User login
SCHOLAR Project
The structure and function of academic hospital medicine programs (AHPs) has evolved significantly with the growth of hospital medicine.[1, 2, 3, 4] Many AHPs formed in response to regulatory and financial changes, which drove demand for increased trainee oversight, improved clinical efficiency, and growth in nonteaching services staffed by hospitalists. Differences in local organizational contexts and needs have contributed to great variability in AHP program design and operations. As AHPs have become more established, the need to engage academic hospitalists in scholarship and activities that support professional development and promotion has been recognized. Defining sustainable and successful positions for academic hospitalists is a priority called for by leaders in the field.[5, 6]
In this rapidly evolving context, AHPs have employed a variety of approaches to organizing clinical and academic faculty roles, without guiding evidence or consensus‐based performance benchmarks. A number of AHPs have achieved success along traditional academic metrics of research, scholarship, and education. Currently, it is not known whether specific approaches to AHP organization, structure, or definition of faculty roles are associated with achievement of more traditional markers of academic success.
The Academic Committee of the Society of Hospital Medicine (SHM), and the Academic Hospitalist Task Force of the Society of General Internal Medicine (SGIM) had separately initiated projects to explore characteristics associated with success in AHPs. In 2012, these organizations combined efforts to jointly develop and implement the SCHOLAR (SuCcessful HOspitaLists in Academics and Research) project. The goals were to identify successful AHPs using objective criteria, and to then study those groups in greater detail to generate insights that would be broadly relevant to the field. Efforts to clarify the factors within AHPs linked to success by traditional academic metrics will benefit hospitalists, their leaders, and key stakeholders striving to achieve optimal balance between clinical and academic roles. We describe the initial work of the SCHOLAR project, our definitions of academic success in AHPs, and the characteristics of a cohort of exemplary AHPs who achieved the highest levels on these metrics.
METHODS
Defining Success
The 11 members of the SCHOLAR project held a variety of clinical and academic roles within a geographically diverse group of AHPs. We sought to create a functional definition of success applicable to AHPs. As no gold standard currently exists, we used a consensus process among task force members to arrive at a definition that was quantifiable, feasible, and meaningful. The first step was brainstorming on conference calls held 1 to 2 times monthly over 4 months. Potential defining characteristics that emerged from these discussions related to research, teaching, and administrative activities. When potential characteristics were proposed, we considered how to operationalize each one. Each characteristic was discussed until there was consensus from the entire group. Those around education and administration were the most complex, as many roles are locally driven and defined, and challenging to quantify. For this reason, we focused on promotion as a more global approach to assessing academic hospitalist success in these areas. Although criteria for academic advancement also vary across institutions, we felt that promotion generally reflected having met some threshold of academic success. We also wanted to recognize that scholarship occurs outside the context of funded research. Ultimately, 3 key domains emerged: research grant funding, faculty promotion, and scholarship.
After these 3 domains were identified, the group sought to define quantitative metrics to assess performance. These discussions occurred on subsequent calls over a 4‐month period. Between calls, group members gathered additional information to facilitate assessment of the feasibility of proposed metrics, reporting on progress via email. Again, group consensus was sought for each metric considered. Data on grant funding and successful promotions were available from a previous survey conducted through the SHM in 2011. Leaders from 170 AHPs were contacted, with 50 providing complete responses to the 21‐item questionnaire (see Supporting Information, Appendix 1, in the online version of this article). Results of the survey, heretofore referred to as the Leaders of Academic Hospitalist Programs survey (LAHP‐50), have been described elsewhere.[7] For the purposes of this study, we used the self‐reported data about grant funding and promotions contained in the survey to reflect the current state of the field. Although the survey response rate was approximately 30%, the survey was not anonymous, and many reputationally prominent academic hospitalist programs were represented. For these reasons, the group members felt that the survey results were relevant for the purposes of assessing academic success.
In the LAHP‐50, funding was defined as principal investigator or coinvestigator roles on federally and nonfederally funded research, clinical trials, internal grants, and any other extramurally funded projects. Mean and median funding for the overall sample was calculated. Through a separate question, each program's total faculty full‐time equivalent (FTE) count was reported, allowing us to adjust for group size by assessing both total funding per group and funding/FTE for each responding AHP.
Promotions were defined by the self‐reported number of faculty at each of the following ranks: instructor, assistant professor, associate professor, full professor, and professor above scale/emeritus. In addition, a category of nonacademic track (eg, adjunct faculty, clinical associate) was included to capture hospitalists that did not fit into the traditional promotions categories. We did not distinguish between tenure‐track and nontenure‐track academic ranks. LAHP‐50 survey respondents reported the number of faculty in their group at each academic rank. Given that the majority of academic hospitalists hold a rank of assistant professor or lower,[6, 8, 9] and that the number of full professors was only 3% in the LAHP‐50 cohort, we combined the faculty at the associate and full professor ranks, defining successfully promoted faculty as the percent of hospitalists above the rank of assistant professor.
We created a new metric to assess scholarly output. We had considerable discussion of ways to assess the numbers of peer‐reviewed manuscripts generated by AHPs. However, the group had concerns about the feasibility of identification and attribution of authors to specific AHPs through literature searches. We considered examining only publications in the Journal of Hospital Medicine and the Journal of General Internal Medicine, but felt that this would exclude significant work published by hospitalists in fields of medical education or health services research that would more likely appear in alternate journals. Instead, we quantified scholarship based on the number of abstracts presented at national meetings. We focused on meetings of the SHM and SGIM as the primary professional societies representing hospital medicine. The group felt that even work published outside of the journals of our professional societies would likely be presented at those meetings. We used the following strategy: We reviewed research abstracts accepted for presentation as posters or oral abstracts at the 2010 and 2011 SHM national meetings, and research abstracts with a primary or secondary category of hospital medicine at the 2010 and 2011 SGIM national meetings. By including submissions at both SGIM and SHM meetings, we accounted for the fact that some programs may gravitate more to one society meeting or another. We did not include abstracts in the clinical vignettes or innovations categories. We tallied the number of abstracts by group affiliation of the authors for each of the 4 meetings above and created a cumulative total per group for the 2‐year period. Abstracts with authors from different AHPs were counted once for each individual group. Members of the study group reviewed abstracts from each of the meetings in pairs. Reviewers worked separately and compared tallies of results to ensure consistent tabulations. Internet searches were conducted to identify or confirm author affiliations if it was not apparent in the abstract author list. Abstract tallies were compiled without regard to whether programs had completed the LAHP‐50 survey; thus, we collected data on programs that did not respond to the LAHP‐50 survey.
Identification of the SCHOLAR Cohort
To identify our cohort of top‐performing AHPs, we combined the funding and promotions data from the LAHP‐50 sample with the abstract data. We limited our sample to adult hospital medicine groups to reduce heterogeneity. We created rank lists of programs in each category (grant funding, successful promotions, and scholarship), using data from the LAHP‐50 survey to rank programs on funding and promotions, and data from our abstract counts to rank on scholarship. We limited the top‐performing list in each category to 10 institutions as a cutoff. Because we set a threshold of at least $1 million in total funding, we identified only 9 top performing AHPs with regard to grant funding. We also calculated mean funding/FTE. We chose to rank programs only by funding/FTE rather than total funding per program to better account for group size. For successful promotions, we ranked programs by the percentage of senior faculty. For abstract counts, we included programs whose faculty presented abstracts at a minimum of 2 separate meetings, and ranked programs based on the total number of abstracts per group.
This process resulted in separate lists of top performing programs in each of the 3 domains we associated with academic success, arranged in descending order by grant dollars/FTE, percent of senior faculty, and abstract counts (Table 1). Seventeen different programs were represented across these 3 top 10 lists. One program appeared on all 3 lists, 8 programs appeared on 2 lists, and the remainder appeared on a single list (Table 2). Seven of these programs were identified solely based on abstract presentations, diversifying our top groups beyond only those who completed the LAHP‐50 survey. We considered all of these programs to represent high performance in academic hospital medicine. The group selected this inclusive approach because we recognized that any 1 metric was potentially limited, and we sought to identify diverse pathways to success.
| Funding | Promotions | Scholarship | |
|---|---|---|---|
| Grant $/FTE | Total Grant $ | Senior Faculty, No. (%) | Total Abstract Count |
| |||
| $1,409,090 | $15,500,000 | 3 (60%) | 23 |
| $1,000,000 | $9,000,000 | 3 (60%) | 21 |
| $750,000 | $8,000,000 | 4 (57%) | 20 |
| $478,609 | $6,700,535 | 9 (53%) | 15 |
| $347,826 | $3,000,000 | 8 (44%) | 11 |
| $86,956 | $3,000,000 | 14 (41%) | 11 |
| $66,666 | $2,000,000 | 17 (36%) | 10 |
| $46,153 | $1,500,000 | 9 (33%) | 10 |
| $38,461 | $1,000,000 | 2 (33%) | 9 |
| 4 (31%) | 9 | ||
| Selection Criteria for SCHOLAR Cohort | No. of Programs |
|---|---|
| |
| Abstracts, funding, and promotions | 1 |
| Abstracts plus promotions | 4 |
| Abstracts plus funding | 3 |
| Funding plus promotion | 1 |
| Funding only | 1 |
| Abstract only | 7 |
| Total | 17 |
| Top 10 abstract count | |
| 4 meetings | 2 |
| 3 meetings | 2 |
| 2 meetings | 6 |
The 17 unique adult AHPs appearing on at least 1 of the top 10 lists comprised the SCHOLAR cohort of programs that we studied in greater detail. Data reflecting program demographics were solicited directly from leaders of the AHPs identified in the SCHOLAR cohort, including size and age of program, reporting structure, number of faculty at various academic ranks (for programs that did not complete the LAHP‐50 survey), and number of faculty with fellowship training (defined as any postresidency fellowship program).
Subsequently, we performed comparative analyses between the programs in the SCHOLAR cohort to the general population of AHPs reflected by the LAHP‐50 sample. Because abstract presentations were not recorded in the original LAHP‐50 survey instrument, it was not possible to perform a benchmarking comparison for the scholarship domain.
Data Analysis
To measure the success of the SCHOLAR cohort we compared the grant funding and proportion of successfully promoted faculty at the SCHOLAR programs to those in the overall LAHP‐50 sample. Differences in mean and median grant funding were compared using t tests and Mann‐Whitney rank sum tests. Proportion of promoted faculty were compared using 2 tests. A 2‐tailed of 0.05 was used to test significance of differences.
RESULTS
Demographics
Among the AHPs in the SCHOLAR cohort, the mean program age was 13.2 years (range, 618 years), and the mean program size was 36 faculty (range, 1895; median, 28). On average, 15% of faculty members at SCHOLAR programs were fellowship trained (range, 0%37%). Reporting structure among the SCHOLAR programs was as follows: 53% were an independent division or section of the department of medicine; 29% were a section within general internal medicine, and 18% were an independent clinical group.
Grant Funding
Table 3 compares grant funding in the SCHOLAR programs to programs in the overall LAHP‐50 sample. Mean funding per group and mean funding per FTE were significantly higher in the SCHOLAR group than in the overall sample.
| Funding (Millions) | ||
|---|---|---|
| LAHP‐50 Overall Sample | SCHOLAR | |
| ||
| Median grant funding/AHP | 0.060 | 1.500* |
| Mean grant funding/AHP | 1.147 (015) | 3.984* (015) |
| Median grant funding/FTE | 0.004 | 0.038* |
| Mean grant funding/FTE | 0.095 (01.4) | 0.364* (01.4) |
Thirteen of the SCHOLAR programs were represented in the initial LAHP‐50, but 2 did not report a dollar amount for grants and contracts. Therefore, data for total grant funding were available for only 65% (11 of 17) of the programs in the SCHOLAR cohort. Of note, 28% of AHPs in the overall LAHP‐50 sample reported no external funding sources.
Faculty Promotion
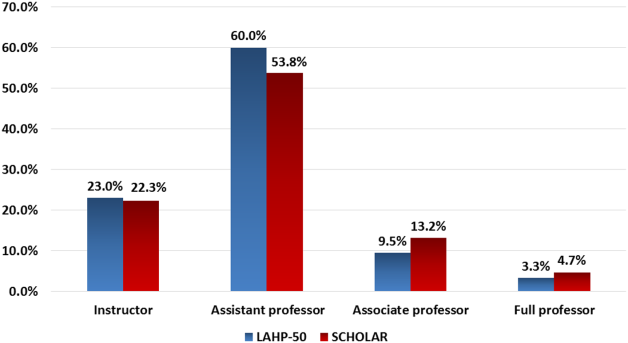
Figure 1 demonstrates the proportion of faculty at various academic ranks. The percent of faculty above the rank of assistant professor in the SCHOLAR programs exceeded those in the overall LAHP‐50 by 5% (17.9% vs 12.8%, P = 0.01). Of note, 6% of the hospitalists at AHPs in the SCHOLAR programs were on nonfaculty tracks.
Scholarship
Mean abstract output over the 2‐year period measured was 10.8 (range, 323) in the SCHOLAR cohort. Because we did not collect these data for the LAHP‐50 group, comparative analyses were not possible.
DISCUSSION
Using a definition of academic success that incorporated metrics of grant funding, faculty promotion, and scholarly output, we identified a unique subset of successful AHPsthe SCHOLAR cohort. The programs represented in the SCHOLAR cohort were generally large and relatively mature. Despite this, the cohort consisted of mostly junior faculty, had a paucity of fellowship‐trained hospitalists, and not all reported grant funding.
Prior published work reported complementary findings.[6, 8, 9] A survey of 20 large, well‐established academic hospitalist programs in 2008 found that the majority of hospitalists were junior faculty with a limited publication portfolio. Of the 266 respondents in that study, 86% reported an academic rank at or below assistant professor; funding was not explored.[9] Our similar findings 4 years later add to this work by demonstrating trends over time, and suggest that progress toward creating successful pathways for academic advancement has been slow. In a 2012 survey of the SHM membership, 28% of hospitalists with academic appointments reported no current or future plans to engage in research.[8] These findings suggest that faculty in AHPs may define scholarship through nontraditional pathways, or in some cases choose not to pursue or prioritize scholarship altogether.
Our findings also add to the literature with regard to our assessment of funding, which was variable across the SCHOLAR group. The broad range of funding in the SCHOLAR programs for which we have data (grant dollars $0$15 million per program) suggests that opportunities to improve supported scholarship remain, even among a selected cohort of successful AHPs. The predominance of junior faculty in the SCHOLAR programs may be a reason for this variation. Junior faculty may be engaged in research with funding directed to senior mentors outside their AHP. Alternatively, they may pursue meaningful local hospital quality improvement or educational innovations not supported by external grants, or hold leadership roles in education, quality, or information technology that allow for advancement and promotion without external grant funding. As the scope and impact of these roles increases, senior leaders with alternate sources of support may rely less on research funds; this too may explain some of the differences. Our findings are congruent with results of a study that reviewed original research published by hospitalists, and concluded that the majority of hospitalist research was not externally funded.[8] Our approach for assessing grant funding by adjusting for FTE had the potential to inadvertently favor smaller well‐funded groups over larger ones; however, programs in our sample were similarly represented when ranked by funding/FTE or total grant dollars. As many successful AHPs do concentrate their research funding among a core of focused hospitalist researchers, our definition may not be the ideal metric for some programs.
We chose to define scholarship based on abstract output, rather than peer‐reviewed publications. Although this choice was necessary from a feasibility perspective, it may have excluded programs that prioritize peer‐reviewed publications over abstracts. Although we were unable to incorporate a search strategy to accurately and comprehensively track the publication output attributed specifically to hospitalist researchers and quantify it by program, others have since defined such an approach.[8] However, tracking abstracts theoretically allowed insights into a larger volume of innovative and creative work generated by top AHPs by potentially including work in the earlier stages of development.
We used a consensus‐based definition of success to define our SCHOLAR cohort. There are other ways to measure academic success, which if applied, may have yielded a different sample of programs. For example, over half of the original research articles published in the Journal of Hospital Medicine over a 7‐year span were generated from 5 academic centers.[8] This definition of success may be equally credible, though we note that 4 of these 5 programs were also included in the SCHOLAR cohort. We feel our broader approach was more reflective of the variety of pathways to success available to academic hospitalists. Before our metrics are applied as a benchmarking tool, however, they should ideally be combined with factors not measured in our study to ensure a more comprehensive or balanced reflection of academic success. Factors such as mentorship, level of hospitalist engagement,[10] prevalence of leadership opportunities, operational and fiscal infrastructure, and the impact of local quality, safety, and value efforts should be considered.
Comparison of successfully promoted faculty at AHPs across the country is inherently limited by the wide variation in promotion standards across different institutions; controlling for such differences was not possible with our methodology. For example, it appears that several programs with relatively few senior faculty may have met metrics leading to their inclusion in the SCHOLAR group because of their small program size. Future benchmarking efforts for promotion at AHPs should take scaling into account and consider both total number as well as percentage of senior faculty when evaluating success.
Our methodology has several limitations. Survey data were self‐reported and not independently validated, and as such are subject to recall and reporting biases. Response bias inherently excluded some AHPs that may have met our grant funding or promotions criteria had they participated in the initial LAHP‐50 survey, though we identified and included additional programs through our scholarship metric, increasing the representativeness of the SCHOLAR cohort. Given the dynamic nature of the field, the age of the data we relied upon for analysis limits the generalizability of our specific benchmarks to current practice. However, the development of academic success occurs over the long‐term, and published data on academic hospitalist productivity are consistent with this slower time course.[8] Despite these limitations, our data inform the general topic of gauging performance of AHPs, underscoring the challenges of developing and applying metrics of success, and highlight the variability of performance on selected metrics even among a relatively small group of 17 programs.
In conclusion, we have created a method to quantify academic success that may be useful to academic hospitalists and their group leaders as they set targets for improvement in the field. Even among our SCHOLAR cohort, room for ongoing improvement in development of funded scholarship and a core of senior faculty exists. Further investigation into the unique features of successful groups will offer insight to leaders in academic hospital medicine regarding infrastructure and processes that should be embraced to raise the bar for all AHPs. In addition, efforts to further define and validate nontraditional approaches to scholarship that allow for successful promotion at AHPs would be informative. We view our work less as a singular approach to benchmarking standards for AHPs, and more a call to action to continue efforts to balance scholarly activity and broad professional development of academic hospitalists with increasing clinical demands.
