User login
Thrombocytopenia signals multiorgan system failure in acute liver failure
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
In patients with acute liver failure (ALF), decreasing platelet counts after hospital admission signaled systemic inflammation and a greater likelihood of systemic complications, such as high-grade hepatic encephalopathy, cardiovascular collapse, the need for liver transplant, and death, according to researchers.
Patients with systemic inflammatory response syndrome (SIRS) had significantly lower platelet counts at admission, compared with those without SIRS, and their platelet counts decreased dramatically (from 182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) compared with stable platelet counts in patients without SIRS. For days 2-7 postadmission, lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
“We hypothesize that the decrease in platelet count represents an integral event in the pathogenesis of the ALF syndrome rather than a nonspecific marker of suppressed bone marrow production. Further studies will be needed to prove the pivotal role of platelets in mediating the proinflammatory and prothrombotic features of the ALF syndrome,” wrote Dr. R. Todd Stravitz, professor of medicine, section of hepatology, Virginia Commonwealth University, Richmond, and his colleagues (Clin Gastroenterol Hepatol. 2016 Feb 25. doi: 10.1016/j.cgh.2015.09.029).
Results showed that SIRS and multiorgan system failure (MOSF) were more closely linked to a decrease in platelet counts than to an increase in the international normalized ratio (INR), a laboratory marker for liver injury. The INR was similar in patients with and without SIRS, in high-grade and low-grade hepatic encephalopathy, and in patients with and without requirements for vasopressor support and renal replacement therapy (indicators of MOSF). Given that both platelet counts and INR were associated with prognosis, the investigators suggest that these laboratory parameters may reflect different types of injury, with INR signaling primary liver injury and platelets reflecting the severity of systemic inflammation secondary to liver injury.
Platelet counts varied according to outcome on day 21. Spontaneous survivors had higher mean platelet counts than patients who underwent liver transplant, and patients who died had the lowest platelet counts. Platelet counts in spontaneous survivors decreased from day 1 to 2, then subsequently recovered; platelets decreased progressively in patients who underwent liver transplant or died. The INR trend over time according to 21-day outcome was similar to that of platelet counts.
The retrospective study evaluated data from 1598 patients who enrolled in the ALF Study Group from 1998 to 2012. The mean age was 41 years, 76% were Caucasian, and 70% were female. Nearly one-half of participants (47%) had ALF due to acetaminophen overdose, 85% had at least one positive element of SIRS on admission, 32% required vasopressors, 33% required renal replacement therapy, and 50% developed high-grade (3 or 4) hepatic encephalopathy. In total, 47% of patients recovered without liver transplant, 24% underwent liver transplant, and 32% died.
The mechanism underlying development of thrombocytopenia in patients with ALF is poorly understood. Previous findings by the investigators showed that platelet-derived, prothrombotic microparticles increased in proportion to SIRS severity, and concentration of microparticles increased in parallel with laboratory markers of poor outcome. Current results suggest the converse to be true as well: platelet counts decreased in proportion to the severity of SIRS, the development of MOSF and poor outcome at 21 days.
The researchers proposed that deficiencies in the number of platelets or in liver-derived, prohemostatic coagulation factors may be compensated by systemic inflammation. Increased levels of platelet-derived microparticles in patients with ALF may overcompensate for thrombocytopenia due to a nearly 40-fold higher prothrombotic potential in tissue factor–dependent assays, compared with healthy control populations.
The findings point to the importance of platelet count in signaling impending complications in patients with ALF.
Dr. Stravitz and his coauthors reported having no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: In patients with acute liver failure (ALF), decreasing platelet counts were associated with systemic inflammation and greater likelihood of serious systemic complications.
Major finding: Platelet counts decreased dramatically in patients with systemic inflammatory response syndrome (SIRS) (182 plus or minus 27 times 109/L on admission to 103 plus or minus 3.20 times 109/L on day 6) but remained stable in patients without SIRS; lower platelet counts were associated with high-grade hepatic encephalopathy, as well as the need for vasopressor support and renal replacement therapy (P less than or equal to .001 for all).
Data source: From 1998 to 2012 the ALF Study Group included 1,598 patients of mean age 41 years; 76% were Caucasian and 70% were female.
Disclosures: Dr. Stravitz and his coauthors reported having no disclosures.
Low serum vitamin D may signal more aggressive prostate cancer
Serum 25-hydroxyviatmin D (25-OH D) deficiency was associated with greater risk for adverse pathology in men with localized prostate cancer undergoing radical prostatectomy, particularly among men with intermediate-risk disease.
Men with adverse pathology at radical prostatectomy had lower median serum 25-OH D (22.7 ng/mL; interquartile range [IQR], 15.9 to 29.0) compared with their counterparts (27.0 ng/mL; IQR, 20.0 to 34.0; P = .007), and they were more likely to have a serum 25-OH D level less than 30 ng/mL (80.5% v. 57.3%; P = .001) (J Clin Oncol. 2016 Feb. 22. doi: 10.1200/JCO.2015.65.1463).
Multiple logistic regression showed that serum 25-OH D level less than 30 ng/mL was associated with adverse pathology independent of age, serum PSA, and abnormal findings on digital rectal examination (odds ratio [OR], 2.51; 95% CI, 1.18 to 5.33; P = .02). Stratified analysis showed that in men with National Comprehensive Cancer Network intermediate prostate cancer at diagnosis, serum 25-OH D less than 30 ng/mL was significantly associated with adverse pathology (OR, 3.62; 95% CI, 1.15 to 11.46; P = .03).
The study cohort from Chicago demonstrated a disparity in vitamin D levels between black men and white men, which may contribute to differences by race observed in adverse pathology at radical prostatectomy.
“The initial assessment of our Chicago cohort demonstrated a significant disparity regarding low vitamin D levels and black race compared with white men in both univariate and multivariate analyses. In fact, greater than 90% of black men in that study had vitamin D levels less than 30 ng/mL,” wrote Dr Yaw Nyame of the Glickman Urological & Kidney Institute, Cleveland Clinic, and colleagues.
The cross-sectional, observational study from 2009 to 2014 included 190 men who underwent radical prostatectomy for localized prostate cancer. Adverse pathology was defined by the presence of dominant Gleason pattern 4, the presence of any pattern 5, and pathologic stage pT3aN0M0 or higher.
In total, 87 men (45.8%) had adverse pathology at radical prostatectomy. The median age of men with adverse pathology was 65.0 years, compared with 62.0 years for their counterparts (P = .005); median BMI was 28.9 kg/m2 vs. 27.7 kg/m2 (P = .04); and serum PSA was 6.8 ng/mL vs. 4.4 ng/mL (P less than .001). Men with adverse pathology at radical prostatectomy were more likely to self identify as black (P = .03).
Studies have shown that prostate cancer cells express the vitamin D receptor, and vitamin D has been shown to inhibit cellular proliferation, differentiation, and apoptosis.
Dr. Yaw Nyame reported having no disclosures. Several of his coauthors reported ties to industry.
Serum 25-hydroxyviatmin D (25-OH D) deficiency was associated with greater risk for adverse pathology in men with localized prostate cancer undergoing radical prostatectomy, particularly among men with intermediate-risk disease.
Men with adverse pathology at radical prostatectomy had lower median serum 25-OH D (22.7 ng/mL; interquartile range [IQR], 15.9 to 29.0) compared with their counterparts (27.0 ng/mL; IQR, 20.0 to 34.0; P = .007), and they were more likely to have a serum 25-OH D level less than 30 ng/mL (80.5% v. 57.3%; P = .001) (J Clin Oncol. 2016 Feb. 22. doi: 10.1200/JCO.2015.65.1463).
Multiple logistic regression showed that serum 25-OH D level less than 30 ng/mL was associated with adverse pathology independent of age, serum PSA, and abnormal findings on digital rectal examination (odds ratio [OR], 2.51; 95% CI, 1.18 to 5.33; P = .02). Stratified analysis showed that in men with National Comprehensive Cancer Network intermediate prostate cancer at diagnosis, serum 25-OH D less than 30 ng/mL was significantly associated with adverse pathology (OR, 3.62; 95% CI, 1.15 to 11.46; P = .03).
