User login
Cardiovascular risk assessment required with use of TKIs for CML
Treatment for chronic myeloid leukemia (CML) entails effective but mostly noncurative long-term use of tyrosine kinase inhibitors (TKIs) that require proactive, rational approaches to minimizing cardiovascular toxicities, according to a recent review.
Survival rates of patients with newly diagnosed CML are about 90%, and in those with a complete cytogenetic response, survival is comparable to that of age-matched controls. Although second-generation TKIs have increased efficacy, survival rates are similar to those of imatinib, possibly due in part to mortality from non-CML causes.
TKIs used in CML therapy target BCR-ABL1, but their potencies vary against other kinases, including receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). The relationship between off-target activities and adverse events (AEs) remains unclear, and AE management is largely empirical, said Dr. Javid Moslehi of Vanderbilt University Medical Center, Nashville, Tenn., and Dr. Michael Deininger, professor at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“Reports of cardiovascular AEs with nilotinib, pulmonary arterial hypertension (PAH) on dasatinib, and frequent cardiovascular AEs with ponatinib have caused a reassessment of the situation,” they noted.
“Given the high population frequency of cardiovascular disease and the increased frequency of vascular events with nilotinib and ponatinib, cardiovascular risk assessment and, if necessary, treatment need to be integrated into the management of patients with CML on TKIs,” they wrote (J Clin Onc. 2015 Dec 10. doi: 10.1200/JCO.2015.62.4718).
Retrospective studies have indicated that imatinib may have favorable metabolic and vascular effects, but prospective controlled trials are lacking. Defining the cardiovascular baseline risk of the specific CML population under study will be crucial in future studies.
Dasatinib was approved for front-line CML treatment based on superior cytogenic response rates, compared with imatinib, but in 2011 the Food and Drug Administration warned against cardiopulmonary risks and recommended that patients be evaluated for signs and symptoms of cardiopulmonary disease before and during dasatinib treatment. Results of DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients) showed that, at 36 months of follow-up, PAH was reported in 3% of patients on dasatinib and 0% on imatinib.
Nilotinib has shown superior efficacy to imatinib and was FDA approved for first-line therapy, with recommendations for arrhythmia monitoring and avoidance of QT interval–prolonging medications. There have been no subsequent reports of ventricular arrhythmias with nilotinib, but 36% of patients on nilotinib experienced hyperglycemia in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, compared with 20% on imatinib. Nilotinib also has been associated with hyperlipidemia and increased body mass. Recent results point to vascular toxicity with nilotinib. At the 6-year follow-up of the ENESTnd study, 10% of patients on nilotinib 300 mg twice per day and 16% on nilotinib 400 mg twice per day had cardiovascular events, compared with 2.5% of patients taking imatinib 400 mg once per day. The dose-dependent increased risk implicates a drug-dependent process.
Ponatinib is the only clinical TKI active against the BCR-ABL1T315I mutation. It is a potent inhibitor of numerous other kinases as well, including VEGF receptors. In the PACE (Ponatinib Ph-positive Acute Lymphoblastic Leukemia and CML Evaluation) study, 26% of patients on ponatinib developed hypertension, and traditional atherosclerosis risk factors (age, hypertension, and diabetes) predisposed patients to serious vascular AEs. Cardiovascular toxicity was shown to be dose dependent, and older patients with history of diabetes or ischemic events are the least tolerant of high dose intensity. A subset of patients will benefit from ponatinib, particularly those with BCR-ABL1T315I, but leukemia-related and cardiovascular risks must both be assessed.
Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
Treatment for chronic myeloid leukemia (CML) entails effective but mostly noncurative long-term use of tyrosine kinase inhibitors (TKIs) that require proactive, rational approaches to minimizing cardiovascular toxicities, according to a recent review.
Survival rates of patients with newly diagnosed CML are about 90%, and in those with a complete cytogenetic response, survival is comparable to that of age-matched controls. Although second-generation TKIs have increased efficacy, survival rates are similar to those of imatinib, possibly due in part to mortality from non-CML causes.
TKIs used in CML therapy target BCR-ABL1, but their potencies vary against other kinases, including receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). The relationship between off-target activities and adverse events (AEs) remains unclear, and AE management is largely empirical, said Dr. Javid Moslehi of Vanderbilt University Medical Center, Nashville, Tenn., and Dr. Michael Deininger, professor at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“Reports of cardiovascular AEs with nilotinib, pulmonary arterial hypertension (PAH) on dasatinib, and frequent cardiovascular AEs with ponatinib have caused a reassessment of the situation,” they noted.
“Given the high population frequency of cardiovascular disease and the increased frequency of vascular events with nilotinib and ponatinib, cardiovascular risk assessment and, if necessary, treatment need to be integrated into the management of patients with CML on TKIs,” they wrote (J Clin Onc. 2015 Dec 10. doi: 10.1200/JCO.2015.62.4718).
Retrospective studies have indicated that imatinib may have favorable metabolic and vascular effects, but prospective controlled trials are lacking. Defining the cardiovascular baseline risk of the specific CML population under study will be crucial in future studies.
Dasatinib was approved for front-line CML treatment based on superior cytogenic response rates, compared with imatinib, but in 2011 the Food and Drug Administration warned against cardiopulmonary risks and recommended that patients be evaluated for signs and symptoms of cardiopulmonary disease before and during dasatinib treatment. Results of DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients) showed that, at 36 months of follow-up, PAH was reported in 3% of patients on dasatinib and 0% on imatinib.
Nilotinib has shown superior efficacy to imatinib and was FDA approved for first-line therapy, with recommendations for arrhythmia monitoring and avoidance of QT interval–prolonging medications. There have been no subsequent reports of ventricular arrhythmias with nilotinib, but 36% of patients on nilotinib experienced hyperglycemia in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, compared with 20% on imatinib. Nilotinib also has been associated with hyperlipidemia and increased body mass. Recent results point to vascular toxicity with nilotinib. At the 6-year follow-up of the ENESTnd study, 10% of patients on nilotinib 300 mg twice per day and 16% on nilotinib 400 mg twice per day had cardiovascular events, compared with 2.5% of patients taking imatinib 400 mg once per day. The dose-dependent increased risk implicates a drug-dependent process.
Ponatinib is the only clinical TKI active against the BCR-ABL1T315I mutation. It is a potent inhibitor of numerous other kinases as well, including VEGF receptors. In the PACE (Ponatinib Ph-positive Acute Lymphoblastic Leukemia and CML Evaluation) study, 26% of patients on ponatinib developed hypertension, and traditional atherosclerosis risk factors (age, hypertension, and diabetes) predisposed patients to serious vascular AEs. Cardiovascular toxicity was shown to be dose dependent, and older patients with history of diabetes or ischemic events are the least tolerant of high dose intensity. A subset of patients will benefit from ponatinib, particularly those with BCR-ABL1T315I, but leukemia-related and cardiovascular risks must both be assessed.
Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
Treatment for chronic myeloid leukemia (CML) entails effective but mostly noncurative long-term use of tyrosine kinase inhibitors (TKIs) that require proactive, rational approaches to minimizing cardiovascular toxicities, according to a recent review.
Survival rates of patients with newly diagnosed CML are about 90%, and in those with a complete cytogenetic response, survival is comparable to that of age-matched controls. Although second-generation TKIs have increased efficacy, survival rates are similar to those of imatinib, possibly due in part to mortality from non-CML causes.
TKIs used in CML therapy target BCR-ABL1, but their potencies vary against other kinases, including receptors for vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). The relationship between off-target activities and adverse events (AEs) remains unclear, and AE management is largely empirical, said Dr. Javid Moslehi of Vanderbilt University Medical Center, Nashville, Tenn., and Dr. Michael Deininger, professor at the University of Utah Huntsman Cancer Institute, Salt Lake City.
“Reports of cardiovascular AEs with nilotinib, pulmonary arterial hypertension (PAH) on dasatinib, and frequent cardiovascular AEs with ponatinib have caused a reassessment of the situation,” they noted.
“Given the high population frequency of cardiovascular disease and the increased frequency of vascular events with nilotinib and ponatinib, cardiovascular risk assessment and, if necessary, treatment need to be integrated into the management of patients with CML on TKIs,” they wrote (J Clin Onc. 2015 Dec 10. doi: 10.1200/JCO.2015.62.4718).
Retrospective studies have indicated that imatinib may have favorable metabolic and vascular effects, but prospective controlled trials are lacking. Defining the cardiovascular baseline risk of the specific CML population under study will be crucial in future studies.
Dasatinib was approved for front-line CML treatment based on superior cytogenic response rates, compared with imatinib, but in 2011 the Food and Drug Administration warned against cardiopulmonary risks and recommended that patients be evaluated for signs and symptoms of cardiopulmonary disease before and during dasatinib treatment. Results of DASISION (Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients) showed that, at 36 months of follow-up, PAH was reported in 3% of patients on dasatinib and 0% on imatinib.
Nilotinib has shown superior efficacy to imatinib and was FDA approved for first-line therapy, with recommendations for arrhythmia monitoring and avoidance of QT interval–prolonging medications. There have been no subsequent reports of ventricular arrhythmias with nilotinib, but 36% of patients on nilotinib experienced hyperglycemia in the ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients) study, compared with 20% on imatinib. Nilotinib also has been associated with hyperlipidemia and increased body mass. Recent results point to vascular toxicity with nilotinib. At the 6-year follow-up of the ENESTnd study, 10% of patients on nilotinib 300 mg twice per day and 16% on nilotinib 400 mg twice per day had cardiovascular events, compared with 2.5% of patients taking imatinib 400 mg once per day. The dose-dependent increased risk implicates a drug-dependent process.
