User login
Therapeutic Interchange From Rosuvastatin to Atorvastatin in a Veteran Population
Patients with known cardiovascular (CV) disease are at greater risk for CV events.1 Hydroxymethylglutaryl-CoA (HMG Co-A) reductase inhibitors, or statins, have been shown to reduce CV events and to reduce all-cause mortality.1,2 Thus, these agents should be a standard approach to secondary prevention of CV events.1,2 Although the main function of statins is to lower total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, trials have shown that other lipid-lowering agents have reduced the incidence of CV events but have failed to show any difference in mortality.1 Thus, the therapeutic effects of statins may be a result of “pleiotropic” effects in addition to a reduction in LDL-C.3
As a result, prescribing practices and professional society guidelines have deferred to statins as a first-line choice for lipid-lowering therapy.1 Although each statin varies in its ability to lower LDL-C and inhibit HMG Co-A reductase, as a class, statins have been proven to be safe and efficacious in reducing LDL-C, decreasing risk of coronary artery disease, and decreasing mortality.
The 2013 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce CV risk in adults resulted in a major shift in clinical practice recommendations. The focus of treatment has changed from LDL-C and TC goals to stratifying patients to either high-intensity or moderate-intensity statin therapy, based on their comorbidities and risk of atherosclerotic CV disease (ASCVD).1 Primary prevention of CV disease has been proposed for patients with diabetes mellitus aged 40 to 75 years, familial hypercholesterolemia (LDL-C > 190 mg/dL), and for patients with an ASCVD risk score > 7.5%. Secondary prevention has been proposed for all patients with a history of ASCVD. Among the available choices for intensive statin therapy, the 2 most potent regimens are atorvastatin (40-80 mg) and rosuvastatin (20-40 mg) daily. The ACC/AHA guideline recommends high-potency therapy with either rosuvastatin or atorvastatin with equal preference.1
Related: New Incentives for Helping Prevent Heart Disease
Statin therapy is generally well tolerated; however, the use of statins is not without risk of adverse drug reactions (ADRs). Skeletal muscle discomfort has been reported in 4% to 10% of patients taking either atorvastatin or rosuvastatin.4,5 Liver enzyme abnormalities are less common, having been reported in only about 2% to 3% of patients taking either atorvastatin or rosuvastatin.4,5 Although muscle-related intolerance and liver enzyme abnormalities are considered to be class effects, research speculates that the therapeutic and safety effects of statins may differ, based on tissue solubility.2,6 Variability in the myotoxic and hepatotoxic effects of statins has been attributed to differences in tissue solubility, hypothesizing that lipophilic statins are more easily taken up into myocytes and hepatocytes, resulting in an increase in toxic effects.2,6
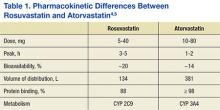
This study assessed differences in therapeutic and safety endpoints resulting from the recent interchange from rosuvastatin to atorvastatin within the North Florida/South Georgia Veterans Health System (NF/SGVHS). Although both these agents are high-potency HMG Co-A reductase inhibitors and share a mechanism of action, they have pharmacokinetic differences, including a key difference in tissue solubility (Table 1).
With the availability of low-cost generic atorvastatin in early 2012, the VA Medical Advisory Panel and Pharmacy Benefits Management (VA MAP/PBM) added atorvastatin to the VA National Formulary as the preferred high-potency statin.7,8 Before October 2012, rosuvastatin had been the preferred high-potency statin within the VA. With support from VA MAP/PBM leadership, NF/SGVHS instituted an interchange from rosuvastatin to atorvastatin for cost-savings purposes.9
Before the interchange, the records of patients were reviewed to determine whether justification existed for continued use of rosuvastatin. Patients were converted to atorvastatin if deemed appropriate and received education and consultation through direct patient contact or a letter regarding the interchange. Justifications for continued use of rosuvastatin included documentation of an atorvastatin ADR, active liver disease, or patients taking cyclosporine or certain protease inhibitors.8
The objective of this retrospective evaluation was to assess the efficacy and safety of the interchange from rosuvastatin to atorvastatin within NF/SGVHS. The results of this review are helpful to confirm the efficacy and safety of the interchange and identify any differences in efficacy and safety that may have occurred as a result of the interchange to a different high-potency statin. For this review, statin efficacy was assessed via review of pre- and postinterchange lipid panel values, assessing for a significant difference between equipotent atorvastatin and rosuvastatin therapy. Similarly, safety was assessed by analysis of pre- and postinterchange liver enzyme panels and assessing for significant differences as a result of the interchange.
Methods
The therapeutic interchange was conducted within the NF/SGVHS, which provides patient care at hospitals in Gainesville, Florida, and Lake City, Florida, and 11 outpatient clinics located throughout North Florida and South Georgia. Like other VA facilities, NF/SGVHS uses Computerized Patient Records System (CPRS) to electronically integrate all clinical patient information, including medical progress notes, consults, admission and discharge summaries, allergies and ADRs, patient problem lists (diagnoses), vital signs, medication orders, and laboratory test results. Approval to conduct this study was granted by the University of Florida Investigational Review Board and the Research and Development Committee at NF/SGVHS.