Acknowledgements
The authors thank all of the AHP leaders who participated in the SCHOLAR project. They also thank the Society of Hospital Medicine and Society of General Internal Medicine and the SHM Academic Committee and SGIM Academic Hospitalist Task Force for their support of this work.
Disclosures
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- , , , , . Characteristics of primary care providers who adopted the hospitalist model from 2001 to 2009. J Hosp Med. 2015;10(2):75–82.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Updating threshold‐based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45–47.
- , , , . Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2).
- , , , , , . Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , , , . The structure of hospital medicine programs at academic medical centers [abstract]. J Hosp Med. 2012;7(suppl 2):s92.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
The structure and function of academic hospital medicine programs (AHPs) has evolved significantly with the growth of hospital medicine.[1, 2, 3, 4] Many AHPs formed in response to regulatory and financial changes, which drove demand for increased trainee oversight, improved clinical efficiency, and growth in nonteaching services staffed by hospitalists. Differences in local organizational contexts and needs have contributed to great variability in AHP program design and operations. As AHPs have become more established, the need to engage academic hospitalists in scholarship and activities that support professional development and promotion has been recognized. Defining sustainable and successful positions for academic hospitalists is a priority called for by leaders in the field.[5, 6]
In this rapidly evolving context, AHPs have employed a variety of approaches to organizing clinical and academic faculty roles, without guiding evidence or consensus‐based performance benchmarks. A number of AHPs have achieved success along traditional academic metrics of research, scholarship, and education. Currently, it is not known whether specific approaches to AHP organization, structure, or definition of faculty roles are associated with achievement of more traditional markers of academic success.
The Academic Committee of the Society of Hospital Medicine (SHM), and the Academic Hospitalist Task Force of the Society of General Internal Medicine (SGIM) had separately initiated projects to explore characteristics associated with success in AHPs. In 2012, these organizations combined efforts to jointly develop and implement the SCHOLAR (SuCcessful HOspitaLists in Academics and Research) project. The goals were to identify successful AHPs using objective criteria, and to then study those groups in greater detail to generate insights that would be broadly relevant to the field. Efforts to clarify the factors within AHPs linked to success by traditional academic metrics will benefit hospitalists, their leaders, and key stakeholders striving to achieve optimal balance between clinical and academic roles. We describe the initial work of the SCHOLAR project, our definitions of academic success in AHPs, and the characteristics of a cohort of exemplary AHPs who achieved the highest levels on these metrics.
METHODS
Defining Success
The 11 members of the SCHOLAR project held a variety of clinical and academic roles within a geographically diverse group of AHPs. We sought to create a functional definition of success applicable to AHPs. As no gold standard currently exists, we used a consensus process among task force members to arrive at a definition that was quantifiable, feasible, and meaningful. The first step was brainstorming on conference calls held 1 to 2 times monthly over 4 months. Potential defining characteristics that emerged from these discussions related to research, teaching, and administrative activities. When potential characteristics were proposed, we considered how to operationalize each one. Each characteristic was discussed until there was consensus from the entire group. Those around education and administration were the most complex, as many roles are locally driven and defined, and challenging to quantify. For this reason, we focused on promotion as a more global approach to assessing academic hospitalist success in these areas. Although criteria for academic advancement also vary across institutions, we felt that promotion generally reflected having met some threshold of academic success. We also wanted to recognize that scholarship occurs outside the context of funded research. Ultimately, 3 key domains emerged: research grant funding, faculty promotion, and scholarship.
After these 3 domains were identified, the group sought to define quantitative metrics to assess performance. These discussions occurred on subsequent calls over a 4‐month period. Between calls, group members gathered additional information to facilitate assessment of the feasibility of proposed metrics, reporting on progress via email. Again, group consensus was sought for each metric considered. Data on grant funding and successful promotions were available from a previous survey conducted through the SHM in 2011. Leaders from 170 AHPs were contacted, with 50 providing complete responses to the 21‐item questionnaire (see Supporting Information, Appendix 1, in the online version of this article). Results of the survey, heretofore referred to as the Leaders of Academic Hospitalist Programs survey (LAHP‐50), have been described elsewhere.[7] For the purposes of this study, we used the self‐reported data about grant funding and promotions contained in the survey to reflect the current state of the field. Although the survey response rate was approximately 30%, the survey was not anonymous, and many reputationally prominent academic hospitalist programs were represented. For these reasons, the group members felt that the survey results were relevant for the purposes of assessing academic success.
In the LAHP‐50, funding was defined as principal investigator or coinvestigator roles on federally and nonfederally funded research, clinical trials, internal grants, and any other extramurally funded projects. Mean and median funding for the overall sample was calculated. Through a separate question, each program's total faculty full‐time equivalent (FTE) count was reported, allowing us to adjust for group size by assessing both total funding per group and funding/FTE for each responding AHP.
Promotions were defined by the self‐reported number of faculty at each of the following ranks: instructor, assistant professor, associate professor, full professor, and professor above scale/emeritus. In addition, a category of nonacademic track (eg, adjunct faculty, clinical associate) was included to capture hospitalists that did not fit into the traditional promotions categories. We did not distinguish between tenure‐track and nontenure‐track academic ranks. LAHP‐50 survey respondents reported the number of faculty in their group at each academic rank. Given that the majority of academic hospitalists hold a rank of assistant professor or lower,[6, 8, 9] and that the number of full professors was only 3% in the LAHP‐50 cohort, we combined the faculty at the associate and full professor ranks, defining successfully promoted faculty as the percent of hospitalists above the rank of assistant professor.
We created a new metric to assess scholarly output. We had considerable discussion of ways to assess the numbers of peer‐reviewed manuscripts generated by AHPs. However, the group had concerns about the feasibility of identification and attribution of authors to specific AHPs through literature searches. We considered examining only publications in the Journal of Hospital Medicine and the Journal of General Internal Medicine, but felt that this would exclude significant work published by hospitalists in fields of medical education or health services research that would more likely appear in alternate journals. Instead, we quantified scholarship based on the number of abstracts presented at national meetings. We focused on meetings of the SHM and SGIM as the primary professional societies representing hospital medicine. The group felt that even work published outside of the journals of our professional societies would likely be presented at those meetings. We used the following strategy: We reviewed research abstracts accepted for presentation as posters or oral abstracts at the 2010 and 2011 SHM national meetings, and research abstracts with a primary or secondary category of hospital medicine at the 2010 and 2011 SGIM national meetings. By including submissions at both SGIM and SHM meetings, we accounted for the fact that some programs may gravitate more to one society meeting or another. We did not include abstracts in the clinical vignettes or innovations categories. We tallied the number of abstracts by group affiliation of the authors for each of the 4 meetings above and created a cumulative total per group for the 2‐year period. Abstracts with authors from different AHPs were counted once for each individual group. Members of the study group reviewed abstracts from each of the meetings in pairs. Reviewers worked separately and compared tallies of results to ensure consistent tabulations. Internet searches were conducted to identify or confirm author affiliations if it was not apparent in the abstract author list. Abstract tallies were compiled without regard to whether programs had completed the LAHP‐50 survey; thus, we collected data on programs that did not respond to the LAHP‐50 survey.
Identification of the SCHOLAR Cohort
To identify our cohort of top‐performing AHPs, we combined the funding and promotions data from the LAHP‐50 sample with the abstract data. We limited our sample to adult hospital medicine groups to reduce heterogeneity. We created rank lists of programs in each category (grant funding, successful promotions, and scholarship), using data from the LAHP‐50 survey to rank programs on funding and promotions, and data from our abstract counts to rank on scholarship. We limited the top‐performing list in each category to 10 institutions as a cutoff. Because we set a threshold of at least $1 million in total funding, we identified only 9 top performing AHPs with regard to grant funding. We also calculated mean funding/FTE. We chose to rank programs only by funding/FTE rather than total funding per program to better account for group size. For successful promotions, we ranked programs by the percentage of senior faculty. For abstract counts, we included programs whose faculty presented abstracts at a minimum of 2 separate meetings, and ranked programs based on the total number of abstracts per group.
This process resulted in separate lists of top performing programs in each of the 3 domains we associated with academic success, arranged in descending order by grant dollars/FTE, percent of senior faculty, and abstract counts (Table 1). Seventeen different programs were represented across these 3 top 10 lists. One program appeared on all 3 lists, 8 programs appeared on 2 lists, and the remainder appeared on a single list (Table 2). Seven of these programs were identified solely based on abstract presentations, diversifying our top groups beyond only those who completed the LAHP‐50 survey. We considered all of these programs to represent high performance in academic hospital medicine. The group selected this inclusive approach because we recognized that any 1 metric was potentially limited, and we sought to identify diverse pathways to success.
| Funding | Promotions | Scholarship | |
|---|---|---|---|
| Grant $/FTE | Total Grant $ | Senior Faculty, No. (%) | Total Abstract Count |
| |||
| $1,409,090 | $15,500,000 | 3 (60%) | 23 |
| $1,000,000 | $9,000,000 | 3 (60%) | 21 |
| $750,000 | $8,000,000 | 4 (57%) | 20 |
| $478,609 | $6,700,535 | 9 (53%) | 15 |
| $347,826 | $3,000,000 | 8 (44%) | 11 |
| $86,956 | $3,000,000 | 14 (41%) | 11 |
| $66,666 | $2,000,000 | 17 (36%) | 10 |
| $46,153 | $1,500,000 | 9 (33%) | 10 |
| $38,461 | $1,000,000 | 2 (33%) | 9 |
| 4 (31%) | 9 | ||
| Selection Criteria for SCHOLAR Cohort | No. of Programs |
|---|---|
| |
| Abstracts, funding, and promotions | 1 |
| Abstracts plus promotions | 4 |
| Abstracts plus funding | 3 |
| Funding plus promotion | 1 |
| Funding only | 1 |
| Abstract only | 7 |
| Total | 17 |
| Top 10 abstract count | |
| 4 meetings | 2 |
| 3 meetings | 2 |
| 2 meetings | 6 |
The 17 unique adult AHPs appearing on at least 1 of the top 10 lists comprised the SCHOLAR cohort of programs that we studied in greater detail. Data reflecting program demographics were solicited directly from leaders of the AHPs identified in the SCHOLAR cohort, including size and age of program, reporting structure, number of faculty at various academic ranks (for programs that did not complete the LAHP‐50 survey), and number of faculty with fellowship training (defined as any postresidency fellowship program).
Subsequently, we performed comparative analyses between the programs in the SCHOLAR cohort to the general population of AHPs reflected by the LAHP‐50 sample. Because abstract presentations were not recorded in the original LAHP‐50 survey instrument, it was not possible to perform a benchmarking comparison for the scholarship domain.
Data Analysis
To measure the success of the SCHOLAR cohort we compared the grant funding and proportion of successfully promoted faculty at the SCHOLAR programs to those in the overall LAHP‐50 sample. Differences in mean and median grant funding were compared using t tests and Mann‐Whitney rank sum tests. Proportion of promoted faculty were compared using 2 tests. A 2‐tailed of 0.05 was used to test significance of differences.
RESULTS
Demographics
Among the AHPs in the SCHOLAR cohort, the mean program age was 13.2 years (range, 618 years), and the mean program size was 36 faculty (range, 1895; median, 28). On average, 15% of faculty members at SCHOLAR programs were fellowship trained (range, 0%37%). Reporting structure among the SCHOLAR programs was as follows: 53% were an independent division or section of the department of medicine; 29% were a section within general internal medicine, and 18% were an independent clinical group.
Grant Funding
Table 3 compares grant funding in the SCHOLAR programs to programs in the overall LAHP‐50 sample. Mean funding per group and mean funding per FTE were significantly higher in the SCHOLAR group than in the overall sample.
| Funding (Millions) | ||
|---|---|---|
| LAHP‐50 Overall Sample | SCHOLAR | |
| ||
| Median grant funding/AHP | 0.060 | 1.500* |
| Mean grant funding/AHP | 1.147 (015) | 3.984* (015) |
| Median grant funding/FTE | 0.004 | 0.038* |
| Mean grant funding/FTE | 0.095 (01.4) | 0.364* (01.4) |
Thirteen of the SCHOLAR programs were represented in the initial LAHP‐50, but 2 did not report a dollar amount for grants and contracts. Therefore, data for total grant funding were available for only 65% (11 of 17) of the programs in the SCHOLAR cohort. Of note, 28% of AHPs in the overall LAHP‐50 sample reported no external funding sources.
Faculty Promotion
Figure 1 demonstrates the proportion of faculty at various academic ranks. The percent of faculty above the rank of assistant professor in the SCHOLAR programs exceeded those in the overall LAHP‐50 by 5% (17.9% vs 12.8%, P = 0.01). Of note, 6% of the hospitalists at AHPs in the SCHOLAR programs were on nonfaculty tracks.

Scholarship
Mean abstract output over the 2‐year period measured was 10.8 (range, 323) in the SCHOLAR cohort. Because we did not collect these data for the LAHP‐50 group, comparative analyses were not possible.
DISCUSSION
Using a definition of academic success that incorporated metrics of grant funding, faculty promotion, and scholarly output, we identified a unique subset of successful AHPsthe SCHOLAR cohort. The programs represented in the SCHOLAR cohort were generally large and relatively mature. Despite this, the cohort consisted of mostly junior faculty, had a paucity of fellowship‐trained hospitalists, and not all reported grant funding.
Prior published work reported complementary findings.[6, 8, 9] A survey of 20 large, well‐established academic hospitalist programs in 2008 found that the majority of hospitalists were junior faculty with a limited publication portfolio. Of the 266 respondents in that study, 86% reported an academic rank at or below assistant professor; funding was not explored.[9] Our similar findings 4 years later add to this work by demonstrating trends over time, and suggest that progress toward creating successful pathways for academic advancement has been slow. In a 2012 survey of the SHM membership, 28% of hospitalists with academic appointments reported no current or future plans to engage in research.[8] These findings suggest that faculty in AHPs may define scholarship through nontraditional pathways, or in some cases choose not to pursue or prioritize scholarship altogether.
Our findings also add to the literature with regard to our assessment of funding, which was variable across the SCHOLAR group. The broad range of funding in the SCHOLAR programs for which we have data (grant dollars $0$15 million per program) suggests that opportunities to improve supported scholarship remain, even among a selected cohort of successful AHPs. The predominance of junior faculty in the SCHOLAR programs may be a reason for this variation. Junior faculty may be engaged in research with funding directed to senior mentors outside their AHP. Alternatively, they may pursue meaningful local hospital quality improvement or educational innovations not supported by external grants, or hold leadership roles in education, quality, or information technology that allow for advancement and promotion without external grant funding. As the scope and impact of these roles increases, senior leaders with alternate sources of support may rely less on research funds; this too may explain some of the differences. Our findings are congruent with results of a study that reviewed original research published by hospitalists, and concluded that the majority of hospitalist research was not externally funded.[8] Our approach for assessing grant funding by adjusting for FTE had the potential to inadvertently favor smaller well‐funded groups over larger ones; however, programs in our sample were similarly represented when ranked by funding/FTE or total grant dollars. As many successful AHPs do concentrate their research funding among a core of focused hospitalist researchers, our definition may not be the ideal metric for some programs.
We chose to define scholarship based on abstract output, rather than peer‐reviewed publications. Although this choice was necessary from a feasibility perspective, it may have excluded programs that prioritize peer‐reviewed publications over abstracts. Although we were unable to incorporate a search strategy to accurately and comprehensively track the publication output attributed specifically to hospitalist researchers and quantify it by program, others have since defined such an approach.[8] However, tracking abstracts theoretically allowed insights into a larger volume of innovative and creative work generated by top AHPs by potentially including work in the earlier stages of development.
We used a consensus‐based definition of success to define our SCHOLAR cohort. There are other ways to measure academic success, which if applied, may have yielded a different sample of programs. For example, over half of the original research articles published in the Journal of Hospital Medicine over a 7‐year span were generated from 5 academic centers.[8] This definition of success may be equally credible, though we note that 4 of these 5 programs were also included in the SCHOLAR cohort. We feel our broader approach was more reflective of the variety of pathways to success available to academic hospitalists. Before our metrics are applied as a benchmarking tool, however, they should ideally be combined with factors not measured in our study to ensure a more comprehensive or balanced reflection of academic success. Factors such as mentorship, level of hospitalist engagement,[10] prevalence of leadership opportunities, operational and fiscal infrastructure, and the impact of local quality, safety, and value efforts should be considered.
Comparison of successfully promoted faculty at AHPs across the country is inherently limited by the wide variation in promotion standards across different institutions; controlling for such differences was not possible with our methodology. For example, it appears that several programs with relatively few senior faculty may have met metrics leading to their inclusion in the SCHOLAR group because of their small program size. Future benchmarking efforts for promotion at AHPs should take scaling into account and consider both total number as well as percentage of senior faculty when evaluating success.
Our methodology has several limitations. Survey data were self‐reported and not independently validated, and as such are subject to recall and reporting biases. Response bias inherently excluded some AHPs that may have met our grant funding or promotions criteria had they participated in the initial LAHP‐50 survey, though we identified and included additional programs through our scholarship metric, increasing the representativeness of the SCHOLAR cohort. Given the dynamic nature of the field, the age of the data we relied upon for analysis limits the generalizability of our specific benchmarks to current practice. However, the development of academic success occurs over the long‐term, and published data on academic hospitalist productivity are consistent with this slower time course.[8] Despite these limitations, our data inform the general topic of gauging performance of AHPs, underscoring the challenges of developing and applying metrics of success, and highlight the variability of performance on selected metrics even among a relatively small group of 17 programs.
