User login
Nonhealing Friable Nodule on the Distal Edge of the Toe
Nonhealing Friable Nodule on the Distal Edge of the Toe
THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
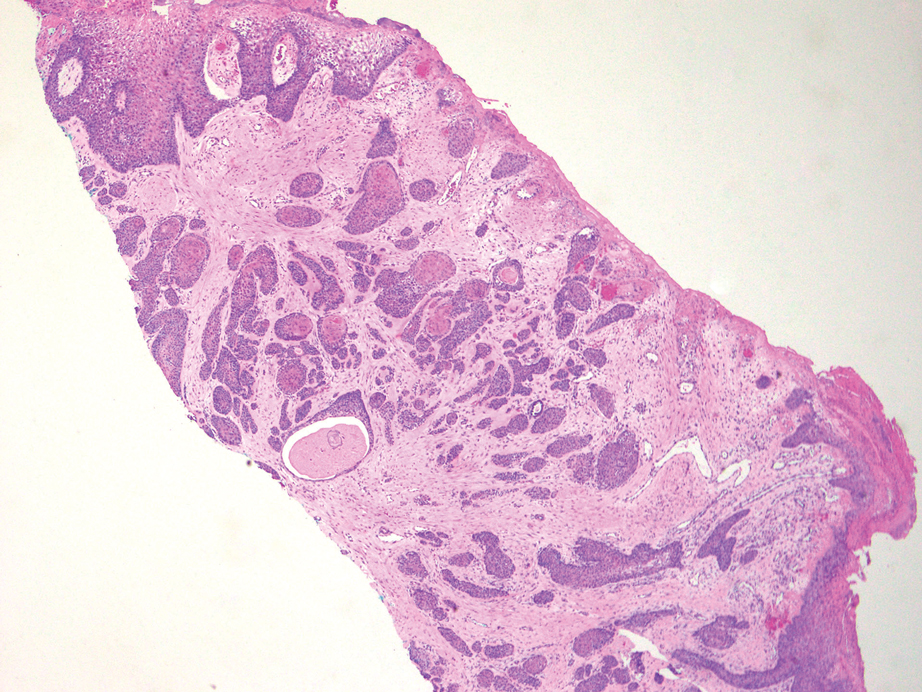
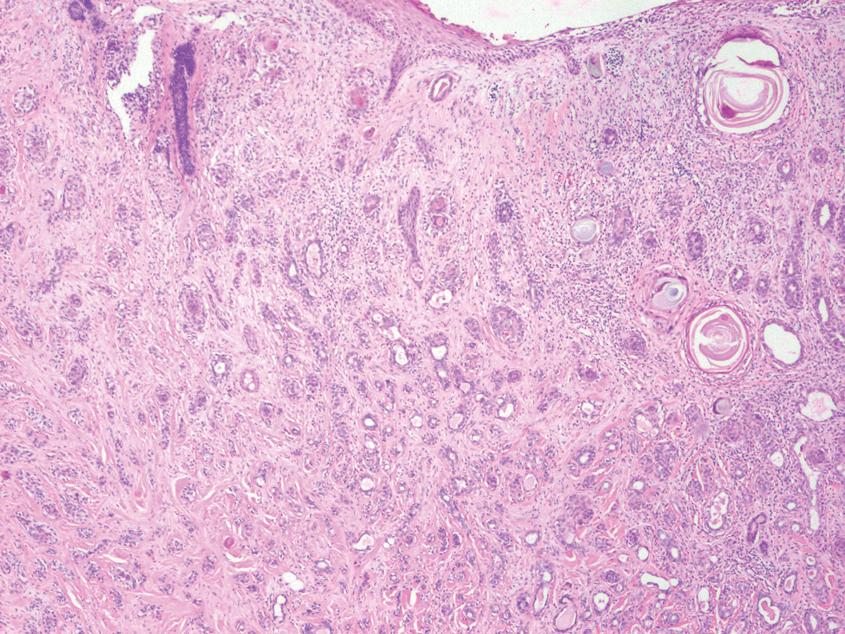
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1
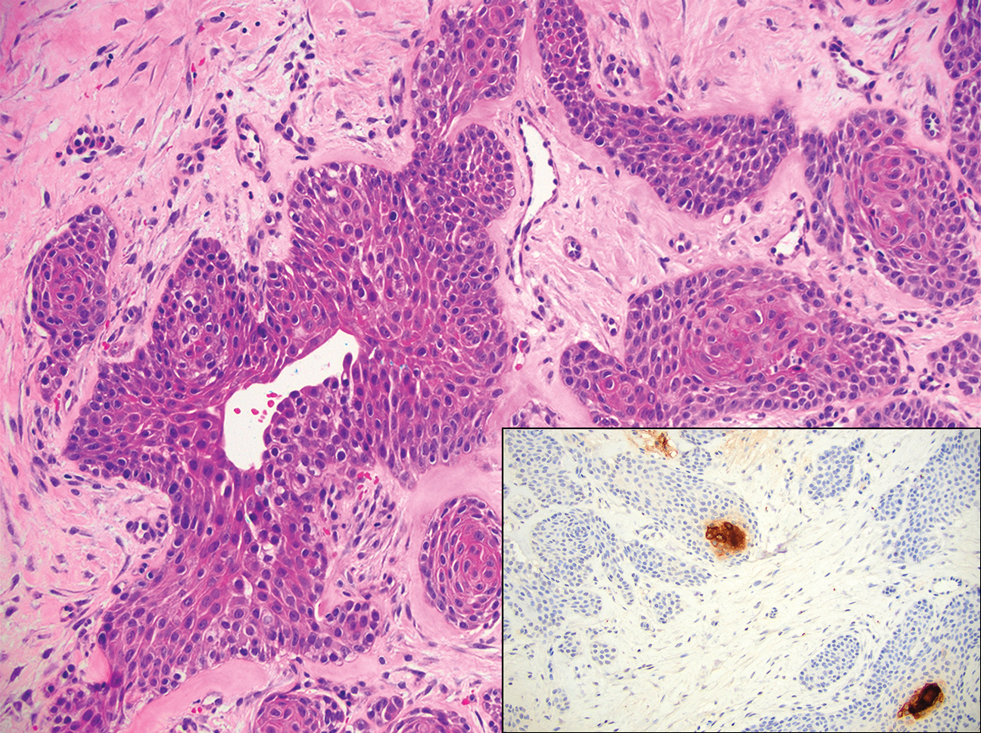
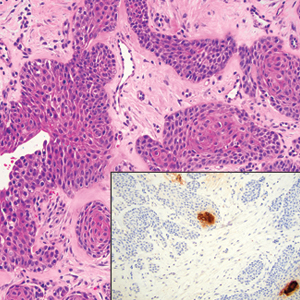
The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
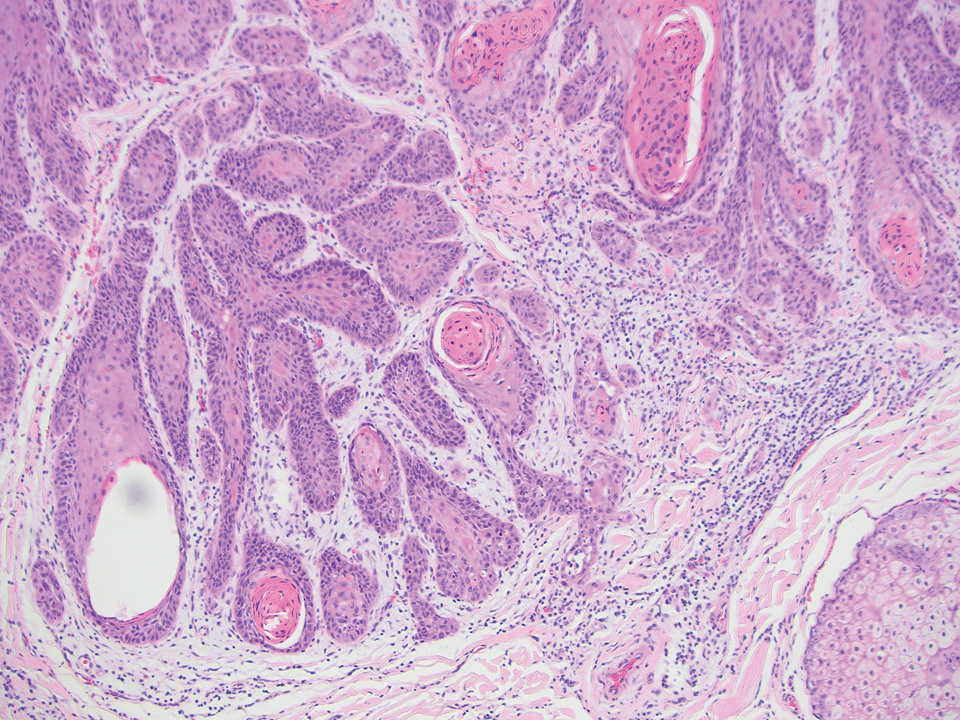
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4
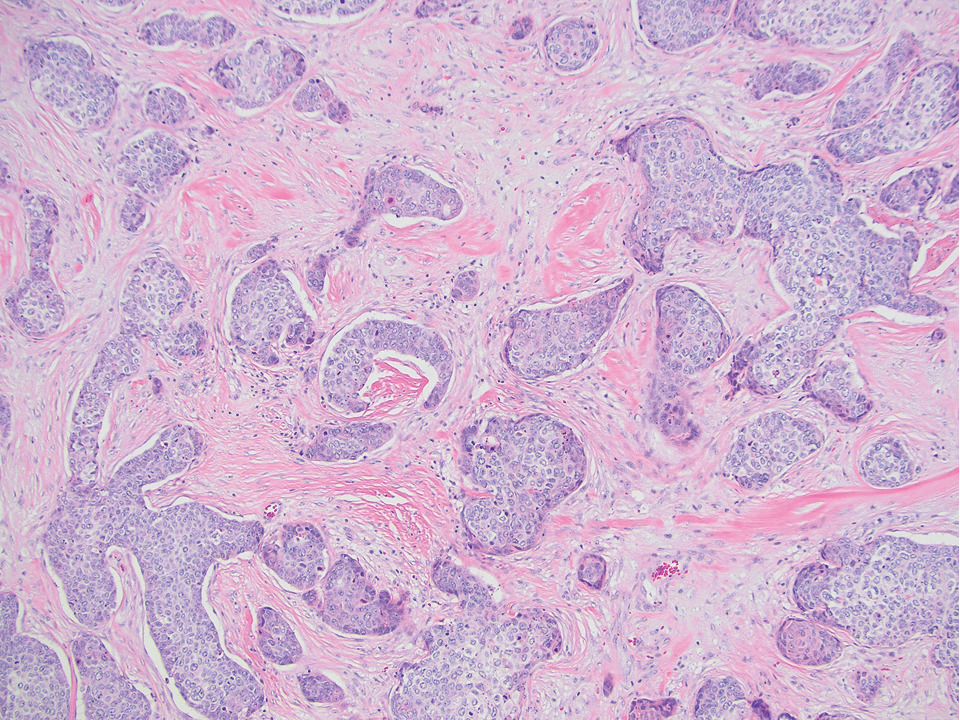
Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5
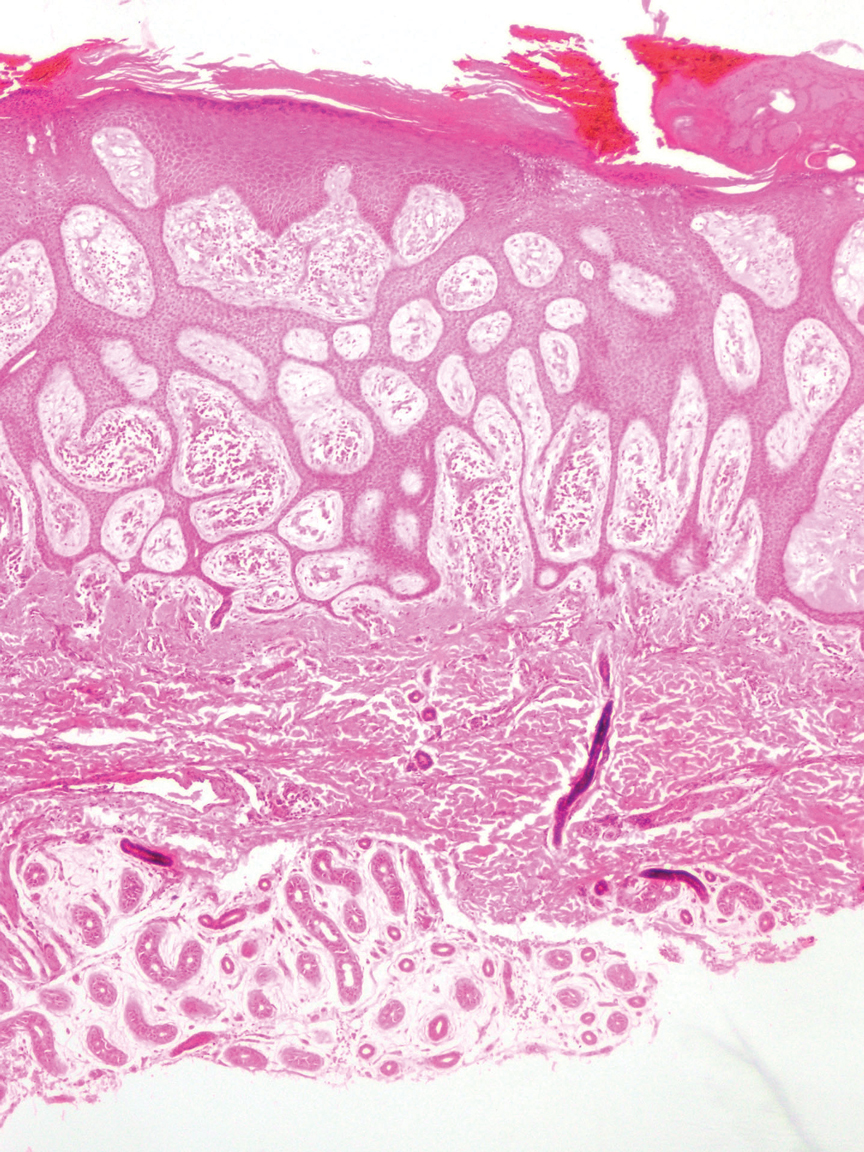
Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.
- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1

The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4

Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5

Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.

THE DIAGNOSIS: Squamoid Eccrine Ductal Carcinoma
Immunohistochemical staining of the biopsy specimen showed neoplastic aggregates that were diffusely positive for pancytokeratin and strongly positive for cytokeratin (CK) 5/6. Epithelial membrane antigen (EMA) and CK7 also were positive, CAM 5.2 was partially positive, and carcinoembryonic antigen (CEA) was focally positive (periluminal); S100 was negative. Given the histologic findings of irregular infiltrative cords and stranding exhibiting ductal differentiation in a fibrotic stroma in combination with the staining pattern, a diagnosis of squamous eccrine ductal carcinoma (SEDC) was made.
Squamoid eccrine ductal carcinoma is a rare primary cutaneous tumor with aggressive features that can be confused both clinically and histologically with squamous cell carcinoma (SCC). Histologically, SEDC is a biphasic tumor. If a shallow histologic specimen is obtained, it may be indistinguishable from a well-differentiated SCC (Figure 1). A deeper biopsy reveals irregular infiltrative cords and strands exhibiting ductal differentiation in a fibrotic stroma.1

The immunohistochemical staining pattern of SEDC is similar to that of SCC, showing diffuse staining with pancytokeratin (AE1/AE3), CK 5/6, CK7, p63, and EMA. What distinguishes SEDC from SCC is that CEA highlights areas of glandular differentiation. An additional histologic feature seen commonly with SEDC is perineural invasion.