Therapeutic Interchange Process
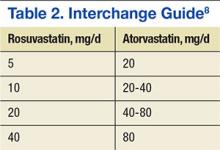
Interchange from rosuvastatin to atorvastatin was expected to provide about $643,000 annually in drug cost savings to NF/SGVHS while providing equivalent therapy. The cost for a 30-day supply of rosuvastatin was about $22.56 at the time of interchange, and a 30-day supply of generic atorvastatin was $1.77.The interchange from rosuvastatin to atorvastatin was approved by the Pharmacy and Therapeutics Committee Meeting on August 8, 2012, and began shortly thereafter. Interchanges were halted temporarily in November 2012 due to a shortage of manufacturer supply, but the process fully resumed in January 2013 once the drug shortage resolved. Direction was provided to VA facilities by a guidance letter issued through PBM leadership.8 The interchange used a standard interchange guide to complete the process (Table 2).
Posttherapeutic Interchange Analysis
Researchers conducted an internal pharmacy computerizedprescription records search to identify VA outpatients who were converted from rosuvastatin to atorvastatin from February 1, 2012, to August 1, 2013. A total of 202 patients were randomly selected and included in this retrospective chart review. Investigators analyzed data points for safety and efficacy, including liver function tests (LFTs), lipid panels, and ADR reports. This information was obtained from laboratory data, vital signs, allergy information, ADR data, and progress notes using CPRS. Two sets of laboratory data were obtained for research purposes, the most recent laboratory values pre-interchange and the most recent laboratory values postinterchange to atorvastatin.
Investigators determined whether patients were converted to an equivalent dose of atorvastatin through an assessment of the most recent dosage of rosuvastatin before the interchange and the dosage of atorvastatin postinterchange. Researchers also analyzed refill history and interacting medications to assess possible confounding factors. Medication adherence was assessed via refill history. Medication adherence was defined as a medication possession ratio of at least 70%, which correlated to receipt of 3 or more 90-day supplies in the year prior to interchange. The above data were collected via retrospective chart review and entered into a spreadsheet.
Statistics
All identifying information was removed from the data set prior to statistical analysis. The data analysis for this project was generated using SAS/STAT software, version 9.3 of the SAS System for Linux x64 (SAS Institute, Cary, North Carolina).
Researchers assumed an equal variance in preinterchange and postinterchange lipid and liver panel values. Preinterchange and postinterchange lipid values (LDL-C, HDL-C, total cholesterol [TC], and triglycerides [TGs]) and liver values (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and creatinine phosphokinase [CPK]) were analyzed by paired t test. All values were reported as mean (SD), and significance was defined as P < .05.
Results
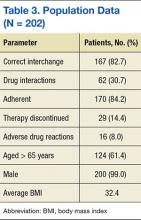
More than 6,000 veterans within the NF/SGVHS were identified as eligible for the interchange. Of those who were converted, 202 patient records were randomly selected and reviewed. Patient population characteristics are summarized in Table 3. Most patients were aged > 65 years (61.4%) with an average body mass index (BMI) of 32.4. Most patients were converted to the correct corresponding dose of atorvastatin (82.7%) and achieved adherence with statin therapy (84.2%). There was no difference in pre- and postinterchange adherence detected as a result of this review.
Related: New Guideline on Dyslipidemia: Less Is More
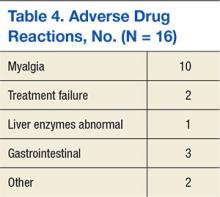
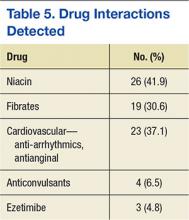
Adverse drug reactions were documented in 16 cases, accounting for 8% of the study population. The most commonly reported ADR was myalgia or arthralgia, which was found in 10 cases (5%). Other ADRs identified in this retrospective review included treatment failure, nausea, vomiting, abdominal discomfort, abnormal liver enzymes, nasopharyngitis, and pruritis (Table 4). Of note, treatment failure was determined on a case-by-case basis but was generally defined as a failure to reach LDL-C goal (< 100 mg/dL or < 70 mg/dL), despite titration of atorvastatin. Interacting medications were identified in 30.7% of patients; however, no reported ADRs were associated with interacting medications. The most common drug interaction was concomitant niacin, followed by antiarrhythmics (ie, amiodarone, diltiazem, etc), and fibrates (ie, gemfibrozil, fenofibrate). All potentially interacting medications identified in this retrospective chart review are compiled in Table 5.
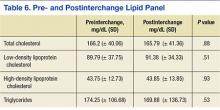
No significant difference between mean pre- and postinterchange lipid panel values was identified in this retrospective chart review (Table 6). In addition, no significant difference was detected in pre- and post-interchange AST, ALT, and CPK values (Table 7). However, a statistically significant increase in ALP was detected, with a mean ALP of 73.33 IU/L prior to interchange and 83.64 IU/L postinterchange (P < .0001).
Discussion
The goal of this retrospective observation was to ensure that safety and efficacy were not compromised as a result of this cost-saving therapeutic interchange. No differences in liver enzymes (safety) and lipid control (effectiveness) were observed in this study. There were no statistically significant changes to the lipid panel or liver panel detected with the exception of ALP. The reason for this statistically significant increase is unknown; however, it may support the hypothesis of variation in hepatocellular effects between the statins due to lipophilic properties.3,6 In general, liver enzymes can be affected by extrahepatic functions. Serum ALP and other liver enzymes can be affected by bone disease, abdominal adiposity, alcoholism, and other concomitant diseases.10 No comorbid conditions were assessed, thus differences in liver enzymes may not be fully attributable to statin therapy. This retrospective review found no clinically significant effect correlated with the increase in ALP.