In conclusion, we have created a method to quantify academic success that may be useful to academic hospitalists and their group leaders as they set targets for improvement in the field. Even among our SCHOLAR cohort, room for ongoing improvement in development of funded scholarship and a core of senior faculty exists. Further investigation into the unique features of successful groups will offer insight to leaders in academic hospital medicine regarding infrastructure and processes that should be embraced to raise the bar for all AHPs. In addition, efforts to further define and validate nontraditional approaches to scholarship that allow for successful promotion at AHPs would be informative. We view our work less as a singular approach to benchmarking standards for AHPs, and more a call to action to continue efforts to balance scholarly activity and broad professional development of academic hospitalists with increasing clinical demands.
Acknowledgements
The authors thank all of the AHP leaders who participated in the SCHOLAR project. They also thank the Society of Hospital Medicine and Society of General Internal Medicine and the SHM Academic Committee and SGIM Academic Hospitalist Task Force for their support of this work.
Disclosures
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
The structure and function of academic hospital medicine programs (AHPs) has evolved significantly with the growth of hospital medicine.[1, 2, 3, 4] Many AHPs formed in response to regulatory and financial changes, which drove demand for increased trainee oversight, improved clinical efficiency, and growth in nonteaching services staffed by hospitalists. Differences in local organizational contexts and needs have contributed to great variability in AHP program design and operations. As AHPs have become more established, the need to engage academic hospitalists in scholarship and activities that support professional development and promotion has been recognized. Defining sustainable and successful positions for academic hospitalists is a priority called for by leaders in the field.[5, 6]
In this rapidly evolving context, AHPs have employed a variety of approaches to organizing clinical and academic faculty roles, without guiding evidence or consensus‐based performance benchmarks. A number of AHPs have achieved success along traditional academic metrics of research, scholarship, and education. Currently, it is not known whether specific approaches to AHP organization, structure, or definition of faculty roles are associated with achievement of more traditional markers of academic success.
The Academic Committee of the Society of Hospital Medicine (SHM), and the Academic Hospitalist Task Force of the Society of General Internal Medicine (SGIM) had separately initiated projects to explore characteristics associated with success in AHPs. In 2012, these organizations combined efforts to jointly develop and implement the SCHOLAR (SuCcessful HOspitaLists in Academics and Research) project. The goals were to identify successful AHPs using objective criteria, and to then study those groups in greater detail to generate insights that would be broadly relevant to the field. Efforts to clarify the factors within AHPs linked to success by traditional academic metrics will benefit hospitalists, their leaders, and key stakeholders striving to achieve optimal balance between clinical and academic roles. We describe the initial work of the SCHOLAR project, our definitions of academic success in AHPs, and the characteristics of a cohort of exemplary AHPs who achieved the highest levels on these metrics.
METHODS
Defining Success
The 11 members of the SCHOLAR project held a variety of clinical and academic roles within a geographically diverse group of AHPs. We sought to create a functional definition of success applicable to AHPs. As no gold standard currently exists, we used a consensus process among task force members to arrive at a definition that was quantifiable, feasible, and meaningful. The first step was brainstorming on conference calls held 1 to 2 times monthly over 4 months. Potential defining characteristics that emerged from these discussions related to research, teaching, and administrative activities. When potential characteristics were proposed, we considered how to operationalize each one. Each characteristic was discussed until there was consensus from the entire group. Those around education and administration were the most complex, as many roles are locally driven and defined, and challenging to quantify. For this reason, we focused on promotion as a more global approach to assessing academic hospitalist success in these areas. Although criteria for academic advancement also vary across institutions, we felt that promotion generally reflected having met some threshold of academic success. We also wanted to recognize that scholarship occurs outside the context of funded research. Ultimately, 3 key domains emerged: research grant funding, faculty promotion, and scholarship.
After these 3 domains were identified, the group sought to define quantitative metrics to assess performance. These discussions occurred on subsequent calls over a 4‐month period. Between calls, group members gathered additional information to facilitate assessment of the feasibility of proposed metrics, reporting on progress via email. Again, group consensus was sought for each metric considered. Data on grant funding and successful promotions were available from a previous survey conducted through the SHM in 2011. Leaders from 170 AHPs were contacted, with 50 providing complete responses to the 21‐item questionnaire (see Supporting Information, Appendix 1, in the online version of this article). Results of the survey, heretofore referred to as the Leaders of Academic Hospitalist Programs survey (LAHP‐50), have been described elsewhere.[7] For the purposes of this study, we used the self‐reported data about grant funding and promotions contained in the survey to reflect the current state of the field. Although the survey response rate was approximately 30%, the survey was not anonymous, and many reputationally prominent academic hospitalist programs were represented. For these reasons, the group members felt that the survey results were relevant for the purposes of assessing academic success.
In the LAHP‐50, funding was defined as principal investigator or coinvestigator roles on federally and nonfederally funded research, clinical trials, internal grants, and any other extramurally funded projects. Mean and median funding for the overall sample was calculated. Through a separate question, each program's total faculty full‐time equivalent (FTE) count was reported, allowing us to adjust for group size by assessing both total funding per group and funding/FTE for each responding AHP.
Promotions were defined by the self‐reported number of faculty at each of the following ranks: instructor, assistant professor, associate professor, full professor, and professor above scale/emeritus. In addition, a category of nonacademic track (eg, adjunct faculty, clinical associate) was included to capture hospitalists that did not fit into the traditional promotions categories. We did not distinguish between tenure‐track and nontenure‐track academic ranks. LAHP‐50 survey respondents reported the number of faculty in their group at each academic rank. Given that the majority of academic hospitalists hold a rank of assistant professor or lower,[6, 8, 9] and that the number of full professors was only 3% in the LAHP‐50 cohort, we combined the faculty at the associate and full professor ranks, defining successfully promoted faculty as the percent of hospitalists above the rank of assistant professor.
We created a new metric to assess scholarly output. We had considerable discussion of ways to assess the numbers of peer‐reviewed manuscripts generated by AHPs. However, the group had concerns about the feasibility of identification and attribution of authors to specific AHPs through literature searches. We considered examining only publications in the Journal of Hospital Medicine and the Journal of General Internal Medicine, but felt that this would exclude significant work published by hospitalists in fields of medical education or health services research that would more likely appear in alternate journals. Instead, we quantified scholarship based on the number of abstracts presented at national meetings. We focused on meetings of the SHM and SGIM as the primary professional societies representing hospital medicine. The group felt that even work published outside of the journals of our professional societies would likely be presented at those meetings. We used the following strategy: We reviewed research abstracts accepted for presentation as posters or oral abstracts at the 2010 and 2011 SHM national meetings, and research abstracts with a primary or secondary category of hospital medicine at the 2010 and 2011 SGIM national meetings. By including submissions at both SGIM and SHM meetings, we accounted for the fact that some programs may gravitate more to one society meeting or another. We did not include abstracts in the clinical vignettes or innovations categories. We tallied the number of abstracts by group affiliation of the authors for each of the 4 meetings above and created a cumulative total per group for the 2‐year period. Abstracts with authors from different AHPs were counted once for each individual group. Members of the study group reviewed abstracts from each of the meetings in pairs. Reviewers worked separately and compared tallies of results to ensure consistent tabulations. Internet searches were conducted to identify or confirm author affiliations if it was not apparent in the abstract author list. Abstract tallies were compiled without regard to whether programs had completed the LAHP‐50 survey; thus, we collected data on programs that did not respond to the LAHP‐50 survey.
Identification of the SCHOLAR Cohort
To identify our cohort of top‐performing AHPs, we combined the funding and promotions data from the LAHP‐50 sample with the abstract data. We limited our sample to adult hospital medicine groups to reduce heterogeneity. We created rank lists of programs in each category (grant funding, successful promotions, and scholarship), using data from the LAHP‐50 survey to rank programs on funding and promotions, and data from our abstract counts to rank on scholarship. We limited the top‐performing list in each category to 10 institutions as a cutoff. Because we set a threshold of at least $1 million in total funding, we identified only 9 top performing AHPs with regard to grant funding. We also calculated mean funding/FTE. We chose to rank programs only by funding/FTE rather than total funding per program to better account for group size. For successful promotions, we ranked programs by the percentage of senior faculty. For abstract counts, we included programs whose faculty presented abstracts at a minimum of 2 separate meetings, and ranked programs based on the total number of abstracts per group.
This process resulted in separate lists of top performing programs in each of the 3 domains we associated with academic success, arranged in descending order by grant dollars/FTE, percent of senior faculty, and abstract counts (Table 1). Seventeen different programs were represented across these 3 top 10 lists. One program appeared on all 3 lists, 8 programs appeared on 2 lists, and the remainder appeared on a single list (Table 2). Seven of these programs were identified solely based on abstract presentations, diversifying our top groups beyond only those who completed the LAHP‐50 survey. We considered all of these programs to represent high performance in academic hospital medicine. The group selected this inclusive approach because we recognized that any 1 metric was potentially limited, and we sought to identify diverse pathways to success.
| Funding | Promotions | Scholarship | |
|---|---|---|---|
| Grant $/FTE | Total Grant $ | Senior Faculty, No. (%) | Total Abstract Count |
| |||
| $1,409,090 | $15,500,000 | 3 (60%) | 23 |
| $1,000,000 | $9,000,000 | 3 (60%) | 21 |
| $750,000 | $8,000,000 | 4 (57%) | 20 |
| $478,609 | $6,700,535 | 9 (53%) | 15 |
| $347,826 | $3,000,000 | 8 (44%) | 11 |
| $86,956 | $3,000,000 | 14 (41%) | 11 |
| $66,666 | $2,000,000 | 17 (36%) | 10 |
| $46,153 | $1,500,000 | 9 (33%) | 10 |
| $38,461 | $1,000,000 | 2 (33%) | 9 |
| 4 (31%) | 9 | ||
| Selection Criteria for SCHOLAR Cohort | No. of Programs |
|---|---|
| |
| Abstracts, funding, and promotions | 1 |
| Abstracts plus promotions | 4 |
| Abstracts plus funding | 3 |
| Funding plus promotion | 1 |
| Funding only | 1 |
| Abstract only | 7 |
| Total | 17 |
| Top 10 abstract count | |
| 4 meetings | 2 |
| 3 meetings | 2 |
| 2 meetings | 6 |
The 17 unique adult AHPs appearing on at least 1 of the top 10 lists comprised the SCHOLAR cohort of programs that we studied in greater detail. Data reflecting program demographics were solicited directly from leaders of the AHPs identified in the SCHOLAR cohort, including size and age of program, reporting structure, number of faculty at various academic ranks (for programs that did not complete the LAHP‐50 survey), and number of faculty with fellowship training (defined as any postresidency fellowship program).
Subsequently, we performed comparative analyses between the programs in the SCHOLAR cohort to the general population of AHPs reflected by the LAHP‐50 sample. Because abstract presentations were not recorded in the original LAHP‐50 survey instrument, it was not possible to perform a benchmarking comparison for the scholarship domain.
Data Analysis
To measure the success of the SCHOLAR cohort we compared the grant funding and proportion of successfully promoted faculty at the SCHOLAR programs to those in the overall LAHP‐50 sample. Differences in mean and median grant funding were compared using t tests and Mann‐Whitney rank sum tests. Proportion of promoted faculty were compared using 2 tests. A 2‐tailed of 0.05 was used to test significance of differences.
RESULTS
Demographics
Among the AHPs in the SCHOLAR cohort, the mean program age was 13.2 years (range, 618 years), and the mean program size was 36 faculty (range, 1895; median, 28). On average, 15% of faculty members at SCHOLAR programs were fellowship trained (range, 0%37%). Reporting structure among the SCHOLAR programs was as follows: 53% were an independent division or section of the department of medicine; 29% were a section within general internal medicine, and 18% were an independent clinical group.
Grant Funding
Table 3 compares grant funding in the SCHOLAR programs to programs in the overall LAHP‐50 sample. Mean funding per group and mean funding per FTE were significantly higher in the SCHOLAR group than in the overall sample.
| Funding (Millions) | ||
|---|---|---|
| LAHP‐50 Overall Sample | SCHOLAR | |
| ||
| Median grant funding/AHP | 0.060 | 1.500* |
| Mean grant funding/AHP | 1.147 (015) | 3.984* (015) |
| Median grant funding/FTE | 0.004 | 0.038* |
| Mean grant funding/FTE | 0.095 (01.4) | 0.364* (01.4) |
Thirteen of the SCHOLAR programs were represented in the initial LAHP‐50, but 2 did not report a dollar amount for grants and contracts. Therefore, data for total grant funding were available for only 65% (11 of 17) of the programs in the SCHOLAR cohort. Of note, 28% of AHPs in the overall LAHP‐50 sample reported no external funding sources.
Faculty Promotion
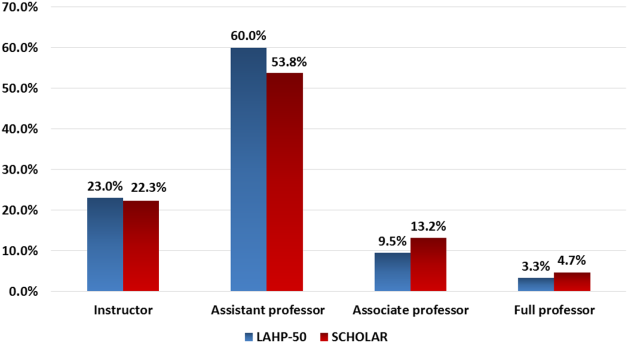
Figure 1 demonstrates the proportion of faculty at various academic ranks. The percent of faculty above the rank of assistant professor in the SCHOLAR programs exceeded those in the overall LAHP‐50 by 5% (17.9% vs 12.8%, P = 0.01). Of note, 6% of the hospitalists at AHPs in the SCHOLAR programs were on nonfaculty tracks.

Scholarship
Mean abstract output over the 2‐year period measured was 10.8 (range, 323) in the SCHOLAR cohort. Because we did not collect these data for the LAHP‐50 group, comparative analyses were not possible.
DISCUSSION
Using a definition of academic success that incorporated metrics of grant funding, faculty promotion, and scholarly output, we identified a unique subset of successful AHPsthe SCHOLAR cohort. The programs represented in the SCHOLAR cohort were generally large and relatively mature. Despite this, the cohort consisted of mostly junior faculty, had a paucity of fellowship‐trained hospitalists, and not all reported grant funding.
Prior published work reported complementary findings.[6, 8, 9] A survey of 20 large, well‐established academic hospitalist programs in 2008 found that the majority of hospitalists were junior faculty with a limited publication portfolio. Of the 266 respondents in that study, 86% reported an academic rank at or below assistant professor; funding was not explored.[9] Our similar findings 4 years later add to this work by demonstrating trends over time, and suggest that progress toward creating successful pathways for academic advancement has been slow. In a 2012 survey of the SHM membership, 28% of hospitalists with academic appointments reported no current or future plans to engage in research.[8] These findings suggest that faculty in AHPs may define scholarship through nontraditional pathways, or in some cases choose not to pursue or prioritize scholarship altogether.
Our findings also add to the literature with regard to our assessment of funding, which was variable across the SCHOLAR group. The broad range of funding in the SCHOLAR programs for which we have data (grant dollars $0$15 million per program) suggests that opportunities to improve supported scholarship remain, even among a selected cohort of successful AHPs. The predominance of junior faculty in the SCHOLAR programs may be a reason for this variation. Junior faculty may be engaged in research with funding directed to senior mentors outside their AHP. Alternatively, they may pursue meaningful local hospital quality improvement or educational innovations not supported by external grants, or hold leadership roles in education, quality, or information technology that allow for advancement and promotion without external grant funding. As the scope and impact of these roles increases, senior leaders with alternate sources of support may rely less on research funds; this too may explain some of the differences. Our findings are congruent with results of a study that reviewed original research published by hospitalists, and concluded that the majority of hospitalist research was not externally funded.[8] Our approach for assessing grant funding by adjusting for FTE had the potential to inadvertently favor smaller well‐funded groups over larger ones; however, programs in our sample were similarly represented when ranked by funding/FTE or total grant dollars. As many successful AHPs do concentrate their research funding among a core of focused hospitalist researchers, our definition may not be the ideal metric for some programs.
We chose to define scholarship based on abstract output, rather than peer‐reviewed publications. Although this choice was necessary from a feasibility perspective, it may have excluded programs that prioritize peer‐reviewed publications over abstracts. Although we were unable to incorporate a search strategy to accurately and comprehensively track the publication output attributed specifically to hospitalist researchers and quantify it by program, others have since defined such an approach.[8] However, tracking abstracts theoretically allowed insights into a larger volume of innovative and creative work generated by top AHPs by potentially including work in the earlier stages of development.
We used a consensus‐based definition of success to define our SCHOLAR cohort. There are other ways to measure academic success, which if applied, may have yielded a different sample of programs. For example, over half of the original research articles published in the Journal of Hospital Medicine over a 7‐year span were generated from 5 academic centers.[8] This definition of success may be equally credible, though we note that 4 of these 5 programs were also included in the SCHOLAR cohort. We feel our broader approach was more reflective of the variety of pathways to success available to academic hospitalists. Before our metrics are applied as a benchmarking tool, however, they should ideally be combined with factors not measured in our study to ensure a more comprehensive or balanced reflection of academic success. Factors such as mentorship, level of hospitalist engagement,[10] prevalence of leadership opportunities, operational and fiscal infrastructure, and the impact of local quality, safety, and value efforts should be considered.
Comparison of successfully promoted faculty at AHPs across the country is inherently limited by the wide variation in promotion standards across different institutions; controlling for such differences was not possible with our methodology. For example, it appears that several programs with relatively few senior faculty may have met metrics leading to their inclusion in the SCHOLAR group because of their small program size. Future benchmarking efforts for promotion at AHPs should take scaling into account and consider both total number as well as percentage of senior faculty when evaluating success.
