User login
How best to manage chronic cholestasis
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.
That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)
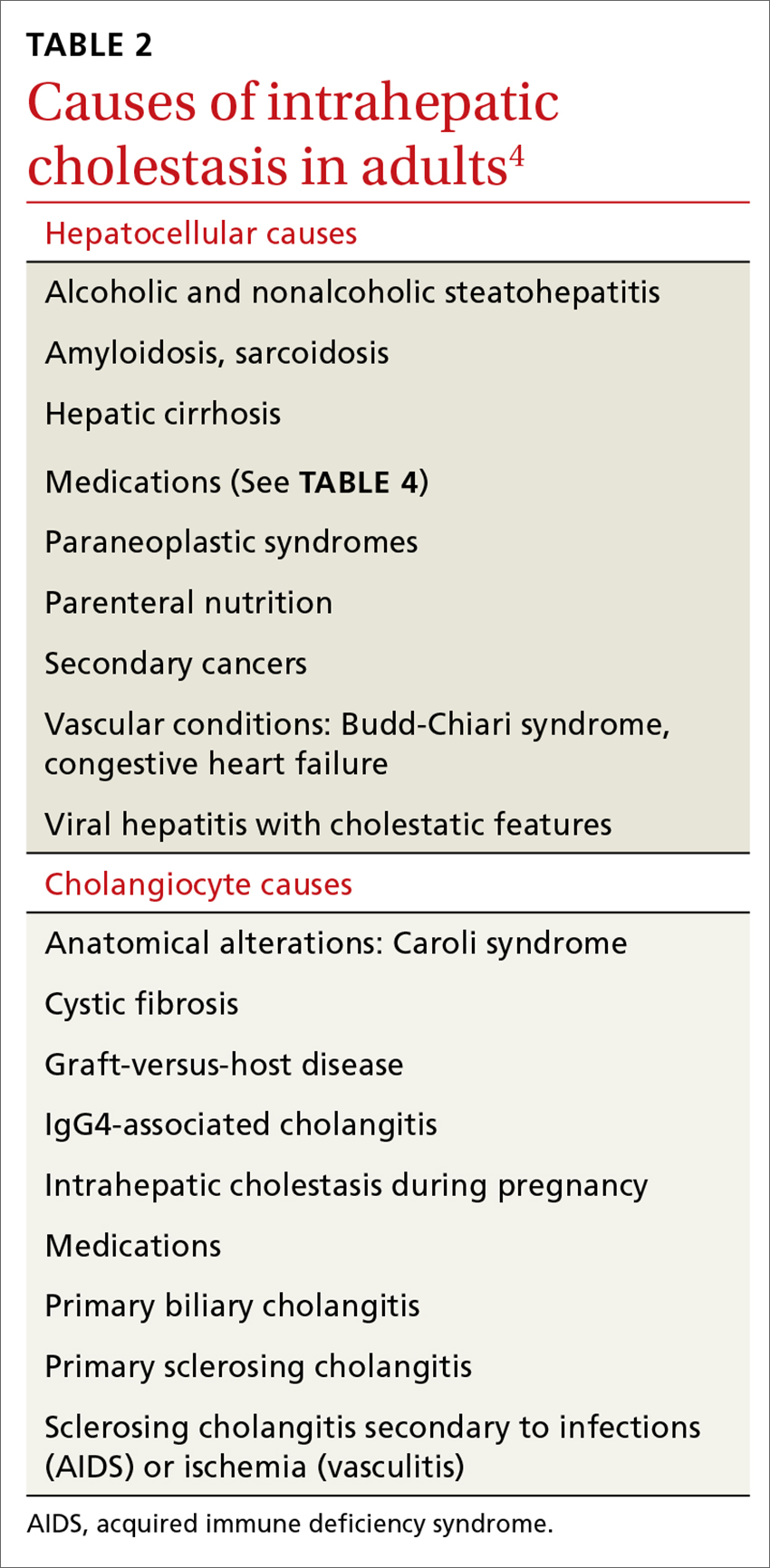
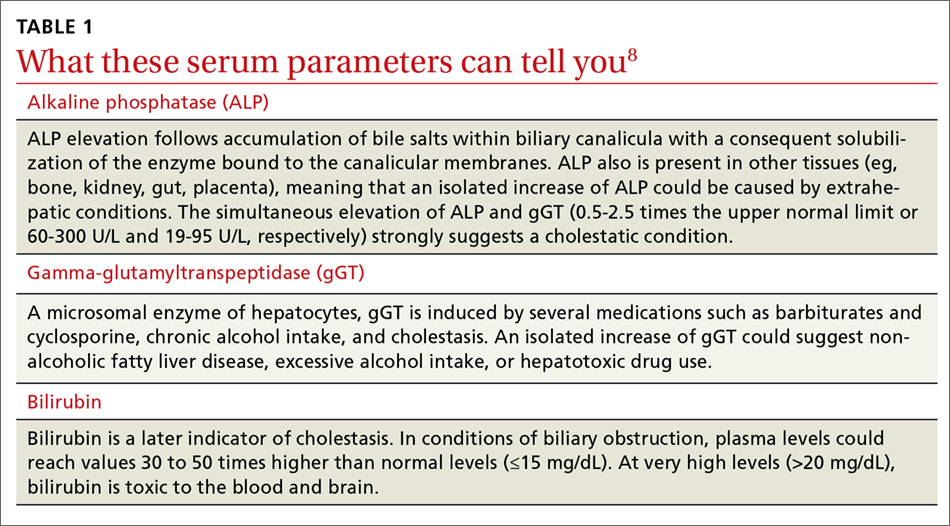
Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
From The Journal of Family Practice | 2018;67(7):E9-E15.
PRACTICE RECOMMENDATIONS
› Suspect intrahepatic cholestasis in a patient with pruritus, normal transaminases, and mildly elevated gamma glutamyl-transpeptidase and alkaline phosphatase levels. A
› Use ultrasonography as a first-line diagnostic tool for cholestasis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Avoiding drug interactions: Here’s help
Be sure to inquire about over-the-counter drugs, herbal remedies, vitamins, and supplements when taking a medication history. A
Use an electronic prescribing software system that flags potential drug-drug interactions. A
Consider adjusting a dosing regimen or temporarily discontinuing a maintenance medication if the drug you are about to prescribe is likely to interact with another agent the patient is taking (and there are no alternatives you can prescribe). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE John L, a 63-year-old man taking lovastatin (40 mg/d) and ramipril (5 mg/d) for hypercholesterolemia and arterial hypertension was hospitalized with atrial fibrillation. Three days later, he was discharged, with a prescription for amiodarone (200 mg/d). After a month, he was readmitted to the hospital with dark urine and intensifying thigh weakness and achiness. Laboratory testing revealed aspartate aminotransferase and alanine aminotransferase levels 10 times the upper limit of normal, and elevated urine and serum myoglobin.
Drug-drug interactions (DDIs) like the one John experienced between lovastatin and amiodarone are a common cause of readmissions, as well as emergency department visits and hospitalizations, for everything from myopathy to electrolyte imbalance, gastrointestinal (GI) bleeding, hepatotoxicity, renal dysfunction, and changes in blood pressure and heart rate.1-3
Yet many, if not most, DDIs can be avoided. With diligence and the right tools, you can do much to reduce the incidence of such interactions and adverse outcomes.
Polypharmacy and age pose the highest risks
The more medications a patient is taking, of course, the greater the likelihood of a clinically significant DDI. According to 1 study, 13% of patients taking 2 drugs develop a DDI; the incidence approaches 40% for patients taking 5 drugs, and exceeds 80% for patients taking 7 or more medications.4
In addition to polypharmacy, age alone is a key risk factor for DDIs.5 Pharmacokinetics and pharmacodynamics are frequently altered in older people, who may have slower intestinal transit time; diminished absorption capacity; decreased liver metabolism, mitochondrial function, and renal excretion; and alterations in volemia and body fat distribution.6 Although the speed at which these changes occur varies, aging is associated with a progressive deficiency in the regulation of most homeo-static mechanisms and an altered response to receptor stimulation.7
Whether age, multiple medications, or both are to blame, the impact on the elderly is striking. One recent retrospective study found 25% of elderly outpatients to be at risk for DDIs.8
Very young patients (<5 years) are also at risk for DDIs because of the immaturity of their enzymatic metabolic system.5,9,10 Additional risk factors, detailed in TABLE 1, include the presence of an infection or other acute medical condition, a metabolic or endocrine disorder, and taking 1 or more drugs with a narrow therapeutic range. Women are also at higher risk for DDIs than their male counterparts, the result of a slower metabolic capacity and interference with sex hormones.3-5,8,9 Pharmacogenetics may also play a big part in DDIs, and more and more studies are focusing on identifying patients at greatest risk.
TABLE 1
Risk factors for drug-drug interactions3-5,8,9
| Risk factor | Potential result |
|---|---|
| Acute medical condition (eg, dehydration, infection, alcoholism) | Augmented risk of elevated plasma drug concentration, increased catabolism, inhibition of hepatic drug metabolism |
| Age (very young [<5 years] and elderly) | Reduced metabolic capacity (greater accumulation of drugs) |
| Decreased renal and/or hepatic function | Decreased drug clearance/elimination; greater accumulation of drugs or their metabolites |
| Drug(s) with narrow therapeutic range | Increased risk for dose-related side effects |
| Female sex | Reduced metabolic capacity, interference with sex hormones |
| Metabolic or endocrine conditions (eg, fatty liver, obesity, hypothyroidism) | Altered hepatic metabolism, increased body distribution volumes, augmented risk of accumulation for hydrophobic molecules |
| Polypharmacy (≥3 medications) | Increased risk of metabolic and/or pharmacodynamic interference |
| Pharmacogenetics | Altered metabolic capacity (greater accumulation of drugs or their metabolites) |
Lack of coordinated care also increases risk
Another risk factor involves the use of multiple providers.9 A woman may be treated by—and receive prescriptions from—an endocrinolo-gist, a gynecologist, and a family physician (FP), for instance, and get medications from a local pharmacy, a nationwide discount chain, and a mail order pharmacy. As with the number of medications being taken, the greater the number of health care professionals a patient sees, the higher the risk.
To mitigate the risk, encourage your patients to fill all their prescriptions at the same pharmacy—and for your part, take a complete medication history before writing a new prescription.
Medication history in doubt? Schedule a “brown bag review”
Ask patients to provide the name and dose of every medication they’re taking. Inquire specifically about over-the-counter (OTC) cough and cold remedies and complementary and alternative medicines, including herbal remedies, vitamins, and supplements. Patients often neglect to mention nonprescription remedies and may not even think of them as medicine, but OTC products with the potential to interact adversely with prescription drugs may otherwise remain undetected.11-13
Consider a “brown bag review” for patients who don’t know what dosage they’re taking or have difficulty identifying the drugs other physicians have prescribed. Ask them to put all their medications in a brown bag and bring them in on their next visit.14,15
Steps to take to reduce risk
Software systems. A number of free and low-cost software systems identify potential DDIs (See “Check for drug interactions: Software programs to consider”). While such electronic programs can indeed lower the risk,16-18 they cannot be counted on to detect or avert every possible adverse interaction.
The downside. One problem is that some software programs fail to distinguish between clinically significant and nonsignificant interactions, causing some prescribers to override system alerts—and possibly miss an important warning.19 Another problem: While most systems do an excellent job of checking to see whether 2 drugs can be safely taken together, few are capable of checking for all potential interactions among multiple medications. What’s more, many drugs have not been evaluated for their potential to interact with other agents, so the absence of reported interactions is no guarantee of a lack of DDIs.
Other strategies to consider:
Minimize the number of prescriptions. While it may not be possible to avoid prescribing a new agent for a patient who is already taking multiple medications, limiting the number of new drugs to those that are absolutely essential will help to minimize DDIs. Whenever possible, select a compound with the desired effects. Prescribe a single agent with antihypertensive as well as uricosuric effects for a patient with elevated blood pressure and uric acid levels rather than 2 different drugs (eg, losartan instead of an anti-hypertensive agent plus allopurinol).
Alter the dosing regimen. Several active molecules may cause DDIs by interfering with intestinal absorption or GI transit time if they’re taken closely together. For example, a quinolone should not be administered at the same time as a cation because of possible chelation in the GI tract. If a patient needs both, however, you may be able to avert a DDI by advising the patient to take them at least 2 hours apart.20 Another possibility is to temporarily discontinue a maintenance medication if it has the potential to interact adversely with a drug that is needed for only a short duration.
Choose a different drug (or drug class). Some drug classes should never be mixed—nitrates and phosphodiesterase type-5 inhibitors, taken together, greatly increase the risk of vasodilation and may result in severe hypotension, for example. There are also drug classes with a low potential for DDIs, including cholinesterase inhibitors and anti-hypertensives (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and thiazides).21
Frequently, though, medications within the same drug class do not share the same potential for DDIs. In such cases, an adverse outcome can often be averted by being aware of combinations likely to result in clinically significant DDIs (TABLE 2) and, whenever possible, prescribing another agent. If a patient taking carbamazepine needs a macro-lide antibiotic, for instance, azithromycin is a better choice than erythromycin. That’s because erythromycin inhibits the hepatic metabolism of the anticonvulsant, increasing the serum level of carbamazepine, while azithromycin does not interfere with carbamazepine metabolism.22
TABLE 2
Clinically significant drug-drug interactions21,22
| Combination (effect) |
|---|
| Allopurinol and captopril (augments allopurinol’s effect) |
| Antidepressants (SSRIs, MAOIs) and antiepileptics (augments antidepressant effect) |
| Clopidogrel and omeprazole or esomeprazole (reduces clopidogrel’s effect) |
| Erythromycin and carbamazepine (augments carbamazepine’s effect) |
| Erythromycin and terfenadine (augments terfenadine’s effect) |
| Ketoconazole and PPIs (reduces ketoconazole’s absorption) |
| Levodopa and metoclopramide (augments levodopa’s effect) |
| MAOIs and narcotic analgesics (augments effects of both drugs) |
| Nitrates and phosphodiesterase type-5 inhibitors (augments effects of both drugs) |
| OCs and penicillins, phenobarbital, or tetracycline (reduces OCs’ effect) |
| Phenobarbital and simvastatin* (reduces simvastatin’s effect) |
| Quinolone and cation (reduces quinolone’s absorption and effect) |
| Repaglinide and diltiazem (augments repaglinide’s effect) |
| Simvastatin* or lovastatin and amiodarone or itraconazole (augments statin’s effect) |
| Theophylline and cimetidine or ciprofloxacin (augments theophylline’s effect) |
| MAOIs, monoamine oxidase inhibitors; OCs, oral contraceptives; PPIs, proton pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. |
| *For a complete list of drugs that may interact with simvastatin, see US Food and Drug Administration.28 |
FREE
- Epocrates Rx
http://www.epocrates.com/products/rx/ - eRx (National ePrescribing Patient Safety Initiative)
http://www.nationalerx.com/
FEE-BASED
- GeneMedRx (Drug-drug and drug-gene interactions)
http://www.genemedrx.com/provider-info.php
$199/year - iFacts (Drug Interaction Facts)
http://www.skyscape.com/EStore/ProductDetail.aspx?ProductID=217
$59.95/year - PEPID Portable Drug Companion
http://www.pepid.com/products/pdc/
$89.95/year
How is the drug metabolized?
DDIs may occur as a result of pharmaco-dynamic interaction (when 2 drugs act on the same receptor, site of action, or physiologic system) or pharmacokinetic changes (interference with absorption, albumin binding, distribution, metabolism, or elimination).23 As already noted, age-related changes in pharmacokinetics and pharmacodynamics contribute to the high prevalence of DDIs in elderly patients.
In the liver, drug metabolism, particularly via the cytochrome P450 (CYP450) system, is the cornerstone of drug transformation.23 Al-though the CYP system consists of “superfami-lies” with more than 100 types of enzymes, only a few are responsible for the majority of biotransformation.23 The CYP system is also subject to genetic polymorphism, making some patients especially prone to DDIs.
P-glycoproteins (PGPs), which regulate drug absorption by transporting the drugs across cell membranes, also play a key role. PGP inhibitors or inducers help determine whether the accumulation of the molecule or the increased delivery of toxic metabolites leads to adverse effects.10
Reviewing the mechanism of action of any drug you prescribe for a patient taking other medications may alert you to a potential DDI—and the need to either switch the newly prescribed agent or alter the individual’s drug regimen in some other way.
