User login
Preventing drinking relapse in patients with alcoholic liver disease
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
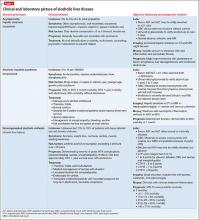
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
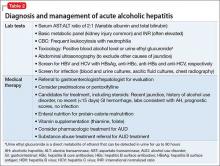
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
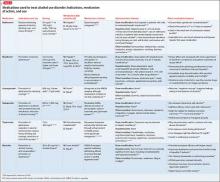
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
Veterans’ Use of Designer Cathinones and Cannabinoids
Although the elevated risks and rates of veterans’ substance abuse patterns are well documented, little has been written about veterans’ use of designer drugs.1-6 In recent months throughout Europe and the U.S., there has been a flurry of media attention for 2 classes of designer drugs: synthetic cathinones and synthetic cannabinoids.7,8 In the U.S., the popularity of these drugs has surged, and a disproportionate amount of use of these 2 drug classes is coming from locations near military instillations.9,10
The purpose of this article is to raise awareness regarding these 2 burgeoning designer drug classes and their impact on veterans. Designer drugs affecting vulnerable populations are not a new phenomenon, yet many providers are unfamiliar with the effects of these unique drugs of abuse on their veteran populations.11-13
Many designer drugs begin their existence as variations of other addictive or psychoactive drugs. Others begin in laboratories as investigative research compounds that end up on the street, often promising a novel mind-altering experience as a “legal high.”14-18 The Designer Drug Enforcement Act of 1986 was an initial attempt in the U.S. to define and control the early rise of copycat drugs that appeared on the streets and mimicked the effects of other illicit substances. More recent legislation enacted in the U.S. has imposed Schedule I controls on the manufacture, distribution, possession, importation, and exportation of these types of drugs, including both synthetic cathinones and synthetic cannabinoids. State laws are perennially in flux trying to keep up with the latest drug trends.19-21
Similar efforts have been made by the European Union to control mephedrone, a synthetic cathinone, citing multiple fatalities, seizures, related crime, lack of medical use, and risk of dependence.22 Although uniform levels of control do not exist in Europe for synthetic cannabinoids, many countries have independently acted to limit their use.23
In its recent World Drug Report 2013, the United Nations Office on Drugs and Crime documents its growing concern about the “new psychoactive substances” category of illicit recreational substances (in which synthetic cannabinoids and cathinones are included) that has increased by 50% since 2009.24 Alone, this category now outnumbers the total number of substances controlled by international drug conventions.
The novelty and variability of designer drugs causes difficulties with detection and regulation. Innovative chemists can legally manufacture new versions of known molecules intended for illicit use with a rapidity that outpaces bureaucratic control. Local law enforcement officials may be unaware of the latest designer drug trends, stifling efforts at public education or restriction. Designer drugs are often deceptively packaged and are available in convenience stores, tobacco outlets, gas stations, pawnshops, tattoo parlors, and truck stops.25-28 The Internet may be the singular reason, however, that designer drugs continue to be widely available to veterans.11,18
Innumerable websites discuss, promote, and sell designer drugs or deceitfully market them as safe, legitimate household products (“not for human consumption”), which can be ordered online and shipped by commercial carriers.12 Little accurate information is known about their effects or about the specific compounds they contain. When the recreational nature of the drugs is actually acknowledged, information on how the buyer can evade prosecution is often provided in tandem. The suppliers’ inventory of the drugs has been shown to be variable and inconsistent, and the product ingredients can be similarly unpredictable despite comparatively more stable naming and labeling.14,29
In the clinical setting, a reliable patient drug history may not be available. This ensures that the diagnosis of designer drug use will be an exclusionary process involving routine laboratory work, physical examination, and at times electroencephalogram and/or neuroimaging. Psychiatric consultation is often useful in this setting. Routine immunoassay tests do not detect either synthetic cathinones or synthetic cannabinoids.30
Both cannabinoids and cathinones can be identified using gas chromatography-mass spectroscopy (GC-MS) or liquid chromatography-mass spectroscopy (LC-MS). However, this technology is limited to specialized laboratories.31,32 The laboratory results often are not immediately available, potentially limiting the tests’ use in emergency or inpatient settings, as the patient may have left the hospital by the time the results are available. Additionally, these drugs’ prevalence of use, while increasing, often does not justify the cost of these tests.
The inability to routinely detect metabolites in urine may increase the enticement of these drugs given the likelihood that active-duty personnel could use them surreptitiously. Further, these compounds are evolving and seemingly limitless in their variability, and there is often a paucity of pure reference materials. As such, it is impossible to guarantee reliable test results.
The following profiles of each of these drug classes will be accompanied by clinical cases depicting the drugs’ effects and how an affected veteran might present clinically. The severe effects of these novel agents illustrate the value in maintaining a functional knowledge base about emerging drug trends. The accuracy of diagnosis as well as the outcome of a veteran’s treatment may depend on the provider’s ability to identify the presence of a drug and manage its effects.
Synthetic Cathinones
Mr. H is a 28-year-old Iraq War veteran with a history of posttraumatic stress disorder (PTSD), alcohol abuse, and opioid dependence who presented for inpatient psychiatric admission after making suicidal statements to his wife in the context of 2 weeks of “bath salts” use. A family member initially introduced him to the drug. His first drug purchase had been 1 gram ($30) at a local movie rental store.
After discharge from the hospital, Mr. H began purchasing increasing amounts online with a credit card. Although he initially had been insufflating and inhaling the substance, he later began injecting it (dissolving it in tap water and loading it through a cotton filter in a syringe). The patient admitted to finding the drug significantly more addictive than any others he had used, and his use resulted in leaving his job and abandoning his family.
Severe cravings and depression were present between episodes of use. He spent $40,000 over 6 months of use. Insomnia lasted for several days, his appearance changed dramatically (including persistent skin infections), and he became paranoid, believing that everyone around him was an undercover police officer. He remained on medications for persistent anxiety. His daily drug cravings continued,
although he remained uncertain about the actual ingredients of bath salts.
Cathinone is a naturally occurring stimulant from the khat plant (Catha edulis), which grows indigenously in Egypt and on the Arabian Peninsula. The recreational and religious use of this plant has occurred for thousands of years, though it is not without risk: The chewing of the leaves containing natural cathinones has been associated with esophagitis, gastritis, oral keratosis, myocardial infarction, dilated cardiomyopathy, hypertension, cerebral ischemia, thromboembolism, diabetes, sexual dysfunction, duodenal ulcer, and hepatitis.33,34
The stimulants known colloquially as bath salts are synthetic cathinones, which have become more widely available within the past 10 years: first in the Middle East, then Europe, and now in the U.S.5,9,10,14,19,25,35-41,44 Although the current rise in use has occurred in the past few years, the first documented abuse of synthetic cathinones in the U.S. dates to the early 1990s in Michigan.42
Bath salts is the most common of the many names used to denote synthetic cathinones. The compounds have no utility when used as such but often are marketed as research chemicals, plant fertilizer, or shoe polish. It is this deliberate counterfeit of household product names that allows many distributors to avoid classifying the compounds according to the true, intended use. More appealing brand names may also be used to entice the user (Table 1).25
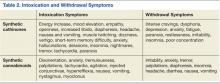
Synthetic cathinones owe their popularity to similarities with cocaine and methamphetamine. They are sympathomimetic with synaptic increases of monoamines after use: Surges in norepinephrine and dopamine account for the stimulant qualities, and serotonergic changes mediate distinct psychoactive effects (Table 2).40 Users are interested in the drugs for many of the same reasons that other recreational stimulants have appeal: euphoria, energy, empathy, heightened sexuality, sociability, and an overall intensification of senses. Synthetic cathinones have become preferred to cocaine for some users.43
The drugs can be used via oral and anal routes. Using methods known as “bombing” or “keystering,” users deliver boluses of the powder wrapped in cigarette paper, which they swallow or insert into the rectum. Insufflation and IV injection are also common methods of administration with a quicker onset of action expected.40 The prices of the drugs range from $25 to $50 per 500-mg packet (though the cost is increasing with more regulation). Users typically use 500 mg to 2 g in one session.
