User login
COVID-19 vaccine insights: The news beyond the headlines
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
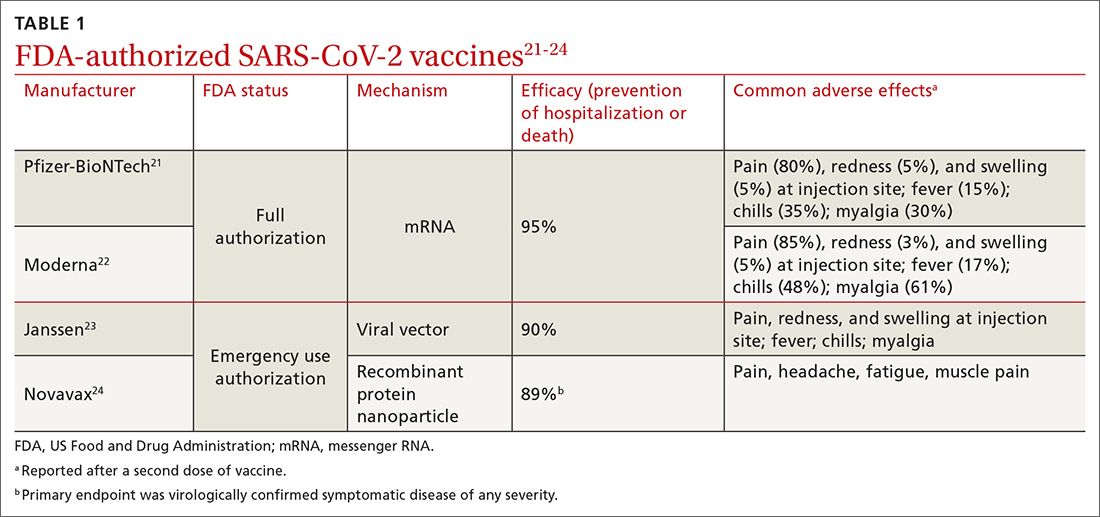
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24
Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
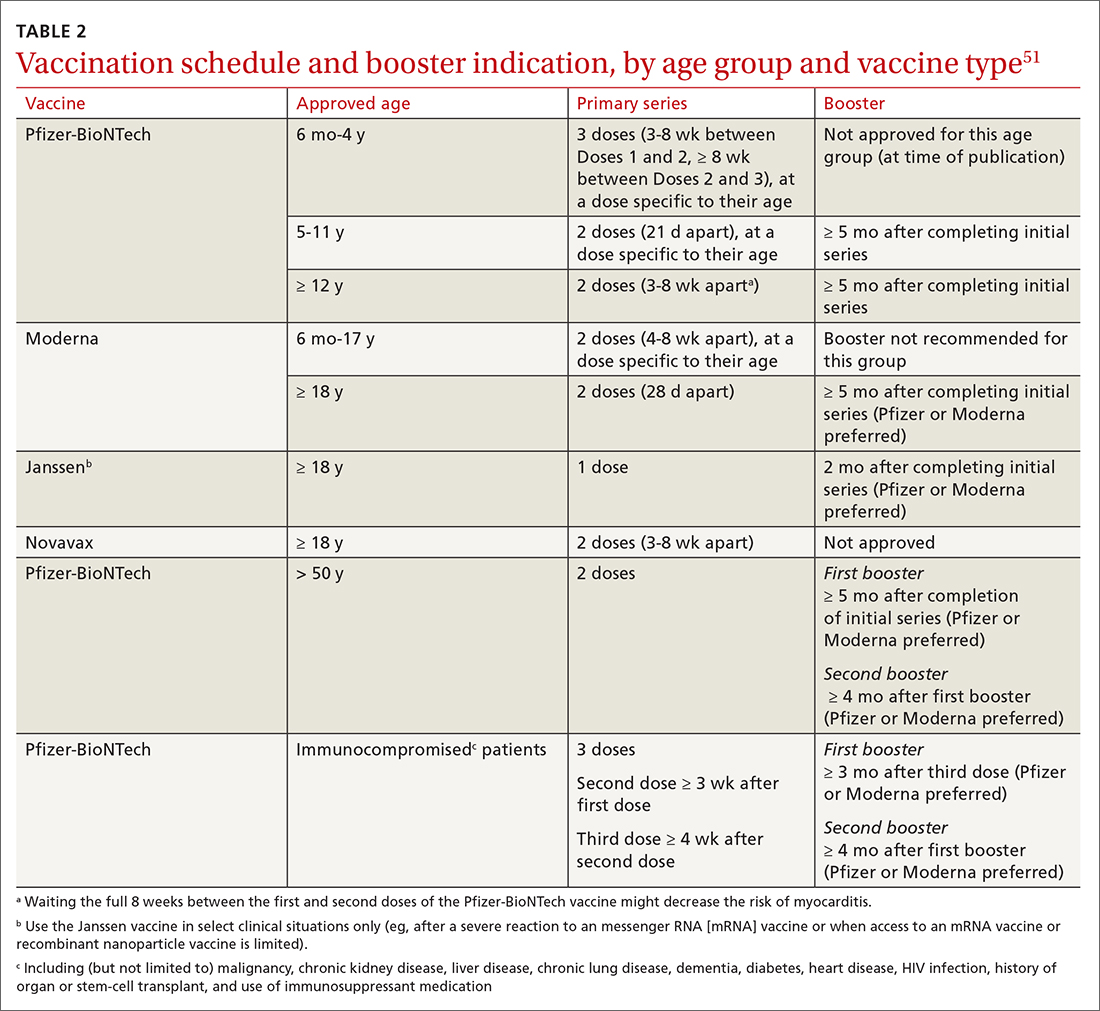
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).
Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
Worldwide and across many diseases, vaccines have been transformative in reducing mortality—an effect that has been sustained with vaccines that protect against COVID-19.1 Since the first cases of SARS-CoV-2 infection were reported in late 2019, the pace of scientific investigation into the virus and the disease—made possible by unprecedented funding, infrastructure, and public and private partnerships—has been explosive. The result? A vast body of clinical and laboratory evidence about the safety and effectiveness of SARS-CoV-2 vaccines, which quickly became widely available.2-4
In this article, we review the basic underlying virology of SARS-CoV-2; the biotechnological basis of vaccines against COVID-19 that are available in the United States; and recommendations on how to provide those vaccines to your patients. Additional guidance for your practice appears in a select online bibliography, “COVID-19 vaccination resources.”
SIDEBAR
COVID-19 vaccination resources
Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States
Centers for Disease Control and Prevention
www.cdc.gov/vaccines/covid-19/clinical-considerations/interimconsiderations-us.html
COVID-19 ACIP vaccine recommendations
Advisory Committee on Immunization Practices (ACIP)
www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
MMWR COVID-19 reports
Morbidity and Mortality Weekly Report
www.cdc.gov/mmwr/Novel_Coronavirus_Reports.html
A literature hub for tracking up-to-date scientific information about the 2019 novel coronavirus
National Center for Biotechnology Information of the National Library of Medicine
www.ncbi.nlm.nih.gov/research/coronavirus
Understanding COVID-19 vaccines
National Institutes of Health COVID-19 Research
https://covid19.nih.gov/treatments-and-vaccines/covid-19-vaccines
How COVID-19 affects pregnancy
National Institutes of Health COVID-19 Research
SARS-CoV-2 virology
As the SARS-CoV-2 virus approaches the host cell, normal cell proteases on the surface membrane cause a change in the shape of the SARS-CoV-2 spike protein. That spike protein conformation change allows the virus to avoid detection by the host’s immune system because its receptor-binding site is effectively hidden until just before entry into the cell.5,6 This process is analogous to a so-called lock-and-key method of entry, in which the key (ie, spike protein conformation) is hidden by the virus until the moment it is needed, thereby minimizing exposure of viral contents to the cell. As the virus spreads through the population, it adapts to improve infectivity and transmissibility and to evade developing immunity.7
After the spike protein changes shape, it attaches to an angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell, allowing the virus to enter that cell. ACE-2 receptors are located in numerous human tissues: nasopharynx, lung, gastrointestinal tract, heart, thymus, lymph nodes, bone marrow, brain, arterial and venous endothelial cells, and testes.5 The variety of tissues that contain ACE-2 receptors explains the many sites of infection and location of symptoms with which SARS-CoV-2 infection can manifest, in addition to the respiratory system.
Basic mRNA vaccine immunology
Although messenger RNA (mRNA) vaccines seem novel, they have been in development for more than 30 years.8
mRNA encodes the protein for the antigen of interest and is delivered to the host muscle tissue. There, mRNA is translated into the antigen, which stimulates an immune response. Host enzymes then rapidly degrade the mRNA in the vaccine, and it is quickly eliminated from the host.
mRNA vaccines are attractive vaccine candidates, particularly in their application to emerging infectious diseases, for several reasons:
- They are nonreplicating.
- They do not integrate into the host genome.
- They are highly effective.
- They can produce antibody and cellular immunity.
- They can be produced (and modified) quickly on a large scale without having to grow the virus in eggs.
Continue to: Vaccines against SARS-CoV-2
Vaccines against SARS-CoV-2
Two vaccines (from Pfizer-BioNTech [Comirnaty] and from Moderna [Spikevax]) are US Food and Drug Administration (FDA)–approved for COVID-19; both utilize mRNA technology. Two other vaccines, which do not use mRNA technology, have an FDA emergency use authorization (from Janssen Biotech, of Johnson & Johnson [Janssen COVID-19 Vaccine] and from Novavax [Novavax COVID-19 Vaccine, Adjuvanted]).9
Pfizer-BioNTech and Moderna vaccines. The mRNA of these vaccines encodes the entire spike protein in its pre-fusion conformation, which is the antigen that is replicated in the host, inducing an immune response.10-12 (Recall the earlier lock-and-key analogy: This conformation structure ingeniously replicates the exposed 3-dimensional key to the host’s immune system.)
The Janssen vaccine utilizes a viral vector (a nonreplicating adenovirus that functions as carrier) to deliver its message to the host for antigen production (again, the spike protein) and an immune response.
The Novavax vaccine uses a recombinant nanoparticle protein composed of the full-length spike protein.13,14 In this review, we focus on the 2 available mRNA vaccines, (1) given their FDA-authorized status and (2) because Centers for Disease Control and Prevention (CDC) recommendations indicate a preference for mRNA vaccination over viral-vectored vaccination. However, we also address key points about the Janssen (Johnson & Johnson) vaccine.
Efficacy of COVID-19 vaccines
The first study to document the safety and efficacy of a SARS-CoV-2 vaccine (the Pfizer-BioNTech vaccine) was published just 12 months after the onset of the pandemic.10 This initial trial demonstrated a 95% efficacy in preventing symptomatic, laboratory-confirmed COVID-19 at 3-month follow-up.10 Clinical trial data on the efficacy of COVID-19 vaccines have continued to be published since that first landmark trial.
Continue to: Data from trials...
Data from trials in Israel that became available early in 2021 showed that, in mRNA-vaccinated adults, mechanical ventilation rates declined strikingly, particularly in patients > 70 years of age.15,16 This finding was corroborated by data from a surveillance study of multiple US hospitals, which showed that mRNA vaccines were > 90% effective in preventing hospitalization in adults > 65 years of age.17
Data published in May 2021 showed that the Pfizer-BioNTech and Moderna vaccines were 94% effective in preventing COVID-19-related hospitalization.18 During the end of the Delta wave of the pandemic and the emergence of the Omicron variant of SARS-CoV-2, unvaccinated people were 5 times as likely to be infected as vaccinated people.19
In March 2022, data from 21 US medical centers in 18 states demonstrated that adults who had received 3 doses of the vaccine were 94% less likely to be intubated or die than those who were unvaccinated.16 A July 2022 retrospective cohort study of 231,037 subjects showed that the risk of hospitalization for acute myocardial infarction or for stroke after COVID-19 infection was reduced by more than half in fully vaccinated (ie, 2 doses of an mRNA vaccine or the viral vector [Janssen/Johnson & Johnson] vaccine) subjects, compared to unvaccinated subjects.20 The efficacy of the vaccines is summarized in TABLE 1.21-24

Even in patients who have natural infection, several studies have shown that COVID-19 vaccination after natural infection increases the level and durability of immune response to infection and reinfection and improves clinical outcomes.9,20,25,26 In summary, published literature shows that (1) mRNA vaccines are highly effective at preventing infection and (2) they augment immunity achieved by infection with circulating virus.
