User login
Reorganizing a Hospital Ward
In 2001, the Institute of Medicine called for a major redesign of the US healthcare system, describing the chasm between the quality of care Americans receive and the quality of healthcare they deserve.[1] The healthcare community recognizes its ongoing quality and value gaps, but progress has been limited by outdated care models, fragmented organizational structures, and insufficient advances in system design.[2] Many healthcare organizations are searching for new care delivery models capable of producing greater value.
A major constraint in hospitals is the persistence of underperforming frontline clinical care teams.[3] Physicians typically travel from 1 unit or patient to the next in unpredictable patterns, resulting in missed opportunities to share perspectives and coordinate care with nurses, discharge planning personnel, pharmacists, therapists, and patients. This geographic fragmentation almost certainly contributes to interprofessional silos and hierarchies, nonspecific care plans, and failure to initiate or intensify therapy when indicated.[4] Modern hospital units could benefit from having a standard care model that synchronizes frontline professionals into teams routinely coordinating and progressing a shared plan of care.
EFFECTIVE CLINICAL MICROSYSTEMS REFLECTED IN THE DESIGN OF THE ACCOUNTABLE CARE UNIT
High‐value healthcare organizations deliberately design clinical microsystems.[5] An effective clinical microsystem combines several traits: (1) a small group of people who work together in a defined setting on a regular basis to provide care, (2) linked care processes and a shared information environment that includes individuals who receive that care, (3) performance outcomes, and (4) set service and care aims.[6] For the accountable care unit (ACU) to reflect the traits of an effective clinical microsystem, we designed it with analogous features: (1) unit‐based teams, (2) structured interdisciplinary bedside rounds (SIBR), (3) unit‐level performance reporting, and (4) unit‐level nurse and physician coleadership. We launched the ACU on September 1, 2010 in a high‐acuity 24‐bed medical unit at Emory University Hospital, a 579‐bed tertiary academic medical center. Herein we provide a brief report of our experience implementing and refining the ACU over a 4‐year period to help others gauge feasibility and sustainability.
FEATURES OF AN ACU
Unit‐Based Teams
Design
Geographic alignment fosters mutual respect, cohesiveness, communication, timeliness, and face‐to‐face problem solving,[7, 8] and has been linked to improved patient satisfaction, decreased length of stay, and reductions in morbidity and mortality.[9, 10, 11] At our hospital, though, patients newly admitted or transferred to the hospital medicine service traditionally had been distributed to physician teams without regard to geography, typically based on physician call schedules or traditions of balancing patient volumes across colleagues. These traditional practices geographically dispersed our teams. Physicians would be forced regularly to travel to 5 to 8 different units each day to see 10 to 18 patients. Nurses might perceive this as a parade of different physician teams coming and going off the unit at unpredictable times. To temporally and spatially align physicians with unit‐based staff, specific physician teams were assigned to the ACU.
Implementation
The first step in implementing unit‐based teams was to identify the smallest number of physician teams that could be assigned to the ACU. Two internal medicine resident teams are assigned to care for all medical patients in the unit. Each resident team consists of 1 hospital medicine attending physician, 1 internal medicine resident, 3 interns (2 covering the day shift and 1 overnight every other night), and up to 2 medical students. The 2 teams alternate a 24‐hour call cycle where the on‐call team admits every patient arriving to the unit. For patients arriving to the unit from 6 pm to 7 am, the on‐call overnight intern admits the patients and hands over care to the team in the morning. The on‐call team becomes aware of an incoming patient once the patient has been assigned a bed in the home unit. Several patients per day may arrive on the unit as transfers from a medical or surgical intensive care unit, but most patients arrive as emergency room or direct admissions. On any given day it is acceptable and typical for a team to have several patients off the ACU. No specific changes were made to nurse staffing, with the unit continuing to have 1 nurse unit manager, 1 charge nurse per shift, and a nurse‐to‐patient ratio of 1 to 4.
Results
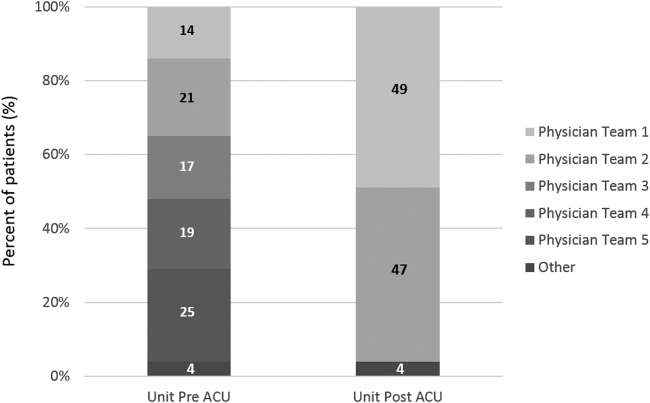
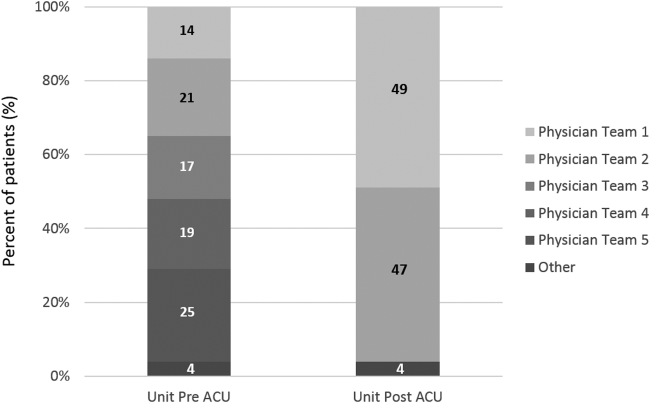
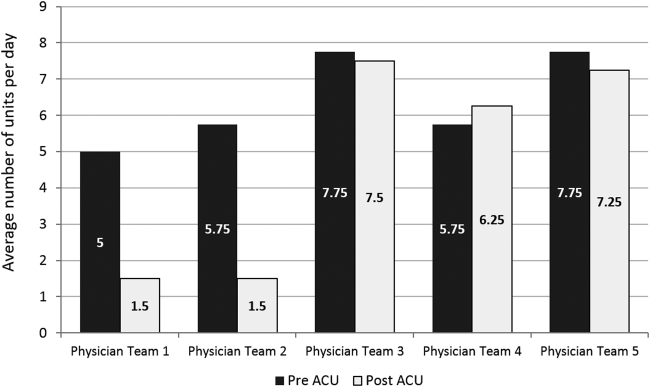
Geographic patient assignment has been successful (Figure 1). Prior to implementing the ACU, more than 5 different hospital medicine physician teams cared for patients on the unit, with no single team caring for more than 25% of them. In the ACU, all medical patients are assigned to 1 of the 2 unit‐based physician teams (physician teams 1 and 2), which regularly represents more than 95% of all patients on the unit. Over the 4 years, these 2 ACU teams have had an average of 12.9 total patient encounters per day (compared to 11.8 in the year before the ACU when these teams were not unit based). The 2 unit‐based teams have over 90% of their patients on the ACU daily. In contrast, 3 attending‐only hospital medicine teams (physician teams 3, 4, and 5) are still dispersed over 6 to 8 units every day (Figure 2), primarily due to high hospital occupancy and a relative scarcity of units eligible to become dedicated hospital medicine units.
Effects of the Change
Through unit‐based teams, the ACU achieves the first trait of an effective clinical microsystem. Although an evaluation of the cultural gains are beyond the scope of this article, the logistical advantages are self‐evident; having the fewest necessary physician teams overseeing care for nearly all patients in 1 unit and where those physician teams simultaneously have nearly all of their patients on that 1 unit, makes it possible to schedule interdisciplinary teamwork activities, such as SIBR, not otherwise feasible.
Structured Interdisciplinary Bedside Rounds
Design
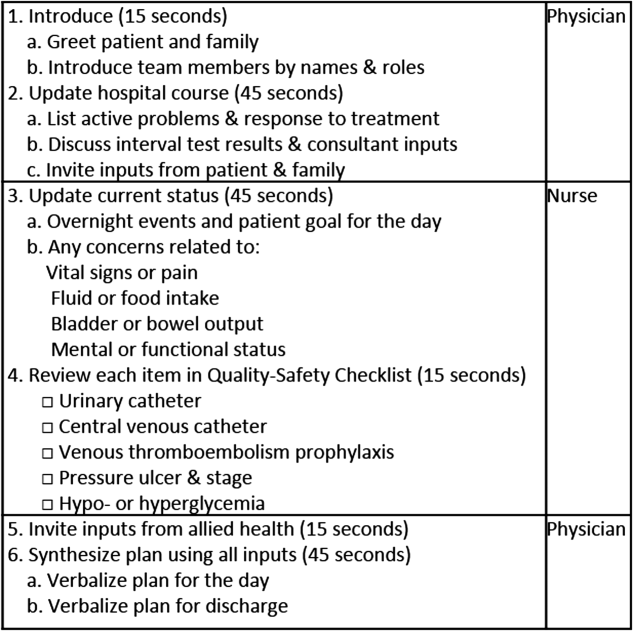
To reflect the second trait of an effective clinical microsystem, a hospital unit should routinely combine best practices for communication, including daily goals sheets,[12] safety checklists,[13] and multidisciplinary rounds.[14, 15] ACU design achieves this through SIBR, a patient‐ and family‐centered, team‐based approach to rounds that brings the nurse, physician, and available allied health professionals to the patient's bedside every day to exchange perspectives using a standard format to cross‐check information with the patient, family, and one another, and articulate a clear plan for the day. Before the SIBR hour starts, physicians and nurses have already performed independent patient assessments through usual activities such as handover, chart review, patient interviews, and physical examinations. Participants in SIBR are expected to give or receive inputs according to the standard SIBR communication protocol (Figure 3), review a quality‐safety checklist together, and ensure the plan of care is verbalized. Including the patient and family allows all parties to hear and be heard, cross‐check information for accuracy, and hold each person accountable for contributions.[16, 17]
Implementation
Each ACU staff member receives orientation to the SIBR communication protocol and is expected to be prepared and punctual for the midmorning start times. The charge nurse serves as the SIBR rounds manager, ensuring physicians waste no time searching for the next nurse and each team's eligible patients are seen in the SIBR hour. For each patient, SIBR begins when the nurse and physician are both present at the bedside. The intern begins SIBR by introducing team members before reviewing the patient's active problem list, response to treatment, and interval test results or consultant inputs. The nurse then relays the patient's goal for the day, overnight events, nursing concerns, and reviews the quality‐safety checklist. The intern then invites allied health professionals to share inputs that might impact medical decision making or discharge planning, before synthesizing all inputs into a shared plan for the day.
Throughout SIBR, the patient and family are encouraged to ask questions or correct misinformation. Although newcomers to SIBR often imagine that inviting patient inputs will disrupt efficiency, we have found teams readily learn to manage this risk, for instance discerning the core question among multiple seemingly disparate ones, or volunteering to return after the SIBR hour to explore a complex issue.
Results
Since the launch of the ACU on September 1, 2010, SIBR has been embedded as a routine on the unit with both physician teams and the nursing staff conducting it every day. Patients not considered eligible for SIBR are those whom the entire physician team has not yet evaluated, typically patients who arrived to the unit overnight. For patients who opt out due to personal preference, or for patients away from the unit for a procedure or a test, SIBR occurs without the patient so the rest of the team can still exchange inputs and formulate a plan of care. A visitor to the unit sees SIBR start punctually at 9 am and 10 am for successive teams, with each completing SIBR on eligible patients in under 60 minutes.
Effects of the Change
The second trait of an effective clinical microsystem is achieved through SIBR's routine forum for staff to share information with each other and the patient. By practicing SIBR every workday, staff are presented with multiple routine opportunities to experience an environment reflective of high‐performing frontline units.[18] We found that SIBR resembled other competencies, with a bell curve of performance. For this reason, by the start of the third year we added a SIBR certification program, a SIBR skills training program where permanent and rotating staff are evaluated through an in vivo observed structured clinical exam, typically with a charge nurse or physician as preceptor. When a nurse, medical student, intern, or resident demonstrates an ability to perform a series of specific high performance SIBR behaviors in 5 of 6 consecutive patients, they can achieve SIBR certification. In the first 2 years of this voluntary certification program, all daytime nursing staff and rotating interns have achieved this demonstration of interdisciplinary teamwork competence.
Unit‐Level Performance Reporting
Design
Hospital outcomes are determined on the clinical frontline. To be effective at managing unit outcomes, performance reports must be made available to unit leadership and staff.[5, 16] However, many hospitals still report performance at the level of the facility or service line. This limits the relevance of reports for the people who directly determine outcomes.
Implementation
For the first year, a data analyst was available to prepare and distribute unit‐level performance reports to unit leaders quarterly, including rates of in‐hospital mortality, blood stream infections, patient satisfaction, length of stay, and 30‐day readmissions. Preparation of these reports was labor intensive, requiring the analyst to acquire raw data from multiple data sources and to build the reports manually.
Results
In an analysis comparing outcomes for every patient spending at least 1 night on the unit in the year before and year after implementation, we observed reductions in in‐hospital mortality and length of stay. Unadjusted in‐hospital mortality decreased from 2.3% to 1.1% (P=0.004), with no change in referrals to hospice (5.4% to 4.5%) (P=0.176), and length‐of‐stay decreased from 5.0 to 4.5 days (P=0.001).[19] A complete report of these findings, including an analysis of concurrent control groups is beyond the scope of this article, but here we highlight an effect we observed on ACU leadership and staff from the reduction in in‐hospital mortality.
Effects of the Change
Noting the apparent mortality reduction, ACU leadership encouraged permanent staff and rotating trainees to consider an unexpected death as a never event. Although perhaps self‐evident, before the ACU we had never been organized to reflect on that concept or to use routines to do something about it. The unit considered an unexpected death one where the patient was not actively receiving comfort measures. At the monthly meet and greet, where ACU leadership bring the permanent staff and new rotating trainees together to introduce themselves by first name, the coleaders proposed that unexpected deaths in the month ahead could represent failures to recognize or respond to deterioration, to consider an alternative or under‐treated process, to transfer the patient to a higher level of care, or to deliver more timely and appropriate end‐of‐life care. It is our impression that this introspection was extraordinarily meaningful and would not have occurred without unit‐based teams, unit‐level performance data, and ACU leadership learning to utilize this rhetoric.
Unit‐Level Nurse and Physician Coleadership
Design
Effective leadership is a major driver of successful clinical microsystems.[20] The ACU is designed to be co‐led by a nurse unit manager and physician medical director. The leadership pair was charged simply with developing patient‐centered teams and ensuring the staff felt connected to the values of the organization and accountable to each other and the outcomes of the unit.
Implementation
Nursing leadership and hospital executives influenced the selection of the physician medical director, which was a way for them to demonstrate support for the care model. Over the first 4 years, the physician medical director position has been afforded a 10% to 20% reduction in clinical duties to fulfill the charge. The leadership pair sets expectations for the ACU's code of conduct, standard operating procedures (eg, SIBR), and best‐practice protocols.
Results
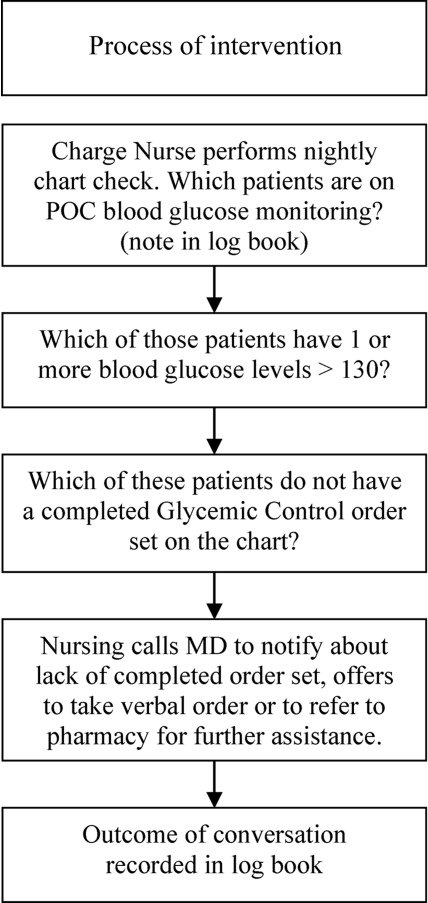
The leadership pair tries explicitly to role model the behaviors enumerated in the ACU's relational covenant, itself the product of a facilitated exercise they commissioned in the first year in which the entire staff drafted and signed a document listing behaviors they wished to see from each other (see Supporting Information, Appendix 1, in the online version of this article). The physician medical director, along with charge nurses, coach staff and trainees wishing to achieve SIBR certification. Over the 4 years, the pair has introduced best‐practice protocols for glycemic control, venous thromboembolism prophylaxis, removal of idle venous and bladder catheters, and bedside goals‐of‐care conversations.
Effects of the Change
Where there had previously been no explicit code of conduct, standard operating procedures such as SIBR, or focused efforts to optimize unit outcomes, the coleadership pair fills a management gap. These coleaders play an essential role in building momentum for the structure and processes of the ACU. The leadership pair has also become a primary resource for intraorganizational spread of the ACU model to medical and surgical wards, as well as geriatric, long‐term acute, and intensive care units.
CHALLENGES
Challenges with implementing the ACU fell into 3 primary categories: (1) performing change management required for a successful launch, (2) solving logistics of maintaining unit‐based physician teams, and (3) training physicians and nurses to perform SIBR at a high level.
For change management, the leadership pair was able to explain the rationale of the model to all staff in sufficient detail to launch the ACU. To build momentum for ACU routines and relationships, the physician leader and the nurse unit manager were both present on the unit daily for the first 100 days. As ACU operations became routine and competencies formed among clinicians, the amount of time spent by these leaders was de‐escalated.
Creating and maintaining unit‐based physician teams required shared understanding and coordination between on‐call hospital medicine physicians and the bed control office so that new admissions or transfers could be consistently assigned to unit‐based teams without adversely affecting patient flow. We found this challenge to be manageable once stakeholders accepted the rationale for the care mode and figured out how to support it.
The challenge of building high‐performance SIBR across the unit, including competence of rotating trainees new to the model, requires individualized assessment and feedback necessary for SIBR certification. We addressed this challenge by creating a SIBR train‐the‐trainer programa list of observable high‐performance SIBR behaviors coupled with a short course about giving effective feedback to learnersand found that once the ACU had several nurse and physician SIBR trainers in the staffing mix every day, the required amount of SIBR coaching expertise was available when needed.
CONCLUSION
Improving value and reliability in hospital care may require new models of care. The ACU is a hospital care model specifically designed to organize physicians, nurses, and allied health professionals into high‐functioning, unit‐based teams. It converges standard workflow, patient‐centered communication, quality‐safety checklists, best‐practice protocols, performance measurement, and progressive leadership. Our experience with the ACU suggests that hospital units can be reorganized as effective clinical microsystems where consistent unit professionals can share time and space, a sense of purpose, code of conduct, shared mental model for teamwork, an interprofessional management structure, and an important level of accountability to each other and their patients.
Disclosures: Jason Stein, MD: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees and royalties for licensed intellectual property to support implementation of the care model described; founder and president of nonprofit Centripital, provider of consulting services to hospital systems implementing the care model described. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Liam Chadwick, PhD, and Diaz Clark, MS, RN: recipients of consulting fees through Centripital to support implementation of the care model described. Bryan W. Castle, MBA, RN: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees through Centripital to support implementation of the care model described. The authors report no other conflicts of interest.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , . The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769.
- . The end of the beginning: patient safety five years after “to err is human”. Health Aff (Millwood). 2004;Suppl Web Exclusives:W4‐534–545.
- , , , et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , . Using a Malcolm Baldrige framework to understand high‐performing clinical microsystems. Qual Saf Health Care. 2007;16(5):334–341.
- , , , . Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18(8):926–937.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Reducing cardiac arrests in the acute admissions unit: a quality improvement journey. BMJ Qual Saf. 2013;22(12):1025–1031.
- , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649–654.
- , . Improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. AHRQ Health Care Innovations Exchange. Available at: https://innovations.ahrq.gov/profiles/improvement‐projects‐led‐unit‐based‐teams‐nurse‐physician‐and‐quality‐leaders‐reduce. Accessed May 4, 2014.
- , , , , . The daily goals communication sheet: a simple and novel tool for improved communication and care. Jt Comm J Qual Patient Saf. 2008;34(10):608–613, 561.
- , , , et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence‐based intensive care unit practices. Crit Care Med. 2009;37(10):2775–2781.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , . Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13(suppl 2):ii34–ii38.
- , , , . Collaborative‐cross checking to enhance resilience. Cogn Tech Work. 2007;9:155–162.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , . Mortality reduction associated with structure process, and management redesign of a hospital medicine unit. J Hosp Med. 2012;7(suppl 2):115.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
In 2001, the Institute of Medicine called for a major redesign of the US healthcare system, describing the chasm between the quality of care Americans receive and the quality of healthcare they deserve.[1] The healthcare community recognizes its ongoing quality and value gaps, but progress has been limited by outdated care models, fragmented organizational structures, and insufficient advances in system design.[2] Many healthcare organizations are searching for new care delivery models capable of producing greater value.
A major constraint in hospitals is the persistence of underperforming frontline clinical care teams.[3] Physicians typically travel from 1 unit or patient to the next in unpredictable patterns, resulting in missed opportunities to share perspectives and coordinate care with nurses, discharge planning personnel, pharmacists, therapists, and patients. This geographic fragmentation almost certainly contributes to interprofessional silos and hierarchies, nonspecific care plans, and failure to initiate or intensify therapy when indicated.[4] Modern hospital units could benefit from having a standard care model that synchronizes frontline professionals into teams routinely coordinating and progressing a shared plan of care.
EFFECTIVE CLINICAL MICROSYSTEMS REFLECTED IN THE DESIGN OF THE ACCOUNTABLE CARE UNIT
High‐value healthcare organizations deliberately design clinical microsystems.[5] An effective clinical microsystem combines several traits: (1) a small group of people who work together in a defined setting on a regular basis to provide care, (2) linked care processes and a shared information environment that includes individuals who receive that care, (3) performance outcomes, and (4) set service and care aims.[6] For the accountable care unit (ACU) to reflect the traits of an effective clinical microsystem, we designed it with analogous features: (1) unit‐based teams, (2) structured interdisciplinary bedside rounds (SIBR), (3) unit‐level performance reporting, and (4) unit‐level nurse and physician coleadership. We launched the ACU on September 1, 2010 in a high‐acuity 24‐bed medical unit at Emory University Hospital, a 579‐bed tertiary academic medical center. Herein we provide a brief report of our experience implementing and refining the ACU over a 4‐year period to help others gauge feasibility and sustainability.
FEATURES OF AN ACU
Unit‐Based Teams
Design
Geographic alignment fosters mutual respect, cohesiveness, communication, timeliness, and face‐to‐face problem solving,[7, 8] and has been linked to improved patient satisfaction, decreased length of stay, and reductions in morbidity and mortality.[9, 10, 11] At our hospital, though, patients newly admitted or transferred to the hospital medicine service traditionally had been distributed to physician teams without regard to geography, typically based on physician call schedules or traditions of balancing patient volumes across colleagues. These traditional practices geographically dispersed our teams. Physicians would be forced regularly to travel to 5 to 8 different units each day to see 10 to 18 patients. Nurses might perceive this as a parade of different physician teams coming and going off the unit at unpredictable times. To temporally and spatially align physicians with unit‐based staff, specific physician teams were assigned to the ACU.
Implementation
The first step in implementing unit‐based teams was to identify the smallest number of physician teams that could be assigned to the ACU. Two internal medicine resident teams are assigned to care for all medical patients in the unit. Each resident team consists of 1 hospital medicine attending physician, 1 internal medicine resident, 3 interns (2 covering the day shift and 1 overnight every other night), and up to 2 medical students. The 2 teams alternate a 24‐hour call cycle where the on‐call team admits every patient arriving to the unit. For patients arriving to the unit from 6 pm to 7 am, the on‐call overnight intern admits the patients and hands over care to the team in the morning. The on‐call team becomes aware of an incoming patient once the patient has been assigned a bed in the home unit. Several patients per day may arrive on the unit as transfers from a medical or surgical intensive care unit, but most patients arrive as emergency room or direct admissions. On any given day it is acceptable and typical for a team to have several patients off the ACU. No specific changes were made to nurse staffing, with the unit continuing to have 1 nurse unit manager, 1 charge nurse per shift, and a nurse‐to‐patient ratio of 1 to 4.
Results
Geographic patient assignment has been successful (Figure 1). Prior to implementing the ACU, more than 5 different hospital medicine physician teams cared for patients on the unit, with no single team caring for more than 25% of them. In the ACU, all medical patients are assigned to 1 of the 2 unit‐based physician teams (physician teams 1 and 2), which regularly represents more than 95% of all patients on the unit. Over the 4 years, these 2 ACU teams have had an average of 12.9 total patient encounters per day (compared to 11.8 in the year before the ACU when these teams were not unit based). The 2 unit‐based teams have over 90% of their patients on the ACU daily. In contrast, 3 attending‐only hospital medicine teams (physician teams 3, 4, and 5) are still dispersed over 6 to 8 units every day (Figure 2), primarily due to high hospital occupancy and a relative scarcity of units eligible to become dedicated hospital medicine units.