The results of this analysis are congruent with similar therapeutic interchange studies, which resulted in cost savings without compromising safety or efficacy.9,11 Unlike other therapeutic interchange studies, this study analyzed both safety and lipid-lowering efficacy outcomes, instead of focusing solely on changes in LDL-C lowering, total cost savings, and/or adherence.12,13 By including the entire lipid panel and liver panel into the review, this study conducted a more inclusive review of interchangeability with statins, addressing issues such as HDL-C lowering, TG changes, and liver enzyme fluctuation on conversion. There had not been a sufficient time to assess efficacy in terms of CV outcomes.
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Two adverse events alluded to therapeutic failure as a reason for discontinuing atorvastatin. In the previous ATP III lipid guidelines, therapeutic failure was achieved when patients did not reach their LDL-C goals despite appropriate titration of statin therapy.2 However, the ACC/AHA lipid guidelines have done away with lipid goals as a measurement of treatment therapy, focusing rather on evidence-based high- or moderate-intensity statin therapy that has been proven in clinical trials to reduce mortality and CV events.1 Although measurement of efficacy via lipid panel values is no longer a guideline recommendation, the results of this chart review have shown no difference in lipid values as a result of the interchange, confirming the interchangeability of rosuvastatin and atorvastatin at their equivalent doses.
Limitations
The interchange of rosuvastatin to atorvastatin was a policy change affecting all patients within the NF/SGVHS. In order to reflect true population data and more accurately predict the effects of such a policy change, this study used intention-to-treat analysis, including all patients, even patients who were found to be nonadherent. This study is also limited by sample size (N = 202). Additionally, the generalizability of these findings may be limited. The study population was mostly males aged > 65 years with an average BMI of 32.4. Researchers did not compile comorbidity, race, or concomitant medication data. Additionally, the duration of statin therapy prior to laboratory value collection was undefined.
A retrospective chart review lends itself to limitations in data collection. Medication adherence is a factor that is assumed to have a significant effect on the results of this interchange. In this review, adherence was assessed via refill history. Researchers were unable to confirm actual consumption of the medication.
Additionally, researchers did not analyze comorbid conditions, which may have had an effect on lipid panel and liver panel values. For those veterans who discontinued atorvastatin therapy, the reason for discontinuation was often not documented. Thus, researchers were unable to assess reasons for discontinuation.
Conclusion
The results generated from a review of the therapeutic exchange of rosuvastatin to atorvastatin within a veteran population affirm that the interchange was not associated with any differences in safety or lipid control, but did result in significant drug cost savings. This study provides support for health care systems considering therapeutic interchange with high-intensity statins safely and effectively.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida. The authors would like to acknowledge Kim Hoang, PharmD, for her contributions to this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
2. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
3. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 suppl 3):III32-III38.
4. Pfizer. Lipitor [package insert]. New York, NY: Pfizer; 2015.
5. Crestor [package insert]. Wilmington, DE:AstraZeneca; 2015.
6. Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408-416.
7. FDA approves first generic version of cholesterol-lowering drug Lipitor [news release]. U.S. Food and Drug Administration; November 30, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed November 11, 2015.
8. U.S. Department of Veterans Affairs. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Interchange to generic atorvastatin-recommendations. Atorvastatin Conversion Guidance. 2012. U.S. Department of Veterans Affairs intranet website. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Therapeutic%20Interchange%20Guidance/Atorvastatin%20Conversion%20Guidance%20(PBM-MAP-VPE)-Final.docx. Accessed November 16, 2015.
9. Pratt DS, Kaplan MM. Evaluation of Liver Function. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill Professional Publishing; 2011:2527-2530.
10. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy . 2001;21(9):1130-1139.
11. Billups SJ, Plushner SL, Olson KI, Koehler TJ, Kerzee J. Clinical and economic outcomes of conversion of simvastatin to lovastatin in a group-model health maintenance organization. J Manag Care Pharm. 2005;11(8):681-686.
12. Schachtner JM, Guharoy R, Medicis JJ, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529-533.
13. Hilleman DE, Wurdeman RL, Lenz TL. Therapeutic change of HMG-CoA reductase inhibitors in patients with coronary artery disease. Pharmacotherapy. 2001;21(4):410-415.
Patients with known cardiovascular (CV) disease are at greater risk for CV events.1 Hydroxymethylglutaryl-CoA (HMG Co-A) reductase inhibitors, or statins, have been shown to reduce CV events and to reduce all-cause mortality.1,2 Thus, these agents should be a standard approach to secondary prevention of CV events.1,2 Although the main function of statins is to lower total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, trials have shown that other lipid-lowering agents have reduced the incidence of CV events but have failed to show any difference in mortality.1 Thus, the therapeutic effects of statins may be a result of “pleiotropic” effects in addition to a reduction in LDL-C.3
As a result, prescribing practices and professional society guidelines have deferred to statins as a first-line choice for lipid-lowering therapy.1 Although each statin varies in its ability to lower LDL-C and inhibit HMG Co-A reductase, as a class, statins have been proven to be safe and efficacious in reducing LDL-C, decreasing risk of coronary artery disease, and decreasing mortality.
