User login
Gender and Patient Satisfaction in a Veterans Health Administration Outpatient Chemotherapy Unit
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
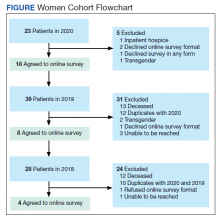
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
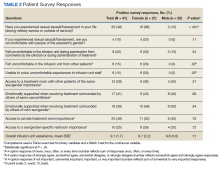
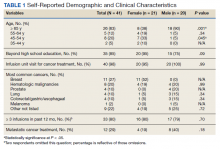
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
Leveraging Veterans Health Administration Clinical and Research Resources to Accelerate Discovery and Testing in Precision Oncology(FULL)
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
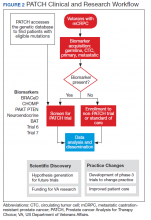
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
In May 2020, the US Food and Drug Administration (FDA) approved the first 2 targeted treatments for prostate cancer, specifically, the poly-(adenosine diphosphate-ribose) polymerase (PARP) inhibitors rucaparib and olaparib.1,2 For these medications to work, the tumor must have a homologous recombination deficiency (HRD), which is a form of DNA repair deficiency. The PARP pathway is important for DNA repair, and PARP inhibition leads to “synthetic lethality” in cancer cells that already are deficient in DNA repair mechanisms.3 Now, there is evidence that patients with prostate cancer who have HRD tumors and receive PARP inhibitors live longer when compared with those who receive standard of care options.4 These findings offer hope for patients with prostate cancer. They also demonstrate the process and potential benefits of precision oncology efforts; namely, targeted treatments for specific tumor types in cancer patients.
This article discusses the challenges and opportunities of precision oncology for US Department of Veterans Affairs (VA) Veterans Health Administration (VHA). First, the article will discuss working with relatively rare mutations. Second, the article will examine how the trials of olaparib and rucaparib illuminate the VHA contribution to research on new therapies for patients with cancer. Finally, the article will explore the ways in which VHA is becoming a major national contributor in drug discovery and approval of precision medications.
Precision Oncology
Despite advances in screening and treatment, an estimated 600,000 people in the US will die of cancer in 2020.5 Meaningful advances in cancer care depend on both laboratory and clinical research. This combination, known as translational research, takes discoveries in the laboratory and applies them to patients and vice versa. Successful translational research requires many components. These include talented scientists to form hypotheses and perform the work; money for supplies and equipment; platforms for timely dissemination of knowledge; well-trained clinicians to treat patients and lead research teams; and patients to participate in clinical trials. In precision oncology, the ability to find patients for the trials can be daunting, particularly in cases where the frequency of the mutation of interest is low.
During the 20th century, with few exceptions, physicians caring for patients with cancer had blunt instruments at their disposal. Surgery and radiation could lead to survival if the cancer was caught early enough. Systemic therapies, such as chemotherapy, rarely cured but could prolong life in some patients. However, chemotherapy is imprecise and targets any cell growing rapidly, including blood, hair, and gastrointestinal tract cells, which often leads to adverse effects. Sometimes complications from chemotherapy may shorten a person’s life, and certainly the quality of life during and after these treatments could be diminished. The improvements in cancer care occurred more rapidly once scientists had the tools to learn about individual tumors.
In the summer of 2000, researchers announced that the human genome had been sequenced.6 The genome (ie, DNA) consists of introns and exons that form a map for human development. Exons can be converted to proteins that carry out specific actions, such as helping in cell growth, cell death, or DNA repair. Solving the human genome itself did not lead directly to cures, but it did represent a huge advance in medical research. As time passed, sequencing genomes became more affordable, and sequencing just the exome alone was even cheaper.7 Treatments for cancer began to expand with the help of these tools, but questions as to the true benefit of targeted therapy also grew.8
Physicians and scientists have amassed more information about cancer cells and have applied this knowledge to active drug development. In 2001, the FDA approved the first targeted therapy, imatinib, for the treatment of chronic myelogenous leukemia (CML). This rapidly improved patient survival through targeting the mutated protein that leads to CML, rather than just aiming for rapidly dividing cells.9 Those mutations for which there is a drug to target, such as the BCR-ABL translocation in CML, are called actionable mutations.
