User login
Isolated oligohydramnios at term: Is induction indicated?
- Isolated term oligohydramnios, as defined by an amniotic fluid index (AFI) of less than 5 cm, has not been shown to be associated with poor maternal or fetal outcomes. Management may be individualized based on factors such as parity, cervical ripeness, and patient preference (SOR: B).
- Maternal hydration with oral water has been shown to increase AFI in a few hours, likely due to improved uteroplacental perfusion. This is a reasonable alternative to immediate labor induction in women with isolated term oligohydramnios (SOR: B).
- An isolated finding of a so-called “border-line” AFI (5–8 cm) is not an indication for labor induction (SOR: B).
Family physicians providing maternity care often face a scenario in which an otherwise low-risk, term patient is incidentally noted to have a low amniotic fluid index (AFI). Common reasons for obtaining an AFI in a woman with a low-risk pregnancy include evaluation of decreased fetal movement, spontaneous variable decelerations during monitoring to evaluate for labor, or an ultrasound evaluation for fundal height measurements discordant with gestational age. How should “isolated” oligohydramnios—an AFI <5 cm—be interpreted, and should immediate induction be recommended for such patients?
Oligohydramnios occurs in about 1% to 5% of pregnancies at term.1,2 Because adverse outcomes occur in high-risk pregnancies complicated by low amniotic fluid volume, oligohydramnios commonly prompts labor induction.1,3,4 At one university center, oligohydramnios is now the leading indication for labor induction.5 Many centers may even induce labor when the AFI is between 5 cm and 8 cm, the so-called borderline AFI.3
Labor induction increases the use of cesarean delivery, particularly for the primiparous woman with an unripe cervix.6 Recent studies questioning the safety of labor induction in women who have had a cesarean may increase the number of elective repeat cesarean procedures when delivery is believed indicated for oligohydramnios.7 (See Underlying causes of oligohydramnios.)
By the second trimester, amniotic fluid is being produced primarily through fetal urine production and is primarily resorbed through fetal swallowing. Significant amounts of amniotic fluid are also produced and resorbed by the fetal lung and directly resorbed from the amniotic cavity by the placenta.8,9 Amniotic fluid volume is affected by the status of maternal hydration and maternal plasma osmolality.10-13
Acute oligohydramnios may occur from ruptured membranes, usually diagnosed by clinical signs and vaginal fluid with altered pH and a ferning pattern on microscopic exam.
Chronic oligohydramnios arises from prerenal, renal, and postrenal causes. The latter 2 groups reflect fetal kidney and urogenital abnormalities, which directly decrease fetal amniotic fluid production. Uteroplacental insufficiency is the most common cause of prerenal oligohydramnios, and the decreased amniotic fluid is a direct result of decreased fetal renal perfusion.14 Uteroplacental insufficiency may result in intrauterine growth restriction as the fetus shunts blood away from the growing torso and limbs and to vital organs such as the brain. Preeclampsia and postdate pregnancies both involve pathologic changes in the placenta that may result in uteroplacental insufficiency and oligohydramnios.
Oligohydramnios is difficult to assess
True oligohydramnios can be difficult to confirm due to the questionable accuracy of amniotic fluid measurement by ultrasound. There is controversy, for example, about whether (and how) to include pockets of amniotic fluid containing umbilical cord.15 The AFI was introduced in 19872 to replace the 2 cm “pocket technique” of fluid assessment, and studies continue to question to what extent the AFI reflects actual amniotic fluid volume.
AFI measurements may vary with the amount of pressure applied to the abdomen and with fetal position or movement.16
Serial measurements taken by the same ultrasound operator have been shown to differ from the true volume by 1 cm, or 10.8%; serial measurements taken by multiple operators have differed by as much as 2 cm, or 15.4%.17,18
O’Reilly-Green compared the diagnosis of oligohydramnios in 449 post-term patients with actual amniotic fluid volume measured at rupture of membranes.19 They found a positive predictive value of 50% for oligohydramnios at an AFI of 5 cm as the lower limit of normal. A study of 144 third trimester patients using the dye-dilution technique found that, to achieve 95% confidence for ruling out oligohydramnios, a cutoff AFI of 30 cm would need to be used, a value consistent with polyhydramnios.20
What is the association between oligohydramnios and poor fetal outcomes?
A number of studies over the past 15 years have shown an association between oligohydramnios and poor fetal outcomes. These were predominantly retrospective studies, which failed to control for the presence of factors known to be associated with oligohydramnios such as intrauterine growth restriction (IUGR) and urogenital malformations.
No studies have directly addressed whether labor induction improves outcomes. A meta-analysis of 18 studies examining outcomes of pregnancies with AFI <5 cm found an increased risk of cesarean delivery for fetal distress and low Apgar scores at 5 minutes. Most of these studies, however, had high-risk patients including IUGR (level of evidence [LOE]: 2).21
A recent study of high-risk patients failed to detect a difference in the incidence of nonreactive nonstress tests, meconium-stained amniotic fluid cesarean delivery for fetal distress, low Apgar scores, or infants with a cord pH of <7.10 when oligohydramnios (AFI <5.0 cm) was present (LOE: 1).2 The patients with oligohydramnios were all induced, while many of the other high-risk patients were expectantly managed. The study therefore provides no guidance on the safety of expectant management for patients with oligohydramnios. To eliminate the potential effect of induction versus expectant management the same authors performed a case-control study of 79 high-risk women with AFI <5 cm matched to 79 women with the same high-risk pregnancy complication who had an AFI >5 cm at the time of induction (LOE: 2).23 They failed to detect any significant differences in neonatal outcomes between the groups.