The etiology of SEDC remains controversial; although it originally was considered an aggressive variant of SCC along the same continuum as adenosquamous carcinoma, the fifth edition of the WHO Classification of Skin Tumors2 has categorized SEDC as an adnexal neoplasm. Our patient demonstrated an atypical presentation of this tumor, which has been most commonly described in the literature as manifesting on the head, neck, or upper extremities in older adults.3 Mohs micrographic surgery is the recommended treatment for this aggressive tumor.3
The differential diagnosis for SEDC includes microcystic adnexal carcinoma, porocarcinoma, and eccrine syringofibroadenoma. Microcystic adnexal carcinoma is a rare, low-grade tumor of the sweat glands that typically manifests as a firm pink papule or plaque in the head and neck region. Microscopically, it demonstrates cords of basaloid cells in a paisley-tie tadpole pattern with a dense pink to red stroma and horn cysts (Figure 2). Histologic differential diagnoses include syringoma, morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and trichoadenoma. Carcinoembryonic antigen stains positive in microcystic adnexal carcinoma, which helps distinguish it from basal cell carcinoma and SCC. Surgical excision or Mohs surgery are recommended for management.4

Porocarcinoma is a malignant skin tumor that originates from the intraepidermal sweat gland ducts. It also has been proposed that porocarcinoma develops from benign eccrine poroma. Porocarcinoma often is seen in elderly individuals, with a predilection for the lower extremities. Porocarcinoma demonstrates diverse clinical and histopathologic features, which can make diagnosis challenging. Histopathologically, porocarcinoma has an infiltrative growth pattern, with large basaloid epithelial cells that demonstrate ductal differentiation, cytologic atypia, increased mitotic activity, and tumor necrosis (Figure 3). Some porocarcinomas may exhibit squamous-cell, spindle-cell, or clear-cell differentiation. Neoplastic cells stain positive for CEA, EMA, and CD117, which can assist in distinguishing porocarcinoma from cutaneous SCC.5

Eccrine syringofibroadenoma is an unusual benign cutaneous adnexal tumor that manifests mostly in individuals aged 40 years or older. It develops as single or multiple lesions that usually affect the lower extremities. Histologically, eccrine syringofibroadenoma demonstrates unique findings of anastomosing ducts and monomorphous epithelial cells within a fibrovascular stroma (Figure 4). On immunohistochemistry, it stains positive for EMA, CEA, high-molecular-weight kininogen, and filaggrin.6 Periodic acid–Schiff staining also is positive.

- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
- Svoboda SA, Rush PS, Garofola CJ, et al. Squamoid eccrine ductal carcinoma. Cutis. 2021;107:E5-E9. doi:10.12788/cutis.0280
- WHO Classification of Tumours Editorial Board. Skin tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2023.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760. doi:10.1097/PAS.0000000000000599
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated April 24, 2023. Accessed August 3, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557857/
- Tsiogka A, Koumaki D, Kyriazopoulou M, et al. Eccrine porocarcinoma: a review of the literature. Diagnostics (Basel). 2023;13:8. doi:10.3390/diagnostics13081431
- Ko EJ, Park KY, Kwon HJ, et al. Eccrine syringofibroadenoma in a patient with long-standing exfoliative dermatitis. Ann Dermatol. 2016;28:765-768. doi:10.5021/ad.2016.28.6.765
Nonhealing Friable Nodule on the Distal Edge of the Toe
Nonhealing Friable Nodule on the Distal Edge of the Toe
A 37-year-old woman with no notable medical history presented to the dermatology clinic with a nonhealing wound on the left fifth toe of 10 month’s duration. The patient reported that the wound developed after burning the toe on an indoor space heater. Physical examination revealed a friable pink papule with a hemorrhagic crust. A biopsy of the lesion was performed.