User login
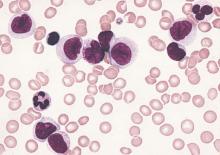
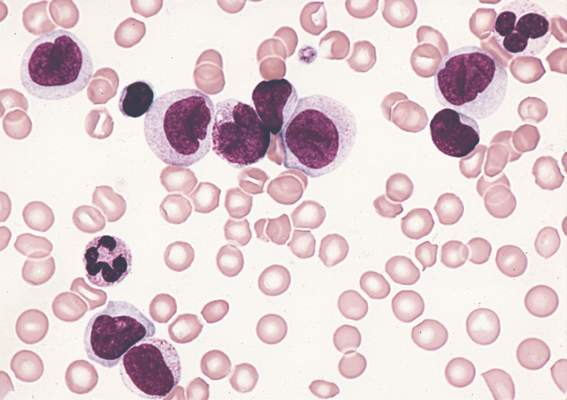
Gemtuzumab ozogamicin boosts overall survival in older AML patients
Older patients with newly diagnosed acute myeloid leukemia who were unsuitable for intensive chemotherapy had significantly longer overall survival with gemtuzumab ozogamicin, compared with best supportive care, according to phase III trial results published Jan. 25.
The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care, including hydroxyurea. The median age was 77 years. In total, 104 of 118 patients in the gemtuzumab ozogamicin arm received the full induction course, and the median number of gemtuzumab ozogamicin infusions was three (range: 1 to 10).
Median overall survival (OS) for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for those who received best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
One-year survival rates were 24.3% (95% CI, 16.9 to 32.4) for gemtuzumab ozogamicin and 9.7% (5.1 to 15.9) for best supportive care (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.0060).
The low intensity gemtuzumab ozogamicin regimen was generally well tolerated, with comparable toxicity between arms. Pancytopenia was observed in nearly all patients during gemtuzumab ozogamicin induction, however.
“Of importance, liver toxicity, a hallmark of [gemtuzumab ozogamicin] safety profile, was not increased in [gemtuzumab ozogamicin] recipients. Furthermore, it appeared to be less frequent and severe than previously reported by our group in a first-line trial, in which a more intensive [gemtuzumab ozogamicin] regimen was used in elderly patients with AML unfit for intensive chemotherapy,” wrote Dr. Sergio Amadori of the Tor Vergata University, Rome, and his colleagues.
Gemtuzumab ozogamicin therapy resulted in an overall complete response (CR) rate of 27% (15.3% CR and 11.7% CRi [incomplete recovery of peripheral blood counts]). The overall clinical benefit rate (CR + CRi+ partial response + stable disease for 30 days) was 56.7%.
Gemtuzumab ozogamicin combines a human monoclonal antibody specific for CD33 on myeloid cells with the DNA intercalator calicheamicin.
Patient characteristics that influenced gemtuzumab ozogamicin treatment effect were CD33 expression status, sex, and cytogenic profile. In patients with more than 80% CD33-positive blasts, gemtuzumab ozogamicin resulted in greater improvements over best supportive care (HR, 0.49; 95% CI, 0.32 to 0.76).
In women, OS was significantly improved (HR, 0.53; 95% CI, 0.35 to 0.79), whereas in men the hazard ratio was near 1. Patients with favorable/intermediate cytogenetic risk profiles had significant gemtuzumab ozogamicin benefit (HR, 0.52; 95% CI, 0.34 to 0.77), and those with adverse risk profiles had no treatment difference between arms.
The research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer . Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Older patients with newly diagnosed acute myeloid leukemia who were unsuitable for intensive chemotherapy had significantly longer overall survival with gemtuzumab ozogamicin, compared with best supportive care, according to phase III trial results published Jan. 25.
The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care, including hydroxyurea. The median age was 77 years. In total, 104 of 118 patients in the gemtuzumab ozogamicin arm received the full induction course, and the median number of gemtuzumab ozogamicin infusions was three (range: 1 to 10).
Median overall survival (OS) for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for those who received best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
One-year survival rates were 24.3% (95% CI, 16.9 to 32.4) for gemtuzumab ozogamicin and 9.7% (5.1 to 15.9) for best supportive care (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.0060).
The low intensity gemtuzumab ozogamicin regimen was generally well tolerated, with comparable toxicity between arms. Pancytopenia was observed in nearly all patients during gemtuzumab ozogamicin induction, however.
“Of importance, liver toxicity, a hallmark of [gemtuzumab ozogamicin] safety profile, was not increased in [gemtuzumab ozogamicin] recipients. Furthermore, it appeared to be less frequent and severe than previously reported by our group in a first-line trial, in which a more intensive [gemtuzumab ozogamicin] regimen was used in elderly patients with AML unfit for intensive chemotherapy,” wrote Dr. Sergio Amadori of the Tor Vergata University, Rome, and his colleagues.
Gemtuzumab ozogamicin therapy resulted in an overall complete response (CR) rate of 27% (15.3% CR and 11.7% CRi [incomplete recovery of peripheral blood counts]). The overall clinical benefit rate (CR + CRi+ partial response + stable disease for 30 days) was 56.7%.
Gemtuzumab ozogamicin combines a human monoclonal antibody specific for CD33 on myeloid cells with the DNA intercalator calicheamicin.
Patient characteristics that influenced gemtuzumab ozogamicin treatment effect were CD33 expression status, sex, and cytogenic profile. In patients with more than 80% CD33-positive blasts, gemtuzumab ozogamicin resulted in greater improvements over best supportive care (HR, 0.49; 95% CI, 0.32 to 0.76).
In women, OS was significantly improved (HR, 0.53; 95% CI, 0.35 to 0.79), whereas in men the hazard ratio was near 1. Patients with favorable/intermediate cytogenetic risk profiles had significant gemtuzumab ozogamicin benefit (HR, 0.52; 95% CI, 0.34 to 0.77), and those with adverse risk profiles had no treatment difference between arms.
The research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer . Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Older patients with newly diagnosed acute myeloid leukemia who were unsuitable for intensive chemotherapy had significantly longer overall survival with gemtuzumab ozogamicin, compared with best supportive care, according to phase III trial results published Jan. 25.
The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care, including hydroxyurea. The median age was 77 years. In total, 104 of 118 patients in the gemtuzumab ozogamicin arm received the full induction course, and the median number of gemtuzumab ozogamicin infusions was three (range: 1 to 10).
Median overall survival (OS) for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for those who received best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
One-year survival rates were 24.3% (95% CI, 16.9 to 32.4) for gemtuzumab ozogamicin and 9.7% (5.1 to 15.9) for best supportive care (J Clin Onc. 2016 Jan 25. doi: 10.1200/JCO.2015.64.0060).
The low intensity gemtuzumab ozogamicin regimen was generally well tolerated, with comparable toxicity between arms. Pancytopenia was observed in nearly all patients during gemtuzumab ozogamicin induction, however.
“Of importance, liver toxicity, a hallmark of [gemtuzumab ozogamicin] safety profile, was not increased in [gemtuzumab ozogamicin] recipients. Furthermore, it appeared to be less frequent and severe than previously reported by our group in a first-line trial, in which a more intensive [gemtuzumab ozogamicin] regimen was used in elderly patients with AML unfit for intensive chemotherapy,” wrote Dr. Sergio Amadori of the Tor Vergata University, Rome, and his colleagues.
Gemtuzumab ozogamicin therapy resulted in an overall complete response (CR) rate of 27% (15.3% CR and 11.7% CRi [incomplete recovery of peripheral blood counts]). The overall clinical benefit rate (CR + CRi+ partial response + stable disease for 30 days) was 56.7%.
Gemtuzumab ozogamicin combines a human monoclonal antibody specific for CD33 on myeloid cells with the DNA intercalator calicheamicin.
Patient characteristics that influenced gemtuzumab ozogamicin treatment effect were CD33 expression status, sex, and cytogenic profile. In patients with more than 80% CD33-positive blasts, gemtuzumab ozogamicin resulted in greater improvements over best supportive care (HR, 0.49; 95% CI, 0.32 to 0.76).
In women, OS was significantly improved (HR, 0.53; 95% CI, 0.35 to 0.79), whereas in men the hazard ratio was near 1. Patients with favorable/intermediate cytogenetic risk profiles had significant gemtuzumab ozogamicin benefit (HR, 0.52; 95% CI, 0.34 to 0.77), and those with adverse risk profiles had no treatment difference between arms.
The research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer . Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
Key clinical point: First-line, low-dose gemtuzumab ozogamicin significantly improved overall survival, compared with best supportive care in patients aged 61 years or older with AML.
Major finding: Median overall survival for patients who received gemtuzumab ozogamicin was 4.9 months, compared with 3.6 months for best supportive care, including hydroxyurea (hazard ratio, 0.69; 95% confidence interval, 0.53-0.90; P = .005).
Data source: The phase III EORTC-GIMEMA AML-19 trial randomly assigned 118 patients to receive gemtuzumab ozogamicin and 119 to receive best supportive care.
Disclosures: Research was supported by Wyeth (Pfizer) and by the European Organisation for Research and Treatment of Cancer. Dr. Amadori reported having no disclosures. Several of his coauthors reported ties to industry.
CD30 identified as novel target in systemic mastocytosis
Mast cells isolated from patients with systemic mastocytosis (SM) often express the Ki-1 antigen CD30 on their surface, and the antibody-conjugate brentuximab-vedotin inhibited growth and promoted apoptosis in neoplastic mast cells expressing CD30.
CD30 cell surface levels roughly correlated with the type of systemic mastocytosis: CD30 was observed in 3 of 25 patients (12%) with indolent SM, 4 of 7 (57%) with aggressive SM, and 4 of 7 (57%) with mast cell leukemia. However, not all patients with aggressive disease exhibited mast cell-surface CD30, and some patients with indolent SM expressed substantial amounts of CD30.
“Our data are in favor of testing for CD30 surface expression on neoplastic [mast cells] by flow cytometry before treatment with brentuximab-vedotin is considered,” wrote Dr. Katharina Blatt of the Medical University of Vienna, and her colleagues (Blood. 2015 Dec 24. doi:10.1182/blood-2015-03-637728).