Our methodology has several limitations. Survey data were self‐reported and not independently validated, and as such are subject to recall and reporting biases. Response bias inherently excluded some AHPs that may have met our grant funding or promotions criteria had they participated in the initial LAHP‐50 survey, though we identified and included additional programs through our scholarship metric, increasing the representativeness of the SCHOLAR cohort. Given the dynamic nature of the field, the age of the data we relied upon for analysis limits the generalizability of our specific benchmarks to current practice. However, the development of academic success occurs over the long‐term, and published data on academic hospitalist productivity are consistent with this slower time course.[8] Despite these limitations, our data inform the general topic of gauging performance of AHPs, underscoring the challenges of developing and applying metrics of success, and highlight the variability of performance on selected metrics even among a relatively small group of 17 programs.
In conclusion, we have created a method to quantify academic success that may be useful to academic hospitalists and their group leaders as they set targets for improvement in the field. Even among our SCHOLAR cohort, room for ongoing improvement in development of funded scholarship and a core of senior faculty exists. Further investigation into the unique features of successful groups will offer insight to leaders in academic hospital medicine regarding infrastructure and processes that should be embraced to raise the bar for all AHPs. In addition, efforts to further define and validate nontraditional approaches to scholarship that allow for successful promotion at AHPs would be informative. We view our work less as a singular approach to benchmarking standards for AHPs, and more a call to action to continue efforts to balance scholarly activity and broad professional development of academic hospitalists with increasing clinical demands.
Acknowledgements
The authors thank all of the AHP leaders who participated in the SCHOLAR project. They also thank the Society of Hospital Medicine and Society of General Internal Medicine and the SHM Academic Committee and SGIM Academic Hospitalist Task Force for their support of this work.
Disclosures
The work reported here was supported by the Department of Veterans Affairs, Veterans Health Administration, South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors report no conflicts of interest.
- , , , , . Characteristics of primary care providers who adopted the hospitalist model from 2001 to 2009. J Hosp Med. 2015;10(2):75–82.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Updating threshold‐based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45–47.
- , , , . Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2).
- , , , , , . Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , , , . The structure of hospital medicine programs at academic medical centers [abstract]. J Hosp Med. 2012;7(suppl 2):s92.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
- , , , , . Characteristics of primary care providers who adopted the hospitalist model from 2001 to 2009. J Hosp Med. 2015;10(2):75–82.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , , , , . Updating threshold‐based identification of hospitalists in 2012 Medicare pay data. J Hosp Med. 2016;11(1):45–47.
- , , , . Use of hospitalists by Medicare beneficiaries: a national picture. Medicare Medicaid Res Rev. 2014;4(2).
- , , , , , . Challenges and opportunities in Academic Hospital Medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240–246.
- , , , . Survey of US academic hospitalist leaders about mentorship and academic activities in hospitalist groups. J Hosp Med. 2011;6(1):5–9.
- , , , , , . The structure of hospital medicine programs at academic medical centers [abstract]. J Hosp Med. 2012;7(suppl 2):s92.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- , , , , , . Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23–27.
- , , , et al. The key principles and characteristics of an effective hospital medicine group: an assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9(2):123–128.
CAP Quality Care / Seymann
The quality movement has spawned efforts to define and measure best practices for clinical conditions commonly cared for by hospitalists. Pneumonia is the most frequent infectious cause of death in the United States, and it accounts for more than 1 million hospitalizations annually at an estimated annual cost of $12.2 billion, most of it incurred by inpatients.1 The morbidity and mortality of the elderly are particularly burdensome.2, 3 For these reasons, attention has been focused on improving the quality of care of inpatients with community‐acquired pneumonia (CAP).
Credentialing agencies such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) require hospitals to report performance on predefined core measures of pneumonia care that they have identified as best practices (see Table 1).4, 5 The performance of individual organizations on these measures is now publicly reported at a website (
|
| Collection of blood cultures before antibiotic therapy. |
| Collection of blood cultures within 24 hours of admission. |
| Mean time of less than 4 hours from arrival to initial administration of antibiotics. |
| Choice of initial antibiotics according to established guidelines.* |
| Pneumococcal screening and vaccination of eligible patients by discharge. |
| Influenza screening and vaccination of eligible patients during flu season. |
| Oxygenation assessment within 24 hours of admission. |
| Smoking cessation counseling to all smokers. |
Performance on core measures for pneumonia is less consistent across hospitals than the other conditions currently being monitored.7 It is instructive, then, to review the evidence base for the existing pneumonia quality measures, which can inform decisions about prioritizing interventions to provide the most effective care for inpatients with CAP.
BLOOD CULTURES
In a large multicenter retrospective study of Medicare patients hospitalized with CAP, Meehan et al.10 found the performance of blood cultures within 24 hours of arrival to be associated with reduced 30‐day mortality. Despite the large sample size of more than 14,000 patients, the risk‐adjusted mortality reduction was of only borderline significance (RR 0.9 [0.81‐1.00]). The unadjusted data did not show a significant mortality reduction. Notably, collection of blood cultures prior to antibiotic administration did not affect mortality, even excluding patients receiving prehospital antibiotics.
A smaller review of 38 U.S. academic medical centers showed relatively high compliance with blood culture performance, but no mortality reduction, even after adjustment for severity of illness. Similarly, performing blood cultures before administration of antibiotics yielded no significant effect.11
Several studies call into question the clinical utility of performing blood cultures drawn from patients with CAP. Combined, these studies evaluated almost 3000 pneumonia patients who had blood cultures drawn; the likelihood of a change in therapy based on results was at most 5%. Among the patients with positive cultures, only 20%‐40% had a treatment change based on the result.1215
The more severely ill patients with CAP may benefit from blood cultures, though the findings reported in the literature vary.12, 16 Using the Pneumonia Severity Index (PSI) score17 to classify severity of illness, an observational study of 209 inpatients with CAP found the yield of blood cultures increased from 10% in the lowest‐risk groups to 27% in the most severely ill.16 In contrast, two larger studies with a combined enrollment of almost 14,000 patients were unable to demonstrate a difference in the incidence of bacteremia despite adjustment for the PSI score.12, 18 It is clear from these and other studies that patients in PSI classes I‐III derive very little benefit from the performance of blood cultures.12, 16, 19
Metersky et al.18 described a prospectively validated risk assessment tool that reliably predicted bacteremia in Medicare patients with CAP and explored its utility in reducing unnecessary blood cultures. Independent risk factors for bacteremia included prior antibiotic use, liver disease, hypotension, tachycardia, fever or hypothermia, BUN > 30 mg/dL, sodium 130 mmol/L, and WBC 5000 or > 20,000/mm2. Use of this tool predicted bacteremia in 89% of patients and avoided 39% of unnecessary blood cultures. The authors also tested a modified version of the tool that excluded the laboratory abnormalities, so rapid assessment could be made at the initial patient presentation. This version advocated a single blood culture for most patients, and 2 blood cultures for patients with 2 or more risk factors. The modified tool accurately identified 88% of the patients with bacteremia and enabled a 44% reduction in unnecessary cultures.
In summary, blood cultures occasionally provide useful clinical information about etiology and resistance patterns, but they do not seem to reliably influence therapeutic decisions. It seems inappropriate to recommend against their use in practice, but they are not a solid benchmark for evidence‐based quality care. Measures that mandate risk assessment of all inpatients with CAP and require blood cultures only for older patients or those judged at high risk by PSI may better reflect quality. Alternatively, performing blood cultures on patients deemed to be high risk by the model of Metersky et al.18 may suffice.
ANTIBIOTIC TIMING
In a study of Medicare patients by Meehan et al.,10 the 30‐day mortality rate was reduced by 15% in the subset of patients who received antibiotics within 8 hours of arrival at the hospital. However, a trend toward mortality reduction was noted for those receiving antibiotics as early as 6 hours after arrival. Rapid administration of antibiotics was thus deemed an important measure of the quality of care of patients with CAP.
Additional studies attempted to confirm this observation. Battleman et al.20 evaluated 700 patients admitted for CAP through the emergency department. They found that a delay of more than 8 hours in the administration of antibiotics was correlated with a prolonged inpatient stay. Mortality rates were not reported. Achieving rapid delivery of antibiotics was closely linked to administration of the first dose of antibiotics in the emergency department.
Conversely, a large retrospective review by Dedier et al.11 found no reduction in inpatient mortality or in length of stay based on rapid antibiotic delivery, despite adjustment for severity of illness. They did not evaluate 30‐day mortality.
The effect of antibiotic timing on the time to clinical stability has also been investigated. Clinical stability was defined as 24 hours of a systolic blood pressure 90 mm Hg, heart rate 100 beats/min, respiratory rate 24 breaths/min, temperature 38.3C (101F), room air oxygen saturation 90%, and the ability to eat. Silber et al.,21 in a review of the records of 409 inpatients with moderate to severe CAP by PSI score, compared patients receiving antibiotics less than 4 hours, between 4 and 8 hours, and more than 8 hours after hospital admission. There was no difference between groups in time to clinical stability, even with adjustment for PSI.
Marrie and Wu22 attempted to define the factors that influenced inpatient mortality of patients with CAP not admitted to the intensive care unit (ICU). In a prospective study of 3043 patients evaluating a clinical pathway, a multivariate analysis showed antibiotic administration within 4 hours was not correlated with reduced mortality.
Although most studies supporting rapid antibiotic delivery used a target of 8 hours, administration in less than 4 hours is the consensus standard for pneumonia care set by CMS and JCAHO.23, 24
A benefit of timing antibiotic administration less than 4 hours after admission has been confirmed by a single, very large retrospective study of Medicare patients at least 65 years old.25 Analysis of a random sample of more than 18,000 patients with CAP who had not received prehospital antibiotics showed that the relative risk reduction for inpatient mortality was 15% in the group receiving antibiotics within 4 hours. Thirty‐day mortality was similarly reduced, and benefits continued for every hour of early antibiotic administration up to 9 hours.
The absolute risk reduction was small, however (0.6%), yielding a number needed to treat of 167 patients to prevent 1 death.
Randomized controlled trials, which would more definitively address the issue of antibiotic timing, are unlikely, as intentionally delaying administration of antibiotics to patients with known CAP is unethical. Hence, reliance on observational data must suffice. Intuitively, it makes sense to begin treatment of a bacterial infection at the earliest time possible. However, it is also known that not all patients present in a typical fashion, and diagnosis is uncertain at least 20% of the time.26 Anecdotal reports suggest that incentivizing physicians on performance measures encourages premature administration of empiric antibiotics to all patients presenting with cough, prior to confirmation of pneumonia.27, 28 Such practices promote further antibiotic resistance, arguably a larger health issue than delay in antibiotic delivery.29, 30
Houck31 offers potential solutions to this problem, such as eliminating the pressure on hospitals to perform at 100% on this measure by reporting performance within acceptable ranges (eg, 70%‐84% and 85%‐100%) Targeting a benchmark of 80% or a duration of 6 hours may also be appropriate. Finally, a 4‐hour benchmark has not been shown to benefit younger patients, so it is important to apply this target only to patients more than 65 years of age.
CHOICE OF ANTIBIOTIC
A retrospective review of 12,945 cases of inpatients with CAP found that, in comparison to ceftriaxone alone, initial antibiotic regimens consisting either of a second‐ or third‐generation cephalosporin plus a macrolide or of a fluoroquinolone alone were associated with an approximately 30% reduction in 30‐day mortality.32 Hence, current guidelines recommend the combination of a B‐lactam and macrolide, a B‐lactam and doxycycline, or a respiratory fluoroquinolone for inpatients with CAP not admitted to the ICU.3335
The results of subsequent studies supported the contention that guideline‐compliant antibiotics improve outcomes. A prospective multicenter study of a clinical pathway that encouraged use of either levofloxacin or cefuroxime plus azithromycin for the initial treatment of inpatient CAP showed significantly reduced mortality. Compared with any other antibiotic regimen, the odds ratio for death was 0.22 with the cephalosporin/macrolide combination and 0.43 with the fluoroquinolone. Of note, early mortality (within 5 days of admission) was not reduced by antibiotic choice.22 Similar results were found in a retrospective analysis, which found the odds of 30‐day mortality increased by 5.7 in patients not receiving guideline‐compliant therapy.36 A third study found guideline‐compliant antibiotics reduced the likelihood of a prolonged length of stay by 45%.20
Of note, data on the effectiveness of the cephalosporin/doxycycline combination are limited, and the major guidelines differ about whether this regimen is appropriate for inpatients with CAP.33, 34 Important findings from a recent retrospective cohort study showed that initial therapy with ceftriaxone plus doxycycline was associated with reduced inpatient mortality (OR = 0.26) as well as reduced 30‐day mortality (OR = 0.37) compared with other guideline‐compliant therapies for CAP.37 When patients who would not have been considered appropriate for initial doxycycline therapy (those resident in nursing homes, with aspiration pneumonia, or in the ICU) were excluded, a large reduction in inpatient mortality remained (OR = 0.17), without any increase in length of stay or readmission rate. Interestingly, this study suggests the potential superiority of this regimen, though a randomized controlled trial is needed to confirm this. The current core measures do include doxycycline as an acceptable option for CAP therapy (see Table 1).
Currently, controversy remains about whether the benefit of these selected regimens results from their activity against atypical pathogens (Mycoplasma, Legionella, Chlamydia) and whether there is additional benefit from using combination antibiotic therapy.38, 39 Waterer40 described 225 inpatients with bacteremic pneumococcal pneumonia, noting the antibiotic regimen received during the first 24 hours of hospitalization. Patients were classified retrospectively into 3 groupssingle effective therapy (SET), dual effective therapy (DET), or more than dual effective therapy (MET)on the basis of the concordance of pneumococcal sensitivity with the initial antibiotics. Patients on 2 antibiotics were classified in the DET group if the organism was sensitive to both and in the SET group if the organism was resistant to 1 of the 2. Those in the MET group were analyzed separately, as they were found to have a higher baseline severity of illness based on the PSI score; the SET and DET groups were equivalent.
Surprisingly, the SET group was found to have a 3‐fold increase in inpatient mortality; adjustment for severity of illness increased the odds ratio for death to 6.4. Of note, all deaths were in the most severely ill patients (PSI IV‐V). The protective effects of receiving DET were not specifically limited to those receiving a macrolide as the second agent, and multivariate analysis did not find coverage of atypical organisms to be an independent predictor of mortality.
A recent prospective multicenter trial of 844 patients with bacteremic pneumococcal pneumonia at 21 hospitals confirmed these findings.41 A significant 14‐day survival advantage (23% versus 55%) was found in the subgroup of critically ill patients who received at least 2 effective antibiotics. Though survival benefit was restricted to the sickest patients, severity of illness was similar among the groups.
The specific importance of macrolides in combination therapy remains under investigation. A review of a database of inpatients with bacteremic pneumococcal pneumonia over a 10‐year period found that 58% received initial empiric therapy with a B‐lactam/macrolide combination and 42% received B‐lactam without a macrolide (though other antibiotic combinations were not excluded).42 After logistic regression analysis, the investigators found a relative reduction in inpatient mortality of 60% in the patients receiving combination therapy with macrolides. Unfortunately, neither comparison to fluoroquinolone monotherapy nor risk stratification by PSI was reported. A similar study from Canada that did stratify for risk confirmed a mortality benefit of combination therapy.43
A subsequent, extremely large study of more than 44,000 patients from a hospital claims‐made database lent support to these findings.44 This study included all CAP patients regardless of microbiology and was not restricted to those with bacteremia. Outcomes among groups receiving monotherapy with any of the standard agents for CAP were compared with those in groups receiving combination therapy with a macrolide as the second agent. Statistically significant reductions in 30‐day mortality were observed in all groups receiving dual therapy with macrolides. Consistent with other studies, the benefit applied only to patients with more severe CAP. The percentage of patients with bacteremia was not specified.
Of note, this study did not allow direct comparison of fluoroquinolone monotherapy to combination therapy with a B‐lactam and a macrolide. However, the fluoroquinolone/macrolide combination conferred no additional benefit beyond fluoroquinolone monotherapy when adjusted for severity of illness or age. This implies that fluoroquinolone monotherapy is adequate, at least in some subpopulations. This is consistent with initial studies that established the superiority of the antibiotic combinations recommended by the guidelines.20, 22, 32
The potential benefit of combination therapy appears limited to patients with higher severity of illness and pneumococcal bacteremia. However, outcomes are affected by the antibiotic regimen selected in the initial 24‐48 hours of hospitalization, before results of blood cultures are routinely available. At present, clinical prediction of patients who will benefit from combination therapy is difficult.
Coverage of undiagnosed mixed infections with atypical organisms is probably not a major factor benefiting patients receiving combination therapy. Several recent meta‐analyses found no reduction in mortality or the rate of clinical failure among patients receiving antibiotics covering atypical organisms compared with those for patients whose regimens did not have such coverage.4547 Subgroups of patients with Legionella pneumonia do benefit from antibiotics with targeted activity against atypical organisms, but fewer than 1% of all patients were so identified. Evidence for antibiotic synergy is similarly lacking.48, 49 The immunomodulatory effects of macrolides, which decrease cytokine production and inflammation and subsequently reduce the severity of lung injury and other complications of sepsis, are considered potential factors in the reduction of mortality.50
The definition of severe CAP and the indications for ICU admission remain controversial, evidence for which is reviewed elsewhere.34, 51, 52 Antibiotic recommendations for ICU patients are included in Table 1 for completeness, but a detailed review of the evidence is lacking because current guidelines are based on consensus opinion.34 The use of fluoroquinolone monotherapy in severe CAP is not currently recommended because of limitations of the existing evidence. The majority of quinolone trials have excluded severely ill patients, and approval trials of newer respiratory fluoroquinolones have used levofloxacin as a comparator. Studies comparing fluoroquinolones typically allowed investigators in the B‐lactam arm the option of adding macrolides or tetracycline at their discretion. In addition, such trials have been designed as noniferiority trials.38 Clearly, randomized controlled trials are needed to resolve this issue.
Currently, selecting appropriate antibiotics should follow established guidelines, with consideration of using combination therapy for patients with a higher severity of illness. Emphasis on this measure should be stronger than that on antibiotic timing, as the bulk of the evidence favors significant mortality reduction from following guidelines for antibiotic therapy.
