User login
How best to manage chronic cholestasis
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.
That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)
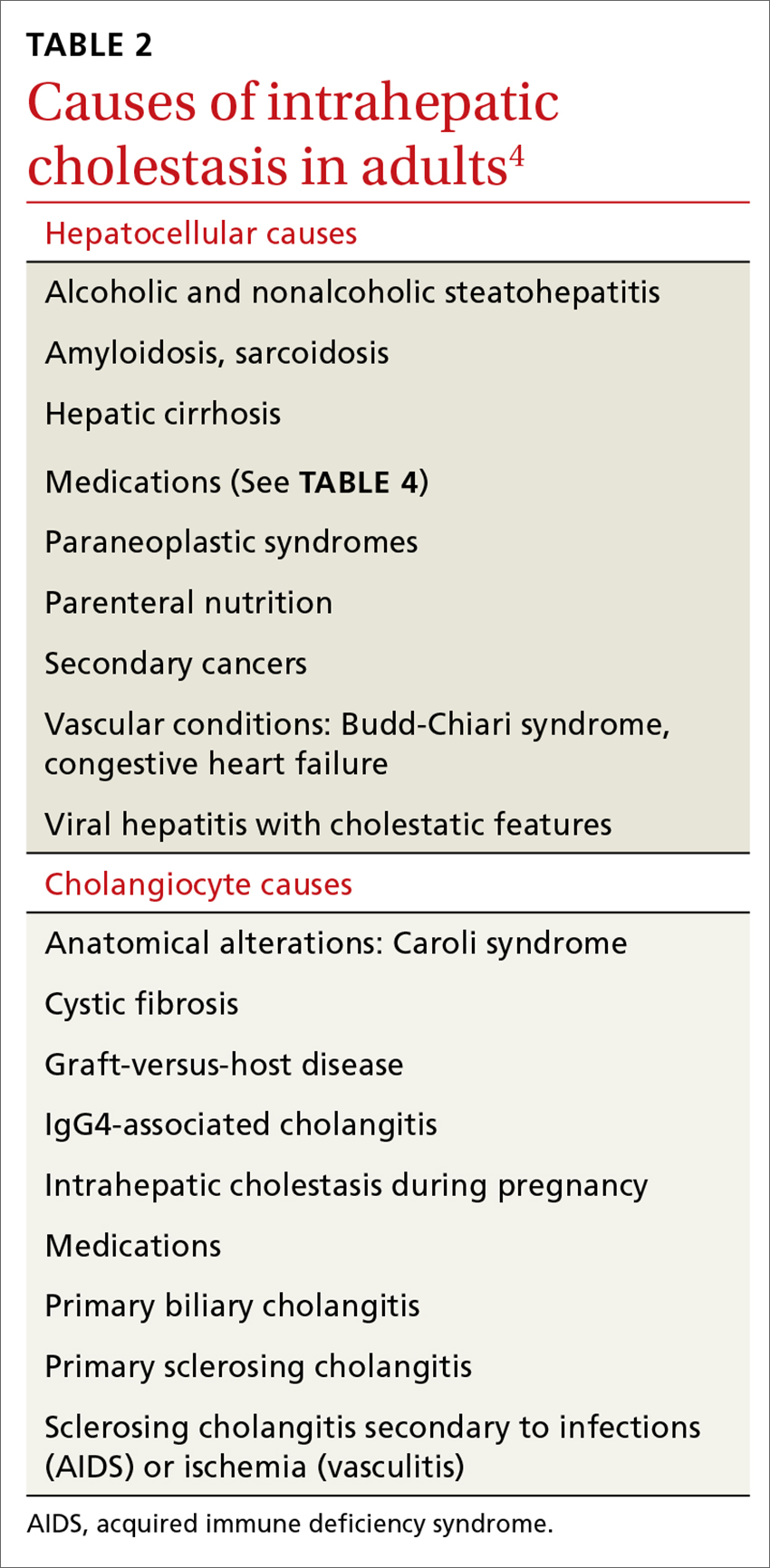
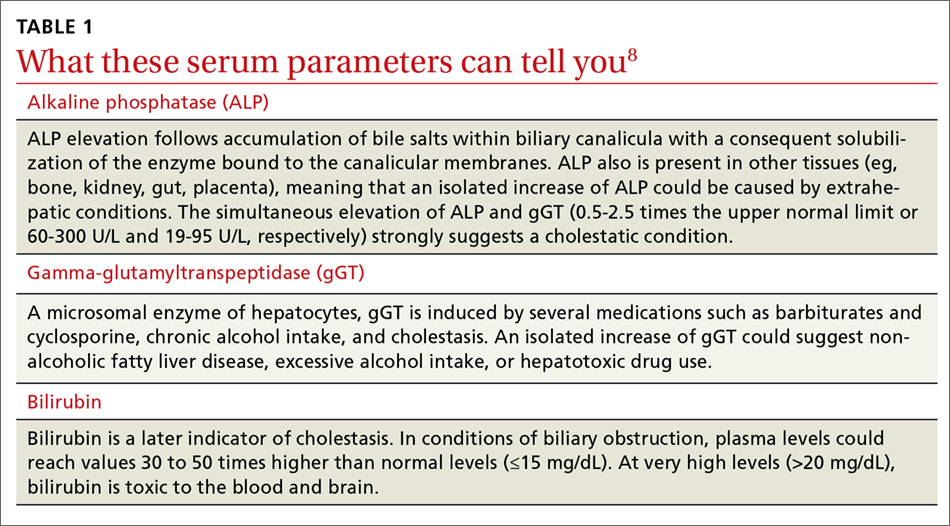
Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
CASE
A 44-year-old nurse describes persistent fatigue and itching over the last 2 months. She is taking ramipril 5 mg/d for hypertension and has a family history of rheumatic disease. Lab tests reveal a recurrent moderate elevation of gamma glutamyl-transpeptidase (gGT; 75 U/L) associated with, on some occasions, mild elevation of alanine aminotransferase (ALT) levels (100 U/L) of unknown origin. She has no history of hepatitis virus infection, hepatotoxic medications, or alcohol intake. She is overweight with a body mass index of 28.5 kg/m2 and a waist circumference of 99 cm (39 inches). Liver ultrasonography detects an enlarged liver with diffuse echostructure dishomogeneity, but no signs of cirrhosis or portal hypertension. The patient’s biliary tree is not dilated.
How would you proceed with the care of this patient?
Cholestasis is characterized by the alteration of bile flow through any part of the biliary system, from the hepatocyte basocellular membrane to the duodenum. The condition is classified as intrahepatic when the cause is a defect of hepatocellular function or obstruction of the biliary tree within the liver. The extrahepatic form includes all conditions obstructing bile flow in the main biliary tract (choledochus, common bile duct).