The 2 most commonly abused synthetic cathinones are mephedrone and MDPV (methylenedioxypyrovalerone). There is some regional variability about which ingredient is present; mephedrone tends to be more prevalent in Europe, whereas MDPV is noted to be more common in the U.S.10,44
When ordering a laboratory test to evaluate for the presence of these drugs, a specific request should be given to the technicians to look for signals of MDPV (most common metabolite is dimethylenyl-methyl-MDPV), mephedrone (4-methylmethcathinone), 3-bromomethcathinone (3-BMC), or 3-fluoromethcathinone (fluphedrone).45-48 The study testing (both in VA and civilian settings) for Mr. H was done by a commercial laboratory several states away where patented techniques can screen for more than 30 compounds via LC-MS. The laboratory offered bath salts panels for urine, serum/plasma, and blood samples.
Synthetic cathinones are dangerous, and as the body of medical literature continues to expand, reports of significant morbidity and death related to their use are appearing. The harmful effects of recreational synthetic cathinone use has been documented across the globe in the form of serotonin syndrome, intoxication delirium, hyperthermia and multi-organ failure, myocarditis, hypo-osmotic hyponatremia with encephalopathy, agitation, psychosis, and death after cardiac arrest.5,12,38,39,49-53 Published treatment methods are largely supportive with the available literature, suggesting that benzodiazepines, antipsychotics (both typical and atypical), restraints to maintain safety, and IV fluids may be indicated.5,9,50
Synthetic Cannabinoids
Mr. W is a 58-year-old veteran with a history of alcohol dependence and PTSD who reported use of the synthetic cannabinoid “Spice” during intake assessment for treatment of alcohol dependence. He reported using Spice about 4 times over a 2-month period. He purchased a small jar of the substance from a party store for $15 per gram and understood its contents to be synthetic marijuana, which he appreciated for its low cost and assumed legality. He denied having any understanding of the package’s contents beyond “synthetic marijuana.”
The patient ingested the drug by smoking and inhaling from a pipe. For the first 3 times that he used the substance, Mr. W reported feeling a pleasant sensation that started quickly and lasted about 30 minutes. The fourth time that he used synthetic cannabis he felt nauseated and vomited several times, had auditory hallucinations, and increased anxiety; he also reported a hangover effect after this use. He identified that the effects may have been different the fourth time “because the brands were changing.”
Mr. W also reported that his neighbor—a daily user of synthetic cannabinoids for several months—became paranoid, suspicious, and developed incomprehensible speech. His neighbor’s symptoms and his own unpleasant experiences prompted a discontinuation of use.
Synthetic cannabinoids are a diverse group of agents numbering in excess of 100 artificial compounds that act as agonists at cannabinoid receptors, mimicking the effects of tetrahydrocannabinol (THC), an active ingredient in marijuana.28,54 The availability of these drugs online and in specialty shops has been documented since the mid-2000s.27,28,32 Their packaging often describes the contents as incense or herbal blends, using various names. Spice is a common name, but these products are also known by a myriad of other designations (Table 1).28 A single packet usually contains several grams of the drug and costs about $30.55
To the user, who may already be familiar with marijuana, the contents intentionally appear similar to the dried buds of cannabis.30,56 In reality, the drug has just been sprayed onto inert plant material.57 The drug is smoked, and the psychoactive dose can be as little as 1 mg.30 Users describe potent drug effects (Table 2). There is a rapid onset of action, and duration of effects last 1 to 2 hours.58
The compounds’ mechanism of action and appeal are derived from their high affinity for the cannabinoid receptors. The CB1 receptor is located primarily in the central nervous system and is responsible for the psychoactive component of the drugs’ actions.27,30,58,59 Two particular synthetic cannabinoids, cannibicyclohexanol and JWH-018, are potent cannabinoid CB1 agonists with affinity exceeding their natural counterparts.27,30, 32,56,58,59
Chemically, these drugs are varied. The largest structural family of these compounds is the JWH group, which includes JWH-018.60 Also common are CP 47,497 and other CP compounds.58 HU compounds, such as HU-210, have also been identified and have been shown to be 100 to 800 times more potent at the CB1 and CB2 receptors than is THC.60,61 A final group includes the benzoylindoles, such as AM-964 and RCS-4, which also bind strongly to CB1 and CB2.60,62
Constitutional symptoms of synthetic cannabinoid intoxication include disorientation, anxiety, tremulousness, palpitations, tachycardia, agitation, injected conjunctivae, hyperreflexia, nausea, vomiting, lateral gaze nystagmus, and myoclonic jerks, which have been mistaken for seizure activity.27,30,55 Pupils are often normal sized.55 Withdrawal phenomena are similar to those of cannabis withdrawal: irritability, anxiety, tremor, palpitations, diaphoresis, insomnia, headache, diarrhea, nausea, and vomiting.59
Given the established link between cannabis use and psychosis, synthetic cannabinoids may stand as a precipitant of psychotic symptoms, which may include visual hallucinations, auditory hallucinations, disorganized speech, paranoia, grandiose delusions, disorganization, or bizarre behavior.58,63-66 These symptoms may represent a relapse of a primary thought disorder or, for some unfortunate individuals, a de novo psychotic illness.58,65,66 Symptoms can linger for months after drug use.65
A key risk in the use of synthetic cannabinoid moieties may involve the absence of cannabidiol. Cannabidiol naturally occurs in many strains of cannabis and is thought to have antipsychotic, neuroprotective properties.67 The absence of this molecule in synthetic cannabinoids may at least partially explain their severe psychoactive effects. Treatment for synthetic cannabinoid intoxication and related psychosis is largely supportive and may include the use of antipsychotic medication.66
Detection of synthetic cannabinoids in urine is difficult, yet many compounds can be detected via GC-MS or LC-MS. Molecules of significance include JWH-018, JWH-073, JWH-015, JWH-250, CP-47 497, HU-210, cannabicyclohexanol, and oleamide; however, these compounds are rarely excreted in urine in their pure form. The many hydroxylated or dealkylated metabolites of these compounds, mostly unnamed, are more consistently detected in urine.68,69 One author has noted that the pentanoic acid metabolite of JWH-018 seems to appear most reliably in urine specimens.68
Many synthetic cannabinoid herbal mixes also contain a detectable compound called tocopherol, seemingly added as an antioxidant.69,70 Synthetic cannabinoids are an evolving drug class, and reliable detection will require that laboratories stay up-to-date in their detection methods. As stated earlier, a commercial laboratory in the region accepted the civilian and veteran patient samples for these case studies. The synthetic cannabinoid panels offered evaluation of the drug itself (GC-MS), an oral fluid screen (LC-MS), and isolation of metabolites in urine (enzyme-linked immunosorbent assay).
Conclusion
Designer drugs will remain a challenge for providers caring for veterans for several key reasons: (1) Veterans are a vulnerable population who abuse substances at higher rates than do their civilian counterparts; (2) Chemists are able to manufacture variations of known habit-forming substances; (3) Modern technology facilitates the purchase and wide distribution of addictive substances; (4) Many designer drugs are deceptively packaged and marketed; (5) The effects of the drugs are often severe; (6) No standardized treatment guidelines exist; and (7) Detection of the drugs is difficult, and new versions of the molecules may evade even cutting-edge techniques.
Due to the high cost of detecting synthetic cathinones and synthetic cannabinoids in body fluids, screening should be considered only in settings where severe symptoms are accompanied by reasonable clinical suspicion of use and an otherwise negative toxicologic workup. As more designer drugs inevitably emerge, research will be needed on their pharmacology, toxidromes, and detection. Military and civilian practitioners must remain abreast of the dynamic trends in designer drugs to ensure that their patients receive the highest level of medical care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. J Drug Alcohol Abuse. 1999;25(2):239-256.
2. Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102(7):1092-1101.
3. Hankin CS, Spiro A 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: The Veterans Health Study. Am J Psychiatry. 1999;156(12):1924-1930.
4. Sirratt D, Ozanian A, Traenkner B. Epidemiology and prevention of substance use disorders in the military. Mil Med. 2012;177(suppl 8):21-28.
5. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat. 2013;44(1):42-45.