Breakthrough infection. COVID-19 mRNA vaccines are associated with breakthrough infection (ie, infections in fully vaccinated people), a phenomenon influenced by the predominant viral variant circulating, the level of vaccine uptake in the studied population, and the timing of vaccination.27,28 Nevertheless, vaccinated people who experience breakthrough infection are much less likely to be hospitalized and die compared to those who are unvaccinated, and vaccination with an mRNA vaccine is more effective than immunity acquired from natural infection.29
Continue to: Vaccine adverse effects
Vaccine adverse effects: Common, rare, myths
Both early mRNA vaccine trials reported common minor adverse effects after vaccination (TABLE 121-24). These included redness and soreness at the injection site, fatigue, myalgias, fever, and nausea, and tended to be more common after the second dose. These adverse effects are similar to common adverse effects seen with other vaccines. Counseling information about adverse effects can be found on the CDC website.a
Two uncommon but serious adverse effects of COVID-19 vaccination are myocarditis or pericarditis after mRNA vaccination and thrombosis with thrombocytopenia syndrome (TTS), which occurs only with the Janssen vaccine.30,31
Myocarditis and pericarditis, particularly in young males (12 to 18 years), and mostly after a second dose of vaccine, was reported in May 2021. Since then, several studies have shown that the risk of myocarditis is slightly higher in males < 40 years of age, with a predicted case rate ranging from 1 to 10 excess cases for every 1 million patients vaccinated.30,32 This risk must be balanced against the rate of myocarditis associated with SARS-CoV-2 infection.
A large study in the United States demonstrated that the risk of myocarditis for those who contract COVID-19 is 16 times higher than it is for those who are disease free.33 Observational safety data from April 2022 showed that men ages 18 to 29 years had 7 to 8 times the risk of heart complications after natural infection, compared to men of those ages who had been vaccinated.34 In this study of 40 US health care systems, the incidence of myocarditis or pericarditis in that age group ranged from 55 to 100 cases for every 100,000 people after infection and from 6 to 15 cases for every 100,000 people after a second dose of an mRNA vaccine.34
A risk–benefit analysis conducted by the Advisory Committee on Immunization Practices (ACIP) ultimately supported the conclusions that (1) the risk of myocarditis secondary to vaccination is small and (2) clear benefits of preventing infection, hospitalization, death, and continued transmission outweigh that risk.35 Study of this question, utilizing vaccine safety and reporting systems around the world, has continued.
Continue to: There is emerging evidence...
There is emerging evidence that extending the interval between the 2 doses of vaccine decreases the risk of myocarditis, particularly in male adolescents.36 That evidence ultimately led the CDC to recommend that it might be optimal that an extended interval (ie, waiting 8 weeks between the first and second dose of vaccine), in particular for males ages 12 to 39 years, could be beneficial in decreasing the risk of myocarditis.
TTS. A population risk–benefit analysis of TTS was conducted by ACIP while use of the Janssen vaccine was paused in the United States in December 2021.36 The analysis determined that, although the risk of TTS was largely in younger women (18 to 49 years; 7 cases for every 1 million vaccine doses administered), benefits of the vaccine in preventing death, hospitalization, and a stay in the intensive care unit (ICU)—particularly if vaccination was delayed or there was a high rate of community infection—clearly outweighed risks. (The CDC estimated an incidence of 2 cases of TTS with more than 3 million doses of Janssen vaccine administered; assuming moderate transmission kinetics, more than 3500 hospitalizations and more than 350 deaths were prevented by vaccination.36) Ultimately, after the CDC analysis was released, vaccination utilizing the Janssen product resumed; however, the CDC offered the caveat that the Janssen vaccine should be used only in specific situations36 (eg, when there has been a severe reaction to mRNA vaccine or when access to mRNA or recombinant nanoparticle vaccine is limited).
Myths surrounding vaccination
Myth #1: SARS-CoV-2 vaccines contain tissue from aborted fetuses. This myth, which emerged during development of the vaccines, is often a conflation of the use of embryonic cell lines obtained decades ago to produce vaccines (a common practice—not only for vaccines but common pharmaceuticals and foods).37 There are no fetal cells or tissue in any SARS-CoV-2 vaccines, and the vaccines have been endorsed by several faith organizations.38
Myth #2: SARS-CoV-2 vaccines can cause sterility in men and women. This myth originated from a report in early December 2020 seeking to link a similarity in a protein involved in placental–uterine binding and a portion of the receptor-binding domain antigen produced by the vaccine.39 No studies support this myth; COVID-19 vaccines are recommended in pregnancy by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine.40,41
Myth #3: mRNA SARS-CoV-2 vaccines alter a recipient’s DNA. mRNA vaccines are broken down by cellular enzymes. They cannot be integrated into the host genome.8
Continue to: Boosters and vaccine mix-and-match
Boosters and vaccine mix-and-match
As the COVID-19 pandemic persists, with new variants of concern emerging, it has also become clear that immunity wanes. In July 2021, the first report was published after a cluster of breakthrough infections occurred in a town in Massachusetts.42 There was no recommendation, at the time, for a booster; the Delta variant was the predominant circulating strain. In this outbreak, there were 469 cases, 74% of which were in people who had received 2 doses of an mRNA vaccine.42 Five patients were hospitalized; none died.42 A key takeaway from this outbreak was that vaccination prevented death, even in the face of fairly wide breakthrough infection.
Newer data show that, although vaccine effectiveness against hospitalization was greater than 90% for the first 2 months after a third dose, it waned to 78% by 4 months.43 Published data, combined with real-world experience, show that boosters provide additional reduction in the risk of death and hospitalization. This has led to a recommendation that all patients ≥ 5 years of age receive a booster.19,26,43-48 The CDC now recommends that people who are ages 12 years and older receive a bivalent booster (containing both wild-type and Omicron-variant antigens) ≥ 2 months after their most recent booster or completed series.
Future booster recommendations will consider the durability of the immune response over time (measured against the original immunizing virus) and the mutation rate of the virus.49
Given the limited supply of vaccine early in the pandemic, and the potential for future limitations, there was early interest in studying so-called mix-and-match SARS-CoV-2 vaccination—that is, receiving one product as a first series and then a different product as a booster, also known as heterologous booster vaccination. Although it is preferred that the 2 doses of the primary series be of the same vaccine product, studies that have examined this question support heterologous boosting as an acceptable approach to protective immunity50 (TABLE 251).

Vaccination in special populations
Three groups of patients have unique host characteristics that are important to consider when providing COVID-19 vaccination in your practice: pregnant patients, children, and patients in the broad category of “immunocompromised status.”
Continue to: Pregnant patients
Pregnant patients with SARS-CoV-2 infection are more likely to be hospitalized and have a higher risk of a stay in the ICU and need for mechanical ventilation. In a study of the course of illness in symptomatic pregnant patients who were hospitalized, 16.2% were admitted to an ICU and 8.5% were mechanically ventilated.52 CDC observational data have consistently supported the finding that (1) pregnant patients infected with SARS-CoV-2 are at increased risk of preterm labor and (2) their newborns are at increased risk of low birth weight and requiring admission to the neonatal ICU.53
A systematic review of 46 studies in pregnant and lactating patients showed no increased risk of adverse effects from COVID-19 vaccination.54 Furthermore, data from multiple studies demonstrate that immunoglobulin G antibodies cross the placenta to protect the infant at birth (ie, are found in umbilical cord blood and neonatal blood) and are found in breast milk. The precise kinetics and durability of these antibodies are unknown.
Pregnant patients were initially excluded from vaccine trials (although there were some patients ultimately found to be pregnant, or who became pregnant, during the trial). Careful examination of vaccine safety and efficacy data has supported the American College of Obstetricians and Gynecologists and European Board and College of Obstetrics and Gynaecology (EBCOG) recommendation that all pregnant patients be vaccinated. Furthermore, EBCOG recommends vaccination during the period of breastfeeding.55
Children. A major challenge during the pandemic has been to understand (1) the effect that infection with SARS-CoV-2 has on children and (2) the role of children in transmission of the virus. Although most children with COVID-19 have mild symptoms, a few require hospitalization and mechanical ventilation and some develop life-threatening multisystem inflammatory syndrome.56 In a large, retrospective study of more than 12,000 children with COVID-19, 5.3% required hospitalization and almost 20% of that subset were admitted to the ICU.57
Various hypotheses have been put forward to describe and explain the differences in disease expression between children and adults. These include:
- the absence of comorbidities often seen in adults
- evidence that pediatric patients might have reduced expression of ACE-2
- a more active T-cell response in infected children, due to an active thymus.56
Continue to: Although the number of children affected...
Although the number of children affected by severe SARS-CoV-2 infection is less than the number of adults, there have been important trends observed in infection and hospitalization as different variants have arisen.58 The Delta and Omicron variants have both been associated with a disturbing trend in the rate of hospitalization of pediatric patients, particularly from birth to 4 years—patients who were ineligible for vaccination at the time of the study.58 Ultimately, these data, combined with multiple studies of vaccine effectiveness in this age group, have led to an emergency use authorization for the Pfizer-BioNTech vaccination in pediatric populations and a recommendation from the American Academy of Pediatrics that all children ages 6 months and older be vaccinated.59,60
Immunocompromised patients. Patients broadly classified as immunocompromised have raised unique concerns. These patients have conditions such as malignancy, primary or secondary immunodeficiency, diabetes, and autoimmune disease; are taking certain classes of medication; or are of older age.61 Early in the pandemic, data showed that immunocompromised hosts could shed virus longer than hosts with an intact immune system—adding to their risk of transmitting SARS-CoV-2 and of viral adaptation for immune escape.62 Antibody response to vaccination was also less robust in this group.
There are limited data that demonstrate a short-lived reduction in risk of infection (in that study, Omicron was the prominent variant) with a fourth dose of an mRNA vaccine.63 Based on these data and FDA approval, the CDC recommends (1) an additional third primary dose and (2) a second booster for people who are moderately or severely immunocompromised. For those ages 50 years or older, a second booster is now required for their vaccination to be considered up to date.b
Predictions (or, why is a COVID-19 vaccine important?)
What does the future hold for our struggle with COVID-19? Perhaps we can learn lessons from the study of the 4 known seasonal coronaviruses, which cause the common cold and circulate annually.64 First, only relative immunity is produced after infection with a seasonal coronavirus.64 Studies of antibodies to seasonal coronaviruses seem to suggest that, although antibody titers remain high, correlation with decreased infection is lacking.65 Second, a dominant strain or 2 emerges each season, probably as a result of genetic variation and selective pressure for immune escape from neutralizing antibodies or cellular immunity.
The complex relationship among competing immune response duration, emergence of viral immune escape, increasing viral transmissibility, and societal viral source control (through vaccination, masking, distancing, testing, isolation, and treatment) widens the confidence bounds on our estimates of what the future holds. Late in 2020, the CDC began reporting wastewater surveillance data as a method for monitoring, and predicting changes in, community spread.66 During Spring 2022, the CDC reported an increase in detection of SARS-CoV-2 from a third of wastewater sampling sites around the United States. This observation coincided with (1) appearance of still more transmissible BA.2 and, later, BA.2.12.1 variants and (2) general relaxing of masking and social distancing guidelines, following the decline of the Omicron variant.
Continue to: At approximately that time...
At approximately that time, application to the FDA for a fourth shot (or a second booster) by Pfizer-BioNTech had been approved for adults > 50 years of age, at > 4 months after their previous vaccination.57 In view of warning signs from wastewater surveillance, priorities for vaccination should be to:
- increase uptake in the hesitant
- get boosters to the eligible
- prepare to tackle either seasonal or sporadic recurrence of COVID-19—whichever scenario the future brings.
As an example of how these priorities have been put into action, in September 2022, the FDA approved, and the CDC recommended, new bivalent boosters for everyone ≥ 12 years of age (Pfizer-BioNTech) or for all those ≥ 18 years of age (Moderna), to be administered ≥ 2 months after receipt of their most recent booster or primary series.
a www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
b Visit www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html for more guidance on COVID-19 vaccination for immunocompromised patients.
