User login
Asymptomatic Hair Loss in a Patient With Systemic Lupus Erythematosus
The Diagnosis: Tinea Capitis
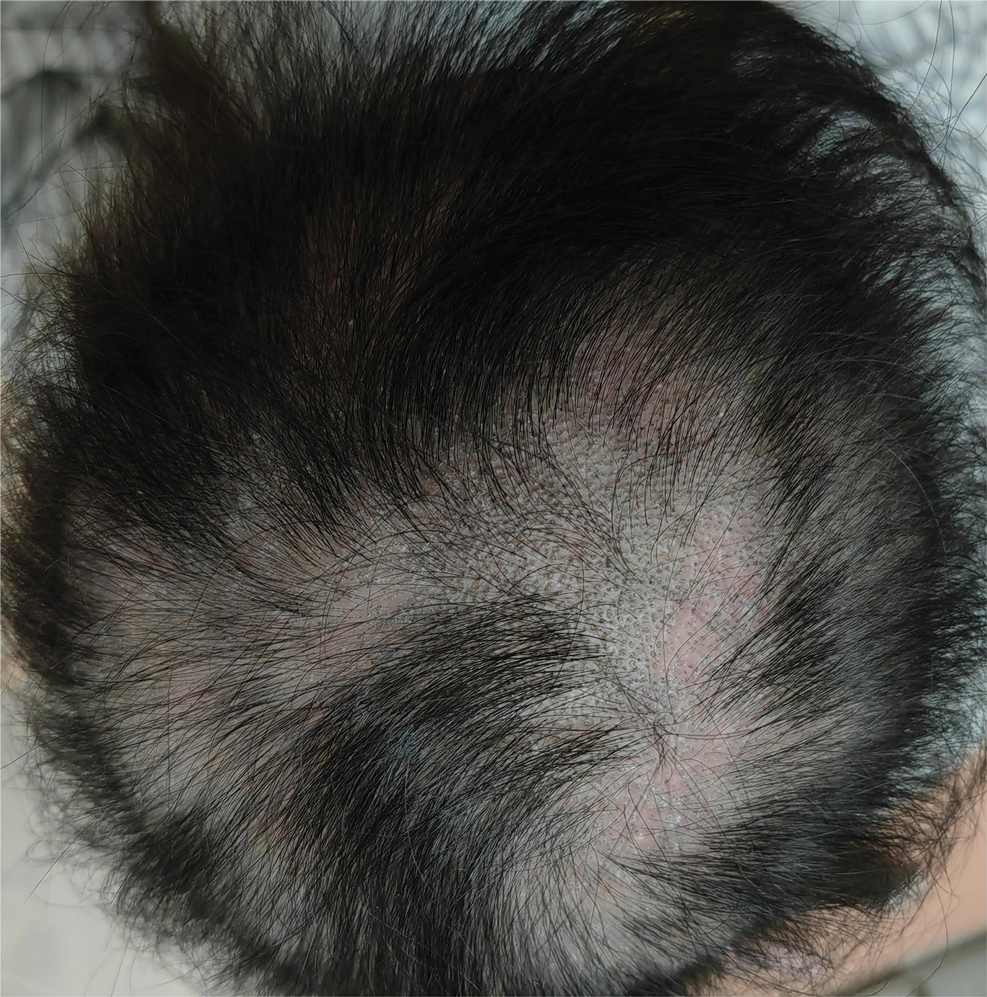
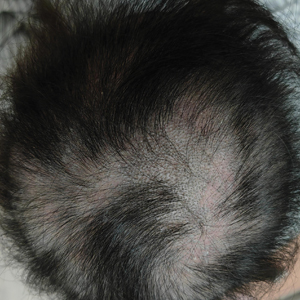
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4
Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
The Diagnosis: Tinea Capitis
Dermoscopy revealed many black spot signs with broken, corkscrew, and comma hairs, as well as increased single hair follicles and focal polymorphic vascular distribution in the scalp (Figure 1). Fungal microscopy showed large round spores within the hair. A fungal culture demonstrated Trichophyton tonsurans growth in the broken hair. Based on the clinical presentation and laboratory findings, a diagnosis of tinea capitis was rendered. Oral terbinafine 250 mg/d was prescribed. At 4-week follow-up, the patient did not report worsening or new symptoms, and there was visible evidence of hair regrowth (Figure 2). There has been no sign of recurrence.

According to the most recent set of classification criteria published by the Systemic Lupus Erythematosus (SLE) International Collaborating Clinics, nonscarring alopecia is now a diagnostic criterion for SLE that has a specificity of 95.7%.1 Although discoid lupus erythematosus presents with diffuse scarring alopecia, SLE manifests as nonscarring alopecia in 1 of 3 patterns: diffuse, patchy, or “lupus hair.”2 It is commonly believed that lupus-related alopecia is a nonspecific symptom of SLE exacerbation and signals that the disease is active.3 Our patient had a history of SLE with no pruritus or pain accompanying the hair loss; however, we considered hair loss due to SLE disease activity, and dermoscopic examination was performed to further rule out the likelihood of SLE alopecia. The dermoscopic characteristics of lupus-related alopecia and tinea capitis vary. For lupusrelated alopecia, alterations to the hair shaft are visible with dermoscopy, including a reduced number or smaller diameter of hairs, hypopigmentation, the black dot sign, brown scattered pigmentation, blue-gray pigmentation, and thick dendritic capillaries.2 Tinea capitis typically displays characteristic dermoscopic manifestations, such as comma, corkscrew, Morse code–like, or jagged hair; black spots; and broken hair.4

Included in the differential diagnosis, androgenetic alopecia dermoscopic findings include hair diameter diversity, perifollicular pigmentation/peripilar sign, and yellow dots.5 The most common vascular patterns present in seborrheic dermatitis are arborizing red lines, twisted red loops, atypical vessels, and glomerular vessels. Perifollicular scaling may be white or yellow and oily.6 There are no specific dermoscopic findings for telogen effluvium; however, the presence of hair regrowth and the predominance of follicular openings with a single sprouting hair shaft may suggest this condition.7 Therefore, dermoscopy can assist clinicians in correctly diagnosing a patient’s condition and determining the its etiology, allowing for early and effective treatment.
