User login
Home apnea monitors—when to discontinue use
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
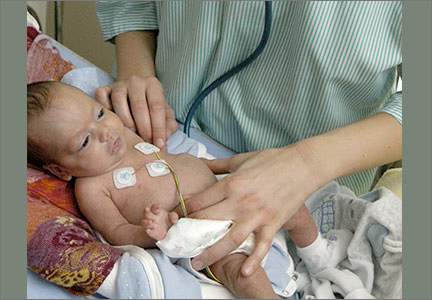
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
› Tell parents that home apnea monitoring has not been shown to prevent sudden unexpected death in infants. C
› Consider discontinuing home apnea monitoring for infants at risk for recurrent apnea at approximately 43 weeks postmenstrual age or after the cessation of extreme episodes. B
› Educate parents about steps they can take to reduce their child’s risk of sudden infant death syndrome, such as putting him to sleep on his back, breastfeeding him, and refraining from sleeping in the same bed with him. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Each year, more than one in every 100 infants are born at less than 32 weeks postmenstrual age.1 In industrialized countries, many of these infants are discharged from the neonatal intensive care unit (NICU) with home apnea monitors,1 which alert caregivers to episodes of apnea and bradycardia, while also capturing and storing data surrounding significant events for later analysis.2
Evidence supporting the use of home apnea monitoring is sparse, and recommendations highlight the need to use this technology sparingly and to discontinue use once it is no longer necessary (TABLE).3 Counseling parents is critical. It’s important to explain that home apnea monitoring can be used to help reduce the likelihood that a child will die in his or her sleep, but it affords users no “guarantees.” In addition, home apnea monitoring can adversely affect parents. Parents who use home apnea monitoring for their infants have been shown to have higher stress scores, greater levels of fatigue, and poorer health than parents of infants without home apnea monitors.4-8
As a family physician, you are likely to encounter home apnea monitoring in one of 3 scenarios: at the first visit after discharge by a premature infant who experienced apnea while hospitalized, at a follow-up visit after discharge from the hospital by an infant who experienced an apparent life-threatening event (ALTE), and at a follow-up visit by an infant whose sibling had died from sudden infant death syndrome (SIDS). This article presents case studies that illustrate each of these scenarios, and explains what to tell parents who ask about how long they should continue home apnea monitoring.
CASE 1 › Apnea of prematurity
Jacob is a newborn who is brought in to your clinic by his parents for an initial visit. The infant was born prematurely at 32 weeks and required a prolonged NICU stay. His mother says that Jacob spent 4 weeks there and was discharged home with home apnea monitoring. On exam, the infant has a monitor attached via a chest band. Jacob appears healthy and his exam is normal. The mother asks you how long her son should use the home monitor.
Pathologic apnea is a respiratory pause that lasts at least 20 seconds or is associated with cyanosis; abrupt, marked pallor or hypotonia; or bradycardia.2 Apnea of prematurity is present in almost all infants born at <29 weeks postmenstrual age or who weigh <1000 g.9 It is found in 54% of infants born at 30 to 31 weeks, 15% born at 32 to 33 weeks, and 7% of infants born at 34 to 35 weeks.10
Apnea of prematurity is primarily due to an immature respiratory control system, which results in impaired breathing regulation, immature respiratory responses to hypercapnia and hypoxia, and an exaggerated inhibitory response to stimulation of airway receptors.11-13 Although apnea of prematurity usually resolves by 36 to 40 weeks postmenstrual age, it often persists beyond 38 to 40 weeks in infants born before 28 weeks.10 In these infants, by 43 to 44 weeks postmenstrual age, the frequency of apneic episodes decreases to that of full-term infants.14
The differences in long-term outcomes of infants with apnea of prematurity vs infants without it are subtle, if present at all.14,15 There does seem to be a correlation between the number of days with apnea and poor outcomes. Neurodevelopmental impairment and death are more likely in neonates who experience a greater number of days with apnea episodes.16,17 However, apnea of prematurity is not associated with an increased risk of SIDS.18
According to the American Academy of Pediatrics (AAP), home apnea monitoring may be warranted for premature infants who are at high risk of recurrent episodes of apnea, bradycardia, and hypoxemia after hospital discharge.3 While there is general consensus that all infants born prior to 29 weeks meet this criterion, the use of home apnea monitors in older preterm infants varies significantly, and the decision to initiate monitoring in these patients is made by the discharging physician.3 Once initiated, the AAP recommends that the use of home apnea monitoring in this population be discontinued after approximately 43 weeks postmenstrual age or after the cessation of extreme episodes, whichever comes last.3
In Jacob’s case, the monitoring should be discontinued at approximately week 12 of life, or about age 3 months.
