User login
The fourth trimester: Achieving improved postpartum care
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.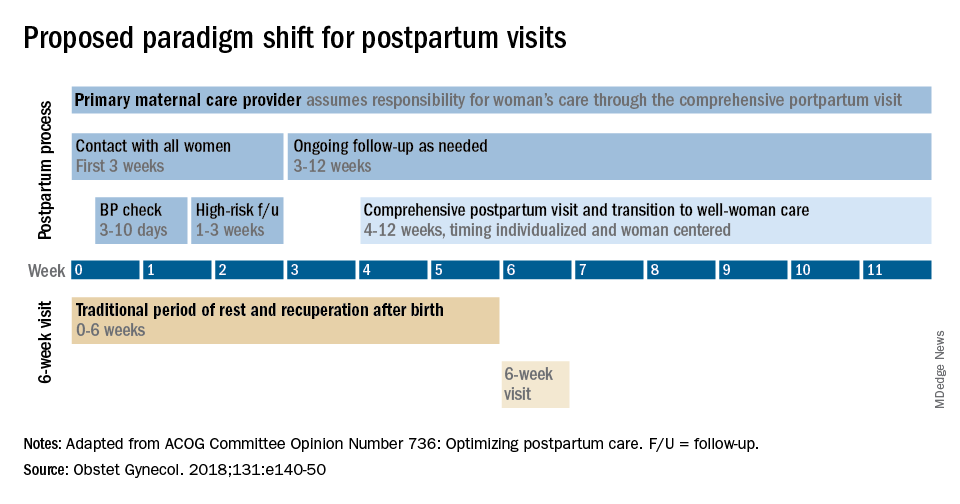
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
A step away from immediate umbilical cord clamping
The common practice of immediate cord clamping, which generally means clamping within 15-20 seconds after birth, was fueled by efforts to reduce the risk of postpartum hemorrhage, a leading cause of maternal death worldwide. Immediate clamping was part of a full active management intervention recommended in 2007 by the World Health Organization, along with the use of uterotonics (generally oxytocin) immediately after birth and controlled cord traction to quickly deliver the placenta.
Adoption of the WHO-recommended “active management during the third stage of labor” (AMTSL) worked, leading to a 70% reduction in postpartum hemorrhage and a 60% reduction in blood transfusion over passive management. However, it appears that immediate cord clamping has not played an important role in these reductions. Several randomized controlled trials have shown that early clamping does not impact the risk of postpartum hemorrhage (> 1000 cc or > 500 cc), nor does it impact the need for manual removal of the placenta or the need for blood transfusion.
Instead, the critical component of the AMTSL package appears to be administration of a uterotonic, as reported in a large WHO-directed multicenter clinical trial published in 2012. The study also found that women who received controlled cord traction bled an average of 11 cc less – an insignificant difference – than did women who delivered their placentas by their own effort. Moreover, they had a third stage of labor that was an average of 6 minutes shorter (Lancet 2012;379:1721-7).

With assurance that the timing of umbilical cord clamping does not impact maternal outcomes, investigators have begun to look more at the impact of immediate versus delayed cord clamping on the health of the baby.
Thus far, the issues in this arena are a bit more complicated than on the maternal side. There are indications, however, that slight delays in umbilical cord clamping may be beneficial for the newborn – particularly for preterm infants, who appear in systemic reviews to have a nearly 50% reduction in intraventricular hemorrhage when clamping is delayed.
Timing in term infants
The theoretical benefits of delayed cord clamping include increased neonatal blood volume (improved perfusion and decreased organ injury), more time for spontaneous breathing (reduced risks of resuscitation and a smoother transition of cardiopulmonary and cerebral circulation), and increased stem cells for the infant (anti-inflammatory, neurotropic, and neuroprotective effects).
Theoretically, delayed clamping will increase the infant’s iron stores and lower the incidence of iron deficiency anemia during infancy. This is particularly relevant in developing countries, where up to 50% of infants have anemia by 1 year of age. Anemia is consistently associated with abnormal neurodevelopment, and treatment may not always reverse developmental issues.
On the negative side, delayed clamping is associated with theoretical concerns about hyperbilirubinemia and jaundice, hypothermia, polycythemia, and delays in the bonding of infants and mothers.
For term infants, our best reading on the benefits and risks of delayed umbilical cord clamping comes from a 2013 Cochrane systematic review that assessed results from 15 randomized controlled trials involving 3,911 women and infant pairs. Early cord clamping was generally carried out within 60 seconds of birth, whereas delayed cord clamping involved clamping the umbilical cord more than 1 minute after birth or when cord pulsation has ceased.
The review found that delayed clamping was associated with a significantly higher neonatal hemoglobin concentration at 24-48 hours postpartum (a weighted mean difference of 2 g/dL) and increased iron reserves up to 6 months after birth. Infants in the early clamping group were more than twice as likely to be iron deficient at 3-6 months compared with infants whose cord clamping was delayed (Cochrane Database Syst. Rev. 2013;7:CD004074)
There were no significant differences between early and late clamping in neonatal mortality or for most other neonatal morbidity outcomes. Delayed clamping also did not increase the risk of severe postpartum hemorrhage, blood loss, or reduced hemoglobin levels in mothers.
The downside to delayed cord clamping was an increased risk of jaundice requiring phototherapy. Infants in the later cord clamping group were 40% more likely to need phototherapy – a difference that equates to 3% of infants in the early clamping group and 5% of infants in the late clamping group.
Data were insufficient in the Cochrane review to draw reliable conclusions about the comparative effects on other short-term outcomes such as symptomatic polycythemia, respiratory problems, hypothermia, and infection, as data were limited on long-term outcomes.
In practice, this means that the risk of jaundice must be weighed against the risk of iron deficiency. In developed countries we have the resources both to increase iron stores of infants and to provide phototherapy. While the WHO recommends umbilical cord clamping after 1-3 minutes to improve an infant’s iron status, I do not believe the evidence is strong enough to universally adopt such delayed cord clamping in the United States.
Considering the risks of jaundice and the relative infrequency of iron deficiency in the United States, we should not routinely delay clamping for term infants at this point.
A recent committee opinion developed by the American College of Obstetricians and Gynecologists and endorsed by the American Academy of Pediatrics (No. 543, December 2012) captures this view by concluding that “insufficient evidence exists to support or to refute the benefits from delayed umbilical cord clamping for term infants that are born in settings with rich resources.” Although the ACOG opinion preceded the Cochrane review, the committee, of which I was a member, reviewed much of the same literature.
Timing in preterm infants
Preterm neonates are at increased risk of temperature dysregulation, hypotension, and the need for rapid initial pediatric care and blood transfusion. The increased risk of intraventricular hemorrhage and necrotizing enterocolitis in preterm infants is possibly related to the increased risk of hypotension.
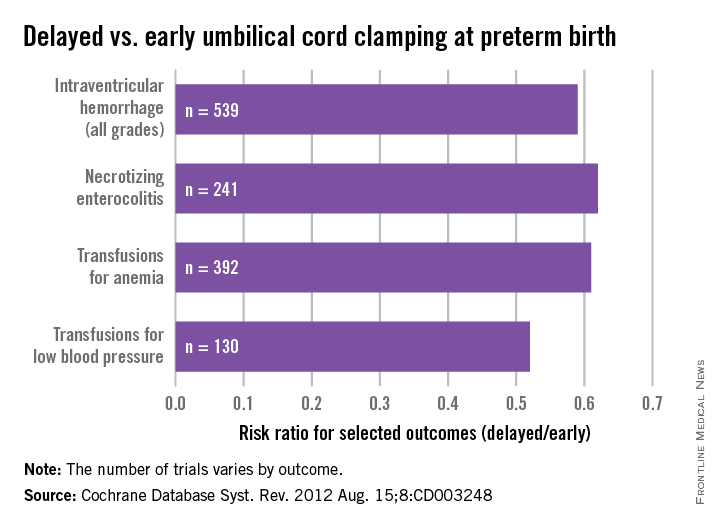
As with term infants, a 2012 Cochrane systematic review offers good insight on our current knowledge. This review of umbilical cord clamping at preterm birth covers 15 studies that included 738 infants delivered between 24 and 36 weeks of gestation. The timing of umbilical cord clamping ranged from 25 seconds to a maximum of 180 seconds (Cochrane Database Syst. Rev. 2012;8:CD003248).