The 2013 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce CV risk in adults resulted in a major shift in clinical practice recommendations. The focus of treatment has changed from LDL-C and TC goals to stratifying patients to either high-intensity or moderate-intensity statin therapy, based on their comorbidities and risk of atherosclerotic CV disease (ASCVD).1 Primary prevention of CV disease has been proposed for patients with diabetes mellitus aged 40 to 75 years, familial hypercholesterolemia (LDL-C > 190 mg/dL), and for patients with an ASCVD risk score > 7.5%. Secondary prevention has been proposed for all patients with a history of ASCVD. Among the available choices for intensive statin therapy, the 2 most potent regimens are atorvastatin (40-80 mg) and rosuvastatin (20-40 mg) daily. The ACC/AHA guideline recommends high-potency therapy with either rosuvastatin or atorvastatin with equal preference.1
Related: New Incentives for Helping Prevent Heart Disease
Statin therapy is generally well tolerated; however, the use of statins is not without risk of adverse drug reactions (ADRs). Skeletal muscle discomfort has been reported in 4% to 10% of patients taking either atorvastatin or rosuvastatin.4,5 Liver enzyme abnormalities are less common, having been reported in only about 2% to 3% of patients taking either atorvastatin or rosuvastatin.4,5 Although muscle-related intolerance and liver enzyme abnormalities are considered to be class effects, research speculates that the therapeutic and safety effects of statins may differ, based on tissue solubility.2,6 Variability in the myotoxic and hepatotoxic effects of statins has been attributed to differences in tissue solubility, hypothesizing that lipophilic statins are more easily taken up into myocytes and hepatocytes, resulting in an increase in toxic effects.2,6
This study assessed differences in therapeutic and safety endpoints resulting from the recent interchange from rosuvastatin to atorvastatin within the North Florida/South Georgia Veterans Health System (NF/SGVHS). Although both these agents are high-potency HMG Co-A reductase inhibitors and share a mechanism of action, they have pharmacokinetic differences, including a key difference in tissue solubility (Table 1).
With the availability of low-cost generic atorvastatin in early 2012, the VA Medical Advisory Panel and Pharmacy Benefits Management (VA MAP/PBM) added atorvastatin to the VA National Formulary as the preferred high-potency statin.7,8 Before October 2012, rosuvastatin had been the preferred high-potency statin within the VA. With support from VA MAP/PBM leadership, NF/SGVHS instituted an interchange from rosuvastatin to atorvastatin for cost-savings purposes.9
Before the interchange, the records of patients were reviewed to determine whether justification existed for continued use of rosuvastatin. Patients were converted to atorvastatin if deemed appropriate and received education and consultation through direct patient contact or a letter regarding the interchange. Justifications for continued use of rosuvastatin included documentation of an atorvastatin ADR, active liver disease, or patients taking cyclosporine or certain protease inhibitors.8
The objective of this retrospective evaluation was to assess the efficacy and safety of the interchange from rosuvastatin to atorvastatin within NF/SGVHS. The results of this review are helpful to confirm the efficacy and safety of the interchange and identify any differences in efficacy and safety that may have occurred as a result of the interchange to a different high-potency statin. For this review, statin efficacy was assessed via review of pre- and postinterchange lipid panel values, assessing for a significant difference between equipotent atorvastatin and rosuvastatin therapy. Similarly, safety was assessed by analysis of pre- and postinterchange liver enzyme panels and assessing for significant differences as a result of the interchange.
Methods
The therapeutic interchange was conducted within the NF/SGVHS, which provides patient care at hospitals in Gainesville, Florida, and Lake City, Florida, and 11 outpatient clinics located throughout North Florida and South Georgia. Like other VA facilities, NF/SGVHS uses Computerized Patient Records System (CPRS) to electronically integrate all clinical patient information, including medical progress notes, consults, admission and discharge summaries, allergies and ADRs, patient problem lists (diagnoses), vital signs, medication orders, and laboratory test results. Approval to conduct this study was granted by the University of Florida Investigational Review Board and the Research and Development Committee at NF/SGVHS.
Therapeutic Interchange Process
Interchange from rosuvastatin to atorvastatin was expected to provide about $643,000 annually in drug cost savings to NF/SGVHS while providing equivalent therapy. The cost for a 30-day supply of rosuvastatin was about $22.56 at the time of interchange, and a 30-day supply of generic atorvastatin was $1.77.The interchange from rosuvastatin to atorvastatin was approved by the Pharmacy and Therapeutics Committee Meeting on August 8, 2012, and began shortly thereafter. Interchanges were halted temporarily in November 2012 due to a shortage of manufacturer supply, but the process fully resumed in January 2013 once the drug shortage resolved. Direction was provided to VA facilities by a guidance letter issued through PBM leadership.8 The interchange used a standard interchange guide to complete the process (Table 2).
Posttherapeutic Interchange Analysis
Researchers conducted an internal pharmacy computerizedprescription records search to identify VA outpatients who were converted from rosuvastatin to atorvastatin from February 1, 2012, to August 1, 2013. A total of 202 patients were randomly selected and included in this retrospective chart review. Investigators analyzed data points for safety and efficacy, including liver function tests (LFTs), lipid panels, and ADR reports. This information was obtained from laboratory data, vital signs, allergy information, ADR data, and progress notes using CPRS. Two sets of laboratory data were obtained for research purposes, the most recent laboratory values pre-interchange and the most recent laboratory values postinterchange to atorvastatin.
Investigators determined whether patients were converted to an equivalent dose of atorvastatin through an assessment of the most recent dosage of rosuvastatin before the interchange and the dosage of atorvastatin postinterchange. Researchers also analyzed refill history and interacting medications to assess possible confounding factors. Medication adherence was assessed via refill history. Medication adherence was defined as a medication possession ratio of at least 70%, which correlated to receipt of 3 or more 90-day supplies in the year prior to interchange. The above data were collected via retrospective chart review and entered into a spreadsheet.
