User login
Relapsing scabies? Nails may hold a clue
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
cautioned Marie Chinazzo, MD, of Centre Hospitalier Régional et Universitaire Tours, France, and her associates.
Nails can harbor mites, representing a potential source for relapse, not only in children, but also in adults.
Few studies have addressed scabies on the nails, which is typically observed in immunocompromised adults with crusted scabies, but also rarely in healthy adults and children.
In an observational, multicenter, prospective study conducted between June 2015 and January 2017, 47 pediatric patients with common scabies, including 3 children under 2 years of age, presented with mites on the first toenail/thumbnail; two of them had already completed treatment and were experiencing relapse. All children with dermatologic diagnosis that was confirmed by visual inspection of “the delta sign” (presence of the mite seen as a triangle representing the head) using dermoscopy or by microscopic identification of Sarcoptes scabiei were included in the study. Dermatologists were required to complete a standardized questionnaire for each participant. Full body inspections and nail samplings also were done.
Clinical nail damage, consisting of hyperkeratosis, onycholysis, onychoschizia, and pachyonychia, appeared in 5 of the 47 patients (11%). No other cause of nail damage was determined in four of the cases, for which mites were not directly visualized, the researchers noted. The report was published in the Journal of Pediatrics.
Of the 47 confirmed cases, 26 were female; 23 were under 2 years of age; 20 were 2-12 years; and 4 were older than 12. Ten cases presented with significant medical history; none were classified as immunocompromised.
Fully 42 of the 47 children (89%) reported pruritus, and of these, 64% also had pruritus present in the family home; 60% of siblings and 45% of parents were affected.
None were diagnosed with crusted scabies. The mean delay from disease onset to diagnosis was 55 days. In 38% of cases, previous treatment for scabies had been rendered.
Treatments varied based on presentation. Ivermectin, esdepallethrin, and 40% urea were repeated after 10 days in at least one case. In another case, an entire family was treated once with topical 5% permethrin; once the child experienced relapse, oral ivermectin was employed. In the case of an 18-month-old girl with pruritus and skin lesions, topical corticosteroid was used for 10 days until such time that dermatoscopy revealed the “delta sign” and 5% topical permethrin was added.
The authors observed that nail scabies in the medical literature is more commonly seen in immunocompromised patients with crusted scabies and higher concentrations of parasites. They were able to locate only three other reports, all in adults, of nail scabies occurring with common scabies.
“Treatment of nail scabies is difficult and is not highly evidence based,” cautioned Dr. Chinazzo and her associates. The primary study limitations were the small patient population and that nail sampling was taken only from the first fingers and toes, which could mean that the number of mites present is actually underestimated, they added.
The authors had no relevant financial disclosures.
SOURCE: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Pediatric relapse estimated as high as 66%.
Major finding: Nail scabies found in great toenail, not fingernails.
Study details: Observational multicenter prospective study of 47 pediatric patients with common scabies.
Disclosures: The authors had no relevant financial disclosures.
Source: Chinazzo M et al. J Pediatr. 2018. doi: 10.1016/j.jpeds.2018.01.038.
Additional training may be warranted for clinicians administering DTaP
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
The Vaccine Adverse Event Reporting System offers confirmation that DTaP vaccines are safe and have a reasonably low frequency of adverse events. Despite this, the U.S.-based resurgence of pertussis shortly after acellular vaccines were introduced legitimately raised concerns over the efficacy of DTaP, which is now known to have a shorter duration of protection than its predecessor, the diphtheria, tetanus toxoids, whole-cell pertussis vaccine. Consequently, older children, adolescents, and adults are left unprotected without periodic booster doses, Flor M. Muñoz, MD, wrote in an editorial accompanying the study by Moro et al.
The World Health Organization’s recommendation to countries that never made the switch to DTaP is to continue using the whole-cell vaccines “because of their consistent higher efficacy” points to “an imperative need to develop more immunogenic pertussis vaccines that are also safe,” she observed.
“Active research is ongoing for the development of novel vaccines, including live attenuated vaccines, whole-cell vaccines with reduced endotoxin content to be less reactogenic, outer membrane vesicles–based vaccines, and acellular vaccine formulations prepared with new adjuvants or additional and novel antigens.
“As we go back to the drawing board in the fight against Bordetella pertussis, much work is needed to learn more about this fascinating pathogen and its interactions with humans to improve our understanding of how immunity and long-lasting protection can be achieved, to engineer and produce novel vaccines, and to design and perform the clinical studies that will eventually lead to the control of pertussis disease and its global impact with safe and effective vaccines for all,” Dr. Muñoz added.
Dr. Muñoz is affiliated with the section of infectious diseases in the department of pediatrics at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. Her comments here were summarized from her editorial accompanying the article by Moro et al (Pediatrics. 2018. doi: 10.1542/peds.2018-1036). Dr. Munoz said she had no relevant financial disclosures and received no external funding.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
Additional training may be needed for providers who administer DTaP vaccine to prevent errors in vaccination, but reported Pedro Moro, MD, MPH, of the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases and his associates in Pediatrics.
After Dr. Moro and his associates performed an automated analysis of all reports included in the Vaccine Adverse Event Reporting System (VAERS), which is coadministered by the CDC and the Food and Drug Administration, as well as a clinical review of reported deaths and a random sampling of serious reports in the database, they concluded that safety findings concerning DTaP were consistent with those from prelicensure trials and postlicensure studies.
DTaP vaccines, which included Infanrix, Daptacel, Pediarix, Kinrix, and Pentacel, were coadministered with one or more other vaccines in 43,984 (88%) of cases reported; of the reports included in the data mining, 5,627 (11%) were classified as serious, including 844 (2%) deaths. Of all reports received in the prelicensure clinical trials, injection site reactions and systemic reactions, such as fever and vomiting, were the most common reactions to DTaP vaccine.
In a 5% random sample of the 4,783 serious nondeath reports included in the study, 25% were neurologic, 23% gastrointestinal, and 20% were caused by general disorders and vaccine site conditions. Fully 80% of those flagged as neurologic were seizure related. In another 79%, for which intussusception was the most common gastrointestinal condition, all but two cases had rotavirus vaccine coadministered with DTaP. Altogether, there were 182 cases of anaphylaxis reported.