The antibody-drug conjugate brentuximab-vedotin targets CD30+ cells in patients with Hodgkin lymphoma and anaplastic large-cell lymphoma. This study demonstrated that brentuximab-vedotin inhibited growth of CD30+ mast cells, as well as proliferation of CD30+ mast cell lines, at concentrations within therapeutic range. Analysis was done on bone marrow samples from 45 patients with systemic mastocytosis, as well as human mast cell lines (HMC-1.1, HMC-1.2, MCPV-1.1, and MCPV-1.4) and canine mastocytoma cell line C2.
Increased numbers and activation of mast cells in patients with SM cause mediator-related symptoms, and these may be reduced in the presence of brentuximab-vedotin, which was shown to counteract IgE-dependent secretion of histamine from basophils and mast cells, according to investigators.
Most patients with aggressive SM or mast cell leukemia show clinically meaningful or complete responses to midostaurin (PKC412), but responses are usually short lived. Brentuximab-vedotin acts synergistically with PKC412 to inhibit growth of CD30+ cells.
“Based on these data it seems tempting to propose a clinical trial exploring antineoplastic effects of the drug combination PKC412 and brentuximab-vedotin in advanced SM,” they wrote. A clinical trial of brentuximab-vedotin in advanced SM was initiated late last year.
Dr. Blatt reported having no disclosures. Several of her coauthors reported ties to industry.
New research provides evidence for CD30 as a therapeutic target for aggressive forms of systemic mastocytosis (SM), for which few targeted drugs exist. The report by Dr. Katharina Blatt and her associates showed that surface CD30 levels were significantly higher in mast cells isolated from patients with advanced disease, compared with indolent SM, and that the growth of CD30+ cells was inhibited by brentuximab-vedotin at therapeutic concentrations. Results show that CD30 is a promising new target for patients with advanced SM. The effects of brentuximab-vedotin appear to depend on the presence of CD30 on the mast cell surface, suggesting the need CD30 testing by flow cytometry, said Dr. Irina Maric, a hematologist affiliated with the National Institutes of Health, Bethesda, Md.
In the future, CD30 expression on mast cells may be a valuable screening tool, prognostic marker, and therapeutic target in advanced forms of mast cell disease, she wrote.
Dr. Maric’s comments were part of an accompanying editorial in Blood (2015 Dec 24. doi: 10.1182/blood-2015-11-678631). She reported having no disclosures.
New research provides evidence for CD30 as a therapeutic target for aggressive forms of systemic mastocytosis (SM), for which few targeted drugs exist. The report by Dr. Katharina Blatt and her associates showed that surface CD30 levels were significantly higher in mast cells isolated from patients with advanced disease, compared with indolent SM, and that the growth of CD30+ cells was inhibited by brentuximab-vedotin at therapeutic concentrations. Results show that CD30 is a promising new target for patients with advanced SM. The effects of brentuximab-vedotin appear to depend on the presence of CD30 on the mast cell surface, suggesting the need CD30 testing by flow cytometry, said Dr. Irina Maric, a hematologist affiliated with the National Institutes of Health, Bethesda, Md.
In the future, CD30 expression on mast cells may be a valuable screening tool, prognostic marker, and therapeutic target in advanced forms of mast cell disease, she wrote.
Dr. Maric’s comments were part of an accompanying editorial in Blood (2015 Dec 24. doi: 10.1182/blood-2015-11-678631). She reported having no disclosures.
New research provides evidence for CD30 as a therapeutic target for aggressive forms of systemic mastocytosis (SM), for which few targeted drugs exist. The report by Dr. Katharina Blatt and her associates showed that surface CD30 levels were significantly higher in mast cells isolated from patients with advanced disease, compared with indolent SM, and that the growth of CD30+ cells was inhibited by brentuximab-vedotin at therapeutic concentrations. Results show that CD30 is a promising new target for patients with advanced SM. The effects of brentuximab-vedotin appear to depend on the presence of CD30 on the mast cell surface, suggesting the need CD30 testing by flow cytometry, said Dr. Irina Maric, a hematologist affiliated with the National Institutes of Health, Bethesda, Md.
In the future, CD30 expression on mast cells may be a valuable screening tool, prognostic marker, and therapeutic target in advanced forms of mast cell disease, she wrote.
Dr. Maric’s comments were part of an accompanying editorial in Blood (2015 Dec 24. doi: 10.1182/blood-2015-11-678631). She reported having no disclosures.
Mast cells isolated from patients with systemic mastocytosis (SM) often express the Ki-1 antigen CD30 on their surface, and the antibody-conjugate brentuximab-vedotin inhibited growth and promoted apoptosis in neoplastic mast cells expressing CD30.
CD30 cell surface levels roughly correlated with the type of systemic mastocytosis: CD30 was observed in 3 of 25 patients (12%) with indolent SM, 4 of 7 (57%) with aggressive SM, and 4 of 7 (57%) with mast cell leukemia. However, not all patients with aggressive disease exhibited mast cell-surface CD30, and some patients with indolent SM expressed substantial amounts of CD30.
“Our data are in favor of testing for CD30 surface expression on neoplastic [mast cells] by flow cytometry before treatment with brentuximab-vedotin is considered,” wrote Dr. Katharina Blatt of the Medical University of Vienna, and her colleagues (Blood. 2015 Dec 24. doi:10.1182/blood-2015-03-637728).
The antibody-drug conjugate brentuximab-vedotin targets CD30+ cells in patients with Hodgkin lymphoma and anaplastic large-cell lymphoma. This study demonstrated that brentuximab-vedotin inhibited growth of CD30+ mast cells, as well as proliferation of CD30+ mast cell lines, at concentrations within therapeutic range. Analysis was done on bone marrow samples from 45 patients with systemic mastocytosis, as well as human mast cell lines (HMC-1.1, HMC-1.2, MCPV-1.1, and MCPV-1.4) and canine mastocytoma cell line C2.
Increased numbers and activation of mast cells in patients with SM cause mediator-related symptoms, and these may be reduced in the presence of brentuximab-vedotin, which was shown to counteract IgE-dependent secretion of histamine from basophils and mast cells, according to investigators.
Most patients with aggressive SM or mast cell leukemia show clinically meaningful or complete responses to midostaurin (PKC412), but responses are usually short lived. Brentuximab-vedotin acts synergistically with PKC412 to inhibit growth of CD30+ cells.
“Based on these data it seems tempting to propose a clinical trial exploring antineoplastic effects of the drug combination PKC412 and brentuximab-vedotin in advanced SM,” they wrote. A clinical trial of brentuximab-vedotin in advanced SM was initiated late last year.
Dr. Blatt reported having no disclosures. Several of her coauthors reported ties to industry.
Mast cells isolated from patients with systemic mastocytosis (SM) often express the Ki-1 antigen CD30 on their surface, and the antibody-conjugate brentuximab-vedotin inhibited growth and promoted apoptosis in neoplastic mast cells expressing CD30.
CD30 cell surface levels roughly correlated with the type of systemic mastocytosis: CD30 was observed in 3 of 25 patients (12%) with indolent SM, 4 of 7 (57%) with aggressive SM, and 4 of 7 (57%) with mast cell leukemia. However, not all patients with aggressive disease exhibited mast cell-surface CD30, and some patients with indolent SM expressed substantial amounts of CD30.
“Our data are in favor of testing for CD30 surface expression on neoplastic [mast cells] by flow cytometry before treatment with brentuximab-vedotin is considered,” wrote Dr. Katharina Blatt of the Medical University of Vienna, and her colleagues (Blood. 2015 Dec 24. doi:10.1182/blood-2015-03-637728).
The antibody-drug conjugate brentuximab-vedotin targets CD30+ cells in patients with Hodgkin lymphoma and anaplastic large-cell lymphoma. This study demonstrated that brentuximab-vedotin inhibited growth of CD30+ mast cells, as well as proliferation of CD30+ mast cell lines, at concentrations within therapeutic range. Analysis was done on bone marrow samples from 45 patients with systemic mastocytosis, as well as human mast cell lines (HMC-1.1, HMC-1.2, MCPV-1.1, and MCPV-1.4) and canine mastocytoma cell line C2.
Increased numbers and activation of mast cells in patients with SM cause mediator-related symptoms, and these may be reduced in the presence of brentuximab-vedotin, which was shown to counteract IgE-dependent secretion of histamine from basophils and mast cells, according to investigators.
Most patients with aggressive SM or mast cell leukemia show clinically meaningful or complete responses to midostaurin (PKC412), but responses are usually short lived. Brentuximab-vedotin acts synergistically with PKC412 to inhibit growth of CD30+ cells.
“Based on these data it seems tempting to propose a clinical trial exploring antineoplastic effects of the drug combination PKC412 and brentuximab-vedotin in advanced SM,” they wrote. A clinical trial of brentuximab-vedotin in advanced SM was initiated late last year.
Dr. Blatt reported having no disclosures. Several of her coauthors reported ties to industry.
FROM BLOOD
Key clinical point: In mast cells isolated from patients with systemic mastocytosis, the antibody-conjugate brentuximab-vedotin inhibited growth and promoted apoptosis of cells expressing CD30 on the cell surface.
Major finding: Brentuximab-vedotin inhibited proliferation of CD30+ mast cells isolated from three patients with systemic mastocytosis but showed weak or no effects on CD30– mast cells.
Data source: Bone marrow samples from 45 patients with systemic mastocytosis and six controls; human mast cell lines HMC-1.1, HMC-1.2, MCPV-1.1, and MCPV-1.4; canine mastocytoma cell line C2.
Disclosures: Dr. Blatt reported having no disclosures. Several of her coauthors reported ties to industry.
Proper hydroxyurea dose tied to better survival in sickle cell anemia
Adults with sickle cell disease who received recommended doses of hydroxyurea had higher fetal hemoglobin (HbF) levels, less organ dysfunction, and improved survival, compared with those who did receive recommended hydroxyurea doses, according to researchers.
“Our data suggest that even moderate increases, and not necessarily maximum HbF induction, may improve survival in patients with sickle cell anemia,” wrote Dr. Courtney D. Fitzhugh, assistant clinical investigator in the Laboratory of Sickle Mortality Prevention at the National Heart, Lung, and Blood Institute, Bethesda, Md., and her colleagues (PLoS One. 2015 Nov 17; doi:10.1371/journal.pone.0141706).
From 2001 to 2010, 383 patients with sickle cell disease underwent data clinical, laboratory, and echocardiographic evaluations every 2 years during a median follow-up of 2.6 years (range, 0.1-11.7).