CASE 2 › Apparent life-threatening event
Sarah is brought to your office after being hospitalized for an ALTE. Her mother reports that she had witnessed her 13-day-old daughter not breathing for “about a minute.” Upon realizing what was happening, she “blew into the baby’s face,” whereupon Sarah awakened. The mother then called 911 and they went by ambulance to the emergency room. The newborn was admitted for observation overnight and received a thorough evaluation. She was discharged with a home apnea monitor.
You review the work-up and find nothing worrisome. Sarah is in a car seat attached to the apnea monitor with a chest strap. An examination of the child is normal. The mother asks you when they should stop using the home monitor.
An ALTE is “an event that is frightening to the observer and ... is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gagging.”2 ALTE is a descriptive term, and not a definitive diagnosis.
The true incidence of ALTE is unknown, but is reported to be 0.5% to 6%; most events occur in children younger than age 1.19,20 The risk for ALTE is increased for premature infants, particularly those with respiratory syncytial virus or who had undergone general anesthesia; infants who feed rapidly, cough frequently, or choke during feeding; and male infants.19,21
The most common causes of ALTE (in descending order) are gastroesophageal reflux, seizure disorder, and lower respiratory tract infection.22 The etiology is unknown for about half of patients with ALTE.23
Tell parents that if their infant experiences an ALTE, they should seek medical attention without delay. The fear is that failing to respond to this concern will ultimately result in a sudden unexpected infant death, specifically as a result of SIDS.24
SIDS is very rare, occurring in only 40 per 100,000 births. One analysis found that children who die from SIDS and those who experience ALTE have very similar histories and clinical factors.25 Approximately 7% of infants who die from SIDS have had an ALTE.2 Overall, the long-term prognosis for infants who have had an ALTE is very good, although it depends on seriousness of the underlying etiology.8,26-28
Guidance on the effective use of home apnea monitors in infants who experience an ALTE is sparse. Despite this, the National Institutes of Health (NIH) Consensus Statement on Infantile Apnea and Home Monitoring2 and the American Academy of Pediatrics policy statement on apnea, sudden infant death syndrome, and home monitoring3 recommend the use of home apnea monitoring for certain infants who’ve had an ALTE. The NIH Consensus Statement specifies home monitoring for infants with one or more severe episodes of ALTEs that require mouth-to-mouth resuscitation or vigorous stimulation.2 There are no specific guidelines regarding the duration of monitoring.2,3
In Sarah’s case, home monitoring should be discontinued as soon as the mother is comfortable with the decision.
CASE 3 › Sudden infant death syndrome
The parents of a 2-month-old boy, Stephen, come to your office to establish care. They recently relocated and their previous care provider had prescribed a home apnea monitor because a child they’d had 3 years ago had died of SIDS. Stephen is in a car seat attached to the apnea monitor with a chest strap. Your examination of him is normal. Stephen’s parents would like to stop using the home monitor, and ask you if it’s safe to do so.