Precision Oncology Program for Prostate Cancer
In 2016, the VA and the Prostate Cancer Foundation (PCF) established the Precision Oncology Program for Prostate Cancer (POPCaP) Centers of Excellence (COE). This partnership was formed to accelerate treatment and cure for veterans with prostate cancer. The VA Greater Los Angeles Healthcare System in California and VA Puget Sound Health Care System in Washington led this effort, and their principal investigators continue to co-lead POPCaP. Since its inception, 9 additional funded POPCaP COEs have joined, each with a mandate to sequence the tumors of men with metastatic prostate cancer.
The more that is learned about a tumor, the more likely it is that researchers can find mutations that are that tumor’s Achilles heel and defeat it. In fact, many drugs that can target mutations are already available. For example, BRCA2 is an actionable mutation that can be exploited by knocking out another key DNA repair mechanism in the cell, PARP. Today, the effort of sequencing has led to a rich database of mutations present in men with metastatic prostate cancer.
Although there are many targeted therapies, most have not been studied formally in prostate cancer. Occasionally, clinicians treating patients will use these drugs in an unapproved way, hoping that there will be anticancer activity. It is difficult to estimate the likelihood of success with a drug in this situation, and the safety profile may not be well described in that setting. Treatment decisions for incurable cancers must be made knowing the risks and benefits. This helps in shared decision making between the clinician and patient and informs choices concerning which laboratory tests to order and how often to see the patient. However, treatment decisions are sometimes made with the hope of activity when a cancer is known to be incurable. Very little data, which are critical to determine whether this helps or hurts patients, support this approach.
Some data suggest that sequencing and giving a drug for an actionable mutation may lead to better outcomes for patients. Sequencing of pancreatic tumors by Pishvaian and colleagues revealed that 282 of 1,082 (26%) samples harbored actionable mutations.10 Those patients who received a drug that targeted their actionable mutation (n = 46; 24%) lived longer when compared with those who had an actionable mutation but did not receive a drug that targeted it (hazard ratio [HR] 0.42 [95% CI, 0.26-0.68; P = .0004]). Additionally, those who received therapy for an actionable mutation lived longer when compared with those who did not have an actionable mutation (HR 0.34 [95% CI, 0.22-0.53; P < .001]). While this finding is intriguing, it does not mean that treating actionable mutations outside of a clinical trial should be done. To this end, VA established Prostate cancer Analysis for Therapy CHoice (PATCH) as a clinical trials network within POPCaP.
Prostate Cancer Analysis
The overall PATCH vision is designed for clinical care and research work to together toward improved care for those with prostate cancer (Figure 1). The resources necessary for successful translational research are substantial, and PATCH aims to streamline those resources. PATCH will support innovative, precision-based clinical research at the POPCaP COEs through its 5 arms.
Arm 1. Dedicated personnel ensure veteran access to trials in PATCH by giving patients and providers accurate information about available trial options; aiding veterans in traveling from home VA to a POPCaP COE for participation on a study; and maintaining the Committee for Veteran Participation in PATCH, where veterans will be represented and asked to provide input into the PATCH process.
Arm 2. Coordinators at the coordinating COE in Portland, Orgeon, train investigators and study staff at the local POPCaP COEs to ensure research can be performed in a safe and responsible way.
Arm 3. Personnel experienced in conducting clinical trials liaise with investigators at VA Central Institutional Review Board, monitor trials, build databases for appropriate and efficient data collection, and manage high-risk studies conducted under an Investigational New Drug application. This group works closely with biostatisticians to choose appropriate trial designs, estimate numbers of patients needed, and interpret data once they are collected.
Arm 4. Protocol development and data dissemination is coordinated by a group to assist investigators in drafting protocols and reviewing abstracts and manuscripts.
Arm 5. A core group manages contracts and budgets, as well as relationships between VA and industry, where funding and drugs may be obtained.
Perhaps most importantly, PATCH leverages the genetic data collected by POPCaP COEs to find patients for clinical trials. For example, the trials that examined olaparib and rucaparib assumed that the prevalence of HRD was about 25% in men with advanced prostate cancer.11 As these trials began enrollment, however, researchers discovered that the prevalence was < 20%. In fact, the study of olaparib screened 4,425 patients at 206 sites in 20 countries to identify 778 (18% of screened) patients with HRD.4 With widespread sequencing within VA, it could be possible to identify a substantial number of patients who are already known to have the mutation of interest (Figure 2).