Serious events were characterized as death, life-threatening illness, hospitalization, lengthening of existing hospital stay, or permanent disability. In cases of death, reports that followed DTaP vaccine were manually reviewed by a physician, who evaluated autopsy report, death certificate, or medical records. The authors also included in their evaluation of records any reports of postvaccine anaphylaxis.
Of the 844 deaths, death certificates, autopsy reports, or medical records were obtained for 86%. Among these, sudden infant death syndrome (SIDS) was found to be the most frequent cause of death in 48%; of these, 62% were male infants, and 91% were infants under 6 months of age.
“It would not be uncommon to observe a coincidental close temporal relationship between vaccination and SIDS because this condition peaks at a time when children receive a relatively large number of recommended vaccinations,” said Dr. Moro and his associates. “There is a large body of evidence in which it is shown that vaccination is not causally associated with SIDS.”
The authors identified disproportional reporting for injection site reactions, as well as other events and conditions, to which they attribute, at least in part, administration of the wrong vaccine or formulation and administration at the wrong site. Such mistakes can be lessened or even prevented with provider education and training on appropriate recommendations and package insert specifications put forth by the CDC’s Advisory Committee on Immunization Practices, they advised.
While the authors praised VAERS for the wealth of timely data it has offered in detecting potential safety issues that may require further investigation, Dr. Moro cautioned that it is a passive surveillance system with limitations that warrant “careful interpretation of its findings.” Its purpose is to improve immunization programs.
Because it does not “meet the definition of research,” the work performed in this study was not subject to institutional review board evaluation and informed consent requirements, the authors added. VAERS generally is not able to assess whether vaccines are the direct cause of adverse events, primarily because of underreporting or overreporting, biased reporting, and inconsistency in quality and completeness of information reported. Because it does not tally number of vaccines administered, it is also unable to provide data needed to calculate incidence rates.
The authors had no relevant financial disclosures. The study was funded by the CDC and the FDA.
SOURCE: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
FROM PEDIATRICS
Key clinical point: No new or unexpected safety issues were found with DTaP.
Major finding: Nearly 90% of adverse events reported were not considered serious.
Study details: Large-scale data mining and records review from the Vaccine Adverse Event Reporting System.
Disclosures: The authors had no relevant financial disclosures. The study was funded by the Centers for Disease Control and Prevention and the Food and Drug Administration.
Source: Moro P et al. Pediatrics. 2018. doi: 10.1542/peds.2017-4171.
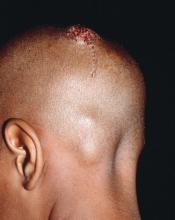
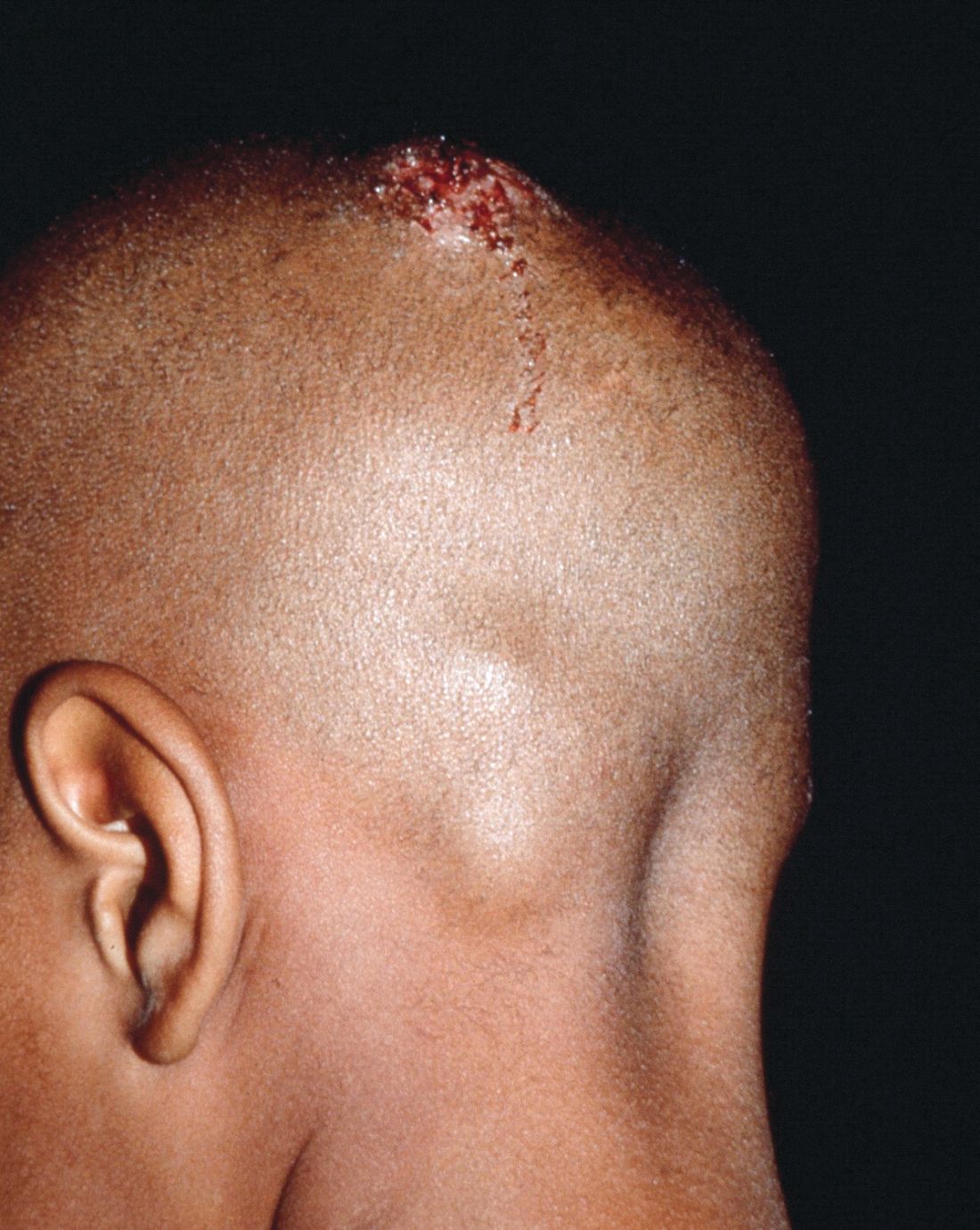
Early diagnosis, treatment key to prevent permanent baldness in tinea capitis
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
and the substantially reduced overall quality of health, reported Aditya K. Gupta, MD, PhD, of Mediprobe Research and the University of Toronto, and his associates.