CORRESPONDENCE
John L. Kiley, MD, 3551 Roger Brooke Drive, Fort Sam Houston, TX 78234; [email protected]
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
1. Orenstein W, Offitt P, Edwards KM, Plotkin S. Plotkin’s Vaccines. 7th ed. Elsevier; 2017:1-15.
2. Operation Warp Speed: implications for global vaccine security. Lancet Glob Health. 2021;9:e1017-e1021. doi: 10.1016/S2214-109X(21)00140-6
3. Lurie N, Saville M, Hatchett R, et al. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi: 10.1056/NEJMp2005630
4. Slaoui M, Hepburn M. Developing safe and effective Covid vaccines—Operation Warp Speed’s strategy and approach. N Engl J Med. 2020;383:1701-1703. doi: 10.1056/NEJMp2027405
5. Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. doi: 10.1038/s41579-020-00459-7
6. Hussain I, Pervaiz N, Khan A, et al. Evolutionary and structural analysis of SARS-CoV-2 specific evasion of host immunity. Genes Immun. 2020;21:409-419. doi: 10.1038/s41435-020-00120-6
7. Rando HM, Wellhausen N, Ghosh S, et al; COVID-19 Review Consortium. Identification and development of therapeutics for COVID-19. mSystems. 2021;6:e0023321. doi: 10.1128/mSystems.00233-21
8. Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-279. doi: 10.1038/nrd.2017.243
9. National Center for Immunization and Respiratory Diseases. Use of COVID-19 vaccines in the United States: interim clinical considerations. Centers for Disease Control and Prevention. Updated August 22, 2022. Accessed August 27, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#references
10. Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577
11. Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines. 2021;6:104. doi: 10.1038/s41541-021-00369-6
12. Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi: 10.1056/NEJMoa2035389
13. Keech C, Albert G, Cho I, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320-2332. doi: 10.1056/NEJMoa2026920
14. Heath PT, Galiza EP, Baxter DN, et al; . Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172-1183. doi: 10.1056/NEJMoa2107659
15. Rinott E, Youngster I, Lewis YE. Reduction in COVID-19 patients requiring mechanical ventilation following implementation of a national COVID-19 vaccination program—Israel, December 2020–February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:326-328. doi: 10.15585/mmwr.mm7009e3
16. Tenforde MW, Self WH, Gaglani M, et al; IVY Network. Effectiveness of mRNA vaccination in preventing COVID-19-associated invasive mechanical ventilation and death—United States, March 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:459-465. doi: 10.15585/mmwr.mm7112e1
17. Moline HL, Whitaker M, Deng L, et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged ≥ 65 years—COVID-NET, 13 States, February–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088-1093. doi: 10.15585/mmwr.mm7032e
18. Tenforde MW, Olson SM, Self WH, et al; ; . Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥ 65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:674-679. doi: 10.15585/mmwr.mm7018e1
19. Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-138. doi: 10.15585/mmwr.mm7104e2
20. Kim Y-E, Huh K, Park Y-J, et al. Association between vaccination and acute myocardial infarction and ischemic stroke after COVID-19 infection. JAMA. Published online July 22, 2022. doi: 10.1001/jama.2022.12992
21. Centers for Disease Control and Prevention. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html
22. Centers for Disease Control and Prevention. The Moderna COVID-19 vaccine’s local reactions, systemic reactions, adverse events, and serious adverse events. Updated June 21, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html
23. Centers for Disease Control and Prevention. The Janssen COVID-19 vaccine’s local Reactions, Systemic reactions, adverse events, and serious adverse events. Updated August 12, 2021. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/janssen/reactogenicity.html
24. Centers for Disease Control and Prevention. Novavax COVID-19 vaccine local reactions, systemic reactions, adverse events, and serious adverse events. Updated August 31, 2022. Accessed September 9, 2022. www.cdc.gov/vaccines/covid-19/info-by-product/novavax/reactogenicity.html
25. Greaney AJ, Loes AN, Gentles LE, et al. Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection. Sci Transl Med. 2021;13:eabi9915. doi: 10.1126/scitranslmed.abi9915
26. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207-1220. doi: 10.1056/NEJMoa2118691
27. Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 avccination. JAMA. 2021;326:2018-2020. doi: 10.1001/jama.2021.19063
28. Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379-1384. doi: 10.1038/s41591-021-01413-7
29. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep. 2022;12:2628. doi: 10.1038/s41598-022-06629-2
30. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977-982. doi: 10.15585/mmwr.mm7027e2
31. MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651-656. doi: 10.15585/mmwr.mm7017e4
32. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410-422. doi: 10.1038/s41591-021-01630-0
33. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
34. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:517-523. doi: 10.15585/mmwr.mm7114e1
35. Rosemblum H. COVID-19 vaccines in adults: benefit–risk discussion. Centers for Disease Control and Prevention. July 22, 2021. Accessed September 21, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
36. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval. medRxiv. 2021:12.02.21267156.
37. Wong A. The ethics of HEK 293. Natl Cathol Bioeth Q. 2006;6:473-495. doi: 10.5840/ncbq20066331
38. North Dakota Health. COVID-19 vaccines & fetal cell lines. Updated December 1, 2021. Accessed September 21, 2022. www.health.nd.gov/sites/www/files/documents/COVID%20Vaccine%20Page/COVID-19_Vaccine_Fetal_Cell_Handout.pdf
39. Abbasi J. Widespread misinformation about infertility continues to create COVID-19 vaccine hesitancy. JAMA. 2022;327:1013-1015. doi: 10.1001/jama.2022.2404
40. Halasa NB, Olson SM, Staat MA, et al; ; . Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged < 6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264-270. doi: 10.15585/mmwr.mm7107e3
41. American College of Obstetricians and Gynecologists. ACOG and SMFM recommend COVID-19 vaccination for pregnant individuals. July 30, 2021. Accessed September 21, 2022. www.acog.org/news/news-releases/2021/07/acog-smfm-recommend-covid-19-vaccination-for-pregnant-individuals#:~:text=%E2%80%9CACOG%20is%20recommending%20vaccination%20of,complications%2C%20and%20because%20it%20isvaccines
42. Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059-1062. doi: 10.15585/mmwr.mm7031e2
43. Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:255-263. doi: 10.15585/mmwr.mm7107e2
44. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804-1816. doi: 10.1056/NEJMoa2200797
45. Arbel R, Hammerman A, Sergienko R, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413-2420. doi: 10.1056/NEJMoa2115624
46. Bar-On YM, Goldberg Y, Mandel M, et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421-2430. doi: 10.1056/NEJMoa2115926
47. Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393-1400. doi: 10.1056/NEJMoa2114255
48. Mbaeyi S, Oliver SE, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1545-1552. doi: 10.15585/mmwr.mm7044e2
49. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin Infect Dis. 2022;74:734-742. doi: 10.1093/cid/ciab646
50. Atmar RL, Lyke KE, Deming ME, et al; . Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046-1057. doi: 10.1056/NEJMoa2116414
51. Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Updated September 2, 2022. Accessed September 21, 2022. www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
52. Ackerman CM, Nguyen JL, Ambati S, et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9:ofab429. doi: 10.1093/ofid/ofab429
53. Osterman MJK, Valenzuela CP, Martin JA. Maternal and infant characteristics among women with confirmed or presumed cases of coronavirus disease (COVID-19) during pregnancy. National Center for Health Statistics. National Vital Statistics System. Updated August 11, 2022. Accessed September 21, 2022. www.cdc.gov/nchs/covid19/technical-linkage.htm
54. De Rose DU, Salvatori G, Dotta A, et al. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14:539. doi: 10.3390/v14030539
55. Martins I, Louwen F, Ayres-de-Campos D, et al. EBCOG position statement on COVID-19 vaccination for pregnant and breastfeeding women. Eur J Obstet Gynecol Reprod Biol. 2021;262:256-258. doi: 10.1016/j.ejogrb.2021.05.021
56. Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177-185. doi: 10.1038/s41590-021-01123-9
57. Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11:10231. doi: 10.1038/s41598-021-89553-1
58. Marks KJ, Whitaker M, Anglin O, et al; . Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271-278. doi: 10.15585/mmwr.mm7107e4
59. Price AM, Olson SM, Newhams MM, et al; . BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899-1909. doi: 10.1056/NEJMoa2202826
60. Maldonado YA, O’Leary ST, Banerjee R, et al; Committee on Infectious Diseases, American Academy of Pediatrics. COVID-19 vaccines in children and adolescents. Pediatrics. 2021;148:e2021052336. doi: 10.1542/peds.2021-052336
61. Lontok K. How effective are COVID-19 vaccines in immunocompromised people? American Society for Microbiology. August 12, 2021. Accessed September 21, 2022. https://asm.org/Articles/2021/August/How-Effective-Are-COVID-19-Vaccines-in-Immunocompr
62. Meiring S, Tempia S, Bhiman JN, et al; . Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus, South Africa. Clin Infect Dis. 2022;75:e144-e156. doi: 10.1093/cid/ciac077
63. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by 4th dose of BNT162b2 against Omicron in Israel. medRxiv. 2022: 02.01.22270232. doi: 10.1101/2022.02.01.22270232
64. Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222:9-16. doi: 10.1093/infdis/jiaa161
65. Petrie JG, Bazzi LA, McDermott AB, et al. Coronavirus occurrence in the Household Influenza Vaccine Evaluation (HIVE) cohort of Michigan households: reinfection frequency and serologic responses to seasonal and severe acute respiratory syndrome coronaviruses. J Infect Dis. 2021;224:49-59. doi: 10.1093/infdis/jiab161
66. Kirby AE, Walters MS, Jennings WC, et al. Using wastewater surveillance data to support the COVID-19 response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70:1242-1244. doi: 10.15585/mmwr.mm7036a2
PRACTICE RECOMMENDATIONS
› Vaccinate all adults (≥ 18 years) against COVID-19, based on recommendations for the initial series and boosters. A
› Vaccinate patients against COVID-19 with evidence-based assurance that doing so reduces disease-related risk of hospitalization, myocardial infarction, stroke, need for mechanical ventilation, and death. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Melanoma: An FP’s guide to diagnosis and management
CASE
A 48-year-old man comes to your clinic with a dark nevus on his right upper arm that appeared 2 months earlier. He says that the lesion has continued to grow and has bled (he thought because he initially picked at it). On exam, there is a 7-mm brown papule with 2 black dots and slightly asymmetric borders.
How would you proceed with this patient?
Melanoma is the fifth leading cause of new cancer cases annually, with > 96,000 new cases in 2019.1 Overall, melanoma is more common in men and in Whites, with 48% diagnosed in people ages 55 to 74.1 The past 2 decades have seen numerous developments in the diagnosis, treatment, and surveillance of melanoma. This article covers recommendations, controversies, and issues that require future study. It does not cover uveal or mucosal melanoma.
Evaluating a patient with a new or changing nevus
Known risk factors for melanoma include a changing nevus, indoor tanning, older age, many melanocytic nevi, history of a dysplastic nevus or of blistering sunburns during teen years, red or blonde hair, large congenital nevus, Fitzpatrick skin type I or II, high socioeconomic status, personal or family history of melanoma, and intermittent high-intensity sun exposure.2-3 Presence of 1 or more of these risk factors should lower the threshold for biopsy.
Worrisome physical exam features (FIGURE) are nevus asymmetry, irregular borders, variegated color, and a diameter > 6 mm (the size of a pencil eraser). Inquire as to whether the nevus’ appearance has evolved and if it has bled without trauma. In a patient with multiple nevi, 1 nevus that looks different than the rest (the so-called “ugly duckling”) is concerning. Accuracy of diagnosis is enhanced with dermoscopy. A Cochrane review showed that skilled use of dermoscopy, in addition to inspection with the naked eye, considerably increases the sensitivity and specificity of diagnosing melanoma.4 Yet a 2017 study of 705 US primary care practitioners showed that only 8.3% of them used dermoscopy to evaluate pigmented lesions.5
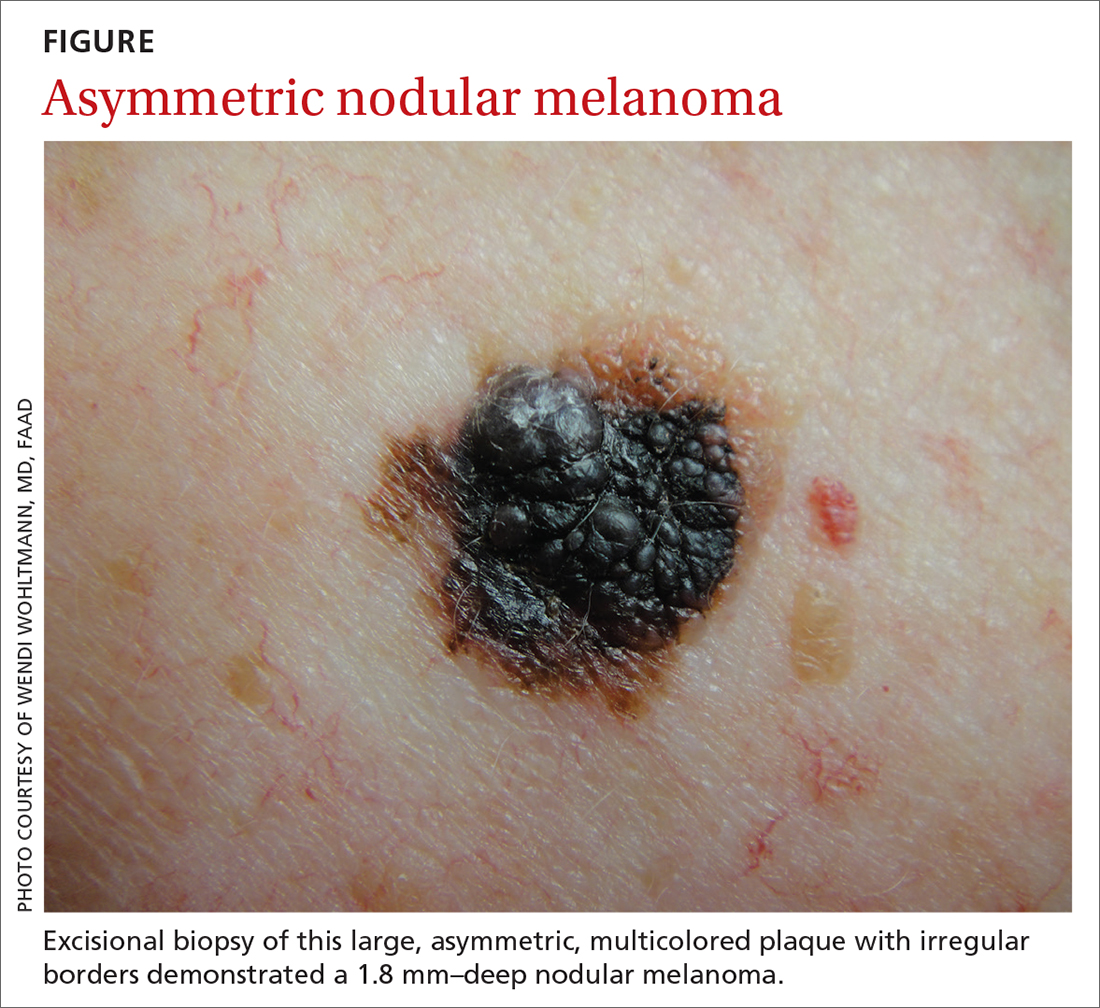
Several published algorithms and checklists can aid clinicians in identifying lesions suggestive of melanoma—eg, ABCDE, CASH, Menzies method, “chaos and clues,” and 2-step and 3- and 7-point checklists.6-10 A simple 3-step algorithm, the TADA (triage amalgamated dermoscopic algorithm) method is available to novice dermoscopy users.11 Experts in pigmented lesions prefer to use pattern analysis, which requires simultaneously assessing multiple lesion patterns that vary according to body site.12,13
Dermoscopic features suggesting melanoma are atypical pigment networks, pseudopods, radial streaking, irregular dots or globules, blue-whitish veil, and granularity or peppering.14 Appropriate and effective use of dermoscopy requires training.15,16 Available methods for learning dermoscopy include online and in-person courses, mentoring by experienced dermoscopists, books and articles, and free apps and online resources.17
Continue to: Perform a skin biopsy, but do this first
Perform a skin biopsy, but do this first
Skin biopsy is the definitive way to diagnose melanoma. Prior to biopsy, take photographs to document the exact location of the lesion and to ensure that the correct area is removed in wide excision (WE). A complete biopsy should include the full depth and breadth of the lesion to ensure there are clinically negative margins. This can be achieved with an elliptical excision (for larger lesions), punch excision (for small lesions), or saucerization (deep shave with 1- to 2-mm peripheral margins, used for intermediate-size lesions).18 Saucerization is distinctly different from a superficial shave biopsy, which is not recommended for lesions with features of melanoma.19
A decision to perform a biopsy on a part of the lesion (partial biopsy) depends on the size of the lesion and its anatomic location, and is best made in agreement with the patient.