Delayed cord clamping was associated with fewer transfusions for anemia or low blood pressure, less intraventricular hemorrhage of all grades (relative risk 0.59), and a lower risk for necrotizing enterocolitis (relative risk 0.62), compared with immediate clamping.
While there were no clear differences with respect to severe intraventricular hemorrhage (grades 3-4), the nearly 50% reduction in intraventricular hemorrhage overall among deliveries with delayed clamping was significant enough to prompt ACOG to conclude that delayed cord clamping should be considered for preterm infants. This reduction in intraventricular hemorrhage appears to be the single most important benefit, based on current findings.
The data on cord clamping in preterm infants are suggestive of benefit, but are not robust. The studies published thus far have been small, and many of them, as the 2012 Cochrane review points out, involved incomplete reporting and wide confidence intervals. Moreover, just as with the studies on term infants, there has been a lack of long-term follow-up in most of the published trials.
When considering delayed cord clamping in preterm infants, as the ACOG Committee Opinion recommends, I urge focusing on earlier gestational ages. Allowing more placental transfusion at births that occur at or after 36 weeks of gestation may not make much sense because by that point the risk of intraventricular hemorrhage is almost nonexistent.
Our practice and the future
At our institution, births that occur at less than 32 weeks of gestation are eligible for delayed umbilical cord clamping, usually at 30-45 seconds after birth. The main contraindications are placental abruption and multiples.
We do not perform any milking or stripping of the umbilical cord, as the risks are unknown and it is not yet clear whether such practices are equivalent to delayed cord clamping. Compared with delayed cord clamping, which is a natural passive transfusion of placental blood to the infant, milking and stripping are not physiologic.
Additional data from an ongoing large international multicenter study, the Australian Placental Transfusion Study, may resolve some of the current controversy. This study is evaluating the cord clamping in neonates < 30 weeks’ gestation. Another study ongoing in Europe should also provide more information.
These studies – and other trials that are larger and longer than the trials published thus far – are necessary to evaluate long-term outcomes and to establish the ideal timing for umbilical cord clamping. Research is also needed to evaluate the management of the third stage of labor relative to umbilical cord clamping as well as the timing in relation to the initiation of voluntary or assisted ventilation.
Dr. Macones said he had no relevant financial disclosures.
Dr. Macones is the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis.
The common practice of immediate cord clamping, which generally means clamping within 15-20 seconds after birth, was fueled by efforts to reduce the risk of postpartum hemorrhage, a leading cause of maternal death worldwide. Immediate clamping was part of a full active management intervention recommended in 2007 by the World Health Organization, along with the use of uterotonics (generally oxytocin) immediately after birth and controlled cord traction to quickly deliver the placenta.
Adoption of the WHO-recommended “active management during the third stage of labor” (AMTSL) worked, leading to a 70% reduction in postpartum hemorrhage and a 60% reduction in blood transfusion over passive management. However, it appears that immediate cord clamping has not played an important role in these reductions. Several randomized controlled trials have shown that early clamping does not impact the risk of postpartum hemorrhage (> 1000 cc or > 500 cc), nor does it impact the need for manual removal of the placenta or the need for blood transfusion.
Instead, the critical component of the AMTSL package appears to be administration of a uterotonic, as reported in a large WHO-directed multicenter clinical trial published in 2012. The study also found that women who received controlled cord traction bled an average of 11 cc less – an insignificant difference – than did women who delivered their placentas by their own effort. Moreover, they had a third stage of labor that was an average of 6 minutes shorter (Lancet 2012;379:1721-7).

With assurance that the timing of umbilical cord clamping does not impact maternal outcomes, investigators have begun to look more at the impact of immediate versus delayed cord clamping on the health of the baby.
Thus far, the issues in this arena are a bit more complicated than on the maternal side. There are indications, however, that slight delays in umbilical cord clamping may be beneficial for the newborn – particularly for preterm infants, who appear in systemic reviews to have a nearly 50% reduction in intraventricular hemorrhage when clamping is delayed.
Timing in term infants
The theoretical benefits of delayed cord clamping include increased neonatal blood volume (improved perfusion and decreased organ injury), more time for spontaneous breathing (reduced risks of resuscitation and a smoother transition of cardiopulmonary and cerebral circulation), and increased stem cells for the infant (anti-inflammatory, neurotropic, and neuroprotective effects).
Theoretically, delayed clamping will increase the infant’s iron stores and lower the incidence of iron deficiency anemia during infancy. This is particularly relevant in developing countries, where up to 50% of infants have anemia by 1 year of age. Anemia is consistently associated with abnormal neurodevelopment, and treatment may not always reverse developmental issues.
On the negative side, delayed clamping is associated with theoretical concerns about hyperbilirubinemia and jaundice, hypothermia, polycythemia, and delays in the bonding of infants and mothers.
For term infants, our best reading on the benefits and risks of delayed umbilical cord clamping comes from a 2013 Cochrane systematic review that assessed results from 15 randomized controlled trials involving 3,911 women and infant pairs. Early cord clamping was generally carried out within 60 seconds of birth, whereas delayed cord clamping involved clamping the umbilical cord more than 1 minute after birth or when cord pulsation has ceased.
The review found that delayed clamping was associated with a significantly higher neonatal hemoglobin concentration at 24-48 hours postpartum (a weighted mean difference of 2 g/dL) and increased iron reserves up to 6 months after birth. Infants in the early clamping group were more than twice as likely to be iron deficient at 3-6 months compared with infants whose cord clamping was delayed (Cochrane Database Syst. Rev. 2013;7:CD004074)
There were no significant differences between early and late clamping in neonatal mortality or for most other neonatal morbidity outcomes. Delayed clamping also did not increase the risk of severe postpartum hemorrhage, blood loss, or reduced hemoglobin levels in mothers.
The downside to delayed cord clamping was an increased risk of jaundice requiring phototherapy. Infants in the later cord clamping group were 40% more likely to need phototherapy – a difference that equates to 3% of infants in the early clamping group and 5% of infants in the late clamping group.
Data were insufficient in the Cochrane review to draw reliable conclusions about the comparative effects on other short-term outcomes such as symptomatic polycythemia, respiratory problems, hypothermia, and infection, as data were limited on long-term outcomes.
In practice, this means that the risk of jaundice must be weighed against the risk of iron deficiency. In developed countries we have the resources both to increase iron stores of infants and to provide phototherapy. While the WHO recommends umbilical cord clamping after 1-3 minutes to improve an infant’s iron status, I do not believe the evidence is strong enough to universally adopt such delayed cord clamping in the United States.
Considering the risks of jaundice and the relative infrequency of iron deficiency in the United States, we should not routinely delay clamping for term infants at this point.
A recent committee opinion developed by the American College of Obstetricians and Gynecologists and endorsed by the American Academy of Pediatrics (No. 543, December 2012) captures this view by concluding that “insufficient evidence exists to support or to refute the benefits from delayed umbilical cord clamping for term infants that are born in settings with rich resources.” Although the ACOG opinion preceded the Cochrane review, the committee, of which I was a member, reviewed much of the same literature.
Timing in preterm infants
Preterm neonates are at increased risk of temperature dysregulation, hypotension, and the need for rapid initial pediatric care and blood transfusion. The increased risk of intraventricular hemorrhage and necrotizing enterocolitis in preterm infants is possibly related to the increased risk of hypotension.
As with term infants, a 2012 Cochrane systematic review offers good insight on our current knowledge. This review of umbilical cord clamping at preterm birth covers 15 studies that included 738 infants delivered between 24 and 36 weeks of gestation. The timing of umbilical cord clamping ranged from 25 seconds to a maximum of 180 seconds (Cochrane Database Syst. Rev. 2012;8:CD003248).
Delayed cord clamping was associated with fewer transfusions for anemia or low blood pressure, less intraventricular hemorrhage of all grades (relative risk 0.59), and a lower risk for necrotizing enterocolitis (relative risk 0.62), compared with immediate clamping.
While there were no clear differences with respect to severe intraventricular hemorrhage (grades 3-4), the nearly 50% reduction in intraventricular hemorrhage overall among deliveries with delayed clamping was significant enough to prompt ACOG to conclude that delayed cord clamping should be considered for preterm infants. This reduction in intraventricular hemorrhage appears to be the single most important benefit, based on current findings.
