User login
Xanthogranulomatous Reaction to Trametinib for Metastatic Malignant Melanoma
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
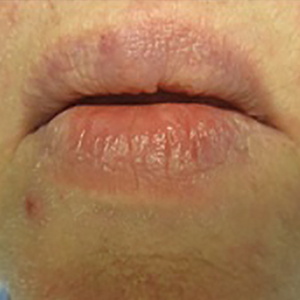
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
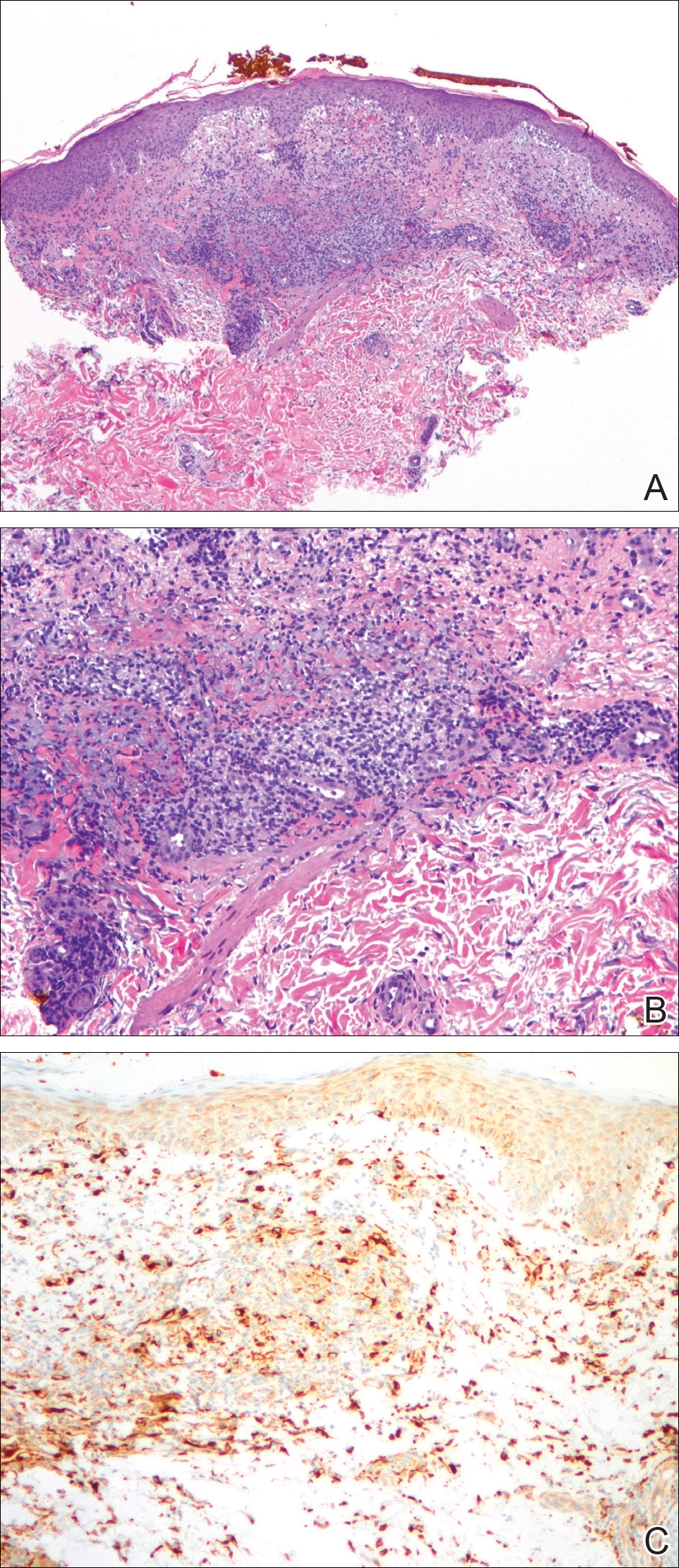
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.
