User login
Review of VTE Prophylaxis Strategies
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
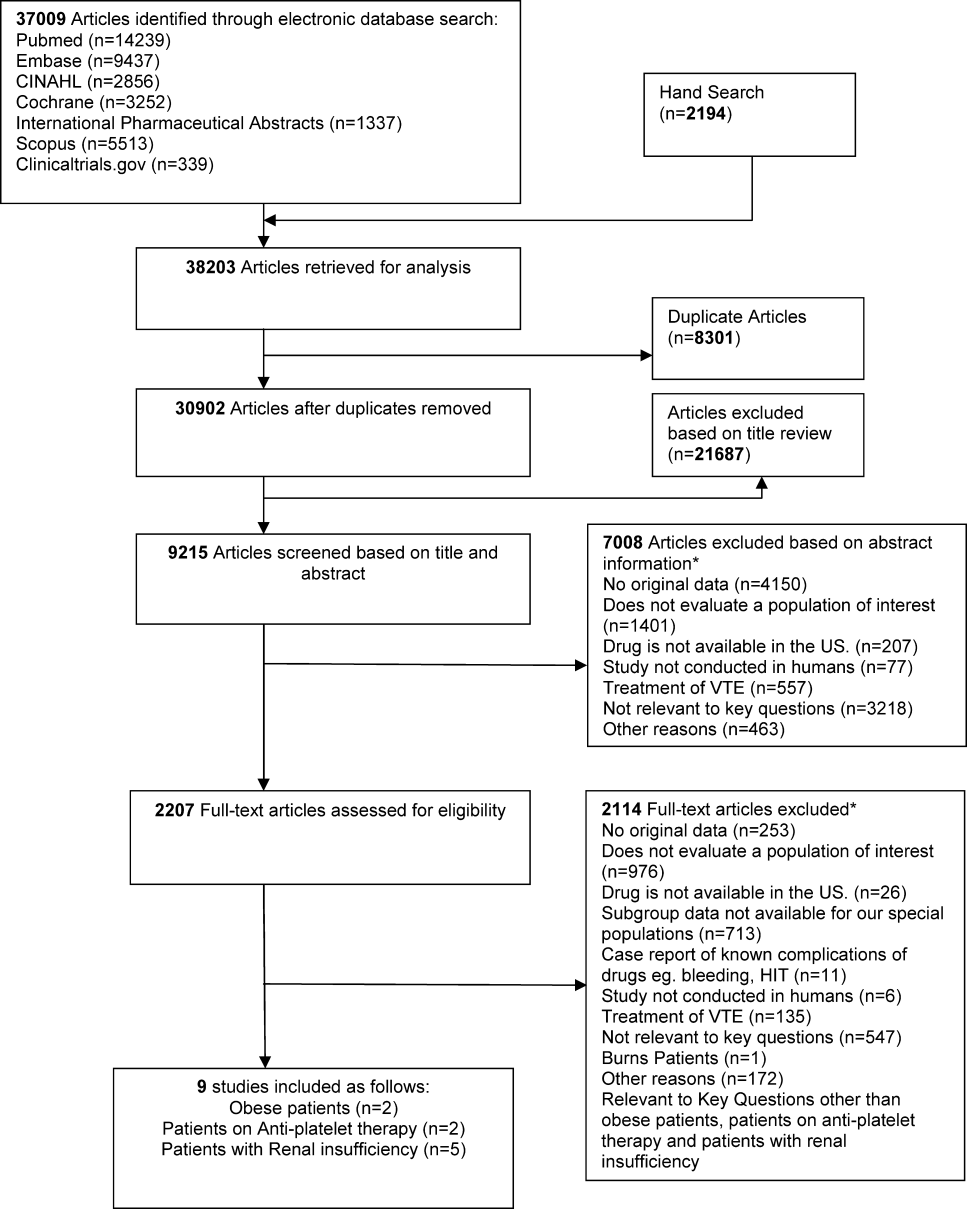
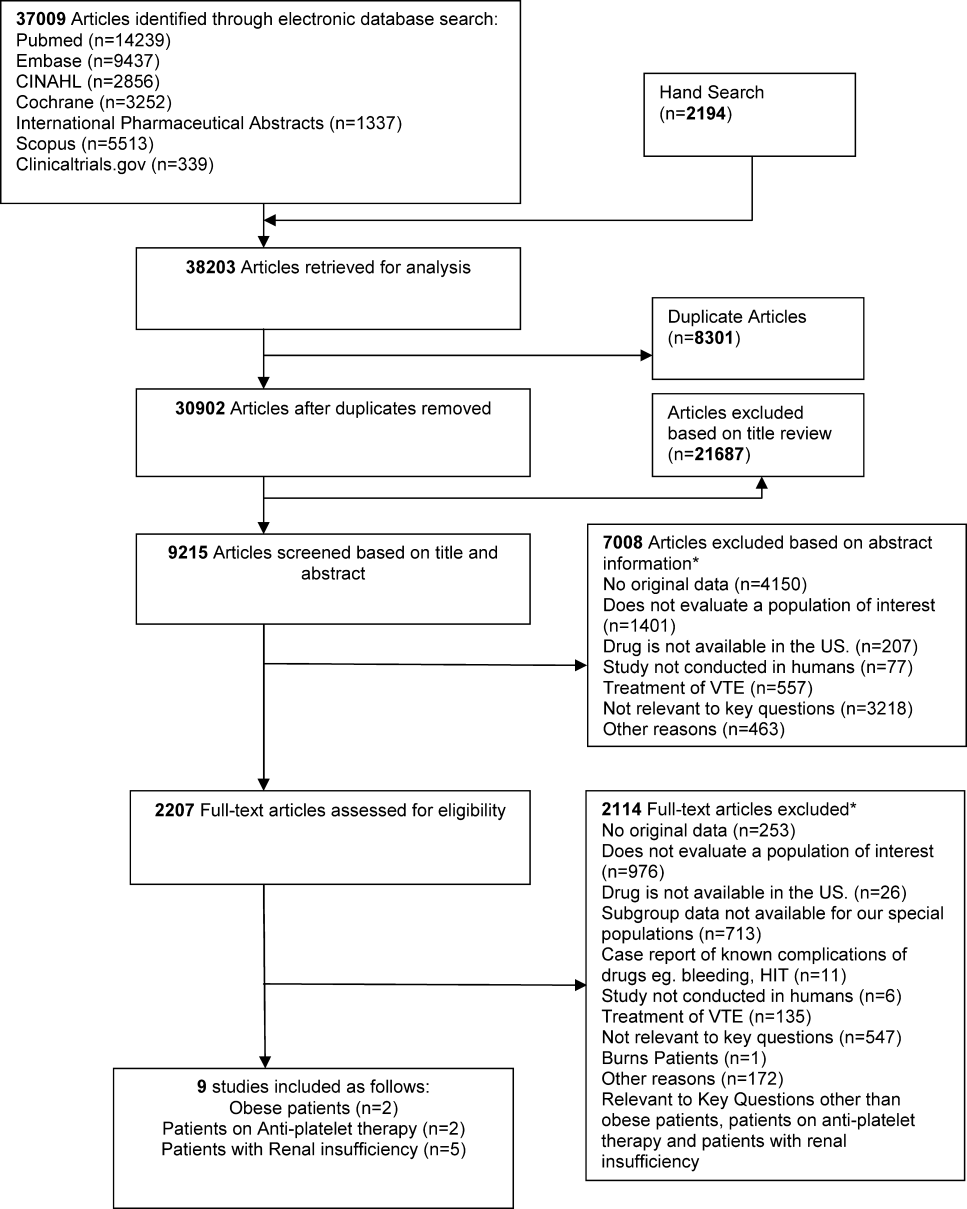
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.
| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.

| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.

| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
The Evidence Regarding the Drugs Used for Ventricular Rate Control
OBJECTIVE: Our goal was to determine what drugs are most effacacious for controlling the ventricular rate in patients with atrial fibrillation.
SEARCH STRATEGY: We conducted a systematic review of the literature published before May 1998, beginning with searches of The Cochrane Collaboration’s CENTRAL database and MEDLINE.
SELECTION CRITERIA: We included English-language articles describing randomized controlled trials of drugs used for heart rate control in adults with atrial fibrillation.
DATA COLLECTION/ANALYSIS: Abstracts of trials were reviewed independently by 2 members of the study team. We reviewed English-language abstracts of non-English-language publications to assess qualitative consistency with our results.
MAIN RESULTS: Forty-five articles evaluating 17 drugs met our criteria for review. In the 5 trials of verapamil and 5 of diltiazem, heart rate was reduced significantly (P<.05), both at rest and with exercise, compared to placebo, with equivalent or improved exercise tolerance in 6 of 7 comparisons. In 7 of 12 comparisons of a beta-blocker with placebo, the beta-blocker was efficacious for control of resting heart rate, with evidence that the effect is drug-specific, as nadolol and atenolol proved to be the most efficacious. All 9 comparisons demonstrated good heart rate control with beta-blockers during exercise, although exercise tolerance was compromised in 3 of 9 comparisons. In 7 of 8 trials, digoxin administered alone slowed the resting heart rate more than placebo, but it did not significantly slow the rate during exercise in 4 studies. The trials evaluating other drugs yielded insufficient evidence to support their use, but those drugs may yet be promising.
CONCLUSIONS: The calcium-channel blockers verapamil or diltiazem, or select {b}-blockers are efficacious for heart rate control at rest and during exercise for patients with atrial fibrillation without a clinically important decrease in exercise tolerance. Digoxin is useful when rate control during exercise is less of a concern.
Despite pharmacologic and electrical interventions, sinus rhythm cannot be restored and maintained in many patients with atrial fibrillation. For these patients, control of the ventricular rate is a primary goal of therapy, since a rapid rate may lead to worsening congestive heart failure, myocardial ischemia, or distressing breathlessness and palpitations.
A number of review articles have described strategies for rate control, principally involving the use of digoxin, calcium-channel blockers, and b-blockers.1-6 A recent analysis of the trends in the use of drugs for ventricular rate control found that the use of digoxin and b-blockers decreased between 1980 and 1981 and 1994 and 1996, and the use of the nondihydropyridine calcium-channel blockers diltiazem and verapamil increased.7 These investigators, however, indicated that “current practices are dictated more by clinical tradition than by clinical science.”7 There has not been a systematic review of the trials evaluating the efficacy of both the familiar and the newer medications for ventricular rate control in atrial fibrillation. It is increasingly clear the drugs that are used most often for heart rate control at rest may not be the most efficacious during exercise, and exercise tolerance is compromised by some drugs.6
The purpose of our review was to characterize the strength of the evidence regarding the efficacy of drugs used for ventricular rate control in atrial fibrillation.
Methods
Study Design
We performed a systematic literature review and synthesis of randomized controlled trials on ventricular rate control in atrial fibrillation. To be eligible for inclusion in our review, trials needed to meet the following criteria: address management of nonpostoperative atrial fibrillation or atrial flutter; include human data; include adult subjects; and present original data. Studies that included patients with postoperative atrial fibrillation were not excluded as long as those patients were only a minority of the included patients.
Literature Identification and Search Strategies
The primary source of literature for our review was the CENTRAL database of The Cochrane Collaboration, a comprehensive collection of controlled clinical trials from 1948 to the present. As a secondary source, we searched MEDLINE from 1966 to May 1998 to ensure completeness. Additionally, we used the related articles feature of PubMed, as well as recent search results submitted to the Baltimore Cochrane Center, the contents pages of recent relevant journals, and programs from recent cardiology meetings. Our search strategy included using the MeSH terms “atrial fibrillation” and “atrial flutter” as subject headings and text words, as well as “random allocation,” “double-blind method,” and “single-blind method.” The publication types were “randomized controlled trials” and “controlled clinical trials.”
Abstracts of the citations of randomized controlled clinical trials were reviewed independently by 2 members of the study team to identify articles that met the inclusion criteria. Only English-language articles were reviewed. However, we reviewed all English-language abstracts of non-English-language publications to assess qualitative consistency with our results.
Data Abstraction
A form was developed to extract information from the eligible articles regarding study quality, characteristics, and findings. The section on study quality was created after our review of forms used in meta-analytic studies8,9 and a literature review10,11 and with the assistance of The Cochrane Collaboration. The resultant form incorporated6 key questions used by The Cochrane Collaboration in its reviews and14 key questions identified by Detsky and colleagues.10 Our form was pilot-tested for clarity and reproducibility and revised as needed. The final version contained 22 questions assessing quality in the following 5 areas: representativeness (how well the study population was described); potential for bias and confounding; description of therapy (eg, how similarly the groups were treated); outcomes and follow-up; and statistical reporting and interpretation [Table 1]. Each question was worth a maximum of 2 points, and the score in each category was the percentage of points received out of the total available. The overall quality score was calculated as the average of the scores for the 5 categories.
The portion of the form for quantitative data abstraction included sections for subject inclusion and exclusion criteria, baseline subject characteristics, therapeutic protocols, and outcomes. We recorded the mean heart rate at rest, the mean maximum heart rate during exercise or immediately after exercise, the proportion of subjects who reached the goal heart rate reduction in each treatment arm, and any measure of exercise tolerance.
The review of study quality was done independently by 2 reviewers, and differences were resolved by consensus. Quantitative data were abstracted by one primary reviewer and then checked for accuracy by a secondary reviewer. The reviewers were not blinded to the author, institution, or journal, since it seemed unlikely that this information would make a significant difference in the results.12
Presentation of the Data
We constructed 3 evidence tables with the trials grouped according to the regimens being compared. [Table 2a][Table 2b] displays the quality scores and key design elements of each trial. [Table 3] contains a listing of the trials of the most frequent comparisons and the absolute differences in heart rates of patients using those therapies. [Table 4] shows the results from the trials for which there were few, if any, given comparisons.
Data was synthesized by creating scatter plots of mean heart rates at rest and with exercise for each of the main drug comparisons (Figures 1-5). The data were not amenable to formal mathematical pooling (ie, meta-analysis) because of significant qualitative heterogeneity among the studies.*
Results
Literature Yield
We retrieved 74 abstracts. Of these, 8 were abstracts of articles from non–English-language literature. Forty-five trials were eligible for inclusion in our review; the authors of those studies evaluated 17 different drugs and several combinations of drugs. Some of the included trials were not designed as rate-control studies; they were studies of pharmacologic conversion that included heart rate data.