In total, 59 patients died, and the median age at death was 46 years for men and 44.5 for women. Deceased subjects had lower fetal hemoglobin (P = .0044), were less likely to have taken hydroxyurea (56% vs. 68%, P = .040), and had a smaller proportion who were prescribed hydroxyurea within the recommended dose range (29% vs. 46%, P = .0039). Study participants who received a dose between 15 and 35 mg/kg/day more likely survived than those who never took hydroxyurea (P = .005). To assess the impact of hydroxyurea-induced HbF on organ injury, the study compared laboratory values from the highest and lowest HbF quartiles. For the lowest HbF quartile, alkaline phosphatase, a marker of organ damage, was consistently lower. “Because organ dysfunction may limit dosing, and hydroxyurea may not reverse severe tissue injury, we recommend treatment before organ damage occurs,” the researchers wrote.
Dr. Fitzhugh reported having no disclosures.
Adults with sickle cell disease who received recommended doses of hydroxyurea had higher fetal hemoglobin (HbF) levels, less organ dysfunction, and improved survival, compared with those who did receive recommended hydroxyurea doses, according to researchers.
“Our data suggest that even moderate increases, and not necessarily maximum HbF induction, may improve survival in patients with sickle cell anemia,” wrote Dr. Courtney D. Fitzhugh, assistant clinical investigator in the Laboratory of Sickle Mortality Prevention at the National Heart, Lung, and Blood Institute, Bethesda, Md., and her colleagues (PLoS One. 2015 Nov 17; doi:10.1371/journal.pone.0141706).
From 2001 to 2010, 383 patients with sickle cell disease underwent data clinical, laboratory, and echocardiographic evaluations every 2 years during a median follow-up of 2.6 years (range, 0.1-11.7).
In total, 59 patients died, and the median age at death was 46 years for men and 44.5 for women. Deceased subjects had lower fetal hemoglobin (P = .0044), were less likely to have taken hydroxyurea (56% vs. 68%, P = .040), and had a smaller proportion who were prescribed hydroxyurea within the recommended dose range (29% vs. 46%, P = .0039). Study participants who received a dose between 15 and 35 mg/kg/day more likely survived than those who never took hydroxyurea (P = .005). To assess the impact of hydroxyurea-induced HbF on organ injury, the study compared laboratory values from the highest and lowest HbF quartiles. For the lowest HbF quartile, alkaline phosphatase, a marker of organ damage, was consistently lower. “Because organ dysfunction may limit dosing, and hydroxyurea may not reverse severe tissue injury, we recommend treatment before organ damage occurs,” the researchers wrote.
Dr. Fitzhugh reported having no disclosures.
Adults with sickle cell disease who received recommended doses of hydroxyurea had higher fetal hemoglobin (HbF) levels, less organ dysfunction, and improved survival, compared with those who did receive recommended hydroxyurea doses, according to researchers.
“Our data suggest that even moderate increases, and not necessarily maximum HbF induction, may improve survival in patients with sickle cell anemia,” wrote Dr. Courtney D. Fitzhugh, assistant clinical investigator in the Laboratory of Sickle Mortality Prevention at the National Heart, Lung, and Blood Institute, Bethesda, Md., and her colleagues (PLoS One. 2015 Nov 17; doi:10.1371/journal.pone.0141706).
From 2001 to 2010, 383 patients with sickle cell disease underwent data clinical, laboratory, and echocardiographic evaluations every 2 years during a median follow-up of 2.6 years (range, 0.1-11.7).
In total, 59 patients died, and the median age at death was 46 years for men and 44.5 for women. Deceased subjects had lower fetal hemoglobin (P = .0044), were less likely to have taken hydroxyurea (56% vs. 68%, P = .040), and had a smaller proportion who were prescribed hydroxyurea within the recommended dose range (29% vs. 46%, P = .0039). Study participants who received a dose between 15 and 35 mg/kg/day more likely survived than those who never took hydroxyurea (P = .005). To assess the impact of hydroxyurea-induced HbF on organ injury, the study compared laboratory values from the highest and lowest HbF quartiles. For the lowest HbF quartile, alkaline phosphatase, a marker of organ damage, was consistently lower. “Because organ dysfunction may limit dosing, and hydroxyurea may not reverse severe tissue injury, we recommend treatment before organ damage occurs,” the researchers wrote.
Dr. Fitzhugh reported having no disclosures.
FROM PLOS ONE
Key clinical point: Proper hydroxyurea dose in adults with sickle cell anemia was linked to higher fetal hemoglobin levels, less organ dysfunction, and improved survival.
Major finding: Patients in the highest fetal hemoglobin quartiles had higher rates of survival, and 75% of patients in the highest quartile received recommended hydroxyurea doses, compared with 18% in the lowest quartile.
Data source: From 2001 to 2010, 383 patients with sickle cell disease underwent data clinical, laboratory, and echocardiographic evaluations at enrollment and every two years subsequently.
Disclosures: Dr. Fitzhugh reported having no disclosures.
Significant risk of relapse remains for ER-positive breast cancer patients beyond 10 years
The risk of breast cancer relapse decreases consistently for 10 years, then remains stable through 25 years, with ER-positive disease carrying higher risk than ER-negative disease from years 5 to 25, according to researchers.
At a median follow up of 24 years, the study reported outcomes of 4,105 patients who were diagnosed from 1978 to 1985 and participated in the International Breast Cancer Study Group Trials I to V. During the first 5 years of follow-up, risk of recurrence was lower for ER-positive compared with ER-negative disease: 9.9% vs. 11.5%. Beyond 5 years, risk was higher: 5-10 years, 5.4% vs. 3.3%; 10-15 years, 2.9% vs. 1.3%, 15-20 years, 2.8% vs. 1.2%. At 20-25 years, risk was 1.3% vs. 1.4% (P less than .001).
“We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied,” wrote Dr. Marco Colleoni of the European Institute of Oncology and International Breast Cancer Study Group, and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.3504).
For the entire patient group, breast cancer recurrence reached a peak at years 1-2 (15.2%), and decreased consistently through year 10 (5-10 years, 4.5%), then remained stable (10-15 years, 2.2%; 15-20, 1.5%; 20-25, 0.7%). Cumulative incidence of distant recurrence for the ER-positive group occurred less frequently than for the ER-negative group during the first 5 years and more frequently from 5 to 25 years: at 5 years, 27.1% vs. 23.4%; at 10 years, 31.9% vs. 31.8%; at 15 years, 35% vs. 33.4%; at 20 years, 37.4% vs. 34.1%; at 25 years, 38.3% vs. 35.3% (P less than .001).
All patients in the trials had undergone mastectomy and axillary clearance with at least eight nodes removed, with no locoregional radiotherapy, as was standard at the time.
Within the ER-positive group, patients who had zero to three positive nodes had had a stable risk of recurrence beyond 10 years, whereas for patients with four or more involved nodes, risk decreased gradually from 10 to 24 years.
Recent studies have shown that 10 years of adjuvant tamoxifen further improves breast cancer survival compared with 5 years of adjuvant therapy, albeit at a cost of 0.4% due to mortality resulting from endometrial carcinoma or pulmonary embolism. The studies underline the critical importance of sufficiently large patient populations and longer follow-up to provide accurate outcome data.
The report by Colleoni et al. describes clinical trial results from a median 24-year follow up. Patients with ER-positive disease require long-term follow up, which should be fundamentally different from those with ER-negative and/or human epidermal growth factor receptor 2–positive disease, who require shorter follow-up. Given these differences, adjuvant clinical studies with shorter follow-up periods will underreport events for ER-positive patients.
The study also reports a secondary malignancy rate of 4.9%, a figure likely to be underreported in studies with shorter follow-up schedules.
Limitations of the study arise from the time period in which it began. Regimens used in the study (different schedules of cyclophosphamide, methotrexate, and fluorouracil, and 1 year of tamoxifen with or without prednisone) have been shown to be inferior to newer regimens. None of the patients received postoperative radiotherapy, in accordance with data available at the time. Today, women with node-positive disease receive locoregional radiotherapy to reduce the risk of locoregional recurrence.
Studies with long-term follow up periods offer insights into both efficacy and safety; however, such studies require extensive resources. The entities that decide which types of cancer care are made available, such as insurance companies, governments, and regional health care funders, must shoulder the responsibility to ensure the required long-term evaluation of these treatments is conducted. Joint projects between pharmaceutical companies and health care providers could identify long-term benefits and adverse effects. The process may be more readily implemented in countries with population-based cancer registries.
Dr. Jonas Bergh is professor in the department of oncology-pathology at Karolinska Institutet and University Hospital, Stockholm. Dr. Kathleen Pritchard is a medical oncologist at Sunnybrook Odette Cancer Centre and professor at the University of Toronto. Dr. David Cameron is clinical director and chair of oncology at the University of Edinburgh Cancer Research Centre, Scotland. These remarks were part of an editorial accompanying the report by Colleoni et al. (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.65.2255). Dr. Bergh reported financial ties to Amgen, AstraZeneca, Bayer HealthCare Pharmaceuticals, Merck, Pfizer, Roche, and Sanofi. Dr. Pritchard reported ties to AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Cameron reported ties to Novartis.
Recent studies have shown that 10 years of adjuvant tamoxifen further improves breast cancer survival compared with 5 years of adjuvant therapy, albeit at a cost of 0.4% due to mortality resulting from endometrial carcinoma or pulmonary embolism. The studies underline the critical importance of sufficiently large patient populations and longer follow-up to provide accurate outcome data.
The report by Colleoni et al. describes clinical trial results from a median 24-year follow up. Patients with ER-positive disease require long-term follow up, which should be fundamentally different from those with ER-negative and/or human epidermal growth factor receptor 2–positive disease, who require shorter follow-up. Given these differences, adjuvant clinical studies with shorter follow-up periods will underreport events for ER-positive patients.
The study also reports a secondary malignancy rate of 4.9%, a figure likely to be underreported in studies with shorter follow-up schedules.
Limitations of the study arise from the time period in which it began. Regimens used in the study (different schedules of cyclophosphamide, methotrexate, and fluorouracil, and 1 year of tamoxifen with or without prednisone) have been shown to be inferior to newer regimens. None of the patients received postoperative radiotherapy, in accordance with data available at the time. Today, women with node-positive disease receive locoregional radiotherapy to reduce the risk of locoregional recurrence.