Ponatinib is the only clinical TKI active against the BCR-ABL1T315I mutation. It is a potent inhibitor of numerous other kinases as well, including VEGF receptors. In the PACE (Ponatinib Ph-positive Acute Lymphoblastic Leukemia and CML Evaluation) study, 26% of patients on ponatinib developed hypertension, and traditional atherosclerosis risk factors (age, hypertension, and diabetes) predisposed patients to serious vascular AEs. Cardiovascular toxicity was shown to be dose dependent, and older patients with history of diabetes or ischemic events are the least tolerant of high dose intensity. A subset of patients will benefit from ponatinib, particularly those with BCR-ABL1T315I, but leukemia-related and cardiovascular risks must both be assessed.
Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Most patients with chronic myeloid leukemia require long-term tyrosine kinase inhibitor (TKI) therapy, and cardiovascular effects are critical factors in treatment decisions.
Major finding: Second- and third-generation TKIs have been associated with more cardiovascular risk than first-generation imatinib.
Data source: Review of current literature on cardiovascular toxicity of BCR-ABL1 TKIs for treatment of chronic myeloid leukemia.
Disclosures: Dr. Moslehi reported financial ties with Novartis, ARIAD, Takeda/Millennium, Bristol-Myers Squibb, and Acceleron Pharma. Dr. Deininger reported ties to Novartis, Bristol-Myers Squibb, Incyte, ARIAD, Pfizer, and Cellgene.
Mutation in ALK-rearranged lung cancer confers resistance to lorlatinib, restores sensitivity to crizotinib
In a patient with metastatic ALK-rearranged non–small-cell lung cancer (NSCLC), resistance to ALK inhibitors began with a founder ALK C1156Y clone resistant to crizotinib, and progressed to a double-mutant C1156Y-L1198F clone that was resistant to lorlatinib but sensitive again to the less potent, first generation crizotinib, according to a case report published Dec. 23 in the New England Journal of Medicine.
Whole genome sequencing of tumor samples suggested that a minor subclone harboring the C1156Y mutation was enriched during crizotinib treatment, and under selective pressure during lorlatinib treatment, acquired the L1198F mutation that conferred resistance to the potent, third-generation ALK inhibitor lorlatinib but restored sensitivity to crizotinib. The patient relapsed after the second response to crizotinib, and tumor analysis did not detect the L1198F mutation (N Engl J Med. 2015 Dec 23. doi:10.1056/NEJMoa1508887).
“These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase,” wrote Dr. Alice Shaw, thoracic oncologist at Massachusetts General Hospital and professor at Harvard Medical School, Boston, and colleagues.
“Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases,” they wrote.
Co-crystal structures of mutant and wild-type ALK kinase domains with bound inhibitors show that the leucine-to-phenylalanine mutation at residue 1198 leads to steric clash with lorlatinib but not with crizotinib. The presence of phenylalanine may result in more favorable binding of crizotinib, which may offset the increased kinase activity due to C1156Y.
The case study analyzed tumor samples from a woman with metastatic ALK-rearranged NSCLC who had received multiple therapies, including crizotinib, ceritinib, and lorlatinib.
Cell survival assays showed that the double mutant ALK C1156Y-L1198F was resistant to lorlatinib, as well as to second-generation ALK inhibitors, and it was sensitive to crizotinib; the single L1198F mutation increased sensitivity to crizotinib. Assays with cell lines expressing additional ALK mutations, including the highly refractory G1202R mutation, demonstrated that in almost all cases, L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors.
The study was funded in part by Pfizer. Dr. Shaw reported personal fees from Pfizer, Novartis, Genentech, Roche, and Ariad during the conduct of the study, and personal fees from Blueprint Medicine, Daiichi-Sankyo, and Ignyta outside the submitted work. Several of her coauthors reported ties to industry.
In a patient with metastatic ALK-rearranged non–small-cell lung cancer (NSCLC), resistance to ALK inhibitors began with a founder ALK C1156Y clone resistant to crizotinib, and progressed to a double-mutant C1156Y-L1198F clone that was resistant to lorlatinib but sensitive again to the less potent, first generation crizotinib, according to a case report published Dec. 23 in the New England Journal of Medicine.
Whole genome sequencing of tumor samples suggested that a minor subclone harboring the C1156Y mutation was enriched during crizotinib treatment, and under selective pressure during lorlatinib treatment, acquired the L1198F mutation that conferred resistance to the potent, third-generation ALK inhibitor lorlatinib but restored sensitivity to crizotinib. The patient relapsed after the second response to crizotinib, and tumor analysis did not detect the L1198F mutation (N Engl J Med. 2015 Dec 23. doi:10.1056/NEJMoa1508887).
“These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase,” wrote Dr. Alice Shaw, thoracic oncologist at Massachusetts General Hospital and professor at Harvard Medical School, Boston, and colleagues.
“Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases,” they wrote.
Co-crystal structures of mutant and wild-type ALK kinase domains with bound inhibitors show that the leucine-to-phenylalanine mutation at residue 1198 leads to steric clash with lorlatinib but not with crizotinib. The presence of phenylalanine may result in more favorable binding of crizotinib, which may offset the increased kinase activity due to C1156Y.
The case study analyzed tumor samples from a woman with metastatic ALK-rearranged NSCLC who had received multiple therapies, including crizotinib, ceritinib, and lorlatinib.
Cell survival assays showed that the double mutant ALK C1156Y-L1198F was resistant to lorlatinib, as well as to second-generation ALK inhibitors, and it was sensitive to crizotinib; the single L1198F mutation increased sensitivity to crizotinib. Assays with cell lines expressing additional ALK mutations, including the highly refractory G1202R mutation, demonstrated that in almost all cases, L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors.
The study was funded in part by Pfizer. Dr. Shaw reported personal fees from Pfizer, Novartis, Genentech, Roche, and Ariad during the conduct of the study, and personal fees from Blueprint Medicine, Daiichi-Sankyo, and Ignyta outside the submitted work. Several of her coauthors reported ties to industry.
In a patient with metastatic ALK-rearranged non–small-cell lung cancer (NSCLC), resistance to ALK inhibitors began with a founder ALK C1156Y clone resistant to crizotinib, and progressed to a double-mutant C1156Y-L1198F clone that was resistant to lorlatinib but sensitive again to the less potent, first generation crizotinib, according to a case report published Dec. 23 in the New England Journal of Medicine.
Whole genome sequencing of tumor samples suggested that a minor subclone harboring the C1156Y mutation was enriched during crizotinib treatment, and under selective pressure during lorlatinib treatment, acquired the L1198F mutation that conferred resistance to the potent, third-generation ALK inhibitor lorlatinib but restored sensitivity to crizotinib. The patient relapsed after the second response to crizotinib, and tumor analysis did not detect the L1198F mutation (N Engl J Med. 2015 Dec 23. doi:10.1056/NEJMoa1508887).
“These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase,” wrote Dr. Alice Shaw, thoracic oncologist at Massachusetts General Hospital and professor at Harvard Medical School, Boston, and colleagues.
“Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases,” they wrote.
Co-crystal structures of mutant and wild-type ALK kinase domains with bound inhibitors show that the leucine-to-phenylalanine mutation at residue 1198 leads to steric clash with lorlatinib but not with crizotinib. The presence of phenylalanine may result in more favorable binding of crizotinib, which may offset the increased kinase activity due to C1156Y.
The case study analyzed tumor samples from a woman with metastatic ALK-rearranged NSCLC who had received multiple therapies, including crizotinib, ceritinib, and lorlatinib.
Cell survival assays showed that the double mutant ALK C1156Y-L1198F was resistant to lorlatinib, as well as to second-generation ALK inhibitors, and it was sensitive to crizotinib; the single L1198F mutation increased sensitivity to crizotinib. Assays with cell lines expressing additional ALK mutations, including the highly refractory G1202R mutation, demonstrated that in almost all cases, L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors.
The study was funded in part by Pfizer. Dr. Shaw reported personal fees from Pfizer, Novartis, Genentech, Roche, and Ariad during the conduct of the study, and personal fees from Blueprint Medicine, Daiichi-Sankyo, and Ignyta outside the submitted work. Several of her coauthors reported ties to industry.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
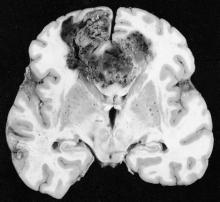
Tumor-treating fields may improve outcomes in glioblastoma
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
FROM JAMA
Key clinical point: Maintenance therapy with tumor-treating fields (TTFields) plus temozolomide resulted in longer survival, compared with temozolomide alone, for patients with glioblastoma.
Major finding: Median progression-free survival for TTFields plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (HR, 0.62; 98.7% CI, 0.43-0.89; P = .001).
Data sources: Interim analysis of the randomized trial included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group.
Disclosures: The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck, and Novartis. Several of his coauthors reported ties to industry.
SLCO2B1 gene variants may influence ADT outcome
Single-nucleotide polymorphisms (SNPs) in the SLCOB1 gene were significantly associated with either gene expression levels or efficiency of dehydroepiandrosterone sulfate transport, which may influence outcomes of androgen deprivation therapy (ADT) in patients with prostate cancer, according to researchers.
The exonic rs12422149 SNP (AA/AG) was associated with a longer median time to progression (TTP) of 27.2 months (95% CI, 18.9-48.9), compared with 20.0 months (95% CI, 16.5-23.0) for the major GG genotype (P = .019). The intronic rs1077858 SNP was associated with a shorter median overall survival (OS) of 5.2 years (95% CI, 4.3-6.8) for the AA/AG variant, compared with 6.7 years (95% CI, 6.2-7.2) for the GG genotype (J Clin Oncol. 2015 Dec 14. doi: 10.1200/JCO.2015.62.5988).