Qualitative Synthesis
The following comparisons were made in the trials: calcium-channel blockers,14-21 b-blockers,19-28 digoxin,14,22,28,30-32,38 or other drugs and combinations compared with placebo;14,28,29,33-38 calcium-channel blockers,14,39-42 b-blockers,23,29,43,44 or other drugs and combinations compared with digoxin;14,27,40,44-49 and other drug comparison trials.18-20,27,42,50-58 Many of the trials involved more than 2 treatment arms. Nearly all of the trials of calcium-channel blockers, b-blockers, and digoxin were designed to evaluate rate control. The studies of the other drugs were principally trials evaluating atrial fibrillation conversion that also reported heart rate data. Two of the digoxin trials were also aimed at evaluating conversion to sinus rhythm.
Study Design
As shown in [Table 2a][Table 2b], there were important similarities and differences among the trials evaluating the same therapies. The duration of the trials and route of administration of the drug are presented to aid in interpretation of the results.
Notably, all of the trials of any given comparison were done within 10 years of each other. This should reduce the likelihood of secular trends affecting the outcomes of the trials. The range in study sizes is extreme, although most of the trials enrolled fewer than 50 patients. Several trials had fewer than 10 participants, and it was anticipated that these small trials would have little power to detect differences between treatments.
The regimens differed among the trials of the same medication. The intravenous diltiazem and verapamil doses were fairly uniform, although the oral dosages and frequency of administration differed. Some of the b-blocker trials involved titration of the medication to effectiveness, and digoxin was often dosed to a target blood level. All these differences may have had an impact on the outcomes. The followup times ranged from minutes to 6 weeks. Short trials may be appropriate for intravenous agents; however, several of the trials assessed the outcome a very short time after a single oral dose of medication, before a therapeutic blood level could be expected.
Another notable feature of these trials was the permissibility of other agents during the trial, as detailed in the evidence table. Permitting the use of digoxin in trials testing other medications, without reporting the number of participants in each arm receiving digoxin, can potentially confound the results.
Although not shown in the evidence table, most trials had explicit inclusion criteria. Some required atrial fibrillation lasting longer than 1 month or longer than 6 months, and several specified a ventricular rate required for entry, such as more than 120 beats per minute or “rapid rate.” Exclusion criteria varied from none to stringent; it was particularly stringent in those studies that involved exercise, from which subjects with angina or significant congestive heart failure were often excluded. None of the trials used echocardiographic data as inclusion or exclusion criteria.
Quality Scores
Many studies were weakest in their description of the participants in the study arms, so it was not always possible to tell if the groups were similar. This can be seen in the exceptionally low scores in the “representativeness” category. The potential for bias and confounding varied markedly across the trials, as did the description of the therapies. It was often unclear which other therapies the patients may have been receiving. Generally, the investigators described the outcomes completely and objectively measured them with Holter monitoring or telemetry. The completeness of statistical reporting was variable, with many studies only reporting a P value without reporting a measure of variability in the outcomes.
The studies published more recently had slightly higher total quality scores. Total quality scores were strongly associated with the size of the study, with the larger studies receiving higher scores (P < .001).
Outcomes
As shown in [Table 3], all of the trials reported either the heart rate reduction [on] for the active drug compared with the comparative drug or the proportion of patients who reached the target heart rate. Many of the trials also evaluated the efficacy of the drugs during exercise. The exercise test itself varied among the trials and included measurement of distance walked on a treadmill, measurement of oxygen consumption, and workload tolerated on a stationary bicycle.
On the basis of our qualitative assessment of the trials, we felt that any mathematical pooling of the results would result in invalid estimates of treatment effects. This was because of the markedly different treatment regimens within each drug class and the differing goals of treatment (acute or chronic management). The mixed quality of the trials also argued against pooling.
Study Results
Calcium-Channel Blockers Versus Placebo for Rate Control. All comparisons of calcium-channel blockers with placebo demonstrated that the calcium-channel blocker was more efficacious than placebo at reducing heart rate both at rest and during exercise. Five of the trials used diltiazem, 4 used verapamil, and 1 evaluated both drugs. An improvement in exercise tolerance was almost always seen, although different measures of tolerance were used. All but 2 of the trials allowed the participants to use digoxin but did not report what percentage of subjects in each treatment group received the drug. Despite different rates in the placebo arms, there was uniformity in treatment effects across the trials [Figure 1].
b-Blockers Versus Placebo for Rate Control. Seven different b-blockers were tested. Only 7 of the 12 comparisons demonstrated efficacy of the b-blocker at rest, although all were efficacious during exercise. The efficacy appears to be medication dependent. Atenolol (at 50 mg daily28 or twice daily20 or 100 mg daily28) performed significantly better than placebo. Timolol (1 mg intravenously) allowed more subjects to reach the target heart rate compared with placebo.24 Pindolol28 (5 mg or 15 mg twice daily) and nadolol25 (titrated dose) significantly reduced mean resting heart rate. The data regarding xamoterol were mixed.20-23 Celiprolol26 and labetalol were no more efficacious than placebo at rest.
All of the tested b-blockers demonstrated a significant reduction in heart rate with exercise compared with placebo; this included atenolol, labetalol, nadolol, celiprolol, and xamoterol.19,20,22,24,25 [Figure 2] shows that the effect of b-blockers on heart rate during exercise was more uniform than their impact on heart rate control at rest. However, these trials suggest that exercise tolerance in patients with atrial fibrillation may be reduced with b-blockers.
As in the calcium-channel blocker studies, most of the trials allowed subjects to continue on digoxin.Digoxin Versus Placebo. The outcomes with digoxin were mixed, as shown in [Table 3] and [Figure 3]. Two of the trials of digoxin versus placebo did not demonstrate a reduction in mean resting heart rate;28,30 in 5 trials, however, there was a reduction.14,22,31,32,38 Two of these studies included patients on verapamil in both arms, so we could not attribute all of the rate reduction to digoxin alone.31,32
Two studies evaluating digoxin during exercise did not find a significant heart rate reduction.14,22 In one trial that suggested a difference, no measure of statistical significance was provided.28 Four of the studies of digoxin and placebo evaluated exercise tolerance.14,22,28,29 In one14 the cardiac output was higher for patients taking digoxin, and in another22 the time on the treadmill was longer with digoxin although the maximal attainable heart rate blood pressure product was higher with placebo.
Calcium-Channel Blockers Versus Digoxin for Rate Control. Three trials compared diltiazem with digoxin,14,19,40 and 3 compared verapamil with digoxin14,41,42 with the outcomes reported in [Table 3] and in [Figure 4]. The scatter plot is most useful for noting the trend toward improved control with calcium-channel blockers both at rest and with exercise. Notably, the cardiac output on digoxin during exercise was greater than in the 2 diltiazem groups (12.6 L/min vs 10.9 L/min and 9.1 L/min for 60 mg and 120 mg, respectively).14 Conversely, the group receiving verapamil was able to exercise longer on the treadmill than the digoxin group.43 This latter study, however, had methodologic flaws, including little description of the participants.
b-Blockers Versus Digoxin for Rate Control. Four trials compared b-blockers with digoxin for rate control in atrial fibrillation, and the outcomes are reported in [Table 3] and in Figure 5.22,28,42,43 Similar to the results of the trials of b-blockers compared with placebo, the efficacy of b-blockers was most convincing in the trials that evaluated their use during exercise. There appeared to be little difference between the efficacy of b-blockers and digoxin at rest.
Other Drugs and Combinations Versus Placebo and Digoxin. [Table 4] summarizes the outcomes for the few trials of other agents. Not surprisingly, of the 8 trials that compared digoxin with a combination of digoxin with a calcium-channel blocker,14,19,22,38,40,41,45,46 only one study did not find a significant decrease in mean resting heart rate with the addition of the calcium-channel blocker.40 In 5 of the 6 studies with an exercise evaluation,14,19,38,41,45 the combination of a calcium-channel blocker and digoxin controlled the heart rate better than digoxin alone, while the sixth trial did not report the statistical significance of this outcome.14 Of the trials of a b}-blocker combined with digoxin, all were more effective than placebo, and all were more effective than digoxin alone except for the combination of digoxin and labetolol.28 During exercise, however, this combination was more effective than either comparison arm.
Other Drugs Evaluated for Rate Control. There were 9 other randomized controlled trials of drugs for rate control in atrial fibrillation.50-58 Two studies compared intravenous magnesium sulfate with intravenous verapamil for acute control.50,51 In both studies, a higher percentage of subjects reached a heart rate of less than 100 beats per minute with verapamil than with magnesium sulfate.
Two studies evaluated rate control with propafenone or flecainide, both at 2 mg per kg intravenously for 1 hour; both significantly reduced the heart rate from baseline.52,53 In both studies, subjects were allowed to continue on digoxin, calcium-channel blockers, and b-blockers. The side effects of flecainide were of more concern than those of propafenone, with conduction abnormalities in the flecainide group. Another study compared propafenone with quinidine for rate control.54 Propafenone significantly slowed the heart rate at rest compared with quinidine. Either drug effectively slowed the heart rate compared with baseline.
Disopyramide did not reduce the mean resting heart rate from baseline.55 The combination of diltiazem and digoxin reduced the mean resting heart rate to a greater degree than the combination of propranolol and digoxin, but all 3 drugs together were even more effective.56 That study also demonstrated that with exercise the combination of propranolol and digoxin was more efficacious for heart rate control than diltiazem and digoxin and that the 3-drug combination was not better than just propranolol and digoxin. The combination of pindolol and digoxin reduced the maximum area under the heart rate curve significantly more than verapamil and digoxin.57 Finally, the combination of amiodarone and digoxin slowed the resting heart rate when compared with baseline, while the combination of quinidine, verapamil, and digoxin did not; this was a small trial, however, and the baseline resting heart rates were not rapid.58
Discussion
The randomized controlled trials of diltiazem and verapamil used by patients with atrial fibrillation provide strong evidence for their efficacy in reducing heart rate both at rest and with exercise when compared with placebo. In all of the studies that evaluated calcium-channel blockers compared with placebo during exercise, the calcium-channel blockers produced either an increase in cardiac output, oxygen consumption, or distance walked. There was also moderate evidence that diltiazem or verapamil was more effective at heart rate control both at rest and during exercise in the direct comparisons with digoxin, with a more rapid onset of action. Although digoxin appeared to increase cardiac output, verapamil prolonged time on the treadmill and increased oxygen consumption. Thus, the evidence strongly supports the use of diltiazem or verapamil for ventricular rate control in atrial fibrillation. Although they have a negative inotropic effect, reflex responses to vasodilatation usually result in a small increase in cardiac output. Therefore, except in moderate to severe heart failure, the negative inotropic effect is often not clinically apparent.60
All of the tested b-blockers successfully reduced heart rate with exercise when compared with placebo, and most of them reduced resting heart rate. The effect on exercise tolerance was variable and may be due to the different receptor specificities of the tested drugs and the varying treatment times before testing.
When administered acutely, b-blocking agents depress myocardial function secondary to the withdrawal of adrenergically mediated inotropic and chronotropic support. However, in patients with congestive heart failure treated for longer than 1 month, b-blockers may improve myocardial function by improving intrinsic systolic function.59,61-63 Extrapolating to patients with atrial fibrillation, b-blocker therapy may increase left ventricular ejection fraction compared with placebo if administered for longer than 1 month. None of the trials in this review lasted for longer than 4 weeks, so it is conceivable that the worsening exercise tolerance was a transient effect of the drug.
When compared with digoxin, the trials favored the use of b-blockers, as digoxin was less efficacious than metoprolol in resting heart rate reduction and less efficacious than labetalol at rate reduction during exercise. Furthermore, time on the treadmill was longer with both labetalol and metoprolol than with digoxin.
It will be interesting to see the effect of the third-generation b-blockers, such as carvedilol, on heart rate control in atrial fibrillation. We anticipate that they will be effective at ventricular rate control, with an improvement in exercise tolerance. Celiprolol and xameterol are not available in the United States and are no longer being evaluated for approval by the Food and Drug Administration.
Although several of the studies comparing digoxin with placebo were limited by subtherapeutic serum levels of digoxin at the time of evaluation, the others did show a resting heart rate reduction. There is little evidence to support the efficacy of digoxin for heart rate control with exercise. However, in the 2 trials that evaluated exercise tolerance on digoxin compared with placebo, cardiac output and time on the treadmill were greater with digoxin.
The trials evaluating other drugs, including propafenone, clonidine, and amiodarone yielded insufficient evidence to support their use for rate control at this time. These drugs appear promising and may prove efficacious when more evidence becomes available.
The optimal degree of heart rate control for patients with atrial fibrillation is unclear, particularly during exercise. Certainly an excessively rapid rate impairs ventricular filling and decreases cardiac output. However, severely limiting the heart rate acceleration that is needed to maintain cardiac output can also limit exercise tolerance.63 There are few empirical trials of this. One recent study found that ventricular rate control in atrial fibrillation had no impact on cardiovascular performance as measured by endurance on a treadmill.63 The studies in our review that report only on heart rate control during exercise without mention of exercise tolerance may be of less value to clinicians.
It is important to recognize that both heart rate control and exercise tolerance are surrogate outcomes for what is truly important to clinicians and their patients: their well-being, ability to conduct their daily activities, and mortality. Although several of the studies did inquire about symptoms such as palpitations or breathlessness, the use of validated quality-of-life questionnaires was rare. A study comparing pharmacologic treatment of atrial fibrillation with atrioventricular junction ablation and pacing demonstrated the use of several different quality-of-life measures: a Quality-of-Life Questionnaire, Specific Symptoms Scale, New York Heart Association Classification, and a Specific Activity Scale.64 The authors of that study commented that they were uncertain whether the sensitivities of these scales were high enough to use as they did.
Our study is the first comprehensive evidence-based review that focused on this aspect of the management of atrial fibrillation. Several recent reviews described ventricular rate control trials in the context of other pharmacologic therapies for managing atrial fibrillation,65,2-4 but those studies did not comprehensively review all the rate control trials. Furthermore, they contained recommendations for drugs that have never been evaluated in controlled trials of patients with atrial fibrillation exclusively (such as intravenous esmolol and intravenous propranolol).2
Limitations
Our systematic review was subject to the same limitations that are common to most reviews. The important differences among the trials preclude mathematical pooling and have to be taken into account when drawing conclusions using these studies. As in most assessments of study quality, our assessment tool was tailored to fit the topic, so the scores cannot be directly compared with quality assessments of studies on other topics. Our intent was to be able to compare the trials.
Few of the studies evaluated adverse effects in any systematic way. This inconsistent reporting of adverse effects limited our ability to comment on them. Many of the trials incompletely described the enrolled participants, so applying these results to all patients with atrial fibrillation should be done cautiously. Also, since results were seldom stratified by the clinical features of the enrolled patients, we could not report on the evidence supporting the use of these drugs in different patient populations.