Studies of the “borderline” AFI (between 5 cm and 8 cm) may also demonstrate an association with adverse neonatal outcomes if researchers include fetuses with IUGR or malformations. In one retrospective case review of 214 women with AFI of 5 cm to 10 cm, the only statistically significant finding was an association with IUGR.3 The authors recommended antepartum surveillance twice a week for mothers with borderline AFI, but they did not comment on induction (LOE: 2). Correspondence regarding this study argued that this recommendation was not supported by the evidence and would lead to unnecessary antenatal testing.24
Studies of isolated oligohydramnios
Investigators have conducted studies (Table 1) excluding fetuses with intrauterine growth restriction or anomalies to try to determine if isolated oligohydramnios is associated with poorer outcomes.25-30
Rainford’s study of outcomes in exclusively term, low-risk patients failed to show significant outcome differences in Apgar scores, NICU admissions, or rates of cesarean delivery for non-reassuring fetal heart rate monitoring (LOE: 2).29 This study was limited due to its retrospective design. The authors comment that the relatively good outcomes in the oligohydramnios group may be due to the widespread practice of inducing such patients.
In a case-control study by Conway, 183 low-risk, term parturients with oligohydramnios were matched to 183 women of similar gestational age and parity who presented in spontaneous labor. The patients with isolated oligohydramnios were induced and showed an increased cesarean delivery rate. The increased rate of cesarean delivery was not due to nonreassuring fetal surveillance and was attributed to the induction process (LOE: 2).25
An analysis of woman diagnosed with isolated oligohydramnios (AFI <5) at any gestational age in the multicenter prospective RADIUS trial demonstrated similar perinatal outcomes and fetal growth compared with pregnancies with a normal amniotic fluid (LOE: 2).30
The only randomized clinical trial of labor induction vs expectant management for term isolated oligohydramnios showed similar outcomes in each group. But this study was small (n=61) and has only been published as an abstract.31
TABLE 1
Isolated oligohydramnios and perinatal outcomes
| Study | Design | Study number n vs controls | Patient-oriented outcomes | Comment | LOE | |
|---|---|---|---|---|---|---|
| Population | Significant findings | Non-significant findings | ||||
| Garmel19 | Prospective cohort | N=187 | Increased preterm birth (OR=3.23; 95% CI, 1.4–7.3) in oligohydramnios group | IUGR, asphyxia, death, NICU admit | Delivery recommended at 37 weeks | 2 |
| 17–37 week with subnormal EFW (>10%) | 65 AFI <8 cm vs 122 AFI >8 cm | |||||
| Conway18 | Prospective cohort | N=366 | Increased CS rate (OR=2.7 95% CI, 1.3–5.4) in oligohydramnios group | CS for fetal distress; all neonatal outcomes | Treatment group induced, controls spontaneous | 2 |
| Term, isolated oligohydramnios undergoing induction | 183 AFI <5 cm vs 183 AFI >5 cm | |||||
| Roberts21 | Prospective cohort | N=206 | Increased IUGR (OR=5.2; 95% CI, 1.6–22), induction (OR=34.4, 95% CI, 4–1425.5), NICU admit (OR=9.8; 95% CI, 1.3–432) | Fetal distress requiring CS | Used >5%ile to exclude IUGR. Included some high-risk pts (diabetes or hypertension) | 2 |
| 3rd trimester, isolated oligohydramnios | 103 AFI 3%ile (N=103) vs matched control | |||||
| Rainford22 | Retrospective cohort | N=232 | Induction rate for AFI <5 = 98% vs 51% AFI >5 P<.001; increased meconium staining in controls without oligohydramnios | NICU 2 admissions, 5-minute Apgar scores | 2 | |
| 37–41 week, low-risk. AFI within 4 days of delivery | AFI <5 (n=44) vs >5 (n=188) | |||||
| Zhang23 | Retrospective nested cohort | N=6657 | Malpresentation (RR=3.5, 95% CI 1.8–6.60) | Fetal growth, CS, low Apgar, overall neonatal morbidity | Benefit of routine ultrasound was the primary study outcome study endpoint | 2 |
| Term or near-term, low-risk | AFI <5 (n=86) vs >5 (n=6571) | |||||
| AFI, amniotic fluid index; CI, confidence interval; CS, cesarean section; EFW, estimated fetal weight; IUGR, intrauterine growth restriction; LOE, level of evidence; NICU, neonatal intensive care unit; OR, odds ratio; RR, relative risk. | ||||||
Effect of maternal hydration
Maternal hydration status and plasma osmolality have an affect on amniotic fluid volume (Table 2). Maternal hydration with oral water or intravenous hypotonic solutions has been shown to increase amniotic fluid volume.8,11-13 Oral hydration with hypotonic fluid has been demonstrated to increase fetal urine production in one observational study.32 Another observational study demonstrated increased amniotic fluid volume and uteroplacental perfusion without alteration of fetal urine production suggesting the possibility that transmembranous fluid shifts from the placenta to the amniotic cavity may be involved.12
Two small, randomized controlled trials (RCTs) demonstrated an increase in amniotic fluid volume in women with oligohydramnios after oral hydration.11,13 Doi demonstrated significant increases in AFI in women with oligohydramnios beyond 35 weeks when given oral hydration with free water (increase of 3.8 cm ± 1.9; P<. 001) or hypotonic intravenous solution (increase of 2.8 cm ± 1.9; P<.001) (LOE: 3).11 Interestingly, this study did not demonstrate an increase in amniotic fluid volume with intravenous hydration with isotonic fluid.