Statistics
All identifying information was removed from the data set prior to statistical analysis. The data analysis for this project was generated using SAS/STAT software, version 9.3 of the SAS System for Linux x64 (SAS Institute, Cary, North Carolina).
Researchers assumed an equal variance in preinterchange and postinterchange lipid and liver panel values. Preinterchange and postinterchange lipid values (LDL-C, HDL-C, total cholesterol [TC], and triglycerides [TGs]) and liver values (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and creatinine phosphokinase [CPK]) were analyzed by paired t test. All values were reported as mean (SD), and significance was defined as P < .05.
Results
More than 6,000 veterans within the NF/SGVHS were identified as eligible for the interchange. Of those who were converted, 202 patient records were randomly selected and reviewed. Patient population characteristics are summarized in Table 3. Most patients were aged > 65 years (61.4%) with an average body mass index (BMI) of 32.4. Most patients were converted to the correct corresponding dose of atorvastatin (82.7%) and achieved adherence with statin therapy (84.2%). There was no difference in pre- and postinterchange adherence detected as a result of this review.
Related: New Guideline on Dyslipidemia: Less Is More
Adverse drug reactions were documented in 16 cases, accounting for 8% of the study population. The most commonly reported ADR was myalgia or arthralgia, which was found in 10 cases (5%). Other ADRs identified in this retrospective review included treatment failure, nausea, vomiting, abdominal discomfort, abnormal liver enzymes, nasopharyngitis, and pruritis (Table 4). Of note, treatment failure was determined on a case-by-case basis but was generally defined as a failure to reach LDL-C goal (< 100 mg/dL or < 70 mg/dL), despite titration of atorvastatin. Interacting medications were identified in 30.7% of patients; however, no reported ADRs were associated with interacting medications. The most common drug interaction was concomitant niacin, followed by antiarrhythmics (ie, amiodarone, diltiazem, etc), and fibrates (ie, gemfibrozil, fenofibrate). All potentially interacting medications identified in this retrospective chart review are compiled in Table 5.
No significant difference between mean pre- and postinterchange lipid panel values was identified in this retrospective chart review (Table 6). In addition, no significant difference was detected in pre- and post-interchange AST, ALT, and CPK values (Table 7). However, a statistically significant increase in ALP was detected, with a mean ALP of 73.33 IU/L prior to interchange and 83.64 IU/L postinterchange (P < .0001).
Discussion
The goal of this retrospective observation was to ensure that safety and efficacy were not compromised as a result of this cost-saving therapeutic interchange. No differences in liver enzymes (safety) and lipid control (effectiveness) were observed in this study. There were no statistically significant changes to the lipid panel or liver panel detected with the exception of ALP. The reason for this statistically significant increase is unknown; however, it may support the hypothesis of variation in hepatocellular effects between the statins due to lipophilic properties.3,6 In general, liver enzymes can be affected by extrahepatic functions. Serum ALP and other liver enzymes can be affected by bone disease, abdominal adiposity, alcoholism, and other concomitant diseases.10 No comorbid conditions were assessed, thus differences in liver enzymes may not be fully attributable to statin therapy. This retrospective review found no clinically significant effect correlated with the increase in ALP.
The results of this analysis are congruent with similar therapeutic interchange studies, which resulted in cost savings without compromising safety or efficacy.9,11 Unlike other therapeutic interchange studies, this study analyzed both safety and lipid-lowering efficacy outcomes, instead of focusing solely on changes in LDL-C lowering, total cost savings, and/or adherence.12,13 By including the entire lipid panel and liver panel into the review, this study conducted a more inclusive review of interchangeability with statins, addressing issues such as HDL-C lowering, TG changes, and liver enzyme fluctuation on conversion. There had not been a sufficient time to assess efficacy in terms of CV outcomes.
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Two adverse events alluded to therapeutic failure as a reason for discontinuing atorvastatin. In the previous ATP III lipid guidelines, therapeutic failure was achieved when patients did not reach their LDL-C goals despite appropriate titration of statin therapy.2 However, the ACC/AHA lipid guidelines have done away with lipid goals as a measurement of treatment therapy, focusing rather on evidence-based high- or moderate-intensity statin therapy that has been proven in clinical trials to reduce mortality and CV events.1 Although measurement of efficacy via lipid panel values is no longer a guideline recommendation, the results of this chart review have shown no difference in lipid values as a result of the interchange, confirming the interchangeability of rosuvastatin and atorvastatin at their equivalent doses.
Limitations
The interchange of rosuvastatin to atorvastatin was a policy change affecting all patients within the NF/SGVHS. In order to reflect true population data and more accurately predict the effects of such a policy change, this study used intention-to-treat analysis, including all patients, even patients who were found to be nonadherent. This study is also limited by sample size (N = 202). Additionally, the generalizability of these findings may be limited. The study population was mostly males aged > 65 years with an average BMI of 32.4. Researchers did not compile comorbidity, race, or concomitant medication data. Additionally, the duration of statin therapy prior to laboratory value collection was undefined.
A retrospective chart review lends itself to limitations in data collection. Medication adherence is a factor that is assumed to have a significant effect on the results of this interchange. In this review, adherence was assessed via refill history. Researchers were unable to confirm actual consumption of the medication.
Additionally, researchers did not analyze comorbid conditions, which may have had an effect on lipid panel and liver panel values. For those veterans who discontinued atorvastatin therapy, the reason for discontinuation was often not documented. Thus, researchers were unable to assess reasons for discontinuation.