CASE When John was readmitted to the hospital, he was taken off both the lovastatin and amiodarone and hydrated with forced alkaline diuresis. After a week, his symptoms resolved, and he was discharged soon after. His blood tests normalized 1 month later. The severe DDI he experienced occurred because lovastatin (which is metabolized primarily by CYP3A4) and amiodarone (a CYP3A4 inhibitor) were taken together. (Statins that are substrates of CYP3A4 have the greatest potential for interacting with drugs known to inhibit the CYP450 system [eg, cyclosporine, morphine derivatives, ketoconazole, and amiodarone].)
This adverse interaction could have been avoided if the physician who started John on amiodarone had been aware of the potential DDI—and switched him to an HMG-CoA inhibitor other than lovastatin. Pravastatin, which is not metabolized via CYP450, would have been an excellent choice.
Warfarin warrants special attention
Medications that have a particularly high potential for adverse interactions require special attention and patient monitoring, warfarin foremost among them. Warfarin metabolism and its anticoagulant effects can be dramatically changed if it is administered with a drug with a higher affinity for PGPs or an agent that competes with it within the CYP450 system.24 Because of warfarin’s narrow therapeutic range, there are many drugs and drug classes that patients on warfarin should avoid (TABLE 3)—a fact that patients as well as their physicians need to be aware of.24 Indeed, warfarin is often involved in drug-related hospital admissions for DDIs, especially in elderly patients and in those who are also taking nonsteroidal anti-inflamma-tory drugs (NSAIDs) or macrolides—2 of the many drug classes that patients taking warfarin should avoid.24
TABLE 3
Patient on warfarin? Steer clear of these drugs10,20,23
| Drug class: agent(s) |
|---|
| Antiarrhythmics: amiodarone, propafenone |
| Antibiotics: ciprofloxacin, metronidazole, rifampin, trimethoprim/sulfamethoxazole |
| Anticonvulsants: carbamazepine, valproate |
| Antidepressants: fluoxetine, fluvoxamine, paroxetine, sertraline, trazodone |
| Antidiabetics: chlorpropamide |
| Antifungals: danazol, fluconazole, itraconazole, miconazole |
| Antimalarial agents: quinidine |
| Antineoplastics: azathioprine, fluorouracil, flutamide, ifosfamide, tamoxifen |
| Antiplatelet agents: ticlopidine |
| Antipsychotics: clozapine |
| Diuretics: spironolactone |
| GI drugs: cimetidine, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, ranitidine |
| Gout treatment: allopurinol |
| Hypolipidemics: atorvastatin, cholestyramine, ezetimibe, fenofibrate, fluvastatin, gemfibrozil, lovastatin, pravastatin, simvastatin |
| NSAIDs: aspirin, celecoxib, diclofenac, ibuprofen, indomethacin, ketoprofen, ketorolac, naproxen, piroxicam, sulindac |
| Thrombolytics: heparin, tissue plasminogen activator |
| Thyroid drugs: methimazole, propylthiouracil |
| Uricosuric agents: sulfinpyrazone |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Keep an eye on these drug combinations, as well
Among the many combinations likely to result in clinically significant DDIs (TABLE 2), the following are worth mentioning:
Clopidogrel + certain proton pump inhibitors. The addition of a PPI to clopidogrel has been associated with a significant increase of recurrent infarction.25 This may occur because clopidogrel is a prodrug and is converted in the liver to its active form by CYP2C19, an enzyme specifically inhibited by various PPIs—thereby altering the effectiveness of the antiplatelet agent. However, a recent analysis suggests that there is no need to avoid the concomitant use of a PPI and clopidogrel—and that the interference appears to be limited to omeprazole and esomeprazole.26
Oral contraceptives (OCs) + penicillins, phenobarbital, or tetracycline. Each of these drugs reduces the effect of OCs, and women who are taking them concomitantly need to be advised to use another means of contraception.
Phenobarbital + simvastatin. Pheno-barbital (a CYP3A4 inducer) may reduce the efficacy of simvastatin.
Repaglinide + diltiazem. Diltiazem inhibits the metabolism of repaglinide (a CYP3A4 substrate), thus increasing the risk of hypoglycemia.
Simvastatin + amiodarone or itraconazole. Either of these antiarrhythmic agents decreases simvastatin metabolism, raising the risk of myopathy; with amiodarone, however, the likelihood of an adverse outcome is especially high. In 2008, the US Food and Drug Administration (FDA) issued a warning to healthcare professionals of the increased risk for rhabdomyolysis when simvastatin doses greater than 20 mg are administered together with amiodarone.27 The agency issued a safety review of simvastatin, warning of its potential for DDIs with amiodarone and numerous other medications, earlier this year.28
As John’s case illustrates, use of lovastatin with amiodarone should be avoided, as well.
Keep others safe: Report adverse events
When a DDI occurs despite your best efforts, you can help ensure that other patients do not experience the same adverse outcome by reporting it to MedWatch, the FDA’s voluntary safety information and adverse event reporting program. Go to https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm to file a report online.
CORRESPONDENCE Ignazio Grattagliano, MD, General Medicine, Department of Internal and Public Medicine, University of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
1. Becker ML, Kallewaard M, Caspers PW, et al. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641-651.
2. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
3. Tulner LR, Frankfort SV, Gijsen GJ, et al. Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging. 2008;25:343-355.
4. Cadieux RJ. Drug interactions in the elderly. How multiple drug use increases risk exponentially. Postgrad Med. 1989;86:179-186.
5. Shapiro LE, Shear NH. Drug interactions: proteins, pumps, and P-450s. J Am Acad Dermatol. 2002;47:467-484.
6. Sitar DS. Aging issues in drug disposition and efficacy. Proc West Pharmacol Soc. 2007;50:16-20.
7. El Desoky ES, Derendorf H, Klotz U. Variability in response to cardiovascular drugs. Curr Clin Pharmacol. 2006;1:35-46.
8. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
9. Becker ML, Kallewaard M, Caspers PW, et al. Potential determinants of drug-drug interaction associated dispensing in community pharmacies. Drug Saf. 2005;28:371-378.
10. Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47:3-10.
11. Buurma H, Bouvy ML, De Smet PA, et al. Prevalence and determinants of pharmacy shopping behaviour. J Clin Pharm Ther. 2008;33:17-23.
12. Russmann S, Barguil Y, Cabalion P, et al. Hepatic injury due to traditional aqueous extracts of kava root in New Caledonia. Eur J Gastroenterol Hepatol. 2003;5:1033-1036.
13. Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901-910.
14. Institute for Safe Medication Practices. ISMP medication safety alert! Available at: http://www.ismp.org/Newsletters/consumer/alerts/BrownBag.asp. Accessed May 5, 2010.
15. National Institute on Aging. Obtaining the medical history. Available at: http://www.nia.nih.gov/Healthinformation/Publications/ClinicianHB/03_history.htm. Accessed May 5, 2010.
16. Glassman PA, Simon B, Belperio P, et al. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40:1161-1171.
17. Goldberg RM, Mabee J, Chan L, et al. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14:447-450.
18. Lapane KL, Waring ME, Schneider KL, et al. A mixed method study of the merits of e-prescribing drug alerts in primary care. J Gen Intern Med. 2008;23:442-446.
19. Magnus D, Rodgers S, Avery AJ. GPs’ views on computerized drug interaction alerts: questionnaire survey. J Clin Pharm Ther. 2002;27:377-382.
20. Shukla UA, Pittman KA, Barbhaiya RH. Pharmacokinetic interactions of cefprozil with food, propantheline, metoclopramide, and probenecid in healthy volunteers. J Clin Pharmacol. 1992;32:725-731.
21. Levy RH, Collins C. Risk and predictability of drug interactions in the elderly. Int Rev Neurobiol. 2007;81:235-251.
22. Pauwels O. Factors contributing to carbamazepine-macrolide interactions. Pharmacol Res. 2002;45:291-298.
23. Beaird SL. HMG-CoA reductase inhibitors: assessing differences in drug interactions and safety profiles. J Am Pharm Assoc (Wash). 2000;40:637-644.
24. Snaith A, Pugh L, Simpson CR, et al. The potential for interaction between warfarin and coprescribed medication: a retrospective study in primary care. Am J Cardiovasc Drugs. 2008;8:207-212.
25. Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713-718.
26. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmaco-dynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
27. US Food and Drug Administration. Serious muscle injury with simvastatin/amiodarone combination. November 2008. Available at: http://www.accessdata.fda.gov/psn/printer.cfm?id=886. Accessed May 5, 2010.
28. US Food and Drug Administration. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. March 19, 2010. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
ucm204882.htm. Accessed May 5, 2010.
Be sure to inquire about over-the-counter drugs, herbal remedies, vitamins, and supplements when taking a medication history. A
Use an electronic prescribing software system that flags potential drug-drug interactions. A
Consider adjusting a dosing regimen or temporarily discontinuing a maintenance medication if the drug you are about to prescribe is likely to interact with another agent the patient is taking (and there are no alternatives you can prescribe). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE John L, a 63-year-old man taking lovastatin (40 mg/d) and ramipril (5 mg/d) for hypercholesterolemia and arterial hypertension was hospitalized with atrial fibrillation. Three days later, he was discharged, with a prescription for amiodarone (200 mg/d). After a month, he was readmitted to the hospital with dark urine and intensifying thigh weakness and achiness. Laboratory testing revealed aspartate aminotransferase and alanine aminotransferase levels 10 times the upper limit of normal, and elevated urine and serum myoglobin.
Drug-drug interactions (DDIs) like the one John experienced between lovastatin and amiodarone are a common cause of readmissions, as well as emergency department visits and hospitalizations, for everything from myopathy to electrolyte imbalance, gastrointestinal (GI) bleeding, hepatotoxicity, renal dysfunction, and changes in blood pressure and heart rate.1-3
Yet many, if not most, DDIs can be avoided. With diligence and the right tools, you can do much to reduce the incidence of such interactions and adverse outcomes.
Polypharmacy and age pose the highest risks
The more medications a patient is taking, of course, the greater the likelihood of a clinically significant DDI. According to 1 study, 13% of patients taking 2 drugs develop a DDI; the incidence approaches 40% for patients taking 5 drugs, and exceeds 80% for patients taking 7 or more medications.4
In addition to polypharmacy, age alone is a key risk factor for DDIs.5 Pharmacokinetics and pharmacodynamics are frequently altered in older people, who may have slower intestinal transit time; diminished absorption capacity; decreased liver metabolism, mitochondrial function, and renal excretion; and alterations in volemia and body fat distribution.6 Although the speed at which these changes occur varies, aging is associated with a progressive deficiency in the regulation of most homeo-static mechanisms and an altered response to receptor stimulation.7
Whether age, multiple medications, or both are to blame, the impact on the elderly is striking. One recent retrospective study found 25% of elderly outpatients to be at risk for DDIs.8
Very young patients (<5 years) are also at risk for DDIs because of the immaturity of their enzymatic metabolic system.5,9,10 Additional risk factors, detailed in TABLE 1, include the presence of an infection or other acute medical condition, a metabolic or endocrine disorder, and taking 1 or more drugs with a narrow therapeutic range. Women are also at higher risk for DDIs than their male counterparts, the result of a slower metabolic capacity and interference with sex hormones.3-5,8,9 Pharmacogenetics may also play a big part in DDIs, and more and more studies are focusing on identifying patients at greatest risk.
TABLE 1
Risk factors for drug-drug interactions3-5,8,9
| Risk factor | Potential result |
|---|---|
| Acute medical condition (eg, dehydration, infection, alcoholism) | Augmented risk of elevated plasma drug concentration, increased catabolism, inhibition of hepatic drug metabolism |
| Age (very young [<5 years] and elderly) | Reduced metabolic capacity (greater accumulation of drugs) |
| Decreased renal and/or hepatic function | Decreased drug clearance/elimination; greater accumulation of drugs or their metabolites |
| Drug(s) with narrow therapeutic range | Increased risk for dose-related side effects |
| Female sex | Reduced metabolic capacity, interference with sex hormones |
| Metabolic or endocrine conditions (eg, fatty liver, obesity, hypothyroidism) | Altered hepatic metabolism, increased body distribution volumes, augmented risk of accumulation for hydrophobic molecules |
| Polypharmacy (≥3 medications) | Increased risk of metabolic and/or pharmacodynamic interference |
| Pharmacogenetics | Altered metabolic capacity (greater accumulation of drugs or their metabolites) |
Lack of coordinated care also increases risk
Another risk factor involves the use of multiple providers.9 A woman may be treated by—and receive prescriptions from—an endocrinolo-gist, a gynecologist, and a family physician (FP), for instance, and get medications from a local pharmacy, a nationwide discount chain, and a mail order pharmacy. As with the number of medications being taken, the greater the number of health care professionals a patient sees, the higher the risk.
To mitigate the risk, encourage your patients to fill all their prescriptions at the same pharmacy—and for your part, take a complete medication history before writing a new prescription.
Medication history in doubt? Schedule a “brown bag review”
Ask patients to provide the name and dose of every medication they’re taking. Inquire specifically about over-the-counter (OTC) cough and cold remedies and complementary and alternative medicines, including herbal remedies, vitamins, and supplements. Patients often neglect to mention nonprescription remedies and may not even think of them as medicine, but OTC products with the potential to interact adversely with prescription drugs may otherwise remain undetected.11-13
Consider a “brown bag review” for patients who don’t know what dosage they’re taking or have difficulty identifying the drugs other physicians have prescribed. Ask them to put all their medications in a brown bag and bring them in on their next visit.14,15
Steps to take to reduce risk
Software systems. A number of free and low-cost software systems identify potential DDIs (See “Check for drug interactions: Software programs to consider”). While such electronic programs can indeed lower the risk,16-18 they cannot be counted on to detect or avert every possible adverse interaction.
The downside. One problem is that some software programs fail to distinguish between clinically significant and nonsignificant interactions, causing some prescribers to override system alerts—and possibly miss an important warning.19 Another problem: While most systems do an excellent job of checking to see whether 2 drugs can be safely taken together, few are capable of checking for all potential interactions among multiple medications. What’s more, many drugs have not been evaluated for their potential to interact with other agents, so the absence of reported interactions is no guarantee of a lack of DDIs.
Other strategies to consider:
Minimize the number of prescriptions. While it may not be possible to avoid prescribing a new agent for a patient who is already taking multiple medications, limiting the number of new drugs to those that are absolutely essential will help to minimize DDIs. Whenever possible, select a compound with the desired effects. Prescribe a single agent with antihypertensive as well as uricosuric effects for a patient with elevated blood pressure and uric acid levels rather than 2 different drugs (eg, losartan instead of an anti-hypertensive agent plus allopurinol).
Alter the dosing regimen. Several active molecules may cause DDIs by interfering with intestinal absorption or GI transit time if they’re taken closely together. For example, a quinolone should not be administered at the same time as a cation because of possible chelation in the GI tract. If a patient needs both, however, you may be able to avert a DDI by advising the patient to take them at least 2 hours apart.20 Another possibility is to temporarily discontinue a maintenance medication if it has the potential to interact adversely with a drug that is needed for only a short duration.