Kirkpatrick demonstrated a 30% increase in amniotic fluid compared with controls in women of unspecified gestational age with oligohydramnios given 2 liters of oral water 2 to 5 hours before repeat amniotic fluid index (LOE: 3).13
A randomized trial in women with normal amniotic fluid demonstrated a 16% increase in amniotic fluid index 4 to 6 hours after hydration with 2 liters of oral water, compared with an 8% decrease after fluid restriction during the same period.8
A recent study of daily oral hydration in women with amniotic fluid volume <10% percentile showed increased amniotic fluid volume at 1 week, suggesting long-term benefit, although the study lacked an appropriate control group (LOE: 3).33
There are no studies of clinical outcomes such as fetal heart rate decelerations during labor, or neonatal outcomes. A Cochrane systematic review concluded that maternal hydration appears to increase amniotic fluid and may be beneficial in management of oligohydramnios; however, it recommended controlled trials to assess clinical outcome benefits (LOE: 3).34
TABLE 2
Effect of hydration on amniotic fluid index
| Study | Design | Population | Intervention | Outcome | Comment | LOE |
|---|---|---|---|---|---|---|
| Kilpatrick32 | RCT | N=40, AFI 2.1–6.0; population of patients referred for antenatal testing | Treatment group drank 2 L water and repeat AFI same or next day | Increase of 1.5 ± 1.4 cm (P<.01) in treatment group | Gestational ages of subjects not stated | 3 |
| Kilpatrick37 | RCT | N=40, AFI 7–24 cm, gestational 28 weeks | Treatment group instructed to drink 2 L and restricted group 0.1 L water. AFI repeated in 4–6 h | Increase of 3.0 ± 2.4 cm (P<.0001) in treatment group; decrease of 1.5 ± 2.7cm in controls (P <.02) | Subjects had normal AFI at entry | 3 |
| Flack36 | Prospective cohort | N=20, 10 w/AFI <5 cm, 10 controls AFI >7, 3rd trimester | 2 L oral water over 2 h for treatment and control groups, repeat AFI at 2 h | Increase in 3.2 cm in AFI (95% CI, 1.1–5.3) in oligohydramnios group but not in normal AFI group | Improved uterine perfusion shown by increased uterine artery velocity only in oligohydramnios group | 3 |
| Doi35 | RCT | N=84, AFI <5, at least 35 wks; randomized three maternal hydration methods (2 L oral water, hypotonic saline IV, or isotonic saline IV) | Hydration with 2 L fluid and AFI repeated in 1 h compared with controls | Significant increases in AFI in oral water and hypotonic IV groups by 3.8 cm and 2.8 cm (P<.001) respectively | IV isotonic solutions did not increase amniotic fluid volume in study population | 3 |
| RCT, randomized controlled trial; AFI, amniotic fluid index; CI, confidence interval. | ||||||
Management recommendations
The AFI has low specificity and positive predictive value for oligohydramnios, and there is scant evidence that isolated term oligohydramnios causes adverse fetal outcomes. We recommend that an AFI under 5 cm should prompt additional antenatal testing rather than immediate induction in low-risk term pregnancies (SOR: B).
Though we acknowledge the lack of high-quality studies with patient-oriented outcomes to support observation and maternal hydration, we have developed a management strategy that does not require immediate induction of labor in women with uncomplicated term pregnancies.
The following recommendations apply to women having oligohydramnios as defined by amniotic fluid volume of less than 5 cm and gestational age between 37 and 41 weeks.
Initial assessment
- Assess for premature rupture of membranes with a thorough history and a sterile speculum exam
- Reassess dating as oligohydramnios in post-dates pregnancy (>41 weeks) is an indication for induction (SOR: C)35
- Perform a nonstress test to assess fetal wellbeing
- Assess for IUGR with an ultrasound for estimated fetal weight and for the ratio of head circumference (HC) to abdominal circumference (AC). A comparison with prior ultrasounds can aid in assessing interval growth. An estimated fetal weight below the 10%, an elevated HC/AC ratio, or poor interval growth would suggest IUGR
- Arrange for an ultrasound anatomic survey for fetal anomalies, if not done previously
- Determine if preeclampsia, chronic hypertension, diabetes, or other maternal conditions associated with uteroplacental insufficiency are present.
Action steps
With any positive findings in the initial evaluation, proceed to labor induction, as the patient does not have isolated, term oligohydramnios (SOR: C). If the initial assessment is unremarkable and the AFI is less than 5, consider hydration with oral water and repeating the AFI 2 to 6 hours later (SOR: B).
Persistent oligohydramnios at term, particularly with a ripe cervix, may lead you to consider labor induction. Continued expectant management of isolated term oligohydramnios with twice weekly fetal surveillance may also be a reasonable option due to the paucity of evidence that oligohydramnios is associated with an adverse outcome in this scenario (SOR: C). Normal results with umbilical artery Doppler flow studies have been used to decrease the need for induction in high-risk pregnancies with oligohydramnios, and this technique may eventually have a role in isolated term oligohydramnios.36
It is essential that patients receive counseling and give informed consent regarding the risks and benefits of observation or induction for isolated term oligohydramnios. The ease of induction based on parity and cervical ripeness should be considered.
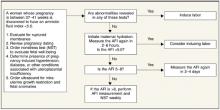
A primiparous woman with an unfavorable cervix who strongly desires a spontaneous, vaginal birth could be told that, although there may be a small risk for her baby, no study has demonstrated any increased long-term morbidity or mortality associated with low fluid in her situation and that labor induction may double her chance of cesarean delivery.37,38 In such a situation, an acceptable approach for mother and clinician may be rehydration followed by a repeat AFI and close follow-up with testing for fetal well-being according to the algorithm (Figure). In a practical sense, rehydration with 2 liters of oral water for oligohydramnios may be done whether or not immediate induction is chosen, as this is a safe measure that has been shown to significantly increase AFI. Alternatively, the preferred approach for a multiparous woman with a ripe cervix by Bishop score may be labor induction.