VACCINATION
Guidelines recommend all eligible adults hospitalized with CAP receive pneumococcal vaccination on discharge,3335, 53 though there is no evidence this reduces the incidence of pneumonia or death.54, 55 Retrospective studies have shown reduced incidence of invasive disease (bacteremia and meningitis), but not of other end points.5457 The estimated mortality from pneumococcal bacteremia remain as high as 20%‐30%, with no evidence that this rate has decreased over the last 30 years.5861 Despite this, a recent meta‐analysis from the Cochrane database that included only randomized, controlled trials (75,197 patients in 15 trials) was unable to show significant reductions in all‐cause pneumonia or mortality for vaccinated subjects.62 Cohort studies, evaluated separately in this analysis, showed an efficacy of 53% in reducing the incidence of invasive pneumococcal disease. Given the relatively low incidence of invasive disease in the general population, the number needed to treat was estimated at 20,000, or 4000 if only older patients were considered. A subsequent retrospective cohort study showed no reduction in pneumonia hospitalizations, cases of outpatient pneumonia, or mortality among 45,365 elderly vaccinees.56 Some specific subgroups may benefit, however. Vaccinated patients with chronic lung disease did show a reduction in hospitalization for pneumonia (RR 0.57 [0.38‐0.84]) and in mortality (RR 0.7 [0.56‐0.9]) in a retrospective study of HMO patients older than age 65.63
It is of interest that since the licensure of the pediatric 7‐valent protein‐polysaccharide conjugate vaccine in 2000, the incidence of invasive pneumococcal disease among adults has dropped significantly. Overall reduction in invasive disease in adults more than 50 years old was 11% from 1998 to 2003 (relative risk reduction [RRR] = 28%). This is likely the result of decreased transmission from colonized or infected children and not a coincidental increase in adult pneumococcal vaccination, as the rates of disease caused by the 16 strains unique to the 23‐valent vaccine did not change.64, 65 The overall reduction in the incidence of invasive disease is still superior with the adult vaccine, up to 30% in vaccinated subjects (RRR = 44%).56 Invasive disease caused specifically by penicillin‐nonsusceptible serotypes has dropped by 49% in the elderly since introduction of the vaccine.66 Thus, the combined impact of the 2 vaccines may be significant. It is not yet clear what effect, if any, the 7‐valent vaccine will have on the hospitalization rate or mortality.
In contrast to the results for pneumococcal vaccination, studies of the benefits of influenza vaccination have shown clear and consistent reductions in mortality, respiratory illness, hospitalization, and pneumonia, especially among patients with comorbidities.6771 Cost effectiveness has been demonstrated for all populations,72, 73 and the reduction in mortality among high‐risk patients younger than age 65 has been estimated to be as high as 78%.68 Among the elderly, reduction in mortality of about 50% has been reported, along with 20%‐30% reductions in hospitalizations for pneumonia, influenza, cardiac disease, and stroke.70 Reduced incidence of pneumonia in vaccinated patients has even been documented among elderly patients without specific comorbidities.67 Annual revaccination has the most significant impact on mortality.74
The pneumococcal vaccine remains important in the effort to reduce the severity of and complications from invasive pneumococcal disease in the elderly, but the lack of significant benefits on hard end points such as mortality or hospitalizations makes it a less robust measure of quality pneumonia care. In contrast, influenza vaccination has a much larger impact on outcomes in the population at risk. Emphasis should be shifted from pneumococcal to influenza vaccine in pneumonia performance measures.
OXYGENATION ASSESSMENT
It seems intuitive that oxygenation assessment is important in the initial evaluation of patients with CAP, though there is not direct evidence to support this. The recommendation for oxygenation assessment in the published guidelines for CAP is by consensus.3335 Documented hypoxemia is associated with increased pneumonia‐related mortality,17, 75 and clinical judgment does not adequately predict hypoxemia.76 Though the assessment of oxygenation has been found to vary widely among practitioners,77 performance has remained consistent since the advent of monitoring and reporting of quality measures, with compliance rates of 99%.4, 7 Monitoring performance of this measure should continue, though high compliance rates limit its ability to discriminate among institutions.
SMOKING CESSATION COUNSELING
Counseling patients to stop smoking was found to be modestly (2%) but statistically significantly effective in promoting abstinence at 1 year.78 In its report on treating tobacco use, the U.S. Public Health Services recommended that all smokers receive hospital‐ and system‐based interventions at every visit.79 As part of the Pneumonia Patient Outcomes Research Team (PORT) study, smokers with pneumonia underwent a tobacco cessation interview. Though only 15% of these patients quit smoking, 93% of those who quit did so at the time they developed CAP.80 A retrospective study of patients with bacteremic pneumococcal pneumonia found tobacco exposure, including passive smoking, to be a strong independent risk factor for invasive disease.81 The most recent CAP guidelines from the Infectious Disease Society of America (IDSA) recommend smoking cessation counseling for all hospitalized patients who smoke.33 However, hospitals are not likely to have the impact that a more comprehensive, outpatient‐based smoking cessation program would. Without ongoing support, counseling, and pharmacotherapy, the effects of an intervention would be expected to be small.79 Though evidence of benefit is limited, smoking cessation interventions should be encouraged at all sites of care. Quality care merits this regardless of admitting diagnosis, but benefits specific to CAP outcomes have not yet been demonstrated.
CONCLUSIONS
The burden of illness caused by CAP mandates that clinicians strive to deliver the highest quality of care to afflicted patients. Critical evaluation of the strength of the evidence will continue to guide such endeavors, and changes in practice will follow as new information surfaces. Standards of care, as adopted by consensus groups such as the IDSA and American Thoracic Society, will continue to inform the practice of hospitalists.
How quality is defined for public reporting requires particularly careful assessment. The definition of quality should be based on evidence more rigorous than that ascribed to consensus guidelines. Within the profession, guidelines offer reasonable standards of care and delineate areas for further research and are invaluable tools for practicing clinicians. In the public arena, however, proclaiming practices as good or bad sets expectations of health care consumers not educated in the nuances of evaluating clinical evidence and can unfairly bias them against conscientious and effective providers whose standards reflect different interpretations of controversial issues. Regulatory agencies should publicly target interventions using only the most solid evidentiary foundation while internally striving to monitor the effects of different practice patterns and report measurable differences in outcomes revealed by careful investigation. Areas where controversy remains should be the primary targets of further research but should not be offered as benchmarks for public scrutiny until the medical community has settled on a position.
Furthermore, when evidence remains questionable, financial incentives should be linked to performance indicators with extreme caution. It would be counterproductive if health care organizations, driven to achieve optimal antibiotic timing to obtain payment updates from CMS, began to administer antibiotics prior to completing workups on all patients with respiratory complaints, as this would likely lead to antibiotic overuse. Similarly, institutions pushed to collect blood cultures before antibiotics are given may inappropriately delay administration in order to perform well on quality measures, resulting in potential harm to patients.
The measures of quality care for CAP for which the evidence on outcomes is the most convincing are antibiotic selection (mortality benefit, reduction in LOS) and influenza vaccination (mortality benefit, reduction in hospitalizations, reduction in respiratory illness). Antibiotic timing also shows a smaller but convincing reduction in mortality, though the advantages of receiving antibiotics within 4 hours instead of 8 hours are not clearly established for younger patients. These measures should be emphasized most heavily in the arena of public reporting and incentives for quality care, with additions and modifications guided by emerging evidence. Revision of the other measures to conform with current evidence would allow public reporting to more accurately reflect quality.
- ,,,.Treatment costs of community‐acquired pneumonia in an employed population.Chest.2004;125:2140–2145.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,,.Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988‐2002.JAMA.2005;294:2712–2719.
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002‐2004.N Engl J Med.2005;353:255–264.
- Performance Measurement: Core Measures. Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/index.htm. Accessed November 7, 2005.
- Medicare Quality Improvement Program Priorities: DRAFT, August2005.Centers for Medicare 353:265–274.
- Medicare demonstration shows hospital quality of care improves with payments tied to quality.Centers for Medicare 278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95(1):78–82.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community‐acquired pneumonia: link between quality of care and resource utilization.Arch Intern Med.2002;162:682–688.
- ,,, et al.Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia.Chest.2003;124:1798–1804.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127:1260–1270.
- Hospital Quality Measures:2004‐2007. Available at: http://www.cms.hhs.gov/quality/hospital/. Accessed November 17, 2005.
- National Hospital Quality Measures Specification Manual version 1.02.Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/aligned_manual.htm.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,,,,.Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours?Chest.2006;130:16–21.
- ,.Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes?Curr Opin Infect Dis.2005;18(2):151–156.
- .The pneumonia controversy: hospitals grapple with 4 hour benchmark.Ann Emerg Med.2006;47:259–261.
- ,,, et al.Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods.Ann Intern Med.2001;134:479–486.
- .The hidden impact of antibacterial resistance in respiratory tract infection. Clinical failures: the tip of the iceberg?Respir Med2001;95Suppl A:S5–11; discussion S26–S27.
- .Antibiotics and pneumonia: is timing everything or just a cause of more problems?Chest.2006;130:1–3.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults.Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,,.Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia.Am J Med.2004;117:726–731.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- .Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia.Curr Opin Infect Dis.2005;18:157–163.
- ,.The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia.Chest.2005;128:940–946.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults.Cochrane Database Syst. Rev.2005:CD004418.
- ,,.Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,.Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro.Antimicrob Agents Chemother.2003;47:1151–1153.
- ,,,,.Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo.J Antimicrob Chemother.2000;46:973–980.
- .The effects of macrolides on inflammatory cells.Chest.2004;125(2 Suppl):41S–50S; quiz 1S.
- ,,, et al.Severe community‐acquired pneumonia. Risk factors and follow‐up epidemiology.Am J Respir Crit Care Med.1999;160:923–929.
- ,,, et al.Severe community‐acquired pneumonia. Assessment of severity criteria.Am J Respir Crit Care Med.1998;158:1102–1108.
- ,,,.Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged 65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services.MMWR Recomm Rep.2005;54(RR‐5):1–11.
- ,,,.The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials.Vaccine.2004;22:3214–3224.
- ,,.Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults.Vaccine.2002;20:2166–2173.
- ,,, et al.Effectiveness of pneumococcal polysaccharide vaccine in older adults.N Engl J Med.2003;348:1747–1755.
- ,,,,,.Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations.JAMA.1993;270:1826–1831.
- ,,,.Early predictors of mortality in pneumococcal bacteraemia.J Infect.2000;40:256–261.
- ,,.Pneumococcal bacteremia in adults: a 14‐year experience in an inner‐city university hospital.Clin Infect Dis.1995;21:345–351.
- ,,,,,.Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995.Commun Dis Public Health.1998;1(1):22–27.
- ,,,,.Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature.Isr J Med Sci.1987;23:174–180.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003:CD000422.
- ,,,.The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease.Arch Intern Med.1999;159:2437–2442.
- ,,, et al.Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.JAMA.2005;294:2043–2051.
- ,,, et al.Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine.N Engl J Med.2003;348:1737–1746.
- ,,, et al.Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae.N Engl J Med.2006;354:1455–1463.
- ,,,,,.Influenza vaccination in community‐dwelling elderly: impact on mortality and influenza‐associated morbidity.Arch Intern Med.2003;163:1089–1094.
- ,,, et al.Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study.Arch Intern Med.2005;165:274–280.
- ,,, et al.Influence of high‐risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed‐care organizations.Clin Infect Dis.2002;35:370–377.
- ,,,,,.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,,,.Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study.Chest.2004;125:2011–2020.
- ,,,,,.Economic analysis of influenza vaccination and antiviral treatment for healthy working adults.Ann Intern Med.2002;137:225–331.
- ,,,,.The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Annual revaccination against influenza and mortality risk in community‐dwelling elderly persons.JAMA.2004;292:2089–2095.
- ,,, et al.Causes of death for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study.Arch Intern Med.2002;162:1059–1064.
- ,,.Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department.Ann Emerg Med.1995;25(1):36–40.
- ,,, et al.Arterial blood gas and pulse oximetry in initial management of patients with community‐acquired pneumonia.J Gen Intern Med.2001;16:590–598.
- ,.An analysis of the effectiveness of interventions intended to help people stop smoking.Arch Intern Med.1995;155:1933–1941.
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.JAMA.2000;283:3244–3254.
- .Quality indicators for the management of pneumonia in vulnerable elders.Ann Intern Med.2001;135:736–743.
- ,,, et al.Cigarette smoking and invasive pneumococcal disease.Active Bacterial Core Surveillance Team.N Engl J Med.2000;342:681–689.
The quality movement has spawned efforts to define and measure best practices for clinical conditions commonly cared for by hospitalists. Pneumonia is the most frequent infectious cause of death in the United States, and it accounts for more than 1 million hospitalizations annually at an estimated annual cost of $12.2 billion, most of it incurred by inpatients.1 The morbidity and mortality of the elderly are particularly burdensome.2, 3 For these reasons, attention has been focused on improving the quality of care of inpatients with community‐acquired pneumonia (CAP).
Credentialing agencies such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) require hospitals to report performance on predefined core measures of pneumonia care that they have identified as best practices (see Table 1).4, 5 The performance of individual organizations on these measures is now publicly reported at a website (
|
| Collection of blood cultures before antibiotic therapy. |
| Collection of blood cultures within 24 hours of admission. |
| Mean time of less than 4 hours from arrival to initial administration of antibiotics. |
| Choice of initial antibiotics according to established guidelines.* |
| Pneumococcal screening and vaccination of eligible patients by discharge. |
| Influenza screening and vaccination of eligible patients during flu season. |
| Oxygenation assessment within 24 hours of admission. |
| Smoking cessation counseling to all smokers. |
Performance on core measures for pneumonia is less consistent across hospitals than the other conditions currently being monitored.7 It is instructive, then, to review the evidence base for the existing pneumonia quality measures, which can inform decisions about prioritizing interventions to provide the most effective care for inpatients with CAP.
BLOOD CULTURES
In a large multicenter retrospective study of Medicare patients hospitalized with CAP, Meehan et al.10 found the performance of blood cultures within 24 hours of arrival to be associated with reduced 30‐day mortality. Despite the large sample size of more than 14,000 patients, the risk‐adjusted mortality reduction was of only borderline significance (RR 0.9 [0.81‐1.00]). The unadjusted data did not show a significant mortality reduction. Notably, collection of blood cultures prior to antibiotic administration did not affect mortality, even excluding patients receiving prehospital antibiotics.
A smaller review of 38 U.S. academic medical centers showed relatively high compliance with blood culture performance, but no mortality reduction, even after adjustment for severity of illness. Similarly, performing blood cultures before administration of antibiotics yielded no significant effect.11
Several studies call into question the clinical utility of performing blood cultures drawn from patients with CAP. Combined, these studies evaluated almost 3000 pneumonia patients who had blood cultures drawn; the likelihood of a change in therapy based on results was at most 5%. Among the patients with positive cultures, only 20%‐40% had a treatment change based on the result.1215
The more severely ill patients with CAP may benefit from blood cultures, though the findings reported in the literature vary.12, 16 Using the Pneumonia Severity Index (PSI) score17 to classify severity of illness, an observational study of 209 inpatients with CAP found the yield of blood cultures increased from 10% in the lowest‐risk groups to 27% in the most severely ill.16 In contrast, two larger studies with a combined enrollment of almost 14,000 patients were unable to demonstrate a difference in the incidence of bacteremia despite adjustment for the PSI score.12, 18 It is clear from these and other studies that patients in PSI classes I‐III derive very little benefit from the performance of blood cultures.12, 16, 19
Metersky et al.18 described a prospectively validated risk assessment tool that reliably predicted bacteremia in Medicare patients with CAP and explored its utility in reducing unnecessary blood cultures. Independent risk factors for bacteremia included prior antibiotic use, liver disease, hypotension, tachycardia, fever or hypothermia, BUN > 30 mg/dL, sodium 130 mmol/L, and WBC 5000 or > 20,000/mm2. Use of this tool predicted bacteremia in 89% of patients and avoided 39% of unnecessary blood cultures. The authors also tested a modified version of the tool that excluded the laboratory abnormalities, so rapid assessment could be made at the initial patient presentation. This version advocated a single blood culture for most patients, and 2 blood cultures for patients with 2 or more risk factors. The modified tool accurately identified 88% of the patients with bacteremia and enabled a 44% reduction in unnecessary cultures.
In summary, blood cultures occasionally provide useful clinical information about etiology and resistance patterns, but they do not seem to reliably influence therapeutic decisions. It seems inappropriate to recommend against their use in practice, but they are not a solid benchmark for evidence‐based quality care. Measures that mandate risk assessment of all inpatients with CAP and require blood cultures only for older patients or those judged at high risk by PSI may better reflect quality. Alternatively, performing blood cultures on patients deemed to be high risk by the model of Metersky et al.18 may suffice.
ANTIBIOTIC TIMING
In a study of Medicare patients by Meehan et al.,10 the 30‐day mortality rate was reduced by 15% in the subset of patients who received antibiotics within 8 hours of arrival at the hospital. However, a trend toward mortality reduction was noted for those receiving antibiotics as early as 6 hours after arrival. Rapid administration of antibiotics was thus deemed an important measure of the quality of care of patients with CAP.
Additional studies attempted to confirm this observation. Battleman et al.20 evaluated 700 patients admitted for CAP through the emergency department. They found that a delay of more than 8 hours in the administration of antibiotics was correlated with a prolonged inpatient stay. Mortality rates were not reported. Achieving rapid delivery of antibiotics was closely linked to administration of the first dose of antibiotics in the emergency department.
Conversely, a large retrospective review by Dedier et al.11 found no reduction in inpatient mortality or in length of stay based on rapid antibiotic delivery, despite adjustment for severity of illness. They did not evaluate 30‐day mortality.
The effect of antibiotic timing on the time to clinical stability has also been investigated. Clinical stability was defined as 24 hours of a systolic blood pressure 90 mm Hg, heart rate 100 beats/min, respiratory rate 24 breaths/min, temperature 38.3C (101F), room air oxygen saturation 90%, and the ability to eat. Silber et al.,21 in a review of the records of 409 inpatients with moderate to severe CAP by PSI score, compared patients receiving antibiotics less than 4 hours, between 4 and 8 hours, and more than 8 hours after hospital admission. There was no difference between groups in time to clinical stability, even with adjustment for PSI.