The SLCO2B1 gene mediates cellular uptake of endogenous sex steroid conjugates, such as DHEAS (dehydroepiandrosterone sulfate), and is highly expressed in castration-resistant prostate cancer tumors, compared with localized prostate cancer primary tumors. A previous study identified three SLCO2B1 SNPs that significantly influenced TTP in patients receiving androgen-deprivation therapy, but the current findings validated only the exonic rs12422149 SNP as having a significant association with TTP.
The intronic rs1077858 SNP correlated with differences in TTP in the initial cohort, but the current study did not find a significant association between rs1077858 and TTP in the validation cohort. The intronic SNP was significantly associated with OS in the combined cohort.
“As such, it is plausible that the rs1077858 GG variant, with its greater SLCO2B1 expression level, results in enhanced DHEAS uptake and increased activation of the AR signaling pathway, with subsequent decreased OS in patients. It is unclear why we observed an OS benefit with no definite impact on TTP with rs1077858 in the validation cohort,” wrote Dr. Xiaodong Wang of the Dana-Farber Cancer Institute, Boston, and colleagues.
The initial and validation cohorts consisted of 478 and 616 patients, respectively, with biochemical recurrence (M0) or radiologically evident metastatic disease (M1) who had received ADT for hormone-sensitive prostate cancer.
The exonic rs12422149 SNP, associated with TTP in both initial and validation cohorts, was not significantly associated with OS in the combined cohort. The investigators proposed a mechanistic explanation that DHEAS uptake may be an important driver in earlier stages of metastatic disease, but later stages might be driven by nonandrogen pathways.
Additional confounders include therapeutic heterogeneity among patients, as well as differences in patient and disease characteristics between the initial and validation cohorts.
Dr. Wang reported having no relevant financial disclosures. Dr. Harshman and Dr. Kantoff reported ties to industry.
Single-nucleotide polymorphisms (SNPs) in the SLCOB1 gene were significantly associated with either gene expression levels or efficiency of dehydroepiandrosterone sulfate transport, which may influence outcomes of androgen deprivation therapy (ADT) in patients with prostate cancer, according to researchers.
The exonic rs12422149 SNP (AA/AG) was associated with a longer median time to progression (TTP) of 27.2 months (95% CI, 18.9-48.9), compared with 20.0 months (95% CI, 16.5-23.0) for the major GG genotype (P = .019). The intronic rs1077858 SNP was associated with a shorter median overall survival (OS) of 5.2 years (95% CI, 4.3-6.8) for the AA/AG variant, compared with 6.7 years (95% CI, 6.2-7.2) for the GG genotype (J Clin Oncol. 2015 Dec 14. doi: 10.1200/JCO.2015.62.5988).
The SLCO2B1 gene mediates cellular uptake of endogenous sex steroid conjugates, such as DHEAS (dehydroepiandrosterone sulfate), and is highly expressed in castration-resistant prostate cancer tumors, compared with localized prostate cancer primary tumors. A previous study identified three SLCO2B1 SNPs that significantly influenced TTP in patients receiving androgen-deprivation therapy, but the current findings validated only the exonic rs12422149 SNP as having a significant association with TTP.
The intronic rs1077858 SNP correlated with differences in TTP in the initial cohort, but the current study did not find a significant association between rs1077858 and TTP in the validation cohort. The intronic SNP was significantly associated with OS in the combined cohort.
“As such, it is plausible that the rs1077858 GG variant, with its greater SLCO2B1 expression level, results in enhanced DHEAS uptake and increased activation of the AR signaling pathway, with subsequent decreased OS in patients. It is unclear why we observed an OS benefit with no definite impact on TTP with rs1077858 in the validation cohort,” wrote Dr. Xiaodong Wang of the Dana-Farber Cancer Institute, Boston, and colleagues.
The initial and validation cohorts consisted of 478 and 616 patients, respectively, with biochemical recurrence (M0) or radiologically evident metastatic disease (M1) who had received ADT for hormone-sensitive prostate cancer.
The exonic rs12422149 SNP, associated with TTP in both initial and validation cohorts, was not significantly associated with OS in the combined cohort. The investigators proposed a mechanistic explanation that DHEAS uptake may be an important driver in earlier stages of metastatic disease, but later stages might be driven by nonandrogen pathways.
Additional confounders include therapeutic heterogeneity among patients, as well as differences in patient and disease characteristics between the initial and validation cohorts.
Dr. Wang reported having no relevant financial disclosures. Dr. Harshman and Dr. Kantoff reported ties to industry.
Single-nucleotide polymorphisms (SNPs) in the SLCOB1 gene were significantly associated with either gene expression levels or efficiency of dehydroepiandrosterone sulfate transport, which may influence outcomes of androgen deprivation therapy (ADT) in patients with prostate cancer, according to researchers.
The exonic rs12422149 SNP (AA/AG) was associated with a longer median time to progression (TTP) of 27.2 months (95% CI, 18.9-48.9), compared with 20.0 months (95% CI, 16.5-23.0) for the major GG genotype (P = .019). The intronic rs1077858 SNP was associated with a shorter median overall survival (OS) of 5.2 years (95% CI, 4.3-6.8) for the AA/AG variant, compared with 6.7 years (95% CI, 6.2-7.2) for the GG genotype (J Clin Oncol. 2015 Dec 14. doi: 10.1200/JCO.2015.62.5988).
The SLCO2B1 gene mediates cellular uptake of endogenous sex steroid conjugates, such as DHEAS (dehydroepiandrosterone sulfate), and is highly expressed in castration-resistant prostate cancer tumors, compared with localized prostate cancer primary tumors. A previous study identified three SLCO2B1 SNPs that significantly influenced TTP in patients receiving androgen-deprivation therapy, but the current findings validated only the exonic rs12422149 SNP as having a significant association with TTP.
The intronic rs1077858 SNP correlated with differences in TTP in the initial cohort, but the current study did not find a significant association between rs1077858 and TTP in the validation cohort. The intronic SNP was significantly associated with OS in the combined cohort.
“As such, it is plausible that the rs1077858 GG variant, with its greater SLCO2B1 expression level, results in enhanced DHEAS uptake and increased activation of the AR signaling pathway, with subsequent decreased OS in patients. It is unclear why we observed an OS benefit with no definite impact on TTP with rs1077858 in the validation cohort,” wrote Dr. Xiaodong Wang of the Dana-Farber Cancer Institute, Boston, and colleagues.
The initial and validation cohorts consisted of 478 and 616 patients, respectively, with biochemical recurrence (M0) or radiologically evident metastatic disease (M1) who had received ADT for hormone-sensitive prostate cancer.
The exonic rs12422149 SNP, associated with TTP in both initial and validation cohorts, was not significantly associated with OS in the combined cohort. The investigators proposed a mechanistic explanation that DHEAS uptake may be an important driver in earlier stages of metastatic disease, but later stages might be driven by nonandrogen pathways.
Additional confounders include therapeutic heterogeneity among patients, as well as differences in patient and disease characteristics between the initial and validation cohorts.
Dr. Wang reported having no relevant financial disclosures. Dr. Harshman and Dr. Kantoff reported ties to industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Single-nucleotide polymorphisms rs12422149 and rs1077858 in the SLCO2B1 gene were associated with time to progression and overall survival, respectively, in prostate cancer patients receiving androgen deprivation therapy.
Major finding: For patients harboring the SNP rs12422149 AA/AG, median time to progression was 27.2 months, compared with 20.0 months for the GG variant; for SNP rs1077858, median overall survival was 5.2 years for the AA/AG variant, compared with 6.7 years for the GG variant.
Data source: Initial and validation cohorts of 478 and 616 patients, respectively, with biochemical recurrence or radiologically evident metastatic disease who had received ADT for hormone-sensitive prostate cancer.
Disclosures: Dr. Wang reported having no relevant financial disclosures. Dr. Harshman and Dr. Kantoff reported ties to industry.
Minimal residual disease predicts outcome of allogeneic HCT in acute myeloid leukemia
Patients with acute myeloid leukemia (AML) who are in remission but with minimal residual disease (MRD) detectable by multiparameter flow cytometry have outcomes similar to those of patients with morphologically detectable disease, and both groups have significantly worse outcomes than patients in MRD-negative remission.
“Is it time to move toward an MRD-based definition of CR [complete remission]? We believe so,” wrote Dr. Daisuke Araki of the University of Washington, Seattle, and colleagues. The researchers suggest that decision algorithms based on “the classic morphologic remission definition are not ideal. Our data support treatment algorithms that use MRD-based (i.e., patients in MRD-negative CR [versus] all other patients), rather than morphology-based disease assessments” (J Clin Oncol. 2015 Dec 12 [doi:10.1200/JCO.2015.63.3826]).
Three-year overall survival estimates for patients with active AML, patients in MRD-positive remission, and patients in MRD-negative remission were 23% (12%-35%), 26% (17%-37%), and 73% (66%-78%), respectively. Progression-free survival estimates showed a similar delineation between patients with morphologically detectable AML and MRD-positive remission compared with MRD-negative remission: 13% (5%-23%) for active AML, 12% (5%-21%) for MRD-positive remission, and 67% (61%-73%) for MRD-negative remission.
