User login
Inhaled Corticosteroids Increase Risk of Serious Pneumonia in Patients with COPD
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents, especially fluticasone and budesonide, were investigated.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990–2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents, especially fluticasone and budesonide, were investigated.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990–2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents, especially fluticasone and budesonide, were investigated.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990–2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Inhaled Corticosteroids Increase Risk of Serious Pneumonia in Patients with COPD
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents were investigated, especially fluticasone and budesonide.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990-2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents were investigated, especially fluticasone and budesonide.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990-2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Clinical question: Does the risk of pneumonia vary for different inhaled agents?
Background: Inhaled corticosteroids (ICS) are known to increase the risk of pneumonia in COPD patients; duration, dosage, and various agents were investigated, especially fluticasone and budesonide.
Study design: Nested, case-control analysis.
Setting: Quebec health insurance database for new users with COPD, 1990-2005, with follow-up through 2007.
Synopsis: Investigators analyzed 163,514 patients, including 20,344 patients with serious pneumonia; current use of ICS was associated with a 69% increase in the rate of serious pneumonia (RR 1.69; 95% CI 1.63-1.75). The increased risk was sustained with long-term use but declined gradually to zero at six months after stopping ICS. The risk of serious pneumonia was higher with fluticasone (RR 2.01; 95% CI 1.93-2.10) than budesonide (RR 1.17; 95% CI 1.09-1.26).
Bottom line: Fluticasone was associated with an increased risk of pneumonia in COPD patients, consistent with earlier clinical trials, but the risk with budesonide was much lower.
Citation: Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029-1036.
Ambulatory Patients with COPD Exacerbations Can Be Managed Without Antibiotics in the Absence of Increased Sputum Purulence, Elevated C-Reactive Protein
Clinical question: Which criteria identify ambulatory patients with exacerbations of mild to moderate COPD who do not need antibiotics?
Background: The Anthonisen criteria (increased dyspnea, sputum volume, sputum purulence) are commonly used to identify which patients with COPD exacerbations would benefit from antibiotics. These criteria, however, were derived in patients with severe COPD. It is unknown whether these criteria are predictive in patients with mild to moderate COPD.
Study design: Multivariate logistic regression analysis of placebo group of a double-blinded RCT.
Setting: Multicenter, ambulatory, primary care clinics in Spain.
Synopsis: The original RCT enrolled 310 ambulatory patients with exacerbations of mild to moderate COPD and tested the efficacy of amoxicillin/clavulanate. Clinical failure without antibiotics was 19.9% compared to 9.5% with antibiotics (P=0.022). Here they analyzed the 152 patients from the placebo group to identify factors associated with increased risk of clinical failure. Only increased sputum purulence (OR 6.1, CI 1.5-25; P=0.005) or C-reactive protein (CRP) >40 mg/L (OR 13.4, CI 4.5-38.8, P<0.001) were independently associated with increased risk of failure. Presence of both predicted a 63.7% failure without antibiotics.
The study did not define “increased sputum purulence,” but this is similar to real-life clinical practice. Placebo effect cannot be ruled out, but correlation of the objective measures with the clinical assessments suggests that the clinical assessments were accurate. The study did not have a protocol for administering co-medications such as steroids and inhalers. Despite these limitations, the criteria of increased sputum purulence and CRP >40 mg/L identified COPD patients likely to have a clinical failure without antibiotics.
Bottom line: Patients with exacerbations of mild to moderate COPD who do not have increased sputum purulence or CRP >40 mg/L can be safely managed without antibiotics.
Citation: Maravitlles M, Moravas A, Hernandez S, Bayona C, Llor C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest. 2013;144(5):1571-1577.
Clinical question: Which criteria identify ambulatory patients with exacerbations of mild to moderate COPD who do not need antibiotics?
Background: The Anthonisen criteria (increased dyspnea, sputum volume, sputum purulence) are commonly used to identify which patients with COPD exacerbations would benefit from antibiotics. These criteria, however, were derived in patients with severe COPD. It is unknown whether these criteria are predictive in patients with mild to moderate COPD.
Study design: Multivariate logistic regression analysis of placebo group of a double-blinded RCT.
Setting: Multicenter, ambulatory, primary care clinics in Spain.
Synopsis: The original RCT enrolled 310 ambulatory patients with exacerbations of mild to moderate COPD and tested the efficacy of amoxicillin/clavulanate. Clinical failure without antibiotics was 19.9% compared to 9.5% with antibiotics (P=0.022). Here they analyzed the 152 patients from the placebo group to identify factors associated with increased risk of clinical failure. Only increased sputum purulence (OR 6.1, CI 1.5-25; P=0.005) or C-reactive protein (CRP) >40 mg/L (OR 13.4, CI 4.5-38.8, P<0.001) were independently associated with increased risk of failure. Presence of both predicted a 63.7% failure without antibiotics.
The study did not define “increased sputum purulence,” but this is similar to real-life clinical practice. Placebo effect cannot be ruled out, but correlation of the objective measures with the clinical assessments suggests that the clinical assessments were accurate. The study did not have a protocol for administering co-medications such as steroids and inhalers. Despite these limitations, the criteria of increased sputum purulence and CRP >40 mg/L identified COPD patients likely to have a clinical failure without antibiotics.
Bottom line: Patients with exacerbations of mild to moderate COPD who do not have increased sputum purulence or CRP >40 mg/L can be safely managed without antibiotics.
Citation: Maravitlles M, Moravas A, Hernandez S, Bayona C, Llor C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest. 2013;144(5):1571-1577.
Clinical question: Which criteria identify ambulatory patients with exacerbations of mild to moderate COPD who do not need antibiotics?
Background: The Anthonisen criteria (increased dyspnea, sputum volume, sputum purulence) are commonly used to identify which patients with COPD exacerbations would benefit from antibiotics. These criteria, however, were derived in patients with severe COPD. It is unknown whether these criteria are predictive in patients with mild to moderate COPD.
Study design: Multivariate logistic regression analysis of placebo group of a double-blinded RCT.
Setting: Multicenter, ambulatory, primary care clinics in Spain.
Synopsis: The original RCT enrolled 310 ambulatory patients with exacerbations of mild to moderate COPD and tested the efficacy of amoxicillin/clavulanate. Clinical failure without antibiotics was 19.9% compared to 9.5% with antibiotics (P=0.022). Here they analyzed the 152 patients from the placebo group to identify factors associated with increased risk of clinical failure. Only increased sputum purulence (OR 6.1, CI 1.5-25; P=0.005) or C-reactive protein (CRP) >40 mg/L (OR 13.4, CI 4.5-38.8, P<0.001) were independently associated with increased risk of failure. Presence of both predicted a 63.7% failure without antibiotics.
The study did not define “increased sputum purulence,” but this is similar to real-life clinical practice. Placebo effect cannot be ruled out, but correlation of the objective measures with the clinical assessments suggests that the clinical assessments were accurate. The study did not have a protocol for administering co-medications such as steroids and inhalers. Despite these limitations, the criteria of increased sputum purulence and CRP >40 mg/L identified COPD patients likely to have a clinical failure without antibiotics.
Bottom line: Patients with exacerbations of mild to moderate COPD who do not have increased sputum purulence or CRP >40 mg/L can be safely managed without antibiotics.
Citation: Maravitlles M, Moravas A, Hernandez S, Bayona C, Llor C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest. 2013;144(5):1571-1577.
Pre-Operative Angiotensin Axis Blockade Increases Risk of Hypotension, Acute Kidney Injury with Major Orthopedic Surgery
Clinical question: Do patients receiving pre-operative angiotensin axis blockade (AAB) prior to elective major orthopedic surgery have an increased risk of peri-operative hypotension and acute kidney injury (AKI)?
Background: Patients with pre-operative AAB from angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have an increased incidence of peri-operative hypotension. Patients undergoing cardiothoracic and vascular surgery with pre-operative AAB have increased incidence of post-operative AKI; however, there is scant literature evaluating the hypotensive and renal effects of pre-operative AAB prior to elective major orthopedic surgery.
Study design: Retrospective, cohort study.
Setting: Academic medical center.
Synopsis: Retrospective review of 922 patients undergoing spinal fusion, total knee arthroplasty, or total hip arthroplasty in one academic medical center in 2010 found that 37% received pre-operative AAB. Post-induction hypotension (systolic blood pressure ≤80 mm Hg for five minutes) was significantly higher in patients receiving AAB (12.2% vs. 6.7%; odds ratio [OR] 1.93, P=0.005). Post-operative AKI was significantly higher in patients receiving AAB (8.3% vs. 1.7%; OR 5.40, P<0.001), remaining significant after adjusting for intra-operative hypotension (OR 2.60, P=0.042). Developing AKI resulted in a significantly higher mean length of stay (5.76 vs. 3.28 days, P<0.001) but no difference in two-year mortality.
The findings suggest an association exists between pre-operative angiotensin-converting enzyme inhibitors/ARB, hypotension, and AKI following major orthopedic surgeries but does not demonstrate causality. A prospective, multi-center, randomized trial is needed to confirm that holding pre-operative AAB would decrease the incidence of AKI in patients undergoing major orthopedic procedures under general anesthesia.
Bottom line: Patients who underwent elective major orthopedic surgery who received pre-operative AAB therapy had an associated increased risk of post-induction hypotension and post-operative AKI, resulting in a greater hospital length of stay.
Citation: Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283-288.
Clinical question: Do patients receiving pre-operative angiotensin axis blockade (AAB) prior to elective major orthopedic surgery have an increased risk of peri-operative hypotension and acute kidney injury (AKI)?
Background: Patients with pre-operative AAB from angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have an increased incidence of peri-operative hypotension. Patients undergoing cardiothoracic and vascular surgery with pre-operative AAB have increased incidence of post-operative AKI; however, there is scant literature evaluating the hypotensive and renal effects of pre-operative AAB prior to elective major orthopedic surgery.