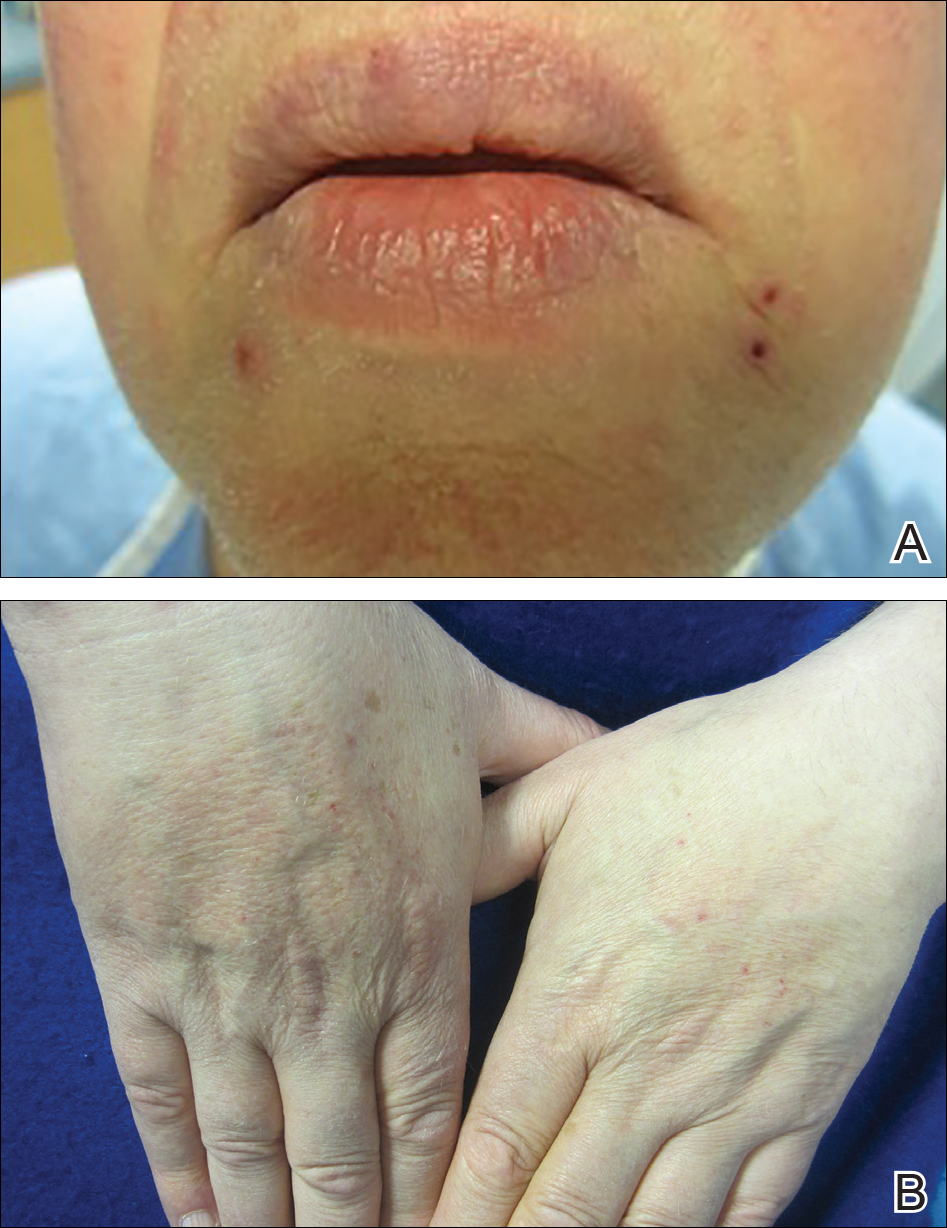
Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.
Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
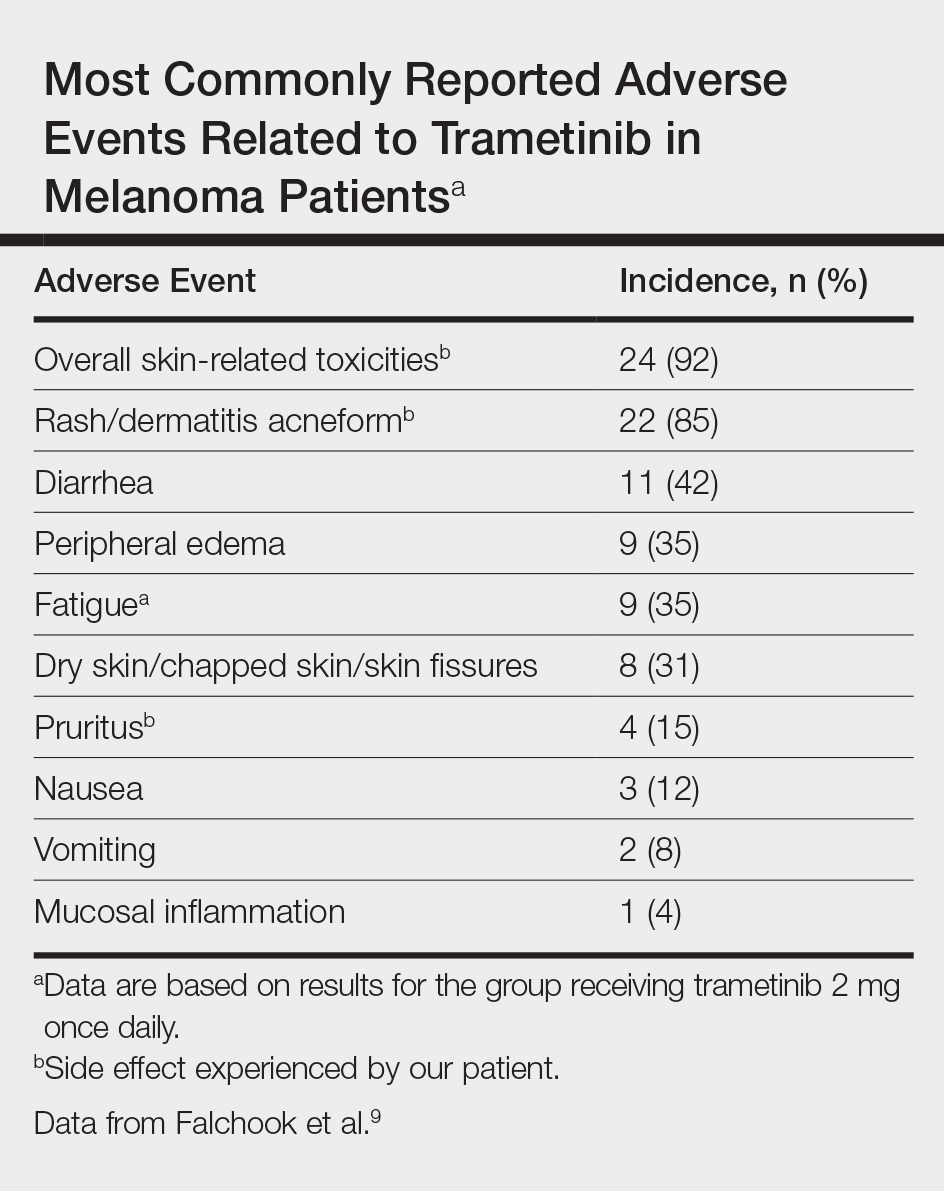
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9
Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9

Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
A decade ago, the few agents approved by the US Food and Drug Administration for treatment of metastatic melanoma demonstrated low therapeutic success rates (ie, <15%–20%).1 Since then, advances in molecular biology have identified oncogenes that contribute to melanoma progression.2 Inhibition of the mitogen-activated protein kinase (MAPK) pathway by targeting mutant BRAF and mitogen-activated extracellular signal-regulated kinase (MEK) has created promising pharmacologic treatment opportunities.3 Due to the recent US Food and Drug Administration approval of these therapies for treatment of melanoma, it is important to better characterize these adverse events (AEs) so that we can manage them. We present the development of an unusual cutaneous reaction to trametinib, a MEK inhibitor, in a man with stage IV M1b malignant melanoma.