The retrospective analysis included 359 patients with AML treated with myeloablative hematopoietic cell transplantation (HCT) at the Fred Hutchinson Cancer Research Center from 2006 to 2014. In total, 311 patients (87%) had morphologically determined CR (less than 5% bone marrow blasts). Of these, 76 patients (24%) had MRD by multiparameter flow cytometry (MRD-positive remission) and 235 (76%) had no flow cytometric evidence of MRD (MRD-negative remission). In the pre-HCT assessment, 48 patients (13%) had 5% or more bone marrow blasts and were classified as having active AML.
Even patients with the lowest detectable amount of MRD had significantly worse outcomes than did patients with no MRD. Among the patients with evidence of leukemia (those in MRD-positive remission and with classified active AML), researchers found no statistically significant differences in outcomes based on MRD levels (less than 0.5%, 0.5%-5%, and greater than 5% abnormal blasts). Sensitivity analysis with different cut points yielded similar findings.
However, in line with previous findings, the data show that a small but significant subset of patients with active leukemia at the time of HCT can achieve long-term disease control with myeloablative conditioning.
No significant associations were found between disease status and non-relapse mortality.
The investigators caution that the results do not necessarily demonstrate that HCT outcomes for patients in MRD-positive remission and those with active disease are identical, because the preparative regimens, in general, were different for the two groups.
Dr. Araki reported having no disclosures. Several of his coauthors reported ties to industry.
For patients in complete remission, with a risk profile that suggests HCT to cure acute myeloid leukemia, MRD should not be ignored. The inexpensive, relatively easy-to-assess analysis provides a sensitive and patient-specific risk indicator, adding information beyond cytogenetic risk class and underlying genetic abnormalities. The results from Araki et al. prompt new clinical studies and, perhaps, a new transplantation strategy. Patients in MRD-positive remission could be combined with patients with active disease in trials aimed primarily at reducing the risk of post-transplant relapse.
In acute lymphoblastic leukemia (ALL), MRD refines individual risk assessment and is crucial to decision making regarding HCT. Its role in AML is similarly growing in importance.
HCT is a powerful therapy commonly prescribed for younger patients in CR with intermediate- and high-risk characteristics, but has significant downsides in terms of economic costs, acute and chronic toxicities, and above all, risk of transplant-related mortality, which averages just below 10%. Use of MRD may profoundly influence the treatment approach for AML by permitting a better definition of CR and risk class, and favoring better risk-adapted treatment strategies oriented to spared the risk of treatment-related mortality and transplant-related morbidity.
One additional note concerns the potential bias in positive results for successful late transplantation in MRD-negative patients (after 6-12 months from CR). Some of these patients may have been cured by prior chemotherapy, particularly if a patient tests negative for MRD soon after achieving CR and prior to HCT. New trials should address this bias by using predefined MRD time points from CR to HCT.
Dr. Renato Bassan is an oncologist at UOC Ematologia, Ospedale dell’Angelo, Mestre-Venezia, Italy. These remarks were part of an editorial accompanying a report in the Journal of Clinical Oncology (2015 Dec 12. doi: 10.1200/JCO.2015.64.8907). Dr. Bassan reported ties to Mundipharma, ARIAD, Roche, and Amgen.
For patients in complete remission, with a risk profile that suggests HCT to cure acute myeloid leukemia, MRD should not be ignored. The inexpensive, relatively easy-to-assess analysis provides a sensitive and patient-specific risk indicator, adding information beyond cytogenetic risk class and underlying genetic abnormalities. The results from Araki et al. prompt new clinical studies and, perhaps, a new transplantation strategy. Patients in MRD-positive remission could be combined with patients with active disease in trials aimed primarily at reducing the risk of post-transplant relapse.
In acute lymphoblastic leukemia (ALL), MRD refines individual risk assessment and is crucial to decision making regarding HCT. Its role in AML is similarly growing in importance.
HCT is a powerful therapy commonly prescribed for younger patients in CR with intermediate- and high-risk characteristics, but has significant downsides in terms of economic costs, acute and chronic toxicities, and above all, risk of transplant-related mortality, which averages just below 10%. Use of MRD may profoundly influence the treatment approach for AML by permitting a better definition of CR and risk class, and favoring better risk-adapted treatment strategies oriented to spared the risk of treatment-related mortality and transplant-related morbidity.
One additional note concerns the potential bias in positive results for successful late transplantation in MRD-negative patients (after 6-12 months from CR). Some of these patients may have been cured by prior chemotherapy, particularly if a patient tests negative for MRD soon after achieving CR and prior to HCT. New trials should address this bias by using predefined MRD time points from CR to HCT.
Dr. Renato Bassan is an oncologist at UOC Ematologia, Ospedale dell’Angelo, Mestre-Venezia, Italy. These remarks were part of an editorial accompanying a report in the Journal of Clinical Oncology (2015 Dec 12. doi: 10.1200/JCO.2015.64.8907). Dr. Bassan reported ties to Mundipharma, ARIAD, Roche, and Amgen.
For patients in complete remission, with a risk profile that suggests HCT to cure acute myeloid leukemia, MRD should not be ignored. The inexpensive, relatively easy-to-assess analysis provides a sensitive and patient-specific risk indicator, adding information beyond cytogenetic risk class and underlying genetic abnormalities. The results from Araki et al. prompt new clinical studies and, perhaps, a new transplantation strategy. Patients in MRD-positive remission could be combined with patients with active disease in trials aimed primarily at reducing the risk of post-transplant relapse.
In acute lymphoblastic leukemia (ALL), MRD refines individual risk assessment and is crucial to decision making regarding HCT. Its role in AML is similarly growing in importance.
HCT is a powerful therapy commonly prescribed for younger patients in CR with intermediate- and high-risk characteristics, but has significant downsides in terms of economic costs, acute and chronic toxicities, and above all, risk of transplant-related mortality, which averages just below 10%. Use of MRD may profoundly influence the treatment approach for AML by permitting a better definition of CR and risk class, and favoring better risk-adapted treatment strategies oriented to spared the risk of treatment-related mortality and transplant-related morbidity.
One additional note concerns the potential bias in positive results for successful late transplantation in MRD-negative patients (after 6-12 months from CR). Some of these patients may have been cured by prior chemotherapy, particularly if a patient tests negative for MRD soon after achieving CR and prior to HCT. New trials should address this bias by using predefined MRD time points from CR to HCT.
Dr. Renato Bassan is an oncologist at UOC Ematologia, Ospedale dell’Angelo, Mestre-Venezia, Italy. These remarks were part of an editorial accompanying a report in the Journal of Clinical Oncology (2015 Dec 12. doi: 10.1200/JCO.2015.64.8907). Dr. Bassan reported ties to Mundipharma, ARIAD, Roche, and Amgen.
Patients with acute myeloid leukemia (AML) who are in remission but with minimal residual disease (MRD) detectable by multiparameter flow cytometry have outcomes similar to those of patients with morphologically detectable disease, and both groups have significantly worse outcomes than patients in MRD-negative remission.
“Is it time to move toward an MRD-based definition of CR [complete remission]? We believe so,” wrote Dr. Daisuke Araki of the University of Washington, Seattle, and colleagues. The researchers suggest that decision algorithms based on “the classic morphologic remission definition are not ideal. Our data support treatment algorithms that use MRD-based (i.e., patients in MRD-negative CR [versus] all other patients), rather than morphology-based disease assessments” (J Clin Oncol. 2015 Dec 12 [doi:10.1200/JCO.2015.63.3826]).
Three-year overall survival estimates for patients with active AML, patients in MRD-positive remission, and patients in MRD-negative remission were 23% (12%-35%), 26% (17%-37%), and 73% (66%-78%), respectively. Progression-free survival estimates showed a similar delineation between patients with morphologically detectable AML and MRD-positive remission compared with MRD-negative remission: 13% (5%-23%) for active AML, 12% (5%-21%) for MRD-positive remission, and 67% (61%-73%) for MRD-negative remission.
The retrospective analysis included 359 patients with AML treated with myeloablative hematopoietic cell transplantation (HCT) at the Fred Hutchinson Cancer Research Center from 2006 to 2014. In total, 311 patients (87%) had morphologically determined CR (less than 5% bone marrow blasts). Of these, 76 patients (24%) had MRD by multiparameter flow cytometry (MRD-positive remission) and 235 (76%) had no flow cytometric evidence of MRD (MRD-negative remission). In the pre-HCT assessment, 48 patients (13%) had 5% or more bone marrow blasts and were classified as having active AML.
Even patients with the lowest detectable amount of MRD had significantly worse outcomes than did patients with no MRD. Among the patients with evidence of leukemia (those in MRD-positive remission and with classified active AML), researchers found no statistically significant differences in outcomes based on MRD levels (less than 0.5%, 0.5%-5%, and greater than 5% abnormal blasts). Sensitivity analysis with different cut points yielded similar findings.
However, in line with previous findings, the data show that a small but significant subset of patients with active leukemia at the time of HCT can achieve long-term disease control with myeloablative conditioning.
No significant associations were found between disease status and non-relapse mortality.
The investigators caution that the results do not necessarily demonstrate that HCT outcomes for patients in MRD-positive remission and those with active disease are identical, because the preparative regimens, in general, were different for the two groups.
Dr. Araki reported having no disclosures. Several of his coauthors reported ties to industry.
Patients with acute myeloid leukemia (AML) who are in remission but with minimal residual disease (MRD) detectable by multiparameter flow cytometry have outcomes similar to those of patients with morphologically detectable disease, and both groups have significantly worse outcomes than patients in MRD-negative remission.
“Is it time to move toward an MRD-based definition of CR [complete remission]? We believe so,” wrote Dr. Daisuke Araki of the University of Washington, Seattle, and colleagues. The researchers suggest that decision algorithms based on “the classic morphologic remission definition are not ideal. Our data support treatment algorithms that use MRD-based (i.e., patients in MRD-negative CR [versus] all other patients), rather than morphology-based disease assessments” (J Clin Oncol. 2015 Dec 12 [doi:10.1200/JCO.2015.63.3826]).