Clinical Trials
There are currently 2 clinical trials in PATCH; 4 additional trials await funding decisions, and more trials are in the concept stage. BRACeD (NCT04038502) is a phase 2 trial examining platinum and taxane chemotherapy in tumors with HRD (specifically, BRCA1, BRCA2, and PALB2). About 15% to 20% of men with advanced prostate cancer will have a DNA repair defect in the tumor that could make them eligible for this study. The primary endpoint is progression-free survival.
A second study, CHOMP (NCT04104893), is a phase 2 trial examining the efficacy of immunotherapy (PD-1 inhibition) in tumors having mismatch repair deficiency or CDK12-/-. Each of those is found in about 7% of men with metastatic prostate cancer, and full accrual of a trial with rare mutations could take 5 to 10 years without a systematic approach of sequencing and identifying potential participants. The primary endpoint is a composite of radiographic response by iRECIST (immune response evaluation criteria in solid tumors), progression-free survival at 6 months and prostate specific antigen reduction by ≥ 50% in ≤ 12 weeks. With 11 POPCaP COEs sequencing the tumors of every man with metastatic prostate cancer, identifying men with the appropriate mutation is possible. PATCH will aid the sites in recruitment through outreach and coordination of travel.
Industry Partnerships
PATCH depends upon pharmaceutical industry partners, as clinical trials of even 40 patients can require significant funding and trial resources to operate. Furthermore, many drugs of interest are not available outside of a clinical trial, and partnerships enable VA researchers to access these medications. PATCH also benefits greatly from foundation partners, such as the PCF, which has made POPCaP possible and will continue to connect talented researchers with VA resources. Finally, access to other publicly available research funds, such as those from VA Office of Research and Development, National Institutes of Health, and US Department of Defense (DoD) Congressionally Directed Research Program are needed for trials.
Funding for these trials remains limited despite public health and broader interests in addressing important questions. Accelerated accrual through PATCH may be an attractive partnership opportunity for companies, foundations and government funding agencies to support the PATCH efforts.
Both POPCaP and PATCH highlight the potential promise of precision oncology within the nation’s largest integrated health care system. The VHA patient population enables prostate cancer researchers to serve an important early target. It also provides a foundational platform for a broader set of activities. These include a tailored approach to identifying tumor profiles and other patient characteristics that may help to elevate standard of care for other common cancers including ones affecting the lungs and/or head and neck.
To this end, VA has been working with the National Cancer Institute (NCI) and DoD to establish a national infrastructure for precision oncology across multiple cancer types.12 In addition to clinical capabilities and the ability to run clinical trials that can accrue sufficient patients to answer key questions, we have developed capabilities for data collection and sharing, and analytical tools to support a learning health care system approach as a core element to precision oncology.
Besides having a research-specific context, such informatics and information technology systems enable clinicians to obtain and apply decision-making data rapidly for a specific patient and cancer type. These systems take particular advantage of the extensive electronic health record that underlies the VHA system, integrating real-world evidence into rigorous trials for precision oncology and other diseases. This is important for facilitating prerequisite activities for quality assessments for incorporation into databases (with appropriate permissions) to enhance treatment options. These activities are a key focus of the APOLLO initiative.13 While a more in-depth discussion of the importance of informatics is beyond the scope of this article, the field represents an important investment that is needed to achieve the goals of precision oncology.
In addition to informatics and data handling capabilities, VA has a longstanding tradition of designing and coordinating multisite clinical trials. This dates to the time of World War II when returning veterans had a high prevalence of tuberculosis. Since then, VA has contributed extensively to landmark findings in cardiovascular disease and surgery, mental health, infectious disease, and cancer. It was a VA study that helped establish colonoscopy as a standard for colorectal cancer screening by detecting colonic neoplasms in asymptomatic patients.14
From such investigations, the VA Cooperative Studies Program (CSP) has developed many strategies to conduct multisite clinical trials. But, CSP also has organized its sites methodically for operational efficiency and the ability to maintain institutional knowledge that crosses different types of studies and diseases. Using its Network of Dedicated Enrollment Sites (NODES) model, VA partnered with NCI to more effectively address administrative and regulatory requirements for initiating trials and recruiting veterans into cancer clinical trials.15 This partnership—the NCI And VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE)—supports 12 sites with a central CSP Coordinating Center (CSPCC).