Conclusion
The results generated from a review of the therapeutic exchange of rosuvastatin to atorvastatin within a veteran population affirm that the interchange was not associated with any differences in safety or lipid control, but did result in significant drug cost savings. This study provides support for health care systems considering therapeutic interchange with high-intensity statins safely and effectively.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida. The authors would like to acknowledge Kim Hoang, PharmD, for her contributions to this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Patients with known cardiovascular (CV) disease are at greater risk for CV events.1 Hydroxymethylglutaryl-CoA (HMG Co-A) reductase inhibitors, or statins, have been shown to reduce CV events and to reduce all-cause mortality.1,2 Thus, these agents should be a standard approach to secondary prevention of CV events.1,2 Although the main function of statins is to lower total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels, trials have shown that other lipid-lowering agents have reduced the incidence of CV events but have failed to show any difference in mortality.1 Thus, the therapeutic effects of statins may be a result of “pleiotropic” effects in addition to a reduction in LDL-C.3
As a result, prescribing practices and professional society guidelines have deferred to statins as a first-line choice for lipid-lowering therapy.1 Although each statin varies in its ability to lower LDL-C and inhibit HMG Co-A reductase, as a class, statins have been proven to be safe and efficacious in reducing LDL-C, decreasing risk of coronary artery disease, and decreasing mortality.
The 2013 American College of Cardiology (ACC) and American Heart Association (AHA) guideline on the treatment of blood cholesterol to reduce CV risk in adults resulted in a major shift in clinical practice recommendations. The focus of treatment has changed from LDL-C and TC goals to stratifying patients to either high-intensity or moderate-intensity statin therapy, based on their comorbidities and risk of atherosclerotic CV disease (ASCVD).1 Primary prevention of CV disease has been proposed for patients with diabetes mellitus aged 40 to 75 years, familial hypercholesterolemia (LDL-C > 190 mg/dL), and for patients with an ASCVD risk score > 7.5%. Secondary prevention has been proposed for all patients with a history of ASCVD. Among the available choices for intensive statin therapy, the 2 most potent regimens are atorvastatin (40-80 mg) and rosuvastatin (20-40 mg) daily. The ACC/AHA guideline recommends high-potency therapy with either rosuvastatin or atorvastatin with equal preference.1
Related: New Incentives for Helping Prevent Heart Disease
Statin therapy is generally well tolerated; however, the use of statins is not without risk of adverse drug reactions (ADRs). Skeletal muscle discomfort has been reported in 4% to 10% of patients taking either atorvastatin or rosuvastatin.4,5 Liver enzyme abnormalities are less common, having been reported in only about 2% to 3% of patients taking either atorvastatin or rosuvastatin.4,5 Although muscle-related intolerance and liver enzyme abnormalities are considered to be class effects, research speculates that the therapeutic and safety effects of statins may differ, based on tissue solubility.2,6 Variability in the myotoxic and hepatotoxic effects of statins has been attributed to differences in tissue solubility, hypothesizing that lipophilic statins are more easily taken up into myocytes and hepatocytes, resulting in an increase in toxic effects.2,6
This study assessed differences in therapeutic and safety endpoints resulting from the recent interchange from rosuvastatin to atorvastatin within the North Florida/South Georgia Veterans Health System (NF/SGVHS). Although both these agents are high-potency HMG Co-A reductase inhibitors and share a mechanism of action, they have pharmacokinetic differences, including a key difference in tissue solubility (Table 1).
With the availability of low-cost generic atorvastatin in early 2012, the VA Medical Advisory Panel and Pharmacy Benefits Management (VA MAP/PBM) added atorvastatin to the VA National Formulary as the preferred high-potency statin.7,8 Before October 2012, rosuvastatin had been the preferred high-potency statin within the VA. With support from VA MAP/PBM leadership, NF/SGVHS instituted an interchange from rosuvastatin to atorvastatin for cost-savings purposes.9
Before the interchange, the records of patients were reviewed to determine whether justification existed for continued use of rosuvastatin. Patients were converted to atorvastatin if deemed appropriate and received education and consultation through direct patient contact or a letter regarding the interchange. Justifications for continued use of rosuvastatin included documentation of an atorvastatin ADR, active liver disease, or patients taking cyclosporine or certain protease inhibitors.8
The objective of this retrospective evaluation was to assess the efficacy and safety of the interchange from rosuvastatin to atorvastatin within NF/SGVHS. The results of this review are helpful to confirm the efficacy and safety of the interchange and identify any differences in efficacy and safety that may have occurred as a result of the interchange to a different high-potency statin. For this review, statin efficacy was assessed via review of pre- and postinterchange lipid panel values, assessing for a significant difference between equipotent atorvastatin and rosuvastatin therapy. Similarly, safety was assessed by analysis of pre- and postinterchange liver enzyme panels and assessing for significant differences as a result of the interchange.
Methods
The therapeutic interchange was conducted within the NF/SGVHS, which provides patient care at hospitals in Gainesville, Florida, and Lake City, Florida, and 11 outpatient clinics located throughout North Florida and South Georgia. Like other VA facilities, NF/SGVHS uses Computerized Patient Records System (CPRS) to electronically integrate all clinical patient information, including medical progress notes, consults, admission and discharge summaries, allergies and ADRs, patient problem lists (diagnoses), vital signs, medication orders, and laboratory test results. Approval to conduct this study was granted by the University of Florida Investigational Review Board and the Research and Development Committee at NF/SGVHS.
