, Thomas J. Fetal alcohol spectrum disorders: research challenges and opportunities. Alcohol Res Health. 2011;34(1):4-15.
32. Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Chamess ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133(2):381-390.
33. Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl estsers in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82(7):487-493.
34. Kulaga V, Pragst F, Fulga N, Koren G. Hair análisis of fatty acid esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261-266.
35. Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Rev. 2006;52(1):107-118.
36. Streissguth A, Bookstein F, Barr H, Sampson P, O’malley K, Young J. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228-238.
37. 19. Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Committee Opinion No. 496: At-risk drinking and alcohol dependence: Obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383-388.
38. Sokol R, Martier S, Ager J. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160(4):863-868.
39. Chan A, Pristach E, Weite J, Russell M. Use of the TWEAK test in screening for alcoholism/ heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17(6):1188-1192.
40. Floyd R, O’Connor M, Bertrand J, Sokol R. Reducing adverse outcomes from prenatal alcohol exposure: a clinical plan of action. Alcohol Clin Exp Res. 2006;30(8):1271-1275.
41. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard GS Nathan PE, eds. Alcohol use and misuse by young adults. Notre Dame, IN: University of Notre Dame Press; 1994:55–81.
42. Center for Disease Control and Prevention. Motivational intervention to reduce alcohol-exposed pregnancies—Florida Texas, and Virginia, 1997–2001. MMWR. 2003;52(19):441-444.
43. Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991-998.
44. Manwell L, Fleming M, Mundt M, Stauffacher E, Barry K. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24(10):1517-1524.
45. O’Connor M, Whaley S. Brief intervention for alcohol use by pregnant women. Am J Pub Health. 2007;97(2):252-258.
46. Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol Drugs. 2011;72(4):530-535.
47. American College of Obstetricians and Gynecologists. At-risk alcohol use screening and intervention. http://198.87.1.43/womenalcohol/index.html. Published 2011. Accessed December 16 2012.
1. Baram M. Moms-to-be get mixed messages on drinking. ABC News. http://abcnews.go.com/Health/story?id=2654849&page=1#.UM9l-RyeARY. Published November 15 2006. Accessed December 14, 2012.
2. Royal College of Obstetricians and Gynaecologists. Alcohol consumption and the outcomes of pregnancy (RCOG Statement No. 5). London UK: Royal College of Obstetricians and Gynaecologists. January 3, 2006.
3. Pearson C. Alcohol during pregnancy: How dangerous is it really? The Huffington Post. http://www.huffingtonpost.com/2011/04/06/alcohol-during-pregnancy_n_845103.html. Published April 6 2011. Updated September 16, 2011. Accessed December 14, 2012.
4. Kelly YJ, Sacker A, Gray R, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Community Health. 2012;66(1):41-48.Epub Oct 5, 2010.
5. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. 2009;38(1):129-140.Epub Oct 30, 2008.
6. Zhou F. Fetal Alcohol Spectrum Disorders Study Group (FASDSG). Research Society on Alcoholism. http://rsoa.org/fas.html. Updated September 9 2010. Accessed December 14, 2012.
7. Kelly S, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22(2):143-149.
8. Vaglenova J, Petkov V. Fetal alcohol effects in rats exposed pre-and postnatally to a low dose of ethanol. Alcohol Clin Exp Res. 1998;22(3):697-703.
9. Schneider M, Moore C, Kraemer G. Moderate alcohol during pregnancy: learning and behavior in adolescent rhesus monkeys. Alcohol Clin Exp Res. 2001;25(9):1383-1392.
10. Centers for Disease Control and Prevention. Fetal alcohol spectrum disorders. Data and statistics in the United States. http://www.cdc.gov/ncbddd/fasd/data.html. Updated August 16 2012. Accessed December 14, 2012.
11. Egeland G, Perham-Hestere KA, Gessner BD, Ingle D, Berner JE, Middaugh J. Fetal alcohol syndrome in Alaska 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Pub Health. 1998;88(5):781-786.
12. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267-1271.
13. Institute of Medicine. Fetal alcohol syndrome: diagnosis epidemiology, prevention, and treatment (1996). http://www.come-over.to/FAS/IOMsummary.htm. Accessed December 14, 2012.
14. Committee of Substance Abuse and Committee on Children with Disabilities. American Academy of Pediatrics. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics. 2000;106(2):358-361.
15. US Department of Health & Human Services. US Surgeon General releases advisory on alcohol use in pregnancy. http://www.surgeongeneral.gov/news/2005/02/sg02222005.html. Published February 21 2005. Accessed December 13, 2012.
16. Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age–United States 2006–2010. MMWR. 2012;61(28):534-538.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6128a4.htm?s_cid=mm6128a4_w. Accessed December 17, 2012.
17. Landgren M, Svensson L, Stromland K, Gronlund M. Prenatal alcohol exposure and neurodvelopmental disorders in children adopted from Eastern Europe. Pediatrics. 2010;125(5):e1178-1185.doi:10.1542/peds.2009-0712.
18. American College of Obstetricians and Gynecologists. Drinking and reproductive health: A fetal alcohol spectrum disorders prevention tool kit. http://www.acog.org/~/media/Departments/Tobacco%20Alcohol%20and%20Substance%20Abuse/FASDToolKit.pdf?dmc=1&ts=20121217T1504384811. Published 2006. Accessed December 14 2012.
19. Anderson B, Dang E, Floyd R, Sokol R, Mahoney J, Schulkin J. Knowledge opinions, and practice patterns of obstetrician-gynecologist regarding their patients’ use of alcohol. J Addiction Med. 2010;4(2):114-121.
20. Abel EL, Kruger M. What do physicians know and say about fetal alcohol syndrome: a survey of obstetricians pediatricians, and family medicine physicians. Alcohol Clin Exp Res. 1998;22(9):1951-1954.
21. Diekman S, Floyd R, Decoufle P, Schulkin J, Ebrahim S, Sokol R. A survey of obstetrician-gynecologists on their patients’ alcohol use during pregnancy. Obstet Gynecol. 2000;95(5):756-763.
22. Strandberg-Larsen K, Gronboek M, Andersen A, Andersen P, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology. 2009;20(6):884-891.
23. Willford J, Leech S, Day N. Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res. 2006;30(6):1051-1059.
24. Richardson G, Ryan C, Willford J, Day N, Goldschmidt. Prenatal alcohol and marijuana exposure: Effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309-320.
25. Feldman H, Jones K, Lindsay S, et al. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: a prospective study. Alcohol Clin Exp Res. 2012;36(4):670-676.
26. O’Leary C, Nassar N, Kurinczuk J, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4):e843-850.doi:10.1542/peds.2010-0256.
27. Abel E, Sokol R. A revised conservative estimate of the incidence of FAS and its economic its impact. Alcohol Clin Exp Res. 1991;15(3):514-524.
28. Lupton C. The financial impact of fetal alcohol syndrome. Fetal Alcohol Spectrum Disorders Center for Excellence. www.fasdcenter.samhsa.gov/publications/cost.cfm. Accessed December 14 2012.
29. Bailey B, Sokol R. Prenatal alcohol exposure and miscarriage stillbirth, preterm delivery, and sudden infant death syndrome. Alcohol Res Health. 2011;34(1):86-91.
30. Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75(5):393-403.
31. Warren K, Hewitt B, Thomas J. Fetal alcohol spectrum disorders: research challenges and opportunities. Alcohol Res Health. 2011;34(1):4-15.
32. Ramanathan R, Wilkemeyer M, Mittal B, Perides G, Chamess ME. Alcohol inhibits cell-cell adhesion mediated by human L1. J Cell Biol. 1996;133(2):381-390.
33. Burd L, Hofer R. Biomarkers for detection of prenatal alcohol exposure: a critical review of fatty acid ethyl estsers in meconium. Birth Defects Res A Clin Mol Teratol. 2008;82(7):487-493.
34. Kulaga V, Pragst F, Fulga N, Koren G. Hair análisis of fatty acid esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261-266.
35. Sari Y, Gozes I. Brain deficits associated with fetal alcohol exposure may be protected in part, by peptides derived from activity-dependent neurotrophic factor and activity-dependent neuroprotective protein. Brain Res Rev. 2006;52(1):107-118.
36. Streissguth A, Bookstein F, Barr H, Sampson P, O’malley K, Young J. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J Dev Behav Pediatr. 2004;25(4):228-238.
37. 19. Committee on Health Care for Underserved Women. American College of Obstetricians and Gynecologists. Committee Opinion No. 496: At-risk drinking and alcohol dependence: Obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383-388.
38. Sokol R, Martier S, Ager J. The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gynecol. 1989;160(4):863-868.
39. Chan A, Pristach E, Weite J, Russell M. Use of the TWEAK test in screening for alcoholism/ heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17(6):1188-1192.
40. Floyd R, O’Connor M, Bertrand J, Sokol R. Reducing adverse outcomes from prenatal alcohol exposure: a clinical plan of action. Alcohol Clin Exp Res. 2006;30(8):1271-1275.
41. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard GS Nathan PE, eds. Alcohol use and misuse by young adults. Notre Dame, IN: University of Notre Dame Press; 1994:55–81.
42. Center for Disease Control and Prevention. Motivational intervention to reduce alcohol-exposed pregnancies—Florida Texas, and Virginia, 1997–2001. MMWR. 2003;52(19):441-444.
43. Chang G, McNamara T, Orav J, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):991-998.
44. Manwell L, Fleming M, Mundt M, Stauffacher E, Barry K. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcohol Clin Exp Res. 2000;24(10):1517-1524.
45. O’Connor M, Whaley S. Brief intervention for alcohol use by pregnant women. Am J Pub Health. 2007;97(2):252-258.
46. Mwansa-Kambafwile J, Rendall-Mkosi K, Jacobs R, Nel E, London L. Evaluation of a service provider short course for prevention of fetal alcohol syndrome. J Stud Alcohol Drugs. 2011;72(4):530-535.
47. American College of Obstetricians and Gynecologists. At-risk alcohol use screening and intervention. http://198.87.1.43/womenalcohol/index.html. Published 2011. Accessed December 16 2012.
Does home birth empower women, or imperil them and their babies?
The author reports no financial relationships relevant to this article.
Few issues in obstetrics spark as much controversy as home birth—and where controversy rages, media attention follows.
Press reports of a 2008 policy statement on home birth issued by the American Medical Association (AMA) and the American College of Obstetricians and Gynecologists (ACOG) highlight the rift between the formal medical establishment and advocates of home birth.1-3
On one side, the AMA and ACOG assert that the hospital or an accredited birthing center “is the safest setting for labor, delivery, and the immediate postpartum period.”1 On the other side, advocates of home birth argue that having the option adds to women’s empowerment and choice.
Some people have accused the medical community of trying to corner the “baby birthing industry.”4 The title of a recent Baltimore Sun article sums up this sentiment: “Home birth battle: Doctors strong-arm women away from healthy alternative to hospital care.”5
Neither ACOG nor the AMA advocates criminalization of home deliveries, but their statements on home birth have generated considerable fear that they will.
This article explores the controversy, focusing on the literature on home birth, gaps in knowledge, the state of regulation, liaison with midwives, and other issues. It also offers suggestions on how to discuss labor and delivery with patients so that they clearly understand the risks involved and do not feel that they have “failed” at meaningful childbirth when they choose hospital delivery.
Did a rise in hospital births reduce maternal mortality?