Similarly, the results were not reported stratified by whether the patient had atrial fibrillation or atrial flutter, thus we cannot report the evidence separately for those 2 conditions. We feel this is appropriate, however, because those 2 arrhythmias frequently coexist.66,67 None of the trials had echocardiographic data as inclusion or exclusion criteria, but that information is more relevant to decisions regarding anticoagulation or cardioversion. We know of no study that associates echocardiographic data with ventricular rate control.
We cannot exclude the presence of publication bias, although we are confident that our search strategy did capture the published literature. In our review of the 8 non–English-language abstracts, we found them in agreement with the articles published in the English literature.
Future research should address the outcomes most relevant to patients — well-being and functionality—particularly since the relationship between heart rate control and exercise tolerance is unclear. We encourage the use of validated instruments for assessment, although which instruments are most appropriate is unknown at this time. Similarly, systematic recording of adverse events should be a regular component of all future trials of these drugs.
Recommendations for clinical practice
For adults with nonpostoperative atrial fibrillation, the evidence supports the following statements. The nondihydropyridine calcium-channel blockers, diltiazem, and verapamil are efficacious for heart rate control at rest and with exercise without decrement in exercise tolerance. Selected b-blockers, such as the noncardioselective b-antagonist nadolol or the second-generation b1-antagonists atenolol and metoprolol, are efficacious at rest and with exercise. There is some evidence, however, that b-blockers cause a transient decrease in exercise tolerance. For patients unlikely to exercise, such as those markedly incapacitated by other illness, digoxin should provide acceptable control.
· Acknowledgments ·
This study was conducted by the Johns Hopkins Evidence-based Practice Center through contract no. 290-97-006 from the Agency for Health Care Policy and Research, Rockville, Maryland. The authors are responsible for its content, including any clinical recommendations. No statement of this article should be construed as an official position of the Agency for Health Care Policy and Research or the US Department of Health and Human Services. We would like to thank Dr Francis Chesley of the Agency for Health Care Policy and Research and Drs Hanan Bell and Michael LeFevre of the American Academy of Family Physicians for their helpful suggestions regarding this project, Dr David Haines of the American College of Cardiology and Drs Ronald Berger and Gary Gerstenblith for their expert advice, Paul Abboud for assistance with data abstraction, and Donna Lea for extensive help with the manuscript.
1. Wakatare JEP, Camm AJ. Acute treatment of atrial fibrillation: why and when to maintain sinus rhythm. Am J Cardiol 1998;81:3C-15C.
2. Kowey PR, Marinchak RA, Rials SJ, Filart RA. Acute treatment of atrial fibrillation. Am J Cardiol 1998;81:16C-22C.
3. Pratt CM. Impact of managed care on the treatment of atrial fibrillation. Am J Cardiol 1998;81:30C-4C.
4. Sopher SM, Camm AJ. Atrial fibrillation; maintenance of sinus rhythm versus rate control. Am J Cardiol 1996;77:24A-37A.
5. Riley RD, Pritchett ELC. Pharmacologic management of atrial fibrillation. J Cardiovasc Electrophysiol 1997;8:818-29.
6. Hohnloser SH, Li Y-G. Drug treatment of atrial fibrillation; what have we learned? Curr Opin Cardiol 1997;12:24-32.
7. Stafford RS, Robson DC, Misra B, Ruskin J, Singer DE. Rate control and sinus rhythm maintenance in atrial fibrillation: national trends in medication use, 1980-1996. Arch Intern Med 1998;158:2144-8.
8. Bass EB, Powe NR, Goodman SN, et al. Efficacy of immune globulin in preventing complications of bone marrow transplantation; a meta-analysis. Bone Marrow Transplant 1993;12:273-82.
9. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Arch Ophthal 1994;112:239-52.
10. Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992;45:255-65.
11. Chalmers TC, Smith H, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49.
12. Berlin JA. University of Pennsylvania. National Technical Information Service accession number PB98-109382.
13. McNamara FL, Miller MR, Segal JB, et al. Evidence report on management of new onset atrial fibrillation: evidence report/technology assessment no.1. AHCPR Publication No. 99-E021. Rockville, Md: Agency for Health Care Policy and Research. In press.
14. Lewis RV, Irvine N, McDevitt DG. Relationships between heart rate, exercise tolerance and cardiac output in atrial fibrillation: the effects of treatment with digoxin, verapamil and diltiazem. Eur Heart J 1988;9:777-81.
15. Salerno DM, Dias VC, Kleiger RE, et al. Efficacy and safety of intravenous diltiazem for treatment of atrial fibrillation and atrial flutter: the diltiazem-atrial fibrillation/flutter study group. Am J Cardiol 1989;63:1046-51
16. Goldenberg IF, Lewis WR, Dias VC, Heywood JT, Pedersen WR. Intravenous diltiazem for the treatment of patients with atrial fibrillation or flutter and moderate to severe congestive heart failure. Am J Cardiol 1994;74:884-9.
17. Ellenbogen K, Dias VC, Plumb VJ, Heywood JT, Mirvis DM. A placebo-controlled trial of continuous intravenous diltiazem infusion for 24-hour heart rate control during atrial fibrillation and atrial flutter: a multicenter study. JACC 1991;18:891-7.
18. Lundstrom T, Ryden L. Ventricular rate control and exercise performance in chronic atrial fibrillation: effects of diltiazem and verapamil. JACC 1990;16:86-90.
19. Lewis RV, McMurray J, McDevitt DG. Effects of atenolol, verapamil, and xamoterol on heart rate and exercise tolerance in digitalised patients with chronic atrial fibrillation. J Cardio Pharm 1989;13:1-6.
20. Lundstrom T, Moor E, Ryden L. Differential effects of xamoterol and verapamil on ventricular rate regulation in patients with chronic atrial fibrillation. Am Heart J 1992;124:917-23.
21. Panidis IP, Morganroth J, Baessler C. Effectiveness and safety of oral verapamil to control exercise-induced tachycardia in patients with atrial fibrillation receiving digitalis. Am J Cardiol 1983;52:1197-201.
22. Ang EL, Chan WL, Cleland JG, et al. Placebo controlled trial of xamoterol versus digoxin in chronic atrial fibrillation. Br Heart J 1990;64:256-60.
23. Sweany AE, Moncloa F, Vickers FF, Zupkis RV. Antiarrhythmic effects of intravenous timolol in supraventricular arrhythmias. Clin Pharmacol Ther 1985;37:124-7.
24. DiBianco R, Morganroth J, Freitag JA, et al. Effects of nadolol on the spontaneous and exercise-provoked heart rate of patients with chronic atrial fibrillation receiving stable dosages of digoxin. Am Heart J 1984;108:1121-7.
25. Myers J, Atwood JE, Sullivan M, et al. Perceived exertion and gas exchange after calcium and beta-blockade in atrial fibrillation. J Appl Physiol 1987;63:97-104.
26. Lin SK, Morganroth J, Heng M, et al. Effect of orally administered celiprolol in patients with chronic atrial fibrillation. J Cardiol Pharm 1986;S112-5.
27. Channer KS, James MA, MacConnell T, Rees JR. Beta-adrenoceptor blockers in atrial fibrillation: the importance of partial agonist activity. Br J Clin Pharm 1994;37:53-7.
28. Wong CK, Lau CP, Leung WH, Cheng CH. Usefulness of labetalol in chronic atrial fibrillation. Am J Cardiol 1990;66:1212-5.
29. Koh KK, Song JH, Kwon KS, et al. Comparative study of efficacy and safety of low-dose diltiazem or betaxolol in combination with digoxin to control ventricular rate in chronic atrial fibrillation: randomized crossover study. Int J Cardiol 1995;52:167-74.
30. Falk RH, Knowlton A A, Bernard SA, Gotlieb NE, Battinelli NJ. Digoxin for converting recent-onset atrial fibrillation to sinus rhythm: a randomized, double-blinded trial. Ann Intern Med 1987;106:503-6.
31. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group Intravenous digoxin in acute atrial fibrillation: results of a randomized, placebo-controlled multicenter trial in 239 patients. Eur Heart J 1997;18:649-54.
32. Jordaens L, Trouerbach J, Calle P, et al. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur Heart J 1997;18:643-8.
33. Roth A, Kaluski E, Felner S, Heller K, Laniado S. Clonidine for patients with rapid atrial fibrillation. Ann Intern Med 1992;116:388-90.
34. Scardi S, Humar F, Pandullo C, Poletti A. Oral clonidine for heart rate control in chronic atrial fibrillation. Letter. Lancet 1993;341:1211-2.
35. Botto GL, Capucci A, Bonini W, et al. Conversion of recent onset atrial fibrillation to sinus rhythm using a single oral loading dose of propafenone: comparison of two regimens. Int J Cardiol 1997;58:55-61.
36. Lok NS, Lau CP. Oxygen uptake kinetics and cardiopulmonary performance in lone atrial fibrillation and the effects of sotalol. Chest 1997;111:934-40.
37. Brodsky MA, Orlov MV, Capparelli EV, et al. Magnesium therapy in new-onset atrial fibrillation. Am J Cardiol 1994;73:1227-9.[Published erratum appears in Am J Cardiol 1994; 74:639.]
38. Koh KK, Kwon KS, Park HB, et al. Efficacy and safety of digoxin alone and in combination with low-dose diltiazem or betaxolol to control ventricular rate in chronic atrial fibrillation. Am J Cardiol 1995;75:88-90.
39. Lewis RV, Laing E, Moreland TA, Service E, McDevitt DG. A comparison of digoxin, diltiazem and their combination in the treatment of atrial fibrillation. Eur Heart J 1988;9:279-83.
40. Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. 1997;29:135-40.
41. Pomfret SM, Beasley CR, Challenor V, Holgate ST. Relative efficacy of oral verapamil and digoxin alone and in combination for the treatment of patients with chronic atrial fibrillation. Clin-Sci 1988;74:351-7.
42. Ahuja RC, Sinha N, Saran RK, Jain AK, Hasan M. Digoxin or verapamil or metoprolol for heart rate control in patients with mitral stenosis: a randomised cross-over study. Int J Cardiol 1989;25:325-31.
43. Lawson-Matthew PJ, McLean KA, Dent M, Austin CA, Channer KS. Xamoterol improves the control of chronic atrial fibrillation in elderly patients. Age Ageing 1995;24:321-5.
44. Brodsky M, Saini R, Bellinger R, Zoble R, Weiss R, Powers L. Comparative effects of the combination of digoxin and dl-sotalol therapy versus digoxin monotherapy for control of ventricular response in chronic atrial fibrillation, dl-Sotalol Atrial Fibrillation Study Group. Am Heart J 1994;127:572-7.
45. Stern EH, Pitchon R, King BD, et al. Clinical use of oral verapamil in chronic and paroxysmal atrial fibrillation. Chest 1982;81:308-11.
46. Lang R, Klein HO, Di Segni E, et al. Verapamil improves exercise capacity in chronic atrial fibrillation: double-blind crossover study. Am Heart J 1983;105:820-5.
47. Zoble RG, Brewington J, Olukotun AY, Gore R. Comparative effects of nadolol-digoxin combination therapy and digoxin monotherapy for chronic atrial fibrillation. Am J Cardiol 1987;60:39D-45D.
48. Hays JV, Gilman JK, Rubal BJ. Effect of magnesium sulfate on ventricular rate control in atrial fibrillation. Ann Emerg Med 1994;24:61-4.
49. Smit AJ, Scaf AH, van Essen LH, Lie KI, Wesseling H. Digoxin infusion versus bolus injection in rapid atrial fibrillation: relation between serum level and response. Eur J Clin Pharm 1990;38:335-41.
50. Joshi PP, Deshmukh PK, Salkar RG. Efficacy of intravenous magnesium sulphate in supraventricular tachyarrhythmias. J Assoc Physicians India 1995;43:529-31.
51. Gullestad L, Birkeland K, Molstad P, et al. The effect of magnesium versus verapamil on supraventricular arrhythmias. Clin Cardiol 1993;16:429-34.
52. Suttorp MJ, Kingma JH, Jessurun ER, et al. The value of class IC antiarrhythmic drugs for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. JACC 1990;16:1722-7.
53. Kingma JH, Suttorp MJ. Acute pharmacologic conversion of atrial fibrillation and flutter: the role of flecainide, propafenone, and verapamil. Am J Cardiol 1992;70:56A-60A.
54. Lee SH, Chen SA, Chiang CE, et al. Comparisons of oral propafenone and quinidine as an initial treatment option in patients with symptomatic paroxysmal atrial fibrillation: a double-blind, randomized trial. J Int Med 1996;239:253-60.
55. Boudonas G, Lefkos N, Efthymiadis A, Styliadis IG, Tsapas G. Intravenous administration of diltiazem in the treatment of supraventricular tachyarrhythmias. Acta Cardiol 1995;50:125-34.
56. Dahlstrom CG, Edvardsson N, Nasheng C, Olsson SB. Effects of diltiazem, propranolol, and their combination in the control of atrial fibrillation. Clin Cardiol 1992;15:280-4.
57. James MA, Channer KS, Papouchado M, Rees JR. Improved control of atrial fibrillation with combined pindolol and digoxin therapy. Eur Heart J 1989;10:83-90.
58. Zehender M, Hohnloser S, Muller B, Meinertz T, Just H. Effects of amiodarone versus quinidine and verapamil in patients with chronic atrial fibrillation: results of a comparative study and a 2-year follow-up. JACC 1992;19:1054-9.
59. Waagstein F, Caidahl K, Wallentin I, Bergh CH, Hjalmarson A. Long-term beta-blockade in dilated cardiomyopathy. Circ 1989;80:551-63.
60. Facts and Comparisons. Drug Facts and Comparisons. St. Louis, Mo: Facts and Comparisons; 1999.
61. Haber HL, Simek CL, Gimple LW, et al. Why do patients with congestive heart failure tolerate the initiation of beta-blocker therapy? Circulation 1993;88:1610-9.
62. Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol 1997;80:26L-40L.
63. Ostermaier RH, Lampert S, Dalla Vecchia L, Ravid S. The effect of atrial fibrillation and the ventricular rate control on exercise capacity. Clin Cardiol 1997;20:23-7.
64. Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological, treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circ 1998;98:953-60.
65. Viskin S, Barron HV, Heller K, Scheinman MM, Olgin JE. The treatment of atrial fibrillation: pharmacologic and nonpharmacologic strategies. Curr Prob Cardiol 1997;22:37-108.