The data on cord clamping in preterm infants are suggestive of benefit, but are not robust. The studies published thus far have been small, and many of them, as the 2012 Cochrane review points out, involved incomplete reporting and wide confidence intervals. Moreover, just as with the studies on term infants, there has been a lack of long-term follow-up in most of the published trials.
When considering delayed cord clamping in preterm infants, as the ACOG Committee Opinion recommends, I urge focusing on earlier gestational ages. Allowing more placental transfusion at births that occur at or after 36 weeks of gestation may not make much sense because by that point the risk of intraventricular hemorrhage is almost nonexistent.
Our practice and the future
At our institution, births that occur at less than 32 weeks of gestation are eligible for delayed umbilical cord clamping, usually at 30-45 seconds after birth. The main contraindications are placental abruption and multiples.
We do not perform any milking or stripping of the umbilical cord, as the risks are unknown and it is not yet clear whether such practices are equivalent to delayed cord clamping. Compared with delayed cord clamping, which is a natural passive transfusion of placental blood to the infant, milking and stripping are not physiologic.
Additional data from an ongoing large international multicenter study, the Australian Placental Transfusion Study, may resolve some of the current controversy. This study is evaluating the cord clamping in neonates < 30 weeks’ gestation. Another study ongoing in Europe should also provide more information.
These studies – and other trials that are larger and longer than the trials published thus far – are necessary to evaluate long-term outcomes and to establish the ideal timing for umbilical cord clamping. Research is also needed to evaluate the management of the third stage of labor relative to umbilical cord clamping as well as the timing in relation to the initiation of voluntary or assisted ventilation.
Dr. Macones said he had no relevant financial disclosures.
Dr. Macones is the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis.
The common practice of immediate cord clamping, which generally means clamping within 15-20 seconds after birth, was fueled by efforts to reduce the risk of postpartum hemorrhage, a leading cause of maternal death worldwide. Immediate clamping was part of a full active management intervention recommended in 2007 by the World Health Organization, along with the use of uterotonics (generally oxytocin) immediately after birth and controlled cord traction to quickly deliver the placenta.
Adoption of the WHO-recommended “active management during the third stage of labor” (AMTSL) worked, leading to a 70% reduction in postpartum hemorrhage and a 60% reduction in blood transfusion over passive management. However, it appears that immediate cord clamping has not played an important role in these reductions. Several randomized controlled trials have shown that early clamping does not impact the risk of postpartum hemorrhage (> 1000 cc or > 500 cc), nor does it impact the need for manual removal of the placenta or the need for blood transfusion.
Instead, the critical component of the AMTSL package appears to be administration of a uterotonic, as reported in a large WHO-directed multicenter clinical trial published in 2012. The study also found that women who received controlled cord traction bled an average of 11 cc less – an insignificant difference – than did women who delivered their placentas by their own effort. Moreover, they had a third stage of labor that was an average of 6 minutes shorter (Lancet 2012;379:1721-7).

With assurance that the timing of umbilical cord clamping does not impact maternal outcomes, investigators have begun to look more at the impact of immediate versus delayed cord clamping on the health of the baby.
Thus far, the issues in this arena are a bit more complicated than on the maternal side. There are indications, however, that slight delays in umbilical cord clamping may be beneficial for the newborn – particularly for preterm infants, who appear in systemic reviews to have a nearly 50% reduction in intraventricular hemorrhage when clamping is delayed.
Timing in term infants
The theoretical benefits of delayed cord clamping include increased neonatal blood volume (improved perfusion and decreased organ injury), more time for spontaneous breathing (reduced risks of resuscitation and a smoother transition of cardiopulmonary and cerebral circulation), and increased stem cells for the infant (anti-inflammatory, neurotropic, and neuroprotective effects).
Theoretically, delayed clamping will increase the infant’s iron stores and lower the incidence of iron deficiency anemia during infancy. This is particularly relevant in developing countries, where up to 50% of infants have anemia by 1 year of age. Anemia is consistently associated with abnormal neurodevelopment, and treatment may not always reverse developmental issues.
On the negative side, delayed clamping is associated with theoretical concerns about hyperbilirubinemia and jaundice, hypothermia, polycythemia, and delays in the bonding of infants and mothers.
For term infants, our best reading on the benefits and risks of delayed umbilical cord clamping comes from a 2013 Cochrane systematic review that assessed results from 15 randomized controlled trials involving 3,911 women and infant pairs. Early cord clamping was generally carried out within 60 seconds of birth, whereas delayed cord clamping involved clamping the umbilical cord more than 1 minute after birth or when cord pulsation has ceased.
The review found that delayed clamping was associated with a significantly higher neonatal hemoglobin concentration at 24-48 hours postpartum (a weighted mean difference of 2 g/dL) and increased iron reserves up to 6 months after birth. Infants in the early clamping group were more than twice as likely to be iron deficient at 3-6 months compared with infants whose cord clamping was delayed (Cochrane Database Syst. Rev. 2013;7:CD004074)
There were no significant differences between early and late clamping in neonatal mortality or for most other neonatal morbidity outcomes. Delayed clamping also did not increase the risk of severe postpartum hemorrhage, blood loss, or reduced hemoglobin levels in mothers.
The downside to delayed cord clamping was an increased risk of jaundice requiring phototherapy. Infants in the later cord clamping group were 40% more likely to need phototherapy – a difference that equates to 3% of infants in the early clamping group and 5% of infants in the late clamping group.
Data were insufficient in the Cochrane review to draw reliable conclusions about the comparative effects on other short-term outcomes such as symptomatic polycythemia, respiratory problems, hypothermia, and infection, as data were limited on long-term outcomes.
In practice, this means that the risk of jaundice must be weighed against the risk of iron deficiency. In developed countries we have the resources both to increase iron stores of infants and to provide phototherapy. While the WHO recommends umbilical cord clamping after 1-3 minutes to improve an infant’s iron status, I do not believe the evidence is strong enough to universally adopt such delayed cord clamping in the United States.
Considering the risks of jaundice and the relative infrequency of iron deficiency in the United States, we should not routinely delay clamping for term infants at this point.
A recent committee opinion developed by the American College of Obstetricians and Gynecologists and endorsed by the American Academy of Pediatrics (No. 543, December 2012) captures this view by concluding that “insufficient evidence exists to support or to refute the benefits from delayed umbilical cord clamping for term infants that are born in settings with rich resources.” Although the ACOG opinion preceded the Cochrane review, the committee, of which I was a member, reviewed much of the same literature.
Timing in preterm infants
Preterm neonates are at increased risk of temperature dysregulation, hypotension, and the need for rapid initial pediatric care and blood transfusion. The increased risk of intraventricular hemorrhage and necrotizing enterocolitis in preterm infants is possibly related to the increased risk of hypotension.
As with term infants, a 2012 Cochrane systematic review offers good insight on our current knowledge. This review of umbilical cord clamping at preterm birth covers 15 studies that included 738 infants delivered between 24 and 36 weeks of gestation. The timing of umbilical cord clamping ranged from 25 seconds to a maximum of 180 seconds (Cochrane Database Syst. Rev. 2012;8:CD003248).
Delayed cord clamping was associated with fewer transfusions for anemia or low blood pressure, less intraventricular hemorrhage of all grades (relative risk 0.59), and a lower risk for necrotizing enterocolitis (relative risk 0.62), compared with immediate clamping.
While there were no clear differences with respect to severe intraventricular hemorrhage (grades 3-4), the nearly 50% reduction in intraventricular hemorrhage overall among deliveries with delayed clamping was significant enough to prompt ACOG to conclude that delayed cord clamping should be considered for preterm infants. This reduction in intraventricular hemorrhage appears to be the single most important benefit, based on current findings.
The data on cord clamping in preterm infants are suggestive of benefit, but are not robust. The studies published thus far have been small, and many of them, as the 2012 Cochrane review points out, involved incomplete reporting and wide confidence intervals. Moreover, just as with the studies on term infants, there has been a lack of long-term follow-up in most of the published trials.
When considering delayed cord clamping in preterm infants, as the ACOG Committee Opinion recommends, I urge focusing on earlier gestational ages. Allowing more placental transfusion at births that occur at or after 36 weeks of gestation may not make much sense because by that point the risk of intraventricular hemorrhage is almost nonexistent.
