User login
An FP’s guide to caring for patients with seizure and epilepsy
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
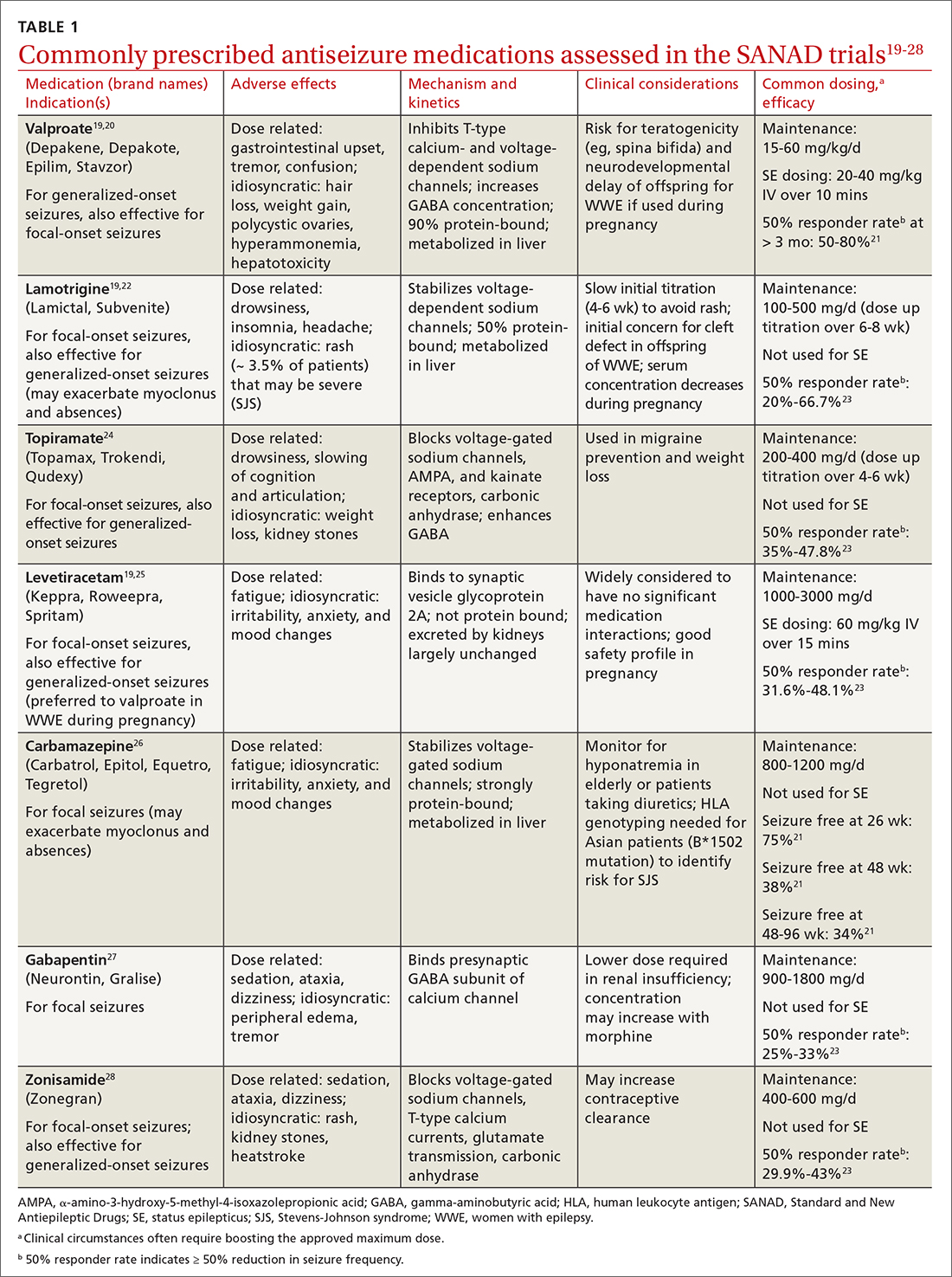
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).
Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
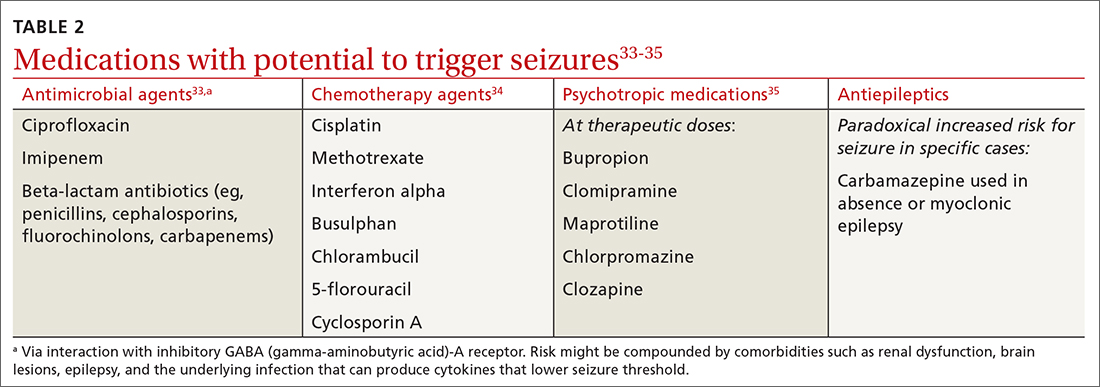
Medications may provoke seizures in susceptible individuals
Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
Managing first-time seizures and epilepsy often requires consultation with a neurologist or epileptologist for diagnosis and subsequent management, including when medical treatment fails or in determining whether patients may benefit from surgery. However, given the high prevalence of epilepsy and even higher incidence of a single seizure, family physicians contribute significantly to the management of these patients. The main issues are managing a first-time seizure, making the diagnosis, establishing a treatment plan, and exploring triggers and mitigating factors.
Seizure vs epilepsy
All patients with epilepsy experience seizures, but not every person who experiences a seizure has (or will develop) epilepsy. Nearly 10% of the population has one seizure during their lifetime, whereas the risk for epilepsy is just 3%.1 Therefore, a first-time seizure may not herald epilepsy, defined as repetitive (≥ 2) unprovoked seizures more than 24 hours apart.2 Seizures can be provoked (acute symptomatic) or unprovoked; a clear distinction between these 2 occurrences—as well as between single and recurrent seizures—is critical for proper management. A close look at the circumstances of a first-time seizure is imperative to define the nature of the event and the possibility of further seizures before devising a treatment plan.
Provoked seizures are due to an acute brain insult such as toxic-metabolic disorders, concussion, alcohol withdrawal, an adverse effect of a medication or its withdrawal, or photic stimulation presumably by disrupting the brain’s metabolic homeostasis or integrity. The key factor is that provoked seizures always happen in close temporal association with an acute insult. A single provoked seizure happens each year in 29 to 39 individuals per 100,000.3 While these seizures typically occur singly, there is a small risk they may recur if the triggering insult persists or repeats.1 Therefore, more than 1 seizure per se may not indicate epilepsy.3
Unprovoked seizures reflect an underlying brain dysfunction. A single unprovoked seizure happens in 23 to 61 individuals per 100,000 per year, often in men in either younger or older age groups.3 Unprovoked seizures may occur only once or may recur (ie, evolve into epilepsy). The latter scenario happens in only about half of cases; the overall risk for a recurrent seizure within 2 years of a first seizure is estimated at 42% (24% to 65%, depending on the etiology and electroencephalogram [EEG] findings).4 More specifically, without treatment the relapse rate will be 36% at 1 year and 47% at 2 years.4 Further, a second unprovoked seizure, if untreated, would increase the risk for third and fourth seizures to 73% and 76%, respectively, within 4 years.3
Evaluating the first-time seizure
Ask the patient or observers about the circumstances of the event to differentiate provoked from unprovoked onset. For one thing, not all “spells” are seizures. The differential diagnoses may include syncope, psychogenic nonepileptic events, drug intoxication or withdrawal, migraine, panic attacks, sleep disorders (parasomnia), transient global amnesia, concussion, and transient ischemic attack. EEG, neuroimaging, and other relevant diagnostic tests often are needed (eg, electrocardiogram/echocardiogram/Holter monitoring to evaluate for syncope/cardiac arrhythmia). Clinically, syncopal episodes tend to be brief with rapid recovery and no confusion, speech problems, aura, or lateralizing signs such as hand posturing or lip smacking that are typical with focal seizures. However, cases of convulsive syncope can be challenging to assess without diagnostic tests.