Effects of the Change
Through unit‐based teams, the ACU achieves the first trait of an effective clinical microsystem. Although an evaluation of the cultural gains are beyond the scope of this article, the logistical advantages are self‐evident; having the fewest necessary physician teams overseeing care for nearly all patients in 1 unit and where those physician teams simultaneously have nearly all of their patients on that 1 unit, makes it possible to schedule interdisciplinary teamwork activities, such as SIBR, not otherwise feasible.
Structured Interdisciplinary Bedside Rounds
Design
To reflect the second trait of an effective clinical microsystem, a hospital unit should routinely combine best practices for communication, including daily goals sheets,[12] safety checklists,[13] and multidisciplinary rounds.[14, 15] ACU design achieves this through SIBR, a patient‐ and family‐centered, team‐based approach to rounds that brings the nurse, physician, and available allied health professionals to the patient's bedside every day to exchange perspectives using a standard format to cross‐check information with the patient, family, and one another, and articulate a clear plan for the day. Before the SIBR hour starts, physicians and nurses have already performed independent patient assessments through usual activities such as handover, chart review, patient interviews, and physical examinations. Participants in SIBR are expected to give or receive inputs according to the standard SIBR communication protocol (Figure 3), review a quality‐safety checklist together, and ensure the plan of care is verbalized. Including the patient and family allows all parties to hear and be heard, cross‐check information for accuracy, and hold each person accountable for contributions.[16, 17]

Implementation
Each ACU staff member receives orientation to the SIBR communication protocol and is expected to be prepared and punctual for the midmorning start times. The charge nurse serves as the SIBR rounds manager, ensuring physicians waste no time searching for the next nurse and each team's eligible patients are seen in the SIBR hour. For each patient, SIBR begins when the nurse and physician are both present at the bedside. The intern begins SIBR by introducing team members before reviewing the patient's active problem list, response to treatment, and interval test results or consultant inputs. The nurse then relays the patient's goal for the day, overnight events, nursing concerns, and reviews the quality‐safety checklist. The intern then invites allied health professionals to share inputs that might impact medical decision making or discharge planning, before synthesizing all inputs into a shared plan for the day.
Throughout SIBR, the patient and family are encouraged to ask questions or correct misinformation. Although newcomers to SIBR often imagine that inviting patient inputs will disrupt efficiency, we have found teams readily learn to manage this risk, for instance discerning the core question among multiple seemingly disparate ones, or volunteering to return after the SIBR hour to explore a complex issue.
Results
Since the launch of the ACU on September 1, 2010, SIBR has been embedded as a routine on the unit with both physician teams and the nursing staff conducting it every day. Patients not considered eligible for SIBR are those whom the entire physician team has not yet evaluated, typically patients who arrived to the unit overnight. For patients who opt out due to personal preference, or for patients away from the unit for a procedure or a test, SIBR occurs without the patient so the rest of the team can still exchange inputs and formulate a plan of care. A visitor to the unit sees SIBR start punctually at 9 am and 10 am for successive teams, with each completing SIBR on eligible patients in under 60 minutes.
Effects of the Change
The second trait of an effective clinical microsystem is achieved through SIBR's routine forum for staff to share information with each other and the patient. By practicing SIBR every workday, staff are presented with multiple routine opportunities to experience an environment reflective of high‐performing frontline units.[18] We found that SIBR resembled other competencies, with a bell curve of performance. For this reason, by the start of the third year we added a SIBR certification program, a SIBR skills training program where permanent and rotating staff are evaluated through an in vivo observed structured clinical exam, typically with a charge nurse or physician as preceptor. When a nurse, medical student, intern, or resident demonstrates an ability to perform a series of specific high performance SIBR behaviors in 5 of 6 consecutive patients, they can achieve SIBR certification. In the first 2 years of this voluntary certification program, all daytime nursing staff and rotating interns have achieved this demonstration of interdisciplinary teamwork competence.
Unit‐Level Performance Reporting
Design
Hospital outcomes are determined on the clinical frontline. To be effective at managing unit outcomes, performance reports must be made available to unit leadership and staff.[5, 16] However, many hospitals still report performance at the level of the facility or service line. This limits the relevance of reports for the people who directly determine outcomes.
Implementation
For the first year, a data analyst was available to prepare and distribute unit‐level performance reports to unit leaders quarterly, including rates of in‐hospital mortality, blood stream infections, patient satisfaction, length of stay, and 30‐day readmissions. Preparation of these reports was labor intensive, requiring the analyst to acquire raw data from multiple data sources and to build the reports manually.
Results
In an analysis comparing outcomes for every patient spending at least 1 night on the unit in the year before and year after implementation, we observed reductions in in‐hospital mortality and length of stay. Unadjusted in‐hospital mortality decreased from 2.3% to 1.1% (P=0.004), with no change in referrals to hospice (5.4% to 4.5%) (P=0.176), and length‐of‐stay decreased from 5.0 to 4.5 days (P=0.001).[19] A complete report of these findings, including an analysis of concurrent control groups is beyond the scope of this article, but here we highlight an effect we observed on ACU leadership and staff from the reduction in in‐hospital mortality.
Effects of the Change
Noting the apparent mortality reduction, ACU leadership encouraged permanent staff and rotating trainees to consider an unexpected death as a never event. Although perhaps self‐evident, before the ACU we had never been organized to reflect on that concept or to use routines to do something about it. The unit considered an unexpected death one where the patient was not actively receiving comfort measures. At the monthly meet and greet, where ACU leadership bring the permanent staff and new rotating trainees together to introduce themselves by first name, the coleaders proposed that unexpected deaths in the month ahead could represent failures to recognize or respond to deterioration, to consider an alternative or under‐treated process, to transfer the patient to a higher level of care, or to deliver more timely and appropriate end‐of‐life care. It is our impression that this introspection was extraordinarily meaningful and would not have occurred without unit‐based teams, unit‐level performance data, and ACU leadership learning to utilize this rhetoric.
Unit‐Level Nurse and Physician Coleadership
Design
Effective leadership is a major driver of successful clinical microsystems.[20] The ACU is designed to be co‐led by a nurse unit manager and physician medical director. The leadership pair was charged simply with developing patient‐centered teams and ensuring the staff felt connected to the values of the organization and accountable to each other and the outcomes of the unit.
Implementation
Nursing leadership and hospital executives influenced the selection of the physician medical director, which was a way for them to demonstrate support for the care model. Over the first 4 years, the physician medical director position has been afforded a 10% to 20% reduction in clinical duties to fulfill the charge. The leadership pair sets expectations for the ACU's code of conduct, standard operating procedures (eg, SIBR), and best‐practice protocols.
Results
The leadership pair tries explicitly to role model the behaviors enumerated in the ACU's relational covenant, itself the product of a facilitated exercise they commissioned in the first year in which the entire staff drafted and signed a document listing behaviors they wished to see from each other (see Supporting Information, Appendix 1, in the online version of this article). The physician medical director, along with charge nurses, coach staff and trainees wishing to achieve SIBR certification. Over the 4 years, the pair has introduced best‐practice protocols for glycemic control, venous thromboembolism prophylaxis, removal of idle venous and bladder catheters, and bedside goals‐of‐care conversations.
Effects of the Change
Where there had previously been no explicit code of conduct, standard operating procedures such as SIBR, or focused efforts to optimize unit outcomes, the coleadership pair fills a management gap. These coleaders play an essential role in building momentum for the structure and processes of the ACU. The leadership pair has also become a primary resource for intraorganizational spread of the ACU model to medical and surgical wards, as well as geriatric, long‐term acute, and intensive care units.
CHALLENGES
Challenges with implementing the ACU fell into 3 primary categories: (1) performing change management required for a successful launch, (2) solving logistics of maintaining unit‐based physician teams, and (3) training physicians and nurses to perform SIBR at a high level.
For change management, the leadership pair was able to explain the rationale of the model to all staff in sufficient detail to launch the ACU. To build momentum for ACU routines and relationships, the physician leader and the nurse unit manager were both present on the unit daily for the first 100 days. As ACU operations became routine and competencies formed among clinicians, the amount of time spent by these leaders was de‐escalated.
Creating and maintaining unit‐based physician teams required shared understanding and coordination between on‐call hospital medicine physicians and the bed control office so that new admissions or transfers could be consistently assigned to unit‐based teams without adversely affecting patient flow. We found this challenge to be manageable once stakeholders accepted the rationale for the care mode and figured out how to support it.
The challenge of building high‐performance SIBR across the unit, including competence of rotating trainees new to the model, requires individualized assessment and feedback necessary for SIBR certification. We addressed this challenge by creating a SIBR train‐the‐trainer programa list of observable high‐performance SIBR behaviors coupled with a short course about giving effective feedback to learnersand found that once the ACU had several nurse and physician SIBR trainers in the staffing mix every day, the required amount of SIBR coaching expertise was available when needed.
CONCLUSION
Improving value and reliability in hospital care may require new models of care. The ACU is a hospital care model specifically designed to organize physicians, nurses, and allied health professionals into high‐functioning, unit‐based teams. It converges standard workflow, patient‐centered communication, quality‐safety checklists, best‐practice protocols, performance measurement, and progressive leadership. Our experience with the ACU suggests that hospital units can be reorganized as effective clinical microsystems where consistent unit professionals can share time and space, a sense of purpose, code of conduct, shared mental model for teamwork, an interprofessional management structure, and an important level of accountability to each other and their patients.
Disclosures: Jason Stein, MD: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees and royalties for licensed intellectual property to support implementation of the care model described; founder and president of nonprofit Centripital, provider of consulting services to hospital systems implementing the care model described. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Liam Chadwick, PhD, and Diaz Clark, MS, RN: recipients of consulting fees through Centripital to support implementation of the care model described. Bryan W. Castle, MBA, RN: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees through Centripital to support implementation of the care model described. The authors report no other conflicts of interest.
In 2001, the Institute of Medicine called for a major redesign of the US healthcare system, describing the chasm between the quality of care Americans receive and the quality of healthcare they deserve.[1] The healthcare community recognizes its ongoing quality and value gaps, but progress has been limited by outdated care models, fragmented organizational structures, and insufficient advances in system design.[2] Many healthcare organizations are searching for new care delivery models capable of producing greater value.
A major constraint in hospitals is the persistence of underperforming frontline clinical care teams.[3] Physicians typically travel from 1 unit or patient to the next in unpredictable patterns, resulting in missed opportunities to share perspectives and coordinate care with nurses, discharge planning personnel, pharmacists, therapists, and patients. This geographic fragmentation almost certainly contributes to interprofessional silos and hierarchies, nonspecific care plans, and failure to initiate or intensify therapy when indicated.[4] Modern hospital units could benefit from having a standard care model that synchronizes frontline professionals into teams routinely coordinating and progressing a shared plan of care.
EFFECTIVE CLINICAL MICROSYSTEMS REFLECTED IN THE DESIGN OF THE ACCOUNTABLE CARE UNIT
High‐value healthcare organizations deliberately design clinical microsystems.[5] An effective clinical microsystem combines several traits: (1) a small group of people who work together in a defined setting on a regular basis to provide care, (2) linked care processes and a shared information environment that includes individuals who receive that care, (3) performance outcomes, and (4) set service and care aims.[6] For the accountable care unit (ACU) to reflect the traits of an effective clinical microsystem, we designed it with analogous features: (1) unit‐based teams, (2) structured interdisciplinary bedside rounds (SIBR), (3) unit‐level performance reporting, and (4) unit‐level nurse and physician coleadership. We launched the ACU on September 1, 2010 in a high‐acuity 24‐bed medical unit at Emory University Hospital, a 579‐bed tertiary academic medical center. Herein we provide a brief report of our experience implementing and refining the ACU over a 4‐year period to help others gauge feasibility and sustainability.
FEATURES OF AN ACU
Unit‐Based Teams
Design
Geographic alignment fosters mutual respect, cohesiveness, communication, timeliness, and face‐to‐face problem solving,[7, 8] and has been linked to improved patient satisfaction, decreased length of stay, and reductions in morbidity and mortality.[9, 10, 11] At our hospital, though, patients newly admitted or transferred to the hospital medicine service traditionally had been distributed to physician teams without regard to geography, typically based on physician call schedules or traditions of balancing patient volumes across colleagues. These traditional practices geographically dispersed our teams. Physicians would be forced regularly to travel to 5 to 8 different units each day to see 10 to 18 patients. Nurses might perceive this as a parade of different physician teams coming and going off the unit at unpredictable times. To temporally and spatially align physicians with unit‐based staff, specific physician teams were assigned to the ACU.
Implementation
The first step in implementing unit‐based teams was to identify the smallest number of physician teams that could be assigned to the ACU. Two internal medicine resident teams are assigned to care for all medical patients in the unit. Each resident team consists of 1 hospital medicine attending physician, 1 internal medicine resident, 3 interns (2 covering the day shift and 1 overnight every other night), and up to 2 medical students. The 2 teams alternate a 24‐hour call cycle where the on‐call team admits every patient arriving to the unit. For patients arriving to the unit from 6 pm to 7 am, the on‐call overnight intern admits the patients and hands over care to the team in the morning. The on‐call team becomes aware of an incoming patient once the patient has been assigned a bed in the home unit. Several patients per day may arrive on the unit as transfers from a medical or surgical intensive care unit, but most patients arrive as emergency room or direct admissions. On any given day it is acceptable and typical for a team to have several patients off the ACU. No specific changes were made to nurse staffing, with the unit continuing to have 1 nurse unit manager, 1 charge nurse per shift, and a nurse‐to‐patient ratio of 1 to 4.
Results
Geographic patient assignment has been successful (Figure 1). Prior to implementing the ACU, more than 5 different hospital medicine physician teams cared for patients on the unit, with no single team caring for more than 25% of them. In the ACU, all medical patients are assigned to 1 of the 2 unit‐based physician teams (physician teams 1 and 2), which regularly represents more than 95% of all patients on the unit. Over the 4 years, these 2 ACU teams have had an average of 12.9 total patient encounters per day (compared to 11.8 in the year before the ACU when these teams were not unit based). The 2 unit‐based teams have over 90% of their patients on the ACU daily. In contrast, 3 attending‐only hospital medicine teams (physician teams 3, 4, and 5) are still dispersed over 6 to 8 units every day (Figure 2), primarily due to high hospital occupancy and a relative scarcity of units eligible to become dedicated hospital medicine units.


