User login
Thromboprophylaxis in Patients with HIV
Patients with human immunodeficiency virus (HIV) are at a 2‐ to 10‐fold greater risk for venous thromboembolism (VTE) compared with the general population.[1] Although antiphospholipid antibodies and protein S deficiency have often been cited as reasons for the thrombophilia associated with HIV, previous studies have also documented an increased risk of VTE with declining CD4+ cell count.[2, 3, 4, 5, 6, 7, 8] Worsening immune function places HIV patients at increased risk for opportunistic and nonopportunistic infections and malignancies, all independently associated with an increased risk of VTE.[5, 9, 10, 11, 12] Although increasing use of antiretroviral therapy has greatly decreased these sequelae, these complications of HIV infection are associated with an increased frequency of hospitalization.[13, 14, 15, 16] HIV infection and associated inflammation has been implicated in cardiovascular conditions such as cardiomyopathy, pulmonary hypertension, and myocardial infarction.[17, 18] Additionally, progression of HIV infection appears to influence T‐cell activation and differentiation in a manner that leads to early immunosenescence in infected individuals.[19, 20]
VTE prophylaxis is effective.[21] Virtually all efforts to decrease VTE have been focused on improving the prescription of prophylaxis with varying degrees of success.[22] These interventions have been employed with the tacit assumption that medication prescribed for inpatients will always be administered. However, at our institution, recent research has demonstrated that a significant proportion of prescribed thromboprophylaxis doses are not administered to hospitalized patients.[23] Refusal by the patient or a family member was the most commonly documented reason for dose nonadministration. In addition, the rate of thromboprophylaxis nonadministration varied greatly between nursing units with distinct patient populations. We hypothesized that nonadministration of VTE prophylaxis may be more common in patients with HIV, and this phenomenon may contribute to their increased risk for VTE.
The purpose of this study was to determine if the proportion of nonadministered thromboprophylaxis is greater among hospitalized patients with HIV and to characterize documented reasons for dose nonadministration.
METHODS
This study was conducted at The Johns Hopkins Hospital (JHH), a large, urban, academic medical center in Baltimore, Maryland. This single‐center retrospective cohort study utilized an existing dataset containing dose administration data extracted from an electronic medication administration record (eMAR). This dataset included information for all prescribed doses of thromboprophylaxis (heparin 5000 U subcutaneously every 8 or 12 hours, heparin 7500 U subcutaneously every 12 hours, enoxaparin 30 mg subcutaneously every 12 hours, or enoxaparin 40 mg subcutaneously daily) for patients hospitalized on medicine units at JHH from November 2007 to December 2008. This time period follows the implementation of an electronic order set for VTE prophylaxis.[24, 25] Data available for each dose included drug name, dose, frequency, patient demographics, and whether or not the dose was administered. Each dose not administered included a reason for nonadministration, which was chosen from a dropdown menu of responses on the eMAR by the nurse at the time the dose was due. A separate electronic report was obtained from an internal administrative database, which identified all patients within the dose administration dataset who had the International Classification of Diseases, 9th Revision code 042 (HIV diagnosis). A report identifying patient history numbers with matching diagnostic code for HIV was appended to the dose administration dataset using a relational database (Microsoft Access; Microsoft Corp., Redmond, WA) prior to analysis. The dose administration data were obtained previously for a separate analysis.[23] Approval for this study was granted from the institutional review board of Johns Hopkins Medicine.
Our analytic plan included comparisons between patients with and without HIV on a dose, patient, and unit level. As JHH operates a nursing unit dedicated to the inpatient care of patients with HIV, we included analyses of dose characteristics between this unit and other medicine units. It should be noted that patients without a diagnosis of HIV are sometimes cared for on this unit. Therefore, the electronic medical record for each patient without the diagnosis code for HIV hospitalized on this unit was reviewed to determine HIV status. An analysis was performed comparing visit identification numbers with diagnosis codes to identify potential seroconversions during the study period. Although we planned to compare nonadministration and documented refusal of doses on the unit level, a lack of patients with HIV on a number of units limited our ability to perform these analyses.
Statistical Analysis
The percent of doses not administered was calculated as the number of doses not administered divided by the number of doses prescribed. Likewise, the percent of prescribed doses documented as refused was calculated as the number of prescribed doses documented as refused divided by the number of doses prescribed. For each comparison, an odds ratio (OR) with 95% confidence interval (CI) was reported. Univariate and multivariate regression analyses were performed to assess the relationship between patient factors and dose nonadministration and documented refusal, respectively. Generalized estimating equations (GEE) using a logit link and an exchangeable correlation structure were used in these analyses. The GEE technique was used to account for within‐individual correlation of administration and documented refusal status.
Categorical data were compared using the two‐sided [2] test. Parametric and nonparametric continuous data were compared using the Student t test and Mann‐Whitney U test, respectively. A P value of <0.05 was considered statistically significant for all analyses. Analyses were performed using Minitab 15 (Minitab Inc., State College, PA) and Stata (StataCorp, College Station, TX).
RESULTS
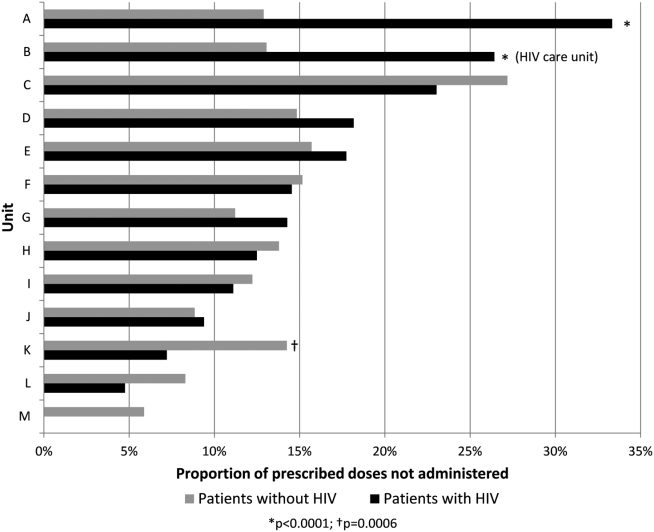
During the 8‐month study period, 42,870 doses of thromboprophylaxis were prescribed during 4947 patient admissions to 13 individual medicine units. Overall, the diagnosis code for HIV was present in 12% of patient visits. The proportion of nonadministered doses per unit ranged from 6% to 27%, whereas the number of doses prescribed per unit ranged from 34 to 7301.
Patient characteristics were described on the visit level (Table 1). Patients with HIV were significantly younger, more often male and black, and had a longer length of stay compared with patients without HIV. Patients hospitalized on the HIV care unit had similar characteristics to the overall population of patients with HIV. It should be noted that not all patients cared for on this unit had a diagnosis of HIV, as patients from other medicine services are sometimes cared for in this location.
| Patients Without HIV | Patients With HIV | P | |
|---|---|---|---|
| |||
| Visits, n | 4,364 | 583 | N/A |
| Male, n (%) | 2,039 (47) | 370 (64) | <0.001 |
| Mean ageSD, y | 5618 | 469 | <0.001 |
| Race, n (%) | |||
| African American | 2,603 (60) | 522 (90) | <0.001 |
| Caucasian | 1,610 (37) | 53 (9) | <0.001 |
| Asian, Pacific Islander, other | 151 (4) | 8 (1) | 0.006 |
| Median length of stay (IQR), d | 3 (15) | 4 (27) | 0.002 |
| Marital status, n (%) | |||
| Single | 2,051 (47) | 471 (81) | <0.001 |
| Married | 1,405 (32) | 71 (12) | <0.001 |
| Widowed | 486 (11) | 10 (1) | <0.001 |
| Divorced | 402 (9) | 28 (5) | <0.001 |
| Separated | 33 (1) | 3 (1) | 0.607 |
| Unknown | 5 (0) | 0 (0) | 0.465 |
| Payor, n (%) | |||
| Medicare | 1,771 (41) | 133 (23) | <0.001 |
| Medicaid | 1,343 (31) | 392 (67) | <0.001 |
| Commercial | 1,181 (27) | 43 (7) | <0.001 |
| Other including self‐pay | 69 (1) | 15 (3) | 0.087 |
Overall, 17% of prescribed prophylaxis doses were not administered. A greater proportion of prescribed doses were not administered to patients with HIV compared with patients without HIV (23.5% vs 16.1%, OR: 1.59, 95% CI: 1.49‐1.70, P<0.001) (Table 2). Using a GEE and univariate regression, HIV diagnosis was associated with nonadministration of doses (OR: 1.37, 95% CI: 1.17‐1.60, P<0.001) (Table 3). Race, age, length of stay, and drug (heparin vs enoxaparin) were each associated with nonadministration. There was no significant association between nonadministration and sex, marital status, or payor. When stratified by nursing unit, there was substantial variation in the proportion of nonadministered doses between units. Within each unit, the proportion of doses not administered varied when stratified by HIV status. For example, on unit A, the proportion of doses not administered was greater for patients with HIV compared with patients without HIV (33.3% vs 12.9%, OR: 3.38, 95% CI: 2.61 to 4.37, P<0.001) (Figure 1). However, on unit K, the proportion of doses not administered to patients with HIV was 2‐fold less than in patients without HIV (7.2% vs 14.3%, OR: 0.47, 95% CI: 0.30‐0.74, P<0.001). Unit‐level analysis was not possible in regression models due to drastic imbalance in the prevalence of HIV across units. When comparing doses prescribed in the HIV care unit to all other medicine units, the proportion not administered (23.9% vs 16.3%, OR: 1.61, 95% CI: 1.49‐1.73, P<0.001) closely resembled the values seen when comparing patients with and without HIV hospital wide (23.5% vs 16.1%). However, when doses on the HIV care unit were stratified by HIV status, the doses not administered were 2‐fold greater, as a proportion, for patients with HIV compared with those without HIV (26.4% vs 13.1%, OR: 2.39, 95% CI: 1.93‐2.96, P<0.001).
| Doses Prescribed | Doses Not Administered (% of Doses Prescribed) | Doses Documented as Refused (% of All Doses Prescribed) | |
|---|---|---|---|
| |||
| All patients with HIV | 5,681 | 1,334 (23.5%)a | 935 (16.5%)a |
| All patients without HIV | 37,189 | 6,005 (16.1%) | 3,935 (10.6%) |
| HIV care unit | 4,452 | 1,063 (23.9%)a | 709 (15.9%)a |
| All other units | 38,418 | 6,276 (16.3%) | 4,161 (10.8%) |
| HIV care unit: patients with HIV | 3,602 | 952 (26.4%)a | 651 (18.1%)a |
| HIV care unit: patients without HIV | 850 | 111 (13.1%) | 58 (6.8%) |
| All other units: patients with HIV | 2,079 | 382 (18.4%)b | 284 (13.7%)a |
| All other units: patients without HIV | 36,339 | 5,894 (16.2%) | 3,877 (10.7%) |
| Nonadministered, n (%) | P | Documented as Refused, n (%) | P | |
|---|---|---|---|---|
| ||||
| Race | 0.001 | 0.072 | ||
| African American | 2,601 (17.8) | 1,708 (11.7) | ||
| Caucasian | 4,379 (16.4) | 2,922 (10.9) | ||
| Asian, Pacific Islander, other | 359 (23.4) | 240 (15.6) | ||
| HIV status | <0.001 | 0.002 | ||
| Negative | 6,005 (16.2) | 3,935 (10.6) | ||
| Positive | 1,344 (23.5) | 935 (16.5) | ||
| Age, y | <0.001 | <0.001 | ||
| 19 | 59 (20.6) | 44 (15.3) | ||
| 2029 | 1,260 (33.8) | 1,000 (26.8) | ||
| 3039 | 1,088 (28.1) | 845 (21.8) | ||
| 4049 | 1,628 (21.0) | 1,104 (14.2) | ||
| 5059 | 1,493 (16.1) | 953 (10.3) | ||
| 6069 | 900 (12.6) | 515 (7.2) | ||
| 7079 | 571 (9.6) | 250 (4.2) | ||
| 8089 | 252 (6.2) | 95 (2.3) | ||
| 90 | 88 (11.5) | 84 (8.4) | ||
| Sex | 0.372 | 0.919 | ||
| Male | 3,689 (17.3) | 2,392 (11.2) | ||
| Female | 3,650 (17.0) | 2,478 (11.5) | ||
| Drug | <0.001 | <0.001 | ||
| Heparin | 6,833 (18.4) | 4,515 (12.2) | ||
| Enoxaparin | 506 (8.9) | 355 (6.2) | ||
| Length of stay, d | <0.001 | <0.001 | ||
| 01 | 446 (24.3) | 282 (15.4) | ||
| 23 | 1,463 (19.4) | 971 (12.9) | ||
| 47 | 2,332 (18.9) | 1,620 (13.1) | ||
| 8 | 3,098 (14.6) | 1,997 (9.4) | ||
The results of the multivariate regression analyses with GEE are displayed in Table 4. HIV diagnosis, non‐African American race, and heparin (as compared with enoxaparin) were associated with increased likelihood of nonadministration. Increasing age and increasing length of stay were associated with decreased likelihood of nonadministration by a small but significant amount.