Tinea capitis is a typical superficial dermatophyte infection that commonly occurs in prepubescent children and is uncommon in adults because the pH level of the scalp shifts during puberty and the amount of sebum that contains saturated fatty acids increases.8 The risk for developing tinea capitis is higher in certain individuals with comorbid systemic immune diseases, such as SLE and diabetes mellitus, among others, as well as in immunocompromised individuals, such as those with AIDS, organ transplant recipients, or patients receiving high doses of steroids or immunosuppressive drugs.9 The type of dermatophyte entering the hair, the level of host resistance, and the intensity of the inflammatory reaction all affect the clinical picture of tinea capitis in adults, which is pleomorphic and atypical.10 Although tinea capitis is not highly prevalent in adults, the fact that our patient had SLE and had been on immunosuppressive therapy to keep the condition stable increased the chance of contracting tinea capitis, underscoring the need for clinicians to be alert for fungal infections in this patient population.
Trichophyton tonsurans is the most prevalent form of microorganism that causes tinea capitis in the United States, the United Kingdom, and France. However, T tonsurans causing tinea capitis is uncommon in China, with one study reporting only 6 cases from 2000 to 2019.11 Tinea capitis caused by T tonsurans typically presents as black spot alopecia with inflammatory erythema and scaling of the scalp.12 Because most T tonsurans infections have few clinical symptoms, it is challenging to make a clinical diagnosis.13 Although not performed in our patient, a potassium hydroxide preparation and direct microscopic inspection of the afflicted hair and scales can help in quickly identifying and treating these infections. Additional fungal cultures can precisely identify the strain and trace its epidemiology, which is clinically significant not only to identify the potential infection source but also to direct the selection of an organized treatment plan.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. doi:10.1002/art.34473
- Desai K, Miteva M. Recent insight on the management of lupus erythematosus alopecia. Clin Cosmet Investig Dermatol. 2021;14:333-347. doi:10.2147/CCID.S269288
- Wysenbeek AJ, Leibovici L, Amit M, et al. Alopecia in systemic lupus erythematosus. relation to disease manifestations. J Rheumatol. 1991;18:1185-1186.
- Lekkas D, Ioannides D, Lazaridou E, et al. Dermatoscopy in tinea capitis: can it provide clues for the responsible fungi? J Eur Acad Dermatol Venereol. 2021;35:E85-E87. doi:10.1111/jdv.16825
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2011;38:71-75. doi:10.1111/j .1346-8138.2010.01119.x
- Golin´ska J, Sar-Pomian M, Rudnicka L. Diagnostic accuracy of trichoscopy in inflammatory scalp diseases: a systematic review. Dermatology. 2022;238:412-421. doi:10.1159/000517516
- Fernández-Domper L, Ballesteros-Redondo M, Vañó-Galván S. Trichoscopy: an update. Actas Dermosifiliogr. 2023;114:327-333. doi:10.1016/j.ad.2022.12.003
- He M, Zeng J, Mao Y, et al. Aetiological changes of tinea capitis in the Hubei area in 60 years: focus on adult tinea capitis. Mycoses. 2021;64:1527-1534. doi:10.1111/myc.13305
- Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181:371-378. doi:10.1007/s11046 -016-0004-9
- Gianni C, Betti R, Perotta E, et al. Tinea capitis in adults. Mycoses. 1995;38:329-331. doi:10.1111/j.1439-0507.1995.tb00417.x
- Liang G, Zheng X, Song G, et al. Adult tinea capitis in China: a retrospective analysis from 2000 to 2019. Mycoses. 2020;63:876-888. doi:10.1111/myc.13102
- Zalewski A, Goldust M, Szepietowski JC. Tinea gladiatorum: epidemiology, clinical aspects, and management. J Clin Med. 2022;11:4066. doi:10.3390/jcm11144066
- Hiruma J, Ogawa Y, Hiruma M. Trichophyton tonsurans infection in Japan: epidemiology, clinical features, diagnosis and infection control. J Dermatol. 2015;42:245-249. doi:10.1111 /1346-8138.12678
A 51-year-old woman residing in the Hainan Province, China, was referred to our hospital for treatment of recurrent joint pain that could not be controlled at the local hospital. She had a history of systemic lupus erythematosus with a Systemic Lupus Erythematosus Disease Activity Index score of 8 (mild activity). Physical examination revealed irregular patches of hair loss on the head. There also were remnants of hair in some areas with black dots at the follicular opening and perifollicular keratotic papules interspersed as well as a few pale erythematous spots and white adherent scales.