SIDS is the death of an infant or young child that is unexplained by history and in which postmortem examination fails to find an adequate explanation of cause of death.2 Since the introduction of the Back to Sleep campaign in the early 1990s, the incidence of SIDS has decreased by more than 50%.8 In 2013, approximately 1500 infant deaths were attributed to SIDS.24 Three-quarters of deaths due to SIDS occur between 2 to 4 months of age, and 95% of deaths occur before 9 months of age.29 Risk factors for SIDS include sleep environment (prone and side sleeping, bed sharing, soft bedding), prenatal and postnatal maternal tobacco use, exposure to tobacco smoke, maternal mental illness or substance abuse, male sex, poverty, prematurity, low birth weight (less than 2500 g), and no or poor prenatal care.30
The etiology of SIDS is unclear.31 The leading hypothesis is the “triple-risk model,” which proposes that death from SIDS is due to 3 overlapping factors: a vulnerable infant, a critical developmental period in homeostatic control, and an exogenous stressor.32
Although the NIH Consensus Statement suggests home apnea monitoring is indicated for infants who are siblings of 2 or more SIDS victims,2 more recent policy statements from the AAP recommend against using home apnea monitors to reduce the incidence of SIDS due to a lack of evidence.3,8
With this in mind, Stephen’s monitor should be discontinued and his parents should be educated on proven methods of preventing SIDS, including placing him on his back to sleep, breastfeeding him, letting him use a pacifier during sleep, and not sleeping in the same bed with him or overdressing him when putting him to sleep.3,8
CORRESPONDENCE
Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604; [email protected].
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
1. Centers for Disease Control and Prevention. Births and natality. Centers for Disease Control and Prevention/National Center for Health Statistics Web site. Available at: http://www.cdc.gov/nchs/fastats/births.htm. Accessed August 18, 2015.
2. National Institutes of Health Consensus Development Program. Infantile apnea and home monitoring. National Institutes of Health Consensus Development Statement. 1986. National Institutes of Health Consensus Development Program Web site. Available at: https://consensus.nih.gov/1986/1986InfantApneaMonitoring058html.htm. Accessed November 2, 2015.
3. Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111:914-917.
4. Kahn A, Sottiaux M, Appelboom-Fondu J, et al. Long-term development of children monitored as infants for an apparent lifethreatening event during sleep: a 10-year follow-up study. Pediatrics. 1989;83:668-673.
5. Vohr BR, Chen A, Garcia Coll C, et al. Mothers of preterm and full-term infants on home apnea monitors. Am J Dis Child. 1988;142:229-231.
6. Williams PD, Press A, Williams AR, et al. Fatigue in mothers of infants discharged to the home on apnea monitors. Appl Nurs Res. 1999;12:69-77.
7. Ahmann E, Wulff L, Meny RG. Home apnea monitoring and disruption in family life: a multidimensional controlled study. Am J Public Health. 1992;82:719-722.
8. Task Force on Sudden Infant Death Syndrome, Moon R. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:1030-1039.
9. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100:354-359.
10. Martin RJ, Abu-Shaweesh JM, Baird TM. Apnoea of prematurity. Paediatr Respir Rev. 2004;5:S377-S382.
11. Miller MJ, Martin RJ. Pathophysiology of apnea of prematurity. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004: 905-918.
12. Miller JM, Haxhiu MA, Martin RJ. Chemical control of breathing from fetal through newborn life. In: Matthew OP, ed. Respiratory Control and Disorders in the Newborn. New York, NY: Marcel Dekker; 2003:83-113.
13. Rigatto H. Control of breathing in fetal life and onset and control of breathing in the neonate. In: Polin RA, Fox WW, Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. Philadelphia, PA: WB Saunders; 2004:890-899.
14. Koons AH, Mojica N, Jadeja N, et al. Neurodevelopmental outcome of infants with apnea of infancy. Am J Perinatol. 1993;10:208-211.
15. Perlman JM. Neurobehavioral deficits in premature graduates of intensive care—potential medical and neonatal environmental risk factors. Pediatrics. 2001;108:1339-1348.