Studies with long-term follow up periods offer insights into both efficacy and safety; however, such studies require extensive resources. The entities that decide which types of cancer care are made available, such as insurance companies, governments, and regional health care funders, must shoulder the responsibility to ensure the required long-term evaluation of these treatments is conducted. Joint projects between pharmaceutical companies and health care providers could identify long-term benefits and adverse effects. The process may be more readily implemented in countries with population-based cancer registries.
Dr. Jonas Bergh is professor in the department of oncology-pathology at Karolinska Institutet and University Hospital, Stockholm. Dr. Kathleen Pritchard is a medical oncologist at Sunnybrook Odette Cancer Centre and professor at the University of Toronto. Dr. David Cameron is clinical director and chair of oncology at the University of Edinburgh Cancer Research Centre, Scotland. These remarks were part of an editorial accompanying the report by Colleoni et al. (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.65.2255). Dr. Bergh reported financial ties to Amgen, AstraZeneca, Bayer HealthCare Pharmaceuticals, Merck, Pfizer, Roche, and Sanofi. Dr. Pritchard reported ties to AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Cameron reported ties to Novartis.
Recent studies have shown that 10 years of adjuvant tamoxifen further improves breast cancer survival compared with 5 years of adjuvant therapy, albeit at a cost of 0.4% due to mortality resulting from endometrial carcinoma or pulmonary embolism. The studies underline the critical importance of sufficiently large patient populations and longer follow-up to provide accurate outcome data.
The report by Colleoni et al. describes clinical trial results from a median 24-year follow up. Patients with ER-positive disease require long-term follow up, which should be fundamentally different from those with ER-negative and/or human epidermal growth factor receptor 2–positive disease, who require shorter follow-up. Given these differences, adjuvant clinical studies with shorter follow-up periods will underreport events for ER-positive patients.
The study also reports a secondary malignancy rate of 4.9%, a figure likely to be underreported in studies with shorter follow-up schedules.
Limitations of the study arise from the time period in which it began. Regimens used in the study (different schedules of cyclophosphamide, methotrexate, and fluorouracil, and 1 year of tamoxifen with or without prednisone) have been shown to be inferior to newer regimens. None of the patients received postoperative radiotherapy, in accordance with data available at the time. Today, women with node-positive disease receive locoregional radiotherapy to reduce the risk of locoregional recurrence.
Studies with long-term follow up periods offer insights into both efficacy and safety; however, such studies require extensive resources. The entities that decide which types of cancer care are made available, such as insurance companies, governments, and regional health care funders, must shoulder the responsibility to ensure the required long-term evaluation of these treatments is conducted. Joint projects between pharmaceutical companies and health care providers could identify long-term benefits and adverse effects. The process may be more readily implemented in countries with population-based cancer registries.
Dr. Jonas Bergh is professor in the department of oncology-pathology at Karolinska Institutet and University Hospital, Stockholm. Dr. Kathleen Pritchard is a medical oncologist at Sunnybrook Odette Cancer Centre and professor at the University of Toronto. Dr. David Cameron is clinical director and chair of oncology at the University of Edinburgh Cancer Research Centre, Scotland. These remarks were part of an editorial accompanying the report by Colleoni et al. (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.65.2255). Dr. Bergh reported financial ties to Amgen, AstraZeneca, Bayer HealthCare Pharmaceuticals, Merck, Pfizer, Roche, and Sanofi. Dr. Pritchard reported ties to AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai. Dr. Cameron reported ties to Novartis.
The risk of breast cancer relapse decreases consistently for 10 years, then remains stable through 25 years, with ER-positive disease carrying higher risk than ER-negative disease from years 5 to 25, according to researchers.
At a median follow up of 24 years, the study reported outcomes of 4,105 patients who were diagnosed from 1978 to 1985 and participated in the International Breast Cancer Study Group Trials I to V. During the first 5 years of follow-up, risk of recurrence was lower for ER-positive compared with ER-negative disease: 9.9% vs. 11.5%. Beyond 5 years, risk was higher: 5-10 years, 5.4% vs. 3.3%; 10-15 years, 2.9% vs. 1.3%, 15-20 years, 2.8% vs. 1.2%. At 20-25 years, risk was 1.3% vs. 1.4% (P less than .001).
“We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied,” wrote Dr. Marco Colleoni of the European Institute of Oncology and International Breast Cancer Study Group, and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.3504).
For the entire patient group, breast cancer recurrence reached a peak at years 1-2 (15.2%), and decreased consistently through year 10 (5-10 years, 4.5%), then remained stable (10-15 years, 2.2%; 15-20, 1.5%; 20-25, 0.7%). Cumulative incidence of distant recurrence for the ER-positive group occurred less frequently than for the ER-negative group during the first 5 years and more frequently from 5 to 25 years: at 5 years, 27.1% vs. 23.4%; at 10 years, 31.9% vs. 31.8%; at 15 years, 35% vs. 33.4%; at 20 years, 37.4% vs. 34.1%; at 25 years, 38.3% vs. 35.3% (P less than .001).
All patients in the trials had undergone mastectomy and axillary clearance with at least eight nodes removed, with no locoregional radiotherapy, as was standard at the time.
Within the ER-positive group, patients who had zero to three positive nodes had had a stable risk of recurrence beyond 10 years, whereas for patients with four or more involved nodes, risk decreased gradually from 10 to 24 years.
The risk of breast cancer relapse decreases consistently for 10 years, then remains stable through 25 years, with ER-positive disease carrying higher risk than ER-negative disease from years 5 to 25, according to researchers.
At a median follow up of 24 years, the study reported outcomes of 4,105 patients who were diagnosed from 1978 to 1985 and participated in the International Breast Cancer Study Group Trials I to V. During the first 5 years of follow-up, risk of recurrence was lower for ER-positive compared with ER-negative disease: 9.9% vs. 11.5%. Beyond 5 years, risk was higher: 5-10 years, 5.4% vs. 3.3%; 10-15 years, 2.9% vs. 1.3%, 15-20 years, 2.8% vs. 1.2%. At 20-25 years, risk was 1.3% vs. 1.4% (P less than .001).
“We identified a population (ER positive) that maintains a significant risk of relapse even after more than 10 years of follow-up. New targeted treatments and different modes of breast cancer surveillance for preventing late recurrences within this population should be studied,” wrote Dr. Marco Colleoni of the European Institute of Oncology and International Breast Cancer Study Group, and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.3504).
For the entire patient group, breast cancer recurrence reached a peak at years 1-2 (15.2%), and decreased consistently through year 10 (5-10 years, 4.5%), then remained stable (10-15 years, 2.2%; 15-20, 1.5%; 20-25, 0.7%). Cumulative incidence of distant recurrence for the ER-positive group occurred less frequently than for the ER-negative group during the first 5 years and more frequently from 5 to 25 years: at 5 years, 27.1% vs. 23.4%; at 10 years, 31.9% vs. 31.8%; at 15 years, 35% vs. 33.4%; at 20 years, 37.4% vs. 34.1%; at 25 years, 38.3% vs. 35.3% (P less than .001).
All patients in the trials had undergone mastectomy and axillary clearance with at least eight nodes removed, with no locoregional radiotherapy, as was standard at the time.
Within the ER-positive group, patients who had zero to three positive nodes had had a stable risk of recurrence beyond 10 years, whereas for patients with four or more involved nodes, risk decreased gradually from 10 to 24 years.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The risk of breast cancer recurrence continues through 24 years after primary treatments, especially for estrogen receptor–positive disease.
Major finding: During the first 5 years, risk of recurrence was lower for ER-positive disease than for ER-negative disease (9.9% vs. 11.5%). Risk was higher 5-10 years later (5.4% vs. 3.3%), at 10-15 years (2.9% vs. 1.3%), and at 15-20 years (2.8% vs. 1.2%). From 20 to 25 years on, the risk was 1.3% vs. 1.4% (P less than .001).
Data sources: The International Breast Cancer Study Group Trials I to V, comprising 4,105 patients with breast cancer diagnosed from 1978 to 1985.
Disclosures: Dr. Colleoni reported financial ties to Novartis, Boehringer Ingelheim, Taiho Pharmaceutical, AbbVie, AstraZeneca, Pierre Fabre, and Pfizer. Several of his coauthors reported ties to industry.
RCTs vs. observational studies: mortality ‘strikingly’ different following RT
Analyses of mortality after breast cancer radiotherapy using randomized clinical trial data versus observational data produced strikingly different results, according to researchers.
Analyses of randomized data indicated radiotherapy reduced mortality after breast-conserving surgery and mastectomy in node-positive disease; by contrast, SEER data analyses showed radiotherapy was associated with a significantly larger reduction in breast cancer mortality after breast-conserving surgery but higher mortality after mastectomy. Among patients with node-positive breast cancer who underwent mastectomy and axillary dissection, radiotherapy was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 1.31-1.37).
Furthermore, analyses of randomized trial data indicated increased mortality from heart disease (1.27; 1.12-1.44) and lung cancer (1.78; 1.30-2.46) following radiotherapy, but analyses of SEER data indicated reduced mortality from heart disease (0.56; 0.53-0.60) and lung cancer (0.86; 0.75-0.99) associated with radiotherapy.
“It is not plausible that these negative associations in the SEER data are causal and, clearly, they are strongly influenced by factors other than the effect or radiotherapy,” wrote Dr. Katherine Henson of the University of Oxford (England), and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
“Randomized trials are needed wherever possible to investigate the effect of treatment on mortality from the original cancer,” according to the investigators, and, “selection biases can be problematic even when analyzing treatment-related toxicities using observational data.”
Analyses of randomized data demonstrated reduced breast cancer mortality associated with radiotherapy after breast-conserving surgery (0.82; 95% CI, 0.75-0.90). Compared with randomized data, analyses of observational data showed a much greater reduction in mortality associated with radiotherapy after breast-conserving surgery (0.64; 95% CI, 0.62-0.66).
Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta analyses of 17 trials (n = 10,801) of radiotherapy after breast-conserving surgery, 14 trials (n = 3,131) of radiotherapy after mastectomy, and 78 trials (n = 42,080) of mortality from causes other than breast cancer. Observational evidence came from the SEER data base (n = 393,840).