As adverse fetal outcomes have not been demonstrated in women with isolated term oligohydramnios, there is no rationale for routinely inducing labor based on an isolated finding of a so called “borderline” amniotic fluid index in the 5-to-8 range. In this situation it is appropriate to perform the initial assessment described above and may be reasonable to repeat the amniotic fluid index in 3 to 4 days to determine if true oligohydramnios has developed.
FIGURE
Assessment of the pregnant woman with oligohydramnios at term
Acknowledgments
We appreciate the assistance of George Gilson MD, Lauren Plante MD, and William Rayburn MD in manuscript review.
Corresponding author
Lawrence Leeman, MD, MPH, University of New Mexico Depts of Family and Community Medicine, Obstetrics and Gynecology, 2400 Tucker NE, 3rd floor, Albuquerque, NM 87131. E-mail: [email protected].
1. Moore TR. Clinical assessment of amniotic fluid. Clin Obstet Gynecol 1997;40:303-313.
2. Phelan JP, Smith CV, Broussard P, Small M. Amniotic fluid volume assessment with the four-quadrant technique at 36–42weeks’ gestation. J Reprod Med 1987;32:540-542.
3. Banks EH. Perinatal risks associated with borderline amniotic fluid index. Am J Obstet Gynecol 1999;180:1461-1463.
4. Casey BM. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks gestation. Am J Obstet Gynecol 2000;182:909-912.
5. Hobbins JC. Oligohydramnios and post-term pregnancy. Ob/Gyn Clinical Alert December 2002;59-60.
6. Johnson DP, Davis NR, Brown AJ. Risk of cesarean delivery after induction at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol 2003;188:1565-1572.
7. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med 2001;345:3-8.
8. Kilpatrick SJ, Safford KL. Maternal hydration increases amniotic fluid index in women with normal amniotic fluid. Obstet Gynecol 1993;81:49-52.
9. Gilbert WM, Brace RA. Amniotic fluid volume and normal flows to and from the amniotic cavity. Seminars in Perinatology 1993;17:150-157.
10. Chandra PC, Schiavello HJ, Lewandowski MA. Effect of oral and intravenous hydration on oligohydramnios. J Reprod Med 2000;45:337-340.
11. Doi S, Osada H, Seki K, Sekiya S. Effect of maternal hydration on oligohydramnios: a comparison of three volume expansion methods. Obstet Gynecol 1998;92:525-529.
12. Flack NJ, Sepulveda W, Bower S, Fisk NM. Acute maternal hydration in third-trimester oligohydramnios: effects on amniotic fluid volume, uteroplacental perfusion, and fetal blood flow and urine output. Am J Obstet Gynecol 1995;173:1186-1191.
13. Kilpatrick SJ, Safford KL, Pomeroy T, Hoedt L, Scheerer L, Laros RK. Maternal hydration increases amniotic fluid index. Obstet Gynecol 1991;78:1098-1102.
14. Larmon JE, Ross BS. Clinical utility of amniotic fluid volume assessment. Obstet Gynecol Clin North Am 1998;25:639-661.
15. Hill LM. Oligohydramnios: sonographic diagnosis and clinical implications. Clin Obstet Gynecol 1997;40:314-327.
16. Flack NJ, Dore C, Southwell D, Kourtis P, Sepulveda W, Fisk NM. The influence of operator transducer pressure on ultrasonographic measurements of amniotic fluid volume. Am J Obstet Gynecol 1994;171:218-222.
17. Rutherford SE, Smith CV, Phelan JP, Kawakami K, Ahn MO. Four-quadrant assessment of amniotic fluid volume: interobserver and intraobserver variation. J Reprod Med 1987;32:587-589.
18. Bruner JP, Reed GW, Sarno AP, Harrington RA, Goodman MA. Intraobserver and interobserver variability of the amniotic fluid index. Am J Obstet Gynecol 1993;168:1309-1313.
19. O’Reilly-Green CP, Divon MY. Predictive value of amniotic fluid index for oligohydramnios in patients with prolonged pregnancies. J Matern Fetal Med 1996;5:218-226.
20. Chauhan SP, Magann EF, Morrison JC, Whitworth NS, Hendrix NW, Devoe LD. Ultrasonographic assessment of amniotic fluid does not reflect actual amniotic fluid volume. Am J Obstet Gynecol 1997;177:291-297.
21. Chauhan SP, Sanderson M, Hendrix NW, Magann EF, Devoe LD. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: a meta-analysis. Am J Obstet Gynecol 1999;181:1473-1478.
22. Magann EF. Antenatal testing among 1001 patients at high risk: the role of ultrasonographic estimate of amniotic fluid volume. Am J Obstet Gynecol 1999;180:1330-1336.
23. Magann EF. Does an amniotic fluid index of 5 cm necessitate delivery in high-risk pregnancies? A case-control study. Am J Obstet Gynecol 1999;180:1354-1359.
24. Nisell H, Ek S. Perinatal risks associated with borderline amniotic fluid index. Am J Obstet Gynecol. 2000;182:750-751.
25. Conway DL, et al. Isolated oligohydramnios in the term pregnancy: Is it a clinical entity. J Matern Fetal Med 1998;7:197-200.
26. Garmel SH, Chelmow D, Sha SJ, Roan JT, D’Alton ME. Oligohydramnios and the appropriately grown fetus. Am J Perinatol 1997;14:359-363.