Our practice and the future
At our institution, births that occur at less than 32 weeks of gestation are eligible for delayed umbilical cord clamping, usually at 30-45 seconds after birth. The main contraindications are placental abruption and multiples.
We do not perform any milking or stripping of the umbilical cord, as the risks are unknown and it is not yet clear whether such practices are equivalent to delayed cord clamping. Compared with delayed cord clamping, which is a natural passive transfusion of placental blood to the infant, milking and stripping are not physiologic.
Additional data from an ongoing large international multicenter study, the Australian Placental Transfusion Study, may resolve some of the current controversy. This study is evaluating the cord clamping in neonates < 30 weeks’ gestation. Another study ongoing in Europe should also provide more information.
These studies – and other trials that are larger and longer than the trials published thus far – are necessary to evaluate long-term outcomes and to establish the ideal timing for umbilical cord clamping. Research is also needed to evaluate the management of the third stage of labor relative to umbilical cord clamping as well as the timing in relation to the initiation of voluntary or assisted ventilation.
Dr. Macones said he had no relevant financial disclosures.
Dr. Macones is the Mitchell and Elaine Yanow Professor and Chair, and director of the division of maternal-fetal medicine and ultrasound in the department of obstetrics and gynecology at Washington University, St. Louis.
Assessing Fetal Heart Rate
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
The majority of women in labor in the United States undergo continuous intrapartum fetal heart rate monitoring. However, the pervasive use of electronic fetal monitoring in obstetric practice has been challenging due to a lack of standardized nomenclature for heart rate assessment and clear guidance about how to interpret and manage various types of tracings.
The issue of nomenclature was a main focus of a 2008 workshop sponsored by the American College of Obstetricians and Gynecologists (ACOG), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Society for Maternal-Fetal Medicine. Workshop participants, myself included, reaffirmed nomenclature for baseline fetal heart rate (FHR) and FHR variability, accelerations, and decelerations. We also recommended new terminology and nomenclature for the description of uterine contractions. We were driven by the need to "speak the same language" – that is, to agree on definitions, lessen ambiguity, and allow for a more evidence-based approach to the management of fetal compromise during labor.
In this light, the workshop also recommended adoption of a three-tier system for categorizing FHR patterns. Under this system, Category I FHR tracings are normal and not associated with fetal acidemia. Category II FHR tracings are indeterminate, and Category III FHR tracings are abnormal, or predictive of abnormal fetal acid-based status (Obstet. Gynecol. 2008;112:661-6).
The recommended adoption of this three-tiered classification system was a significant outcome of the workshop and a useful first step in bringing more clarity and meaning to challenges of electronic fetal monitoring.
There was a problem, however: There are very few tracings that do not fall into Category II. Indeed, a growing number of studies have indicated that a significant fraction of all FHR tracings encountered in clinical care – as many as 85% of tracings, it is believed – are of an "indeterminate" nature. Thus, a major task after the 2008 workshop became one of digging more deeply into the Category II tracings to give practicing physicians more meaningful guidance on how to manage this diverse spectrum of abnormal FHR patterns.
The ACOG Practice Bulletin issued in 2010 on "Management of Intrapartum Fetal Heart Rate Tracings" (No. 116) delves into the challenging issue of Category II FHR tracings. It essentially addresses the question, how does one manage the woman who has variable decelerations?
Variable Decelerations
Although intermittent variable decelerations (those that occur with less than 50% of contractions) most often do not require any treatment and are associated with normal perinatal outcomes, recurrent variable decelerations (those occurring with 50% or more of contractions) can be more indicative of impending fetal acidemia.
The evidence is fairly strong that recurrent variable decelerations result from umbilical cord compression. Changing maternal position is a good first step to alleviate some of that compression. Amnioinfusion, that is, the infusion of a solution into the uterine cavity to cushion the umbilical cord – also may be used in managing umbilical cord compression.
A meta-analysis reviewed and reprinted this year in the Cochrane Database of Systematic Reviews concluded that the use of amnioinfusion for "potential or suspected umbilical cord compression" may reduce the occurrence of variable FHR decelerations, improve short-term measures of neonatal outcome, reduce maternal postpartum endometritis, and lower the rate of cesarean delivery (Cochrane Database Syst. Rev. 2012 Jan. 18;1:CD000013).
One important overarching principle is that in FHR tracings with recurrent variable decelerations – as in other types of Category II tracings – the presence of FHR accelerations (either spontaneous or induced) or FHR variability that is good ("moderate" FHR variability) is significantly predictive of a fetus that is not acidemic.
Recurrent variable decelerations should, of course, be evaluated for frequency, depth, and duration; uterine contraction pattern; and other FHR characteristics. If variable decelerations get deeper and/or last for longer periods of time, one has to be more concerned than if decelerations are not lasting as long, or if FHR is not dropping as much.
Other Category II Tracings
• Recurrent Late Decelerations. These patterns are believed to reflect uteroplacental insufficiency, which is usually caused by uterine tachysystole, maternal hypoxia, or maternal hypotension, the latter of which often occurs after the administration of regional anesthesia. Measures to improve perfusion to the placenta include the administration of intravenous fluid boluses, maternal oxygen administration, and steps to reduce uterine activity when the uterus is contracting too frequently. One important initial measure is to check the blood pressure, and if it is low (likely due to regional anesthesia), to work with the anesthesiologist on appropriate medical management of hypotension.
As is the case with variable recurrent decelerations, the presence of accelerations or moderate FHR variability, or both, provides reassurance that the baby is doing well at that point. Evaluating for the presence of these factors is important and can be most helpful with recurrent late decelerations, as these patterns in general are poorly predictive for acidemia.
If late decelerations continue in the setting of minimal FHR variability and absent accelerations, despite intrauterine resuscitation efforts, the presence of fetal acidemia should be considered and the potential need for expedited delivery should be evaluated.
• Fetal Tachycardia. Intrapartum fetal tachycardia, defined as a baseline heart rate greater than 160 beats/min for at least 10 minutes, is a fairly common occurrence in labor. When the FHR is extremely high – greater than 200 beats/min – one should consider fetal tachyarrhythmias. These are uncommon, and it is somewhat unlikely for them to occur for the first time during labor (they are more commonly identified antepartum).
When the FHR is high but in the range of approximately 160-180 beats/min, evaluation for chorioamnionitis and other maternal infections is important. If a fever is present, broad-spectrum antibiotics should be administered.
Again, even in the case of fetal tachycardia, the presence of minimal variability and/or accelerations tells us that the baby is probably not acidemic.
• Bradycardia, Prolonged Decelerations. Intrapartum bradycardia and prolonged decelerations differ mainly in their duration. The patterns are similarly managed since, as the practice bulletin says, clinical intervention is often indicated before a distinction between the two can be made.
In either case, we need to be concerned about the possibility of maternal hypotension (for example, postepidural), umbilical cord prolapse or occlusion, placental abruption, rapid fetal descent, tachysystole, or uterine rupture. Essentially, we must evaluate all these potential causes by performing a vaginal exam to determine whether the baby’s head has descended quickly or whether the umbilical cord has fallen ahead of the baby, for example, and by checking the mother’s blood pressure. Significant bleeding would signal placental abruption. And, in the case of significant bradycardia, uterine rupture becomes a major concern, especially in women with a prior cesarean.
Management is directed at the underlying cause and at resuscitating and supporting the baby with the use of fluid, oxygen, and other targeted measures.
Category III: Timing of Delivery
As part of its framework for managing FHR patterns based on the three-tiered categorization, ACOG’s 2010 Practice Bulletin also addresses the critical question of the timing of emergent cesarean delivery. Category III tracings most often require prompt delivery when intrauterine resuscitation measures are unsuccessful. Multiple studies, however, have called into question the 30-minute decision-to-incision time that historically has been the guiding principle in the setting of abnormal Category III patterns.
In one study published in 2006 involving 2,808 women who had cesarean deliveries for emergency indications including umbilical cord prolapse, placental abruption, and "nonreassuring fetal heart rate pattern," adverse neonatal outcomes were not increased among infants delivered after more than 30 minutes. (Approximately one-third of the emergency cesarean deliveries began more than 30 minutes after the decision to operate was made, and most of these deliveries were for nonreassuring FHR tracings.)