Effects of the Change
Through unit‐based teams, the ACU achieves the first trait of an effective clinical microsystem. Although an evaluation of the cultural gains are beyond the scope of this article, the logistical advantages are self‐evident; having the fewest necessary physician teams overseeing care for nearly all patients in 1 unit and where those physician teams simultaneously have nearly all of their patients on that 1 unit, makes it possible to schedule interdisciplinary teamwork activities, such as SIBR, not otherwise feasible.
Structured Interdisciplinary Bedside Rounds
Design
To reflect the second trait of an effective clinical microsystem, a hospital unit should routinely combine best practices for communication, including daily goals sheets,[12] safety checklists,[13] and multidisciplinary rounds.[14, 15] ACU design achieves this through SIBR, a patient‐ and family‐centered, team‐based approach to rounds that brings the nurse, physician, and available allied health professionals to the patient's bedside every day to exchange perspectives using a standard format to cross‐check information with the patient, family, and one another, and articulate a clear plan for the day. Before the SIBR hour starts, physicians and nurses have already performed independent patient assessments through usual activities such as handover, chart review, patient interviews, and physical examinations. Participants in SIBR are expected to give or receive inputs according to the standard SIBR communication protocol (Figure 3), review a quality‐safety checklist together, and ensure the plan of care is verbalized. Including the patient and family allows all parties to hear and be heard, cross‐check information for accuracy, and hold each person accountable for contributions.[16, 17]

Implementation
Each ACU staff member receives orientation to the SIBR communication protocol and is expected to be prepared and punctual for the midmorning start times. The charge nurse serves as the SIBR rounds manager, ensuring physicians waste no time searching for the next nurse and each team's eligible patients are seen in the SIBR hour. For each patient, SIBR begins when the nurse and physician are both present at the bedside. The intern begins SIBR by introducing team members before reviewing the patient's active problem list, response to treatment, and interval test results or consultant inputs. The nurse then relays the patient's goal for the day, overnight events, nursing concerns, and reviews the quality‐safety checklist. The intern then invites allied health professionals to share inputs that might impact medical decision making or discharge planning, before synthesizing all inputs into a shared plan for the day.
Throughout SIBR, the patient and family are encouraged to ask questions or correct misinformation. Although newcomers to SIBR often imagine that inviting patient inputs will disrupt efficiency, we have found teams readily learn to manage this risk, for instance discerning the core question among multiple seemingly disparate ones, or volunteering to return after the SIBR hour to explore a complex issue.
Results
Since the launch of the ACU on September 1, 2010, SIBR has been embedded as a routine on the unit with both physician teams and the nursing staff conducting it every day. Patients not considered eligible for SIBR are those whom the entire physician team has not yet evaluated, typically patients who arrived to the unit overnight. For patients who opt out due to personal preference, or for patients away from the unit for a procedure or a test, SIBR occurs without the patient so the rest of the team can still exchange inputs and formulate a plan of care. A visitor to the unit sees SIBR start punctually at 9 am and 10 am for successive teams, with each completing SIBR on eligible patients in under 60 minutes.
Effects of the Change
The second trait of an effective clinical microsystem is achieved through SIBR's routine forum for staff to share information with each other and the patient. By practicing SIBR every workday, staff are presented with multiple routine opportunities to experience an environment reflective of high‐performing frontline units.[18] We found that SIBR resembled other competencies, with a bell curve of performance. For this reason, by the start of the third year we added a SIBR certification program, a SIBR skills training program where permanent and rotating staff are evaluated through an in vivo observed structured clinical exam, typically with a charge nurse or physician as preceptor. When a nurse, medical student, intern, or resident demonstrates an ability to perform a series of specific high performance SIBR behaviors in 5 of 6 consecutive patients, they can achieve SIBR certification. In the first 2 years of this voluntary certification program, all daytime nursing staff and rotating interns have achieved this demonstration of interdisciplinary teamwork competence.
Unit‐Level Performance Reporting
Design
Hospital outcomes are determined on the clinical frontline. To be effective at managing unit outcomes, performance reports must be made available to unit leadership and staff.[5, 16] However, many hospitals still report performance at the level of the facility or service line. This limits the relevance of reports for the people who directly determine outcomes.
Implementation
For the first year, a data analyst was available to prepare and distribute unit‐level performance reports to unit leaders quarterly, including rates of in‐hospital mortality, blood stream infections, patient satisfaction, length of stay, and 30‐day readmissions. Preparation of these reports was labor intensive, requiring the analyst to acquire raw data from multiple data sources and to build the reports manually.
Results
In an analysis comparing outcomes for every patient spending at least 1 night on the unit in the year before and year after implementation, we observed reductions in in‐hospital mortality and length of stay. Unadjusted in‐hospital mortality decreased from 2.3% to 1.1% (P=0.004), with no change in referrals to hospice (5.4% to 4.5%) (P=0.176), and length‐of‐stay decreased from 5.0 to 4.5 days (P=0.001).[19] A complete report of these findings, including an analysis of concurrent control groups is beyond the scope of this article, but here we highlight an effect we observed on ACU leadership and staff from the reduction in in‐hospital mortality.
Effects of the Change
Noting the apparent mortality reduction, ACU leadership encouraged permanent staff and rotating trainees to consider an unexpected death as a never event. Although perhaps self‐evident, before the ACU we had never been organized to reflect on that concept or to use routines to do something about it. The unit considered an unexpected death one where the patient was not actively receiving comfort measures. At the monthly meet and greet, where ACU leadership bring the permanent staff and new rotating trainees together to introduce themselves by first name, the coleaders proposed that unexpected deaths in the month ahead could represent failures to recognize or respond to deterioration, to consider an alternative or under‐treated process, to transfer the patient to a higher level of care, or to deliver more timely and appropriate end‐of‐life care. It is our impression that this introspection was extraordinarily meaningful and would not have occurred without unit‐based teams, unit‐level performance data, and ACU leadership learning to utilize this rhetoric.
Unit‐Level Nurse and Physician Coleadership
Design
Effective leadership is a major driver of successful clinical microsystems.[20] The ACU is designed to be co‐led by a nurse unit manager and physician medical director. The leadership pair was charged simply with developing patient‐centered teams and ensuring the staff felt connected to the values of the organization and accountable to each other and the outcomes of the unit.
Implementation
Nursing leadership and hospital executives influenced the selection of the physician medical director, which was a way for them to demonstrate support for the care model. Over the first 4 years, the physician medical director position has been afforded a 10% to 20% reduction in clinical duties to fulfill the charge. The leadership pair sets expectations for the ACU's code of conduct, standard operating procedures (eg, SIBR), and best‐practice protocols.
Results
The leadership pair tries explicitly to role model the behaviors enumerated in the ACU's relational covenant, itself the product of a facilitated exercise they commissioned in the first year in which the entire staff drafted and signed a document listing behaviors they wished to see from each other (see Supporting Information, Appendix 1, in the online version of this article). The physician medical director, along with charge nurses, coach staff and trainees wishing to achieve SIBR certification. Over the 4 years, the pair has introduced best‐practice protocols for glycemic control, venous thromboembolism prophylaxis, removal of idle venous and bladder catheters, and bedside goals‐of‐care conversations.
Effects of the Change
Where there had previously been no explicit code of conduct, standard operating procedures such as SIBR, or focused efforts to optimize unit outcomes, the coleadership pair fills a management gap. These coleaders play an essential role in building momentum for the structure and processes of the ACU. The leadership pair has also become a primary resource for intraorganizational spread of the ACU model to medical and surgical wards, as well as geriatric, long‐term acute, and intensive care units.
CHALLENGES
Challenges with implementing the ACU fell into 3 primary categories: (1) performing change management required for a successful launch, (2) solving logistics of maintaining unit‐based physician teams, and (3) training physicians and nurses to perform SIBR at a high level.
For change management, the leadership pair was able to explain the rationale of the model to all staff in sufficient detail to launch the ACU. To build momentum for ACU routines and relationships, the physician leader and the nurse unit manager were both present on the unit daily for the first 100 days. As ACU operations became routine and competencies formed among clinicians, the amount of time spent by these leaders was de‐escalated.
Creating and maintaining unit‐based physician teams required shared understanding and coordination between on‐call hospital medicine physicians and the bed control office so that new admissions or transfers could be consistently assigned to unit‐based teams without adversely affecting patient flow. We found this challenge to be manageable once stakeholders accepted the rationale for the care mode and figured out how to support it.
The challenge of building high‐performance SIBR across the unit, including competence of rotating trainees new to the model, requires individualized assessment and feedback necessary for SIBR certification. We addressed this challenge by creating a SIBR train‐the‐trainer programa list of observable high‐performance SIBR behaviors coupled with a short course about giving effective feedback to learnersand found that once the ACU had several nurse and physician SIBR trainers in the staffing mix every day, the required amount of SIBR coaching expertise was available when needed.
CONCLUSION
Improving value and reliability in hospital care may require new models of care. The ACU is a hospital care model specifically designed to organize physicians, nurses, and allied health professionals into high‐functioning, unit‐based teams. It converges standard workflow, patient‐centered communication, quality‐safety checklists, best‐practice protocols, performance measurement, and progressive leadership. Our experience with the ACU suggests that hospital units can be reorganized as effective clinical microsystems where consistent unit professionals can share time and space, a sense of purpose, code of conduct, shared mental model for teamwork, an interprofessional management structure, and an important level of accountability to each other and their patients.
Disclosures: Jason Stein, MD: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees and royalties for licensed intellectual property to support implementation of the care model described; founder and president of nonprofit Centripital, provider of consulting services to hospital systems implementing the care model described. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. Liam Chadwick, PhD, and Diaz Clark, MS, RN: recipients of consulting fees through Centripital to support implementation of the care model described. Bryan W. Castle, MBA, RN: grant support from the US Health & Resources Services Administration to support organizational implementation of the care model described; recipient of consulting fees through Centripital to support implementation of the care model described. The authors report no other conflicts of interest.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , . The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769.
- . The end of the beginning: patient safety five years after “to err is human”. Health Aff (Millwood). 2004;Suppl Web Exclusives:W4‐534–545.
- , , , et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , . Using a Malcolm Baldrige framework to understand high‐performing clinical microsystems. Qual Saf Health Care. 2007;16(5):334–341.
- , , , . Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18(8):926–937.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Reducing cardiac arrests in the acute admissions unit: a quality improvement journey. BMJ Qual Saf. 2013;22(12):1025–1031.
- , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649–654.
- , . Improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. AHRQ Health Care Innovations Exchange. Available at: https://innovations.ahrq.gov/profiles/improvement‐projects‐led‐unit‐based‐teams‐nurse‐physician‐and‐quality‐leaders‐reduce. Accessed May 4, 2014.
- , , , , . The daily goals communication sheet: a simple and novel tool for improved communication and care. Jt Comm J Qual Patient Saf. 2008;34(10):608–613, 561.
- , , , et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence‐based intensive care unit practices. Crit Care Med. 2009;37(10):2775–2781.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , . Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13(suppl 2):ii34–ii38.
- , , , . Collaborative‐cross checking to enhance resilience. Cogn Tech Work. 2007;9:155–162.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , . Mortality reduction associated with structure process, and management redesign of a hospital medicine unit. J Hosp Med. 2012;7(suppl 2):115.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , . The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769.
- . The end of the beginning: patient safety five years after “to err is human”. Health Aff (Millwood). 2004;Suppl Web Exclusives:W4‐534–545.
- , , , et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–834.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , . Using a Malcolm Baldrige framework to understand high‐performing clinical microsystems. Qual Saf Health Care. 2007;16(5):334–341.
- , , , . Relational coordination among nurses and other providers: impact on the quality of patient care. J Nurs Manag. 2010;18(8):926–937.
- , , , et al. Unit‐based care teams and the frequency and quality of physician‐nurse communications. Arch Pediatr Adolesc Med. 2011;165(5):424–428.
- , , , et al. Reducing cardiac arrests in the acute admissions unit: a quality improvement journey. BMJ Qual Saf. 2013;22(12):1025–1031.
- , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7(8):649–654.
- , . Improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. AHRQ Health Care Innovations Exchange. Available at: https://innovations.ahrq.gov/profiles/improvement‐projects‐led‐unit‐based‐teams‐nurse‐physician‐and‐quality‐leaders‐reduce. Accessed May 4, 2014.
- , , , , . The daily goals communication sheet: a simple and novel tool for improved communication and care. Jt Comm J Qual Patient Saf. 2008;34(10):608–613, 561.
- , , , et al. Implementation of a mandatory checklist of protocols and objectives improves compliance with a wide range of evidence‐based intensive care unit practices. Crit Care Med. 2009;37(10):2775–2781.
- , , , et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678–684.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6(2):88–93.
- , , . Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13(suppl 2):ii34–ii38.
- , , , . Collaborative‐cross checking to enhance resilience. Cogn Tech Work. 2007;9:155–162.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , . Mortality reduction associated with structure process, and management redesign of a hospital medicine unit. J Hosp Med. 2012;7(suppl 2):115.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
Hospital Unit‐Based Leadership Models
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
RTNI to Safely Reduce Dysglycemia
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).
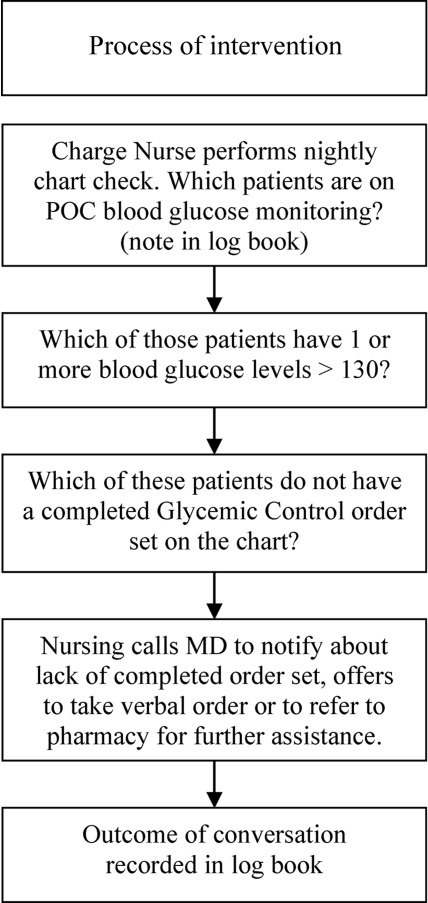
The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).
Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.

| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).

The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).

Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.



| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
Dysglycemia, defined as a random blood glucose value >180 mg/dL or <70 mg/dL, is present in 25% to 28% of hospitalized patients.1, 2 It is associated with poor clinical outcomes, such as increased hospital‐acquired infection rates, increased hospital length of stay, and higher mortality rates.25 Although optimal targets for glycemic control remain unknown for non‐criticallyill patients, adverse effects of hyperglycemia remain very clear.3, 5, 6 The American Diabetes Association and various medical societies have published recommendations and position statements urging better management of hyperglycemia even in stabilized patients on general medical floors.7 Effective options for managing inpatient dysglycemia are available,8 but still remain underutilized. Despite increasing questions about its clinical benefit, lone correctional insulin (LCI) therapy, commonly known as sliding scale insulin, remains a common approach for glycemic control.9, 10 Explanations for this clinical inertia to utilize best practices range from fears of causing hypoglycemia, to a shortage of glycemic control specialists.11
Along with LCI therapy, use of potentially inappropriate oral hypoglycemic medications (PIOHMs) during hospitalization remains common. Scotton et al.12 reports that up to 68% of hospitalized patients on metformin were continued on the drug despite contraindications to its use.1214 Surgical intervention, intravenous contrast use, and elevated creatinine accounted for the majority of contraindications.12 Unfortunately, an educational memo mailed to physicians as well as a computer alert regarding the contraindications to metformin use failed to decrease the inappropriate use of metformin on an inpatient basis.12 Notably, the computer alert appeared whenever metformin was prescribed but did not require the clinician to actively acknowledge the statement. One study has found that inpatient metformin use did not result in increased mortality or adverse events.
Research has shown that implementation of best practices increases with a team approach, or when specialist oversight follows educational efforts.15, 16 Elinav et al.15 describe the difficulty in maintaining specialist oversight, which was essential to provide enhanced glycemic control for inpatients. To capture the potential strengths of team‐based care and specialist oversight, we hypothesized that a glycemic control order set combined with a real‐time nursing intervention (RTNI) could improve best‐practice utilization for glycemic control among hospitalized patients. This intervention likely has the capability to be sustainable, as it is modeled to be incorporated into the frontline workflow. This pilot study depicts the effects of a comprehensive effort to improve glycemic control catalyzed by the RTNI.
Materials and Methods
This study was carried out in a new 110‐bed exurban community teaching hospital, where patients with dysglycemia are primarily treated by family medicine residents, academic hospitalists, private generalists, and bariatric surgeons. Several months prior to the beginning of the study, a glycemic control task force was formed and supported as part of the strategic plan for this new hospital. The interventions in this study were approved by the task force as part of a quality improvement program (QI). Institutional review board (IRB) approval for this study was obtained through Emory University.
A total of 653 patients qualified to participate in this study (Table 1). The analysis was retrospective, using the hospital's electronic health record. Patients were included based on the frequency of blood glucose values obtained. Consent was not required nor obtained for this analysis.
| Baseline (n = 142) | Education (n = 153) | RTNI (n = 183) | Post‐RTNI (n = 175) | P value | |
|---|---|---|---|---|---|
| |||||
| Age (years) | 61 | 60 | 64 | 64 | 0.0606 |
| Weight (lb) | 202 | 207 | 188 | 200 | 0.0378 |
| Serum creatinine | 1.31 | 1.40 | 1.43 | 1.65 | 0.3161 |
| Sex (% male) | 46 | 47 | 40 | 38 | 0.3067 |
| WBC | 9.2 | 9.5 | 9.9 | 9.4 | 0.7249 |
Prior to the RTNI, several educational programs were undertaken from mid‐September 2007 to early November 2007. The glycemic control task force conducted physician education through: 1‐on‐1 physician office visits; phone conferences for hospital‐based physicians; and mailed letters to physicians informing them of available protocols. All 40 physicians who manage dysglycemia at this hospital were contacted by the principal investigator (PI), with 2 exceptions, due to logistic difficulties. We posted clinical guidelines to treat dysglycemia and glucometric performance data in physician workstations. In addition, we developed and conducted a mandatory educational session for nurses. The session lasted 6 hours, and consisted of literature review, pathophysiology, hospital metrics, diabetic pharmacology, and dietary education. All nurses who work on the medical and surgical floors of our hospital were required to attend. Nurses hired after the live educational sessions were required to watch a videotape. Finally, we compiled, distributed, and publicized a paper‐based glycemic control order set for non‐critically‐ill patients. The glycemic control protocol (GCP) contained prompts to encourage key elements of best practices, such as basal insulin, use of prandial insulin for patients who were eating, automatic orders for nurses to address nutritional interruptions, and a hypoglycemic protocol (see Appendix A: Glycemic Control Protocol).
After these educational measures, the RTNI ran for 2 months (December 1, 2007 to January 31, 2008). The charge nurse of each floor identified patients with point of care (POC) glucose monitoring who had any glucose level >130mg/dL. When any such patient did not have a physician‐completed GCP, the charge nurse called the attending physician to remind them of the availability and likely appropriateness of initiating the GCP. The nurses offered to take verbal orders for the GCP and referred the physicians to the hospital pharmacist for any dosing questions. This information was recorded on log sheets and stored in a secure office by the charge nurses. After 2 months, the RTNI was removed as scheduled (Figure 1).

The hospital's electronic clinical information system was used to extract information on all noncritical and nonobstetric adult patients having 2 recorded blood glucose values per day for at least 2 days during the admission. Both serum glucose and POC glucose values were sufficient for inclusion. This level of glucose monitoring was the only qualifying criteria. Serum glucose testing was performed on the Siemens RXL MAX (Siemens, Deerfield, IL), and POC glucose values were obtained using the Roche Accuchek (Roche, Nutley, NJ).
One laboratory technician was trained to conduct this data extraction. This work was reviewed by the PI to assure data integrity. Our analysis included data on qualifying patients from the following time periods: (1) patients hospitalized during the 2‐month period prior to the initiation of educational programs (baseline); (2) patients hospitalized during the 2 months of education; (3) patients hospitalized during the RTNI; and (4) patients admitted for 2 months after the RTNI was removed (post‐RTNI). Between the RTNI and the post‐RTNI groups, 1 month's data were discarded as a washout period.
Five metrics were tracked for all patients. The first metric, the overall patient day‐weighted mean glucose (PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days) value, was calculated using a method similar to a previously validated technique.17 We excluded all values <70 mg/dL, all values after day 10 of the hospitalization, and all values within 90 minutes of the previous value. Using the remaining values, the mean for each day was calculated. With each patient having 1 such value per patient‐day, we then calculated the individual PDWMG as the mean of all these patient‐days (1 value per qualifying patient per admission). The overall PDWMG was an average of the PDWMGs for all study patients in a particular time period.
The second metric was the percentage of qualifying patients with PDWMGs >180 mg/dL. The third metric was the percentage of patients who were administered PIOHMs (metformin or sulfonylureas). The fourth metric was the percentage of study patients who were administered correctional insulin without scheduled insulin. Fifth, we calculated the percentage of patients with severe recurrent hypoglycemia (glucose <50 mg/dL on more than 1 occasion separated by 30 minutes). We tracked patient data on a monthly basis and used 1‐way analysis of variance (ANOVA) to analyze the data (Figure 2).