27. Kreiser D, el-Sayed YY, Sorem KA, Chitkara U, Holbrook RH, Jr, Druzin ML. Decreased amniotic fluid index in low-risk pregnancy. J Reprod Med 2001;46:743-746.
28. Roberts D, Nwosu EC, Walkinshaw SA. The fetal outcome in pregnancies with isolated reduced amniotic fluid volume in the third trimester. J Perinat Med 1998;26:390-395.
29. Rainford M, Adair R, Scialli AR, Ghidini A, Spong CY. Amniotic fluid index in the uncomplicated term pregnancy. Prediction of outcome. J Reprod Med 2001;46:589-592.
30. Zhang J, Troendle J, Meikle S, Klebanoff MA, Rayburn WF. Isolated oligohydramnios is not associated with adverse perinatal outcomes. BJOG 2004;111:220-225.
31. Conway DL, Groth S, Adkins WB, Langer O. Management of isolated oligohyramnios in the term pregnancy: a randomized clinical trial. Am J Obstet Gynecol 2000;182:S21.-
32. Oosterhof H, Haak MC, Aarnoudse JG. Acute maternal rehydration increases the urine production rate in the near-term human fetus. Am J Obstet Gynecol 2000;183:226-229.
33. Fait G, Pauzner D, Gull I, Lessing JB, Jaffa AJ, Wolman I. Effect of 1 week or oral hydration on the amniotic fluid index. J Reprod Med 2003;48:187-190.
34. Hofmeyr GJ, Gulmezoglu AM. Maternal hydration for increasing amniotic fluid volume in oligohydramnios and normal amniotic fluid volute (Cochrane Review). In: The Cochrane Library. Issue 4, 2002. Oxford: Update Software.
35. Sherer DM, Langer O. Oligohydramnios: use and misuse in clinical management. Ultrasound Obstet Gynecol 2001;18:411-419.
36. Carroll BC, Bruner JP. Umbilical artery Doppler velocimetry in pregnancies complicated by oligohydramnios. J Reprod Med 2000;45:562-566.
37. Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol 1999;94:600-607.
38. Yeast JD, Jones A, Poskin M. Induction of labor and the relationship to cesarean delivery: A review of 7001 consecutive inductions. Am J Obstet Gynecol 1999;180:628-633.
- Isolated term oligohydramnios, as defined by an amniotic fluid index (AFI) of less than 5 cm, has not been shown to be associated with poor maternal or fetal outcomes. Management may be individualized based on factors such as parity, cervical ripeness, and patient preference (SOR: B).
- Maternal hydration with oral water has been shown to increase AFI in a few hours, likely due to improved uteroplacental perfusion. This is a reasonable alternative to immediate labor induction in women with isolated term oligohydramnios (SOR: B).
- An isolated finding of a so-called “border-line” AFI (5–8 cm) is not an indication for labor induction (SOR: B).
Family physicians providing maternity care often face a scenario in which an otherwise low-risk, term patient is incidentally noted to have a low amniotic fluid index (AFI). Common reasons for obtaining an AFI in a woman with a low-risk pregnancy include evaluation of decreased fetal movement, spontaneous variable decelerations during monitoring to evaluate for labor, or an ultrasound evaluation for fundal height measurements discordant with gestational age. How should “isolated” oligohydramnios—an AFI <5 cm—be interpreted, and should immediate induction be recommended for such patients?
Oligohydramnios occurs in about 1% to 5% of pregnancies at term.1,2 Because adverse outcomes occur in high-risk pregnancies complicated by low amniotic fluid volume, oligohydramnios commonly prompts labor induction.1,3,4 At one university center, oligohydramnios is now the leading indication for labor induction.5 Many centers may even induce labor when the AFI is between 5 cm and 8 cm, the so-called borderline AFI.3
Labor induction increases the use of cesarean delivery, particularly for the primiparous woman with an unripe cervix.6 Recent studies questioning the safety of labor induction in women who have had a cesarean may increase the number of elective repeat cesarean procedures when delivery is believed indicated for oligohydramnios.7 (See Underlying causes of oligohydramnios.)
By the second trimester, amniotic fluid is being produced primarily through fetal urine production and is primarily resorbed through fetal swallowing. Significant amounts of amniotic fluid are also produced and resorbed by the fetal lung and directly resorbed from the amniotic cavity by the placenta.8,9 Amniotic fluid volume is affected by the status of maternal hydration and maternal plasma osmolality.10-13
Acute oligohydramnios may occur from ruptured membranes, usually diagnosed by clinical signs and vaginal fluid with altered pH and a ferning pattern on microscopic exam.
Chronic oligohydramnios arises from prerenal, renal, and postrenal causes. The latter 2 groups reflect fetal kidney and urogenital abnormalities, which directly decrease fetal amniotic fluid production. Uteroplacental insufficiency is the most common cause of prerenal oligohydramnios, and the decreased amniotic fluid is a direct result of decreased fetal renal perfusion.14 Uteroplacental insufficiency may result in intrauterine growth restriction as the fetus shunts blood away from the growing torso and limbs and to vital organs such as the brain. Preeclampsia and postdate pregnancies both involve pathologic changes in the placenta that may result in uteroplacental insufficiency and oligohydramnios.
Oligohydramnios is difficult to assess
True oligohydramnios can be difficult to confirm due to the questionable accuracy of amniotic fluid measurement by ultrasound. There is controversy, for example, about whether (and how) to include pockets of amniotic fluid containing umbilical cord.15 The AFI was introduced in 19872 to replace the 2 cm “pocket technique” of fluid assessment, and studies continue to question to what extent the AFI reflects actual amniotic fluid volume.