Marrie and Wu22 attempted to define the factors that influenced inpatient mortality of patients with CAP not admitted to the intensive care unit (ICU). In a prospective study of 3043 patients evaluating a clinical pathway, a multivariate analysis showed antibiotic administration within 4 hours was not correlated with reduced mortality.
Although most studies supporting rapid antibiotic delivery used a target of 8 hours, administration in less than 4 hours is the consensus standard for pneumonia care set by CMS and JCAHO.23, 24
A benefit of timing antibiotic administration less than 4 hours after admission has been confirmed by a single, very large retrospective study of Medicare patients at least 65 years old.25 Analysis of a random sample of more than 18,000 patients with CAP who had not received prehospital antibiotics showed that the relative risk reduction for inpatient mortality was 15% in the group receiving antibiotics within 4 hours. Thirty‐day mortality was similarly reduced, and benefits continued for every hour of early antibiotic administration up to 9 hours.
The absolute risk reduction was small, however (0.6%), yielding a number needed to treat of 167 patients to prevent 1 death.
Randomized controlled trials, which would more definitively address the issue of antibiotic timing, are unlikely, as intentionally delaying administration of antibiotics to patients with known CAP is unethical. Hence, reliance on observational data must suffice. Intuitively, it makes sense to begin treatment of a bacterial infection at the earliest time possible. However, it is also known that not all patients present in a typical fashion, and diagnosis is uncertain at least 20% of the time.26 Anecdotal reports suggest that incentivizing physicians on performance measures encourages premature administration of empiric antibiotics to all patients presenting with cough, prior to confirmation of pneumonia.27, 28 Such practices promote further antibiotic resistance, arguably a larger health issue than delay in antibiotic delivery.29, 30
Houck31 offers potential solutions to this problem, such as eliminating the pressure on hospitals to perform at 100% on this measure by reporting performance within acceptable ranges (eg, 70%‐84% and 85%‐100%) Targeting a benchmark of 80% or a duration of 6 hours may also be appropriate. Finally, a 4‐hour benchmark has not been shown to benefit younger patients, so it is important to apply this target only to patients more than 65 years of age.
CHOICE OF ANTIBIOTIC
A retrospective review of 12,945 cases of inpatients with CAP found that, in comparison to ceftriaxone alone, initial antibiotic regimens consisting either of a second‐ or third‐generation cephalosporin plus a macrolide or of a fluoroquinolone alone were associated with an approximately 30% reduction in 30‐day mortality.32 Hence, current guidelines recommend the combination of a B‐lactam and macrolide, a B‐lactam and doxycycline, or a respiratory fluoroquinolone for inpatients with CAP not admitted to the ICU.3335
The results of subsequent studies supported the contention that guideline‐compliant antibiotics improve outcomes. A prospective multicenter study of a clinical pathway that encouraged use of either levofloxacin or cefuroxime plus azithromycin for the initial treatment of inpatient CAP showed significantly reduced mortality. Compared with any other antibiotic regimen, the odds ratio for death was 0.22 with the cephalosporin/macrolide combination and 0.43 with the fluoroquinolone. Of note, early mortality (within 5 days of admission) was not reduced by antibiotic choice.22 Similar results were found in a retrospective analysis, which found the odds of 30‐day mortality increased by 5.7 in patients not receiving guideline‐compliant therapy.36 A third study found guideline‐compliant antibiotics reduced the likelihood of a prolonged length of stay by 45%.20
Of note, data on the effectiveness of the cephalosporin/doxycycline combination are limited, and the major guidelines differ about whether this regimen is appropriate for inpatients with CAP.33, 34 Important findings from a recent retrospective cohort study showed that initial therapy with ceftriaxone plus doxycycline was associated with reduced inpatient mortality (OR = 0.26) as well as reduced 30‐day mortality (OR = 0.37) compared with other guideline‐compliant therapies for CAP.37 When patients who would not have been considered appropriate for initial doxycycline therapy (those resident in nursing homes, with aspiration pneumonia, or in the ICU) were excluded, a large reduction in inpatient mortality remained (OR = 0.17), without any increase in length of stay or readmission rate. Interestingly, this study suggests the potential superiority of this regimen, though a randomized controlled trial is needed to confirm this. The current core measures do include doxycycline as an acceptable option for CAP therapy (see Table 1).
Currently, controversy remains about whether the benefit of these selected regimens results from their activity against atypical pathogens (Mycoplasma, Legionella, Chlamydia) and whether there is additional benefit from using combination antibiotic therapy.38, 39 Waterer40 described 225 inpatients with bacteremic pneumococcal pneumonia, noting the antibiotic regimen received during the first 24 hours of hospitalization. Patients were classified retrospectively into 3 groupssingle effective therapy (SET), dual effective therapy (DET), or more than dual effective therapy (MET)on the basis of the concordance of pneumococcal sensitivity with the initial antibiotics. Patients on 2 antibiotics were classified in the DET group if the organism was sensitive to both and in the SET group if the organism was resistant to 1 of the 2. Those in the MET group were analyzed separately, as they were found to have a higher baseline severity of illness based on the PSI score; the SET and DET groups were equivalent.
Surprisingly, the SET group was found to have a 3‐fold increase in inpatient mortality; adjustment for severity of illness increased the odds ratio for death to 6.4. Of note, all deaths were in the most severely ill patients (PSI IV‐V). The protective effects of receiving DET were not specifically limited to those receiving a macrolide as the second agent, and multivariate analysis did not find coverage of atypical organisms to be an independent predictor of mortality.
A recent prospective multicenter trial of 844 patients with bacteremic pneumococcal pneumonia at 21 hospitals confirmed these findings.41 A significant 14‐day survival advantage (23% versus 55%) was found in the subgroup of critically ill patients who received at least 2 effective antibiotics. Though survival benefit was restricted to the sickest patients, severity of illness was similar among the groups.
The specific importance of macrolides in combination therapy remains under investigation. A review of a database of inpatients with bacteremic pneumococcal pneumonia over a 10‐year period found that 58% received initial empiric therapy with a B‐lactam/macrolide combination and 42% received B‐lactam without a macrolide (though other antibiotic combinations were not excluded).42 After logistic regression analysis, the investigators found a relative reduction in inpatient mortality of 60% in the patients receiving combination therapy with macrolides. Unfortunately, neither comparison to fluoroquinolone monotherapy nor risk stratification by PSI was reported. A similar study from Canada that did stratify for risk confirmed a mortality benefit of combination therapy.43
A subsequent, extremely large study of more than 44,000 patients from a hospital claims‐made database lent support to these findings.44 This study included all CAP patients regardless of microbiology and was not restricted to those with bacteremia. Outcomes among groups receiving monotherapy with any of the standard agents for CAP were compared with those in groups receiving combination therapy with a macrolide as the second agent. Statistically significant reductions in 30‐day mortality were observed in all groups receiving dual therapy with macrolides. Consistent with other studies, the benefit applied only to patients with more severe CAP. The percentage of patients with bacteremia was not specified.
Of note, this study did not allow direct comparison of fluoroquinolone monotherapy to combination therapy with a B‐lactam and a macrolide. However, the fluoroquinolone/macrolide combination conferred no additional benefit beyond fluoroquinolone monotherapy when adjusted for severity of illness or age. This implies that fluoroquinolone monotherapy is adequate, at least in some subpopulations. This is consistent with initial studies that established the superiority of the antibiotic combinations recommended by the guidelines.20, 22, 32
The potential benefit of combination therapy appears limited to patients with higher severity of illness and pneumococcal bacteremia. However, outcomes are affected by the antibiotic regimen selected in the initial 24‐48 hours of hospitalization, before results of blood cultures are routinely available. At present, clinical prediction of patients who will benefit from combination therapy is difficult.
Coverage of undiagnosed mixed infections with atypical organisms is probably not a major factor benefiting patients receiving combination therapy. Several recent meta‐analyses found no reduction in mortality or the rate of clinical failure among patients receiving antibiotics covering atypical organisms compared with those for patients whose regimens did not have such coverage.4547 Subgroups of patients with Legionella pneumonia do benefit from antibiotics with targeted activity against atypical organisms, but fewer than 1% of all patients were so identified. Evidence for antibiotic synergy is similarly lacking.48, 49 The immunomodulatory effects of macrolides, which decrease cytokine production and inflammation and subsequently reduce the severity of lung injury and other complications of sepsis, are considered potential factors in the reduction of mortality.50
The definition of severe CAP and the indications for ICU admission remain controversial, evidence for which is reviewed elsewhere.34, 51, 52 Antibiotic recommendations for ICU patients are included in Table 1 for completeness, but a detailed review of the evidence is lacking because current guidelines are based on consensus opinion.34 The use of fluoroquinolone monotherapy in severe CAP is not currently recommended because of limitations of the existing evidence. The majority of quinolone trials have excluded severely ill patients, and approval trials of newer respiratory fluoroquinolones have used levofloxacin as a comparator. Studies comparing fluoroquinolones typically allowed investigators in the B‐lactam arm the option of adding macrolides or tetracycline at their discretion. In addition, such trials have been designed as noniferiority trials.38 Clearly, randomized controlled trials are needed to resolve this issue.
Currently, selecting appropriate antibiotics should follow established guidelines, with consideration of using combination therapy for patients with a higher severity of illness. Emphasis on this measure should be stronger than that on antibiotic timing, as the bulk of the evidence favors significant mortality reduction from following guidelines for antibiotic therapy.
VACCINATION
Guidelines recommend all eligible adults hospitalized with CAP receive pneumococcal vaccination on discharge,3335, 53 though there is no evidence this reduces the incidence of pneumonia or death.54, 55 Retrospective studies have shown reduced incidence of invasive disease (bacteremia and meningitis), but not of other end points.5457 The estimated mortality from pneumococcal bacteremia remain as high as 20%‐30%, with no evidence that this rate has decreased over the last 30 years.5861 Despite this, a recent meta‐analysis from the Cochrane database that included only randomized, controlled trials (75,197 patients in 15 trials) was unable to show significant reductions in all‐cause pneumonia or mortality for vaccinated subjects.62 Cohort studies, evaluated separately in this analysis, showed an efficacy of 53% in reducing the incidence of invasive pneumococcal disease. Given the relatively low incidence of invasive disease in the general population, the number needed to treat was estimated at 20,000, or 4000 if only older patients were considered. A subsequent retrospective cohort study showed no reduction in pneumonia hospitalizations, cases of outpatient pneumonia, or mortality among 45,365 elderly vaccinees.56 Some specific subgroups may benefit, however. Vaccinated patients with chronic lung disease did show a reduction in hospitalization for pneumonia (RR 0.57 [0.38‐0.84]) and in mortality (RR 0.7 [0.56‐0.9]) in a retrospective study of HMO patients older than age 65.63
It is of interest that since the licensure of the pediatric 7‐valent protein‐polysaccharide conjugate vaccine in 2000, the incidence of invasive pneumococcal disease among adults has dropped significantly. Overall reduction in invasive disease in adults more than 50 years old was 11% from 1998 to 2003 (relative risk reduction [RRR] = 28%). This is likely the result of decreased transmission from colonized or infected children and not a coincidental increase in adult pneumococcal vaccination, as the rates of disease caused by the 16 strains unique to the 23‐valent vaccine did not change.64, 65 The overall reduction in the incidence of invasive disease is still superior with the adult vaccine, up to 30% in vaccinated subjects (RRR = 44%).56 Invasive disease caused specifically by penicillin‐nonsusceptible serotypes has dropped by 49% in the elderly since introduction of the vaccine.66 Thus, the combined impact of the 2 vaccines may be significant. It is not yet clear what effect, if any, the 7‐valent vaccine will have on the hospitalization rate or mortality.
In contrast to the results for pneumococcal vaccination, studies of the benefits of influenza vaccination have shown clear and consistent reductions in mortality, respiratory illness, hospitalization, and pneumonia, especially among patients with comorbidities.6771 Cost effectiveness has been demonstrated for all populations,72, 73 and the reduction in mortality among high‐risk patients younger than age 65 has been estimated to be as high as 78%.68 Among the elderly, reduction in mortality of about 50% has been reported, along with 20%‐30% reductions in hospitalizations for pneumonia, influenza, cardiac disease, and stroke.70 Reduced incidence of pneumonia in vaccinated patients has even been documented among elderly patients without specific comorbidities.67 Annual revaccination has the most significant impact on mortality.74
The pneumococcal vaccine remains important in the effort to reduce the severity of and complications from invasive pneumococcal disease in the elderly, but the lack of significant benefits on hard end points such as mortality or hospitalizations makes it a less robust measure of quality pneumonia care. In contrast, influenza vaccination has a much larger impact on outcomes in the population at risk. Emphasis should be shifted from pneumococcal to influenza vaccine in pneumonia performance measures.
OXYGENATION ASSESSMENT
It seems intuitive that oxygenation assessment is important in the initial evaluation of patients with CAP, though there is not direct evidence to support this. The recommendation for oxygenation assessment in the published guidelines for CAP is by consensus.3335 Documented hypoxemia is associated with increased pneumonia‐related mortality,17, 75 and clinical judgment does not adequately predict hypoxemia.76 Though the assessment of oxygenation has been found to vary widely among practitioners,77 performance has remained consistent since the advent of monitoring and reporting of quality measures, with compliance rates of 99%.4, 7 Monitoring performance of this measure should continue, though high compliance rates limit its ability to discriminate among institutions.
SMOKING CESSATION COUNSELING
Counseling patients to stop smoking was found to be modestly (2%) but statistically significantly effective in promoting abstinence at 1 year.78 In its report on treating tobacco use, the U.S. Public Health Services recommended that all smokers receive hospital‐ and system‐based interventions at every visit.79 As part of the Pneumonia Patient Outcomes Research Team (PORT) study, smokers with pneumonia underwent a tobacco cessation interview. Though only 15% of these patients quit smoking, 93% of those who quit did so at the time they developed CAP.80 A retrospective study of patients with bacteremic pneumococcal pneumonia found tobacco exposure, including passive smoking, to be a strong independent risk factor for invasive disease.81 The most recent CAP guidelines from the Infectious Disease Society of America (IDSA) recommend smoking cessation counseling for all hospitalized patients who smoke.33 However, hospitals are not likely to have the impact that a more comprehensive, outpatient‐based smoking cessation program would. Without ongoing support, counseling, and pharmacotherapy, the effects of an intervention would be expected to be small.79 Though evidence of benefit is limited, smoking cessation interventions should be encouraged at all sites of care. Quality care merits this regardless of admitting diagnosis, but benefits specific to CAP outcomes have not yet been demonstrated.
CONCLUSIONS
The burden of illness caused by CAP mandates that clinicians strive to deliver the highest quality of care to afflicted patients. Critical evaluation of the strength of the evidence will continue to guide such endeavors, and changes in practice will follow as new information surfaces. Standards of care, as adopted by consensus groups such as the IDSA and American Thoracic Society, will continue to inform the practice of hospitalists.
How quality is defined for public reporting requires particularly careful assessment. The definition of quality should be based on evidence more rigorous than that ascribed to consensus guidelines. Within the profession, guidelines offer reasonable standards of care and delineate areas for further research and are invaluable tools for practicing clinicians. In the public arena, however, proclaiming practices as good or bad sets expectations of health care consumers not educated in the nuances of evaluating clinical evidence and can unfairly bias them against conscientious and effective providers whose standards reflect different interpretations of controversial issues. Regulatory agencies should publicly target interventions using only the most solid evidentiary foundation while internally striving to monitor the effects of different practice patterns and report measurable differences in outcomes revealed by careful investigation. Areas where controversy remains should be the primary targets of further research but should not be offered as benchmarks for public scrutiny until the medical community has settled on a position.
Furthermore, when evidence remains questionable, financial incentives should be linked to performance indicators with extreme caution. It would be counterproductive if health care organizations, driven to achieve optimal antibiotic timing to obtain payment updates from CMS, began to administer antibiotics prior to completing workups on all patients with respiratory complaints, as this would likely lead to antibiotic overuse. Similarly, institutions pushed to collect blood cultures before antibiotics are given may inappropriately delay administration in order to perform well on quality measures, resulting in potential harm to patients.
The measures of quality care for CAP for which the evidence on outcomes is the most convincing are antibiotic selection (mortality benefit, reduction in LOS) and influenza vaccination (mortality benefit, reduction in hospitalizations, reduction in respiratory illness). Antibiotic timing also shows a smaller but convincing reduction in mortality, though the advantages of receiving antibiotics within 4 hours instead of 8 hours are not clearly established for younger patients. These measures should be emphasized most heavily in the arena of public reporting and incentives for quality care, with additions and modifications guided by emerging evidence. Revision of the other measures to conform with current evidence would allow public reporting to more accurately reflect quality.
The quality movement has spawned efforts to define and measure best practices for clinical conditions commonly cared for by hospitalists. Pneumonia is the most frequent infectious cause of death in the United States, and it accounts for more than 1 million hospitalizations annually at an estimated annual cost of $12.2 billion, most of it incurred by inpatients.1 The morbidity and mortality of the elderly are particularly burdensome.2, 3 For these reasons, attention has been focused on improving the quality of care of inpatients with community‐acquired pneumonia (CAP).
Credentialing agencies such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) require hospitals to report performance on predefined core measures of pneumonia care that they have identified as best practices (see Table 1).4, 5 The performance of individual organizations on these measures is now publicly reported at a website (
|
| Collection of blood cultures before antibiotic therapy. |
| Collection of blood cultures within 24 hours of admission. |
| Mean time of less than 4 hours from arrival to initial administration of antibiotics. |
| Choice of initial antibiotics according to established guidelines.* |
| Pneumococcal screening and vaccination of eligible patients by discharge. |
| Influenza screening and vaccination of eligible patients during flu season. |
| Oxygenation assessment within 24 hours of admission. |
| Smoking cessation counseling to all smokers. |
Performance on core measures for pneumonia is less consistent across hospitals than the other conditions currently being monitored.7 It is instructive, then, to review the evidence base for the existing pneumonia quality measures, which can inform decisions about prioritizing interventions to provide the most effective care for inpatients with CAP.
