User login
Is NPH associated with fewer adverse events than analog basal insulin for adults with T2D?
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
EVIDENCE-BASED ANSWER:
NO. Insulin glargine may lead to less patient-reported, symptomatic, and nocturnal hypoglycemia, although overall, there may not be a difference in the risk for severe hypoglycemia or hypoglycemia-related emergency department (ED) visits and hospitalizations (strength of recommendation [SOR]: B, systematic review of randomized controlled trials [RCTs], individual RCTs, and observational study).
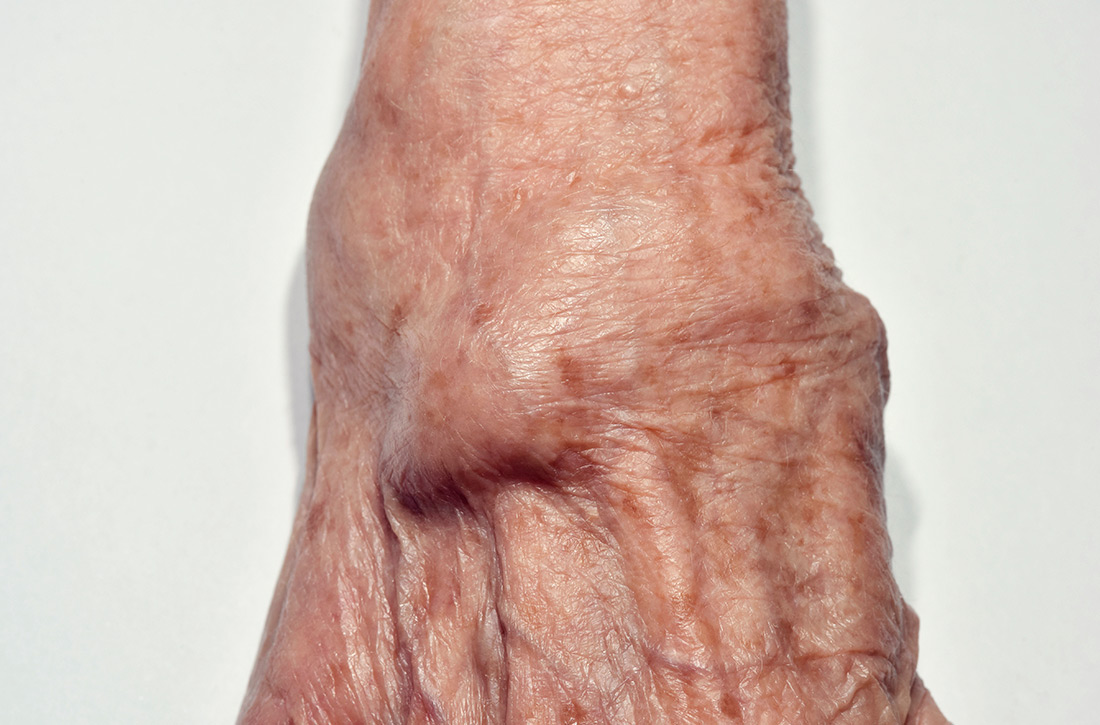
What is the best treatment for wrist ganglion cysts?
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
EVIDENCE-BASED ANSWER:
Open surgical excision of wrist ganglion cysts is associated with a lower recurrence rate than aspiration with or without corticosteroid injection (strength of recommendation [SOR]: B, systematic review of randomized clinical trials [RCTs] and observational trials and RCT).
Even though the recurrence rate with aspiration is about 50%, most patients are satisfied with aspiration and report a decrease in symptoms involving pain, function, and range of motion (SOR: B, individual cohort and case series).
How do hyaluronic acid and corticosteroid injections compare for knee OA relief?
EVIDENCE SUMMARY
A 2015 network meta-analysis of 137 RCTs with 33,243 patients (ages 45-76 years) with knee OA compared the effectiveness of a variety of treatments including intra-articular CS and HA.1 At 3 months, the effect on pain was not significantly different between the CS and HA groups (12 trials; effect size [ES]=0.02; 95% confidence interval [CI], -0.12 to 0.17). However, a small but significant improvement in function was noted (scoring system not defined) at 3 months favoring HA (ES=0.24; 95% CI, 0.06-0.43; number of trials not specified).