Obstetric care changed dramatically in the mid-20th century. In 1940, 55.8% of deliveries occurred in the hospital, but that percentage rose to 99.4 by 1970 and hasn’t changed appreciably since.6
Some proponents of hospital delivery note that, in 1940, when 44% of births occurred outside the hospital, the maternal mortality rate was 608 deaths for every 100,000 live births, compared with 37 deaths for every 100,000 live births in 1960, when fewer than 4% of deliveries occurred outside the hospital.6 And in 2003, with only 1% of deliveries occurring in a home setting, the maternal mortality rate was even lower: 12 deaths for every 100,000 live births.7
Others argue that this sharp decrease in maternal mortality cannot be attributed solely to the change in location of the delivery (and subsequent availability of services and personnel), but reflects universal advancement in safe practices such as aseptic technique.8
What do the data show? All studies of home birth have serious methodologic flaws, thanks largely to the nature of the subject matter. A recent Cochrane review observes that there is only one randomized, controlled trial—with a sample size of only 11 women—from which to draw conclusions.9 The review concludes that “there is no strong evidence to favour either home or hospital birth for selected, low-risk pregnant women.”10
Most data come from abroad
Much of the literature on home birth comes from international sites because of the higher prevalence of home delivery in other countries. These data reveal that:
- Two percent of deliveries in the United Kingdom occur in the home.11 The British National Institute for Health and Clinical Excellence recommended that all women be offered the option to have their baby at home or in the hospital, although, depending on the “trust” (a geographically based public-system cooperative that provides care), 8% to 76% of women weren’t given this choice formally.12
- One study conducted in Switzerland involved 489 women who opted for home birth and 385 who chose hospital birth. Of the former, 37 were referred to a specialist during pregnancy, and 70 were referred during labor. The groups had similar birth weights, gestational ages, and clinical conditions.13
- In the Netherlands, 30% of infants are born at home.14 If a woman has an uncomplicated pregnancy, she remains under midwifery care and can decide where to deliver. A study of 280,000 “low-risk” women under primary midwifery care found that 68.1% completed childbirth under that care, 3.6% were referred urgently, and 28.3% were referred without urgency.14 When referrals were considered as a whole, 11.2% involved urgency, primarily for fetal distress (50.2%) and postpartum hemorrhage (33%). Adverse neonatal outcomes were most common in urgently referred cases, followed by nonurgent referrals. The authors acknowledge the importance of transport time once a referral is initiated, stating that, “The Netherlands is a very densely populated country where the average distance to the hospital is relatively short.” (The same cannot be said of many parts of rural America.)
- A study involving home deliveries in Australia from 1985 to 1990 identified 50 perinatal deaths out of 7,002 planned home births.15 The perinatal death rate of infants weighing more than 2,500 g exceeded the national average (5.7 versus 3.6 for every 1,000 deliveries), with a relative risk (RR) of 1.6 (95% confidence interval [CI], 1.1–1.4). Intrapartum death not attributable to prematurity or fetal malformation was also higher (2.7 versus 0.9 for every 1,000 deliveries), with a RR of 3.0 (95% CI, 1.9–4.8). According to the authors, the main contributors to excess mortality were underestimation of the risks associated with post-term birth, twin pregnancy, and breech presentation, and a lack of response to fetal distress.
In the summer of 1999, a woman delivered a 7.7-lb infant after 42 weeks of gestation. The birth took place in the woman’s home in Japan, and the baby was delivered in a bathtub of warm water. The woman had had an uneventful pregnancy, and the baby appeared to be perfectly normal.
Four days later, the infant developed fever and jaundice and was admitted to the hospital, where she was treated with phototherapy. She improved, but her symptoms recurred 3 days later, and she began to vomit. Eight days after birth, she suffered cardiopulmonary arrest and died. An autopsy revealed the cause of death to be legionellosis—infection with Legionella pneumonia. The most likely source was the bathtub in which she was born.43
Other case reports describe similar tragedies associated with water birth (among them, drowning, infection, and a snapped umbilical cord), but no randomized, clinical trial has systematically compared delivery in water with conventional land-based birth.
The death, morbidity, and lack of data so troubled members of the American Academy of Pediatrics that the Committee on Fetus and Newborn issued an advisory in 2005:
- The safety and efficacy of underwater birth for the newborn has not been established. There is no convincing evidence of benefit to the neonate but some concern for serious harm. Therefore, underwater birth should be considered an experimental procedure that should not be performed except within the context of an appropriately designed randomized clinical trial after informed parental consent.44
This statement contrasts the conclusion of the most recent Cochrane review of the subject, which found that, “Immersion in water during the first stage of labour significantly reduces women’s perception of pain and use of epidural/spinal analgesia.”45 The review also noted, however, that, “No trials could be located that assessed the immersion of women in water during the third stage of labour.”45
No studies have explored immersion in water during the third stage of labor.
What’s in that water?
Amy Tuteur, MD, an ObGyn who publishes a popular blog (“The Skeptical OB”), focused on the topic of water birth earlier this year. “What’s in the water at waterbirth?” she asks.46
To answer the question, Dr. Tuteur cites a 1999 study of 4,030 deliveries in water, which found that 35 infants suffered serious morbidity and three died—although it is unclear if any of the deaths were a direct result of water birth. “However, of the 32 survivors who were admitted to the NICU,” writes Dr. Tuteur, “13 had significant respiratory problems, including pneumonia, meconium aspiration, water aspiration, and drowning. Other complications attributable to water birth include five babies who had significant hemorrhage due to snapped umbilical cord. In all, 18 babies had serious complications directly attributable to waterbirth.”47
Dr. Tuteur also points to the poor quality of the water in birthing pools, arguing that it is “essentially toilet water.”46 “The water in a birth pool, conveniently heated to body temperature, the optimum temperature for bacterial growth, is a microbial paradise,” she writes.46 She cites a study of 1,500 water births that included analysis of the water found in the birthing pools (before anyone entered the water) and identified:
- coliforms in 21% of samples
- enterococcus in 19% of samples
- Escherichia coli in 10% of samples
- Legionella pneumophila in 12% of samples
- Pseudomonas aeruginosa in 11% of samples.48
After a special water filter was installed, contamination diminished but did not disappear completely.
Pools in the home setting were not the only ones implicated in contamination; some hospital pools also were affected.
What’s the bottom line?
The American College of Obstetricians and Gynecologists has yet to weigh in on the matter. Until it does, ObGyns may be wise to heed the words of Ruth Gilbert, MD, of the Centre for Paediatric Epidemiology and Biostatistics at the Institute of Child Health in London.
“Can delivery in water cause serious adverse outcomes?” she asks, rhetorically, it turns out.
“Undoubtedly, the answer is ‘yes.’”49 —JANELLE YATES, SENIOR EDITOR
The data we do have are difficult to interpret
Among the limitations of studies of home birth are:
- lack of follow-up after the delivery
- varying definitions of perinatal mortality internationally
- lack of clarity regarding the identity and education of delivering providers
- the fact that there are often “too few neonatal deaths from which to extrapolate reliable rate calculations.”16
One meta-analysis found a rate of intrapartum transfer ranging from 7.4% to 16.5%, and a rate of primary cesarean delivery of 1.4% to 17.7% (it was 13.8% to 28.25% in the “comparison group”).16
A challenge inherent in many of these studies is identifying exactly what the comparison group is. In addition, some of the data are obtained from discharge summary records, which don’t always reflect the level of risk or acuity.
Oft-cited study has weaknesses
The study that many advocates of home birth cite was conducted in the United States and Canada and published in 2005.17 It evaluated “all 5,418 women expecting to deliver in 2000 supported by midwives with a common certification [certified professional midwives] and who planned to deliver at home when labour began.” The hospital transfer rate was 12.1%, in line with other studies. The risk of adverse outcomes was lower in the group that planned to have home delivery, compared with a “relatively low-risk hospital group.”
The study focused on:
- electronic fetal monitoring, used in 9.6% of deliveries in the home-birth group, versus 84.3% of the hospital group
- episiotomy, performed in 2.1% of home deliveries, compared with 33% of hospital births
- cesarean delivery, 3.7% of planned home deliveries, versus 19% of hospital births
- vacuum-assisted vaginal delivery, performed in 0.6% of planned home deliveries, versus 5.5% of hospital births
- neonatal death, at a rate of 2.0 deaths for every 1,000 intended home births. No comparison figure was cited.
One of the weaknesses of this study, as of others, was identification of a comparison group as a “low-risk” population without data to back up that designation. In addition, this study derived its data from birth certificates for 3,360,868 singleton, vertex births at 37 weeks or more of gestation. Data from birth certificates are limited as a basis for accurate risk assessment. Moreover, although the authors of this study asserted that they had no conflict of interest, the investigation was funded by The Foundation for the Advancement of Midwifery.
Study cited by advocates of hospital birth is also flawed
One of the studies many hospital and birthing center advocates cite was published in 2002.18 It involved an analysis of birth registry information on uncomplicated singleton pregnancies at 34 weeks or more of gestation in Washington state between 1989 and 1996. These pregnancies were either:
- delivered at home by a health professional (n=5,854)
- transferred to medical facilities after attempted home delivery (n=279)
- planned to be delivered in the hospital (n=10,593).
Infants whose mothers planned to deliver at home had a higher risk of neonatal death (RR, 1.99; 95% CI, 1.06–3.73) and a higher risk of having a 5-minute Apgar score of less than 3 (RR, 2.31; 95% CI, 1.29–4.16). After adjustment for a gestational-age cutoff of 37 weeks, these risks remained similar.
Nulliparous women, in particular, had a higher risk for prolonged labor (RR, 1.73; 95% CI, 1.28–2.34) and postpartum bleeding (RR, 2.76; 95% CI, 1.74–4.36).
The authors themselves point out a potential flaw in this study: the use of data from birth certificates. These data create “the potential for misclassifying unplanned home births as planned home births.” The difference in outcomes could be significant. For example, the neonatal death rate for unplanned home deliveries in North Carolina and Kentucky was 18 to 20 times higher than the rate for planned home births in these states.19,20
A study from Missouri observes that neonatal mortality was elevated for both planned and unplanned home birth, compared with physician-attended hospital birth.21
Selection bias is a concern
Selection bias is an inherent difficulty in many of these studies. Except for one previously mentioned paper—a very small study—none of the investigations involve randomization. As a result, we cannot exclude the possibility that “women who choose to deliver at home or in a birth center are likely to be different in terms of expectations and approach from women choosing to deliver in hospitals.”22
Risk level can escalate rapidly
What is potentially troubling about home birth is the fact that a low-risk pregnancy that was complication-free during antepartum care can become a high-risk pregnancy in a matter of minutes, necessitating urgent, appropriate obstetric care. Some classic examples of urgent events include cord prolapse, postpartum hemorrhage, bleeding from vasa previa, and shoulder dystocia.
Let’s focus on shoulder dystocia, which occurs in 1.4% of all vaginal deliveries. The authors of one study point out that “most of the traditional risk factors for shoulder dystocia have no predictive value, shoulder dystocia itself is an unpredictable event, and infants at risk for permanent injury are virtually impossible to predict.”23 This may make delivery in the home a high-risk endeavor because of the inability to mobilize an obstetric team to assist with shoulder dystocia maneuvers or perform a Zavanelli delivery.
Although the American College of Obstetricians and Gynecologists (ACOG) reiterated its opposition to home birth in early 2008, its stance on the matter has not shifted since 1979.50 In a news release describing that position, ACOG acknowledged “a woman’s right to make informed decisions regarding her delivery and to have a choice in choosing her health-care provider,” but made it clear that ACOG “does not support programs that advocate for, or individuals who provide, home births.”3
It emphasized its opposition pointedly, saying: “Choosing to deliver a baby at home…is to place the process of giving birth over the goal of having a healthy baby.”3
AMA resolution includes the reasoning behind the opposition
The American Medical Association (AMA) listed several variables that underscore the need for a clear-cut policy on home birth:
- the fact that 21 states “currently license midwives to attend home births, all using the certified professional midwife credential (CPM or ‘lay’ midwives), not the certified midwives (CM) credential which both the American College of Obstetricians and Gynecologists and American College of Nurse Midwives recognize”
- considerable media attention to celebrities who have given birth at home
- the fact that “an apparently uncomplicated pregnancy or delivery can quickly become very complicated in the setting of maternal hemorrhage, shoulder dystocia, eclampsia, or other obstetric emergencies.”1
Both ACOG and the AMA consider the following to fall within the category of “hospital”:
- a birthing center situated “within a hospital complex, that meets standards jointly outlined by the American Academy of Pediatrics and ACOG”
- “a freestanding birthing center that meets the standards of the Accreditation Association for Ambulatory Health Care, The Joint Commission, or the American Association of Birth Centers.”3
Another variable overlooked in most studies is the speed of transfer and the outcomes of pregnancies in which the women intended to deliver at home but ended up requiring urgent transfer. One study that did examine this scenario found that “women who had booked for a home birth, but later needed to transfer their care for a hospital birth, appeared to have the highest risk of intrapartum-related perinatal mortality.”24
There is also some controversy regarding the delivery of women who are pregnant with twins, who have a fetus in breech presentation, or who have a history of cesarean delivery. One study examined outcomes for intended home delivery of 57 women who had a prior abdominal delivery.25 Fifty of these women delivered vaginally in the home, and seven (12.3%) delivered in the hospital. One hospital transfer was urgent for fetal distress. One baby was stillborn, delivered at home.