BLOOD CULTURES
In a large multicenter retrospective study of Medicare patients hospitalized with CAP, Meehan et al.10 found the performance of blood cultures within 24 hours of arrival to be associated with reduced 30‐day mortality. Despite the large sample size of more than 14,000 patients, the risk‐adjusted mortality reduction was of only borderline significance (RR 0.9 [0.81‐1.00]). The unadjusted data did not show a significant mortality reduction. Notably, collection of blood cultures prior to antibiotic administration did not affect mortality, even excluding patients receiving prehospital antibiotics.
A smaller review of 38 U.S. academic medical centers showed relatively high compliance with blood culture performance, but no mortality reduction, even after adjustment for severity of illness. Similarly, performing blood cultures before administration of antibiotics yielded no significant effect.11
Several studies call into question the clinical utility of performing blood cultures drawn from patients with CAP. Combined, these studies evaluated almost 3000 pneumonia patients who had blood cultures drawn; the likelihood of a change in therapy based on results was at most 5%. Among the patients with positive cultures, only 20%‐40% had a treatment change based on the result.1215
The more severely ill patients with CAP may benefit from blood cultures, though the findings reported in the literature vary.12, 16 Using the Pneumonia Severity Index (PSI) score17 to classify severity of illness, an observational study of 209 inpatients with CAP found the yield of blood cultures increased from 10% in the lowest‐risk groups to 27% in the most severely ill.16 In contrast, two larger studies with a combined enrollment of almost 14,000 patients were unable to demonstrate a difference in the incidence of bacteremia despite adjustment for the PSI score.12, 18 It is clear from these and other studies that patients in PSI classes I‐III derive very little benefit from the performance of blood cultures.12, 16, 19
Metersky et al.18 described a prospectively validated risk assessment tool that reliably predicted bacteremia in Medicare patients with CAP and explored its utility in reducing unnecessary blood cultures. Independent risk factors for bacteremia included prior antibiotic use, liver disease, hypotension, tachycardia, fever or hypothermia, BUN > 30 mg/dL, sodium 130 mmol/L, and WBC 5000 or > 20,000/mm2. Use of this tool predicted bacteremia in 89% of patients and avoided 39% of unnecessary blood cultures. The authors also tested a modified version of the tool that excluded the laboratory abnormalities, so rapid assessment could be made at the initial patient presentation. This version advocated a single blood culture for most patients, and 2 blood cultures for patients with 2 or more risk factors. The modified tool accurately identified 88% of the patients with bacteremia and enabled a 44% reduction in unnecessary cultures.
In summary, blood cultures occasionally provide useful clinical information about etiology and resistance patterns, but they do not seem to reliably influence therapeutic decisions. It seems inappropriate to recommend against their use in practice, but they are not a solid benchmark for evidence‐based quality care. Measures that mandate risk assessment of all inpatients with CAP and require blood cultures only for older patients or those judged at high risk by PSI may better reflect quality. Alternatively, performing blood cultures on patients deemed to be high risk by the model of Metersky et al.18 may suffice.
ANTIBIOTIC TIMING
In a study of Medicare patients by Meehan et al.,10 the 30‐day mortality rate was reduced by 15% in the subset of patients who received antibiotics within 8 hours of arrival at the hospital. However, a trend toward mortality reduction was noted for those receiving antibiotics as early as 6 hours after arrival. Rapid administration of antibiotics was thus deemed an important measure of the quality of care of patients with CAP.
Additional studies attempted to confirm this observation. Battleman et al.20 evaluated 700 patients admitted for CAP through the emergency department. They found that a delay of more than 8 hours in the administration of antibiotics was correlated with a prolonged inpatient stay. Mortality rates were not reported. Achieving rapid delivery of antibiotics was closely linked to administration of the first dose of antibiotics in the emergency department.
Conversely, a large retrospective review by Dedier et al.11 found no reduction in inpatient mortality or in length of stay based on rapid antibiotic delivery, despite adjustment for severity of illness. They did not evaluate 30‐day mortality.
The effect of antibiotic timing on the time to clinical stability has also been investigated. Clinical stability was defined as 24 hours of a systolic blood pressure 90 mm Hg, heart rate 100 beats/min, respiratory rate 24 breaths/min, temperature 38.3C (101F), room air oxygen saturation 90%, and the ability to eat. Silber et al.,21 in a review of the records of 409 inpatients with moderate to severe CAP by PSI score, compared patients receiving antibiotics less than 4 hours, between 4 and 8 hours, and more than 8 hours after hospital admission. There was no difference between groups in time to clinical stability, even with adjustment for PSI.
Marrie and Wu22 attempted to define the factors that influenced inpatient mortality of patients with CAP not admitted to the intensive care unit (ICU). In a prospective study of 3043 patients evaluating a clinical pathway, a multivariate analysis showed antibiotic administration within 4 hours was not correlated with reduced mortality.
Although most studies supporting rapid antibiotic delivery used a target of 8 hours, administration in less than 4 hours is the consensus standard for pneumonia care set by CMS and JCAHO.23, 24
A benefit of timing antibiotic administration less than 4 hours after admission has been confirmed by a single, very large retrospective study of Medicare patients at least 65 years old.25 Analysis of a random sample of more than 18,000 patients with CAP who had not received prehospital antibiotics showed that the relative risk reduction for inpatient mortality was 15% in the group receiving antibiotics within 4 hours. Thirty‐day mortality was similarly reduced, and benefits continued for every hour of early antibiotic administration up to 9 hours.
The absolute risk reduction was small, however (0.6%), yielding a number needed to treat of 167 patients to prevent 1 death.
Randomized controlled trials, which would more definitively address the issue of antibiotic timing, are unlikely, as intentionally delaying administration of antibiotics to patients with known CAP is unethical. Hence, reliance on observational data must suffice. Intuitively, it makes sense to begin treatment of a bacterial infection at the earliest time possible. However, it is also known that not all patients present in a typical fashion, and diagnosis is uncertain at least 20% of the time.26 Anecdotal reports suggest that incentivizing physicians on performance measures encourages premature administration of empiric antibiotics to all patients presenting with cough, prior to confirmation of pneumonia.27, 28 Such practices promote further antibiotic resistance, arguably a larger health issue than delay in antibiotic delivery.29, 30
Houck31 offers potential solutions to this problem, such as eliminating the pressure on hospitals to perform at 100% on this measure by reporting performance within acceptable ranges (eg, 70%‐84% and 85%‐100%) Targeting a benchmark of 80% or a duration of 6 hours may also be appropriate. Finally, a 4‐hour benchmark has not been shown to benefit younger patients, so it is important to apply this target only to patients more than 65 years of age.
CHOICE OF ANTIBIOTIC
A retrospective review of 12,945 cases of inpatients with CAP found that, in comparison to ceftriaxone alone, initial antibiotic regimens consisting either of a second‐ or third‐generation cephalosporin plus a macrolide or of a fluoroquinolone alone were associated with an approximately 30% reduction in 30‐day mortality.32 Hence, current guidelines recommend the combination of a B‐lactam and macrolide, a B‐lactam and doxycycline, or a respiratory fluoroquinolone for inpatients with CAP not admitted to the ICU.3335
The results of subsequent studies supported the contention that guideline‐compliant antibiotics improve outcomes. A prospective multicenter study of a clinical pathway that encouraged use of either levofloxacin or cefuroxime plus azithromycin for the initial treatment of inpatient CAP showed significantly reduced mortality. Compared with any other antibiotic regimen, the odds ratio for death was 0.22 with the cephalosporin/macrolide combination and 0.43 with the fluoroquinolone. Of note, early mortality (within 5 days of admission) was not reduced by antibiotic choice.22 Similar results were found in a retrospective analysis, which found the odds of 30‐day mortality increased by 5.7 in patients not receiving guideline‐compliant therapy.36 A third study found guideline‐compliant antibiotics reduced the likelihood of a prolonged length of stay by 45%.20
Of note, data on the effectiveness of the cephalosporin/doxycycline combination are limited, and the major guidelines differ about whether this regimen is appropriate for inpatients with CAP.33, 34 Important findings from a recent retrospective cohort study showed that initial therapy with ceftriaxone plus doxycycline was associated with reduced inpatient mortality (OR = 0.26) as well as reduced 30‐day mortality (OR = 0.37) compared with other guideline‐compliant therapies for CAP.37 When patients who would not have been considered appropriate for initial doxycycline therapy (those resident in nursing homes, with aspiration pneumonia, or in the ICU) were excluded, a large reduction in inpatient mortality remained (OR = 0.17), without any increase in length of stay or readmission rate. Interestingly, this study suggests the potential superiority of this regimen, though a randomized controlled trial is needed to confirm this. The current core measures do include doxycycline as an acceptable option for CAP therapy (see Table 1).
Currently, controversy remains about whether the benefit of these selected regimens results from their activity against atypical pathogens (Mycoplasma, Legionella, Chlamydia) and whether there is additional benefit from using combination antibiotic therapy.38, 39 Waterer40 described 225 inpatients with bacteremic pneumococcal pneumonia, noting the antibiotic regimen received during the first 24 hours of hospitalization. Patients were classified retrospectively into 3 groupssingle effective therapy (SET), dual effective therapy (DET), or more than dual effective therapy (MET)on the basis of the concordance of pneumococcal sensitivity with the initial antibiotics. Patients on 2 antibiotics were classified in the DET group if the organism was sensitive to both and in the SET group if the organism was resistant to 1 of the 2. Those in the MET group were analyzed separately, as they were found to have a higher baseline severity of illness based on the PSI score; the SET and DET groups were equivalent.
Surprisingly, the SET group was found to have a 3‐fold increase in inpatient mortality; adjustment for severity of illness increased the odds ratio for death to 6.4. Of note, all deaths were in the most severely ill patients (PSI IV‐V). The protective effects of receiving DET were not specifically limited to those receiving a macrolide as the second agent, and multivariate analysis did not find coverage of atypical organisms to be an independent predictor of mortality.
A recent prospective multicenter trial of 844 patients with bacteremic pneumococcal pneumonia at 21 hospitals confirmed these findings.41 A significant 14‐day survival advantage (23% versus 55%) was found in the subgroup of critically ill patients who received at least 2 effective antibiotics. Though survival benefit was restricted to the sickest patients, severity of illness was similar among the groups.
The specific importance of macrolides in combination therapy remains under investigation. A review of a database of inpatients with bacteremic pneumococcal pneumonia over a 10‐year period found that 58% received initial empiric therapy with a B‐lactam/macrolide combination and 42% received B‐lactam without a macrolide (though other antibiotic combinations were not excluded).42 After logistic regression analysis, the investigators found a relative reduction in inpatient mortality of 60% in the patients receiving combination therapy with macrolides. Unfortunately, neither comparison to fluoroquinolone monotherapy nor risk stratification by PSI was reported. A similar study from Canada that did stratify for risk confirmed a mortality benefit of combination therapy.43
A subsequent, extremely large study of more than 44,000 patients from a hospital claims‐made database lent support to these findings.44 This study included all CAP patients regardless of microbiology and was not restricted to those with bacteremia. Outcomes among groups receiving monotherapy with any of the standard agents for CAP were compared with those in groups receiving combination therapy with a macrolide as the second agent. Statistically significant reductions in 30‐day mortality were observed in all groups receiving dual therapy with macrolides. Consistent with other studies, the benefit applied only to patients with more severe CAP. The percentage of patients with bacteremia was not specified.
Of note, this study did not allow direct comparison of fluoroquinolone monotherapy to combination therapy with a B‐lactam and a macrolide. However, the fluoroquinolone/macrolide combination conferred no additional benefit beyond fluoroquinolone monotherapy when adjusted for severity of illness or age. This implies that fluoroquinolone monotherapy is adequate, at least in some subpopulations. This is consistent with initial studies that established the superiority of the antibiotic combinations recommended by the guidelines.20, 22, 32
The potential benefit of combination therapy appears limited to patients with higher severity of illness and pneumococcal bacteremia. However, outcomes are affected by the antibiotic regimen selected in the initial 24‐48 hours of hospitalization, before results of blood cultures are routinely available. At present, clinical prediction of patients who will benefit from combination therapy is difficult.
Coverage of undiagnosed mixed infections with atypical organisms is probably not a major factor benefiting patients receiving combination therapy. Several recent meta‐analyses found no reduction in mortality or the rate of clinical failure among patients receiving antibiotics covering atypical organisms compared with those for patients whose regimens did not have such coverage.4547 Subgroups of patients with Legionella pneumonia do benefit from antibiotics with targeted activity against atypical organisms, but fewer than 1% of all patients were so identified. Evidence for antibiotic synergy is similarly lacking.48, 49 The immunomodulatory effects of macrolides, which decrease cytokine production and inflammation and subsequently reduce the severity of lung injury and other complications of sepsis, are considered potential factors in the reduction of mortality.50
The definition of severe CAP and the indications for ICU admission remain controversial, evidence for which is reviewed elsewhere.34, 51, 52 Antibiotic recommendations for ICU patients are included in Table 1 for completeness, but a detailed review of the evidence is lacking because current guidelines are based on consensus opinion.34 The use of fluoroquinolone monotherapy in severe CAP is not currently recommended because of limitations of the existing evidence. The majority of quinolone trials have excluded severely ill patients, and approval trials of newer respiratory fluoroquinolones have used levofloxacin as a comparator. Studies comparing fluoroquinolones typically allowed investigators in the B‐lactam arm the option of adding macrolides or tetracycline at their discretion. In addition, such trials have been designed as noniferiority trials.38 Clearly, randomized controlled trials are needed to resolve this issue.
Currently, selecting appropriate antibiotics should follow established guidelines, with consideration of using combination therapy for patients with a higher severity of illness. Emphasis on this measure should be stronger than that on antibiotic timing, as the bulk of the evidence favors significant mortality reduction from following guidelines for antibiotic therapy.
VACCINATION
Guidelines recommend all eligible adults hospitalized with CAP receive pneumococcal vaccination on discharge,3335, 53 though there is no evidence this reduces the incidence of pneumonia or death.54, 55 Retrospective studies have shown reduced incidence of invasive disease (bacteremia and meningitis), but not of other end points.5457 The estimated mortality from pneumococcal bacteremia remain as high as 20%‐30%, with no evidence that this rate has decreased over the last 30 years.5861 Despite this, a recent meta‐analysis from the Cochrane database that included only randomized, controlled trials (75,197 patients in 15 trials) was unable to show significant reductions in all‐cause pneumonia or mortality for vaccinated subjects.62 Cohort studies, evaluated separately in this analysis, showed an efficacy of 53% in reducing the incidence of invasive pneumococcal disease. Given the relatively low incidence of invasive disease in the general population, the number needed to treat was estimated at 20,000, or 4000 if only older patients were considered. A subsequent retrospective cohort study showed no reduction in pneumonia hospitalizations, cases of outpatient pneumonia, or mortality among 45,365 elderly vaccinees.56 Some specific subgroups may benefit, however. Vaccinated patients with chronic lung disease did show a reduction in hospitalization for pneumonia (RR 0.57 [0.38‐0.84]) and in mortality (RR 0.7 [0.56‐0.9]) in a retrospective study of HMO patients older than age 65.63
It is of interest that since the licensure of the pediatric 7‐valent protein‐polysaccharide conjugate vaccine in 2000, the incidence of invasive pneumococcal disease among adults has dropped significantly. Overall reduction in invasive disease in adults more than 50 years old was 11% from 1998 to 2003 (relative risk reduction [RRR] = 28%). This is likely the result of decreased transmission from colonized or infected children and not a coincidental increase in adult pneumococcal vaccination, as the rates of disease caused by the 16 strains unique to the 23‐valent vaccine did not change.64, 65 The overall reduction in the incidence of invasive disease is still superior with the adult vaccine, up to 30% in vaccinated subjects (RRR = 44%).56 Invasive disease caused specifically by penicillin‐nonsusceptible serotypes has dropped by 49% in the elderly since introduction of the vaccine.66 Thus, the combined impact of the 2 vaccines may be significant. It is not yet clear what effect, if any, the 7‐valent vaccine will have on the hospitalization rate or mortality.
In contrast to the results for pneumococcal vaccination, studies of the benefits of influenza vaccination have shown clear and consistent reductions in mortality, respiratory illness, hospitalization, and pneumonia, especially among patients with comorbidities.6771 Cost effectiveness has been demonstrated for all populations,72, 73 and the reduction in mortality among high‐risk patients younger than age 65 has been estimated to be as high as 78%.68 Among the elderly, reduction in mortality of about 50% has been reported, along with 20%‐30% reductions in hospitalizations for pneumonia, influenza, cardiac disease, and stroke.70 Reduced incidence of pneumonia in vaccinated patients has even been documented among elderly patients without specific comorbidities.67 Annual revaccination has the most significant impact on mortality.74
The pneumococcal vaccine remains important in the effort to reduce the severity of and complications from invasive pneumococcal disease in the elderly, but the lack of significant benefits on hard end points such as mortality or hospitalizations makes it a less robust measure of quality pneumonia care. In contrast, influenza vaccination has a much larger impact on outcomes in the population at risk. Emphasis should be shifted from pneumococcal to influenza vaccine in pneumonia performance measures.
OXYGENATION ASSESSMENT
It seems intuitive that oxygenation assessment is important in the initial evaluation of patients with CAP, though there is not direct evidence to support this. The recommendation for oxygenation assessment in the published guidelines for CAP is by consensus.3335 Documented hypoxemia is associated with increased pneumonia‐related mortality,17, 75 and clinical judgment does not adequately predict hypoxemia.76 Though the assessment of oxygenation has been found to vary widely among practitioners,77 performance has remained consistent since the advent of monitoring and reporting of quality measures, with compliance rates of 99%.4, 7 Monitoring performance of this measure should continue, though high compliance rates limit its ability to discriminate among institutions.