At 3 and 6 months, HA improves pain, but not function, more than CS
Another meta-analysis published in 2015 examined the effectiveness of intra-articular CS and HA in 7 RCTs with 583 patients with knee OA.2 All 7 trials were included in the network meta-analysis and discussed separately to evaluate different time points.
Pain at one month wasn’t significantly different using a visual analog score (VAS) of one to 100 (4 trials; 245 patients; mean difference [MD]=1.66 points; 95% CI, -0.90 to 4.23). At 3 and 6 months, the HA group reported significantly reduced pain compared with the CS group (3 months: 3 trials; 320 patients; MD=12.58 points; 95% CI, -17.76 to -7.40; 6 months: 5 trials; 411 patients; MD=9.01 points; 95% CI, -12.62 to -5.40). There were no significant differences in function outcomes (Index of severity for OA of the knee by Lequesne et al; The Knee Society Clinical Rating System), maximum flexion, or adverse events.
Triamcinolone improves pain, function, but not for long
A 2016 double-blind RCT of 110 patients with knee OA compared intra-articular HA and triamcinolone, assessing pain and function at intervals between 24 hours and 6 months.3 Patients in the HA group received a single injection of 6 mL hylan G-F 20 (Synvisc); patients in the CS group received 1 mL of triamcinolone acetonide 40 mg and 5 mL of 1% lidocaine with epinephrine.
The CS group reported significantly less pain (VAS score 1 to 100) at 24 hours than the HA group (24 points vs 36 points; P=.002); relief lasted as long as one week (14 points vs 23 points; P=.018). After the first week, no difference was seen in pain between groups for as long as 6 months.
Function, assessed by a modified Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC 1 to 100; higher score indicates worse pain, stiffness, and function) showed a significant improvement with CS at 2 weeks (25 points vs 31 points; P=.03), but no difference at any other time point up to 6 months.
HA (mostly) improves pain, function more than betamethasone
A 2015 RCT of 200 patients with knee OA compared the effectiveness of intra-articular HA and betamethasone.4 Evaluators were blinded and assessments were made at 3, 6, 9, and 12 months. The HA group received 2.5 mL of 1% HA (Suprahyal); the CS group received betamethasone dipropionate 5 mg plus betamethasone sodium phosphate 2 mg in 1 mL.
The CS group had significantly less pain (VAS 1 to 10) at 3 months compared with the HA group (2.2 points vs 3.1 points; P=.004), but the HA group had less pain at all other time points (6 months: 3.9 points vs 2.4 points; P=.0001; 9 months: 5.5 points vs 3.6 points; P=.0001; 12 months: 6 points vs 4.1 points; P=.0001).
The WOMAC function subscores (0 to 68; lower indicates more function) were significantly better at all follow-up points in the HA group compared with the CS group (3 months: 19 vs 25; P=.0001; 6 months: 17 vs 29; P=.0001; 9 months: 25 vs 42; P=.0001; 12 months: 28 vs 42; P=.0001).4
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons 2013 work group couldn’t recommend for or against using intra-articular CS for patients with symptomatic knee OA based on inconclusive evidence.5 They also couldn’t recommend using HA (SOR: strong).
The National Institute for Health and Care Excellence (NICE) stated in 2008 that intra-articular CS injections should be considered as an adjunct to core treatments for the relief of moderate to severe pain in people with OA.6 In 2014, NICE recommended against offering intra-articular HA injections for managing OA.
The US Veterans Administration and Department of Defense have issued guidelines stating that clinicians may consider intra-articular CS injections for patients with symptomatic knee OA (US Preventive Services Task Force [USPSTF] Grade B).7 They report insufficient evidence to recommend for or against the use of intra-articular HA with the caveat that HA may be considered for patients who don’t respond adequately to nonpharmacologic measures and who have an inadequate response, intolerable adverse events, or contraindications to other pharmacologic therapies (USPSTF Grade I).
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9:493-500.