If you are untrained or uncomfortable performing the biopsy, contact a dermatologist immediately. In many communities, such referrals are subject to long delays, which further supports the advisability of family physicians doing their own biopsies after photographing the suspicious lesion. Many resources are available to help family physicians learn to do biopsies proficiently (www.mdedge.com/familymedicine/article/164358/oncology/biopsies-skin-cancer-detection-dispelling-myths).19
What to communicate to the pathologist. At a minimum, the biopsy request form should include patient age, sex, biopsy type (punch, excisional, or scoop shave), intention (complete or partial sample), exact site of the biopsy with laterality, and clinical details. These details should include the lesion size and clinical description, the suspected diagnosis, and clinical information, such as whether there is a history of bleeding or changing color, size, or symmetry. In standard biopsy specimens, the pathologist is only examining a portion of the lesion. Communicating clearly to the pathologist may lead to a request for deeper or additional sections or special stains.
If the biopsy results do not match the clinical impression, a phone call to the pathologist is warranted. In addition, evaluation by a dermatopathologist may be merited as pathologic diagnosis of melanoma can be quite challenging. Newer molecular tests, such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH), can assist in the histologic evaluation of complex pigmented lesions.
Continue to: CASE
CASE
You perform an elliptical excisional biopsy on your patient. The biopsy report comes back as a nodular malignant melanoma, Breslow depth 2.5 mm without ulceration, and no evidence of lymphovascular invasion or microsatellitosis. The report states that the biopsy margins appear clear of tumor involvement.
Further evaluation when the biopsy result is positive
Key steps in initial patient care include relaying pathology results to the patient, conducting (as needed) a more extensive evaluation, and obtaining appropriate consultation.
Clearly explain the diagnosis and convey an accurate reading of the pathology report. The vital pieces of information in the biopsy report are the Breslow depth and presence of ulceration, as evidence shows these 2 factors to be important independent predictors of outcome.22,23 Also important are the presence of microsatellitosis (essential for staging purposes), pathologic stage, and the status of the peripheral and deep biopsy margins. Review Breslow depth with the patient as this largely dictates treatment options and prognosis.
Evaluate for possible metastatic disease. Obtain a complete history from every patient with cutaneous melanoma, looking for any positive review of systems as a harbinger of metastatic disease. A full-body skin and lymph node exam is vital, given that melanoma can arise anywhere including on the scalp, in the gluteal cleft, and beneath nails. If the lymph node exam is worrisome, conduct an ultrasound exam, even while referring to specialty care. Treating a patient with melanoma requires a multidisciplinary approach that may include dermatologists, surgeons, and oncologists based on the stage of disease. A challenge for family physicians is knowing which consultation to prioritize and how to counsel the patient to schedule these for the most cost-effective and timely evaluation.
Expedite a dermatology consultation. If the melanoma is deep or appears advanced based on size or palpable lymph nodes, contact the dermatologist immediately by phone to set up a rapid referral. Delays in the definitive management of thick melanomas can negatively affect outcome. Paper, facsimile, or electronic referrals can get lost in the system and are not reliable methods for referring patients for a melanoma consultation. One benefit of the family physician performing the initial biopsy is that a confirmed melanoma diagnosis will almost certainly get an expedited dermatology appointment.
Continue to: Wide excision and sentinel node biopsy
Wide excision and sentinel node biopsy
Wide excision of a primary melanoma is standard practice, with evidence favoring the following surgical margins: 0.5 to 1 cm for melanoma in situ, 1 cm for tumors up to 1 mm in thickness, 1 to 2 cm for tumors > 1 to 2 mm thick, and 2 cm for tumors > 2 mm thick.18 WE is often performed by dermatologists for nonulcerated tumors < 0.8 mm thick (T1a) without adverse features. If trained in cutaneous surgery, you can also choose to excise these thin melanomas in your office. Otherwise refer all patients with biopsy-proven melanoma to dermatologists to perform an adequate WE.
Refer patients who have tumors ≥ 0.8 mm thick to the appropriate surgical specialty (surgical oncology, if available) for consultation on sentinel lymph node biopsy. SLNB, when indicated, should be performed prior to WE of the primary tumor, and whenever possible in the same surgical setting, to maximize lymphatic drainage mapping techniques.18 Medical oncology referral, if needed, is usually made after WE.
SLNB remains the standard for lymph node staging. It is controversial mainly in its use for very thin or very thick lesions. Randomized controlled trials, including the Multicenter Selective Lymphadenectomy Trial,24 have shown no difference in melanoma-specific survival for patients with intermediate-thickness melanomas who had undergone SLNB.24
Many professional organizations consider SLNB to be the most significant prognostic indicator of disease recurrence. With a negative SLNB result, the risk of regional node recurrence is 5% or lower.18,25 In addition, sentinel lymph node status is a critical determinant for systemic adjuvant therapy consideration and clinical trial eligibility. For patients who have primary cutaneous melanoma without clinical lymphadenopathy, an online tool is available for patients to use with their physician in predicting the likelihood of SLNB positivity.26
Recommendations for SLNB, supported by multidisciplinary consensus:18
- Do not pursue SLNB for melanoma in situ or most cutaneous melanomas < 0.8 mm without ulceration (T1a). (See TABLE 127)
- Discuss SLNB with patients who have T1a melanoma and additional adverse features: young age, high mitotic rate, lymphovascular invasion, and nevus depth close to 0.8 mm with positive deep biopsy margins.
- Discuss SLNB with patients who have T1b disease (< 0.8 mm with ulceration, or 0.8-1 mm), although rates of SLNB positivity are low.
- Offer SLNB to patients with T2a and higher disease (> 1 mm).18
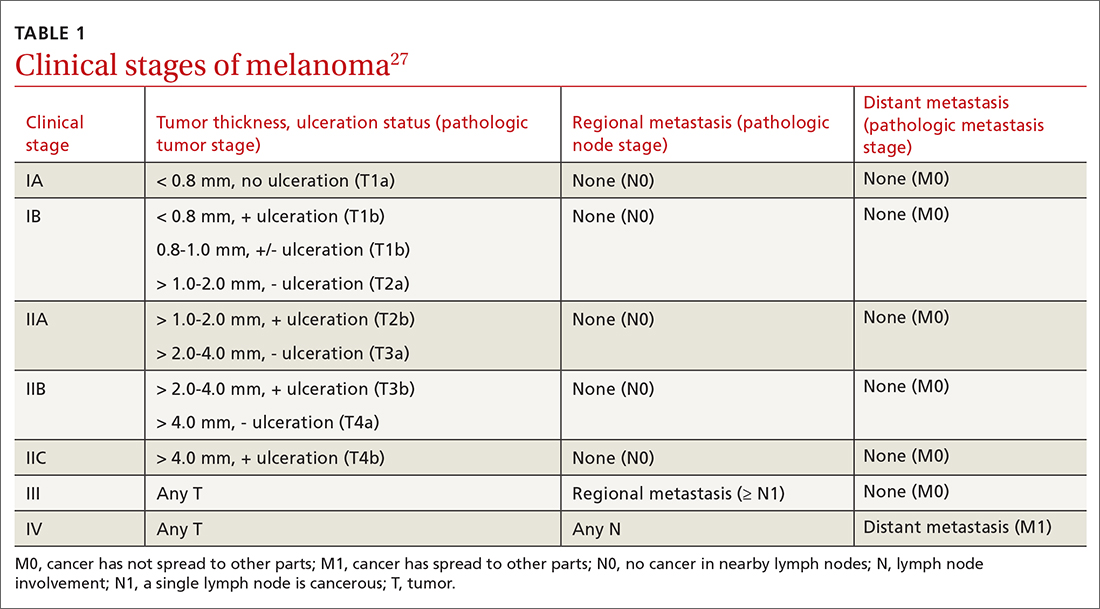
Continue to: Patients who have...
Patients who have clinical Stage I or II disease (TABLE 127)
Melanoma in women: Considerations to keep in mind
Hormonal influences of pregnancy, lactation, contraception, and menopause introduce special considerations regarding melanoma, which is the most common cancer occurring during pregnancy, accounting for 31% of new malignancies.33 Risk of melanoma lessens, however, for women who first give birth at a younger age or who have had > 5 live births.18,34,35 There is no evidence that nevi darken during pregnancy, although nevi on the breast and abdomen may seem to enlarge due to skin stretching.18 All changing nevi in pregnancy warrant an examination, preferably with dermoscopy, and patients should be offered biopsy if there are any nevus characteristics associated with melanoma.18
The effect of pregnancy on an existing melanoma is not fully understood, but evidence from controlled studies shows no negative effect. Recent working group guidelines advise WE with local anesthesia without delay in pregnant patients.18 Definitive treatment after melanoma diagnosis should take a multidisciplinary approach involving obstetric care coordinated with Dermatology, Surgery, and Medical Oncology.18
Most recommendations on the timing of pregnancy following a melanoma diagnosis have limited evidence. One meta-analysis concluded that pregnancy occurring after successful treatment of melanoma did not change a woman’s prognosis.36 Current guidelines do not recommend delaying future pregnancy if a woman had an early-stage melanoma. For melanomas deemed higher risk, a woman could consider a 2- to 3-year delay in the next planned pregnancy, owing to current data on recurrence rates.18
A systematic review of women who used hormonal contraception or postmenopausal hormone replacement therapy (HRT) showed no associated increased risk of melanoma.35 An additional randomized trial showed no effect of HRT on melanoma risk.37
Continue to: Systemic melanoma treatment and common adverse effects
Systemic melanoma treatment and common adverse effects
Multiple systemic therapies have been approved for the treatment of advanced or unresectable cutaneous melanomas. While these treatments are managed primarily by Oncology in concert with Dermatology, an awareness of the medications’ common dermatologic toxicities is important for the primary care provider. The 2 broad categories of FDA-approved systemic medications for advanced melanoma are mitogen-activated protein kinase (MAPK) inhibitors and immune checkpoint inhibitors, each having its own set of adverse cutaneous effects.