The key to successfully managing cholestasis lies in the early identification of subtle signs and symptoms before serious complications can arise. In the review that follows, we provide guidance for evaluating laboratory and imaging results that are vital to the accurate diagnosis of intrahepatic and extrahepatic cholestasis. We also detail treatment recommendations.
Clues—subtle and otherwise—of cholestasis
Clinical features of cholestasis include fatigue and itching all over the skin. The latter likely is caused by induction of the enzyme autotaxin, which produces the neuronal activator lysophosphatidic acid. Retention of pruritogenic substances that normally are excreted into bile might contribute to pruritus as well.1 Jaundice, dark urine, and pale and fatty stools occur with advanced disease. However, a cholestatic condition can be detected in asymptomatic patients with elevated biochemical markers.
Continue to: Mildly elevated gGT and/or alkaline phosphatase (ALP)
Mildly elevated gGT and/or alkaline phosphatase (ALP) (0.5-2.5 times the upper normal limit [UNL] or 19-95 U/L and 60-300 U/L, respectively2) in the presence of normal transaminase levels (<20 U/L) in an asymptomatic patient can indicate chronic liver disease. Signs suggestive of significant liver disease have been reported in many patients with gGT or ALP elevation with good sensitivity (65%) and specificity (83%) for a diagnosis of intrahepatic cholestasis.3 However, because abnormal gGT values are common and often resolve spontaneously, family physicians (FPs) may pay little attention to this finding, thus missing an opportunity for early identification and treatment.

