User login
Thiazide Diuretic Utilization Within the VA
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
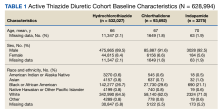
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
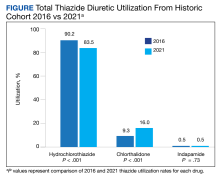
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205