3. Tammachote N, Kanitnate S, Yakumpor T, et al. Intra-articular, single-shot Hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885-892.
4. Trueba Davalillo CA, Trueba Vasavilbaso C, Navarrete Alvarez JM, et al. Clinical efficacy of intra-articular injections in knee osteoarthritis: a prospective randomized study comparing hyaluronic acid and betamethasone. Open Access Rheumatol Res Rev. 2015;7:9-18.
5. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd ed. Available at: http://www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Accessed May 15, 2016.
6. National Institute for Health and Care Excellence. Osteoarthritis: Care and Management. Available at: https://www.nice.org.uk/guidance/cg177/chapter/1-recommendations. Accessed May 15, 2016.
7. United States Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Non-Surgical Management of Hip and Knee Osteoarthritis. Available at: http://www.healthquality.va.gov/guidelines/CD/OA/VADoDOACPGFINAL090214.pdf. Accessed May 15, 2016.
EVIDENCE SUMMARY
A 2015 network meta-analysis of 137 RCTs with 33,243 patients (ages 45-76 years) with knee OA compared the effectiveness of a variety of treatments including intra-articular CS and HA.1 At 3 months, the effect on pain was not significantly different between the CS and HA groups (12 trials; effect size [ES]=0.02; 95% confidence interval [CI], -0.12 to 0.17). However, a small but significant improvement in function was noted (scoring system not defined) at 3 months favoring HA (ES=0.24; 95% CI, 0.06-0.43; number of trials not specified).
At 3 and 6 months, HA improves pain, but not function, more than CS
Another meta-analysis published in 2015 examined the effectiveness of intra-articular CS and HA in 7 RCTs with 583 patients with knee OA.2 All 7 trials were included in the network meta-analysis and discussed separately to evaluate different time points.
Pain at one month wasn’t significantly different using a visual analog score (VAS) of one to 100 (4 trials; 245 patients; mean difference [MD]=1.66 points; 95% CI, -0.90 to 4.23). At 3 and 6 months, the HA group reported significantly reduced pain compared with the CS group (3 months: 3 trials; 320 patients; MD=12.58 points; 95% CI, -17.76 to -7.40; 6 months: 5 trials; 411 patients; MD=9.01 points; 95% CI, -12.62 to -5.40). There were no significant differences in function outcomes (Index of severity for OA of the knee by Lequesne et al; The Knee Society Clinical Rating System), maximum flexion, or adverse events.
Triamcinolone improves pain, function, but not for long
A 2016 double-blind RCT of 110 patients with knee OA compared intra-articular HA and triamcinolone, assessing pain and function at intervals between 24 hours and 6 months.3 Patients in the HA group received a single injection of 6 mL hylan G-F 20 (Synvisc); patients in the CS group received 1 mL of triamcinolone acetonide 40 mg and 5 mL of 1% lidocaine with epinephrine.
The CS group reported significantly less pain (VAS score 1 to 100) at 24 hours than the HA group (24 points vs 36 points; P=.002); relief lasted as long as one week (14 points vs 23 points; P=.018). After the first week, no difference was seen in pain between groups for as long as 6 months.
Function, assessed by a modified Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC 1 to 100; higher score indicates worse pain, stiffness, and function) showed a significant improvement with CS at 2 weeks (25 points vs 31 points; P=.03), but no difference at any other time point up to 6 months.
HA (mostly) improves pain, function more than betamethasone
A 2015 RCT of 200 patients with knee OA compared the effectiveness of intra-articular HA and betamethasone.4 Evaluators were blinded and assessments were made at 3, 6, 9, and 12 months. The HA group received 2.5 mL of 1% HA (Suprahyal); the CS group received betamethasone dipropionate 5 mg plus betamethasone sodium phosphate 2 mg in 1 mL.
The CS group had significantly less pain (VAS 1 to 10) at 3 months compared with the HA group (2.2 points vs 3.1 points; P=.004), but the HA group had less pain at all other time points (6 months: 3.9 points vs 2.4 points; P=.0001; 9 months: 5.5 points vs 3.6 points; P=.0001; 12 months: 6 points vs 4.1 points; P=.0001).