16. Janvier A, Khairy M, Kokkotis A, et al. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763-768.
17. Pillekamp F, Hermann C, Keller T, et al. Factors influencing apnea and bradycardia of prematurity—implications for neurodevelopment. Neonatology. 2007;91:155-161.
18. Hoffman HJ, Damus K, Hillman L, et al. Risk factors for SIDS. Results for the National Institutes of Child Health and Human Development SIDS Cooperative Epidemiological Study. Ann N Y Acad Sci. 1988;533:13-30.
19. Brooks JG. Apparent life-threatening events and apnea of infancy. Clin Perinatol. 1992;19:809-838.
20. Semmekrot BA, van Sleuwan BE, Engelberts AC, et al. Surveillance study of apparent life-threatening events (ALTE) in the Netherlands. Eur J Pediatr. 2010;169:229-236.
21. Carroll JL. Apparent Life Threatening Event (ALTE) assessment. Pediatr Pulmonol Suppl. 2004;26:108-109.
22. McGovern MC, Smith MB. Causes of apparent life threatening events in infants: a systematic review. Arch Dis Child. 2004;89:1043-1048.
23. Hall KL, Zalman B. Evaluation and management of apparent life-threatening events in children. Am Fam Physician. 2005;71:2301-2308.
24. Centers for Disease Control and Prevention. Sudden unexpected infant death and sudden infant death syndrome. Updated May 11, 2015. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/sids/data.htm. Accessed June 30, 2015.
25. Kahn A, Blum D, Hennart P, et al. A critical comparison of the history of sudden-death infants and infants hospitalized for nearmiss for SIDS. Eur J Pediatr. 1984;143:103-107.
26. Baroni MA. Apparent life-threatening events during infancy: a follow-up study of subsequent growth and development. J Dev Behav Pediatr. 1991;12:154-161.
27. Tirosh E, Kessel A, Jaffe M, et al. Outcome of idiopathic apparent life-threatening events: infant and mother perspectives. Pediatr Pulmonol. 1999;28:47-52.
28. Koons AH. Neurodevelopmental outcome in infants with apnea. N J Med. 1992;89:688-690.
29. Poets CF. Apnea of prematurity, sudden infant death syndrome, and apparent life-threatening events. In: Taussig LM, ed. Pediatric Respiratory Medicine. Philadelphia, PA: Mosby; 2008:413-434.
30. Adams SM, Ward CE, Garcia KL. Sudden infant death syndrome. Am Fam Physician. 2015;91:778-783.
31. Goldwater PN. A perspective on SIDS pathogenesis. The hypothesis: plausibility and evidence. BMC Med. 2011;9:64.
32. Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol Neonate. 1994;65:194-197.
Improving our approach to preventive care
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
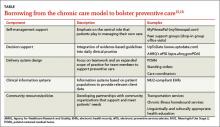
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; [email protected]
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; [email protected]
› Avoid scheduling annual visits exclusively for preventive care. A
› Institute simple practice changes to improve the preventive services you provide, such as implementing standing orders for influenza vaccines. A
› Adopt components of the chronic care model for preventive services wherever possible—using ancillary providers to remind patients to undergo colorectal cancer screening and recommending apps that support self-management, for example. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For well over a century, the periodic health exam has been associated with the delivery of preventive services1—a model widely accepted by physicians and patients alike. Approximately 8% of ambulatory care visits are for check-ups, and more than 20% of US residents schedule a health exam annually.2
Periodic health exams, however, do not result in optimal preventive care. Evidence suggests that important preventive services, such as dietary counseling, occur at only 8% of such visits; that just 10 seconds, on average, is devoted to smoking cessation; and that 80% of the preventive services patients receive are delivered outside of scheduled health exams.2 Because physicians and patients alike understandably prioritize acute problems, any discussion of health maintenance issues during a periodic check-up is likely to occur despite the visit’s agenda, not as part of it.3-7
Primary care physicians and practices are increasingly being held accountable for their performance on preventive measures. With that in mind, being familiar with evidence-based guidelines relating to the delivery of preventive services in ambulatory care settings, such as those developed by the US Preventive Services Task Force (USPSTF),8 is crucial. Identifying elements of the chronic care model that can be effectively applied to preventive care—and recognizing that the reactive acute care model is ineffective for both chronic illness and preventive services—is essential, as well.