Therapeutic Interchange Process
Interchange from rosuvastatin to atorvastatin was expected to provide about $643,000 annually in drug cost savings to NF/SGVHS while providing equivalent therapy. The cost for a 30-day supply of rosuvastatin was about $22.56 at the time of interchange, and a 30-day supply of generic atorvastatin was $1.77.The interchange from rosuvastatin to atorvastatin was approved by the Pharmacy and Therapeutics Committee Meeting on August 8, 2012, and began shortly thereafter. Interchanges were halted temporarily in November 2012 due to a shortage of manufacturer supply, but the process fully resumed in January 2013 once the drug shortage resolved. Direction was provided to VA facilities by a guidance letter issued through PBM leadership.8 The interchange used a standard interchange guide to complete the process (Table 2).
Posttherapeutic Interchange Analysis
Researchers conducted an internal pharmacy computerizedprescription records search to identify VA outpatients who were converted from rosuvastatin to atorvastatin from February 1, 2012, to August 1, 2013. A total of 202 patients were randomly selected and included in this retrospective chart review. Investigators analyzed data points for safety and efficacy, including liver function tests (LFTs), lipid panels, and ADR reports. This information was obtained from laboratory data, vital signs, allergy information, ADR data, and progress notes using CPRS. Two sets of laboratory data were obtained for research purposes, the most recent laboratory values pre-interchange and the most recent laboratory values postinterchange to atorvastatin.
Investigators determined whether patients were converted to an equivalent dose of atorvastatin through an assessment of the most recent dosage of rosuvastatin before the interchange and the dosage of atorvastatin postinterchange. Researchers also analyzed refill history and interacting medications to assess possible confounding factors. Medication adherence was assessed via refill history. Medication adherence was defined as a medication possession ratio of at least 70%, which correlated to receipt of 3 or more 90-day supplies in the year prior to interchange. The above data were collected via retrospective chart review and entered into a spreadsheet.
Statistics
All identifying information was removed from the data set prior to statistical analysis. The data analysis for this project was generated using SAS/STAT software, version 9.3 of the SAS System for Linux x64 (SAS Institute, Cary, North Carolina).
Researchers assumed an equal variance in preinterchange and postinterchange lipid and liver panel values. Preinterchange and postinterchange lipid values (LDL-C, HDL-C, total cholesterol [TC], and triglycerides [TGs]) and liver values (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and creatinine phosphokinase [CPK]) were analyzed by paired t test. All values were reported as mean (SD), and significance was defined as P < .05.
Results
More than 6,000 veterans within the NF/SGVHS were identified as eligible for the interchange. Of those who were converted, 202 patient records were randomly selected and reviewed. Patient population characteristics are summarized in Table 3. Most patients were aged > 65 years (61.4%) with an average body mass index (BMI) of 32.4. Most patients were converted to the correct corresponding dose of atorvastatin (82.7%) and achieved adherence with statin therapy (84.2%). There was no difference in pre- and postinterchange adherence detected as a result of this review.
Related: New Guideline on Dyslipidemia: Less Is More
Adverse drug reactions were documented in 16 cases, accounting for 8% of the study population. The most commonly reported ADR was myalgia or arthralgia, which was found in 10 cases (5%). Other ADRs identified in this retrospective review included treatment failure, nausea, vomiting, abdominal discomfort, abnormal liver enzymes, nasopharyngitis, and pruritis (Table 4). Of note, treatment failure was determined on a case-by-case basis but was generally defined as a failure to reach LDL-C goal (< 100 mg/dL or < 70 mg/dL), despite titration of atorvastatin. Interacting medications were identified in 30.7% of patients; however, no reported ADRs were associated with interacting medications. The most common drug interaction was concomitant niacin, followed by antiarrhythmics (ie, amiodarone, diltiazem, etc), and fibrates (ie, gemfibrozil, fenofibrate). All potentially interacting medications identified in this retrospective chart review are compiled in Table 5.
No significant difference between mean pre- and postinterchange lipid panel values was identified in this retrospective chart review (Table 6). In addition, no significant difference was detected in pre- and post-interchange AST, ALT, and CPK values (Table 7). However, a statistically significant increase in ALP was detected, with a mean ALP of 73.33 IU/L prior to interchange and 83.64 IU/L postinterchange (P < .0001).
Discussion
The goal of this retrospective observation was to ensure that safety and efficacy were not compromised as a result of this cost-saving therapeutic interchange. No differences in liver enzymes (safety) and lipid control (effectiveness) were observed in this study. There were no statistically significant changes to the lipid panel or liver panel detected with the exception of ALP. The reason for this statistically significant increase is unknown; however, it may support the hypothesis of variation in hepatocellular effects between the statins due to lipophilic properties.3,6 In general, liver enzymes can be affected by extrahepatic functions. Serum ALP and other liver enzymes can be affected by bone disease, abdominal adiposity, alcoholism, and other concomitant diseases.10 No comorbid conditions were assessed, thus differences in liver enzymes may not be fully attributable to statin therapy. This retrospective review found no clinically significant effect correlated with the increase in ALP.
The results of this analysis are congruent with similar therapeutic interchange studies, which resulted in cost savings without compromising safety or efficacy.9,11 Unlike other therapeutic interchange studies, this study analyzed both safety and lipid-lowering efficacy outcomes, instead of focusing solely on changes in LDL-C lowering, total cost savings, and/or adherence.12,13 By including the entire lipid panel and liver panel into the review, this study conducted a more inclusive review of interchangeability with statins, addressing issues such as HDL-C lowering, TG changes, and liver enzyme fluctuation on conversion. There had not been a sufficient time to assess efficacy in terms of CV outcomes.