MAPK pathway–targeting drugs include the B-Raf proto-oncogene serine/threonine-kinase inhibitors (BRAFIs) vemurafenib and dabrafenib, and the MAPK inhibitors (MEKIs) trametinib and cobimetinib. The most common adverse skin effects in MAPK pathway–targeting drugs are severe ultraviolet photosensitivity, cutaneous epidermal neoplasms (particularly squamous cell carcinoma, keratoacanthoma-type), thick actinic keratosis, wart-like keratosis, painful palmoplantar keratosis, and dry skin.38 These effects are most commonly seen with BRAFI monotherapy and can be abated with the addition of a MEKI. MEKI therapy can cause acneiform eruptions and paronychia.39 Additional adverse effects include diarrhea, pyrexia, arthralgias, and fatigue for BRAFIs and diarrhea, fatigue, and peripheral edema for MEKIs.40
Immune checkpoint inhibitors include anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab and nivolumab), and anti-PDL-1 (atezolizumab). Adverse skin effects include morbilliform rash with or without an associated itch, itch with or without an associated rash, vitiligo, and lichenoid skin rashes. PD-1 and PDL-1 inhibitors have been associated with flares or unmasking of atopic dermatitis, psoriasis, sarcoidosis, and autoimmune bullous disease.18 Diarrhea, colitis, hepatitis, elevated liver enzymes, hypophysitis, and thyroiditis are some of the more common noncutaneous adverse effects reported with CTLA-4 inhibitors, while fatigue, diarrhea, nausea, pneumonitis, and thyroid disease are seen with anti-PD-1/PDL-1 therapy.3
A look at the prognosis
For patients diagnosed with primary cutaneous melanoma between 2011 and 2017, the 5-year survival rate for localized disease (Stages I-II) was 99%.1 For regional (Stage III) and distant (Stage IV) disease, the 5-year survival rates were 68% and 30%, respectively.1 With the advent of adjuvant systemic therapy, 5-year overall survival rates for metastatic melanoma have markedly improved from < 10% to up to 40% to 50%.41 The 3-year survival rate for patients with high tumor burden, brain metastasis, and elevated lactate dehydrogenase remains at < 10%.42 Relative survival decreases with increased age, although survival is higher in women than in men.43 Risk of melanoma recurrence after surgical excision is high in patients with stage IIB, IIC, III and IV (resectable) disease. The most important risk factor for recurrence is primary tumor thickness.44 The most common site of first recurrence in stage I-II disease is regional lymph node metastasis (42.8%), closely followed by distant metastasis (37.6%).44
Long-term follow-up and surveillance
Recommendations for long-term care of patients with melanoma have evolved with advances in treatment, prognostication, and imaging. Caring for these patients requires a multidisciplinary approach wherein the family physician provides frontline care and team coordination. Since most recurrences are discovered by the patient or the patient’s family, patient education and self-examination are the cost-effective foundation for recurrence screening. In a trial of patients and partners, a 30-minute structured session on skin examination followed by physician reminders every 4 months increased the detection of melanoma recurrence without significant increases in patient visits.45
Continue to: Patient education should include sun safety...
Patient education should include sun safety (wearing sun-protective clothing, using broad-spectrum sunscreen, and avoiding sun exposure during peak times of the day). The US Preventive Services Task Force (USPSTF) says the level of evidence is insufficient to support routine skin cancer screening in adults.46 However, the USPSTF recommends discussing efforts to minimize UV radiation exposure to prevent skin cancer in fair-skinned individuals 10 to 24 years of age.
Current National Comprehensive Cancer Network (NCCN) guidelines have outlined the follow-up frequency for all melanoma patients. TABLE 232 outlines those recommendations in addition to self-examination and patient education.
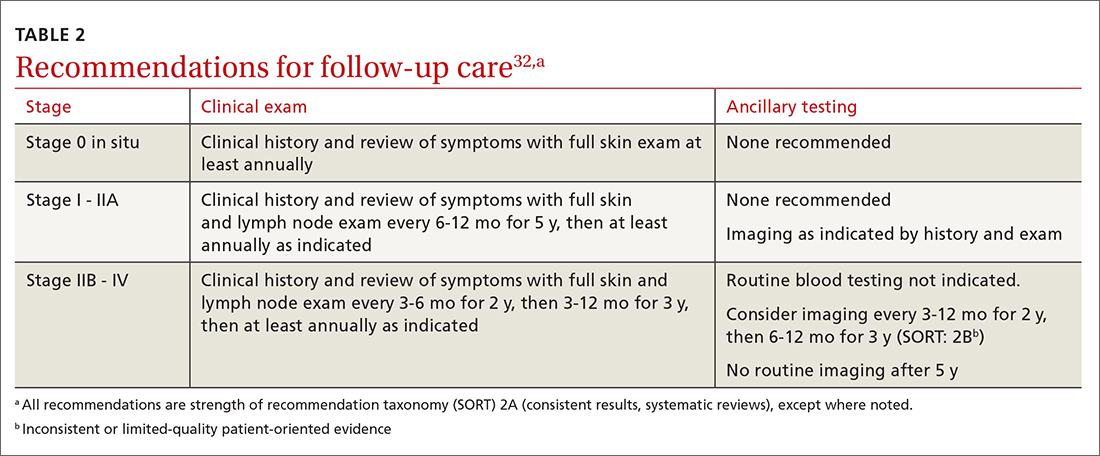
Melanoma epidemic or overdiagnosis?
Over the past 2 decades, a marked rise in the incidence of melanoma has been reported in developed countries worldwide, although melanoma mortality rates have not increased as rapidly, with melanoma-specific survival stable in most groups.47-50 Due to conflicting evidence, significant disagreement exists as to whether this is an actual epidemic caused by a true rise in disease burden or is merely an artifact stemming from overdiagnosis.47
Evidence supporting a true melanoma epidemic includes population-based studies demonstrating greater UV radiation–induced carcinogenesis (from the sun and tanning bed use), a larger aging population, and increased incidence regardless of socioeconomic status.47 Those challenging the validity of an epidemic instead attribute the rising incidence to early-detection public awareness campaigns, expanded screenings, improved diagnostic modalities, and increased biopsies. They also credit lower pathologic thresholds that help identify thinner tumors with little to no metastatic potential.48 Additionally, multiple studies report an increased incidence in melanomas of all histologic subtypes and thicknesses, not just thinner, more curable tumors.49,51,52 Although increased screening and biopsies are effective, they alone cannot account for the sharp rise in melanoma cases.47 This “melanoma paradox” of increasing incidence without a parallel increase in mortality remains unsettled.47
CASE
Your patient had Stage IIA disease and a WE was performed with 1-cm margins. Ultrasound of the axilla identified an enlarged node, which was removed and found not to be diseased. He has now returned to have you look at another lesion identified by his spouse. His review of symptoms is negative. His initial melanoma was removed 2 years earlier, and his last dermatology skin exam was 5 months prior. You look at the lesion using a dermatoscope and do not note any worrisome features. You recommend that the patient photograph the area for reexamination and follow-up with his dermatologist next month for a 6-month follow-up.
CORRESPONDENCE
Jessica Servey, MD, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. NIH. Cancer stat facts: melanoma of the skin. 2018. Accessed May 13, 2021. https://seer.cancer.gov/statfacts/html/melan.html
2. Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
3. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392:971-984.
4. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018(12):CD011902.
5. Morris JB, Alfonso SV, Hernandez N, et al. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7:63-70.
6. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
7. Rosendahl C, Cameron A, McColl I, et al. Dermatoscopy in routine practice — “chaos and clues”. Aust Fam Physician. 2012;41:482-487.
8. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy: a new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
9. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
10. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
11. Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29:694-701.
12. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-93.
13. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981-984.
14. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80:365-377.
15. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
16. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
17. Usatine RP, Shama LK, Marghoob AA, et al. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67:E1-E11.
18. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
19. Seiverling EV, Ahrns HT, Bacik LC, et al. Biopsies for skin cancer detection: dispelling the myths. J Fam Pract. 2018;67:270-274.
20. Martin RCG, Scoggins CR, Ross MI, et al. Is incisional biopsy of melanoma harmful? Am J Surg. 2005;190:913-917.
21. Mir M, Chan CS, Khan F, et al. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J Am Acad Dermatol. 2013;68:452-458.
22. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902-908
23. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer 8th ed cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
24. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
25. Valsecchi ME, Silbermins D, de Rosa N, et al. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29:1479-1487.
26. Memorial Sloan Kettering Cancer Center. Risk of sentinel lymph node metastasis nomogram. Accessed May 13, 2021. www.mskcc.org/nomograms/melanoma/sentinel_lymph_node_metastasis
27. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017:563-581.
28. Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129-142.
29. Tsao H, Feldman M, Fullerton JE, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol. 2004;140:67-70.
30. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
31. Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007; 110:1107-1114.
32. Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines: cutaneous melanoma, version 4.2020. Accessed June 7, 2021. http://medi-guide.meditool.cn/ymtpdf/ACC90A18-6CDF-9443-BF3F-E29394D495E8.pdf
33. Stensheim H, Møller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45-51.
34. Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22:4369-4375.
35. Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607-2617.
36. Byrom L, Olsen CM, Knight L, et al. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol Surg. 2015;41:875-882.
37. Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: women’s health initiative randomized trials. J Natl Cancer Inst. 2011;103:1469-1475.
38. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
39. Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-218.
40. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
41. Kandolf Sekulovic L, Peris K, Hauschild A, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer. 2017;75:313-322.
42. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743-1754.
43. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396-1414.
44. Lyth J, Falk M, Maroti M, et al. Prognostic risk factors of first recurrence in patients with primary stages I–II cutaneous malignant melanoma – from the population‐based Swedish melanoma register. J Eur Acad Dermatol Venereol. 2017;31:1468-1474.
45. Robinson JK, Wayne JD, Martini MC, et al. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152:979-985.
Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
47. Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1-12.
48. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75:698-705.
49. Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-1674.
50. Curchin DJ, Forward E, Dickison P, et al. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985-2015. J Am Acad Dermatol. 2019;80:1791-1793.
51. Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275-280.
52. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17-S25.
CASE
A 48-year-old man comes to your clinic with a dark nevus on his right upper arm that appeared 2 months earlier. He says that the lesion has continued to grow and has bled (he thought because he initially picked at it). On exam, there is a 7-mm brown papule with 2 black dots and slightly asymmetric borders.
How would you proceed with this patient?
Melanoma is the fifth leading cause of new cancer cases annually, with > 96,000 new cases in 2019.1 Overall, melanoma is more common in men and in Whites, with 48% diagnosed in people ages 55 to 74.1 The past 2 decades have seen numerous developments in the diagnosis, treatment, and surveillance of melanoma. This article covers recommendations, controversies, and issues that require future study. It does not cover uveal or mucosal melanoma.
Evaluating a patient with a new or changing nevus
Known risk factors for melanoma include a changing nevus, indoor tanning, older age, many melanocytic nevi, history of a dysplastic nevus or of blistering sunburns during teen years, red or blonde hair, large congenital nevus, Fitzpatrick skin type I or II, high socioeconomic status, personal or family history of melanoma, and intermittent high-intensity sun exposure.2-3 Presence of 1 or more of these risk factors should lower the threshold for biopsy.
Worrisome physical exam features (FIGURE) are nevus asymmetry, irregular borders, variegated color, and a diameter > 6 mm (the size of a pencil eraser). Inquire as to whether the nevus’ appearance has evolved and if it has bled without trauma. In a patient with multiple nevi, 1 nevus that looks different than the rest (the so-called “ugly duckling”) is concerning. Accuracy of diagnosis is enhanced with dermoscopy. A Cochrane review showed that skilled use of dermoscopy, in addition to inspection with the naked eye, considerably increases the sensitivity and specificity of diagnosing melanoma.4 Yet a 2017 study of 705 US primary care practitioners showed that only 8.3% of them used dermoscopy to evaluate pigmented lesions.5

Several published algorithms and checklists can aid clinicians in identifying lesions suggestive of melanoma—eg, ABCDE, CASH, Menzies method, “chaos and clues,” and 2-step and 3- and 7-point checklists.6-10 A simple 3-step algorithm, the TADA (triage amalgamated dermoscopic algorithm) method is available to novice dermoscopy users.11 Experts in pigmented lesions prefer to use pattern analysis, which requires simultaneously assessing multiple lesion patterns that vary according to body site.12,13
Dermoscopic features suggesting melanoma are atypical pigment networks, pseudopods, radial streaking, irregular dots or globules, blue-whitish veil, and granularity or peppering.14 Appropriate and effective use of dermoscopy requires training.15,16 Available methods for learning dermoscopy include online and in-person courses, mentoring by experienced dermoscopists, books and articles, and free apps and online resources.17
Continue to: Perform a skin biopsy, but do this first
Perform a skin biopsy, but do this first
Skin biopsy is the definitive way to diagnose melanoma. Prior to biopsy, take photographs to document the exact location of the lesion and to ensure that the correct area is removed in wide excision (WE). A complete biopsy should include the full depth and breadth of the lesion to ensure there are clinically negative margins. This can be achieved with an elliptical excision (for larger lesions), punch excision (for small lesions), or saucerization (deep shave with 1- to 2-mm peripheral margins, used for intermediate-size lesions).18 Saucerization is distinctly different from a superficial shave biopsy, which is not recommended for lesions with features of melanoma.19
A decision to perform a biopsy on a part of the lesion (partial biopsy) depends on the size of the lesion and its anatomic location, and is best made in agreement with the patient.