| OR of Nonadministration | 95% CI, P | OR of Documented Refusal | 95% CI, P | |
|---|---|---|---|---|
| ||||
| Race | ||||
| African American | 1.00 | Reference | 1.00 | Reference |
| Caucasian | 1.62 | 1.44‐1.81, <0.001 | 1.53 | 1.32‐1.77, <0.001 |
| Asian, Pacific Islander, Other | 1.54 | 1.19‐2.00, 0.001 | 1.48 | 1.07‐2.01, 0.019 |
| HIV status | ||||
| Negative | 1.00 | Reference | 1.00 | Reference |
| Positive | 1.21 | 1.001.45, 0.039 | 1.29 | 1.06‐1.56, 0.012 |
| Age, per year | 0.97 | 0.97‐0.98, <0.001 | 0.97 | 0.96‐0.97, <0.001 |
| Drug | ||||
| Heparin | 1.00 | Reference | 1.00 | Reference |
| Enoxaparin | 0.45 | 0.40‐0.51, <0.001 | 0.53 | 0.47‐0.61, <0.001 |
| Length of stay, per day | 0.991 | 0.987‐0.995, <0.001 | 0.989 | 0.983‐0.993, <0.001 |
The most commonly documented reason for nonadministration was refusal by the patient or family member (66% of all doses not administered). The second most common reason, patient condition not appropriate, accounted for an additional 10% of doses. Across all nursing units, the proportion of prescribed doses that were documented as refused was significantly greater for patients with HIV compared with patients without HIV (16.5% vs 10.6%, OR: 1.66, 95% CI: 1.54‐1.80, P<0.0001) (Table 2). Using the GEE and multivariate regression, HIV diagnosis, non‐African American race, and heparin were associated with increased risk of documented dose refusal. Age and length of stay were inversely related to the likelihood of documented dose refusal. When all administered doses were excluded from the analysis, the association between these variables and documented dose refusal were not as strong. Age and length of stay remained significantly inversely related; however, the other factors were no longer significantly positively associated with documented dose refusal.
Within the HIV care unit, the proportion of prescribed doses documented as refused was greater for patients with HIV compared with patients without HIV (18.1% vs 6.8%, OR: 3.01, 95% CI: 2.28‐3.99, P<0.0001). For all other medicine units, the proportion of nonadministered doses documented as refused was also greater for patients with HIV compared with patients without HIV (13.7% vs 10.7%, OR: 1.32, 95% CI: 1.16‐1.51, P<0.0001).
DISCUSSION
We have identified that nonadministration of thromboprophylaxis was more common among patients with HIV at our institution. Substantial variation in the proportion of doses not administered existed on the nursing unit level, as well as within each unit when stratified by HIV status. This disparity in dose administration was observed on the HIV care unit as well, as the proportion not administered was about 2‐fold greater for patients with HIV compared with those without HIV. Documented dose refusal appeared to account for the majority of nonadministered doses in our cohort. Our analysis also demonstrated that HIV diagnosis is significantly associated with both dose nonadministration and documented dose refusal at our institution.
Medication refusal is a well‐recognized phenomenon among hospitalized patients. A recent study of medication administration in hospitalized patients in the United Kingdom noted that refusal accounted for about 45% of omitted doses.[26] Fanikos et al. also found that documented refusal of doses contributed significantly to the overall number of VTE prophylaxis doses not administered to patients.[27] In our study, the proportion of nonadministered doses documented as refused by the patient or family member was significantly greater in patients with HIV than in patients without HIV across all units. Interestingly, the difference was greater on the HIV care unit when doses were stratified by HIV status. This observation leads us to hypothesize that specific hospital care environments may influence dose nonadministration and refusal rates among our patient population.
Based on regression analyses, increasing age and length of stay were associated with a decreased likelihood of any particular dose not being administered and with any particular dose being documented as refused. It is important to note that our GEE did not take into account date or time of each dose, and therefore we cannot make conclusions as to the likelihood of dose nonadministration or refusal of doses in relation to each other on a time scale. One cannot assume that a dose due later in a hospital course was more or less likely to be given than a dose due on the first hospital day. Although we did not expect these findings, one can hypothesize that patients who are older or have longer stays may be perceived to have more severe illness, and therefore greater need for prophylaxis, from nursing staff and others involved in their care. The associations were small but significant and warrant future investigation.
To our knowledge, this is the first investigation comparing the proportion of nonadministered doses of thromboprophylaxis between patients with and without HIV. Our data show that nonadministered doses and refused doses of thromboprophylaxis are more frequent among patients with HIV. In addition, we noted that nonadministration was more common on the dedicated HIV care unit compared with other units. We cannot currently offer a clear explanation for the disparity observed between units, and more specifically, within the HIV care unit. However, it is possible that a unique culture of care and provider‐specific factors may contribute.
Our study was limited by a number of factors. Seroconversion among patients during the study period was possible; however, our analysis revealed only 2 instances among nearly 4000 unique patients. A more significant limitation was the level of analysis allowed by the dataset. We examined dose characteristics on a dose and unit level, but the ability to analyze doses based on the prescriber and nurse level may have provided valuable insight into the specific reasons behind the observations presented here. Additionally, the specific unit assigned to a given dose in our dataset represented the discharge location for the corresponding patient, making it possible that some amount of nonadministered doses may be attributed to the incorrect unit. However, we do not believe that unit‐to‐unit transfers would be frequent enough to influence the overall results. In addition, we did not link nonadministration of thromboprophylaxis with VTE events, as these data were not present in the current dataset. Although this is a limitation of the current study, we believe that the notion that missed doses of thromboprophylaxis place patients at higher risk for VTE is plausible, as the efficacy of thromboprophylaxis is well established.[28, 29, 30] It is important to note that the reason for nonadministration selected by the nurse on the eMAR may not always represent the only reason or even the true reason for dose nonadministration. It is possible that dose refusal may be over‐represented in our sample, in part due to inaccurate documentation. Recent investigations at JHH have identified varying attitudes on the part of the patient and the nurse regarding thromboprophylaxis. A questionnaire and interview of patients showed a large knowledge gap regarding thromboprophylaxis, with many individuals unable to explain its role or significance in their medical care.[31] A common theme was also observed in a survey of nurses regarding VTE prophylaxis: doses were sometimes considered optional for reasons such as ambulation status, perceived severity of illness, or reason for hospitalization. Some nurses also reported that after an initial refused dose, they may continue to document subsequent doses as refused, sometimes without offering the dose to the patient.[32] As variation in practice was observed between individual nurses, it is also likely that the culture of care may vary between units, influencing thromboprophylaxis nonadministration rates as well as documentation of doses as refused. The dose‐level data used for the GEE analyses did not include date and time of administration, which limited the ability of the GEE to more completely account for autocorrelation.
To further investigate the findings of this and related studies, we intend to more closely analyze data at multiple levels with the goal of identifying an appropriate and feasible target for intervention. Additionally, further investigation should be performed with the goal of determining the relationship between decreased exposure to thromboprophylaxis and VTE. However, as patients with HIV appear to be at increased risk of VTE, ensuring that thromboprophylaxis is delivered appropriately and consistently should be an important goal for all who provide care to this population.
- , , , , . Venous thromboembolism in patients with HIV/AIDS: a case‐control study. J Acquir Immune Defic Syndr. 2008;48(3):310–314.
- , , . AIDS and thrombosis: retrospective study of 131 HIV‐infected patients. AIDS Patient Care STDS. 2001;15(6):311–320.
- , , , et al. HIV and risk of venous thromboembolism: a Danish nationwide population‐based cohort study. HIV Med. 2011;12(4):202–210.
- , , , . Epidemiology of thrombosis in HIV‐infected individuals. The adult/adolescent spectrum of HIV disease project. AIDS. 2000;14(3):321–324.
- , , . Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis. 2004;39(8):1214–1222.
- , , . Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(2):175–180.
- , . The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044.
- , , , . Acquired protein C and protein S deficiency in HIV‐infected patients. Clin Appl Thromb Hemost. 2003;9(4):325–331.
- , , , et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24(2):197–200.
- , . Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263.
- , . AIDS‐defining and non‐AIDS‐defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446–451.
- , , , , . Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29–38.
- , , , et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV‐positive adults in 2001. Med Care. 2005;43(9 suppl):III3–III14.
- , , , et al. Opportunistic infections as causes of death in HIV‐infected patients in the HAART era in France. Scand J Infect Dis. 2005;37(6‐7):482–487.
- , , , et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354.
- , , . Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40(5):609–616.
- , , , , , . Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155.
- , , , et al. Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.
- , , , et al. Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLoS Biol. 2004;2(2):E20.
- , , , , . CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–3406.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , . Practices to prevent venous thromboembolism [published online ahead of print May 24, 2013]. BMJ Qual Saf. doi:10.1136/bmjqs‐2012‐001782.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Lessons from The Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events [published online ahead of print April 4, 2013]. Am J Hematol. doi: 10.1002/ajh.23450.
- , , . Dose omissions in hospitalized patients in a UK hospital: an analysis of the relative contribution of adverse drug reactions. Drug Saf. 2012;35(8):677–683.
- , , , et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123(6):536–541.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , et al. Patient perspectives on pharmacological venous thromboembolism prophylaxis at The Johns Hopkins Hospital. J Thromb Thrombolysis. 2013;35(3):416.
- , , , et al. Culture of care and documented patient refusal of pharmacologic venous thromboembolism prophylaxis. J Thromb Thrombolysis. 2011;31(3):367–400.
Patients with human immunodeficiency virus (HIV) are at a 2‐ to 10‐fold greater risk for venous thromboembolism (VTE) compared with the general population.[1] Although antiphospholipid antibodies and protein S deficiency have often been cited as reasons for the thrombophilia associated with HIV, previous studies have also documented an increased risk of VTE with declining CD4+ cell count.[2, 3, 4, 5, 6, 7, 8] Worsening immune function places HIV patients at increased risk for opportunistic and nonopportunistic infections and malignancies, all independently associated with an increased risk of VTE.[5, 9, 10, 11, 12] Although increasing use of antiretroviral therapy has greatly decreased these sequelae, these complications of HIV infection are associated with an increased frequency of hospitalization.[13, 14, 15, 16] HIV infection and associated inflammation has been implicated in cardiovascular conditions such as cardiomyopathy, pulmonary hypertension, and myocardial infarction.[17, 18] Additionally, progression of HIV infection appears to influence T‐cell activation and differentiation in a manner that leads to early immunosenescence in infected individuals.[19, 20]
VTE prophylaxis is effective.[21] Virtually all efforts to decrease VTE have been focused on improving the prescription of prophylaxis with varying degrees of success.[22] These interventions have been employed with the tacit assumption that medication prescribed for inpatients will always be administered. However, at our institution, recent research has demonstrated that a significant proportion of prescribed thromboprophylaxis doses are not administered to hospitalized patients.[23] Refusal by the patient or a family member was the most commonly documented reason for dose nonadministration. In addition, the rate of thromboprophylaxis nonadministration varied greatly between nursing units with distinct patient populations. We hypothesized that nonadministration of VTE prophylaxis may be more common in patients with HIV, and this phenomenon may contribute to their increased risk for VTE.
The purpose of this study was to determine if the proportion of nonadministered thromboprophylaxis is greater among hospitalized patients with HIV and to characterize documented reasons for dose nonadministration.
METHODS
This study was conducted at The Johns Hopkins Hospital (JHH), a large, urban, academic medical center in Baltimore, Maryland. This single‐center retrospective cohort study utilized an existing dataset containing dose administration data extracted from an electronic medication administration record (eMAR). This dataset included information for all prescribed doses of thromboprophylaxis (heparin 5000 U subcutaneously every 8 or 12 hours, heparin 7500 U subcutaneously every 12 hours, enoxaparin 30 mg subcutaneously every 12 hours, or enoxaparin 40 mg subcutaneously daily) for patients hospitalized on medicine units at JHH from November 2007 to December 2008. This time period follows the implementation of an electronic order set for VTE prophylaxis.[24, 25] Data available for each dose included drug name, dose, frequency, patient demographics, and whether or not the dose was administered. Each dose not administered included a reason for nonadministration, which was chosen from a dropdown menu of responses on the eMAR by the nurse at the time the dose was due. A separate electronic report was obtained from an internal administrative database, which identified all patients within the dose administration dataset who had the International Classification of Diseases, 9th Revision code 042 (HIV diagnosis). A report identifying patient history numbers with matching diagnostic code for HIV was appended to the dose administration dataset using a relational database (Microsoft Access; Microsoft Corp., Redmond, WA) prior to analysis. The dose administration data were obtained previously for a separate analysis.[23] Approval for this study was granted from the institutional review board of Johns Hopkins Medicine.