Preventive care that's evidence-based
Good practice guidelines should have the following features, according to the Institute of Medicine:9
• Validity. Application would lead to the desired health and cost outcomes.
• Reliability/reproducibility. Others using the same data/interpretation would reach the same conclusion.
• Clinical applicability. Patient populations are appropriately defined.
• Clinical flexibility. Known or generally expected exceptions are identified.
• Clarity. Guideline uses unambiguous language with precise definitions.
• Multidisciplinary process. Developed with the participation of all key stakeholders.
• Scheduled review. Periodically reviewed and revised to incorporate new evidence/changing consensus.
• Documentation. Procedures used in development are well documented.
The USPSTF guidelines are consistent with these attributes.8
Some brief interventions fail
Guidelines are useful as practice standards for establishing preventive care goals, but their existence alone does not ensure the delivery of high-quality preventive services in an office setting. One key factor is time. It is estimated that a primary care physician with an average-sized patient panel would require an additional 7.4 hours per day to achieve 100% compliance with all of the USPSTF recommendations.10
Counseling, in particular, is an intervention that may be more effective in theory than in practice. Evidence suggests that even when primary care providers are trained in preventive counseling (and many are not), many brief interventions are not effective at creating sustained behavior change or health improvement.11
Others are effective
That’s not to say that there’s little that can be done. Use of standing orders for influenza vaccination is one example of an effective and easily implemented preventive measure that requires little or no additional physician time. Yet some doctors are resistant, fearing loss of control or lawsuits. In fact, the National Vaccine Injury Compensation Program specifically provides protection against vaccinerelated malpractice claims.12 A standing order for office staff to arrange a screening mammogram is another effective intervention; it has been shown to improve screening rates by as much as 30%.13
Lessons from chronic illness management
Chronic illness care and preventive care have much in common.14 Both acknowledge the need for proactive screening and counseling to bring about behavior change. In addition, both require ongoing care and follow-up, as well as depend on links to community resources. And finally, both are resource-intensive—too resource-intensive, some say, to be delivered in a cost-effective manner.
The Chronic Care Model has been proposed as a framework for improving preventive services (TABLE).15,16 Evidence suggests adopting some key components of chronic care can lead to significant gains in the delivery of preventive care, with the largest improvement seen when multiple components are used simultaneously.17-20
Self-management support. A growing body of evidence shows that self-management activities are associated with improved outcomes.21 Instituting a peer support group for pregnant women at a federally qualified health center, for example, led to a 7% absolute risk reduction in preterm births.22
Not all efforts to encourage self-management are effective, however, and evaluation is crucial to determine what works. In one large-scale trial, the use of patient reminder cards to facilitate a reduction in health risk behaviors such as tobacco use, risky drinking, unhealthy dietary patterns, and physical inactivity led to fewer health risk assessments being performed, fewer individual counseling encounters, and no change in these behaviors.23
Decision support. Clinical decision support systems, which generate patient-specific evidence-based assessments and/or recommendations that are actionable as part of the workflow at the point of care, have been found to improve care.24,25 An example of this is a prompt that reminds the physician to discuss chemoprevention with a patient at high risk for breast cancer.