Choose a different drug (or drug class). Some drug classes should never be mixed—nitrates and phosphodiesterase type-5 inhibitors, taken together, greatly increase the risk of vasodilation and may result in severe hypotension, for example. There are also drug classes with a low potential for DDIs, including cholinesterase inhibitors and anti-hypertensives (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and thiazides).21
Frequently, though, medications within the same drug class do not share the same potential for DDIs. In such cases, an adverse outcome can often be averted by being aware of combinations likely to result in clinically significant DDIs (TABLE 2) and, whenever possible, prescribing another agent. If a patient taking carbamazepine needs a macro-lide antibiotic, for instance, azithromycin is a better choice than erythromycin. That’s because erythromycin inhibits the hepatic metabolism of the anticonvulsant, increasing the serum level of carbamazepine, while azithromycin does not interfere with carbamazepine metabolism.22
TABLE 2
Clinically significant drug-drug interactions21,22
| Combination (effect) |
|---|
| Allopurinol and captopril (augments allopurinol’s effect) |
| Antidepressants (SSRIs, MAOIs) and antiepileptics (augments antidepressant effect) |
| Clopidogrel and omeprazole or esomeprazole (reduces clopidogrel’s effect) |
| Erythromycin and carbamazepine (augments carbamazepine’s effect) |
| Erythromycin and terfenadine (augments terfenadine’s effect) |
| Ketoconazole and PPIs (reduces ketoconazole’s absorption) |
| Levodopa and metoclopramide (augments levodopa’s effect) |
| MAOIs and narcotic analgesics (augments effects of both drugs) |
| Nitrates and phosphodiesterase type-5 inhibitors (augments effects of both drugs) |
| OCs and penicillins, phenobarbital, or tetracycline (reduces OCs’ effect) |
| Phenobarbital and simvastatin* (reduces simvastatin’s effect) |
| Quinolone and cation (reduces quinolone’s absorption and effect) |
| Repaglinide and diltiazem (augments repaglinide’s effect) |
| Simvastatin* or lovastatin and amiodarone or itraconazole (augments statin’s effect) |
| Theophylline and cimetidine or ciprofloxacin (augments theophylline’s effect) |
| MAOIs, monoamine oxidase inhibitors; OCs, oral contraceptives; PPIs, proton pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. |
| *For a complete list of drugs that may interact with simvastatin, see US Food and Drug Administration.28 |
FREE
- Epocrates Rx
http://www.epocrates.com/products/rx/ - eRx (National ePrescribing Patient Safety Initiative)
http://www.nationalerx.com/
FEE-BASED
- GeneMedRx (Drug-drug and drug-gene interactions)
http://www.genemedrx.com/provider-info.php
$199/year - iFacts (Drug Interaction Facts)
http://www.skyscape.com/EStore/ProductDetail.aspx?ProductID=217
$59.95/year - PEPID Portable Drug Companion
http://www.pepid.com/products/pdc/
$89.95/year
How is the drug metabolized?
DDIs may occur as a result of pharmaco-dynamic interaction (when 2 drugs act on the same receptor, site of action, or physiologic system) or pharmacokinetic changes (interference with absorption, albumin binding, distribution, metabolism, or elimination).23 As already noted, age-related changes in pharmacokinetics and pharmacodynamics contribute to the high prevalence of DDIs in elderly patients.
In the liver, drug metabolism, particularly via the cytochrome P450 (CYP450) system, is the cornerstone of drug transformation.23 Al-though the CYP system consists of “superfami-lies” with more than 100 types of enzymes, only a few are responsible for the majority of biotransformation.23 The CYP system is also subject to genetic polymorphism, making some patients especially prone to DDIs.
P-glycoproteins (PGPs), which regulate drug absorption by transporting the drugs across cell membranes, also play a key role. PGP inhibitors or inducers help determine whether the accumulation of the molecule or the increased delivery of toxic metabolites leads to adverse effects.10
Reviewing the mechanism of action of any drug you prescribe for a patient taking other medications may alert you to a potential DDI—and the need to either switch the newly prescribed agent or alter the individual’s drug regimen in some other way.
CASE When John was readmitted to the hospital, he was taken off both the lovastatin and amiodarone and hydrated with forced alkaline diuresis. After a week, his symptoms resolved, and he was discharged soon after. His blood tests normalized 1 month later. The severe DDI he experienced occurred because lovastatin (which is metabolized primarily by CYP3A4) and amiodarone (a CYP3A4 inhibitor) were taken together. (Statins that are substrates of CYP3A4 have the greatest potential for interacting with drugs known to inhibit the CYP450 system [eg, cyclosporine, morphine derivatives, ketoconazole, and amiodarone].)
This adverse interaction could have been avoided if the physician who started John on amiodarone had been aware of the potential DDI—and switched him to an HMG-CoA inhibitor other than lovastatin. Pravastatin, which is not metabolized via CYP450, would have been an excellent choice.
Warfarin warrants special attention
Medications that have a particularly high potential for adverse interactions require special attention and patient monitoring, warfarin foremost among them. Warfarin metabolism and its anticoagulant effects can be dramatically changed if it is administered with a drug with a higher affinity for PGPs or an agent that competes with it within the CYP450 system.24 Because of warfarin’s narrow therapeutic range, there are many drugs and drug classes that patients on warfarin should avoid (TABLE 3)—a fact that patients as well as their physicians need to be aware of.24 Indeed, warfarin is often involved in drug-related hospital admissions for DDIs, especially in elderly patients and in those who are also taking nonsteroidal anti-inflamma-tory drugs (NSAIDs) or macrolides—2 of the many drug classes that patients taking warfarin should avoid.24
TABLE 3
Patient on warfarin? Steer clear of these drugs10,20,23
| Drug class: agent(s) |
|---|
| Antiarrhythmics: amiodarone, propafenone |
| Antibiotics: ciprofloxacin, metronidazole, rifampin, trimethoprim/sulfamethoxazole |
| Anticonvulsants: carbamazepine, valproate |
| Antidepressants: fluoxetine, fluvoxamine, paroxetine, sertraline, trazodone |
| Antidiabetics: chlorpropamide |
| Antifungals: danazol, fluconazole, itraconazole, miconazole |
| Antimalarial agents: quinidine |
| Antineoplastics: azathioprine, fluorouracil, flutamide, ifosfamide, tamoxifen |
| Antiplatelet agents: ticlopidine |
| Antipsychotics: clozapine |
| Diuretics: spironolactone |
| GI drugs: cimetidine, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, ranitidine |
| Gout treatment: allopurinol |
| Hypolipidemics: atorvastatin, cholestyramine, ezetimibe, fenofibrate, fluvastatin, gemfibrozil, lovastatin, pravastatin, simvastatin |
| NSAIDs: aspirin, celecoxib, diclofenac, ibuprofen, indomethacin, ketoprofen, ketorolac, naproxen, piroxicam, sulindac |
| Thrombolytics: heparin, tissue plasminogen activator |
| Thyroid drugs: methimazole, propylthiouracil |
| Uricosuric agents: sulfinpyrazone |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Keep an eye on these drug combinations, as well
Among the many combinations likely to result in clinically significant DDIs (TABLE 2), the following are worth mentioning:
Clopidogrel + certain proton pump inhibitors. The addition of a PPI to clopidogrel has been associated with a significant increase of recurrent infarction.25 This may occur because clopidogrel is a prodrug and is converted in the liver to its active form by CYP2C19, an enzyme specifically inhibited by various PPIs—thereby altering the effectiveness of the antiplatelet agent. However, a recent analysis suggests that there is no need to avoid the concomitant use of a PPI and clopidogrel—and that the interference appears to be limited to omeprazole and esomeprazole.26
Oral contraceptives (OCs) + penicillins, phenobarbital, or tetracycline. Each of these drugs reduces the effect of OCs, and women who are taking them concomitantly need to be advised to use another means of contraception.
Phenobarbital + simvastatin. Pheno-barbital (a CYP3A4 inducer) may reduce the efficacy of simvastatin.
Repaglinide + diltiazem. Diltiazem inhibits the metabolism of repaglinide (a CYP3A4 substrate), thus increasing the risk of hypoglycemia.
Simvastatin + amiodarone or itraconazole. Either of these antiarrhythmic agents decreases simvastatin metabolism, raising the risk of myopathy; with amiodarone, however, the likelihood of an adverse outcome is especially high. In 2008, the US Food and Drug Administration (FDA) issued a warning to healthcare professionals of the increased risk for rhabdomyolysis when simvastatin doses greater than 20 mg are administered together with amiodarone.27 The agency issued a safety review of simvastatin, warning of its potential for DDIs with amiodarone and numerous other medications, earlier this year.28
As John’s case illustrates, use of lovastatin with amiodarone should be avoided, as well.
Keep others safe: Report adverse events
When a DDI occurs despite your best efforts, you can help ensure that other patients do not experience the same adverse outcome by reporting it to MedWatch, the FDA’s voluntary safety information and adverse event reporting program. Go to https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm to file a report online.
CORRESPONDENCE Ignazio Grattagliano, MD, General Medicine, Department of Internal and Public Medicine, University of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
Be sure to inquire about over-the-counter drugs, herbal remedies, vitamins, and supplements when taking a medication history. A
Use an electronic prescribing software system that flags potential drug-drug interactions. A
Consider adjusting a dosing regimen or temporarily discontinuing a maintenance medication if the drug you are about to prescribe is likely to interact with another agent the patient is taking (and there are no alternatives you can prescribe). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE John L, a 63-year-old man taking lovastatin (40 mg/d) and ramipril (5 mg/d) for hypercholesterolemia and arterial hypertension was hospitalized with atrial fibrillation. Three days later, he was discharged, with a prescription for amiodarone (200 mg/d). After a month, he was readmitted to the hospital with dark urine and intensifying thigh weakness and achiness. Laboratory testing revealed aspartate aminotransferase and alanine aminotransferase levels 10 times the upper limit of normal, and elevated urine and serum myoglobin.
Drug-drug interactions (DDIs) like the one John experienced between lovastatin and amiodarone are a common cause of readmissions, as well as emergency department visits and hospitalizations, for everything from myopathy to electrolyte imbalance, gastrointestinal (GI) bleeding, hepatotoxicity, renal dysfunction, and changes in blood pressure and heart rate.1-3
Yet many, if not most, DDIs can be avoided. With diligence and the right tools, you can do much to reduce the incidence of such interactions and adverse outcomes.
Polypharmacy and age pose the highest risks
The more medications a patient is taking, of course, the greater the likelihood of a clinically significant DDI. According to 1 study, 13% of patients taking 2 drugs develop a DDI; the incidence approaches 40% for patients taking 5 drugs, and exceeds 80% for patients taking 7 or more medications.4
In addition to polypharmacy, age alone is a key risk factor for DDIs.5 Pharmacokinetics and pharmacodynamics are frequently altered in older people, who may have slower intestinal transit time; diminished absorption capacity; decreased liver metabolism, mitochondrial function, and renal excretion; and alterations in volemia and body fat distribution.6 Although the speed at which these changes occur varies, aging is associated with a progressive deficiency in the regulation of most homeo-static mechanisms and an altered response to receptor stimulation.7
Whether age, multiple medications, or both are to blame, the impact on the elderly is striking. One recent retrospective study found 25% of elderly outpatients to be at risk for DDIs.8
Very young patients (<5 years) are also at risk for DDIs because of the immaturity of their enzymatic metabolic system.5,9,10 Additional risk factors, detailed in TABLE 1, include the presence of an infection or other acute medical condition, a metabolic or endocrine disorder, and taking 1 or more drugs with a narrow therapeutic range. Women are also at higher risk for DDIs than their male counterparts, the result of a slower metabolic capacity and interference with sex hormones.3-5,8,9 Pharmacogenetics may also play a big part in DDIs, and more and more studies are focusing on identifying patients at greatest risk.
TABLE 1
Risk factors for drug-drug interactions3-5,8,9
| Risk factor | Potential result |
|---|---|
| Acute medical condition (eg, dehydration, infection, alcoholism) | Augmented risk of elevated plasma drug concentration, increased catabolism, inhibition of hepatic drug metabolism |
| Age (very young [<5 years] and elderly) | Reduced metabolic capacity (greater accumulation of drugs) |
| Decreased renal and/or hepatic function | Decreased drug clearance/elimination; greater accumulation of drugs or their metabolites |
| Drug(s) with narrow therapeutic range | Increased risk for dose-related side effects |
| Female sex | Reduced metabolic capacity, interference with sex hormones |
| Metabolic or endocrine conditions (eg, fatty liver, obesity, hypothyroidism) | Altered hepatic metabolism, increased body distribution volumes, augmented risk of accumulation for hydrophobic molecules |
| Polypharmacy (≥3 medications) | Increased risk of metabolic and/or pharmacodynamic interference |
| Pharmacogenetics | Altered metabolic capacity (greater accumulation of drugs or their metabolites) |
Lack of coordinated care also increases risk
Another risk factor involves the use of multiple providers.9 A woman may be treated by—and receive prescriptions from—an endocrinolo-gist, a gynecologist, and a family physician (FP), for instance, and get medications from a local pharmacy, a nationwide discount chain, and a mail order pharmacy. As with the number of medications being taken, the greater the number of health care professionals a patient sees, the higher the risk.
To mitigate the risk, encourage your patients to fill all their prescriptions at the same pharmacy—and for your part, take a complete medication history before writing a new prescription.
Medication history in doubt? Schedule a “brown bag review”
Ask patients to provide the name and dose of every medication they’re taking. Inquire specifically about over-the-counter (OTC) cough and cold remedies and complementary and alternative medicines, including herbal remedies, vitamins, and supplements. Patients often neglect to mention nonprescription remedies and may not even think of them as medicine, but OTC products with the potential to interact adversely with prescription drugs may otherwise remain undetected.11-13
Consider a “brown bag review” for patients who don’t know what dosage they’re taking or have difficulty identifying the drugs other physicians have prescribed. Ask them to put all their medications in a brown bag and bring them in on their next visit.14,15
Steps to take to reduce risk
Software systems. A number of free and low-cost software systems identify potential DDIs (See “Check for drug interactions: Software programs to consider”). While such electronic programs can indeed lower the risk,16-18 they cannot be counted on to detect or avert every possible adverse interaction.
The downside. One problem is that some software programs fail to distinguish between clinically significant and nonsignificant interactions, causing some prescribers to override system alerts—and possibly miss an important warning.19 Another problem: While most systems do an excellent job of checking to see whether 2 drugs can be safely taken together, few are capable of checking for all potential interactions among multiple medications. What’s more, many drugs have not been evaluated for their potential to interact with other agents, so the absence of reported interactions is no guarantee of a lack of DDIs.
Other strategies to consider:
Minimize the number of prescriptions. While it may not be possible to avoid prescribing a new agent for a patient who is already taking multiple medications, limiting the number of new drugs to those that are absolutely essential will help to minimize DDIs. Whenever possible, select a compound with the desired effects. Prescribe a single agent with antihypertensive as well as uricosuric effects for a patient with elevated blood pressure and uric acid levels rather than 2 different drugs (eg, losartan instead of an anti-hypertensive agent plus allopurinol).
Alter the dosing regimen. Several active molecules may cause DDIs by interfering with intestinal absorption or GI transit time if they’re taken closely together. For example, a quinolone should not be administered at the same time as a cation because of possible chelation in the GI tract. If a patient needs both, however, you may be able to avert a DDI by advising the patient to take them at least 2 hours apart.20 Another possibility is to temporarily discontinue a maintenance medication if it has the potential to interact adversely with a drug that is needed for only a short duration.
Choose a different drug (or drug class). Some drug classes should never be mixed—nitrates and phosphodiesterase type-5 inhibitors, taken together, greatly increase the risk of vasodilation and may result in severe hypotension, for example. There are also drug classes with a low potential for DDIs, including cholinesterase inhibitors and anti-hypertensives (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and thiazides).21
Frequently, though, medications within the same drug class do not share the same potential for DDIs. In such cases, an adverse outcome can often be averted by being aware of combinations likely to result in clinically significant DDIs (TABLE 2) and, whenever possible, prescribing another agent. If a patient taking carbamazepine needs a macro-lide antibiotic, for instance, azithromycin is a better choice than erythromycin. That’s because erythromycin inhibits the hepatic metabolism of the anticonvulsant, increasing the serum level of carbamazepine, while azithromycin does not interfere with carbamazepine metabolism.22
TABLE 2
Clinically significant drug-drug interactions21,22
| Combination (effect) |
|---|
| Allopurinol and captopril (augments allopurinol’s effect) |
| Antidepressants (SSRIs, MAOIs) and antiepileptics (augments antidepressant effect) |
| Clopidogrel and omeprazole or esomeprazole (reduces clopidogrel’s effect) |
| Erythromycin and carbamazepine (augments carbamazepine’s effect) |
| Erythromycin and terfenadine (augments terfenadine’s effect) |
| Ketoconazole and PPIs (reduces ketoconazole’s absorption) |
| Levodopa and metoclopramide (augments levodopa’s effect) |
| MAOIs and narcotic analgesics (augments effects of both drugs) |
| Nitrates and phosphodiesterase type-5 inhibitors (augments effects of both drugs) |
| OCs and penicillins, phenobarbital, or tetracycline (reduces OCs’ effect) |
| Phenobarbital and simvastatin* (reduces simvastatin’s effect) |
| Quinolone and cation (reduces quinolone’s absorption and effect) |
| Repaglinide and diltiazem (augments repaglinide’s effect) |
| Simvastatin* or lovastatin and amiodarone or itraconazole (augments statin’s effect) |
| Theophylline and cimetidine or ciprofloxacin (augments theophylline’s effect) |
| MAOIs, monoamine oxidase inhibitors; OCs, oral contraceptives; PPIs, proton pump inhibitors; SSRIs, selective serotonin reuptake inhibitors. |
| *For a complete list of drugs that may interact with simvastatin, see US Food and Drug Administration.28 |
FREE
- Epocrates Rx
http://www.epocrates.com/products/rx/ - eRx (National ePrescribing Patient Safety Initiative)
http://www.nationalerx.com/
FEE-BASED
- GeneMedRx (Drug-drug and drug-gene interactions)
http://www.genemedrx.com/provider-info.php
$199/year - iFacts (Drug Interaction Facts)
http://www.skyscape.com/EStore/ProductDetail.aspx?ProductID=217
$59.95/year - PEPID Portable Drug Companion
http://www.pepid.com/products/pdc/
$89.95/year
How is the drug metabolized?