Results
There were 1902 nonobstetric, noncritical, adult admissions to our facility during the entire study period. A total of 521 patients were admitted during the RTNI period. A total of 653 patients met inclusion criteria during the entire study. During the RTNI period, 183 patients met inclusion criteria. Forty‐nine patients met criteria for an RTNI call. The number of patients who had an RTNI call done was 25. The number of patients placed on the order set after an RTNI call was 12.
The study was designed to elucidate whether or not our RTNI was effective in improving best practices and glycemic control in a hospital that provided its staff with education to effectively treat dysglycemia. Compared to baseline, the use of LCIn regimens decreased from 48.2% to 31.3% (P < 0.01) during the RTNI period and the rate of PIOHM usage was reduced from 28.5% to 13.3% (P < 0.01).
We observed a decrease in PDWMG from 166 mg/dL to 156 mg/dL (P = 0.04) and found a trend toward a reduced rate of patients with PDWMG > 180 mg/dL, from 27.0% to 21.7% (P = 0.28). After removal of the intervention, all 4 glycemic control metrics trended back toward and were not significantly different from the baseline, with the exception of PIOHM use. The PIOHM remained significantly lower, from 28.5% in the baseline group, to 19.4% in the postintervention group (P = 0.039) (Table 2). The prevalence of severe recurrent hypoglycemia was not significantly different in 7 of the 8 months. The exception was in the first month of the RTNI, when we observed a spike to 10%. Figures 3 to 5 depict some of these findings using annotated statistical process control charts.