AFI measurements may vary with the amount of pressure applied to the abdomen and with fetal position or movement.16
Serial measurements taken by the same ultrasound operator have been shown to differ from the true volume by 1 cm, or 10.8%; serial measurements taken by multiple operators have differed by as much as 2 cm, or 15.4%.17,18
O’Reilly-Green compared the diagnosis of oligohydramnios in 449 post-term patients with actual amniotic fluid volume measured at rupture of membranes.19 They found a positive predictive value of 50% for oligohydramnios at an AFI of 5 cm as the lower limit of normal. A study of 144 third trimester patients using the dye-dilution technique found that, to achieve 95% confidence for ruling out oligohydramnios, a cutoff AFI of 30 cm would need to be used, a value consistent with polyhydramnios.20
What is the association between oligohydramnios and poor fetal outcomes?
A number of studies over the past 15 years have shown an association between oligohydramnios and poor fetal outcomes. These were predominantly retrospective studies, which failed to control for the presence of factors known to be associated with oligohydramnios such as intrauterine growth restriction (IUGR) and urogenital malformations.
No studies have directly addressed whether labor induction improves outcomes. A meta-analysis of 18 studies examining outcomes of pregnancies with AFI <5 cm found an increased risk of cesarean delivery for fetal distress and low Apgar scores at 5 minutes. Most of these studies, however, had high-risk patients including IUGR (level of evidence [LOE]: 2).21
A recent study of high-risk patients failed to detect a difference in the incidence of nonreactive nonstress tests, meconium-stained amniotic fluid cesarean delivery for fetal distress, low Apgar scores, or infants with a cord pH of <7.10 when oligohydramnios (AFI <5.0 cm) was present (LOE: 1).2 The patients with oligohydramnios were all induced, while many of the other high-risk patients were expectantly managed. The study therefore provides no guidance on the safety of expectant management for patients with oligohydramnios. To eliminate the potential effect of induction versus expectant management the same authors performed a case-control study of 79 high-risk women with AFI <5 cm matched to 79 women with the same high-risk pregnancy complication who had an AFI >5 cm at the time of induction (LOE: 2).23 They failed to detect any significant differences in neonatal outcomes between the groups.
Studies of the “borderline” AFI (between 5 cm and 8 cm) may also demonstrate an association with adverse neonatal outcomes if researchers include fetuses with IUGR or malformations. In one retrospective case review of 214 women with AFI of 5 cm to 10 cm, the only statistically significant finding was an association with IUGR.3 The authors recommended antepartum surveillance twice a week for mothers with borderline AFI, but they did not comment on induction (LOE: 2). Correspondence regarding this study argued that this recommendation was not supported by the evidence and would lead to unnecessary antenatal testing.24
Studies of isolated oligohydramnios
Investigators have conducted studies (Table 1) excluding fetuses with intrauterine growth restriction or anomalies to try to determine if isolated oligohydramnios is associated with poorer outcomes.25-30
Rainford’s study of outcomes in exclusively term, low-risk patients failed to show significant outcome differences in Apgar scores, NICU admissions, or rates of cesarean delivery for non-reassuring fetal heart rate monitoring (LOE: 2).29 This study was limited due to its retrospective design. The authors comment that the relatively good outcomes in the oligohydramnios group may be due to the widespread practice of inducing such patients.
In a case-control study by Conway, 183 low-risk, term parturients with oligohydramnios were matched to 183 women of similar gestational age and parity who presented in spontaneous labor. The patients with isolated oligohydramnios were induced and showed an increased cesarean delivery rate. The increased rate of cesarean delivery was not due to nonreassuring fetal surveillance and was attributed to the induction process (LOE: 2).25
An analysis of woman diagnosed with isolated oligohydramnios (AFI <5) at any gestational age in the multicenter prospective RADIUS trial demonstrated similar perinatal outcomes and fetal growth compared with pregnancies with a normal amniotic fluid (LOE: 2).30
The only randomized clinical trial of labor induction vs expectant management for term isolated oligohydramnios showed similar outcomes in each group. But this study was small (n=61) and has only been published as an abstract.31
TABLE 1
Isolated oligohydramnios and perinatal outcomes
| Study | Design | Study number n vs controls | Patient-oriented outcomes | Comment | LOE | |
|---|---|---|---|---|---|---|
| Population | Significant findings | Non-significant findings | ||||
| Garmel19 | Prospective cohort | N=187 | Increased preterm birth (OR=3.23; 95% CI, 1.4–7.3) in oligohydramnios group | IUGR, asphyxia, death, NICU admit | Delivery recommended at 37 weeks | 2 |
| 17–37 week with subnormal EFW (>10%) | 65 AFI <8 cm vs 122 AFI >8 cm | |||||
| Conway18 | Prospective cohort | N=366 | Increased CS rate (OR=2.7 95% CI, 1.3–5.4) in oligohydramnios group | CS for fetal distress; all neonatal outcomes | Treatment group induced, controls spontaneous | 2 |
| Term, isolated oligohydramnios undergoing induction | 183 AFI <5 cm vs 183 AFI >5 cm | |||||
| Roberts21 | Prospective cohort | N=206 | Increased IUGR (OR=5.2; 95% CI, 1.6–22), induction (OR=34.4, 95% CI, 4–1425.5), NICU admit (OR=9.8; 95% CI, 1.3–432) | Fetal distress requiring CS | Used >5%ile to exclude IUGR. Included some high-risk pts (diabetes or hypertension) | 2 |