Three-year overall survival estimates for patients with active AML, patients in MRD-positive remission, and patients in MRD-negative remission were 23% (12%-35%), 26% (17%-37%), and 73% (66%-78%), respectively. Progression-free survival estimates showed a similar delineation between patients with morphologically detectable AML and MRD-positive remission compared with MRD-negative remission: 13% (5%-23%) for active AML, 12% (5%-21%) for MRD-positive remission, and 67% (61%-73%) for MRD-negative remission.
The retrospective analysis included 359 patients with AML treated with myeloablative hematopoietic cell transplantation (HCT) at the Fred Hutchinson Cancer Research Center from 2006 to 2014. In total, 311 patients (87%) had morphologically determined CR (less than 5% bone marrow blasts). Of these, 76 patients (24%) had MRD by multiparameter flow cytometry (MRD-positive remission) and 235 (76%) had no flow cytometric evidence of MRD (MRD-negative remission). In the pre-HCT assessment, 48 patients (13%) had 5% or more bone marrow blasts and were classified as having active AML.
Even patients with the lowest detectable amount of MRD had significantly worse outcomes than did patients with no MRD. Among the patients with evidence of leukemia (those in MRD-positive remission and with classified active AML), researchers found no statistically significant differences in outcomes based on MRD levels (less than 0.5%, 0.5%-5%, and greater than 5% abnormal blasts). Sensitivity analysis with different cut points yielded similar findings.
However, in line with previous findings, the data show that a small but significant subset of patients with active leukemia at the time of HCT can achieve long-term disease control with myeloablative conditioning.
No significant associations were found between disease status and non-relapse mortality.
The investigators caution that the results do not necessarily demonstrate that HCT outcomes for patients in MRD-positive remission and those with active disease are identical, because the preparative regimens, in general, were different for the two groups.
Dr. Araki reported having no disclosures. Several of his coauthors reported ties to industry.
Key clinical point: Allogeneic hematopoietic cell transplantation (HCT) outcomes for patients in minimal residual disease (MRD)–positive remission were similar to outcomes of patients with morphologically detectable acute myeloid leukemia (AML), and significantly worse than outcomes of patients in MRD-negative remission.
Major finding: Three-year overall survival estimates for patients with active AML, patients in MRD-positive remission, and patients in MRD-negative remission were 23% (12%-35%), 26% (17%-37%), and 73% (66%-78%), respectively.
Data sources: A retrospective analysis of 359 patients with AML treated with myeloablative HCT at the Fred Hutchinson Cancer Research Center from 2006 to 2014.
Disclosures: Dr. Araki reported having no disclosures. Several of his coauthors reported ties to industry.
Treatment delay linked with worse outcome for head and neck cancer
An analysis of more than 50,000 patients with head and neck squamous cell carcinoma (HNSCC) found that prolonged time to treatment initiation (TTI) was an independent predictor of worse mortality.
Median overall survival for TTI of 67 days or fewer was 71 months compared with 49 months for TTI less than 67 days (P less than .001). For TTI of 46 to 52 days, median overall survival (OS) was 72 months; for TTI of 53 to 67 days, 61 months, and for TTI of greater than 67 days, 47 months (P less than .001).
The results provide strong evidence that “TTI greater than 67 days is too long and should be considered unacceptable,” wrote Dr. Colin Murphy, a radiation oncologist at Fox Chase Cancer Center, Philadelphia, and his colleagues.
“The current analysis suggests that increasing TTI beyond the threshold established in this monograph alters HNSCC survival and represents a public health issue,” the researchers stated (J Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.5906).
Data from the National Cancer Data Base pertained to 51,655 patients with head and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, during 1998-2011; median follow-up time was 84 months.
Academic institutions had significantly higher median TTI (28 days), compared with community programs (22-23 days), probably because of patients transitioning care, which was an independently associated factor in higher TTI. Despite higher median TTI, academic institutions were associated with improved overall survival, compared with community hospitals, as were care transitions.
Despite rapid tumor proliferation in HNSCC that can result in stage progression, 9.6% of all patients in 2011 had TTI of greater than 67 days, and 25% (29% at academic institutions) had TTI of greater than 46 days, another benchmark level identified in the study.
Mortality risk, according to TTI, was greater for patients with stage I or II disease, compared with stage III or IV disease, a finding that may be because of lymph node involvement. Development of nodal disease at stage III is a significant risk factor for mortality.
The investigators note that health systems elsewhere, in Denmark for example, have addressed the problem of prolonged TTI. Such efforts require coordination among providers and mandate expedited appointments for a patients with a new cancer diagnosis.
“Recently piloted programs offering next-day appointments with cancer specialists address this reversible predictor of mortality and may partially alleviate increasing TTI. Without such reforms, it is conceivable that outcomes will continue to worsen because of prolonged TTI,” they wrote.
An analysis of more than 50,000 patients with head and neck squamous cell carcinoma (HNSCC) found that prolonged time to treatment initiation (TTI) was an independent predictor of worse mortality.
Median overall survival for TTI of 67 days or fewer was 71 months compared with 49 months for TTI less than 67 days (P less than .001). For TTI of 46 to 52 days, median overall survival (OS) was 72 months; for TTI of 53 to 67 days, 61 months, and for TTI of greater than 67 days, 47 months (P less than .001).
The results provide strong evidence that “TTI greater than 67 days is too long and should be considered unacceptable,” wrote Dr. Colin Murphy, a radiation oncologist at Fox Chase Cancer Center, Philadelphia, and his colleagues.
“The current analysis suggests that increasing TTI beyond the threshold established in this monograph alters HNSCC survival and represents a public health issue,” the researchers stated (J Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.5906).
Data from the National Cancer Data Base pertained to 51,655 patients with head and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, during 1998-2011; median follow-up time was 84 months.
Academic institutions had significantly higher median TTI (28 days), compared with community programs (22-23 days), probably because of patients transitioning care, which was an independently associated factor in higher TTI. Despite higher median TTI, academic institutions were associated with improved overall survival, compared with community hospitals, as were care transitions.
Despite rapid tumor proliferation in HNSCC that can result in stage progression, 9.6% of all patients in 2011 had TTI of greater than 67 days, and 25% (29% at academic institutions) had TTI of greater than 46 days, another benchmark level identified in the study.
Mortality risk, according to TTI, was greater for patients with stage I or II disease, compared with stage III or IV disease, a finding that may be because of lymph node involvement. Development of nodal disease at stage III is a significant risk factor for mortality.
The investigators note that health systems elsewhere, in Denmark for example, have addressed the problem of prolonged TTI. Such efforts require coordination among providers and mandate expedited appointments for a patients with a new cancer diagnosis.
“Recently piloted programs offering next-day appointments with cancer specialists address this reversible predictor of mortality and may partially alleviate increasing TTI. Without such reforms, it is conceivable that outcomes will continue to worsen because of prolonged TTI,” they wrote.
An analysis of more than 50,000 patients with head and neck squamous cell carcinoma (HNSCC) found that prolonged time to treatment initiation (TTI) was an independent predictor of worse mortality.
Median overall survival for TTI of 67 days or fewer was 71 months compared with 49 months for TTI less than 67 days (P less than .001). For TTI of 46 to 52 days, median overall survival (OS) was 72 months; for TTI of 53 to 67 days, 61 months, and for TTI of greater than 67 days, 47 months (P less than .001).
The results provide strong evidence that “TTI greater than 67 days is too long and should be considered unacceptable,” wrote Dr. Colin Murphy, a radiation oncologist at Fox Chase Cancer Center, Philadelphia, and his colleagues.
“The current analysis suggests that increasing TTI beyond the threshold established in this monograph alters HNSCC survival and represents a public health issue,” the researchers stated (J Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.5906).
Data from the National Cancer Data Base pertained to 51,655 patients with head and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, during 1998-2011; median follow-up time was 84 months.
Academic institutions had significantly higher median TTI (28 days), compared with community programs (22-23 days), probably because of patients transitioning care, which was an independently associated factor in higher TTI. Despite higher median TTI, academic institutions were associated with improved overall survival, compared with community hospitals, as were care transitions.
Despite rapid tumor proliferation in HNSCC that can result in stage progression, 9.6% of all patients in 2011 had TTI of greater than 67 days, and 25% (29% at academic institutions) had TTI of greater than 46 days, another benchmark level identified in the study.
Mortality risk, according to TTI, was greater for patients with stage I or II disease, compared with stage III or IV disease, a finding that may be because of lymph node involvement. Development of nodal disease at stage III is a significant risk factor for mortality.
The investigators note that health systems elsewhere, in Denmark for example, have addressed the problem of prolonged TTI. Such efforts require coordination among providers and mandate expedited appointments for a patients with a new cancer diagnosis.
“Recently piloted programs offering next-day appointments with cancer specialists address this reversible predictor of mortality and may partially alleviate increasing TTI. Without such reforms, it is conceivable that outcomes will continue to worsen because of prolonged TTI,” they wrote.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with time to treatment initiation (TTI) greater than 46-52 days had increased risk of mortality, with greatest risk increases for early-stage disease.
Major finding: Median overall survival was 72 months for TTI of 46-52 days, 61 months for 53-67 days, and 47 months for greater than 67 days (P less than .001).
Data source: Data from the National Cancer Data Base pertained to 51,655 patients with had and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, from 1998 to 2011; median follow-up time was 84 months.