66. Della Bella P, Riva S, Galimberti P. Should ablation of atrial flutter be discouraged in patients with documented atrial fibrillation? Cardiologia 1999;44:439-42.
67. Roithinger FX, Lesh MD. What is the relationship of atrial flutter and fibrillation? Pacing Clin Electrophysiol 1999;22:643-54.
OBJECTIVE: Our goal was to determine what drugs are most effacacious for controlling the ventricular rate in patients with atrial fibrillation.
SEARCH STRATEGY: We conducted a systematic review of the literature published before May 1998, beginning with searches of The Cochrane Collaboration’s CENTRAL database and MEDLINE.
SELECTION CRITERIA: We included English-language articles describing randomized controlled trials of drugs used for heart rate control in adults with atrial fibrillation.
DATA COLLECTION/ANALYSIS: Abstracts of trials were reviewed independently by 2 members of the study team. We reviewed English-language abstracts of non-English-language publications to assess qualitative consistency with our results.
MAIN RESULTS: Forty-five articles evaluating 17 drugs met our criteria for review. In the 5 trials of verapamil and 5 of diltiazem, heart rate was reduced significantly (P<.05), both at rest and with exercise, compared to placebo, with equivalent or improved exercise tolerance in 6 of 7 comparisons. In 7 of 12 comparisons of a beta-blocker with placebo, the beta-blocker was efficacious for control of resting heart rate, with evidence that the effect is drug-specific, as nadolol and atenolol proved to be the most efficacious. All 9 comparisons demonstrated good heart rate control with beta-blockers during exercise, although exercise tolerance was compromised in 3 of 9 comparisons. In 7 of 8 trials, digoxin administered alone slowed the resting heart rate more than placebo, but it did not significantly slow the rate during exercise in 4 studies. The trials evaluating other drugs yielded insufficient evidence to support their use, but those drugs may yet be promising.
CONCLUSIONS: The calcium-channel blockers verapamil or diltiazem, or select {b}-blockers are efficacious for heart rate control at rest and during exercise for patients with atrial fibrillation without a clinically important decrease in exercise tolerance. Digoxin is useful when rate control during exercise is less of a concern.
Despite pharmacologic and electrical interventions, sinus rhythm cannot be restored and maintained in many patients with atrial fibrillation. For these patients, control of the ventricular rate is a primary goal of therapy, since a rapid rate may lead to worsening congestive heart failure, myocardial ischemia, or distressing breathlessness and palpitations.
A number of review articles have described strategies for rate control, principally involving the use of digoxin, calcium-channel blockers, and b-blockers.1-6 A recent analysis of the trends in the use of drugs for ventricular rate control found that the use of digoxin and b-blockers decreased between 1980 and 1981 and 1994 and 1996, and the use of the nondihydropyridine calcium-channel blockers diltiazem and verapamil increased.7 These investigators, however, indicated that “current practices are dictated more by clinical tradition than by clinical science.”7 There has not been a systematic review of the trials evaluating the efficacy of both the familiar and the newer medications for ventricular rate control in atrial fibrillation. It is increasingly clear the drugs that are used most often for heart rate control at rest may not be the most efficacious during exercise, and exercise tolerance is compromised by some drugs.6
The purpose of our review was to characterize the strength of the evidence regarding the efficacy of drugs used for ventricular rate control in atrial fibrillation.
Methods
Study Design
We performed a systematic literature review and synthesis of randomized controlled trials on ventricular rate control in atrial fibrillation. To be eligible for inclusion in our review, trials needed to meet the following criteria: address management of nonpostoperative atrial fibrillation or atrial flutter; include human data; include adult subjects; and present original data. Studies that included patients with postoperative atrial fibrillation were not excluded as long as those patients were only a minority of the included patients.
Literature Identification and Search Strategies
The primary source of literature for our review was the CENTRAL database of The Cochrane Collaboration, a comprehensive collection of controlled clinical trials from 1948 to the present. As a secondary source, we searched MEDLINE from 1966 to May 1998 to ensure completeness. Additionally, we used the related articles feature of PubMed, as well as recent search results submitted to the Baltimore Cochrane Center, the contents pages of recent relevant journals, and programs from recent cardiology meetings. Our search strategy included using the MeSH terms “atrial fibrillation” and “atrial flutter” as subject headings and text words, as well as “random allocation,” “double-blind method,” and “single-blind method.” The publication types were “randomized controlled trials” and “controlled clinical trials.”
Abstracts of the citations of randomized controlled clinical trials were reviewed independently by 2 members of the study team to identify articles that met the inclusion criteria. Only English-language articles were reviewed. However, we reviewed all English-language abstracts of non-English-language publications to assess qualitative consistency with our results.
Data Abstraction
A form was developed to extract information from the eligible articles regarding study quality, characteristics, and findings. The section on study quality was created after our review of forms used in meta-analytic studies8,9 and a literature review10,11 and with the assistance of The Cochrane Collaboration. The resultant form incorporated6 key questions used by The Cochrane Collaboration in its reviews and14 key questions identified by Detsky and colleagues.10 Our form was pilot-tested for clarity and reproducibility and revised as needed. The final version contained 22 questions assessing quality in the following 5 areas: representativeness (how well the study population was described); potential for bias and confounding; description of therapy (eg, how similarly the groups were treated); outcomes and follow-up; and statistical reporting and interpretation [Table 1]. Each question was worth a maximum of 2 points, and the score in each category was the percentage of points received out of the total available. The overall quality score was calculated as the average of the scores for the 5 categories.
The portion of the form for quantitative data abstraction included sections for subject inclusion and exclusion criteria, baseline subject characteristics, therapeutic protocols, and outcomes. We recorded the mean heart rate at rest, the mean maximum heart rate during exercise or immediately after exercise, the proportion of subjects who reached the goal heart rate reduction in each treatment arm, and any measure of exercise tolerance.
The review of study quality was done independently by 2 reviewers, and differences were resolved by consensus. Quantitative data were abstracted by one primary reviewer and then checked for accuracy by a secondary reviewer. The reviewers were not blinded to the author, institution, or journal, since it seemed unlikely that this information would make a significant difference in the results.12
Presentation of the Data
We constructed 3 evidence tables with the trials grouped according to the regimens being compared. [Table 2a][Table 2b] displays the quality scores and key design elements of each trial. [Table 3] contains a listing of the trials of the most frequent comparisons and the absolute differences in heart rates of patients using those therapies. [Table 4] shows the results from the trials for which there were few, if any, given comparisons.
Data was synthesized by creating scatter plots of mean heart rates at rest and with exercise for each of the main drug comparisons (Figures 1-5). The data were not amenable to formal mathematical pooling (ie, meta-analysis) because of significant qualitative heterogeneity among the studies.*
Results
Literature Yield
We retrieved 74 abstracts. Of these, 8 were abstracts of articles from non–English-language literature. Forty-five trials were eligible for inclusion in our review; the authors of those studies evaluated 17 different drugs and several combinations of drugs. Some of the included trials were not designed as rate-control studies; they were studies of pharmacologic conversion that included heart rate data.
Qualitative Synthesis
The following comparisons were made in the trials: calcium-channel blockers,14-21 b-blockers,19-28 digoxin,14,22,28,30-32,38 or other drugs and combinations compared with placebo;14,28,29,33-38 calcium-channel blockers,14,39-42 b-blockers,23,29,43,44 or other drugs and combinations compared with digoxin;14,27,40,44-49 and other drug comparison trials.18-20,27,42,50-58 Many of the trials involved more than 2 treatment arms. Nearly all of the trials of calcium-channel blockers, b-blockers, and digoxin were designed to evaluate rate control. The studies of the other drugs were principally trials evaluating atrial fibrillation conversion that also reported heart rate data. Two of the digoxin trials were also aimed at evaluating conversion to sinus rhythm.
Study Design
As shown in [Table 2a][Table 2b], there were important similarities and differences among the trials evaluating the same therapies. The duration of the trials and route of administration of the drug are presented to aid in interpretation of the results.
Notably, all of the trials of any given comparison were done within 10 years of each other. This should reduce the likelihood of secular trends affecting the outcomes of the trials. The range in study sizes is extreme, although most of the trials enrolled fewer than 50 patients. Several trials had fewer than 10 participants, and it was anticipated that these small trials would have little power to detect differences between treatments.
The regimens differed among the trials of the same medication. The intravenous diltiazem and verapamil doses were fairly uniform, although the oral dosages and frequency of administration differed. Some of the b-blocker trials involved titration of the medication to effectiveness, and digoxin was often dosed to a target blood level. All these differences may have had an impact on the outcomes. The followup times ranged from minutes to 6 weeks. Short trials may be appropriate for intravenous agents; however, several of the trials assessed the outcome a very short time after a single oral dose of medication, before a therapeutic blood level could be expected.
Another notable feature of these trials was the permissibility of other agents during the trial, as detailed in the evidence table. Permitting the use of digoxin in trials testing other medications, without reporting the number of participants in each arm receiving digoxin, can potentially confound the results.
Although not shown in the evidence table, most trials had explicit inclusion criteria. Some required atrial fibrillation lasting longer than 1 month or longer than 6 months, and several specified a ventricular rate required for entry, such as more than 120 beats per minute or “rapid rate.” Exclusion criteria varied from none to stringent; it was particularly stringent in those studies that involved exercise, from which subjects with angina or significant congestive heart failure were often excluded. None of the trials used echocardiographic data as inclusion or exclusion criteria.
Quality Scores
Many studies were weakest in their description of the participants in the study arms, so it was not always possible to tell if the groups were similar. This can be seen in the exceptionally low scores in the “representativeness” category. The potential for bias and confounding varied markedly across the trials, as did the description of the therapies. It was often unclear which other therapies the patients may have been receiving. Generally, the investigators described the outcomes completely and objectively measured them with Holter monitoring or telemetry. The completeness of statistical reporting was variable, with many studies only reporting a P value without reporting a measure of variability in the outcomes.
The studies published more recently had slightly higher total quality scores. Total quality scores were strongly associated with the size of the study, with the larger studies receiving higher scores (P < .001).
Outcomes
As shown in [Table 3], all of the trials reported either the heart rate reduction [on] for the active drug compared with the comparative drug or the proportion of patients who reached the target heart rate. Many of the trials also evaluated the efficacy of the drugs during exercise. The exercise test itself varied among the trials and included measurement of distance walked on a treadmill, measurement of oxygen consumption, and workload tolerated on a stationary bicycle.
On the basis of our qualitative assessment of the trials, we felt that any mathematical pooling of the results would result in invalid estimates of treatment effects. This was because of the markedly different treatment regimens within each drug class and the differing goals of treatment (acute or chronic management). The mixed quality of the trials also argued against pooling.
Study Results
Calcium-Channel Blockers Versus Placebo for Rate Control. All comparisons of calcium-channel blockers with placebo demonstrated that the calcium-channel blocker was more efficacious than placebo at reducing heart rate both at rest and during exercise. Five of the trials used diltiazem, 4 used verapamil, and 1 evaluated both drugs. An improvement in exercise tolerance was almost always seen, although different measures of tolerance were used. All but 2 of the trials allowed the participants to use digoxin but did not report what percentage of subjects in each treatment group received the drug. Despite different rates in the placebo arms, there was uniformity in treatment effects across the trials [Figure 1].
b-Blockers Versus Placebo for Rate Control. Seven different b-blockers were tested. Only 7 of the 12 comparisons demonstrated efficacy of the b-blocker at rest, although all were efficacious during exercise. The efficacy appears to be medication dependent. Atenolol (at 50 mg daily28 or twice daily20 or 100 mg daily28) performed significantly better than placebo. Timolol (1 mg intravenously) allowed more subjects to reach the target heart rate compared with placebo.24 Pindolol28 (5 mg or 15 mg twice daily) and nadolol25 (titrated dose) significantly reduced mean resting heart rate. The data regarding xamoterol were mixed.20-23 Celiprolol26 and labetalol were no more efficacious than placebo at rest.
All of the tested b-blockers demonstrated a significant reduction in heart rate with exercise compared with placebo; this included atenolol, labetalol, nadolol, celiprolol, and xamoterol.19,20,22,24,25 [Figure 2] shows that the effect of b-blockers on heart rate during exercise was more uniform than their impact on heart rate control at rest. However, these trials suggest that exercise tolerance in patients with atrial fibrillation may be reduced with b-blockers.
As in the calcium-channel blocker studies, most of the trials allowed subjects to continue on digoxin.Digoxin Versus Placebo. The outcomes with digoxin were mixed, as shown in [Table 3] and [Figure 3]. Two of the trials of digoxin versus placebo did not demonstrate a reduction in mean resting heart rate;28,30 in 5 trials, however, there was a reduction.14,22,31,32,38 Two of these studies included patients on verapamil in both arms, so we could not attribute all of the rate reduction to digoxin alone.31,32
Two studies evaluating digoxin during exercise did not find a significant heart rate reduction.14,22 In one trial that suggested a difference, no measure of statistical significance was provided.28 Four of the studies of digoxin and placebo evaluated exercise tolerance.14,22,28,29 In one14 the cardiac output was higher for patients taking digoxin, and in another22 the time on the treadmill was longer with digoxin although the maximal attainable heart rate blood pressure product was higher with placebo.
Calcium-Channel Blockers Versus Digoxin for Rate Control. Three trials compared diltiazem with digoxin,14,19,40 and 3 compared verapamil with digoxin14,41,42 with the outcomes reported in [Table 3] and in [Figure 4]. The scatter plot is most useful for noting the trend toward improved control with calcium-channel blockers both at rest and with exercise. Notably, the cardiac output on digoxin during exercise was greater than in the 2 diltiazem groups (12.6 L/min vs 10.9 L/min and 9.1 L/min for 60 mg and 120 mg, respectively).14 Conversely, the group receiving verapamil was able to exercise longer on the treadmill than the digoxin group.43 This latter study, however, had methodologic flaws, including little description of the participants.
b-Blockers Versus Digoxin for Rate Control. Four trials compared b-blockers with digoxin for rate control in atrial fibrillation, and the outcomes are reported in [Table 3] and in Figure 5.22,28,42,43 Similar to the results of the trials of b-blockers compared with placebo, the efficacy of b-blockers was most convincing in the trials that evaluated their use during exercise. There appeared to be little difference between the efficacy of b-blockers and digoxin at rest.