| 3rd trimester, isolated oligohydramnios | 103 AFI 3%ile (N=103) vs matched control | |||||
| Rainford22 | Retrospective cohort | N=232 | Induction rate for AFI <5 = 98% vs 51% AFI >5 P<.001; increased meconium staining in controls without oligohydramnios | NICU 2 admissions, 5-minute Apgar scores | 2 | |
| 37–41 week, low-risk. AFI within 4 days of delivery | AFI <5 (n=44) vs >5 (n=188) | |||||
| Zhang23 | Retrospective nested cohort | N=6657 | Malpresentation (RR=3.5, 95% CI 1.8–6.60) | Fetal growth, CS, low Apgar, overall neonatal morbidity | Benefit of routine ultrasound was the primary study outcome study endpoint | 2 |
| Term or near-term, low-risk | AFI <5 (n=86) vs >5 (n=6571) | |||||
| AFI, amniotic fluid index; CI, confidence interval; CS, cesarean section; EFW, estimated fetal weight; IUGR, intrauterine growth restriction; LOE, level of evidence; NICU, neonatal intensive care unit; OR, odds ratio; RR, relative risk. | ||||||
Effect of maternal hydration
Maternal hydration status and plasma osmolality have an affect on amniotic fluid volume (Table 2). Maternal hydration with oral water or intravenous hypotonic solutions has been shown to increase amniotic fluid volume.8,11-13 Oral hydration with hypotonic fluid has been demonstrated to increase fetal urine production in one observational study.32 Another observational study demonstrated increased amniotic fluid volume and uteroplacental perfusion without alteration of fetal urine production suggesting the possibility that transmembranous fluid shifts from the placenta to the amniotic cavity may be involved.12
Two small, randomized controlled trials (RCTs) demonstrated an increase in amniotic fluid volume in women with oligohydramnios after oral hydration.11,13 Doi demonstrated significant increases in AFI in women with oligohydramnios beyond 35 weeks when given oral hydration with free water (increase of 3.8 cm ± 1.9; P<. 001) or hypotonic intravenous solution (increase of 2.8 cm ± 1.9; P<.001) (LOE: 3).11 Interestingly, this study did not demonstrate an increase in amniotic fluid volume with intravenous hydration with isotonic fluid.
Kirkpatrick demonstrated a 30% increase in amniotic fluid compared with controls in women of unspecified gestational age with oligohydramnios given 2 liters of oral water 2 to 5 hours before repeat amniotic fluid index (LOE: 3).13
A randomized trial in women with normal amniotic fluid demonstrated a 16% increase in amniotic fluid index 4 to 6 hours after hydration with 2 liters of oral water, compared with an 8% decrease after fluid restriction during the same period.8
A recent study of daily oral hydration in women with amniotic fluid volume <10% percentile showed increased amniotic fluid volume at 1 week, suggesting long-term benefit, although the study lacked an appropriate control group (LOE: 3).33
There are no studies of clinical outcomes such as fetal heart rate decelerations during labor, or neonatal outcomes. A Cochrane systematic review concluded that maternal hydration appears to increase amniotic fluid and may be beneficial in management of oligohydramnios; however, it recommended controlled trials to assess clinical outcome benefits (LOE: 3).34
TABLE 2
Effect of hydration on amniotic fluid index
| Study | Design | Population | Intervention | Outcome | Comment | LOE |
|---|---|---|---|---|---|---|
| Kilpatrick32 | RCT | N=40, AFI 2.1–6.0; population of patients referred for antenatal testing | Treatment group drank 2 L water and repeat AFI same or next day | Increase of 1.5 ± 1.4 cm (P<.01) in treatment group | Gestational ages of subjects not stated | 3 |
| Kilpatrick37 | RCT | N=40, AFI 7–24 cm, gestational 28 weeks | Treatment group instructed to drink 2 L and restricted group 0.1 L water. AFI repeated in 4–6 h | Increase of 3.0 ± 2.4 cm (P<.0001) in treatment group; decrease of 1.5 ± 2.7cm in controls (P <.02) | Subjects had normal AFI at entry | 3 |
| Flack36 | Prospective cohort | N=20, 10 w/AFI <5 cm, 10 controls AFI >7, 3rd trimester | 2 L oral water over 2 h for treatment and control groups, repeat AFI at 2 h | Increase in 3.2 cm in AFI (95% CI, 1.1–5.3) in oligohydramnios group but not in normal AFI group | Improved uterine perfusion shown by increased uterine artery velocity only in oligohydramnios group | 3 |
| Doi35 | RCT | N=84, AFI <5, at least 35 wks; randomized three maternal hydration methods (2 L oral water, hypotonic saline IV, or isotonic saline IV) | Hydration with 2 L fluid and AFI repeated in 1 h compared with controls | Significant increases in AFI in oral water and hypotonic IV groups by 3.8 cm and 2.8 cm (P<.001) respectively | IV isotonic solutions did not increase amniotic fluid volume in study population | 3 |
| RCT, randomized controlled trial; AFI, amniotic fluid index; CI, confidence interval. | ||||||
Management recommendations
The AFI has low specificity and positive predictive value for oligohydramnios, and there is scant evidence that isolated term oligohydramnios causes adverse fetal outcomes. We recommend that an AFI under 5 cm should prompt additional antenatal testing rather than immediate induction in low-risk term pregnancies (SOR: B).
Though we acknowledge the lack of high-quality studies with patient-oriented outcomes to support observation and maternal hydration, we have developed a management strategy that does not require immediate induction of labor in women with uncomplicated term pregnancies.
The following recommendations apply to women having oligohydramnios as defined by amniotic fluid volume of less than 5 cm and gestational age between 37 and 41 weeks.