Our analytic plan included comparisons between patients with and without HIV on a dose, patient, and unit level. As JHH operates a nursing unit dedicated to the inpatient care of patients with HIV, we included analyses of dose characteristics between this unit and other medicine units. It should be noted that patients without a diagnosis of HIV are sometimes cared for on this unit. Therefore, the electronic medical record for each patient without the diagnosis code for HIV hospitalized on this unit was reviewed to determine HIV status. An analysis was performed comparing visit identification numbers with diagnosis codes to identify potential seroconversions during the study period. Although we planned to compare nonadministration and documented refusal of doses on the unit level, a lack of patients with HIV on a number of units limited our ability to perform these analyses.
Statistical Analysis
The percent of doses not administered was calculated as the number of doses not administered divided by the number of doses prescribed. Likewise, the percent of prescribed doses documented as refused was calculated as the number of prescribed doses documented as refused divided by the number of doses prescribed. For each comparison, an odds ratio (OR) with 95% confidence interval (CI) was reported. Univariate and multivariate regression analyses were performed to assess the relationship between patient factors and dose nonadministration and documented refusal, respectively. Generalized estimating equations (GEE) using a logit link and an exchangeable correlation structure were used in these analyses. The GEE technique was used to account for within‐individual correlation of administration and documented refusal status.
Categorical data were compared using the two‐sided [2] test. Parametric and nonparametric continuous data were compared using the Student t test and Mann‐Whitney U test, respectively. A P value of <0.05 was considered statistically significant for all analyses. Analyses were performed using Minitab 15 (Minitab Inc., State College, PA) and Stata (StataCorp, College Station, TX).
RESULTS
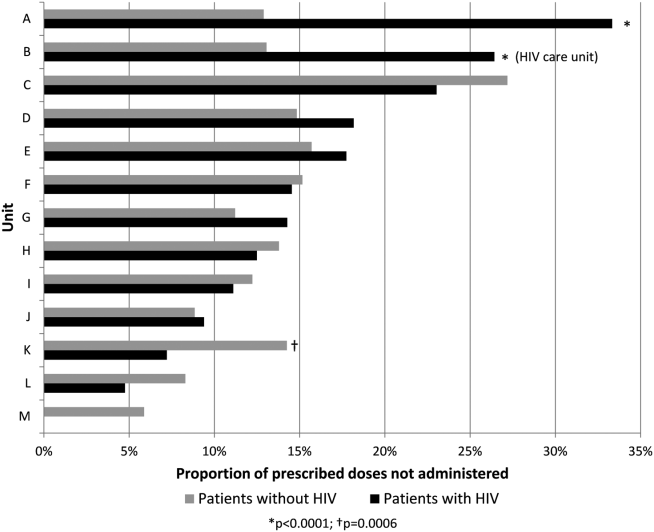
During the 8‐month study period, 42,870 doses of thromboprophylaxis were prescribed during 4947 patient admissions to 13 individual medicine units. Overall, the diagnosis code for HIV was present in 12% of patient visits. The proportion of nonadministered doses per unit ranged from 6% to 27%, whereas the number of doses prescribed per unit ranged from 34 to 7301.
Patient characteristics were described on the visit level (Table 1). Patients with HIV were significantly younger, more often male and black, and had a longer length of stay compared with patients without HIV. Patients hospitalized on the HIV care unit had similar characteristics to the overall population of patients with HIV. It should be noted that not all patients cared for on this unit had a diagnosis of HIV, as patients from other medicine services are sometimes cared for in this location.
| Patients Without HIV | Patients With HIV | P | |
|---|---|---|---|
| |||
| Visits, n | 4,364 | 583 | N/A |
| Male, n (%) | 2,039 (47) | 370 (64) | <0.001 |
| Mean ageSD, y | 5618 | 469 | <0.001 |
| Race, n (%) | |||
| African American | 2,603 (60) | 522 (90) | <0.001 |
| Caucasian | 1,610 (37) | 53 (9) | <0.001 |
| Asian, Pacific Islander, other | 151 (4) | 8 (1) | 0.006 |
| Median length of stay (IQR), d | 3 (15) | 4 (27) | 0.002 |
| Marital status, n (%) | |||
| Single | 2,051 (47) | 471 (81) | <0.001 |
| Married | 1,405 (32) | 71 (12) | <0.001 |
| Widowed | 486 (11) | 10 (1) | <0.001 |
| Divorced | 402 (9) | 28 (5) | <0.001 |
| Separated | 33 (1) | 3 (1) | 0.607 |
| Unknown | 5 (0) | 0 (0) | 0.465 |
| Payor, n (%) | |||
| Medicare | 1,771 (41) | 133 (23) | <0.001 |
| Medicaid | 1,343 (31) | 392 (67) | <0.001 |
| Commercial | 1,181 (27) | 43 (7) | <0.001 |
| Other including self‐pay | 69 (1) | 15 (3) | 0.087 |
Overall, 17% of prescribed prophylaxis doses were not administered. A greater proportion of prescribed doses were not administered to patients with HIV compared with patients without HIV (23.5% vs 16.1%, OR: 1.59, 95% CI: 1.49‐1.70, P<0.001) (Table 2). Using a GEE and univariate regression, HIV diagnosis was associated with nonadministration of doses (OR: 1.37, 95% CI: 1.17‐1.60, P<0.001) (Table 3). Race, age, length of stay, and drug (heparin vs enoxaparin) were each associated with nonadministration. There was no significant association between nonadministration and sex, marital status, or payor. When stratified by nursing unit, there was substantial variation in the proportion of nonadministered doses between units. Within each unit, the proportion of doses not administered varied when stratified by HIV status. For example, on unit A, the proportion of doses not administered was greater for patients with HIV compared with patients without HIV (33.3% vs 12.9%, OR: 3.38, 95% CI: 2.61 to 4.37, P<0.001) (Figure 1). However, on unit K, the proportion of doses not administered to patients with HIV was 2‐fold less than in patients without HIV (7.2% vs 14.3%, OR: 0.47, 95% CI: 0.30‐0.74, P<0.001). Unit‐level analysis was not possible in regression models due to drastic imbalance in the prevalence of HIV across units. When comparing doses prescribed in the HIV care unit to all other medicine units, the proportion not administered (23.9% vs 16.3%, OR: 1.61, 95% CI: 1.49‐1.73, P<0.001) closely resembled the values seen when comparing patients with and without HIV hospital wide (23.5% vs 16.1%). However, when doses on the HIV care unit were stratified by HIV status, the doses not administered were 2‐fold greater, as a proportion, for patients with HIV compared with those without HIV (26.4% vs 13.1%, OR: 2.39, 95% CI: 1.93‐2.96, P<0.001).
| Doses Prescribed | Doses Not Administered (% of Doses Prescribed) | Doses Documented as Refused (% of All Doses Prescribed) | |
|---|---|---|---|
| |||
| All patients with HIV | 5,681 | 1,334 (23.5%)a | 935 (16.5%)a |
| All patients without HIV | 37,189 | 6,005 (16.1%) | 3,935 (10.6%) |
| HIV care unit | 4,452 | 1,063 (23.9%)a | 709 (15.9%)a |
| All other units | 38,418 | 6,276 (16.3%) | 4,161 (10.8%) |
| HIV care unit: patients with HIV | 3,602 | 952 (26.4%)a | 651 (18.1%)a |
| HIV care unit: patients without HIV | 850 | 111 (13.1%) | 58 (6.8%) |
| All other units: patients with HIV | 2,079 | 382 (18.4%)b | 284 (13.7%)a |
| All other units: patients without HIV | 36,339 | 5,894 (16.2%) | 3,877 (10.7%) |
| Nonadministered, n (%) | P | Documented as Refused, n (%) | P | |
|---|---|---|---|---|
| ||||
| Race | 0.001 | 0.072 | ||
| African American | 2,601 (17.8) | 1,708 (11.7) | ||
| Caucasian | 4,379 (16.4) | 2,922 (10.9) | ||
| Asian, Pacific Islander, other | 359 (23.4) | 240 (15.6) | ||
| HIV status | <0.001 | 0.002 | ||
| Negative | 6,005 (16.2) | 3,935 (10.6) | ||
| Positive | 1,344 (23.5) | 935 (16.5) | ||
| Age, y | <0.001 | <0.001 | ||
| 19 | 59 (20.6) | 44 (15.3) | ||
| 2029 | 1,260 (33.8) | 1,000 (26.8) | ||
| 3039 | 1,088 (28.1) | 845 (21.8) | ||
| 4049 | 1,628 (21.0) | 1,104 (14.2) | ||
| 5059 | 1,493 (16.1) | 953 (10.3) | ||
| 6069 | 900 (12.6) | 515 (7.2) | ||
| 7079 | 571 (9.6) | 250 (4.2) | ||
| 8089 | 252 (6.2) | 95 (2.3) | ||
| 90 | 88 (11.5) | 84 (8.4) | ||
| Sex | 0.372 | 0.919 | ||
| Male | 3,689 (17.3) | 2,392 (11.2) | ||
| Female | 3,650 (17.0) | 2,478 (11.5) | ||
| Drug | <0.001 | <0.001 | ||
| Heparin | 6,833 (18.4) | 4,515 (12.2) | ||
| Enoxaparin | 506 (8.9) | 355 (6.2) | ||
| Length of stay, d | <0.001 | <0.001 | ||
| 01 | 446 (24.3) | 282 (15.4) | ||
| 23 | 1,463 (19.4) | 971 (12.9) | ||
| 47 | 2,332 (18.9) | 1,620 (13.1) | ||
| 8 | 3,098 (14.6) | 1,997 (9.4) | ||

The results of the multivariate regression analyses with GEE are displayed in Table 4. HIV diagnosis, non‐African American race, and heparin (as compared with enoxaparin) were associated with increased likelihood of nonadministration. Increasing age and increasing length of stay were associated with decreased likelihood of nonadministration by a small but significant amount.
| OR of Nonadministration | 95% CI, P | OR of Documented Refusal | 95% CI, P | |
|---|---|---|---|---|
| ||||
| Race | ||||
| African American | 1.00 | Reference | 1.00 | Reference |
| Caucasian | 1.62 | 1.44‐1.81, <0.001 | 1.53 | 1.32‐1.77, <0.001 |
| Asian, Pacific Islander, Other | 1.54 | 1.19‐2.00, 0.001 | 1.48 | 1.07‐2.01, 0.019 |
| HIV status | ||||
| Negative | 1.00 | Reference | 1.00 | Reference |
| Positive | 1.21 | 1.001.45, 0.039 | 1.29 | 1.06‐1.56, 0.012 |
| Age, per year | 0.97 | 0.97‐0.98, <0.001 | 0.97 | 0.96‐0.97, <0.001 |
| Drug | ||||
| Heparin | 1.00 | Reference | 1.00 | Reference |
| Enoxaparin | 0.45 | 0.40‐0.51, <0.001 | 0.53 | 0.47‐0.61, <0.001 |
| Length of stay, per day | 0.991 | 0.987‐0.995, <0.001 | 0.989 | 0.983‐0.993, <0.001 |
The most commonly documented reason for nonadministration was refusal by the patient or family member (66% of all doses not administered). The second most common reason, patient condition not appropriate, accounted for an additional 10% of doses. Across all nursing units, the proportion of prescribed doses that were documented as refused was significantly greater for patients with HIV compared with patients without HIV (16.5% vs 10.6%, OR: 1.66, 95% CI: 1.54‐1.80, P<0.0001) (Table 2). Using the GEE and multivariate regression, HIV diagnosis, non‐African American race, and heparin were associated with increased risk of documented dose refusal. Age and length of stay were inversely related to the likelihood of documented dose refusal. When all administered doses were excluded from the analysis, the association between these variables and documented dose refusal were not as strong. Age and length of stay remained significantly inversely related; however, the other factors were no longer significantly positively associated with documented dose refusal.
Within the HIV care unit, the proportion of prescribed doses documented as refused was greater for patients with HIV compared with patients without HIV (18.1% vs 6.8%, OR: 3.01, 95% CI: 2.28‐3.99, P<0.0001). For all other medicine units, the proportion of nonadministered doses documented as refused was also greater for patients with HIV compared with patients without HIV (13.7% vs 10.7%, OR: 1.32, 95% CI: 1.16‐1.51, P<0.0001).