In a systematic review of both randomized, controlled trials and clinical trials published before June 1, 2017, the authors sought to identify differences between treatment medications and significant adverse side effects, and to evaluate the most effective methods for diagnosis. The study criteria included trials with clinical and mycologic diagnosis of tinea capitis, evaluation of efficacy rates and/or safety measures in participants aged 18 years or younger, yielded a total of 4,190 studies in this article published in Pediatric Dermatology.
Dr. Gupta and his colleagues evaluated efficacy rates that reported on mycologic cure (negative mycologic testing), clinical cure (complete absence of signs and symptoms), and complete cure (both mycologic and clinical cure). Trichophyton tonsurans was the most common organism reported in North America, and Wood’s light examination/light microscopic examination was the most common hair sample collection method identified.
In a population of 3,998 children who received treatment across all studies, five oral antifungals were used (terbinafine, griseofulvin, itraconazole, ketoconazole, and fluconazole). In addition, several studies examined the safety and effectiveness of combined oral and topical treatment in 833 children, while 25 children received topical-only therapy.
Although topical treatment may be useful adjunctively, some studies noted that oral treatment is necessary for effective resolution of tinea capitis. While some experts recommend continuing topical treatment until clinical and mycologic cure are achieved, the authors cautioned that “the presence of a topical antifungal in a culture media would likely lead to a false negative result, so a clinical confirmation is necessary.”
Adverse events
Altogether, 295 drug-related adverse effects were reported: 51.2% from terbinafine, 26.8% from griseofulvin, 12.2% from fluconazole, 8.5% from itraconazole, and 1.4% from ketoconazole; all were transient and mild to moderate in severity.
Of the total population observed, just 50 children (1.3% of 3,998) ceased treatment because of adverse effects of the medication.
Therapy choices
Of the 75 antifungal treatment combinations identified, cure rates were highest with continuous itraconazole and terbinafine (mycologic), griseofulvin and terbinafine (clinical), and griseofulvin and terbinafine (complete). Griseofulvin was more effective at treating Microsporum than Trichophyton infections, fluconazole was comparably effective in treating both Microsporum and Trichophyton infections, and continuous itraconazole and terbinafine were more effective at curing Trichophyton infections than Microsporum, noted Dr. Gupta and his associates.
Terbinafine treatment for Trichophyton infections was found to be effective at just 4 weeks, however, oral terbinafine was singularly responsible for more than half of adverse events reported. The authors suggested that this might be from its extensive biodistribution. In such cases, the authors recommended baseline monitoring of transaminase.
Although griseofulvin is the most widely prescribed medication for pediatric tinea capitis, primarily because of its cost effectiveness and accessibility, a 2016 Cochrane review found that newer treatments – terbinafine, itraconazole and fluconazole – offer comparative effectiveness in cases of Trichophyton infection. The relatively higher cost of these treatments and the prevalence of tinea capitis in lower socioeconomic populations, however, may render them impractical, the authors noted. As recent clinical trials have suggested significantly larger, weight-normalized doses are required in children to approximate the exposure estimates of adults, this should be of key consideration when choosing appropriate, cost-effective treatments.
Diagnostic issues
T. tonsurans cases of tinea capitis are most prevalent in North America, and recent data suggest they are on the rise. The organism typically infects human skin and hair, and can to survive for lengthy periods on inanimate objects, including combs, brushes, sheets, and blankets. Researchers credit the growing number of cases in North America to several factors. Infections from the fungus have become increasingly common in the United States and Canada as a consequence of changing travel and immigration patterns. In addition, many physicians still turn to fluorescence (Wood’s light examination) in diagnosing tinea capitis, but T. tonsurans does not show up with fluorescence and typically does not present with the classic black dots characteristic of other fungal species. As a result, many cases in North America are misdiagnosed as seborrhea, dandruff, and impetigo, and subsequently undertreated, leading to spread of the infection. It was noted that more than half of the included studies used some form of Wood’s light examination.
Of all the techniques addressed, microscopy was found to be the fastest, but not always the most accurate, means of diagnosing tinea capitis. Dr. Gupta and his associates advised that diagnosis confirmation and precise species identification is best obtained with cultured scrapings, but this process can take 3 weeks or longer.
While fomites and hair care practices play a key role in tinea capitis infection, large family size, crowded living conditions, and low socioeconomic status are predisposing factors. Those who come in contact with infected patients should be considered possible asymptomatic carriers and be evaluated accordingly for treatment and to prevent spread of infection, the authors advised. Furthermore, recent studies recognized the impracticality of isolating children recently treated with oral therapy from classrooms since shedding of spores can continue for months.
The researchers had no relevant financial disclosures to report.
SOURCE: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The psychosocial impact and overall lower quality of health associated with tinea capitis is significant.
Major finding: Wood’s light should not be the only method of organism identification.
Study details: A systematic literature review of 4,190 studies.
Disclosures: The researchers had no relevant financial disclosures.
Source: Gupta AK et al. Ped Dermatol. 2018 May 24. doi: 10.1111/jdv.15088.