The study cohort from Chicago demonstrated a disparity in vitamin D levels between black men and white men, which may contribute to differences by race observed in adverse pathology at radical prostatectomy.
“The initial assessment of our Chicago cohort demonstrated a significant disparity regarding low vitamin D levels and black race compared with white men in both univariate and multivariate analyses. In fact, greater than 90% of black men in that study had vitamin D levels less than 30 ng/mL,” wrote Dr Yaw Nyame of the Glickman Urological & Kidney Institute, Cleveland Clinic, and colleagues.
The cross-sectional, observational study from 2009 to 2014 included 190 men who underwent radical prostatectomy for localized prostate cancer. Adverse pathology was defined by the presence of dominant Gleason pattern 4, the presence of any pattern 5, and pathologic stage pT3aN0M0 or higher.
In total, 87 men (45.8%) had adverse pathology at radical prostatectomy. The median age of men with adverse pathology was 65.0 years, compared with 62.0 years for their counterparts (P = .005); median BMI was 28.9 kg/m2 vs. 27.7 kg/m2 (P = .04); and serum PSA was 6.8 ng/mL vs. 4.4 ng/mL (P less than .001). Men with adverse pathology at radical prostatectomy were more likely to self identify as black (P = .03).
Studies have shown that prostate cancer cells express the vitamin D receptor, and vitamin D has been shown to inhibit cellular proliferation, differentiation, and apoptosis.
Dr. Yaw Nyame reported having no disclosures. Several of his coauthors reported ties to industry.
Serum 25-hydroxyviatmin D (25-OH D) deficiency was associated with greater risk for adverse pathology in men with localized prostate cancer undergoing radical prostatectomy, particularly among men with intermediate-risk disease.
Men with adverse pathology at radical prostatectomy had lower median serum 25-OH D (22.7 ng/mL; interquartile range [IQR], 15.9 to 29.0) compared with their counterparts (27.0 ng/mL; IQR, 20.0 to 34.0; P = .007), and they were more likely to have a serum 25-OH D level less than 30 ng/mL (80.5% v. 57.3%; P = .001) (J Clin Oncol. 2016 Feb. 22. doi: 10.1200/JCO.2015.65.1463).
Multiple logistic regression showed that serum 25-OH D level less than 30 ng/mL was associated with adverse pathology independent of age, serum PSA, and abnormal findings on digital rectal examination (odds ratio [OR], 2.51; 95% CI, 1.18 to 5.33; P = .02). Stratified analysis showed that in men with National Comprehensive Cancer Network intermediate prostate cancer at diagnosis, serum 25-OH D less than 30 ng/mL was significantly associated with adverse pathology (OR, 3.62; 95% CI, 1.15 to 11.46; P = .03).
The study cohort from Chicago demonstrated a disparity in vitamin D levels between black men and white men, which may contribute to differences by race observed in adverse pathology at radical prostatectomy.
“The initial assessment of our Chicago cohort demonstrated a significant disparity regarding low vitamin D levels and black race compared with white men in both univariate and multivariate analyses. In fact, greater than 90% of black men in that study had vitamin D levels less than 30 ng/mL,” wrote Dr Yaw Nyame of the Glickman Urological & Kidney Institute, Cleveland Clinic, and colleagues.
The cross-sectional, observational study from 2009 to 2014 included 190 men who underwent radical prostatectomy for localized prostate cancer. Adverse pathology was defined by the presence of dominant Gleason pattern 4, the presence of any pattern 5, and pathologic stage pT3aN0M0 or higher.
In total, 87 men (45.8%) had adverse pathology at radical prostatectomy. The median age of men with adverse pathology was 65.0 years, compared with 62.0 years for their counterparts (P = .005); median BMI was 28.9 kg/m2 vs. 27.7 kg/m2 (P = .04); and serum PSA was 6.8 ng/mL vs. 4.4 ng/mL (P less than .001). Men with adverse pathology at radical prostatectomy were more likely to self identify as black (P = .03).
Studies have shown that prostate cancer cells express the vitamin D receptor, and vitamin D has been shown to inhibit cellular proliferation, differentiation, and apoptosis.
Dr. Yaw Nyame reported having no disclosures. Several of his coauthors reported ties to industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Serum 25-hydroxyvitamin D (25-OH D) deficiency was associated with greater risk for adverse pathology in men with localized prostate cancer undergoing radical prostatectomy.
Major finding: Men with adverse pathology at radical prostatectomy were more likely to have a serum 25-OH D level less than 30 ng/mL (80.5% v. 57.3%; P = .001).
Data source: Cross-sectional, observational study of 190 men who underwent radical prostatectomy for localized prostate cancer.
Disclosures: Dr. Yaw Nyame reported having no disclosures. Several of his coauthors reported ties to industry.
SIRT plus FOLFOX chemo for mCRC improved progression-free survival in liver only
The addition of selective internal radiation therapy (SIRT) to FOLFOX-based first-line chemotherapy in patients with metastatic colorectal cancer (mCRC) failed to improve progression-free survival (PFS) at any site, but significantly improved median PFS in the liver, according to results of SIRFLOX, the first large, randomized controlled trial of liver-directed therapy in mCRC.
Median PFS at any site was similar for the SIRT and control arms at 10.7 and 10.2 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.77-1.12; P = .43). Median PFS in the liver was significantly greater in the SIRT than the control arm: 20.5 vs. 12.6 months (HR, 0.69; 95% CI, 0.55-0.90; P = .002) (J Clin Oncol. 2016 Feb 22. doi: 10.1200/JCO.2015.66.1181).
Fewer SIRT patients had the first disease progression occur in the liver (SIRT: 52.4%, control: 77%; P less than .001), and a correspondingly higher proportion of the SIRT arm had first progression outside the liver (SIRT: 27.7%, control 7.9%; P less than .001).
“Whereas control of intra- and extrahepatic disease is required to achieve a benefit in PFS at any site, the analysis of first progression site suggests that progressive disease in nonliver sites … may mitigate the benefit of controlling liver disease with SIRT. Furthermore, the appearance of new lesions accounted for a substantially greater proportion of first progressions in the liver in SIRT,” wrote Dr. Guy van Hazel of the University of Western Australia, and his colleagues.
To determine whether control of liver metastasis translates into survival gains, analysis of overall survival data from SIRFLOX combined with two other first-line studies are ongoing.
Only 84% of patients assigned to the SIRT arm received SIRT per protocol, which may have compromised the observed affect of SIRT on PFS at any site, according to investigators. In addition, a larger than expected number of patients (about 45%) had an intact primary tumor, which has an uncertain impact on PFS at any site but is reported to be associated with poorer survival.
Adverse events (AEs) were increased in the SIRT arm, both those associated with chemotherapy (for example, neutropenia, febrile neutropenia, and thrombocytopenia) and AEs associated with SIRT (for example, nausea, vomiting, abdominal pain, and fatigue).
In total, 530 chemotherapy-naïve patients with adenocarcinoma of the colon or rectum and proven liver metastases were enrolled in the randomized, controlled phase III SIRFLOX from 2006 to 2013. The control arm included 263 patients who received modified FOLFOX (mFOLFOX6), and the treatment arm included 267 patients who received mFOLFOX6 plus SIRT. Bevacizumab combined with mFOLFOX6 was allowed at the investigator’s discretion.*
*A change was made to this story on 2/25.
The addition of selective internal radiation therapy (SIRT) to FOLFOX-based first-line chemotherapy in patients with metastatic colorectal cancer (mCRC) failed to improve progression-free survival (PFS) at any site, but significantly improved median PFS in the liver, according to results of SIRFLOX, the first large, randomized controlled trial of liver-directed therapy in mCRC.
Median PFS at any site was similar for the SIRT and control arms at 10.7 and 10.2 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.77-1.12; P = .43). Median PFS in the liver was significantly greater in the SIRT than the control arm: 20.5 vs. 12.6 months (HR, 0.69; 95% CI, 0.55-0.90; P = .002) (J Clin Oncol. 2016 Feb 22. doi: 10.1200/JCO.2015.66.1181).