Disclosures: Dr. Murphy reported having no disclosures. Several of his coauthors reported ties to industry.
ADT linked to increased risk of Alzheimer’s disease
The use of androgen deprivation therapy (ADT) for treatment of prostate cancer was associated with increased risk of Alzheimer’s disease, and patients with greater duration of ADT use had higher risks, according to medical records data analysis.
ADT use was significantly associated with Alzheimer’s disease risk, with a hazard ratio (HR) of 1.88 by propensity score–matched Cox regression analysis (95% confidence interval, 1.10-3.20; P = .021), and HR of 1.66 by traditional multivariable-adjusted Cox regression analysis (95% CI, 1.05-2.64; P = .031).
Patients who used ADT for 12 months or more had the greatest risk observed (HR, 2.12; 95% CI, 1.11-4.03; P = .011), and the risk increased by category of ADT duration (P for trend = .016).
Investigators used a novel text-processing pipeline to analyze clinical data, extracting disease and terminology codes, medication lists, and positive-present mentions of drug and disease concepts from clinical notes.
“Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcome, including those unlikely to be seen in smaller clinical trials,” wrote Dr. Kevin T. Nead of the University of Pennsylvania, Philadelphia, and his colleagues (J Clin Oncol. 2015 Dec 7. doi: 10.1200/JCO.2015.63.6266).
The study evaluated 16,888 patients with prostate cancer; in total, 2,397 received ADT and 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years. The median time to Alzheimer’s disease diagnosis was 4 years.
The analysis replicated previously known associations between Alzheimer’s disease and age (HR, 1.06; P less than .001) and cardiovascular disease (HR, 1.60; P = .031), supporting the validity of the method, according to the researchers.
The use of androgen deprivation therapy (ADT) for treatment of prostate cancer was associated with increased risk of Alzheimer’s disease, and patients with greater duration of ADT use had higher risks, according to medical records data analysis.
ADT use was significantly associated with Alzheimer’s disease risk, with a hazard ratio (HR) of 1.88 by propensity score–matched Cox regression analysis (95% confidence interval, 1.10-3.20; P = .021), and HR of 1.66 by traditional multivariable-adjusted Cox regression analysis (95% CI, 1.05-2.64; P = .031).
Patients who used ADT for 12 months or more had the greatest risk observed (HR, 2.12; 95% CI, 1.11-4.03; P = .011), and the risk increased by category of ADT duration (P for trend = .016).
Investigators used a novel text-processing pipeline to analyze clinical data, extracting disease and terminology codes, medication lists, and positive-present mentions of drug and disease concepts from clinical notes.
“Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcome, including those unlikely to be seen in smaller clinical trials,” wrote Dr. Kevin T. Nead of the University of Pennsylvania, Philadelphia, and his colleagues (J Clin Oncol. 2015 Dec 7. doi: 10.1200/JCO.2015.63.6266).
The study evaluated 16,888 patients with prostate cancer; in total, 2,397 received ADT and 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years. The median time to Alzheimer’s disease diagnosis was 4 years.
The analysis replicated previously known associations between Alzheimer’s disease and age (HR, 1.06; P less than .001) and cardiovascular disease (HR, 1.60; P = .031), supporting the validity of the method, according to the researchers.
The use of androgen deprivation therapy (ADT) for treatment of prostate cancer was associated with increased risk of Alzheimer’s disease, and patients with greater duration of ADT use had higher risks, according to medical records data analysis.
ADT use was significantly associated with Alzheimer’s disease risk, with a hazard ratio (HR) of 1.88 by propensity score–matched Cox regression analysis (95% confidence interval, 1.10-3.20; P = .021), and HR of 1.66 by traditional multivariable-adjusted Cox regression analysis (95% CI, 1.05-2.64; P = .031).
Patients who used ADT for 12 months or more had the greatest risk observed (HR, 2.12; 95% CI, 1.11-4.03; P = .011), and the risk increased by category of ADT duration (P for trend = .016).
Investigators used a novel text-processing pipeline to analyze clinical data, extracting disease and terminology codes, medication lists, and positive-present mentions of drug and disease concepts from clinical notes.
“Use of the electronic medical record in this way allows rapid investigation of a rich data source to study a broad range of postmarketing outcome, including those unlikely to be seen in smaller clinical trials,” wrote Dr. Kevin T. Nead of the University of Pennsylvania, Philadelphia, and his colleagues (J Clin Oncol. 2015 Dec 7. doi: 10.1200/JCO.2015.63.6266).
The study evaluated 16,888 patients with prostate cancer; in total, 2,397 received ADT and 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years. The median time to Alzheimer’s disease diagnosis was 4 years.
The analysis replicated previously known associations between Alzheimer’s disease and age (HR, 1.06; P less than .001) and cardiovascular disease (HR, 1.60; P = .031), supporting the validity of the method, according to the researchers.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients who underwent ADT for prostate cancer had significantly increased risk of future Alzheimer’s disease diagnosis.
Major finding: ADT use was significantly associated with Alzheimer’s disease risk, with an HR of 1.88 by propensity score–matched Cox regression analysis (95% CI, 1.10-3.20; P = .021).
Data source: The electronic medical record analysis evaluated 16,888 patients with prostate cancer. Of 2,397 patients who received ADT, 125 were diagnosed with Alzheimer’s disease during a median follow-up of 2.7 years.
Disclosures: Research was supported by grants from the National Institutes of Health, National Library of Medicine, and National Institute of General Medical Sciences, which owns the patent by Stanford on data mining techniques. Dr. Nead reported having no disclosures.
High risk for getting breast cancer linked with low risk of metastasis
Breast cancer prediction tools were associated with tumor prognosticators such that women with calculated high risk were significantly more likely to be diagnosed with low-grade, estrogen receptor (ER)–positive cancers, according to researchers.
The Tyrer-Cuzick model incorporates hormonal, lifestyle, and reproductive risk factors, as well as family history, into breast cancer risk estimates and was associated with ER status (ER-negative odds ratio, 0.80), grade (grade 3: OR, 0.79), and lymph node involvement (lymph node positive: OR, 0.77), but not tumor size. A polygenic risk score based on 77 single-nucleotide polymorphisms (SNP) variants was associated with ER status (ER-negative: OR, 0.80), tumor size (greater than 40 mm: OR, 0.86), and grade (grade 3: OR, 0.86). Tyrer-Cuzick model associations were observed in women younger than age 50, but not in older women. The polygenic risk score associations held for all age groups.
“Our results support the hypothesis that breast cancer subtypes have different etiologies and highlight the need to identify risk factors separately for distinct breast cancer subtypes and ages of onset. Better knowledge of subtype-specific risk factors and understanding of disease etiology may be vital for the success of primary prevention and screening programs aimed at lowering mortality,” wrote Johanna Holm of the Karolinska Institute, Stockholm, and colleagues (Journ Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.63.0624).
The study evaluated 5,500 female breast cancer patients younger than 80 years diagnosed between 2001 and 2008 in Sweden. In total, 5,232 participants had information on the Tyrer-Cuzick score, 4,927 had information on the polygenic risk score, and 3,488 had a prediagnostic mammographic density measurement.
The researchers assessed three breast cancer prediction tools, the Tyrer-Cuzick–predicted 10-year breast cancer risk score, the polygenic risk score, and mammographic density, to determine if risk estimates differed according to tumor prognosticators and metastasis. Unlike the Tyrer-Cuzick score and polygenic risk score, mammographic densities did not vary according to tumor prognosticators and therefore seem to indicate general breast cancer risk, according to investigators.
Both the Tyrer-Cuzick and polygenic risk scores were associated with favorable tumor prognosticators, and women with high scores in both may be even more likely to have favorable disease outcomes, according to researchers. In support of this finding, the survival analysis showed that women above the median in both risk calculators had decreased risk of distant metastasis.
Breast cancer prediction tools were associated with tumor prognosticators such that women with calculated high risk were significantly more likely to be diagnosed with low-grade, estrogen receptor (ER)–positive cancers, according to researchers.
The Tyrer-Cuzick model incorporates hormonal, lifestyle, and reproductive risk factors, as well as family history, into breast cancer risk estimates and was associated with ER status (ER-negative odds ratio, 0.80), grade (grade 3: OR, 0.79), and lymph node involvement (lymph node positive: OR, 0.77), but not tumor size. A polygenic risk score based on 77 single-nucleotide polymorphisms (SNP) variants was associated with ER status (ER-negative: OR, 0.80), tumor size (greater than 40 mm: OR, 0.86), and grade (grade 3: OR, 0.86). Tyrer-Cuzick model associations were observed in women younger than age 50, but not in older women. The polygenic risk score associations held for all age groups.
“Our results support the hypothesis that breast cancer subtypes have different etiologies and highlight the need to identify risk factors separately for distinct breast cancer subtypes and ages of onset. Better knowledge of subtype-specific risk factors and understanding of disease etiology may be vital for the success of primary prevention and screening programs aimed at lowering mortality,” wrote Johanna Holm of the Karolinska Institute, Stockholm, and colleagues (Journ Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.63.0624).
The study evaluated 5,500 female breast cancer patients younger than 80 years diagnosed between 2001 and 2008 in Sweden. In total, 5,232 participants had information on the Tyrer-Cuzick score, 4,927 had information on the polygenic risk score, and 3,488 had a prediagnostic mammographic density measurement.
The researchers assessed three breast cancer prediction tools, the Tyrer-Cuzick–predicted 10-year breast cancer risk score, the polygenic risk score, and mammographic density, to determine if risk estimates differed according to tumor prognosticators and metastasis. Unlike the Tyrer-Cuzick score and polygenic risk score, mammographic densities did not vary according to tumor prognosticators and therefore seem to indicate general breast cancer risk, according to investigators.