Study design: Retrospective, cohort study.
Setting: Academic medical center.
Synopsis: Retrospective review of 922 patients undergoing spinal fusion, total knee arthroplasty, or total hip arthroplasty in one academic medical center in 2010 found that 37% received pre-operative AAB. Post-induction hypotension (systolic blood pressure ≤80 mm Hg for five minutes) was significantly higher in patients receiving AAB (12.2% vs. 6.7%; odds ratio [OR] 1.93, P=0.005). Post-operative AKI was significantly higher in patients receiving AAB (8.3% vs. 1.7%; OR 5.40, P<0.001), remaining significant after adjusting for intra-operative hypotension (OR 2.60, P=0.042). Developing AKI resulted in a significantly higher mean length of stay (5.76 vs. 3.28 days, P<0.001) but no difference in two-year mortality.
The findings suggest an association exists between pre-operative angiotensin-converting enzyme inhibitors/ARB, hypotension, and AKI following major orthopedic surgeries but does not demonstrate causality. A prospective, multi-center, randomized trial is needed to confirm that holding pre-operative AAB would decrease the incidence of AKI in patients undergoing major orthopedic procedures under general anesthesia.
Bottom line: Patients who underwent elective major orthopedic surgery who received pre-operative AAB therapy had an associated increased risk of post-induction hypotension and post-operative AKI, resulting in a greater hospital length of stay.
Citation: Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283-288.
Clinical question: Do patients receiving pre-operative angiotensin axis blockade (AAB) prior to elective major orthopedic surgery have an increased risk of peri-operative hypotension and acute kidney injury (AKI)?
Background: Patients with pre-operative AAB from angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have an increased incidence of peri-operative hypotension. Patients undergoing cardiothoracic and vascular surgery with pre-operative AAB have increased incidence of post-operative AKI; however, there is scant literature evaluating the hypotensive and renal effects of pre-operative AAB prior to elective major orthopedic surgery.
Study design: Retrospective, cohort study.
Setting: Academic medical center.
Synopsis: Retrospective review of 922 patients undergoing spinal fusion, total knee arthroplasty, or total hip arthroplasty in one academic medical center in 2010 found that 37% received pre-operative AAB. Post-induction hypotension (systolic blood pressure ≤80 mm Hg for five minutes) was significantly higher in patients receiving AAB (12.2% vs. 6.7%; odds ratio [OR] 1.93, P=0.005). Post-operative AKI was significantly higher in patients receiving AAB (8.3% vs. 1.7%; OR 5.40, P<0.001), remaining significant after adjusting for intra-operative hypotension (OR 2.60, P=0.042). Developing AKI resulted in a significantly higher mean length of stay (5.76 vs. 3.28 days, P<0.001) but no difference in two-year mortality.
The findings suggest an association exists between pre-operative angiotensin-converting enzyme inhibitors/ARB, hypotension, and AKI following major orthopedic surgeries but does not demonstrate causality. A prospective, multi-center, randomized trial is needed to confirm that holding pre-operative AAB would decrease the incidence of AKI in patients undergoing major orthopedic procedures under general anesthesia.
Bottom line: Patients who underwent elective major orthopedic surgery who received pre-operative AAB therapy had an associated increased risk of post-induction hypotension and post-operative AKI, resulting in a greater hospital length of stay.
Citation: Nielson E, Hennrikus E, Lehman E, Mets B. Angiotensin axis blockade, hypotension, and acute kidney injury in elective major orthopedic surgery. J Hosp Med. 2014;9(5):283-288.
American College of Physicians Releases Clinical Practice Guideline for Treating Anemia in Heart Disease Patients
Clinical question: What is the recommended threshold for red blood cell (RBC) transfusion and erythropoiesis-stimulating agents (ESA) in anemic hospitalized patients with coronary heart disease?
Background: Anemia can worsen cardiac function and is associated with increased risk of hospitalization and death in patients with coronary heart disease (CHD) or congestive heart failure (CHF). It is unclear if treatments such as RBC transfusion, ESA, or iron replacement improve outcomes in patients with heart disease.
Study design: Systematic review.
Setting: Studies of hospitalized medical and surgical patients.
Synopsis: The guideline was developed by reviewing studies evaluating anemia treatment outcomes, including mortality, hospitalization, exercise tolerance, quality of life, and cardiovascular events. Six studies evaluated the benefits and harms resulting from RBC transfusion, each determined to be low-quality evidence. The current evidence showed no benefit when comparing liberal (hemoglobin (Hgb) >10 g/dL) versus restrictive (Hgb <10 g/dL) transfusion thresholds. Potential harms of transfusion included fever, transfusion-related acute lung injury, and CHF.
Given the low-quality evidence, the American College of Physicians (ACP) makes a weak recommendation for a restrictive transfusion strategy of Hgb 7-8 g/dL in patients with CHD.
A review of 16 RCTs (moderate-quality evidence) evaluated the effects of ESAs in mild to moderate anemia and showed no difference in outcomes for patients with CHD and CHF. Harms associated with ESA therapy included hypertension and venous thrombosis. The ACP makes a strong recommendation not to use ESAs in patients with heart disease.
Bottom line: The ACP recommends restrictive transfusion with hemoglobin threshold of 7-8 g/dL in hospitalized patients with CHD (weak recommendation, low-quality evidence) and recommends against using erythropoiesis-stimulating agents for mild to moderate anemia in patients with CHF or CHD (strong recommendation, moderate-quality evidence).
Citation: Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(11):770-779.
Clinical question: What is the recommended threshold for red blood cell (RBC) transfusion and erythropoiesis-stimulating agents (ESA) in anemic hospitalized patients with coronary heart disease?
Background: Anemia can worsen cardiac function and is associated with increased risk of hospitalization and death in patients with coronary heart disease (CHD) or congestive heart failure (CHF). It is unclear if treatments such as RBC transfusion, ESA, or iron replacement improve outcomes in patients with heart disease.
Study design: Systematic review.
Setting: Studies of hospitalized medical and surgical patients.
Synopsis: The guideline was developed by reviewing studies evaluating anemia treatment outcomes, including mortality, hospitalization, exercise tolerance, quality of life, and cardiovascular events. Six studies evaluated the benefits and harms resulting from RBC transfusion, each determined to be low-quality evidence. The current evidence showed no benefit when comparing liberal (hemoglobin (Hgb) >10 g/dL) versus restrictive (Hgb <10 g/dL) transfusion thresholds. Potential harms of transfusion included fever, transfusion-related acute lung injury, and CHF.
Given the low-quality evidence, the American College of Physicians (ACP) makes a weak recommendation for a restrictive transfusion strategy of Hgb 7-8 g/dL in patients with CHD.
A review of 16 RCTs (moderate-quality evidence) evaluated the effects of ESAs in mild to moderate anemia and showed no difference in outcomes for patients with CHD and CHF. Harms associated with ESA therapy included hypertension and venous thrombosis. The ACP makes a strong recommendation not to use ESAs in patients with heart disease.
Bottom line: The ACP recommends restrictive transfusion with hemoglobin threshold of 7-8 g/dL in hospitalized patients with CHD (weak recommendation, low-quality evidence) and recommends against using erythropoiesis-stimulating agents for mild to moderate anemia in patients with CHF or CHD (strong recommendation, moderate-quality evidence).
Citation: Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(11):770-779.
Clinical question: What is the recommended threshold for red blood cell (RBC) transfusion and erythropoiesis-stimulating agents (ESA) in anemic hospitalized patients with coronary heart disease?
Background: Anemia can worsen cardiac function and is associated with increased risk of hospitalization and death in patients with coronary heart disease (CHD) or congestive heart failure (CHF). It is unclear if treatments such as RBC transfusion, ESA, or iron replacement improve outcomes in patients with heart disease.
Study design: Systematic review.
Setting: Studies of hospitalized medical and surgical patients.
Synopsis: The guideline was developed by reviewing studies evaluating anemia treatment outcomes, including mortality, hospitalization, exercise tolerance, quality of life, and cardiovascular events. Six studies evaluated the benefits and harms resulting from RBC transfusion, each determined to be low-quality evidence. The current evidence showed no benefit when comparing liberal (hemoglobin (Hgb) >10 g/dL) versus restrictive (Hgb <10 g/dL) transfusion thresholds. Potential harms of transfusion included fever, transfusion-related acute lung injury, and CHF.
Given the low-quality evidence, the American College of Physicians (ACP) makes a weak recommendation for a restrictive transfusion strategy of Hgb 7-8 g/dL in patients with CHD.
A review of 16 RCTs (moderate-quality evidence) evaluated the effects of ESAs in mild to moderate anemia and showed no difference in outcomes for patients with CHD and CHF. Harms associated with ESA therapy included hypertension and venous thrombosis. The ACP makes a strong recommendation not to use ESAs in patients with heart disease.
Bottom line: The ACP recommends restrictive transfusion with hemoglobin threshold of 7-8 g/dL in hospitalized patients with CHD (weak recommendation, low-quality evidence) and recommends against using erythropoiesis-stimulating agents for mild to moderate anemia in patients with CHF or CHD (strong recommendation, moderate-quality evidence).
Citation: Qaseem A, Humphrey LL, Fitterman N, Starkey M, Shekelle P. Treatment of anemia in patients with heart disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159(11):770-779.
Risk Stratification Model Predicts Continued Stability in Patients with Pulmonary Embolism
Clinical question: Can right ventricular (RV) dysfunction and troponin levels be used to risk stratify hemodynamically stable patients with acute pulmonary embolism (PE)?
Background: PE can present with varying degrees of clinical severity, necessitating appropriate risk stratification for decision making and management. Observational studies have demonstrated that RV dysfunction and injury provide important prognostic information in patients with acute PE, but this has not been confirmed in large prospective studies.
Study design: Prospective, cohort study.
Setting: Multicenter registry of academic and community hospitals in Italy.