SMOKING CESSATION COUNSELING
Counseling patients to stop smoking was found to be modestly (2%) but statistically significantly effective in promoting abstinence at 1 year.78 In its report on treating tobacco use, the U.S. Public Health Services recommended that all smokers receive hospital‐ and system‐based interventions at every visit.79 As part of the Pneumonia Patient Outcomes Research Team (PORT) study, smokers with pneumonia underwent a tobacco cessation interview. Though only 15% of these patients quit smoking, 93% of those who quit did so at the time they developed CAP.80 A retrospective study of patients with bacteremic pneumococcal pneumonia found tobacco exposure, including passive smoking, to be a strong independent risk factor for invasive disease.81 The most recent CAP guidelines from the Infectious Disease Society of America (IDSA) recommend smoking cessation counseling for all hospitalized patients who smoke.33 However, hospitals are not likely to have the impact that a more comprehensive, outpatient‐based smoking cessation program would. Without ongoing support, counseling, and pharmacotherapy, the effects of an intervention would be expected to be small.79 Though evidence of benefit is limited, smoking cessation interventions should be encouraged at all sites of care. Quality care merits this regardless of admitting diagnosis, but benefits specific to CAP outcomes have not yet been demonstrated.
CONCLUSIONS
The burden of illness caused by CAP mandates that clinicians strive to deliver the highest quality of care to afflicted patients. Critical evaluation of the strength of the evidence will continue to guide such endeavors, and changes in practice will follow as new information surfaces. Standards of care, as adopted by consensus groups such as the IDSA and American Thoracic Society, will continue to inform the practice of hospitalists.
How quality is defined for public reporting requires particularly careful assessment. The definition of quality should be based on evidence more rigorous than that ascribed to consensus guidelines. Within the profession, guidelines offer reasonable standards of care and delineate areas for further research and are invaluable tools for practicing clinicians. In the public arena, however, proclaiming practices as good or bad sets expectations of health care consumers not educated in the nuances of evaluating clinical evidence and can unfairly bias them against conscientious and effective providers whose standards reflect different interpretations of controversial issues. Regulatory agencies should publicly target interventions using only the most solid evidentiary foundation while internally striving to monitor the effects of different practice patterns and report measurable differences in outcomes revealed by careful investigation. Areas where controversy remains should be the primary targets of further research but should not be offered as benchmarks for public scrutiny until the medical community has settled on a position.
Furthermore, when evidence remains questionable, financial incentives should be linked to performance indicators with extreme caution. It would be counterproductive if health care organizations, driven to achieve optimal antibiotic timing to obtain payment updates from CMS, began to administer antibiotics prior to completing workups on all patients with respiratory complaints, as this would likely lead to antibiotic overuse. Similarly, institutions pushed to collect blood cultures before antibiotics are given may inappropriately delay administration in order to perform well on quality measures, resulting in potential harm to patients.
The measures of quality care for CAP for which the evidence on outcomes is the most convincing are antibiotic selection (mortality benefit, reduction in LOS) and influenza vaccination (mortality benefit, reduction in hospitalizations, reduction in respiratory illness). Antibiotic timing also shows a smaller but convincing reduction in mortality, though the advantages of receiving antibiotics within 4 hours instead of 8 hours are not clearly established for younger patients. These measures should be emphasized most heavily in the arena of public reporting and incentives for quality care, with additions and modifications guided by emerging evidence. Revision of the other measures to conform with current evidence would allow public reporting to more accurately reflect quality.
- ,,,.Treatment costs of community‐acquired pneumonia in an employed population.Chest.2004;125:2140–2145.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,,.Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988‐2002.JAMA.2005;294:2712–2719.
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002‐2004.N Engl J Med.2005;353:255–264.
- Performance Measurement: Core Measures. Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/index.htm. Accessed November 7, 2005.
- Medicare Quality Improvement Program Priorities: DRAFT, August2005.Centers for Medicare 353:265–274.
- Medicare demonstration shows hospital quality of care improves with payments tied to quality.Centers for Medicare 278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95(1):78–82.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community‐acquired pneumonia: link between quality of care and resource utilization.Arch Intern Med.2002;162:682–688.
- ,,, et al.Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia.Chest.2003;124:1798–1804.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127:1260–1270.
- Hospital Quality Measures:2004‐2007. Available at: http://www.cms.hhs.gov/quality/hospital/. Accessed November 17, 2005.
- National Hospital Quality Measures Specification Manual version 1.02.Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/aligned_manual.htm.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,,,,.Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours?Chest.2006;130:16–21.
- ,.Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes?Curr Opin Infect Dis.2005;18(2):151–156.
- .The pneumonia controversy: hospitals grapple with 4 hour benchmark.Ann Emerg Med.2006;47:259–261.
- ,,, et al.Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods.Ann Intern Med.2001;134:479–486.
- .The hidden impact of antibacterial resistance in respiratory tract infection. Clinical failures: the tip of the iceberg?Respir Med2001;95Suppl A:S5–11; discussion S26–S27.
- .Antibiotics and pneumonia: is timing everything or just a cause of more problems?Chest.2006;130:1–3.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults.Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,,.Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia.Am J Med.2004;117:726–731.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- .Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia.Curr Opin Infect Dis.2005;18:157–163.
- ,.The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia.Chest.2005;128:940–946.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults.Cochrane Database Syst. Rev.2005:CD004418.
- ,,.Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,.Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro.Antimicrob Agents Chemother.2003;47:1151–1153.
- ,,,,.Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo.J Antimicrob Chemother.2000;46:973–980.
- .The effects of macrolides on inflammatory cells.Chest.2004;125(2 Suppl):41S–50S; quiz 1S.
- ,,, et al.Severe community‐acquired pneumonia. Risk factors and follow‐up epidemiology.Am J Respir Crit Care Med.1999;160:923–929.
- ,,, et al.Severe community‐acquired pneumonia. Assessment of severity criteria.Am J Respir Crit Care Med.1998;158:1102–1108.
- ,,,.Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged 65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services.MMWR Recomm Rep.2005;54(RR‐5):1–11.
- ,,,.The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials.Vaccine.2004;22:3214–3224.
- ,,.Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults.Vaccine.2002;20:2166–2173.
- ,,, et al.Effectiveness of pneumococcal polysaccharide vaccine in older adults.N Engl J Med.2003;348:1747–1755.
- ,,,,,.Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations.JAMA.1993;270:1826–1831.
- ,,,.Early predictors of mortality in pneumococcal bacteraemia.J Infect.2000;40:256–261.
- ,,.Pneumococcal bacteremia in adults: a 14‐year experience in an inner‐city university hospital.Clin Infect Dis.1995;21:345–351.
- ,,,,,.Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995.Commun Dis Public Health.1998;1(1):22–27.
- ,,,,.Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature.Isr J Med Sci.1987;23:174–180.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003:CD000422.
- ,,,.The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease.Arch Intern Med.1999;159:2437–2442.
- ,,, et al.Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.JAMA.2005;294:2043–2051.
- ,,, et al.Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine.N Engl J Med.2003;348:1737–1746.
- ,,, et al.Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae.N Engl J Med.2006;354:1455–1463.
- ,,,,,.Influenza vaccination in community‐dwelling elderly: impact on mortality and influenza‐associated morbidity.Arch Intern Med.2003;163:1089–1094.
- ,,, et al.Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study.Arch Intern Med.2005;165:274–280.
- ,,, et al.Influence of high‐risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed‐care organizations.Clin Infect Dis.2002;35:370–377.
- ,,,,,.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,,,.Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study.Chest.2004;125:2011–2020.
- ,,,,,.Economic analysis of influenza vaccination and antiviral treatment for healthy working adults.Ann Intern Med.2002;137:225–331.
- ,,,,.The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Annual revaccination against influenza and mortality risk in community‐dwelling elderly persons.JAMA.2004;292:2089–2095.
- ,,, et al.Causes of death for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study.Arch Intern Med.2002;162:1059–1064.
- ,,.Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department.Ann Emerg Med.1995;25(1):36–40.
- ,,, et al.Arterial blood gas and pulse oximetry in initial management of patients with community‐acquired pneumonia.J Gen Intern Med.2001;16:590–598.
- ,.An analysis of the effectiveness of interventions intended to help people stop smoking.Arch Intern Med.1995;155:1933–1941.
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.JAMA.2000;283:3244–3254.
- .Quality indicators for the management of pneumonia in vulnerable elders.Ann Intern Med.2001;135:736–743.
- ,,, et al.Cigarette smoking and invasive pneumococcal disease.Active Bacterial Core Surveillance Team.N Engl J Med.2000;342:681–689.
- ,,,.Treatment costs of community‐acquired pneumonia in an employed population.Chest.2004;125:2140–2145.
- ,,, et al.Pneumonia: still the old man's friend?Arch Intern Med.2003;163:317–323.
- ,,,.Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988‐2002.JAMA.2005;294:2712–2719.
- ,,,,.Quality of care in U.S. hospitals as reflected by standardized measures, 2002‐2004.N Engl J Med.2005;353:255–264.
- Performance Measurement: Core Measures. Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/index.htm. Accessed November 7, 2005.
- Medicare Quality Improvement Program Priorities: DRAFT, August2005.Centers for Medicare 353:265–274.
- Medicare demonstration shows hospital quality of care improves with payments tied to quality.Centers for Medicare 278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community‐acquired pneumonia: a prospective observational study.Chest.2003;123:1142–1150.
- ,,,,.Clinical utility of blood cultures in adult patients with community‐acquired pneumonia without defined underlying risks.Chest.1995;108:932–936.
- ,,,.Limited usefulness of initial blood cultures in community acquired pneumonia.Emerg Med J.2004;21:446–448.
- ,,.The impact of blood cultures on antibiotic therapy in pneumococcal pneumonia.Chest.1999;116:1278–1281.
- ,.The influence of the severity of community‐acquired pneumonia on the usefulness of blood cultures.Respir Med.2001;95(1):78–82.
- ,,, et al.A prediction rule to identify low‐risk patients with community‐acquired pneumonia.N Engl J Med.1997;336:243–250.
- ,,,.Predicting bacteremia in patients with community‐acquired pneumonia.Am J Respir Crit Care Med.2004;169:342–347.
- ,,,,.Nonvalue of the initial microbiological studies in the management of nonsevere community‐acquired pneumonia.Chest.2001;119:181–184.
- ,,.Rapid antibiotic delivery and appropriate antibiotic selection reduce length of hospital stay of patients with community‐acquired pneumonia: link between quality of care and resource utilization.Arch Intern Med.2002;162:682–688.
- ,,, et al.Early administration of antibiotics does not shorten time to clinical stability in patients with moderate‐to‐severe community‐acquired pneumonia.Chest.2003;124:1798–1804.
- ,.Factors influencing in‐hospital mortality in community‐acquired pneumonia: a prospective study of patients not initially admitted to the ICU.Chest.2005;127:1260–1270.
- Hospital Quality Measures:2004‐2007. Available at: http://www.cms.hhs.gov/quality/hospital/. Accessed November 17, 2005.
- National Hospital Quality Measures Specification Manual version 1.02.Joint Commission on Accreditation of Healthcare Organizations,2005. Available at: http://www.jcaho.org/pms/core+measures/aligned_manual.htm.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,,,,.Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours?Chest.2006;130:16–21.
- ,.Administration of first hospital antibiotics for community‐acquired pneumonia: does timeliness affect outcomes?Curr Opin Infect Dis.2005;18(2):151–156.
- .The pneumonia controversy: hospitals grapple with 4 hour benchmark.Ann Emerg Med.2006;47:259–261.
- ,,, et al.Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims, and methods.Ann Intern Med.2001;134:479–486.
- .The hidden impact of antibacterial resistance in respiratory tract infection. Clinical failures: the tip of the iceberg?Respir Med2001;95Suppl A:S5–11; discussion S26–S27.
- .Antibiotics and pneumonia: is timing everything or just a cause of more problems?Chest.2006;130:1–3.
- ,,,,.Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia.Arch Intern Med.1999;159:2562–2572.
- ,,,,,.Update of practice guidelines for the management of community‐acquired pneumonia in immunocompetent adults.Clin Infect Dis.2003;37:1405–1433.
- ,,, et al.Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention.Am J Respir Crit Care Med.2001;163:1730–1754.
- ,,,,,.Practice guidelines for the management of community‐acquired pneumonia in adults.Infectious Diseases Society of America.Clin Infect Dis.2000;31:347–382.
- ,,,.Effects of guideline‐concordant antimicrobial therapy on mortality among patients with community‐acquired pneumonia.Am J Med.2004;117:726–731.
- ,,,,.Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community‐acquired pneumonia.J Hosp Med.2006;1:7–12.
- .Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia.Curr Opin Infect Dis.2005;18:157–163.
- ,.The controversy of combination vs monotherapy in the treatment of hospitalized community‐acquired pneumonia.Chest.2005;128:940–946.
- ,,.Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia.Arch Intern Med.2001;161:1837–1842.
- ,,, et al.Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia.Am J Respir Crit Care Med.2004;170:440–444.
- ,,, et al.Addition of a macrolide to a beta‐lactam‐based empirical antibiotic regimen is associated with lower in‐hospital mortality for patients with bacteremic pneumococcal pneumonia.Clin Infect Dis.2003;36:389–395.
- ,,, et al.Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults.Can Respir J.2004;11:589–593.
- ,,,.Impact of initial antibiotic choice on clinical outcomes in community‐acquired pneumonia: analysis of a hospital claims‐made database.Chest.2003;123:1503–1511.
- ,,,.Empirical atypical coverage for inpatients with community‐acquired pneumonia: systematic review of randomized controlled trials.Arch Intern Med.2005;165:1992–2000.
- ,,,.Empiric antibiotic coverage of atypical pathogens for community acquired pneumonia in hospitalized adults.Cochrane Database Syst. Rev.2005:CD004418.
- ,,.Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non‐severe community acquired pneumonia: meta‐analysis.Br Med J.2005;330:456.
- ,,.Lack of synergy of erythromycin combined with penicillin or cefotaxime against Streptococcus pneumoniae in vitro.Antimicrob Agents Chemother.2003;47:1151–1153.
- ,,,,.Antagonism between penicillin and erythromycin against Streptococcus pneumoniae in vitro and in vivo.J Antimicrob Chemother.2000;46:973–980.
- .The effects of macrolides on inflammatory cells.Chest.2004;125(2 Suppl):41S–50S; quiz 1S.
- ,,, et al.Severe community‐acquired pneumonia. Risk factors and follow‐up epidemiology.Am J Respir Crit Care Med.1999;160:923–929.
- ,,, et al.Severe community‐acquired pneumonia. Assessment of severity criteria.Am J Respir Crit Care Med.1998;158:1102–1108.
- ,,,.Improving influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among adults aged 65 years at high risk: a report on recommendations of the Task Force on Community Preventive Services.MMWR Recomm Rep.2005;54(RR‐5):1–11.
- ,,,.The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials.Vaccine.2004;22:3214–3224.
- ,,.Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults.Vaccine.2002;20:2166–2173.
- ,,, et al.Effectiveness of pneumococcal polysaccharide vaccine in older adults.N Engl J Med.2003;348:1747–1755.
- ,,,,,.Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations.JAMA.1993;270:1826–1831.
- ,,,.Early predictors of mortality in pneumococcal bacteraemia.J Infect.2000;40:256–261.
- ,,.Pneumococcal bacteremia in adults: a 14‐year experience in an inner‐city university hospital.Clin Infect Dis.1995;21:345–351.
- ,,,,,.Pneumococcal bacteraemia and meningitis in England and Wales, 1993 to 1995.Commun Dis Public Health.1998;1(1):22–27.
- ,,,,.Pneumococcal bacteremia—no change in mortality in 30 years: analysis of 104 cases and review of the literature.Isr J Med Sci.1987;23:174–180.
- ,,,.Vaccines for preventing pneumococcal infection in adults.Cochrane Database Syst Rev.2003:CD000422.
- ,,,.The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease.Arch Intern Med.1999;159:2437–2442.
- ,,, et al.Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine.JAMA.2005;294:2043–2051.
- ,,, et al.Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine.N Engl J Med.2003;348:1737–1746.
- ,,, et al.Effect of introduction of the pneumococcal conjugate vaccine on drug‐resistant Streptococcus pneumoniae.N Engl J Med.2006;354:1455–1463.
- ,,,,,.Influenza vaccination in community‐dwelling elderly: impact on mortality and influenza‐associated morbidity.Arch Intern Med.2003;163:1089–1094.
- ,,, et al.Clinical effectiveness of influenza vaccination in persons younger than 65 years with high‐risk medical conditions: the PRISMA study.Arch Intern Med.2005;165:274–280.
- ,,, et al.Influence of high‐risk medical conditions on the effectiveness of influenza vaccination among elderly members of 3 large managed‐care organizations.Clin Infect Dis.2002;35:370–377.
- ,,,,,.Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly.N Engl J Med.2003;348:1322–1332.
- ,,,,,.Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study.Chest.2004;125:2011–2020.
- ,,,,,.Economic analysis of influenza vaccination and antiviral treatment for healthy working adults.Ann Intern Med.2002;137:225–331.
- ,,,,.The efficacy of influenza vaccine in elderly persons. A meta‐analysis and review of the literature.Ann Intern Med.1995;123:518–527.
- ,,, et al.Annual revaccination against influenza and mortality risk in community‐dwelling elderly persons.JAMA.2004;292:2089–2095.
- ,,, et al.Causes of death for patients with community‐acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study.Arch Intern Med.2002;162:1059–1064.
- ,,.Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department.Ann Emerg Med.1995;25(1):36–40.
- ,,, et al.Arterial blood gas and pulse oximetry in initial management of patients with community‐acquired pneumonia.J Gen Intern Med.2001;16:590–598.
- ,.An analysis of the effectiveness of interventions intended to help people stop smoking.Arch Intern Med.1995;155:1933–1941.
- A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.JAMA.2000;283:3244–3254.
- .Quality indicators for the management of pneumonia in vulnerable elders.Ann Intern Med.2001;135:736–743.
- ,,, et al.Cigarette smoking and invasive pneumococcal disease.Active Bacterial Core Surveillance Team.N Engl J Med.2000;342:681–689.