The researchers offered plausible explanations for selection bias that may have resulted in the divergent results calculated using observational data. Because radiotherapy following mastectomy is indicated only in the presence of adverse disease characteristics, patients who did not receive radiotherapy may have survived longer because of especially favorable characteristics, despite of lack of radiotherapy.
“When evaluating rare late effects for which sufficient randomized evidence cannot reasonably be obtained, analyses of observational data comparing treated and untreated patients may often be the only source of information, but they must always be interpreted with considerable caution,” wrote the investigators.
The hierarchy of evidence establishes that meta-analyses and randomized clinical trials (RCTs) offer the highest quality of evidence, and RCTs clearly offer the best assessment of effects of therapy. Observational studies, on the other hand, have considerable limitations, yet they have helped establish key causal relationships. Large prospective cohort studies, such as Framingham Heart Study, the National Child Development Study, and the Nurse’s Health Study, among others, continue to provide important data.
The study by Henson et al. reopens the debate over the value of RCTs versus observational studies (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
It is well known the nonrandomized studies can produce misleading results due to selection bias. Selection bias favoring treatment for healthier patients produces improved survival among treated patients. On the other hand, selection bias disfavoring treatment occurs in studies of patients with more aggressive tumors who receive more treatment, and despite interventions, have worse outcomes.
The observation that postmastectomy radiotherapy is associated with worse outcomes reflects a real phenomenon, likely explained by confounding by indication. Patients treated with radiotherapy likely have worse prognoses, and despite therapy, had worse outcomes.
In the Henson et al. study, detailed data on treatment, comorbid conditions, or other health determinants were not available. In recognizing the importance of observational data for comparative effectiveness research, we must also understand and account for the limitations.
There should be no battle between RCTs and observational data, as both can provide valid and important knowledge to help clinicians make decisions and deliver evidence-based compassionate care.
Dr. Mariana Chavez-MacGregor is assistant professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston. Dr. Sharon Giordano is associate professor in the department of breast medical oncology at the university. These remarks were part of an editorial (J Clin Oncol. 2016 Jan. 18. doi: 10.1200/JCO.2015.64.7487). Dr. Chavez-MacGregor reported financial ties with Roche, Novarits, InVitae, Pfizer, Genomic Health, and Genentech/Roche. Dr. Giordano reported having no disclosures.
The hierarchy of evidence establishes that meta-analyses and randomized clinical trials (RCTs) offer the highest quality of evidence, and RCTs clearly offer the best assessment of effects of therapy. Observational studies, on the other hand, have considerable limitations, yet they have helped establish key causal relationships. Large prospective cohort studies, such as Framingham Heart Study, the National Child Development Study, and the Nurse’s Health Study, among others, continue to provide important data.
The study by Henson et al. reopens the debate over the value of RCTs versus observational studies (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
It is well known the nonrandomized studies can produce misleading results due to selection bias. Selection bias favoring treatment for healthier patients produces improved survival among treated patients. On the other hand, selection bias disfavoring treatment occurs in studies of patients with more aggressive tumors who receive more treatment, and despite interventions, have worse outcomes.
The observation that postmastectomy radiotherapy is associated with worse outcomes reflects a real phenomenon, likely explained by confounding by indication. Patients treated with radiotherapy likely have worse prognoses, and despite therapy, had worse outcomes.
In the Henson et al. study, detailed data on treatment, comorbid conditions, or other health determinants were not available. In recognizing the importance of observational data for comparative effectiveness research, we must also understand and account for the limitations.
There should be no battle between RCTs and observational data, as both can provide valid and important knowledge to help clinicians make decisions and deliver evidence-based compassionate care.
Dr. Mariana Chavez-MacGregor is assistant professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston. Dr. Sharon Giordano is associate professor in the department of breast medical oncology at the university. These remarks were part of an editorial (J Clin Oncol. 2016 Jan. 18. doi: 10.1200/JCO.2015.64.7487). Dr. Chavez-MacGregor reported financial ties with Roche, Novarits, InVitae, Pfizer, Genomic Health, and Genentech/Roche. Dr. Giordano reported having no disclosures.
The hierarchy of evidence establishes that meta-analyses and randomized clinical trials (RCTs) offer the highest quality of evidence, and RCTs clearly offer the best assessment of effects of therapy. Observational studies, on the other hand, have considerable limitations, yet they have helped establish key causal relationships. Large prospective cohort studies, such as Framingham Heart Study, the National Child Development Study, and the Nurse’s Health Study, among others, continue to provide important data.
The study by Henson et al. reopens the debate over the value of RCTs versus observational studies (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
It is well known the nonrandomized studies can produce misleading results due to selection bias. Selection bias favoring treatment for healthier patients produces improved survival among treated patients. On the other hand, selection bias disfavoring treatment occurs in studies of patients with more aggressive tumors who receive more treatment, and despite interventions, have worse outcomes.
The observation that postmastectomy radiotherapy is associated with worse outcomes reflects a real phenomenon, likely explained by confounding by indication. Patients treated with radiotherapy likely have worse prognoses, and despite therapy, had worse outcomes.
In the Henson et al. study, detailed data on treatment, comorbid conditions, or other health determinants were not available. In recognizing the importance of observational data for comparative effectiveness research, we must also understand and account for the limitations.
There should be no battle between RCTs and observational data, as both can provide valid and important knowledge to help clinicians make decisions and deliver evidence-based compassionate care.
Dr. Mariana Chavez-MacGregor is assistant professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston. Dr. Sharon Giordano is associate professor in the department of breast medical oncology at the university. These remarks were part of an editorial (J Clin Oncol. 2016 Jan. 18. doi: 10.1200/JCO.2015.64.7487). Dr. Chavez-MacGregor reported financial ties with Roche, Novarits, InVitae, Pfizer, Genomic Health, and Genentech/Roche. Dr. Giordano reported having no disclosures.
Analyses of mortality after breast cancer radiotherapy using randomized clinical trial data versus observational data produced strikingly different results, according to researchers.
Analyses of randomized data indicated radiotherapy reduced mortality after breast-conserving surgery and mastectomy in node-positive disease; by contrast, SEER data analyses showed radiotherapy was associated with a significantly larger reduction in breast cancer mortality after breast-conserving surgery but higher mortality after mastectomy. Among patients with node-positive breast cancer who underwent mastectomy and axillary dissection, radiotherapy was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 1.31-1.37).
Furthermore, analyses of randomized trial data indicated increased mortality from heart disease (1.27; 1.12-1.44) and lung cancer (1.78; 1.30-2.46) following radiotherapy, but analyses of SEER data indicated reduced mortality from heart disease (0.56; 0.53-0.60) and lung cancer (0.86; 0.75-0.99) associated with radiotherapy.
“It is not plausible that these negative associations in the SEER data are causal and, clearly, they are strongly influenced by factors other than the effect or radiotherapy,” wrote Dr. Katherine Henson of the University of Oxford (England), and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
“Randomized trials are needed wherever possible to investigate the effect of treatment on mortality from the original cancer,” according to the investigators, and, “selection biases can be problematic even when analyzing treatment-related toxicities using observational data.”
Analyses of randomized data demonstrated reduced breast cancer mortality associated with radiotherapy after breast-conserving surgery (0.82; 95% CI, 0.75-0.90). Compared with randomized data, analyses of observational data showed a much greater reduction in mortality associated with radiotherapy after breast-conserving surgery (0.64; 95% CI, 0.62-0.66).
Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta analyses of 17 trials (n = 10,801) of radiotherapy after breast-conserving surgery, 14 trials (n = 3,131) of radiotherapy after mastectomy, and 78 trials (n = 42,080) of mortality from causes other than breast cancer. Observational evidence came from the SEER data base (n = 393,840).
The researchers offered plausible explanations for selection bias that may have resulted in the divergent results calculated using observational data. Because radiotherapy following mastectomy is indicated only in the presence of adverse disease characteristics, patients who did not receive radiotherapy may have survived longer because of especially favorable characteristics, despite of lack of radiotherapy.
“When evaluating rare late effects for which sufficient randomized evidence cannot reasonably be obtained, analyses of observational data comparing treated and untreated patients may often be the only source of information, but they must always be interpreted with considerable caution,” wrote the investigators.
Analyses of mortality after breast cancer radiotherapy using randomized clinical trial data versus observational data produced strikingly different results, according to researchers.
Analyses of randomized data indicated radiotherapy reduced mortality after breast-conserving surgery and mastectomy in node-positive disease; by contrast, SEER data analyses showed radiotherapy was associated with a significantly larger reduction in breast cancer mortality after breast-conserving surgery but higher mortality after mastectomy. Among patients with node-positive breast cancer who underwent mastectomy and axillary dissection, radiotherapy was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 1.31-1.37).
Furthermore, analyses of randomized trial data indicated increased mortality from heart disease (1.27; 1.12-1.44) and lung cancer (1.78; 1.30-2.46) following radiotherapy, but analyses of SEER data indicated reduced mortality from heart disease (0.56; 0.53-0.60) and lung cancer (0.86; 0.75-0.99) associated with radiotherapy.
“It is not plausible that these negative associations in the SEER data are causal and, clearly, they are strongly influenced by factors other than the effect or radiotherapy,” wrote Dr. Katherine Henson of the University of Oxford (England), and colleagues (J Clin Oncol. 2016 Jan 18. doi: 10.1200/JCO.2015.62.0294).
“Randomized trials are needed wherever possible to investigate the effect of treatment on mortality from the original cancer,” according to the investigators, and, “selection biases can be problematic even when analyzing treatment-related toxicities using observational data.”
Analyses of randomized data demonstrated reduced breast cancer mortality associated with radiotherapy after breast-conserving surgery (0.82; 95% CI, 0.75-0.90). Compared with randomized data, analyses of observational data showed a much greater reduction in mortality associated with radiotherapy after breast-conserving surgery (0.64; 95% CI, 0.62-0.66).
Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta analyses of 17 trials (n = 10,801) of radiotherapy after breast-conserving surgery, 14 trials (n = 3,131) of radiotherapy after mastectomy, and 78 trials (n = 42,080) of mortality from causes other than breast cancer. Observational evidence came from the SEER data base (n = 393,840).