The WOMAC function subscores (0 to 68; lower indicates more function) were significantly better at all follow-up points in the HA group compared with the CS group (3 months: 19 vs 25; P=.0001; 6 months: 17 vs 29; P=.0001; 9 months: 25 vs 42; P=.0001; 12 months: 28 vs 42; P=.0001).4
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons 2013 work group couldn’t recommend for or against using intra-articular CS for patients with symptomatic knee OA based on inconclusive evidence.5 They also couldn’t recommend using HA (SOR: strong).
The National Institute for Health and Care Excellence (NICE) stated in 2008 that intra-articular CS injections should be considered as an adjunct to core treatments for the relief of moderate to severe pain in people with OA.6 In 2014, NICE recommended against offering intra-articular HA injections for managing OA.
The US Veterans Administration and Department of Defense have issued guidelines stating that clinicians may consider intra-articular CS injections for patients with symptomatic knee OA (US Preventive Services Task Force [USPSTF] Grade B).7 They report insufficient evidence to recommend for or against the use of intra-articular HA with the caveat that HA may be considered for patients who don’t respond adequately to nonpharmacologic measures and who have an inadequate response, intolerable adverse events, or contraindications to other pharmacologic therapies (USPSTF Grade I).
EVIDENCE SUMMARY
A 2015 network meta-analysis of 137 RCTs with 33,243 patients (ages 45-76 years) with knee OA compared the effectiveness of a variety of treatments including intra-articular CS and HA.1 At 3 months, the effect on pain was not significantly different between the CS and HA groups (12 trials; effect size [ES]=0.02; 95% confidence interval [CI], -0.12 to 0.17). However, a small but significant improvement in function was noted (scoring system not defined) at 3 months favoring HA (ES=0.24; 95% CI, 0.06-0.43; number of trials not specified).
At 3 and 6 months, HA improves pain, but not function, more than CS
Another meta-analysis published in 2015 examined the effectiveness of intra-articular CS and HA in 7 RCTs with 583 patients with knee OA.2 All 7 trials were included in the network meta-analysis and discussed separately to evaluate different time points.
Pain at one month wasn’t significantly different using a visual analog score (VAS) of one to 100 (4 trials; 245 patients; mean difference [MD]=1.66 points; 95% CI, -0.90 to 4.23). At 3 and 6 months, the HA group reported significantly reduced pain compared with the CS group (3 months: 3 trials; 320 patients; MD=12.58 points; 95% CI, -17.76 to -7.40; 6 months: 5 trials; 411 patients; MD=9.01 points; 95% CI, -12.62 to -5.40). There were no significant differences in function outcomes (Index of severity for OA of the knee by Lequesne et al; The Knee Society Clinical Rating System), maximum flexion, or adverse events.
Triamcinolone improves pain, function, but not for long
A 2016 double-blind RCT of 110 patients with knee OA compared intra-articular HA and triamcinolone, assessing pain and function at intervals between 24 hours and 6 months.3 Patients in the HA group received a single injection of 6 mL hylan G-F 20 (Synvisc); patients in the CS group received 1 mL of triamcinolone acetonide 40 mg and 5 mL of 1% lidocaine with epinephrine.
The CS group reported significantly less pain (VAS score 1 to 100) at 24 hours than the HA group (24 points vs 36 points; P=.002); relief lasted as long as one week (14 points vs 23 points; P=.018). After the first week, no difference was seen in pain between groups for as long as 6 months.
Function, assessed by a modified Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC 1 to 100; higher score indicates worse pain, stiffness, and function) showed a significant improvement with CS at 2 weeks (25 points vs 31 points; P=.03), but no difference at any other time point up to 6 months.
HA (mostly) improves pain, function more than betamethasone
A 2015 RCT of 200 patients with knee OA compared the effectiveness of intra-articular HA and betamethasone.4 Evaluators were blinded and assessments were made at 3, 6, 9, and 12 months. The HA group received 2.5 mL of 1% HA (Suprahyal); the CS group received betamethasone dipropionate 5 mg plus betamethasone sodium phosphate 2 mg in 1 mL.