Both the Tyrer-Cuzick and polygenic risk scores were associated with favorable tumor prognosticators, and women with high scores in both may be even more likely to have favorable disease outcomes, according to researchers. In support of this finding, the survival analysis showed that women above the median in both risk calculators had decreased risk of distant metastasis.
Breast cancer prediction tools were associated with tumor prognosticators such that women with calculated high risk were significantly more likely to be diagnosed with low-grade, estrogen receptor (ER)–positive cancers, according to researchers.
The Tyrer-Cuzick model incorporates hormonal, lifestyle, and reproductive risk factors, as well as family history, into breast cancer risk estimates and was associated with ER status (ER-negative odds ratio, 0.80), grade (grade 3: OR, 0.79), and lymph node involvement (lymph node positive: OR, 0.77), but not tumor size. A polygenic risk score based on 77 single-nucleotide polymorphisms (SNP) variants was associated with ER status (ER-negative: OR, 0.80), tumor size (greater than 40 mm: OR, 0.86), and grade (grade 3: OR, 0.86). Tyrer-Cuzick model associations were observed in women younger than age 50, but not in older women. The polygenic risk score associations held for all age groups.
“Our results support the hypothesis that breast cancer subtypes have different etiologies and highlight the need to identify risk factors separately for distinct breast cancer subtypes and ages of onset. Better knowledge of subtype-specific risk factors and understanding of disease etiology may be vital for the success of primary prevention and screening programs aimed at lowering mortality,” wrote Johanna Holm of the Karolinska Institute, Stockholm, and colleagues (Journ Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.63.0624).
The study evaluated 5,500 female breast cancer patients younger than 80 years diagnosed between 2001 and 2008 in Sweden. In total, 5,232 participants had information on the Tyrer-Cuzick score, 4,927 had information on the polygenic risk score, and 3,488 had a prediagnostic mammographic density measurement.
The researchers assessed three breast cancer prediction tools, the Tyrer-Cuzick–predicted 10-year breast cancer risk score, the polygenic risk score, and mammographic density, to determine if risk estimates differed according to tumor prognosticators and metastasis. Unlike the Tyrer-Cuzick score and polygenic risk score, mammographic densities did not vary according to tumor prognosticators and therefore seem to indicate general breast cancer risk, according to investigators.
Both the Tyrer-Cuzick and polygenic risk scores were associated with favorable tumor prognosticators, and women with high scores in both may be even more likely to have favorable disease outcomes, according to researchers. In support of this finding, the survival analysis showed that women above the median in both risk calculators had decreased risk of distant metastasis.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: High risk of breast cancer based on specific prediction models was associated with favorable tumor prognosticators and reduced risk of distant metastasis.
Major finding: The Tyrer-Cuzick model was associated with ER status (ER-negative: OR, 0.80), grade (grade 3: OR, 0.79), and lymph node involvement (lymph node positive: OR, 0.77), but not tumor size; The polygenic risk score was associated with ER status, tumor size, and grade.
Data source: The study evaluated 5,500 female breast cancer patients younger than 80 years diagnosed between 2001 and 2008 in Sweden.
Disclosures: Ms. Holm reported having no disclosures. One of her coauthors reported ties to industry.
SBRT may be better than RFA for large hepatocellular carcinoma lesions
A retrospective data analysis showed that stereotactic body radiotherapy (SBRT) outperformed radiofrequency ablation (RFA) on tumors larger than 2 cm in patients with hepatocellular carcinoma.
For all tumors treated with RFA, 1- and 2-year freedom from local progression (FFLP) were 83.6% and 80.2%, and for tumors treated with SBRT, rates were 97.4% and 83.8%. On tumors smaller than 2 cm, FFLP was similar for the two methods (HR, 2.50; 95% confidence interval, 0.72-8.67; P = .15) but was significantly worse for RFA treatment of larger tumors (HR, 3.35; 95% CI, 1.17-9.62, P = .025).
“These results suggest that both SBRT and RFA are excellent choices for smaller tumors but that SBRT may be preferred for larger tumors. Prospective, randomized clinical trials are needed to compare these two modalities, especially for larger tumors, although we are unaware of any such trials,” wrote Dr. Daniel R. Wahl, radiation oncologist at the University of Michigan (J Clin Oncol. 2015 Dec. 2. doi:10.1200/JCO.2015.61.4925).
The retrospective study evaluated 224 patients with nonmetastatic hepatocellular carcinoma – 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012. Patients treated with RFA had higher rates of cirrhosis (95% vs. 78%; P less than .001), lower AFP levels (8.8 vs. 18.6; P = .04), and fewer prior liver-directed treatments compared with patients treated with SBRT.
To investigate the impact of fiducial use for image guidance in SBRT, the researchers examined treatment failures. Of the 21 treatments that used fiducials, none had local failure, compared with six failures in 62 treatments without fiducials.
Both methods had similar low rates of late adverse events. Acute adverse events and treatment-related deaths were nonsignificantly greater with RFA, which may suggest SBRT as a better option for medically unfit patients who may not tolerate invasive procedures such as RFA.
The study included only three tumors larger than 5 cm in diameter, so rates of local control for this size tumor cannot be estimated reliably, reported Dr. Wahl and his colleagues.
The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
A retrospective data analysis showed that stereotactic body radiotherapy (SBRT) outperformed radiofrequency ablation (RFA) on tumors larger than 2 cm in patients with hepatocellular carcinoma.
For all tumors treated with RFA, 1- and 2-year freedom from local progression (FFLP) were 83.6% and 80.2%, and for tumors treated with SBRT, rates were 97.4% and 83.8%. On tumors smaller than 2 cm, FFLP was similar for the two methods (HR, 2.50; 95% confidence interval, 0.72-8.67; P = .15) but was significantly worse for RFA treatment of larger tumors (HR, 3.35; 95% CI, 1.17-9.62, P = .025).
“These results suggest that both SBRT and RFA are excellent choices for smaller tumors but that SBRT may be preferred for larger tumors. Prospective, randomized clinical trials are needed to compare these two modalities, especially for larger tumors, although we are unaware of any such trials,” wrote Dr. Daniel R. Wahl, radiation oncologist at the University of Michigan (J Clin Oncol. 2015 Dec. 2. doi:10.1200/JCO.2015.61.4925).
The retrospective study evaluated 224 patients with nonmetastatic hepatocellular carcinoma – 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012. Patients treated with RFA had higher rates of cirrhosis (95% vs. 78%; P less than .001), lower AFP levels (8.8 vs. 18.6; P = .04), and fewer prior liver-directed treatments compared with patients treated with SBRT.
To investigate the impact of fiducial use for image guidance in SBRT, the researchers examined treatment failures. Of the 21 treatments that used fiducials, none had local failure, compared with six failures in 62 treatments without fiducials.
Both methods had similar low rates of late adverse events. Acute adverse events and treatment-related deaths were nonsignificantly greater with RFA, which may suggest SBRT as a better option for medically unfit patients who may not tolerate invasive procedures such as RFA.
The study included only three tumors larger than 5 cm in diameter, so rates of local control for this size tumor cannot be estimated reliably, reported Dr. Wahl and his colleagues.
The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
A retrospective data analysis showed that stereotactic body radiotherapy (SBRT) outperformed radiofrequency ablation (RFA) on tumors larger than 2 cm in patients with hepatocellular carcinoma.
For all tumors treated with RFA, 1- and 2-year freedom from local progression (FFLP) were 83.6% and 80.2%, and for tumors treated with SBRT, rates were 97.4% and 83.8%. On tumors smaller than 2 cm, FFLP was similar for the two methods (HR, 2.50; 95% confidence interval, 0.72-8.67; P = .15) but was significantly worse for RFA treatment of larger tumors (HR, 3.35; 95% CI, 1.17-9.62, P = .025).
“These results suggest that both SBRT and RFA are excellent choices for smaller tumors but that SBRT may be preferred for larger tumors. Prospective, randomized clinical trials are needed to compare these two modalities, especially for larger tumors, although we are unaware of any such trials,” wrote Dr. Daniel R. Wahl, radiation oncologist at the University of Michigan (J Clin Oncol. 2015 Dec. 2. doi:10.1200/JCO.2015.61.4925).
The retrospective study evaluated 224 patients with nonmetastatic hepatocellular carcinoma – 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012. Patients treated with RFA had higher rates of cirrhosis (95% vs. 78%; P less than .001), lower AFP levels (8.8 vs. 18.6; P = .04), and fewer prior liver-directed treatments compared with patients treated with SBRT.
To investigate the impact of fiducial use for image guidance in SBRT, the researchers examined treatment failures. Of the 21 treatments that used fiducials, none had local failure, compared with six failures in 62 treatments without fiducials.
Both methods had similar low rates of late adverse events. Acute adverse events and treatment-related deaths were nonsignificantly greater with RFA, which may suggest SBRT as a better option for medically unfit patients who may not tolerate invasive procedures such as RFA.
The study included only three tumors larger than 5 cm in diameter, so rates of local control for this size tumor cannot be estimated reliably, reported Dr. Wahl and his colleagues.
The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Stereotactic body radiotherapy (SBRT) and radiofrequency ablation (RFA) both achieved good local control for small hepatocellular carcinoma lesions, but for tumors larger than 2 cm, SBRT showed significantly better freedom from local progression (FFLP).
Major finding: For tumors larger than 2 cm, FFLP was worse for RFA compared with SBRT (hazard ratio, 3.35; 95% CI, 1.17-9.62, P = .025).
Data source: The retrospective study evaluated 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012.