DDIs may occur as a result of pharmaco-dynamic interaction (when 2 drugs act on the same receptor, site of action, or physiologic system) or pharmacokinetic changes (interference with absorption, albumin binding, distribution, metabolism, or elimination).23 As already noted, age-related changes in pharmacokinetics and pharmacodynamics contribute to the high prevalence of DDIs in elderly patients.
In the liver, drug metabolism, particularly via the cytochrome P450 (CYP450) system, is the cornerstone of drug transformation.23 Al-though the CYP system consists of “superfami-lies” with more than 100 types of enzymes, only a few are responsible for the majority of biotransformation.23 The CYP system is also subject to genetic polymorphism, making some patients especially prone to DDIs.
P-glycoproteins (PGPs), which regulate drug absorption by transporting the drugs across cell membranes, also play a key role. PGP inhibitors or inducers help determine whether the accumulation of the molecule or the increased delivery of toxic metabolites leads to adverse effects.10
Reviewing the mechanism of action of any drug you prescribe for a patient taking other medications may alert you to a potential DDI—and the need to either switch the newly prescribed agent or alter the individual’s drug regimen in some other way.
CASE When John was readmitted to the hospital, he was taken off both the lovastatin and amiodarone and hydrated with forced alkaline diuresis. After a week, his symptoms resolved, and he was discharged soon after. His blood tests normalized 1 month later. The severe DDI he experienced occurred because lovastatin (which is metabolized primarily by CYP3A4) and amiodarone (a CYP3A4 inhibitor) were taken together. (Statins that are substrates of CYP3A4 have the greatest potential for interacting with drugs known to inhibit the CYP450 system [eg, cyclosporine, morphine derivatives, ketoconazole, and amiodarone].)
This adverse interaction could have been avoided if the physician who started John on amiodarone had been aware of the potential DDI—and switched him to an HMG-CoA inhibitor other than lovastatin. Pravastatin, which is not metabolized via CYP450, would have been an excellent choice.
Warfarin warrants special attention
Medications that have a particularly high potential for adverse interactions require special attention and patient monitoring, warfarin foremost among them. Warfarin metabolism and its anticoagulant effects can be dramatically changed if it is administered with a drug with a higher affinity for PGPs or an agent that competes with it within the CYP450 system.24 Because of warfarin’s narrow therapeutic range, there are many drugs and drug classes that patients on warfarin should avoid (TABLE 3)—a fact that patients as well as their physicians need to be aware of.24 Indeed, warfarin is often involved in drug-related hospital admissions for DDIs, especially in elderly patients and in those who are also taking nonsteroidal anti-inflamma-tory drugs (NSAIDs) or macrolides—2 of the many drug classes that patients taking warfarin should avoid.24
TABLE 3
Patient on warfarin? Steer clear of these drugs10,20,23
| Drug class: agent(s) |
|---|
| Antiarrhythmics: amiodarone, propafenone |
| Antibiotics: ciprofloxacin, metronidazole, rifampin, trimethoprim/sulfamethoxazole |
| Anticonvulsants: carbamazepine, valproate |
| Antidepressants: fluoxetine, fluvoxamine, paroxetine, sertraline, trazodone |
| Antidiabetics: chlorpropamide |
| Antifungals: danazol, fluconazole, itraconazole, miconazole |
| Antimalarial agents: quinidine |
| Antineoplastics: azathioprine, fluorouracil, flutamide, ifosfamide, tamoxifen |
| Antiplatelet agents: ticlopidine |
| Antipsychotics: clozapine |
| Diuretics: spironolactone |
| GI drugs: cimetidine, esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, ranitidine |
| Gout treatment: allopurinol |
| Hypolipidemics: atorvastatin, cholestyramine, ezetimibe, fenofibrate, fluvastatin, gemfibrozil, lovastatin, pravastatin, simvastatin |
| NSAIDs: aspirin, celecoxib, diclofenac, ibuprofen, indomethacin, ketoprofen, ketorolac, naproxen, piroxicam, sulindac |
| Thrombolytics: heparin, tissue plasminogen activator |
| Thyroid drugs: methimazole, propylthiouracil |
| Uricosuric agents: sulfinpyrazone |
| GI, gastrointestinal; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Keep an eye on these drug combinations, as well
Among the many combinations likely to result in clinically significant DDIs (TABLE 2), the following are worth mentioning:
Clopidogrel + certain proton pump inhibitors. The addition of a PPI to clopidogrel has been associated with a significant increase of recurrent infarction.25 This may occur because clopidogrel is a prodrug and is converted in the liver to its active form by CYP2C19, an enzyme specifically inhibited by various PPIs—thereby altering the effectiveness of the antiplatelet agent. However, a recent analysis suggests that there is no need to avoid the concomitant use of a PPI and clopidogrel—and that the interference appears to be limited to omeprazole and esomeprazole.26
Oral contraceptives (OCs) + penicillins, phenobarbital, or tetracycline. Each of these drugs reduces the effect of OCs, and women who are taking them concomitantly need to be advised to use another means of contraception.
Phenobarbital + simvastatin. Pheno-barbital (a CYP3A4 inducer) may reduce the efficacy of simvastatin.
Repaglinide + diltiazem. Diltiazem inhibits the metabolism of repaglinide (a CYP3A4 substrate), thus increasing the risk of hypoglycemia.
Simvastatin + amiodarone or itraconazole. Either of these antiarrhythmic agents decreases simvastatin metabolism, raising the risk of myopathy; with amiodarone, however, the likelihood of an adverse outcome is especially high. In 2008, the US Food and Drug Administration (FDA) issued a warning to healthcare professionals of the increased risk for rhabdomyolysis when simvastatin doses greater than 20 mg are administered together with amiodarone.27 The agency issued a safety review of simvastatin, warning of its potential for DDIs with amiodarone and numerous other medications, earlier this year.28
As John’s case illustrates, use of lovastatin with amiodarone should be avoided, as well.
Keep others safe: Report adverse events
When a DDI occurs despite your best efforts, you can help ensure that other patients do not experience the same adverse outcome by reporting it to MedWatch, the FDA’s voluntary safety information and adverse event reporting program. Go to https://www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm to file a report online.
CORRESPONDENCE Ignazio Grattagliano, MD, General Medicine, Department of Internal and Public Medicine, University of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
1. Becker ML, Kallewaard M, Caspers PW, et al. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641-651.
2. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
3. Tulner LR, Frankfort SV, Gijsen GJ, et al. Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging. 2008;25:343-355.
4. Cadieux RJ. Drug interactions in the elderly. How multiple drug use increases risk exponentially. Postgrad Med. 1989;86:179-186.
5. Shapiro LE, Shear NH. Drug interactions: proteins, pumps, and P-450s. J Am Acad Dermatol. 2002;47:467-484.
6. Sitar DS. Aging issues in drug disposition and efficacy. Proc West Pharmacol Soc. 2007;50:16-20.
7. El Desoky ES, Derendorf H, Klotz U. Variability in response to cardiovascular drugs. Curr Clin Pharmacol. 2006;1:35-46.
8. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
9. Becker ML, Kallewaard M, Caspers PW, et al. Potential determinants of drug-drug interaction associated dispensing in community pharmacies. Drug Saf. 2005;28:371-378.
10. Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47:3-10.
11. Buurma H, Bouvy ML, De Smet PA, et al. Prevalence and determinants of pharmacy shopping behaviour. J Clin Pharm Ther. 2008;33:17-23.
12. Russmann S, Barguil Y, Cabalion P, et al. Hepatic injury due to traditional aqueous extracts of kava root in New Caledonia. Eur J Gastroenterol Hepatol. 2003;5:1033-1036.
13. Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901-910.
14. Institute for Safe Medication Practices. ISMP medication safety alert! Available at: http://www.ismp.org/Newsletters/consumer/alerts/BrownBag.asp. Accessed May 5, 2010.
15. National Institute on Aging. Obtaining the medical history. Available at: http://www.nia.nih.gov/Healthinformation/Publications/ClinicianHB/03_history.htm. Accessed May 5, 2010.
16. Glassman PA, Simon B, Belperio P, et al. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40:1161-1171.
17. Goldberg RM, Mabee J, Chan L, et al. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14:447-450.
18. Lapane KL, Waring ME, Schneider KL, et al. A mixed method study of the merits of e-prescribing drug alerts in primary care. J Gen Intern Med. 2008;23:442-446.
19. Magnus D, Rodgers S, Avery AJ. GPs’ views on computerized drug interaction alerts: questionnaire survey. J Clin Pharm Ther. 2002;27:377-382.
20. Shukla UA, Pittman KA, Barbhaiya RH. Pharmacokinetic interactions of cefprozil with food, propantheline, metoclopramide, and probenecid in healthy volunteers. J Clin Pharmacol. 1992;32:725-731.
21. Levy RH, Collins C. Risk and predictability of drug interactions in the elderly. Int Rev Neurobiol. 2007;81:235-251.
22. Pauwels O. Factors contributing to carbamazepine-macrolide interactions. Pharmacol Res. 2002;45:291-298.
23. Beaird SL. HMG-CoA reductase inhibitors: assessing differences in drug interactions and safety profiles. J Am Pharm Assoc (Wash). 2000;40:637-644.
24. Snaith A, Pugh L, Simpson CR, et al. The potential for interaction between warfarin and coprescribed medication: a retrospective study in primary care. Am J Cardiovasc Drugs. 2008;8:207-212.
25. Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713-718.
26. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmaco-dynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
27. US Food and Drug Administration. Serious muscle injury with simvastatin/amiodarone combination. November 2008. Available at: http://www.accessdata.fda.gov/psn/printer.cfm?id=886. Accessed May 5, 2010.
28. US Food and Drug Administration. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. March 19, 2010. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
ucm204882.htm. Accessed May 5, 2010.
1. Becker ML, Kallewaard M, Caspers PW, et al. Hospitalisations and emergency department visits due to drug-drug interactions: a literature review. Pharmacoepidemiol Drug Saf. 2007;16:641-651.
2. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
3. Tulner LR, Frankfort SV, Gijsen GJ, et al. Drug-drug interactions in a geriatric outpatient cohort: prevalence and relevance. Drugs Aging. 2008;25:343-355.
4. Cadieux RJ. Drug interactions in the elderly. How multiple drug use increases risk exponentially. Postgrad Med. 1989;86:179-186.
5. Shapiro LE, Shear NH. Drug interactions: proteins, pumps, and P-450s. J Am Acad Dermatol. 2002;47:467-484.
6. Sitar DS. Aging issues in drug disposition and efficacy. Proc West Pharmacol Soc. 2007;50:16-20.
7. El Desoky ES, Derendorf H, Klotz U. Variability in response to cardiovascular drugs. Curr Clin Pharmacol. 2006;1:35-46.
8. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
9. Becker ML, Kallewaard M, Caspers PW, et al. Potential determinants of drug-drug interaction associated dispensing in community pharmacies. Drug Saf. 2005;28:371-378.
10. Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47:3-10.
11. Buurma H, Bouvy ML, De Smet PA, et al. Prevalence and determinants of pharmacy shopping behaviour. J Clin Pharm Ther. 2008;33:17-23.
12. Russmann S, Barguil Y, Cabalion P, et al. Hepatic injury due to traditional aqueous extracts of kava root in New Caledonia. Eur J Gastroenterol Hepatol. 2003;5:1033-1036.
13. Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901-910.
14. Institute for Safe Medication Practices. ISMP medication safety alert! Available at: http://www.ismp.org/Newsletters/consumer/alerts/BrownBag.asp. Accessed May 5, 2010.
15. National Institute on Aging. Obtaining the medical history. Available at: http://www.nia.nih.gov/Healthinformation/Publications/ClinicianHB/03_history.htm. Accessed May 5, 2010.
16. Glassman PA, Simon B, Belperio P, et al. Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care. 2002;40:1161-1171.
17. Goldberg RM, Mabee J, Chan L, et al. Drug-drug and drug-disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med. 1996;14:447-450.
18. Lapane KL, Waring ME, Schneider KL, et al. A mixed method study of the merits of e-prescribing drug alerts in primary care. J Gen Intern Med. 2008;23:442-446.
19. Magnus D, Rodgers S, Avery AJ. GPs’ views on computerized drug interaction alerts: questionnaire survey. J Clin Pharm Ther. 2002;27:377-382.
20. Shukla UA, Pittman KA, Barbhaiya RH. Pharmacokinetic interactions of cefprozil with food, propantheline, metoclopramide, and probenecid in healthy volunteers. J Clin Pharmacol. 1992;32:725-731.
21. Levy RH, Collins C. Risk and predictability of drug interactions in the elderly. Int Rev Neurobiol. 2007;81:235-251.
22. Pauwels O. Factors contributing to carbamazepine-macrolide interactions. Pharmacol Res. 2002;45:291-298.
23. Beaird SL. HMG-CoA reductase inhibitors: assessing differences in drug interactions and safety profiles. J Am Pharm Assoc (Wash). 2000;40:637-644.
24. Snaith A, Pugh L, Simpson CR, et al. The potential for interaction between warfarin and coprescribed medication: a retrospective study in primary care. Am J Cardiovasc Drugs. 2008;8:207-212.
25. Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713-718.
26. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmaco-dynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
27. US Food and Drug Administration. Serious muscle injury with simvastatin/amiodarone combination. November 2008. Available at: http://www.accessdata.fda.gov/psn/printer.cfm?id=886. Accessed May 5, 2010.
28. US Food and Drug Administration. FDA drug safety communication: ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury. March 19, 2010. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
ucm204882.htm. Accessed May 5, 2010.
Liver disease: Early signs you may be missing
• Suspect compensated liver cirrhosis in a patient with abnormal liver function tests, a low platelet count, and prolonged prothrombin time. C
• Use ultrasonography as a first-line diagnostic tool for liver cirrhosis. C
• Prescribe beta-blockers as prophylaxis for patients at risk for variceal bleeding. A
• Work collaboratively with hepatic specialists to manage the care of patients with cirrhosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: A patient with mildly elevated ALT and AST
John M., a 63-year-old truck driver with a family history of diabetes and arterial hypertension, is complaining of persistent fatigue—again. He has type 2 diabetes and takes metformin and repaglinide, but his blood pressure is normal. Lab tests reveal a recurrent mild elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of unknown origin; Mr. M. has no history of virus or hepatotoxic drugs, and reports only modest alcohol intake. He is obese, however, with a BMI of 34.5 and a waist circumference of 41 inches.
CASE 2: A patient with abdominal swelling
Anna B., a slim 68-year-old, comes in with 2 acute conditions: About a week ago, she noticed abdominal swelling and low back pain that began suddenly, after she carried a heavy load. She’s taken nonsteroidal anti-inflammatory drugs for 6 days, but the pain has not improved. The patient’s only significant clinical history is a hysterectomy, with oophorectomy, at age 38, and a recurrent elevation of serum transaminase levels that has never been investigated. Examination reveals an important kyphosis, and finger pressure on the vertebral spine or a position change exacerbates the pain. Her abdomen is swollen and tense, with a tympanic sound on the upper abdomen and a dull sound on the lower portion.
If John M. and Anna B. were your patients, would you suspect that they both have advanced liver disease? What diagnostic tests would you order, and how would you manage their care?
Cirrhosis has always been associated with high rates of morbidity and mortality. It is the 12th most common cause of death in the United States;1 in some parts of the world, its ranking as a cause of death is considerably higher.2,3 In recent years, however, cirrhosis has become the focus of greater attention both here and abroad, for 2 reasons: The first is the increasing prevalence of viral hepatitis and steatohepatitis, both of which are prominent causes of cirrhosis. The second is the improvement we’ve made in treatment: Not only can we slow the progression of cirrhosis, but in some cases, we can even restore hepatic function.4
The key to successful management of cirrhosis lies in spotting subtle signs and symptoms well in advance of the serious complications that can arise down the road. Here’s what to look for.