The CS group had significantly less pain (VAS 1 to 10) at 3 months compared with the HA group (2.2 points vs 3.1 points; P=.004), but the HA group had less pain at all other time points (6 months: 3.9 points vs 2.4 points; P=.0001; 9 months: 5.5 points vs 3.6 points; P=.0001; 12 months: 6 points vs 4.1 points; P=.0001).
The WOMAC function subscores (0 to 68; lower indicates more function) were significantly better at all follow-up points in the HA group compared with the CS group (3 months: 19 vs 25; P=.0001; 6 months: 17 vs 29; P=.0001; 9 months: 25 vs 42; P=.0001; 12 months: 28 vs 42; P=.0001).4
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons 2013 work group couldn’t recommend for or against using intra-articular CS for patients with symptomatic knee OA based on inconclusive evidence.5 They also couldn’t recommend using HA (SOR: strong).
The National Institute for Health and Care Excellence (NICE) stated in 2008 that intra-articular CS injections should be considered as an adjunct to core treatments for the relief of moderate to severe pain in people with OA.6 In 2014, NICE recommended against offering intra-articular HA injections for managing OA.
The US Veterans Administration and Department of Defense have issued guidelines stating that clinicians may consider intra-articular CS injections for patients with symptomatic knee OA (US Preventive Services Task Force [USPSTF] Grade B).7 They report insufficient evidence to recommend for or against the use of intra-articular HA with the caveat that HA may be considered for patients who don’t respond adequately to nonpharmacologic measures and who have an inadequate response, intolerable adverse events, or contraindications to other pharmacologic therapies (USPSTF Grade I).
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9:493-500.
3. Tammachote N, Kanitnate S, Yakumpor T, et al. Intra-articular, single-shot Hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885-892.
4. Trueba Davalillo CA, Trueba Vasavilbaso C, Navarrete Alvarez JM, et al. Clinical efficacy of intra-articular injections in knee osteoarthritis: a prospective randomized study comparing hyaluronic acid and betamethasone. Open Access Rheumatol Res Rev. 2015;7:9-18.
5. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd ed. Available at: http://www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Accessed May 15, 2016.
6. National Institute for Health and Care Excellence. Osteoarthritis: Care and Management. Available at: https://www.nice.org.uk/guidance/cg177/chapter/1-recommendations. Accessed May 15, 2016.
7. United States Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Non-Surgical Management of Hip and Knee Osteoarthritis. Available at: http://www.healthquality.va.gov/guidelines/CD/OA/VADoDOACPGFINAL090214.pdf. Accessed May 15, 2016.
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. Wang F, He X. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp Ther Med. 2015;9:493-500.
3. Tammachote N, Kanitnate S, Yakumpor T, et al. Intra-articular, single-shot Hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885-892.
4. Trueba Davalillo CA, Trueba Vasavilbaso C, Navarrete Alvarez JM, et al. Clinical efficacy of intra-articular injections in knee osteoarthritis: a prospective randomized study comparing hyaluronic acid and betamethasone. Open Access Rheumatol Res Rev. 2015;7:9-18.
5. American Academy of Orthopaedic Surgeons. Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline. 2nd ed. Available at: http://www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Accessed May 15, 2016.
6. National Institute for Health and Care Excellence. Osteoarthritis: Care and Management. Available at: https://www.nice.org.uk/guidance/cg177/chapter/1-recommendations. Accessed May 15, 2016.
7. United States Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Non-Surgical Management of Hip and Knee Osteoarthritis. Available at: http://www.healthquality.va.gov/guidelines/CD/OA/VADoDOACPGFINAL090214.pdf. Accessed May 15, 2016.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Inconsistent evidence shows a small amount of pain relief early (one week to 3 months) with corticosteroid (CS) injections and an equally small improvement in pain relief and function later (3 to 12 months) with hyaluronic acid (HA) injections (strength of recommendation [SOR]: B, meta-analysis of a randomized controlled trial [RCT] and inconsistent RCTs).
Guidelines state that CS injections can be considered for symptomatic knee osteoarthritis (OA), but that insufficient evidence exists to recommend HA injections (SOR: B, evidence-based guidelines).