Related: Poor Outcomes for African Americans in Cardiac Rehabilitation
Two adverse events alluded to therapeutic failure as a reason for discontinuing atorvastatin. In the previous ATP III lipid guidelines, therapeutic failure was achieved when patients did not reach their LDL-C goals despite appropriate titration of statin therapy.2 However, the ACC/AHA lipid guidelines have done away with lipid goals as a measurement of treatment therapy, focusing rather on evidence-based high- or moderate-intensity statin therapy that has been proven in clinical trials to reduce mortality and CV events.1 Although measurement of efficacy via lipid panel values is no longer a guideline recommendation, the results of this chart review have shown no difference in lipid values as a result of the interchange, confirming the interchangeability of rosuvastatin and atorvastatin at their equivalent doses.
Limitations
The interchange of rosuvastatin to atorvastatin was a policy change affecting all patients within the NF/SGVHS. In order to reflect true population data and more accurately predict the effects of such a policy change, this study used intention-to-treat analysis, including all patients, even patients who were found to be nonadherent. This study is also limited by sample size (N = 202). Additionally, the generalizability of these findings may be limited. The study population was mostly males aged > 65 years with an average BMI of 32.4. Researchers did not compile comorbidity, race, or concomitant medication data. Additionally, the duration of statin therapy prior to laboratory value collection was undefined.
A retrospective chart review lends itself to limitations in data collection. Medication adherence is a factor that is assumed to have a significant effect on the results of this interchange. In this review, adherence was assessed via refill history. Researchers were unable to confirm actual consumption of the medication.
Additionally, researchers did not analyze comorbid conditions, which may have had an effect on lipid panel and liver panel values. For those veterans who discontinued atorvastatin therapy, the reason for discontinuation was often not documented. Thus, researchers were unable to assess reasons for discontinuation.
Conclusion
The results generated from a review of the therapeutic exchange of rosuvastatin to atorvastatin within a veteran population affirm that the interchange was not associated with any differences in safety or lipid control, but did result in significant drug cost savings. This study provides support for health care systems considering therapeutic interchange with high-intensity statins safely and effectively.
Acknowledgements
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida. The authors would like to acknowledge Kim Hoang, PharmD, for her contributions to this project.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
2. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
3. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 suppl 3):III32-III38.
4. Pfizer. Lipitor [package insert]. New York, NY: Pfizer; 2015.
5. Crestor [package insert]. Wilmington, DE:AstraZeneca; 2015.
6. Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408-416.
7. FDA approves first generic version of cholesterol-lowering drug Lipitor [news release]. U.S. Food and Drug Administration; November 30, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed November 11, 2015.
8. U.S. Department of Veterans Affairs. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Interchange to generic atorvastatin-recommendations. Atorvastatin Conversion Guidance. 2012. U.S. Department of Veterans Affairs intranet website. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Therapeutic%20Interchange%20Guidance/Atorvastatin%20Conversion%20Guidance%20(PBM-MAP-VPE)-Final.docx. Accessed November 16, 2015.
9. Pratt DS, Kaplan MM. Evaluation of Liver Function. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill Professional Publishing; 2011:2527-2530.
10. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy . 2001;21(9):1130-1139.
11. Billups SJ, Plushner SL, Olson KI, Koehler TJ, Kerzee J. Clinical and economic outcomes of conversion of simvastatin to lovastatin in a group-model health maintenance organization. J Manag Care Pharm. 2005;11(8):681-686.
12. Schachtner JM, Guharoy R, Medicis JJ, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529-533.
13. Hilleman DE, Wurdeman RL, Lenz TL. Therapeutic change of HMG-CoA reductase inhibitors in patients with coronary artery disease. Pharmacotherapy. 2001;21(4):410-415.
1. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 pt B):2889-2934.
2. Grundy SM, Cleeman JI, Merz CN, et al; National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239.
3. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 suppl 3):III32-III38.
4. Pfizer. Lipitor [package insert]. New York, NY: Pfizer; 2015.
5. Crestor [package insert]. Wilmington, DE:AstraZeneca; 2015.
6. Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116(6):408-416.
7. FDA approves first generic version of cholesterol-lowering drug Lipitor [news release]. U.S. Food and Drug Administration; November 30, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm281817.htm. Accessed November 11, 2015.
8. U.S. Department of Veterans Affairs. VHA Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. Interchange to generic atorvastatin-recommendations. Atorvastatin Conversion Guidance. 2012. U.S. Department of Veterans Affairs intranet website. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Therapeutic%20Interchange%20Guidance/Atorvastatin%20Conversion%20Guidance%20(PBM-MAP-VPE)-Final.docx. Accessed November 16, 2015.
9. Pratt DS, Kaplan MM. Evaluation of Liver Function. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 18th ed. New York: McGraw Hill Professional Publishing; 2011:2527-2530.
10. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutic statin formulary conversion program. Pharmacotherapy . 2001;21(9):1130-1139.
11. Billups SJ, Plushner SL, Olson KI, Koehler TJ, Kerzee J. Clinical and economic outcomes of conversion of simvastatin to lovastatin in a group-model health maintenance organization. J Manag Care Pharm. 2005;11(8):681-686.
12. Schachtner JM, Guharoy R, Medicis JJ, Newman N, Speizer R. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm. 2002;59(6):529-533.
13. Hilleman DE, Wurdeman RL, Lenz TL. Therapeutic change of HMG-CoA reductase inhibitors in patients with coronary artery disease. Pharmacotherapy. 2001;21(4):410-415.