Early warnings you can’t afford to overlook
While the clinical presentation of a patient with liver cirrhosis is often asymptomatic, serum transminases—included in many standard laboratory tests as part of a routine examination—often provide the first sign of a problem.
Mildly elevated ALT in an asymptomatic patient may be transient and benign, or an indication of chronic liver disease.5 In fact, signs suggestive of significant liver disease have been reported in more than 20% of patients with ALT elevation.2 But because abnormal ALT values are common and frequently resolve, many primary care physicians pay little attention to this potentially important finding—and miss a key opportunity for early identification and treatment.6
Look at other lab values, risk factors, as well
Additional lab values that suggest the possibility of cirrhosis include an elevated AST/ALT ratio, a low platelet count (<150,000/L), elevated alkaline phosphatase, elevated bilirubin (>1.1 mg/dL), low serum albumin (<2.5 g/dL), and decreased prothrombin time (<100%). Potential causes include viral hepatitis, heavy alcohol use, hepatotoxic drugs, steatosis, and steatohepatitis.
The next step for a patient with any of these abnormal values is a thorough medication review and medical history. Identify all prescription and nonprescription drugs the patient is taking, as well as any herbal products and supplements, in search of hepatotoxic agents. Amiodarone and valproic acid, among other drugs, may cause steatosis, and some herbal products—particularly kava kava extract, used to treat anxiety and insomnia—have been linked to hepatitis and even liver failure.7 Question the patient about alcohol consumption and a history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders, as well.
At a minimum, schedule follow-up testing of asymptomatic patients with abnormal laboratory findings in no more than 6 months. Persistent ALT elevation in such patients is most commonly caused by major viruses, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH).8 Nonalcoholic fatty liver is especially likely in patients with clinical and demographic risk factors—those who, like John M., suffer from obesity or diabetes, or both.
Ultrasound yields further information
Further screening should be limited to patients who continue to have abnormal test results for 6 months or more or have multiple risk factors. While biopsy is still considered the gold standard for diagnosing and staging chronic liver disease, it should be considered, according to the American Gastroenterological Association, only if ultrasound and other tests have not been helpful in reaching a diagnosis.9
Often, though, ultrasonography aids in diagnosis. In the case of John M., ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity and signs of severe steatosis and mild splenomegaly, but no increase in portal vein diameter and no ascites. For asymptomatic patients with cirrhosis or an earlier stage of liver disease, ultrasound at 6-month intervals, combined with blood alpha-fetoprotein measurement, can be used to track disease progression and screen for hepatocellular carcinoma.10
Newer, noninvasive methods aid in diagnosis
Noninvasive means of evaluating the presence and extent of liver fibrosis and differentiating cirrhosis from noncirrhosis, developed in recent years, have been found to have positive predictive values greater than 85% to 90%.11 Transient elastography (FibroScan, London, England), which assesses liver stiffness, is 1 such method. Although it is often used successfully, however, morbid obesity, small intercostal spaces, and ascites limit the diagnostic capability of this medical device.12
Fibrosis can also be detected with the use of 1 or more algorithms—each testing blood samples for a different combination of serum surrogate markers for liver disease. Some widely used algorithms include the APRI (AST-to-platelets ratio index), the Fibrotest (aptoglobin, alpha-2 macroglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and bilirubin), the Hepascore (bilirubin, gamma-glutamyl transpeptidase, haluronic acid, alpha-2 macroglobulin, age, sex), and the BARD (BMI, AST/ALT ratio, diabetes).
Hepatologists often use the results of ultrasonography, followed by transient elastography in conjunction with findings from 1 or more of these algorithms, to determine which patients are candidates for liver biopsy.11,12
Staging is crucial, with or without biopsy
The decision to perform a liver biopsy should be based on a number of factors, including the patient’s age, lifestyle, liver chemistry abnormalities, desire for prognostic information, and associated comorbidities.9 Despite the value of biopsy, it is a costly procedure with potentially serious side effects and risks—and not always accepted by patients. In a recent survey of 1177 primary care physicians in France, as many as 59% of patients with chronic hepatitis C refused to undergo liver biopsy; what’s more, 22% of the doctors surveyed shared the patients’ hesitancy.13 Whether patients refuse biopsy or it is deemed unnecessary because ultrasound and other noninvasive tests result in a probable diagnosis, staging is necessary, both to guide therapy and to arrive at a prognosis.
Liver enzyme levels reveal little about organ integrity and are not useful for staging. But other parameters (specifically, bilirubin, albumin, and prothrombin time), combined with the presence (or absence) and severity of physical findings such as encephalopathy and ascites, are included in the Child-Pugh classification system ( TABLE 1 ),14 a widely used system that roughly indicates disease severity.15
The Model for End-stage Liver Disease (MELD)—and PELD, the pediatric model—use bilirubin, creatinine, and international normalized ratio values to classify disease severity. MELD and PELD scores are considered more accurate than the Child-Pugh score in determining short-term mortality,16 and are used by the United Network of Organ Sharing (UNOS) for liver allocation. You’ll find a calculator at http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=97.17
Despite the progress in diagnostic techniques, the life expectancy and quality of life for patients with advanced cirrhosis remains poor. Patients routinely experience fatigue, pruritus, ascites, encephalopathy, and bleeding; dyspepsia and malnutrition are common, as well. Cirrhosis also carries the risk of life-threatening complications, partly due to comorbidities—most notably, osteoporosis, malabsorption, and rheumatic diseases. Liver transplantation has the potential to change the life expectancy of these patients, but because of the extensive waiting lists, candidates for transplant often die before a liver becomes available.
But for many patients who are in stable condition—those with compensated cirrhosis, that is—the prognosis is far more hopeful: In addition to providing standard medical care, including immunization, if necessary, and nutritional counseling, targeted therapy is crucial to slow, or stop, disease progression.
TABLE 1
Child-Pugh: Classifying cirrhosis, predicting survival*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Prothrombin time (INR) | <4 sec (<1.7) | 4-6 sec (1.7-2.3) | > 6 sec (>2.3) |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Ascites | Absent | Mild | Severe |
| Encephalopathy | Absent | Mild | Severe |
| INR, international normalized ratio. | |||
| * Total the number of points for all 5 indicators (1 point for every answer in column 1, 2 points for every answer in column 2, and 3 points for every answer in column 3). Patients with ≤6 points (Grade A) have an estimated 1-year survival rate of 100%; patients with 7-9 points (Grade B) have an estimated 1-year survival rate of 80%; and patients with ≥10 points (Grade C) have an estimated 1-year surival rate of 45%. | |||
| Adapted from: Infante-Rivard C, et al. Hepatology. 1987.14 | |||
Treatment for cirrhosis depends on the cause
Although primary care physicians can often provide most, or all, of the care for those in stable condition, a specialist may be helpful in determining further testing to identify the underlying cause of the cirrhosis, which is essential to determining the most appropriate treatment. What’s more, research has shown that patients with cirrhosis whose care is managed by a primary care physician and a hepatologist have better outcomes than those who are treated by a primary care doctor alone.18
What to test for?
Tests to determine the cause of cirrhosis are listed in TABLE 2 . For an individual patient, diagnostic tests would be based on the suspected cause. A patient with a family history of hereditary hemochromatosis would be tested for elevated serum ferritin levels and hepatic iron content on liver biopsy sample; the transferrin saturation index would also be obtained, and the patient might be tested for specific gene mutations. A patient who drinks heavily would be tested for elevated gamma-glutamyl transpeptidase and mean corpuscular volume. For someone with obesity, diabetes, and an enlarged liver, standard lab tests, including high-density lipoprotein (HDL) cholesterol, glucose, and triglycerides, may be sufficient.
Keep in mind, however, that cirrhosis may have more than 1 contributing factor—obesity or chronic alcohol use and a virus, for example; alcohol abuse and metabolic fatty liver; or virus and hemochromatosis. Thus, it may require more than 1 type of treatment.
Alcohol abuse is the cause of 25% of cases of liver cirrhosis, and a contributor to another 25% to 50%.19 The key treatment here—and an ideal role for a family physician—is to refer the patient to a detoxification and treatment program and provide ongoing monitoring and support. Antiviral treatment may be helpful for a recovering alcoholic who also tests positive for hepatitis B or C virus, but because of potential problems with compliance, some physicians delay therapy until the patient has had at least 6 months of continuous abstinence. Although this is not an absolute criterion, the same period of abstinence may be required before a patient becomes eligible for a liver transplant.
NAFLD/NASH is typically diagnosed on the basis of lab values and physical presentation. For a stable patient, the primary treatment includes lifestyle change—a low-calorie, low-carbohydrate diet and an exercise regimen—and a possible switch to insulin for better glycemic control.
For patients who are not candidates for such targeted treatments, either because their disease is too advanced or they’re unable to tolerate the recommended therapy, numerous pharmaceutical preparations claiming antioxidant or anti-inflammatory properties are available. But only 1—an herbal extract known as silymarin, derived from the milk thistle plant and taken with vitamin E—has been found to have some protective effects.20
TABLE 2
Liver cirrhosis: Common causes, diagnostic tests, and treatments4,34-38
| Cause | Test (result) | Therapy |
|---|---|---|
| Alcohol | GGT (↑), MCV (↑) | Abstinence |
| HBV + delta virus infection | HBsAg (+) HBV-DNA(+) HBc-IgM (+) HDV-RNA (+) | Interferon alpha-2b, nucleoside (lamivudine, telbivudine, entecavir) and nucleotide (adefovir, tenofovir) analogs |
| HCV infection | HCV-RNA (+) | Interferon + ribavirin |
| Primary biliary cirrhosis | GGT (↑) Alkaline phosphatase (↑) AMA (+) | Ursodeoxycholate |
| Autoimmune hepatitis | ANA (+) ASMA (+) LKM (+) | Prednisone, azathioprine |
| Hemochromatosis | Ferritin (↑) Transferrin saturation index (>45%) Hepatic iron content (↑) HFE gene mutation (C282Y, H63D) | Phlebotomy, chelating agents |
| Wilson’s disease | Ceruloplasmin (↓) Serum copper (↓) 24h urinary copper excretion (↑) | D-penicillamine, zinc |
| NAFLD/NASH | HDL cholesterol (↓) Glucose (↑) Triglycerides (↑) | Low-calorie diet, physical activity, insulin-sensitizer drugs or insulin |
| AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth-muscle antibody; GGT, gamma–glutamyl transpeptidase; HBc-IgM, immunoglobulin M antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; HCV-RNA, hepatitis C virus RNA; HDL, high-density lipoprotein; HDV-RNA, hepatitis delta virus RNA; LKM, liver kidney microsomes; MCV, mean corpuscular volume, NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. | ||
Address systemic problems along with targeted treatment
Malnutrition is a serious problem for many patients with cirrhosis. Causes range from poor oral intake or malabsorption to ongoing alcohol use, chronic nausea, or early satiety because of compression from ascites. Dental problems that prevent the patient from chewing properly may be a contributing factor, as well.
Regardless of the cause, malnutrition is associated with muscle wasting, hypoalbuminemia, decreased resistance to infections, and variceal bleeding, and addressing it is a key part of treatment. Assess the nutritional status of every patient with cirrhosis, and stress the importance of multivitamin supplementation.21 If dental care is needed, take steps to see that the patient receives it.
Nutritional support, however, should be reserved for severely malnourished patients awaiting transplantation.22
Osteoporosis. Reduced bone formation—the result of vitamin D deficiency, hypoparathyroidism, and hypogonadism—is a well-known complication of end-stage cirrhosis. However, osteopenia may occur in an earlier stage of disease, especially in patients with cholestatic disease and those receiving antiviral therapy. Prescribe bisphosphonates, together with calcium and vitamin D3, to improve bone mineral density.23
Diabetes. The relationship between diabetes and cirrhosis is particularly complex, because diabetes can be both a causal factor and a consequence of cirrhosis. Diabetes is common in patients with NASH, and prevalent among those with hepatitis C and hemochromatosis. Multivariate analyses have found that diabetes has an independent negative effect on the progression of liver disease.24
Diet remains the first-line treatment for hyperglycemia, with metformin as the drug of choice if diet alone is unsuccessful. Sulfonylureas can be used, but require caution to avoid hypoglycemia. Glitazones are a newer alternative, but their value in patients with liver cirrhosis has not been studied. However, the use of any oral antidiabetic agent requires extra caution in patients with cirrhosis, and should be avoided in those with advanced liver disease. Although insulin requires intense self-monitoring of serum glucose levels, it is preferable to oral agents for this patient population.25
Managing complications of cirrhosis
Hospital, home, or long-term care? Whether patients with advanced cirrhosis can be maintained at home or require hospitalization or long-term care is best decided in consultation with patient, family, and other members of the health care team. One helpful tool is the Karnofsky Performance Scale Index (http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf), which scores patients from 0 to 100 based on their functional impairment.26 (Patients with decompensated liver cirrhosis and limited self-sufficiency typically score <50, indicating that they require home health care, hospice, or institutional care.) Whatever the outcome, the patient may need to be reevaluated as the disease progresses and complications occur.
Ascites, the most common complication of cirrhosis,27 is a primary reason for hospitalization, but may be managed on an outpatient basis, depending on the patient presentation. Determining factors include the presence or absence of portal hypertension, impaired albumin synthesis, decreased plasma oncotic pressure, and sodium retention. Diagnosis is based on physical exam and ultrasonography.
Initial treatment for ascites includes salt restriction28,29 and avoidance of NSAIDs, which promote renal sodium retention, followed by spironolactone (100–400 mg/d). Add furosemide (40-160 mg/d) if the fluid retention does not begin to resolve after 3 to 5 days of treatment. If the condition persists despite maximum tolerable doses of diuretics, large-volume paracentesis to remove transudative fluid (albumin <1 g/dL; serum/ascites albumin gradient >1.1) may be needed. A patient with recurrent or refractory ascites should see a specialist for further evaluation and the possibility of a transjugular intrahepatic portosystemic shunt (TIPS).
Abdominal pain and an ascitic granulocyte count >250/mm3 suggest spontaneous bacterial peritonitis (SBP)—a severe complication of ascites that can result in renal and liver failure. In addition to pain, patients may present with tense ascites and fever, followed by encephalopathy, shock, and increased serum creatinine levels. Hospitalization is required for SBP; therapy includes high-dose albumin and intravenous antibiotics, typically cephalosporin. Long-term prophylaxis with norfloxacin to prevent the recurrence of SBP is indicated.30
If your patient has ascites and is being cared for at home, talk to the patient and his or her family about the importance of a daily weight check. Tell them to contact you if the patient gains more than 4 to 8 lbs within a few days. Frequent electrolyte checks are needed, as well. An albumin infusion is required when serum levels are particularly low, or after large-volume paracentesis.31 Patients with SBP or refractory ascites generally have more advanced disease and a poor prognosis.
Portal hypertension/esophageal varices. The main aim of treating portal hypertension is to prevent esophageal variceal bleeding. The appearance of varices should be checked by endoscopy every 2 to 3 years, or yearly for patients at high risk of bleeding. Patients with varices can be managed with nonselective beta-blockers at doses that are sufficient to elicit a 25% reduction in resting heart rate. Those at high risk for bleeding and patients who have already had esophageal bleeding may require endoscopic band ligation.32 TIPS is an alternative for those whose previous treatments have failed.33
Hepatic encephalopathy. This potentially reversible decrease in neuropsychiatric function mainly affects patients with portal hypertension. Caused by reduced hepatic clearance of gut-deriving neurotoxins, hepatic encephalopathy is associated with a range of signs and symptoms—from subtle personality changes to coma, with flapping tremor as a frequent initial finding. Acid-base and electrolyte disturbances, constipation, infections, gastrointestinal bleeding, and sedatives can precipitate encephalopathy. Hepatic encephalopathy is a diagnosis of exclusion, however, requiring the exclusion of all other etiologies of altered mental status.
Treatment consists of identifying and correcting the precipitating factors, and includes electrolyte correction, colon cleansing, and acidification with lactulose. Dietary protein restriction is no longer advocated, because it may facilitate malnutrition and complications. Oral rifaximine is useful and well tolerated for suppression of intestinal bacterial flora. Venous infusion of branched-chain amino acids or flumazenil may be effective in case of coma.