Fewer SIRT patients had the first disease progression occur in the liver (SIRT: 52.4%, control: 77%; P less than .001), and a correspondingly higher proportion of the SIRT arm had first progression outside the liver (SIRT: 27.7%, control 7.9%; P less than .001).
“Whereas control of intra- and extrahepatic disease is required to achieve a benefit in PFS at any site, the analysis of first progression site suggests that progressive disease in nonliver sites … may mitigate the benefit of controlling liver disease with SIRT. Furthermore, the appearance of new lesions accounted for a substantially greater proportion of first progressions in the liver in SIRT,” wrote Dr. Guy van Hazel of the University of Western Australia, and his colleagues.
To determine whether control of liver metastasis translates into survival gains, analysis of overall survival data from SIRFLOX combined with two other first-line studies are ongoing.
Only 84% of patients assigned to the SIRT arm received SIRT per protocol, which may have compromised the observed affect of SIRT on PFS at any site, according to investigators. In addition, a larger than expected number of patients (about 45%) had an intact primary tumor, which has an uncertain impact on PFS at any site but is reported to be associated with poorer survival.
Adverse events (AEs) were increased in the SIRT arm, both those associated with chemotherapy (for example, neutropenia, febrile neutropenia, and thrombocytopenia) and AEs associated with SIRT (for example, nausea, vomiting, abdominal pain, and fatigue).
In total, 530 chemotherapy-naïve patients with adenocarcinoma of the colon or rectum and proven liver metastases were enrolled in the randomized, controlled phase III SIRFLOX from 2006 to 2013. The control arm included 263 patients who received modified FOLFOX (mFOLFOX6), and the treatment arm included 267 patients who received mFOLFOX6 plus SIRT. Bevacizumab combined with mFOLFOX6 was allowed at the investigator’s discretion.*
*A change was made to this story on 2/25.
The addition of selective internal radiation therapy (SIRT) to FOLFOX-based first-line chemotherapy in patients with metastatic colorectal cancer (mCRC) failed to improve progression-free survival (PFS) at any site, but significantly improved median PFS in the liver, according to results of SIRFLOX, the first large, randomized controlled trial of liver-directed therapy in mCRC.
Median PFS at any site was similar for the SIRT and control arms at 10.7 and 10.2 months, respectively (hazard ratio, 0.93; 95% confidence interval, 0.77-1.12; P = .43). Median PFS in the liver was significantly greater in the SIRT than the control arm: 20.5 vs. 12.6 months (HR, 0.69; 95% CI, 0.55-0.90; P = .002) (J Clin Oncol. 2016 Feb 22. doi: 10.1200/JCO.2015.66.1181).
Fewer SIRT patients had the first disease progression occur in the liver (SIRT: 52.4%, control: 77%; P less than .001), and a correspondingly higher proportion of the SIRT arm had first progression outside the liver (SIRT: 27.7%, control 7.9%; P less than .001).
“Whereas control of intra- and extrahepatic disease is required to achieve a benefit in PFS at any site, the analysis of first progression site suggests that progressive disease in nonliver sites … may mitigate the benefit of controlling liver disease with SIRT. Furthermore, the appearance of new lesions accounted for a substantially greater proportion of first progressions in the liver in SIRT,” wrote Dr. Guy van Hazel of the University of Western Australia, and his colleagues.
To determine whether control of liver metastasis translates into survival gains, analysis of overall survival data from SIRFLOX combined with two other first-line studies are ongoing.
Only 84% of patients assigned to the SIRT arm received SIRT per protocol, which may have compromised the observed affect of SIRT on PFS at any site, according to investigators. In addition, a larger than expected number of patients (about 45%) had an intact primary tumor, which has an uncertain impact on PFS at any site but is reported to be associated with poorer survival.
Adverse events (AEs) were increased in the SIRT arm, both those associated with chemotherapy (for example, neutropenia, febrile neutropenia, and thrombocytopenia) and AEs associated with SIRT (for example, nausea, vomiting, abdominal pain, and fatigue).
In total, 530 chemotherapy-naïve patients with adenocarcinoma of the colon or rectum and proven liver metastases were enrolled in the randomized, controlled phase III SIRFLOX from 2006 to 2013. The control arm included 263 patients who received modified FOLFOX (mFOLFOX6), and the treatment arm included 267 patients who received mFOLFOX6 plus SIRT. Bevacizumab combined with mFOLFOX6 was allowed at the investigator’s discretion.*
*A change was made to this story on 2/25.
Key clinical point: Selective internal radiation therapy (SIRT) combined with FOLFOX-based chemotherapy in patients with metastatic colorectal cancer failed to improve progression-free survival (PFS) at any site, but significantly improved median PFS in the liver.
Major finding: Median PFS at any site for the SIRT and control arms were 10.7 and 10.2 months, respectively (HR, 0.93; 95% CI, 0.77-1.12; P = .43); median PFS in the liver for the SIRT and control arms were 20.5 and 12.6 months, respectively (HR, 0.69; 95% CI, 0.55-0.90; P = .002).
Data source: From 2006 to 2013, the randomized phase III SIRFLOX trial assigned 263 patients to control and 267 to SIRT.
Disclosures: Dr. van Hazel reported financial ties to Sirtex, Roche, Merck, and Boehringer Ingelheim. Several of his coauthors reported ties to industry.
Muscle loss during chemotherapy predicts poor survival in metastatic colorectal cancer
Among patients with metastatic colorectal cancer, muscle loss of 9% or greater during chemotherapy predicted significantly worse survival than did loss of less than 9%, independent of known prognostic covariates.
The proportion of patients with muscle loss of 9% or more (lowest tertile) who survived 6 months was 33%, compared with 69% for patients with less muscle loss (tertiles 2 and 3). The proportion of those who survived 1 year were 17% vs. 49% (log-rank P = .001). The association remained significant when adjusted for sex, age, baseline LDH concentration, comorbidity, mono-organ or multiorgan metastases, treatment line, and tumor progression (hazard ratio [HR], 4.47; 95% CI, 2.21 to 9.05; P less than .01).
“Muscle loss during chemotherapy was associated with worse overall survival, independent of important prognostic covariates. This is a new finding and raises the question whether interventions aimed at preservation of muscle mass during treatment are effective in improving outcome,” wrote Dr. Susanne Blauwhoff-Buskermolen of VU University Medical Center, Amsterdam, and colleagues (J Clin Oncol. 2016 Feb 22. doi: 10.1200/JCO.2015.63.6043).
Previous studies demonstrated that low muscle mass is associated with poor survival and greater treatment toxicity. The current study examined the effect of muscle mass change during chemotherapy. Low muscle density at baseline was significantly associated with shorter survival (HR, 2.38; 95% CI, 1.16 to 4.87; P = .018), but low skeletal muscle index was not.
The prospective study evaluated muscle mass change in 67 patients with mCRC via single computed tomography images of the abdominal region (L3). The mean age of patients was 66.4 years; 82% had multiorgan metastases, and 78% received first-line chemotherapy. At baseline, 55% of patients were overweight, 8% were obese, and 57% had low skeletal muscle index.
Toxicity-related treatment modifications occurred in 29 patients (43%), but in contrast to previous studies, no association was observed between change in muscle mass and treatment modification. Heterogeneity in treatment regimens represented (six in total) may account for the lack of association between muscle mass change and treatment modification.
Dr. Susanne Blauwhoff-Buskermolen reported having no disclosures. Several of her coauthors reported ties to industry.
Among patients with metastatic colorectal cancer, muscle loss of 9% or greater during chemotherapy predicted significantly worse survival than did loss of less than 9%, independent of known prognostic covariates.
The proportion of patients with muscle loss of 9% or more (lowest tertile) who survived 6 months was 33%, compared with 69% for patients with less muscle loss (tertiles 2 and 3). The proportion of those who survived 1 year were 17% vs. 49% (log-rank P = .001). The association remained significant when adjusted for sex, age, baseline LDH concentration, comorbidity, mono-organ or multiorgan metastases, treatment line, and tumor progression (hazard ratio [HR], 4.47; 95% CI, 2.21 to 9.05; P less than .01).