Other Drugs and Combinations Versus Placebo and Digoxin. [Table 4] summarizes the outcomes for the few trials of other agents. Not surprisingly, of the 8 trials that compared digoxin with a combination of digoxin with a calcium-channel blocker,14,19,22,38,40,41,45,46 only one study did not find a significant decrease in mean resting heart rate with the addition of the calcium-channel blocker.40 In 5 of the 6 studies with an exercise evaluation,14,19,38,41,45 the combination of a calcium-channel blocker and digoxin controlled the heart rate better than digoxin alone, while the sixth trial did not report the statistical significance of this outcome.14 Of the trials of a b}-blocker combined with digoxin, all were more effective than placebo, and all were more effective than digoxin alone except for the combination of digoxin and labetolol.28 During exercise, however, this combination was more effective than either comparison arm.
Other Drugs Evaluated for Rate Control. There were 9 other randomized controlled trials of drugs for rate control in atrial fibrillation.50-58 Two studies compared intravenous magnesium sulfate with intravenous verapamil for acute control.50,51 In both studies, a higher percentage of subjects reached a heart rate of less than 100 beats per minute with verapamil than with magnesium sulfate.
Two studies evaluated rate control with propafenone or flecainide, both at 2 mg per kg intravenously for 1 hour; both significantly reduced the heart rate from baseline.52,53 In both studies, subjects were allowed to continue on digoxin, calcium-channel blockers, and b-blockers. The side effects of flecainide were of more concern than those of propafenone, with conduction abnormalities in the flecainide group. Another study compared propafenone with quinidine for rate control.54 Propafenone significantly slowed the heart rate at rest compared with quinidine. Either drug effectively slowed the heart rate compared with baseline.
Disopyramide did not reduce the mean resting heart rate from baseline.55 The combination of diltiazem and digoxin reduced the mean resting heart rate to a greater degree than the combination of propranolol and digoxin, but all 3 drugs together were even more effective.56 That study also demonstrated that with exercise the combination of propranolol and digoxin was more efficacious for heart rate control than diltiazem and digoxin and that the 3-drug combination was not better than just propranolol and digoxin. The combination of pindolol and digoxin reduced the maximum area under the heart rate curve significantly more than verapamil and digoxin.57 Finally, the combination of amiodarone and digoxin slowed the resting heart rate when compared with baseline, while the combination of quinidine, verapamil, and digoxin did not; this was a small trial, however, and the baseline resting heart rates were not rapid.58
Discussion
The randomized controlled trials of diltiazem and verapamil used by patients with atrial fibrillation provide strong evidence for their efficacy in reducing heart rate both at rest and with exercise when compared with placebo. In all of the studies that evaluated calcium-channel blockers compared with placebo during exercise, the calcium-channel blockers produced either an increase in cardiac output, oxygen consumption, or distance walked. There was also moderate evidence that diltiazem or verapamil was more effective at heart rate control both at rest and during exercise in the direct comparisons with digoxin, with a more rapid onset of action. Although digoxin appeared to increase cardiac output, verapamil prolonged time on the treadmill and increased oxygen consumption. Thus, the evidence strongly supports the use of diltiazem or verapamil for ventricular rate control in atrial fibrillation. Although they have a negative inotropic effect, reflex responses to vasodilatation usually result in a small increase in cardiac output. Therefore, except in moderate to severe heart failure, the negative inotropic effect is often not clinically apparent.60
All of the tested b-blockers successfully reduced heart rate with exercise when compared with placebo, and most of them reduced resting heart rate. The effect on exercise tolerance was variable and may be due to the different receptor specificities of the tested drugs and the varying treatment times before testing.
When administered acutely, b-blocking agents depress myocardial function secondary to the withdrawal of adrenergically mediated inotropic and chronotropic support. However, in patients with congestive heart failure treated for longer than 1 month, b-blockers may improve myocardial function by improving intrinsic systolic function.59,61-63 Extrapolating to patients with atrial fibrillation, b-blocker therapy may increase left ventricular ejection fraction compared with placebo if administered for longer than 1 month. None of the trials in this review lasted for longer than 4 weeks, so it is conceivable that the worsening exercise tolerance was a transient effect of the drug.
When compared with digoxin, the trials favored the use of b-blockers, as digoxin was less efficacious than metoprolol in resting heart rate reduction and less efficacious than labetalol at rate reduction during exercise. Furthermore, time on the treadmill was longer with both labetalol and metoprolol than with digoxin.
It will be interesting to see the effect of the third-generation b-blockers, such as carvedilol, on heart rate control in atrial fibrillation. We anticipate that they will be effective at ventricular rate control, with an improvement in exercise tolerance. Celiprolol and xameterol are not available in the United States and are no longer being evaluated for approval by the Food and Drug Administration.
Although several of the studies comparing digoxin with placebo were limited by subtherapeutic serum levels of digoxin at the time of evaluation, the others did show a resting heart rate reduction. There is little evidence to support the efficacy of digoxin for heart rate control with exercise. However, in the 2 trials that evaluated exercise tolerance on digoxin compared with placebo, cardiac output and time on the treadmill were greater with digoxin.
The trials evaluating other drugs, including propafenone, clonidine, and amiodarone yielded insufficient evidence to support their use for rate control at this time. These drugs appear promising and may prove efficacious when more evidence becomes available.
The optimal degree of heart rate control for patients with atrial fibrillation is unclear, particularly during exercise. Certainly an excessively rapid rate impairs ventricular filling and decreases cardiac output. However, severely limiting the heart rate acceleration that is needed to maintain cardiac output can also limit exercise tolerance.63 There are few empirical trials of this. One recent study found that ventricular rate control in atrial fibrillation had no impact on cardiovascular performance as measured by endurance on a treadmill.63 The studies in our review that report only on heart rate control during exercise without mention of exercise tolerance may be of less value to clinicians.
It is important to recognize that both heart rate control and exercise tolerance are surrogate outcomes for what is truly important to clinicians and their patients: their well-being, ability to conduct their daily activities, and mortality. Although several of the studies did inquire about symptoms such as palpitations or breathlessness, the use of validated quality-of-life questionnaires was rare. A study comparing pharmacologic treatment of atrial fibrillation with atrioventricular junction ablation and pacing demonstrated the use of several different quality-of-life measures: a Quality-of-Life Questionnaire, Specific Symptoms Scale, New York Heart Association Classification, and a Specific Activity Scale.64 The authors of that study commented that they were uncertain whether the sensitivities of these scales were high enough to use as they did.
Our study is the first comprehensive evidence-based review that focused on this aspect of the management of atrial fibrillation. Several recent reviews described ventricular rate control trials in the context of other pharmacologic therapies for managing atrial fibrillation,65,2-4 but those studies did not comprehensively review all the rate control trials. Furthermore, they contained recommendations for drugs that have never been evaluated in controlled trials of patients with atrial fibrillation exclusively (such as intravenous esmolol and intravenous propranolol).2
Limitations
Our systematic review was subject to the same limitations that are common to most reviews. The important differences among the trials preclude mathematical pooling and have to be taken into account when drawing conclusions using these studies. As in most assessments of study quality, our assessment tool was tailored to fit the topic, so the scores cannot be directly compared with quality assessments of studies on other topics. Our intent was to be able to compare the trials.
Few of the studies evaluated adverse effects in any systematic way. This inconsistent reporting of adverse effects limited our ability to comment on them. Many of the trials incompletely described the enrolled participants, so applying these results to all patients with atrial fibrillation should be done cautiously. Also, since results were seldom stratified by the clinical features of the enrolled patients, we could not report on the evidence supporting the use of these drugs in different patient populations.
Similarly, the results were not reported stratified by whether the patient had atrial fibrillation or atrial flutter, thus we cannot report the evidence separately for those 2 conditions. We feel this is appropriate, however, because those 2 arrhythmias frequently coexist.66,67 None of the trials had echocardiographic data as inclusion or exclusion criteria, but that information is more relevant to decisions regarding anticoagulation or cardioversion. We know of no study that associates echocardiographic data with ventricular rate control.
We cannot exclude the presence of publication bias, although we are confident that our search strategy did capture the published literature. In our review of the 8 non–English-language abstracts, we found them in agreement with the articles published in the English literature.
Future research should address the outcomes most relevant to patients — well-being and functionality—particularly since the relationship between heart rate control and exercise tolerance is unclear. We encourage the use of validated instruments for assessment, although which instruments are most appropriate is unknown at this time. Similarly, systematic recording of adverse events should be a regular component of all future trials of these drugs.
Recommendations for clinical practice
For adults with nonpostoperative atrial fibrillation, the evidence supports the following statements. The nondihydropyridine calcium-channel blockers, diltiazem, and verapamil are efficacious for heart rate control at rest and with exercise without decrement in exercise tolerance. Selected b-blockers, such as the noncardioselective b-antagonist nadolol or the second-generation b1-antagonists atenolol and metoprolol, are efficacious at rest and with exercise. There is some evidence, however, that b-blockers cause a transient decrease in exercise tolerance. For patients unlikely to exercise, such as those markedly incapacitated by other illness, digoxin should provide acceptable control.
· Acknowledgments ·
This study was conducted by the Johns Hopkins Evidence-based Practice Center through contract no. 290-97-006 from the Agency for Health Care Policy and Research, Rockville, Maryland. The authors are responsible for its content, including any clinical recommendations. No statement of this article should be construed as an official position of the Agency for Health Care Policy and Research or the US Department of Health and Human Services. We would like to thank Dr Francis Chesley of the Agency for Health Care Policy and Research and Drs Hanan Bell and Michael LeFevre of the American Academy of Family Physicians for their helpful suggestions regarding this project, Dr David Haines of the American College of Cardiology and Drs Ronald Berger and Gary Gerstenblith for their expert advice, Paul Abboud for assistance with data abstraction, and Donna Lea for extensive help with the manuscript.
OBJECTIVE: Our goal was to determine what drugs are most effacacious for controlling the ventricular rate in patients with atrial fibrillation.
SEARCH STRATEGY: We conducted a systematic review of the literature published before May 1998, beginning with searches of The Cochrane Collaboration’s CENTRAL database and MEDLINE.
SELECTION CRITERIA: We included English-language articles describing randomized controlled trials of drugs used for heart rate control in adults with atrial fibrillation.
DATA COLLECTION/ANALYSIS: Abstracts of trials were reviewed independently by 2 members of the study team. We reviewed English-language abstracts of non-English-language publications to assess qualitative consistency with our results.
MAIN RESULTS: Forty-five articles evaluating 17 drugs met our criteria for review. In the 5 trials of verapamil and 5 of diltiazem, heart rate was reduced significantly (P<.05), both at rest and with exercise, compared to placebo, with equivalent or improved exercise tolerance in 6 of 7 comparisons. In 7 of 12 comparisons of a beta-blocker with placebo, the beta-blocker was efficacious for control of resting heart rate, with evidence that the effect is drug-specific, as nadolol and atenolol proved to be the most efficacious. All 9 comparisons demonstrated good heart rate control with beta-blockers during exercise, although exercise tolerance was compromised in 3 of 9 comparisons. In 7 of 8 trials, digoxin administered alone slowed the resting heart rate more than placebo, but it did not significantly slow the rate during exercise in 4 studies. The trials evaluating other drugs yielded insufficient evidence to support their use, but those drugs may yet be promising.
CONCLUSIONS: The calcium-channel blockers verapamil or diltiazem, or select {b}-blockers are efficacious for heart rate control at rest and during exercise for patients with atrial fibrillation without a clinically important decrease in exercise tolerance. Digoxin is useful when rate control during exercise is less of a concern.
Despite pharmacologic and electrical interventions, sinus rhythm cannot be restored and maintained in many patients with atrial fibrillation. For these patients, control of the ventricular rate is a primary goal of therapy, since a rapid rate may lead to worsening congestive heart failure, myocardial ischemia, or distressing breathlessness and palpitations.
A number of review articles have described strategies for rate control, principally involving the use of digoxin, calcium-channel blockers, and b-blockers.1-6 A recent analysis of the trends in the use of drugs for ventricular rate control found that the use of digoxin and b-blockers decreased between 1980 and 1981 and 1994 and 1996, and the use of the nondihydropyridine calcium-channel blockers diltiazem and verapamil increased.7 These investigators, however, indicated that “current practices are dictated more by clinical tradition than by clinical science.”7 There has not been a systematic review of the trials evaluating the efficacy of both the familiar and the newer medications for ventricular rate control in atrial fibrillation. It is increasingly clear the drugs that are used most often for heart rate control at rest may not be the most efficacious during exercise, and exercise tolerance is compromised by some drugs.6
The purpose of our review was to characterize the strength of the evidence regarding the efficacy of drugs used for ventricular rate control in atrial fibrillation.
Methods
Study Design
We performed a systematic literature review and synthesis of randomized controlled trials on ventricular rate control in atrial fibrillation. To be eligible for inclusion in our review, trials needed to meet the following criteria: address management of nonpostoperative atrial fibrillation or atrial flutter; include human data; include adult subjects; and present original data. Studies that included patients with postoperative atrial fibrillation were not excluded as long as those patients were only a minority of the included patients.
Literature Identification and Search Strategies
The primary source of literature for our review was the CENTRAL database of The Cochrane Collaboration, a comprehensive collection of controlled clinical trials from 1948 to the present. As a secondary source, we searched MEDLINE from 1966 to May 1998 to ensure completeness. Additionally, we used the related articles feature of PubMed, as well as recent search results submitted to the Baltimore Cochrane Center, the contents pages of recent relevant journals, and programs from recent cardiology meetings. Our search strategy included using the MeSH terms “atrial fibrillation” and “atrial flutter” as subject headings and text words, as well as “random allocation,” “double-blind method,” and “single-blind method.” The publication types were “randomized controlled trials” and “controlled clinical trials.”
Abstracts of the citations of randomized controlled clinical trials were reviewed independently by 2 members of the study team to identify articles that met the inclusion criteria. Only English-language articles were reviewed. However, we reviewed all English-language abstracts of non-English-language publications to assess qualitative consistency with our results.
Data Abstraction
A form was developed to extract information from the eligible articles regarding study quality, characteristics, and findings. The section on study quality was created after our review of forms used in meta-analytic studies8,9 and a literature review10,11 and with the assistance of The Cochrane Collaboration. The resultant form incorporated6 key questions used by The Cochrane Collaboration in its reviews and14 key questions identified by Detsky and colleagues.10 Our form was pilot-tested for clarity and reproducibility and revised as needed. The final version contained 22 questions assessing quality in the following 5 areas: representativeness (how well the study population was described); potential for bias and confounding; description of therapy (eg, how similarly the groups were treated); outcomes and follow-up; and statistical reporting and interpretation [Table 1]. Each question was worth a maximum of 2 points, and the score in each category was the percentage of points received out of the total available. The overall quality score was calculated as the average of the scores for the 5 categories.