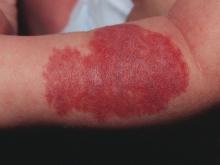
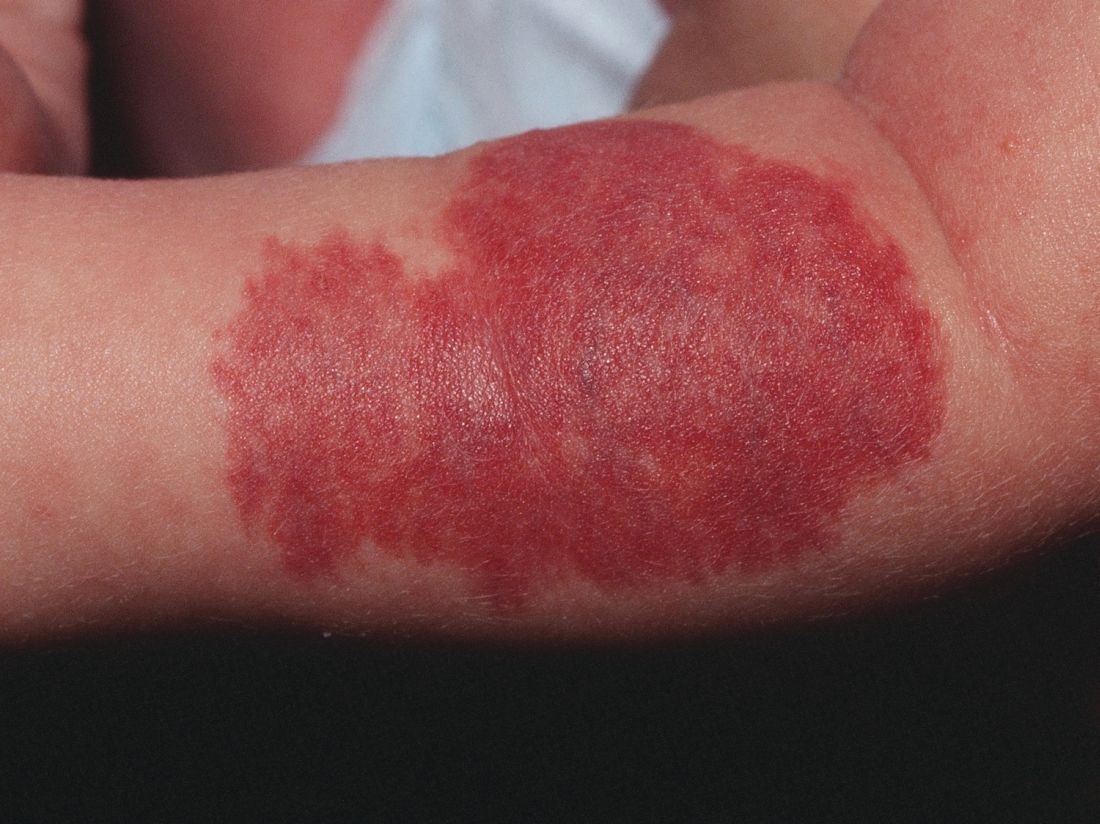
Pain relievers, bed rest may be sufficient to manage PEH
Although skin biopsy and the “presence of deep dermal mixed infiltrate with abundant neutrophils surrounding eccrine sweat glands” is considered the preferred method for diagnosing palmoplantar eccrine hidradenitis (PEH), Paola Piccini, MD, and her colleagues at the University of Florence (Italy) caution that biopsy frequently is not needed.
In the days prior to the appearance of erythematous and painful nodules on the soles of his feet, a healthy 8-year-old boy sustained “thermal and mechanical trauma playing football and cycling,” that made walking difficult. The week prior to the injury, he had complained of diarrhea in the absence of fever.
Given results of testing and the patient’s overall good health, he received a diagnosis of PEH. Dr. Piccini and her colleagues chose not to biopsy the nodules because they typically resolve on their own within a few weeks. Instead, he was prescribed pain relievers and bed rest. Within 2 weeks, the nodules were completely healed, and no further relapse was reported, the authors noted in the Journal of Pediatrics.
All previous case studies cited in the literature reported complete resolution without treatment within 4 weeks, said Dr. Piccini and her colleagues. They added that correct diagnosis is key to avoiding inappropriate medical treatments given the benign course of PEH.
Common presenting risk factors tend to include local thermal and mechanical trauma plus intense physical activity and recent infection. It is these risk factors that cause rupture of the eccrine gland and resulting infiltration of neutrophils to the site.
Conditions that share common symptoms with PEH include erythema multiforme, nodular erythema, cellulitis, and viral infections.
No disclosures were noted.
SOURCE: Piccini, P et al. J. Pediatr. 2018. doi: 10.1016/j.peds.2018.03.017.
Although skin biopsy and the “presence of deep dermal mixed infiltrate with abundant neutrophils surrounding eccrine sweat glands” is considered the preferred method for diagnosing palmoplantar eccrine hidradenitis (PEH), Paola Piccini, MD, and her colleagues at the University of Florence (Italy) caution that biopsy frequently is not needed.
In the days prior to the appearance of erythematous and painful nodules on the soles of his feet, a healthy 8-year-old boy sustained “thermal and mechanical trauma playing football and cycling,” that made walking difficult. The week prior to the injury, he had complained of diarrhea in the absence of fever.
Given results of testing and the patient’s overall good health, he received a diagnosis of PEH. Dr. Piccini and her colleagues chose not to biopsy the nodules because they typically resolve on their own within a few weeks. Instead, he was prescribed pain relievers and bed rest. Within 2 weeks, the nodules were completely healed, and no further relapse was reported, the authors noted in the Journal of Pediatrics.
All previous case studies cited in the literature reported complete resolution without treatment within 4 weeks, said Dr. Piccini and her colleagues. They added that correct diagnosis is key to avoiding inappropriate medical treatments given the benign course of PEH.
Common presenting risk factors tend to include local thermal and mechanical trauma plus intense physical activity and recent infection. It is these risk factors that cause rupture of the eccrine gland and resulting infiltration of neutrophils to the site.
Conditions that share common symptoms with PEH include erythema multiforme, nodular erythema, cellulitis, and viral infections.
No disclosures were noted.
SOURCE: Piccini, P et al. J. Pediatr. 2018. doi: 10.1016/j.peds.2018.03.017.