Many policy makers decry the high prevalence of cesarean delivery in the United States and argue that providers who don’t perform this procedure offer a low-cost alternative for obstetric care.36 Some proponents of elective primary cesarean argue that it protects the perineum, but this issue is largely absent from the debate on home birth. Nor have I seen any study that addresses long-term outcomes in women who deliver at home, as most data collection ends after the delivery.
This oversight concerns me when I see interviews of midwives who doubt the existence of fetopelvic disproportion, who make statements such as, “You can get a baby through a knothole” and “I’ve never seen [a pelvis] that isn’t large enough.”37
If patients are encouraged to have a prolonged second stage of labor, does it have a harmful effect on their pelvic floor in later years? This important question merits further discussion.—ERIN E. TRACY, MD, MPH
EDITOR’S NOTE: See the related item, “ Award-winning video urges women to avoid cesarean delivery.”
A 10-year prospective study of vaginal birth after cesarean (VBAC) in birth centers found that more than 50% of uterine ruptures and 57% of perinatal deaths involved the 10% of women who had more than one prior cesarean delivery or who had reached a gestational age of more than 42 weeks.26
Skill of the caregiver is important
The training and qualifications of the obstetric care provider are incredibly important. One study evaluated 4,361 home births attended by “apprentice-trained midwives from 1970 to 1985 and 4,107 home births attended by family physicians from 1969 to 1981.”27 The perinatal mortality rate for the midwife-attended births was 14 for every 1,000 births, in contrast to the rate of 5 for every 1,000 physician-attended births.
Three types of midwife are credentialed in this country:
- certified nurse-midwife (CNM)
- certified midwife (CM)
- certified professional midwife (CPM).
The first two categories are certified by the American Midwifery Certification Board (AMCB). CNMs and CMs undergo rigorous training and examination, and this designation will require a graduate degree within the next few years. The CPM category, however, requires much less rigorous training. Its midwives are certified by the North American Registry of Midwives. The clinical requirements for certification as a CPM include:
- attending a “minimum of 20 births”
- managing at least 20 additional births, at least half of them in the home or another out-of-hospital setting
- performing a small number of prenatal, newborn, and postpartum exams.28
A high school diploma is not required.
I suspect that concerns about this lax certification process contributed to ACOG’s decision to issue a statement from its executive board in 2006: “While ACOG supports women having a choice in determining their providers of care, ACOG does not support the provision of care by lay midwives or other midwives who are not certified by the American College of Nurse-Midwives (ACNM) or AMCB.”29
A number of midwifery advocates have made a legislative push to expand licensure for CPMs in this country, and the debate continues on a state-by-state basis.30
Economics and other variables affect delivery decision
Some advocates of home birth note that the “average uncomplicated vaginal birth costs 68% less in a home than in a hospital.”31 Others try to organize support for women who want to give birth at home, such as the Home Birth Hotline, a voluntary, UK-based organization.32
Some articles suggest that patient satisfaction is of significant importance in the decision about where to deliver. One noted that women who delivered where they had planned had higher overall satisfaction when that place was in the home (P<.01).33
A randomized, controlled trial (n=3,510) simulated home delivery in a hospital, with “home delivery” patients having midwifery care in a room “similar to one in one’s own home” and the others having “consultant-led care” in rooms in the delivery suite that contained equipment to resuscitate both mother and baby, as well as monitors and other technology.34 This study found no significant differences in measured outcomes, but “generally higher levels of satisfaction” among the women who had simulated home delivery.
A study from “remote and rural Scotland” found that most women “expressed a preference to give birth in hospital and have consultant-led care because they felt safer.”35
Does the rhetoric surrounding home birth “empower” women?
Another frequently overlooked issue is the passionate rhetoric used to describe home birth—and the effect of that passion on women whose birth plan doesn’t play out as expected. Words such as “choice” and “empowerment” are often used. Regrettably, there is considerable mistrust of the medical system.
One woman describes how her planned home delivery, “influenced by the feminist literature,” went awry.38 After a long labor, she wrote, she “just wanted the baby out, safe and healthy. It no longer mattered how it happened….I couldn’t get rid of the underlying feeling that I had ‘failed’ in some way….”38
Because of her strong desire for home delivery, this woman was deeply affected when the delivery became difficult: “I did not have the authority to proclaim whether or not various medical interventions were necessary, or whether my case actually did constitute a medical emergency….Faced with these ‘options’—safe birth or potential death—how could I be said to be making a ‘choice’?…The obstetrician has more power than the woman because s/he has more knowledge.”38
Despite having come to this realization, and delivering a healthy baby, she still experienced “a sense of disappointment and anger” and “traumatic flashbacks.”
I worry that patients may become so caught up in the rhetoric of their own power and choice that, when uncontrollable events occur, the happiness of a healthy delivery is overshadowed by deep disappointment.
Heated debate isn’t helpful
An unfortunate rift seems to have developed between some members of the midwifery community and some physicians. ACOG and the ACNM have a longstanding policy that: “In those circumstances in which obstetrician/gynecologists and certified nurse-midwives/certified midwives collaborate in the care of women, the quality of those practices is enhanced by a working relationship characterized by mutual respect and trust.”39
Whether individual physicians agree with the practice of planned home birth or not, the health and welfare of the patient must be paramount. The American Public Health Association and the ACNM support home birth.40,41
When obstetric emergencies do arise in the home setting, necessitating emergent transfer, it is critical that the transfer be managed in a way that ensures the best outcome.
One disturbing article describes both “disarticulations” that occur “when there is no correspondence of information or action between the midwife and the hospital staff” and “fractured articulations” that arise from “partial and incomplete correspondence.”42 A number of midwives were interviewed who no longer feel comfortable bringing patients to certain hospitals because of the negative response they received from health-care providers, sometimes to the detriment of the patient.
Can we improve the situation?
First, we need to choose our words carefully when we counsel women about labor and delivery, in recognition of the buzzwords used by advocates of home birth (“empowerment,” “choice”) and the sense of failure and distress some women feel when they eventually require heightened medical intervention.
Perhaps we should dispense with the term “failure,” as in failure to progress, failure to dilate, and so on, to avoid implying that this “failure” is the woman’s fault. And instead of saying that a patient’s pelvis is “adequate,” implying that another woman’s pelvis isn’t, we could use a term that sounds less judgmental.
We can also make the hospital environment more nurturing and supportive of women’s choices for labor, as long as safety isn’t compromised. And when we receive a transfer of a patient whose home delivery has gone awry, we should openly, efficiently, and professionally communicate with the home-delivery provider to best benefit the patient, regardless of our feelings on the subject.
Home birth isn’t going away
That’s my take on the literature. There are certainly data supporting the safety of home birth for the vast majority of women who choose it, but there is also a significant number of women who will experience unpredictable events that could be fatal if blood products or surgery isn’t rapidly available. For that reason, and in light of the very high stakes involved, I wonder: Why take that chance?
1. American Medical Association. Resolution on home deliveries. April 28, 2008. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/471/205.doc. Accessed July 1, 2009.
2. Boyle C. Ricki Lake’s home-birth film upsets AMA. New York Daily News. June 17, 2008. Available at: http://www.nydailynews.com/entertainment/2008/06/17/2008-06-17_ricki_lakes_homebirth_film_upsets_ama.html. Accessed July 1, 2009.
3. American College of Obstetricians and Gynecologists. ACOG statement on home births [press release]. Washington, DC: ACOG; Feb. 6, 2008. Available at: http://www.acog.org/from_home/publications/press_releases/nr02-06-08-2.cfm. Accessed July 1, 2009.
4. Celizic M. Ricki Lake takes on baby birthing industry. Available at: http://www.msnbc.msn.com/id/22592397/. Accessed June 29, 2009.
5. http://www.chicagotribune.com/news/opinion/oped/bal-op.homebirth13jul13,0,6603392.story. Accessed July 23, 2008.
6. National Center for Health Statistics. Vital statistics rates in the United States 1940–1960. Washington, DC: NCHS; 1968.
7. Hoyert DL. Maternal mortality and related concepts. National Center for Health Statistics. Vital Health Stat. 2007;3(33). Available at: http://www.cdc.gov/nchs/data/series/sr_03/sr03_033.pdf. Accessed July 9, 2009.
8. Högberg U. The decline in maternal mortality in Sweden: the role of community midwifery. Am J Public Health. 2004;94:1312-1320.
9. MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomized controlled trial. Br J Obstet Gynaecol. 1993;100:316-323.
10. Olsen O, Jewell MD. Home versus hospital birth. Cochrane Database Syst Rev. 2000;(2):CD000352.-
11. Newburn M. Culture, control and the birth environment. Pract Midwife. 2003;6:20-25.
12. Kmietowicz A. More than four in 10 women were not offered the choice of a home birth, report says. BMJ. 2007;335:112.-
13. Ackermann-Liebrich U, Voegeli T, Günter-Witt K, et al. Home versus hospital deliveries: follow up study of matched pairs for procedure and outcome. BMJ. 1996;313:1313-1318.
14. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: a descriptive study. BJOG. 2008;115:570-578.
15. Bastian H, Keirse MJ, Lancaster PA. Perinatal death associated with planned home birth in Australia: population based study. BMJ. 1998;317:384-388.
16. Fullerton JT, Navarro AM, Young SH. Outcomes of planned home birth: an integrative review. J Midwifery Womens Health. 2007;52:323-333.
17. Johnson KC, Daviss BA. Outcomes of planned home births with certified professional midwives: large prospective study in North America. BMJ. 2005;330:1416-1422.
18. Pang JW, Heffelfinger JD, Huang GJ, Benedetti TJ, Weiss NS. Outcomes of planned home births in Washington State: 1989-1996. Obstet Gynecol. 2002;100:253-259.
19. Burnett CA, 3rd, Jones JA, Rooks J, Chen CH, Tyler CW, Jr, Miller CA. Home delivery and neonatal mortality in North Carolina. JAMA. 1980;244:2741-2745.
20. Hinds MW, Bergeisen GH, Allen DT. Neonatal outcome in planned v unplanned out-of-hospital births in Kentucky. JAMA. 1985;253:1578-1582.
21. Schramm WF, Barnes DE, Bakewell JM. Neonatal mortality in Missouri home births, 1978–84. Am J Public Health. 1987;77:930-935.
22. Henderson J, Petrou S. Economic implications of home births and birth centers: a structured review. Birth. 2008;35:136-146.
23. Nocon JJ, McKenzie DK, Thomas LJ, Hansell RS. Shoulder dystocia: an analysis of risks and obstetric maneuvers. Am J Obstet Gynecol. 1993;168(6 Pt 1):1732-1739.
24. Mori R, Dougherty M, Whittle M. An estimation of intrapartum-related perinatal mortality rates for booked home births in England and Wales between 1994 and 2003. BJOG. 2008;115:554-559.
25. Latendresse G, Murphy PA, Fullerton JT. A description of the management and outcomes of vaginal birth after cesarean birth in the homebirth setting. J Midwifery Womens Health. 2005;50:386-391.
26. Lieberman E, Ernst EK, Rooks JP, Stapleton S, Flamm B. Results of the national study of vaginal birth after cesarean in birth centers. Obstet Gynecol. 2004;104(5 Pt 1):933-942.
27. Mehl-Madrona L, Mehl-Madrona MM. Physician and midwife-attended home births. Effects of breech, twin, and post-dates outcome data on mortality rates. J Nurse Midwifery. 1997;42:91-98.
28. How to become a NARM certified professional midwife (CPM). North American Registry of Midwives. Available at: http://www.narm.org/htb.htm. Accessed June 29,2009.
29. http://www.acog.org/publications/policy_statements/sop0602.cfm. Accessed August 26. 2008.
30. Reed A, Roberts E. State regulation of midwives: issues and options. J Midwifery Womens Health. 2000;45:130-149.
31. Anderson RE, Anderson DA. The cost-effectiveness of home birth. J Nurse Midwifery. 1999;44:30-35.
32. Shaw R, Kitzinger C. Calls to a home birth helpline: empowerment in childbirth. Soc Sci Med. 2005;61:2374-2383.
33. Janssen PA, Carty EA, Reime B. Satisfaction with planned place of birth among midwifery clients in British Columbia. J Midwifery Womens Health. 2006;51:91-97.
34. MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomized controlled trial. Br J Obstet Gynaecol. 1993;100:316-323.
35. Pitchforth E, Watson V, Tucker J, et al. Models of intrapartum care and women’s trade-offs in remote and rural Scotland: a mixed-methods study. BJOG. 2007;115:560-569.