6. Bobes J, Sáiz PA, González MP, et al. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8(3):147-154.
7. D.E.A. Cracks Down on Designer Drug Operations. The New York Times Website. http://www.nytimes.com/2013/06/27/us/dea-cracks-down-on-designer-drug-operations.html. Published June 26, 2013. Accessed October 6, 2014.
8. Travis A. Mushrooming legal highs leave drug control system floundering, UN warns. The Guardian Website. http://www.guardian.co.uk/world/2013/jun/26/legal-highs-drug-control. Published June 26, 2013. Accessed October 6, 2014.
9. Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med. 2012;79(4):258-264.
10. Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath salts” and “plant food” products: The experience of one regional US poison center. J Med Toxicol. 2013;9(1):42-48.
11. Wax PM. Just a click away: Recreational drug web sites on the Internet. Pediatrics. 2002;109(6):e96.
12. Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201(3):191-195.
13. Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: Deadly new Internet drugs. Pediatrics. 2000;106(4):829-830.
14. Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1-3):59-66.
15. Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18-38.
16. Christophersen AS. Amphetamine designer drugs—An overview and epidemiology. Toxicol Lett. 2000;112-113:127-131.
17. Buchanan JF, Brown CR. ‘Designer drugs.’ A problem in clinical toxicology. Med Toxicol Adverse Drug Exp. 1988;3(1):1-17.
18. Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105(6):951-953.
19. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76(204):65371-65375.
20. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Placement of five synthetic cannabinoids into Schedule I. Fed Regist. 2012;77(41):12508-12514.
21. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into Schedule I. Fed Regist. 2013;78(95):28735-28739.
22. Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. EUR-Lex Website. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:322:0044:0045:en:PDF. Published December 8, 2010. Accessed October 7, 2014.
23. European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘spice’. European Monitoring Centre for Drugs and Drug Addiction Website. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids. Updated September 15, 2011. Accessed October 7, 2014.
24. United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Office on Drugs and Crime Website. http://www.unodc.org/wdr2014. Accessed October 3, 2014.
25. Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436-441.
26. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
27. Loeffler G, Hurst D, Penn A, Yung K. Spice, bath salts, and the U.S. military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil Med. 2012;177(9):1041-1048.
28. European Monitoring Centre for Drugs and Drug Addiction. Understanding the spice phenomenon. The National Documentation Centre on Drug Use Website. http://www.drugsandalcohol.ie/12597/1/Understanding_the_Spice_phenomenon.pdf. Published 2009. Accessed October 7, 2014.
29. Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—Is there consistency in what you get? QJM. 2010;103(7):489-493.
30. Harris CR, Brown A. Synthetic cannabinoid intoxication: A case series and review. J Emerg Med. 2013;44(2):360-366.
31. Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2--Designer cathinones. J Anal Toxicol. 2012;36(6):381-389.
32. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3(7-8):466-478.
33. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145-1154.
34. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540-548.
35. Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal. 2011;3(7-8):454-463.
36. Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210(1-3):195-200.
37. Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375(9723):1333-1334.
38. Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010.
39. Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742.
40. Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8(1):33-42.
41. Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310-313.
42. Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22(12):1897-1903.
43. Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addict. 2011;106(1):154-161.
44. Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49(6):499-505.
45. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther Drug Monit. 2011;33(2):257-263.
46. Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. JMS. 2010;45(12):1426-1442.
47. Santali EY, Cadogan AK, Daeid NN, Savage KA, Sutcliffe OB. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J Pharm Biomed Anal. 2011;56(2):246-255.
48. Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: Studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. JMS. 2012;47(2):253-262.
49. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
50. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: Two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
51. Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34(6):647-650.
52. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: Acute mephedrone ‘meow’ myocarditis. Heart. 2010;96(24):2051-2052.
53. Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8(1):69-75.
54. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
55. Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299.
56. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585-593.
57. Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198(1-3):31-38.
58. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117(2-3):152-157.
59. Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Deutsches Ärzteblatt Int. 2009;106(27):464-467.
60. Loeffler G, Hurst D, Penn A, Yung K. Spice, Bath salts, and the US military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the US armed forces. Mil Med. 2012;177(9):1041-1048.
61. Devane WA, Breuer A, Sheskin T, Jäerbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35(11):2065-2069.
62. Gottardo R, Chiarini A, Dal Prà I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. JMS. 2012;47(1):141-146.
63. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440-447.
64. Every-Palmer S. Warning: Legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addict. 2010;105(10):1859-1860.
65. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am J Psychiatry. 2011;168(10):1119.
66. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287-288.
67. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
68. ElSohly MA, Gul W, ElSohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35(7):487-495.
69. Grigoryev A, Savchuk S, Melnik A, et al. Chromatography–mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15-16):1126-1136.
70. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1):141-147.
Although the elevated risks and rates of veterans’ substance abuse patterns are well documented, little has been written about veterans’ use of designer drugs.1-6 In recent months throughout Europe and the U.S., there has been a flurry of media attention for 2 classes of designer drugs: synthetic cathinones and synthetic cannabinoids.7,8 In the U.S., the popularity of these drugs has surged, and a disproportionate amount of use of these 2 drug classes is coming from locations near military instillations.9,10
The purpose of this article is to raise awareness regarding these 2 burgeoning designer drug classes and their impact on veterans. Designer drugs affecting vulnerable populations are not a new phenomenon, yet many providers are unfamiliar with the effects of these unique drugs of abuse on their veteran populations.11-13
Many designer drugs begin their existence as variations of other addictive or psychoactive drugs. Others begin in laboratories as investigative research compounds that end up on the street, often promising a novel mind-altering experience as a “legal high.”14-18 The Designer Drug Enforcement Act of 1986 was an initial attempt in the U.S. to define and control the early rise of copycat drugs that appeared on the streets and mimicked the effects of other illicit substances. More recent legislation enacted in the U.S. has imposed Schedule I controls on the manufacture, distribution, possession, importation, and exportation of these types of drugs, including both synthetic cathinones and synthetic cannabinoids. State laws are perennially in flux trying to keep up with the latest drug trends.19-21
Similar efforts have been made by the European Union to control mephedrone, a synthetic cathinone, citing multiple fatalities, seizures, related crime, lack of medical use, and risk of dependence.22 Although uniform levels of control do not exist in Europe for synthetic cannabinoids, many countries have independently acted to limit their use.23
In its recent World Drug Report 2013, the United Nations Office on Drugs and Crime documents its growing concern about the “new psychoactive substances” category of illicit recreational substances (in which synthetic cannabinoids and cathinones are included) that has increased by 50% since 2009.24 Alone, this category now outnumbers the total number of substances controlled by international drug conventions.
The novelty and variability of designer drugs causes difficulties with detection and regulation. Innovative chemists can legally manufacture new versions of known molecules intended for illicit use with a rapidity that outpaces bureaucratic control. Local law enforcement officials may be unaware of the latest designer drug trends, stifling efforts at public education or restriction. Designer drugs are often deceptively packaged and are available in convenience stores, tobacco outlets, gas stations, pawnshops, tattoo parlors, and truck stops.25-28 The Internet may be the singular reason, however, that designer drugs continue to be widely available to veterans.11,18
Innumerable websites discuss, promote, and sell designer drugs or deceitfully market them as safe, legitimate household products (“not for human consumption”), which can be ordered online and shipped by commercial carriers.12 Little accurate information is known about their effects or about the specific compounds they contain. When the recreational nature of the drugs is actually acknowledged, information on how the buyer can evade prosecution is often provided in tandem. The suppliers’ inventory of the drugs has been shown to be variable and inconsistent, and the product ingredients can be similarly unpredictable despite comparatively more stable naming and labeling.14,29
In the clinical setting, a reliable patient drug history may not be available. This ensures that the diagnosis of designer drug use will be an exclusionary process involving routine laboratory work, physical examination, and at times electroencephalogram and/or neuroimaging. Psychiatric consultation is often useful in this setting. Routine immunoassay tests do not detect either synthetic cathinones or synthetic cannabinoids.30
Both cannabinoids and cathinones can be identified using gas chromatography-mass spectroscopy (GC-MS) or liquid chromatography-mass spectroscopy (LC-MS). However, this technology is limited to specialized laboratories.31,32 The laboratory results often are not immediately available, potentially limiting the tests’ use in emergency or inpatient settings, as the patient may have left the hospital by the time the results are available. Additionally, these drugs’ prevalence of use, while increasing, often does not justify the cost of these tests.