Although skin biopsy and the “presence of deep dermal mixed infiltrate with abundant neutrophils surrounding eccrine sweat glands” is considered the preferred method for diagnosing palmoplantar eccrine hidradenitis (PEH), Paola Piccini, MD, and her colleagues at the University of Florence (Italy) caution that biopsy frequently is not needed.
In the days prior to the appearance of erythematous and painful nodules on the soles of his feet, a healthy 8-year-old boy sustained “thermal and mechanical trauma playing football and cycling,” that made walking difficult. The week prior to the injury, he had complained of diarrhea in the absence of fever.
Given results of testing and the patient’s overall good health, he received a diagnosis of PEH. Dr. Piccini and her colleagues chose not to biopsy the nodules because they typically resolve on their own within a few weeks. Instead, he was prescribed pain relievers and bed rest. Within 2 weeks, the nodules were completely healed, and no further relapse was reported, the authors noted in the Journal of Pediatrics.
All previous case studies cited in the literature reported complete resolution without treatment within 4 weeks, said Dr. Piccini and her colleagues. They added that correct diagnosis is key to avoiding inappropriate medical treatments given the benign course of PEH.
Common presenting risk factors tend to include local thermal and mechanical trauma plus intense physical activity and recent infection. It is these risk factors that cause rupture of the eccrine gland and resulting infiltration of neutrophils to the site.
Conditions that share common symptoms with PEH include erythema multiforme, nodular erythema, cellulitis, and viral infections.
No disclosures were noted.
SOURCE: Piccini, P et al. J. Pediatr. 2018. doi: 10.1016/j.peds.2018.03.017.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Complete resolution within 4 weeks is common without treatment.
Major finding: .
Study details: Case study.
Disclosures: No disclosures were noted.
Source: Lund, E et al. Ped. Dermatol. 2018. doi: 10.1111/pde.13508.
Pretreatment ECG unwarranted for most infantile hemangioma patients starting propranolol
reported Emily B. Lund, MD, of the University of Chicago, and her associates.
This finding supports previously published studies that pretreatment ECG is not necessary, despite consensus guidelines published in 2013 that recommend ECG screening of high-risk infants presenting with below-normal heart rate, arrhythmia, or family history of either arrhythmia or congenital heart disease.
Among the 6% of patients included in the study who had a positive personal cardiac history, congenital heart disease was most common; coronary artery disease was most prevalent among the 41% with a positive family cardiac history. Baseline vital signs revealed no hypotension or bradycardia.
All patients prescribed propranolol were routinely screened with ECG prior to therapy during the study period. Baseline heart rate and blood pressure were observed for abnormalities; patients also were observed during follow-up for possible propranolol side effects.
A total of 43% of ECG screenings performed were found to be abnormal; left ventricular hypertrophy was the most common abnormality. Despite further cardiac evaluation of all but one patient with abnormal ECG, no contraindications to treatment were identified, Dr. Lund and her colleagues reported in Pediatric Dermatology.
Ultimately, 96% of patients observed started treatment with propranolol; of the remaining 4% who did not, the authors cited parental preference and lack of follow-up as the primary reasons for nontreatment.
The researchers found no association between reported side effects and abnormal ECG, a positive personal history of cardiac problems, or a positive family history of cardiac problems.
Dr. Lund and her associates suggested that future revision of the guidelines should emphasize the absence of significant positive predictive value of ECG abnormalities for treatment-related side effects.
The researchers reported no relevant financial disclosures.
SOURCE: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
reported Emily B. Lund, MD, of the University of Chicago, and her associates.
This finding supports previously published studies that pretreatment ECG is not necessary, despite consensus guidelines published in 2013 that recommend ECG screening of high-risk infants presenting with below-normal heart rate, arrhythmia, or family history of either arrhythmia or congenital heart disease.
Among the 6% of patients included in the study who had a positive personal cardiac history, congenital heart disease was most common; coronary artery disease was most prevalent among the 41% with a positive family cardiac history. Baseline vital signs revealed no hypotension or bradycardia.
All patients prescribed propranolol were routinely screened with ECG prior to therapy during the study period. Baseline heart rate and blood pressure were observed for abnormalities; patients also were observed during follow-up for possible propranolol side effects.
A total of 43% of ECG screenings performed were found to be abnormal; left ventricular hypertrophy was the most common abnormality. Despite further cardiac evaluation of all but one patient with abnormal ECG, no contraindications to treatment were identified, Dr. Lund and her colleagues reported in Pediatric Dermatology.
Ultimately, 96% of patients observed started treatment with propranolol; of the remaining 4% who did not, the authors cited parental preference and lack of follow-up as the primary reasons for nontreatment.
The researchers found no association between reported side effects and abnormal ECG, a positive personal history of cardiac problems, or a positive family history of cardiac problems.
Dr. Lund and her associates suggested that future revision of the guidelines should emphasize the absence of significant positive predictive value of ECG abnormalities for treatment-related side effects.
The researchers reported no relevant financial disclosures.
SOURCE: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
reported Emily B. Lund, MD, of the University of Chicago, and her associates.
This finding supports previously published studies that pretreatment ECG is not necessary, despite consensus guidelines published in 2013 that recommend ECG screening of high-risk infants presenting with below-normal heart rate, arrhythmia, or family history of either arrhythmia or congenital heart disease.
Among the 6% of patients included in the study who had a positive personal cardiac history, congenital heart disease was most common; coronary artery disease was most prevalent among the 41% with a positive family cardiac history. Baseline vital signs revealed no hypotension or bradycardia.
All patients prescribed propranolol were routinely screened with ECG prior to therapy during the study period. Baseline heart rate and blood pressure were observed for abnormalities; patients also were observed during follow-up for possible propranolol side effects.
A total of 43% of ECG screenings performed were found to be abnormal; left ventricular hypertrophy was the most common abnormality. Despite further cardiac evaluation of all but one patient with abnormal ECG, no contraindications to treatment were identified, Dr. Lund and her colleagues reported in Pediatric Dermatology.