| Outcome Measure | Baseline | Education | Intervention | Postintervention |
|---|---|---|---|---|
| ||||
| Mean PDWMG (mg/dL) | 166.1 | 162.8 (P = 0.52) | 156.4 (P = 0.04) | 167.0 (P = 0.15) |
| Patients with PDWMG > 180 mg/dL (%) | 27.0 | 27.3 (P = 0.075) | 21.7 (P = 0.28) | 24.6 (P = 0.49) |
| Patients on correctional insulin only (%) | 48.2 | 37.9 (P = 0.075) | 31.1 (P = 0.0006) | 37.7 (P = 0.49) |
| Patients on potentially inappropriate medications (%) | 28.5 | 24.2 (P = 0.42) | 13.3 (P = 0.0005) | 19.4 (P = 0.039) |
| Number of patients | 142 | 153 | 183 | 175 |
Discussion
Glucometrics are useful in monitoring changes during a glycemic control QI program.17 Our study was designed to explore the glucometric effect of a RTNI when preceded by staff education and a best‐practice glycemic control order set. In this study, after identifying patients with dysglycemia, charge nurses personally encouraged physicians to use a paper‐based best‐practice order set. During the 2 months of the RTNI, we observed a significant corresponding improvement in many metrics. This improvement largely disappeared following removal of the RTNI. We postulate that the RTNI triggered clinically important moments of awareness or accountability to overcome clinical inertia. The total number of calls was only a fraction of the total patients who met inclusion criteria. We postulate that the publicized RTNI program created a level of awareness for many providers, who came to anticipate phone call reminders regarding use of the GCP. Clinical inertia has been described as the failure of health care providers to initiate or intensify therapy when indicated,18 and thereby represents a plausible explanation for underutilizing best‐practice guidelines.
PIOHM usage decreased and stayed low after withdrawal of the intervention. The literature is not conclusive with regard to the inappropriateness of oral medication use in hospitals, but avoiding these oral medications is espoused by experts in the field.19
Because our RTNI did not include a focused insulin titration component we did not demonstrate a vast improvement in glycemic control itself, the metric with the greatest association with morbid events.3 We theorize that the addition of a focused titration component to an RTNI may address this issue.
There was a concerning rise in hypoglycemic events initially, which completely returned to pre‐RTNI levels in 1 month. Although the reason for the increased hypoglycemia is not clear, we speculate that the lack of physician familiarity with insulin dosing played a large role. Since this problem did not persist after the first RTNI month, despite the same study conditions, we speculate that the physicians responsible adapted by learning to make the appropriate dose adjustments. In these patients, no intensive care unit (ICU) transfers or seizures resulted from the hypoglycemia.
Hypoglycemia is a common problem encountered even in several studies on intensive glucose control in both an inpatient and outpatient setting. In medical ICU patients, the rate of hypoglycemia was shown be 18.7% in the intensive treatment group as compared with 3.1% in the control group.20 Hypoglycemia is also the reason one clinical trial on intensive insulin therapy in critically ill patients was stopped.21 However, one study of 302 ICU patients found no association between hypoglycemia and short‐term (within 5 days of the event) or late (hospital) mortality.22 The Normoglycaemia in Intensive Care Evaluation and Survival Using Glucose Algorithm Regulation (NICE‐SUGAR) study found a lower overall incidence of severe hypoglycemia in its study of critically ill patients, but the tight glycemic control group had a 2.6% higher mortality rate, and the number needed to harm was only 38.23
In February 2008, the outpatient glycemic control study of Evaluating How the Treatments in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) was halted due to the finding of an increased rate of mortality in the intensive arm compared with the standard arm. In both study arms, participants with severe hypoglycemia had higher mortality than those without severe hypoglycemia. Controversy still remains secondary to the inability of the ACCORD, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), and Veterans Administration Diabetes Trial (VADT) trials to demonstrate significant reduction of cardiovascular disease (CVD) with intensive glycemic control in outpatients and recently the American Diabetes Association (ADA) came out with a position statement,24 in which it concludes the evidence obtained from the ACCORD, ADVANCE, and VADT trials do not suggest the need for total abandonment of, or major changes in glycemic control targets. The statement stresses on individualization as the benefits of intensive glycemic control on microvascular and neuropathic complications are well established for both type 1 and type 2 diabetes. This controversy is all the more reason to properly address dysglycemia. LCI places patients at risk for both hyperglycemia from lack of basal insulin, and hypoglycemia from insulin stacking. A proactive strategy of appropriately dosed scheduled insulin via a defined protocol is therefore recommended.
Our study demonstrates that a relatively simple intervention can create the situational awareness to overcome clinical inertia in appropriately treating hyperglycemia. However, it clearly warns glycemic control QI leaders of the need to diligently monitor for hypoglycemia as improvement efforts begin. A system devised to formally check insulin dosing may be warranted. Healthcare providers new to practicing proactive glycemic control with basal/bolus insulin regimens may require close oversight, especially early in the Do phase of the Plan Do Study Act (PDSA) cycle. The Randomized Study of Basal Bolus Insulin Therapy in the Inpatient Management of Patients with Type 2 Diabetes (RABBIT 2) trial randomized insulin naive diabetic patients to weight based scheduled insulin dosing and an adjustable LCI regimen, and found no difference in rates of hypoglycemia, while substantially reducing hyperglycemia with scheduled insulin.25 Our study included patients with advanced age and renal dysfunction who require decreased insulin dosing, the initial increase of hypoglycemia highlights the need for further research in this area.
Study Limitations
This study does have limitations. First, it is not clear how much the improvement in glucometrics was due to the RTNI alone. In fact, it is likely that there was a carryover effect from the education period. A longer time series might make the relative contributions clearer. Second, routine glycosylated hemoglobin (HbA1c) values, severity of illness, patient mix, and mortality were not assessed in this study. It is difficult to generalize the results of this single‐center study. Finally, our method of tracking glycemic control was limited by evaluating patient stay (patient day‐weighted mean glucose, PDWMG; ie, mean glucose for each hospital day, averaged across all hospital days), rather than the patient day mean glucose (PDMG). Mean glucose changes in short hospital patient stay may be highly blunted by using this method. Rigorous analyses in future QI studies using PDMG may be done by excluding the PDMG values for the first hospital day in all patients. This would yield a greater number of meaningful data points, enabling a more clear and rapid realization of results.
Conclusions
An RTNI coupled with a GCP significantly improved best‐practices for hospitalized patients with dysglycemia and may have modestly improved glycemic control. The RTNI accommodates normal clinical workflow and therefore is likely to be sustainable. Additional study should gauge the effect of a focused insulin titration component and further investigation is needed to gauge sustainability, transferability across nursing units and hospitals, and scalability of the underlying concept to additional inpatient care metrics. Vigilant monitoring of hypoglycemia is necessary as glycemic control QI initiatives are undertaken.
Acknowledgements
The authors thank Jennifer H. Eig, MPH; Alicia Fish, MT; Emily O'Malley, MSPH; Moges S. Ido, MPH; John D. Quinlivan, MHA; Kimberly Bentley, MS; Gloria Nunn, PhD; Laurie Hansen, MS; Beth Delrossi, PharmD; Roland Tam, PharmD; Christina Ostrowski, BS; and all others who contributed to the study.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2(4):203–211.
- ,.Inpatient diabetes management in non‐ICU settings: evidence and strategies.Curr Diabetes Rev.2007;3(4):239–243.
- ,,, et al.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87(3):978–982.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1(3):141–144.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(3):43–48.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enter Nutr.1998;22:77–81.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:856.
- ,.Point: inpatient glucose management: the emperor finally has clothes.Diabetes Care.2005;28(4):973–975.
- ,. Sliding‐scale insulin therapy: an ineffective option for inpatient glycemic control. Available at:http://www.residentandstaff.com/issues/articles/2007–02_08.asp. Accessed July 2009.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,, et al.Improving glycemic control in medical inpatients: a pilot study.J Hosp Med.2008;3(1):55–63.
- ,,,,.Assessing the appropriate use of metformin in an inpatient setting and the effectiveness of two pharmacy‐based measures to improve guideline adherence.Qual Manag Health Care.2009;18(1):71–76.
- ,,,,.Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy.Arch Intern Med.2002;162(4):434–437.
- ,,,,,Retrospective review of metformin in inpatients and outpatients at the University of Michigan.Diabetes Care.2006;29(1):170–171.
- ,,, et al.In‐hospital treatment of hyperglycemia: effects of intensified subcutaneous insulin treatment.Curr Med Res Opin.2007;23(4):757–765.
- ,,, et al.An institutional process to improve inpatient glycemic control.Qual Manag Health Care.2007;16(3):239–249.
- ,,, et al.“Glucometrics”—assessing the quality of inpatient glucose management.Diabetes Technol Ther.2006;8(5):560–569.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135(9):825–834.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3(S5):17–18.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med2006;354:449–461.
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis (VISEP).N Engl J Med.2008;358(2):125–139.
- ,,, et al.Evaluation of short‐term consequences of hypoglycemia in an intensive care unit.Crit Care Med.2006;34:2714–2718.
- ,,, et al.Intensive versus conventional glucose control in critically ill patients (NICE SUGAR).N Engl J Med.2009;26;360(13):1283–1297.
- ,,, et al.Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association.Circulation.2009;119:351–357.
- ,,.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30(9):2181–2186.
Copyright © 2010 Society of Hospital Medicine
Editorial
As we approach the 6th year since the Institute of Medicine's Crossing the Quality Chasm offered a new vision for the American health care system, we still have a marked mismatch between the demand for health care quality and the supply of know‐how to deliver it. What the field of quality improvement (QI) still needs is merely this: QI practitioners in every care setting, a working vocabulary, a predictive framework for the mechanisms of reliable care, and rational therapies rigorously studied.13 Fortunately, the field of QI has attracted enough empiricistsworking in the lab of the hospital and other care settingsto lurch forward. But few would argue that we still have far less insight into the delivery of quality care than into the delivery of myocardial blood flow.
For ischemic heart disease we have classes of therapies, each of which is grounded in basic and clinical science: antiplatelets, beta‐blockers, vasodilators, lipid‐lowering agents. For care delivery we have the makings of analogous therapy classes, derived and introduced rather recently in a large review, Closing the Quality Gap: A Critical Analysis of the Quality Improvement Literature.4, 5 To facilitate their review of the evidence, the authors, including 2 prominent hospitalists, developed a new taxonomy of QI strategies (see Table 1). Though their effect size, relative efficacy, and interactions are not yet clear, many of these strategies can be applied to the inpatient setting, perhaps no less rationally than a well‐constructed antianginal regimen.
| QI Strategies | Examples |
|---|---|
| |
| Provider education | Conferences and workshops |
| Educational outreach visits (eg, academic detailing) | |
| Distributed educational materials | |
| Provider reminder systems | Reminders in charts for providers |
| Computer‐based reminders for providers | |
| Computer‐based decision support | |
| Facilitated relay of clinical data to providers | Transmission of clinical data from data source to hospital physician by means other than medical record, eg, page, e‐mail, phone call to hospitalist about clinically significant findings in postdischarge period |
| Audit and feedback of performance to providers | Feedback of performance to individual providers |
| Quality indicators and reports | |
| National/state quality report cards | |
| Publicly released performance data | |
| Benchmarkingprovision of outcomes data from top performers for comparison with provider's own data | |
| Patient education | Classes |
| Parent and family education | |
| Patient pamphlets | |
| Intensive education strategies promoting self‐management of chronic conditions | |
| Promotion of self‐management | Materials and devices promoting self‐management, eg, diabetes educator, pharmacist‐facilitated teaching of discharge medications |
| Patient reminder systems | Postcards or calls to patients |
| Organizational or team change | Case management, disease management |
| Multidisciplinary teams | |
| Change from paper to computer‐based records | |
| Increased staffing | |
| Skill mix changes | |
| Continuous quality improvement | Interventions using an iterative process for assessing quality problems, developing solutions, testing their impacts, and then reassessing the need for further action, eg, PlanDoStudyAct |
| Financial incentives, regulation, and policy | Provider directed: |
| Financial incentives based on achievement of performance goals | |
| Alternative reimbursement systems (eg, fee‐for‐service, capitated payments) | |
| Licensure requirements | |
| Health system directed: | |
| Initiatives by accreditation bodies (eg, residency work hour limits) | |
| Changes in reimbursement schemes (eg, capitation, prospective payment, salaried providers) | |
Where in the pathophysiology of a hospital do these QI therapies act? A plurality target the level of the provider: provider education, provider reminders, audit‐and‐feedback of provider performance, and facilitated relay of clinical data to providers. Remaining strategies target the patient (patient education, promotion of self‐management, and patient reminders), the immediate system within which care is delivered (organizational change), and the methodology of problem solving (continuous quality improvement). Only one strategy (financial incentives, regulation, and policy) fails to act directly at the level of the patient or provider, arguably the only level at which care actually can be improved.6
The value of the Quality Gap taxonomy is still largely untapped. If QI researchers and practitioners were to adopt its language as a standard, we could ramp up the power with which we communicate, interpret, and ultimately conduct improvement initiatives. In this issue of the Journal of Hospital Medicine, Cohn and colleagues profile a quality improvement initiative that achieved an impressive new level of performance. For an inpatient metric with a baseline institutional performance of 47%and an international benchmark of 39%the investigators executed a QI initiative that appears to have raised the rate of VTE prophylaxis to 85%.7 Despite a study design that weakens validity (beforeafter without controls) and a setting that diminishes applicability (medical patients in a single academic center), the authors have made a solid contribution to the QI literature simply by using the Quality Gap taxonomy. The authors specifically name and profile at least 3 distinct classes of QI strategies: provider education, a provider reminder element (ie, decision support), and an audit‐and‐feedback layer.
Even though provider education is unlikely to be sufficient as a lone QI strategya large review showed consistent but only modest benefitsit is often necessary.8 The provider education executed by Cohn and colleagues was frequent and regular. In the beginning of each month the chief resident oriented incoming house staff about venous thromboembolism (VTE) risk factors and the need for prophylaxis. They were given decision support pocket cards. Posters on display in nurse and physician work areas highlighted VTE risk factors. The provider education element also included discussions with the division chief about the topic. As robust as it was, however, the provider education was just a single component of the larger QI effort.
The second element, decision support, included VTE risk factor pocket cards with prophylaxis options listed. Introduced initially with the provider education, the pocket cards were handed out monthly by the chief resident. It is critical to recognize this decision support layer as a distinct core QI strategyand that it may even be fundamental to the success of the other strategies. Placed into the clinical workflow as a durable item, the decision support pocket card has the power to overcome provider uncertainty at moments of medical decision making. Generally speaking, a decision support layer, whether a pocket card, computer alert, or algorithm on a preprinted order form, can function as a shared baseline. Shared baselines or protocols reduce unnecessary variation in practice, a common source of poor quality care. Any mechanism that encourages groups of providers to deliver the same recommended care to groups of similarly at‐risk patients, while allowing customization of the protocol to meet the special needs of any individual patient, will have the net effect of raising overall quality of care.
This QI initiative may have achieved its greatest performance gainsas well as its greatest loss in terms of applicability to other settingsfrom its third facet, the audit‐and‐feedback layer. As a QI strategy audit‐and‐feedback has been defined as a summary of clinical performance for health care providers or institutions, performed for a specific period of time and reported either publicly or confidentially.1 It has demonstrated small to moderate benefits, with variations in effect most likely related to the format.9 As profiled in this study, it is hard to imagine a more powerful audit‐and‐feedback arrangement. The division chief of General Internal Medicine not only performed the audits, but also directly delivered the feedback to the house staff. In a deliberate, systematic, and successful way the investigators constructively used an existing authority gradient to leverage the Hawthorne effect, a change in worker behavior triggered by knowledge of being observed. Although it contributed to the impressive new VTE prophylaxis rates, this component did diminish generalizability and sustainability. Nonacademic centers may struggle to replicate these results, a point the authors dutifully point out. But even other academic centers might struggle in the absence of an authority figure with comparable influence and dedication to VTE prophylaxis. At the study hospital itself, similar rates of improvement would not be expected in patient populations outside the purview of the division chief.
Several alternatives to the before‐after study design could have produced richer information. Simultaneous data on VTE prophylaxis rates in a nonintervention population in the same or a similar hospital could have controlled for background or secular effects. An interrupted time series design may even have been feasible and could have provided more confidence in causality and more information on effect size. For example, what would be the effect on performance, if any, with removal of the decision support pocket card at 10 or 15 months? How much would performance rebound after its reintroduction? What could we have learned had the authors chosen instead to measure performance after sequentially introducing each component?
Using the language of the Quality Gap taxonomy, what conclusions can we draw from this improvement initiative? The introduction of a portable provider reminder (the decision support pocket card), when preceded by a program of provider education and followed by high‐intensity audit‐and‐feedback within an existing provider hierarchy, may have the power to raise VTE prophylaxis rates to 85% over an 18‐month period. With the large effect size somewhat mitigating the design flaws that weaken causality, we might risk an inference that these 3 classes of QI strategies can be reasonably successful in combination. But would we introduce them in our own medical centers? Using the clarity afforded by the taxonomy, we can identify several potential limitations, all attributable to the specifics of the audit‐and‐feedback arrangement: stringent preconditions of the practice setting, guaranteed inability to spread the initiative to other patient populations within the same medical center, limited scalability to include other QI projects, and reliance on the role of a single individual. Although clopidogrel 300 mg daily in the last week of every month is one way to pursue antiplatelet activity, other schedules or alternative agents may be preferable for the vast majority of patients.
The taxonomy can be used to compare, contrast, and more fully understand other QI studies. For example, among acutely ill medical inpatients not receiving VTE prophylaxis, Kucher and colleagues found that an electronic alert nearly doubled prophylaxis rates compared to those in a control group.10 Before trying to emulate their experience, a similarly equipped hospital would do well to recognize that the electronic alert was deployed as a composite of provider education, provider reminder, and facilitated relay of clinical information strategies, increasing prophylaxis rates for high risk patients from a baseline of 85% to 88%.
On the 21st‐century side of the quality chasm there is still something to be learned from QI research that falls short of recently proposed standards.3 This may be true as long as the key question remains: what are the mechanisms of reliable and sustainable performance improvement? We have not yet reached the day where a predictive framework, the clarity of our inquiry, the rigor of our study design, and the strength of our evidence churn out coherent answers. But we do have insights from a wealth of ongoing QI activity triggered by such forces as the Institute for Healthcare Improvement's 100,000 Lives Campaign and the advent of mandatory public reporting of hospital performance measures. By adding to this primordial mix the taxonomy offered by Closing the Quality Gap and its uptake into our vernacular by reports such as the one by Cohn in this issue of the Journal of Hospital Medicine, we are acquiring the language and experience to conduct intelligent and intelligible QI research.
- ,,.Designing a quality improvement intervention: a systematic approach.Qual Saf Health Care.2003;12;215–220.
- ,,.Implementing clinical guidelines: current evidence and future implications.J Contin Educ Health Prof.2004;24(Suppl 1):S31–S37.
- ,.Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project.Qual Saf Health Care.2005;14:319–325.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol.1, Series Overview and Methodology. Technical Review 9 (Contract No. 290‐02‐0017 to the Stanford University–UCSF Evidence‐based Practices Center). AHRQ Publication No. 04‐0051‐1.Rockville, MD:Agency for Healthcare Research and Quality,2004.
- ,,, et al.Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis.JAMA.2006;296:427–440.
- ,,, et al.Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units.Jt Comm J Qual Improv.2002;28:472–493.
- ,,, et al.A multinational observational cohort study in acutely ill medical patients of Pharmacological thromboembolic prophylaxis practices in prevention of venous thromboembolism: findings of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE).Blood.2003;102(Suppl):321a.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;6:1–84.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977
As we approach the 6th year since the Institute of Medicine's Crossing the Quality Chasm offered a new vision for the American health care system, we still have a marked mismatch between the demand for health care quality and the supply of know‐how to deliver it. What the field of quality improvement (QI) still needs is merely this: QI practitioners in every care setting, a working vocabulary, a predictive framework for the mechanisms of reliable care, and rational therapies rigorously studied.13 Fortunately, the field of QI has attracted enough empiricistsworking in the lab of the hospital and other care settingsto lurch forward. But few would argue that we still have far less insight into the delivery of quality care than into the delivery of myocardial blood flow.
For ischemic heart disease we have classes of therapies, each of which is grounded in basic and clinical science: antiplatelets, beta‐blockers, vasodilators, lipid‐lowering agents. For care delivery we have the makings of analogous therapy classes, derived and introduced rather recently in a large review, Closing the Quality Gap: A Critical Analysis of the Quality Improvement Literature.4, 5 To facilitate their review of the evidence, the authors, including 2 prominent hospitalists, developed a new taxonomy of QI strategies (see Table 1). Though their effect size, relative efficacy, and interactions are not yet clear, many of these strategies can be applied to the inpatient setting, perhaps no less rationally than a well‐constructed antianginal regimen.
| QI Strategies | Examples |
|---|---|
| |
| Provider education | Conferences and workshops |
| Educational outreach visits (eg, academic detailing) | |
| Distributed educational materials | |
| Provider reminder systems | Reminders in charts for providers |
| Computer‐based reminders for providers | |
| Computer‐based decision support | |
| Facilitated relay of clinical data to providers | Transmission of clinical data from data source to hospital physician by means other than medical record, eg, page, e‐mail, phone call to hospitalist about clinically significant findings in postdischarge period |
| Audit and feedback of performance to providers | Feedback of performance to individual providers |
| Quality indicators and reports | |
| National/state quality report cards | |
| Publicly released performance data | |
| Benchmarkingprovision of outcomes data from top performers for comparison with provider's own data | |
| Patient education | Classes |
| Parent and family education | |
| Patient pamphlets | |
| Intensive education strategies promoting self‐management of chronic conditions | |
| Promotion of self‐management | Materials and devices promoting self‐management, eg, diabetes educator, pharmacist‐facilitated teaching of discharge medications |
| Patient reminder systems | Postcards or calls to patients |
| Organizational or team change | Case management, disease management |
| Multidisciplinary teams | |
| Change from paper to computer‐based records | |
| Increased staffing | |
| Skill mix changes | |
| Continuous quality improvement | Interventions using an iterative process for assessing quality problems, developing solutions, testing their impacts, and then reassessing the need for further action, eg, PlanDoStudyAct |
| Financial incentives, regulation, and policy | Provider directed: |
| Financial incentives based on achievement of performance goals | |
| Alternative reimbursement systems (eg, fee‐for‐service, capitated payments) | |
| Licensure requirements | |
| Health system directed: | |
| Initiatives by accreditation bodies (eg, residency work hour limits) | |
| Changes in reimbursement schemes (eg, capitation, prospective payment, salaried providers) | |
Where in the pathophysiology of a hospital do these QI therapies act? A plurality target the level of the provider: provider education, provider reminders, audit‐and‐feedback of provider performance, and facilitated relay of clinical data to providers. Remaining strategies target the patient (patient education, promotion of self‐management, and patient reminders), the immediate system within which care is delivered (organizational change), and the methodology of problem solving (continuous quality improvement). Only one strategy (financial incentives, regulation, and policy) fails to act directly at the level of the patient or provider, arguably the only level at which care actually can be improved.6
The value of the Quality Gap taxonomy is still largely untapped. If QI researchers and practitioners were to adopt its language as a standard, we could ramp up the power with which we communicate, interpret, and ultimately conduct improvement initiatives. In this issue of the Journal of Hospital Medicine, Cohn and colleagues profile a quality improvement initiative that achieved an impressive new level of performance. For an inpatient metric with a baseline institutional performance of 47%and an international benchmark of 39%the investigators executed a QI initiative that appears to have raised the rate of VTE prophylaxis to 85%.7 Despite a study design that weakens validity (beforeafter without controls) and a setting that diminishes applicability (medical patients in a single academic center), the authors have made a solid contribution to the QI literature simply by using the Quality Gap taxonomy. The authors specifically name and profile at least 3 distinct classes of QI strategies: provider education, a provider reminder element (ie, decision support), and an audit‐and‐feedback layer.
Even though provider education is unlikely to be sufficient as a lone QI strategya large review showed consistent but only modest benefitsit is often necessary.8 The provider education executed by Cohn and colleagues was frequent and regular. In the beginning of each month the chief resident oriented incoming house staff about venous thromboembolism (VTE) risk factors and the need for prophylaxis. They were given decision support pocket cards. Posters on display in nurse and physician work areas highlighted VTE risk factors. The provider education element also included discussions with the division chief about the topic. As robust as it was, however, the provider education was just a single component of the larger QI effort.
The second element, decision support, included VTE risk factor pocket cards with prophylaxis options listed. Introduced initially with the provider education, the pocket cards were handed out monthly by the chief resident. It is critical to recognize this decision support layer as a distinct core QI strategyand that it may even be fundamental to the success of the other strategies. Placed into the clinical workflow as a durable item, the decision support pocket card has the power to overcome provider uncertainty at moments of medical decision making. Generally speaking, a decision support layer, whether a pocket card, computer alert, or algorithm on a preprinted order form, can function as a shared baseline. Shared baselines or protocols reduce unnecessary variation in practice, a common source of poor quality care. Any mechanism that encourages groups of providers to deliver the same recommended care to groups of similarly at‐risk patients, while allowing customization of the protocol to meet the special needs of any individual patient, will have the net effect of raising overall quality of care.
This QI initiative may have achieved its greatest performance gainsas well as its greatest loss in terms of applicability to other settingsfrom its third facet, the audit‐and‐feedback layer. As a QI strategy audit‐and‐feedback has been defined as a summary of clinical performance for health care providers or institutions, performed for a specific period of time and reported either publicly or confidentially.1 It has demonstrated small to moderate benefits, with variations in effect most likely related to the format.9 As profiled in this study, it is hard to imagine a more powerful audit‐and‐feedback arrangement. The division chief of General Internal Medicine not only performed the audits, but also directly delivered the feedback to the house staff. In a deliberate, systematic, and successful way the investigators constructively used an existing authority gradient to leverage the Hawthorne effect, a change in worker behavior triggered by knowledge of being observed. Although it contributed to the impressive new VTE prophylaxis rates, this component did diminish generalizability and sustainability. Nonacademic centers may struggle to replicate these results, a point the authors dutifully point out. But even other academic centers might struggle in the absence of an authority figure with comparable influence and dedication to VTE prophylaxis. At the study hospital itself, similar rates of improvement would not be expected in patient populations outside the purview of the division chief.
Several alternatives to the before‐after study design could have produced richer information. Simultaneous data on VTE prophylaxis rates in a nonintervention population in the same or a similar hospital could have controlled for background or secular effects. An interrupted time series design may even have been feasible and could have provided more confidence in causality and more information on effect size. For example, what would be the effect on performance, if any, with removal of the decision support pocket card at 10 or 15 months? How much would performance rebound after its reintroduction? What could we have learned had the authors chosen instead to measure performance after sequentially introducing each component?
Using the language of the Quality Gap taxonomy, what conclusions can we draw from this improvement initiative? The introduction of a portable provider reminder (the decision support pocket card), when preceded by a program of provider education and followed by high‐intensity audit‐and‐feedback within an existing provider hierarchy, may have the power to raise VTE prophylaxis rates to 85% over an 18‐month period. With the large effect size somewhat mitigating the design flaws that weaken causality, we might risk an inference that these 3 classes of QI strategies can be reasonably successful in combination. But would we introduce them in our own medical centers? Using the clarity afforded by the taxonomy, we can identify several potential limitations, all attributable to the specifics of the audit‐and‐feedback arrangement: stringent preconditions of the practice setting, guaranteed inability to spread the initiative to other patient populations within the same medical center, limited scalability to include other QI projects, and reliance on the role of a single individual. Although clopidogrel 300 mg daily in the last week of every month is one way to pursue antiplatelet activity, other schedules or alternative agents may be preferable for the vast majority of patients.
The taxonomy can be used to compare, contrast, and more fully understand other QI studies. For example, among acutely ill medical inpatients not receiving VTE prophylaxis, Kucher and colleagues found that an electronic alert nearly doubled prophylaxis rates compared to those in a control group.10 Before trying to emulate their experience, a similarly equipped hospital would do well to recognize that the electronic alert was deployed as a composite of provider education, provider reminder, and facilitated relay of clinical information strategies, increasing prophylaxis rates for high risk patients from a baseline of 85% to 88%.
On the 21st‐century side of the quality chasm there is still something to be learned from QI research that falls short of recently proposed standards.3 This may be true as long as the key question remains: what are the mechanisms of reliable and sustainable performance improvement? We have not yet reached the day where a predictive framework, the clarity of our inquiry, the rigor of our study design, and the strength of our evidence churn out coherent answers. But we do have insights from a wealth of ongoing QI activity triggered by such forces as the Institute for Healthcare Improvement's 100,000 Lives Campaign and the advent of mandatory public reporting of hospital performance measures. By adding to this primordial mix the taxonomy offered by Closing the Quality Gap and its uptake into our vernacular by reports such as the one by Cohn in this issue of the Journal of Hospital Medicine, we are acquiring the language and experience to conduct intelligent and intelligible QI research.
As we approach the 6th year since the Institute of Medicine's Crossing the Quality Chasm offered a new vision for the American health care system, we still have a marked mismatch between the demand for health care quality and the supply of know‐how to deliver it. What the field of quality improvement (QI) still needs is merely this: QI practitioners in every care setting, a working vocabulary, a predictive framework for the mechanisms of reliable care, and rational therapies rigorously studied.13 Fortunately, the field of QI has attracted enough empiricistsworking in the lab of the hospital and other care settingsto lurch forward. But few would argue that we still have far less insight into the delivery of quality care than into the delivery of myocardial blood flow.
For ischemic heart disease we have classes of therapies, each of which is grounded in basic and clinical science: antiplatelets, beta‐blockers, vasodilators, lipid‐lowering agents. For care delivery we have the makings of analogous therapy classes, derived and introduced rather recently in a large review, Closing the Quality Gap: A Critical Analysis of the Quality Improvement Literature.4, 5 To facilitate their review of the evidence, the authors, including 2 prominent hospitalists, developed a new taxonomy of QI strategies (see Table 1). Though their effect size, relative efficacy, and interactions are not yet clear, many of these strategies can be applied to the inpatient setting, perhaps no less rationally than a well‐constructed antianginal regimen.
| QI Strategies | Examples |
|---|---|
| |
| Provider education | Conferences and workshops |
| Educational outreach visits (eg, academic detailing) | |
| Distributed educational materials | |
| Provider reminder systems | Reminders in charts for providers |
| Computer‐based reminders for providers | |
| Computer‐based decision support | |
| Facilitated relay of clinical data to providers | Transmission of clinical data from data source to hospital physician by means other than medical record, eg, page, e‐mail, phone call to hospitalist about clinically significant findings in postdischarge period |
| Audit and feedback of performance to providers | Feedback of performance to individual providers |
| Quality indicators and reports | |
| National/state quality report cards | |
| Publicly released performance data | |
| Benchmarkingprovision of outcomes data from top performers for comparison with provider's own data | |
| Patient education | Classes |
| Parent and family education | |
| Patient pamphlets | |
| Intensive education strategies promoting self‐management of chronic conditions | |
| Promotion of self‐management | Materials and devices promoting self‐management, eg, diabetes educator, pharmacist‐facilitated teaching of discharge medications |
| Patient reminder systems | Postcards or calls to patients |
| Organizational or team change | Case management, disease management |
| Multidisciplinary teams | |
| Change from paper to computer‐based records | |
| Increased staffing | |
| Skill mix changes | |
| Continuous quality improvement | Interventions using an iterative process for assessing quality problems, developing solutions, testing their impacts, and then reassessing the need for further action, eg, PlanDoStudyAct |
| Financial incentives, regulation, and policy | Provider directed: |
| Financial incentives based on achievement of performance goals | |
| Alternative reimbursement systems (eg, fee‐for‐service, capitated payments) | |
| Licensure requirements | |
| Health system directed: | |
| Initiatives by accreditation bodies (eg, residency work hour limits) | |
| Changes in reimbursement schemes (eg, capitation, prospective payment, salaried providers) | |
Where in the pathophysiology of a hospital do these QI therapies act? A plurality target the level of the provider: provider education, provider reminders, audit‐and‐feedback of provider performance, and facilitated relay of clinical data to providers. Remaining strategies target the patient (patient education, promotion of self‐management, and patient reminders), the immediate system within which care is delivered (organizational change), and the methodology of problem solving (continuous quality improvement). Only one strategy (financial incentives, regulation, and policy) fails to act directly at the level of the patient or provider, arguably the only level at which care actually can be improved.6
The value of the Quality Gap taxonomy is still largely untapped. If QI researchers and practitioners were to adopt its language as a standard, we could ramp up the power with which we communicate, interpret, and ultimately conduct improvement initiatives. In this issue of the Journal of Hospital Medicine, Cohn and colleagues profile a quality improvement initiative that achieved an impressive new level of performance. For an inpatient metric with a baseline institutional performance of 47%and an international benchmark of 39%the investigators executed a QI initiative that appears to have raised the rate of VTE prophylaxis to 85%.7 Despite a study design that weakens validity (beforeafter without controls) and a setting that diminishes applicability (medical patients in a single academic center), the authors have made a solid contribution to the QI literature simply by using the Quality Gap taxonomy. The authors specifically name and profile at least 3 distinct classes of QI strategies: provider education, a provider reminder element (ie, decision support), and an audit‐and‐feedback layer.
Even though provider education is unlikely to be sufficient as a lone QI strategya large review showed consistent but only modest benefitsit is often necessary.8 The provider education executed by Cohn and colleagues was frequent and regular. In the beginning of each month the chief resident oriented incoming house staff about venous thromboembolism (VTE) risk factors and the need for prophylaxis. They were given decision support pocket cards. Posters on display in nurse and physician work areas highlighted VTE risk factors. The provider education element also included discussions with the division chief about the topic. As robust as it was, however, the provider education was just a single component of the larger QI effort.
The second element, decision support, included VTE risk factor pocket cards with prophylaxis options listed. Introduced initially with the provider education, the pocket cards were handed out monthly by the chief resident. It is critical to recognize this decision support layer as a distinct core QI strategyand that it may even be fundamental to the success of the other strategies. Placed into the clinical workflow as a durable item, the decision support pocket card has the power to overcome provider uncertainty at moments of medical decision making. Generally speaking, a decision support layer, whether a pocket card, computer alert, or algorithm on a preprinted order form, can function as a shared baseline. Shared baselines or protocols reduce unnecessary variation in practice, a common source of poor quality care. Any mechanism that encourages groups of providers to deliver the same recommended care to groups of similarly at‐risk patients, while allowing customization of the protocol to meet the special needs of any individual patient, will have the net effect of raising overall quality of care.
This QI initiative may have achieved its greatest performance gainsas well as its greatest loss in terms of applicability to other settingsfrom its third facet, the audit‐and‐feedback layer. As a QI strategy audit‐and‐feedback has been defined as a summary of clinical performance for health care providers or institutions, performed for a specific period of time and reported either publicly or confidentially.1 It has demonstrated small to moderate benefits, with variations in effect most likely related to the format.9 As profiled in this study, it is hard to imagine a more powerful audit‐and‐feedback arrangement. The division chief of General Internal Medicine not only performed the audits, but also directly delivered the feedback to the house staff. In a deliberate, systematic, and successful way the investigators constructively used an existing authority gradient to leverage the Hawthorne effect, a change in worker behavior triggered by knowledge of being observed. Although it contributed to the impressive new VTE prophylaxis rates, this component did diminish generalizability and sustainability. Nonacademic centers may struggle to replicate these results, a point the authors dutifully point out. But even other academic centers might struggle in the absence of an authority figure with comparable influence and dedication to VTE prophylaxis. At the study hospital itself, similar rates of improvement would not be expected in patient populations outside the purview of the division chief.
Several alternatives to the before‐after study design could have produced richer information. Simultaneous data on VTE prophylaxis rates in a nonintervention population in the same or a similar hospital could have controlled for background or secular effects. An interrupted time series design may even have been feasible and could have provided more confidence in causality and more information on effect size. For example, what would be the effect on performance, if any, with removal of the decision support pocket card at 10 or 15 months? How much would performance rebound after its reintroduction? What could we have learned had the authors chosen instead to measure performance after sequentially introducing each component?
Using the language of the Quality Gap taxonomy, what conclusions can we draw from this improvement initiative? The introduction of a portable provider reminder (the decision support pocket card), when preceded by a program of provider education and followed by high‐intensity audit‐and‐feedback within an existing provider hierarchy, may have the power to raise VTE prophylaxis rates to 85% over an 18‐month period. With the large effect size somewhat mitigating the design flaws that weaken causality, we might risk an inference that these 3 classes of QI strategies can be reasonably successful in combination. But would we introduce them in our own medical centers? Using the clarity afforded by the taxonomy, we can identify several potential limitations, all attributable to the specifics of the audit‐and‐feedback arrangement: stringent preconditions of the practice setting, guaranteed inability to spread the initiative to other patient populations within the same medical center, limited scalability to include other QI projects, and reliance on the role of a single individual. Although clopidogrel 300 mg daily in the last week of every month is one way to pursue antiplatelet activity, other schedules or alternative agents may be preferable for the vast majority of patients.
The taxonomy can be used to compare, contrast, and more fully understand other QI studies. For example, among acutely ill medical inpatients not receiving VTE prophylaxis, Kucher and colleagues found that an electronic alert nearly doubled prophylaxis rates compared to those in a control group.10 Before trying to emulate their experience, a similarly equipped hospital would do well to recognize that the electronic alert was deployed as a composite of provider education, provider reminder, and facilitated relay of clinical information strategies, increasing prophylaxis rates for high risk patients from a baseline of 85% to 88%.
On the 21st‐century side of the quality chasm there is still something to be learned from QI research that falls short of recently proposed standards.3 This may be true as long as the key question remains: what are the mechanisms of reliable and sustainable performance improvement? We have not yet reached the day where a predictive framework, the clarity of our inquiry, the rigor of our study design, and the strength of our evidence churn out coherent answers. But we do have insights from a wealth of ongoing QI activity triggered by such forces as the Institute for Healthcare Improvement's 100,000 Lives Campaign and the advent of mandatory public reporting of hospital performance measures. By adding to this primordial mix the taxonomy offered by Closing the Quality Gap and its uptake into our vernacular by reports such as the one by Cohn in this issue of the Journal of Hospital Medicine, we are acquiring the language and experience to conduct intelligent and intelligible QI research.
- ,,.Designing a quality improvement intervention: a systematic approach.Qual Saf Health Care.2003;12;215–220.
- ,,.Implementing clinical guidelines: current evidence and future implications.J Contin Educ Health Prof.2004;24(Suppl 1):S31–S37.
- ,.Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project.Qual Saf Health Care.2005;14:319–325.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol.1, Series Overview and Methodology. Technical Review 9 (Contract No. 290‐02‐0017 to the Stanford University–UCSF Evidence‐based Practices Center). AHRQ Publication No. 04‐0051‐1.Rockville, MD:Agency for Healthcare Research and Quality,2004.
- ,,, et al.Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis.JAMA.2006;296:427–440.
- ,,, et al.Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units.Jt Comm J Qual Improv.2002;28:472–493.
- ,,, et al.A multinational observational cohort study in acutely ill medical patients of Pharmacological thromboembolic prophylaxis practices in prevention of venous thromboembolism: findings of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE).Blood.2003;102(Suppl):321a.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;6:1–84.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977
- ,,.Designing a quality improvement intervention: a systematic approach.Qual Saf Health Care.2003;12;215–220.
- ,,.Implementing clinical guidelines: current evidence and future implications.J Contin Educ Health Prof.2004;24(Suppl 1):S31–S37.
- ,.Toward stronger evidence on quality improvement. Draft publication guidelines: the beginning of a consensus project.Qual Saf Health Care.2005;14:319–325.
- ,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Vol.1, Series Overview and Methodology. Technical Review 9 (Contract No. 290‐02‐0017 to the Stanford University–UCSF Evidence‐based Practices Center). AHRQ Publication No. 04‐0051‐1.Rockville, MD:Agency for Healthcare Research and Quality,2004.
- ,,, et al.Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta‐regression analysis.JAMA.2006;296:427–440.
- ,,, et al.Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units.Jt Comm J Qual Improv.2002;28:472–493.
- ,,, et al.A multinational observational cohort study in acutely ill medical patients of Pharmacological thromboembolic prophylaxis practices in prevention of venous thromboembolism: findings of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE).Blood.2003;102(Suppl):321a.
- ,,, et al.Effectiveness and efficiency of guideline dissemination and implementation strategies.Health Technol Assess.2004;6:1–84.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969–977
The Venous Thromboembolism Quality Improvement Resource Room
The goal of this article is to explain how the first in a series of online resource rooms provides trainees and hospitalists with quality improvement tools that can be applied locally to improve inpatient care.1 During the emergence and explosive growth of hospital medicine, the SHM recognized the need to revise training relating to inpatient care and hospital process design to meet the evolving expectation of hospitalists that their performance will be measured, to actively set quality parameters, and to lead multidisciplinary teams to improve hospital performance.2 Armed with the appropriate skill set, hospitalists would be uniquely situated to lead and manage improvements in processes in the hospitals in which they work.
The content of the first Society of Hospital Medicine (SHM) Quality Improvement Resource Room (QI RR) supports hospitalists leading a multidisciplinary team dedicated to improving inpatient outcomes by preventing hospital‐acquired venous thromboembolism (VTE), a common cause of morbidity and mortality in hospitalized patients.3 The SHM developed this educational resource in the context of numerous reports on the incidence of medical errors in US hospitals and calls for action to improve the quality of health care.'47 Hospital report cards on quality measures are now public record, and hospitals will require uniformity in practice among physicians. Hospitalists are increasingly expected to lead initiatives that will implement national standards in key practices such as VTE prophylaxis2.
The QI RRs of the SHM are a collection of electronic tools accessible through the SHM Web site. They are designed to enhance the readiness of hospitalists and members of the multidisciplinary inpatient team to redesign care at the institutional level. Although all performance improvement is ultimately occurs locally, many QI methods and tools transcend hospital geography and disease topic. Leveraging a Web‐based platform, the SHM QI RRs present hospitalists with a general approach to QI, enriched by customizable workbooks that can be downloaded to best meet user needs. This resource is an innovation in practice‐based learning, quality improvement, and systems‐based practice.
METHODS
Development of the first QI RR followed a series of steps described in Curriculum Development for Medical Education8 (for process and timeline, see Table 1). Inadequate VTE prophylaxis was identified as an ongoing widespread problem of health care underutilization despite randomized clinical trials supporting the efficacy of prophylaxis.9, 10 Mirroring the AHRQ's assessment of underutilization of VTE prophylaxis as the single most important safety priority,6 the first QI RR focused on VTE, with plans to cover additional clinical conditions over time. As experts in the care of inpatients, hospitalists should be able to take custody of predictable complications of serious illness, identify and lower barriers to prevention, critically review prophylaxis options, utilize hospital‐specific data, and devise strategies to bridge the gap between knowledge and practice. Already leaders of multidisciplinary care teams, hospitalists are primed to lead multidisciplinary improvement teams as well.
| Phase 1 (January 2005April 2005): Executing the educational strategy |
|---|
| One‐hour conference calls |
| Curricular, clinical, technical, and creative aspects of production |
| Additional communication between members of working group between calls |
| Development of questionnaire for SHM membership, board, education, and hospital quality patient safety (HQPS) committees |
| Content freeze: fourth month of development |
| Implementation of revisions prior to April 2005 SHM Annual Meeting |
| Phase 2 (April 2005August 2005): revision based on feedback |
| Analysis of formative evaluation from Phase 1 |
| Launch of the VTE QI RR August 2005 |
| Secondary phases and venues for implementation |
| Workshops at hospital medicine educational events |
| SHM Quality course |
| Formal recognition of the learning, experience, or proficiency acquired by users |
| The working editorial team for the first resource room |
| Dedicated project manager (SHM staff) |
| Senior adviser for planning and development (SHM staff) |
| Senior adviser for education (SHM staff) |
| Content expert |
| Education editor |
| Hospital quality editor |
| Managing editor |
Available data on the demographics of hospitalists and feedback from the SHM membership, leadership, and committees indicated that most learners would have minimal previous exposure to QI concepts and only a few years of management experience. Any previous quality improvement initiatives would tend to have been isolated, experimental, or smaller in scale. The resource rooms are designed to facilitate quality improvement learning among hospitalists that is practice‐based and immediately relevant to patient care. Measurable improvement in particular care processes or outcomes should correlate with actual learning.
The educational strategy of the SHM was predicated on ensuring that a quality and patient safety curriculum would retain clinical applicability in the hospital setting. This approach, grounded in adult learning principles and common to medical education, teaches general principles by framing the learning experience as problem centered.11 Several domains were identified as universally important to any quality improvement effort: raising awareness of a local performance gap, applying the best current evidence to practice, tapping the experience of others leading QI efforts, and using measurements derived from rapid‐cycle tests of change. Such a template delineates the components of successful QI planning, implementation, and evaluation and provides users with a familiar RR format applicable to improving any care process, not just VTE.
The Internet was chosen as the mechanism for delivering training on the basis of previous surveys of the SHM membership in which members expressed a preference for electronic and Web‐based forms of educational content delivery. Drawing from the example of other organizations teaching quality improvement, including the Institute for Healthcare Improvement and Intermountain Health Care, the SHM valued the ubiquity of a Web‐based educational resource. To facilitate on‐the‐job training, the first SHM QI RR provides a comprehensive tool kit to guide hospitalists through the process of advocating, developing, implementing, and evaluating a QI initiative for VTE.
Prior to launching the resource room, formative input was collected from SHM leaders, a panel of education and QI experts, and attendees of the society's annual meetings. Such input followed each significant step in the development of the RR curricula. For example, visitors at a kiosk at the 2005 SHM annual meeting completed surveys as they navigated through the VTE QI RR. This focused feedback shaped prelaunch development. The ultimate performance evaluation and feedback for the QI RR curricula will be gauged by user reports of measurable improvement in specific hospital process or outcomes measures. The VTE QI RR was launched in August 2005 and promoted at the SHM Web site.
RESULTS
The content and layout of the VTE QI RR are depicted in Figure 1. The self‐directed learner may navigate through the entire resource room or just select areas for study. Those likely to visit only a single area are individuals looking for guidance to support discrete roles on the improvement team: champion, clinical leader, facilitator of the QI process, or educator of staff or patient audiences (see Figure 2).