Synopsis: Using the Italian Pulmonary Embolism Registry database of 860 hemodynamically stable patients with PE who underwent troponin measurement and echocardiogram, this study demonstrated that a model including RV dysfunction and injury has an incremental prognostic value for risk stratification of in-hospital death or clinical deterioration. The negative predictive value of negative echocardiography with negative troponin was 100% for in-hospital death and 99% for in-hospital clinical deterioration.
This study was limited by its observational approach; in addition, it did not provide its metrics for defining hemodynamic stability and did not standardize or report treatment strategies for these patients.
The predictive value of this study is impressive and could lead to the identification of patients in whom early discharge or brief hospitalization is appropriate; however, its application could be challenging in clinical environments where timely echocardiography resources are not readily available to a hemodynamically stable patient population.
Bottom line: Negative troponin and absence of RV dysfunction predict clinically stable pulmonary embolism.
Citation: Becattini C, Casazza F, Forgione C, et al. Acute pulmonary embolism: external validation of an integrated risk stratification model. Chest. 2013;144(5):1539-1545.
Clinical question: Can right ventricular (RV) dysfunction and troponin levels be used to risk stratify hemodynamically stable patients with acute pulmonary embolism (PE)?
Background: PE can present with varying degrees of clinical severity, necessitating appropriate risk stratification for decision making and management. Observational studies have demonstrated that RV dysfunction and injury provide important prognostic information in patients with acute PE, but this has not been confirmed in large prospective studies.
Study design: Prospective, cohort study.
Setting: Multicenter registry of academic and community hospitals in Italy.
Synopsis: Using the Italian Pulmonary Embolism Registry database of 860 hemodynamically stable patients with PE who underwent troponin measurement and echocardiogram, this study demonstrated that a model including RV dysfunction and injury has an incremental prognostic value for risk stratification of in-hospital death or clinical deterioration. The negative predictive value of negative echocardiography with negative troponin was 100% for in-hospital death and 99% for in-hospital clinical deterioration.
This study was limited by its observational approach; in addition, it did not provide its metrics for defining hemodynamic stability and did not standardize or report treatment strategies for these patients.
The predictive value of this study is impressive and could lead to the identification of patients in whom early discharge or brief hospitalization is appropriate; however, its application could be challenging in clinical environments where timely echocardiography resources are not readily available to a hemodynamically stable patient population.
Bottom line: Negative troponin and absence of RV dysfunction predict clinically stable pulmonary embolism.
Citation: Becattini C, Casazza F, Forgione C, et al. Acute pulmonary embolism: external validation of an integrated risk stratification model. Chest. 2013;144(5):1539-1545.
Clinical question: Can right ventricular (RV) dysfunction and troponin levels be used to risk stratify hemodynamically stable patients with acute pulmonary embolism (PE)?
Background: PE can present with varying degrees of clinical severity, necessitating appropriate risk stratification for decision making and management. Observational studies have demonstrated that RV dysfunction and injury provide important prognostic information in patients with acute PE, but this has not been confirmed in large prospective studies.
Study design: Prospective, cohort study.
Setting: Multicenter registry of academic and community hospitals in Italy.
Synopsis: Using the Italian Pulmonary Embolism Registry database of 860 hemodynamically stable patients with PE who underwent troponin measurement and echocardiogram, this study demonstrated that a model including RV dysfunction and injury has an incremental prognostic value for risk stratification of in-hospital death or clinical deterioration. The negative predictive value of negative echocardiography with negative troponin was 100% for in-hospital death and 99% for in-hospital clinical deterioration.
This study was limited by its observational approach; in addition, it did not provide its metrics for defining hemodynamic stability and did not standardize or report treatment strategies for these patients.
The predictive value of this study is impressive and could lead to the identification of patients in whom early discharge or brief hospitalization is appropriate; however, its application could be challenging in clinical environments where timely echocardiography resources are not readily available to a hemodynamically stable patient population.
Bottom line: Negative troponin and absence of RV dysfunction predict clinically stable pulmonary embolism.
Citation: Becattini C, Casazza F, Forgione C, et al. Acute pulmonary embolism: external validation of an integrated risk stratification model. Chest. 2013;144(5):1539-1545.
Accelerated Diagnostic Protocol for Chest Pain Results in Earlier Discharge of Low-Risk Patients
Clinical question: In patients with possible cardiac chest pain, does an accelerated diagnostic protocol result in higher rates of successful early discharge when compared with the standard care pathway?
Background: An accelerated diagnostic pathway (ADP), which combines TIMI [thrombolysis in myocardial infarction] score, EKG, and zero- and two-hour troponin levels, can identify low-risk patients presenting with chest pain; however, little is known about its effect on the rates of early, successful discharge in a real-world population.
Study design: RCT.
Setting: An academic general and tertiary care hospital in Christchurch, New Zealand.
Synopsis: This study of 542 patients ages 18 years and older who presented with chest pain demonstrated that when compared with a conventional pathway, an ADP results in nearly twice the proportion of patients (19.3% vs. 11.0%; P=0.008) achieving safe, early discharge from the ED, defined as discharge within six hours and no major adverse cardiac events within 30 days. This was a single-center trial in New Zealand, which limits its sample size and its generalizability; however, if larger studies led to the implementation of ADP by ED physicians, there would likely be fewer low-risk patients admitted to hospitalist services for evaluation of chest pain.
Bottom line: An accelerated diagnostic chest pain protocol results in earlier discharge of low-risk patients.
Citation: Than M, Aldous S, Lord SJ, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. 2014;174(1):51-58.
Clinical question: In patients with possible cardiac chest pain, does an accelerated diagnostic protocol result in higher rates of successful early discharge when compared with the standard care pathway?
Background: An accelerated diagnostic pathway (ADP), which combines TIMI [thrombolysis in myocardial infarction] score, EKG, and zero- and two-hour troponin levels, can identify low-risk patients presenting with chest pain; however, little is known about its effect on the rates of early, successful discharge in a real-world population.
Study design: RCT.
Setting: An academic general and tertiary care hospital in Christchurch, New Zealand.
Synopsis: This study of 542 patients ages 18 years and older who presented with chest pain demonstrated that when compared with a conventional pathway, an ADP results in nearly twice the proportion of patients (19.3% vs. 11.0%; P=0.008) achieving safe, early discharge from the ED, defined as discharge within six hours and no major adverse cardiac events within 30 days. This was a single-center trial in New Zealand, which limits its sample size and its generalizability; however, if larger studies led to the implementation of ADP by ED physicians, there would likely be fewer low-risk patients admitted to hospitalist services for evaluation of chest pain.
Bottom line: An accelerated diagnostic chest pain protocol results in earlier discharge of low-risk patients.
Citation: Than M, Aldous S, Lord SJ, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. 2014;174(1):51-58.
Clinical question: In patients with possible cardiac chest pain, does an accelerated diagnostic protocol result in higher rates of successful early discharge when compared with the standard care pathway?
Background: An accelerated diagnostic pathway (ADP), which combines TIMI [thrombolysis in myocardial infarction] score, EKG, and zero- and two-hour troponin levels, can identify low-risk patients presenting with chest pain; however, little is known about its effect on the rates of early, successful discharge in a real-world population.
Study design: RCT.
Setting: An academic general and tertiary care hospital in Christchurch, New Zealand.
Synopsis: This study of 542 patients ages 18 years and older who presented with chest pain demonstrated that when compared with a conventional pathway, an ADP results in nearly twice the proportion of patients (19.3% vs. 11.0%; P=0.008) achieving safe, early discharge from the ED, defined as discharge within six hours and no major adverse cardiac events within 30 days. This was a single-center trial in New Zealand, which limits its sample size and its generalizability; however, if larger studies led to the implementation of ADP by ED physicians, there would likely be fewer low-risk patients admitted to hospitalist services for evaluation of chest pain.
Bottom line: An accelerated diagnostic chest pain protocol results in earlier discharge of low-risk patients.
Citation: Than M, Aldous S, Lord SJ, et al. A 2-hour diagnostic protocol for possible cardiac chest pain in the emergency department: a randomized clinical trial. JAMA Intern Med. 2014;174(1):51-58.
Restrictive Blood Transfusion Strategy with Trigger Hemoglobin
Clinical question: What effect does a restrictive hemoglobin transfusion trigger of <7 g/dL have on clinical outcomes compared to more liberal transfusion triggers?
Background: Blood transfusions are standard of care in managing anemia despite sparse evidence that they improve clinical outcomes. As more evidence mounts that blood transfusions are associated with worse outcomes, the threshold hemoglobin level for transfusion has decreased. The safest hemoglobin trigger for blood transfusion remains unknown.
Study design: Meta-analysis of randomized control trials (RCTs) and systematic review of observational studies.
Setting: Data from MEDLINE.
Synopsis: Pooled data from three RCTs, including 2364 patients, compared outcomes between a restrictive hemoglobin transfusion trigger of <7 g/dL with a more liberal trigger. The restrictive strategy resulted in decreased in-hospital mortality (RR 0.74, CI 0.6-0.92), total mortality (RR 0.8, CI 0.65-0.98), rebleeding (RR 0.64, CI 0.45-0.9), acute coronary syndrome (RR 0.44, CI 0.22-0.89), and pulmonary edema (RR 0.48, CI 0.33-0.72). Using the restrictive strategy, the number needed to treat to prevent one death was 33.
A second meta-analysis of 16 RCTs found that less restrictive transfusion strategies (triggers of 7.5 to 10 g/dL) were not effective in improving outcomes. A systematic review of observational studies suggested that normovolemic patients can tolerate hemoglobins of 5-6 g/dL without affecting oxygen delivery.
The primary meta-analysis was limited by the inclusion of adult and pediatric patients and different indications for transfusion (critical illness, gastrointestinal bleed), which could have introduced some bias. Although the safest transfusion trigger remains unknown, this study demonstrates that <7 g/dL is safer than more liberal triggers.