Case Report
A 66-year-old man with stage IV M1b malignant melanoma with metastases to the brain and lungs presented with recurring pruritic erythematous papules on the face and bilateral forearms that began shortly after initiating therapy with trametinib. The cutaneous eruption had initially presented on the face, forearms, and dorsal hands when trametinib was used in combination with vemurafenib, a BRAF inhibitor, and ipilimumab, a human cytotoxic T-lymphocyte antigen 4–blocking antibody; however, lesions initially were minimal and self-resolving. When trametinib was reintroduced as monotherapy due to fever attributed to the combination treatment regimen, the cutaneous eruption recurred more severely. Physical examination revealed erythematous scaly papules limited to the face and bilateral upper extremities, including the flexural surfaces.
A biopsy from the flexural surface of the right forearm revealed a dense perivascular lymphoid and xanthomatous infiltrate in the dermis (Figure 1). Poorly formed granulomas within the mid reticular dermis demonstrated focal palisading of histiocytes with prominent giant cells at the periphery. Histiocytes and giant cells showed foamy or xanthomatous cytoplasm. Within the reaction, degenerative and swollen collagen fibers were noted with no mucin deposition, which was confirmed with negative colloidal iron staining.

Brief cessation of trametinib along with application of clobetasol propionate ointment 0.05% resulted in resolution of the cutaneous eruption. Later, trametinib was reintroduced in combination with vemurafenib, though therapy was intermittently discontinued due to various side effects. Skin lesions continued to recur (Figure 2) while the patient was on trametinib but remained minimal and continued to respond to topical clobetasol propionate. One year later, the patient continues to tolerate combination therapy with trametinib and vemurafenib.

Comment
BRAF Inhibitors
Normally, activated BRAF phosphorylates and stimulates MEK proteins, ultimately influencing cell proliferation, survival, and differentiation.3-5 BRAF mutations that constitutively activate this pathway have been detected in several malignancies, including papillary thyroid cancer, colorectal cancer, and brain tumors, but they are particularly prevalent in melanoma.4,6 The majority of BRAF-positive malignant melanomas are associated with V600E, in which valine is substituted for glutamic acid at codon 600. The next most common BRAF mutation is V600K, in which valine is substituted for lysine.2,7 Together these constitute approximately 95% of BRAF mutations in melanoma patients.5
MEK Inhibitors
Initially, BRAF inhibitors (BRAFi) were introduced to the market for treating melanoma with great success; however, resistance to BRAFi therapy quickly was identified within months of initiating therapy, leading to investigations for combination therapy with MEK inhibitors (MEKi).2,5 MEK inhibition decreases cellular proliferation and also leads to apoptosis of melanoma cells in patients with BRAF V600E or V600K mutations.2,8 Trametinib, in particular, is a reversible, highly selective allosteric inhibitor of both MEK1 and MEK2. While on trametinib, patients with metastatic melanoma have experienced 3 times as long progression-free survival as well as 81% overall survival compared to 67% overall survival at 6 months in patients on chemotherapy, dacarbazine, or paclitaxel.5 However, AEs are quite common with trametinib, with cutaneous AEs being a leading side effect. Several large trials have reported that 57% to 92% of patients on trametinib report cutaneous AEs, with the majority of cases being described as papulopustular or acneform (Table).5,9

Combination Therapy
Fortunately, combination treatment with a BRAFi may alleviate MEKi-induced cutaneous drug reactions. In one study, acneform eruptions were identified in only 10% of those on combination therapy—trametinib with the BRAFi dabrafenib—compared to 77% of patients on trametinib monotherapy.10 Strikingly, cutaneous AEs occurred in 100% of trametinib-treated mice compared to 30% of combination-treated mice in another study, while the benefits of MEKi remained similar in both groups.11 Because BRAFi and MEKi combination therapy improves progression-free survival while minimizing AEs, we support the use of combination therapy instead of BRAFi or MEKi monotherapy.5