Ultimately, 96% of patients observed started treatment with propranolol; of the remaining 4% who did not, the authors cited parental preference and lack of follow-up as the primary reasons for nontreatment.
The researchers found no association between reported side effects and abnormal ECG, a positive personal history of cardiac problems, or a positive family history of cardiac problems.
Dr. Lund and her associates suggested that future revision of the guidelines should emphasize the absence of significant positive predictive value of ECG abnormalities for treatment-related side effects.
The researchers reported no relevant financial disclosures.
SOURCE: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: There was no association between side effects, abnormal ECG, and personal or family history of cardiac problems in children with infantile hemangioma who underwent propranolol therapy.
Major finding: Despite the fact that 43% of ECG screenings were abnormal, 96% of patients started treatment with propranolol, with no side effects related to abnormal ECG or to personal or family history of cardiac problems.
Study details: A retrospective chart review of 272 patients with infantile hemangioma.
Disclosures: The researchers reported no relevant financial disclosures.
Source: Lund EB et al. Ped Dermatol. 2018. doi: 10.1111/pde.13508.
Low incidence of HS in children does not diminish importance of early diagnosis
a study has found.
“The relatively low disease burden must not overshadow the extreme quality of life impact this disease has on those afflicted with it,” noted Amit Garg, MD, and associates at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hyde Park, N.Y.
The clinical term “hidradenitis’ was used to locate pediatric patients within a multi-institutional database of 55 million patients participating in 27 integrated health care organizations whose records were active in the database between March 2014 and March 2017.
The standardized prevalence of HS among girls was 3.75 times greater than in boys (P less than .0001), and the condition was most common in those aged 15-17 years (72%) across each racial group. “HS disproportionately affects African American children and adolescents, who have a 3.5-fold greater standardized prevalence than do Caucasians,” the authors wrote. The report was published in the Journal of Investigative Dermatology. Specifically, the highest prevalence by race was found in females aged 15-17 years who were African American (525 per 100,000) and biracial (253 per 100,000).
The authors acknowledged the availability of limited existing HS pediatric data from case reports and small series, none of which provided descriptions of subgroups by gender, age, or race.
In their review of the existing literature, Dr. Garg and his associates noted several key observations that may further aid in clinical diagnosis of pediatric patients at greater risk of developing HS:
- HS appears most likely to be a post-adrenarche disease; children with the disease more frequently present with a hormonal imbalance compared with adults. In fact, HS in children may be a marker of precocious puberty, as noted in those presenting with adrenal hyperplasia and premature adrenarche.
- A separate population-based analysis revealed an association between HS and polycystic ovary syndrome.
- Pediatric patients diagnosed with HS are more likely to present with a family history of the condition, and those experiencing early onset appear likely to develop more widespread HS.
- A fivefold likelihood of HS in pediatric Down syndrome patients is also attributed to genetic mutations.
The higher incidence of HS among adults (0.1%) is likely due to largely postpubertal disease onset, the authors speculated. They acknowledged that delays in diagnosing adolescent HS could account for the difference in prevalence between pediatric and adult populations. According to one study cited by Dr. Garg and his colleagues, adults with HS may have symptoms as many as 7 years prior to receiving a diagnosis.
Findings in Dr. Garg’s study serve to reinforce those of previous studies on adult HS populations, which also cited higher prevalence among females, especially African American females.
The research was funded by an unrestricted educational grant from AbbVie. Dr. Garg has served as an adviser for and received honoraria from AbbVie; the remaining researchers had no relevant financial disclosures.
SOURCE: Garg A et al. J Investig Dermatol. 2018 Apr 2. doi: 10.1016/j.jid.2018.04.001.
a study has found.
“The relatively low disease burden must not overshadow the extreme quality of life impact this disease has on those afflicted with it,” noted Amit Garg, MD, and associates at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hyde Park, N.Y.
The clinical term “hidradenitis’ was used to locate pediatric patients within a multi-institutional database of 55 million patients participating in 27 integrated health care organizations whose records were active in the database between March 2014 and March 2017.
The standardized prevalence of HS among girls was 3.75 times greater than in boys (P less than .0001), and the condition was most common in those aged 15-17 years (72%) across each racial group. “HS disproportionately affects African American children and adolescents, who have a 3.5-fold greater standardized prevalence than do Caucasians,” the authors wrote. The report was published in the Journal of Investigative Dermatology. Specifically, the highest prevalence by race was found in females aged 15-17 years who were African American (525 per 100,000) and biracial (253 per 100,000).
The authors acknowledged the availability of limited existing HS pediatric data from case reports and small series, none of which provided descriptions of subgroups by gender, age, or race.
In their review of the existing literature, Dr. Garg and his associates noted several key observations that may further aid in clinical diagnosis of pediatric patients at greater risk of developing HS:
- HS appears most likely to be a post-adrenarche disease; children with the disease more frequently present with a hormonal imbalance compared with adults. In fact, HS in children may be a marker of precocious puberty, as noted in those presenting with adrenal hyperplasia and premature adrenarche.
- A separate population-based analysis revealed an association between HS and polycystic ovary syndrome.
- Pediatric patients diagnosed with HS are more likely to present with a family history of the condition, and those experiencing early onset appear likely to develop more widespread HS.
- A fivefold likelihood of HS in pediatric Down syndrome patients is also attributed to genetic mutations.
The higher incidence of HS among adults (0.1%) is likely due to largely postpubertal disease onset, the authors speculated. They acknowledged that delays in diagnosing adolescent HS could account for the difference in prevalence between pediatric and adult populations. According to one study cited by Dr. Garg and his colleagues, adults with HS may have symptoms as many as 7 years prior to receiving a diagnosis.
Findings in Dr. Garg’s study serve to reinforce those of previous studies on adult HS populations, which also cited higher prevalence among females, especially African American females.
The research was funded by an unrestricted educational grant from AbbVie. Dr. Garg has served as an adviser for and received honoraria from AbbVie; the remaining researchers had no relevant financial disclosures.
SOURCE: Garg A et al. J Investig Dermatol. 2018 Apr 2. doi: 10.1016/j.jid.2018.04.001.
a study has found.