Bottom line: A restrictive blood transfusion strategy with hemoglobin trigger of <7 g/dL in patients with critical illness or bleeding significantly decreases rebleeding, cardiac events, and total mortality.
Citation: Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127(2): 124-131.
Clinical question: What effect does a restrictive hemoglobin transfusion trigger of <7 g/dL have on clinical outcomes compared to more liberal transfusion triggers?
Background: Blood transfusions are standard of care in managing anemia despite sparse evidence that they improve clinical outcomes. As more evidence mounts that blood transfusions are associated with worse outcomes, the threshold hemoglobin level for transfusion has decreased. The safest hemoglobin trigger for blood transfusion remains unknown.
Study design: Meta-analysis of randomized control trials (RCTs) and systematic review of observational studies.
Setting: Data from MEDLINE.
Synopsis: Pooled data from three RCTs, including 2364 patients, compared outcomes between a restrictive hemoglobin transfusion trigger of <7 g/dL with a more liberal trigger. The restrictive strategy resulted in decreased in-hospital mortality (RR 0.74, CI 0.6-0.92), total mortality (RR 0.8, CI 0.65-0.98), rebleeding (RR 0.64, CI 0.45-0.9), acute coronary syndrome (RR 0.44, CI 0.22-0.89), and pulmonary edema (RR 0.48, CI 0.33-0.72). Using the restrictive strategy, the number needed to treat to prevent one death was 33.
A second meta-analysis of 16 RCTs found that less restrictive transfusion strategies (triggers of 7.5 to 10 g/dL) were not effective in improving outcomes. A systematic review of observational studies suggested that normovolemic patients can tolerate hemoglobins of 5-6 g/dL without affecting oxygen delivery.
The primary meta-analysis was limited by the inclusion of adult and pediatric patients and different indications for transfusion (critical illness, gastrointestinal bleed), which could have introduced some bias. Although the safest transfusion trigger remains unknown, this study demonstrates that <7 g/dL is safer than more liberal triggers.
Bottom line: A restrictive blood transfusion strategy with hemoglobin trigger of <7 g/dL in patients with critical illness or bleeding significantly decreases rebleeding, cardiac events, and total mortality.
Citation: Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127(2): 124-131.
Clinical question: What effect does a restrictive hemoglobin transfusion trigger of <7 g/dL have on clinical outcomes compared to more liberal transfusion triggers?
Background: Blood transfusions are standard of care in managing anemia despite sparse evidence that they improve clinical outcomes. As more evidence mounts that blood transfusions are associated with worse outcomes, the threshold hemoglobin level for transfusion has decreased. The safest hemoglobin trigger for blood transfusion remains unknown.
Study design: Meta-analysis of randomized control trials (RCTs) and systematic review of observational studies.
Setting: Data from MEDLINE.
Synopsis: Pooled data from three RCTs, including 2364 patients, compared outcomes between a restrictive hemoglobin transfusion trigger of <7 g/dL with a more liberal trigger. The restrictive strategy resulted in decreased in-hospital mortality (RR 0.74, CI 0.6-0.92), total mortality (RR 0.8, CI 0.65-0.98), rebleeding (RR 0.64, CI 0.45-0.9), acute coronary syndrome (RR 0.44, CI 0.22-0.89), and pulmonary edema (RR 0.48, CI 0.33-0.72). Using the restrictive strategy, the number needed to treat to prevent one death was 33.
A second meta-analysis of 16 RCTs found that less restrictive transfusion strategies (triggers of 7.5 to 10 g/dL) were not effective in improving outcomes. A systematic review of observational studies suggested that normovolemic patients can tolerate hemoglobins of 5-6 g/dL without affecting oxygen delivery.
The primary meta-analysis was limited by the inclusion of adult and pediatric patients and different indications for transfusion (critical illness, gastrointestinal bleed), which could have introduced some bias. Although the safest transfusion trigger remains unknown, this study demonstrates that <7 g/dL is safer than more liberal triggers.
Bottom line: A restrictive blood transfusion strategy with hemoglobin trigger of <7 g/dL in patients with critical illness or bleeding significantly decreases rebleeding, cardiac events, and total mortality.
Citation: Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127(2): 124-131.
ED Observation Syncope Protocol Reduces Resource Use Without Compromising Safety
Clinical question: Can an ED observation syncope protocol decrease resource utilization without adversely affecting outcomes?
Background: Syncope is a common complaint in EDs. Uncertainty in the best way to manage older adults at intermediate risk for adverse outcomes results in frequent, costly hospitalizations without clear improvement in outcomes. An observation syncope protocol may standardize care and reduce admissions without worsening safety.
Study design: Randomized, non-blinded, clinical trial.
Setting: Five EDs in the United States.
Synopsis: One hundred twenty-four intermediate-risk adults (≥50 years) presenting with syncope or near syncope were randomized to an ED observation syncope protocol vs. routine admission. The observation protocol resulted in a lower admission rate (15% vs. 92%), shorter stay (29 vs. 47 hours), and decreased index hospital costs ($629); however, there were no significant differences in serious outcomes after discharge at 30 days (3% vs. 0%) or six months (8% vs. 10%), 30-day quality of life scores, or patient satisfaction. Limitations include the small study size, the fact that half of eligible patients were excluded as high risk, higher number of abnormal ECGs in the control group, and a cost savings that was attenuated at 30 days.
This ED observation syncope protocol demonstrated an impressive decrease in admission rates and length of stay without compromising safety, but only a small fraction of patients met the inclusion criteria, and the cost savings were small and transient.
Bottom line: An ED observation syncope protocol for intermediate-risk patients decreased admission rates, length of stay, and index hospitalization costs without a difference in safety events, patient satisfaction, or quality of life.
Citation: Sun BC, McCreath H, Liang L, et al. Randomized clinical trial of an emergency department observation syncope protocol versus routine inpatient admission [published online ahead of print November 13, 2013]. Ann Emerg Med.
Clinical question: Can an ED observation syncope protocol decrease resource utilization without adversely affecting outcomes?
Background: Syncope is a common complaint in EDs. Uncertainty in the best way to manage older adults at intermediate risk for adverse outcomes results in frequent, costly hospitalizations without clear improvement in outcomes. An observation syncope protocol may standardize care and reduce admissions without worsening safety.
Study design: Randomized, non-blinded, clinical trial.
Setting: Five EDs in the United States.
Synopsis: One hundred twenty-four intermediate-risk adults (≥50 years) presenting with syncope or near syncope were randomized to an ED observation syncope protocol vs. routine admission. The observation protocol resulted in a lower admission rate (15% vs. 92%), shorter stay (29 vs. 47 hours), and decreased index hospital costs ($629); however, there were no significant differences in serious outcomes after discharge at 30 days (3% vs. 0%) or six months (8% vs. 10%), 30-day quality of life scores, or patient satisfaction. Limitations include the small study size, the fact that half of eligible patients were excluded as high risk, higher number of abnormal ECGs in the control group, and a cost savings that was attenuated at 30 days.
This ED observation syncope protocol demonstrated an impressive decrease in admission rates and length of stay without compromising safety, but only a small fraction of patients met the inclusion criteria, and the cost savings were small and transient.
Bottom line: An ED observation syncope protocol for intermediate-risk patients decreased admission rates, length of stay, and index hospitalization costs without a difference in safety events, patient satisfaction, or quality of life.
Citation: Sun BC, McCreath H, Liang L, et al. Randomized clinical trial of an emergency department observation syncope protocol versus routine inpatient admission [published online ahead of print November 13, 2013]. Ann Emerg Med.
Clinical question: Can an ED observation syncope protocol decrease resource utilization without adversely affecting outcomes?
Background: Syncope is a common complaint in EDs. Uncertainty in the best way to manage older adults at intermediate risk for adverse outcomes results in frequent, costly hospitalizations without clear improvement in outcomes. An observation syncope protocol may standardize care and reduce admissions without worsening safety.
Study design: Randomized, non-blinded, clinical trial.
Setting: Five EDs in the United States.
Synopsis: One hundred twenty-four intermediate-risk adults (≥50 years) presenting with syncope or near syncope were randomized to an ED observation syncope protocol vs. routine admission. The observation protocol resulted in a lower admission rate (15% vs. 92%), shorter stay (29 vs. 47 hours), and decreased index hospital costs ($629); however, there were no significant differences in serious outcomes after discharge at 30 days (3% vs. 0%) or six months (8% vs. 10%), 30-day quality of life scores, or patient satisfaction. Limitations include the small study size, the fact that half of eligible patients were excluded as high risk, higher number of abnormal ECGs in the control group, and a cost savings that was attenuated at 30 days.
This ED observation syncope protocol demonstrated an impressive decrease in admission rates and length of stay without compromising safety, but only a small fraction of patients met the inclusion criteria, and the cost savings were small and transient.
Bottom line: An ED observation syncope protocol for intermediate-risk patients decreased admission rates, length of stay, and index hospitalization costs without a difference in safety events, patient satisfaction, or quality of life.
Citation: Sun BC, McCreath H, Liang L, et al. Randomized clinical trial of an emergency department observation syncope protocol versus routine inpatient admission [published online ahead of print November 13, 2013]. Ann Emerg Med.
How Is SIADH Diagnosed and Managed?
Case
A 70-year-old woman with hypertension presents after a fall. Her medications include hydrochlorothiazide. Her blood pressure is 130/70 mm/Hg, with heart rate of 86. She has normal orthostatic vital signs. Her mucus membranes are moist and she has no jugular venous distension, edema, or ascites. Her plasma sodium (PNa) is 125 mmol/L, potassium 3.6 mmol/L, blood urea nitrogen (BUN) 30 mg/dL, and creatinine 0.8 mg/dL. Additional labs include serum thyroid stimulating hormone 1.12 mIU/L, cortisol 15 mcg/dL, serum osmolality 270 mOsm/kg, uric acid 4 mg/dL, urine osmolality 300 mOsm/kg, urine sodium (UNa) 40 mmol/L, fractional excretion of sodium 1.0%, and fractional excretion of urate (FEUrate) 13%. She receives 2 L isotonic saline intravenously over 24 hours, with resulting PNa of 127.