True convulsive seizures do not have the variability in clinical signs seen with psychogenic nonepileptic events (eg, alternating body parts involved or direction of movements). Transient global amnesia is a rare condition with no established diagnostic test and is considered a diagnosis of exclusion, although bitemporal hyperintensities on magnetic resonance imaging (MRI) may appear 12 to 48 hours after the clinical episode.5 Blood work is needed in patients with medical issues treated with multiple medications to evaluate for metabolic derangements; otherwise, routine blood work provides minimal information in stable patients.
Region-specific causes. Neurocysticercosis is common in some regions, such as Latin America; therefore, attention should be paid to this aspect of patient history.
Continue to: Is it really a first-time seizure?
Is it really a first-time seizure? A “first,” usually dramatic, generalized tonic-clonic seizure that triggers the diagnostic work-up may not be the very first seizure. Evidence suggests that many patients have experienced prior undiagnosed seizures. Subtle prior events often missed include episodes of deja vu, transient feelings of fear or unusual smells, speech difficulties, staring spells, or myoclonic jerks.1 A routine EEG to record epileptiform discharges and a high-resolution brain MRI to rule out any intracranial pathology are indicated. However, if the EEG indicates a primary generalized (as opposed to focal-onset) epilepsy, a brain MRI may not be needed. If a routine EEG is unrevealing, long-term video-EEG monitoring may be needed to detect an abnormality.
Accuracy of EEG and MRI. Following a first unprovoked seizure, routine EEG to detect epileptiform discharges in adults has yielded a sensitivity of 17.3% and specificity of 94.7%. In evaluating children, these values are 57.8% and 69.6%, respectively.6 If results are equivocal, a 24-hour EEG can increase the likelihood of detecting epileptiform discharges to 89% of patients.7 Brain MRI may detect an abnormality in 12% to 14% of patients with newly diagnosed epilepsy, and in up to 80% of those with recurrent seizures.8 In confirming hippocampus sclerosis, MRI has demonstrated a sensitivity of 93% and specificity of 86%.9
When to treat a first-time seizure. Available data and prediction models identify risk factors that would help determine whether to start an antiseizure medication after a first unprovoked seizure:
Epilepsy diagnosis
The International League Against Epilepsy (ILAE) previously defined epilepsy as 2 unprovoked seizures more than 24 hours apart. However, a more recent ILAE task force modified this definition: even a single unprovoked seizure would be enough to diagnose epilepsy if there is high probability of further seizures—eg, in the presence of definitive epileptiform discharges on EEG or presence of a brain tumor or a remote brain insult on imaging, since such conditions induce an enduring predisposition to generate epileptic seizures. 2 Also, a single unprovoked seizure is enough to diagnose epilepsy if it is part of an epileptic syndrome such as juvenile myoclonic epilepsy. Further, a time limit was added to the definition—ie, epilepsy is considered resolved if a patient remains seizure free for 10 years without use of antiseizure medications during the past 5 years. However, given the multitude of variables and evidence, the task force acknowledged the need for individualized considerations. 2
Seizure classification
Classification of seizure type is based on the site of seizure onset and its spread pattern—ie, focal, generalized, or unknown onset.
