User login
Hepatocellular carcinoma: Options for diagnosing and managing a deadly disease
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
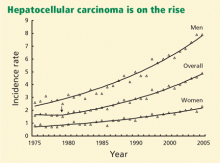
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
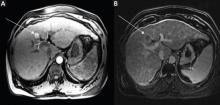
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
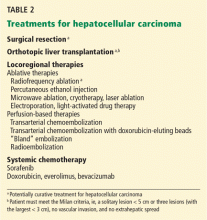
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
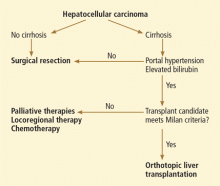
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. However, it can be detected early in high-risk individuals by using effective screening strategies, resulting in the ability to provide curative treatment.
Here, we review the risk factors for HCC, strategies for surveillance and diagnosis, and therapies that can be used.
EPIDEMIOLOGY
HCC is the most common primary malignancy of the liver. Overall, it is the fifth most common type of cancer in men and the seventh most common in women.1
Cirrhosis is present in 80% to 90% of patients with HCC.
Male sex. The male-to-female ratio is from 2:1 to 4:1, depending on the region.2 In the United States, the overall male-to-female ratio has been reported2 as 2.4:1. In another report,3 the incidence rate of HCC per 100,000 person-years was 3.7 for men and 2.0 for women.
Geographic areas with a high incidence of HCC include sub-Saharan Africa and eastern Asia, whereas Canada and the United States are low-incidence areas. The difference has been because of a lower prevalence of hepatitis B virus infection in North America. However, recent data show a downward trend in incidence of HCC in eastern Asia and an upward trend in North America (Figure 1).3,4
Viral hepatitis (ie, hepatitis B or hepatitis C) is the main risk factor for cirrhosis and HCC.
Diabetes mellitus can predispose to nonalcoholic steatohepatitis, which can subsequently progress to cirrhosis. Thus, it increases the risk of HCC.
Obesity increases the risk of death from liver cancer, with obese people (body mass index ≥ 30 kg/m2) having a higher HCC-related death rate than leaner individuals.5 And as obesity becomes more prevalent, the number of deaths from HCC could increase.
Other diseases that predispose to HCC include alcohol abuse, hereditary hemochromatosis, alpha-1-antitrypsin deficiency, and glycogen storage disease.
SURVEILLANCE OF PATIENTS AT RISK
Patients at high risk of developing liver cancer require frequent screening (Table 1).
Patients with cirrhosis. Sarasin et al6 calculated that surveillance is cost-effective and increases the odds of survival in patients with cirrhosis if the incidence of HCC exceeds 1.5% per year (which it does). In view of this finding, all patients with cirrhosis should be screened every 6 months, irrespective of the cause of the cirrhosis.
Hepatitis B carriers. Surveillance is also indicated in some hepatitis B carriers (Table 1), eg, those with a family history of HCC in a first-degree relative (an independent risk factor for developing the disease in this group).7 Also, Africans with hepatitis B tend to develop HCC early in life.8 Though it has been recommended that surveillance be started at a younger age in these patients,9 the age at which it should begin has not been clearly established. In addition, it is not clear if black people born outside Africa are at higher risk.
Benefit of surveillance
HCC surveillance has shown to lower the death rate. A randomized controlled trial in China compared screening (with abdominal ultrasonography and alpha-fetoprotein levels) vs no screening in patients with hepatitis B. It showed that screening led to a 37% decrease in the death rate.12 Studies have also established that patients with early-stage HCC have a better survival rate than patients with more-advanced disease.10,11 This survival benefit is largely explained by the availability of effective treatments for early-stage cancer, including liver transplantation. Therefore, early-stage asymptomatic patients diagnosed by a surveillance program should have a better survival rate than symptomatic patients.
Surveillance methods
The tests most often used in surveillance for HCC are serum alpha-fetoprotein levels and liver ultrasonography.
Serum alpha-fetoprotein levels by themselves have not been shown to be useful, whereas the combination of alpha-fetoprotein levels and ultrasonography has been shown to reduce the death rate when used for surveillance in a randomized trial.12 A 2012 study reported that the combination of alpha-fetoprotein testing and ultrasonography had a higher sensitivity (90%) than ultrasonography alone (58%), but at the expense of a lower specificity.13
Alpha-fetoprotein has a low sensitivity (ie, 54%) for HCC.14 Tumor size is one of the factors limiting the sensitivity of alpha-fetoprotein, 14 and this would imply that this test may not be helpful in detecting HCC at an early stage. Alpha-fetoprotein L3, an isoform of alpha-fetoprotein, may be helpful in patients with alpha-fetoprotein levels in the intermediate range, and it is currently being studied.