What is the cause of her hyponatremia, and how should her hyponatremia be managed?
Overview
Hyponatremia is one of the most common electrolyte abnormalities; it has a prevalence as high as 30% upon admission to the hospital.1 Hyponatremia is important clinically because of its high risk of mortality in the acute and symptomatic setting, and the risk of central pontine myelinolysis (CPM), or death with too rapid correction.2 Even so-called “asymptomatic” mild hyponatremia is associated with increased falls and impairments in gait and attention in the elderly.3
Hyponatremia is a state of excess water compared with the amount of solute in the extracellular fluid. To aid in diagnosing the etiology of hypotonic hyponatremia, the differential is traditionally divided into categories based on extracellular fluid volume (ECV) status, as shown in Table 1 (below), with syndrome of inappropriate antidiuretic hormone secretion (SIADH) being the most common cause of euvolemic hyponatremia.2 However, data show that clinical determination of volume status is often flawed,4 and an algorithmic approach to diagnosis and treatment yields improved results.5
Review of the Data
Diagnosis of SIADH. The original diagnostic criteria for SIADH, with minor modifications, are presented in Table 2, page 18).6,7,8 However, applying these criteria in clinical settings presents several difficulties, most notably a determination of ECV. The gold standard for assessing ECV status is by radioisotope, which is not practically feasible.9 Therefore, clinicians must rely on surrogate clinical markers of ECV (orthostatic hypotension, skin turgor, mucus membrane dryness, central venous pressure, BUN, BUN-creatinine ratio, and serum uric acid levels), which lack both sensitivity and specificity.4 Astoundingly, clinical assessment of ECV has been demonstrated to be accurate only 50% of the time when differentiating euvolemic patients from those with hypovolemia.4
Another challenge lies in the interpretation of UNa, which frequently is used as a surrogate for extra-arterial blood volume (EABV) status.10 Unfortunately, in the setting of diuretic use, UNa becomes inaccurate. The FEUrate, however, is unaffected by diuretic use and can be helpful in distinguishing between etiologies of hyponatremia with UNa greater than 30 mmol/L.11 The FEUrate is about 10% in normal euvolemic subjects and is reduced (usually <8%) in patients with low effective arterial blood volume.11,12 A trial of 86 patients demonstrated that a FEUrate of 12% had a specificity and positive predictive value of 100% in accurately identifying SIADH from diuretic-induced hyponatremia in patients on diuretics.11,12 Therefore, the UNa is a valid marker of EABV status when patients are not on diuretics; however, the FEUrate should be used in the setting of diuretic use.
Yet another pitfall is differentiating patients with salt depletion from those with SIADH. In these situations, measurement of the change in PNa concentration after a test infusion of isotonic saline is helpful. In salt depletion, PNa usually increases ≥5 mmol/L after 2 L saline infusion, which is not the case with SIADH.13 Incorrectly diagnosing renal salt wasting (RSW) as SIADH results in fluid restriction and, consequently, ECV depletion and increased morbidity.14 The persistence of hypouricemia and elevated FEUrate after correction of the hyponatremia in RSW differentiates it from SIADH.13, 14
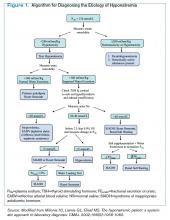
Given these challenges, recommendations to use an algorithmic approach for the evaluation and diagnosis of hyponatremia have surfaced. In a study of 121 patients admitted with hyponatremia, an algorithm-based approach to the diagnosis of hyponatremia yielded an overall diagnostic accuracy of 71%, compared with an accuracy of 32% by experienced clinicians.5 This study also highlighted SIADH as the most frequent false-positive diagnosis that was expected whenever the combination of euvolemia and a UNa >30 mmol/L was present.5 Cases of diuretic-induced hyponatremia often were misclassified due to errors in the accurate assessment of ECV status, as most of these patients appeared clinically euvolemic or hypervolemic.5 Therefore, it is important to use an algorithm in identifying SIADH and to use one that does not rely solely on clinical estimation of ECV status (see Figure 1, below).
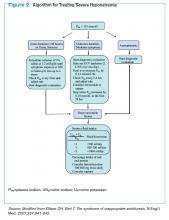
Management of acute and symptomatic hyponatremia. When hyponatremia develops acutely, urgent treatment is required (see Figure 2, below).15 Hyponatremia is considered acute when the onset is within 48 hours.15 Acute hyponatremia is most easily identified in the hospital and is commonly iatrogenic. Small case reviews in the 1980s began to associate postoperative deaths with the administration of hypotonic fluids.16 Asymptomatic patients with hyponatremia presenting from home should be considered chronic hyponatremias as the duration often is unclear.
Acute hyponatremia or neurologically symptomatic hyponatremia regardless of duration requires the use of hypertonic saline.15 Traditional sodium correction algorithms are based on early case series, which were focused on limiting neurologic complications from sodium overcorrection.17 This resulted in protocols recommending a conservative rate of correction spread over a 24- to 48-hour period.17 Infusing 3% saline at a rate of 1 ml/kg/hr to 2 ml/kg/hr results in a 1 mmol/L/hr to 2 mmol/L/hr increase in PNa.15 This simplified formula results in similar correction rates as more complex calculations.15 Correction should not exceed 8 mmol/L to 10 mmol/L within the first 24 hours, and 18 mmol/L to 25 mmol/L by 48 hours to avoid CPM.15 PNa should be checked every two hours to ensure that the correction rate is not exceeding the predicted rate, as the formulas do not take into account oral intake and ongoing losses.15
Recent observations focused on the initial four hours from onset of hyponatremia suggest a higher rate of correction can be tolerated without complications.18 Rapid sodium correction of 4 mmol/L to 6 mmol/L often is enough to stop neurologic complications.18 This can be accomplished with a bolus infusion of 100 mL of 3% saline.19 This may be repeated twice at 10-minute intervals until there is neurologic improvement.19 This might sound aggressive, but this would correspond to a rise in PNa of 5 mmol/L to 6 mmol/L in a 50 kg woman. Subsequent treatment with hypertonic fluid might not be needed if symptoms resolve.
Management of chronic hyponatremia. Hyponatremia secondary to SIADH improves with the treatment of the underlying cause, thus an active search for a causative medication or condition should be sought (see Table 1, p. 17).20
Water restriction. Restriction of fluid intake is the first-line treatment for SIADH in patients without hypovolemia. The severity of fluid restriction is guided by the concentration of the urinary solutes.15 Restriction of water intake to 500 ml/day to 1,000 ml/day is generally advised for many patients, as losses from the skin, lungs, and urine exceed this amount, leading to a gradual reduction in total body water.21 The main drawback of fluid restriction is poor compliance due to an intact thirst mechanism.
Saline infusion. The infusion of normal saline theoretically worsens hyponatremia due to SIADH because the water is retained while the salt is excreted. However, a trial of normal saline sometimes is attempted in patients in whom the differentiation between hypovolemia and euvolemia is difficult. From a study of a series of 17 patients with chronic SIADH, Musch and Decaux concluded that the infusion of intravenous normal (0.9%) saline raises PNa when the urine osmolality is less than 530 mosm/L.22
Oral solutes (urea and salt). The oral intake of salt augments water excretion23, and salt tablets are used as a second-line agent in patients with persistent hyponatremia despite fluid restriction.23 The oral administration of urea also results in increased free-water excretion via osmotic diuresis,24 but its poor palatability, lack of availability in the U.S., and limited user experience has restricted its usage.24
Demeclocycline. Demeclo-cycline is a tetracycline derivative that causes a partial nephrogenic diabetes insipidus.25 Its limitations include a slow onset of action (two to five days) and an unpredictable treatment effect with the possibility of causing profound polyuria and hypernatremia. It is also associated with reversible azotemia and sometimes nephrotoxicity, especially in patients with cirrhosis.
Lithium. Lithium also causes nephrogenic diabetes insipidus by downregulating vasopressin-stimulated aquaporin-2 expression and thus improves hyponatremia in SIADH.26 However, its use is significantly limited by its unpredictable response and the risks of interstitial nephritis and end-stage renal disease with chronic use. Therefore, it is no longer recommended for the treatment of SIADH.
Vasopressin receptor antagonists. Due to the role of excessive levels of vasopressin in the pathophysiology of most types of SIADH, antagonists of the vasopressin receptor were developed with the goal of preventing the excess water absorption that causes hyponatremia. Two vasopressin receptor antagonists, or vaptans, have been approved by the FDA for the treatment of nonemergent euvolemic and hypervolemic hyponatremia. Conivaptan is a nonselective vasopressin receptor antagonist that is for IV use only. Tolvaptan is a selective V2 receptor antagonist that is taken orally. Both conivaptan and tolvaptan successfully increase PNa levels while the drugs are being taken.27,28,29,30 Tolvaptan increases PNa levels in hyponatremia due to SIADH and CHF, and modestly so in cirrhosis.30
The most common side effects of the vaptans include dry mouth, increased thirst, and increased urination, although serious side effects (hypernatremia or too-rapid rate of increase in PNa) are possible.29 It is unclear if treating stable, asymptomatic hyponatremia with vaptans has any reduction in morbidity or mortality. One study found that tolvaptan increased the patients’ self-evaluations of mental functioning, but a study of tolvaptan used in combination with diuretics in the setting of CHF did not result in decreased mortality.29,31 Due to their expense, necessity of being started in the hospital, and unclear long-term benefit, the vaptans are only recommended when traditional measures such as fluid restriction and salt tablets have been unsuccessful.