The portion of the form for quantitative data abstraction included sections for subject inclusion and exclusion criteria, baseline subject characteristics, therapeutic protocols, and outcomes. We recorded the mean heart rate at rest, the mean maximum heart rate during exercise or immediately after exercise, the proportion of subjects who reached the goal heart rate reduction in each treatment arm, and any measure of exercise tolerance.
The review of study quality was done independently by 2 reviewers, and differences were resolved by consensus. Quantitative data were abstracted by one primary reviewer and then checked for accuracy by a secondary reviewer. The reviewers were not blinded to the author, institution, or journal, since it seemed unlikely that this information would make a significant difference in the results.12
Presentation of the Data
We constructed 3 evidence tables with the trials grouped according to the regimens being compared. [Table 2a][Table 2b] displays the quality scores and key design elements of each trial. [Table 3] contains a listing of the trials of the most frequent comparisons and the absolute differences in heart rates of patients using those therapies. [Table 4] shows the results from the trials for which there were few, if any, given comparisons.
Data was synthesized by creating scatter plots of mean heart rates at rest and with exercise for each of the main drug comparisons (Figures 1-5). The data were not amenable to formal mathematical pooling (ie, meta-analysis) because of significant qualitative heterogeneity among the studies.*
Results
Literature Yield
We retrieved 74 abstracts. Of these, 8 were abstracts of articles from non–English-language literature. Forty-five trials were eligible for inclusion in our review; the authors of those studies evaluated 17 different drugs and several combinations of drugs. Some of the included trials were not designed as rate-control studies; they were studies of pharmacologic conversion that included heart rate data.
Qualitative Synthesis
The following comparisons were made in the trials: calcium-channel blockers,14-21 b-blockers,19-28 digoxin,14,22,28,30-32,38 or other drugs and combinations compared with placebo;14,28,29,33-38 calcium-channel blockers,14,39-42 b-blockers,23,29,43,44 or other drugs and combinations compared with digoxin;14,27,40,44-49 and other drug comparison trials.18-20,27,42,50-58 Many of the trials involved more than 2 treatment arms. Nearly all of the trials of calcium-channel blockers, b-blockers, and digoxin were designed to evaluate rate control. The studies of the other drugs were principally trials evaluating atrial fibrillation conversion that also reported heart rate data. Two of the digoxin trials were also aimed at evaluating conversion to sinus rhythm.
Study Design
As shown in [Table 2a][Table 2b], there were important similarities and differences among the trials evaluating the same therapies. The duration of the trials and route of administration of the drug are presented to aid in interpretation of the results.
Notably, all of the trials of any given comparison were done within 10 years of each other. This should reduce the likelihood of secular trends affecting the outcomes of the trials. The range in study sizes is extreme, although most of the trials enrolled fewer than 50 patients. Several trials had fewer than 10 participants, and it was anticipated that these small trials would have little power to detect differences between treatments.
The regimens differed among the trials of the same medication. The intravenous diltiazem and verapamil doses were fairly uniform, although the oral dosages and frequency of administration differed. Some of the b-blocker trials involved titration of the medication to effectiveness, and digoxin was often dosed to a target blood level. All these differences may have had an impact on the outcomes. The followup times ranged from minutes to 6 weeks. Short trials may be appropriate for intravenous agents; however, several of the trials assessed the outcome a very short time after a single oral dose of medication, before a therapeutic blood level could be expected.
Another notable feature of these trials was the permissibility of other agents during the trial, as detailed in the evidence table. Permitting the use of digoxin in trials testing other medications, without reporting the number of participants in each arm receiving digoxin, can potentially confound the results.
Although not shown in the evidence table, most trials had explicit inclusion criteria. Some required atrial fibrillation lasting longer than 1 month or longer than 6 months, and several specified a ventricular rate required for entry, such as more than 120 beats per minute or “rapid rate.” Exclusion criteria varied from none to stringent; it was particularly stringent in those studies that involved exercise, from which subjects with angina or significant congestive heart failure were often excluded. None of the trials used echocardiographic data as inclusion or exclusion criteria.
Quality Scores
Many studies were weakest in their description of the participants in the study arms, so it was not always possible to tell if the groups were similar. This can be seen in the exceptionally low scores in the “representativeness” category. The potential for bias and confounding varied markedly across the trials, as did the description of the therapies. It was often unclear which other therapies the patients may have been receiving. Generally, the investigators described the outcomes completely and objectively measured them with Holter monitoring or telemetry. The completeness of statistical reporting was variable, with many studies only reporting a P value without reporting a measure of variability in the outcomes.
The studies published more recently had slightly higher total quality scores. Total quality scores were strongly associated with the size of the study, with the larger studies receiving higher scores (P < .001).
Outcomes
As shown in [Table 3], all of the trials reported either the heart rate reduction [on] for the active drug compared with the comparative drug or the proportion of patients who reached the target heart rate. Many of the trials also evaluated the efficacy of the drugs during exercise. The exercise test itself varied among the trials and included measurement of distance walked on a treadmill, measurement of oxygen consumption, and workload tolerated on a stationary bicycle.
On the basis of our qualitative assessment of the trials, we felt that any mathematical pooling of the results would result in invalid estimates of treatment effects. This was because of the markedly different treatment regimens within each drug class and the differing goals of treatment (acute or chronic management). The mixed quality of the trials also argued against pooling.
Study Results
Calcium-Channel Blockers Versus Placebo for Rate Control. All comparisons of calcium-channel blockers with placebo demonstrated that the calcium-channel blocker was more efficacious than placebo at reducing heart rate both at rest and during exercise. Five of the trials used diltiazem, 4 used verapamil, and 1 evaluated both drugs. An improvement in exercise tolerance was almost always seen, although different measures of tolerance were used. All but 2 of the trials allowed the participants to use digoxin but did not report what percentage of subjects in each treatment group received the drug. Despite different rates in the placebo arms, there was uniformity in treatment effects across the trials [Figure 1].
b-Blockers Versus Placebo for Rate Control. Seven different b-blockers were tested. Only 7 of the 12 comparisons demonstrated efficacy of the b-blocker at rest, although all were efficacious during exercise. The efficacy appears to be medication dependent. Atenolol (at 50 mg daily28 or twice daily20 or 100 mg daily28) performed significantly better than placebo. Timolol (1 mg intravenously) allowed more subjects to reach the target heart rate compared with placebo.24 Pindolol28 (5 mg or 15 mg twice daily) and nadolol25 (titrated dose) significantly reduced mean resting heart rate. The data regarding xamoterol were mixed.20-23 Celiprolol26 and labetalol were no more efficacious than placebo at rest.
All of the tested b-blockers demonstrated a significant reduction in heart rate with exercise compared with placebo; this included atenolol, labetalol, nadolol, celiprolol, and xamoterol.19,20,22,24,25 [Figure 2] shows that the effect of b-blockers on heart rate during exercise was more uniform than their impact on heart rate control at rest. However, these trials suggest that exercise tolerance in patients with atrial fibrillation may be reduced with b-blockers.
As in the calcium-channel blocker studies, most of the trials allowed subjects to continue on digoxin.Digoxin Versus Placebo. The outcomes with digoxin were mixed, as shown in [Table 3] and [Figure 3]. Two of the trials of digoxin versus placebo did not demonstrate a reduction in mean resting heart rate;28,30 in 5 trials, however, there was a reduction.14,22,31,32,38 Two of these studies included patients on verapamil in both arms, so we could not attribute all of the rate reduction to digoxin alone.31,32
Two studies evaluating digoxin during exercise did not find a significant heart rate reduction.14,22 In one trial that suggested a difference, no measure of statistical significance was provided.28 Four of the studies of digoxin and placebo evaluated exercise tolerance.14,22,28,29 In one14 the cardiac output was higher for patients taking digoxin, and in another22 the time on the treadmill was longer with digoxin although the maximal attainable heart rate blood pressure product was higher with placebo.
Calcium-Channel Blockers Versus Digoxin for Rate Control. Three trials compared diltiazem with digoxin,14,19,40 and 3 compared verapamil with digoxin14,41,42 with the outcomes reported in [Table 3] and in [Figure 4]. The scatter plot is most useful for noting the trend toward improved control with calcium-channel blockers both at rest and with exercise. Notably, the cardiac output on digoxin during exercise was greater than in the 2 diltiazem groups (12.6 L/min vs 10.9 L/min and 9.1 L/min for 60 mg and 120 mg, respectively).14 Conversely, the group receiving verapamil was able to exercise longer on the treadmill than the digoxin group.43 This latter study, however, had methodologic flaws, including little description of the participants.
b-Blockers Versus Digoxin for Rate Control. Four trials compared b-blockers with digoxin for rate control in atrial fibrillation, and the outcomes are reported in [Table 3] and in Figure 5.22,28,42,43 Similar to the results of the trials of b-blockers compared with placebo, the efficacy of b-blockers was most convincing in the trials that evaluated their use during exercise. There appeared to be little difference between the efficacy of b-blockers and digoxin at rest.
Other Drugs and Combinations Versus Placebo and Digoxin. [Table 4] summarizes the outcomes for the few trials of other agents. Not surprisingly, of the 8 trials that compared digoxin with a combination of digoxin with a calcium-channel blocker,14,19,22,38,40,41,45,46 only one study did not find a significant decrease in mean resting heart rate with the addition of the calcium-channel blocker.40 In 5 of the 6 studies with an exercise evaluation,14,19,38,41,45 the combination of a calcium-channel blocker and digoxin controlled the heart rate better than digoxin alone, while the sixth trial did not report the statistical significance of this outcome.14 Of the trials of a b}-blocker combined with digoxin, all were more effective than placebo, and all were more effective than digoxin alone except for the combination of digoxin and labetolol.28 During exercise, however, this combination was more effective than either comparison arm.
Other Drugs Evaluated for Rate Control. There were 9 other randomized controlled trials of drugs for rate control in atrial fibrillation.50-58 Two studies compared intravenous magnesium sulfate with intravenous verapamil for acute control.50,51 In both studies, a higher percentage of subjects reached a heart rate of less than 100 beats per minute with verapamil than with magnesium sulfate.
Two studies evaluated rate control with propafenone or flecainide, both at 2 mg per kg intravenously for 1 hour; both significantly reduced the heart rate from baseline.52,53 In both studies, subjects were allowed to continue on digoxin, calcium-channel blockers, and b-blockers. The side effects of flecainide were of more concern than those of propafenone, with conduction abnormalities in the flecainide group. Another study compared propafenone with quinidine for rate control.54 Propafenone significantly slowed the heart rate at rest compared with quinidine. Either drug effectively slowed the heart rate compared with baseline.
Disopyramide did not reduce the mean resting heart rate from baseline.55 The combination of diltiazem and digoxin reduced the mean resting heart rate to a greater degree than the combination of propranolol and digoxin, but all 3 drugs together were even more effective.56 That study also demonstrated that with exercise the combination of propranolol and digoxin was more efficacious for heart rate control than diltiazem and digoxin and that the 3-drug combination was not better than just propranolol and digoxin. The combination of pindolol and digoxin reduced the maximum area under the heart rate curve significantly more than verapamil and digoxin.57 Finally, the combination of amiodarone and digoxin slowed the resting heart rate when compared with baseline, while the combination of quinidine, verapamil, and digoxin did not; this was a small trial, however, and the baseline resting heart rates were not rapid.58
Discussion
The randomized controlled trials of diltiazem and verapamil used by patients with atrial fibrillation provide strong evidence for their efficacy in reducing heart rate both at rest and with exercise when compared with placebo. In all of the studies that evaluated calcium-channel blockers compared with placebo during exercise, the calcium-channel blockers produced either an increase in cardiac output, oxygen consumption, or distance walked. There was also moderate evidence that diltiazem or verapamil was more effective at heart rate control both at rest and during exercise in the direct comparisons with digoxin, with a more rapid onset of action. Although digoxin appeared to increase cardiac output, verapamil prolonged time on the treadmill and increased oxygen consumption. Thus, the evidence strongly supports the use of diltiazem or verapamil for ventricular rate control in atrial fibrillation. Although they have a negative inotropic effect, reflex responses to vasodilatation usually result in a small increase in cardiac output. Therefore, except in moderate to severe heart failure, the negative inotropic effect is often not clinically apparent.60
All of the tested b-blockers successfully reduced heart rate with exercise when compared with placebo, and most of them reduced resting heart rate. The effect on exercise tolerance was variable and may be due to the different receptor specificities of the tested drugs and the varying treatment times before testing.
When administered acutely, b-blocking agents depress myocardial function secondary to the withdrawal of adrenergically mediated inotropic and chronotropic support. However, in patients with congestive heart failure treated for longer than 1 month, b-blockers may improve myocardial function by improving intrinsic systolic function.59,61-63 Extrapolating to patients with atrial fibrillation, b-blocker therapy may increase left ventricular ejection fraction compared with placebo if administered for longer than 1 month. None of the trials in this review lasted for longer than 4 weeks, so it is conceivable that the worsening exercise tolerance was a transient effect of the drug.
When compared with digoxin, the trials favored the use of b-blockers, as digoxin was less efficacious than metoprolol in resting heart rate reduction and less efficacious than labetalol at rate reduction during exercise. Furthermore, time on the treadmill was longer with both labetalol and metoprolol than with digoxin.
It will be interesting to see the effect of the third-generation b-blockers, such as carvedilol, on heart rate control in atrial fibrillation. We anticipate that they will be effective at ventricular rate control, with an improvement in exercise tolerance. Celiprolol and xameterol are not available in the United States and are no longer being evaluated for approval by the Food and Drug Administration.
Although several of the studies comparing digoxin with placebo were limited by subtherapeutic serum levels of digoxin at the time of evaluation, the others did show a resting heart rate reduction. There is little evidence to support the efficacy of digoxin for heart rate control with exercise. However, in the 2 trials that evaluated exercise tolerance on digoxin compared with placebo, cardiac output and time on the treadmill were greater with digoxin.
The trials evaluating other drugs, including propafenone, clonidine, and amiodarone yielded insufficient evidence to support their use for rate control at this time. These drugs appear promising and may prove efficacious when more evidence becomes available.
The optimal degree of heart rate control for patients with atrial fibrillation is unclear, particularly during exercise. Certainly an excessively rapid rate impairs ventricular filling and decreases cardiac output. However, severely limiting the heart rate acceleration that is needed to maintain cardiac output can also limit exercise tolerance.63 There are few empirical trials of this. One recent study found that ventricular rate control in atrial fibrillation had no impact on cardiovascular performance as measured by endurance on a treadmill.63 The studies in our review that report only on heart rate control during exercise without mention of exercise tolerance may be of less value to clinicians.