The researchers offered plausible explanations for selection bias that may have resulted in the divergent results calculated using observational data. Because radiotherapy following mastectomy is indicated only in the presence of adverse disease characteristics, patients who did not receive radiotherapy may have survived longer because of especially favorable characteristics, despite of lack of radiotherapy.
“When evaluating rare late effects for which sufficient randomized evidence cannot reasonably be obtained, analyses of observational data comparing treated and untreated patients may often be the only source of information, but they must always be interpreted with considerable caution,” wrote the investigators.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Analysis of randomized trial data compared with observational data on mortality after breast cancer radiotherapy shows strikingly different results.
Major finding: Radiotherapy after mastectomy and axillary dissection was associated with lower mortality by clinical trial data (rate ratio, 0.84; 95% CI, 0.76-0.94) but was associated with higher mortality by observational data (1.34; 95% CI, 1.31-1.37).
Data sources: Randomized evidence came from the Early Breast Cancer Trialists’ Collaborative Group, meta-analyses of 31 trials (n = 13,932); observational evidence came from the SEER data base (n = 393,840).
Disclosures: Dr. Henson reported having no disclosures. One of her coauthors reported ties to industry.
Death from late effects of childhood cancer on decline
The rate of death from treatment-related late effects such as subsequent cancers and cardiopulmonary conditions has decreased among childhood cancer survivors, according to researchers.
At 15 years post diagnosis, the cumulative incidence of death from any cause for survivors diagnosed in the 1970s was 10.7%, 7.9% for those diagnosed in the 1980s, and 5.8% for those diagnosed in the 1990s (P less than .001). The cumulative incidence of death due to health-related causes, which include late effects of cancer therapy, were 3.1%, 2.4%, and 1.9%, respectively (P less than .001).
Results indicate that “the strategy of reducing treatment exposures in order to decrease the frequency of late effects is translating into a significant reduction in observed late mortality and an extension of the life span of children and adolescents who are successfully treated for cancer,” wrote Dr. Gregory Armstrong of St. Jude Children’s Research Hospital, Memphis, Tenn., and colleagues (N Engl J Med. 2016 Jan 13. doi: 10.1056/NEJMoa1510795).
A multivariate model showed that more recent treatment eras were associated with a reduced rate of death. The adjusted relative rate per every 5 years for death due to subsequent neoplasms was 0.83 (95% CI, 0.78-0.88), for cardiac causes, 0.77 (0.68-0.86), and for pulmonary causes, 0.77 (0.66-0.89).
Reductions across treatment eras in the rate of death from health-related causes were observed among survivors of acute lymphoblastic leukemia (3.2% in the early 1970s to 2.1% in the 1990s, P less than .001), Hodgkin lymphoma (5.3% to 2.6%, P = .006), Wilms tumor (2.6% to 0.4%, P = .005), and astrocytoma (4.7% to 1.8%, P = .02).
Temporal reductions in exposure to radiotherapy and anthracyclines occurred in treatment for acute lymphoblastic leukemia, Hodgkin lymphoma, Wilms tumor, and astrocytoma. Health-related mortality reductions were observed concurrently with reduced therapeutic exposures for acute lymphoblastic leukemia and Wilms tumor. For Hodgkin lymphoma and astrocytoma, other factors such as improved screening for late effects of cancer treatment appear to account for reductions in late health-related mortality.
For certain cancers, primarily neuroblastoma, late mortality has increased in recent decades. Increased therapeutic intensity has improved 5-year survival but increased the risk of late effects.
The reduced rate of death from recurrence or progression of primary cancers is the main driver to reductions in all-cause mortality, consistent with results from previous studies.
The retrospective Childhood Cancer Survivor Study evaluated 34,033 patients diagnosed at 31 hospitals in the United States and Canada from 1970 through 1999. In total, 3,958 deaths occurred, 2,002 due to primary cancer and 1,618 due to health-related causes: 746 subsequent neoplasms, 241 cardiac causes, 137 pulmonary causes, and 494 from other causes.
The rate of death from treatment-related late effects such as subsequent cancers and cardiopulmonary conditions has decreased among childhood cancer survivors, according to researchers.
At 15 years post diagnosis, the cumulative incidence of death from any cause for survivors diagnosed in the 1970s was 10.7%, 7.9% for those diagnosed in the 1980s, and 5.8% for those diagnosed in the 1990s (P less than .001). The cumulative incidence of death due to health-related causes, which include late effects of cancer therapy, were 3.1%, 2.4%, and 1.9%, respectively (P less than .001).
Results indicate that “the strategy of reducing treatment exposures in order to decrease the frequency of late effects is translating into a significant reduction in observed late mortality and an extension of the life span of children and adolescents who are successfully treated for cancer,” wrote Dr. Gregory Armstrong of St. Jude Children’s Research Hospital, Memphis, Tenn., and colleagues (N Engl J Med. 2016 Jan 13. doi: 10.1056/NEJMoa1510795).
A multivariate model showed that more recent treatment eras were associated with a reduced rate of death. The adjusted relative rate per every 5 years for death due to subsequent neoplasms was 0.83 (95% CI, 0.78-0.88), for cardiac causes, 0.77 (0.68-0.86), and for pulmonary causes, 0.77 (0.66-0.89).
Reductions across treatment eras in the rate of death from health-related causes were observed among survivors of acute lymphoblastic leukemia (3.2% in the early 1970s to 2.1% in the 1990s, P less than .001), Hodgkin lymphoma (5.3% to 2.6%, P = .006), Wilms tumor (2.6% to 0.4%, P = .005), and astrocytoma (4.7% to 1.8%, P = .02).
Temporal reductions in exposure to radiotherapy and anthracyclines occurred in treatment for acute lymphoblastic leukemia, Hodgkin lymphoma, Wilms tumor, and astrocytoma. Health-related mortality reductions were observed concurrently with reduced therapeutic exposures for acute lymphoblastic leukemia and Wilms tumor. For Hodgkin lymphoma and astrocytoma, other factors such as improved screening for late effects of cancer treatment appear to account for reductions in late health-related mortality.
For certain cancers, primarily neuroblastoma, late mortality has increased in recent decades. Increased therapeutic intensity has improved 5-year survival but increased the risk of late effects.
The reduced rate of death from recurrence or progression of primary cancers is the main driver to reductions in all-cause mortality, consistent with results from previous studies.
The retrospective Childhood Cancer Survivor Study evaluated 34,033 patients diagnosed at 31 hospitals in the United States and Canada from 1970 through 1999. In total, 3,958 deaths occurred, 2,002 due to primary cancer and 1,618 due to health-related causes: 746 subsequent neoplasms, 241 cardiac causes, 137 pulmonary causes, and 494 from other causes.
The rate of death from treatment-related late effects such as subsequent cancers and cardiopulmonary conditions has decreased among childhood cancer survivors, according to researchers.
At 15 years post diagnosis, the cumulative incidence of death from any cause for survivors diagnosed in the 1970s was 10.7%, 7.9% for those diagnosed in the 1980s, and 5.8% for those diagnosed in the 1990s (P less than .001). The cumulative incidence of death due to health-related causes, which include late effects of cancer therapy, were 3.1%, 2.4%, and 1.9%, respectively (P less than .001).
Results indicate that “the strategy of reducing treatment exposures in order to decrease the frequency of late effects is translating into a significant reduction in observed late mortality and an extension of the life span of children and adolescents who are successfully treated for cancer,” wrote Dr. Gregory Armstrong of St. Jude Children’s Research Hospital, Memphis, Tenn., and colleagues (N Engl J Med. 2016 Jan 13. doi: 10.1056/NEJMoa1510795).
A multivariate model showed that more recent treatment eras were associated with a reduced rate of death. The adjusted relative rate per every 5 years for death due to subsequent neoplasms was 0.83 (95% CI, 0.78-0.88), for cardiac causes, 0.77 (0.68-0.86), and for pulmonary causes, 0.77 (0.66-0.89).
Reductions across treatment eras in the rate of death from health-related causes were observed among survivors of acute lymphoblastic leukemia (3.2% in the early 1970s to 2.1% in the 1990s, P less than .001), Hodgkin lymphoma (5.3% to 2.6%, P = .006), Wilms tumor (2.6% to 0.4%, P = .005), and astrocytoma (4.7% to 1.8%, P = .02).
Temporal reductions in exposure to radiotherapy and anthracyclines occurred in treatment for acute lymphoblastic leukemia, Hodgkin lymphoma, Wilms tumor, and astrocytoma. Health-related mortality reductions were observed concurrently with reduced therapeutic exposures for acute lymphoblastic leukemia and Wilms tumor. For Hodgkin lymphoma and astrocytoma, other factors such as improved screening for late effects of cancer treatment appear to account for reductions in late health-related mortality.
For certain cancers, primarily neuroblastoma, late mortality has increased in recent decades. Increased therapeutic intensity has improved 5-year survival but increased the risk of late effects.
The reduced rate of death from recurrence or progression of primary cancers is the main driver to reductions in all-cause mortality, consistent with results from previous studies.
The retrospective Childhood Cancer Survivor Study evaluated 34,033 patients diagnosed at 31 hospitals in the United States and Canada from 1970 through 1999. In total, 3,958 deaths occurred, 2,002 due to primary cancer and 1,618 due to health-related causes: 746 subsequent neoplasms, 241 cardiac causes, 137 pulmonary causes, and 494 from other causes.
Key clinical point: Five-year survivors of childhood cancers have increased lifespans due in part to reduced rates of treatment-related late effects.
Major finding: At 15 years post diagnosis, the cumulative incidence of death from any cause for survivors diagnosed in the 1970s was 10.7%; in the 1980s, 7.9%; and in the 1990s, 5.8% (P less than .001). The cumulative incidence of death due to health-related causes, which include late effects of cancer therapy, were 3.1%, 2.4%, and 1.9%, respectively (P less than .001).
Data source: The retrospective Childhood Cancer Survivor Study, evaluating 34,033 patients diagnosed at 31 hospitals in the United States and Canada from 1970 through 1999; 3,958 deaths occurred.
Disclosures: Dr. Armstrong and coauthors reported having no disclosures.
Volasertib active against platinum-resistant, refractory ovarian cancer
In patients with recurrent platinum-resistant or platinum-refractory ovarian cancer, volasertib demonstrated activity and resulted in manageable adverse effects, which were mostly hematologic, according to phase II trial results.