“Muscle loss during chemotherapy was associated with worse overall survival, independent of important prognostic covariates. This is a new finding and raises the question whether interventions aimed at preservation of muscle mass during treatment are effective in improving outcome,” wrote Dr. Susanne Blauwhoff-Buskermolen of VU University Medical Center, Amsterdam, and colleagues (J Clin Oncol. 2016 Feb 22. doi: 10.1200/JCO.2015.63.6043).
Previous studies demonstrated that low muscle mass is associated with poor survival and greater treatment toxicity. The current study examined the effect of muscle mass change during chemotherapy. Low muscle density at baseline was significantly associated with shorter survival (HR, 2.38; 95% CI, 1.16 to 4.87; P = .018), but low skeletal muscle index was not.
The prospective study evaluated muscle mass change in 67 patients with mCRC via single computed tomography images of the abdominal region (L3). The mean age of patients was 66.4 years; 82% had multiorgan metastases, and 78% received first-line chemotherapy. At baseline, 55% of patients were overweight, 8% were obese, and 57% had low skeletal muscle index.
Toxicity-related treatment modifications occurred in 29 patients (43%), but in contrast to previous studies, no association was observed between change in muscle mass and treatment modification. Heterogeneity in treatment regimens represented (six in total) may account for the lack of association between muscle mass change and treatment modification.
Dr. Susanne Blauwhoff-Buskermolen reported having no disclosures. Several of her coauthors reported ties to industry.
Among patients with metastatic colorectal cancer, muscle loss of 9% or greater during chemotherapy predicted significantly worse survival than did loss of less than 9%, independent of known prognostic covariates.
The proportion of patients with muscle loss of 9% or more (lowest tertile) who survived 6 months was 33%, compared with 69% for patients with less muscle loss (tertiles 2 and 3). The proportion of those who survived 1 year were 17% vs. 49% (log-rank P = .001). The association remained significant when adjusted for sex, age, baseline LDH concentration, comorbidity, mono-organ or multiorgan metastases, treatment line, and tumor progression (hazard ratio [HR], 4.47; 95% CI, 2.21 to 9.05; P less than .01).
“Muscle loss during chemotherapy was associated with worse overall survival, independent of important prognostic covariates. This is a new finding and raises the question whether interventions aimed at preservation of muscle mass during treatment are effective in improving outcome,” wrote Dr. Susanne Blauwhoff-Buskermolen of VU University Medical Center, Amsterdam, and colleagues (J Clin Oncol. 2016 Feb 22. doi: 10.1200/JCO.2015.63.6043).
Previous studies demonstrated that low muscle mass is associated with poor survival and greater treatment toxicity. The current study examined the effect of muscle mass change during chemotherapy. Low muscle density at baseline was significantly associated with shorter survival (HR, 2.38; 95% CI, 1.16 to 4.87; P = .018), but low skeletal muscle index was not.
The prospective study evaluated muscle mass change in 67 patients with mCRC via single computed tomography images of the abdominal region (L3). The mean age of patients was 66.4 years; 82% had multiorgan metastases, and 78% received first-line chemotherapy. At baseline, 55% of patients were overweight, 8% were obese, and 57% had low skeletal muscle index.
Toxicity-related treatment modifications occurred in 29 patients (43%), but in contrast to previous studies, no association was observed between change in muscle mass and treatment modification. Heterogeneity in treatment regimens represented (six in total) may account for the lack of association between muscle mass change and treatment modification.
Dr. Susanne Blauwhoff-Buskermolen reported having no disclosures. Several of her coauthors reported ties to industry.
Key clinical point: Patients with metastatic colorectal cancer (mCRC) whose muscle mass dropped by 9% or more during chemotherapy had significantly worse survival than did those with less muscle loss.
Major finding: The proportion of patients with muscle loss of 9% or more who survived 6 months was 33%, compared with 69% of those with less muscle loss; 1-year survival was 17% vs. 49% (log-rank P = .001).
Data source: The prospective study evaluated muscle mass change in 67 patients with mCRC via single computed tomography (CT) images of the abdominal region (L3).
Disclosures: Dr. Susanne Blauwhoff-Buskermolen reported having no disclosures. Several of her coauthors reported ties to industry.
Ponatinib effective in chronic phase CML regardless of baseline mutation status
In heavily pretreated patients with chronic phase chronic myeloid leukemia (CP-CML), response to the tyrosine kinase inhibitor ponatinib did not depend on baseline mutation status, and no single or compound mutation was a major driver of primary or secondary resistance to ponatinib, according to researchers.
Irrespective of baseline mutation status, responses to ponatinib were durable. As determined by next-generation sequencing (NGS), patients with zero, one, or two or more BCR-ABL1 mutations had rates of 50%-61% for major cytogenetic response (MCyR) by 1 year and 29%-45% for major molecular response (MMR) at any time. The rates were similar to those observed with mutation status determined by Sanger sequencing. Rates of sustained response at 2 years for MCyR and MMR were 87% and 65%, respectively (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-08-660977).
Sanger sequencing typically is used to identify BCR-ABL1 mutations associated with tyrosine kinase inhibitor (TKI) resistance, but the method fails to detect low-level mutations that occur in less than 10%-20% of cells. Researchers used NGS to determine the impact of low-level mutations, as well as compound mutations, on the efficacy of the third generation TKI ponatinib.
Ponatinib is the most potent BCR-ABL1 TKI but is associated with considerable cardiovascular toxicity.
“The role of NGS in this setting may be to identify patients with (low level) T315I who are unlikely to derive lasting benefit from second-generation TKIs, but have a high likelihood of achieving durable cytogenetic and molecular responses to ponatinib, an important factor for balancing risks and benefits of salvage therapy selection,” wrote Dr. Michael W. Deininger, Chief of Hematology at the Huntsman Cancer Institute at the University of Utah, Salt Lake City, and his colleagues.
Patients with low-level mutations had similar response rates to those with no mutations: MCyR by one year and MMR at any time were 43% and 31%, respectively, compared with 50% and 29%. Response rates were higher in patients with compound mutations (64% and 52%) or one or more mutation (57% and 64%). The researchers speculated that the lower response rates in patients with low level or no mutations may reflect resistance mechanisms independent of BCR-ABL1.
Analysis of postbaseline samples from 127 patients (24 of whom had discontinued ponatinib for at least 1 month) determined the impact of acquired resistance. At a median follow-up of 30.1 months, emergence of previously undetected single and compound mutants during ponatinib therapy was observed in 8 patients.
The study analyzed patients from the PACE trial who had CP-CML with resistance or intolerance to dasatinib or nilotinib, or with a T315I mutation, and who were treated with ponatinib. All 267 patients had baseline mutation status determined by Sanger sequencing, with 161 mutations detected in 131 patients. NGS identified these and 105 additional mutations. Consistent with greater sensitivity of NGS, the proportion of patients with no baseline mutations by 39% by NGS vs. 51% by Sanger sequencing, and the proportion with multiple mutations was 23% vs. 10%.
The study was funded by ARIAD Pharmaceuticals. Dr. Deininger reported financial ties to ARIAD, Bristol Myers-Squibb, Novartis, Celgene, Genzyme, Gilead, Incyte, and Pfizer. Several of his coauthors reported ties to industry.
In heavily pretreated patients with chronic phase chronic myeloid leukemia (CP-CML), response to the tyrosine kinase inhibitor ponatinib did not depend on baseline mutation status, and no single or compound mutation was a major driver of primary or secondary resistance to ponatinib, according to researchers.
Irrespective of baseline mutation status, responses to ponatinib were durable. As determined by next-generation sequencing (NGS), patients with zero, one, or two or more BCR-ABL1 mutations had rates of 50%-61% for major cytogenetic response (MCyR) by 1 year and 29%-45% for major molecular response (MMR) at any time. The rates were similar to those observed with mutation status determined by Sanger sequencing. Rates of sustained response at 2 years for MCyR and MMR were 87% and 65%, respectively (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-08-660977).