36. Barbieri RL. How will we know it when we’ve got the right cesarean rate? OBG Management. 2008;20(6):10-15.
37. Sakala C. Midwifery care and out-of-hospital birth settings: how do they reduce unnecessary cesarean section births? Soc Sci Med. 1993;37:1233-1250.
38. Crossley ML. Childbirth, complications, and the illusion of “choice”: a case study. Fem Psychol. 2007;17:543-563.
39. http://www.acog.org/publications/policy_statements/sop0210.htm. Accessed September 4, 2008.
40. American Public Health Association. Increasing access to out-of-hospital maternity care services through state-regulated and nationally certified direct-entry midwives. January 1, 2001. Available at: http://www.apha.org/advocacy/policy/policysearch/default.htm?id=242. Accessed June 29, 2009.
41. American College of Nurse-Midwives. Backgrounds of CNMs/CMs rich in diversity. Available at: http://www.midwife.org/background_of_cnms.cfm. Accessed June 29, 2009.
42. Davis-Floyd R. Home-birth emergencies in the US and Mexico: the trouble with transport. Soc Sci Med. 2003;56:1911-1931.
43. Nagai T, Sobajima H, Iwasa M, et al. Neonatal sudden death due to Legionella pneumonia associated with water birth in a domestic spa bath. J Clin Microbiol. 2003;41:2227-2229.
44. Batton DG, Blackmon LR, Adamkin DH, et al. Committee on Fetus and Newborn, 2004–2005, American Academy of Pediatrics. Underwater births. Pediatrics. 2005;115:1413-1414.
45. Cluett ER, Nikodem VC, McCandlish RE, Burns EE. Immersion in water in pregnancy, labour and birth. Cochrane Database Syst Rev. 2004;(2):CD000111.-
46. Tuteur A. What’s in the water at waterbirth? Skeptical OB. February 19, 2009. Available at: http://skepticalob.blogspot.com/2009/02/whats-in-water-at-waterbirth.html. Accessed July 7, 2009.
47. Gilbert RE, Tookey PA. Perinatal mortality and morbidity among babies delivered in water: surveillance study and postal survey. BMJ. 1999;319:483-487.
48. Thoeni A, Zech N, Moroder L. Water birth and the risk of infection: experience after 1,500 water births. Pol J Gyn Invest. 2004;7(1/4):21-26.
49. Gilbert R. Water birth—a near-drowning experience. Pediatrics. 2002;110(2 Pt 1):409.-
50. E-mail correspondence from American College of Obstetrics and Gynecology staff. July 22, 2008.
The author reports no financial relationships relevant to this article.
Few issues in obstetrics spark as much controversy as home birth—and where controversy rages, media attention follows.
Press reports of a 2008 policy statement on home birth issued by the American Medical Association (AMA) and the American College of Obstetricians and Gynecologists (ACOG) highlight the rift between the formal medical establishment and advocates of home birth.1-3
On one side, the AMA and ACOG assert that the hospital or an accredited birthing center “is the safest setting for labor, delivery, and the immediate postpartum period.”1 On the other side, advocates of home birth argue that having the option adds to women’s empowerment and choice.
Some people have accused the medical community of trying to corner the “baby birthing industry.”4 The title of a recent Baltimore Sun article sums up this sentiment: “Home birth battle: Doctors strong-arm women away from healthy alternative to hospital care.”5
Neither ACOG nor the AMA advocates criminalization of home deliveries, but their statements on home birth have generated considerable fear that they will.
This article explores the controversy, focusing on the literature on home birth, gaps in knowledge, the state of regulation, liaison with midwives, and other issues. It also offers suggestions on how to discuss labor and delivery with patients so that they clearly understand the risks involved and do not feel that they have “failed” at meaningful childbirth when they choose hospital delivery.
Did a rise in hospital births reduce maternal mortality?
Obstetric care changed dramatically in the mid-20th century. In 1940, 55.8% of deliveries occurred in the hospital, but that percentage rose to 99.4 by 1970 and hasn’t changed appreciably since.6
Some proponents of hospital delivery note that, in 1940, when 44% of births occurred outside the hospital, the maternal mortality rate was 608 deaths for every 100,000 live births, compared with 37 deaths for every 100,000 live births in 1960, when fewer than 4% of deliveries occurred outside the hospital.6 And in 2003, with only 1% of deliveries occurring in a home setting, the maternal mortality rate was even lower: 12 deaths for every 100,000 live births.7
Others argue that this sharp decrease in maternal mortality cannot be attributed solely to the change in location of the delivery (and subsequent availability of services and personnel), but reflects universal advancement in safe practices such as aseptic technique.8
What do the data show? All studies of home birth have serious methodologic flaws, thanks largely to the nature of the subject matter. A recent Cochrane review observes that there is only one randomized, controlled trial—with a sample size of only 11 women—from which to draw conclusions.9 The review concludes that “there is no strong evidence to favour either home or hospital birth for selected, low-risk pregnant women.”10
Most data come from abroad
Much of the literature on home birth comes from international sites because of the higher prevalence of home delivery in other countries. These data reveal that:
- Two percent of deliveries in the United Kingdom occur in the home.11 The British National Institute for Health and Clinical Excellence recommended that all women be offered the option to have their baby at home or in the hospital, although, depending on the “trust” (a geographically based public-system cooperative that provides care), 8% to 76% of women weren’t given this choice formally.12
- One study conducted in Switzerland involved 489 women who opted for home birth and 385 who chose hospital birth. Of the former, 37 were referred to a specialist during pregnancy, and 70 were referred during labor. The groups had similar birth weights, gestational ages, and clinical conditions.13
- In the Netherlands, 30% of infants are born at home.14 If a woman has an uncomplicated pregnancy, she remains under midwifery care and can decide where to deliver. A study of 280,000 “low-risk” women under primary midwifery care found that 68.1% completed childbirth under that care, 3.6% were referred urgently, and 28.3% were referred without urgency.14 When referrals were considered as a whole, 11.2% involved urgency, primarily for fetal distress (50.2%) and postpartum hemorrhage (33%). Adverse neonatal outcomes were most common in urgently referred cases, followed by nonurgent referrals. The authors acknowledge the importance of transport time once a referral is initiated, stating that, “The Netherlands is a very densely populated country where the average distance to the hospital is relatively short.” (The same cannot be said of many parts of rural America.)
- A study involving home deliveries in Australia from 1985 to 1990 identified 50 perinatal deaths out of 7,002 planned home births.15 The perinatal death rate of infants weighing more than 2,500 g exceeded the national average (5.7 versus 3.6 for every 1,000 deliveries), with a relative risk (RR) of 1.6 (95% confidence interval [CI], 1.1–1.4). Intrapartum death not attributable to prematurity or fetal malformation was also higher (2.7 versus 0.9 for every 1,000 deliveries), with a RR of 3.0 (95% CI, 1.9–4.8). According to the authors, the main contributors to excess mortality were underestimation of the risks associated with post-term birth, twin pregnancy, and breech presentation, and a lack of response to fetal distress.
In the summer of 1999, a woman delivered a 7.7-lb infant after 42 weeks of gestation. The birth took place in the woman’s home in Japan, and the baby was delivered in a bathtub of warm water. The woman had had an uneventful pregnancy, and the baby appeared to be perfectly normal.
Four days later, the infant developed fever and jaundice and was admitted to the hospital, where she was treated with phototherapy. She improved, but her symptoms recurred 3 days later, and she began to vomit. Eight days after birth, she suffered cardiopulmonary arrest and died. An autopsy revealed the cause of death to be legionellosis—infection with Legionella pneumonia. The most likely source was the bathtub in which she was born.43
Other case reports describe similar tragedies associated with water birth (among them, drowning, infection, and a snapped umbilical cord), but no randomized, clinical trial has systematically compared delivery in water with conventional land-based birth.
The death, morbidity, and lack of data so troubled members of the American Academy of Pediatrics that the Committee on Fetus and Newborn issued an advisory in 2005:
- The safety and efficacy of underwater birth for the newborn has not been established. There is no convincing evidence of benefit to the neonate but some concern for serious harm. Therefore, underwater birth should be considered an experimental procedure that should not be performed except within the context of an appropriately designed randomized clinical trial after informed parental consent.44
This statement contrasts the conclusion of the most recent Cochrane review of the subject, which found that, “Immersion in water during the first stage of labour significantly reduces women’s perception of pain and use of epidural/spinal analgesia.”45 The review also noted, however, that, “No trials could be located that assessed the immersion of women in water during the third stage of labour.”45
No studies have explored immersion in water during the third stage of labor.
What’s in that water?
Amy Tuteur, MD, an ObGyn who publishes a popular blog (“The Skeptical OB”), focused on the topic of water birth earlier this year. “What’s in the water at waterbirth?” she asks.46
To answer the question, Dr. Tuteur cites a 1999 study of 4,030 deliveries in water, which found that 35 infants suffered serious morbidity and three died—although it is unclear if any of the deaths were a direct result of water birth. “However, of the 32 survivors who were admitted to the NICU,” writes Dr. Tuteur, “13 had significant respiratory problems, including pneumonia, meconium aspiration, water aspiration, and drowning. Other complications attributable to water birth include five babies who had significant hemorrhage due to snapped umbilical cord. In all, 18 babies had serious complications directly attributable to waterbirth.”47
Dr. Tuteur also points to the poor quality of the water in birthing pools, arguing that it is “essentially toilet water.”46 “The water in a birth pool, conveniently heated to body temperature, the optimum temperature for bacterial growth, is a microbial paradise,” she writes.46 She cites a study of 1,500 water births that included analysis of the water found in the birthing pools (before anyone entered the water) and identified:
- coliforms in 21% of samples
- enterococcus in 19% of samples
- Escherichia coli in 10% of samples
- Legionella pneumophila in 12% of samples
- Pseudomonas aeruginosa in 11% of samples.48
After a special water filter was installed, contamination diminished but did not disappear completely.
Pools in the home setting were not the only ones implicated in contamination; some hospital pools also were affected.
What’s the bottom line?
The American College of Obstetricians and Gynecologists has yet to weigh in on the matter. Until it does, ObGyns may be wise to heed the words of Ruth Gilbert, MD, of the Centre for Paediatric Epidemiology and Biostatistics at the Institute of Child Health in London.
“Can delivery in water cause serious adverse outcomes?” she asks, rhetorically, it turns out.
“Undoubtedly, the answer is ‘yes.’”49 —JANELLE YATES, SENIOR EDITOR
The data we do have are difficult to interpret
Among the limitations of studies of home birth are:
- lack of follow-up after the delivery
- varying definitions of perinatal mortality internationally
- lack of clarity regarding the identity and education of delivering providers
- the fact that there are often “too few neonatal deaths from which to extrapolate reliable rate calculations.”16
One meta-analysis found a rate of intrapartum transfer ranging from 7.4% to 16.5%, and a rate of primary cesarean delivery of 1.4% to 17.7% (it was 13.8% to 28.25% in the “comparison group”).16
A challenge inherent in many of these studies is identifying exactly what the comparison group is. In addition, some of the data are obtained from discharge summary records, which don’t always reflect the level of risk or acuity.
Oft-cited study has weaknesses
The study that many advocates of home birth cite was conducted in the United States and Canada and published in 2005.17 It evaluated “all 5,418 women expecting to deliver in 2000 supported by midwives with a common certification [certified professional midwives] and who planned to deliver at home when labour began.” The hospital transfer rate was 12.1%, in line with other studies. The risk of adverse outcomes was lower in the group that planned to have home delivery, compared with a “relatively low-risk hospital group.”
The study focused on:
- electronic fetal monitoring, used in 9.6% of deliveries in the home-birth group, versus 84.3% of the hospital group
- episiotomy, performed in 2.1% of home deliveries, compared with 33% of hospital births
- cesarean delivery, 3.7% of planned home deliveries, versus 19% of hospital births
- vacuum-assisted vaginal delivery, performed in 0.6% of planned home deliveries, versus 5.5% of hospital births
- neonatal death, at a rate of 2.0 deaths for every 1,000 intended home births. No comparison figure was cited.
One of the weaknesses of this study, as of others, was identification of a comparison group as a “low-risk” population without data to back up that designation. In addition, this study derived its data from birth certificates for 3,360,868 singleton, vertex births at 37 weeks or more of gestation. Data from birth certificates are limited as a basis for accurate risk assessment. Moreover, although the authors of this study asserted that they had no conflict of interest, the investigation was funded by The Foundation for the Advancement of Midwifery.