Continue to: Focal-onset seizures
Focal-onset seizures originate “within networks limited to one hemisphere,” although possibly in more than 1 region (ie, multifocal, and presence or absence of loss of awareness). 12 Focal seizures may then be further classified into “motor onset” or “nonmotor onset” (eg, autonomic, emotional, sensory). 2
Generalized seizures are those “originating at some point within, and rapidly engaging, bilaterally distributed networks.” 13 Unlike focal-onset seizures, generalized seizures are not classified based on awareness, as most generalized seizures involve loss of awareness (absence) or total loss of consciousness (generalized tonic-clonic). They are instead categorized based on the presence of motor vs nonmotor features (eg, tonic-clonic, myoclonic, atonic). Epilepsy classification is quite dynamic and constantly updated based on new genetic, electroencephalographic, and neuroimaging discoveries.
Treatment of epilepsy
Antiseizure medications
Treatment with antiseizure medications (ASMs; formerly known as antiepileptic drugs ) is the mainstay of epilepsy management. Achieving efficacy (seizure freedom) and tolerability (minimal adverse effects) are the primary goals of treatment. Factors that should govern the selection of an ASM include the seizure type/epilepsy syndrome, adverse effect profile of the ASM, pharmacodynamic/pharmacokinetic considerations, and patient comorbidities.
The Standard and New Antiepileptic Drugs (SANAD I and II) trials provide data from direct, unblinded, and longitudinal comparisons of existing and new ASMs and their utility in different seizure types. In the SANAD I cohort of patients with generalized and unclassified epilepsies, valproate was superior to lamotrigine and topiramate for 12-month remission and treatment failure rates, respectively.14 However, valproate generally is avoided in women of childbearing age due its potential adverse effects during pregnancy. In focal epilepsies, lamotrigine was superior to carbamazepine, gabapentin, and topiramate with respect to treatment failure, and noninferior to carbamazepine for 12-month remission.15 In the SANAD II trial, levetiracetam was noninferior to valproate for incidence of adverse events in patients with generalized and unclassified epilepsies although was found to be neither more clinically effective nor more cost effective.16 For patients of childbearing potential with generalized and unclassified epilepsies, there is evidence to support the safe and effective use of levetiracetam.17 In focal epilepsies, lamotrigine was superior to levetiracetam and zonisamide with respect to treatment failures and adverse events and was noninferior to zonisamide for 12-month remission.18 In summary, levetiracetam and valproate (not to be used in women of childbearing potential) are considered appropriate first-line agents for generalized and unclassified epilepsies while lamotrigine is deemed an appropriate first-line agent for focal epilepsies (TABLE 119-28).

Drug level monitoring. It is standard practice to periodically monitor serum levels in patients taking first-generation ASMs such as phenytoin, carbamazepine, phenobarbital, and valproic acid because of their narrow therapeutic range and the potential for overdose or interaction with other medications or foods (eg, grapefruit juice may increase carbamazepine serum level by inhibiting CYP3A4, the enzyme that metabolizes the drug). Patients taking newer ASMs may not require regular serum level monitoring except during titration, with hepatic or renal dosing, when concomitantly used with estrogen-based oral contraceptives (eg, lamotrigine), before or during pregnancy, or when nonadherence is suspected.
Continue to: Can antiseizure treatment be stopped?
Can antiseizure treatment be stopped?
Current evidence favors continuing ASM therapy in patients whose seizures are under control, although the decision should be tailored to an individual’s circumstances. According to the 2021 American Academy of Neurology (AAN) guidelines, adults who have been seizure free for at least 2 years and discontinue ASMs are possibly still at higher risk for seizure recurrence in the long term (24-60 months), compared with those who continue treatment.29 On the other hand, for adults who have been seizure free for at least 12 months, ASM withdrawal may not increase their risk for status epilepticus, and there are insufficient data to support or refute an effect on mortality or quality of life with ASM withdrawal in this population. The decision to taper or maintain ASM therapy in seizure-free patients also should take into consideration other clinically relevant outcome measures such as the patient’s lifestyle and medication adverse effects. Therefore, this decision should be made after sufficient discussion with patients and their caregivers. (Information for patients can be found at: www.epilepsy.com/treatment/medicines/stopping-medication.)