Liver ultrasonography is operator-dependent, and it may not be as accurate in overweight or obese people.
Computed tomography (CT) and magnetic resonance imaging (MRI) are not recommended for surveillance. Serial CT poses risks of radiation-induced damage, contrast-related anaphylaxis, and renal failure, and MRI is not cost-effective and can also lead to gadolinium-induced nephrogenic systemic fibrosis in patients with renal failure.
Currently, the American Association for the Study of Liver Diseases9 recommends ultrasonography only, every 6 months, for surveillance for HCC. However, it may be premature to conclude that alpha-fetoprotein measurement is no longer required for surveillance, and if new data emerge that support its role, it may be reincorporated into the guidelines.
DIAGNOSING HEPATOCELLULAR CARCINOMA
Lesions larger than 1 cm on ultrasonography
The finding of a liver lesion larger than 1 cm on ultrasonography during surveillance warrants further testing.
Noninvasive testing with four-phase multidetector CT or dynamic contrast-enhanced MRI is the next step. Typical findings on either of these imaging studies are sufficient to make a diagnosis of HCC, as they have a high specificity and positive predictive value.15 Arterial hyperenhancement with a venous-phase or delayed-phase washout of contrast medium confirms the diagnosis (Figure 2).9 If one of the two imaging studies is typical for HCC, liver biopsy is not needed.
Other imaging studies, including contrast-enhanced ultrasonography, have not been shown to be specific for this diagnosis.16
Liver biopsy is indicated in patients in whom the imaging findings are atypical for HCC.9,17 Biopsy has very good sensitivity and specificity for cancer, but false-negative findings do occur.18 Therefore, a negative biopsy does not entirely exclude HCC. In this situation, patients should be followed by serial ultrasonography, and any further growth or change in character should be reevaluated.
Lesions smaller than 1 cm
For lesions smaller than 1 cm, the incidence of HCC is low, and currently available diagnostic tests are not reliable.15,19 Lesions of this size should be followed by serial ultrasonography every 3 to 4 months until they either enlarge to greater than 1 cm or remain stable at 2 years.9 If they remain stable at the end of 2 years, regular surveillance ultrasonography once every 6 months can be continued.
CURATIVE AND PALLIATIVE THERAPIES
Therapies for HCC (Table 2) can be divided into two categories: curative and palliative.
Curative treatments include surgical resection, liver transplantation, and radiofrequency ablation. All other treatments are palliative, including transarterial chemoembolization and medical therapy with sorafenib.
The choice of treatment depends on the characteristics of the tumor, the degree of liver dysfunction, and the patient’s current level of function. The Barcelona Clinic Liver Cancer classification is widely used in making these decisions, as it incorporates both clinical features and tumor stage.9 Figure 3 shows a simplified management algorithm.
SURGICAL RESECTION
Surgical resection is the preferred treatment for patients who have a solitary HCC lesion without cirrhosis.9 It is also indicated in patients with well-compensated cirrhosis who have normal portal pressure, a normal serum bilirubin level, and a platelet count greater than 100 × 109/L.20,21 In such patients, the 5-year survival rate is about 74%, compared with 25% in patients with portal hypertension and serum bilirubin levels higher than 1 mg/dL.21
Surgical resection is not recommended for patients with decompensated cirrhosis, as it can worsen liver function postoperatively and increase the risk of death.19,20 In Western countries, where cirrhosis from hepatitis C is the commonest cause of HCC, most patients have poorly preserved hepatic function at the time of diagnosis, leaving only a minority of patients as candidates for surgical resection.
After surgical resection of HCC, the recurrence rate can be as high as 70% to 80% at 5 years.22,23 Studies have consistently found larger tumor size and vascular invasion to be factors that predict recurrence.24,25 Vascular invasion was also found to predict poor survival after recurrence.24 Studies have so far not shown any conclusive benefit from post-surgical adjuvant chemotherapy in reducing the rate of recurrence of HCC.26,27
How to treat recurrent HCC after surgical resection has not been clearly established. Radiofrequency ablation, transarterial chemoembolization, repeat resection, and liver transplantation have all improved survival when used alone or in combination.28 However, randomized controlled trials are needed to establish the effective treatment strategy and the benefit of multimodal treatment of recurrent HCC.