Study cited by advocates of hospital birth is also flawed
One of the studies many hospital and birthing center advocates cite was published in 2002.18 It involved an analysis of birth registry information on uncomplicated singleton pregnancies at 34 weeks or more of gestation in Washington state between 1989 and 1996. These pregnancies were either:
- delivered at home by a health professional (n=5,854)
- transferred to medical facilities after attempted home delivery (n=279)
- planned to be delivered in the hospital (n=10,593).
Infants whose mothers planned to deliver at home had a higher risk of neonatal death (RR, 1.99; 95% CI, 1.06–3.73) and a higher risk of having a 5-minute Apgar score of less than 3 (RR, 2.31; 95% CI, 1.29–4.16). After adjustment for a gestational-age cutoff of 37 weeks, these risks remained similar.
Nulliparous women, in particular, had a higher risk for prolonged labor (RR, 1.73; 95% CI, 1.28–2.34) and postpartum bleeding (RR, 2.76; 95% CI, 1.74–4.36).
The authors themselves point out a potential flaw in this study: the use of data from birth certificates. These data create “the potential for misclassifying unplanned home births as planned home births.” The difference in outcomes could be significant. For example, the neonatal death rate for unplanned home deliveries in North Carolina and Kentucky was 18 to 20 times higher than the rate for planned home births in these states.19,20
A study from Missouri observes that neonatal mortality was elevated for both planned and unplanned home birth, compared with physician-attended hospital birth.21
Selection bias is a concern
Selection bias is an inherent difficulty in many of these studies. Except for one previously mentioned paper—a very small study—none of the investigations involve randomization. As a result, we cannot exclude the possibility that “women who choose to deliver at home or in a birth center are likely to be different in terms of expectations and approach from women choosing to deliver in hospitals.”22
Risk level can escalate rapidly
What is potentially troubling about home birth is the fact that a low-risk pregnancy that was complication-free during antepartum care can become a high-risk pregnancy in a matter of minutes, necessitating urgent, appropriate obstetric care. Some classic examples of urgent events include cord prolapse, postpartum hemorrhage, bleeding from vasa previa, and shoulder dystocia.
Let’s focus on shoulder dystocia, which occurs in 1.4% of all vaginal deliveries. The authors of one study point out that “most of the traditional risk factors for shoulder dystocia have no predictive value, shoulder dystocia itself is an unpredictable event, and infants at risk for permanent injury are virtually impossible to predict.”23 This may make delivery in the home a high-risk endeavor because of the inability to mobilize an obstetric team to assist with shoulder dystocia maneuvers or perform a Zavanelli delivery.
Although the American College of Obstetricians and Gynecologists (ACOG) reiterated its opposition to home birth in early 2008, its stance on the matter has not shifted since 1979.50 In a news release describing that position, ACOG acknowledged “a woman’s right to make informed decisions regarding her delivery and to have a choice in choosing her health-care provider,” but made it clear that ACOG “does not support programs that advocate for, or individuals who provide, home births.”3
It emphasized its opposition pointedly, saying: “Choosing to deliver a baby at home…is to place the process of giving birth over the goal of having a healthy baby.”3
AMA resolution includes the reasoning behind the opposition
The American Medical Association (AMA) listed several variables that underscore the need for a clear-cut policy on home birth:
- the fact that 21 states “currently license midwives to attend home births, all using the certified professional midwife credential (CPM or ‘lay’ midwives), not the certified midwives (CM) credential which both the American College of Obstetricians and Gynecologists and American College of Nurse Midwives recognize”
- considerable media attention to celebrities who have given birth at home
- the fact that “an apparently uncomplicated pregnancy or delivery can quickly become very complicated in the setting of maternal hemorrhage, shoulder dystocia, eclampsia, or other obstetric emergencies.”1
Both ACOG and the AMA consider the following to fall within the category of “hospital”:
- a birthing center situated “within a hospital complex, that meets standards jointly outlined by the American Academy of Pediatrics and ACOG”
- “a freestanding birthing center that meets the standards of the Accreditation Association for Ambulatory Health Care, The Joint Commission, or the American Association of Birth Centers.”3
Another variable overlooked in most studies is the speed of transfer and the outcomes of pregnancies in which the women intended to deliver at home but ended up requiring urgent transfer. One study that did examine this scenario found that “women who had booked for a home birth, but later needed to transfer their care for a hospital birth, appeared to have the highest risk of intrapartum-related perinatal mortality.”24
There is also some controversy regarding the delivery of women who are pregnant with twins, who have a fetus in breech presentation, or who have a history of cesarean delivery. One study examined outcomes for intended home delivery of 57 women who had a prior abdominal delivery.25 Fifty of these women delivered vaginally in the home, and seven (12.3%) delivered in the hospital. One hospital transfer was urgent for fetal distress. One baby was stillborn, delivered at home.
Many policy makers decry the high prevalence of cesarean delivery in the United States and argue that providers who don’t perform this procedure offer a low-cost alternative for obstetric care.36 Some proponents of elective primary cesarean argue that it protects the perineum, but this issue is largely absent from the debate on home birth. Nor have I seen any study that addresses long-term outcomes in women who deliver at home, as most data collection ends after the delivery.
This oversight concerns me when I see interviews of midwives who doubt the existence of fetopelvic disproportion, who make statements such as, “You can get a baby through a knothole” and “I’ve never seen [a pelvis] that isn’t large enough.”37
If patients are encouraged to have a prolonged second stage of labor, does it have a harmful effect on their pelvic floor in later years? This important question merits further discussion.—ERIN E. TRACY, MD, MPH
EDITOR’S NOTE: See the related item, “ Award-winning video urges women to avoid cesarean delivery.”
A 10-year prospective study of vaginal birth after cesarean (VBAC) in birth centers found that more than 50% of uterine ruptures and 57% of perinatal deaths involved the 10% of women who had more than one prior cesarean delivery or who had reached a gestational age of more than 42 weeks.26
Skill of the caregiver is important
The training and qualifications of the obstetric care provider are incredibly important. One study evaluated 4,361 home births attended by “apprentice-trained midwives from 1970 to 1985 and 4,107 home births attended by family physicians from 1969 to 1981.”27 The perinatal mortality rate for the midwife-attended births was 14 for every 1,000 births, in contrast to the rate of 5 for every 1,000 physician-attended births.
Three types of midwife are credentialed in this country:
- certified nurse-midwife (CNM)
- certified midwife (CM)
- certified professional midwife (CPM).
The first two categories are certified by the American Midwifery Certification Board (AMCB). CNMs and CMs undergo rigorous training and examination, and this designation will require a graduate degree within the next few years. The CPM category, however, requires much less rigorous training. Its midwives are certified by the North American Registry of Midwives. The clinical requirements for certification as a CPM include:
- attending a “minimum of 20 births”
- managing at least 20 additional births, at least half of them in the home or another out-of-hospital setting
- performing a small number of prenatal, newborn, and postpartum exams.28
A high school diploma is not required.
I suspect that concerns about this lax certification process contributed to ACOG’s decision to issue a statement from its executive board in 2006: “While ACOG supports women having a choice in determining their providers of care, ACOG does not support the provision of care by lay midwives or other midwives who are not certified by the American College of Nurse-Midwives (ACNM) or AMCB.”29
A number of midwifery advocates have made a legislative push to expand licensure for CPMs in this country, and the debate continues on a state-by-state basis.30
Economics and other variables affect delivery decision
Some advocates of home birth note that the “average uncomplicated vaginal birth costs 68% less in a home than in a hospital.”31 Others try to organize support for women who want to give birth at home, such as the Home Birth Hotline, a voluntary, UK-based organization.32
Some articles suggest that patient satisfaction is of significant importance in the decision about where to deliver. One noted that women who delivered where they had planned had higher overall satisfaction when that place was in the home (P<.01).33
A randomized, controlled trial (n=3,510) simulated home delivery in a hospital, with “home delivery” patients having midwifery care in a room “similar to one in one’s own home” and the others having “consultant-led care” in rooms in the delivery suite that contained equipment to resuscitate both mother and baby, as well as monitors and other technology.34 This study found no significant differences in measured outcomes, but “generally higher levels of satisfaction” among the women who had simulated home delivery.
A study from “remote and rural Scotland” found that most women “expressed a preference to give birth in hospital and have consultant-led care because they felt safer.”35
Does the rhetoric surrounding home birth “empower” women?
Another frequently overlooked issue is the passionate rhetoric used to describe home birth—and the effect of that passion on women whose birth plan doesn’t play out as expected. Words such as “choice” and “empowerment” are often used. Regrettably, there is considerable mistrust of the medical system.
One woman describes how her planned home delivery, “influenced by the feminist literature,” went awry.38 After a long labor, she wrote, she “just wanted the baby out, safe and healthy. It no longer mattered how it happened….I couldn’t get rid of the underlying feeling that I had ‘failed’ in some way….”38
Because of her strong desire for home delivery, this woman was deeply affected when the delivery became difficult: “I did not have the authority to proclaim whether or not various medical interventions were necessary, or whether my case actually did constitute a medical emergency….Faced with these ‘options’—safe birth or potential death—how could I be said to be making a ‘choice’?…The obstetrician has more power than the woman because s/he has more knowledge.”38
Despite having come to this realization, and delivering a healthy baby, she still experienced “a sense of disappointment and anger” and “traumatic flashbacks.”
I worry that patients may become so caught up in the rhetoric of their own power and choice that, when uncontrollable events occur, the happiness of a healthy delivery is overshadowed by deep disappointment.
Heated debate isn’t helpful
An unfortunate rift seems to have developed between some members of the midwifery community and some physicians. ACOG and the ACNM have a longstanding policy that: “In those circumstances in which obstetrician/gynecologists and certified nurse-midwives/certified midwives collaborate in the care of women, the quality of those practices is enhanced by a working relationship characterized by mutual respect and trust.”39
Whether individual physicians agree with the practice of planned home birth or not, the health and welfare of the patient must be paramount. The American Public Health Association and the ACNM support home birth.40,41
When obstetric emergencies do arise in the home setting, necessitating emergent transfer, it is critical that the transfer be managed in a way that ensures the best outcome.
One disturbing article describes both “disarticulations” that occur “when there is no correspondence of information or action between the midwife and the hospital staff” and “fractured articulations” that arise from “partial and incomplete correspondence.”42 A number of midwives were interviewed who no longer feel comfortable bringing patients to certain hospitals because of the negative response they received from health-care providers, sometimes to the detriment of the patient.
Can we improve the situation?
First, we need to choose our words carefully when we counsel women about labor and delivery, in recognition of the buzzwords used by advocates of home birth (“empowerment,” “choice”) and the sense of failure and distress some women feel when they eventually require heightened medical intervention.
Perhaps we should dispense with the term “failure,” as in failure to progress, failure to dilate, and so on, to avoid implying that this “failure” is the woman’s fault. And instead of saying that a patient’s pelvis is “adequate,” implying that another woman’s pelvis isn’t, we could use a term that sounds less judgmental.
We can also make the hospital environment more nurturing and supportive of women’s choices for labor, as long as safety isn’t compromised. And when we receive a transfer of a patient whose home delivery has gone awry, we should openly, efficiently, and professionally communicate with the home-delivery provider to best benefit the patient, regardless of our feelings on the subject.
Home birth isn’t going away
That’s my take on the literature. There are certainly data supporting the safety of home birth for the vast majority of women who choose it, but there is also a significant number of women who will experience unpredictable events that could be fatal if blood products or surgery isn’t rapidly available. For that reason, and in light of the very high stakes involved, I wonder: Why take that chance?
The author reports no financial relationships relevant to this article.
Few issues in obstetrics spark as much controversy as home birth—and where controversy rages, media attention follows.
Press reports of a 2008 policy statement on home birth issued by the American Medical Association (AMA) and the American College of Obstetricians and Gynecologists (ACOG) highlight the rift between the formal medical establishment and advocates of home birth.1-3
On one side, the AMA and ACOG assert that the hospital or an accredited birthing center “is the safest setting for labor, delivery, and the immediate postpartum period.”1 On the other side, advocates of home birth argue that having the option adds to women’s empowerment and choice.
Some people have accused the medical community of trying to corner the “baby birthing industry.”4 The title of a recent Baltimore Sun article sums up this sentiment: “Home birth battle: Doctors strong-arm women away from healthy alternative to hospital care.”5
Neither ACOG nor the AMA advocates criminalization of home deliveries, but their statements on home birth have generated considerable fear that they will.