DISCUSSION
We have identified that nonadministration of thromboprophylaxis was more common among patients with HIV at our institution. Substantial variation in the proportion of doses not administered existed on the nursing unit level, as well as within each unit when stratified by HIV status. This disparity in dose administration was observed on the HIV care unit as well, as the proportion not administered was about 2‐fold greater for patients with HIV compared with those without HIV. Documented dose refusal appeared to account for the majority of nonadministered doses in our cohort. Our analysis also demonstrated that HIV diagnosis is significantly associated with both dose nonadministration and documented dose refusal at our institution.
Medication refusal is a well‐recognized phenomenon among hospitalized patients. A recent study of medication administration in hospitalized patients in the United Kingdom noted that refusal accounted for about 45% of omitted doses.[26] Fanikos et al. also found that documented refusal of doses contributed significantly to the overall number of VTE prophylaxis doses not administered to patients.[27] In our study, the proportion of nonadministered doses documented as refused by the patient or family member was significantly greater in patients with HIV than in patients without HIV across all units. Interestingly, the difference was greater on the HIV care unit when doses were stratified by HIV status. This observation leads us to hypothesize that specific hospital care environments may influence dose nonadministration and refusal rates among our patient population.
Based on regression analyses, increasing age and length of stay were associated with a decreased likelihood of any particular dose not being administered and with any particular dose being documented as refused. It is important to note that our GEE did not take into account date or time of each dose, and therefore we cannot make conclusions as to the likelihood of dose nonadministration or refusal of doses in relation to each other on a time scale. One cannot assume that a dose due later in a hospital course was more or less likely to be given than a dose due on the first hospital day. Although we did not expect these findings, one can hypothesize that patients who are older or have longer stays may be perceived to have more severe illness, and therefore greater need for prophylaxis, from nursing staff and others involved in their care. The associations were small but significant and warrant future investigation.
To our knowledge, this is the first investigation comparing the proportion of nonadministered doses of thromboprophylaxis between patients with and without HIV. Our data show that nonadministered doses and refused doses of thromboprophylaxis are more frequent among patients with HIV. In addition, we noted that nonadministration was more common on the dedicated HIV care unit compared with other units. We cannot currently offer a clear explanation for the disparity observed between units, and more specifically, within the HIV care unit. However, it is possible that a unique culture of care and provider‐specific factors may contribute.
Our study was limited by a number of factors. Seroconversion among patients during the study period was possible; however, our analysis revealed only 2 instances among nearly 4000 unique patients. A more significant limitation was the level of analysis allowed by the dataset. We examined dose characteristics on a dose and unit level, but the ability to analyze doses based on the prescriber and nurse level may have provided valuable insight into the specific reasons behind the observations presented here. Additionally, the specific unit assigned to a given dose in our dataset represented the discharge location for the corresponding patient, making it possible that some amount of nonadministered doses may be attributed to the incorrect unit. However, we do not believe that unit‐to‐unit transfers would be frequent enough to influence the overall results. In addition, we did not link nonadministration of thromboprophylaxis with VTE events, as these data were not present in the current dataset. Although this is a limitation of the current study, we believe that the notion that missed doses of thromboprophylaxis place patients at higher risk for VTE is plausible, as the efficacy of thromboprophylaxis is well established.[28, 29, 30] It is important to note that the reason for nonadministration selected by the nurse on the eMAR may not always represent the only reason or even the true reason for dose nonadministration. It is possible that dose refusal may be over‐represented in our sample, in part due to inaccurate documentation. Recent investigations at JHH have identified varying attitudes on the part of the patient and the nurse regarding thromboprophylaxis. A questionnaire and interview of patients showed a large knowledge gap regarding thromboprophylaxis, with many individuals unable to explain its role or significance in their medical care.[31] A common theme was also observed in a survey of nurses regarding VTE prophylaxis: doses were sometimes considered optional for reasons such as ambulation status, perceived severity of illness, or reason for hospitalization. Some nurses also reported that after an initial refused dose, they may continue to document subsequent doses as refused, sometimes without offering the dose to the patient.[32] As variation in practice was observed between individual nurses, it is also likely that the culture of care may vary between units, influencing thromboprophylaxis nonadministration rates as well as documentation of doses as refused. The dose‐level data used for the GEE analyses did not include date and time of administration, which limited the ability of the GEE to more completely account for autocorrelation.
To further investigate the findings of this and related studies, we intend to more closely analyze data at multiple levels with the goal of identifying an appropriate and feasible target for intervention. Additionally, further investigation should be performed with the goal of determining the relationship between decreased exposure to thromboprophylaxis and VTE. However, as patients with HIV appear to be at increased risk of VTE, ensuring that thromboprophylaxis is delivered appropriately and consistently should be an important goal for all who provide care to this population.
Patients with human immunodeficiency virus (HIV) are at a 2‐ to 10‐fold greater risk for venous thromboembolism (VTE) compared with the general population.[1] Although antiphospholipid antibodies and protein S deficiency have often been cited as reasons for the thrombophilia associated with HIV, previous studies have also documented an increased risk of VTE with declining CD4+ cell count.[2, 3, 4, 5, 6, 7, 8] Worsening immune function places HIV patients at increased risk for opportunistic and nonopportunistic infections and malignancies, all independently associated with an increased risk of VTE.[5, 9, 10, 11, 12] Although increasing use of antiretroviral therapy has greatly decreased these sequelae, these complications of HIV infection are associated with an increased frequency of hospitalization.[13, 14, 15, 16] HIV infection and associated inflammation has been implicated in cardiovascular conditions such as cardiomyopathy, pulmonary hypertension, and myocardial infarction.[17, 18] Additionally, progression of HIV infection appears to influence T‐cell activation and differentiation in a manner that leads to early immunosenescence in infected individuals.[19, 20]
VTE prophylaxis is effective.[21] Virtually all efforts to decrease VTE have been focused on improving the prescription of prophylaxis with varying degrees of success.[22] These interventions have been employed with the tacit assumption that medication prescribed for inpatients will always be administered. However, at our institution, recent research has demonstrated that a significant proportion of prescribed thromboprophylaxis doses are not administered to hospitalized patients.[23] Refusal by the patient or a family member was the most commonly documented reason for dose nonadministration. In addition, the rate of thromboprophylaxis nonadministration varied greatly between nursing units with distinct patient populations. We hypothesized that nonadministration of VTE prophylaxis may be more common in patients with HIV, and this phenomenon may contribute to their increased risk for VTE.
The purpose of this study was to determine if the proportion of nonadministered thromboprophylaxis is greater among hospitalized patients with HIV and to characterize documented reasons for dose nonadministration.
METHODS
This study was conducted at The Johns Hopkins Hospital (JHH), a large, urban, academic medical center in Baltimore, Maryland. This single‐center retrospective cohort study utilized an existing dataset containing dose administration data extracted from an electronic medication administration record (eMAR). This dataset included information for all prescribed doses of thromboprophylaxis (heparin 5000 U subcutaneously every 8 or 12 hours, heparin 7500 U subcutaneously every 12 hours, enoxaparin 30 mg subcutaneously every 12 hours, or enoxaparin 40 mg subcutaneously daily) for patients hospitalized on medicine units at JHH from November 2007 to December 2008. This time period follows the implementation of an electronic order set for VTE prophylaxis.[24, 25] Data available for each dose included drug name, dose, frequency, patient demographics, and whether or not the dose was administered. Each dose not administered included a reason for nonadministration, which was chosen from a dropdown menu of responses on the eMAR by the nurse at the time the dose was due. A separate electronic report was obtained from an internal administrative database, which identified all patients within the dose administration dataset who had the International Classification of Diseases, 9th Revision code 042 (HIV diagnosis). A report identifying patient history numbers with matching diagnostic code for HIV was appended to the dose administration dataset using a relational database (Microsoft Access; Microsoft Corp., Redmond, WA) prior to analysis. The dose administration data were obtained previously for a separate analysis.[23] Approval for this study was granted from the institutional review board of Johns Hopkins Medicine.
Our analytic plan included comparisons between patients with and without HIV on a dose, patient, and unit level. As JHH operates a nursing unit dedicated to the inpatient care of patients with HIV, we included analyses of dose characteristics between this unit and other medicine units. It should be noted that patients without a diagnosis of HIV are sometimes cared for on this unit. Therefore, the electronic medical record for each patient without the diagnosis code for HIV hospitalized on this unit was reviewed to determine HIV status. An analysis was performed comparing visit identification numbers with diagnosis codes to identify potential seroconversions during the study period. Although we planned to compare nonadministration and documented refusal of doses on the unit level, a lack of patients with HIV on a number of units limited our ability to perform these analyses.
Statistical Analysis
The percent of doses not administered was calculated as the number of doses not administered divided by the number of doses prescribed. Likewise, the percent of prescribed doses documented as refused was calculated as the number of prescribed doses documented as refused divided by the number of doses prescribed. For each comparison, an odds ratio (OR) with 95% confidence interval (CI) was reported. Univariate and multivariate regression analyses were performed to assess the relationship between patient factors and dose nonadministration and documented refusal, respectively. Generalized estimating equations (GEE) using a logit link and an exchangeable correlation structure were used in these analyses. The GEE technique was used to account for within‐individual correlation of administration and documented refusal status.
Categorical data were compared using the two‐sided [2] test. Parametric and nonparametric continuous data were compared using the Student t test and Mann‐Whitney U test, respectively. A P value of <0.05 was considered statistically significant for all analyses. Analyses were performed using Minitab 15 (Minitab Inc., State College, PA) and Stata (StataCorp, College Station, TX).
RESULTS
During the 8‐month study period, 42,870 doses of thromboprophylaxis were prescribed during 4947 patient admissions to 13 individual medicine units. Overall, the diagnosis code for HIV was present in 12% of patient visits. The proportion of nonadministered doses per unit ranged from 6% to 27%, whereas the number of doses prescribed per unit ranged from 34 to 7301.
Patient characteristics were described on the visit level (Table 1). Patients with HIV were significantly younger, more often male and black, and had a longer length of stay compared with patients without HIV. Patients hospitalized on the HIV care unit had similar characteristics to the overall population of patients with HIV. It should be noted that not all patients cared for on this unit had a diagnosis of HIV, as patients from other medicine services are sometimes cared for in this location.
| Patients Without HIV | Patients With HIV | P | |
|---|---|---|---|
| |||
| Visits, n | 4,364 | 583 | N/A |
| Male, n (%) | 2,039 (47) | 370 (64) | <0.001 |
| Mean ageSD, y | 5618 | 469 | <0.001 |
| Race, n (%) | |||
| African American | 2,603 (60) | 522 (90) | <0.001 |
| Caucasian | 1,610 (37) | 53 (9) | <0.001 |
| Asian, Pacific Islander, other | 151 (4) | 8 (1) | 0.006 |
| Median length of stay (IQR), d | 3 (15) | 4 (27) | 0.002 |
| Marital status, n (%) | |||
| Single | 2,051 (47) | 471 (81) | <0.001 |
| Married | 1,405 (32) | 71 (12) | <0.001 |
| Widowed | 486 (11) | 10 (1) | <0.001 |
| Divorced | 402 (9) | 28 (5) | <0.001 |
| Separated | 33 (1) | 3 (1) | 0.607 |
| Unknown | 5 (0) | 0 (0) | 0.465 |
| Payor, n (%) | |||
| Medicare | 1,771 (41) | 133 (23) | <0.001 |
| Medicaid | 1,343 (31) | 392 (67) | <0.001 |
| Commercial | 1,181 (27) | 43 (7) | <0.001 |
| Other including self‐pay | 69 (1) | 15 (3) | 0.087 |
Overall, 17% of prescribed prophylaxis doses were not administered. A greater proportion of prescribed doses were not administered to patients with HIV compared with patients without HIV (23.5% vs 16.1%, OR: 1.59, 95% CI: 1.49‐1.70, P<0.001) (Table 2). Using a GEE and univariate regression, HIV diagnosis was associated with nonadministration of doses (OR: 1.37, 95% CI: 1.17‐1.60, P<0.001) (Table 3). Race, age, length of stay, and drug (heparin vs enoxaparin) were each associated with nonadministration. There was no significant association between nonadministration and sex, marital status, or payor. When stratified by nursing unit, there was substantial variation in the proportion of nonadministered doses between units. Within each unit, the proportion of doses not administered varied when stratified by HIV status. For example, on unit A, the proportion of doses not administered was greater for patients with HIV compared with patients without HIV (33.3% vs 12.9%, OR: 3.38, 95% CI: 2.61 to 4.37, P<0.001) (Figure 1). However, on unit K, the proportion of doses not administered to patients with HIV was 2‐fold less than in patients without HIV (7.2% vs 14.3%, OR: 0.47, 95% CI: 0.30‐0.74, P<0.001). Unit‐level analysis was not possible in regression models due to drastic imbalance in the prevalence of HIV across units. When comparing doses prescribed in the HIV care unit to all other medicine units, the proportion not administered (23.9% vs 16.3%, OR: 1.61, 95% CI: 1.49‐1.73, P<0.001) closely resembled the values seen when comparing patients with and without HIV hospital wide (23.5% vs 16.1%). However, when doses on the HIV care unit were stratified by HIV status, the doses not administered were 2‐fold greater, as a proportion, for patients with HIV compared with those without HIV (26.4% vs 13.1%, OR: 2.39, 95% CI: 1.93‐2.96, P<0.001).