LIVER TRANSPLANTATION
Orthotopic liver transplantation is the preferred treatment for patients with HCC complicated by cirrhosis and portal hypertension. It has the advantage not only of being potentially curative, but also of overcoming liver cirrhosis by replacing the liver.
To qualify for liver transplantation, patients must meet the Milan criteria (ie, have a single nodule less than 5 cm in diameter or up to three nodules, with the largest being less than 3 cm in diameter, with no evidence of vascular invasion or distant metastasis). These patients have an expected 4-year survival rate of 85% and a recurrence-free survival rate of 92% after transplantation, compared with 50% and 59%, respectively, in patients whose tumors exceeded these criteria.29
Some believe that the Milan criteria are too restrictive and could be expanded. Yao et al at the University of California-San Francisco30 reported that patients with HCC meeting the criteria of having a solitary tumor smaller than 6.5 cm or having up to three nodules, with the largest smaller than 4.5 cm, and total tumor diameter less than 8 cm, had survival rates of 90% at 1 year and 75.2% at 5 years after liver transplantation, compared with 50% at 1 year for patients with tumors exceeding these limits. (These have come to be known as the UCSF criteria.) However, the United Network for Organ Sharing (UNOS) has not adopted these expanded criteria. UNOS has a point system for allocating livers for transplant called the Model for End-Stage Liver Disease (MELD). Patients who meet the Milan criteria receive extra points, putting them higher on the transplant list. This allows for early transplantation, thus reducing tumor progression and dropout from the transplant list. UNOS allocates a MELD score of 22 to all patients who meet the Milan criteria, and the score is further adjusted once every 3 months to reflect a 10% increase in the mortality rate. However, patients who have a single lesion smaller than 2 cm and are candidates for liver transplantation are not assigned additional MELD points per UNOS policy, as the risk of tumor progression beyond the Milan criteria in these patients is deemed to be low.
Therapies while awaiting transplantation
Even if they receive additional MELD points to give them priority on the waiting list, patients face a considerable wait before transplantation because of the limited availability of donor organs. In the interim, they have a risk of tumor progression beyond the Milan criteria and subsequent dropout from the transplant list.31 Patients on the waiting list may therefore undergo a locoregional therapy such as transarterial chemoembolization or radiofrequency ablation as bridging therapy.
These therapies have been shown to decrease dropout from the waiting list.31 A prospective study showed that in 48 patients who underwent transarterial chemoembolization while awaiting liver transplantation, none had tumor progression, and 41 did receive a transplant, with excellent posttransplantation survival rates.32 Similarly, radioembolization using yttrium-90-labeled microspheres or radiofrequency ablation while on the waiting list has been shown to significantly decrease the rate of dropout, with good posttransplantation outcomes.33,34
However, in spite of these benefits, these bridging therapies do not increase survival rates after transplantation. It is also unclear whether they are useful in regions with short waiting times for liver transplantation.
Adjuvant systemic chemotherapy has not been shown to improve survival in patients undergoing liver transplantation. For example, in a randomized controlled trial of doxorubicin given before, during, and after surgery, the survival rate at 5 years was 38% with doxorubicin and 40% without.35
ABLATIVE LOCOREGIONAL THERAPIES
Locoregional therapies play an important role in managing HCC. They are classified as ablative and perfusion-based.
Ablative locoregional therapies include chemical modalities such as percutaneous ethanol injection; thermal therapies such as radiofrequency ablation, microwave ablation, laser ablation, and cryotherapy; and newer methods such as irreversible electroporation and light-activated drug therapy. Of these, radiofrequency ablation is the most widely used.
Radiofrequency ablation
Radiofrequency ablation induces thermal injury, resulting in tumor necrosis. It can be used as an alternative to surgery in patients who have a single HCC lesion less than 3 to 5 cm in diameter, confined to the liver, and in a site amenable to this procedure and who have a reasonable coagulation profile. The procedure can be performed percutaneously or via laparoscopy.
Radiofrequency ablation is contraindicated in patients with decompensated cirrhosis, Child-Pugh class C cirrhosis (the most severe category), vascular or bile duct invasion, extrahepatic disease, or lesions that are not accessible or are adjacent to structures such as the gall bladder, bowel, stomach, or diaphragm.
Radiofrequency ablation has been compared with surgical resection in patients who had small tumors. Though a randomized controlled trial did not show any difference between the two treatment groups in terms of survival at 5 years and recurrence rates,36 a meta-analysis showed that overall survival rates at 3 years and 5 years were significantly higher with surgical resection than with radiofrequency ablation.37 Patients also had a higher rate of local recurrence with radiofrequency ablation than with surgical resection.37 In addition, radiofrequency ablation has been shown to be effective only in small tumors and does not perform as well in lesions larger than 2 or 3 cm.