That’s why it’s important to schedule follow-up testing within 6 months for asymptomatic patients with abnormal laboratory findings. Persistent elevation of gGT alone or accompanied by ALP and ALT elevation (ALT >0.5 times the UNL or >18 U/L) is the most common feature of a chronic (>6 months) cholestatic condition.4 (In particular, elevated ALP levels appear to be associated with more aggressive disease and predict risk of liver transplantation or death in patients with primary biliary cholangitis (PBC).5,6 Lowering ALP levels is associated with improved disease outcomes, including transplant-free survival rates.5,7)

Elevated serum aminotransferase levels (aspartate aminotransferase [AST] >0.5 times the UNL or 17.5 U/L; ALT >0.5 times the UNL or >18 U/L) and bilirubin (>1.1 mg/dL), with predominance of the conjugated form (TABLE 18), suggest possible cholestasis. In light of such findings, a clinician’s next step should be to distinguish intrahepatic from extrahepatic conditions. (For a detailed list of the causes of intra- and extrahepatic cholestasis, see TABLES 24 and 3.9)

Patient’s history can provide important clues
A thorough patient history is especially important when cholestasis is suspected. Details about the patient’s occupation, environment, and lifestyle are key, as are the specifics of prescribed or over-the-counter medications and supplements that could be hepatotoxic (TABLE 410). A number of exogenous substances can cause liver injury, and the use of some herbal products (senna, black cohosh, greater celandine, kava) have been linked to hepatitis and cholestasis.11 Ask patients about alcohol use and history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders.

Continue to: Indicators pointing to cholestasis? It's time for ultrasonography
Indicators pointing to cholestasis? It’s time for ultrasonography
While biopsy is considered the gold standard for diagnosing and staging chronic cholestatic liver disease and can exclude an extrahepatic obstruction, it should be employed only if blood tests have been confirmed, second-level tests have been performed, and ultrasound is inconclusive.12 (More on biopsy in a bit.)
Ultrasonography is a low-cost, widely available, noninvasive test that allows easy identification of extrahepatic dilatation of the biliary tree and sometimes the underlying cause, as well. Ultrasonography identifies extrahepatic cholestasis by allowing visualization of an enlarged choledochus (>7 mm) or common hepatic duct (>5 mm) and an intrahepatic bile duct diameter that is more than 40% larger than adjacent branches of the portal vein.13 However, ultrasonography has a low diagnostic sensitivity for many conditions (eg, 15% to 89% for detecting common bile duct stones),14 requiring other diagnostic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) or magnetic resonance cholangiopancreatography (MRCP), before reaching a diagnosis.
For asymptomatic patients with cirrhosis or those at an early stage of liver disease, ultrasound at 6-month intervals combined with serum liver function tests can be useful to track disease progression and screen for hepatocellular carcinoma or cholangiocarcinoma.15,16
New noninvasive methods. Noninvasive tools for evaluating the presence and severity of liver fibrosis and for differentiating cirrhosis from noncirrhotic conditions have positive predictive values >85% to 90% for some chronic liver diseases.17 Transient elastography, which assesses liver stiffness, is one such method. Although it is often used successfully, morbid obesity, small intercostal spaces, and ascites limit its diagnostic capability.18 Recently, some questions about the validity of elastography to assess the extent of fibrosis in patients with chronic cholestatic conditions have been reported.19,20
Suspect intrahepatic cholestasis? Your next steps
If imaging techniques do not show bile duct obstruction and you suspect the intrahepatic form, second-level tests could have strategic importance. This is where antimitochondrial antibodies (AMAs) come in. AMAs are immunoglobulins (IgG and IgM) directed against mitochondrial antigens. They are important markers for PBC, which is a T-lymphocyte-mediated attack on small intralobular bile ducts resulting in their gradual destruction and eventual disappearance. The sustained loss of intralobular bile ducts leads to signs and symptoms of cholestasis and eventually results in cirrhosis and liver failure.
AMA serum levels show high sensitivity and specificity (90% and 95%, respectively) for PBC.21 Some PBC patients (<5%) show histologic confirmation of the disease, but have negative AMA tests (AMA negative PBC or autoimmune cholangitis).22 Therefore, according to the American Association for the Study of Liver Diseases, diagnosis of PBC is guided by the combination of serologic, biochemical, and histologic criteria.23 Many PBC patients with or without a positive AMA (≥1:40) also have positive circulating antinuclear antibodies (ANA; ≥1:80). The recent availability of lab tests for antibodies (anti-M2, anti-gp120, anti-sp100) has allowed identification of subgroups of patients who have a more aggressive form of PBC. Patients with PBC often have elevated levels of circulating IgM (>280 mg/dL).
Continue to: Other circulating antibodies
Other circulating antibodies can help discriminate among cholestatic disorders. In particular, positive tests for perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) are found in 25% to 95% of patients with primary sclerosing cholangitis (PSC), a chronic progressive disorder of unknown etiology that is characterized by inflammation, fibrosis, and stricturing of medium and large ducts of the intrahepatic and extrahepatic biliary tree.24 Anti-smooth muscle antibodies (SMA) can be observed in both PSC and autoimmune hepatitis.