It is important to recognize that both heart rate control and exercise tolerance are surrogate outcomes for what is truly important to clinicians and their patients: their well-being, ability to conduct their daily activities, and mortality. Although several of the studies did inquire about symptoms such as palpitations or breathlessness, the use of validated quality-of-life questionnaires was rare. A study comparing pharmacologic treatment of atrial fibrillation with atrioventricular junction ablation and pacing demonstrated the use of several different quality-of-life measures: a Quality-of-Life Questionnaire, Specific Symptoms Scale, New York Heart Association Classification, and a Specific Activity Scale.64 The authors of that study commented that they were uncertain whether the sensitivities of these scales were high enough to use as they did.
Our study is the first comprehensive evidence-based review that focused on this aspect of the management of atrial fibrillation. Several recent reviews described ventricular rate control trials in the context of other pharmacologic therapies for managing atrial fibrillation,65,2-4 but those studies did not comprehensively review all the rate control trials. Furthermore, they contained recommendations for drugs that have never been evaluated in controlled trials of patients with atrial fibrillation exclusively (such as intravenous esmolol and intravenous propranolol).2
Limitations
Our systematic review was subject to the same limitations that are common to most reviews. The important differences among the trials preclude mathematical pooling and have to be taken into account when drawing conclusions using these studies. As in most assessments of study quality, our assessment tool was tailored to fit the topic, so the scores cannot be directly compared with quality assessments of studies on other topics. Our intent was to be able to compare the trials.
Few of the studies evaluated adverse effects in any systematic way. This inconsistent reporting of adverse effects limited our ability to comment on them. Many of the trials incompletely described the enrolled participants, so applying these results to all patients with atrial fibrillation should be done cautiously. Also, since results were seldom stratified by the clinical features of the enrolled patients, we could not report on the evidence supporting the use of these drugs in different patient populations.
Similarly, the results were not reported stratified by whether the patient had atrial fibrillation or atrial flutter, thus we cannot report the evidence separately for those 2 conditions. We feel this is appropriate, however, because those 2 arrhythmias frequently coexist.66,67 None of the trials had echocardiographic data as inclusion or exclusion criteria, but that information is more relevant to decisions regarding anticoagulation or cardioversion. We know of no study that associates echocardiographic data with ventricular rate control.
We cannot exclude the presence of publication bias, although we are confident that our search strategy did capture the published literature. In our review of the 8 non–English-language abstracts, we found them in agreement with the articles published in the English literature.
Future research should address the outcomes most relevant to patients — well-being and functionality—particularly since the relationship between heart rate control and exercise tolerance is unclear. We encourage the use of validated instruments for assessment, although which instruments are most appropriate is unknown at this time. Similarly, systematic recording of adverse events should be a regular component of all future trials of these drugs.
Recommendations for clinical practice
For adults with nonpostoperative atrial fibrillation, the evidence supports the following statements. The nondihydropyridine calcium-channel blockers, diltiazem, and verapamil are efficacious for heart rate control at rest and with exercise without decrement in exercise tolerance. Selected b-blockers, such as the noncardioselective b-antagonist nadolol or the second-generation b1-antagonists atenolol and metoprolol, are efficacious at rest and with exercise. There is some evidence, however, that b-blockers cause a transient decrease in exercise tolerance. For patients unlikely to exercise, such as those markedly incapacitated by other illness, digoxin should provide acceptable control.
· Acknowledgments ·
This study was conducted by the Johns Hopkins Evidence-based Practice Center through contract no. 290-97-006 from the Agency for Health Care Policy and Research, Rockville, Maryland. The authors are responsible for its content, including any clinical recommendations. No statement of this article should be construed as an official position of the Agency for Health Care Policy and Research or the US Department of Health and Human Services. We would like to thank Dr Francis Chesley of the Agency for Health Care Policy and Research and Drs Hanan Bell and Michael LeFevre of the American Academy of Family Physicians for their helpful suggestions regarding this project, Dr David Haines of the American College of Cardiology and Drs Ronald Berger and Gary Gerstenblith for their expert advice, Paul Abboud for assistance with data abstraction, and Donna Lea for extensive help with the manuscript.
1. Wakatare JEP, Camm AJ. Acute treatment of atrial fibrillation: why and when to maintain sinus rhythm. Am J Cardiol 1998;81:3C-15C.
2. Kowey PR, Marinchak RA, Rials SJ, Filart RA. Acute treatment of atrial fibrillation. Am J Cardiol 1998;81:16C-22C.
3. Pratt CM. Impact of managed care on the treatment of atrial fibrillation. Am J Cardiol 1998;81:30C-4C.
4. Sopher SM, Camm AJ. Atrial fibrillation; maintenance of sinus rhythm versus rate control. Am J Cardiol 1996;77:24A-37A.
5. Riley RD, Pritchett ELC. Pharmacologic management of atrial fibrillation. J Cardiovasc Electrophysiol 1997;8:818-29.
6. Hohnloser SH, Li Y-G. Drug treatment of atrial fibrillation; what have we learned? Curr Opin Cardiol 1997;12:24-32.
7. Stafford RS, Robson DC, Misra B, Ruskin J, Singer DE. Rate control and sinus rhythm maintenance in atrial fibrillation: national trends in medication use, 1980-1996. Arch Intern Med 1998;158:2144-8.
8. Bass EB, Powe NR, Goodman SN, et al. Efficacy of immune globulin in preventing complications of bone marrow transplantation; a meta-analysis. Bone Marrow Transplant 1993;12:273-82.
9. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Arch Ophthal 1994;112:239-52.
10. Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992;45:255-65.
11. Chalmers TC, Smith H, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49.
12. Berlin JA. University of Pennsylvania. National Technical Information Service accession number PB98-109382.
13. McNamara FL, Miller MR, Segal JB, et al. Evidence report on management of new onset atrial fibrillation: evidence report/technology assessment no.1. AHCPR Publication No. 99-E021. Rockville, Md: Agency for Health Care Policy and Research. In press.
14. Lewis RV, Irvine N, McDevitt DG. Relationships between heart rate, exercise tolerance and cardiac output in atrial fibrillation: the effects of treatment with digoxin, verapamil and diltiazem. Eur Heart J 1988;9:777-81.
15. Salerno DM, Dias VC, Kleiger RE, et al. Efficacy and safety of intravenous diltiazem for treatment of atrial fibrillation and atrial flutter: the diltiazem-atrial fibrillation/flutter study group. Am J Cardiol 1989;63:1046-51
16. Goldenberg IF, Lewis WR, Dias VC, Heywood JT, Pedersen WR. Intravenous diltiazem for the treatment of patients with atrial fibrillation or flutter and moderate to severe congestive heart failure. Am J Cardiol 1994;74:884-9.
17. Ellenbogen K, Dias VC, Plumb VJ, Heywood JT, Mirvis DM. A placebo-controlled trial of continuous intravenous diltiazem infusion for 24-hour heart rate control during atrial fibrillation and atrial flutter: a multicenter study. JACC 1991;18:891-7.
18. Lundstrom T, Ryden L. Ventricular rate control and exercise performance in chronic atrial fibrillation: effects of diltiazem and verapamil. JACC 1990;16:86-90.
19. Lewis RV, McMurray J, McDevitt DG. Effects of atenolol, verapamil, and xamoterol on heart rate and exercise tolerance in digitalised patients with chronic atrial fibrillation. J Cardio Pharm 1989;13:1-6.
20. Lundstrom T, Moor E, Ryden L. Differential effects of xamoterol and verapamil on ventricular rate regulation in patients with chronic atrial fibrillation. Am Heart J 1992;124:917-23.
21. Panidis IP, Morganroth J, Baessler C. Effectiveness and safety of oral verapamil to control exercise-induced tachycardia in patients with atrial fibrillation receiving digitalis. Am J Cardiol 1983;52:1197-201.
22. Ang EL, Chan WL, Cleland JG, et al. Placebo controlled trial of xamoterol versus digoxin in chronic atrial fibrillation. Br Heart J 1990;64:256-60.
23. Sweany AE, Moncloa F, Vickers FF, Zupkis RV. Antiarrhythmic effects of intravenous timolol in supraventricular arrhythmias. Clin Pharmacol Ther 1985;37:124-7.
24. DiBianco R, Morganroth J, Freitag JA, et al. Effects of nadolol on the spontaneous and exercise-provoked heart rate of patients with chronic atrial fibrillation receiving stable dosages of digoxin. Am Heart J 1984;108:1121-7.
25. Myers J, Atwood JE, Sullivan M, et al. Perceived exertion and gas exchange after calcium and beta-blockade in atrial fibrillation. J Appl Physiol 1987;63:97-104.
26. Lin SK, Morganroth J, Heng M, et al. Effect of orally administered celiprolol in patients with chronic atrial fibrillation. J Cardiol Pharm 1986;S112-5.
27. Channer KS, James MA, MacConnell T, Rees JR. Beta-adrenoceptor blockers in atrial fibrillation: the importance of partial agonist activity. Br J Clin Pharm 1994;37:53-7.
28. Wong CK, Lau CP, Leung WH, Cheng CH. Usefulness of labetalol in chronic atrial fibrillation. Am J Cardiol 1990;66:1212-5.
29. Koh KK, Song JH, Kwon KS, et al. Comparative study of efficacy and safety of low-dose diltiazem or betaxolol in combination with digoxin to control ventricular rate in chronic atrial fibrillation: randomized crossover study. Int J Cardiol 1995;52:167-74.
30. Falk RH, Knowlton A A, Bernard SA, Gotlieb NE, Battinelli NJ. Digoxin for converting recent-onset atrial fibrillation to sinus rhythm: a randomized, double-blinded trial. Ann Intern Med 1987;106:503-6.
31. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group Intravenous digoxin in acute atrial fibrillation: results of a randomized, placebo-controlled multicenter trial in 239 patients. Eur Heart J 1997;18:649-54.
32. Jordaens L, Trouerbach J, Calle P, et al. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur Heart J 1997;18:643-8.
33. Roth A, Kaluski E, Felner S, Heller K, Laniado S. Clonidine for patients with rapid atrial fibrillation. Ann Intern Med 1992;116:388-90.
34. Scardi S, Humar F, Pandullo C, Poletti A. Oral clonidine for heart rate control in chronic atrial fibrillation. Letter. Lancet 1993;341:1211-2.
35. Botto GL, Capucci A, Bonini W, et al. Conversion of recent onset atrial fibrillation to sinus rhythm using a single oral loading dose of propafenone: comparison of two regimens. Int J Cardiol 1997;58:55-61.
36. Lok NS, Lau CP. Oxygen uptake kinetics and cardiopulmonary performance in lone atrial fibrillation and the effects of sotalol. Chest 1997;111:934-40.
37. Brodsky MA, Orlov MV, Capparelli EV, et al. Magnesium therapy in new-onset atrial fibrillation. Am J Cardiol 1994;73:1227-9.[Published erratum appears in Am J Cardiol 1994; 74:639.]
38. Koh KK, Kwon KS, Park HB, et al. Efficacy and safety of digoxin alone and in combination with low-dose diltiazem or betaxolol to control ventricular rate in chronic atrial fibrillation. Am J Cardiol 1995;75:88-90.
39. Lewis RV, Laing E, Moreland TA, Service E, McDevitt DG. A comparison of digoxin, diltiazem and their combination in the treatment of atrial fibrillation. Eur Heart J 1988;9:279-83.
40. Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. 1997;29:135-40.
41. Pomfret SM, Beasley CR, Challenor V, Holgate ST. Relative efficacy of oral verapamil and digoxin alone and in combination for the treatment of patients with chronic atrial fibrillation. Clin-Sci 1988;74:351-7.
42. Ahuja RC, Sinha N, Saran RK, Jain AK, Hasan M. Digoxin or verapamil or metoprolol for heart rate control in patients with mitral stenosis: a randomised cross-over study. Int J Cardiol 1989;25:325-31.
43. Lawson-Matthew PJ, McLean KA, Dent M, Austin CA, Channer KS. Xamoterol improves the control of chronic atrial fibrillation in elderly patients. Age Ageing 1995;24:321-5.
44. Brodsky M, Saini R, Bellinger R, Zoble R, Weiss R, Powers L. Comparative effects of the combination of digoxin and dl-sotalol therapy versus digoxin monotherapy for control of ventricular response in chronic atrial fibrillation, dl-Sotalol Atrial Fibrillation Study Group. Am Heart J 1994;127:572-7.
45. Stern EH, Pitchon R, King BD, et al. Clinical use of oral verapamil in chronic and paroxysmal atrial fibrillation. Chest 1982;81:308-11.
46. Lang R, Klein HO, Di Segni E, et al. Verapamil improves exercise capacity in chronic atrial fibrillation: double-blind crossover study. Am Heart J 1983;105:820-5.
47. Zoble RG, Brewington J, Olukotun AY, Gore R. Comparative effects of nadolol-digoxin combination therapy and digoxin monotherapy for chronic atrial fibrillation. Am J Cardiol 1987;60:39D-45D.
48. Hays JV, Gilman JK, Rubal BJ. Effect of magnesium sulfate on ventricular rate control in atrial fibrillation. Ann Emerg Med 1994;24:61-4.
49. Smit AJ, Scaf AH, van Essen LH, Lie KI, Wesseling H. Digoxin infusion versus bolus injection in rapid atrial fibrillation: relation between serum level and response. Eur J Clin Pharm 1990;38:335-41.
50. Joshi PP, Deshmukh PK, Salkar RG. Efficacy of intravenous magnesium sulphate in supraventricular tachyarrhythmias. J Assoc Physicians India 1995;43:529-31.
51. Gullestad L, Birkeland K, Molstad P, et al. The effect of magnesium versus verapamil on supraventricular arrhythmias. Clin Cardiol 1993;16:429-34.
52. Suttorp MJ, Kingma JH, Jessurun ER, et al. The value of class IC antiarrhythmic drugs for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. JACC 1990;16:1722-7.
53. Kingma JH, Suttorp MJ. Acute pharmacologic conversion of atrial fibrillation and flutter: the role of flecainide, propafenone, and verapamil. Am J Cardiol 1992;70:56A-60A.
54. Lee SH, Chen SA, Chiang CE, et al. Comparisons of oral propafenone and quinidine as an initial treatment option in patients with symptomatic paroxysmal atrial fibrillation: a double-blind, randomized trial. J Int Med 1996;239:253-60.
55. Boudonas G, Lefkos N, Efthymiadis A, Styliadis IG, Tsapas G. Intravenous administration of diltiazem in the treatment of supraventricular tachyarrhythmias. Acta Cardiol 1995;50:125-34.