The inability to routinely detect metabolites in urine may increase the enticement of these drugs given the likelihood that active-duty personnel could use them surreptitiously. Further, these compounds are evolving and seemingly limitless in their variability, and there is often a paucity of pure reference materials. As such, it is impossible to guarantee reliable test results.
The following profiles of each of these drug classes will be accompanied by clinical cases depicting the drugs’ effects and how an affected veteran might present clinically. The severe effects of these novel agents illustrate the value in maintaining a functional knowledge base about emerging drug trends. The accuracy of diagnosis as well as the outcome of a veteran’s treatment may depend on the provider’s ability to identify the presence of a drug and manage its effects.
Synthetic Cathinones
Mr. H is a 28-year-old Iraq War veteran with a history of posttraumatic stress disorder (PTSD), alcohol abuse, and opioid dependence who presented for inpatient psychiatric admission after making suicidal statements to his wife in the context of 2 weeks of “bath salts” use. A family member initially introduced him to the drug. His first drug purchase had been 1 gram ($30) at a local movie rental store.
After discharge from the hospital, Mr. H began purchasing increasing amounts online with a credit card. Although he initially had been insufflating and inhaling the substance, he later began injecting it (dissolving it in tap water and loading it through a cotton filter in a syringe). The patient admitted to finding the drug significantly more addictive than any others he had used, and his use resulted in leaving his job and abandoning his family.
Severe cravings and depression were present between episodes of use. He spent $40,000 over 6 months of use. Insomnia lasted for several days, his appearance changed dramatically (including persistent skin infections), and he became paranoid, believing that everyone around him was an undercover police officer. He remained on medications for persistent anxiety. His daily drug cravings continued,
although he remained uncertain about the actual ingredients of bath salts.
Cathinone is a naturally occurring stimulant from the khat plant (Catha edulis), which grows indigenously in Egypt and on the Arabian Peninsula. The recreational and religious use of this plant has occurred for thousands of years, though it is not without risk: The chewing of the leaves containing natural cathinones has been associated with esophagitis, gastritis, oral keratosis, myocardial infarction, dilated cardiomyopathy, hypertension, cerebral ischemia, thromboembolism, diabetes, sexual dysfunction, duodenal ulcer, and hepatitis.33,34
The stimulants known colloquially as bath salts are synthetic cathinones, which have become more widely available within the past 10 years: first in the Middle East, then Europe, and now in the U.S.5,9,10,14,19,25,35-41,44 Although the current rise in use has occurred in the past few years, the first documented abuse of synthetic cathinones in the U.S. dates to the early 1990s in Michigan.42
Bath salts is the most common of the many names used to denote synthetic cathinones. The compounds have no utility when used as such but often are marketed as research chemicals, plant fertilizer, or shoe polish. It is this deliberate counterfeit of household product names that allows many distributors to avoid classifying the compounds according to the true, intended use. More appealing brand names may also be used to entice the user (Table 1).25
Synthetic cathinones owe their popularity to similarities with cocaine and methamphetamine. They are sympathomimetic with synaptic increases of monoamines after use: Surges in norepinephrine and dopamine account for the stimulant qualities, and serotonergic changes mediate distinct psychoactive effects (Table 2).40 Users are interested in the drugs for many of the same reasons that other recreational stimulants have appeal: euphoria, energy, empathy, heightened sexuality, sociability, and an overall intensification of senses. Synthetic cathinones have become preferred to cocaine for some users.43
The drugs can be used via oral and anal routes. Using methods known as “bombing” or “keystering,” users deliver boluses of the powder wrapped in cigarette paper, which they swallow or insert into the rectum. Insufflation and IV injection are also common methods of administration with a quicker onset of action expected.40 The prices of the drugs range from $25 to $50 per 500-mg packet (though the cost is increasing with more regulation). Users typically use 500 mg to 2 g in one session.
The 2 most commonly abused synthetic cathinones are mephedrone and MDPV (methylenedioxypyrovalerone). There is some regional variability about which ingredient is present; mephedrone tends to be more prevalent in Europe, whereas MDPV is noted to be more common in the U.S.10,44
When ordering a laboratory test to evaluate for the presence of these drugs, a specific request should be given to the technicians to look for signals of MDPV (most common metabolite is dimethylenyl-methyl-MDPV), mephedrone (4-methylmethcathinone), 3-bromomethcathinone (3-BMC), or 3-fluoromethcathinone (fluphedrone).45-48 The study testing (both in VA and civilian settings) for Mr. H was done by a commercial laboratory several states away where patented techniques can screen for more than 30 compounds via LC-MS. The laboratory offered bath salts panels for urine, serum/plasma, and blood samples.
Synthetic cathinones are dangerous, and as the body of medical literature continues to expand, reports of significant morbidity and death related to their use are appearing. The harmful effects of recreational synthetic cathinone use has been documented across the globe in the form of serotonin syndrome, intoxication delirium, hyperthermia and multi-organ failure, myocarditis, hypo-osmotic hyponatremia with encephalopathy, agitation, psychosis, and death after cardiac arrest.5,12,38,39,49-53 Published treatment methods are largely supportive with the available literature, suggesting that benzodiazepines, antipsychotics (both typical and atypical), restraints to maintain safety, and IV fluids may be indicated.5,9,50
Synthetic Cannabinoids
Mr. W is a 58-year-old veteran with a history of alcohol dependence and PTSD who reported use of the synthetic cannabinoid “Spice” during intake assessment for treatment of alcohol dependence. He reported using Spice about 4 times over a 2-month period. He purchased a small jar of the substance from a party store for $15 per gram and understood its contents to be synthetic marijuana, which he appreciated for its low cost and assumed legality. He denied having any understanding of the package’s contents beyond “synthetic marijuana.”
The patient ingested the drug by smoking and inhaling from a pipe. For the first 3 times that he used the substance, Mr. W reported feeling a pleasant sensation that started quickly and lasted about 30 minutes. The fourth time that he used synthetic cannabis he felt nauseated and vomited several times, had auditory hallucinations, and increased anxiety; he also reported a hangover effect after this use. He identified that the effects may have been different the fourth time “because the brands were changing.”
Mr. W also reported that his neighbor—a daily user of synthetic cannabinoids for several months—became paranoid, suspicious, and developed incomprehensible speech. His neighbor’s symptoms and his own unpleasant experiences prompted a discontinuation of use.