Finally, there are syndromes with serologic and histologic overlap that are characterized by the simultaneous presence of PBC with autoimmune hepatitis or PSC or overlap of PSC with autoimmune hepatitis.
Liver biopsy fills in the rest of the diagnostic picture
Unfortunately, blood tests reveal little about organ integrity and are not useful for disease staging. The decision to perform a liver biopsy should be based on several factors, including the patient’s age, serum parameters, the need to stage the disease, therapy choices, and prognosis.12 One should also consider that biopsy is a costly procedure with potentially serious adverse effects; it should not be repeated frequently. However, when a biopsy is done, it provides critical information, including damage to medium-sized intrahepatic bile ducts with neoductular formation or bile duct scars and strictures.
Treating intrahepatic cholestasis
Although FPs often can provide most—or even all—of the care for patients with stable conditions, a specialist consultation might recommend further testing to identify the underlying disease, which is essential to establish the most appropriate treatment.
Treatment of patients with PBC is based on administering hydrophilic secondary bile salt ursodeoxycholic acid (UDCA) 15 mg/kg/d, which is used to equilibrate the ratio between hydrophilic and hydrophobic bile salts in the liver and bile,25 and is the only treatment approved by the US Food and Drug Administration (FDA) for PBC.4 Tauroursodeoxycholate is better absorbed than UDCA, and, although partially deconjugated and reconjugated with glycine, it undergoes reduced biotransformation to more hydrophobic metabolites and has benefits, including antioxidant, immunomodulation, and neuroprotective effects over UDCA—especially for long-term therapy in PBC.26 However, it is not used often in clinical practice.
Continue to: Bile acid administration counters the cytotoxic effect...
Bile acid administration counters the cytotoxic effect of hydrophobic bile salts. Although it seems that UDCA might improve biochemical and histologic features of the disease at earlier stages (I-II), it fails in patients with more advanced disease.27 In addition, monitoring and defining response to UDCA is inconsistent, partly because of variations in guideline criteria.28,29
Recently a new molecule, obeticholic acid (OCA), has been approved by the FDA. A farnesoid X receptor agonist, OCA is indicated for treating patients who do not tolerate UDCA or as an adjunct to UDCA in those with a partial response to UDCA, defined as lowering ALP levels by <1.5 times the baseline value after 12 months of treatment.
Treating PSC is more complex. Combination therapy with prednisone and azathioprine is recommended only when there is an overlap syndrome between PSC and autoimmune hepatitis.4 UDCA at a high dosage (15-20 mg/kg/d) is used to facilitate long-lasting biochemical remission. These patients also need to be monitored for inflammatory bowel diseases, which affect up to 75% of patients,30 and for cholangiocarcinoma, which is a life-limiting complication because of a lack of therapy options. Finally, these patients might need endoscopic-guided dilatation of the biliary tree when they have evidence of dominant fibrotic strictures of the greater bile ducts.14,31
Addressing the systemic effects of intrahepatic cholestasis
Pruritus. A number of potential pruritogens, including bile salts, endogenous opioids, histamine, serotonin, and lisophosphatidic acid (LPA), can be targeted to relieve pruritus.
- Bile acid resin binders such as cholestyramine are the first step for treating pruritus. UDCA also can be useful, mainly for intrahepatic cholestasis during pregnancy. Rifampicin, 300 mg/d, improves cholestatic pruritus, but is associated with hepatotoxicity and a number of severe reactions, such as nausea, loss of appetite, hemolytic anemia, and thrombocytopenia.31
- Most evidence favors a role for opioids in relieving itch, and micro-opioid receptor antagonists (naltrexone, naloxone, nalmefene) that exert an antipruritic effect can be effective.
- Sertraline (a selective serotonin reuptake inhibitor), 50 to 75 mg/d, usually is well tolerated in patients with chronic cholestasis and exerts a beneficial effect on pruritus in approximately 40% of patients.32
- Extracorporeal albumin dialysis removes albumin-bound pruritogens and has been found to be effective in patients with liver failure. Steroids and UV light also can be used in select patients.