Why Should You Act?
The visual center of the QI RR layout presents sobering statisticsalthough pulmonary embolism from deep vein thrombosis is the most common cause of preventable hospital death, most hospitalized medical patients at risk do not receive appropriate prophylaxisand then encourages hospitalist‐led action to reduce hospital‐acquired VTE. The role of the hospitalist is extracted from the competencies articulated in the Venous Thromboembolism, Quality Improvement, and Hospitalist as Teacher chapters of The Core Competencies in Hospital Medicine.2
Awareness
In the Awareness area of the VTE QI RR, materials to raise clinician, hospital staff, and patient awareness are suggested and made available. Through the SHM's lead sponsorship of the national DVT Awareness Month campaign, suggested Steps to Action depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem.
Evidence
The Evidence section aggregates a list of the most pertinent VTE prophylaxis literature to help ground any QI effort firmly in the evidence base. Through an agreement with the American College of Physicians (ACP), VTE prophylaxis articles reviewed in the ACP Journal Club are presented here.12 Although the listed literature focuses on prophylaxis, plans are in place to include references on diagnosis and treatment.
Experience
Resource room visitors interested in tapping into the experience of hospitalists and other leaders of QI efforts can navigate directly to this area. Interactive resources here include downloadable and adaptable protocols for VTE prophylaxis and, most importantly, improvement stories profiling actual QI successes. The Experience section features comments from an author of a seminal trial that studied computer alerts for high‐risk patients not receiving prophylaxis.10 The educational goal of this section of the QI RR is to provide opportunities to learn from successful QI projects, from the composition of the improvement team to the relevant metrics, implementation plan, and next steps.
Ask the Expert
The most interactive part of the resource room, the Ask the Expert forum, provides a hybrid of experience and evidence. A visitor who posts a clinical or improvement question to this discussion community receives a multidisciplinary response. For each question posted, a hospitalist moderator collects and aggregates responses from a panel of VTE experts, QI experts, hospitalist teachers, and pharmacists. The online exchange permitted by this forum promotes wider debate and learning. The questions and responses are archived and thus are available for subsequent users to read.
Improve
This area features the focal point of the entire resource room, the VTE QI workbook, which was written and designed to provide action‐oriented learning in quality improvement. The workbook is a downloadable project outline to guide and document efforts aimed at reducing rates of hospital‐acquired VTE. Hospitalists who complete the workbook should have acquired familiarity with and a working proficiency in leading system‐level efforts to drive better patient care. Users new to the theory and practice of QI can also review key concepts from a slide presentation in this part of the resource room.
Educate
This content area profiles the hospital medicine core competencies that relate to VTE and QI while also offering teaching materials and advice for teachers of VTE or QI. Teaching resources for clinician educators include online CME and an up‐to‐date slide lecture about VTE prophylaxis. The lecture presentation can be downloaded and customized to serve the needs of the speaker and the audience, whether students, residents, or other hospital staff. Clinician educators can also share or review teaching pearls used by hospitalist colleagues who serve as ward attendings.
DISCUSSION
A case example, shown in Figure 3, demonstrates how content accessible through the SHM VTE QI RR may be used to catalyze a local quality improvement effort.
Hospitals will be measured on rates of VTE prophylaxis on medical and surgical services. Failure to standardize prophylaxis among different physician groups may adversely affect overall performance, with implications for both patient care and accreditation. The lack of a agreed‐on gold standard of what constitutes appropriate prophylaxis for a given patient does not absolve an institution of the duty to implement its own standards. The challenge of achieving local consensus on appropriate prophylaxis should not outweigh the urgency to address preventable in‐hospital deaths. In caring for increasing numbers of general medical and surgical patients, hospitalists are likely to be asked to develop and implement a protocol for VTE prophylaxis that can be used hospitalwide. In many instances hospitalists will accept this charge in the aftermath of previous hospital failures in which admission order sets or VTE assessment protocols were launched but never widely implemented. As the National Quality Forum or JCAHO regulations for uniformity among hospitals shift VTE prophylaxis from being voluntary to compulsory, hospitalists will need to develop improvement strategies that have greater reliability.
Hospitalists with no formal training in either vascular medicine or quality improvement may not be able to immediately cite the most current data about VTE prophylaxis rates and regimens and may not have the time to enroll in a training course on quality improvement. How would hospitalists determine baseline rates of appropriate VTE prophylaxis? How can medical education be used to build consensus and recruit support from other physicians? What should be the scope of the QI initiative, and what patient population should be targeted for intervention?
The goal of the SHM QI RR is to provide the tools and the framework to help hospitalists develop, implement, and manage a VTE prophylaxis quality improvement initiative. Suggested Steps to Action in the Awareness section depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem. Hospital quality officers can direct the hospital's public relations department to the Awareness section for DVT Awareness Month materials, including public service announcements in audio, visual, and print formats. The hold music at the hospital can be temporarily replaced, television kiosks can be set up to run video loops, and banners can be printed and hung in central locations, all to get out the message simultaneously to patients and medical staff.
The Evidence section of the VTE QI RR references a key benchmark study, the DVT‐Free Prospective Registry.9 This study reported that at 183 sites in North America and Europe, more than twice as many medical patients as surgical patients failed to receive prophylaxis. The Evidence section includes the 7th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy and also highlights 3 randomized placebo‐controlled clinical trials (MEDENOX 1999, ARTEMIS 2003, and PREVENT 2004) that have reported significant reduction of risk of VTE (50%‐60%) from pharmacologic prophylaxis in moderate‐risk medical inpatients.1315 Review of the data helps to determine which patient population to study first, which prophylaxis options a hospital could deploy appropriately, and the expected magnitude of the effect. Because the literature has already been narrowed and is kept current, hospitalists can save time in answering a range of questions, from the most commonly agreed‐on factors to stratify risk to which populations require alternative interventions.
The Experience section references the first clinical trial demonstrating improved patient outcomes from a quality improvement initiative aimed at improving utilization of VTE prophylaxis.10 At the large teaching hospital where the electronic alerts were studied, a preexisting wealth of educational information on the hospital Web site, in the form of multiple seminars and lectures on VTE prophylaxis by opinion leaders and international experts, had little impact on practice. For this reason, the investigators implemented a trial of how to change physician behavior by introducing a point‐of‐care intervention, the computer alerts. Clinicians prompted by an electronic alert to consider DVT prophylaxis for at‐risk patients employed nearly double the rate of pharmacologic prophylaxis and reduced the incidence of DVT or pulmonary embolism (PE) by 41%. This study suggests that a change introduced to the clinical workflow can improve evidence‐based VTE prophylaxis and also can reduce the incidence of VTE in acutely ill hospitalized patients.
We believe that if hospitalists use the current evidence and experience assembled in the VTE QI RR, they could develop and lead a systematic approach to improving utilization of VTE prophylaxis. Although there is no gold standard method for integrating VTE risk assessment into clinical workflow, the VTE QI RR presents key lessons both from the literature and real world experiences. The crucial take‐home message is that hospitalists can facilitate implementation of VTE risk assessments if they stress simplicity (ie, the sick, old, surgery benefit), link the risk assessment to a menu of evidence‐based prophylaxis options, and require assessment of VTE risk as part of a regular routine (on admission and at regular intervals). Although many hospitals do not yet have computerized entry of physician orders, the simple 4‐point VTE risk assessment described by Kucher et al might be applied to other hospitals.10 The 4‐point system would identify the patients at highest risk, a reasonable starting point for a QI initiative. Whatever the modelCPOE alerts of very high‐risk patients, CPOE‐forced VTE risk assessments, nursing assessments, or paper‐based order setsregular VTE risk assessment can be incorporated into the daily routine of hospital care.
The QI workbook sequences the steps of a multidisciplinary improvement team and prompts users to set specific goals, collect practical metrics, and conduct plan‐do‐study‐act (PDSA) cycles of learning and action (Figure 4). Hospitalists and other team members can use the information in the workbook to estimate the prevalence of use of the appropriate VTE prophylaxis and the incidence of hospital‐acquired VTE at their medical centers, develop a suitable VTE risk assessment model, and plan interventions. Starting with all patients admitted to one nurse on one unit, then expanding to an entire nursing unit, an improvement team could implement rapid PDSA cycles to iron out the wrinkles of a risk assessment protocol. After demonstrating a measurable benefit for the patients at highest risk, the team would then be expected to capture more patients at risk for VTE by modifying the risk assessment protocol to identify moderate‐risk patients (hospitalized patients with one risk factor), as in the MEDENOX, ARTEMIS, and PREVENT clinical trials. Within the first several months, the QI intervention could be expanded to more nursing units. An improvement report profiling a clinically important increase in the rate of appropriate VTE prophylaxis would advocate for additional local resources and projects.

As questions arise in assembling an improvement team, setting useful aims and metrics, choosing interventions, implementing and studying change, or collecting performance data, hospitalists can review answers to questions already posted and post their own questions in the Ask the Expert area. For example, one user asked whether there was a standard risk assessment tool for identifying patients at high risk of VTE. Another asked about the use of unfractionated heparin as a low‐cost alternative to low‐molecular‐weight heparin. Both these questions were answered within 24 hours by the content editor of the VTE QI RR and, for one question, also by 2 pharmacists and an international expert in VTE.
As other hospitalists begin de novo efforts of their own, success stories and strategies posted in the online forums of the VTE QI RR will be an evolving resource for basic know‐how and innovation.
Suggestions from a community of resource room users will be solicited, evaluated, and incorporated into the QI RR in order to improve its educational value and utility. The curricula could also be adapted or refined by others with an interest in systems‐based care or practice‐based learning, such as directors of residency training programs.
CONCLUSIONS
The QI RRs bring QI theory and practice to the hospitalist, when and wherever it is wanted, minimizing time away from patient care. The workbook links theory to practice and can be used to launch, sustain, and document a local VTE‐specific QI initiative. A range of experience is accommodated. Content is provided in a way that enables the user to immediately apply and adapt it to a local contextusers can access and download the subset of tools that best meet their needs. For practicing hospitalists, this QI resource offers an opportunity to bridge the training gap in systems‐based hospital care and should increase the quality and quantity of and support for opportunities to lead successful QI projects.
The Accreditation Council of Graduate Medical Education (ACGME) now requires education in health care systems, a requirement not previously mandated for traditional medical residency programs.17 Because the resource rooms should increase the number of hospitalists competently leading local efforts that achieve measurable gains in hospital outcomes, a wider potential constituency also includes residency program directors, internal medicine residents, physician assistants and nurse‐practitioners, nurses, hospital quality officers, and hospital medicine practice leaders.
Further research is needed to determine the clinical impact of the VTE QI workbook on outcomes for hospitalized patients. The effectiveness of such an educational method should be evaluated, at least in part, by documenting changes in clinically important process and outcome measures, in this case those specific to hospital‐acquired VTE. Investigation also will need to generate an impact assessment to see if the curricula are effective in meeting the strategic educational goals of the Society of Hospital Medicine. Further investigation will examine whether this resource can help residency training programs achieve ACGME goals for practice‐based learning and systems‐based care.
- Society of Hospital Medicine Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement_Resource_Rooms1(suppl 1).
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Arch Intern Med.1991;151:933–938.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicinehttp://www.iom.edu/CMS/3718.aspx
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices.Agency for Healthcare Research and Quality, Publication 01‐E058;2001.
- Joint Commission on the Accreditation of Health Care Organizations. Public policy initiatives. Available at: http://www.jcaho.org/about+us/public+policy+initiatives/pay_for_performance.htm
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, Md:Johns Hopkins University Press;1998.
- ,;DVT FREE Steering Committee.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969.
- ,,.Teaching the Case Method.3rd ed.Cambridge, Mass :Harvard Business School.
- American College of Physicians. Available at: http://www.acpjc.org/?hp
- ,,, et al.MEDENOX trial.N Engl J Med.1999;341:793–800.
- ,,.Fondaparinux versus placebo for the prevention of VTE in acutely ill medical patients (ARTEMIS).J Thromb Haemost.2003;1(suppl 1):2046.
- ,,,,,.PREVENT Medical Thromboprophylaxis Study Group.Circulation.2004;110:874–879.
- ,.Comparing the costs, risks and benefits of competing strategies for the primary prevention of VTE.Circulation.2004;110:IV25–IV32.
- Accreditation Council for Graduate Medical Education. Available at: http://www.acgme.org/acWebsite/programDir/pd_index.asp.
The goal of this article is to explain how the first in a series of online resource rooms provides trainees and hospitalists with quality improvement tools that can be applied locally to improve inpatient care.1 During the emergence and explosive growth of hospital medicine, the SHM recognized the need to revise training relating to inpatient care and hospital process design to meet the evolving expectation of hospitalists that their performance will be measured, to actively set quality parameters, and to lead multidisciplinary teams to improve hospital performance.2 Armed with the appropriate skill set, hospitalists would be uniquely situated to lead and manage improvements in processes in the hospitals in which they work.
The content of the first Society of Hospital Medicine (SHM) Quality Improvement Resource Room (QI RR) supports hospitalists leading a multidisciplinary team dedicated to improving inpatient outcomes by preventing hospital‐acquired venous thromboembolism (VTE), a common cause of morbidity and mortality in hospitalized patients.3 The SHM developed this educational resource in the context of numerous reports on the incidence of medical errors in US hospitals and calls for action to improve the quality of health care.'47 Hospital report cards on quality measures are now public record, and hospitals will require uniformity in practice among physicians. Hospitalists are increasingly expected to lead initiatives that will implement national standards in key practices such as VTE prophylaxis2.
The QI RRs of the SHM are a collection of electronic tools accessible through the SHM Web site. They are designed to enhance the readiness of hospitalists and members of the multidisciplinary inpatient team to redesign care at the institutional level. Although all performance improvement is ultimately occurs locally, many QI methods and tools transcend hospital geography and disease topic. Leveraging a Web‐based platform, the SHM QI RRs present hospitalists with a general approach to QI, enriched by customizable workbooks that can be downloaded to best meet user needs. This resource is an innovation in practice‐based learning, quality improvement, and systems‐based practice.
METHODS
Development of the first QI RR followed a series of steps described in Curriculum Development for Medical Education8 (for process and timeline, see Table 1). Inadequate VTE prophylaxis was identified as an ongoing widespread problem of health care underutilization despite randomized clinical trials supporting the efficacy of prophylaxis.9, 10 Mirroring the AHRQ's assessment of underutilization of VTE prophylaxis as the single most important safety priority,6 the first QI RR focused on VTE, with plans to cover additional clinical conditions over time. As experts in the care of inpatients, hospitalists should be able to take custody of predictable complications of serious illness, identify and lower barriers to prevention, critically review prophylaxis options, utilize hospital‐specific data, and devise strategies to bridge the gap between knowledge and practice. Already leaders of multidisciplinary care teams, hospitalists are primed to lead multidisciplinary improvement teams as well.
| Phase 1 (January 2005April 2005): Executing the educational strategy |
|---|
| One‐hour conference calls |
| Curricular, clinical, technical, and creative aspects of production |
| Additional communication between members of working group between calls |
| Development of questionnaire for SHM membership, board, education, and hospital quality patient safety (HQPS) committees |
| Content freeze: fourth month of development |
| Implementation of revisions prior to April 2005 SHM Annual Meeting |
| Phase 2 (April 2005August 2005): revision based on feedback |
| Analysis of formative evaluation from Phase 1 |
| Launch of the VTE QI RR August 2005 |
| Secondary phases and venues for implementation |
| Workshops at hospital medicine educational events |
| SHM Quality course |
| Formal recognition of the learning, experience, or proficiency acquired by users |
| The working editorial team for the first resource room |
| Dedicated project manager (SHM staff) |
| Senior adviser for planning and development (SHM staff) |
| Senior adviser for education (SHM staff) |
| Content expert |
| Education editor |
| Hospital quality editor |
| Managing editor |
Available data on the demographics of hospitalists and feedback from the SHM membership, leadership, and committees indicated that most learners would have minimal previous exposure to QI concepts and only a few years of management experience. Any previous quality improvement initiatives would tend to have been isolated, experimental, or smaller in scale. The resource rooms are designed to facilitate quality improvement learning among hospitalists that is practice‐based and immediately relevant to patient care. Measurable improvement in particular care processes or outcomes should correlate with actual learning.
The educational strategy of the SHM was predicated on ensuring that a quality and patient safety curriculum would retain clinical applicability in the hospital setting. This approach, grounded in adult learning principles and common to medical education, teaches general principles by framing the learning experience as problem centered.11 Several domains were identified as universally important to any quality improvement effort: raising awareness of a local performance gap, applying the best current evidence to practice, tapping the experience of others leading QI efforts, and using measurements derived from rapid‐cycle tests of change. Such a template delineates the components of successful QI planning, implementation, and evaluation and provides users with a familiar RR format applicable to improving any care process, not just VTE.
The Internet was chosen as the mechanism for delivering training on the basis of previous surveys of the SHM membership in which members expressed a preference for electronic and Web‐based forms of educational content delivery. Drawing from the example of other organizations teaching quality improvement, including the Institute for Healthcare Improvement and Intermountain Health Care, the SHM valued the ubiquity of a Web‐based educational resource. To facilitate on‐the‐job training, the first SHM QI RR provides a comprehensive tool kit to guide hospitalists through the process of advocating, developing, implementing, and evaluating a QI initiative for VTE.
Prior to launching the resource room, formative input was collected from SHM leaders, a panel of education and QI experts, and attendees of the society's annual meetings. Such input followed each significant step in the development of the RR curricula. For example, visitors at a kiosk at the 2005 SHM annual meeting completed surveys as they navigated through the VTE QI RR. This focused feedback shaped prelaunch development. The ultimate performance evaluation and feedback for the QI RR curricula will be gauged by user reports of measurable improvement in specific hospital process or outcomes measures. The VTE QI RR was launched in August 2005 and promoted at the SHM Web site.
RESULTS
The content and layout of the VTE QI RR are depicted in Figure 1. The self‐directed learner may navigate through the entire resource room or just select areas for study. Those likely to visit only a single area are individuals looking for guidance to support discrete roles on the improvement team: champion, clinical leader, facilitator of the QI process, or educator of staff or patient audiences (see Figure 2).