Back to the Case
Our patient has hypotonic hyponatremia based on her low serum osmolality. The duration of her hyponatremia is unclear, but the patient is not experiencing seizures or coma. Therefore, her hyponatremia should be corrected slowly, and hypertonic saline is not indicated.
As is common in clinical practice, her true volume status is difficult to clinically ascertain. By physical exam, she appears euvolemic, but because she is on hydrochlorothiazide, she might be subtly hypovolemic. The UNa of 40 mmol/L is not consistent with hypovolemia, but its accuracy is limited in the setting of diuretics. The failure to improve her sodium by at least 5 mmol/L after a 2 L normal saline infusion argues against low effective arterial blood volume and indicates that the hydrochlorothiazide is unlikely to be the cause of her hyponatremia.
Therefore, the most likely cause of the hyponatremia is SIADH, a diagnosis further corroborated by the elevated FEUrate of 13%. Her chronic hyponatremia should be managed initially with fluid restriction while an investigation for an underlying cause of SIADH is initiated.
Bottom Line
The diagnosis of SIADH relies on the careful evaluation of laboratory values, use of an algorithm, and recognizing the limitations of clinically assessing volume status. The underlying cause of SIADH must also be sought and treated. TH
Dr. Grant is a clinical lecturer in internal medicine, Dr. Cho is a clinical instructor in internal medicine, and Dr. Nichani is an assistant professor of internal medicine at the University of Michigan Hospital and Health Systems in Ann Arbor.
References
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-21.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e71-78.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806.
- Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678.
- Verbalis JG. Hyponatraemia. Baillieres Clin Endocrinol Metab. Aug 1989;3(2):499-530.
- Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934-938.
- Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471-503.
- Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997.
- Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917-933.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
- Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072.
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535.
- Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190-1195.
- Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29(3):282-299.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121.
- List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4(8):1191-1198.
- Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. J Hosp Med. 2010;5 Suppl 3:S18-S26.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM. 1998;91(11):749-753.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078.
- Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. 1980;69(1):99-106.
- Forrest JN Jr., Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978;298(4):173-177.
- Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634-3639.
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457.
- Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159-168.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712.
- Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.
Case
A 70-year-old woman with hypertension presents after a fall. Her medications include hydrochlorothiazide. Her blood pressure is 130/70 mm/Hg, with heart rate of 86. She has normal orthostatic vital signs. Her mucus membranes are moist and she has no jugular venous distension, edema, or ascites. Her plasma sodium (PNa) is 125 mmol/L, potassium 3.6 mmol/L, blood urea nitrogen (BUN) 30 mg/dL, and creatinine 0.8 mg/dL. Additional labs include serum thyroid stimulating hormone 1.12 mIU/L, cortisol 15 mcg/dL, serum osmolality 270 mOsm/kg, uric acid 4 mg/dL, urine osmolality 300 mOsm/kg, urine sodium (UNa) 40 mmol/L, fractional excretion of sodium 1.0%, and fractional excretion of urate (FEUrate) 13%. She receives 2 L isotonic saline intravenously over 24 hours, with resulting PNa of 127.
What is the cause of her hyponatremia, and how should her hyponatremia be managed?
Overview
Hyponatremia is one of the most common electrolyte abnormalities; it has a prevalence as high as 30% upon admission to the hospital.1 Hyponatremia is important clinically because of its high risk of mortality in the acute and symptomatic setting, and the risk of central pontine myelinolysis (CPM), or death with too rapid correction.2 Even so-called “asymptomatic” mild hyponatremia is associated with increased falls and impairments in gait and attention in the elderly.3
Hyponatremia is a state of excess water compared with the amount of solute in the extracellular fluid. To aid in diagnosing the etiology of hypotonic hyponatremia, the differential is traditionally divided into categories based on extracellular fluid volume (ECV) status, as shown in Table 1 (below), with syndrome of inappropriate antidiuretic hormone secretion (SIADH) being the most common cause of euvolemic hyponatremia.2 However, data show that clinical determination of volume status is often flawed,4 and an algorithmic approach to diagnosis and treatment yields improved results.5
Review of the Data
Diagnosis of SIADH. The original diagnostic criteria for SIADH, with minor modifications, are presented in Table 2, page 18).6,7,8 However, applying these criteria in clinical settings presents several difficulties, most notably a determination of ECV. The gold standard for assessing ECV status is by radioisotope, which is not practically feasible.9 Therefore, clinicians must rely on surrogate clinical markers of ECV (orthostatic hypotension, skin turgor, mucus membrane dryness, central venous pressure, BUN, BUN-creatinine ratio, and serum uric acid levels), which lack both sensitivity and specificity.4 Astoundingly, clinical assessment of ECV has been demonstrated to be accurate only 50% of the time when differentiating euvolemic patients from those with hypovolemia.4
Another challenge lies in the interpretation of UNa, which frequently is used as a surrogate for extra-arterial blood volume (EABV) status.10 Unfortunately, in the setting of diuretic use, UNa becomes inaccurate. The FEUrate, however, is unaffected by diuretic use and can be helpful in distinguishing between etiologies of hyponatremia with UNa greater than 30 mmol/L.11 The FEUrate is about 10% in normal euvolemic subjects and is reduced (usually <8%) in patients with low effective arterial blood volume.11,12 A trial of 86 patients demonstrated that a FEUrate of 12% had a specificity and positive predictive value of 100% in accurately identifying SIADH from diuretic-induced hyponatremia in patients on diuretics.11,12 Therefore, the UNa is a valid marker of EABV status when patients are not on diuretics; however, the FEUrate should be used in the setting of diuretic use.
Yet another pitfall is differentiating patients with salt depletion from those with SIADH. In these situations, measurement of the change in PNa concentration after a test infusion of isotonic saline is helpful. In salt depletion, PNa usually increases ≥5 mmol/L after 2 L saline infusion, which is not the case with SIADH.13 Incorrectly diagnosing renal salt wasting (RSW) as SIADH results in fluid restriction and, consequently, ECV depletion and increased morbidity.14 The persistence of hypouricemia and elevated FEUrate after correction of the hyponatremia in RSW differentiates it from SIADH.13, 14
Given these challenges, recommendations to use an algorithmic approach for the evaluation and diagnosis of hyponatremia have surfaced. In a study of 121 patients admitted with hyponatremia, an algorithm-based approach to the diagnosis of hyponatremia yielded an overall diagnostic accuracy of 71%, compared with an accuracy of 32% by experienced clinicians.5 This study also highlighted SIADH as the most frequent false-positive diagnosis that was expected whenever the combination of euvolemia and a UNa >30 mmol/L was present.5 Cases of diuretic-induced hyponatremia often were misclassified due to errors in the accurate assessment of ECV status, as most of these patients appeared clinically euvolemic or hypervolemic.5 Therefore, it is important to use an algorithm in identifying SIADH and to use one that does not rely solely on clinical estimation of ECV status (see Figure 1, below).
Management of acute and symptomatic hyponatremia. When hyponatremia develops acutely, urgent treatment is required (see Figure 2, below).15 Hyponatremia is considered acute when the onset is within 48 hours.15 Acute hyponatremia is most easily identified in the hospital and is commonly iatrogenic. Small case reviews in the 1980s began to associate postoperative deaths with the administration of hypotonic fluids.16 Asymptomatic patients with hyponatremia presenting from home should be considered chronic hyponatremias as the duration often is unclear.
Acute hyponatremia or neurologically symptomatic hyponatremia regardless of duration requires the use of hypertonic saline.15 Traditional sodium correction algorithms are based on early case series, which were focused on limiting neurologic complications from sodium overcorrection.17 This resulted in protocols recommending a conservative rate of correction spread over a 24- to 48-hour period.17 Infusing 3% saline at a rate of 1 ml/kg/hr to 2 ml/kg/hr results in a 1 mmol/L/hr to 2 mmol/L/hr increase in PNa.15 This simplified formula results in similar correction rates as more complex calculations.15 Correction should not exceed 8 mmol/L to 10 mmol/L within the first 24 hours, and 18 mmol/L to 25 mmol/L by 48 hours to avoid CPM.15 PNa should be checked every two hours to ensure that the correction rate is not exceeding the predicted rate, as the formulas do not take into account oral intake and ongoing losses.15
Recent observations focused on the initial four hours from onset of hyponatremia suggest a higher rate of correction can be tolerated without complications.18 Rapid sodium correction of 4 mmol/L to 6 mmol/L often is enough to stop neurologic complications.18 This can be accomplished with a bolus infusion of 100 mL of 3% saline.19 This may be repeated twice at 10-minute intervals until there is neurologic improvement.19 This might sound aggressive, but this would correspond to a rise in PNa of 5 mmol/L to 6 mmol/L in a 50 kg woman. Subsequent treatment with hypertonic fluid might not be needed if symptoms resolve.
Management of chronic hyponatremia. Hyponatremia secondary to SIADH improves with the treatment of the underlying cause, thus an active search for a causative medication or condition should be sought (see Table 1, p. 17).20
Water restriction. Restriction of fluid intake is the first-line treatment for SIADH in patients without hypovolemia. The severity of fluid restriction is guided by the concentration of the urinary solutes.15 Restriction of water intake to 500 ml/day to 1,000 ml/day is generally advised for many patients, as losses from the skin, lungs, and urine exceed this amount, leading to a gradual reduction in total body water.21 The main drawback of fluid restriction is poor compliance due to an intact thirst mechanism.