Sanger sequencing typically is used to identify BCR-ABL1 mutations associated with tyrosine kinase inhibitor (TKI) resistance, but the method fails to detect low-level mutations that occur in less than 10%-20% of cells. Researchers used NGS to determine the impact of low-level mutations, as well as compound mutations, on the efficacy of the third generation TKI ponatinib.
Ponatinib is the most potent BCR-ABL1 TKI but is associated with considerable cardiovascular toxicity.
“The role of NGS in this setting may be to identify patients with (low level) T315I who are unlikely to derive lasting benefit from second-generation TKIs, but have a high likelihood of achieving durable cytogenetic and molecular responses to ponatinib, an important factor for balancing risks and benefits of salvage therapy selection,” wrote Dr. Michael W. Deininger, Chief of Hematology at the Huntsman Cancer Institute at the University of Utah, Salt Lake City, and his colleagues.
Patients with low-level mutations had similar response rates to those with no mutations: MCyR by one year and MMR at any time were 43% and 31%, respectively, compared with 50% and 29%. Response rates were higher in patients with compound mutations (64% and 52%) or one or more mutation (57% and 64%). The researchers speculated that the lower response rates in patients with low level or no mutations may reflect resistance mechanisms independent of BCR-ABL1.
Analysis of postbaseline samples from 127 patients (24 of whom had discontinued ponatinib for at least 1 month) determined the impact of acquired resistance. At a median follow-up of 30.1 months, emergence of previously undetected single and compound mutants during ponatinib therapy was observed in 8 patients.
The study analyzed patients from the PACE trial who had CP-CML with resistance or intolerance to dasatinib or nilotinib, or with a T315I mutation, and who were treated with ponatinib. All 267 patients had baseline mutation status determined by Sanger sequencing, with 161 mutations detected in 131 patients. NGS identified these and 105 additional mutations. Consistent with greater sensitivity of NGS, the proportion of patients with no baseline mutations by 39% by NGS vs. 51% by Sanger sequencing, and the proportion with multiple mutations was 23% vs. 10%.
The study was funded by ARIAD Pharmaceuticals. Dr. Deininger reported financial ties to ARIAD, Bristol Myers-Squibb, Novartis, Celgene, Genzyme, Gilead, Incyte, and Pfizer. Several of his coauthors reported ties to industry.
In heavily pretreated patients with chronic phase chronic myeloid leukemia (CP-CML), response to the tyrosine kinase inhibitor ponatinib did not depend on baseline mutation status, and no single or compound mutation was a major driver of primary or secondary resistance to ponatinib, according to researchers.
Irrespective of baseline mutation status, responses to ponatinib were durable. As determined by next-generation sequencing (NGS), patients with zero, one, or two or more BCR-ABL1 mutations had rates of 50%-61% for major cytogenetic response (MCyR) by 1 year and 29%-45% for major molecular response (MMR) at any time. The rates were similar to those observed with mutation status determined by Sanger sequencing. Rates of sustained response at 2 years for MCyR and MMR were 87% and 65%, respectively (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-08-660977).
Sanger sequencing typically is used to identify BCR-ABL1 mutations associated with tyrosine kinase inhibitor (TKI) resistance, but the method fails to detect low-level mutations that occur in less than 10%-20% of cells. Researchers used NGS to determine the impact of low-level mutations, as well as compound mutations, on the efficacy of the third generation TKI ponatinib.
Ponatinib is the most potent BCR-ABL1 TKI but is associated with considerable cardiovascular toxicity.
“The role of NGS in this setting may be to identify patients with (low level) T315I who are unlikely to derive lasting benefit from second-generation TKIs, but have a high likelihood of achieving durable cytogenetic and molecular responses to ponatinib, an important factor for balancing risks and benefits of salvage therapy selection,” wrote Dr. Michael W. Deininger, Chief of Hematology at the Huntsman Cancer Institute at the University of Utah, Salt Lake City, and his colleagues.
Patients with low-level mutations had similar response rates to those with no mutations: MCyR by one year and MMR at any time were 43% and 31%, respectively, compared with 50% and 29%. Response rates were higher in patients with compound mutations (64% and 52%) or one or more mutation (57% and 64%). The researchers speculated that the lower response rates in patients with low level or no mutations may reflect resistance mechanisms independent of BCR-ABL1.
Analysis of postbaseline samples from 127 patients (24 of whom had discontinued ponatinib for at least 1 month) determined the impact of acquired resistance. At a median follow-up of 30.1 months, emergence of previously undetected single and compound mutants during ponatinib therapy was observed in 8 patients.
The study analyzed patients from the PACE trial who had CP-CML with resistance or intolerance to dasatinib or nilotinib, or with a T315I mutation, and who were treated with ponatinib. All 267 patients had baseline mutation status determined by Sanger sequencing, with 161 mutations detected in 131 patients. NGS identified these and 105 additional mutations. Consistent with greater sensitivity of NGS, the proportion of patients with no baseline mutations by 39% by NGS vs. 51% by Sanger sequencing, and the proportion with multiple mutations was 23% vs. 10%.
The study was funded by ARIAD Pharmaceuticals. Dr. Deininger reported financial ties to ARIAD, Bristol Myers-Squibb, Novartis, Celgene, Genzyme, Gilead, Incyte, and Pfizer. Several of his coauthors reported ties to industry.
FROM BLOOD
Key clinical point: Baseline mutation status had little impact on ponatinib response, and no single or compound mutation was a major driver of primary or secondary resistance to ponatinib in patients with chronic phase chronic myeloid leukemia (CP-CML).
Major finding: In patients with zero, one, or two or more BCR-ABL1 mutations at baseline by next-generation sequencing were 50%-61% for major cytogenetic response (MCyR) by 1 year and 29%-45% for major molecular response (MMR) at any time; rates of sustained response at 2 years for MCyR and MMR were 87% and 65%, respectively.
Data source: From the PACE trial, 267 patients with CP-CML with resistance or intolerance to dasatinib or nilotinib, or with a T315I mutation, were treated with ponatinib.
Disclosures: The study was funded by ARIAD Pharmaceuticals. Dr. Deininger reported financial ties to ARIAD, Bristol Myers-Squibb, Novartis, Celgene, Genzyme, Gilead, Incyte, and Pfizer. Several of his coauthors reported ties to industry.
Panobinostat plus bortezomib and dexamethasone improved outcomes in previously treated multiple myeloma
The addition of panobinostat to bortezomib and dexamethasone (PAN-BTZ-Dex) improved outcomes in patients with multiple myeloma who had received prior treatment with immunomodulatory drugs (IMiDs), prior bortezomib plus an IMiD, and two or more prior regimens including bortezomib and an IMiD.
Subgroup analysis of the PANORAMA phase III trial of patients with relapsed or relapsed and refractory multiple myeloma (MM) reported median progression-free survival (PFS) with PAN-BTZ-Dex versus placebo-BTZ-Dex (Pbo-BTZ-Dex) in groups defined by prior treatment: prior IMiD (12.3 vs. 7.4 months; hazard ratio, 0.54; 95% confidence interval, 0.43-0.68), prior bortezomib plus IMiD (10.6 vs. 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and two or more prior regimens including bortezomib and an IMiD (12.5 vs. 4.7 months; HR, 0.47; 95% CI, 0.31-0.72) (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-09-665018).
The greatest difference in median PFS between the panobinostat and placebo arms (7.8 months) was observed among patients who had received two or more prior regimens including bortezomib and an IMiD, a population with a poorer prognosis and an urgent unmet need, according to investigators.
“Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action. Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance,” wrote Dr. Paul G. Richardson of the Dana Farber Cancer Institute, Boston, and his colleagues, adding that since the deacetylase inhibitor “acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.”
The analysis examined treatment outcomes from the PANORAMA trial, including 485 patients who had received prior IMiD (63% of total population), 193 who received prior bortezomib plus IMiD (25% of total population), and 147 who received two or more prior regimens including bortezomib and an IMiD (19% of total population).