If you are untrained or uncomfortable performing the biopsy, contact a dermatologist immediately. In many communities, such referrals are subject to long delays, which further supports the advisability of family physicians doing their own biopsies after photographing the suspicious lesion. Many resources are available to help family physicians learn to do biopsies proficiently (www.mdedge.com/familymedicine/article/164358/oncology/biopsies-skin-cancer-detection-dispelling-myths).19
What to communicate to the pathologist. At a minimum, the biopsy request form should include patient age, sex, biopsy type (punch, excisional, or scoop shave), intention (complete or partial sample), exact site of the biopsy with laterality, and clinical details. These details should include the lesion size and clinical description, the suspected diagnosis, and clinical information, such as whether there is a history of bleeding or changing color, size, or symmetry. In standard biopsy specimens, the pathologist is only examining a portion of the lesion. Communicating clearly to the pathologist may lead to a request for deeper or additional sections or special stains.
If the biopsy results do not match the clinical impression, a phone call to the pathologist is warranted. In addition, evaluation by a dermatopathologist may be merited as pathologic diagnosis of melanoma can be quite challenging. Newer molecular tests, such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH), can assist in the histologic evaluation of complex pigmented lesions.
Continue to: CASE
CASE
You perform an elliptical excisional biopsy on your patient. The biopsy report comes back as a nodular malignant melanoma, Breslow depth 2.5 mm without ulceration, and no evidence of lymphovascular invasion or microsatellitosis. The report states that the biopsy margins appear clear of tumor involvement.
Further evaluation when the biopsy result is positive
Key steps in initial patient care include relaying pathology results to the patient, conducting (as needed) a more extensive evaluation, and obtaining appropriate consultation.
Clearly explain the diagnosis and convey an accurate reading of the pathology report. The vital pieces of information in the biopsy report are the Breslow depth and presence of ulceration, as evidence shows these 2 factors to be important independent predictors of outcome.22,23 Also important are the presence of microsatellitosis (essential for staging purposes), pathologic stage, and the status of the peripheral and deep biopsy margins. Review Breslow depth with the patient as this largely dictates treatment options and prognosis.
Evaluate for possible metastatic disease. Obtain a complete history from every patient with cutaneous melanoma, looking for any positive review of systems as a harbinger of metastatic disease. A full-body skin and lymph node exam is vital, given that melanoma can arise anywhere including on the scalp, in the gluteal cleft, and beneath nails. If the lymph node exam is worrisome, conduct an ultrasound exam, even while referring to specialty care. Treating a patient with melanoma requires a multidisciplinary approach that may include dermatologists, surgeons, and oncologists based on the stage of disease. A challenge for family physicians is knowing which consultation to prioritize and how to counsel the patient to schedule these for the most cost-effective and timely evaluation.
Expedite a dermatology consultation. If the melanoma is deep or appears advanced based on size or palpable lymph nodes, contact the dermatologist immediately by phone to set up a rapid referral. Delays in the definitive management of thick melanomas can negatively affect outcome. Paper, facsimile, or electronic referrals can get lost in the system and are not reliable methods for referring patients for a melanoma consultation. One benefit of the family physician performing the initial biopsy is that a confirmed melanoma diagnosis will almost certainly get an expedited dermatology appointment.
Continue to: Wide excision and sentinel node biopsy
Wide excision and sentinel node biopsy
Wide excision of a primary melanoma is standard practice, with evidence favoring the following surgical margins: 0.5 to 1 cm for melanoma in situ, 1 cm for tumors up to 1 mm in thickness, 1 to 2 cm for tumors > 1 to 2 mm thick, and 2 cm for tumors > 2 mm thick.18 WE is often performed by dermatologists for nonulcerated tumors < 0.8 mm thick (T1a) without adverse features. If trained in cutaneous surgery, you can also choose to excise these thin melanomas in your office. Otherwise refer all patients with biopsy-proven melanoma to dermatologists to perform an adequate WE.
Refer patients who have tumors ≥ 0.8 mm thick to the appropriate surgical specialty (surgical oncology, if available) for consultation on sentinel lymph node biopsy. SLNB, when indicated, should be performed prior to WE of the primary tumor, and whenever possible in the same surgical setting, to maximize lymphatic drainage mapping techniques.18 Medical oncology referral, if needed, is usually made after WE.
SLNB remains the standard for lymph node staging. It is controversial mainly in its use for very thin or very thick lesions. Randomized controlled trials, including the Multicenter Selective Lymphadenectomy Trial,24 have shown no difference in melanoma-specific survival for patients with intermediate-thickness melanomas who had undergone SLNB.24
Many professional organizations consider SLNB to be the most significant prognostic indicator of disease recurrence. With a negative SLNB result, the risk of regional node recurrence is 5% or lower.18,25 In addition, sentinel lymph node status is a critical determinant for systemic adjuvant therapy consideration and clinical trial eligibility. For patients who have primary cutaneous melanoma without clinical lymphadenopathy, an online tool is available for patients to use with their physician in predicting the likelihood of SLNB positivity.26
Recommendations for SLNB, supported by multidisciplinary consensus:18
- Do not pursue SLNB for melanoma in situ or most cutaneous melanomas < 0.8 mm without ulceration (T1a). (See TABLE 127)
- Discuss SLNB with patients who have T1a melanoma and additional adverse features: young age, high mitotic rate, lymphovascular invasion, and nevus depth close to 0.8 mm with positive deep biopsy margins.
- Discuss SLNB with patients who have T1b disease (< 0.8 mm with ulceration, or 0.8-1 mm), although rates of SLNB positivity are low.
- Offer SLNB to patients with T2a and higher disease (> 1 mm).18

Continue to: Patients who have...
Patients who have clinical Stage I or II disease (TABLE 127)
Melanoma in women: Considerations to keep in mind
Hormonal influences of pregnancy, lactation, contraception, and menopause introduce special considerations regarding melanoma, which is the most common cancer occurring during pregnancy, accounting for 31% of new malignancies.33 Risk of melanoma lessens, however, for women who first give birth at a younger age or who have had > 5 live births.18,34,35 There is no evidence that nevi darken during pregnancy, although nevi on the breast and abdomen may seem to enlarge due to skin stretching.18 All changing nevi in pregnancy warrant an examination, preferably with dermoscopy, and patients should be offered biopsy if there are any nevus characteristics associated with melanoma.18
The effect of pregnancy on an existing melanoma is not fully understood, but evidence from controlled studies shows no negative effect. Recent working group guidelines advise WE with local anesthesia without delay in pregnant patients.18 Definitive treatment after melanoma diagnosis should take a multidisciplinary approach involving obstetric care coordinated with Dermatology, Surgery, and Medical Oncology.18
Most recommendations on the timing of pregnancy following a melanoma diagnosis have limited evidence. One meta-analysis concluded that pregnancy occurring after successful treatment of melanoma did not change a woman’s prognosis.36 Current guidelines do not recommend delaying future pregnancy if a woman had an early-stage melanoma. For melanomas deemed higher risk, a woman could consider a 2- to 3-year delay in the next planned pregnancy, owing to current data on recurrence rates.18
A systematic review of women who used hormonal contraception or postmenopausal hormone replacement therapy (HRT) showed no associated increased risk of melanoma.35 An additional randomized trial showed no effect of HRT on melanoma risk.37
Continue to: Systemic melanoma treatment and common adverse effects
Systemic melanoma treatment and common adverse effects
Multiple systemic therapies have been approved for the treatment of advanced or unresectable cutaneous melanomas. While these treatments are managed primarily by Oncology in concert with Dermatology, an awareness of the medications’ common dermatologic toxicities is important for the primary care provider. The 2 broad categories of FDA-approved systemic medications for advanced melanoma are mitogen-activated protein kinase (MAPK) inhibitors and immune checkpoint inhibitors, each having its own set of adverse cutaneous effects.
MAPK pathway–targeting drugs include the B-Raf proto-oncogene serine/threonine-kinase inhibitors (BRAFIs) vemurafenib and dabrafenib, and the MAPK inhibitors (MEKIs) trametinib and cobimetinib. The most common adverse skin effects in MAPK pathway–targeting drugs are severe ultraviolet photosensitivity, cutaneous epidermal neoplasms (particularly squamous cell carcinoma, keratoacanthoma-type), thick actinic keratosis, wart-like keratosis, painful palmoplantar keratosis, and dry skin.38 These effects are most commonly seen with BRAFI monotherapy and can be abated with the addition of a MEKI. MEKI therapy can cause acneiform eruptions and paronychia.39 Additional adverse effects include diarrhea, pyrexia, arthralgias, and fatigue for BRAFIs and diarrhea, fatigue, and peripheral edema for MEKIs.40
Immune checkpoint inhibitors include anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab and nivolumab), and anti-PDL-1 (atezolizumab). Adverse skin effects include morbilliform rash with or without an associated itch, itch with or without an associated rash, vitiligo, and lichenoid skin rashes. PD-1 and PDL-1 inhibitors have been associated with flares or unmasking of atopic dermatitis, psoriasis, sarcoidosis, and autoimmune bullous disease.18 Diarrhea, colitis, hepatitis, elevated liver enzymes, hypophysitis, and thyroiditis are some of the more common noncutaneous adverse effects reported with CTLA-4 inhibitors, while fatigue, diarrhea, nausea, pneumonitis, and thyroid disease are seen with anti-PD-1/PDL-1 therapy.3
A look at the prognosis
For patients diagnosed with primary cutaneous melanoma between 2011 and 2017, the 5-year survival rate for localized disease (Stages I-II) was 99%.1 For regional (Stage III) and distant (Stage IV) disease, the 5-year survival rates were 68% and 30%, respectively.1 With the advent of adjuvant systemic therapy, 5-year overall survival rates for metastatic melanoma have markedly improved from < 10% to up to 40% to 50%.41 The 3-year survival rate for patients with high tumor burden, brain metastasis, and elevated lactate dehydrogenase remains at < 10%.42 Relative survival decreases with increased age, although survival is higher in women than in men.43 Risk of melanoma recurrence after surgical excision is high in patients with stage IIB, IIC, III and IV (resectable) disease. The most important risk factor for recurrence is primary tumor thickness.44 The most common site of first recurrence in stage I-II disease is regional lymph node metastasis (42.8%), closely followed by distant metastasis (37.6%).44
Long-term follow-up and surveillance
Recommendations for long-term care of patients with melanoma have evolved with advances in treatment, prognostication, and imaging. Caring for these patients requires a multidisciplinary approach wherein the family physician provides frontline care and team coordination. Since most recurrences are discovered by the patient or the patient’s family, patient education and self-examination are the cost-effective foundation for recurrence screening. In a trial of patients and partners, a 30-minute structured session on skin examination followed by physician reminders every 4 months increased the detection of melanoma recurrence without significant increases in patient visits.45
Continue to: Patient education should include sun safety...
Patient education should include sun safety (wearing sun-protective clothing, using broad-spectrum sunscreen, and avoiding sun exposure during peak times of the day). The US Preventive Services Task Force (USPSTF) says the level of evidence is insufficient to support routine skin cancer screening in adults.46 However, the USPSTF recommends discussing efforts to minimize UV radiation exposure to prevent skin cancer in fair-skinned individuals 10 to 24 years of age.
Current National Comprehensive Cancer Network (NCCN) guidelines have outlined the follow-up frequency for all melanoma patients. TABLE 232 outlines those recommendations in addition to self-examination and patient education.

Melanoma epidemic or overdiagnosis?
Over the past 2 decades, a marked rise in the incidence of melanoma has been reported in developed countries worldwide, although melanoma mortality rates have not increased as rapidly, with melanoma-specific survival stable in most groups.47-50 Due to conflicting evidence, significant disagreement exists as to whether this is an actual epidemic caused by a true rise in disease burden or is merely an artifact stemming from overdiagnosis.47
Evidence supporting a true melanoma epidemic includes population-based studies demonstrating greater UV radiation–induced carcinogenesis (from the sun and tanning bed use), a larger aging population, and increased incidence regardless of socioeconomic status.47 Those challenging the validity of an epidemic instead attribute the rising incidence to early-detection public awareness campaigns, expanded screenings, improved diagnostic modalities, and increased biopsies. They also credit lower pathologic thresholds that help identify thinner tumors with little to no metastatic potential.48 Additionally, multiple studies report an increased incidence in melanomas of all histologic subtypes and thicknesses, not just thinner, more curable tumors.49,51,52 Although increased screening and biopsies are effective, they alone cannot account for the sharp rise in melanoma cases.47 This “melanoma paradox” of increasing incidence without a parallel increase in mortality remains unsettled.47
CASE
Your patient had Stage IIA disease and a WE was performed with 1-cm margins. Ultrasound of the axilla identified an enlarged node, which was removed and found not to be diseased. He has now returned to have you look at another lesion identified by his spouse. His review of symptoms is negative. His initial melanoma was removed 2 years earlier, and his last dermatology skin exam was 5 months prior. You look at the lesion using a dermatoscope and do not note any worrisome features. You recommend that the patient photograph the area for reexamination and follow-up with his dermatologist next month for a 6-month follow-up.
CORRESPONDENCE
Jessica Servey, MD, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
CASE
A 48-year-old man comes to your clinic with a dark nevus on his right upper arm that appeared 2 months earlier. He says that the lesion has continued to grow and has bled (he thought because he initially picked at it). On exam, there is a 7-mm brown papule with 2 black dots and slightly asymmetric borders.