Why Should You Act?
The visual center of the QI RR layout presents sobering statisticsalthough pulmonary embolism from deep vein thrombosis is the most common cause of preventable hospital death, most hospitalized medical patients at risk do not receive appropriate prophylaxisand then encourages hospitalist‐led action to reduce hospital‐acquired VTE. The role of the hospitalist is extracted from the competencies articulated in the Venous Thromboembolism, Quality Improvement, and Hospitalist as Teacher chapters of The Core Competencies in Hospital Medicine.2
Awareness
In the Awareness area of the VTE QI RR, materials to raise clinician, hospital staff, and patient awareness are suggested and made available. Through the SHM's lead sponsorship of the national DVT Awareness Month campaign, suggested Steps to Action depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem.
Evidence
The Evidence section aggregates a list of the most pertinent VTE prophylaxis literature to help ground any QI effort firmly in the evidence base. Through an agreement with the American College of Physicians (ACP), VTE prophylaxis articles reviewed in the ACP Journal Club are presented here.12 Although the listed literature focuses on prophylaxis, plans are in place to include references on diagnosis and treatment.
Experience
Resource room visitors interested in tapping into the experience of hospitalists and other leaders of QI efforts can navigate directly to this area. Interactive resources here include downloadable and adaptable protocols for VTE prophylaxis and, most importantly, improvement stories profiling actual QI successes. The Experience section features comments from an author of a seminal trial that studied computer alerts for high‐risk patients not receiving prophylaxis.10 The educational goal of this section of the QI RR is to provide opportunities to learn from successful QI projects, from the composition of the improvement team to the relevant metrics, implementation plan, and next steps.
Ask the Expert
The most interactive part of the resource room, the Ask the Expert forum, provides a hybrid of experience and evidence. A visitor who posts a clinical or improvement question to this discussion community receives a multidisciplinary response. For each question posted, a hospitalist moderator collects and aggregates responses from a panel of VTE experts, QI experts, hospitalist teachers, and pharmacists. The online exchange permitted by this forum promotes wider debate and learning. The questions and responses are archived and thus are available for subsequent users to read.
Improve
This area features the focal point of the entire resource room, the VTE QI workbook, which was written and designed to provide action‐oriented learning in quality improvement. The workbook is a downloadable project outline to guide and document efforts aimed at reducing rates of hospital‐acquired VTE. Hospitalists who complete the workbook should have acquired familiarity with and a working proficiency in leading system‐level efforts to drive better patient care. Users new to the theory and practice of QI can also review key concepts from a slide presentation in this part of the resource room.
Educate
This content area profiles the hospital medicine core competencies that relate to VTE and QI while also offering teaching materials and advice for teachers of VTE or QI. Teaching resources for clinician educators include online CME and an up‐to‐date slide lecture about VTE prophylaxis. The lecture presentation can be downloaded and customized to serve the needs of the speaker and the audience, whether students, residents, or other hospital staff. Clinician educators can also share or review teaching pearls used by hospitalist colleagues who serve as ward attendings.
DISCUSSION
A case example, shown in Figure 3, demonstrates how content accessible through the SHM VTE QI RR may be used to catalyze a local quality improvement effort.

Hospitals will be measured on rates of VTE prophylaxis on medical and surgical services. Failure to standardize prophylaxis among different physician groups may adversely affect overall performance, with implications for both patient care and accreditation. The lack of a agreed‐on gold standard of what constitutes appropriate prophylaxis for a given patient does not absolve an institution of the duty to implement its own standards. The challenge of achieving local consensus on appropriate prophylaxis should not outweigh the urgency to address preventable in‐hospital deaths. In caring for increasing numbers of general medical and surgical patients, hospitalists are likely to be asked to develop and implement a protocol for VTE prophylaxis that can be used hospitalwide. In many instances hospitalists will accept this charge in the aftermath of previous hospital failures in which admission order sets or VTE assessment protocols were launched but never widely implemented. As the National Quality Forum or JCAHO regulations for uniformity among hospitals shift VTE prophylaxis from being voluntary to compulsory, hospitalists will need to develop improvement strategies that have greater reliability.
Hospitalists with no formal training in either vascular medicine or quality improvement may not be able to immediately cite the most current data about VTE prophylaxis rates and regimens and may not have the time to enroll in a training course on quality improvement. How would hospitalists determine baseline rates of appropriate VTE prophylaxis? How can medical education be used to build consensus and recruit support from other physicians? What should be the scope of the QI initiative, and what patient population should be targeted for intervention?
The goal of the SHM QI RR is to provide the tools and the framework to help hospitalists develop, implement, and manage a VTE prophylaxis quality improvement initiative. Suggested Steps to Action in the Awareness section depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem. Hospital quality officers can direct the hospital's public relations department to the Awareness section for DVT Awareness Month materials, including public service announcements in audio, visual, and print formats. The hold music at the hospital can be temporarily replaced, television kiosks can be set up to run video loops, and banners can be printed and hung in central locations, all to get out the message simultaneously to patients and medical staff.
The Evidence section of the VTE QI RR references a key benchmark study, the DVT‐Free Prospective Registry.9 This study reported that at 183 sites in North America and Europe, more than twice as many medical patients as surgical patients failed to receive prophylaxis. The Evidence section includes the 7th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy and also highlights 3 randomized placebo‐controlled clinical trials (MEDENOX 1999, ARTEMIS 2003, and PREVENT 2004) that have reported significant reduction of risk of VTE (50%‐60%) from pharmacologic prophylaxis in moderate‐risk medical inpatients.1315 Review of the data helps to determine which patient population to study first, which prophylaxis options a hospital could deploy appropriately, and the expected magnitude of the effect. Because the literature has already been narrowed and is kept current, hospitalists can save time in answering a range of questions, from the most commonly agreed‐on factors to stratify risk to which populations require alternative interventions.
The Experience section references the first clinical trial demonstrating improved patient outcomes from a quality improvement initiative aimed at improving utilization of VTE prophylaxis.10 At the large teaching hospital where the electronic alerts were studied, a preexisting wealth of educational information on the hospital Web site, in the form of multiple seminars and lectures on VTE prophylaxis by opinion leaders and international experts, had little impact on practice. For this reason, the investigators implemented a trial of how to change physician behavior by introducing a point‐of‐care intervention, the computer alerts. Clinicians prompted by an electronic alert to consider DVT prophylaxis for at‐risk patients employed nearly double the rate of pharmacologic prophylaxis and reduced the incidence of DVT or pulmonary embolism (PE) by 41%. This study suggests that a change introduced to the clinical workflow can improve evidence‐based VTE prophylaxis and also can reduce the incidence of VTE in acutely ill hospitalized patients.
We believe that if hospitalists use the current evidence and experience assembled in the VTE QI RR, they could develop and lead a systematic approach to improving utilization of VTE prophylaxis. Although there is no gold standard method for integrating VTE risk assessment into clinical workflow, the VTE QI RR presents key lessons both from the literature and real world experiences. The crucial take‐home message is that hospitalists can facilitate implementation of VTE risk assessments if they stress simplicity (ie, the sick, old, surgery benefit), link the risk assessment to a menu of evidence‐based prophylaxis options, and require assessment of VTE risk as part of a regular routine (on admission and at regular intervals). Although many hospitals do not yet have computerized entry of physician orders, the simple 4‐point VTE risk assessment described by Kucher et al might be applied to other hospitals.10 The 4‐point system would identify the patients at highest risk, a reasonable starting point for a QI initiative. Whatever the modelCPOE alerts of very high‐risk patients, CPOE‐forced VTE risk assessments, nursing assessments, or paper‐based order setsregular VTE risk assessment can be incorporated into the daily routine of hospital care.
The QI workbook sequences the steps of a multidisciplinary improvement team and prompts users to set specific goals, collect practical metrics, and conduct plan‐do‐study‐act (PDSA) cycles of learning and action (Figure 4). Hospitalists and other team members can use the information in the workbook to estimate the prevalence of use of the appropriate VTE prophylaxis and the incidence of hospital‐acquired VTE at their medical centers, develop a suitable VTE risk assessment model, and plan interventions. Starting with all patients admitted to one nurse on one unit, then expanding to an entire nursing unit, an improvement team could implement rapid PDSA cycles to iron out the wrinkles of a risk assessment protocol. After demonstrating a measurable benefit for the patients at highest risk, the team would then be expected to capture more patients at risk for VTE by modifying the risk assessment protocol to identify moderate‐risk patients (hospitalized patients with one risk factor), as in the MEDENOX, ARTEMIS, and PREVENT clinical trials. Within the first several months, the QI intervention could be expanded to more nursing units. An improvement report profiling a clinically important increase in the rate of appropriate VTE prophylaxis would advocate for additional local resources and projects.

As questions arise in assembling an improvement team, setting useful aims and metrics, choosing interventions, implementing and studying change, or collecting performance data, hospitalists can review answers to questions already posted and post their own questions in the Ask the Expert area. For example, one user asked whether there was a standard risk assessment tool for identifying patients at high risk of VTE. Another asked about the use of unfractionated heparin as a low‐cost alternative to low‐molecular‐weight heparin. Both these questions were answered within 24 hours by the content editor of the VTE QI RR and, for one question, also by 2 pharmacists and an international expert in VTE.
As other hospitalists begin de novo efforts of their own, success stories and strategies posted in the online forums of the VTE QI RR will be an evolving resource for basic know‐how and innovation.
Suggestions from a community of resource room users will be solicited, evaluated, and incorporated into the QI RR in order to improve its educational value and utility. The curricula could also be adapted or refined by others with an interest in systems‐based care or practice‐based learning, such as directors of residency training programs.
CONCLUSIONS
The QI RRs bring QI theory and practice to the hospitalist, when and wherever it is wanted, minimizing time away from patient care. The workbook links theory to practice and can be used to launch, sustain, and document a local VTE‐specific QI initiative. A range of experience is accommodated. Content is provided in a way that enables the user to immediately apply and adapt it to a local contextusers can access and download the subset of tools that best meet their needs. For practicing hospitalists, this QI resource offers an opportunity to bridge the training gap in systems‐based hospital care and should increase the quality and quantity of and support for opportunities to lead successful QI projects.
The Accreditation Council of Graduate Medical Education (ACGME) now requires education in health care systems, a requirement not previously mandated for traditional medical residency programs.17 Because the resource rooms should increase the number of hospitalists competently leading local efforts that achieve measurable gains in hospital outcomes, a wider potential constituency also includes residency program directors, internal medicine residents, physician assistants and nurse‐practitioners, nurses, hospital quality officers, and hospital medicine practice leaders.
Further research is needed to determine the clinical impact of the VTE QI workbook on outcomes for hospitalized patients. The effectiveness of such an educational method should be evaluated, at least in part, by documenting changes in clinically important process and outcome measures, in this case those specific to hospital‐acquired VTE. Investigation also will need to generate an impact assessment to see if the curricula are effective in meeting the strategic educational goals of the Society of Hospital Medicine. Further investigation will examine whether this resource can help residency training programs achieve ACGME goals for practice‐based learning and systems‐based care.
The goal of this article is to explain how the first in a series of online resource rooms provides trainees and hospitalists with quality improvement tools that can be applied locally to improve inpatient care.1 During the emergence and explosive growth of hospital medicine, the SHM recognized the need to revise training relating to inpatient care and hospital process design to meet the evolving expectation of hospitalists that their performance will be measured, to actively set quality parameters, and to lead multidisciplinary teams to improve hospital performance.2 Armed with the appropriate skill set, hospitalists would be uniquely situated to lead and manage improvements in processes in the hospitals in which they work.
The content of the first Society of Hospital Medicine (SHM) Quality Improvement Resource Room (QI RR) supports hospitalists leading a multidisciplinary team dedicated to improving inpatient outcomes by preventing hospital‐acquired venous thromboembolism (VTE), a common cause of morbidity and mortality in hospitalized patients.3 The SHM developed this educational resource in the context of numerous reports on the incidence of medical errors in US hospitals and calls for action to improve the quality of health care.'47 Hospital report cards on quality measures are now public record, and hospitals will require uniformity in practice among physicians. Hospitalists are increasingly expected to lead initiatives that will implement national standards in key practices such as VTE prophylaxis2.
The QI RRs of the SHM are a collection of electronic tools accessible through the SHM Web site. They are designed to enhance the readiness of hospitalists and members of the multidisciplinary inpatient team to redesign care at the institutional level. Although all performance improvement is ultimately occurs locally, many QI methods and tools transcend hospital geography and disease topic. Leveraging a Web‐based platform, the SHM QI RRs present hospitalists with a general approach to QI, enriched by customizable workbooks that can be downloaded to best meet user needs. This resource is an innovation in practice‐based learning, quality improvement, and systems‐based practice.
METHODS
Development of the first QI RR followed a series of steps described in Curriculum Development for Medical Education8 (for process and timeline, see Table 1). Inadequate VTE prophylaxis was identified as an ongoing widespread problem of health care underutilization despite randomized clinical trials supporting the efficacy of prophylaxis.9, 10 Mirroring the AHRQ's assessment of underutilization of VTE prophylaxis as the single most important safety priority,6 the first QI RR focused on VTE, with plans to cover additional clinical conditions over time. As experts in the care of inpatients, hospitalists should be able to take custody of predictable complications of serious illness, identify and lower barriers to prevention, critically review prophylaxis options, utilize hospital‐specific data, and devise strategies to bridge the gap between knowledge and practice. Already leaders of multidisciplinary care teams, hospitalists are primed to lead multidisciplinary improvement teams as well.
| Phase 1 (January 2005April 2005): Executing the educational strategy |
|---|
| One‐hour conference calls |
| Curricular, clinical, technical, and creative aspects of production |
| Additional communication between members of working group between calls |
| Development of questionnaire for SHM membership, board, education, and hospital quality patient safety (HQPS) committees |
| Content freeze: fourth month of development |
| Implementation of revisions prior to April 2005 SHM Annual Meeting |
| Phase 2 (April 2005August 2005): revision based on feedback |
| Analysis of formative evaluation from Phase 1 |
| Launch of the VTE QI RR August 2005 |
| Secondary phases and venues for implementation |
| Workshops at hospital medicine educational events |
| SHM Quality course |
| Formal recognition of the learning, experience, or proficiency acquired by users |
| The working editorial team for the first resource room |
| Dedicated project manager (SHM staff) |
| Senior adviser for planning and development (SHM staff) |
| Senior adviser for education (SHM staff) |
| Content expert |
| Education editor |
| Hospital quality editor |
| Managing editor |
Available data on the demographics of hospitalists and feedback from the SHM membership, leadership, and committees indicated that most learners would have minimal previous exposure to QI concepts and only a few years of management experience. Any previous quality improvement initiatives would tend to have been isolated, experimental, or smaller in scale. The resource rooms are designed to facilitate quality improvement learning among hospitalists that is practice‐based and immediately relevant to patient care. Measurable improvement in particular care processes or outcomes should correlate with actual learning.
The educational strategy of the SHM was predicated on ensuring that a quality and patient safety curriculum would retain clinical applicability in the hospital setting. This approach, grounded in adult learning principles and common to medical education, teaches general principles by framing the learning experience as problem centered.11 Several domains were identified as universally important to any quality improvement effort: raising awareness of a local performance gap, applying the best current evidence to practice, tapping the experience of others leading QI efforts, and using measurements derived from rapid‐cycle tests of change. Such a template delineates the components of successful QI planning, implementation, and evaluation and provides users with a familiar RR format applicable to improving any care process, not just VTE.
The Internet was chosen as the mechanism for delivering training on the basis of previous surveys of the SHM membership in which members expressed a preference for electronic and Web‐based forms of educational content delivery. Drawing from the example of other organizations teaching quality improvement, including the Institute for Healthcare Improvement and Intermountain Health Care, the SHM valued the ubiquity of a Web‐based educational resource. To facilitate on‐the‐job training, the first SHM QI RR provides a comprehensive tool kit to guide hospitalists through the process of advocating, developing, implementing, and evaluating a QI initiative for VTE.
Prior to launching the resource room, formative input was collected from SHM leaders, a panel of education and QI experts, and attendees of the society's annual meetings. Such input followed each significant step in the development of the RR curricula. For example, visitors at a kiosk at the 2005 SHM annual meeting completed surveys as they navigated through the VTE QI RR. This focused feedback shaped prelaunch development. The ultimate performance evaluation and feedback for the QI RR curricula will be gauged by user reports of measurable improvement in specific hospital process or outcomes measures. The VTE QI RR was launched in August 2005 and promoted at the SHM Web site.
RESULTS
The content and layout of the VTE QI RR are depicted in Figure 1. The self‐directed learner may navigate through the entire resource room or just select areas for study. Those likely to visit only a single area are individuals looking for guidance to support discrete roles on the improvement team: champion, clinical leader, facilitator of the QI process, or educator of staff or patient audiences (see Figure 2).


Why Should You Act?
The visual center of the QI RR layout presents sobering statisticsalthough pulmonary embolism from deep vein thrombosis is the most common cause of preventable hospital death, most hospitalized medical patients at risk do not receive appropriate prophylaxisand then encourages hospitalist‐led action to reduce hospital‐acquired VTE. The role of the hospitalist is extracted from the competencies articulated in the Venous Thromboembolism, Quality Improvement, and Hospitalist as Teacher chapters of The Core Competencies in Hospital Medicine.2
Awareness
In the Awareness area of the VTE QI RR, materials to raise clinician, hospital staff, and patient awareness are suggested and made available. Through the SHM's lead sponsorship of the national DVT Awareness Month campaign, suggested Steps to Action depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem.
Evidence
The Evidence section aggregates a list of the most pertinent VTE prophylaxis literature to help ground any QI effort firmly in the evidence base. Through an agreement with the American College of Physicians (ACP), VTE prophylaxis articles reviewed in the ACP Journal Club are presented here.12 Although the listed literature focuses on prophylaxis, plans are in place to include references on diagnosis and treatment.
Experience
Resource room visitors interested in tapping into the experience of hospitalists and other leaders of QI efforts can navigate directly to this area. Interactive resources here include downloadable and adaptable protocols for VTE prophylaxis and, most importantly, improvement stories profiling actual QI successes. The Experience section features comments from an author of a seminal trial that studied computer alerts for high‐risk patients not receiving prophylaxis.10 The educational goal of this section of the QI RR is to provide opportunities to learn from successful QI projects, from the composition of the improvement team to the relevant metrics, implementation plan, and next steps.
Ask the Expert
The most interactive part of the resource room, the Ask the Expert forum, provides a hybrid of experience and evidence. A visitor who posts a clinical or improvement question to this discussion community receives a multidisciplinary response. For each question posted, a hospitalist moderator collects and aggregates responses from a panel of VTE experts, QI experts, hospitalist teachers, and pharmacists. The online exchange permitted by this forum promotes wider debate and learning. The questions and responses are archived and thus are available for subsequent users to read.
Improve
This area features the focal point of the entire resource room, the VTE QI workbook, which was written and designed to provide action‐oriented learning in quality improvement. The workbook is a downloadable project outline to guide and document efforts aimed at reducing rates of hospital‐acquired VTE. Hospitalists who complete the workbook should have acquired familiarity with and a working proficiency in leading system‐level efforts to drive better patient care. Users new to the theory and practice of QI can also review key concepts from a slide presentation in this part of the resource room.
Educate
This content area profiles the hospital medicine core competencies that relate to VTE and QI while also offering teaching materials and advice for teachers of VTE or QI. Teaching resources for clinician educators include online CME and an up‐to‐date slide lecture about VTE prophylaxis. The lecture presentation can be downloaded and customized to serve the needs of the speaker and the audience, whether students, residents, or other hospital staff. Clinician educators can also share or review teaching pearls used by hospitalist colleagues who serve as ward attendings.
DISCUSSION
A case example, shown in Figure 3, demonstrates how content accessible through the SHM VTE QI RR may be used to catalyze a local quality improvement effort.