Synthetic cannabinoids are a diverse group of agents numbering in excess of 100 artificial compounds that act as agonists at cannabinoid receptors, mimicking the effects of tetrahydrocannabinol (THC), an active ingredient in marijuana.28,54 The availability of these drugs online and in specialty shops has been documented since the mid-2000s.27,28,32 Their packaging often describes the contents as incense or herbal blends, using various names. Spice is a common name, but these products are also known by a myriad of other designations (Table 1).28 A single packet usually contains several grams of the drug and costs about $30.55
To the user, who may already be familiar with marijuana, the contents intentionally appear similar to the dried buds of cannabis.30,56 In reality, the drug has just been sprayed onto inert plant material.57 The drug is smoked, and the psychoactive dose can be as little as 1 mg.30 Users describe potent drug effects (Table 2). There is a rapid onset of action, and duration of effects last 1 to 2 hours.58
The compounds’ mechanism of action and appeal are derived from their high affinity for the cannabinoid receptors. The CB1 receptor is located primarily in the central nervous system and is responsible for the psychoactive component of the drugs’ actions.27,30,58,59 Two particular synthetic cannabinoids, cannibicyclohexanol and JWH-018, are potent cannabinoid CB1 agonists with affinity exceeding their natural counterparts.27,30, 32,56,58,59
Chemically, these drugs are varied. The largest structural family of these compounds is the JWH group, which includes JWH-018.60 Also common are CP 47,497 and other CP compounds.58 HU compounds, such as HU-210, have also been identified and have been shown to be 100 to 800 times more potent at the CB1 and CB2 receptors than is THC.60,61 A final group includes the benzoylindoles, such as AM-964 and RCS-4, which also bind strongly to CB1 and CB2.60,62
Constitutional symptoms of synthetic cannabinoid intoxication include disorientation, anxiety, tremulousness, palpitations, tachycardia, agitation, injected conjunctivae, hyperreflexia, nausea, vomiting, lateral gaze nystagmus, and myoclonic jerks, which have been mistaken for seizure activity.27,30,55 Pupils are often normal sized.55 Withdrawal phenomena are similar to those of cannabis withdrawal: irritability, anxiety, tremor, palpitations, diaphoresis, insomnia, headache, diarrhea, nausea, and vomiting.59
Given the established link between cannabis use and psychosis, synthetic cannabinoids may stand as a precipitant of psychotic symptoms, which may include visual hallucinations, auditory hallucinations, disorganized speech, paranoia, grandiose delusions, disorganization, or bizarre behavior.58,63-66 These symptoms may represent a relapse of a primary thought disorder or, for some unfortunate individuals, a de novo psychotic illness.58,65,66 Symptoms can linger for months after drug use.65
A key risk in the use of synthetic cannabinoid moieties may involve the absence of cannabidiol. Cannabidiol naturally occurs in many strains of cannabis and is thought to have antipsychotic, neuroprotective properties.67 The absence of this molecule in synthetic cannabinoids may at least partially explain their severe psychoactive effects. Treatment for synthetic cannabinoid intoxication and related psychosis is largely supportive and may include the use of antipsychotic medication.66
Detection of synthetic cannabinoids in urine is difficult, yet many compounds can be detected via GC-MS or LC-MS. Molecules of significance include JWH-018, JWH-073, JWH-015, JWH-250, CP-47 497, HU-210, cannabicyclohexanol, and oleamide; however, these compounds are rarely excreted in urine in their pure form. The many hydroxylated or dealkylated metabolites of these compounds, mostly unnamed, are more consistently detected in urine.68,69 One author has noted that the pentanoic acid metabolite of JWH-018 seems to appear most reliably in urine specimens.68
Many synthetic cannabinoid herbal mixes also contain a detectable compound called tocopherol, seemingly added as an antioxidant.69,70 Synthetic cannabinoids are an evolving drug class, and reliable detection will require that laboratories stay up-to-date in their detection methods. As stated earlier, a commercial laboratory in the region accepted the civilian and veteran patient samples for these case studies. The synthetic cannabinoid panels offered evaluation of the drug itself (GC-MS), an oral fluid screen (LC-MS), and isolation of metabolites in urine (enzyme-linked immunosorbent assay).
Conclusion
Designer drugs will remain a challenge for providers caring for veterans for several key reasons: (1) Veterans are a vulnerable population who abuse substances at higher rates than do their civilian counterparts; (2) Chemists are able to manufacture variations of known habit-forming substances; (3) Modern technology facilitates the purchase and wide distribution of addictive substances; (4) Many designer drugs are deceptively packaged and marketed; (5) The effects of the drugs are often severe; (6) No standardized treatment guidelines exist; and (7) Detection of the drugs is difficult, and new versions of the molecules may evade even cutting-edge techniques.
Due to the high cost of detecting synthetic cathinones and synthetic cannabinoids in body fluids, screening should be considered only in settings where severe symptoms are accompanied by reasonable clinical suspicion of use and an otherwise negative toxicologic workup. As more designer drugs inevitably emerge, research will be needed on their pharmacology, toxidromes, and detection. Military and civilian practitioners must remain abreast of the dynamic trends in designer drugs to ensure that their patients receive the highest level of medical care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Although the elevated risks and rates of veterans’ substance abuse patterns are well documented, little has been written about veterans’ use of designer drugs.1-6 In recent months throughout Europe and the U.S., there has been a flurry of media attention for 2 classes of designer drugs: synthetic cathinones and synthetic cannabinoids.7,8 In the U.S., the popularity of these drugs has surged, and a disproportionate amount of use of these 2 drug classes is coming from locations near military instillations.9,10
The purpose of this article is to raise awareness regarding these 2 burgeoning designer drug classes and their impact on veterans. Designer drugs affecting vulnerable populations are not a new phenomenon, yet many providers are unfamiliar with the effects of these unique drugs of abuse on their veteran populations.11-13
Many designer drugs begin their existence as variations of other addictive or psychoactive drugs. Others begin in laboratories as investigative research compounds that end up on the street, often promising a novel mind-altering experience as a “legal high.”14-18 The Designer Drug Enforcement Act of 1986 was an initial attempt in the U.S. to define and control the early rise of copycat drugs that appeared on the streets and mimicked the effects of other illicit substances. More recent legislation enacted in the U.S. has imposed Schedule I controls on the manufacture, distribution, possession, importation, and exportation of these types of drugs, including both synthetic cathinones and synthetic cannabinoids. State laws are perennially in flux trying to keep up with the latest drug trends.19-21
Similar efforts have been made by the European Union to control mephedrone, a synthetic cathinone, citing multiple fatalities, seizures, related crime, lack of medical use, and risk of dependence.22 Although uniform levels of control do not exist in Europe for synthetic cannabinoids, many countries have independently acted to limit their use.23
In its recent World Drug Report 2013, the United Nations Office on Drugs and Crime documents its growing concern about the “new psychoactive substances” category of illicit recreational substances (in which synthetic cannabinoids and cathinones are included) that has increased by 50% since 2009.24 Alone, this category now outnumbers the total number of substances controlled by international drug conventions.
The novelty and variability of designer drugs causes difficulties with detection and regulation. Innovative chemists can legally manufacture new versions of known molecules intended for illicit use with a rapidity that outpaces bureaucratic control. Local law enforcement officials may be unaware of the latest designer drug trends, stifling efforts at public education or restriction. Designer drugs are often deceptively packaged and are available in convenience stores, tobacco outlets, gas stations, pawnshops, tattoo parlors, and truck stops.25-28 The Internet may be the singular reason, however, that designer drugs continue to be widely available to veterans.11,18
Innumerable websites discuss, promote, and sell designer drugs or deceitfully market them as safe, legitimate household products (“not for human consumption”), which can be ordered online and shipped by commercial carriers.12 Little accurate information is known about their effects or about the specific compounds they contain. When the recreational nature of the drugs is actually acknowledged, information on how the buyer can evade prosecution is often provided in tandem. The suppliers’ inventory of the drugs has been shown to be variable and inconsistent, and the product ingredients can be similarly unpredictable despite comparatively more stable naming and labeling.14,29
In the clinical setting, a reliable patient drug history may not be available. This ensures that the diagnosis of designer drug use will be an exclusionary process involving routine laboratory work, physical examination, and at times electroencephalogram and/or neuroimaging. Psychiatric consultation is often useful in this setting. Routine immunoassay tests do not detect either synthetic cathinones or synthetic cannabinoids.30
Both cannabinoids and cathinones can be identified using gas chromatography-mass spectroscopy (GC-MS) or liquid chromatography-mass spectroscopy (LC-MS). However, this technology is limited to specialized laboratories.31,32 The laboratory results often are not immediately available, potentially limiting the tests’ use in emergency or inpatient settings, as the patient may have left the hospital by the time the results are available. Additionally, these drugs’ prevalence of use, while increasing, often does not justify the cost of these tests.
The inability to routinely detect metabolites in urine may increase the enticement of these drugs given the likelihood that active-duty personnel could use them surreptitiously. Further, these compounds are evolving and seemingly limitless in their variability, and there is often a paucity of pure reference materials. As such, it is impossible to guarantee reliable test results.
The following profiles of each of these drug classes will be accompanied by clinical cases depicting the drugs’ effects and how an affected veteran might present clinically. The severe effects of these novel agents illustrate the value in maintaining a functional knowledge base about emerging drug trends. The accuracy of diagnosis as well as the outcome of a veteran’s treatment may depend on the provider’s ability to identify the presence of a drug and manage its effects.