| Doses Prescribed | Doses Not Administered (% of Doses Prescribed) | Doses Documented as Refused (% of All Doses Prescribed) | |
|---|---|---|---|
| |||
| All patients with HIV | 5,681 | 1,334 (23.5%)a | 935 (16.5%)a |
| All patients without HIV | 37,189 | 6,005 (16.1%) | 3,935 (10.6%) |
| HIV care unit | 4,452 | 1,063 (23.9%)a | 709 (15.9%)a |
| All other units | 38,418 | 6,276 (16.3%) | 4,161 (10.8%) |
| HIV care unit: patients with HIV | 3,602 | 952 (26.4%)a | 651 (18.1%)a |
| HIV care unit: patients without HIV | 850 | 111 (13.1%) | 58 (6.8%) |
| All other units: patients with HIV | 2,079 | 382 (18.4%)b | 284 (13.7%)a |
| All other units: patients without HIV | 36,339 | 5,894 (16.2%) | 3,877 (10.7%) |
| Nonadministered, n (%) | P | Documented as Refused, n (%) | P | |
|---|---|---|---|---|
| ||||
| Race | 0.001 | 0.072 | ||
| African American | 2,601 (17.8) | 1,708 (11.7) | ||
| Caucasian | 4,379 (16.4) | 2,922 (10.9) | ||
| Asian, Pacific Islander, other | 359 (23.4) | 240 (15.6) | ||
| HIV status | <0.001 | 0.002 | ||
| Negative | 6,005 (16.2) | 3,935 (10.6) | ||
| Positive | 1,344 (23.5) | 935 (16.5) | ||
| Age, y | <0.001 | <0.001 | ||
| 19 | 59 (20.6) | 44 (15.3) | ||
| 2029 | 1,260 (33.8) | 1,000 (26.8) | ||
| 3039 | 1,088 (28.1) | 845 (21.8) | ||
| 4049 | 1,628 (21.0) | 1,104 (14.2) | ||
| 5059 | 1,493 (16.1) | 953 (10.3) | ||
| 6069 | 900 (12.6) | 515 (7.2) | ||
| 7079 | 571 (9.6) | 250 (4.2) | ||
| 8089 | 252 (6.2) | 95 (2.3) | ||
| 90 | 88 (11.5) | 84 (8.4) | ||
| Sex | 0.372 | 0.919 | ||
| Male | 3,689 (17.3) | 2,392 (11.2) | ||
| Female | 3,650 (17.0) | 2,478 (11.5) | ||
| Drug | <0.001 | <0.001 | ||
| Heparin | 6,833 (18.4) | 4,515 (12.2) | ||
| Enoxaparin | 506 (8.9) | 355 (6.2) | ||
| Length of stay, d | <0.001 | <0.001 | ||
| 01 | 446 (24.3) | 282 (15.4) | ||
| 23 | 1,463 (19.4) | 971 (12.9) | ||
| 47 | 2,332 (18.9) | 1,620 (13.1) | ||
| 8 | 3,098 (14.6) | 1,997 (9.4) | ||

The results of the multivariate regression analyses with GEE are displayed in Table 4. HIV diagnosis, non‐African American race, and heparin (as compared with enoxaparin) were associated with increased likelihood of nonadministration. Increasing age and increasing length of stay were associated with decreased likelihood of nonadministration by a small but significant amount.
| OR of Nonadministration | 95% CI, P | OR of Documented Refusal | 95% CI, P | |
|---|---|---|---|---|
| ||||
| Race | ||||
| African American | 1.00 | Reference | 1.00 | Reference |
| Caucasian | 1.62 | 1.44‐1.81, <0.001 | 1.53 | 1.32‐1.77, <0.001 |
| Asian, Pacific Islander, Other | 1.54 | 1.19‐2.00, 0.001 | 1.48 | 1.07‐2.01, 0.019 |
| HIV status | ||||
| Negative | 1.00 | Reference | 1.00 | Reference |
| Positive | 1.21 | 1.001.45, 0.039 | 1.29 | 1.06‐1.56, 0.012 |
| Age, per year | 0.97 | 0.97‐0.98, <0.001 | 0.97 | 0.96‐0.97, <0.001 |
| Drug | ||||
| Heparin | 1.00 | Reference | 1.00 | Reference |
| Enoxaparin | 0.45 | 0.40‐0.51, <0.001 | 0.53 | 0.47‐0.61, <0.001 |
| Length of stay, per day | 0.991 | 0.987‐0.995, <0.001 | 0.989 | 0.983‐0.993, <0.001 |
The most commonly documented reason for nonadministration was refusal by the patient or family member (66% of all doses not administered). The second most common reason, patient condition not appropriate, accounted for an additional 10% of doses. Across all nursing units, the proportion of prescribed doses that were documented as refused was significantly greater for patients with HIV compared with patients without HIV (16.5% vs 10.6%, OR: 1.66, 95% CI: 1.54‐1.80, P<0.0001) (Table 2). Using the GEE and multivariate regression, HIV diagnosis, non‐African American race, and heparin were associated with increased risk of documented dose refusal. Age and length of stay were inversely related to the likelihood of documented dose refusal. When all administered doses were excluded from the analysis, the association between these variables and documented dose refusal were not as strong. Age and length of stay remained significantly inversely related; however, the other factors were no longer significantly positively associated with documented dose refusal.
Within the HIV care unit, the proportion of prescribed doses documented as refused was greater for patients with HIV compared with patients without HIV (18.1% vs 6.8%, OR: 3.01, 95% CI: 2.28‐3.99, P<0.0001). For all other medicine units, the proportion of nonadministered doses documented as refused was also greater for patients with HIV compared with patients without HIV (13.7% vs 10.7%, OR: 1.32, 95% CI: 1.16‐1.51, P<0.0001).
DISCUSSION
We have identified that nonadministration of thromboprophylaxis was more common among patients with HIV at our institution. Substantial variation in the proportion of doses not administered existed on the nursing unit level, as well as within each unit when stratified by HIV status. This disparity in dose administration was observed on the HIV care unit as well, as the proportion not administered was about 2‐fold greater for patients with HIV compared with those without HIV. Documented dose refusal appeared to account for the majority of nonadministered doses in our cohort. Our analysis also demonstrated that HIV diagnosis is significantly associated with both dose nonadministration and documented dose refusal at our institution.
Medication refusal is a well‐recognized phenomenon among hospitalized patients. A recent study of medication administration in hospitalized patients in the United Kingdom noted that refusal accounted for about 45% of omitted doses.[26] Fanikos et al. also found that documented refusal of doses contributed significantly to the overall number of VTE prophylaxis doses not administered to patients.[27] In our study, the proportion of nonadministered doses documented as refused by the patient or family member was significantly greater in patients with HIV than in patients without HIV across all units. Interestingly, the difference was greater on the HIV care unit when doses were stratified by HIV status. This observation leads us to hypothesize that specific hospital care environments may influence dose nonadministration and refusal rates among our patient population.
Based on regression analyses, increasing age and length of stay were associated with a decreased likelihood of any particular dose not being administered and with any particular dose being documented as refused. It is important to note that our GEE did not take into account date or time of each dose, and therefore we cannot make conclusions as to the likelihood of dose nonadministration or refusal of doses in relation to each other on a time scale. One cannot assume that a dose due later in a hospital course was more or less likely to be given than a dose due on the first hospital day. Although we did not expect these findings, one can hypothesize that patients who are older or have longer stays may be perceived to have more severe illness, and therefore greater need for prophylaxis, from nursing staff and others involved in their care. The associations were small but significant and warrant future investigation.
To our knowledge, this is the first investigation comparing the proportion of nonadministered doses of thromboprophylaxis between patients with and without HIV. Our data show that nonadministered doses and refused doses of thromboprophylaxis are more frequent among patients with HIV. In addition, we noted that nonadministration was more common on the dedicated HIV care unit compared with other units. We cannot currently offer a clear explanation for the disparity observed between units, and more specifically, within the HIV care unit. However, it is possible that a unique culture of care and provider‐specific factors may contribute.
Our study was limited by a number of factors. Seroconversion among patients during the study period was possible; however, our analysis revealed only 2 instances among nearly 4000 unique patients. A more significant limitation was the level of analysis allowed by the dataset. We examined dose characteristics on a dose and unit level, but the ability to analyze doses based on the prescriber and nurse level may have provided valuable insight into the specific reasons behind the observations presented here. Additionally, the specific unit assigned to a given dose in our dataset represented the discharge location for the corresponding patient, making it possible that some amount of nonadministered doses may be attributed to the incorrect unit. However, we do not believe that unit‐to‐unit transfers would be frequent enough to influence the overall results. In addition, we did not link nonadministration of thromboprophylaxis with VTE events, as these data were not present in the current dataset. Although this is a limitation of the current study, we believe that the notion that missed doses of thromboprophylaxis place patients at higher risk for VTE is plausible, as the efficacy of thromboprophylaxis is well established.[28, 29, 30] It is important to note that the reason for nonadministration selected by the nurse on the eMAR may not always represent the only reason or even the true reason for dose nonadministration. It is possible that dose refusal may be over‐represented in our sample, in part due to inaccurate documentation. Recent investigations at JHH have identified varying attitudes on the part of the patient and the nurse regarding thromboprophylaxis. A questionnaire and interview of patients showed a large knowledge gap regarding thromboprophylaxis, with many individuals unable to explain its role or significance in their medical care.[31] A common theme was also observed in a survey of nurses regarding VTE prophylaxis: doses were sometimes considered optional for reasons such as ambulation status, perceived severity of illness, or reason for hospitalization. Some nurses also reported that after an initial refused dose, they may continue to document subsequent doses as refused, sometimes without offering the dose to the patient.[32] As variation in practice was observed between individual nurses, it is also likely that the culture of care may vary between units, influencing thromboprophylaxis nonadministration rates as well as documentation of doses as refused. The dose‐level data used for the GEE analyses did not include date and time of administration, which limited the ability of the GEE to more completely account for autocorrelation.
To further investigate the findings of this and related studies, we intend to more closely analyze data at multiple levels with the goal of identifying an appropriate and feasible target for intervention. Additionally, further investigation should be performed with the goal of determining the relationship between decreased exposure to thromboprophylaxis and VTE. However, as patients with HIV appear to be at increased risk of VTE, ensuring that thromboprophylaxis is delivered appropriately and consistently should be an important goal for all who provide care to this population.
- , , , , . Venous thromboembolism in patients with HIV/AIDS: a case‐control study. J Acquir Immune Defic Syndr. 2008;48(3):310–314.
- , , . AIDS and thrombosis: retrospective study of 131 HIV‐infected patients. AIDS Patient Care STDS. 2001;15(6):311–320.
- , , , et al. HIV and risk of venous thromboembolism: a Danish nationwide population‐based cohort study. HIV Med. 2011;12(4):202–210.
- , , , . Epidemiology of thrombosis in HIV‐infected individuals. The adult/adolescent spectrum of HIV disease project. AIDS. 2000;14(3):321–324.
- , , . Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis. 2004;39(8):1214–1222.
- , , . Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(2):175–180.
- , . The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044.
- , , , . Acquired protein C and protein S deficiency in HIV‐infected patients. Clin Appl Thromb Hemost. 2003;9(4):325–331.
- , , , et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24(2):197–200.
- , . Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263.
- , . AIDS‐defining and non‐AIDS‐defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446–451.
- , , , , . Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29–38.
- , , , et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV‐positive adults in 2001. Med Care. 2005;43(9 suppl):III3–III14.
- , , , et al. Opportunistic infections as causes of death in HIV‐infected patients in the HAART era in France. Scand J Infect Dis. 2005;37(6‐7):482–487.
- , , , et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354.
- , , . Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40(5):609–616.
- , , , , , . Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155.
- , , , et al. Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.
- , , , et al. Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLoS Biol. 2004;2(2):E20.
- , , , , . CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–3406.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , . Practices to prevent venous thromboembolism [published online ahead of print May 24, 2013]. BMJ Qual Saf. doi:10.1136/bmjqs‐2012‐001782.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Lessons from The Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events [published online ahead of print April 4, 2013]. Am J Hematol. doi: 10.1002/ajh.23450.
- , , . Dose omissions in hospitalized patients in a UK hospital: an analysis of the relative contribution of adverse drug reactions. Drug Saf. 2012;35(8):677–683.