In fact, the vast majority of those delivered after 30 minutes – 95% – did not experience a measure of newborn compromise (Obstet. Gynecol. 2006;108:6-11). Other studies have similarly failed to show any association of increased adverse outcomes with a 30-minute time frame.
Rather than thinking about deliveries within 30 minutes, we should deliver based on timing that best incorporates maternal and fetal risks and benefits, as the practice bulletin states. The bottom line, in other words, is to accomplish delivery both as expeditiously as possible and as safely as possible for the baby and for the mother. There is no single optimal time frame. A mother with morbid obesity and significant anesthesia risk, for instance, may require more stabilization or surgical preparation than a mother without such a high-risk condition.
Dr. Macones is the Mitchell and Elaine Yanow professor and chair of the department of obstetrics and gynecology at Washington University in St. Louis. Dr. Macones said he had no relevant financial disclosures.
Is routine sampling of fetal fibronectin justified?
- Spontaneous preterm birth is associated with elevated cervicovaginal fetal fibronectin levels, especially in women who deliver at an early gestational age.
- A recent study suggests that antibiotic therapy in women with a positive fetal fibronectin screening test does not reduce the incidence of spontaneous preterm birth or improve neonatal outcomes.
- Fetal fibronectin cannot be recommended as a screening test for preterm birth.
This article reviews the landmark studies of fetal fibronectin testing, as well as findings of a recent multicenter double-blind, place-bo-controlled trial of antibiotic therapy.
Approximately two thirds of preterm births are “spontaneous,” associated with preterm premature rupture of the membranes (PPROM) or preterm labor. The remainder have been linked to a variety of other maternal and fetal conditions, such as preeclampsia remote from term, and fetal growth restriction.
Because most studies have focused on identifying women at risk of spontaneous preterm birth, our discussion is limited to this group.
Requirements for routine screening
A screening test is not meant to be diagnostic. Persons with positive findings require more conclusive testing, followed by treatment or another intervention.1
A screening program must meet specific criteria before widespread implementation can be recommended:
- The screened condition poses a significant burden.
- The test is sensitive and specific.
- It is inexpensive and easy to perform.
- It is safe and acceptable to patients.
- Effective treatment is available for patients who test positive.
Cervicovaginal fibronectin is elevated in women who deliver preterm
Fetal fibronectin is a glycoprotein produced by many cell types, including those of the fetal amnion. Indeed, high concentrations of the protein are found in amniotic fluid and maternal plasma.2
Fetal fibronectin acts as an intracellular adhesive; it “glues” the blastocyst to the uterine endometrium. In addition, the glycoprotein is secreted throughout pregnancy, bonding the placenta to the uterus until parturition.
Normally, cervicovaginal secretions contain increased amounts of fetal fibronectin at the beginning of gestation and 1 to 2 weeks before the onset of labor at term. However, in women who deliver preterm, these levels are elevated as early as the second trimester. This observation led early investigators to postulate that fibronectin sampling might be a suitable screening test for increased risk of spontaneous preterm delivery.
Possible causes. It is not clear precisely what causes cervicovaginal fetal fibronectin levels to increase prematurely in women at risk of preterm delivery.
One possibility might be infection: A large percentage of preterm births are associated with subclinical infection and bacterial colonization of the fetal membranes; inflammation associated with such infection may lead to disruption of the extracellular matrix, causing release of fetal fibronectin into the cervix and vagina.
Landmark studies confirm predictive role
In 1991, Lockwood et al2 published one of the first studies to find a predictive association between fetal fibronectin levels and preterm delivery. In 144 women with uncomplicated pregnancies, cervicovaginal concentrations of fetal fibronectin were rarely greater than 50 ng/mL between 21 and 37 weeks of gestation. In contrast, high levels were detected in 95% of 65 patients with PPROM and in 50% of 117 patients with preterm uterine contractions and intact membranes. The 3 groups were well matched for age, race, gravidity, and parity. An elevated fetal fibronectin level identified 60 women who delivered before term with a sensitivity of 81.7% and specificity of 82.5%.
From 1996 to 1998, Goldenberg et al3-5 published several reports on a large multicenter observational study of 2,929 women. In contrast to the Lockwood study, these women were asymptomatic—there was no indication that any would deliver prematurely. Cervicovaginal fetal fibronectin was measured every 2 weeks between weeks 22 to 24 and 30; a test was considered positive if the concentration was 50 ng/mL or greater. The primary outcome was spontaneous delivery associated with PPROM or preterm labor before 35 weeks. The investigators found a significant association between abnormal fetal fibronectin levels and preterm birth (TABLE 1).
Sensitivity was greater for earlier than for later preterm delivery; thus, a positive test would be more useful for identifying women who would deliver before 28 weeks.
Specificity and negative predictive values were much greater than sensitivity and positive predictive values, suggesting that a negative test might be more informative clinically than a positive one. Additionally, sensitivity for earlier preterm delivery (24 to 26 weeks) was greater than for later preterm delivery (28 to 30 weeks); thus, a positive fetal fibronectin test would be more useful for identifying women who would deliver before 28 weeks’ gestation.
Because the Goldenberg study was a large multicenter trial that included women across a broad spectrum of age, race, and socioeconomic status, its results could be applied to all US women. Indeed, this study provided a compelling incentive to use fetal fibronectin sampling as a screening test for risk of preterm delivery.
Other studies6-8 corroborated the landmark Lockwood and Goldenberg studies, providing an affirmative answer to the question “Can we screen for preterm delivery?”
Some experts argue that a negative fibronectin test is useful in identifying those at low risk of delivery, thereby avoiding tocolysis and hospitalization.
TABLE 1
Fetal fibronectin as a predictor of spontaneous preterm delivery: Detection at 22-24 weeks’ gestation
| TIMING OF SPONTANEOUS PRETERM DELIVERY | SENSITIVITY (%) | SPECIFICITY (%) | POSITIVE PREDICTIVE VALUE (%) | NEGATIVE PREDICTIVE VALUE (%) |
|---|---|---|---|---|
| 50 | 94 | 13 | 99.9 | |
| 32 | 95 | 21 | 97 | |
| 19 | 95 | 29 | 91 | |
| Data from Goldenberg et al.3-5 | ||||
Does fibronectin sampling meet the standard for screening?
Significant burden. There is no question that preterm birth represents a significant burden.9,10 For one, it is common. Recent data indicate that approximately 10% of deliveries in the United States occur before 37 weeks of gestation, which translates to more than 400,000 preterm births annually. Of these, 2% to 3% occur before 32 weeks’ gestation.
Preterm infants are at a greatly increased risk of serious complications (eg, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage) and death.11 In fact, preterm delivery is perhaps the most common cause of neonatal death.
Good marks for accuracy, cost, ease of use, safety, acceptability. As noted, a positive result is strongly associated with preterm birth. Further, this noninvasive test poses little to no threat to women, is simple for the clinician to perform, is economical, and is well received by patients.
Availability of effective treatment. Of the 5 conditions a widely used screening test must possess, this is the only one in which fetal fibronectin sampling is lacking. No effective interventions exist to decrease preterm birth in women with positive tests.
Antibiotic therapy fails to reduce preterm births
In May 2003, Andrews et al12 published the results of a multicenter, double-blind, placebo-controlled trial in which asymptomatic women with fetal fibronectin levels over 50 ng/mL (6.6% of the 16,317 women screened) received antibiotic therapy.
The final study population included 347 women given a 10-day course of metronidazole (250 mg 3 times daily) plus erythromycin (250 mg once daily) and 356 women taking placebo. The 2 groups were well matched for age, ethnicity, marital status, education, average gestational age, and incidence of bacterial vaginosis.
Treatment did not reduce the rate of spontaneous preterm birth. No differences were seen in the incidence of delivery before 32, 35, or 37 weeks, or in birth weight (TABLE 2). Nor was there improvement in any health parameters in the infants delivered by women in the antibiotic group.
TABLE 2
Effect of antibiotic therapy on spontaneous preterm delivery
| OUTCOMES | METRONIDAZOLE/ERYTHROMYCIN | PLACEBO | RELATIVE RISK (95% CI) |
|---|---|---|---|
| Spontaneous preterm delivery | |||
| 4.3% | 2.2% | 1.94 (0.83-4.52) | |
| 6.9% | 7.5% | 0.92 (0.54-1.56) | |
| 14.4% | 12.4% | 1.17 (0.80-1.70) | |
| Birth weight | |||
| 3.5% | 3.2% | 1.12 (0.50-2.50) | |
| 12.7% | 14.3% | 0.88 (0.60-1.29) | |
| CI = confidence interval | |||
| Data from Andrews et al.12 | |||
Was the study flawed?