Synthetic Cathinones
Mr. H is a 28-year-old Iraq War veteran with a history of posttraumatic stress disorder (PTSD), alcohol abuse, and opioid dependence who presented for inpatient psychiatric admission after making suicidal statements to his wife in the context of 2 weeks of “bath salts” use. A family member initially introduced him to the drug. His first drug purchase had been 1 gram ($30) at a local movie rental store.
After discharge from the hospital, Mr. H began purchasing increasing amounts online with a credit card. Although he initially had been insufflating and inhaling the substance, he later began injecting it (dissolving it in tap water and loading it through a cotton filter in a syringe). The patient admitted to finding the drug significantly more addictive than any others he had used, and his use resulted in leaving his job and abandoning his family.
Severe cravings and depression were present between episodes of use. He spent $40,000 over 6 months of use. Insomnia lasted for several days, his appearance changed dramatically (including persistent skin infections), and he became paranoid, believing that everyone around him was an undercover police officer. He remained on medications for persistent anxiety. His daily drug cravings continued,
although he remained uncertain about the actual ingredients of bath salts.
Cathinone is a naturally occurring stimulant from the khat plant (Catha edulis), which grows indigenously in Egypt and on the Arabian Peninsula. The recreational and religious use of this plant has occurred for thousands of years, though it is not without risk: The chewing of the leaves containing natural cathinones has been associated with esophagitis, gastritis, oral keratosis, myocardial infarction, dilated cardiomyopathy, hypertension, cerebral ischemia, thromboembolism, diabetes, sexual dysfunction, duodenal ulcer, and hepatitis.33,34
The stimulants known colloquially as bath salts are synthetic cathinones, which have become more widely available within the past 10 years: first in the Middle East, then Europe, and now in the U.S.5,9,10,14,19,25,35-41,44 Although the current rise in use has occurred in the past few years, the first documented abuse of synthetic cathinones in the U.S. dates to the early 1990s in Michigan.42
Bath salts is the most common of the many names used to denote synthetic cathinones. The compounds have no utility when used as such but often are marketed as research chemicals, plant fertilizer, or shoe polish. It is this deliberate counterfeit of household product names that allows many distributors to avoid classifying the compounds according to the true, intended use. More appealing brand names may also be used to entice the user (Table 1).25
Synthetic cathinones owe their popularity to similarities with cocaine and methamphetamine. They are sympathomimetic with synaptic increases of monoamines after use: Surges in norepinephrine and dopamine account for the stimulant qualities, and serotonergic changes mediate distinct psychoactive effects (Table 2).40 Users are interested in the drugs for many of the same reasons that other recreational stimulants have appeal: euphoria, energy, empathy, heightened sexuality, sociability, and an overall intensification of senses. Synthetic cathinones have become preferred to cocaine for some users.43
The drugs can be used via oral and anal routes. Using methods known as “bombing” or “keystering,” users deliver boluses of the powder wrapped in cigarette paper, which they swallow or insert into the rectum. Insufflation and IV injection are also common methods of administration with a quicker onset of action expected.40 The prices of the drugs range from $25 to $50 per 500-mg packet (though the cost is increasing with more regulation). Users typically use 500 mg to 2 g in one session.
The 2 most commonly abused synthetic cathinones are mephedrone and MDPV (methylenedioxypyrovalerone). There is some regional variability about which ingredient is present; mephedrone tends to be more prevalent in Europe, whereas MDPV is noted to be more common in the U.S.10,44
When ordering a laboratory test to evaluate for the presence of these drugs, a specific request should be given to the technicians to look for signals of MDPV (most common metabolite is dimethylenyl-methyl-MDPV), mephedrone (4-methylmethcathinone), 3-bromomethcathinone (3-BMC), or 3-fluoromethcathinone (fluphedrone).45-48 The study testing (both in VA and civilian settings) for Mr. H was done by a commercial laboratory several states away where patented techniques can screen for more than 30 compounds via LC-MS. The laboratory offered bath salts panels for urine, serum/plasma, and blood samples.
Synthetic cathinones are dangerous, and as the body of medical literature continues to expand, reports of significant morbidity and death related to their use are appearing. The harmful effects of recreational synthetic cathinone use has been documented across the globe in the form of serotonin syndrome, intoxication delirium, hyperthermia and multi-organ failure, myocarditis, hypo-osmotic hyponatremia with encephalopathy, agitation, psychosis, and death after cardiac arrest.5,12,38,39,49-53 Published treatment methods are largely supportive with the available literature, suggesting that benzodiazepines, antipsychotics (both typical and atypical), restraints to maintain safety, and IV fluids may be indicated.5,9,50
Synthetic Cannabinoids
Mr. W is a 58-year-old veteran with a history of alcohol dependence and PTSD who reported use of the synthetic cannabinoid “Spice” during intake assessment for treatment of alcohol dependence. He reported using Spice about 4 times over a 2-month period. He purchased a small jar of the substance from a party store for $15 per gram and understood its contents to be synthetic marijuana, which he appreciated for its low cost and assumed legality. He denied having any understanding of the package’s contents beyond “synthetic marijuana.”
The patient ingested the drug by smoking and inhaling from a pipe. For the first 3 times that he used the substance, Mr. W reported feeling a pleasant sensation that started quickly and lasted about 30 minutes. The fourth time that he used synthetic cannabis he felt nauseated and vomited several times, had auditory hallucinations, and increased anxiety; he also reported a hangover effect after this use. He identified that the effects may have been different the fourth time “because the brands were changing.”
Mr. W also reported that his neighbor—a daily user of synthetic cannabinoids for several months—became paranoid, suspicious, and developed incomprehensible speech. His neighbor’s symptoms and his own unpleasant experiences prompted a discontinuation of use.
Synthetic cannabinoids are a diverse group of agents numbering in excess of 100 artificial compounds that act as agonists at cannabinoid receptors, mimicking the effects of tetrahydrocannabinol (THC), an active ingredient in marijuana.28,54 The availability of these drugs online and in specialty shops has been documented since the mid-2000s.27,28,32 Their packaging often describes the contents as incense or herbal blends, using various names. Spice is a common name, but these products are also known by a myriad of other designations (Table 1).28 A single packet usually contains several grams of the drug and costs about $30.55
To the user, who may already be familiar with marijuana, the contents intentionally appear similar to the dried buds of cannabis.30,56 In reality, the drug has just been sprayed onto inert plant material.57 The drug is smoked, and the psychoactive dose can be as little as 1 mg.30 Users describe potent drug effects (Table 2). There is a rapid onset of action, and duration of effects last 1 to 2 hours.58
The compounds’ mechanism of action and appeal are derived from their high affinity for the cannabinoid receptors. The CB1 receptor is located primarily in the central nervous system and is responsible for the psychoactive component of the drugs’ actions.27,30,58,59 Two particular synthetic cannabinoids, cannibicyclohexanol and JWH-018, are potent cannabinoid CB1 agonists with affinity exceeding their natural counterparts.27,30, 32,56,58,59
Chemically, these drugs are varied. The largest structural family of these compounds is the JWH group, which includes JWH-018.60 Also common are CP 47,497 and other CP compounds.58 HU compounds, such as HU-210, have also been identified and have been shown to be 100 to 800 times more potent at the CB1 and CB2 receptors than is THC.60,61 A final group includes the benzoylindoles, such as AM-964 and RCS-4, which also bind strongly to CB1 and CB2.60,62
Constitutional symptoms of synthetic cannabinoid intoxication include disorientation, anxiety, tremulousness, palpitations, tachycardia, agitation, injected conjunctivae, hyperreflexia, nausea, vomiting, lateral gaze nystagmus, and myoclonic jerks, which have been mistaken for seizure activity.27,30,55 Pupils are often normal sized.55 Withdrawal phenomena are similar to those of cannabis withdrawal: irritability, anxiety, tremor, palpitations, diaphoresis, insomnia, headache, diarrhea, nausea, and vomiting.59
Given the established link between cannabis use and psychosis, synthetic cannabinoids may stand as a precipitant of psychotic symptoms, which may include visual hallucinations, auditory hallucinations, disorganized speech, paranoia, grandiose delusions, disorganization, or bizarre behavior.58,63-66 These symptoms may represent a relapse of a primary thought disorder or, for some unfortunate individuals, a de novo psychotic illness.58,65,66 Symptoms can linger for months after drug use.65
A key risk in the use of synthetic cannabinoid moieties may involve the absence of cannabidiol. Cannabidiol naturally occurs in many strains of cannabis and is thought to have antipsychotic, neuroprotective properties.67 The absence of this molecule in synthetic cannabinoids may at least partially explain their severe psychoactive effects. Treatment for synthetic cannabinoid intoxication and related psychosis is largely supportive and may include the use of antipsychotic medication.66
Detection of synthetic cannabinoids in urine is difficult, yet many compounds can be detected via GC-MS or LC-MS. Molecules of significance include JWH-018, JWH-073, JWH-015, JWH-250, CP-47 497, HU-210, cannabicyclohexanol, and oleamide; however, these compounds are rarely excreted in urine in their pure form. The many hydroxylated or dealkylated metabolites of these compounds, mostly unnamed, are more consistently detected in urine.68,69 One author has noted that the pentanoic acid metabolite of JWH-018 seems to appear most reliably in urine specimens.68
Many synthetic cannabinoid herbal mixes also contain a detectable compound called tocopherol, seemingly added as an antioxidant.69,70 Synthetic cannabinoids are an evolving drug class, and reliable detection will require that laboratories stay up-to-date in their detection methods. As stated earlier, a commercial laboratory in the region accepted the civilian and veteran patient samples for these case studies. The synthetic cannabinoid panels offered evaluation of the drug itself (GC-MS), an oral fluid screen (LC-MS), and isolation of metabolites in urine (enzyme-linked immunosorbent assay).