Thus, based on current evidence, surgical resection is preferable to radiofrequency ablation as first-line treatment. The latter, however, is also used as a bridging therapy in patients awaiting liver transplantation.
Percutaneous ethanol injection
Percutaneous ethanol injection is used less frequently than radiofrequency ablation, as studies have shown the latter to be superior in regard to local recurrence-free survival rates.38 However, percutaneous ethanol injection is used instead of radiofrequency ablation in a small number of patients, when the lesion is very close to organs such as the bile duct (which could be damaged by radiofrequency ablation) or the large vessels (which may make radiofrequency ablation less effective, since heat may dissipate as a result of excessive blood flow in this region).
Microwave ablation
Microwave ablation is an emerging therapy for HCC. Its advantage over radiofrequency ablation is that its use is not limited by blood vessels in close proximity to the ablation site.
Earlier studies did not show microwave ablation to be superior to radiofrequency ablation.39,40 However, current studies involving newer techniques of microwave ablation are more promising.41
PERFUSION-BASED LOCOREGIONAL THERAPIES
Perfusion-based locoregional therapies deliver embolic particles, chemotherapeutic agents, or radioactive materials into the artery feeding the tumor. The portal blood flow allows for preservation of vital liver tissue during arterial embolization of liver tumors. Perfusionbased therapies include transarterial chemoembolization, transarterial chemoembolization with doxorubicin-eluting beads (DEB-TACE), “bland” embolization, and radioembolization.
Transarterial chemoembolization
Transarterial chemoembolization is a minimally invasive procedure in which the hepatic artery is cannulated through a percutaneous puncture, the branches of the hepatic artery supplying the tumor are identified, and then embolic particles and chemotherapeutic agents are injected. This serves a dual purpose: it embolizes the feeding vessel that supplies the tumor, causing tumor necrosis, and it focuses the chemotherapy on the tumor and thus minimizes the systemic effects of the chemotherapeutic agent.
This therapy is contraindicated in patients with portal vein thrombosis, advanced liver dysfunction, or a transjugular intrahepatic portosystemic shunt. Side effects of the procedure include a postembolization syndrome of abdominal pain and fever (occurring in about 50% of patients from ischemic injury to the liver), hepatic abscesses, injury to the hepatic artery, development of ascites, liver dysfunction, and contrast-induced renal failure.
In addition to bridging patients to liver transplantation, transarterial chemoembolization is recommended as palliative treatment to prolong survival in patients with HCC who are not candidates for liver transplantation, surgical resection, or radiofrequency ablation.9,42 Patients who have Child-Pugh grade A or B cirrhosis but do not have main portal vein thrombosis or extrahepatic spread are candidates for this therapy. Patients such as these who undergo this therapy have a better survival rate at 2 years compared with untreated patients.43,44
Transarterial chemoembolization has also been used to reduce the size of (ie, to “downstage”) tumors that are outside the Milan criteria in patients who are otherwise candidates for liver transplantation. It induces tumor necrosis and has been shown to decrease the tumor size in a selected group of patients and to bring them within the Milan criteria, thus potentially enabling them to be put on the transplant list.45 Studies have shown that patients who receive a transplant after successful down-staging may achieve a 5-year survival rate comparable with that of patients who were initially within the Milan criteria and received a transplant without the need for down-staging.45 However, factors that predict successful down-staging have not been clearly established.
Newer techniques have been developed. A randomized controlled trial found transarterial chemoembolization with doxorubicin-eluting beads to be safer and better tolerated than conventional transarterial chemembolization.46
Bland embolization is transarterial embolization without chemotherapeutic agents and is performed in patients with significant liver dysfunction who might not tolerate chemotherapy. The benefits of this approach are yet to be determined.
Radioembolization
Radioembolization with yttrium-90 microspheres has recently been introduced as an alternative to transarterial chemoembolization, especially in patients with portal vein thrombosis, a portocaval shunt, or a transjugular intrahepatic portosystemic shunt.
In observational studies, radioembolization was as effective as transarterial chemoembolization, with a similar survival benefit.47 However, significant pulmonary shunting must be ruled out before radioembolization, as it would lead to radiation-induced pulmonary disease. Randomized controlled trials are under way to compare the efficacy of the two methods.