Saline infusion. The infusion of normal saline theoretically worsens hyponatremia due to SIADH because the water is retained while the salt is excreted. However, a trial of normal saline sometimes is attempted in patients in whom the differentiation between hypovolemia and euvolemia is difficult. From a study of a series of 17 patients with chronic SIADH, Musch and Decaux concluded that the infusion of intravenous normal (0.9%) saline raises PNa when the urine osmolality is less than 530 mosm/L.22
Oral solutes (urea and salt). The oral intake of salt augments water excretion23, and salt tablets are used as a second-line agent in patients with persistent hyponatremia despite fluid restriction.23 The oral administration of urea also results in increased free-water excretion via osmotic diuresis,24 but its poor palatability, lack of availability in the U.S., and limited user experience has restricted its usage.24
Demeclocycline. Demeclo-cycline is a tetracycline derivative that causes a partial nephrogenic diabetes insipidus.25 Its limitations include a slow onset of action (two to five days) and an unpredictable treatment effect with the possibility of causing profound polyuria and hypernatremia. It is also associated with reversible azotemia and sometimes nephrotoxicity, especially in patients with cirrhosis.
Lithium. Lithium also causes nephrogenic diabetes insipidus by downregulating vasopressin-stimulated aquaporin-2 expression and thus improves hyponatremia in SIADH.26 However, its use is significantly limited by its unpredictable response and the risks of interstitial nephritis and end-stage renal disease with chronic use. Therefore, it is no longer recommended for the treatment of SIADH.
Vasopressin receptor antagonists. Due to the role of excessive levels of vasopressin in the pathophysiology of most types of SIADH, antagonists of the vasopressin receptor were developed with the goal of preventing the excess water absorption that causes hyponatremia. Two vasopressin receptor antagonists, or vaptans, have been approved by the FDA for the treatment of nonemergent euvolemic and hypervolemic hyponatremia. Conivaptan is a nonselective vasopressin receptor antagonist that is for IV use only. Tolvaptan is a selective V2 receptor antagonist that is taken orally. Both conivaptan and tolvaptan successfully increase PNa levels while the drugs are being taken.27,28,29,30 Tolvaptan increases PNa levels in hyponatremia due to SIADH and CHF, and modestly so in cirrhosis.30
The most common side effects of the vaptans include dry mouth, increased thirst, and increased urination, although serious side effects (hypernatremia or too-rapid rate of increase in PNa) are possible.29 It is unclear if treating stable, asymptomatic hyponatremia with vaptans has any reduction in morbidity or mortality. One study found that tolvaptan increased the patients’ self-evaluations of mental functioning, but a study of tolvaptan used in combination with diuretics in the setting of CHF did not result in decreased mortality.29,31 Due to their expense, necessity of being started in the hospital, and unclear long-term benefit, the vaptans are only recommended when traditional measures such as fluid restriction and salt tablets have been unsuccessful.
Back to the Case
Our patient has hypotonic hyponatremia based on her low serum osmolality. The duration of her hyponatremia is unclear, but the patient is not experiencing seizures or coma. Therefore, her hyponatremia should be corrected slowly, and hypertonic saline is not indicated.
As is common in clinical practice, her true volume status is difficult to clinically ascertain. By physical exam, she appears euvolemic, but because she is on hydrochlorothiazide, she might be subtly hypovolemic. The UNa of 40 mmol/L is not consistent with hypovolemia, but its accuracy is limited in the setting of diuretics. The failure to improve her sodium by at least 5 mmol/L after a 2 L normal saline infusion argues against low effective arterial blood volume and indicates that the hydrochlorothiazide is unlikely to be the cause of her hyponatremia.
Therefore, the most likely cause of the hyponatremia is SIADH, a diagnosis further corroborated by the elevated FEUrate of 13%. Her chronic hyponatremia should be managed initially with fluid restriction while an investigation for an underlying cause of SIADH is initiated.
Bottom Line
The diagnosis of SIADH relies on the careful evaluation of laboratory values, use of an algorithm, and recognizing the limitations of clinically assessing volume status. The underlying cause of SIADH must also be sought and treated. TH
Dr. Grant is a clinical lecturer in internal medicine, Dr. Cho is a clinical instructor in internal medicine, and Dr. Nichani is an assistant professor of internal medicine at the University of Michigan Hospital and Health Systems in Ann Arbor.
References
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-21.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e71-78.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806.
- Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678.
- Verbalis JG. Hyponatraemia. Baillieres Clin Endocrinol Metab. Aug 1989;3(2):499-530.
- Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934-938.
- Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471-503.
- Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997.
- Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917-933.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
- Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072.
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535.
- Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190-1195.
- Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29(3):282-299.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121.
- List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4(8):1191-1198.
- Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. J Hosp Med. 2010;5 Suppl 3:S18-S26.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM. 1998;91(11):749-753.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078.
- Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. 1980;69(1):99-106.
- Forrest JN Jr., Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978;298(4):173-177.
- Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634-3639.
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457.
- Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159-168.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712.
- Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.
Case
A 70-year-old woman with hypertension presents after a fall. Her medications include hydrochlorothiazide. Her blood pressure is 130/70 mm/Hg, with heart rate of 86. She has normal orthostatic vital signs. Her mucus membranes are moist and she has no jugular venous distension, edema, or ascites. Her plasma sodium (PNa) is 125 mmol/L, potassium 3.6 mmol/L, blood urea nitrogen (BUN) 30 mg/dL, and creatinine 0.8 mg/dL. Additional labs include serum thyroid stimulating hormone 1.12 mIU/L, cortisol 15 mcg/dL, serum osmolality 270 mOsm/kg, uric acid 4 mg/dL, urine osmolality 300 mOsm/kg, urine sodium (UNa) 40 mmol/L, fractional excretion of sodium 1.0%, and fractional excretion of urate (FEUrate) 13%. She receives 2 L isotonic saline intravenously over 24 hours, with resulting PNa of 127.
What is the cause of her hyponatremia, and how should her hyponatremia be managed?
Overview
Hyponatremia is one of the most common electrolyte abnormalities; it has a prevalence as high as 30% upon admission to the hospital.1 Hyponatremia is important clinically because of its high risk of mortality in the acute and symptomatic setting, and the risk of central pontine myelinolysis (CPM), or death with too rapid correction.2 Even so-called “asymptomatic” mild hyponatremia is associated with increased falls and impairments in gait and attention in the elderly.3
Hyponatremia is a state of excess water compared with the amount of solute in the extracellular fluid. To aid in diagnosing the etiology of hypotonic hyponatremia, the differential is traditionally divided into categories based on extracellular fluid volume (ECV) status, as shown in Table 1 (below), with syndrome of inappropriate antidiuretic hormone secretion (SIADH) being the most common cause of euvolemic hyponatremia.2 However, data show that clinical determination of volume status is often flawed,4 and an algorithmic approach to diagnosis and treatment yields improved results.5
Review of the Data
Diagnosis of SIADH. The original diagnostic criteria for SIADH, with minor modifications, are presented in Table 2, page 18).6,7,8 However, applying these criteria in clinical settings presents several difficulties, most notably a determination of ECV. The gold standard for assessing ECV status is by radioisotope, which is not practically feasible.9 Therefore, clinicians must rely on surrogate clinical markers of ECV (orthostatic hypotension, skin turgor, mucus membrane dryness, central venous pressure, BUN, BUN-creatinine ratio, and serum uric acid levels), which lack both sensitivity and specificity.4 Astoundingly, clinical assessment of ECV has been demonstrated to be accurate only 50% of the time when differentiating euvolemic patients from those with hypovolemia.4
Another challenge lies in the interpretation of UNa, which frequently is used as a surrogate for extra-arterial blood volume (EABV) status.10 Unfortunately, in the setting of diuretic use, UNa becomes inaccurate. The FEUrate, however, is unaffected by diuretic use and can be helpful in distinguishing between etiologies of hyponatremia with UNa greater than 30 mmol/L.11 The FEUrate is about 10% in normal euvolemic subjects and is reduced (usually <8%) in patients with low effective arterial blood volume.11,12 A trial of 86 patients demonstrated that a FEUrate of 12% had a specificity and positive predictive value of 100% in accurately identifying SIADH from diuretic-induced hyponatremia in patients on diuretics.11,12 Therefore, the UNa is a valid marker of EABV status when patients are not on diuretics; however, the FEUrate should be used in the setting of diuretic use.
Yet another pitfall is differentiating patients with salt depletion from those with SIADH. In these situations, measurement of the change in PNa concentration after a test infusion of isotonic saline is helpful. In salt depletion, PNa usually increases ≥5 mmol/L after 2 L saline infusion, which is not the case with SIADH.13 Incorrectly diagnosing renal salt wasting (RSW) as SIADH results in fluid restriction and, consequently, ECV depletion and increased morbidity.14 The persistence of hypouricemia and elevated FEUrate after correction of the hyponatremia in RSW differentiates it from SIADH.13, 14
Given these challenges, recommendations to use an algorithmic approach for the evaluation and diagnosis of hyponatremia have surfaced. In a study of 121 patients admitted with hyponatremia, an algorithm-based approach to the diagnosis of hyponatremia yielded an overall diagnostic accuracy of 71%, compared with an accuracy of 32% by experienced clinicians.5 This study also highlighted SIADH as the most frequent false-positive diagnosis that was expected whenever the combination of euvolemia and a UNa >30 mmol/L was present.5 Cases of diuretic-induced hyponatremia often were misclassified due to errors in the accurate assessment of ECV status, as most of these patients appeared clinically euvolemic or hypervolemic.5 Therefore, it is important to use an algorithm in identifying SIADH and to use one that does not rely solely on clinical estimation of ECV status (see Figure 1, below).
Management of acute and symptomatic hyponatremia. When hyponatremia develops acutely, urgent treatment is required (see Figure 2, below).15 Hyponatremia is considered acute when the onset is within 48 hours.15 Acute hyponatremia is most easily identified in the hospital and is commonly iatrogenic. Small case reviews in the 1980s began to associate postoperative deaths with the administration of hypotonic fluids.16 Asymptomatic patients with hyponatremia presenting from home should be considered chronic hyponatremias as the duration often is unclear.