How would you proceed with this patient?
Melanoma is the fifth leading cause of new cancer cases annually, with > 96,000 new cases in 2019.1 Overall, melanoma is more common in men and in Whites, with 48% diagnosed in people ages 55 to 74.1 The past 2 decades have seen numerous developments in the diagnosis, treatment, and surveillance of melanoma. This article covers recommendations, controversies, and issues that require future study. It does not cover uveal or mucosal melanoma.
Evaluating a patient with a new or changing nevus
Known risk factors for melanoma include a changing nevus, indoor tanning, older age, many melanocytic nevi, history of a dysplastic nevus or of blistering sunburns during teen years, red or blonde hair, large congenital nevus, Fitzpatrick skin type I or II, high socioeconomic status, personal or family history of melanoma, and intermittent high-intensity sun exposure.2-3 Presence of 1 or more of these risk factors should lower the threshold for biopsy.
Worrisome physical exam features (FIGURE) are nevus asymmetry, irregular borders, variegated color, and a diameter > 6 mm (the size of a pencil eraser). Inquire as to whether the nevus’ appearance has evolved and if it has bled without trauma. In a patient with multiple nevi, 1 nevus that looks different than the rest (the so-called “ugly duckling”) is concerning. Accuracy of diagnosis is enhanced with dermoscopy. A Cochrane review showed that skilled use of dermoscopy, in addition to inspection with the naked eye, considerably increases the sensitivity and specificity of diagnosing melanoma.4 Yet a 2017 study of 705 US primary care practitioners showed that only 8.3% of them used dermoscopy to evaluate pigmented lesions.5

Several published algorithms and checklists can aid clinicians in identifying lesions suggestive of melanoma—eg, ABCDE, CASH, Menzies method, “chaos and clues,” and 2-step and 3- and 7-point checklists.6-10 A simple 3-step algorithm, the TADA (triage amalgamated dermoscopic algorithm) method is available to novice dermoscopy users.11 Experts in pigmented lesions prefer to use pattern analysis, which requires simultaneously assessing multiple lesion patterns that vary according to body site.12,13
Dermoscopic features suggesting melanoma are atypical pigment networks, pseudopods, radial streaking, irregular dots or globules, blue-whitish veil, and granularity or peppering.14 Appropriate and effective use of dermoscopy requires training.15,16 Available methods for learning dermoscopy include online and in-person courses, mentoring by experienced dermoscopists, books and articles, and free apps and online resources.17
Continue to: Perform a skin biopsy, but do this first
Perform a skin biopsy, but do this first
Skin biopsy is the definitive way to diagnose melanoma. Prior to biopsy, take photographs to document the exact location of the lesion and to ensure that the correct area is removed in wide excision (WE). A complete biopsy should include the full depth and breadth of the lesion to ensure there are clinically negative margins. This can be achieved with an elliptical excision (for larger lesions), punch excision (for small lesions), or saucerization (deep shave with 1- to 2-mm peripheral margins, used for intermediate-size lesions).18 Saucerization is distinctly different from a superficial shave biopsy, which is not recommended for lesions with features of melanoma.19
A decision to perform a biopsy on a part of the lesion (partial biopsy) depends on the size of the lesion and its anatomic location, and is best made in agreement with the patient.
If you are untrained or uncomfortable performing the biopsy, contact a dermatologist immediately. In many communities, such referrals are subject to long delays, which further supports the advisability of family physicians doing their own biopsies after photographing the suspicious lesion. Many resources are available to help family physicians learn to do biopsies proficiently (www.mdedge.com/familymedicine/article/164358/oncology/biopsies-skin-cancer-detection-dispelling-myths).19
What to communicate to the pathologist. At a minimum, the biopsy request form should include patient age, sex, biopsy type (punch, excisional, or scoop shave), intention (complete or partial sample), exact site of the biopsy with laterality, and clinical details. These details should include the lesion size and clinical description, the suspected diagnosis, and clinical information, such as whether there is a history of bleeding or changing color, size, or symmetry. In standard biopsy specimens, the pathologist is only examining a portion of the lesion. Communicating clearly to the pathologist may lead to a request for deeper or additional sections or special stains.
If the biopsy results do not match the clinical impression, a phone call to the pathologist is warranted. In addition, evaluation by a dermatopathologist may be merited as pathologic diagnosis of melanoma can be quite challenging. Newer molecular tests, such as fluorescence in situ hybridization (FISH) and comparative genomic hybridization (CGH), can assist in the histologic evaluation of complex pigmented lesions.
Continue to: CASE
CASE
You perform an elliptical excisional biopsy on your patient. The biopsy report comes back as a nodular malignant melanoma, Breslow depth 2.5 mm without ulceration, and no evidence of lymphovascular invasion or microsatellitosis. The report states that the biopsy margins appear clear of tumor involvement.
Further evaluation when the biopsy result is positive
Key steps in initial patient care include relaying pathology results to the patient, conducting (as needed) a more extensive evaluation, and obtaining appropriate consultation.
Clearly explain the diagnosis and convey an accurate reading of the pathology report. The vital pieces of information in the biopsy report are the Breslow depth and presence of ulceration, as evidence shows these 2 factors to be important independent predictors of outcome.22,23 Also important are the presence of microsatellitosis (essential for staging purposes), pathologic stage, and the status of the peripheral and deep biopsy margins. Review Breslow depth with the patient as this largely dictates treatment options and prognosis.
Evaluate for possible metastatic disease. Obtain a complete history from every patient with cutaneous melanoma, looking for any positive review of systems as a harbinger of metastatic disease. A full-body skin and lymph node exam is vital, given that melanoma can arise anywhere including on the scalp, in the gluteal cleft, and beneath nails. If the lymph node exam is worrisome, conduct an ultrasound exam, even while referring to specialty care. Treating a patient with melanoma requires a multidisciplinary approach that may include dermatologists, surgeons, and oncologists based on the stage of disease. A challenge for family physicians is knowing which consultation to prioritize and how to counsel the patient to schedule these for the most cost-effective and timely evaluation.
Expedite a dermatology consultation. If the melanoma is deep or appears advanced based on size or palpable lymph nodes, contact the dermatologist immediately by phone to set up a rapid referral. Delays in the definitive management of thick melanomas can negatively affect outcome. Paper, facsimile, or electronic referrals can get lost in the system and are not reliable methods for referring patients for a melanoma consultation. One benefit of the family physician performing the initial biopsy is that a confirmed melanoma diagnosis will almost certainly get an expedited dermatology appointment.
Continue to: Wide excision and sentinel node biopsy
Wide excision and sentinel node biopsy
Wide excision of a primary melanoma is standard practice, with evidence favoring the following surgical margins: 0.5 to 1 cm for melanoma in situ, 1 cm for tumors up to 1 mm in thickness, 1 to 2 cm for tumors > 1 to 2 mm thick, and 2 cm for tumors > 2 mm thick.18 WE is often performed by dermatologists for nonulcerated tumors < 0.8 mm thick (T1a) without adverse features. If trained in cutaneous surgery, you can also choose to excise these thin melanomas in your office. Otherwise refer all patients with biopsy-proven melanoma to dermatologists to perform an adequate WE.
Refer patients who have tumors ≥ 0.8 mm thick to the appropriate surgical specialty (surgical oncology, if available) for consultation on sentinel lymph node biopsy. SLNB, when indicated, should be performed prior to WE of the primary tumor, and whenever possible in the same surgical setting, to maximize lymphatic drainage mapping techniques.18 Medical oncology referral, if needed, is usually made after WE.
SLNB remains the standard for lymph node staging. It is controversial mainly in its use for very thin or very thick lesions. Randomized controlled trials, including the Multicenter Selective Lymphadenectomy Trial,24 have shown no difference in melanoma-specific survival for patients with intermediate-thickness melanomas who had undergone SLNB.24
Many professional organizations consider SLNB to be the most significant prognostic indicator of disease recurrence. With a negative SLNB result, the risk of regional node recurrence is 5% or lower.18,25 In addition, sentinel lymph node status is a critical determinant for systemic adjuvant therapy consideration and clinical trial eligibility. For patients who have primary cutaneous melanoma without clinical lymphadenopathy, an online tool is available for patients to use with their physician in predicting the likelihood of SLNB positivity.26
Recommendations for SLNB, supported by multidisciplinary consensus:18
- Do not pursue SLNB for melanoma in situ or most cutaneous melanomas < 0.8 mm without ulceration (T1a). (See TABLE 127)
- Discuss SLNB with patients who have T1a melanoma and additional adverse features: young age, high mitotic rate, lymphovascular invasion, and nevus depth close to 0.8 mm with positive deep biopsy margins.
- Discuss SLNB with patients who have T1b disease (< 0.8 mm with ulceration, or 0.8-1 mm), although rates of SLNB positivity are low.
- Offer SLNB to patients with T2a and higher disease (> 1 mm).18

Continue to: Patients who have...
Patients who have clinical Stage I or II disease (TABLE 127)
Melanoma in women: Considerations to keep in mind
Hormonal influences of pregnancy, lactation, contraception, and menopause introduce special considerations regarding melanoma, which is the most common cancer occurring during pregnancy, accounting for 31% of new malignancies.33 Risk of melanoma lessens, however, for women who first give birth at a younger age or who have had > 5 live births.18,34,35 There is no evidence that nevi darken during pregnancy, although nevi on the breast and abdomen may seem to enlarge due to skin stretching.18 All changing nevi in pregnancy warrant an examination, preferably with dermoscopy, and patients should be offered biopsy if there are any nevus characteristics associated with melanoma.18
The effect of pregnancy on an existing melanoma is not fully understood, but evidence from controlled studies shows no negative effect. Recent working group guidelines advise WE with local anesthesia without delay in pregnant patients.18 Definitive treatment after melanoma diagnosis should take a multidisciplinary approach involving obstetric care coordinated with Dermatology, Surgery, and Medical Oncology.18
Most recommendations on the timing of pregnancy following a melanoma diagnosis have limited evidence. One meta-analysis concluded that pregnancy occurring after successful treatment of melanoma did not change a woman’s prognosis.36 Current guidelines do not recommend delaying future pregnancy if a woman had an early-stage melanoma. For melanomas deemed higher risk, a woman could consider a 2- to 3-year delay in the next planned pregnancy, owing to current data on recurrence rates.18
A systematic review of women who used hormonal contraception or postmenopausal hormone replacement therapy (HRT) showed no associated increased risk of melanoma.35 An additional randomized trial showed no effect of HRT on melanoma risk.37
Continue to: Systemic melanoma treatment and common adverse effects
Systemic melanoma treatment and common adverse effects
Multiple systemic therapies have been approved for the treatment of advanced or unresectable cutaneous melanomas. While these treatments are managed primarily by Oncology in concert with Dermatology, an awareness of the medications’ common dermatologic toxicities is important for the primary care provider. The 2 broad categories of FDA-approved systemic medications for advanced melanoma are mitogen-activated protein kinase (MAPK) inhibitors and immune checkpoint inhibitors, each having its own set of adverse cutaneous effects.
MAPK pathway–targeting drugs include the B-Raf proto-oncogene serine/threonine-kinase inhibitors (BRAFIs) vemurafenib and dabrafenib, and the MAPK inhibitors (MEKIs) trametinib and cobimetinib. The most common adverse skin effects in MAPK pathway–targeting drugs are severe ultraviolet photosensitivity, cutaneous epidermal neoplasms (particularly squamous cell carcinoma, keratoacanthoma-type), thick actinic keratosis, wart-like keratosis, painful palmoplantar keratosis, and dry skin.38 These effects are most commonly seen with BRAFI monotherapy and can be abated with the addition of a MEKI. MEKI therapy can cause acneiform eruptions and paronychia.39 Additional adverse effects include diarrhea, pyrexia, arthralgias, and fatigue for BRAFIs and diarrhea, fatigue, and peripheral edema for MEKIs.40
Immune checkpoint inhibitors include anti-CTLA-4 (ipilimumab), anti-PD-1 (pembrolizumab and nivolumab), and anti-PDL-1 (atezolizumab). Adverse skin effects include morbilliform rash with or without an associated itch, itch with or without an associated rash, vitiligo, and lichenoid skin rashes. PD-1 and PDL-1 inhibitors have been associated with flares or unmasking of atopic dermatitis, psoriasis, sarcoidosis, and autoimmune bullous disease.18 Diarrhea, colitis, hepatitis, elevated liver enzymes, hypophysitis, and thyroiditis are some of the more common noncutaneous adverse effects reported with CTLA-4 inhibitors, while fatigue, diarrhea, nausea, pneumonitis, and thyroid disease are seen with anti-PD-1/PDL-1 therapy.3
A look at the prognosis
For patients diagnosed with primary cutaneous melanoma between 2011 and 2017, the 5-year survival rate for localized disease (Stages I-II) was 99%.1 For regional (Stage III) and distant (Stage IV) disease, the 5-year survival rates were 68% and 30%, respectively.1 With the advent of adjuvant systemic therapy, 5-year overall survival rates for metastatic melanoma have markedly improved from < 10% to up to 40% to 50%.41 The 3-year survival rate for patients with high tumor burden, brain metastasis, and elevated lactate dehydrogenase remains at < 10%.42 Relative survival decreases with increased age, although survival is higher in women than in men.43 Risk of melanoma recurrence after surgical excision is high in patients with stage IIB, IIC, III and IV (resectable) disease. The most important risk factor for recurrence is primary tumor thickness.44 The most common site of first recurrence in stage I-II disease is regional lymph node metastasis (42.8%), closely followed by distant metastasis (37.6%).44
Long-term follow-up and surveillance
Recommendations for long-term care of patients with melanoma have evolved with advances in treatment, prognostication, and imaging. Caring for these patients requires a multidisciplinary approach wherein the family physician provides frontline care and team coordination. Since most recurrences are discovered by the patient or the patient’s family, patient education and self-examination are the cost-effective foundation for recurrence screening. In a trial of patients and partners, a 30-minute structured session on skin examination followed by physician reminders every 4 months increased the detection of melanoma recurrence without significant increases in patient visits.45
Continue to: Patient education should include sun safety...