56. Dahlstrom CG, Edvardsson N, Nasheng C, Olsson SB. Effects of diltiazem, propranolol, and their combination in the control of atrial fibrillation. Clin Cardiol 1992;15:280-4.
57. James MA, Channer KS, Papouchado M, Rees JR. Improved control of atrial fibrillation with combined pindolol and digoxin therapy. Eur Heart J 1989;10:83-90.
58. Zehender M, Hohnloser S, Muller B, Meinertz T, Just H. Effects of amiodarone versus quinidine and verapamil in patients with chronic atrial fibrillation: results of a comparative study and a 2-year follow-up. JACC 1992;19:1054-9.
59. Waagstein F, Caidahl K, Wallentin I, Bergh CH, Hjalmarson A. Long-term beta-blockade in dilated cardiomyopathy. Circ 1989;80:551-63.
60. Facts and Comparisons. Drug Facts and Comparisons. St. Louis, Mo: Facts and Comparisons; 1999.
61. Haber HL, Simek CL, Gimple LW, et al. Why do patients with congestive heart failure tolerate the initiation of beta-blocker therapy? Circulation 1993;88:1610-9.
62. Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol 1997;80:26L-40L.
63. Ostermaier RH, Lampert S, Dalla Vecchia L, Ravid S. The effect of atrial fibrillation and the ventricular rate control on exercise capacity. Clin Cardiol 1997;20:23-7.
64. Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological, treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circ 1998;98:953-60.
65. Viskin S, Barron HV, Heller K, Scheinman MM, Olgin JE. The treatment of atrial fibrillation: pharmacologic and nonpharmacologic strategies. Curr Prob Cardiol 1997;22:37-108.
66. Della Bella P, Riva S, Galimberti P. Should ablation of atrial flutter be discouraged in patients with documented atrial fibrillation? Cardiologia 1999;44:439-42.
67. Roithinger FX, Lesh MD. What is the relationship of atrial flutter and fibrillation? Pacing Clin Electrophysiol 1999;22:643-54.
1. Wakatare JEP, Camm AJ. Acute treatment of atrial fibrillation: why and when to maintain sinus rhythm. Am J Cardiol 1998;81:3C-15C.
2. Kowey PR, Marinchak RA, Rials SJ, Filart RA. Acute treatment of atrial fibrillation. Am J Cardiol 1998;81:16C-22C.
3. Pratt CM. Impact of managed care on the treatment of atrial fibrillation. Am J Cardiol 1998;81:30C-4C.
4. Sopher SM, Camm AJ. Atrial fibrillation; maintenance of sinus rhythm versus rate control. Am J Cardiol 1996;77:24A-37A.
5. Riley RD, Pritchett ELC. Pharmacologic management of atrial fibrillation. J Cardiovasc Electrophysiol 1997;8:818-29.
6. Hohnloser SH, Li Y-G. Drug treatment of atrial fibrillation; what have we learned? Curr Opin Cardiol 1997;12:24-32.
7. Stafford RS, Robson DC, Misra B, Ruskin J, Singer DE. Rate control and sinus rhythm maintenance in atrial fibrillation: national trends in medication use, 1980-1996. Arch Intern Med 1998;158:2144-8.
8. Bass EB, Powe NR, Goodman SN, et al. Efficacy of immune globulin in preventing complications of bone marrow transplantation; a meta-analysis. Bone Marrow Transplant 1993;12:273-82.
9. Powe NR, Schein OD, Gieser SC, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Arch Ophthal 1994;112:239-52.
10. Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’abbe KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol 1992;45:255-65.
11. Chalmers TC, Smith H, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49.
12. Berlin JA. University of Pennsylvania. National Technical Information Service accession number PB98-109382.
13. McNamara FL, Miller MR, Segal JB, et al. Evidence report on management of new onset atrial fibrillation: evidence report/technology assessment no.1. AHCPR Publication No. 99-E021. Rockville, Md: Agency for Health Care Policy and Research. In press.
14. Lewis RV, Irvine N, McDevitt DG. Relationships between heart rate, exercise tolerance and cardiac output in atrial fibrillation: the effects of treatment with digoxin, verapamil and diltiazem. Eur Heart J 1988;9:777-81.
15. Salerno DM, Dias VC, Kleiger RE, et al. Efficacy and safety of intravenous diltiazem for treatment of atrial fibrillation and atrial flutter: the diltiazem-atrial fibrillation/flutter study group. Am J Cardiol 1989;63:1046-51
16. Goldenberg IF, Lewis WR, Dias VC, Heywood JT, Pedersen WR. Intravenous diltiazem for the treatment of patients with atrial fibrillation or flutter and moderate to severe congestive heart failure. Am J Cardiol 1994;74:884-9.
17. Ellenbogen K, Dias VC, Plumb VJ, Heywood JT, Mirvis DM. A placebo-controlled trial of continuous intravenous diltiazem infusion for 24-hour heart rate control during atrial fibrillation and atrial flutter: a multicenter study. JACC 1991;18:891-7.
18. Lundstrom T, Ryden L. Ventricular rate control and exercise performance in chronic atrial fibrillation: effects of diltiazem and verapamil. JACC 1990;16:86-90.
19. Lewis RV, McMurray J, McDevitt DG. Effects of atenolol, verapamil, and xamoterol on heart rate and exercise tolerance in digitalised patients with chronic atrial fibrillation. J Cardio Pharm 1989;13:1-6.
20. Lundstrom T, Moor E, Ryden L. Differential effects of xamoterol and verapamil on ventricular rate regulation in patients with chronic atrial fibrillation. Am Heart J 1992;124:917-23.
21. Panidis IP, Morganroth J, Baessler C. Effectiveness and safety of oral verapamil to control exercise-induced tachycardia in patients with atrial fibrillation receiving digitalis. Am J Cardiol 1983;52:1197-201.
22. Ang EL, Chan WL, Cleland JG, et al. Placebo controlled trial of xamoterol versus digoxin in chronic atrial fibrillation. Br Heart J 1990;64:256-60.
23. Sweany AE, Moncloa F, Vickers FF, Zupkis RV. Antiarrhythmic effects of intravenous timolol in supraventricular arrhythmias. Clin Pharmacol Ther 1985;37:124-7.
24. DiBianco R, Morganroth J, Freitag JA, et al. Effects of nadolol on the spontaneous and exercise-provoked heart rate of patients with chronic atrial fibrillation receiving stable dosages of digoxin. Am Heart J 1984;108:1121-7.
25. Myers J, Atwood JE, Sullivan M, et al. Perceived exertion and gas exchange after calcium and beta-blockade in atrial fibrillation. J Appl Physiol 1987;63:97-104.
26. Lin SK, Morganroth J, Heng M, et al. Effect of orally administered celiprolol in patients with chronic atrial fibrillation. J Cardiol Pharm 1986;S112-5.
27. Channer KS, James MA, MacConnell T, Rees JR. Beta-adrenoceptor blockers in atrial fibrillation: the importance of partial agonist activity. Br J Clin Pharm 1994;37:53-7.
28. Wong CK, Lau CP, Leung WH, Cheng CH. Usefulness of labetalol in chronic atrial fibrillation. Am J Cardiol 1990;66:1212-5.
29. Koh KK, Song JH, Kwon KS, et al. Comparative study of efficacy and safety of low-dose diltiazem or betaxolol in combination with digoxin to control ventricular rate in chronic atrial fibrillation: randomized crossover study. Int J Cardiol 1995;52:167-74.
30. Falk RH, Knowlton A A, Bernard SA, Gotlieb NE, Battinelli NJ. Digoxin for converting recent-onset atrial fibrillation to sinus rhythm: a randomized, double-blinded trial. Ann Intern Med 1987;106:503-6.
31. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group Intravenous digoxin in acute atrial fibrillation: results of a randomized, placebo-controlled multicenter trial in 239 patients. Eur Heart J 1997;18:649-54.
32. Jordaens L, Trouerbach J, Calle P, et al. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur Heart J 1997;18:643-8.
33. Roth A, Kaluski E, Felner S, Heller K, Laniado S. Clonidine for patients with rapid atrial fibrillation. Ann Intern Med 1992;116:388-90.
34. Scardi S, Humar F, Pandullo C, Poletti A. Oral clonidine for heart rate control in chronic atrial fibrillation. Letter. Lancet 1993;341:1211-2.
35. Botto GL, Capucci A, Bonini W, et al. Conversion of recent onset atrial fibrillation to sinus rhythm using a single oral loading dose of propafenone: comparison of two regimens. Int J Cardiol 1997;58:55-61.
36. Lok NS, Lau CP. Oxygen uptake kinetics and cardiopulmonary performance in lone atrial fibrillation and the effects of sotalol. Chest 1997;111:934-40.
37. Brodsky MA, Orlov MV, Capparelli EV, et al. Magnesium therapy in new-onset atrial fibrillation. Am J Cardiol 1994;73:1227-9.[Published erratum appears in Am J Cardiol 1994; 74:639.]
38. Koh KK, Kwon KS, Park HB, et al. Efficacy and safety of digoxin alone and in combination with low-dose diltiazem or betaxolol to control ventricular rate in chronic atrial fibrillation. Am J Cardiol 1995;75:88-90.
39. Lewis RV, Laing E, Moreland TA, Service E, McDevitt DG. A comparison of digoxin, diltiazem and their combination in the treatment of atrial fibrillation. Eur Heart J 1988;9:279-83.
40. Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. 1997;29:135-40.
41. Pomfret SM, Beasley CR, Challenor V, Holgate ST. Relative efficacy of oral verapamil and digoxin alone and in combination for the treatment of patients with chronic atrial fibrillation. Clin-Sci 1988;74:351-7.
42. Ahuja RC, Sinha N, Saran RK, Jain AK, Hasan M. Digoxin or verapamil or metoprolol for heart rate control in patients with mitral stenosis: a randomised cross-over study. Int J Cardiol 1989;25:325-31.
43. Lawson-Matthew PJ, McLean KA, Dent M, Austin CA, Channer KS. Xamoterol improves the control of chronic atrial fibrillation in elderly patients. Age Ageing 1995;24:321-5.
44. Brodsky M, Saini R, Bellinger R, Zoble R, Weiss R, Powers L. Comparative effects of the combination of digoxin and dl-sotalol therapy versus digoxin monotherapy for control of ventricular response in chronic atrial fibrillation, dl-Sotalol Atrial Fibrillation Study Group. Am Heart J 1994;127:572-7.
45. Stern EH, Pitchon R, King BD, et al. Clinical use of oral verapamil in chronic and paroxysmal atrial fibrillation. Chest 1982;81:308-11.
46. Lang R, Klein HO, Di Segni E, et al. Verapamil improves exercise capacity in chronic atrial fibrillation: double-blind crossover study. Am Heart J 1983;105:820-5.
47. Zoble RG, Brewington J, Olukotun AY, Gore R. Comparative effects of nadolol-digoxin combination therapy and digoxin monotherapy for chronic atrial fibrillation. Am J Cardiol 1987;60:39D-45D.
48. Hays JV, Gilman JK, Rubal BJ. Effect of magnesium sulfate on ventricular rate control in atrial fibrillation. Ann Emerg Med 1994;24:61-4.
49. Smit AJ, Scaf AH, van Essen LH, Lie KI, Wesseling H. Digoxin infusion versus bolus injection in rapid atrial fibrillation: relation between serum level and response. Eur J Clin Pharm 1990;38:335-41.
50. Joshi PP, Deshmukh PK, Salkar RG. Efficacy of intravenous magnesium sulphate in supraventricular tachyarrhythmias. J Assoc Physicians India 1995;43:529-31.
51. Gullestad L, Birkeland K, Molstad P, et al. The effect of magnesium versus verapamil on supraventricular arrhythmias. Clin Cardiol 1993;16:429-34.
52. Suttorp MJ, Kingma JH, Jessurun ER, et al. The value of class IC antiarrhythmic drugs for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. JACC 1990;16:1722-7.
53. Kingma JH, Suttorp MJ. Acute pharmacologic conversion of atrial fibrillation and flutter: the role of flecainide, propafenone, and verapamil. Am J Cardiol 1992;70:56A-60A.
54. Lee SH, Chen SA, Chiang CE, et al. Comparisons of oral propafenone and quinidine as an initial treatment option in patients with symptomatic paroxysmal atrial fibrillation: a double-blind, randomized trial. J Int Med 1996;239:253-60.
55. Boudonas G, Lefkos N, Efthymiadis A, Styliadis IG, Tsapas G. Intravenous administration of diltiazem in the treatment of supraventricular tachyarrhythmias. Acta Cardiol 1995;50:125-34.
56. Dahlstrom CG, Edvardsson N, Nasheng C, Olsson SB. Effects of diltiazem, propranolol, and their combination in the control of atrial fibrillation. Clin Cardiol 1992;15:280-4.
57. James MA, Channer KS, Papouchado M, Rees JR. Improved control of atrial fibrillation with combined pindolol and digoxin therapy. Eur Heart J 1989;10:83-90.
58. Zehender M, Hohnloser S, Muller B, Meinertz T, Just H. Effects of amiodarone versus quinidine and verapamil in patients with chronic atrial fibrillation: results of a comparative study and a 2-year follow-up. JACC 1992;19:1054-9.
59. Waagstein F, Caidahl K, Wallentin I, Bergh CH, Hjalmarson A. Long-term beta-blockade in dilated cardiomyopathy. Circ 1989;80:551-63.
60. Facts and Comparisons. Drug Facts and Comparisons. St. Louis, Mo: Facts and Comparisons; 1999.
61. Haber HL, Simek CL, Gimple LW, et al. Why do patients with congestive heart failure tolerate the initiation of beta-blocker therapy? Circulation 1993;88:1610-9.
62. Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol 1997;80:26L-40L.
63. Ostermaier RH, Lampert S, Dalla Vecchia L, Ravid S. The effect of atrial fibrillation and the ventricular rate control on exercise capacity. Clin Cardiol 1997;20:23-7.
64. Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological, treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circ 1998;98:953-60.
65. Viskin S, Barron HV, Heller K, Scheinman MM, Olgin JE. The treatment of atrial fibrillation: pharmacologic and nonpharmacologic strategies. Curr Prob Cardiol 1997;22:37-108.
66. Della Bella P, Riva S, Galimberti P. Should ablation of atrial flutter be discouraged in patients with documented atrial fibrillation? Cardiologia 1999;44:439-42.
67. Roithinger FX, Lesh MD. What is the relationship of atrial flutter and fibrillation? Pacing Clin Electrophysiol 1999;22:643-54.