This article explores the controversy, focusing on the literature on home birth, gaps in knowledge, the state of regulation, liaison with midwives, and other issues. It also offers suggestions on how to discuss labor and delivery with patients so that they clearly understand the risks involved and do not feel that they have “failed” at meaningful childbirth when they choose hospital delivery.
Did a rise in hospital births reduce maternal mortality?
Obstetric care changed dramatically in the mid-20th century. In 1940, 55.8% of deliveries occurred in the hospital, but that percentage rose to 99.4 by 1970 and hasn’t changed appreciably since.6
Some proponents of hospital delivery note that, in 1940, when 44% of births occurred outside the hospital, the maternal mortality rate was 608 deaths for every 100,000 live births, compared with 37 deaths for every 100,000 live births in 1960, when fewer than 4% of deliveries occurred outside the hospital.6 And in 2003, with only 1% of deliveries occurring in a home setting, the maternal mortality rate was even lower: 12 deaths for every 100,000 live births.7
Others argue that this sharp decrease in maternal mortality cannot be attributed solely to the change in location of the delivery (and subsequent availability of services and personnel), but reflects universal advancement in safe practices such as aseptic technique.8
What do the data show? All studies of home birth have serious methodologic flaws, thanks largely to the nature of the subject matter. A recent Cochrane review observes that there is only one randomized, controlled trial—with a sample size of only 11 women—from which to draw conclusions.9 The review concludes that “there is no strong evidence to favour either home or hospital birth for selected, low-risk pregnant women.”10
Most data come from abroad
Much of the literature on home birth comes from international sites because of the higher prevalence of home delivery in other countries. These data reveal that:
- Two percent of deliveries in the United Kingdom occur in the home.11 The British National Institute for Health and Clinical Excellence recommended that all women be offered the option to have their baby at home or in the hospital, although, depending on the “trust” (a geographically based public-system cooperative that provides care), 8% to 76% of women weren’t given this choice formally.12
- One study conducted in Switzerland involved 489 women who opted for home birth and 385 who chose hospital birth. Of the former, 37 were referred to a specialist during pregnancy, and 70 were referred during labor. The groups had similar birth weights, gestational ages, and clinical conditions.13
- In the Netherlands, 30% of infants are born at home.14 If a woman has an uncomplicated pregnancy, she remains under midwifery care and can decide where to deliver. A study of 280,000 “low-risk” women under primary midwifery care found that 68.1% completed childbirth under that care, 3.6% were referred urgently, and 28.3% were referred without urgency.14 When referrals were considered as a whole, 11.2% involved urgency, primarily for fetal distress (50.2%) and postpartum hemorrhage (33%). Adverse neonatal outcomes were most common in urgently referred cases, followed by nonurgent referrals. The authors acknowledge the importance of transport time once a referral is initiated, stating that, “The Netherlands is a very densely populated country where the average distance to the hospital is relatively short.” (The same cannot be said of many parts of rural America.)
- A study involving home deliveries in Australia from 1985 to 1990 identified 50 perinatal deaths out of 7,002 planned home births.15 The perinatal death rate of infants weighing more than 2,500 g exceeded the national average (5.7 versus 3.6 for every 1,000 deliveries), with a relative risk (RR) of 1.6 (95% confidence interval [CI], 1.1–1.4). Intrapartum death not attributable to prematurity or fetal malformation was also higher (2.7 versus 0.9 for every 1,000 deliveries), with a RR of 3.0 (95% CI, 1.9–4.8). According to the authors, the main contributors to excess mortality were underestimation of the risks associated with post-term birth, twin pregnancy, and breech presentation, and a lack of response to fetal distress.
In the summer of 1999, a woman delivered a 7.7-lb infant after 42 weeks of gestation. The birth took place in the woman’s home in Japan, and the baby was delivered in a bathtub of warm water. The woman had had an uneventful pregnancy, and the baby appeared to be perfectly normal.
Four days later, the infant developed fever and jaundice and was admitted to the hospital, where she was treated with phototherapy. She improved, but her symptoms recurred 3 days later, and she began to vomit. Eight days after birth, she suffered cardiopulmonary arrest and died. An autopsy revealed the cause of death to be legionellosis—infection with Legionella pneumonia. The most likely source was the bathtub in which she was born.43
Other case reports describe similar tragedies associated with water birth (among them, drowning, infection, and a snapped umbilical cord), but no randomized, clinical trial has systematically compared delivery in water with conventional land-based birth.
The death, morbidity, and lack of data so troubled members of the American Academy of Pediatrics that the Committee on Fetus and Newborn issued an advisory in 2005:
- The safety and efficacy of underwater birth for the newborn has not been established. There is no convincing evidence of benefit to the neonate but some concern for serious harm. Therefore, underwater birth should be considered an experimental procedure that should not be performed except within the context of an appropriately designed randomized clinical trial after informed parental consent.44
This statement contrasts the conclusion of the most recent Cochrane review of the subject, which found that, “Immersion in water during the first stage of labour significantly reduces women’s perception of pain and use of epidural/spinal analgesia.”45 The review also noted, however, that, “No trials could be located that assessed the immersion of women in water during the third stage of labour.”45
No studies have explored immersion in water during the third stage of labor.
What’s in that water?
Amy Tuteur, MD, an ObGyn who publishes a popular blog (“The Skeptical OB”), focused on the topic of water birth earlier this year. “What’s in the water at waterbirth?” she asks.46
To answer the question, Dr. Tuteur cites a 1999 study of 4,030 deliveries in water, which found that 35 infants suffered serious morbidity and three died—although it is unclear if any of the deaths were a direct result of water birth. “However, of the 32 survivors who were admitted to the NICU,” writes Dr. Tuteur, “13 had significant respiratory problems, including pneumonia, meconium aspiration, water aspiration, and drowning. Other complications attributable to water birth include five babies who had significant hemorrhage due to snapped umbilical cord. In all, 18 babies had serious complications directly attributable to waterbirth.”47
Dr. Tuteur also points to the poor quality of the water in birthing pools, arguing that it is “essentially toilet water.”46 “The water in a birth pool, conveniently heated to body temperature, the optimum temperature for bacterial growth, is a microbial paradise,” she writes.46 She cites a study of 1,500 water births that included analysis of the water found in the birthing pools (before anyone entered the water) and identified:
- coliforms in 21% of samples
- enterococcus in 19% of samples
- Escherichia coli in 10% of samples
- Legionella pneumophila in 12% of samples
- Pseudomonas aeruginosa in 11% of samples.48
After a special water filter was installed, contamination diminished but did not disappear completely.
Pools in the home setting were not the only ones implicated in contamination; some hospital pools also were affected.
What’s the bottom line?
The American College of Obstetricians and Gynecologists has yet to weigh in on the matter. Until it does, ObGyns may be wise to heed the words of Ruth Gilbert, MD, of the Centre for Paediatric Epidemiology and Biostatistics at the Institute of Child Health in London.
“Can delivery in water cause serious adverse outcomes?” she asks, rhetorically, it turns out.
“Undoubtedly, the answer is ‘yes.’”49 —JANELLE YATES, SENIOR EDITOR
The data we do have are difficult to interpret
Among the limitations of studies of home birth are:
- lack of follow-up after the delivery
- varying definitions of perinatal mortality internationally
- lack of clarity regarding the identity and education of delivering providers
- the fact that there are often “too few neonatal deaths from which to extrapolate reliable rate calculations.”16
One meta-analysis found a rate of intrapartum transfer ranging from 7.4% to 16.5%, and a rate of primary cesarean delivery of 1.4% to 17.7% (it was 13.8% to 28.25% in the “comparison group”).16
A challenge inherent in many of these studies is identifying exactly what the comparison group is. In addition, some of the data are obtained from discharge summary records, which don’t always reflect the level of risk or acuity.
Oft-cited study has weaknesses
The study that many advocates of home birth cite was conducted in the United States and Canada and published in 2005.17 It evaluated “all 5,418 women expecting to deliver in 2000 supported by midwives with a common certification [certified professional midwives] and who planned to deliver at home when labour began.” The hospital transfer rate was 12.1%, in line with other studies. The risk of adverse outcomes was lower in the group that planned to have home delivery, compared with a “relatively low-risk hospital group.”
The study focused on:
- electronic fetal monitoring, used in 9.6% of deliveries in the home-birth group, versus 84.3% of the hospital group
- episiotomy, performed in 2.1% of home deliveries, compared with 33% of hospital births
- cesarean delivery, 3.7% of planned home deliveries, versus 19% of hospital births
- vacuum-assisted vaginal delivery, performed in 0.6% of planned home deliveries, versus 5.5% of hospital births
- neonatal death, at a rate of 2.0 deaths for every 1,000 intended home births. No comparison figure was cited.
One of the weaknesses of this study, as of others, was identification of a comparison group as a “low-risk” population without data to back up that designation. In addition, this study derived its data from birth certificates for 3,360,868 singleton, vertex births at 37 weeks or more of gestation. Data from birth certificates are limited as a basis for accurate risk assessment. Moreover, although the authors of this study asserted that they had no conflict of interest, the investigation was funded by The Foundation for the Advancement of Midwifery.
Study cited by advocates of hospital birth is also flawed
One of the studies many hospital and birthing center advocates cite was published in 2002.18 It involved an analysis of birth registry information on uncomplicated singleton pregnancies at 34 weeks or more of gestation in Washington state between 1989 and 1996. These pregnancies were either:
- delivered at home by a health professional (n=5,854)
- transferred to medical facilities after attempted home delivery (n=279)
- planned to be delivered in the hospital (n=10,593).
Infants whose mothers planned to deliver at home had a higher risk of neonatal death (RR, 1.99; 95% CI, 1.06–3.73) and a higher risk of having a 5-minute Apgar score of less than 3 (RR, 2.31; 95% CI, 1.29–4.16). After adjustment for a gestational-age cutoff of 37 weeks, these risks remained similar.
Nulliparous women, in particular, had a higher risk for prolonged labor (RR, 1.73; 95% CI, 1.28–2.34) and postpartum bleeding (RR, 2.76; 95% CI, 1.74–4.36).
The authors themselves point out a potential flaw in this study: the use of data from birth certificates. These data create “the potential for misclassifying unplanned home births as planned home births.” The difference in outcomes could be significant. For example, the neonatal death rate for unplanned home deliveries in North Carolina and Kentucky was 18 to 20 times higher than the rate for planned home births in these states.19,20
A study from Missouri observes that neonatal mortality was elevated for both planned and unplanned home birth, compared with physician-attended hospital birth.21
Selection bias is a concern
Selection bias is an inherent difficulty in many of these studies. Except for one previously mentioned paper—a very small study—none of the investigations involve randomization. As a result, we cannot exclude the possibility that “women who choose to deliver at home or in a birth center are likely to be different in terms of expectations and approach from women choosing to deliver in hospitals.”22
Risk level can escalate rapidly
What is potentially troubling about home birth is the fact that a low-risk pregnancy that was complication-free during antepartum care can become a high-risk pregnancy in a matter of minutes, necessitating urgent, appropriate obstetric care. Some classic examples of urgent events include cord prolapse, postpartum hemorrhage, bleeding from vasa previa, and shoulder dystocia.
Let’s focus on shoulder dystocia, which occurs in 1.4% of all vaginal deliveries. The authors of one study point out that “most of the traditional risk factors for shoulder dystocia have no predictive value, shoulder dystocia itself is an unpredictable event, and infants at risk for permanent injury are virtually impossible to predict.”23 This may make delivery in the home a high-risk endeavor because of the inability to mobilize an obstetric team to assist with shoulder dystocia maneuvers or perform a Zavanelli delivery.