CHEMOTHERAPY
Sorafenib
Sorafenib is an oral antiangiogenic agent. A kinase inhibitor, it interacts with multiple intracellular and cell-surface kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and Raf proto-oncogene, inhibiting tumor cell proliferation and angiogenesis.
Sorafenib has been shown to prolong survival in patients with advanced-stage HCC.48 A randomized placebo-controlled trial in patients with Child-Pugh grade A cirrhosis and advanced HCC who had not received chemotherapy showed that sorafenib increased the life expectancy by nearly 3 months compared with placebo.47 Sorafenib therapy is very expensive, but it is usually covered by insurance.
Sorafenib is recommended in patients who have advanced HCC with vascular invasion, extrahepatic dissemination, or minimal constitutional symptoms. It is not recommended for patients with severe advanced liver disease who have moderate to severe tumor-related constitutional symptoms or Child-Pugh grade C cirrhosis, or for patients with a life expectancy of less than 3 months.
The most common side effects of sorafenib are diarrhea, weight loss, and skin reactions on the hands and feet. These commonly lead to decreased tolerability and dose reductions.47 Doses should be adjusted on the basis of the bilirubin and albumin levels.49
Other chemotherapeutic agents
Several molecular targeted agents are undergoing clinical trials for the treatment of HCC. These include bevacizumab, erlotinib, brivanib, and ramucirumab. Chemotherapeutic agents such as doxorubicin and everolimus are also being studied.
PALLIATIVE TREATMENT
Patients with end-stage HCC with moderate to severe constitutional symptoms, extrahepatic disease progression, and decompensated liver disease have a survival of less than 3 months and are treated for pain and symptom control.9
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557–2576.
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273.e1.
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485–1491.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003; 348:1625–1638.
- Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med 1996; 101:422–434.
- Yu MW, Chang HC, Liaw YF, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst 2000; 92:1159–1164.
- Kew MC, Macerollo P. Effect of age on the etiologic role of the hepatitis B virus in hepatocellular carcinoma in blacks. Gastroenterology 1988; 94:439–442.
- Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009; 373:614–616.
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012; 104:298–304.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004; 130:417–422.
- Giannini EG, Erroi V, Trevisani F. Effectiveness of a-fetoprotein for hepatocellular carcinoma surveillance: the return of the living-dead? Expert Rev Gastroenterol Hepatol 2012; 6:441–444.
- Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006; 101:524–532.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47:97–104.
- Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51:2020–2029.
- Kojiro M. Pathological diagnosis at early stage: reaching international consensus. Oncology 2010; 78(suppl 1):31–35.
- Schölmerich J, Schacherer D. Diagnostic biopsy for hepatocellular carcinoma in cirrhosis: useful, necessary, dangerous, or academic sport? Gut 2004; 53:1224–1226.
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001; 35:254–258.
- Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996; 111:1018–1022.
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999; 30:1434–1440.
- Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology 1993; 105:488–494.
- Arii S, Tanaka J, Yamazoe Y, et al. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer 1992; 69:913–919.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg 2003; 197:753–758.
- Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339.
- Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43:1405–1409.
- Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24(suppl 6):S6–25.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–222.
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334:693–699.
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33:1394–1403.
- Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl 2011; 17(suppl 2):S98–S108.
- Graziadei IW, Sandmueller H, Waldenberger P, et al. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl 2003; 9:557–563.
- Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol 2006; 94:572–586.
- Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology 2005; 41:1130–1137.
- Pokorny H, Gnant M, Rasoul-Rockenschaub S, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005; 5:788–794.
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012; 57:794–802.
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010; 10:78.
- Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology 2003; 228:235–240.
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009; 24:223–227.
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223:331–337.
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol 2012; 22:1983–1990.
- Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol 2012; 56:1330–1335.
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002; 224:47–54.
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37:429–442.
- Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology 2008; 48:819–827.
- Ferrer Puchol MD, la Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEBTACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma (article in Spanish). Radiologia 2011; 53:246–253.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011; 140:497–507.e2.
- Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378–390.
- Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol 2009; 27:1800–1805.
KEY POINTS
- Surveillance for HCC is indicated in all patients with cirrhosis, regardless of the cause of the cirrhosis.
- Liver biopsy is not needed to make the diagnosis if the findings on four-phase multidetector computed tomography or dynamic contrast-enhanced magnetic resonance imaging are typical of HCC (arterial hyperenhancement with venous-phase or delayed-phase washout).
- Many treatments are available, including surgical resection, liver transplantation, ablative therapy, perfusion-based therapy, chemotherapy, and palliative therapy.