User login
Abdominal pain in a 20-year-old woman
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
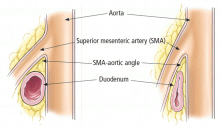
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
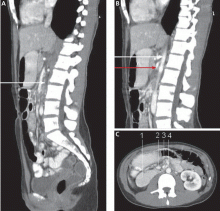
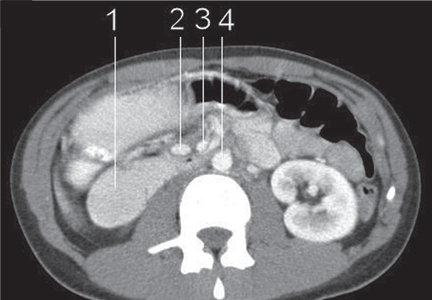
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.