- , , , et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123(6):536–541.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , et al. Patient perspectives on pharmacological venous thromboembolism prophylaxis at The Johns Hopkins Hospital. J Thromb Thrombolysis. 2013;35(3):416.
- , , , et al. Culture of care and documented patient refusal of pharmacologic venous thromboembolism prophylaxis. J Thromb Thrombolysis. 2011;31(3):367–400.
- , , , , . Venous thromboembolism in patients with HIV/AIDS: a case‐control study. J Acquir Immune Defic Syndr. 2008;48(3):310–314.
- , , . AIDS and thrombosis: retrospective study of 131 HIV‐infected patients. AIDS Patient Care STDS. 2001;15(6):311–320.
- , , , et al. HIV and risk of venous thromboembolism: a Danish nationwide population‐based cohort study. HIV Med. 2011;12(4):202–210.
- , , , . Epidemiology of thrombosis in HIV‐infected individuals. The adult/adolescent spectrum of HIV disease project. AIDS. 2000;14(3):321–324.
- , , . Thrombotic complications in patients infected with HIV in the era of highly active antiretroviral therapy: a case series. Clin Infect Dis. 2004;39(8):1214–1222.
- , , . Circulating coagulation inhibitors in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(2):175–180.
- , . The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044.
- , , , . Acquired protein C and protein S deficiency in HIV‐infected patients. Clin Appl Thromb Hemost. 2003;9(4):325–331.
- , , , et al. Antiphospholipid syndrome associated with cytomegalovirus infection: case report and review. Clin Infect Dis. 1997;24(2):197–200.
- , . Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31(4):256–263.
- , . AIDS‐defining and non‐AIDS‐defining malignancies: cancer occurrence in the antiretroviral therapy era. Curr Opin Oncol. 2007;19(5):446–451.
- , , , , . Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29–38.
- , , , et al. Patterns of diagnoses in hospital admissions in a multistate cohort of HIV‐positive adults in 2001. Med Care. 2005;43(9 suppl):III3–III14.
- , , , et al. Opportunistic infections as causes of death in HIV‐infected patients in the HAART era in France. Scand J Infect Dis. 2005;37(6‐7):482–487.
- , , , et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. 2008;22(11):1345–1354.
- , , . Hospitalizations for metabolic conditions, opportunistic infections, and injection drug use among HIV patients: trends between 1996 and 2000 in 12 states. J Acquir Immune Defic Syndr. 2005;40(5):609–616.
- , , , , , . Cardiovascular disease in HIV infection. Am Heart J. 2006;151:1147–1155.
- , , , et al. Epidemiological evidence for cardiovascular disease in HIV‐infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35.
- , , , et al. Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLoS Biol. 2004;2(2):E20.
- , , , , . CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV‐1 and HIV‐2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–3406.
- , . Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637.
- , . Practices to prevent venous thromboembolism [published online ahead of print May 24, 2013]. BMJ Qual Saf. doi:10.1136/bmjqs‐2012‐001782.
- , , , et al. Patterns of non‐administration of ordered doses of venous thromboembolism prophylaxis: implications for novel intervention strategies. PLoS One. 2013;8(6):e66311.
- , , , et al. Lessons from The Johns Hopkins multi‐disciplinary venous thromboembolism (VTE) prevention collaborative. BMJ. 2012;344:e3935.
- , , , et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events [published online ahead of print April 4, 2013]. Am J Hematol. doi: 10.1002/ajh.23450.
- , , . Dose omissions in hospitalized patients in a UK hospital: an analysis of the relative contribution of adverse drug reactions. Drug Saf. 2012;35(8):677–683.
- , , , et al. Adherence to pharmacological thromboprophylaxis orders in hospitalized patients. Am J Med. 2010;123(6):536–541.
- , , , et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793–800.
- , , , et al. Randomized, placebo‐controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874–879.
- , , , et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325–329.
- , , , et al. Patient perspectives on pharmacological venous thromboembolism prophylaxis at The Johns Hopkins Hospital. J Thromb Thrombolysis. 2013;35(3):416.
- , , , et al. Culture of care and documented patient refusal of pharmacologic venous thromboembolism prophylaxis. J Thromb Thrombolysis. 2011;31(3):367–400.
© 2014 Society of Hospital Medicine
Review of VTE Prophylaxis Strategies
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.
| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.

| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is estimated to affect 900,000 Americans each year and is a cause of significant morbidity and mortality with associated high healthcare costs.[1] Accordingly, the comparative effectiveness and safety of interventions for the prevention and treatment of VTE are among the national priorities for comparative effectiveness research.[2] Whereas we have evidence‐based guidelines for the prophylaxis of VTE in the general population, there are no guidelines informing the care of select patient populations. Select populations are those patients in whom there is decisional uncertainty about the optimal choice, timing, and dose of VTE prophylaxis. Not only do these patients have an increased risk of DVT and PE, but most are also at high risk of bleeding, the most important complication of VTE prophylaxis.[3, 4, 5, 6]
The objectives of this systematic review were to define the comparative effectiveness and safety of pharmacologic and mechanical strategies for VTE prevention in some of these select medical populations including obese patients, patients on concomitant antiplatelet therapy, patients with renal insufficiency, patients who are underweight, and patients with coagulopathy due to liver disease.
METHODS
The methods for this comparative effectiveness review (CER) follow the guidelines suggested in the Agency for Healthcare Research and Quality (AHRQ) Methods Guide for Effectiveness and Comparative Effectiveness Reviews.[7] The protocol was publically posted.[8]
Search Strategy
We searched MEDLINE, EMBASE, and SCOPUS through August 2011, CINAHL, International Pharmaceutical Abstracts,
Study Selection
We reviewed titles followed by abstracts to identify randomized controlled trials (RCTs) or observational studies with comparison groups reporting on the effectiveness or safety of VTE prevention in our populations. Two investigators independently reviewed abstracts, and we excluded the abstracts if both investigators agreed that the article met 1 or more of the exclusion criteria. We included only English‐language articles that evaluated the effectiveness of pharmacological or mechanical interventions that have been approved for clinical use in the United States. To be eligible, the studies must have addressed relevant key questions in the population of our interest. We resolved disagreements by consensus. We used DistillerSR (Evidence Partners Inc., Ottawa, Ontario, Canada), a Web‐based database management program to manage the review process. Two investigators assessed the risk of bias in each study independently, using the Downs and Black instrument for observational studies and trials.[10]
Data Synthesis
For each select population, we created detailed evidence tables containing the information abstracted from the eligible studies. After synthesizing the evidence, we graded the quantity, quality, and consistency of the best available evidence for each select population by adapting an evidence‐grading scheme recommended in the Methods Guide for Conducting Comparative Effectiveness Reviews.[7]
RESULTS
We identified 30,902 unique citations and included 9 studies (Figure 1). There were 5 RCTs with relevant subgroups and 4 observational studies (Table 1). Two studies reported on the risk of bleeding in patients given pharmacologic prophylaxis while they are concomitantly taking nonsteroidal anti‐inflammatory drugs (NSAIDS) or antiplatelet agents/aspirin, 1 RCT and 1 prospective observational study reported on obese patients, and 5 studies described outcomes of patients with renal insufficiency (see Supporting Information, Table 1, in the online version of this article). No study tested prophylaxis in underweight patients or those with liver disease.

| Study | Arm, n | Total VTE (DVT and PE) | Bleeding | Other Outcomes | |
|---|---|---|---|---|---|
| |||||
| Obese patients | |||||
| Kucher et al., 2005[11] | Arm 1 (dalteparin), 558 | 2.8% (95% CI: 1.34.3) | 0% | Mortality at 21 days: 4.6% | |
| Arm 2 (placebo), 560 | 4.3% (95% CI: 2.56.2) | 0.7% | Mortality at 21 days: 2.7% | ||
| Freeman et al., [12] | Arm 1 (fixed‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 19 % | |
| Arm 2 (lower‐dose enoxaparin), 9 | NR | NR | Peak anti‐factor Xa level 32 % | ||
| Arm 3 (higher‐dose enoxaparin), 11 | NR | NR | Peak anti‐factor Xa level 86 % | ||
| Patients on antiplatelet agents | |||||
| Eriksson et al., 2012[14] | Arm 1 (rivaroxaban), 563 | NR | 20 (3.6%), rate ratio for use vs nonuse: 1.32 (95% CI: 0.85‐2.05) | NR | |
| Arm 2 (enoxaparin/placebo), 526 | NR | 17 (3.2%), rate ratio for use vs nonuse: 1.40 (95% CI: 0.87‐2.25) | NR | ||
| Friedman et al., 2012[15] | Arm 2 (150 mg dabigatran, no ASA), 1149 | NR | 11 (1.0%)a | NR | |
| Arm 5 (150 mg dabigatran+ASA), 128 | NR | 2 (1.6%)a | NR | ||
| Arm 3 (enoxaparin, no ASA), 1167 | NR | 14 (1.2%)a | NR | ||
| Arm 6 (enoxaparin+ASA), 132 | NR | 4 (3.0%) | NR | ||
| 150 mg dabigatran compared with enoxaparinNo concomitant ASA therapy | NR | RR: 0.82 (95% CI: 0.37‐1.84) | NR | ||
| 150 mg dabigatran compared with enoxaparinWith concomitant ASA therapy | NR | RR: 0.55 (95% CI: 0.11‐2.78) | NR | ||
| Patients with renal insufficiency | |||||
| Bauersachs et al., 2011[16] | Arm 2 (GFR 30), 92 | Total DVT: 11.11%; Total PE: 0% | Major bleeding: 4/92 (4.35%), minor bleeding: 9/92 (9.78%) | Mortality: 5.81% | |
| Mah et al., 2007[17] | Arm 2 (tinzaparin), 27 | NR | Major bleeding: 2/27 (7.4%), minor bleeding: 3/27 (11.1%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.05 | |
| Arm 3 (enoxaparin), 28 | NR | Major bleeding: 1/28 (3.6%), minor bleeding: 3/28 (10.7%) | Factor Xa level: AF: CmaxD8/Cmax D1=1.22 | ||
| Dahl et al., 2012[18] | Arm 1 (enoxaparin), 332 | Major VTE: 8 (9.0%) | Major bleeding: 6 (4.7%) | Infections and infestations: 25 (7.5%), Wound infection: 4 (1.2%) | |
| Arm 2 (dabigatran), 300 | Major VTE: 3 (4.3%) | Major bleeding: 0 (0%) | Infections and infestations: 21 (7.0%), Wound Infection: 3 (1.0%) | ||
| Shorr et al., 2012[19] | Arm 1 (enoxaparin, CrCL 60 mL/min), 353 | Total VTE: 17/275 (6.2%) | Major bleeding: 0/351 (0%) | NR | |
| Arm 2 (desirudin, CrCL 60 mL/min), 353 | Total VTE: 13/284 (4.3%) | Major bleeding: 2/349 (0.27%) | NR | ||
| Arm 3 (enoxaparin, CrCL 4559 mL/min), 369 | Total VTE: 18/282 (6.2%) | Major bleeding: 1/365 (0.27%) | NR | ||
| Arm 4 (desirudin, CrCL 4559 mL/min), 395 | Total VTE: 17/303 (5.6%) | Major bleeding: 1/393 (0.25%) | NR | ||
| Arm 5 (enoxaparin, CrCL 45 mL/min), 298 | Total VTE: 24/216 (11.1%) | Major bleeding: 1/294 (0.34%) | NR | ||
| Arm 6 (desirudin, CrCL 45 mL/min), 279 | Total VTE: 7/205 (3.4%) | Major bleeding: 5/275 (1.82%) | NR | ||
| Elsaid et al., 2012[20] | Arm 1 (enoxaparin, CrCL 60 mL/min), 17 | NR | Major bleeding: 2 (11.8%) | NR | |
| Arm 2 (enoxaparin, CrCL 3059 mL/min), 86 | NR | Major bleeding: 9 (10.5%) | NR | ||
| Arm 3 (enoxaparin, CrCL 30 mL/min), 53 | NR | Major bleeding: 10 (18.9%) | NR | ||
| Arm 4 (UFH, CrCL 60 mL/min), 19 | NR | Major bleeding: 2 (10.5%) | NR | ||
| Arm 5 (UFH, CrCL 3059 mL/min), 99 | NR | Major bleeding: 3 (3%) | NR | ||
| Arm 6 (UFH, CrCL 30 mL/min), 49 | NR | Major bleeding: 2 (4.1%) | NR | ||
Obese Patients
We found 1 subgroup analysis of an RCT (total 3706 patients, 2563 nonobese and 1118 obese patients) that reported on the comparative effectiveness and safety of fixed low‐dose dalteparin 5000 IU/day compared to placebo among 1118 hospitalized medically ill patients with body mass indices (BMI) greater than 30 kg/m2.11 Neither group received additional concurrent prophylactic therapies. The 3 most prevalent medical diagnoses prompting hospitalization were congestive heart failure, respiratory failure, and infectious diseases. Compression ultrasound was performed in all patients by day 21 of hospitalization. The primary end point was the composite of VTE, fatal PE, and sudden death, and secondary end points included DVT, bleeding, and thrombocytopenia by day 21 (Table 1). In obese patients, the primary end point occurred in 2.8% (95% confidence interval [CI]: 1.34.3) of the dalteparin group and in 4.3% (95% CI: 2.56.2) of the placebo group (relative risk [RR]: 0.64; 95% CI: 0.32‐1.28). In nonobese patients, the primary end point occurred in 2.8% (95% CI: 1.8‐3.8) and 5.2% (95% CI: 3.9‐6.6) of the dalteparin and placebo groups, respectively (RR: 0.53; 95% CI: 0.34‐0.82). When weight was modeled as a continuous variable, no statistically significant interaction between weight and dalteparin efficacy was observed (P=0.97). The authors calculated the RR in predefined BMI subgroups and found that dalteparin was effective in reducing VTE in patients with BMIs up to 40, with RRs of 1.0 for all (approximate range, 0.20.8). However, a fixed dose of dalteparin 5000 IU/day was not better than placebo for individuals with BMI >40 kg/m2. There was no significant difference in mortality or major hemorrhage by day 21 between treatment and placebo groups.
