User login
Recurrent Soft Tissue Rosai Dorfman Disease of Right Medial Thigh Lipoma With Lymph Node Involvement
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
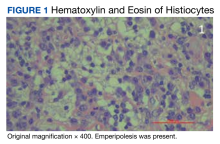
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
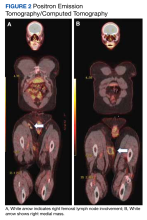
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531