“The relatively low disease burden must not overshadow the extreme quality of life impact this disease has on those afflicted with it,” noted Amit Garg, MD, and associates at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hyde Park, N.Y.
The clinical term “hidradenitis’ was used to locate pediatric patients within a multi-institutional database of 55 million patients participating in 27 integrated health care organizations whose records were active in the database between March 2014 and March 2017.
The standardized prevalence of HS among girls was 3.75 times greater than in boys (P less than .0001), and the condition was most common in those aged 15-17 years (72%) across each racial group. “HS disproportionately affects African American children and adolescents, who have a 3.5-fold greater standardized prevalence than do Caucasians,” the authors wrote. The report was published in the Journal of Investigative Dermatology. Specifically, the highest prevalence by race was found in females aged 15-17 years who were African American (525 per 100,000) and biracial (253 per 100,000).
The authors acknowledged the availability of limited existing HS pediatric data from case reports and small series, none of which provided descriptions of subgroups by gender, age, or race.
In their review of the existing literature, Dr. Garg and his associates noted several key observations that may further aid in clinical diagnosis of pediatric patients at greater risk of developing HS:
- HS appears most likely to be a post-adrenarche disease; children with the disease more frequently present with a hormonal imbalance compared with adults. In fact, HS in children may be a marker of precocious puberty, as noted in those presenting with adrenal hyperplasia and premature adrenarche.
- A separate population-based analysis revealed an association between HS and polycystic ovary syndrome.
- Pediatric patients diagnosed with HS are more likely to present with a family history of the condition, and those experiencing early onset appear likely to develop more widespread HS.
- A fivefold likelihood of HS in pediatric Down syndrome patients is also attributed to genetic mutations.
The higher incidence of HS among adults (0.1%) is likely due to largely postpubertal disease onset, the authors speculated. They acknowledged that delays in diagnosing adolescent HS could account for the difference in prevalence between pediatric and adult populations. According to one study cited by Dr. Garg and his colleagues, adults with HS may have symptoms as many as 7 years prior to receiving a diagnosis.
Findings in Dr. Garg’s study serve to reinforce those of previous studies on adult HS populations, which also cited higher prevalence among females, especially African American females.
The research was funded by an unrestricted educational grant from AbbVie. Dr. Garg has served as an adviser for and received honoraria from AbbVie; the remaining researchers had no relevant financial disclosures.
SOURCE: Garg A et al. J Investig Dermatol. 2018 Apr 2. doi: 10.1016/j.jid.2018.04.001.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Onset of HS prior to age 10 years is extremely rate; more than two-thirds of cases occurred in teens aged 15-17 years.
Major finding: HS rates among girls are almost four times higher than those of boys; more than 70% of cases occurred in teens aged 15-17 years.
Study details: Cross-sectional population analysis of 1,240 pediatric patients sampled from a database of 55 million unique records.
Disclosures: The research was funded by an unrestricted educational grant from AbbVie. Dr. Garg has served as an adviser for and received honoraria from AbbVie; the remaining researchers had no relevant financial disclosures.
Source: Garg A et al. J Invest Dermatol. 2018 Apr 2. doi: 10.1016/j.jid.2018.04.001.
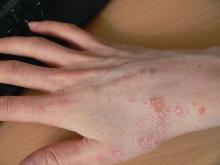
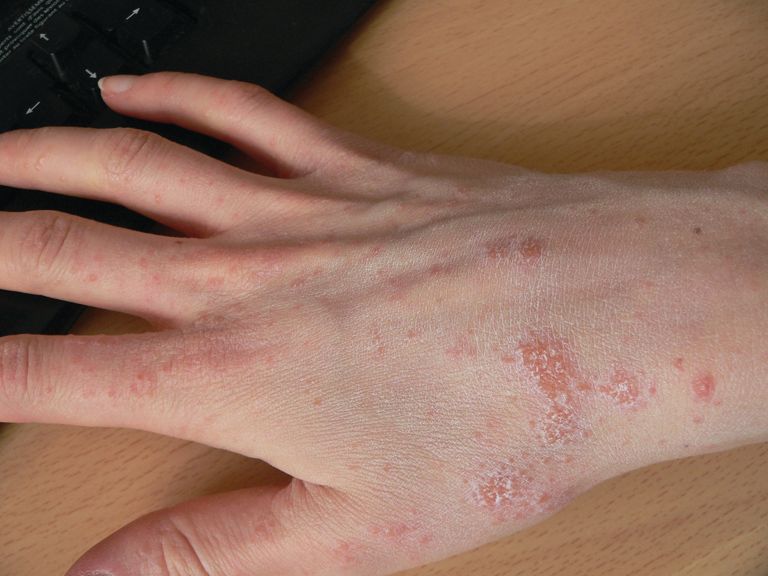
Nickel allergy common in children, significantly higher in girls
said Erin M. Warshaw, MD, MS, of the University of Minnesota, Minneapolis, and her associates.
“Although nickel sensitivity is reported to be problematic in children, the pediatric population is often underrepresented in large-scale epidemiologic studies,” they added.
In this retrospective, cross-sectional study of 1,894 children aged 18 years or younger tested by the North American Contact Dermatitis Group (NACDG) between 1994 and 2014, 23.7% of those patch tested were found to be sensitive to nickel. This included 6.5% who were 5 years or younger, 34.2% who were 6-12 years, and 59.4% who were 13-18 years.
Among all three patient groups, jewelry was the most common source of nickel sensitivity (36.4%), and this sensitivity was found to increase with age (5 years and younger, 20.7%; 6-12 years, 28.3%; and 13-18 years, 42.9%; P = .0006).
More than two-thirds of positive patch test reactions to nickel were found to be extreme or strong, Dr. Warshaw and her colleagues reported in the Journal of the American Academy of Dermatology.
Notably, girls were significantly more likely to exhibit nickel sensitivity than boys, a result the authors credit to “trends and social norms. ”
Citing a separate study conducted recently by NACDG on the correlation between piercing and nickel sensitivity across all ages, researchers found that females were significantly more likely to have piercings than were males, and with age, they speculated, “girls may be more likely to encounter high nickel release through piercing jewelry, bracelets, necklaces, hair clips, etc., resulting in higher proportions of girls than boys with nickel allergy.”