Fever and sepsis. Infection is a high-risk factor for mortality in patients with cirrhosis, as it can lead to renal and liver failure, variceal bleeding, and hepatic encephalopathy. However, individuals with cirrhosis often do not develop the typical signs and symptoms of infection; leukocytosis may be absent because of severe leukopenia, for instance, and patients may be afebrile.
Thus, the general appearance of systemic illness is an indication for antibiotics, with quinolones and cephalosporins as first-line agents. Infections most commonly involve the urinary tract (25%-55%) or the respiratory tract (20%), or are related to SBP (10%-30%).33 Hospitalization is suggested in case of poor general health status or the appearance of organ dysfunction.
When medical therapy and other interventions fail to control complications, transplantation is the only alternative. Primary care physicians can play a role here, too, in referring potential candidates for liver transplants to specialists for further consideration.
CASE 1: Resolution
As we’ve already seen, John M.’s ultrasound revealed an enlarged liver. The results led to a probable diagnosis of an advanced form of NASH. Other lab tests indicated that he had poorly controlled diabetes, high triglyceride levels, and—for the first time—a low platelet count. His physician stressed the importance of following a low-calorie, low-carbohydrate diet and exercising regularly, prescribed insulin, and referred the patient to a hepatologist for further noninvasive evaluation of fibrosis and to determine whether liver biopsy was needed.
CASE 2: Resolution
Blood tests revealed that Anna B. had a low platelet count (64,000/mm3), elevated liver enzymes (AST 2× upper limit of normal [ULN], ALT 1.5× ULN, GGT 2.5× ULN), and high gamma-globulins (33.6%) with no monoclonal bands. Ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity, portal vein dilatation, and moderate ascites. She also tested positive for HCV and had an HCV-RNA reading of 15×106 IU/mL. No other cause of chronic liver disease emerged. Ms. B.’s physician told her that she had an osteoporotic vertebral fracture—a frequent comorbidity in patients with liver cirrhosis—and decompensated liver cirrhosis from an old HCV infection. He added that her abdomen was distended because of fluid retention. The physician recommended bed rest, prescribed paracetamol (1 g tid) and spironolactone (100 mg/d), and referred the patient to an orthopedist for treatment of the fracture and to a hepatologist to be evaluated for transplantation.
CORRESPONDENCE
Ignazio Grattagliano, MD, Department of Internal Medicine, University Medical School of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
1. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National Vital Stat Rep. 2009;57:(14):1-135.Available at: www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. Accessed September 16, 2009.
2. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442-1449.
3. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851.
5. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110.
6. Sherwood P, Lyburn I, Brown S, et al. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ. 2001;322:276-278.
7. US Food and Drug Administration. Food. Consumer advisory: Kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. Accessed September 11, 2009.
8. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863.
9. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
10. Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465-1473.
11. Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
12. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
13. Bonny C, Rayssiguier R, Ughetto S, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region [In French]. Gastroenterol Clin Biol. 2003;27:1021-1025.
14. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
15. Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;Aug. 20 [Epub ahead of print].
16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96.
17. United Network of Organ Sharing. Resources. Meld/Peld calculator. Available at: http://www.unos.org/resources/meldPeldCalculator.asp. Accessed September 11, 2009.
18. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
19. Habib A, Bond WM, Heuman DM. Long-term management of cirrhosis. Appropriate supportive care is both critical and difficult. Postgrad Med. 2001;109:101-103.
20. Flora K, Hahn M, Rosen H, et al. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143.
21. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis [In French]. Gastroenterol Clin Biol. 2008;32:265-273.
22. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55.
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(suppl 1):i1-i9.
24. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75.
25. Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288.
26. Karnofsky Performance Scale Index. Available at: http://www.medal.org/visitor/www%5CActive%5Cch1%5Cch1.01%5Cch1.01.01.aspx. Accessed September 11, 2009.
27. Gentilini P, Bernardi M, Bolondi L, et al. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546.
28. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
29. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856.
30. Gines P, et al. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1:129-155.
31. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
32. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091.
33. McCormick PA, Greenslade L, Kibbler CC, et al. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology. 1997;25:833-836.
34. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
35. European Association tor the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242.
36. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
37. Portincasa P, Grattagliano I, Palmieri VO, et al. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889-2900.
38. Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328-338.
• Suspect compensated liver cirrhosis in a patient with abnormal liver function tests, a low platelet count, and prolonged prothrombin time. C
• Use ultrasonography as a first-line diagnostic tool for liver cirrhosis. C
• Prescribe beta-blockers as prophylaxis for patients at risk for variceal bleeding. A
• Work collaboratively with hepatic specialists to manage the care of patients with cirrhosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: A patient with mildly elevated ALT and AST
John M., a 63-year-old truck driver with a family history of diabetes and arterial hypertension, is complaining of persistent fatigue—again. He has type 2 diabetes and takes metformin and repaglinide, but his blood pressure is normal. Lab tests reveal a recurrent mild elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of unknown origin; Mr. M. has no history of virus or hepatotoxic drugs, and reports only modest alcohol intake. He is obese, however, with a BMI of 34.5 and a waist circumference of 41 inches.
CASE 2: A patient with abdominal swelling
Anna B., a slim 68-year-old, comes in with 2 acute conditions: About a week ago, she noticed abdominal swelling and low back pain that began suddenly, after she carried a heavy load. She’s taken nonsteroidal anti-inflammatory drugs for 6 days, but the pain has not improved. The patient’s only significant clinical history is a hysterectomy, with oophorectomy, at age 38, and a recurrent elevation of serum transaminase levels that has never been investigated. Examination reveals an important kyphosis, and finger pressure on the vertebral spine or a position change exacerbates the pain. Her abdomen is swollen and tense, with a tympanic sound on the upper abdomen and a dull sound on the lower portion.
If John M. and Anna B. were your patients, would you suspect that they both have advanced liver disease? What diagnostic tests would you order, and how would you manage their care?
Cirrhosis has always been associated with high rates of morbidity and mortality. It is the 12th most common cause of death in the United States;1 in some parts of the world, its ranking as a cause of death is considerably higher.2,3 In recent years, however, cirrhosis has become the focus of greater attention both here and abroad, for 2 reasons: The first is the increasing prevalence of viral hepatitis and steatohepatitis, both of which are prominent causes of cirrhosis. The second is the improvement we’ve made in treatment: Not only can we slow the progression of cirrhosis, but in some cases, we can even restore hepatic function.4
The key to successful management of cirrhosis lies in spotting subtle signs and symptoms well in advance of the serious complications that can arise down the road. Here’s what to look for.
Early warnings you can’t afford to overlook
While the clinical presentation of a patient with liver cirrhosis is often asymptomatic, serum transminases—included in many standard laboratory tests as part of a routine examination—often provide the first sign of a problem.
Mildly elevated ALT in an asymptomatic patient may be transient and benign, or an indication of chronic liver disease.5 In fact, signs suggestive of significant liver disease have been reported in more than 20% of patients with ALT elevation.2 But because abnormal ALT values are common and frequently resolve, many primary care physicians pay little attention to this potentially important finding—and miss a key opportunity for early identification and treatment.6
Look at other lab values, risk factors, as well
Additional lab values that suggest the possibility of cirrhosis include an elevated AST/ALT ratio, a low platelet count (<150,000/L), elevated alkaline phosphatase, elevated bilirubin (>1.1 mg/dL), low serum albumin (<2.5 g/dL), and decreased prothrombin time (<100%). Potential causes include viral hepatitis, heavy alcohol use, hepatotoxic drugs, steatosis, and steatohepatitis.
The next step for a patient with any of these abnormal values is a thorough medication review and medical history. Identify all prescription and nonprescription drugs the patient is taking, as well as any herbal products and supplements, in search of hepatotoxic agents. Amiodarone and valproic acid, among other drugs, may cause steatosis, and some herbal products—particularly kava kava extract, used to treat anxiety and insomnia—have been linked to hepatitis and even liver failure.7 Question the patient about alcohol consumption and a history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders, as well.
At a minimum, schedule follow-up testing of asymptomatic patients with abnormal laboratory findings in no more than 6 months. Persistent ALT elevation in such patients is most commonly caused by major viruses, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH).8 Nonalcoholic fatty liver is especially likely in patients with clinical and demographic risk factors—those who, like John M., suffer from obesity or diabetes, or both.
Ultrasound yields further information
Further screening should be limited to patients who continue to have abnormal test results for 6 months or more or have multiple risk factors. While biopsy is still considered the gold standard for diagnosing and staging chronic liver disease, it should be considered, according to the American Gastroenterological Association, only if ultrasound and other tests have not been helpful in reaching a diagnosis.9
Often, though, ultrasonography aids in diagnosis. In the case of John M., ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity and signs of severe steatosis and mild splenomegaly, but no increase in portal vein diameter and no ascites. For asymptomatic patients with cirrhosis or an earlier stage of liver disease, ultrasound at 6-month intervals, combined with blood alpha-fetoprotein measurement, can be used to track disease progression and screen for hepatocellular carcinoma.10
Newer, noninvasive methods aid in diagnosis
Noninvasive means of evaluating the presence and extent of liver fibrosis and differentiating cirrhosis from noncirrhosis, developed in recent years, have been found to have positive predictive values greater than 85% to 90%.11 Transient elastography (FibroScan, London, England), which assesses liver stiffness, is 1 such method. Although it is often used successfully, however, morbid obesity, small intercostal spaces, and ascites limit the diagnostic capability of this medical device.12
Fibrosis can also be detected with the use of 1 or more algorithms—each testing blood samples for a different combination of serum surrogate markers for liver disease. Some widely used algorithms include the APRI (AST-to-platelets ratio index), the Fibrotest (aptoglobin, alpha-2 macroglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and bilirubin), the Hepascore (bilirubin, gamma-glutamyl transpeptidase, haluronic acid, alpha-2 macroglobulin, age, sex), and the BARD (BMI, AST/ALT ratio, diabetes).
Hepatologists often use the results of ultrasonography, followed by transient elastography in conjunction with findings from 1 or more of these algorithms, to determine which patients are candidates for liver biopsy.11,12
Staging is crucial, with or without biopsy
The decision to perform a liver biopsy should be based on a number of factors, including the patient’s age, lifestyle, liver chemistry abnormalities, desire for prognostic information, and associated comorbidities.9 Despite the value of biopsy, it is a costly procedure with potentially serious side effects and risks—and not always accepted by patients. In a recent survey of 1177 primary care physicians in France, as many as 59% of patients with chronic hepatitis C refused to undergo liver biopsy; what’s more, 22% of the doctors surveyed shared the patients’ hesitancy.13 Whether patients refuse biopsy or it is deemed unnecessary because ultrasound and other noninvasive tests result in a probable diagnosis, staging is necessary, both to guide therapy and to arrive at a prognosis.
Liver enzyme levels reveal little about organ integrity and are not useful for staging. But other parameters (specifically, bilirubin, albumin, and prothrombin time), combined with the presence (or absence) and severity of physical findings such as encephalopathy and ascites, are included in the Child-Pugh classification system ( TABLE 1 ),14 a widely used system that roughly indicates disease severity.15
The Model for End-stage Liver Disease (MELD)—and PELD, the pediatric model—use bilirubin, creatinine, and international normalized ratio values to classify disease severity. MELD and PELD scores are considered more accurate than the Child-Pugh score in determining short-term mortality,16 and are used by the United Network of Organ Sharing (UNOS) for liver allocation. You’ll find a calculator at http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=97.17
Despite the progress in diagnostic techniques, the life expectancy and quality of life for patients with advanced cirrhosis remains poor. Patients routinely experience fatigue, pruritus, ascites, encephalopathy, and bleeding; dyspepsia and malnutrition are common, as well. Cirrhosis also carries the risk of life-threatening complications, partly due to comorbidities—most notably, osteoporosis, malabsorption, and rheumatic diseases. Liver transplantation has the potential to change the life expectancy of these patients, but because of the extensive waiting lists, candidates for transplant often die before a liver becomes available.
But for many patients who are in stable condition—those with compensated cirrhosis, that is—the prognosis is far more hopeful: In addition to providing standard medical care, including immunization, if necessary, and nutritional counseling, targeted therapy is crucial to slow, or stop, disease progression.
TABLE 1
Child-Pugh: Classifying cirrhosis, predicting survival*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Prothrombin time (INR) | <4 sec (<1.7) | 4-6 sec (1.7-2.3) | > 6 sec (>2.3) |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Ascites | Absent | Mild | Severe |
| Encephalopathy | Absent | Mild | Severe |
| INR, international normalized ratio. | |||
| * Total the number of points for all 5 indicators (1 point for every answer in column 1, 2 points for every answer in column 2, and 3 points for every answer in column 3). Patients with ≤6 points (Grade A) have an estimated 1-year survival rate of 100%; patients with 7-9 points (Grade B) have an estimated 1-year survival rate of 80%; and patients with ≥10 points (Grade C) have an estimated 1-year surival rate of 45%. | |||
| Adapted from: Infante-Rivard C, et al. Hepatology. 1987.14 | |||
Treatment for cirrhosis depends on the cause
Although primary care physicians can often provide most, or all, of the care for those in stable condition, a specialist may be helpful in determining further testing to identify the underlying cause of the cirrhosis, which is essential to determining the most appropriate treatment. What’s more, research has shown that patients with cirrhosis whose care is managed by a primary care physician and a hepatologist have better outcomes than those who are treated by a primary care doctor alone.18
What to test for?
Tests to determine the cause of cirrhosis are listed in TABLE 2 . For an individual patient, diagnostic tests would be based on the suspected cause. A patient with a family history of hereditary hemochromatosis would be tested for elevated serum ferritin levels and hepatic iron content on liver biopsy sample; the transferrin saturation index would also be obtained, and the patient might be tested for specific gene mutations. A patient who drinks heavily would be tested for elevated gamma-glutamyl transpeptidase and mean corpuscular volume. For someone with obesity, diabetes, and an enlarged liver, standard lab tests, including high-density lipoprotein (HDL) cholesterol, glucose, and triglycerides, may be sufficient.
Keep in mind, however, that cirrhosis may have more than 1 contributing factor—obesity or chronic alcohol use and a virus, for example; alcohol abuse and metabolic fatty liver; or virus and hemochromatosis. Thus, it may require more than 1 type of treatment.
Alcohol abuse is the cause of 25% of cases of liver cirrhosis, and a contributor to another 25% to 50%.19 The key treatment here—and an ideal role for a family physician—is to refer the patient to a detoxification and treatment program and provide ongoing monitoring and support. Antiviral treatment may be helpful for a recovering alcoholic who also tests positive for hepatitis B or C virus, but because of potential problems with compliance, some physicians delay therapy until the patient has had at least 6 months of continuous abstinence. Although this is not an absolute criterion, the same period of abstinence may be required before a patient becomes eligible for a liver transplant.
NAFLD/NASH is typically diagnosed on the basis of lab values and physical presentation. For a stable patient, the primary treatment includes lifestyle change—a low-calorie, low-carbohydrate diet and an exercise regimen—and a possible switch to insulin for better glycemic control.
For patients who are not candidates for such targeted treatments, either because their disease is too advanced or they’re unable to tolerate the recommended therapy, numerous pharmaceutical preparations claiming antioxidant or anti-inflammatory properties are available. But only 1—an herbal extract known as silymarin, derived from the milk thistle plant and taken with vitamin E—has been found to have some protective effects.20
TABLE 2
Liver cirrhosis: Common causes, diagnostic tests, and treatments4,34-38
| Cause | Test (result) | Therapy |
|---|---|---|
| Alcohol | GGT (↑), MCV (↑) | Abstinence |
| HBV + delta virus infection | HBsAg (+) HBV-DNA(+) HBc-IgM (+) HDV-RNA (+) | Interferon alpha-2b, nucleoside (lamivudine, telbivudine, entecavir) and nucleotide (adefovir, tenofovir) analogs |
| HCV infection | HCV-RNA (+) | Interferon + ribavirin |
| Primary biliary cirrhosis | GGT (↑) Alkaline phosphatase (↑) AMA (+) | Ursodeoxycholate |
| Autoimmune hepatitis | ANA (+) ASMA (+) LKM (+) | Prednisone, azathioprine |
| Hemochromatosis | Ferritin (↑) Transferrin saturation index (>45%) Hepatic iron content (↑) HFE gene mutation (C282Y, H63D) | Phlebotomy, chelating agents |
| Wilson’s disease | Ceruloplasmin (↓) Serum copper (↓) 24h urinary copper excretion (↑) | D-penicillamine, zinc |
| NAFLD/NASH | HDL cholesterol (↓) Glucose (↑) Triglycerides (↑) | Low-calorie diet, physical activity, insulin-sensitizer drugs or insulin |
| AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth-muscle antibody; GGT, gamma–glutamyl transpeptidase; HBc-IgM, immunoglobulin M antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; HCV-RNA, hepatitis C virus RNA; HDL, high-density lipoprotein; HDV-RNA, hepatitis delta virus RNA; LKM, liver kidney microsomes; MCV, mean corpuscular volume, NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. | ||
Address systemic problems along with targeted treatment
Malnutrition is a serious problem for many patients with cirrhosis. Causes range from poor oral intake or malabsorption to ongoing alcohol use, chronic nausea, or early satiety because of compression from ascites. Dental problems that prevent the patient from chewing properly may be a contributing factor, as well.