Hospitals will be measured on rates of VTE prophylaxis on medical and surgical services. Failure to standardize prophylaxis among different physician groups may adversely affect overall performance, with implications for both patient care and accreditation. The lack of a agreed‐on gold standard of what constitutes appropriate prophylaxis for a given patient does not absolve an institution of the duty to implement its own standards. The challenge of achieving local consensus on appropriate prophylaxis should not outweigh the urgency to address preventable in‐hospital deaths. In caring for increasing numbers of general medical and surgical patients, hospitalists are likely to be asked to develop and implement a protocol for VTE prophylaxis that can be used hospitalwide. In many instances hospitalists will accept this charge in the aftermath of previous hospital failures in which admission order sets or VTE assessment protocols were launched but never widely implemented. As the National Quality Forum or JCAHO regulations for uniformity among hospitals shift VTE prophylaxis from being voluntary to compulsory, hospitalists will need to develop improvement strategies that have greater reliability.
Hospitalists with no formal training in either vascular medicine or quality improvement may not be able to immediately cite the most current data about VTE prophylaxis rates and regimens and may not have the time to enroll in a training course on quality improvement. How would hospitalists determine baseline rates of appropriate VTE prophylaxis? How can medical education be used to build consensus and recruit support from other physicians? What should be the scope of the QI initiative, and what patient population should be targeted for intervention?
The goal of the SHM QI RR is to provide the tools and the framework to help hospitalists develop, implement, and manage a VTE prophylaxis quality improvement initiative. Suggested Steps to Action in the Awareness section depict exactly how a hospital medicine service can use the campaign's materials to raise institutional support for tackling this preventable problem. Hospital quality officers can direct the hospital's public relations department to the Awareness section for DVT Awareness Month materials, including public service announcements in audio, visual, and print formats. The hold music at the hospital can be temporarily replaced, television kiosks can be set up to run video loops, and banners can be printed and hung in central locations, all to get out the message simultaneously to patients and medical staff.
The Evidence section of the VTE QI RR references a key benchmark study, the DVT‐Free Prospective Registry.9 This study reported that at 183 sites in North America and Europe, more than twice as many medical patients as surgical patients failed to receive prophylaxis. The Evidence section includes the 7th American College of Chest Physicians Consensus Conference on Antithrombotic and Thrombolytic Therapy and also highlights 3 randomized placebo‐controlled clinical trials (MEDENOX 1999, ARTEMIS 2003, and PREVENT 2004) that have reported significant reduction of risk of VTE (50%‐60%) from pharmacologic prophylaxis in moderate‐risk medical inpatients.1315 Review of the data helps to determine which patient population to study first, which prophylaxis options a hospital could deploy appropriately, and the expected magnitude of the effect. Because the literature has already been narrowed and is kept current, hospitalists can save time in answering a range of questions, from the most commonly agreed‐on factors to stratify risk to which populations require alternative interventions.
The Experience section references the first clinical trial demonstrating improved patient outcomes from a quality improvement initiative aimed at improving utilization of VTE prophylaxis.10 At the large teaching hospital where the electronic alerts were studied, a preexisting wealth of educational information on the hospital Web site, in the form of multiple seminars and lectures on VTE prophylaxis by opinion leaders and international experts, had little impact on practice. For this reason, the investigators implemented a trial of how to change physician behavior by introducing a point‐of‐care intervention, the computer alerts. Clinicians prompted by an electronic alert to consider DVT prophylaxis for at‐risk patients employed nearly double the rate of pharmacologic prophylaxis and reduced the incidence of DVT or pulmonary embolism (PE) by 41%. This study suggests that a change introduced to the clinical workflow can improve evidence‐based VTE prophylaxis and also can reduce the incidence of VTE in acutely ill hospitalized patients.
We believe that if hospitalists use the current evidence and experience assembled in the VTE QI RR, they could develop and lead a systematic approach to improving utilization of VTE prophylaxis. Although there is no gold standard method for integrating VTE risk assessment into clinical workflow, the VTE QI RR presents key lessons both from the literature and real world experiences. The crucial take‐home message is that hospitalists can facilitate implementation of VTE risk assessments if they stress simplicity (ie, the sick, old, surgery benefit), link the risk assessment to a menu of evidence‐based prophylaxis options, and require assessment of VTE risk as part of a regular routine (on admission and at regular intervals). Although many hospitals do not yet have computerized entry of physician orders, the simple 4‐point VTE risk assessment described by Kucher et al might be applied to other hospitals.10 The 4‐point system would identify the patients at highest risk, a reasonable starting point for a QI initiative. Whatever the modelCPOE alerts of very high‐risk patients, CPOE‐forced VTE risk assessments, nursing assessments, or paper‐based order setsregular VTE risk assessment can be incorporated into the daily routine of hospital care.
The QI workbook sequences the steps of a multidisciplinary improvement team and prompts users to set specific goals, collect practical metrics, and conduct plan‐do‐study‐act (PDSA) cycles of learning and action (Figure 4). Hospitalists and other team members can use the information in the workbook to estimate the prevalence of use of the appropriate VTE prophylaxis and the incidence of hospital‐acquired VTE at their medical centers, develop a suitable VTE risk assessment model, and plan interventions. Starting with all patients admitted to one nurse on one unit, then expanding to an entire nursing unit, an improvement team could implement rapid PDSA cycles to iron out the wrinkles of a risk assessment protocol. After demonstrating a measurable benefit for the patients at highest risk, the team would then be expected to capture more patients at risk for VTE by modifying the risk assessment protocol to identify moderate‐risk patients (hospitalized patients with one risk factor), as in the MEDENOX, ARTEMIS, and PREVENT clinical trials. Within the first several months, the QI intervention could be expanded to more nursing units. An improvement report profiling a clinically important increase in the rate of appropriate VTE prophylaxis would advocate for additional local resources and projects.

As questions arise in assembling an improvement team, setting useful aims and metrics, choosing interventions, implementing and studying change, or collecting performance data, hospitalists can review answers to questions already posted and post their own questions in the Ask the Expert area. For example, one user asked whether there was a standard risk assessment tool for identifying patients at high risk of VTE. Another asked about the use of unfractionated heparin as a low‐cost alternative to low‐molecular‐weight heparin. Both these questions were answered within 24 hours by the content editor of the VTE QI RR and, for one question, also by 2 pharmacists and an international expert in VTE.
As other hospitalists begin de novo efforts of their own, success stories and strategies posted in the online forums of the VTE QI RR will be an evolving resource for basic know‐how and innovation.
Suggestions from a community of resource room users will be solicited, evaluated, and incorporated into the QI RR in order to improve its educational value and utility. The curricula could also be adapted or refined by others with an interest in systems‐based care or practice‐based learning, such as directors of residency training programs.
CONCLUSIONS
The QI RRs bring QI theory and practice to the hospitalist, when and wherever it is wanted, minimizing time away from patient care. The workbook links theory to practice and can be used to launch, sustain, and document a local VTE‐specific QI initiative. A range of experience is accommodated. Content is provided in a way that enables the user to immediately apply and adapt it to a local contextusers can access and download the subset of tools that best meet their needs. For practicing hospitalists, this QI resource offers an opportunity to bridge the training gap in systems‐based hospital care and should increase the quality and quantity of and support for opportunities to lead successful QI projects.
The Accreditation Council of Graduate Medical Education (ACGME) now requires education in health care systems, a requirement not previously mandated for traditional medical residency programs.17 Because the resource rooms should increase the number of hospitalists competently leading local efforts that achieve measurable gains in hospital outcomes, a wider potential constituency also includes residency program directors, internal medicine residents, physician assistants and nurse‐practitioners, nurses, hospital quality officers, and hospital medicine practice leaders.
Further research is needed to determine the clinical impact of the VTE QI workbook on outcomes for hospitalized patients. The effectiveness of such an educational method should be evaluated, at least in part, by documenting changes in clinically important process and outcome measures, in this case those specific to hospital‐acquired VTE. Investigation also will need to generate an impact assessment to see if the curricula are effective in meeting the strategic educational goals of the Society of Hospital Medicine. Further investigation will examine whether this resource can help residency training programs achieve ACGME goals for practice‐based learning and systems‐based care.
- Society of Hospital Medicine Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement_Resource_Rooms1(suppl 1).
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Arch Intern Med.1991;151:933–938.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicinehttp://www.iom.edu/CMS/3718.aspx
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices.Agency for Healthcare Research and Quality, Publication 01‐E058;2001.
- Joint Commission on the Accreditation of Health Care Organizations. Public policy initiatives. Available at: http://www.jcaho.org/about+us/public+policy+initiatives/pay_for_performance.htm
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, Md:Johns Hopkins University Press;1998.
- ,;DVT FREE Steering Committee.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969.
- ,,.Teaching the Case Method.3rd ed.Cambridge, Mass :Harvard Business School.
- American College of Physicians. Available at: http://www.acpjc.org/?hp
- ,,, et al.MEDENOX trial.N Engl J Med.1999;341:793–800.
- ,,.Fondaparinux versus placebo for the prevention of VTE in acutely ill medical patients (ARTEMIS).J Thromb Haemost.2003;1(suppl 1):2046.
- ,,,,,.PREVENT Medical Thromboprophylaxis Study Group.Circulation.2004;110:874–879.
- ,.Comparing the costs, risks and benefits of competing strategies for the primary prevention of VTE.Circulation.2004;110:IV25–IV32.
- Accreditation Council for Graduate Medical Education. Available at: http://www.acgme.org/acWebsite/programDir/pd_index.asp.
- Society of Hospital Medicine Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Quality_Improvement_Resource_Rooms1(suppl 1).
- ,,,,,.Physician practices in the prevention of venous thromboembolism.Arch Intern Med.1991;151:933–938.
- Kohn LT,Corrigan JM,Donaldson MS, eds.To Err Is Human.Washington, DC:National Academy Press;2000.
- Institute of Medicinehttp://www.iom.edu/CMS/3718.aspx
- Shojania KG,Duncan BW,McDonald KM,Wachter RM, eds.Making health care safer: a critical analysis of patient safety practices.Agency for Healthcare Research and Quality, Publication 01‐E058;2001.
- Joint Commission on the Accreditation of Health Care Organizations. Public policy initiatives. Available at: http://www.jcaho.org/about+us/public+policy+initiatives/pay_for_performance.htm
- .Curriculum Development for Medical Education: A Six‐Step Approach.Baltimore, Md:Johns Hopkins University Press;1998.
- ,;DVT FREE Steering Committee.A prospective registry of 5,451 patients with ultrasound‐confirmed deep vein thrombosis.Am J Cardiol.2004;93:259.
- ,,, et al.Electronic alerts to prevent venous thromboembolism among hospitalized patients.N Engl J Med.2005;352:969.
- ,,.Teaching the Case Method.3rd ed.Cambridge, Mass :Harvard Business School.
- American College of Physicians. Available at: http://www.acpjc.org/?hp
- ,,, et al.MEDENOX trial.N Engl J Med.1999;341:793–800.
- ,,.Fondaparinux versus placebo for the prevention of VTE in acutely ill medical patients (ARTEMIS).J Thromb Haemost.2003;1(suppl 1):2046.
- ,,,,,.PREVENT Medical Thromboprophylaxis Study Group.Circulation.2004;110:874–879.
- ,.Comparing the costs, risks and benefits of competing strategies for the primary prevention of VTE.Circulation.2004;110:IV25–IV32.
- Accreditation Council for Graduate Medical Education. Available at: http://www.acgme.org/acWebsite/programDir/pd_index.asp.
Copyright © 2006 Society of Hospital Medicine
Preventing Surgical Site Infections
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at [email protected].
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at [email protected].
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.
Background
An appropriately feared complication of operations, surgical site infections (SSIs) are infections associated with high economic costs and significantly worse clinical outcomes (1). Defined as infections of the superficial incision site, deep incision space, or organ space, SSIs add additional cost ranging from $2,700 to $26,000 per episode according to CDC’s National Nosocomial Infections Surveillance System. Patients who develop an SSI have hospital lengths of stay (LOS) in excess of 7 days longer and are 60% more likely to spend time in the intensive care unit than are patients without an SSI. A patient with an SSI is five times more likely to be readmitted to the hospital and is twice as likely to die (2).
Unfortunately, surgical site infections are common. Among healthcare-acquired infections, SSIs rank second only to urinary tract infections in frequency, making them more common than bloodstream infections and nosocomial pneumonia (3). There are approximately 30 million operations annually in the United States and an SSI complicates 2–5% of clean extra-abdominal sites. The rate is much higher for intra-abdominal operations, approaching 20% (1). Because most SSIs begin within 2 hours of contamination, the perioperative period is the most crucial for development of an SSI (4). By offering clinical expertise in the practice guidelines that reduce the risk of SSIs, hospital medicine programs can help patients and hospital systems lower morbidity, mortality, and costs associated with this complication. Adherence to best practices will likely require coordinated, multidisciplinary process improvement.
Several important interventions fall directly under the control of the anesthesia and surgical teams, such as administering perioperative oxygen, ensuring perioperative normothermia, and avoiding shaving of the surgical site. In coordinated quality improvement efforts, members of the operative team should assume direct responsibility for the performance of these measures. But the performance of two important interventions in this decisive period is likely to be significantly enhanced by the presence of focused hospitalist surgical co-management: antimicrobial prophylaxis and perioperative glycemic control (Table 1).
Antimicrobial Prophylaxis
Studies overwhelmingly show a marked reduction in the relative risk of SSIs with the use of antibiotic prophylaxis (1). In June 2004, the National Surgical Infection Prevention Project (NSIPP) published an advisory statement on antimicrobial prophylaxis in which it outlined three performance measures for quality improvement in prevention of SSIs:
- The proportion of patients who have parenteral antimicrobial prophylaxis initiated within 1 hour before surgical incision
- The proportion of patients provided with a prophylactic antimicrobial agent that is consistent with currently published guidelines, and
- The proportion of patients whose prophylactic antimicrobial therapy is discontinued within 24 hours after the end of surgery (5)
Pooled data suggest that attention to timing makes a favorable difference in SSI rates (1). Fully administering the appropriate antibiotic within 60 minutes of incision ensures that serum and tissue drug levels exceed the MICs of the most likely contaminating organisms. Dosing the antibiotic immediately prior to the start of surgery also provides the best opportunity to extend therapeutic levels for the duration of the surgery. The fact that anesthesia and surgical teams are in the most practical time-space positions to apply this measure underscores the multi-disciplinary and process-level efforts necessary to reduce SSI rates.
When it comes to the choice of antimicrobial and the duration of its use, hospitalists may find themselves in superior positions of impact. Familiarity with recommendations of the NSIPP advisory statement (summarized in Table 2) promotes evidence-based selection of antibiotic prophylaxis based on patient-specific factors: type of operation and presence of true drug allergies (5). Compared with other members of the surgical co-management team, hospitalists are more likely to be aware of relevant patient-specific risk factors such as the likelihood of colonization with methicillin-resistant Staphylococcus aureus (MRSA). For example, in patients colonized with MRSA, hospitalists might consider vancomycin as the alternative agent for prophylaxis. Free access to the NSIPP advisory statement is available at www.journals.uchicago.edu/CID/journal/issues/v38n12/33257/33257.html.
Antimicrobial prophylaxis after wound closure is unnecessary; published evidence demonstrates the non-inferiority of single dose prophylaxis when compared with multiple dose prophylaxis (5). Further, prolonged use of antimicrobial prophylaxis is associated with the emergence of resistant organisms (6-8). By ensuring that the duration of prophylaxis does not exceed 24 hours past the end of the operation, hospitalists can make valuable contributions to public health and cost containment.
Non-Antimicrobial Measures
Several non-antimicrobial measures also significantly reduce SSI rates. Those that fall outside the domain of the hospitalist and into the direct purview of the operative team include high levels of inspired oxygen, maintenance of perioperative normothermia, and use of clippers rather than a razor when hair removal is necessary. The risk of SSIs is directly related to tissue oxygenation. Bacterial infectivity is enhanced and cellular immunity is compromised in hypoperfused, poorly oxygenated tissue (9). The practice of administering perioperative supplemental oxygen (at least 80% FIO2 in intubated patients) reduces the risk of SSI by nearly one-half (1). For non-intubated patients, oxygen at 12 L/min by non-rebreathing face mask applied intra-operatively and for at least 2 hours following surgery leads to similar reductions of SSI rates. Besides being effective, this intervention is inexpensive, has no recognized adverse effects, and carries the added benefit of significantly reducing post-operative nausea and vomiting (4).
Hypothermia also predisposes the surgical wound to infection. Even mild perioperative hypothermia (i.e., core temperature 35-36.5°C) typically occurs in the absence of specific measures to prevent net heat loss. Perioperative hypothermia is the combined result of exposure and anesthetic-induced thermo-dysregulation, with redistribution of core body heat to the periphery (4). Even mild hypothermia causes vasoconstriction which diminishes perfusion, dropping tissue oxygen tension which impairs phagocytosis and oxidative killing by neutrophils (10). Hypothermia also blunts scar formation which further diminishes wound integrity. Active warming of the patient to maintain a core temperature near 36.5°C constitutes the intra-operative standard of care and is effective at reducing the risk of SSIs by as much as two-thirds (1).
Hyperglycemia, an established independent risk factor for an array of adverse outcomes in hospitalized patients, is also an independent risk factor for SSIs across a range of surgical patients (1). Short-term hyperglycemia depresses immune function through nonenzymatic glycosylation of immunoglobulin and by impairing normal leukocyte performance (11). Among diabetic cardiac surgery patients, reduction of hyperglycemia with an intravenous insulin infusion lowered the incidence of deep sternal wound infection by as much as two-thirds (12). While the value of achieving glycemic targets has already been established for a variety of important endpoints and across a range of inpatient populations, hospitalists should stay tuned. As high quality studies emerge proving that glycemic control lowers SSIs among non-cardiac surgical subpopulations, hospitalists may increasingly be relied upon to achieve strict glycemic targets.
By recognizing and coordinating practices known to reduce SSIs, hospitalists can elevate the level of care provided for surgical patients. At the same time, hospitalists can help lower costs and keep the hospital system mindful of public health goals, such as prevention of antimicrobial resistance. While individual hospitalists have key roles to play, the overall approach to SSI reduction calls for a coordinated, multidisciplinary team approach with process and system-level efforts.
Dr. Stein can be contacted at [email protected].
References
- Auerbach AD. Prevention of surgical site infections. In: Shojania KG, Duncan BW, McDonald KM, et al., eds. Making health care safer: a critical analysis of patient safety practices. Evidence report/technology assessment no. 43. AHRQ publication no. 01-E058. Rockville, MD: Agency for Healthcare Research and Quality, 20 July 2001:221-44.
- Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-30.
- National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1996, issued May 1996: a report from the National Nosocomial Infections Surveillance (NNIS) system. Am J Infect Control. 1996;24:380-8.
- Sessler DI, Akca O. Nonpharmacologic prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397 404.
- Bratzler D, Houck PM. Surgical Infection Prevention Guidelines Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis. 2004;Jun15;38(12):1706-15. E-pub 2004 May 26.
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21.
- Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93.
- Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8.
- Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997-1005.
- Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-15.
- Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology Task Force on Inpatient Diabetes Metabolic Control. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract. 2004;Mar-Apr;10Suppl2:4-9.
- Furnary AP, Zerr K, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [with discussion]. Ann Thorac Surg. 1999;67:352-62.