User login
Granulomatous Dermatitis in a Patient With Cholangiocarcinoma Treated With BRAF and MEK Inhibitors
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
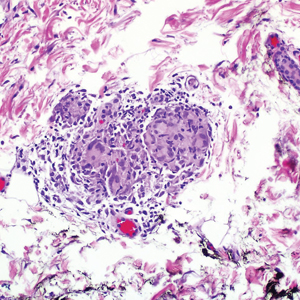
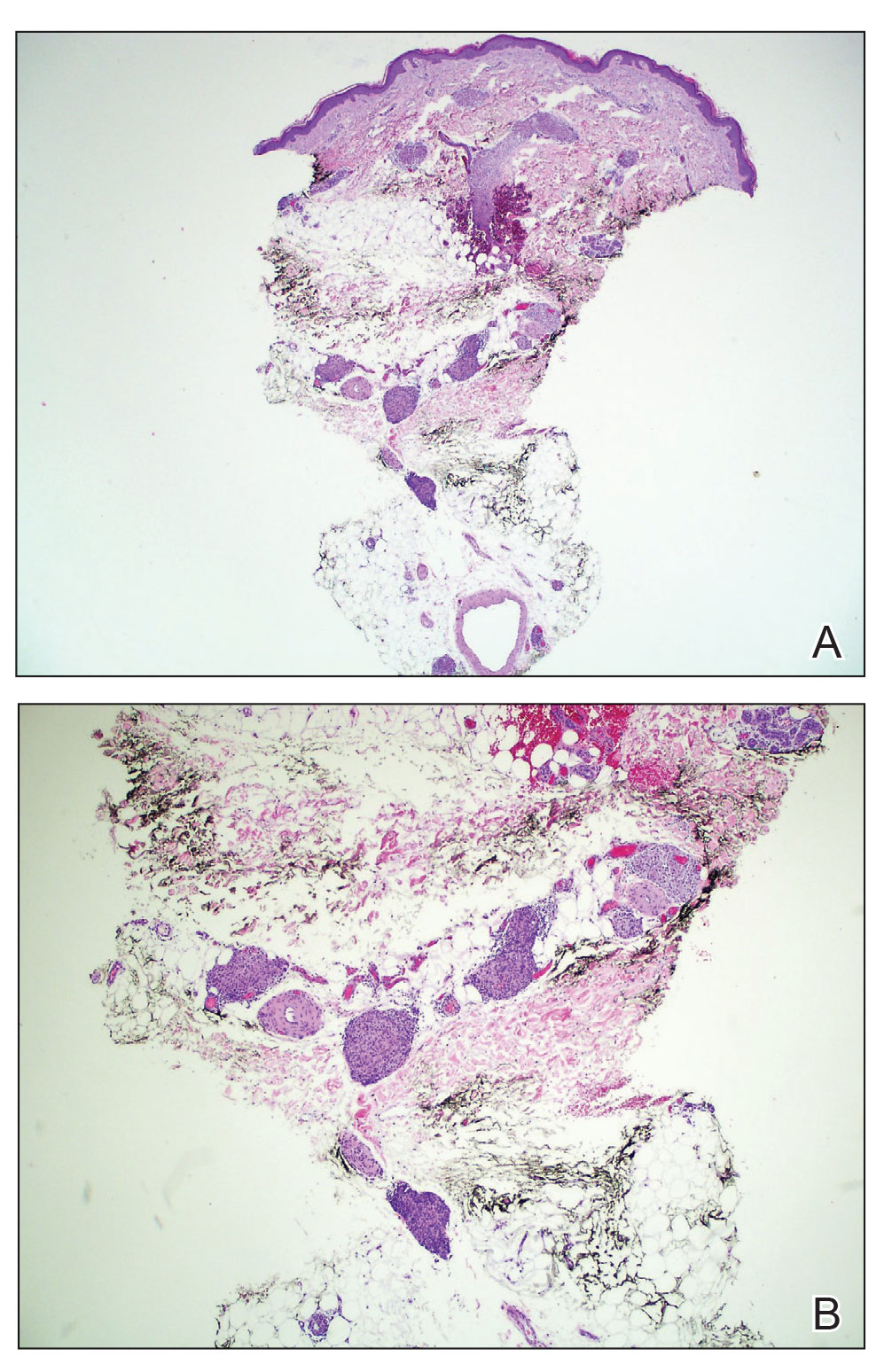
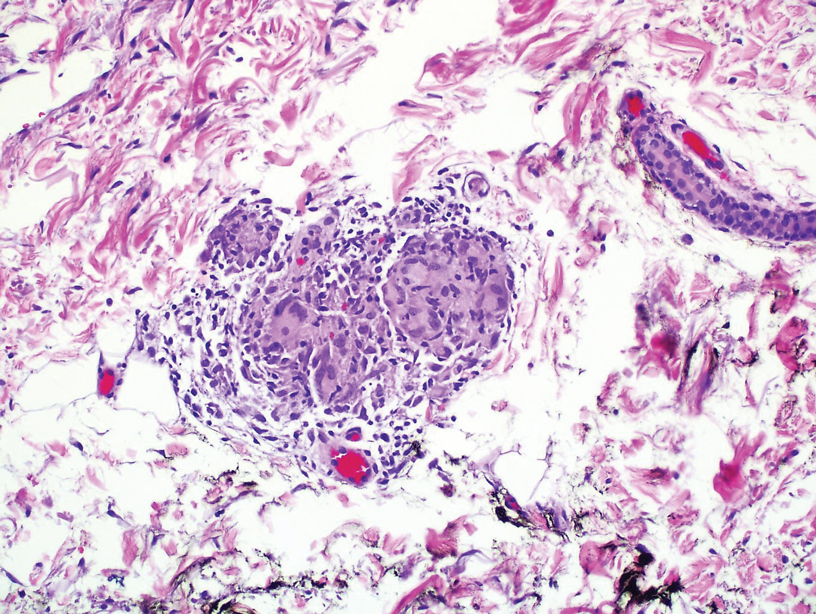
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.
A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.
BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
Practice Points
- Granulomatous dermatitis (GD) is a potential rare side effect of the use of BRAF and MEK inhibitors for the treatment of BRAF V600 mutation–positive cancers, including metastatic cholangiocarcinoma.
- Granulomatous dermatitis can resolve despite continuation of BRAF and MEK inhibitor therapies.
- Histologically, GD can appear similar to disease recurrence. It is imperative that clinicians and pathologists recognize the cutaneous manifestations of BRAF and MEK inhibitors.