Conclusion
Designer drugs will remain a challenge for providers caring for veterans for several key reasons: (1) Veterans are a vulnerable population who abuse substances at higher rates than do their civilian counterparts; (2) Chemists are able to manufacture variations of known habit-forming substances; (3) Modern technology facilitates the purchase and wide distribution of addictive substances; (4) Many designer drugs are deceptively packaged and marketed; (5) The effects of the drugs are often severe; (6) No standardized treatment guidelines exist; and (7) Detection of the drugs is difficult, and new versions of the molecules may evade even cutting-edge techniques.
Due to the high cost of detecting synthetic cathinones and synthetic cannabinoids in body fluids, screening should be considered only in settings where severe symptoms are accompanied by reasonable clinical suspicion of use and an otherwise negative toxicologic workup. As more designer drugs inevitably emerge, research will be needed on their pharmacology, toxidromes, and detection. Military and civilian practitioners must remain abreast of the dynamic trends in designer drugs to ensure that their patients receive the highest level of medical care.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. J Drug Alcohol Abuse. 1999;25(2):239-256.
2. Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102(7):1092-1101.
3. Hankin CS, Spiro A 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: The Veterans Health Study. Am J Psychiatry. 1999;156(12):1924-1930.
4. Sirratt D, Ozanian A, Traenkner B. Epidemiology and prevention of substance use disorders in the military. Mil Med. 2012;177(suppl 8):21-28.
5. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat. 2013;44(1):42-45.
6. Bobes J, Sáiz PA, González MP, et al. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8(3):147-154.
7. D.E.A. Cracks Down on Designer Drug Operations. The New York Times Website. http://www.nytimes.com/2013/06/27/us/dea-cracks-down-on-designer-drug-operations.html. Published June 26, 2013. Accessed October 6, 2014.
8. Travis A. Mushrooming legal highs leave drug control system floundering, UN warns. The Guardian Website. http://www.guardian.co.uk/world/2013/jun/26/legal-highs-drug-control. Published June 26, 2013. Accessed October 6, 2014.
9. Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med. 2012;79(4):258-264.
10. Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath salts” and “plant food” products: The experience of one regional US poison center. J Med Toxicol. 2013;9(1):42-48.
11. Wax PM. Just a click away: Recreational drug web sites on the Internet. Pediatrics. 2002;109(6):e96.
12. Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201(3):191-195.
13. Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: Deadly new Internet drugs. Pediatrics. 2000;106(4):829-830.
14. Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1-3):59-66.
15. Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18-38.
16. Christophersen AS. Amphetamine designer drugs—An overview and epidemiology. Toxicol Lett. 2000;112-113:127-131.
17. Buchanan JF, Brown CR. ‘Designer drugs.’ A problem in clinical toxicology. Med Toxicol Adverse Drug Exp. 1988;3(1):1-17.
18. Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105(6):951-953.
19. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76(204):65371-65375.
20. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Placement of five synthetic cannabinoids into Schedule I. Fed Regist. 2012;77(41):12508-12514.
21. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into Schedule I. Fed Regist. 2013;78(95):28735-28739.
22. Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. EUR-Lex Website. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:322:0044:0045:en:PDF. Published December 8, 2010. Accessed October 7, 2014.
23. European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘spice’. European Monitoring Centre for Drugs and Drug Addiction Website. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids. Updated September 15, 2011. Accessed October 7, 2014.
24. United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Office on Drugs and Crime Website. http://www.unodc.org/wdr2014. Accessed October 3, 2014.
25. Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436-441.
26. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
27. Loeffler G, Hurst D, Penn A, Yung K. Spice, bath salts, and the U.S. military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil Med. 2012;177(9):1041-1048.
28. European Monitoring Centre for Drugs and Drug Addiction. Understanding the spice phenomenon. The National Documentation Centre on Drug Use Website. http://www.drugsandalcohol.ie/12597/1/Understanding_the_Spice_phenomenon.pdf. Published 2009. Accessed October 7, 2014.
29. Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—Is there consistency in what you get? QJM. 2010;103(7):489-493.
30. Harris CR, Brown A. Synthetic cannabinoid intoxication: A case series and review. J Emerg Med. 2013;44(2):360-366.
31. Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2--Designer cathinones. J Anal Toxicol. 2012;36(6):381-389.
32. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3(7-8):466-478.
33. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145-1154.
34. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540-548.
35. Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal. 2011;3(7-8):454-463.
36. Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210(1-3):195-200.
37. Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375(9723):1333-1334.
38. Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010.
39. Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742.
40. Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8(1):33-42.
41. Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310-313.
42. Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22(12):1897-1903.
43. Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addict. 2011;106(1):154-161.
44. Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49(6):499-505.
45. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther Drug Monit. 2011;33(2):257-263.
46. Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. JMS. 2010;45(12):1426-1442.
47. Santali EY, Cadogan AK, Daeid NN, Savage KA, Sutcliffe OB. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J Pharm Biomed Anal. 2011;56(2):246-255.
48. Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: Studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. JMS. 2012;47(2):253-262.
49. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
50. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: Two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
51. Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34(6):647-650.
52. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: Acute mephedrone ‘meow’ myocarditis. Heart. 2010;96(24):2051-2052.
53. Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8(1):69-75.
54. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
55. Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299.
56. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585-593.
57. Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198(1-3):31-38.
58. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117(2-3):152-157.
59. Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Deutsches Ärzteblatt Int. 2009;106(27):464-467.
60. Loeffler G, Hurst D, Penn A, Yung K. Spice, Bath salts, and the US military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the US armed forces. Mil Med. 2012;177(9):1041-1048.
61. Devane WA, Breuer A, Sheskin T, Jäerbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35(11):2065-2069.
62. Gottardo R, Chiarini A, Dal Prà I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. JMS. 2012;47(1):141-146.
63. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440-447.
64. Every-Palmer S. Warning: Legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addict. 2010;105(10):1859-1860.
65. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am J Psychiatry. 2011;168(10):1119.
66. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287-288.
67. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
68. ElSohly MA, Gul W, ElSohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35(7):487-495.
69. Grigoryev A, Savchuk S, Melnik A, et al. Chromatography–mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15-16):1126-1136.
70. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1):141-147.
1. Bray RM, Fairbank JA, Marsden ME. Stress and substance use among military women and men. J Drug Alcohol Abuse. 1999;25(2):239-256.
2. Bray RM, Hourani LL. Substance use trends among active duty military personnel: Findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102(7):1092-1101.
3. Hankin CS, Spiro A 3rd, Miller DR, Kazis L. Mental disorders and mental health treatment among U.S. Department of Veterans Affairs outpatients: The Veterans Health Study. Am J Psychiatry. 1999;156(12):1924-1930.