Common adverse events (AEs) by treatment subgroups were similar to those of the overall trial population, which were increased in the PAN-BTZ-Dex compared with the placebo arm. The most common nonhematologic AE was diarrhea and the most common hematologic abnormality was thrombocytopenia.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
The addition of panobinostat to bortezomib and dexamethasone (PAN-BTZ-Dex) improved outcomes in patients with multiple myeloma who had received prior treatment with immunomodulatory drugs (IMiDs), prior bortezomib plus an IMiD, and two or more prior regimens including bortezomib and an IMiD.
Subgroup analysis of the PANORAMA phase III trial of patients with relapsed or relapsed and refractory multiple myeloma (MM) reported median progression-free survival (PFS) with PAN-BTZ-Dex versus placebo-BTZ-Dex (Pbo-BTZ-Dex) in groups defined by prior treatment: prior IMiD (12.3 vs. 7.4 months; hazard ratio, 0.54; 95% confidence interval, 0.43-0.68), prior bortezomib plus IMiD (10.6 vs. 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and two or more prior regimens including bortezomib and an IMiD (12.5 vs. 4.7 months; HR, 0.47; 95% CI, 0.31-0.72) (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-09-665018).
The greatest difference in median PFS between the panobinostat and placebo arms (7.8 months) was observed among patients who had received two or more prior regimens including bortezomib and an IMiD, a population with a poorer prognosis and an urgent unmet need, according to investigators.
“Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action. Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance,” wrote Dr. Paul G. Richardson of the Dana Farber Cancer Institute, Boston, and his colleagues, adding that since the deacetylase inhibitor “acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.”
The analysis examined treatment outcomes from the PANORAMA trial, including 485 patients who had received prior IMiD (63% of total population), 193 who received prior bortezomib plus IMiD (25% of total population), and 147 who received two or more prior regimens including bortezomib and an IMiD (19% of total population).
Common adverse events (AEs) by treatment subgroups were similar to those of the overall trial population, which were increased in the PAN-BTZ-Dex compared with the placebo arm. The most common nonhematologic AE was diarrhea and the most common hematologic abnormality was thrombocytopenia.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
The addition of panobinostat to bortezomib and dexamethasone (PAN-BTZ-Dex) improved outcomes in patients with multiple myeloma who had received prior treatment with immunomodulatory drugs (IMiDs), prior bortezomib plus an IMiD, and two or more prior regimens including bortezomib and an IMiD.
Subgroup analysis of the PANORAMA phase III trial of patients with relapsed or relapsed and refractory multiple myeloma (MM) reported median progression-free survival (PFS) with PAN-BTZ-Dex versus placebo-BTZ-Dex (Pbo-BTZ-Dex) in groups defined by prior treatment: prior IMiD (12.3 vs. 7.4 months; hazard ratio, 0.54; 95% confidence interval, 0.43-0.68), prior bortezomib plus IMiD (10.6 vs. 5.8 months; HR, 0.52; 95% CI, 0.36-0.76), and two or more prior regimens including bortezomib and an IMiD (12.5 vs. 4.7 months; HR, 0.47; 95% CI, 0.31-0.72) (Blood. 2016 Feb 11. doi: 10.1182/blood-2015-09-665018).
The greatest difference in median PFS between the panobinostat and placebo arms (7.8 months) was observed among patients who had received two or more prior regimens including bortezomib and an IMiD, a population with a poorer prognosis and an urgent unmet need, according to investigators.
“Panobinostat represents a novel addition to the MM treatment armamentarium by introducing an agent with a novel mechanism of action. Novel agents are needed to address the ongoing unmet need in patients who progress on bortezomib and IMiDs as a strategy to overcome therapeutic resistance,” wrote Dr. Paul G. Richardson of the Dana Farber Cancer Institute, Boston, and his colleagues, adding that since the deacetylase inhibitor “acts on distinct epigenetic and protein metabolism pathways, it is uniquely suited to provide benefit in patients previously treated with proteasome inhibitors and/or IMiDs.”
The analysis examined treatment outcomes from the PANORAMA trial, including 485 patients who had received prior IMiD (63% of total population), 193 who received prior bortezomib plus IMiD (25% of total population), and 147 who received two or more prior regimens including bortezomib and an IMiD (19% of total population).
Common adverse events (AEs) by treatment subgroups were similar to those of the overall trial population, which were increased in the PAN-BTZ-Dex compared with the placebo arm. The most common nonhematologic AE was diarrhea and the most common hematologic abnormality was thrombocytopenia.
The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
FROM BLOOD
Key clinical point: Panobinostat plus bortezomib and dexamethasone improved outcomes in patients with previously treated multiple myeloma, particularly those with two or more prior regimens including immunomodulatory drugs (IMiDs) and bortezomib.
Major finding: In patients with two or more prior regimens including IMiDs and bortezomib, progression-free survival for the panobinostat plus bortezomib and dexamethasone arm, compared with placebo plus bortezomib and dexamethasone arm, was 12.5 months (95% CI, 7.3-14.0) vs. 4.7 months (95% CI, 3.7-6.1); hazard ratio, 0.47 (95% CI, 0.31-0.72).
Data source: Subgroup analysis of the phase III PANORAMA trial evaluating panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma.
Disclosures: The PANORAMA 1 study was funded by Novartis Pharmaceuticals. Dr. Richardson reported having no disclosures. Several of his coauthors reported ties to industry.
Breast cancer patients at increased risk of colorectal cancer
Women previously diagnosed with breast cancer had a 60% increased risk of colorectal cancer, according to analysis of Swedish cancer registries, and breast cancer therapy had no effect on risk.
Women previously diagnosed with breast cancer had a higher risk of colorectal adenocarcinoma, compared with the general population (standardized incidence ratio, 1.59; 95% confidence interval, 1.53-1.65). Higher risk was observed for adenocarcinoma of the proximal colon, compared with the distal colon (SIR, 1.72; 95% CI, 1.61-1.82 vs. SIR, 1.46; 95% CI, 1.34-1.58).
Risk for adenocarcinoma of the proximal colon, the predominant subtype in women, increased with age at breast cancer diagnosis, from an SIR of 1.60 for women diagnosed between the ages of 15 and 49 years, to an SIR of 1.51 for 50-59 years, to an SIR of 1.82 for age 60 years or greater. By contrast, the SIR for general colorectal adenocarcinoma dipped in the 50-59 age group, a finding that may point to the role of estrogen in colorectal carcinoma (Cancer Epidemiol. 2016 Feb 15. doi: 10.1016/j.canep.2016.01.006).
“It is well known that estrogen levels are elevated in breast cancer patients, which may contribute to the increased risk of colorectal cancer in this population. Interestingly, our results showed that the SIRs of colorectal adenocarcinoma changed in different age groups, compared with the general population. Specifically, the SIRs showed a drop in the 50-59 age group but increased again after the age of 60,” wrote Dr. Yunxia Lu, associate professor in the department of molecular medicine and surgery, Karolinska Institute, Stockholm, and colleagues. The researchers noted previous reports of endogenous estrogen levels decreasing after menopause for a short period, while exogenous hormones may take effect some time after menopause.
The study found no association between different types of breast cancer treatment, including antihormonal therapy, and the risk of colorectal cancer.
The retrospective analysis used data from the Swedish Cancer Register, including 179,733 patients diagnosed with breast cancer from 1961 to 2010, and 2,571 colorectal cancers identified. Analysis of treatment effects on colorectal cancer risk used data from the Stockholm-Gotland Breast Cancer Register of 20,171 patients with breast cancer and relevant treatment information.
Dr. Lu and coauthors reported having no disclosures.
Women previously diagnosed with breast cancer had a 60% increased risk of colorectal cancer, according to analysis of Swedish cancer registries, and breast cancer therapy had no effect on risk.