Histologic Evidence of AEs
Histology of trametinib-associated cutaneous reactions is not well characterized, which is in contrast to our understanding of cutaneous AEs associated with BRAFi in which transient acantholytic dermatosis (seen in 45% of patients) and verrucal keratosis (seen in 18% of patients) have been well characterized on histology.12 Interestingly, cutaneous granulomatous eruptions have been attributed to BRAFi therapy in 4 patients.13,14 One patient was on monotherapy with vemurafenib and granulomatous dermatitis with focal necrosis was seen on histology.13 The other 3 patients were on combination therapy with trametinib; 2 had histology-proven sarcoidal granulomatous inflammation, and 1 demonstrated perifollicular granulomatous inflammation and granulomatous inflammation surrounding a focus of melanoma cells.13,14 Although these granulomatous reactions were attributed to BRAFi or combination therapy, the association with trametinib remains unclear. On the other hand, our patient’s granulomatous reaction was exacerbated on trametinib monotherapy, suggesting a relationship to trametinib itself rather than BRAFi.
Conclusion
With the discovery of molecular targeting in melanoma, BRAFi and MEKi therapies provide major milestones in metastatic melanoma management. As more patients are treated with these agents, it is important that we better characterize their associated side effects. Our case of an unusual xanthogranulomatous reaction to trametinib adds to the knowledge base of possible cutaneous reactions caused by this drug. We hope that prospective studies will further investigate and differentiate the cutaneous AEs described so that we can better manage these patients.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Eggermont AM, Schadendorf D. Melanoma and immunotherapy. Hematol Oncol Clin North Am. 2009;23:547-564.
- Chung C, Reilly S. Trametinib: a novel signal transduction inhibitors for the treatment of metastatic cutaneous melanoma. Am J Health Syst Pharm. 2015;72:101-110.
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy [published online February 12, 2009]. Cancer Lett. 2009;283:125-134.
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy [published online December 8, 2013]. Pharmacol Ther. 2014;142:176-182.
- Flaherty KT, Robert C, Hersey P, et al; METRIC Study Group. Improved survival with MEK inhibition in BRAF-mutated melanoma [published online June 4, 2012]. N Engl J Med. 2012;367:107-114.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer [published online June 9, 2002]. Nature. 2002;417:949-954.
- Houben R, Becker JC, Kappel A, et al. Constitutive activation of the Ras-Raf signaling pathway in metastatic melanoma is associated with poor prognosis. J Carcinog. 2004;3:6.
- Roberts PF, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291-3310.
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 2 dose-escalation trial [published online July 16, 2012]. Lancet Oncol. 2012;13:782-789.
- Anforth R, Liu M, Nguyen B, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib [published online December 9, 2013]. Australas J Dermatol. 2014;55:250-254.
- Gadiot J, Hooijkaas AI, Deken MA, et al. Synchronous BRAF(V600E) and MEK inhibition leads to superior control of murine melanoma by limiting MEK inhibitor induced skin toxicity. Onco Targets Ther. 2013;6:1649-1658.
- Anforth R, Carlos G, Clements A, et al. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks [published online November 21, 2014]. Br J Dermatol. 2015;172:239-243.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307-311.
- Green JS, Norris DA, Wisell K. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
Practice Points
- With the discovery of molecular targeting in melanoma, BRAF and MEK inhibitors have been increasingly utilized as therapies in metastatic melanoma management.
- Trametinib, a MEK inhibitor, is commonly associated with cutaneous adverse reactions, particularly acneform eruptions.
- We report a patient on trametinib who developed an eruption with an unusual xanthogranulomatous reaction pattern noted on histology.