Delivery system design. The patient-centered medical home (PCMH) is a well-known example of a redesign of health care delivery.26 Conversion to this model is associated with a small positive effect on preventive interventions.27 However, the persistence of a fee-for-service payment system—which does not include physician reimbursement for some of the added services incorporated into the PCMH—limits the implementation of the PCMH model.28
Many practices are improving health care delivery by using nonphysicians for various tasks related to preventive care. Care managers, for example, typically have smaller caseloads and focus on reducing unnecessary treatment for patients with high-risk conditions, such as congestive heart failure, while patient navigators generally have less clinical expertise but more knowledge of community services.
In one study, practice-initiated phone conversations with nonphysicians increased colorectal cancer screening by up to 40%.29 And in one pilot program, the use of ancillary providers led to an increase in colorectal cancer screening by as much as 123%.30 The human touch seems to be key to the success of these interventions. Passive reminders, such as videos being shown on waiting room televisions, have not proven to be effective.31
Clinical information systems. Early on, the power of electronic health records (EHRs) to improve practices’ delivery of preventive services was recognized. As early as 1995, the use of a reminder system to highlight such services during acute care visits was linked to improvements in counseling about smoking cessation and higher rates of cervical cancer screening, among other preventive measures.32,33 Overall, the use of EHRs alone has been shown to improve rates of preventive services by as much as 66%, with most practices reporting improvements of at least 20%.34
Today, EHRs that are Meaningful Use Stage 2-compliant have the tools needed to improve care. Requirements include the ability to generate patient registries of all those with a given disease and to identify patients on the registry who have not received needed care.35 To improve preventive care, registries should focus on the mitigation of risk factors, such as identifying—and contacting—patients with diabetes who are in need of, or overdue for, an annual eye exam.
Trials using the registry function, in combination with automated messaging to deliver targeted information to various patient groups (identified by the demographic information available from the EHR), are ongoing.
Community resources. Many clinicians have informal referral relationships with community organizations, such as the YMCA. Physician practices that establish links to community resources have the potential to have a large effect on unhealthy behaviors. (See “Putting theory into practice: 2 cases”.) However, a systematic review found that, while evidence to support such connections is mainly positive, research is limited and further evaluation is needed.36
The Practical Playbook (practicalplaybook.org)37 developed by the deBeaumont Foundation, Duke Department of Community and Family Medicine, and the Centers for Disease Control and Prevention, offers concrete examples of how physician practices are linking with community resources to improve the health of the population. For example, Duke University’s “Just for Us” program provides in-home chronic illness care to 350 high-risk elderly individuals. The LATCH program connects thousands of Latino immigrants to health care services and culturally and linguistically appropriate health education classes.37
CASE 1
Dominic B, a 53-year-old patient, has scheduled a visit for a cough that has persisted for 4 weeks. The patient is a nonsmoker, is married, and has no first-degree relatives with cancer. But when you review his chart before the visit, you note that he missed his 6-month check-up for hypertension and hyperlipidemia. In addition, your electronic health record (EHR ) flags the fact that he has not undergone colorectal screening and that his immunizations are not current. Because you have standing orders in place, your medical assistant gives him a flu shot and a pamphlet providing information on colorectal cancer screening before you enter the room.
During the visit, Mr. B mentions that his father, age 82, recently had a heart attack. This event—reinforced by the postcards and phone messages he received from your office after he missed his 6-month follow-up—prompted him to reluctantly admit it was “time for a check-up.” You take the patient’s blood pressure (BP) and review his lipid panel (blood work was ordered prior to his visit) with him. He is relieved to know that he will not need to be on a statin and agrees to be screened for colorectal cancer using a sensitive stool study.
Before he leaves, the patient requests medication for erectile dysfunction—a problem he never reported before. You ask him to keep a diary and return in 2 months, and promise to discuss his erectile dysfunction at that time.
CASE 2
A review of your practice’s patient registry reveals that Gladys P, age 55, is behind on breast and cervical cancer screening. She has had only a few sporadic office visits, the last of which was for bronchitis 18 months ago. At that time, the patient’s systolic BP was 162 mm Hg. You told her you would recheck it in 6 weeks, but she failed to return for follow-up.