4. Sirratt D, Ozanian A, Traenkner B. Epidemiology and prevention of substance use disorders in the military. Mil Med. 2012;177(suppl 8):21-28.
5. Winder GS, Stern N, Hosanagar A. Are “bath salts” the next generation of stimulant abuse? J Subst Abuse Treat. 2013;44(1):42-45.
6. Bobes J, Sáiz PA, González MP, et al. Use of MDMA and other illicit drugs by young adult males in northern Spain. A five-year study. Eur Addict Res. 2002;8(3):147-154.
7. D.E.A. Cracks Down on Designer Drug Operations. The New York Times Website. http://www.nytimes.com/2013/06/27/us/dea-cracks-down-on-designer-drug-operations.html. Published June 26, 2013. Accessed October 6, 2014.
8. Travis A. Mushrooming legal highs leave drug control system floundering, UN warns. The Guardian Website. http://www.guardian.co.uk/world/2013/jun/26/legal-highs-drug-control. Published June 26, 2013. Accessed October 6, 2014.
9. Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: The emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med. 2012;79(4):258-264.
10. Murphy CM, Dulaney AR, Beuhler MC, Kacinko S. “Bath salts” and “plant food” products: The experience of one regional US poison center. J Med Toxicol. 2013;9(1):42-48.
11. Wax PM. Just a click away: Recreational drug web sites on the Internet. Pediatrics. 2002;109(6):e96.
12. Vardakou I, Pistos C, Spiliopoulou C. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201(3):191-195.
13. Winickoff JP, Houck CS, Rothman EL, Bauchner H. Verve and jolt: Deadly new Internet drugs. Pediatrics. 2000;106(4):829-830.
14. Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DG. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1-3):59-66.
15. Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: A medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18-38.
16. Christophersen AS. Amphetamine designer drugs—An overview and epidemiology. Toxicol Lett. 2000;112-113:127-131.
17. Buchanan JF, Brown CR. ‘Designer drugs.’ A problem in clinical toxicology. Med Toxicol Adverse Drug Exp. 1988;3(1):1-17.
18. Griffiths P, Sedefov R, Gallegos A, Lopez D. How globalization and market innovation challenge how we think about and respond to drug use: ‘Spice’ a case study. Addiction. 2010;105(6):951-953.
19. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cathinones in Schedule I. Final Order. Fed Regist. 2011;76(204):65371-65375.
20. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Placement of five synthetic cannabinoids into Schedule I. Fed Regist. 2012;77(41):12508-12514.
21. Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of three synthetic cannabinoids into Schedule I. Fed Regist. 2013;78(95):28735-28739.
22. Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures. EUR-Lex Website. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:322:0044:0045:en:PDF. Published December 8, 2010. Accessed October 7, 2014.
23. European Monitoring Centre for Drugs and Drug Addiction. Synthetic cannabinoids and ‘spice’. European Monitoring Centre for Drugs and Drug Addiction Website. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids. Updated September 15, 2011. Accessed October 7, 2014.
24. United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Office on Drugs and Crime Website. http://www.unodc.org/wdr2014. Accessed October 3, 2014.
25. Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): Legal status and patterns of abuse. Ann Pharmacother. 2012;46(3):436-441.
26. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
27. Loeffler G, Hurst D, Penn A, Yung K. Spice, bath salts, and the U.S. military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the U.S. Armed Forces. Mil Med. 2012;177(9):1041-1048.
28. European Monitoring Centre for Drugs and Drug Addiction. Understanding the spice phenomenon. The National Documentation Centre on Drug Use Website. http://www.drugsandalcohol.ie/12597/1/Understanding_the_Spice_phenomenon.pdf. Published 2009. Accessed October 7, 2014.
29. Davies S, Wood DM, Smith G, et al. Purchasing ‘legal highs’ on the Internet—Is there consistency in what you get? QJM. 2010;103(7):489-493.
30. Harris CR, Brown A. Synthetic cannabinoid intoxication: A case series and review. J Emerg Med. 2013;44(2):360-366.
31. Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2--Designer cathinones. J Anal Toxicol. 2012;36(6):381-389.
32. Hudson S, Ramsey J. The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 2011;3(7-8):466-478.
33. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. 2005;4(6):1145-1154.
34. Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: The current situation and directions for future research. J Ethnopharmacol. 2010;132(3):540-548.
35. Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone). Drug Test Anal. 2011;3(7-8):454-463.
36. Kriikku P, Wilhelm L, Schwarz O, Rintatalo J. New designer drug of abuse: 3,4-Methylenedioxypyrovalerone (MDPV). Findings from apprehended drivers in Finland. Forensic Sci Int. 2011;210(1-3):195-200.
37. Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375(9723):1333-1334.
38. Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010.
39. Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan JI. A harmless high? Lancet. 2010;376(9742):742.
40. Prosser JM, Nelson LS. The toxicology of bath salts: A review of synthetic cathinones. J Med Toxicol. 2012;8(1):33-42.
41. Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8(3):310-313.
42. Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22(12):1897-1903.
43. Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addict. 2011;106(1):154-161.
44. Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila). 2011;49(6):499-505.
45. Ojanperä IA, Heikman PK, Rasanen IJ. Urine analysis of 3,4-methylenedioxypyrovalerone in opioid-dependent patients by gas chromatography-mass spectrometry. Ther Drug Monit. 2011;33(2):257-263.
46. Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the alpha-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. JMS. 2010;45(12):1426-1442.
47. Santali EY, Cadogan AK, Daeid NN, Savage KA, Sutcliffe OB. Synthesis, full chemical characterisation and development of validated methods for the quantification of (+/-)-4’-methylmethcathinone (mephedrone): A new “legal high”. J Pharm Biomed Anal. 2011;56(2):246-255.
48. Meyer MR, Vollmar C, Schwaninger AE, Wolf E, Maurer HH. New cathinone-derived designer drugs 3-bromomethcathinone and 3-fluoromethcathinone: Studies on their metabolism in rat urine and human liver microsomes using GC-MS and LC-high-resolution MS and their detectability in urine. JMS. 2012;47(2):253-262.
49. Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60(1):103-105.
50. Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: Two cases and a brief review of the emergence of mephedrone use. Am J Drug Alcohol Abuse. 2012;38(2):176-180.
51. Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34(6):647-650.
52. Nicholson PJ, Quinn MJ, Dodd JD. Headshop heartache: Acute mephedrone ‘meow’ myocarditis. Heart. 2010;96(24):2051-2052.
53. Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8(1):69-75.
54. Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. Spice” and “K2” herbal highs: A case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320-326.
55. Schneir AB, Cullen J, Ly BT. “Spice” girls: Synthetic cannabinoid intoxication. J Emerg Med. 2011;40(3):296-299.
56. Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585-593.
57. Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198(1-3):31-38.
58. Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011;117(2-3):152-157.
59. Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Deutsches Ärzteblatt Int. 2009;106(27):464-467.
60. Loeffler G, Hurst D, Penn A, Yung K. Spice, Bath salts, and the US military: The emergence of synthetic cannabinoid receptor agonists and cathinones in the US armed forces. Mil Med. 2012;177(9):1041-1048.
61. Devane WA, Breuer A, Sheskin T, Jäerbe TU, Eisen MS, Mechoulam R. A novel probe for the cannabinoid receptor. J Med Chem. 1992;35(11):2065-2069.
62. Gottardo R, Chiarini A, Dal Prà I, et al. Direct screening of herbal blends for new synthetic cannabinoids by MALDI-TOF MS. JMS. 2012;47(1):141-146.
63. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67(5):440-447.
64. Every-Palmer S. Warning: Legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addict. 2010;105(10):1859-1860.
65. Hurst D, Loeffler G, McLay R. Psychosis associated with synthetic cannabinoid agonists: A case series. Am J Psychiatry. 2011;168(10):1119.
66. Peglow S, Buchner J, Briscoe G. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287-288.
67. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. 2008;192(4):306-307.
68. ElSohly MA, Gul W, ElSohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J Anal Toxicol. 2011;35(7):487-495.
69. Grigoryev A, Savchuk S, Melnik A, et al. Chromatography–mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15-16):1126-1136.
70. Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci Int. 2010;200(1):141-147.