Nickel release, not nickel content, is an important factor in cases of nickel allergic contact dermatitis, the authors added. For nickel release to occur, prolonged skin contact is required. According to the European Chemicals Agency, prolonged exposure is defined as more than 10 minutes over three or more occasions within a 2-week period or more than 30 minutes over one or more occasion within the same 2 weeks.
The research was funded by the Minneapolis Veterans Affairs Medical Center, and in part, by the Nickel Producers Environmental Research Association. Three of the researchers have ties to various pharmaceutical companies and other organizations. Dr. Warshaw and the remaining researchers had no relevant financial disclosures.
SOURCE: Warshaw EM et al. J Am Acad Dermatol. 2018 Apr 14. doi: 10.1016/j.jaad.2018.02.071.
said Erin M. Warshaw, MD, MS, of the University of Minnesota, Minneapolis, and her associates.
“Although nickel sensitivity is reported to be problematic in children, the pediatric population is often underrepresented in large-scale epidemiologic studies,” they added.
In this retrospective, cross-sectional study of 1,894 children aged 18 years or younger tested by the North American Contact Dermatitis Group (NACDG) between 1994 and 2014, 23.7% of those patch tested were found to be sensitive to nickel. This included 6.5% who were 5 years or younger, 34.2% who were 6-12 years, and 59.4% who were 13-18 years.
Among all three patient groups, jewelry was the most common source of nickel sensitivity (36.4%), and this sensitivity was found to increase with age (5 years and younger, 20.7%; 6-12 years, 28.3%; and 13-18 years, 42.9%; P = .0006).
More than two-thirds of positive patch test reactions to nickel were found to be extreme or strong, Dr. Warshaw and her colleagues reported in the Journal of the American Academy of Dermatology.
Notably, girls were significantly more likely to exhibit nickel sensitivity than boys, a result the authors credit to “trends and social norms. ”
Citing a separate study conducted recently by NACDG on the correlation between piercing and nickel sensitivity across all ages, researchers found that females were significantly more likely to have piercings than were males, and with age, they speculated, “girls may be more likely to encounter high nickel release through piercing jewelry, bracelets, necklaces, hair clips, etc., resulting in higher proportions of girls than boys with nickel allergy.”
Nickel release, not nickel content, is an important factor in cases of nickel allergic contact dermatitis, the authors added. For nickel release to occur, prolonged skin contact is required. According to the European Chemicals Agency, prolonged exposure is defined as more than 10 minutes over three or more occasions within a 2-week period or more than 30 minutes over one or more occasion within the same 2 weeks.
The research was funded by the Minneapolis Veterans Affairs Medical Center, and in part, by the Nickel Producers Environmental Research Association. Three of the researchers have ties to various pharmaceutical companies and other organizations. Dr. Warshaw and the remaining researchers had no relevant financial disclosures.
SOURCE: Warshaw EM et al. J Am Acad Dermatol. 2018 Apr 14. doi: 10.1016/j.jaad.2018.02.071.
said Erin M. Warshaw, MD, MS, of the University of Minnesota, Minneapolis, and her associates.
“Although nickel sensitivity is reported to be problematic in children, the pediatric population is often underrepresented in large-scale epidemiologic studies,” they added.
In this retrospective, cross-sectional study of 1,894 children aged 18 years or younger tested by the North American Contact Dermatitis Group (NACDG) between 1994 and 2014, 23.7% of those patch tested were found to be sensitive to nickel. This included 6.5% who were 5 years or younger, 34.2% who were 6-12 years, and 59.4% who were 13-18 years.
Among all three patient groups, jewelry was the most common source of nickel sensitivity (36.4%), and this sensitivity was found to increase with age (5 years and younger, 20.7%; 6-12 years, 28.3%; and 13-18 years, 42.9%; P = .0006).
More than two-thirds of positive patch test reactions to nickel were found to be extreme or strong, Dr. Warshaw and her colleagues reported in the Journal of the American Academy of Dermatology.
Notably, girls were significantly more likely to exhibit nickel sensitivity than boys, a result the authors credit to “trends and social norms. ”
Citing a separate study conducted recently by NACDG on the correlation between piercing and nickel sensitivity across all ages, researchers found that females were significantly more likely to have piercings than were males, and with age, they speculated, “girls may be more likely to encounter high nickel release through piercing jewelry, bracelets, necklaces, hair clips, etc., resulting in higher proportions of girls than boys with nickel allergy.”
Nickel release, not nickel content, is an important factor in cases of nickel allergic contact dermatitis, the authors added. For nickel release to occur, prolonged skin contact is required. According to the European Chemicals Agency, prolonged exposure is defined as more than 10 minutes over three or more occasions within a 2-week period or more than 30 minutes over one or more occasion within the same 2 weeks.
The research was funded by the Minneapolis Veterans Affairs Medical Center, and in part, by the Nickel Producers Environmental Research Association. Three of the researchers have ties to various pharmaceutical companies and other organizations. Dr. Warshaw and the remaining researchers had no relevant financial disclosures.
SOURCE: Warshaw EM et al. J Am Acad Dermatol. 2018 Apr 14. doi: 10.1016/j.jaad.2018.02.071.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: This U.S. study reinforces other recent investigations citing high occurrence of pediatric nickel-associated allergic contact dermatitis.
Major finding: Almost one-quarter (23.7%) of almost 2,000 children patch tested were found to have nickel sensitivity.
Study details: Retrospective, cross-sectional study of 1,894 pediatric patients.
Disclosures: The research was funded by the Minneapolis Veterans Affairs Medical Center, and in part by the Nickel Producers Environmental Research Association. Three of the researchers have ties to various pharmaceutical companies and other organizations. Dr. Warshaw and the remaining researchers had no relevant financial disclosures.
Source: Warshaw EM et al. J Am Acad Dermatol. 2018 Apr 14. doi: 10.1016/j.jaad.2018.02.071.