- The potent neuronal activator LPA and its converting enzyme autotaxin have been identified in the serum of patients with cholestatic pruritus; experimental modalities using LPA antagonists are ongoing for treating pruritus in patients who do not respond to other medications.33
Continue to: Malnutrition
Malnutrition. Many patients with cholestasis are at risk for malnutrition, which can be exacerbated in those with cirrhosis. Causes of malnutrition include poor oral intake, malabsorption, or dental problems that prevent the patient from chewing. Assess the nutritional status of every patient with chronic cholestasis, and stress the importance of multivitamin supplementation to reverse systemic alterations caused by malnutrition.34
When the patient has advanced disease
Despite progress in diagnostic techniques, life expectancy and quality of life for patients with advanced cholestatic conditions remain poor. Patients routinely experience fatigue, pruritus, and complications of cirrhosis including ascites, encephalopathy, and bleeding. Cholestasis also carries the risk of life-threatening complications, partly because of comorbidities such as osteoporosis and malabsorption.
Liver transplantation can improve the life expectancy of patients with advanced disease, but because of long waiting lists, candidates for transplant often die before an organ becomes available. For many patients who are not in end-stage condition, targeted therapy is crucial to slow disease progression and is recommended along with hepatitis A and B vaccinations and nutritional counseling.35
Extrahepatic cholestasis is suspected? How to proceed
Computer tomography (CT) is recommended for better identification of neoplastic causes of biliary obstruction and for staging purposes. MRCP is an excellent noninvasive imaging technique for evaluating biliary ducts.36
MRCP has 92% to 93% sensitivity and 97% to 98% specificity for diagnosing biliary duct stones.37 MRCP also is the first-choice modality for evaluating bile ducts in patients with suspected PSC. If performed in expert centers, the diagnostic accuracy reaches that of ERCP. A meta-analysis of studies from 2000 to 2006 has shown a sensitivity of 86% and specificity of 94% for diagnosing PSC.38
Endoscopic ultrasonography, which uses an ultrasonographic probe, allows clinicians to evaluate the integrity of the biliary and pancreatic ducts and is effective for diagnosing and staging cancer of the ampulla of Vater (sensitivity 93% vs 7% for abdominal ultrasonography and 29% for CT), and identifying biliary stones and biliary tree strictures.
Continue to: ERCP
ERCP is widely employed for diagnosing and treating pancreatobiliary diseases; however, its use has dropped over the last 10 years because of the risk of complications. ERCP is nearly exclusively used as a therapeutic procedure for pancreatic sphincterotomy, biliary dilatations, and removing biliary stones. It also has a diagnostic role in dominant stenosis or suspected biliary malignancy using brushing cytology and sampling biopsies of the bile ducts.
Treating extrahepatic cholestasis
Treatment of the different underlying conditions that cause extrahepatic cholestasis is surgical. Thus, the potential surgical techniques that can resolve or improve an extrahepatic cholestatic condition are guided by the surgeon and beyond the scope of this article.
Treating osteopenia: A concern for intra- and extrahepatic cholestasis
Vitamin D deficiency as a consequence of reduced intestinal absorption (poor availability of bile salts) or decreased hepatic activation to 25,OH-cholecalcipherol in both intrahepatic and extrahepatic cholestasis can lead to reduced bone formation.39 However, osteopenia can occur even in early stages of the disease. Prescribing bisphosphonates, in combination with calcium and vitamin D3, to improve bone mineral density is a good practice.40
CASE
Blood tests and ultrasound imaging suggest the presence of a chronic liver disease. Other lab tests indicate that the patient has an ALP level 3 times normal. This finding, together with the other tests, points to a likely diagnosis of intrahepatic cholestatic liver disease. Serology confirms positivity for ANA (1:160) and AMA (1:640). The clinician suspects PBC, so the patient is referred to a liver specialist for further evaluation and to determine whether a liver biopsy is needed.
The liver specialist confirms the diagnosis of PBC, performs a transient elastographym, which indicates a low-grade liver fibrosis (F1 out of 4), and starts therapy with UDCA.
CORRESPONDENCE
Ignazio Grattagliano, MD, Italian College of General Practitioners and Primary Care, Via del Sansovino 179, 50142, Florence, Italy; [email protected].
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
1. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
2. Deska Pagana K, Pagana TJ. Mosby’s Diagnostic and Laboratory Test Reference. 13th ed. St. Louis, MO: Elsevier; 2017.
3. Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5’ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259-263.