Regardless of the cause, malnutrition is associated with muscle wasting, hypoalbuminemia, decreased resistance to infections, and variceal bleeding, and addressing it is a key part of treatment. Assess the nutritional status of every patient with cirrhosis, and stress the importance of multivitamin supplementation.21 If dental care is needed, take steps to see that the patient receives it.
Nutritional support, however, should be reserved for severely malnourished patients awaiting transplantation.22
Osteoporosis. Reduced bone formation—the result of vitamin D deficiency, hypoparathyroidism, and hypogonadism—is a well-known complication of end-stage cirrhosis. However, osteopenia may occur in an earlier stage of disease, especially in patients with cholestatic disease and those receiving antiviral therapy. Prescribe bisphosphonates, together with calcium and vitamin D3, to improve bone mineral density.23
Diabetes. The relationship between diabetes and cirrhosis is particularly complex, because diabetes can be both a causal factor and a consequence of cirrhosis. Diabetes is common in patients with NASH, and prevalent among those with hepatitis C and hemochromatosis. Multivariate analyses have found that diabetes has an independent negative effect on the progression of liver disease.24
Diet remains the first-line treatment for hyperglycemia, with metformin as the drug of choice if diet alone is unsuccessful. Sulfonylureas can be used, but require caution to avoid hypoglycemia. Glitazones are a newer alternative, but their value in patients with liver cirrhosis has not been studied. However, the use of any oral antidiabetic agent requires extra caution in patients with cirrhosis, and should be avoided in those with advanced liver disease. Although insulin requires intense self-monitoring of serum glucose levels, it is preferable to oral agents for this patient population.25
Managing complications of cirrhosis
Hospital, home, or long-term care? Whether patients with advanced cirrhosis can be maintained at home or require hospitalization or long-term care is best decided in consultation with patient, family, and other members of the health care team. One helpful tool is the Karnofsky Performance Scale Index (http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf), which scores patients from 0 to 100 based on their functional impairment.26 (Patients with decompensated liver cirrhosis and limited self-sufficiency typically score <50, indicating that they require home health care, hospice, or institutional care.) Whatever the outcome, the patient may need to be reevaluated as the disease progresses and complications occur.
Ascites, the most common complication of cirrhosis,27 is a primary reason for hospitalization, but may be managed on an outpatient basis, depending on the patient presentation. Determining factors include the presence or absence of portal hypertension, impaired albumin synthesis, decreased plasma oncotic pressure, and sodium retention. Diagnosis is based on physical exam and ultrasonography.
Initial treatment for ascites includes salt restriction28,29 and avoidance of NSAIDs, which promote renal sodium retention, followed by spironolactone (100–400 mg/d). Add furosemide (40-160 mg/d) if the fluid retention does not begin to resolve after 3 to 5 days of treatment. If the condition persists despite maximum tolerable doses of diuretics, large-volume paracentesis to remove transudative fluid (albumin <1 g/dL; serum/ascites albumin gradient >1.1) may be needed. A patient with recurrent or refractory ascites should see a specialist for further evaluation and the possibility of a transjugular intrahepatic portosystemic shunt (TIPS).
Abdominal pain and an ascitic granulocyte count >250/mm3 suggest spontaneous bacterial peritonitis (SBP)—a severe complication of ascites that can result in renal and liver failure. In addition to pain, patients may present with tense ascites and fever, followed by encephalopathy, shock, and increased serum creatinine levels. Hospitalization is required for SBP; therapy includes high-dose albumin and intravenous antibiotics, typically cephalosporin. Long-term prophylaxis with norfloxacin to prevent the recurrence of SBP is indicated.30
If your patient has ascites and is being cared for at home, talk to the patient and his or her family about the importance of a daily weight check. Tell them to contact you if the patient gains more than 4 to 8 lbs within a few days. Frequent electrolyte checks are needed, as well. An albumin infusion is required when serum levels are particularly low, or after large-volume paracentesis.31 Patients with SBP or refractory ascites generally have more advanced disease and a poor prognosis.
Portal hypertension/esophageal varices. The main aim of treating portal hypertension is to prevent esophageal variceal bleeding. The appearance of varices should be checked by endoscopy every 2 to 3 years, or yearly for patients at high risk of bleeding. Patients with varices can be managed with nonselective beta-blockers at doses that are sufficient to elicit a 25% reduction in resting heart rate. Those at high risk for bleeding and patients who have already had esophageal bleeding may require endoscopic band ligation.32 TIPS is an alternative for those whose previous treatments have failed.33
Hepatic encephalopathy. This potentially reversible decrease in neuropsychiatric function mainly affects patients with portal hypertension. Caused by reduced hepatic clearance of gut-deriving neurotoxins, hepatic encephalopathy is associated with a range of signs and symptoms—from subtle personality changes to coma, with flapping tremor as a frequent initial finding. Acid-base and electrolyte disturbances, constipation, infections, gastrointestinal bleeding, and sedatives can precipitate encephalopathy. Hepatic encephalopathy is a diagnosis of exclusion, however, requiring the exclusion of all other etiologies of altered mental status.
Treatment consists of identifying and correcting the precipitating factors, and includes electrolyte correction, colon cleansing, and acidification with lactulose. Dietary protein restriction is no longer advocated, because it may facilitate malnutrition and complications. Oral rifaximine is useful and well tolerated for suppression of intestinal bacterial flora. Venous infusion of branched-chain amino acids or flumazenil may be effective in case of coma.
Fever and sepsis. Infection is a high-risk factor for mortality in patients with cirrhosis, as it can lead to renal and liver failure, variceal bleeding, and hepatic encephalopathy. However, individuals with cirrhosis often do not develop the typical signs and symptoms of infection; leukocytosis may be absent because of severe leukopenia, for instance, and patients may be afebrile.
Thus, the general appearance of systemic illness is an indication for antibiotics, with quinolones and cephalosporins as first-line agents. Infections most commonly involve the urinary tract (25%-55%) or the respiratory tract (20%), or are related to SBP (10%-30%).33 Hospitalization is suggested in case of poor general health status or the appearance of organ dysfunction.
When medical therapy and other interventions fail to control complications, transplantation is the only alternative. Primary care physicians can play a role here, too, in referring potential candidates for liver transplants to specialists for further consideration.
CASE 1: Resolution
As we’ve already seen, John M.’s ultrasound revealed an enlarged liver. The results led to a probable diagnosis of an advanced form of NASH. Other lab tests indicated that he had poorly controlled diabetes, high triglyceride levels, and—for the first time—a low platelet count. His physician stressed the importance of following a low-calorie, low-carbohydrate diet and exercising regularly, prescribed insulin, and referred the patient to a hepatologist for further noninvasive evaluation of fibrosis and to determine whether liver biopsy was needed.
CASE 2: Resolution
Blood tests revealed that Anna B. had a low platelet count (64,000/mm3), elevated liver enzymes (AST 2× upper limit of normal [ULN], ALT 1.5× ULN, GGT 2.5× ULN), and high gamma-globulins (33.6%) with no monoclonal bands. Ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity, portal vein dilatation, and moderate ascites. She also tested positive for HCV and had an HCV-RNA reading of 15×106 IU/mL. No other cause of chronic liver disease emerged. Ms. B.’s physician told her that she had an osteoporotic vertebral fracture—a frequent comorbidity in patients with liver cirrhosis—and decompensated liver cirrhosis from an old HCV infection. He added that her abdomen was distended because of fluid retention. The physician recommended bed rest, prescribed paracetamol (1 g tid) and spironolactone (100 mg/d), and referred the patient to an orthopedist for treatment of the fracture and to a hepatologist to be evaluated for transplantation.
CORRESPONDENCE
Ignazio Grattagliano, MD, Department of Internal Medicine, University Medical School of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
• Suspect compensated liver cirrhosis in a patient with abnormal liver function tests, a low platelet count, and prolonged prothrombin time. C
• Use ultrasonography as a first-line diagnostic tool for liver cirrhosis. C
• Prescribe beta-blockers as prophylaxis for patients at risk for variceal bleeding. A
• Work collaboratively with hepatic specialists to manage the care of patients with cirrhosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: A patient with mildly elevated ALT and AST
John M., a 63-year-old truck driver with a family history of diabetes and arterial hypertension, is complaining of persistent fatigue—again. He has type 2 diabetes and takes metformin and repaglinide, but his blood pressure is normal. Lab tests reveal a recurrent mild elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of unknown origin; Mr. M. has no history of virus or hepatotoxic drugs, and reports only modest alcohol intake. He is obese, however, with a BMI of 34.5 and a waist circumference of 41 inches.
CASE 2: A patient with abdominal swelling
Anna B., a slim 68-year-old, comes in with 2 acute conditions: About a week ago, she noticed abdominal swelling and low back pain that began suddenly, after she carried a heavy load. She’s taken nonsteroidal anti-inflammatory drugs for 6 days, but the pain has not improved. The patient’s only significant clinical history is a hysterectomy, with oophorectomy, at age 38, and a recurrent elevation of serum transaminase levels that has never been investigated. Examination reveals an important kyphosis, and finger pressure on the vertebral spine or a position change exacerbates the pain. Her abdomen is swollen and tense, with a tympanic sound on the upper abdomen and a dull sound on the lower portion.
If John M. and Anna B. were your patients, would you suspect that they both have advanced liver disease? What diagnostic tests would you order, and how would you manage their care?
Cirrhosis has always been associated with high rates of morbidity and mortality. It is the 12th most common cause of death in the United States;1 in some parts of the world, its ranking as a cause of death is considerably higher.2,3 In recent years, however, cirrhosis has become the focus of greater attention both here and abroad, for 2 reasons: The first is the increasing prevalence of viral hepatitis and steatohepatitis, both of which are prominent causes of cirrhosis. The second is the improvement we’ve made in treatment: Not only can we slow the progression of cirrhosis, but in some cases, we can even restore hepatic function.4
The key to successful management of cirrhosis lies in spotting subtle signs and symptoms well in advance of the serious complications that can arise down the road. Here’s what to look for.
Early warnings you can’t afford to overlook
While the clinical presentation of a patient with liver cirrhosis is often asymptomatic, serum transminases—included in many standard laboratory tests as part of a routine examination—often provide the first sign of a problem.
Mildly elevated ALT in an asymptomatic patient may be transient and benign, or an indication of chronic liver disease.5 In fact, signs suggestive of significant liver disease have been reported in more than 20% of patients with ALT elevation.2 But because abnormal ALT values are common and frequently resolve, many primary care physicians pay little attention to this potentially important finding—and miss a key opportunity for early identification and treatment.6
Look at other lab values, risk factors, as well
Additional lab values that suggest the possibility of cirrhosis include an elevated AST/ALT ratio, a low platelet count (<150,000/L), elevated alkaline phosphatase, elevated bilirubin (>1.1 mg/dL), low serum albumin (<2.5 g/dL), and decreased prothrombin time (<100%). Potential causes include viral hepatitis, heavy alcohol use, hepatotoxic drugs, steatosis, and steatohepatitis.
The next step for a patient with any of these abnormal values is a thorough medication review and medical history. Identify all prescription and nonprescription drugs the patient is taking, as well as any herbal products and supplements, in search of hepatotoxic agents. Amiodarone and valproic acid, among other drugs, may cause steatosis, and some herbal products—particularly kava kava extract, used to treat anxiety and insomnia—have been linked to hepatitis and even liver failure.7 Question the patient about alcohol consumption and a history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders, as well.
At a minimum, schedule follow-up testing of asymptomatic patients with abnormal laboratory findings in no more than 6 months. Persistent ALT elevation in such patients is most commonly caused by major viruses, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH).8 Nonalcoholic fatty liver is especially likely in patients with clinical and demographic risk factors—those who, like John M., suffer from obesity or diabetes, or both.
Ultrasound yields further information
Further screening should be limited to patients who continue to have abnormal test results for 6 months or more or have multiple risk factors. While biopsy is still considered the gold standard for diagnosing and staging chronic liver disease, it should be considered, according to the American Gastroenterological Association, only if ultrasound and other tests have not been helpful in reaching a diagnosis.9
Often, though, ultrasonography aids in diagnosis. In the case of John M., ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity and signs of severe steatosis and mild splenomegaly, but no increase in portal vein diameter and no ascites. For asymptomatic patients with cirrhosis or an earlier stage of liver disease, ultrasound at 6-month intervals, combined with blood alpha-fetoprotein measurement, can be used to track disease progression and screen for hepatocellular carcinoma.10
Newer, noninvasive methods aid in diagnosis
Noninvasive means of evaluating the presence and extent of liver fibrosis and differentiating cirrhosis from noncirrhosis, developed in recent years, have been found to have positive predictive values greater than 85% to 90%.11 Transient elastography (FibroScan, London, England), which assesses liver stiffness, is 1 such method. Although it is often used successfully, however, morbid obesity, small intercostal spaces, and ascites limit the diagnostic capability of this medical device.12
Fibrosis can also be detected with the use of 1 or more algorithms—each testing blood samples for a different combination of serum surrogate markers for liver disease. Some widely used algorithms include the APRI (AST-to-platelets ratio index), the Fibrotest (aptoglobin, alpha-2 macroglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and bilirubin), the Hepascore (bilirubin, gamma-glutamyl transpeptidase, haluronic acid, alpha-2 macroglobulin, age, sex), and the BARD (BMI, AST/ALT ratio, diabetes).
Hepatologists often use the results of ultrasonography, followed by transient elastography in conjunction with findings from 1 or more of these algorithms, to determine which patients are candidates for liver biopsy.11,12
Staging is crucial, with or without biopsy
The decision to perform a liver biopsy should be based on a number of factors, including the patient’s age, lifestyle, liver chemistry abnormalities, desire for prognostic information, and associated comorbidities.9 Despite the value of biopsy, it is a costly procedure with potentially serious side effects and risks—and not always accepted by patients. In a recent survey of 1177 primary care physicians in France, as many as 59% of patients with chronic hepatitis C refused to undergo liver biopsy; what’s more, 22% of the doctors surveyed shared the patients’ hesitancy.13 Whether patients refuse biopsy or it is deemed unnecessary because ultrasound and other noninvasive tests result in a probable diagnosis, staging is necessary, both to guide therapy and to arrive at a prognosis.
Liver enzyme levels reveal little about organ integrity and are not useful for staging. But other parameters (specifically, bilirubin, albumin, and prothrombin time), combined with the presence (or absence) and severity of physical findings such as encephalopathy and ascites, are included in the Child-Pugh classification system ( TABLE 1 ),14 a widely used system that roughly indicates disease severity.15
The Model for End-stage Liver Disease (MELD)—and PELD, the pediatric model—use bilirubin, creatinine, and international normalized ratio values to classify disease severity. MELD and PELD scores are considered more accurate than the Child-Pugh score in determining short-term mortality,16 and are used by the United Network of Organ Sharing (UNOS) for liver allocation. You’ll find a calculator at http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=97.17
Despite the progress in diagnostic techniques, the life expectancy and quality of life for patients with advanced cirrhosis remains poor. Patients routinely experience fatigue, pruritus, ascites, encephalopathy, and bleeding; dyspepsia and malnutrition are common, as well. Cirrhosis also carries the risk of life-threatening complications, partly due to comorbidities—most notably, osteoporosis, malabsorption, and rheumatic diseases. Liver transplantation has the potential to change the life expectancy of these patients, but because of the extensive waiting lists, candidates for transplant often die before a liver becomes available.
But for many patients who are in stable condition—those with compensated cirrhosis, that is—the prognosis is far more hopeful: In addition to providing standard medical care, including immunization, if necessary, and nutritional counseling, targeted therapy is crucial to slow, or stop, disease progression.