For children, the AAN guideline panel recommends discussing with family the small risk (2%) for becoming medication resistant if seizures recur during or after ASM withdrawal. 29 For children who have been seizure free for 18 to 24 months, there is probably not a significant long-term (24-48 months) difference in seizure recurrence in those who taper ASMs vs those who do not. However, presence of epileptiform discharges on EEG before discontinuation of an ASM indicates increased risk for seizure recurrence. 29
Intractable (refractory) epilepsy
While most patients with epilepsy attain complete seizure control with appropriate drug therapy, approximately 30% continue to experience seizures (“drug-resistant” epilepsy, also termed intractable or refractory ). 30 In 2010, the ILAE defined drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used anti-epileptic drug schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom” (defined as cessation of seizures for at least 3 times the longest pre-intervention inter-seizure interval or 12 months, whichever is longer). 21,31 It should be noted that drug withdrawal due to adverse effects is not counted as failure of that ASM. Recognition of drug-resistant epilepsy may prompt referral to an epileptologist who can consider rational combination drug therapy or surgical resection of the seizure focus, vagus nerve stimulation, electrical stimulation of the seizure focus, or deep brain (thalamic) stimulation.
Seizure triggers and mitigating factors
Epilepsy mostly affects patients during seizure episodes; however, the unpredictability of these events adds significantly to the burden of disease. There are no reliable methods for predicting seizure other than knowing of the several potential risks and recognizing and avoiding these triggers.
Noncompliance with antiseizure medications is a common seizure trigger affecting up to one-half of patients with epilepsy.32
Continue to: Medications
Medications may provoke seizures in susceptible individuals

Sleep deprivation is a potential seizure trigger in people with epilepsy based on observational studies, case reports, patient surveys, and EEG-based studies, although data from randomized controlled studies are limited.36 The standard best practice is to encourage appropriate sleep hygiene, which involves getting at least 7 hours of sleep per night.37
Alcohol is a GABAergic substance like benzodiazepines with antiseizure effects. However, it acts as a potential precipitant of seizures in cases of withdrawal or acute intoxication, or when it leads to sleep disruption or nonadherence to antiseizure medications. Therefore, advise patients with alcohol use disorder to slowly taper consumption (best done through a support program) and avoid sudden withdrawal. However, complete abstinence from alcohol use is not often recommended except in special circumstances (eg, a history of alcohol-related seizures). Several studies have demonstrated that modest alcohol use (1-2 drinks per occasion) does not increase seizure frequency or significantly alter serum concentrations of commonly used ASMs.38
Cannabis and other substances. The 2 main biologically active components of marijuana are delta-9-tetrahydrocannibinol (THC), the main psychoactive constituent, and cannabidiol (CBD). Animal and human studies have demonstrated anticonvulsant properties of THC and CBD. But THC, in high amounts, can result in adverse cognitive effects and worsening seizures.39 A purified 98% oil-based CBD extract (Epidiolex) has been approved as an adjunctive treatment for certain medically refractory epilepsy syndromes in children and young adults—ie, Dravet syndrome, Lennox-Gastaut syndrome, and tuberous sclerosis complex syndrome.40 There are no reliable data on the effect of recreational use of marijuana on seizure control. Other illicit substances such as cocaine may lower seizure threshold by their stimulatory and disruptive effects on sleep, diet, and healthy routines.
Special clinical cases
Pregnancy and epilepsy
Despite the potential adverse effects of ASMs on fetal health, the current global consensus is to continue treatment during pregnancy, given that the potential harm of convulsive seizures outweighs the potential risks associated with in-utero exposure to ASMs. There is not enough evidence to indicate significant harm to the fetus caused by focal, absence, or myoclonic seizures. Low-dose folic acid is used to minimize the risks of ASMs during pregnancy.
Continue to: As the fetus develops...