Women previously diagnosed with breast cancer had a higher risk of colorectal adenocarcinoma, compared with the general population (standardized incidence ratio, 1.59; 95% confidence interval, 1.53-1.65). Higher risk was observed for adenocarcinoma of the proximal colon, compared with the distal colon (SIR, 1.72; 95% CI, 1.61-1.82 vs. SIR, 1.46; 95% CI, 1.34-1.58).
Risk for adenocarcinoma of the proximal colon, the predominant subtype in women, increased with age at breast cancer diagnosis, from an SIR of 1.60 for women diagnosed between the ages of 15 and 49 years, to an SIR of 1.51 for 50-59 years, to an SIR of 1.82 for age 60 years or greater. By contrast, the SIR for general colorectal adenocarcinoma dipped in the 50-59 age group, a finding that may point to the role of estrogen in colorectal carcinoma (Cancer Epidemiol. 2016 Feb 15. doi: 10.1016/j.canep.2016.01.006).
“It is well known that estrogen levels are elevated in breast cancer patients, which may contribute to the increased risk of colorectal cancer in this population. Interestingly, our results showed that the SIRs of colorectal adenocarcinoma changed in different age groups, compared with the general population. Specifically, the SIRs showed a drop in the 50-59 age group but increased again after the age of 60,” wrote Dr. Yunxia Lu, associate professor in the department of molecular medicine and surgery, Karolinska Institute, Stockholm, and colleagues. The researchers noted previous reports of endogenous estrogen levels decreasing after menopause for a short period, while exogenous hormones may take effect some time after menopause.
The study found no association between different types of breast cancer treatment, including antihormonal therapy, and the risk of colorectal cancer.
The retrospective analysis used data from the Swedish Cancer Register, including 179,733 patients diagnosed with breast cancer from 1961 to 2010, and 2,571 colorectal cancers identified. Analysis of treatment effects on colorectal cancer risk used data from the Stockholm-Gotland Breast Cancer Register of 20,171 patients with breast cancer and relevant treatment information.
Dr. Lu and coauthors reported having no disclosures.
Women previously diagnosed with breast cancer had a 60% increased risk of colorectal cancer, according to analysis of Swedish cancer registries, and breast cancer therapy had no effect on risk.
Women previously diagnosed with breast cancer had a higher risk of colorectal adenocarcinoma, compared with the general population (standardized incidence ratio, 1.59; 95% confidence interval, 1.53-1.65). Higher risk was observed for adenocarcinoma of the proximal colon, compared with the distal colon (SIR, 1.72; 95% CI, 1.61-1.82 vs. SIR, 1.46; 95% CI, 1.34-1.58).
Risk for adenocarcinoma of the proximal colon, the predominant subtype in women, increased with age at breast cancer diagnosis, from an SIR of 1.60 for women diagnosed between the ages of 15 and 49 years, to an SIR of 1.51 for 50-59 years, to an SIR of 1.82 for age 60 years or greater. By contrast, the SIR for general colorectal adenocarcinoma dipped in the 50-59 age group, a finding that may point to the role of estrogen in colorectal carcinoma (Cancer Epidemiol. 2016 Feb 15. doi: 10.1016/j.canep.2016.01.006).
“It is well known that estrogen levels are elevated in breast cancer patients, which may contribute to the increased risk of colorectal cancer in this population. Interestingly, our results showed that the SIRs of colorectal adenocarcinoma changed in different age groups, compared with the general population. Specifically, the SIRs showed a drop in the 50-59 age group but increased again after the age of 60,” wrote Dr. Yunxia Lu, associate professor in the department of molecular medicine and surgery, Karolinska Institute, Stockholm, and colleagues. The researchers noted previous reports of endogenous estrogen levels decreasing after menopause for a short period, while exogenous hormones may take effect some time after menopause.
The study found no association between different types of breast cancer treatment, including antihormonal therapy, and the risk of colorectal cancer.
The retrospective analysis used data from the Swedish Cancer Register, including 179,733 patients diagnosed with breast cancer from 1961 to 2010, and 2,571 colorectal cancers identified. Analysis of treatment effects on colorectal cancer risk used data from the Stockholm-Gotland Breast Cancer Register of 20,171 patients with breast cancer and relevant treatment information.
Dr. Lu and coauthors reported having no disclosures.
FROM CANCER EPIDEMIOLOGY
Key clinical point: Colorectal cancer risk was significantly increased in patients previously diagnosed with breast cancer.
Major finding: Standardized incidence ratio for the breast cancer cohort compared with the general population was 1.59 (95% CI, 1.53-1.65).
Data source: The Swedish Cancer Register included 179,733 patients with breast cancer; the Stockholm-Gotland Breast Cancer Register included 20,171 patients with breast cancer and relevant treatment information.
Disclosures: Dr. Lu and coauthors reported having no disclosures.
Nanoparticles deliver Aurora kinase inhibitor with increased safety and efficacy
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
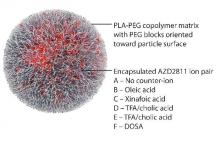
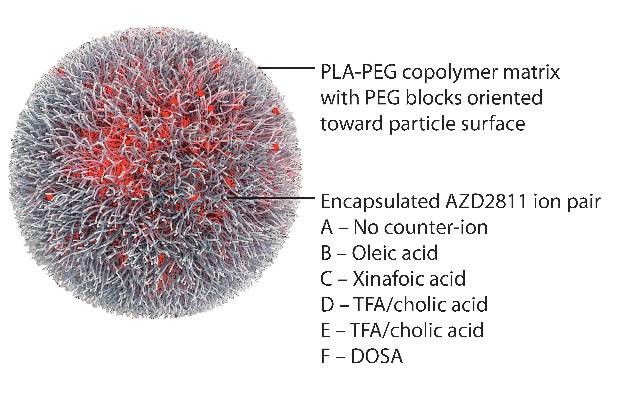
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Key clinical point: Aurora B kinase inhibitor nanoparticles displayed accumulation and retention in tumors with improved efficacy and minimal bone marrow pathology in animal models.
Major finding: Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity; the free drug was undetected in tumors 24 hours after administration, and nanoparticle-delivered drug was detectable up to 6 days.
Data sources: Nude rats and nude mice bearing human colorectal adenocarcinoma SW620 xenografts.
Disclosures: AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Lysolipid antigens prominent in MGUS and myeloma
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In patients with Gaucher disease and in nearly one-third of patients with MGUS or myeloma, clonal immunoglobulins reacted with lysolipid antigens.
Major finding: Clonal immunoglobulin from 17 of 20 patients with Gaucher disease and from 22 of 66 patients with sporadic myeloma were reactive to lyso-glucosylceramide.
Data source: Peripheral blood or bone marrow samples from 25 healthy donors, 20 patients with Gaucher disease, and 66 patients with sporadic monoclonal gammopathy.
Disclosures: Dr. Nair reported having no disclosures. One of her coauthors reported ties to industry.
Defibrotide offers benefit for severe veno-occlusive disease and multiorgan failure
Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Of the 102 patients in the defibrotide group, 39 were alive 100 days after HSCT (38.2%), compared with 8 of 32 (25.0%) in the historical control group. The propensity-adjusted, between-group difference was 23.0% (95.1% confidence interval, 5.2%-40.8%; P = .0109). At 180 days post-HSCT, the difference in survival between the groups was not significant (Blood. 2016 Feb 3. doi: 10.1182/blood-2015-10-676924).
Defibrotide has Fast Track designation from the FDA and the new drug application is currently under Priority Review with a decision expected by March 31, 2016. A potentially fatal complication of HSCT, hepatic veno-occlusive disease is characterized by hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites. There are no approved therapies.
“In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need,” wrote Dr. Paul G. Richardson of Dana-Farber Cancer Institute in Boston and his colleagues.
At day 100 post-HSCT, complete response was seen in 25.5% of the defibrotide group and in 12.5% of the historical control group. The propensity-adjusted, between-group difference was 19% (95.1% CI, 3.5-34.6%; P = .0160). The complete response was durable in 22 of the 26 patients. In the control group, complete response was limited in two patients, impossible to assess in one patient, and durable in one patient.