Disclosures: The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
Study finds no benefit from lapatinib for HER2-positive gastric cancer patients
Addition of the anti-HER2 antibody lapatinib to capecitabine and oxaliplatin (CapeOx) as first-line treatment for advanced gastroesophageal adenocarcinoma did not significantly improve overall survival (OS) in the total study population, but subgroup analysis showed benefits for patients from Asia and those younger than 60 years.
The primary endpoint, median OS, was similar in the lapatinib and placebo arms at 12.2 months and 10.5 months (hazard ratio, 0.91; 95% confidence interval, 0.73-1.12; P = .35). Median PFS was significantly improved in the lapatinib arm, compared with placebo at 6.0 months vs. 5.4 months (HR, 0.82; 95% CI, 0.68-1.00; P = .04).
Preplanned exploratory analysis of OS by geographic location showed that patients from Asia had significantly improved OS with lapatinib: 16. 5 months vs. 10.9 months (HR, 0.68; 95% CI, 0.48-0.96; P = .03). Patients younger than 60 years also benefited, with median OS of 12.9 months vs. 9.0 months for lapatinib and placebo arms, respectively (HR, 0.69; 95% CI, 0.51-0.94; P = .01)
Patients in the lapatinib arm experienced a slightly higher incidence of adverse events (94% vs. 88%), with the most common events in both arms being diarrhea, nausea, vomiting, and decreased appetite.
“The use of lapatinib in combination with CapeOx in patients with HER2-positive gastric cancer cannot be recommended. Future research may identify a subgroup of patients who benefit from such treatment, although there are several new anti-HER2 agents, such as pertuzumab and ado-trastuzumab, that are being tested in HER2-positive gastric cancer,” wrote Dr. J. Randolph Hecht, professor of clinical medicine in the University of California, Los Angeles, and director of the UCLA Gastrointestinal Oncology Program (J Clin Oncol. 2015 Dec 2. doi: 10.1200/JCO.2015.62.6598).
The randomized phase III TRIO-013/LOGiC trial evaluated 545 patients with unresectable adenocarcinoma of the stomach, esophagus, or gastroesophageal junction with confirmed HER2 amplification.
Given that anti-HER2 antibodies (lapatinib- and trastuzumab-based regimens) have improved outcomes in patients with HER2-positive breast cancer, and trastuzumab plus cisplatin-based therapy significantly improved OS among of patients with HER2-positive gastric cancer in the ToGA trial, the researchers offered several explanations for the nonsignificant improvement reported here. The toxicity profiles differ between lapatinib and trastuzumab, particularly with regard to diarrhea, which may affect patients with gastroesophageal cancer more than other populations. There are concerns about absorption with the use of oral agents, especially among the 23% of patients in the study who had their pylorus removed.
GlaxoSmithKline, in collaboration with Translational Research in Oncology (TRIO), funded the work. Dr. Hecht disclosed ties with Amgen, Roche/Genentech, Pfizer, Immunomedics, Gilead Sciences, Celgene, and OncoMed. Several of his coauthors reported ties to industry.
Addition of the anti-HER2 antibody lapatinib to capecitabine and oxaliplatin (CapeOx) as first-line treatment for advanced gastroesophageal adenocarcinoma did not significantly improve overall survival (OS) in the total study population, but subgroup analysis showed benefits for patients from Asia and those younger than 60 years.
The primary endpoint, median OS, was similar in the lapatinib and placebo arms at 12.2 months and 10.5 months (hazard ratio, 0.91; 95% confidence interval, 0.73-1.12; P = .35). Median PFS was significantly improved in the lapatinib arm, compared with placebo at 6.0 months vs. 5.4 months (HR, 0.82; 95% CI, 0.68-1.00; P = .04).
Preplanned exploratory analysis of OS by geographic location showed that patients from Asia had significantly improved OS with lapatinib: 16. 5 months vs. 10.9 months (HR, 0.68; 95% CI, 0.48-0.96; P = .03). Patients younger than 60 years also benefited, with median OS of 12.9 months vs. 9.0 months for lapatinib and placebo arms, respectively (HR, 0.69; 95% CI, 0.51-0.94; P = .01)
Patients in the lapatinib arm experienced a slightly higher incidence of adverse events (94% vs. 88%), with the most common events in both arms being diarrhea, nausea, vomiting, and decreased appetite.
“The use of lapatinib in combination with CapeOx in patients with HER2-positive gastric cancer cannot be recommended. Future research may identify a subgroup of patients who benefit from such treatment, although there are several new anti-HER2 agents, such as pertuzumab and ado-trastuzumab, that are being tested in HER2-positive gastric cancer,” wrote Dr. J. Randolph Hecht, professor of clinical medicine in the University of California, Los Angeles, and director of the UCLA Gastrointestinal Oncology Program (J Clin Oncol. 2015 Dec 2. doi: 10.1200/JCO.2015.62.6598).
The randomized phase III TRIO-013/LOGiC trial evaluated 545 patients with unresectable adenocarcinoma of the stomach, esophagus, or gastroesophageal junction with confirmed HER2 amplification.
Given that anti-HER2 antibodies (lapatinib- and trastuzumab-based regimens) have improved outcomes in patients with HER2-positive breast cancer, and trastuzumab plus cisplatin-based therapy significantly improved OS among of patients with HER2-positive gastric cancer in the ToGA trial, the researchers offered several explanations for the nonsignificant improvement reported here. The toxicity profiles differ between lapatinib and trastuzumab, particularly with regard to diarrhea, which may affect patients with gastroesophageal cancer more than other populations. There are concerns about absorption with the use of oral agents, especially among the 23% of patients in the study who had their pylorus removed.
GlaxoSmithKline, in collaboration with Translational Research in Oncology (TRIO), funded the work. Dr. Hecht disclosed ties with Amgen, Roche/Genentech, Pfizer, Immunomedics, Gilead Sciences, Celgene, and OncoMed. Several of his coauthors reported ties to industry.
Addition of the anti-HER2 antibody lapatinib to capecitabine and oxaliplatin (CapeOx) as first-line treatment for advanced gastroesophageal adenocarcinoma did not significantly improve overall survival (OS) in the total study population, but subgroup analysis showed benefits for patients from Asia and those younger than 60 years.
The primary endpoint, median OS, was similar in the lapatinib and placebo arms at 12.2 months and 10.5 months (hazard ratio, 0.91; 95% confidence interval, 0.73-1.12; P = .35). Median PFS was significantly improved in the lapatinib arm, compared with placebo at 6.0 months vs. 5.4 months (HR, 0.82; 95% CI, 0.68-1.00; P = .04).
Preplanned exploratory analysis of OS by geographic location showed that patients from Asia had significantly improved OS with lapatinib: 16. 5 months vs. 10.9 months (HR, 0.68; 95% CI, 0.48-0.96; P = .03). Patients younger than 60 years also benefited, with median OS of 12.9 months vs. 9.0 months for lapatinib and placebo arms, respectively (HR, 0.69; 95% CI, 0.51-0.94; P = .01)
Patients in the lapatinib arm experienced a slightly higher incidence of adverse events (94% vs. 88%), with the most common events in both arms being diarrhea, nausea, vomiting, and decreased appetite.
“The use of lapatinib in combination with CapeOx in patients with HER2-positive gastric cancer cannot be recommended. Future research may identify a subgroup of patients who benefit from such treatment, although there are several new anti-HER2 agents, such as pertuzumab and ado-trastuzumab, that are being tested in HER2-positive gastric cancer,” wrote Dr. J. Randolph Hecht, professor of clinical medicine in the University of California, Los Angeles, and director of the UCLA Gastrointestinal Oncology Program (J Clin Oncol. 2015 Dec 2. doi: 10.1200/JCO.2015.62.6598).
The randomized phase III TRIO-013/LOGiC trial evaluated 545 patients with unresectable adenocarcinoma of the stomach, esophagus, or gastroesophageal junction with confirmed HER2 amplification.
Given that anti-HER2 antibodies (lapatinib- and trastuzumab-based regimens) have improved outcomes in patients with HER2-positive breast cancer, and trastuzumab plus cisplatin-based therapy significantly improved OS among of patients with HER2-positive gastric cancer in the ToGA trial, the researchers offered several explanations for the nonsignificant improvement reported here. The toxicity profiles differ between lapatinib and trastuzumab, particularly with regard to diarrhea, which may affect patients with gastroesophageal cancer more than other populations. There are concerns about absorption with the use of oral agents, especially among the 23% of patients in the study who had their pylorus removed.
GlaxoSmithKline, in collaboration with Translational Research in Oncology (TRIO), funded the work. Dr. Hecht disclosed ties with Amgen, Roche/Genentech, Pfizer, Immunomedics, Gilead Sciences, Celgene, and OncoMed. Several of his coauthors reported ties to industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Addition of lapatinib to capecitabine and oxaliplatin as first-line treatment for HER2-positive advanced gastroesophageal adenocarcinoma did not significantly improve overall survival (OS).
Major finding: Median OS was 12.2 months for the lapatinib arm vs. 10.5 months for the placebo arm (hazard ratio, 0.91; 95% CI, 0.73-1.12; P = .35).
Data source: The randomized phase III TRIO-013/LOGiC trial, evaluating 545 patients with unresectable adenocarcinoma of the stomach, esophagus, or gastroesophageal junction with confirmed HER2 amplification.
Disclosures: GlaxoSmithKline, in collaboration with Translational Research in Oncology (TRIO), funded the work. Dr. Hecht disclosed ties with Amgen, Roche/Genentech, Pfizer, Immunomedics, Gilead Sciences, Celgene, and OncoMed. Several of his coauthors reported ties to industry.