As the fetus develops, there are changes in volume of ASM distribution, renal clearance, protein binding, and hepatic metabolism, which require checking serum levels at regular intervals and making dosage adjustments.
The ongoing study evaluating Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD)41 has led to multiple landmark studies guiding the choice of preferred ASMs during pregnancy in patients with epilepsy.42,43 This has culminated in today’s use of lamotrigine and levetiracetam as the 2 preferred agents (while avoiding valproate) in pregnant patients with epilepsy.44
Psychogenic nonepileptic seizures
A form of conversion disorder, psychogenic nonepileptic seizures (PNES) manifests as abnormal motor or behavioral events mimicking seizures but without associated epileptiform discharges on EEG. This is observed in 10% of patients seen in epilepsy clinics and even more often in those admitted to epilepsy monitoring units (25%-40%).45 Diagnosis of PNES requires EEG monitoring both for confirmation and for discernment from true epileptic seizures, in particular frontal lobe epilepsy that may clinically mimic PNES. PNES often is associated with underlying psychological tensions or comorbid conditions such as depression, anxiety, or traumatic life experiences. There is no treatment for PNES per se, and its management is focused on controlling any underlying psychological comorbidities that may not always be obvious. There is some evidence suggesting that these patients experience an innate inability to verbally express their emotions and instead subconsciously resort to psychosomatics to express them in a somatic dimension.46,47
Status epilepticus
Defined as prolonged seizures (> 5 min) or 2 consecutive seizures without regaining aware ness in between, status epilepticus (SE) is a potentially fatal condition. Subclinical nonconvulsive SE, especially in comatose patients, can be diagnosed only via EEG monitoring. Untreated SE may manifest as a diagnostic dilemma in unresponsive or critically ill patients and can increase the risk for mortality. 48
Febrile seizures
Febrile seizures affect 2% to 5% of children most often in the second year of life.49 The use of preventive antiseizure medication is not recommended; instead, the key is to investigate the underlying febrile illness. Lumbar puncture is indicated if there are signs and symptoms of meningitis (25% of children with bacterial meningitis present with seizures).49 Febrile seizures often are self-limited, but there is risk for SE in up to 15% of cases.50 If convulsive febrile seizures last longer than 5 minutes, initiate benzodiazepines followed by the standard protocol used for the management of SE.51
Continue to: Epilepsy as a spectrum disorder
Epilepsy as a spectrum disorder
The higher prevalence of comorbid cognitive and psychiatric conditions in patients with epilepsy, affecting about half of patients, 52 suggests that seizures may constitute only one aspect of a multifaceted disease that otherwise should be considered a spectrum disorder. Among such conditions are memory deficits, depression, and anxiety. Conversely, epilepsy is more common in patients with depression than in those without. 52
Social impact of epilepsy
Vehicle driving regulations. Patients with epilepsy are required to follow state law regarding driving restrictions. Different states have different rules and regulations about driving restrictions and reporting requirements (by patients or their physicians). Refer patients to the Department of Motor Vehicles (DMV) in their state of residence for up-to-date instructions.53 The Epilepsy Foundation (epilepsy.com) can serve as a resource for each state’s DMV website.
Employment assistance. Having epilepsy should not preclude patients from seeking employment and pursuing meaningful careers. The Americans with Disabilities Act (ADA) and the US Equal Employment Opportunity Commission (EEOC) forbid discrimination against qualified people with disabilities, including those with epilepsy, and require reasonable accommodations in the workplace (www.eeoc.gov/laws/guidance/epilepsy-workplace-and-ada).54
CORRESPONDENCE
Gholam K. Motamedi, MD, Department of Neurology, PHC 7, Georgetown University Hospital, 3800 Reservoir Road, NW, Washington, DC 20007; [email protected]
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
1. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc. 1996;71:576-586. doi: 10.4065/71.6.576
2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475-482. doi: 10.1111/epi.12550.
3. Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8-12. doi: 10.1111/j.1528-1167.2008.01443.x
4. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology. 1991;41:965-972. doi: 10.1212/wnl.41.7.965
5. Ropper AH. Transient global amnesia. N Engl J Med. 2023;388:635-540. doi: 10.1056/NEJMra2213867
6. Bouma HK, Labos C, Gore GC, et al. The diagnostic accuracy of routine electroencephalography after a first unprovoked seizure. Eur J Neurol. 2016;23:455-463. doi: 10.1111/ene.12739
7. Narayanan JT, Labar DR, Schaul N. Latency to first spike in the EEG of epilepsy patients. Seizure. 2008;17:34-41. doi: 10.1016/j.seizure.2007.06.003
8. Salmenpera TM, Duncan JS. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76:iii2-iii10. doi: 10.1136/jnnp.2005.075135
9. Jackson GD, Berkovic SF, Tress , et al Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869-1875. doi: 10.1212/wnl.40.12.1869
10. Bonnett LJ, Kim, L, Johnson A, et al. Risk of seizure recurrence in people with single seizures and early epilepsy - model development and external validation. Seizure. 2022;94:26-32. doi: 10.1016/j.seizure.2021.11.007
11. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: Report of the Guideline Development Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2015;84:1705-1713. doi: 10.1212/WNL.0000000000001487
12. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and terminology. Epilepsia. 2017;58:522-530. doi: 10.1111/epi.13670
13. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsy: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51:676-685. doi: 10.1111/j.1528-1167.2010.02522.x
14. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalized and unclassifiable epilepsy: an unblinded randomized controlled trial. Lancet. 2007;369:1016-1026. doi: 10.1016/S0140-6736(07)60461-9
15. Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 2007;369:1000-1015. doi: 10.1016/S0140-6736(07)60460-7
16. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalized and unclassified epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1375-1386. doi: 10.1016/S0140-6736(21)00246-4
17. Mawhinney E, Craig J, Morrow J. Levetiracetam in pregnancy: results from the UK and Ireland epilepsy and pregnancy registers. Neurology. 2013;80:400-405.
18. Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet. 2021;397:1363-1374. doi: 10.1016/S0140-6736(21)00247-6
19. Smith PE. Initial management of seizure in adults. N Engl J Med. 2021;385:251-263. doi: 10.1056/NEJMcp2024526
20. Depakene (valproic acid). Package insert. Abbott Laboratories; 2011. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2011/018081s046_18082s031lbl.pdf
21. Greenberg RG, Melloni C, Wu H, et al. Therapeutic index estimation of antiepileptic drugs: a systematic literature review approach. Clin Neuropharmacol. 2016;39:232-240. doi: 10.1097/WNF.0000000000000172
22. Lamictal (lamotrigine). Package insert. GlaxoSmithKline; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/020241s037s038,020764s030s031lbl.pdf
23. LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605-614. doi: 10.1001/jama.291.5.605
24. Topamax (topiramate). Package insert. Janssen Pharmaceuticals, Inc. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf
25. Keppra (levetiracetam). Package insert. UCB, Inc.; 2009. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080%2C021505s021s024lbl.pdf
26. Carbatrol (carbamazepine). Package insert. Shire US Inc; 2013. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2013/020712s032s035lbl.pdf
27.Neurontin (gabapentin). Package insert. Pfizer; 2017. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf
28.Zonegran (zonisamide). Package insert. Eisai Inc; 2006. Accessed October 6, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2006/020789s019lbl.pdf
29.Gloss D, Paragon K, Pack A, et al. Antiseizure medication withdrawal in seizure-free patients: practice advisory update. Report of the AAN Guideline Subcommittee. Neurology. 2021;97:1072-1081. doi: 10.1212/WNL.0000000000012944
30.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000:342:314-319. doi: 10.1056/NEJM200002033420503
31.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069-1077. doi: 10.1111/j.1528-1167.2009.02397.x
Compliance during treatment of epilepsy. Epilepsia 1988;29(suppl 2):S79-S84.