The multicenter, open-label, phase III trial prospectively enrolled 102 patients with hepatic veno-occlusive disease from 2006 to 2008. Defibrotide was administered intravenously at 25 mg/kg/day in 4 divided doses for a minimum of 21 days. Treatment continued beyond 21 days until resolution of veno-occlusive disease or until the patient was discharged from the hospital.
To identify the historical controls, 6,867 medical charts of HSCT patients hospitalized from 1995 to 2007 were reviewed, and 32 historical control patients were selected. Most (21 of 32) were diagnosed with during 2000-2006, and 11 were diagnosed before 2000. The historical controls were selected by an independent medical review committee, and met the same entry criteria as the defibrotide group.
Because recruiting for the defibrotide group and screening for historical controls occurred at the same institutions during similar time periods, patient management and supportive care were likely similar for the two groups. Propensity scores were included in the analysis to adjust for prognostic factors that were unbalanced between treatment and control groups, including ventilator and dialysis dependency at study entry, age greater or less than 16 years, prior HSCT (0 vs. 1), and allogeneic or autologous transplant.
Hypotension was the most common adverse event reported in the defibrotide and control groups (39% and 50%, respectively), followed by diarrhea (23.5% and 37.5%, respectively). The defibrotide and control groups had similar incidences of common hemorrhagic adverse events (64% and 75%, respectively). Fatal adverse events occurred in 64% of the defibrotide group and 69% of the control group, and fatal hemorrhagic events occurred in 14.7% of the defibrotide group and 6.3% of the control group.
Approved by the European Union, defibrotide is a single-stranded, deoxyribonucleic acid derivative that stabilizes damaged endothelial cells and prevents further endothelial cell damage.
Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Of the 102 patients in the defibrotide group, 39 were alive 100 days after HSCT (38.2%), compared with 8 of 32 (25.0%) in the historical control group. The propensity-adjusted, between-group difference was 23.0% (95.1% confidence interval, 5.2%-40.8%; P = .0109). At 180 days post-HSCT, the difference in survival between the groups was not significant (Blood. 2016 Feb 3. doi: 10.1182/blood-2015-10-676924).
Defibrotide has Fast Track designation from the FDA and the new drug application is currently under Priority Review with a decision expected by March 31, 2016. A potentially fatal complication of HSCT, hepatic veno-occlusive disease is characterized by hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites. There are no approved therapies.
“In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need,” wrote Dr. Paul G. Richardson of Dana-Farber Cancer Institute in Boston and his colleagues.
At day 100 post-HSCT, complete response was seen in 25.5% of the defibrotide group and in 12.5% of the historical control group. The propensity-adjusted, between-group difference was 19% (95.1% CI, 3.5-34.6%; P = .0160). The complete response was durable in 22 of the 26 patients. In the control group, complete response was limited in two patients, impossible to assess in one patient, and durable in one patient.
The multicenter, open-label, phase III trial prospectively enrolled 102 patients with hepatic veno-occlusive disease from 2006 to 2008. Defibrotide was administered intravenously at 25 mg/kg/day in 4 divided doses for a minimum of 21 days. Treatment continued beyond 21 days until resolution of veno-occlusive disease or until the patient was discharged from the hospital.
To identify the historical controls, 6,867 medical charts of HSCT patients hospitalized from 1995 to 2007 were reviewed, and 32 historical control patients were selected. Most (21 of 32) were diagnosed with during 2000-2006, and 11 were diagnosed before 2000. The historical controls were selected by an independent medical review committee, and met the same entry criteria as the defibrotide group.
Because recruiting for the defibrotide group and screening for historical controls occurred at the same institutions during similar time periods, patient management and supportive care were likely similar for the two groups. Propensity scores were included in the analysis to adjust for prognostic factors that were unbalanced between treatment and control groups, including ventilator and dialysis dependency at study entry, age greater or less than 16 years, prior HSCT (0 vs. 1), and allogeneic or autologous transplant.
Hypotension was the most common adverse event reported in the defibrotide and control groups (39% and 50%, respectively), followed by diarrhea (23.5% and 37.5%, respectively). The defibrotide and control groups had similar incidences of common hemorrhagic adverse events (64% and 75%, respectively). Fatal adverse events occurred in 64% of the defibrotide group and 69% of the control group, and fatal hemorrhagic events occurred in 14.7% of the defibrotide group and 6.3% of the control group.
Approved by the European Union, defibrotide is a single-stranded, deoxyribonucleic acid derivative that stabilizes damaged endothelial cells and prevents further endothelial cell damage.
Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Of the 102 patients in the defibrotide group, 39 were alive 100 days after HSCT (38.2%), compared with 8 of 32 (25.0%) in the historical control group. The propensity-adjusted, between-group difference was 23.0% (95.1% confidence interval, 5.2%-40.8%; P = .0109). At 180 days post-HSCT, the difference in survival between the groups was not significant (Blood. 2016 Feb 3. doi: 10.1182/blood-2015-10-676924).
Defibrotide has Fast Track designation from the FDA and the new drug application is currently under Priority Review with a decision expected by March 31, 2016. A potentially fatal complication of HSCT, hepatic veno-occlusive disease is characterized by hepatomegaly, jaundice, rapid weight gain, fluid retention, and ascites. There are no approved therapies.
“In this context, defibrotide provides a promising treatment option for patients with a high unmet medical need,” wrote Dr. Paul G. Richardson of Dana-Farber Cancer Institute in Boston and his colleagues.
At day 100 post-HSCT, complete response was seen in 25.5% of the defibrotide group and in 12.5% of the historical control group. The propensity-adjusted, between-group difference was 19% (95.1% CI, 3.5-34.6%; P = .0160). The complete response was durable in 22 of the 26 patients. In the control group, complete response was limited in two patients, impossible to assess in one patient, and durable in one patient.
The multicenter, open-label, phase III trial prospectively enrolled 102 patients with hepatic veno-occlusive disease from 2006 to 2008. Defibrotide was administered intravenously at 25 mg/kg/day in 4 divided doses for a minimum of 21 days. Treatment continued beyond 21 days until resolution of veno-occlusive disease or until the patient was discharged from the hospital.
To identify the historical controls, 6,867 medical charts of HSCT patients hospitalized from 1995 to 2007 were reviewed, and 32 historical control patients were selected. Most (21 of 32) were diagnosed with during 2000-2006, and 11 were diagnosed before 2000. The historical controls were selected by an independent medical review committee, and met the same entry criteria as the defibrotide group.
Because recruiting for the defibrotide group and screening for historical controls occurred at the same institutions during similar time periods, patient management and supportive care were likely similar for the two groups. Propensity scores were included in the analysis to adjust for prognostic factors that were unbalanced between treatment and control groups, including ventilator and dialysis dependency at study entry, age greater or less than 16 years, prior HSCT (0 vs. 1), and allogeneic or autologous transplant.
Hypotension was the most common adverse event reported in the defibrotide and control groups (39% and 50%, respectively), followed by diarrhea (23.5% and 37.5%, respectively). The defibrotide and control groups had similar incidences of common hemorrhagic adverse events (64% and 75%, respectively). Fatal adverse events occurred in 64% of the defibrotide group and 69% of the control group, and fatal hemorrhagic events occurred in 14.7% of the defibrotide group and 6.3% of the control group.
Approved by the European Union, defibrotide is a single-stranded, deoxyribonucleic acid derivative that stabilizes damaged endothelial cells and prevents further endothelial cell damage.
Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Defibrotide improved survival at 100 days after hematopoietic stem cell transplantation (HSCT) in patients with hepatic veno-occlusive disease, based on an open-label trial that compared trial participants with historical controls.
Major finding: At day 100 post-HSCT, survival was 38.2% for the treatment group and 25.0% for historical controls; propensity-adjusted between-group difference, 23.0%; 95.1% CI, 5.2%-40.8%; P = .0109).
Data source: The multicenter phase III trial included 102 patients with hepatic veno-occlusive disease in the defibrotide group and 32 patients selected for the historical control group.
Disclosures: Dr. Richardson reported consulting or advisory roles with Gentium/Jazz Pharmaceuticals, the maker of defibrotide.