Although the American College of Obstetricians and Gynecologists (ACOG) reiterated its opposition to home birth in early 2008, its stance on the matter has not shifted since 1979.50 In a news release describing that position, ACOG acknowledged “a woman’s right to make informed decisions regarding her delivery and to have a choice in choosing her health-care provider,” but made it clear that ACOG “does not support programs that advocate for, or individuals who provide, home births.”3
It emphasized its opposition pointedly, saying: “Choosing to deliver a baby at home…is to place the process of giving birth over the goal of having a healthy baby.”3
AMA resolution includes the reasoning behind the opposition
The American Medical Association (AMA) listed several variables that underscore the need for a clear-cut policy on home birth:
- the fact that 21 states “currently license midwives to attend home births, all using the certified professional midwife credential (CPM or ‘lay’ midwives), not the certified midwives (CM) credential which both the American College of Obstetricians and Gynecologists and American College of Nurse Midwives recognize”
- considerable media attention to celebrities who have given birth at home
- the fact that “an apparently uncomplicated pregnancy or delivery can quickly become very complicated in the setting of maternal hemorrhage, shoulder dystocia, eclampsia, or other obstetric emergencies.”1
Both ACOG and the AMA consider the following to fall within the category of “hospital”:
- a birthing center situated “within a hospital complex, that meets standards jointly outlined by the American Academy of Pediatrics and ACOG”
- “a freestanding birthing center that meets the standards of the Accreditation Association for Ambulatory Health Care, The Joint Commission, or the American Association of Birth Centers.”3
Another variable overlooked in most studies is the speed of transfer and the outcomes of pregnancies in which the women intended to deliver at home but ended up requiring urgent transfer. One study that did examine this scenario found that “women who had booked for a home birth, but later needed to transfer their care for a hospital birth, appeared to have the highest risk of intrapartum-related perinatal mortality.”24
There is also some controversy regarding the delivery of women who are pregnant with twins, who have a fetus in breech presentation, or who have a history of cesarean delivery. One study examined outcomes for intended home delivery of 57 women who had a prior abdominal delivery.25 Fifty of these women delivered vaginally in the home, and seven (12.3%) delivered in the hospital. One hospital transfer was urgent for fetal distress. One baby was stillborn, delivered at home.
Many policy makers decry the high prevalence of cesarean delivery in the United States and argue that providers who don’t perform this procedure offer a low-cost alternative for obstetric care.36 Some proponents of elective primary cesarean argue that it protects the perineum, but this issue is largely absent from the debate on home birth. Nor have I seen any study that addresses long-term outcomes in women who deliver at home, as most data collection ends after the delivery.
This oversight concerns me when I see interviews of midwives who doubt the existence of fetopelvic disproportion, who make statements such as, “You can get a baby through a knothole” and “I’ve never seen [a pelvis] that isn’t large enough.”37
If patients are encouraged to have a prolonged second stage of labor, does it have a harmful effect on their pelvic floor in later years? This important question merits further discussion.—ERIN E. TRACY, MD, MPH
EDITOR’S NOTE: See the related item, “ Award-winning video urges women to avoid cesarean delivery.”
A 10-year prospective study of vaginal birth after cesarean (VBAC) in birth centers found that more than 50% of uterine ruptures and 57% of perinatal deaths involved the 10% of women who had more than one prior cesarean delivery or who had reached a gestational age of more than 42 weeks.26
Skill of the caregiver is important
The training and qualifications of the obstetric care provider are incredibly important. One study evaluated 4,361 home births attended by “apprentice-trained midwives from 1970 to 1985 and 4,107 home births attended by family physicians from 1969 to 1981.”27 The perinatal mortality rate for the midwife-attended births was 14 for every 1,000 births, in contrast to the rate of 5 for every 1,000 physician-attended births.
Three types of midwife are credentialed in this country:
- certified nurse-midwife (CNM)
- certified midwife (CM)
- certified professional midwife (CPM).
The first two categories are certified by the American Midwifery Certification Board (AMCB). CNMs and CMs undergo rigorous training and examination, and this designation will require a graduate degree within the next few years. The CPM category, however, requires much less rigorous training. Its midwives are certified by the North American Registry of Midwives. The clinical requirements for certification as a CPM include:
- attending a “minimum of 20 births”
- managing at least 20 additional births, at least half of them in the home or another out-of-hospital setting
- performing a small number of prenatal, newborn, and postpartum exams.28
A high school diploma is not required.
I suspect that concerns about this lax certification process contributed to ACOG’s decision to issue a statement from its executive board in 2006: “While ACOG supports women having a choice in determining their providers of care, ACOG does not support the provision of care by lay midwives or other midwives who are not certified by the American College of Nurse-Midwives (ACNM) or AMCB.”29
A number of midwifery advocates have made a legislative push to expand licensure for CPMs in this country, and the debate continues on a state-by-state basis.30
Economics and other variables affect delivery decision
Some advocates of home birth note that the “average uncomplicated vaginal birth costs 68% less in a home than in a hospital.”31 Others try to organize support for women who want to give birth at home, such as the Home Birth Hotline, a voluntary, UK-based organization.32
Some articles suggest that patient satisfaction is of significant importance in the decision about where to deliver. One noted that women who delivered where they had planned had higher overall satisfaction when that place was in the home (P<.01).33
A randomized, controlled trial (n=3,510) simulated home delivery in a hospital, with “home delivery” patients having midwifery care in a room “similar to one in one’s own home” and the others having “consultant-led care” in rooms in the delivery suite that contained equipment to resuscitate both mother and baby, as well as monitors and other technology.34 This study found no significant differences in measured outcomes, but “generally higher levels of satisfaction” among the women who had simulated home delivery.
A study from “remote and rural Scotland” found that most women “expressed a preference to give birth in hospital and have consultant-led care because they felt safer.”35
Does the rhetoric surrounding home birth “empower” women?
Another frequently overlooked issue is the passionate rhetoric used to describe home birth—and the effect of that passion on women whose birth plan doesn’t play out as expected. Words such as “choice” and “empowerment” are often used. Regrettably, there is considerable mistrust of the medical system.
One woman describes how her planned home delivery, “influenced by the feminist literature,” went awry.38 After a long labor, she wrote, she “just wanted the baby out, safe and healthy. It no longer mattered how it happened….I couldn’t get rid of the underlying feeling that I had ‘failed’ in some way….”38
Because of her strong desire for home delivery, this woman was deeply affected when the delivery became difficult: “I did not have the authority to proclaim whether or not various medical interventions were necessary, or whether my case actually did constitute a medical emergency….Faced with these ‘options’—safe birth or potential death—how could I be said to be making a ‘choice’?…The obstetrician has more power than the woman because s/he has more knowledge.”38
Despite having come to this realization, and delivering a healthy baby, she still experienced “a sense of disappointment and anger” and “traumatic flashbacks.”
I worry that patients may become so caught up in the rhetoric of their own power and choice that, when uncontrollable events occur, the happiness of a healthy delivery is overshadowed by deep disappointment.
Heated debate isn’t helpful
An unfortunate rift seems to have developed between some members of the midwifery community and some physicians. ACOG and the ACNM have a longstanding policy that: “In those circumstances in which obstetrician/gynecologists and certified nurse-midwives/certified midwives collaborate in the care of women, the quality of those practices is enhanced by a working relationship characterized by mutual respect and trust.”39
Whether individual physicians agree with the practice of planned home birth or not, the health and welfare of the patient must be paramount. The American Public Health Association and the ACNM support home birth.40,41
When obstetric emergencies do arise in the home setting, necessitating emergent transfer, it is critical that the transfer be managed in a way that ensures the best outcome.
One disturbing article describes both “disarticulations” that occur “when there is no correspondence of information or action between the midwife and the hospital staff” and “fractured articulations” that arise from “partial and incomplete correspondence.”42 A number of midwives were interviewed who no longer feel comfortable bringing patients to certain hospitals because of the negative response they received from health-care providers, sometimes to the detriment of the patient.
Can we improve the situation?
First, we need to choose our words carefully when we counsel women about labor and delivery, in recognition of the buzzwords used by advocates of home birth (“empowerment,” “choice”) and the sense of failure and distress some women feel when they eventually require heightened medical intervention.
Perhaps we should dispense with the term “failure,” as in failure to progress, failure to dilate, and so on, to avoid implying that this “failure” is the woman’s fault. And instead of saying that a patient’s pelvis is “adequate,” implying that another woman’s pelvis isn’t, we could use a term that sounds less judgmental.
We can also make the hospital environment more nurturing and supportive of women’s choices for labor, as long as safety isn’t compromised. And when we receive a transfer of a patient whose home delivery has gone awry, we should openly, efficiently, and professionally communicate with the home-delivery provider to best benefit the patient, regardless of our feelings on the subject.
Home birth isn’t going away
That’s my take on the literature. There are certainly data supporting the safety of home birth for the vast majority of women who choose it, but there is also a significant number of women who will experience unpredictable events that could be fatal if blood products or surgery isn’t rapidly available. For that reason, and in light of the very high stakes involved, I wonder: Why take that chance?
1. American Medical Association. Resolution on home deliveries. April 28, 2008. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/471/205.doc. Accessed July 1, 2009.
2. Boyle C. Ricki Lake’s home-birth film upsets AMA. New York Daily News. June 17, 2008. Available at: http://www.nydailynews.com/entertainment/2008/06/17/2008-06-17_ricki_lakes_homebirth_film_upsets_ama.html. Accessed July 1, 2009.
3. American College of Obstetricians and Gynecologists. ACOG statement on home births [press release]. Washington, DC: ACOG; Feb. 6, 2008. Available at: http://www.acog.org/from_home/publications/press_releases/nr02-06-08-2.cfm. Accessed July 1, 2009.
4. Celizic M. Ricki Lake takes on baby birthing industry. Available at: http://www.msnbc.msn.com/id/22592397/. Accessed June 29, 2009.
5. http://www.chicagotribune.com/news/opinion/oped/bal-op.homebirth13jul13,0,6603392.story. Accessed July 23, 2008.
6. National Center for Health Statistics. Vital statistics rates in the United States 1940–1960. Washington, DC: NCHS; 1968.
7. Hoyert DL. Maternal mortality and related concepts. National Center for Health Statistics. Vital Health Stat. 2007;3(33). Available at: http://www.cdc.gov/nchs/data/series/sr_03/sr03_033.pdf. Accessed July 9, 2009.
8. Högberg U. The decline in maternal mortality in Sweden: the role of community midwifery. Am J Public Health. 2004;94:1312-1320.
9. MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomized controlled trial. Br J Obstet Gynaecol. 1993;100:316-323.
10. Olsen O, Jewell MD. Home versus hospital birth. Cochrane Database Syst Rev. 2000;(2):CD000352.-
11. Newburn M. Culture, control and the birth environment. Pract Midwife. 2003;6:20-25.
12. Kmietowicz A. More than four in 10 women were not offered the choice of a home birth, report says. BMJ. 2007;335:112.-
13. Ackermann-Liebrich U, Voegeli T, Günter-Witt K, et al. Home versus hospital deliveries: follow up study of matched pairs for procedure and outcome. BMJ. 1996;313:1313-1318.
14. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: a descriptive study. BJOG. 2008;115:570-578.
15. Bastian H, Keirse MJ, Lancaster PA. Perinatal death associated with planned home birth in Australia: population based study. BMJ. 1998;317:384-388.
16. Fullerton JT, Navarro AM, Young SH. Outcomes of planned home birth: an integrative review. J Midwifery Womens Health. 2007;52:323-333.
17. Johnson KC, Daviss BA. Outcomes of planned home births with certified professional midwives: large prospective study in North America. BMJ. 2005;330:1416-1422.
18. Pang JW, Heffelfinger JD, Huang GJ, Benedetti TJ, Weiss NS. Outcomes of planned home births in Washington State: 1989-1996. Obstet Gynecol. 2002;100:253-259.
19. Burnett CA, 3rd, Jones JA, Rooks J, Chen CH, Tyler CW, Jr, Miller CA. Home delivery and neonatal mortality in North Carolina. JAMA. 1980;244:2741-2745.
20. Hinds MW, Bergeisen GH, Allen DT. Neonatal outcome in planned v unplanned out-of-hospital births in Kentucky. JAMA. 1985;253:1578-1582.
21. Schramm WF, Barnes DE, Bakewell JM. Neonatal mortality in Missouri home births, 1978–84. Am J Public Health. 1987;77:930-935.
22. Henderson J, Petrou S. Economic implications of home births and birth centers: a structured review. Birth. 2008;35:136-146.
23. Nocon JJ, McKenzie DK, Thomas LJ, Hansell RS. Shoulder dystocia: an analysis of risks and obstetric maneuvers. Am J Obstet Gynecol. 1993;168(6 Pt 1):1732-1739.
24. Mori R, Dougherty M, Whittle M. An estimation of intrapartum-related perinatal mortality rates for booked home births in England and Wales between 1994 and 2003. BJOG. 2008;115:554-559.
25. Latendresse G, Murphy PA, Fullerton JT. A description of the management and outcomes of vaginal birth after cesarean birth in the homebirth setting. J Midwifery Womens Health. 2005;50:386-391.
26. Lieberman E, Ernst EK, Rooks JP, Stapleton S, Flamm B. Results of the national study of vaginal birth after cesarean in birth centers. Obstet Gynecol. 2004;104(5 Pt 1):933-942.