One could argue that the Andrews study had some limitations. First, it is possible that the sample size was too small to detect differences between the treatment groups. Second, screening was performed at a mean gestational age of 23 weeks (range, 21 to 26 weeks), which may not have been early enough. Third, patient compliance was poor (only about 50% of the antibiotic group took all of their medication); thus, treatment efficacy may have been inadequately assessed. In addition, the 10-day treatment regimen may have been too brief.
Could other agents yield better results?
It is possible that metronidazole and erythromycin were not the appropriate antibiotics for treating the subclinical infection associated with preterm delivery. In fact, an entirely different class of agents may be needed.
Is inflammation a factor? If inflammation rather than subclinical infection is the primary precipitant of fetal fibronectin release into the cervix and vagina prior to preterm labor, antiinflammatory therapy may have a role in its prevention.
Clinical experience
We do not use fetal fibronectin as a screening test in asymptomatic women since there is no established intervention. There is more debate about whether it can be used as a diagnostic test. Some experts argue that, in patients with “threatened” preterm labor, a negative fetal fibronectin test may be useful in identifying those at low risk of delivery, thereby avoiding tocolysis and hospitalization.
However, since this benefit has not been demonstrated in clinical trials or observational studies, it should not alter our practice. Until it has been proven, we will continue to manage women with symptoms of preterm labor without the use of fetal fibronectin.
The authors report no financial relationships with companies whose products are mentioned in this article.
1. Last JL. A Dictionary of Epidemiology. New York: Oxford University Press; 1995.
2. Lockwood C, Senyel A, Dische R, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669-674.
3. Goldenberg R, Mercer B, Meis P, Copper R, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:643-648.
4. Goldenberg R, Mercer B, Iams J, et al. The preterm prediction study: patterns of cervicovaginal fetal fibronectin as predictors of spontaneous preterm delivery. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1997;177:8-12.
5. Goldenberg R, Iams J, Mercer B, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD Maternal Fetal Medicine Units Network. Am J Public Health. 1998;88:233-238.
6. Bartnicki J, Casal D, Kreaden US, Saling E, Vetter K. Fetal fibronectin in vaginal specimens predicts preterm delivery and very-low-birthweight infants. Am J Obstet Gynecol. 1996;174:971-974.
7. Hellemans P, Gerris J, Verdonk P. Fetal fibronectin detection for prediction of preterm birth in low risk women. Br J Obstet Gynaecol. 1995;102:207-212.
8. Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervicovaginal fetal fibronectin as a marker for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 1999;180:1169-1176.
9. Morrison J. Preterm birth: a puzzle worth solving. Obstet Gynecol. 1990;76:5-11.
10. Holzman C, Paneth N. Preterm birth: from prediction to prevention. Am J Public Health. 1998;88:183-184.
11. Copper R, Goldenberg R, Creasy R, et al. A multicenter study of preterm birth weight and gestational age specific mortality. Am J Obstet Gynecol. 1993;168:78-84.
12. Andrews W, Sibai B, Thom E, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin-positive women. Obstet Gynecol. 2003;101:847-855.
- Spontaneous preterm birth is associated with elevated cervicovaginal fetal fibronectin levels, especially in women who deliver at an early gestational age.
- A recent study suggests that antibiotic therapy in women with a positive fetal fibronectin screening test does not reduce the incidence of spontaneous preterm birth or improve neonatal outcomes.
- Fetal fibronectin cannot be recommended as a screening test for preterm birth.
This article reviews the landmark studies of fetal fibronectin testing, as well as findings of a recent multicenter double-blind, place-bo-controlled trial of antibiotic therapy.
Approximately two thirds of preterm births are “spontaneous,” associated with preterm premature rupture of the membranes (PPROM) or preterm labor. The remainder have been linked to a variety of other maternal and fetal conditions, such as preeclampsia remote from term, and fetal growth restriction.
Because most studies have focused on identifying women at risk of spontaneous preterm birth, our discussion is limited to this group.
Requirements for routine screening
A screening test is not meant to be diagnostic. Persons with positive findings require more conclusive testing, followed by treatment or another intervention.1
A screening program must meet specific criteria before widespread implementation can be recommended:
- The screened condition poses a significant burden.
- The test is sensitive and specific.
- It is inexpensive and easy to perform.
- It is safe and acceptable to patients.
- Effective treatment is available for patients who test positive.
Cervicovaginal fibronectin is elevated in women who deliver preterm
Fetal fibronectin is a glycoprotein produced by many cell types, including those of the fetal amnion. Indeed, high concentrations of the protein are found in amniotic fluid and maternal plasma.2
Fetal fibronectin acts as an intracellular adhesive; it “glues” the blastocyst to the uterine endometrium. In addition, the glycoprotein is secreted throughout pregnancy, bonding the placenta to the uterus until parturition.
Normally, cervicovaginal secretions contain increased amounts of fetal fibronectin at the beginning of gestation and 1 to 2 weeks before the onset of labor at term. However, in women who deliver preterm, these levels are elevated as early as the second trimester. This observation led early investigators to postulate that fibronectin sampling might be a suitable screening test for increased risk of spontaneous preterm delivery.
Possible causes. It is not clear precisely what causes cervicovaginal fetal fibronectin levels to increase prematurely in women at risk of preterm delivery.
One possibility might be infection: A large percentage of preterm births are associated with subclinical infection and bacterial colonization of the fetal membranes; inflammation associated with such infection may lead to disruption of the extracellular matrix, causing release of fetal fibronectin into the cervix and vagina.
Landmark studies confirm predictive role
In 1991, Lockwood et al2 published one of the first studies to find a predictive association between fetal fibronectin levels and preterm delivery. In 144 women with uncomplicated pregnancies, cervicovaginal concentrations of fetal fibronectin were rarely greater than 50 ng/mL between 21 and 37 weeks of gestation. In contrast, high levels were detected in 95% of 65 patients with PPROM and in 50% of 117 patients with preterm uterine contractions and intact membranes. The 3 groups were well matched for age, race, gravidity, and parity. An elevated fetal fibronectin level identified 60 women who delivered before term with a sensitivity of 81.7% and specificity of 82.5%.
From 1996 to 1998, Goldenberg et al3-5 published several reports on a large multicenter observational study of 2,929 women. In contrast to the Lockwood study, these women were asymptomatic—there was no indication that any would deliver prematurely. Cervicovaginal fetal fibronectin was measured every 2 weeks between weeks 22 to 24 and 30; a test was considered positive if the concentration was 50 ng/mL or greater. The primary outcome was spontaneous delivery associated with PPROM or preterm labor before 35 weeks. The investigators found a significant association between abnormal fetal fibronectin levels and preterm birth (TABLE 1).
Sensitivity was greater for earlier than for later preterm delivery; thus, a positive test would be more useful for identifying women who would deliver before 28 weeks.
Specificity and negative predictive values were much greater than sensitivity and positive predictive values, suggesting that a negative test might be more informative clinically than a positive one. Additionally, sensitivity for earlier preterm delivery (24 to 26 weeks) was greater than for later preterm delivery (28 to 30 weeks); thus, a positive fetal fibronectin test would be more useful for identifying women who would deliver before 28 weeks’ gestation.
Because the Goldenberg study was a large multicenter trial that included women across a broad spectrum of age, race, and socioeconomic status, its results could be applied to all US women. Indeed, this study provided a compelling incentive to use fetal fibronectin sampling as a screening test for risk of preterm delivery.
Other studies6-8 corroborated the landmark Lockwood and Goldenberg studies, providing an affirmative answer to the question “Can we screen for preterm delivery?”
Some experts argue that a negative fibronectin test is useful in identifying those at low risk of delivery, thereby avoiding tocolysis and hospitalization.
TABLE 1
Fetal fibronectin as a predictor of spontaneous preterm delivery: Detection at 22-24 weeks’ gestation
| TIMING OF SPONTANEOUS PRETERM DELIVERY | SENSITIVITY (%) | SPECIFICITY (%) | POSITIVE PREDICTIVE VALUE (%) | NEGATIVE PREDICTIVE VALUE (%) |
|---|---|---|---|---|
| 50 | 94 | 13 | 99.9 | |
| 32 | 95 | 21 | 97 | |
| 19 | 95 | 29 | 91 | |
| Data from Goldenberg et al.3-5 | ||||
Does fibronectin sampling meet the standard for screening?