Freeman and colleagues prospectively assigned 31 medically ill patients with extreme obesity (BMI >40 kg/m2) to 1 of 3 dosing regimens of enoxaparin: a fixed dose of 40 mg daily enoxaparin (control group, n=11), enoxaparin at 0.4 mg/kg (n=9), or enoxaparin at 0.5 mg/kg (n=11).[12] The average BMI of the entire cohort was 62.1 kg/m2 (range, 40.582.4). All patients had anti‐factor Xa levels drawn on the day of enrollment and daily for 3 days (Table 2). The relationship between anti‐factor Xa levels and clinical efficacy of low‐molecular weight heparin (LMWH) in VTE prophylaxis is still unclear; however, an anti‐factor Xa level of 0.2 to 0.5 IU/mL, measured 4 hours after the fourth dose of LMWH, is the target level recommended for VTE prophylaxis.[13] Patients who received weight‐based enoxaparin at 0.5mg/kg achieved target anti‐factor Xa level 86% of the time compared to 32% of the time in those receiving 0.4 mg/kg and 19% of the time for those in the fixed‐dose group (P0.001). No clinical outcomes were reported in this study.
| Intervention | Outcome | Risk of Bias | Evidence Statement and Magnitude of Effect |
|---|---|---|---|
| |||
| Patients on antiplatelet agents | |||
| Rivaroxaban vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic rivaroxaban or enoxaparin in patients concomitantly treated with antiplatelet agents; 3.6% vs 3.25% |
| Dabigatran vs enoxaparin | Major bleeding | Low | Insufficient to support no difference in rates of major bleeding with prophylactic dabigatran or enoxaparin in patients concomitantly treated with aspirin; 1.6% vs 3.0% |
| Obese patients | |||
| Dalteparin vs placebo | VTE | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing total VTE in obese patients; 2.8% vs 4.3%, RR: 0.64, 95% CI: 0.32‐1.28 |
| Dalteparin vs placebo | Mortality | Moderate | Insufficient evidence for effectiveness of dalteparin vs placebo in reducing mortality in obese patients; 9.9% vs 8.6%, P=0.36 |
| Dalteparin vs placebo | Major bleeding | Moderate | Insufficient evidence for safety of dalteparin vs placebo in reducing major bleeding in obese patients; 0% vs 0.7%, P>0.99 |
| Enoxaparin 40 mg daily vs 0.4 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.4 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 32%, P=NR |
| Enoxaparin 40 mg daily vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 40 mg daily versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 19% vs 86%, P0.001 |
| Enoxaparin 0.4 mg/kg vs 0.5 mg/kg | Percentage of patients achieving target anti‐factor Xa level | Moderate | Insufficient evidence for effectiveness of enoxaparin 0.4 mg/kg versus 0.5 mg/kg in achieving peak anti‐factor Xa level in obese patients; 32% vs 86%, P=NR |
| Patients with renal insufficiency | |||
| Tinzaparin vs enoxaparin | VTE | High | Insufficient evidence about superiority of either drug for preventing VTE in patients with renal insufficiency, 0/27 vs 0/28* |
| Tinzaparin vs enoxaparin | Bleeding | High | Insufficient evidence about safety of either drug in patients with renal insufficiency; 5/27 vs 4/28, P=0.67 |
| Dabigatran vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of dabigatran in reducing VTE in severe renal compromise patients vs enoxaparin; 4.3% vs 9%, OR: 0.48, 95% CI: 0.13‐1.73, P=0.271 |
| Dabigatran vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of dabigatran vs enoxaparin in patients with renal impairment; 0 vs 4.7%, P=0.039 |
| Desirudin vs enoxaparin | VTE | Moderate | Insufficient evidence for effectiveness of desirudin vs enoxaparin in reducing VTE in patients with renal impairment; 4.9% vs 7.6%, P=0.019 |
| Desirudin vs enoxaparin | Bleeding | Moderate | Insufficient evidence for safety of desirudin vs enoxaparin in patients with renal impairment; 0.8% vs 0.2%, P=0.109 |
| Enoxaparin vs UFH | Bleeding | High | Insufficient evidence for increased risk of bleeding with enoxaparin vs unfractionated heparin in patients with all levels of renal impairment, 13.5% vs 4.2%, RR: 3.2, 95% CI: 1.47.3; and for the subgroup of patients with creatinine clearance 30 mL/min; 18.9% vs 4.1%, RR: 4.68, 95% CI: 1.120.6 |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | VTE | Moderate | Insufficient evidence regarding differential benefit of unfractionated heparin by renal function; 2.6% of patients had a VTE event |
| UFH in severe renal compromise vs all other renal status (undifferentiated) | Bleeding | Moderate | Insufficient evidence for differential harm from unfractionated heparin by renal function; 13 events in 92 patients |
Patients on Antiplatelet Drugs
We did not find studies that directly looked at the comparative effectiveness of VTE prophylaxis in patients who were on antiplatelet drugs including aspirin. However, there were 2 studies that looked at the risk of bleeding in patients who received VTE pharmacologic prophylaxis while concurrently taking antiplatelet agents including aspirin. Both studies used pooled data from large phase III trials.
The study by Eriksson et al. used data from the RECORD (Regulation of Coagulation in Orthopedic Surgery to Prevent Deep Venous Thrombosis and Pulmonary Embolism) trial where over 12,000 patients undergoing elective total knee or hip replacement were randomized to receive VTE prophylaxis with oral rivaroxaban or subcutaneous enoxaparin.[14] Nine percent of participants in each arm (563 in rivaroxaban and 526 in enoxaparin/placebo) were concomitantly using antiplatelet agents or aspirin at least once during the at risk period, defined as starting at day 1 of surgery up to 2 days after the last intake of the study drug. The only end point evaluated was bleeding, and the authors found no statistically significant bleeding difference among the 2 arms (Table 1). Any bleeding event in the rivaroxaban with antiplatelets or aspirin arm was found in 20 (3.6%) patients, whereas in those on enoxaparin/placebo with antiplatelets or aspirin arm it was 17 (3.2%). The relative rate of bleeding among users versus nonusers of antiplatelet drugs or aspirin was 1.32 (95% CI: 0.85‐2.05) in the rivaroxaban group and 1.40 (95% CI: 0.87‐2.25) in the enoxaparin arm (Table 1).
Friedman et al. used pooled data from the RE‐MODEL, RENOVATE, and REMOBILIZE trials, where patients who were undergoing hip or knee arthroplasty were randomized to 220 mg of dabigatran once daily, 150 mg of dabigatran once daily (we focused on this lower dosage as this is the only available dose used in the US), 40 mg of enoxaparin once daily, or 30 mg of enoxaparin twice a day.[15] Of the 8135 patients, 4.7% were on concomitant aspirin. The baseline characteristics of those on aspirin were similar to the other enrollees. The primary outcome was major bleeding events requiring transfusion, symptomatic internal bleeding, or bleeding requiring surgery. Among patients receiving 150 mg of dabigatran, bleeding events with and without concomitant aspirin occurred in 1.6% and 1.0%, respectively (odds ratio [OR]: 1.64; 95% CI: 0.36‐7.49; P=0.523). The percentages of participants with bleeding who received enoxaparin, with and without aspirin, were 3.0% and 1.2%, respectively (OR: 2.57; 95% CI: 0.83‐7.94; P=0.101). The RR of bleeding on dabigatran compared to enoxaparin with and without aspirin therapy was 0.55 (95% CI: 0.11‐2.78) and 0.82 (95% CI: 0.37‐1.84), respectively (Table 1).
Patients With Renal Insufficiency
We found 5 studies that evaluated the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE in patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, or patients receiving dialysis. Four studies were RCTs,[16, 17, 18, 19] and 1 used a cohort design assessing separate cohorts before and after a quality improvement intervention.[20] Bauersachs and colleagues conducted an RCT comparing unfractionated heparin at 5000 IU, 3 times daily to certoparin, which is not approved in the United States and is not further discussed here.[16] The rate of DVT among patients treated with unfractionated heparin in patients with a glomerular filtration rate >30 mL/min was marginally lower than those with severe renal dysfunction (10.3 vs 11.1%) (Table 1).
Patients with severe renal dysfunction who received 5000 IU of unfractionated heparin 3 times a day were at increased risk of all bleeds (RR: 3.4; 95% CI: 2.05.9), major bleeds (RR: 7.3; 95% CI: 3.316), and minor bleeds (RR: 2.6; 95% CI: 1.4‐4.9) compared to patients treated with unfractionated heparin without severe renal dysfunction.[16]
A randomized trial by Mah and colleagues compared drug accumulation and anti‐Xa activity in elderly patients with renal dysfunction (defined as a glomerular filtration rate of 20 to 50 mL/min) who received either tinzaparin at 4500 IU once daily or enoxaparin at 4000 IU once daily.[17] Enoxaparin accumulated to a greater extent from day 1 to day 8 than did tinzaparin; the ratio of maximum concentration on day 8 compared to day 1 was 1.22 for enoxaparin and 1.05 for tinzaparin (P=0.016). No VTE events were reported in patients who received tinzaparin or enoxaparin. There was no statistical difference in the incidence of bleeding events between patients receiving tinzaparin (5, including 2 major events) and enoxaparin (4, including 3 major events, P=0.67) (Table 1).
The trial by Dahl and colleagues randomly assigned patients who were over 75 years of age and/or who had moderate renal dysfunction (defined as creatinine clearance between 30 and 49 mL/min) to receive enoxaparin 40 mg daily or dabigatran 150 mg daily.[18] There was no significant difference in the rate of major VTE events between patients receiving dabigatran (4.3%) and enoxaparin (9%) (OR: 0.48; 95% CI: 0.13‐1.73; P=0.271) (Table 1). The rate of major bleeding was significantly higher among patients randomly assigned to receive enoxaparin (4.7%) versus dabigatran (0%) (P=0.039).[18]
Shorr and colleagues published a post hoc subgroup analysis of a multicenter trial in which orthopedic patients were randomly assigned to receive desirudin 15 mg twice daily or enoxaparin 40 mg once daily.[19] Evaluable patients (1565 of the 2079 patients randomized in the trial) receiving desirudin experienced a significantly lower rate of major VTE compared with patients receiving enoxaparin (4.9% vs 7.6%, P=0.019). This relationship was particularly pronounced for evaluable patients whose creatinine clearance was between 30 and 44 mL/min. In evaluable patients with this degree of renal dysfunction, 11% of patients taking enoxaparin compared to 3.4% of those taking desirudin had a major VTE (OR: 3.52; 95% CI: 1.48‐8.4; P=0.004). There was no significant difference in the rates of major bleeding among a subset of patients assessed for safety outcomes (2078 of the 2079 patients randomized in the trial) who received desirudin (0.8%) or enoxaparin (0.2%) (Table 1).