Ms. P smokes, but has no other chronic diseases and takes no medications. There is no record of a mammogram or Pap smear, and you don’t know whether she sees a gynecologist routinely. Your office contacts her and discovers that you are the only doctor she sees. The patient tells the medical assistant who placed the call that her car broke down but she has not had money to repair or replace it, so she has had no way to get to your office.
Your staff arranges for her to get a ride from a community volunteer group, first to the nearby hospital for a mammogram and then to your office, where you perform a Pap smear and address her elevated BP and smoking. You are rewarded for the counseling and preventive care with a letter and a bonus check from Ms. P’s insurance carrier, congratulating you on your quality improvement efforts.
Growing emphasis on quality
Systemic changes in the US health care system are occurring rapidly, with an emphasis on quality and improved outcomes. Many physicians are now required to submit data to external agencies for payment, and much of the data is grounded in preventive standards. Medicare’s Physicians Quality Reporting System requires that all Medicare providers provide data on preventive and chronic illness care. Rates of vaccination, obesity screening, and tobacco use screening are examples of preventive services that will be reported publicly on the Centers for Medicare & Medicaid Services’ Physician Compare Web site.38
Physicians who work in accountable care organizations are required to meet quality standards on the delivery of certain preventive services, including breast cancer screening, colorectal cancer screening, influenza and pneumonia immunization, body mass index screening and follow-up, tobacco use screening and cessation intervention, screening for high blood pressure and followup, and screening for clinical depression and follow-up.39 As patients discover that the Affordable Care Act mandates that preventive services be covered with no cost sharing, they are likely to become more receptive to physician attempts to provide them.40,41
CORRESPONDENCE
Gerald Liu, MD, 1504 Springhill Avenue, Suite 3414, Mobile, AL 36604-3207; [email protected]
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.
1. Han P. Historical changes in the objectives of the periodic health exam. Ann Intern Med. 1997;127:910-917.
2. Mehrota A, Zaslavsky A, Anyanian J. Preventive health examinations and preventive gynecologic examinations in the United States. Arch Intern Med. 2007;167:1876-1883.
3. Jean CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. J Fam Pract. 1994;38:166-171.
4. McGinnis JM, Foege WH. The immediate versus the important. JAMA. 2004;291:1263-1264.
5. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
6. US Department of Health and Human Services. August 28, 2013. Healthy People 2020. Available at: www.healthypeople.gov. Accessed September 13, 2013.
7. Pollak KI, Krause KM, Yarnall KS, et al. Estimated time spent on preventive services by primary care physicians. BMC Health Serv Res. 2008;8:245.
8. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Washington, DC: Office of Disease Prevention and Health Promotion; 1996.
9. Field MJ, Lohr KN, Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academies Press; 1990.
10. Yarnell K, Pollak K, Ostbye T, et al. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635-641.
11. Butler CC, Simpson SA, Hood K, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomized trial. BMJ. 2013;346:f1191.
12. Yonas M, Nowalk M, Zimmerman R, et al. Examining structural and clinical factors associated with implementation of standing orders for adult immunization. J Healthcare Qual. 2012;34:34-42.
13. Donahue K, Plescia M, Stafford K. Do standing orders help with chronic disease care and health maintenance in ambulatory practice? J Fam Pract. 2010;59:226-227.
14. Glasgow R, Orleans T, Wagner E. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2002;79.
15. Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effective Clin Pract. 1998;1:2-4.
16. Barr V, Robinson S, Marin-Link B, et al. Chronic care model: an integration of concepts and strategies from population health promotion and the chronic care model. Hosp Q. 2003;7:73-83.
17. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775-1779.
18. Moore LG. Escaping the tyranny of the urgent by delivering planned care. Fam Pract Manage. 2006;13:37-40.
19. Tsai AC, Morton SC, Mangione CM, et al. A meta-analysis of interventions to improve care for chronic illnesses. Am J Managed Care. 2005;11:478-488.