27. Mehl-Madrona L, Mehl-Madrona MM. Physician and midwife-attended home births. Effects of breech, twin, and post-dates outcome data on mortality rates. J Nurse Midwifery. 1997;42:91-98.
28. How to become a NARM certified professional midwife (CPM). North American Registry of Midwives. Available at: http://www.narm.org/htb.htm. Accessed June 29,2009.
29. http://www.acog.org/publications/policy_statements/sop0602.cfm. Accessed August 26. 2008.
30. Reed A, Roberts E. State regulation of midwives: issues and options. J Midwifery Womens Health. 2000;45:130-149.
31. Anderson RE, Anderson DA. The cost-effectiveness of home birth. J Nurse Midwifery. 1999;44:30-35.
32. Shaw R, Kitzinger C. Calls to a home birth helpline: empowerment in childbirth. Soc Sci Med. 2005;61:2374-2383.
33. Janssen PA, Carty EA, Reime B. Satisfaction with planned place of birth among midwifery clients in British Columbia. J Midwifery Womens Health. 2006;51:91-97.
34. MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomized controlled trial. Br J Obstet Gynaecol. 1993;100:316-323.
35. Pitchforth E, Watson V, Tucker J, et al. Models of intrapartum care and women’s trade-offs in remote and rural Scotland: a mixed-methods study. BJOG. 2007;115:560-569.
36. Barbieri RL. How will we know it when we’ve got the right cesarean rate? OBG Management. 2008;20(6):10-15.
37. Sakala C. Midwifery care and out-of-hospital birth settings: how do they reduce unnecessary cesarean section births? Soc Sci Med. 1993;37:1233-1250.
38. Crossley ML. Childbirth, complications, and the illusion of “choice”: a case study. Fem Psychol. 2007;17:543-563.
39. http://www.acog.org/publications/policy_statements/sop0210.htm. Accessed September 4, 2008.
40. American Public Health Association. Increasing access to out-of-hospital maternity care services through state-regulated and nationally certified direct-entry midwives. January 1, 2001. Available at: http://www.apha.org/advocacy/policy/policysearch/default.htm?id=242. Accessed June 29, 2009.
41. American College of Nurse-Midwives. Backgrounds of CNMs/CMs rich in diversity. Available at: http://www.midwife.org/background_of_cnms.cfm. Accessed June 29, 2009.
42. Davis-Floyd R. Home-birth emergencies in the US and Mexico: the trouble with transport. Soc Sci Med. 2003;56:1911-1931.
43. Nagai T, Sobajima H, Iwasa M, et al. Neonatal sudden death due to Legionella pneumonia associated with water birth in a domestic spa bath. J Clin Microbiol. 2003;41:2227-2229.
44. Batton DG, Blackmon LR, Adamkin DH, et al. Committee on Fetus and Newborn, 2004–2005, American Academy of Pediatrics. Underwater births. Pediatrics. 2005;115:1413-1414.
45. Cluett ER, Nikodem VC, McCandlish RE, Burns EE. Immersion in water in pregnancy, labour and birth. Cochrane Database Syst Rev. 2004;(2):CD000111.-
46. Tuteur A. What’s in the water at waterbirth? Skeptical OB. February 19, 2009. Available at: http://skepticalob.blogspot.com/2009/02/whats-in-water-at-waterbirth.html. Accessed July 7, 2009.
47. Gilbert RE, Tookey PA. Perinatal mortality and morbidity among babies delivered in water: surveillance study and postal survey. BMJ. 1999;319:483-487.
48. Thoeni A, Zech N, Moroder L. Water birth and the risk of infection: experience after 1,500 water births. Pol J Gyn Invest. 2004;7(1/4):21-26.
49. Gilbert R. Water birth—a near-drowning experience. Pediatrics. 2002;110(2 Pt 1):409.-
50. E-mail correspondence from American College of Obstetrics and Gynecology staff. July 22, 2008.
1. American Medical Association. Resolution on home deliveries. April 28, 2008. Available at: http://www.ama-assn.org/ama1/pub/upload/mm/471/205.doc. Accessed July 1, 2009.
2. Boyle C. Ricki Lake’s home-birth film upsets AMA. New York Daily News. June 17, 2008. Available at: http://www.nydailynews.com/entertainment/2008/06/17/2008-06-17_ricki_lakes_homebirth_film_upsets_ama.html. Accessed July 1, 2009.
3. American College of Obstetricians and Gynecologists. ACOG statement on home births [press release]. Washington, DC: ACOG; Feb. 6, 2008. Available at: http://www.acog.org/from_home/publications/press_releases/nr02-06-08-2.cfm. Accessed July 1, 2009.
4. Celizic M. Ricki Lake takes on baby birthing industry. Available at: http://www.msnbc.msn.com/id/22592397/. Accessed June 29, 2009.
5. http://www.chicagotribune.com/news/opinion/oped/bal-op.homebirth13jul13,0,6603392.story. Accessed July 23, 2008.
6. National Center for Health Statistics. Vital statistics rates in the United States 1940–1960. Washington, DC: NCHS; 1968.
7. Hoyert DL. Maternal mortality and related concepts. National Center for Health Statistics. Vital Health Stat. 2007;3(33). Available at: http://www.cdc.gov/nchs/data/series/sr_03/sr03_033.pdf. Accessed July 9, 2009.
8. Högberg U. The decline in maternal mortality in Sweden: the role of community midwifery. Am J Public Health. 2004;94:1312-1320.
9. MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomized controlled trial. Br J Obstet Gynaecol. 1993;100:316-323.
10. Olsen O, Jewell MD. Home versus hospital birth. Cochrane Database Syst Rev. 2000;(2):CD000352.-
11. Newburn M. Culture, control and the birth environment. Pract Midwife. 2003;6:20-25.
12. Kmietowicz A. More than four in 10 women were not offered the choice of a home birth, report says. BMJ. 2007;335:112.-
13. Ackermann-Liebrich U, Voegeli T, Günter-Witt K, et al. Home versus hospital deliveries: follow up study of matched pairs for procedure and outcome. BMJ. 1996;313:1313-1318.
14. Amelink-Verburg MP, Verloove-Vanhorick SP, Hakkenberg RM, Veldhuijzen IM, Bennebroek Gravenhorst J, Buitendijk SE. Evaluation of 280,000 cases in Dutch midwifery practices: a descriptive study. BJOG. 2008;115:570-578.
15. Bastian H, Keirse MJ, Lancaster PA. Perinatal death associated with planned home birth in Australia: population based study. BMJ. 1998;317:384-388.
16. Fullerton JT, Navarro AM, Young SH. Outcomes of planned home birth: an integrative review. J Midwifery Womens Health. 2007;52:323-333.
17. Johnson KC, Daviss BA. Outcomes of planned home births with certified professional midwives: large prospective study in North America. BMJ. 2005;330:1416-1422.
18. Pang JW, Heffelfinger JD, Huang GJ, Benedetti TJ, Weiss NS. Outcomes of planned home births in Washington State: 1989-1996. Obstet Gynecol. 2002;100:253-259.
19. Burnett CA, 3rd, Jones JA, Rooks J, Chen CH, Tyler CW, Jr, Miller CA. Home delivery and neonatal mortality in North Carolina. JAMA. 1980;244:2741-2745.
20. Hinds MW, Bergeisen GH, Allen DT. Neonatal outcome in planned v unplanned out-of-hospital births in Kentucky. JAMA. 1985;253:1578-1582.
21. Schramm WF, Barnes DE, Bakewell JM. Neonatal mortality in Missouri home births, 1978–84. Am J Public Health. 1987;77:930-935.
22. Henderson J, Petrou S. Economic implications of home births and birth centers: a structured review. Birth. 2008;35:136-146.
23. Nocon JJ, McKenzie DK, Thomas LJ, Hansell RS. Shoulder dystocia: an analysis of risks and obstetric maneuvers. Am J Obstet Gynecol. 1993;168(6 Pt 1):1732-1739.
24. Mori R, Dougherty M, Whittle M. An estimation of intrapartum-related perinatal mortality rates for booked home births in England and Wales between 1994 and 2003. BJOG. 2008;115:554-559.
25. Latendresse G, Murphy PA, Fullerton JT. A description of the management and outcomes of vaginal birth after cesarean birth in the homebirth setting. J Midwifery Womens Health. 2005;50:386-391.
26. Lieberman E, Ernst EK, Rooks JP, Stapleton S, Flamm B. Results of the national study of vaginal birth after cesarean in birth centers. Obstet Gynecol. 2004;104(5 Pt 1):933-942.
27. Mehl-Madrona L, Mehl-Madrona MM. Physician and midwife-attended home births. Effects of breech, twin, and post-dates outcome data on mortality rates. J Nurse Midwifery. 1997;42:91-98.
28. How to become a NARM certified professional midwife (CPM). North American Registry of Midwives. Available at: http://www.narm.org/htb.htm. Accessed June 29,2009.
29. http://www.acog.org/publications/policy_statements/sop0602.cfm. Accessed August 26. 2008.
30. Reed A, Roberts E. State regulation of midwives: issues and options. J Midwifery Womens Health. 2000;45:130-149.
31. Anderson RE, Anderson DA. The cost-effectiveness of home birth. J Nurse Midwifery. 1999;44:30-35.
32. Shaw R, Kitzinger C. Calls to a home birth helpline: empowerment in childbirth. Soc Sci Med. 2005;61:2374-2383.
33. Janssen PA, Carty EA, Reime B. Satisfaction with planned place of birth among midwifery clients in British Columbia. J Midwifery Womens Health. 2006;51:91-97.
34. MacVicar J, Dobbie G, Owen-Johnstone L, Jagger C, Hopkins M, Kennedy J. Simulated home delivery in hospital: a randomized controlled trial. Br J Obstet Gynaecol. 1993;100:316-323.
35. Pitchforth E, Watson V, Tucker J, et al. Models of intrapartum care and women’s trade-offs in remote and rural Scotland: a mixed-methods study. BJOG. 2007;115:560-569.
36. Barbieri RL. How will we know it when we’ve got the right cesarean rate? OBG Management. 2008;20(6):10-15.
37. Sakala C. Midwifery care and out-of-hospital birth settings: how do they reduce unnecessary cesarean section births? Soc Sci Med. 1993;37:1233-1250.
38. Crossley ML. Childbirth, complications, and the illusion of “choice”: a case study. Fem Psychol. 2007;17:543-563.
39. http://www.acog.org/publications/policy_statements/sop0210.htm. Accessed September 4, 2008.
40. American Public Health Association. Increasing access to out-of-hospital maternity care services through state-regulated and nationally certified direct-entry midwives. January 1, 2001. Available at: http://www.apha.org/advocacy/policy/policysearch/default.htm?id=242. Accessed June 29, 2009.
41. American College of Nurse-Midwives. Backgrounds of CNMs/CMs rich in diversity. Available at: http://www.midwife.org/background_of_cnms.cfm. Accessed June 29, 2009.
42. Davis-Floyd R. Home-birth emergencies in the US and Mexico: the trouble with transport. Soc Sci Med. 2003;56:1911-1931.
43. Nagai T, Sobajima H, Iwasa M, et al. Neonatal sudden death due to Legionella pneumonia associated with water birth in a domestic spa bath. J Clin Microbiol. 2003;41:2227-2229.
44. Batton DG, Blackmon LR, Adamkin DH, et al. Committee on Fetus and Newborn, 2004–2005, American Academy of Pediatrics. Underwater births. Pediatrics. 2005;115:1413-1414.
45. Cluett ER, Nikodem VC, McCandlish RE, Burns EE. Immersion in water in pregnancy, labour and birth. Cochrane Database Syst Rev. 2004;(2):CD000111.-
46. Tuteur A. What’s in the water at waterbirth? Skeptical OB. February 19, 2009. Available at: http://skepticalob.blogspot.com/2009/02/whats-in-water-at-waterbirth.html. Accessed July 7, 2009.
47. Gilbert RE, Tookey PA. Perinatal mortality and morbidity among babies delivered in water: surveillance study and postal survey. BMJ. 1999;319:483-487.
48. Thoeni A, Zech N, Moroder L. Water birth and the risk of infection: experience after 1,500 water births. Pol J Gyn Invest. 2004;7(1/4):21-26.
49. Gilbert R. Water birth—a near-drowning experience. Pediatrics. 2002;110(2 Pt 1):409.-
50. E-mail correspondence from American College of Obstetrics and Gynecology staff. July 22, 2008.