CSPCC provides support, shares best practices and provides organizational commitment at the senior levels of both agencies to overcome potential barriers. The goals and strategies are described by Schiller and colleagues.16 While still in its early stage as a cancer research network, NAVIGATE may be integrated with POPCaP and other parts of VA clinical research enterprise. This would allow us to specialize in advancing oncology care and to leverage capabilities more specifically to precision oncology. With an emphasis on recruitment, NAVIGATE has established capabilities with VA Informatics and Computing Infrastructure to quickly identify patients who may be eligible for particular clinical trials. We envision further refining these capabilities for precision oncology trials that incorporate genetic and other information for individual patients. VA also hopes to inform trial sponsors about design considerations. This is important since networked investigators will have direct insights into patient-level factors, which may help with more effectively identifying and enrolling them into trials for their particular cancers.
Conclusions
VA may have an opportunity to reach out to veterans who may not have immediate access to facilities running clinical trials. As it develops capabilities to bring the trial to the veteran, VA could have more virtual and/or centralized recruitment strategies. This would broaden opportunities for considering novel approaches that may not rely on a more traditional facility-based recruitment approach.
Ultimately, VA can be a critical part of a national effort to fight and, perhaps even, defeat cancers. With its extensive resources and capabilities, VA has the ability to advance a precision oncology agenda that provides veterans with the highest standard of care. It has built upon many key elements in clinical, technological and scientific fields of study that would challenge most health care systems given the extensive costs involved. In addition, creating strong partnerships with organizations such as PCF, NCI, and DoD that are complementary in resources and expertise will help VA to build a national network for cancer care. Putting this all together will support and facilitate a vision for more precise care for any veteran with cancer by more rapidly enabling the testing and approval of medications developed for this purpose.
Acknowledgments
The authors would like to thank Daphne Swancutt for comments and edits on drafts of this article.
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005
1. Lynparza (Olaparib) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP Inc, 2019.
2. Rubraca (rucaparib) [package insert]: Clovis Oncology, Inc., Boulder, CO: 2018.
3. McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med. 2014;371(18):1725-1735. doi:10.1056/NEJMra1407390
4. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
6. Bentley DR. Decoding the human genome sequence. Hum Mol Genet. 2000;9(16):2353-2358. doi:10.1093/hmg/9.16.2353
7. National Human Genome Research institute. The cost of sequencing a human genome. https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost. Updated October 30, 2019. Accessed July 31, 2020. 8. Paggio JCD, Sullivan R, Booth CM. Targeting the value of targeted therapy. Oncotarget. 2017;8(53):90612-90613. Published 2017 Oct 7. doi:10.18632/oncotarget.21596
9. Druker BJ, Guilhot F, O’Brien SG, et al; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. doi:10.1056/NEJMoa062867
10. Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial [published correction appears in Lancet Oncol. 2020 Apr;21(4):e182]. Lancet Oncol. 2020;21(4):508-518. doi:10.1016/S1470-2045(20)30074-7
11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
12. Fiore LD, Brophy MT, Ferguson RE, et al. Data sharing, clinical trials, and biomarkers in precision oncology: challenges, opportunities, and programs at the Department of Veterans Affairs. Clin Pharmacol Ther. 2017;101(5):586-589. doi:10.1002/cpt.660
13. Lee JSH, Darcy KM, Hu H, et al. From discovery to practice and survivorship: building a national real-world data learning healthcare framework for military and veteran cancer patients. Clin Pharmacol Ther. 2019;106(1):52-57. doi:10.1002/cpt.1425
14. Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380 [published correction appears in N Engl J Med 2000 Oct 19;343(16):1204]. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
15. Condon DL, Beck D, Kenworthy-Heinige T, et al. A cross-cutting approach to enhancing clinical trial site success: The Department of Veterans Affairs’ Network of Dedicated Enrollment Sites (NODES) model. Contemp Clin Trials Commun. 2017;6:78-84. Published 2017 Mar 29. doi:10.1016/j.conctc.2017.03.006
16. Schiller SJ, Shannon C, Brophy MT, et al. The National Cancer Institute and Department of Veterans Affairs Interagency Group to Accelerate Trials Enrollment (NAVIGATE): A federal collaboration to improve cancer care. Semin Oncol. 2019;46(4-5):308-313. doi:10.1053/j.seminoncol.2019.09.005