The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and with single-agent chemotherapy was 43.1% (95% CI, 29.5-56.7). The median progression-free survival (PFS) for volasertib was 13.1 weeks (interquartile range, 6.6-30.1) and was 20.6 weeks (11.6-30.7) for chemotherapy (hazard ratio, 1.01; 95% CI, 0.66-1.53). In the volasertib arm, six patients (11%) achieved PFS greater than or equal to 1 year, in contrast to no patients in the chemotherapy arm.
“This suggests that volasertib might achieve a durable response in a subpopulation of patients with heavily pretreated resistant/refractory ovarian cancer,” wrote Dr. Eric Pujade-Lauraine of the Centre des Cancers de la Femme et Recherche Clinique, Paris, and head of the Group d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens (GINECO), and his colleagues (J Clin Onc. 2016 Jan 11. doi: 10.1200/JCO.2015.62.1474).
“Further clinical development of volasertib in this setting should [be pursued only] after a biomarker is identified to select for patients with a greater chance of response to monotherapy or combination therapies,” they wrote.
Immunohistochemical analysis of potential biomarkers, including Plk1, Ki-67, Aurora kinases A and B, and pHH3, showed no relationship between biomarker expression and drug response.
The phase II open-label, randomized controlled trial evaluated 109 patients with ovarian cancer who had undergone two or three prior lines of treatment. Patients were randomized 1:1 to receive volasertib, a cell cycle kinase inhibitor selective for Plk, or an investigator’s choice of single-agent, nonplatinum, cytotoxic chemotherapy.
The volasertib arm had a higher rate of hematologic adverse events than did the chemotherapy arm, with 33 patients (61.1%) experiencing grade 3 or greater toxicity. Nonhematologic drug–related toxicity, such as nausea, fatigue, and peripheral neuropathy, occurred more frequently in the chemotherapy arm. The rate of treatment discontinuation because of adverse events was higher in the chemotherapy vs. volasertib arm (27.3% vs. 13.0%, respectively).
In patients with recurrent platinum-resistant or platinum-refractory ovarian cancer, volasertib demonstrated activity and resulted in manageable adverse effects, which were mostly hematologic, according to phase II trial results.
The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and with single-agent chemotherapy was 43.1% (95% CI, 29.5-56.7). The median progression-free survival (PFS) for volasertib was 13.1 weeks (interquartile range, 6.6-30.1) and was 20.6 weeks (11.6-30.7) for chemotherapy (hazard ratio, 1.01; 95% CI, 0.66-1.53). In the volasertib arm, six patients (11%) achieved PFS greater than or equal to 1 year, in contrast to no patients in the chemotherapy arm.
“This suggests that volasertib might achieve a durable response in a subpopulation of patients with heavily pretreated resistant/refractory ovarian cancer,” wrote Dr. Eric Pujade-Lauraine of the Centre des Cancers de la Femme et Recherche Clinique, Paris, and head of the Group d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens (GINECO), and his colleagues (J Clin Onc. 2016 Jan 11. doi: 10.1200/JCO.2015.62.1474).
“Further clinical development of volasertib in this setting should [be pursued only] after a biomarker is identified to select for patients with a greater chance of response to monotherapy or combination therapies,” they wrote.
Immunohistochemical analysis of potential biomarkers, including Plk1, Ki-67, Aurora kinases A and B, and pHH3, showed no relationship between biomarker expression and drug response.
The phase II open-label, randomized controlled trial evaluated 109 patients with ovarian cancer who had undergone two or three prior lines of treatment. Patients were randomized 1:1 to receive volasertib, a cell cycle kinase inhibitor selective for Plk, or an investigator’s choice of single-agent, nonplatinum, cytotoxic chemotherapy.
The volasertib arm had a higher rate of hematologic adverse events than did the chemotherapy arm, with 33 patients (61.1%) experiencing grade 3 or greater toxicity. Nonhematologic drug–related toxicity, such as nausea, fatigue, and peripheral neuropathy, occurred more frequently in the chemotherapy arm. The rate of treatment discontinuation because of adverse events was higher in the chemotherapy vs. volasertib arm (27.3% vs. 13.0%, respectively).
In patients with recurrent platinum-resistant or platinum-refractory ovarian cancer, volasertib demonstrated activity and resulted in manageable adverse effects, which were mostly hematologic, according to phase II trial results.
The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and with single-agent chemotherapy was 43.1% (95% CI, 29.5-56.7). The median progression-free survival (PFS) for volasertib was 13.1 weeks (interquartile range, 6.6-30.1) and was 20.6 weeks (11.6-30.7) for chemotherapy (hazard ratio, 1.01; 95% CI, 0.66-1.53). In the volasertib arm, six patients (11%) achieved PFS greater than or equal to 1 year, in contrast to no patients in the chemotherapy arm.
“This suggests that volasertib might achieve a durable response in a subpopulation of patients with heavily pretreated resistant/refractory ovarian cancer,” wrote Dr. Eric Pujade-Lauraine of the Centre des Cancers de la Femme et Recherche Clinique, Paris, and head of the Group d’Investigateurs Nationaux pour l’Étude des Cancers Ovariens (GINECO), and his colleagues (J Clin Onc. 2016 Jan 11. doi: 10.1200/JCO.2015.62.1474).
“Further clinical development of volasertib in this setting should [be pursued only] after a biomarker is identified to select for patients with a greater chance of response to monotherapy or combination therapies,” they wrote.
Immunohistochemical analysis of potential biomarkers, including Plk1, Ki-67, Aurora kinases A and B, and pHH3, showed no relationship between biomarker expression and drug response.
The phase II open-label, randomized controlled trial evaluated 109 patients with ovarian cancer who had undergone two or three prior lines of treatment. Patients were randomized 1:1 to receive volasertib, a cell cycle kinase inhibitor selective for Plk, or an investigator’s choice of single-agent, nonplatinum, cytotoxic chemotherapy.
The volasertib arm had a higher rate of hematologic adverse events than did the chemotherapy arm, with 33 patients (61.1%) experiencing grade 3 or greater toxicity. Nonhematologic drug–related toxicity, such as nausea, fatigue, and peripheral neuropathy, occurred more frequently in the chemotherapy arm. The rate of treatment discontinuation because of adverse events was higher in the chemotherapy vs. volasertib arm (27.3% vs. 13.0%, respectively).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Volasertib showed activity against platinum-resistant or -refractory ovarian cancer; adverse effects were mostly hematologic and manageable.
Major finding: The 24-week disease control rate with volasertib was 30.6% (95% confidence interval, 18.0-43.2) and 43.1% (29.5-56.7) with chemotherapy.
Data source: The phase II randomized controlled trial evaluated 109 patients with platinum-resistant or -refractory ovarian cancer who received volasertib or single-agent chemotherapy.
Disclosures: Research was supported by Boehringer Ingelheim. Dr. Pujade-Lauraine reported having no disclosures. Several of his coauthors reported ties to industry.
Antilymphocyte globulin curbs chronic graft-versus-host disease
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
FROM NEJM
Key clinical point: Chronic graft-versus-host disease (GVHD) after allogeneic stem cell transplantation with peripheral blood stem cells was less than half as frequent with antilymphocyte globulin added to the conditioning regimen.
Major finding: Cumulative incidence of chronic GVHD at 2 years was 32.2% with antilymphocyte globulin vs 68.7% without it.
Data source: The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012.
Disclosures: Research was supported in part by Neovii Biotech. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Daratumumab clinically active, well tolerated in heavily treated multiple myeloma
In patients with multiple myeloma who were treated with at least three prior therapies (median five), daratumumab demonstrated substantial clinical activity and was well tolerated, investigators reported in the Lancet.
Overall response rates were observed in 31 of 106 people (ORR 29.2%; 95% confidence interval, 20.8-38.9), stringent complete responses in 3, and very good partial responses in 10 people. In total, 87 patients (82%) had received more than three lines of therapy: all patients had been treated previously with proteasome inhibitors and immunomodulatory drugs, and dexamethasone. In addition, 103 (97%) were refractory to the last line of therapy before study enrollment, and 95% were refractory to the most recent proteasome inhibitors and immunomodulatory drugs.
“Resistance to any previous therapy had no effect on the activity of daratumumab, lending support to a novel mechanism of action, but these findings need to be confirmed in larger studies,” wrote Dr. Sagar Lonial, executive vice chair of the department of hematology medical oncology, Emory University, Atlanta, and colleagues (Lancet. 2016 Jan 7. doi: 10.1016/S0140-6736[15]01120-4).
Daratumumab was well tolerated. The most common hematologic treatment-emergent adverse events of any grade were anemia (33%), thrombocytopenia (25%), and neutropenia (23%). The overall favorable safety profile makes it a promising candidate for combination regimens, and the monoclonal IgG1 antibody has shown early activity in combination with lenalidomide and dexamethasone, according to the researchers.
The open-label, multicenter, phase II trial included 106 patients who received daratumumab 16 mg/kg. The median time since initial diagnosis was 4.8 years (1.1-23.8 years), median number of previous therapies was 5 (2-14), and 80% of patients had received autologous stem cell transplantation.
In patients with multiple myeloma who were treated with at least three prior therapies (median five), daratumumab demonstrated substantial clinical activity and was well tolerated, investigators reported in the Lancet.
Overall response rates were observed in 31 of 106 people (ORR 29.2%; 95% confidence interval, 20.8-38.9), stringent complete responses in 3, and very good partial responses in 10 people. In total, 87 patients (82%) had received more than three lines of therapy: all patients had been treated previously with proteasome inhibitors and immunomodulatory drugs, and dexamethasone. In addition, 103 (97%) were refractory to the last line of therapy before study enrollment, and 95% were refractory to the most recent proteasome inhibitors and immunomodulatory drugs.
“Resistance to any previous therapy had no effect on the activity of daratumumab, lending support to a novel mechanism of action, but these findings need to be confirmed in larger studies,” wrote Dr. Sagar Lonial, executive vice chair of the department of hematology medical oncology, Emory University, Atlanta, and colleagues (Lancet. 2016 Jan 7. doi: 10.1016/S0140-6736[15]01120-4).
Daratumumab was well tolerated. The most common hematologic treatment-emergent adverse events of any grade were anemia (33%), thrombocytopenia (25%), and neutropenia (23%). The overall favorable safety profile makes it a promising candidate for combination regimens, and the monoclonal IgG1 antibody has shown early activity in combination with lenalidomide and dexamethasone, according to the researchers.