33.utter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;13;85:1332-1341. doi: 10.1212/WNL.0000000000002023
34.Singh G, Rees JH, Sander JW. Seizures and epilepsy in oncological practice: causes, course, mechanisms and treatment. JNNP. 2007;78:342-349. doi: 10.1136/jnnp.2006.106211
35.Pisani F, Oteri G, Costa C., et al. Effects of psychotropic drugs on seizure threshold. Drug Safety. 2002;25:91-110.
36.Rossi KC, Joe J, Makhjia M, et al. Insufficient sleep, electroencephalogram activation, and seizure risk: re-evaluating the evidence. Ann Neurol. 2020;86:798-806. doi: 10.1002/ana.25710
37.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-844. doi: 10.5665/sleep.4716
38.Höppener RJ, Kuyer A, van der Lugt PJ. Epilepsy and alcohol: the influence of social alcohol intake on seizures and treatment in epilepsy. Epilepsia. 1983;24:459-471. doi: 10.1111/j.1528-1157.1983.tb04917.x
39.Keeler MH, Reifler CB. Grand mal convulsions subsequent to marijuana use. Case report. Dis Nerv Syst. 1967:28:474-475.
40.Epidiolex (cannabidiol). Package insert. Greenwich Biosciences Inc; 2018. Accessed September 27, 2023. www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf
41.ClinicalTrials.gov. Maternal Outcomes and Neurodevelopmental Effects of Antiseizure Drugs (MONEAD). Accessed September 24, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT01730170
42.Meador KJ, Baker GA, Finnell RH, et al. In utero antiepileptic drug exposure: fetal death and malformations. Neurology. 2006;67:407-412. doi: 10.1212/01.wnl.0000227919.81208.b2
43.Meador K, Reynolds MW, Crean S. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1-13. doi:10.1016/j.eplepsyres.2008.04.022
44.Marxer CA, Rüegg S, Rauch A review of the evidence on the risk of congenital malformations and neurodevelopmental disorders in association with antiseizure medications during pregnancy. Expert Opin Drug Saf. 2021;20:1487-1499. doi: 10.1080/14740338.2021.1943355
Asadi-Pooya AA, Sperling MR. Epidemiology of psychogenic nonepileptic seizures. Epilepsy Behav. 2015;46:60-65. doi: 10.1016/j.yebeh.2015.03.015
Evaluation and treatment of psychogenic nonepileptic seizures. Neurol Clin. 2022;40:799-820. doi: 10.1016/j.ncl.2022.03.017
47.Motamedi GK. Psychogenic nonepileptic seizures: a disconnect between body and mind. Epilepsy Behav. 2018;78:293-294. doi: 10.1016/j.yebeh.2017.10.016
, Nonconvulsive status epilepticus. Emerg Med Clin North Am. 2011;29:65-72. doi: 10.1016/j.emc.2010.08.006
doi: 10.1542/peds.2010-3318
Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Sys Rev. 2018;1(1):CD001905. doi: 10.1002/14651858.CD001905.pub3
52.Jensen FE. Epilepsy as a spectrum disorder: implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(suppl 1):1-6. doi: 10.1111/j.1528-1167.2010.02904.x
53.Kass JS, Rose RV. Driving and epilepsy: ethical, legal, and health care policy challenges. Continuum (Minneap Minn). 2019;25:537-542. doi: 10.1212/CON.0000000000000714
54.Troxell J. Epilepsy and employment: the Americans with Disabilities Act and its protections against employment discrimination. Med Law. 1997;16:375-384.
PRACTICE RECOMMENDATIONS
› Consider treating a first-time seizure if electroencephalography shows particular epileptiform activity, if the neurologic exam or computerized tomography or magnetic resonance imaging results are abnormal, if the seizure is focal or nocturnal, or if there is a family history of seizures. A
› Consider valproate (except for women of childbearing age) and levetiracetam as first-line agents for generalized or unclassified epilepsy, and lamotrigine for focal epilepsies. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Daily episodes of confusion • altered behavior • chronic sleep deprivation • Dx?
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.