TABLE 1
Child-Pugh: Classifying cirrhosis, predicting survival*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Prothrombin time (INR) | <4 sec (<1.7) | 4-6 sec (1.7-2.3) | > 6 sec (>2.3) |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Ascites | Absent | Mild | Severe |
| Encephalopathy | Absent | Mild | Severe |
| INR, international normalized ratio. | |||
| * Total the number of points for all 5 indicators (1 point for every answer in column 1, 2 points for every answer in column 2, and 3 points for every answer in column 3). Patients with ≤6 points (Grade A) have an estimated 1-year survival rate of 100%; patients with 7-9 points (Grade B) have an estimated 1-year survival rate of 80%; and patients with ≥10 points (Grade C) have an estimated 1-year surival rate of 45%. | |||
| Adapted from: Infante-Rivard C, et al. Hepatology. 1987.14 | |||
Treatment for cirrhosis depends on the cause
Although primary care physicians can often provide most, or all, of the care for those in stable condition, a specialist may be helpful in determining further testing to identify the underlying cause of the cirrhosis, which is essential to determining the most appropriate treatment. What’s more, research has shown that patients with cirrhosis whose care is managed by a primary care physician and a hepatologist have better outcomes than those who are treated by a primary care doctor alone.18
What to test for?
Tests to determine the cause of cirrhosis are listed in TABLE 2 . For an individual patient, diagnostic tests would be based on the suspected cause. A patient with a family history of hereditary hemochromatosis would be tested for elevated serum ferritin levels and hepatic iron content on liver biopsy sample; the transferrin saturation index would also be obtained, and the patient might be tested for specific gene mutations. A patient who drinks heavily would be tested for elevated gamma-glutamyl transpeptidase and mean corpuscular volume. For someone with obesity, diabetes, and an enlarged liver, standard lab tests, including high-density lipoprotein (HDL) cholesterol, glucose, and triglycerides, may be sufficient.
Keep in mind, however, that cirrhosis may have more than 1 contributing factor—obesity or chronic alcohol use and a virus, for example; alcohol abuse and metabolic fatty liver; or virus and hemochromatosis. Thus, it may require more than 1 type of treatment.
Alcohol abuse is the cause of 25% of cases of liver cirrhosis, and a contributor to another 25% to 50%.19 The key treatment here—and an ideal role for a family physician—is to refer the patient to a detoxification and treatment program and provide ongoing monitoring and support. Antiviral treatment may be helpful for a recovering alcoholic who also tests positive for hepatitis B or C virus, but because of potential problems with compliance, some physicians delay therapy until the patient has had at least 6 months of continuous abstinence. Although this is not an absolute criterion, the same period of abstinence may be required before a patient becomes eligible for a liver transplant.
NAFLD/NASH is typically diagnosed on the basis of lab values and physical presentation. For a stable patient, the primary treatment includes lifestyle change—a low-calorie, low-carbohydrate diet and an exercise regimen—and a possible switch to insulin for better glycemic control.
For patients who are not candidates for such targeted treatments, either because their disease is too advanced or they’re unable to tolerate the recommended therapy, numerous pharmaceutical preparations claiming antioxidant or anti-inflammatory properties are available. But only 1—an herbal extract known as silymarin, derived from the milk thistle plant and taken with vitamin E—has been found to have some protective effects.20
TABLE 2
Liver cirrhosis: Common causes, diagnostic tests, and treatments4,34-38
| Cause | Test (result) | Therapy |
|---|---|---|
| Alcohol | GGT (↑), MCV (↑) | Abstinence |
| HBV + delta virus infection | HBsAg (+) HBV-DNA(+) HBc-IgM (+) HDV-RNA (+) | Interferon alpha-2b, nucleoside (lamivudine, telbivudine, entecavir) and nucleotide (adefovir, tenofovir) analogs |
| HCV infection | HCV-RNA (+) | Interferon + ribavirin |
| Primary biliary cirrhosis | GGT (↑) Alkaline phosphatase (↑) AMA (+) | Ursodeoxycholate |
| Autoimmune hepatitis | ANA (+) ASMA (+) LKM (+) | Prednisone, azathioprine |
| Hemochromatosis | Ferritin (↑) Transferrin saturation index (>45%) Hepatic iron content (↑) HFE gene mutation (C282Y, H63D) | Phlebotomy, chelating agents |
| Wilson’s disease | Ceruloplasmin (↓) Serum copper (↓) 24h urinary copper excretion (↑) | D-penicillamine, zinc |
| NAFLD/NASH | HDL cholesterol (↓) Glucose (↑) Triglycerides (↑) | Low-calorie diet, physical activity, insulin-sensitizer drugs or insulin |
| AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth-muscle antibody; GGT, gamma–glutamyl transpeptidase; HBc-IgM, immunoglobulin M antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; HCV-RNA, hepatitis C virus RNA; HDL, high-density lipoprotein; HDV-RNA, hepatitis delta virus RNA; LKM, liver kidney microsomes; MCV, mean corpuscular volume, NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. | ||
Address systemic problems along with targeted treatment
Malnutrition is a serious problem for many patients with cirrhosis. Causes range from poor oral intake or malabsorption to ongoing alcohol use, chronic nausea, or early satiety because of compression from ascites. Dental problems that prevent the patient from chewing properly may be a contributing factor, as well.
Regardless of the cause, malnutrition is associated with muscle wasting, hypoalbuminemia, decreased resistance to infections, and variceal bleeding, and addressing it is a key part of treatment. Assess the nutritional status of every patient with cirrhosis, and stress the importance of multivitamin supplementation.21 If dental care is needed, take steps to see that the patient receives it.
Nutritional support, however, should be reserved for severely malnourished patients awaiting transplantation.22
Osteoporosis. Reduced bone formation—the result of vitamin D deficiency, hypoparathyroidism, and hypogonadism—is a well-known complication of end-stage cirrhosis. However, osteopenia may occur in an earlier stage of disease, especially in patients with cholestatic disease and those receiving antiviral therapy. Prescribe bisphosphonates, together with calcium and vitamin D3, to improve bone mineral density.23
Diabetes. The relationship between diabetes and cirrhosis is particularly complex, because diabetes can be both a causal factor and a consequence of cirrhosis. Diabetes is common in patients with NASH, and prevalent among those with hepatitis C and hemochromatosis. Multivariate analyses have found that diabetes has an independent negative effect on the progression of liver disease.24
Diet remains the first-line treatment for hyperglycemia, with metformin as the drug of choice if diet alone is unsuccessful. Sulfonylureas can be used, but require caution to avoid hypoglycemia. Glitazones are a newer alternative, but their value in patients with liver cirrhosis has not been studied. However, the use of any oral antidiabetic agent requires extra caution in patients with cirrhosis, and should be avoided in those with advanced liver disease. Although insulin requires intense self-monitoring of serum glucose levels, it is preferable to oral agents for this patient population.25
Managing complications of cirrhosis
Hospital, home, or long-term care? Whether patients with advanced cirrhosis can be maintained at home or require hospitalization or long-term care is best decided in consultation with patient, family, and other members of the health care team. One helpful tool is the Karnofsky Performance Scale Index (http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf), which scores patients from 0 to 100 based on their functional impairment.26 (Patients with decompensated liver cirrhosis and limited self-sufficiency typically score <50, indicating that they require home health care, hospice, or institutional care.) Whatever the outcome, the patient may need to be reevaluated as the disease progresses and complications occur.
Ascites, the most common complication of cirrhosis,27 is a primary reason for hospitalization, but may be managed on an outpatient basis, depending on the patient presentation. Determining factors include the presence or absence of portal hypertension, impaired albumin synthesis, decreased plasma oncotic pressure, and sodium retention. Diagnosis is based on physical exam and ultrasonography.
Initial treatment for ascites includes salt restriction28,29 and avoidance of NSAIDs, which promote renal sodium retention, followed by spironolactone (100–400 mg/d). Add furosemide (40-160 mg/d) if the fluid retention does not begin to resolve after 3 to 5 days of treatment. If the condition persists despite maximum tolerable doses of diuretics, large-volume paracentesis to remove transudative fluid (albumin <1 g/dL; serum/ascites albumin gradient >1.1) may be needed. A patient with recurrent or refractory ascites should see a specialist for further evaluation and the possibility of a transjugular intrahepatic portosystemic shunt (TIPS).
Abdominal pain and an ascitic granulocyte count >250/mm3 suggest spontaneous bacterial peritonitis (SBP)—a severe complication of ascites that can result in renal and liver failure. In addition to pain, patients may present with tense ascites and fever, followed by encephalopathy, shock, and increased serum creatinine levels. Hospitalization is required for SBP; therapy includes high-dose albumin and intravenous antibiotics, typically cephalosporin. Long-term prophylaxis with norfloxacin to prevent the recurrence of SBP is indicated.30
If your patient has ascites and is being cared for at home, talk to the patient and his or her family about the importance of a daily weight check. Tell them to contact you if the patient gains more than 4 to 8 lbs within a few days. Frequent electrolyte checks are needed, as well. An albumin infusion is required when serum levels are particularly low, or after large-volume paracentesis.31 Patients with SBP or refractory ascites generally have more advanced disease and a poor prognosis.
Portal hypertension/esophageal varices. The main aim of treating portal hypertension is to prevent esophageal variceal bleeding. The appearance of varices should be checked by endoscopy every 2 to 3 years, or yearly for patients at high risk of bleeding. Patients with varices can be managed with nonselective beta-blockers at doses that are sufficient to elicit a 25% reduction in resting heart rate. Those at high risk for bleeding and patients who have already had esophageal bleeding may require endoscopic band ligation.32 TIPS is an alternative for those whose previous treatments have failed.33
Hepatic encephalopathy. This potentially reversible decrease in neuropsychiatric function mainly affects patients with portal hypertension. Caused by reduced hepatic clearance of gut-deriving neurotoxins, hepatic encephalopathy is associated with a range of signs and symptoms—from subtle personality changes to coma, with flapping tremor as a frequent initial finding. Acid-base and electrolyte disturbances, constipation, infections, gastrointestinal bleeding, and sedatives can precipitate encephalopathy. Hepatic encephalopathy is a diagnosis of exclusion, however, requiring the exclusion of all other etiologies of altered mental status.
Treatment consists of identifying and correcting the precipitating factors, and includes electrolyte correction, colon cleansing, and acidification with lactulose. Dietary protein restriction is no longer advocated, because it may facilitate malnutrition and complications. Oral rifaximine is useful and well tolerated for suppression of intestinal bacterial flora. Venous infusion of branched-chain amino acids or flumazenil may be effective in case of coma.
Fever and sepsis. Infection is a high-risk factor for mortality in patients with cirrhosis, as it can lead to renal and liver failure, variceal bleeding, and hepatic encephalopathy. However, individuals with cirrhosis often do not develop the typical signs and symptoms of infection; leukocytosis may be absent because of severe leukopenia, for instance, and patients may be afebrile.
Thus, the general appearance of systemic illness is an indication for antibiotics, with quinolones and cephalosporins as first-line agents. Infections most commonly involve the urinary tract (25%-55%) or the respiratory tract (20%), or are related to SBP (10%-30%).33 Hospitalization is suggested in case of poor general health status or the appearance of organ dysfunction.
When medical therapy and other interventions fail to control complications, transplantation is the only alternative. Primary care physicians can play a role here, too, in referring potential candidates for liver transplants to specialists for further consideration.
CASE 1: Resolution
As we’ve already seen, John M.’s ultrasound revealed an enlarged liver. The results led to a probable diagnosis of an advanced form of NASH. Other lab tests indicated that he had poorly controlled diabetes, high triglyceride levels, and—for the first time—a low platelet count. His physician stressed the importance of following a low-calorie, low-carbohydrate diet and exercising regularly, prescribed insulin, and referred the patient to a hepatologist for further noninvasive evaluation of fibrosis and to determine whether liver biopsy was needed.
CASE 2: Resolution
Blood tests revealed that Anna B. had a low platelet count (64,000/mm3), elevated liver enzymes (AST 2× upper limit of normal [ULN], ALT 1.5× ULN, GGT 2.5× ULN), and high gamma-globulins (33.6%) with no monoclonal bands. Ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity, portal vein dilatation, and moderate ascites. She also tested positive for HCV and had an HCV-RNA reading of 15×106 IU/mL. No other cause of chronic liver disease emerged. Ms. B.’s physician told her that she had an osteoporotic vertebral fracture—a frequent comorbidity in patients with liver cirrhosis—and decompensated liver cirrhosis from an old HCV infection. He added that her abdomen was distended because of fluid retention. The physician recommended bed rest, prescribed paracetamol (1 g tid) and spironolactone (100 mg/d), and referred the patient to an orthopedist for treatment of the fracture and to a hepatologist to be evaluated for transplantation.
CORRESPONDENCE
Ignazio Grattagliano, MD, Department of Internal Medicine, University Medical School of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
1. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National Vital Stat Rep. 2009;57:(14):1-135.Available at: www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. Accessed September 16, 2009.
2. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442-1449.
3. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851.
5. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110.
6. Sherwood P, Lyburn I, Brown S, et al. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ. 2001;322:276-278.
7. US Food and Drug Administration. Food. Consumer advisory: Kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. Accessed September 11, 2009.
8. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863.
9. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
10. Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465-1473.
11. Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
12. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
13. Bonny C, Rayssiguier R, Ughetto S, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region [In French]. Gastroenterol Clin Biol. 2003;27:1021-1025.
14. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
15. Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;Aug. 20 [Epub ahead of print].
16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96.
17. United Network of Organ Sharing. Resources. Meld/Peld calculator. Available at: http://www.unos.org/resources/meldPeldCalculator.asp. Accessed September 11, 2009.
18. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
19. Habib A, Bond WM, Heuman DM. Long-term management of cirrhosis. Appropriate supportive care is both critical and difficult. Postgrad Med. 2001;109:101-103.
20. Flora K, Hahn M, Rosen H, et al. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143.
21. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis [In French]. Gastroenterol Clin Biol. 2008;32:265-273.
22. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55.
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(suppl 1):i1-i9.
24. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75.
25. Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288.
26. Karnofsky Performance Scale Index. Available at: http://www.medal.org/visitor/www%5CActive%5Cch1%5Cch1.01%5Cch1.01.01.aspx. Accessed September 11, 2009.
27. Gentilini P, Bernardi M, Bolondi L, et al. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546.
28. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
29. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856.
30. Gines P, et al. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1:129-155.
31. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
32. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091.
33. McCormick PA, Greenslade L, Kibbler CC, et al. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology. 1997;25:833-836.
34. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
35. European Association tor the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242.
36. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
37. Portincasa P, Grattagliano I, Palmieri VO, et al. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889-2900.
38. Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328-338.
1. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National Vital Stat Rep. 2009;57:(14):1-135.Available at: www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. Accessed September 16, 2009.
2. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442-1449.
3. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851.
5. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110.
6. Sherwood P, Lyburn I, Brown S, et al. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ. 2001;322:276-278.
7. US Food and Drug Administration. Food. Consumer advisory: Kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. Accessed September 11, 2009.
8. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863.
9. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
10. Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465-1473.
11. Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
12. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
13. Bonny C, Rayssiguier R, Ughetto S, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region [In French]. Gastroenterol Clin Biol. 2003;27:1021-1025.
14. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
15. Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;Aug. 20 [Epub ahead of print].
16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96.
17. United Network of Organ Sharing. Resources. Meld/Peld calculator. Available at: http://www.unos.org/resources/meldPeldCalculator.asp. Accessed September 11, 2009.
18. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
19. Habib A, Bond WM, Heuman DM. Long-term management of cirrhosis. Appropriate supportive care is both critical and difficult. Postgrad Med. 2001;109:101-103.
20. Flora K, Hahn M, Rosen H, et al. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143.
21. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis [In French]. Gastroenterol Clin Biol. 2008;32:265-273.
22. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55.
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(suppl 1):i1-i9.
24. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75.
25. Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288.
26. Karnofsky Performance Scale Index. Available at: http://www.medal.org/visitor/www%5CActive%5Cch1%5Cch1.01%5Cch1.01.01.aspx. Accessed September 11, 2009.
27. Gentilini P, Bernardi M, Bolondi L, et al. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546.
28. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
29. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856.
30. Gines P, et al. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1:129-155.
31. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
32. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091.
33. McCormick PA, Greenslade L, Kibbler CC, et al. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology. 1997;25:833-836.
34. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
35. European Association tor the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242.
36. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
37. Portincasa P, Grattagliano I, Palmieri VO, et al. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889-2900.
38. Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328-338.