Significant burden. There is no question that preterm birth represents a significant burden.9,10 For one, it is common. Recent data indicate that approximately 10% of deliveries in the United States occur before 37 weeks of gestation, which translates to more than 400,000 preterm births annually. Of these, 2% to 3% occur before 32 weeks’ gestation.
Preterm infants are at a greatly increased risk of serious complications (eg, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage) and death.11 In fact, preterm delivery is perhaps the most common cause of neonatal death.
Good marks for accuracy, cost, ease of use, safety, acceptability. As noted, a positive result is strongly associated with preterm birth. Further, this noninvasive test poses little to no threat to women, is simple for the clinician to perform, is economical, and is well received by patients.
Availability of effective treatment. Of the 5 conditions a widely used screening test must possess, this is the only one in which fetal fibronectin sampling is lacking. No effective interventions exist to decrease preterm birth in women with positive tests.
Antibiotic therapy fails to reduce preterm births
In May 2003, Andrews et al12 published the results of a multicenter, double-blind, placebo-controlled trial in which asymptomatic women with fetal fibronectin levels over 50 ng/mL (6.6% of the 16,317 women screened) received antibiotic therapy.
The final study population included 347 women given a 10-day course of metronidazole (250 mg 3 times daily) plus erythromycin (250 mg once daily) and 356 women taking placebo. The 2 groups were well matched for age, ethnicity, marital status, education, average gestational age, and incidence of bacterial vaginosis.
Treatment did not reduce the rate of spontaneous preterm birth. No differences were seen in the incidence of delivery before 32, 35, or 37 weeks, or in birth weight (TABLE 2). Nor was there improvement in any health parameters in the infants delivered by women in the antibiotic group.
TABLE 2
Effect of antibiotic therapy on spontaneous preterm delivery
| OUTCOMES | METRONIDAZOLE/ERYTHROMYCIN | PLACEBO | RELATIVE RISK (95% CI) |
|---|---|---|---|
| Spontaneous preterm delivery | |||
| 4.3% | 2.2% | 1.94 (0.83-4.52) | |
| 6.9% | 7.5% | 0.92 (0.54-1.56) | |
| 14.4% | 12.4% | 1.17 (0.80-1.70) | |
| Birth weight | |||
| 3.5% | 3.2% | 1.12 (0.50-2.50) | |
| 12.7% | 14.3% | 0.88 (0.60-1.29) | |
| CI = confidence interval | |||
| Data from Andrews et al.12 | |||
Was the study flawed?
One could argue that the Andrews study had some limitations. First, it is possible that the sample size was too small to detect differences between the treatment groups. Second, screening was performed at a mean gestational age of 23 weeks (range, 21 to 26 weeks), which may not have been early enough. Third, patient compliance was poor (only about 50% of the antibiotic group took all of their medication); thus, treatment efficacy may have been inadequately assessed. In addition, the 10-day treatment regimen may have been too brief.
Could other agents yield better results?
It is possible that metronidazole and erythromycin were not the appropriate antibiotics for treating the subclinical infection associated with preterm delivery. In fact, an entirely different class of agents may be needed.
Is inflammation a factor? If inflammation rather than subclinical infection is the primary precipitant of fetal fibronectin release into the cervix and vagina prior to preterm labor, antiinflammatory therapy may have a role in its prevention.
Clinical experience
We do not use fetal fibronectin as a screening test in asymptomatic women since there is no established intervention. There is more debate about whether it can be used as a diagnostic test. Some experts argue that, in patients with “threatened” preterm labor, a negative fetal fibronectin test may be useful in identifying those at low risk of delivery, thereby avoiding tocolysis and hospitalization.
However, since this benefit has not been demonstrated in clinical trials or observational studies, it should not alter our practice. Until it has been proven, we will continue to manage women with symptoms of preterm labor without the use of fetal fibronectin.
The authors report no financial relationships with companies whose products are mentioned in this article.
- Spontaneous preterm birth is associated with elevated cervicovaginal fetal fibronectin levels, especially in women who deliver at an early gestational age.
- A recent study suggests that antibiotic therapy in women with a positive fetal fibronectin screening test does not reduce the incidence of spontaneous preterm birth or improve neonatal outcomes.
- Fetal fibronectin cannot be recommended as a screening test for preterm birth.
This article reviews the landmark studies of fetal fibronectin testing, as well as findings of a recent multicenter double-blind, place-bo-controlled trial of antibiotic therapy.
Approximately two thirds of preterm births are “spontaneous,” associated with preterm premature rupture of the membranes (PPROM) or preterm labor. The remainder have been linked to a variety of other maternal and fetal conditions, such as preeclampsia remote from term, and fetal growth restriction.
Because most studies have focused on identifying women at risk of spontaneous preterm birth, our discussion is limited to this group.
Requirements for routine screening
A screening test is not meant to be diagnostic. Persons with positive findings require more conclusive testing, followed by treatment or another intervention.1
A screening program must meet specific criteria before widespread implementation can be recommended:
- The screened condition poses a significant burden.
- The test is sensitive and specific.
- It is inexpensive and easy to perform.
- It is safe and acceptable to patients.
- Effective treatment is available for patients who test positive.
Cervicovaginal fibronectin is elevated in women who deliver preterm
Fetal fibronectin is a glycoprotein produced by many cell types, including those of the fetal amnion. Indeed, high concentrations of the protein are found in amniotic fluid and maternal plasma.2
Fetal fibronectin acts as an intracellular adhesive; it “glues” the blastocyst to the uterine endometrium. In addition, the glycoprotein is secreted throughout pregnancy, bonding the placenta to the uterus until parturition.
Normally, cervicovaginal secretions contain increased amounts of fetal fibronectin at the beginning of gestation and 1 to 2 weeks before the onset of labor at term. However, in women who deliver preterm, these levels are elevated as early as the second trimester. This observation led early investigators to postulate that fibronectin sampling might be a suitable screening test for increased risk of spontaneous preterm delivery.
Possible causes. It is not clear precisely what causes cervicovaginal fetal fibronectin levels to increase prematurely in women at risk of preterm delivery.
One possibility might be infection: A large percentage of preterm births are associated with subclinical infection and bacterial colonization of the fetal membranes; inflammation associated with such infection may lead to disruption of the extracellular matrix, causing release of fetal fibronectin into the cervix and vagina.
Landmark studies confirm predictive role
In 1991, Lockwood et al2 published one of the first studies to find a predictive association between fetal fibronectin levels and preterm delivery. In 144 women with uncomplicated pregnancies, cervicovaginal concentrations of fetal fibronectin were rarely greater than 50 ng/mL between 21 and 37 weeks of gestation. In contrast, high levels were detected in 95% of 65 patients with PPROM and in 50% of 117 patients with preterm uterine contractions and intact membranes. The 3 groups were well matched for age, race, gravidity, and parity. An elevated fetal fibronectin level identified 60 women who delivered before term with a sensitivity of 81.7% and specificity of 82.5%.
From 1996 to 1998, Goldenberg et al3-5 published several reports on a large multicenter observational study of 2,929 women. In contrast to the Lockwood study, these women were asymptomatic—there was no indication that any would deliver prematurely. Cervicovaginal fetal fibronectin was measured every 2 weeks between weeks 22 to 24 and 30; a test was considered positive if the concentration was 50 ng/mL or greater. The primary outcome was spontaneous delivery associated with PPROM or preterm labor before 35 weeks. The investigators found a significant association between abnormal fetal fibronectin levels and preterm birth (TABLE 1).
Sensitivity was greater for earlier than for later preterm delivery; thus, a positive test would be more useful for identifying women who would deliver before 28 weeks.
Specificity and negative predictive values were much greater than sensitivity and positive predictive values, suggesting that a negative test might be more informative clinically than a positive one. Additionally, sensitivity for earlier preterm delivery (24 to 26 weeks) was greater than for later preterm delivery (28 to 30 weeks); thus, a positive fetal fibronectin test would be more useful for identifying women who would deliver before 28 weeks’ gestation.