4. European Association for the Study of the Liver. EASL Clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267.
5. Lammers WJ, van Buuren HR, Hirschfield GM, et al; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-1349.
6. Trivedi PJ, Corpechot C, Pares A, et al. Risk stratification in autoimmune cholestatic liver diseases: opportunities for clinicians and trialists. Hepatology. 2016;63:644-659.
7. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804-1812.
8. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223-2230.
9. Assy N, Jacob G, Spira G, et al. Diagnostic approach to patients with cholestatic jaundice. World J Gastroenterol. 1999;5:252-262.
10. Padda MS, Sanchez M, Akhtar AJ, et al. Drug-induced cholestasis. Hepatology. 2011;53:1377-1387.
11. US Food and Drug Administration. Food. Consumer advisory: kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://wayback.archive-it.org/7993/20171114232640/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/SafetyAlertsAdvisories/ucm085482.htm. Accessed June 19, 2018.
12. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
13. Rogoveanu I, Gheonea DI, Saftoiu A, et al. The role of imaging methods in identifying the causes of extrahepatic cholestasis. J Gastrointestin Liver Dis. 2006;15:265-271.
14. Gotthardt DN, Rudolph G, Klöters-Plachky P, et al. Endoscopic dilation of dominant stenoses in primary sclerosing cholangitis: outcome after long-term treatment. Gastrointest Endosc. 2010;71:527-534.
15. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2015;11:38-46.
16. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022.
17. Pinzani M, Vizzutti F, Arena U, et al. Technology insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
18. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
19. Van Gossum A, Pironi L, Messing B, et al. Transient elastography (FibroScan) is not correlated with liver fibrosis but with cholestasis in patients with long-term home parenteral nutrition. JPEN. 2015;39:719-724.
20. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723.
21. European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
22. Ozaslan E, Efe C, Gokbulut Ozaslan N. The diagnosis of antimitochondrial antibody-negative primary biliary cholangitis. Clin Res Hepatol Gastroenterol. 2016;40:553-561.
23. Lindor KD, Gershwin ME, Poupon R, et al; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308.
24. Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3781-3791.
25. Dilger K, Hohenester S, Winkler-Budenhofer U, et al. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133-140.
26. Invernizzi P, Setchell KD, Crosignani A, et al. Differences in the metabolism and disposition of ursodeoxycholic acid and of its taurine-conjugated species in patients with primary biliary cirrhosis. Hepatology. 1999;29:320-327.
27. Jorgensen R, Angulo P, Dickson ER, et al. Results of long-term ursodiol treatment for patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97:2647-2650.
28. Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130:715-720.
29. Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877.
30. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (NY). 2011;7:235-241.
31. Rodriguez HJ, Bass NM. Primary sclerosing cholangitis. Semin Gastrointest Dis. 2003;14:189-198.
32. Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23:3418-3426.
33. Kremer AE, Namer B, Bolier R, et al. Pathogenesis and management of pruritus in PBC and PSC. Dig Dis. 2015;33(suppl 2):164-175.
34. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis. Gastroenterol Clin Biol. 2008;32:265-273.
35. Fagiuoli S, Colli A, Bruno R, et al; 2011 AISF Single Topic Group. Management of infections pre- and post-liver transplantation: report of an AISF consensus conference. J Hepatol. 2014;60:1075-1089.
36. Kanaan Z, Antaki F. Magnetic resonance cholangiopancreatography still plays a role in the preoperative evaluation of choledocholithiasis and biliary pathology. J Am Coll Surg. 2016;222:325-326.
37. McMahon CJ. The relative roles of magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound in diagnosis of common bile duct calculi: a critically appraised topic. Abdom Imaging. 2008;33:6-9.
38. Njei B, McCarty TR, Varadarajulu S, et al. Systematic review with meta-analysis: endoscopic retrograde cholangiopancreatography-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;44:1139-1151.
39. Wimalawansa SJ, Razzaque DMS, Al-Daghri NM. Calcium and vitamin D in human health: hype or real? J Steroid Biochem Mol Biol. 2017. doi: 10.1016/j.jsbmb.2017.12.009.
40. Yadav A, Carey EJ. Osteoporosis in chronic liver disease. Nutr Clin Pract. 2013;28:52-64.
From The Journal of Family Practice | 2018;67(7):E9-E15.
PRACTICE RECOMMENDATIONS
› Suspect intrahepatic cholestasis in a patient with pruritus, normal transaminases, and mildly elevated gamma glutamyl-transpeptidase and alkaline phosphatase levels. A
› Use ultrasonography as a first-line diagnostic tool for cholestasis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series