The open-label, multicenter, phase II trial included 106 patients who received daratumumab 16 mg/kg. The median time since initial diagnosis was 4.8 years (1.1-23.8 years), median number of previous therapies was 5 (2-14), and 80% of patients had received autologous stem cell transplantation.
In patients with multiple myeloma who were treated with at least three prior therapies (median five), daratumumab demonstrated substantial clinical activity and was well tolerated, investigators reported in the Lancet.
Overall response rates were observed in 31 of 106 people (ORR 29.2%; 95% confidence interval, 20.8-38.9), stringent complete responses in 3, and very good partial responses in 10 people. In total, 87 patients (82%) had received more than three lines of therapy: all patients had been treated previously with proteasome inhibitors and immunomodulatory drugs, and dexamethasone. In addition, 103 (97%) were refractory to the last line of therapy before study enrollment, and 95% were refractory to the most recent proteasome inhibitors and immunomodulatory drugs.
“Resistance to any previous therapy had no effect on the activity of daratumumab, lending support to a novel mechanism of action, but these findings need to be confirmed in larger studies,” wrote Dr. Sagar Lonial, executive vice chair of the department of hematology medical oncology, Emory University, Atlanta, and colleagues (Lancet. 2016 Jan 7. doi: 10.1016/S0140-6736[15]01120-4).
Daratumumab was well tolerated. The most common hematologic treatment-emergent adverse events of any grade were anemia (33%), thrombocytopenia (25%), and neutropenia (23%). The overall favorable safety profile makes it a promising candidate for combination regimens, and the monoclonal IgG1 antibody has shown early activity in combination with lenalidomide and dexamethasone, according to the researchers.
The open-label, multicenter, phase II trial included 106 patients who received daratumumab 16 mg/kg. The median time since initial diagnosis was 4.8 years (1.1-23.8 years), median number of previous therapies was 5 (2-14), and 80% of patients had received autologous stem cell transplantation.
FROM THE LANCET
Key clinical point: Daratumumab monotherapy was clinically active and well tolerated in patients with multiple myeloma who were treated with at least three prior therapies.
Major finding: In the 16 mg/kg group, 31 of 106 patients achieved an overall response rate (ORR 29.2%; 95% confidence interval, 20.8-38.9); 3 achieved a stringent complete response; 10 achieved a very good partial response.
Data source: The open-label, multicenter, phase II trial included 106 patients who received daratumumab 16 mg/kg.
Disclosures: Janssen Research & Development contributed to the design of the study. Dr. Lonial reported ties Bristol-Myers Squibb, Celgene, Janssen, Millennium, Novartis, and Onyx. Several of his coauthors reported ties to industry.
KIR2DL5B genotype predicts outcome in chronic phase–CML
The presence of KIR2DL5B was associated with lower rates of major molecular response (MMR), transformation-free survival, and event-free survival (but not overall survival) in patients with chronic phase–chronic myeloid leukemia (CP-CML) treated with sequential imatinib/nilotinib, according to researchers.
Univariate analysis demonstrated a significant association between KIR2DL5B and achievement of a major molecular response, with hazard ratio 0.423 (95% CI, 0.262-0.682; P less than .001). Other KIR genotypes, KIR2DL2pos and KIR2DS3pos, were also associated with inferior achievement of MMR, probably because of their association with KIR2DL5B due to linkage disequilibrium among KIR genes, according to the investigators.
“Our findings suggest that even with the potent second-generation TKI [tyrosine kinase inhibitor] nilotinib, KIR genotypes, a predetermined genetic host factor, may still be one of the most discriminatory prognostic markers available at baseline,” wrote Dr. David T. Yeung of the department of genetics and molecular pathology, Centre for Cancer Biology and the University of Adelaide, South Australia, and colleagues (Blood 2015 Dec 17. doi:10.1182/blood-2015-07-655589).
Killer immunoglobulin-like receptors (KIRs) contribute to natural killer (NK) cell–mediated killing of tumor cells, in both activating and inhibitory roles. Normal cells are spared through actions of inhibitory KIRs. Although the mechanism underlying the association between KIR2DL5B and CP-CML treatment outcomes is still unclear, the gene encodes an inhibitory KIR receptor, the absence of which may increase efficiency of NK-mediated killing of leukemic stem cells, researchers suggested.
The Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study included 210 patients with CP-CML who were treated with imatinib initially, and nilotinib subsequently if predetermined molecular targets were not met. The KIR substudy included 148 patients with samples available for genotyping.
KIR genotype frequencies observed in this study were similar to other white populations reported in the Allele Frequency Database.
Early molecular response was also significantly associated with treatment outcomes, independent of KIR prognostic significance, and may add additional prognostic information, available 3 months after treatment commences.
“In contrast, KIR2DL5B can identify, at baseline, the 20% of patients with a transformation risk of [about] 10% over 2 years versus the 80% of patients with a transformation risk of less than 3%,” the authors wrote. They suggest that KIR2DL5B, combined with other predictive markers, may enable targeted early interventions to improve outcomes.
The presence of KIR2DL5B was associated with lower rates of major molecular response (MMR), transformation-free survival, and event-free survival (but not overall survival) in patients with chronic phase–chronic myeloid leukemia (CP-CML) treated with sequential imatinib/nilotinib, according to researchers.
Univariate analysis demonstrated a significant association between KIR2DL5B and achievement of a major molecular response, with hazard ratio 0.423 (95% CI, 0.262-0.682; P less than .001). Other KIR genotypes, KIR2DL2pos and KIR2DS3pos, were also associated with inferior achievement of MMR, probably because of their association with KIR2DL5B due to linkage disequilibrium among KIR genes, according to the investigators.
“Our findings suggest that even with the potent second-generation TKI [tyrosine kinase inhibitor] nilotinib, KIR genotypes, a predetermined genetic host factor, may still be one of the most discriminatory prognostic markers available at baseline,” wrote Dr. David T. Yeung of the department of genetics and molecular pathology, Centre for Cancer Biology and the University of Adelaide, South Australia, and colleagues (Blood 2015 Dec 17. doi:10.1182/blood-2015-07-655589).
Killer immunoglobulin-like receptors (KIRs) contribute to natural killer (NK) cell–mediated killing of tumor cells, in both activating and inhibitory roles. Normal cells are spared through actions of inhibitory KIRs. Although the mechanism underlying the association between KIR2DL5B and CP-CML treatment outcomes is still unclear, the gene encodes an inhibitory KIR receptor, the absence of which may increase efficiency of NK-mediated killing of leukemic stem cells, researchers suggested.
The Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study included 210 patients with CP-CML who were treated with imatinib initially, and nilotinib subsequently if predetermined molecular targets were not met. The KIR substudy included 148 patients with samples available for genotyping.
KIR genotype frequencies observed in this study were similar to other white populations reported in the Allele Frequency Database.
Early molecular response was also significantly associated with treatment outcomes, independent of KIR prognostic significance, and may add additional prognostic information, available 3 months after treatment commences.
“In contrast, KIR2DL5B can identify, at baseline, the 20% of patients with a transformation risk of [about] 10% over 2 years versus the 80% of patients with a transformation risk of less than 3%,” the authors wrote. They suggest that KIR2DL5B, combined with other predictive markers, may enable targeted early interventions to improve outcomes.
The presence of KIR2DL5B was associated with lower rates of major molecular response (MMR), transformation-free survival, and event-free survival (but not overall survival) in patients with chronic phase–chronic myeloid leukemia (CP-CML) treated with sequential imatinib/nilotinib, according to researchers.
Univariate analysis demonstrated a significant association between KIR2DL5B and achievement of a major molecular response, with hazard ratio 0.423 (95% CI, 0.262-0.682; P less than .001). Other KIR genotypes, KIR2DL2pos and KIR2DS3pos, were also associated with inferior achievement of MMR, probably because of their association with KIR2DL5B due to linkage disequilibrium among KIR genes, according to the investigators.
“Our findings suggest that even with the potent second-generation TKI [tyrosine kinase inhibitor] nilotinib, KIR genotypes, a predetermined genetic host factor, may still be one of the most discriminatory prognostic markers available at baseline,” wrote Dr. David T. Yeung of the department of genetics and molecular pathology, Centre for Cancer Biology and the University of Adelaide, South Australia, and colleagues (Blood 2015 Dec 17. doi:10.1182/blood-2015-07-655589).
Killer immunoglobulin-like receptors (KIRs) contribute to natural killer (NK) cell–mediated killing of tumor cells, in both activating and inhibitory roles. Normal cells are spared through actions of inhibitory KIRs. Although the mechanism underlying the association between KIR2DL5B and CP-CML treatment outcomes is still unclear, the gene encodes an inhibitory KIR receptor, the absence of which may increase efficiency of NK-mediated killing of leukemic stem cells, researchers suggested.
The Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study included 210 patients with CP-CML who were treated with imatinib initially, and nilotinib subsequently if predetermined molecular targets were not met. The KIR substudy included 148 patients with samples available for genotyping.
KIR genotype frequencies observed in this study were similar to other white populations reported in the Allele Frequency Database.
Early molecular response was also significantly associated with treatment outcomes, independent of KIR prognostic significance, and may add additional prognostic information, available 3 months after treatment commences.
“In contrast, KIR2DL5B can identify, at baseline, the 20% of patients with a transformation risk of [about] 10% over 2 years versus the 80% of patients with a transformation risk of less than 3%,” the authors wrote. They suggest that KIR2DL5B, combined with other predictive markers, may enable targeted early interventions to improve outcomes.
FROM BLOOD
Key clinical point: The presence of KIR2DL5B was associated with worse outcomes in patients with chronic phase–chronic myeloid leukemia treated with sequential imatinib/nilotinib.
Major finding: Achievement of a major molecular response was associated with the KIR2DL5B genotype (HR, 0.423; 95% CI, 0.262-0.682; P less than .001).
Data source: A substudy of the Therapeutic Intensification in De Novo Leukaemia (TIDEL-II) study that included 148 patients with KIR genotype data available.
Disclosures: Support for the study was provided in part by Novartis. Dr. Yeung reported consulting or advisory roles with Novartis, BMS, and Ariad. Several coauthors reported ties to industry.