Because the Goldenberg study was a large multicenter trial that included women across a broad spectrum of age, race, and socioeconomic status, its results could be applied to all US women. Indeed, this study provided a compelling incentive to use fetal fibronectin sampling as a screening test for risk of preterm delivery.
Other studies6-8 corroborated the landmark Lockwood and Goldenberg studies, providing an affirmative answer to the question “Can we screen for preterm delivery?”
Some experts argue that a negative fibronectin test is useful in identifying those at low risk of delivery, thereby avoiding tocolysis and hospitalization.
TABLE 1
Fetal fibronectin as a predictor of spontaneous preterm delivery: Detection at 22-24 weeks’ gestation
| TIMING OF SPONTANEOUS PRETERM DELIVERY | SENSITIVITY (%) | SPECIFICITY (%) | POSITIVE PREDICTIVE VALUE (%) | NEGATIVE PREDICTIVE VALUE (%) |
|---|---|---|---|---|
| 50 | 94 | 13 | 99.9 | |
| 32 | 95 | 21 | 97 | |
| 19 | 95 | 29 | 91 | |
| Data from Goldenberg et al.3-5 | ||||
Does fibronectin sampling meet the standard for screening?
Significant burden. There is no question that preterm birth represents a significant burden.9,10 For one, it is common. Recent data indicate that approximately 10% of deliveries in the United States occur before 37 weeks of gestation, which translates to more than 400,000 preterm births annually. Of these, 2% to 3% occur before 32 weeks’ gestation.
Preterm infants are at a greatly increased risk of serious complications (eg, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage) and death.11 In fact, preterm delivery is perhaps the most common cause of neonatal death.
Good marks for accuracy, cost, ease of use, safety, acceptability. As noted, a positive result is strongly associated with preterm birth. Further, this noninvasive test poses little to no threat to women, is simple for the clinician to perform, is economical, and is well received by patients.
Availability of effective treatment. Of the 5 conditions a widely used screening test must possess, this is the only one in which fetal fibronectin sampling is lacking. No effective interventions exist to decrease preterm birth in women with positive tests.
Antibiotic therapy fails to reduce preterm births
In May 2003, Andrews et al12 published the results of a multicenter, double-blind, placebo-controlled trial in which asymptomatic women with fetal fibronectin levels over 50 ng/mL (6.6% of the 16,317 women screened) received antibiotic therapy.
The final study population included 347 women given a 10-day course of metronidazole (250 mg 3 times daily) plus erythromycin (250 mg once daily) and 356 women taking placebo. The 2 groups were well matched for age, ethnicity, marital status, education, average gestational age, and incidence of bacterial vaginosis.
Treatment did not reduce the rate of spontaneous preterm birth. No differences were seen in the incidence of delivery before 32, 35, or 37 weeks, or in birth weight (TABLE 2). Nor was there improvement in any health parameters in the infants delivered by women in the antibiotic group.
TABLE 2
Effect of antibiotic therapy on spontaneous preterm delivery
| OUTCOMES | METRONIDAZOLE/ERYTHROMYCIN | PLACEBO | RELATIVE RISK (95% CI) |
|---|---|---|---|
| Spontaneous preterm delivery | |||
| 4.3% | 2.2% | 1.94 (0.83-4.52) | |
| 6.9% | 7.5% | 0.92 (0.54-1.56) | |
| 14.4% | 12.4% | 1.17 (0.80-1.70) | |
| Birth weight | |||
| 3.5% | 3.2% | 1.12 (0.50-2.50) | |
| 12.7% | 14.3% | 0.88 (0.60-1.29) | |
| CI = confidence interval | |||
| Data from Andrews et al.12 | |||
Was the study flawed?
One could argue that the Andrews study had some limitations. First, it is possible that the sample size was too small to detect differences between the treatment groups. Second, screening was performed at a mean gestational age of 23 weeks (range, 21 to 26 weeks), which may not have been early enough. Third, patient compliance was poor (only about 50% of the antibiotic group took all of their medication); thus, treatment efficacy may have been inadequately assessed. In addition, the 10-day treatment regimen may have been too brief.
Could other agents yield better results?
It is possible that metronidazole and erythromycin were not the appropriate antibiotics for treating the subclinical infection associated with preterm delivery. In fact, an entirely different class of agents may be needed.
Is inflammation a factor? If inflammation rather than subclinical infection is the primary precipitant of fetal fibronectin release into the cervix and vagina prior to preterm labor, antiinflammatory therapy may have a role in its prevention.
Clinical experience
We do not use fetal fibronectin as a screening test in asymptomatic women since there is no established intervention. There is more debate about whether it can be used as a diagnostic test. Some experts argue that, in patients with “threatened” preterm labor, a negative fetal fibronectin test may be useful in identifying those at low risk of delivery, thereby avoiding tocolysis and hospitalization.
However, since this benefit has not been demonstrated in clinical trials or observational studies, it should not alter our practice. Until it has been proven, we will continue to manage women with symptoms of preterm labor without the use of fetal fibronectin.
The authors report no financial relationships with companies whose products are mentioned in this article.
1. Last JL. A Dictionary of Epidemiology. New York: Oxford University Press; 1995.
2. Lockwood C, Senyel A, Dische R, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669-674.
3. Goldenberg R, Mercer B, Meis P, Copper R, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:643-648.
4. Goldenberg R, Mercer B, Iams J, et al. The preterm prediction study: patterns of cervicovaginal fetal fibronectin as predictors of spontaneous preterm delivery. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1997;177:8-12.
5. Goldenberg R, Iams J, Mercer B, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD Maternal Fetal Medicine Units Network. Am J Public Health. 1998;88:233-238.
6. Bartnicki J, Casal D, Kreaden US, Saling E, Vetter K. Fetal fibronectin in vaginal specimens predicts preterm delivery and very-low-birthweight infants. Am J Obstet Gynecol. 1996;174:971-974.
7. Hellemans P, Gerris J, Verdonk P. Fetal fibronectin detection for prediction of preterm birth in low risk women. Br J Obstet Gynaecol. 1995;102:207-212.
8. Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervicovaginal fetal fibronectin as a marker for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 1999;180:1169-1176.
9. Morrison J. Preterm birth: a puzzle worth solving. Obstet Gynecol. 1990;76:5-11.
10. Holzman C, Paneth N. Preterm birth: from prediction to prevention. Am J Public Health. 1998;88:183-184.
11. Copper R, Goldenberg R, Creasy R, et al. A multicenter study of preterm birth weight and gestational age specific mortality. Am J Obstet Gynecol. 1993;168:78-84.
12. Andrews W, Sibai B, Thom E, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin-positive women. Obstet Gynecol. 2003;101:847-855.
1. Last JL. A Dictionary of Epidemiology. New York: Oxford University Press; 1995.
2. Lockwood C, Senyel A, Dische R, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med. 1991;325:669-674.
3. Goldenberg R, Mercer B, Meis P, Copper R, Das A, McNellis D. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996;87:643-648.
4. Goldenberg R, Mercer B, Iams J, et al. The preterm prediction study: patterns of cervicovaginal fetal fibronectin as predictors of spontaneous preterm delivery. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1997;177:8-12.
5. Goldenberg R, Iams J, Mercer B, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD Maternal Fetal Medicine Units Network. Am J Public Health. 1998;88:233-238.
6. Bartnicki J, Casal D, Kreaden US, Saling E, Vetter K. Fetal fibronectin in vaginal specimens predicts preterm delivery and very-low-birthweight infants. Am J Obstet Gynecol. 1996;174:971-974.
7. Hellemans P, Gerris J, Verdonk P. Fetal fibronectin detection for prediction of preterm birth in low risk women. Br J Obstet Gynaecol. 1995;102:207-212.
8. Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervicovaginal fetal fibronectin as a marker for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 1999;180:1169-1176.
9. Morrison J. Preterm birth: a puzzle worth solving. Obstet Gynecol. 1990;76:5-11.
10. Holzman C, Paneth N. Preterm birth: from prediction to prevention. Am J Public Health. 1998;88:183-184.
11. Copper R, Goldenberg R, Creasy R, et al. A multicenter study of preterm birth weight and gestational age specific mortality. Am J Obstet Gynecol. 1993;168:78-84.
12. Andrews W, Sibai B, Thom E, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin-positive women. Obstet Gynecol. 2003;101:847-855.