Elsaid and Collins assessed VTE and bleeding events associated with the use of unfractionated heparin 5000U either 2 or 3 times daily and enoxaparin 30 mg once or twice daily across patients stratified by renal function (creatinine clearance 30, 3059, and 60 mL/min). The investigators made assessments before and after a quality improvement intervention that was designed to eliminate the use of enoxaparin in patients whose creatinine clearance was 30 mL/min. No VTE events were reported. Patients receiving enoxaparin were significantly more likely to experience a major bleeding episode compared with patients receiving unfractionated heparin (overall rates for all levels of renal function: 13.5% vs 4. 2%; RR: 3.2; 95% CI: 1.47.3) (Table 2). This association was largely driven by the subgroup of patients with a creatinine clearance 30 mL/min. For this subgroup with severe renal insufficiency, patients receiving enoxaparin were significantly more likely to have a bleed compared with patients receiving unfractionated heparin (18.9% vs 4.1%; RR: 4.68; 95% CI: 1.120.6) (Tables 1 and 2). There was no difference in the bleeding rates for patients whose creatinine clearances were >60 mL/min.[20]
Strength of Evidence
Obese Patients
Overall, we found that the strength of evidence was insufficient regarding the composite end point of DVT, PE, and sudden death, and the outcomes of mortality and bleeding (Table 2). This was based on a paucity of available data, and a moderate risk of bias in the reviewed studies. Additionally, 92% of the enrolled patients in the studies were white, limiting the generalizability of the results to other ethnic groups.
Patients on Antiplatelets
The strength of evidence was insufficient in the studies reviewed here to conclude that there is no difference in rates of bleeding in patients who are concomitantly taking antiplatelet drugs while getting VTE prophylaxis with rivaroxaban, dabigatran, or enoxaparin. We based this rating because of the imprecision of results and unknown consistencies across multiple studies.
Patients With Renal Insufficiency
One RCT had a high risk of bias for our key question because data from only 1 study arm were useful for our review.[16] The other RCTs were judged to have a moderate risk of bias. The analyses led by Dahl and Shorr[18, 19] were based on post hoc (ie, not prespecified) analysis of data from RCTs. Additionally, outcomes in the Shorr et al. trial were reported for evaluable subpopulations of the cohort that was initially randomized in the clinical trial.
We rated the strength of evidence as insufficient to know the comparative effectiveness and safety of pharmacologic prophylaxis for prevention of VTE during hospitalization of patients with acute kidney injury, moderate renal insufficiency, severe renal insufficiency not undergoing dialysis, and patients receiving dialysis. We based this rating on the risk of bias associated with published studies and a lack of consistent evidence regarding associations that were reported. Similarly, we rated the strength of evidence as insufficient that 5000 U of unfractionated heparin 3 times daily increases the risk of major and minor bleeding events in patients with severely compromised renal function compared to this dose in patients without severely compromised renal function. We based this rating on a high risk of bias of included studies and inconsistent evidence. Likewise, we rated the strength of evidence as insufficient that enoxaparin significantly increases the risk of major bleeding compared with unfractionated heparin in patients with severe renal insufficiency. We based this rating on a high risk of bias and inconsistent published evidence.
We similarly found insufficient evidence to guide treatment decisions for patients with renal insufficiency. Our findings are consistent with other recent reviews. The American College of Chest Physicians (ACCP) practice guidelines[21] make dosing recommendations for the therapeutic use of enoxaparin. However, their assessment is that the data are insufficient to make direct recommendations about prophylaxis. Their assessment of the indirect evidence regarding bioaccumulation and increased anti‐factor Xa levels are consistent with ours. The ACCP guidelines also suggest that decreased clearance of enoxaparin has been associated with increased risk of bleeding events for patients with severe renal insufficiency. However, the cited study[20] compares patients with and without severe renal dysfunction who received the same therapy. Therefore, it is not possible to determine the additional risk conveyed by enoxaparin therapy, that is, above the baseline increased risk of bleeding among patients with renal insufficiency, particularly those receiving an alternate pharmacologic VTE prevention strategy, such as unfractionated heparin.
DISCUSSION
We found that the evidence was very limited about prevention of VTE in these select and yet prevalent patient populations. Despite the fact that there is an increasing number of obese patients and patients who are on antiplatelet therapies, most clinical practice guidelines do not address the care of these populations, which may be entirely appropriate given the state of the evidence.
The ACCP practice guidelines[21] suggest using a higher dose of enoxaparin for the prevention of VTE in obese patients. The subgroup analysis by Kucher et al.[11] showed effect attenuation of dalteparin when given at a fixed dose of 5000 IU/mL to patients with a BMI of >40 kg/m2. The Freeman study[12] showed that extremely obese patients (average BMI >62.1 kg/m2) who are given a fixed dose of enoxaparin achieved target anti‐factor Xa levels significantly less often than those who received a higher dose of enoxaparin. The 2 separate findings, although not conclusive, lend some credence to the current ACCP guidelines.[21]
The studies we reviewed on VTE prophylaxis in patients who are concomitantly on antiplatelets including aspirin reported no major increased risk of bleeding; however, in the Friedman et al. study,[15] 3.0% of patients who were put on enoxaparin while still on aspirin had a bleeding event compared to 1.2% of those on enoxaparin alone. This difference is not statistically significant but is a trend possibly worth noting, especially when one looks at the lower RR of bleeding at 0.55 compared to 0.82 when dabigatran is compared with enoxaparin with and without concomitant aspirin therapy, respectively (Table 1). The highest dose of aspirin used in either of the studies was 160 mg/day, and neither study addressed other potent antiplatelets such as clopidogrel or ticlopidine separately, which limits the generalizability of the finding to all antiplatelets. Current ACCP guidelines do not recommend aspirin as a sole option for the prevention of VTE in orthopedic surgery patients.[22] Concerns remain among clinicians that antiplatelets, including aspirin, on their own are unlikely to be fully effective to thwart venous thrombotic processes for most patients, and yet the risk of bleeding is not fully known when these agents are combined with other anticoagulants for VTE prophylaxis.
Our review has several limitations, including the possibility that we may have missed some observational studies, as the identification of relevant observational studies in electronic searches is more challenging than that of RCTs. The few studies made it impossible to quantitatively pool results. These results, however, have important implications, namely that additional research on the comparative effectiveness and safety of pharmacologic and mechanical strategies to prevent VTE is needed for the optimal care of these patient subgroups. This might be achieved with trials dedicated to enrolling these patients or prespecified subgroup analyses within larger trials. Observational data may be appropriate as long as attention is paid to confounding.
APPENDIX
MEDLINE Search Strategy
((pulmonary embolism[mh] OR PE[tiab] OR Pulmonary embolism[tiab] OR thromboembolism[mh] OR thromboembolism[tiab] OR thromboembolisms[tiab] OR Thrombosis[mh] OR thrombosis[tiab] OR DVT[tiab] OR VTE[tiab] OR clot[tiab]) AND (Anticoagulants[mh] OR Anticoagulants[tiab] OR Anticoagulant[tiab] OR thrombin inhibitors[tiab] OR Aspirin[mh] or aspirin[tiab] OR aspirins[tiab] or clopidogrel[nm] OR clopidogrel[tiab] OR Plavix[tiab] or ticlopidine[mh] or ticlopidine[tiab]OR ticlid[tiab] OR prasugrel[nm]Or prasugrel[tiab]OR effient[tiab]OR ticagrelor[NM] OR ticagrelor[tiab]OR Brilinta[tiab] OR cilostazol[NM] OR cilostazol[tiab]OR pletal[tiab] OR warfarin[mh]OR warfarin[tiab]OR coumadin[tiab] OR coumadine[tiab] OR Dipyridamole[mh]OR dipyridamole[tiab]OR persantine[tiab] OR dicoumarol[MH] OR dicoumarol[tiab] OR dicumarol[tiab] OR Dextran sulfate[mh] OR dextran sulfate[tiab] ORthrombin inhibitors[tiab] OR thrombin inhibitor[tiab] OR heparin[mh] OR Heparin[tiab] OR Heparins[tiab] OR LMWH[tiab] OR LDUH[tiab] OR Enoxaparin[mh] OR Enoxaparin[tiab] OR Lovenox[tiab] OR Dalteparin[tiab] OR Fragmin[tiab] OR Tinzaparin[tiab] OR innohep[tiab] OR Nadroparin[tiab] OR Fondaparinux[nm] OR Fondaparinux[tiab] OR Arixtra[tiab] OR Idraparinux[nm] OR Idraparinux[tiab] OR Rivaroxaban[nm] OR Rivaroxaban[tiab] OR novastan[tiab] OR Desirudin[nm] OR Desirudin[tiab] OR Iprivask[tiab]OR direct thrombin inhibitor[tiab] OR Argatroban[nm] OR Argatroban[tiab] OR Acova[tiab] OR Bivalirudin[nm] OR Bivalirudin[tiab] OR Angiomax[tiab] OR Lepirudin[nm] OR Lepirudin[tiab] OR Refludan[tiab] OR Dabigatran[nm] OR Dabigatran[tiab] OR Pradaxa[tiab] OR factor xa[mh] OR factor Xa[tiab] OR vena cava filters[mh] OR filters[tiab] OR filter[tiab] OR compression stockings[mh] OR intermittent pneumatic compression devices[mh] OR compression [tiab] OR Venous foot pump[tiab])) AND(prevent*[tiab] OR prophyla*[tiab] OR prevention and control[subheading]) NOT (animals[mh] NOT humans[mh]) NOT (editorial[pt] OR comment[pt]) NOT ((infant[mh] OR infant[tiab] OR child[mh] OR child[tiab] OR children[tiab] OR adolescent[mh] OR adolescent[tiab] OR teen‐age[tiab] OR pediatric[tiab] OR perinatal[tiab]) NOT (adult[tiab] OR adults[tiab] OR adult[mh])) NOT (mechanical valve[tiab] OR heart valve[tiab] OR atrial fibrillation[mh] OR atrial fibrillation[tiab] OR thrombophilia[mh] OR thrombophilia[tiab] OR pregnancy[mh])
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.
- , , . Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood. 2005;106:910.
- Institute of Medicine. Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009.
- Lovenox (enoxaparin sodium injection for subcutaneous and intravenous use: prescribing information). Bridgewater, NJ: SanofiAventis; 2011. Available at: http://products.sanofi.us/lovenox/lovenox.html. Accessed October 17, 2012.
- Innohep (tinzaparin sodium injection). Ballerup, Denmark: LEO Pharmaceutical Products; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020484s011lbl.pdf. Accessed October 17, 2012.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐ the IRIS trial. [50th ASH Annual Meeting and Exposition abstract 434]. Blood. 2008;11:112.
- Fragmin (dalteparin sodium injection). New York, NY: Pfizer Inc.; 2007. Available at: http://www.pfizer.com/files/products/uspi_fragmin.pdf. Accessed October 17, 2012.
- Methods guide for effectiveness and comparative effectiveness reviews. Rockville, MD: Agency for Healthcare Research and Quality; August 2011. AHRQ publication No. 10 (11)‐EHC063‐EF. Available at: http://www.effectivehealthcare.ahrq.gov. Accessed October 17, 2012.
- Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/928/VTE‐Special‐Populations_Protocol_20120112.pdf. Accessed April 17, 2012.
- , , , et al. Comparative effectiveness of pharmacologic and mechanical prophylaxis of venous thromboembolism among special populations. Evidence Report/Technology Assessment (AHRQ). Available at: http://effectivehealthcare.ahrq.gov/ehc/products/341/1501/venous‐thromboembolism‐special‐populations‐report‐130529.pdf. 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Efficacy and safety of fixed low‐dose dalteparin in preventing venous thromboembolism among obese or elderly hospitalized patients: a subgroup analysis of the PREVENT trial. Arch Intern Med. 2005;165(3):341–345.
- , , , . Prospective comparison of three enoxaparin dosing regimens to achieve target anti‐factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–743.
- , , . Effect of prophylactic dalteparin on anti‐factor xa levels in morbidly obese patients after bariatric surgery. Obes Surg. 2010;20(4):487–491.
- , , , , . Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res. 2012;130(2):147–151.
- , , , , , . Dabigatran etexilate and concomitant use of non‐steroidal anti‐inflammatory drugs or acetylsalicylic acid in patients undergoing total hip and total knee arthroplasty: No increased risk of bleeding. Thromb Haemost. 2012;108(1):183–190.
- , , , et al. CERTIFY: prophylaxis of venous thromboembolism in patients with severe renal insufficiency. Thromb Haemost. 2011;105(6):981–988.
- , , , et al. Tinzaparin and enoxaparin given at prophylactic dose for eight days in medical elderly patients with impaired renal function: a comparative pharmacokinetic study. Thromb Haemost. 2007;97(4):581–586.
- , , , , , . Thromboprophylaxis in patients older than 75 years or with moderate renal impairment undergoing knee or hip replacement surgery [published correction appears in Int Orthop. 2012;36(5):1113]. Int Orthop. 2012;36(4):741–748.
- , , , . Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thromb Haemost. 2012;10(8):1515–1520.
- , . Initiative to improve thromboprophylactic enoxaparin exposure in hospitalized patients with renal impairment. Am J Health Syst Pharm. 2012;69(5):390–396.
- , , , , ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):7S–47S.
- , . Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74.