Patient education should include sun safety (wearing sun-protective clothing, using broad-spectrum sunscreen, and avoiding sun exposure during peak times of the day). The US Preventive Services Task Force (USPSTF) says the level of evidence is insufficient to support routine skin cancer screening in adults.46 However, the USPSTF recommends discussing efforts to minimize UV radiation exposure to prevent skin cancer in fair-skinned individuals 10 to 24 years of age.
Current National Comprehensive Cancer Network (NCCN) guidelines have outlined the follow-up frequency for all melanoma patients. TABLE 232 outlines those recommendations in addition to self-examination and patient education.

Melanoma epidemic or overdiagnosis?
Over the past 2 decades, a marked rise in the incidence of melanoma has been reported in developed countries worldwide, although melanoma mortality rates have not increased as rapidly, with melanoma-specific survival stable in most groups.47-50 Due to conflicting evidence, significant disagreement exists as to whether this is an actual epidemic caused by a true rise in disease burden or is merely an artifact stemming from overdiagnosis.47
Evidence supporting a true melanoma epidemic includes population-based studies demonstrating greater UV radiation–induced carcinogenesis (from the sun and tanning bed use), a larger aging population, and increased incidence regardless of socioeconomic status.47 Those challenging the validity of an epidemic instead attribute the rising incidence to early-detection public awareness campaigns, expanded screenings, improved diagnostic modalities, and increased biopsies. They also credit lower pathologic thresholds that help identify thinner tumors with little to no metastatic potential.48 Additionally, multiple studies report an increased incidence in melanomas of all histologic subtypes and thicknesses, not just thinner, more curable tumors.49,51,52 Although increased screening and biopsies are effective, they alone cannot account for the sharp rise in melanoma cases.47 This “melanoma paradox” of increasing incidence without a parallel increase in mortality remains unsettled.47
CASE
Your patient had Stage IIA disease and a WE was performed with 1-cm margins. Ultrasound of the axilla identified an enlarged node, which was removed and found not to be diseased. He has now returned to have you look at another lesion identified by his spouse. His review of symptoms is negative. His initial melanoma was removed 2 years earlier, and his last dermatology skin exam was 5 months prior. You look at the lesion using a dermatoscope and do not note any worrisome features. You recommend that the patient photograph the area for reexamination and follow-up with his dermatologist next month for a 6-month follow-up.
CORRESPONDENCE
Jessica Servey, MD, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. NIH. Cancer stat facts: melanoma of the skin. 2018. Accessed May 13, 2021. https://seer.cancer.gov/statfacts/html/melan.html
2. Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
3. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392:971-984.
4. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018(12):CD011902.
5. Morris JB, Alfonso SV, Hernandez N, et al. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7:63-70.
6. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
7. Rosendahl C, Cameron A, McColl I, et al. Dermatoscopy in routine practice — “chaos and clues”. Aust Fam Physician. 2012;41:482-487.
8. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy: a new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
9. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
10. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
11. Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29:694-701.
12. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-93.
13. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981-984.
14. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80:365-377.
15. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
16. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
17. Usatine RP, Shama LK, Marghoob AA, et al. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67:E1-E11.
18. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
19. Seiverling EV, Ahrns HT, Bacik LC, et al. Biopsies for skin cancer detection: dispelling the myths. J Fam Pract. 2018;67:270-274.
20. Martin RCG, Scoggins CR, Ross MI, et al. Is incisional biopsy of melanoma harmful? Am J Surg. 2005;190:913-917.
21. Mir M, Chan CS, Khan F, et al. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J Am Acad Dermatol. 2013;68:452-458.
22. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902-908
23. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer 8th ed cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
24. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
25. Valsecchi ME, Silbermins D, de Rosa N, et al. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29:1479-1487.
26. Memorial Sloan Kettering Cancer Center. Risk of sentinel lymph node metastasis nomogram. Accessed May 13, 2021. www.mskcc.org/nomograms/melanoma/sentinel_lymph_node_metastasis
27. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017:563-581.
28. Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129-142.
29. Tsao H, Feldman M, Fullerton JE, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol. 2004;140:67-70.
30. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
31. Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007; 110:1107-1114.
32. Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines: cutaneous melanoma, version 4.2020. Accessed June 7, 2021. http://medi-guide.meditool.cn/ymtpdf/ACC90A18-6CDF-9443-BF3F-E29394D495E8.pdf
33. Stensheim H, Møller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45-51.
34. Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22:4369-4375.
35. Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607-2617.
36. Byrom L, Olsen CM, Knight L, et al. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol Surg. 2015;41:875-882.
37. Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: women’s health initiative randomized trials. J Natl Cancer Inst. 2011;103:1469-1475.
38. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
39. Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-218.
40. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
41. Kandolf Sekulovic L, Peris K, Hauschild A, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer. 2017;75:313-322.
42. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743-1754.
43. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396-1414.
44. Lyth J, Falk M, Maroti M, et al. Prognostic risk factors of first recurrence in patients with primary stages I–II cutaneous malignant melanoma – from the population‐based Swedish melanoma register. J Eur Acad Dermatol Venereol. 2017;31:1468-1474.
45. Robinson JK, Wayne JD, Martini MC, et al. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152:979-985.
Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
47. Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1-12.
48. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75:698-705.
49. Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-1674.
50. Curchin DJ, Forward E, Dickison P, et al. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985-2015. J Am Acad Dermatol. 2019;80:1791-1793.
51. Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275-280.
52. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17-S25.
1. NIH. Cancer stat facts: melanoma of the skin. 2018. Accessed May 13, 2021. https://seer.cancer.gov/statfacts/html/melan.html
2. Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
3. Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392:971-984.
4. Dinnes J, Deeks JJ, Chuchu N, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database Syst Rev. 2018(12):CD011902.
5. Morris JB, Alfonso SV, Hernandez N, et al. Examining the factors associated with past and present dermoscopy use among family physicians. Dermatol Pract Concept. 2017;7:63-70.
6. Henning JS, Dusza SW, Wang SQ, et al. The CASH (color, architecture, symmetry, and homogeneity) algorithm for dermoscopy. J Am Acad Dermatol. 2007;56:45-52.
7. Rosendahl C, Cameron A, McColl I, et al. Dermatoscopy in routine practice — “chaos and clues”. Aust Fam Physician. 2012;41:482-487.
8. Soyer HP, Argenziano G, Zalaudek I, et al. Three-point checklist of dermoscopy: a new screening method for early detection of melanoma. Dermatology. 2004;208:27-31.
9. Argenziano G, Fabbrocini G, Carli P, et al. Epiluminescence microscopy for the diagnosis of doubtful melanocytic skin lesions. Comparison of the ABCD rule of dermatoscopy and a new 7-point checklist based on pattern analysis. Arch Dermatol. 1998;134:1563-1570.
10. Marghoob AA, Usatine RP, Jaimes N. Dermoscopy for the family physician. Am Fam Physician. 2013;88:441-450.
11. Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29:694-701.
12. Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679-93.
13. Carli P, Quercioli E, Sestini S, et al. Pattern analysis, not simplified algorithms, is the most reliable method for teaching dermoscopy for melanoma diagnosis to residents in dermatology. Br J Dermatol. 2003;148:981-984.
14. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80:365-377.
15. Westerhoff K, McCarthy WH, Menzies SW. Increase in the sensitivity for melanoma diagnosis by primary care physicians using skin surface microscopy. Br J Dermatol. 2000;143:1016-1020.
16. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
17. Usatine RP, Shama LK, Marghoob AA, et al. Dermoscopy in family medicine: a primer. J Fam Pract. 2018;67:E1-E11.
18. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208-250.
19. Seiverling EV, Ahrns HT, Bacik LC, et al. Biopsies for skin cancer detection: dispelling the myths. J Fam Pract. 2018;67:270-274.
20. Martin RCG, Scoggins CR, Ross MI, et al. Is incisional biopsy of melanoma harmful? Am J Surg. 2005;190:913-917.
21. Mir M, Chan CS, Khan F, et al. The rate of melanoma transection with various biopsy techniques and the influence of tumor transection on patient survival. J Am Acad Dermatol. 2013;68:452-458.
22. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902-908
23. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer 8th ed cancer staging manual. CA Cancer J Clin. 2017;67:472-492.
24. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599-609.
25. Valsecchi ME, Silbermins D, de Rosa N, et al. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta-analysis. J Clin Oncol. 2011;29:1479-1487.
26. Memorial Sloan Kettering Cancer Center. Risk of sentinel lymph node metastasis nomogram. Accessed May 13, 2021. www.mskcc.org/nomograms/melanoma/sentinel_lymph_node_metastasis
27. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge SB, Greene FL, eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017:563-581.
28. Xing Y, Bronstein Y, Ross MI, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103:129-142.
29. Tsao H, Feldman M, Fullerton JE, et al. Early detection of asymptomatic pulmonary melanoma metastases by routine chest radiographs is not associated with improved survival. Arch Dermatol. 2004;140:67-70.
30. Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
31. Yancovitz M, Finelt N, Warycha MA, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b-T3b melanoma. Cancer. 2007; 110:1107-1114.
32. Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines: cutaneous melanoma, version 4.2020. Accessed June 7, 2021. http://medi-guide.meditool.cn/ymtpdf/ACC90A18-6CDF-9443-BF3F-E29394D495E8.pdf
33. Stensheim H, Møller B, van Dijk T, et al. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27:45-51.
34. Lens MB, Rosdahl I, Ahlbom A, et al. Effect of pregnancy on survival in women with cutaneous malignant melanoma. J Clin Oncol. 2004;22:4369-4375.
35. Gandini S, Iodice S, Koomen E, et al. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607-2617.
36. Byrom L, Olsen CM, Knight L, et al. Does pregnancy after a diagnosis of melanoma affect prognosis? Systematic review and meta-analysis. Dermatol Surg. 2015;41:875-882.
37. Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: women’s health initiative randomized trials. J Natl Cancer Inst. 2011;103:1469-1475.
38. Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
39. Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: part I: inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203-218.
40. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015;7:122-136.
41. Kandolf Sekulovic L, Peris K, Hauschild A, et al. More than 5000 patients with metastatic melanoma in Europe per year do not have access to recommended first-line innovative treatments. Eur J Cancer. 2017;75:313-322.
42. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743-1754.
43. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9:1396-1414.
44. Lyth J, Falk M, Maroti M, et al. Prognostic risk factors of first recurrence in patients with primary stages I–II cutaneous malignant melanoma – from the population‐based Swedish melanoma register. J Eur Acad Dermatol Venereol. 2017;31:1468-1474.
45. Robinson JK, Wayne JD, Martini MC, et al. Early detection of new melanomas by patients with melanoma and their partners using a structured skin self-examination skills training intervention: a randomized clinical trial. JAMA Dermatol. 2016;152:979-985.
Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
47. Gardner LJ, Strunck JL, Wu YP, et al. Current controversies in early-stage melanoma: questions on incidence, screening, and histologic regression. J Am Acad Dermatol. 2019;80:1-12.
48. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75:698-705.
49. Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666-1674.
50. Curchin DJ, Forward E, Dickison P, et al. The acceleration of melanoma in situ: a population-based study of melanoma incidence trends from Victoria, Australia, 1985-2015. J Am Acad Dermatol. 2019;80:1791-1793.
51. Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275-280.
52. Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65:S17-S25.
PRACTICE RECOMMENDATIONS
› Consider adding dermoscopy to the physical exam to increase sensitivity and specificity in diagnosing melanoma. A
› Perform wide local excision for invasive cutaneous melanoma: 1-cm margin for tumors up to 1 mm thick; 1 to 2 cm for tumors > 1 mm to 2 mm thick; and 2 cm for tumors > 2 mm thick. A
› Do not hesitate to consider, as needed, hormone replacement therapy or hormonal contraception for women with a prior diagnosis of melanoma, as this form of contraception does not confer an increased risk of melanoma. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series