20. Coleman K, Austin BT, Brach C, et al. Evidence on the chronic care model in the new millenium. Health Affairs. 2009;28:75-85.
21. Pearson ML, Mattke S, Shaw R, et al. Patient Self-Management Support Programs: An Evaluation. Final Contract Report. Publication No. 08-0011. Rockville, MD: Agency for Healthcare Research and Quality. November 2007.
22. Feder J. Restructuring care in a federally qualified health center to better meet patients’ needs. Health Affairs. 2011. Available at: http://content.healthaffairs.org/content/30/3/419.full.html. Accessed February 13, 2014.
23. Hung D, Rundall T, Tallia A, et al. Rethinking prevention in primary care: applying the chronic care model to address health risk behaviors. Milbank Q. 2007;85:69-91.
24. Lobach D, Sanders GD, Bright TJ, et al. Enabling Health Care Decisionmaking Through Clinical Decision Support and Knowledge Management. Evidence Report No. 203. Publication No. 12-E001-EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012.
25. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systemic review of trials to identify features critical to success. BMJ. 2005;330:765.
26. American Academy of Family Physicians, American Academy of Pediatrics, American College of Physicians, American Osteopathic Association. Joint Principles of the Patient-Centered Medical Home. March 2007. American Academy of Family Physicians Web site. Available at: http://www.aafp.org/dam/AAFP/documents/practice_management/pcmh/initiatives/PCMHJoint.pdf. Accessed May 7, 2015.
27. Agency for Healthcare Research and Quality. The Patient-Centered Medical Home. Closing the Quality Gap: Revisiting the State of the Science: Evidence Report/Technology Assessment Executive Summary No. 208. Agency for Healthcare Research and Quality Web site. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/391/1178/EvidReport208_CQGPatientCenteredMedicalHome_FinalReport_20120703.pdf. Accessed May 7, 2015.
28. Peikes D, Zutshi A, Genevro J, et al. Early Evidence on the Patient-Centered Medical Home. Final Report. Publication No. 12-0020-EF. Rockville, MD: Agency for Healthcare Research and Quality. February 2012.
29. Liu G, Perkins A. Using a lay cancer screening navigator to increase colorectal cancer screening rates. J Am Board Fam Med. 2015;28:280-282.
30. Baker N, Parsons M, Donnelly S, et al. Improving colon cancer screening rates in primary care: a pilot study emphasising the role of the medical assistant. Qual Saf Health Care. 2009;18:355-359.
31. Holden D, Jonas D, Portersfield D. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668-676.
32. Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inf Assoc. 2008;15:311-320.
33. Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inf Assoc. 1996;3:399-409.
34. Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144:742-752.
35. Future of Family Medicine Leadership Committee. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(Suppl 1):S3-S32.
36. Porterfield D, Hinnant L, Kane H, et al. Linkages Between Clinical Practices and Community Organizations for Prevention: Final Report. Rockville, MD: Agency for Healthcare Research and Quality. October 2010.
37. deBeaumont Foundation, Duke Community and Family Medicine, Centers for Disease Control and Prevention. A Practical Playbook. Available at: https://practicalplaybook.org. Accessed March 29, 2015.
38. Physician Quality Reporting System. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS. Accessed March 29, 2015.
39. RTI International. Accountable Care Organization 2014 Program Analysis Quality Performance Standards Narrative Measure Specifications. 2014. Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/Medicare/Medicare-Feefor-Service-Payment/sharedsavingsprogram/Downloads/ACONarrativeMeasures-Specs.pdf. Accessed May 7, 2015.
40. Koh K, Sebelius K. Promoting prevention through the Affordable Care Act. N Engl J Med. 2010;363:1296-1299.
41. Rosenbaum S. The patient protection and affordable care act: Implications for public health policy and practice. Public Health Rep. 2011;126:130-135.