Acute hyponatremia or neurologically symptomatic hyponatremia regardless of duration requires the use of hypertonic saline.15 Traditional sodium correction algorithms are based on early case series, which were focused on limiting neurologic complications from sodium overcorrection.17 This resulted in protocols recommending a conservative rate of correction spread over a 24- to 48-hour period.17 Infusing 3% saline at a rate of 1 ml/kg/hr to 2 ml/kg/hr results in a 1 mmol/L/hr to 2 mmol/L/hr increase in PNa.15 This simplified formula results in similar correction rates as more complex calculations.15 Correction should not exceed 8 mmol/L to 10 mmol/L within the first 24 hours, and 18 mmol/L to 25 mmol/L by 48 hours to avoid CPM.15 PNa should be checked every two hours to ensure that the correction rate is not exceeding the predicted rate, as the formulas do not take into account oral intake and ongoing losses.15
Recent observations focused on the initial four hours from onset of hyponatremia suggest a higher rate of correction can be tolerated without complications.18 Rapid sodium correction of 4 mmol/L to 6 mmol/L often is enough to stop neurologic complications.18 This can be accomplished with a bolus infusion of 100 mL of 3% saline.19 This may be repeated twice at 10-minute intervals until there is neurologic improvement.19 This might sound aggressive, but this would correspond to a rise in PNa of 5 mmol/L to 6 mmol/L in a 50 kg woman. Subsequent treatment with hypertonic fluid might not be needed if symptoms resolve.
Management of chronic hyponatremia. Hyponatremia secondary to SIADH improves with the treatment of the underlying cause, thus an active search for a causative medication or condition should be sought (see Table 1, p. 17).20
Water restriction. Restriction of fluid intake is the first-line treatment for SIADH in patients without hypovolemia. The severity of fluid restriction is guided by the concentration of the urinary solutes.15 Restriction of water intake to 500 ml/day to 1,000 ml/day is generally advised for many patients, as losses from the skin, lungs, and urine exceed this amount, leading to a gradual reduction in total body water.21 The main drawback of fluid restriction is poor compliance due to an intact thirst mechanism.
Saline infusion. The infusion of normal saline theoretically worsens hyponatremia due to SIADH because the water is retained while the salt is excreted. However, a trial of normal saline sometimes is attempted in patients in whom the differentiation between hypovolemia and euvolemia is difficult. From a study of a series of 17 patients with chronic SIADH, Musch and Decaux concluded that the infusion of intravenous normal (0.9%) saline raises PNa when the urine osmolality is less than 530 mosm/L.22
Oral solutes (urea and salt). The oral intake of salt augments water excretion23, and salt tablets are used as a second-line agent in patients with persistent hyponatremia despite fluid restriction.23 The oral administration of urea also results in increased free-water excretion via osmotic diuresis,24 but its poor palatability, lack of availability in the U.S., and limited user experience has restricted its usage.24
Demeclocycline. Demeclo-cycline is a tetracycline derivative that causes a partial nephrogenic diabetes insipidus.25 Its limitations include a slow onset of action (two to five days) and an unpredictable treatment effect with the possibility of causing profound polyuria and hypernatremia. It is also associated with reversible azotemia and sometimes nephrotoxicity, especially in patients with cirrhosis.
Lithium. Lithium also causes nephrogenic diabetes insipidus by downregulating vasopressin-stimulated aquaporin-2 expression and thus improves hyponatremia in SIADH.26 However, its use is significantly limited by its unpredictable response and the risks of interstitial nephritis and end-stage renal disease with chronic use. Therefore, it is no longer recommended for the treatment of SIADH.
Vasopressin receptor antagonists. Due to the role of excessive levels of vasopressin in the pathophysiology of most types of SIADH, antagonists of the vasopressin receptor were developed with the goal of preventing the excess water absorption that causes hyponatremia. Two vasopressin receptor antagonists, or vaptans, have been approved by the FDA for the treatment of nonemergent euvolemic and hypervolemic hyponatremia. Conivaptan is a nonselective vasopressin receptor antagonist that is for IV use only. Tolvaptan is a selective V2 receptor antagonist that is taken orally. Both conivaptan and tolvaptan successfully increase PNa levels while the drugs are being taken.27,28,29,30 Tolvaptan increases PNa levels in hyponatremia due to SIADH and CHF, and modestly so in cirrhosis.30
The most common side effects of the vaptans include dry mouth, increased thirst, and increased urination, although serious side effects (hypernatremia or too-rapid rate of increase in PNa) are possible.29 It is unclear if treating stable, asymptomatic hyponatremia with vaptans has any reduction in morbidity or mortality. One study found that tolvaptan increased the patients’ self-evaluations of mental functioning, but a study of tolvaptan used in combination with diuretics in the setting of CHF did not result in decreased mortality.29,31 Due to their expense, necessity of being started in the hospital, and unclear long-term benefit, the vaptans are only recommended when traditional measures such as fluid restriction and salt tablets have been unsuccessful.
Back to the Case
Our patient has hypotonic hyponatremia based on her low serum osmolality. The duration of her hyponatremia is unclear, but the patient is not experiencing seizures or coma. Therefore, her hyponatremia should be corrected slowly, and hypertonic saline is not indicated.
As is common in clinical practice, her true volume status is difficult to clinically ascertain. By physical exam, she appears euvolemic, but because she is on hydrochlorothiazide, she might be subtly hypovolemic. The UNa of 40 mmol/L is not consistent with hypovolemia, but its accuracy is limited in the setting of diuretics. The failure to improve her sodium by at least 5 mmol/L after a 2 L normal saline infusion argues against low effective arterial blood volume and indicates that the hydrochlorothiazide is unlikely to be the cause of her hyponatremia.
Therefore, the most likely cause of the hyponatremia is SIADH, a diagnosis further corroborated by the elevated FEUrate of 13%. Her chronic hyponatremia should be managed initially with fluid restriction while an investigation for an underlying cause of SIADH is initiated.
Bottom Line
The diagnosis of SIADH relies on the careful evaluation of laboratory values, use of an algorithm, and recognizing the limitations of clinically assessing volume status. The underlying cause of SIADH must also be sought and treated. TH
Dr. Grant is a clinical lecturer in internal medicine, Dr. Cho is a clinical instructor in internal medicine, and Dr. Nichani is an assistant professor of internal medicine at the University of Michigan Hospital and Health Systems in Ann Arbor.
References
- Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119(7 Suppl 1):S30-35.
- Verbalis JG, Goldsmith SR, Greenberg A, Schrier RW, Sterns RH. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am J Med. 2007;120(11 Suppl 1):S1-21.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e71-78.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Fenske W, Maier SK, Blechschmidt A, Allolio B, Störk S. Utility and limitations of the traditional diagnostic approach to hyponatremia: a diagnostic study. Am J Med. 2010;123(7):652-657.
- Bartter FC, Schwartz WB. The syndrome of inappropriate secretion of antidiuretic hormone. Am J Med. 1967;42(5):790-806.
- Smith DM, McKenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol (Oxf). 2000;52(6):667-678.
- Verbalis JG. Hyponatraemia. Baillieres Clin Endocrinol Metab. Aug 1989;3(2):499-530.
- Maesaka JK, Imbriano LJ, Ali NM, Ilamathi E. Is it cerebral or renal salt wasting? Kidney Int. 2009;76(9):934-938.
- Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17(4):471-503.
- Fenske W, Störk S, Koschker AC, et al. Value of fractional uric acid excretion in differential diagnosis of hyponatremic patients on diuretics. J Clin Endocrinol Metab. 2008;93(8):2991-2997.
- Maesaka JK, Fishbane S. Regulation of renal urate excretion: a critical review. Am J Kidney Dis. 1998;32(6):917-933.
- Milionis HJ, Liamis GL, Elisaf MS. The hyponatremic patient: a systematic approach to laboratory diagnosis. CMAJ. 2002;166(8):1056-1062.
- Bitew S, Imbriano L, Miyawaki N, Fishbane S, Maesaka JK. More on renal salt wasting without cerebral disease: response to saline infusion. Clin J Am Soc Nephrol. 2009;4(2):309-315.
- Ellison DH, Berl T. Clinical practice. The syndrome of inappropriate antidiuresis. N Engl J Med. 2007;356(20):2064-2072.
- Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314(24):1529-1535.
- Ayus JC, Krothapalli RK, Arieff AI. Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med. 1987;317(19):1190-1195.
- Sterns RH, Nigwekar SU, Hix JK. The treatment of hyponatremia. Semin Nephrol. 2009;29(3):282-299.
- Hew-Butler T, Ayus JC, Kipps C, et al. Statement of the Second International Exercise-Associated Hyponatremia Consensus Development Conference, New Zealand, 2007. Clin J Sport Med. 2008;18(2):111-121.
- List AF, Hainsworth JD, Davis BW, Hande KR, Greco FA, Johnson DH. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4(8):1191-1198.
- Verbalis JG. Managing hyponatremia in patients with syndrome of inappropriate antidiuretic hormone secretion. J Hosp Med. 2010;5 Suppl 3:S18-S26.
- Musch W, Decaux G. Treating the syndrome of inappropriate ADH secretion with isotonic saline. QJM. 1998;91(11):749-753.
- Berl T. Impact of solute intake on urine flow and water excretion. J Am Soc Nephrol. 2008;19(6):1076-1078.
- Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. 1980;69(1):99-106.
- Forrest JN Jr., Cox M, Hong C, Morrison G, Bia M, Singer I. Superiority of demeclocycline over lithium in the treatment of chronic syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med. 1978;298(4):173-177.
- Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA. Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A. 2008;105(9):3634-3639.
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007;27(5):447-457.
- Verbalis JG, Zeltser D, Smith N, Barve A, Andoh M. Assessment of the efficacy and safety of intravenous conivaptan in patients with euvolaemic hyponatraemia: subgroup analysis of a randomized, controlled study. Clin Endocrinol (Oxf). 2008;69(1):159-168.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21(4):705-712.
- Konstam MA, Gheorghiade M, Burnett JC Jr., et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.