Initial assessment
- Assess for premature rupture of membranes with a thorough history and a sterile speculum exam
- Reassess dating as oligohydramnios in post-dates pregnancy (>41 weeks) is an indication for induction (SOR: C)35
- Perform a nonstress test to assess fetal wellbeing
- Assess for IUGR with an ultrasound for estimated fetal weight and for the ratio of head circumference (HC) to abdominal circumference (AC). A comparison with prior ultrasounds can aid in assessing interval growth. An estimated fetal weight below the 10%, an elevated HC/AC ratio, or poor interval growth would suggest IUGR
- Arrange for an ultrasound anatomic survey for fetal anomalies, if not done previously
- Determine if preeclampsia, chronic hypertension, diabetes, or other maternal conditions associated with uteroplacental insufficiency are present.
Action steps
With any positive findings in the initial evaluation, proceed to labor induction, as the patient does not have isolated, term oligohydramnios (SOR: C). If the initial assessment is unremarkable and the AFI is less than 5, consider hydration with oral water and repeating the AFI 2 to 6 hours later (SOR: B).
Persistent oligohydramnios at term, particularly with a ripe cervix, may lead you to consider labor induction. Continued expectant management of isolated term oligohydramnios with twice weekly fetal surveillance may also be a reasonable option due to the paucity of evidence that oligohydramnios is associated with an adverse outcome in this scenario (SOR: C). Normal results with umbilical artery Doppler flow studies have been used to decrease the need for induction in high-risk pregnancies with oligohydramnios, and this technique may eventually have a role in isolated term oligohydramnios.36
It is essential that patients receive counseling and give informed consent regarding the risks and benefits of observation or induction for isolated term oligohydramnios. The ease of induction based on parity and cervical ripeness should be considered.
A primiparous woman with an unfavorable cervix who strongly desires a spontaneous, vaginal birth could be told that, although there may be a small risk for her baby, no study has demonstrated any increased long-term morbidity or mortality associated with low fluid in her situation and that labor induction may double her chance of cesarean delivery.37,38 In such a situation, an acceptable approach for mother and clinician may be rehydration followed by a repeat AFI and close follow-up with testing for fetal well-being according to the algorithm (Figure). In a practical sense, rehydration with 2 liters of oral water for oligohydramnios may be done whether or not immediate induction is chosen, as this is a safe measure that has been shown to significantly increase AFI. Alternatively, the preferred approach for a multiparous woman with a ripe cervix by Bishop score may be labor induction.
As adverse fetal outcomes have not been demonstrated in women with isolated term oligohydramnios, there is no rationale for routinely inducing labor based on an isolated finding of a so called “borderline” amniotic fluid index in the 5-to-8 range. In this situation it is appropriate to perform the initial assessment described above and may be reasonable to repeat the amniotic fluid index in 3 to 4 days to determine if true oligohydramnios has developed.
FIGURE
Assessment of the pregnant woman with oligohydramnios at term
Acknowledgments
We appreciate the assistance of George Gilson MD, Lauren Plante MD, and William Rayburn MD in manuscript review.
Corresponding author
Lawrence Leeman, MD, MPH, University of New Mexico Depts of Family and Community Medicine, Obstetrics and Gynecology, 2400 Tucker NE, 3rd floor, Albuquerque, NM 87131. E-mail: [email protected].
- Isolated term oligohydramnios, as defined by an amniotic fluid index (AFI) of less than 5 cm, has not been shown to be associated with poor maternal or fetal outcomes. Management may be individualized based on factors such as parity, cervical ripeness, and patient preference (SOR: B).
- Maternal hydration with oral water has been shown to increase AFI in a few hours, likely due to improved uteroplacental perfusion. This is a reasonable alternative to immediate labor induction in women with isolated term oligohydramnios (SOR: B).
- An isolated finding of a so-called “border-line” AFI (5–8 cm) is not an indication for labor induction (SOR: B).
Family physicians providing maternity care often face a scenario in which an otherwise low-risk, term patient is incidentally noted to have a low amniotic fluid index (AFI). Common reasons for obtaining an AFI in a woman with a low-risk pregnancy include evaluation of decreased fetal movement, spontaneous variable decelerations during monitoring to evaluate for labor, or an ultrasound evaluation for fundal height measurements discordant with gestational age. How should “isolated” oligohydramnios—an AFI <5 cm—be interpreted, and should immediate induction be recommended for such patients?
Oligohydramnios occurs in about 1% to 5% of pregnancies at term.1,2 Because adverse outcomes occur in high-risk pregnancies complicated by low amniotic fluid volume, oligohydramnios commonly prompts labor induction.1,3,4 At one university center, oligohydramnios is now the leading indication for labor induction.5 Many centers may even induce labor when the AFI is between 5 cm and 8 cm, the so-called borderline AFI.3
Labor induction increases the use of cesarean delivery, particularly for the primiparous woman with an unripe cervix.6 Recent studies questioning the safety of labor induction in women who have had a cesarean may increase the number of elective repeat cesarean procedures when delivery is believed indicated for oligohydramnios.7 (See Underlying causes of oligohydramnios.)
By the second trimester, amniotic fluid is being produced primarily through fetal urine production and is primarily resorbed through fetal swallowing. Significant amounts of amniotic fluid are also produced and resorbed by the fetal lung and directly resorbed from the amniotic cavity by the placenta.8,9 Amniotic fluid volume is affected by the status of maternal hydration and maternal plasma osmolality.10-13
Acute oligohydramnios may occur from ruptured membranes, usually diagnosed by clinical signs and vaginal fluid with altered pH and a ferning pattern on microscopic exam.
Chronic oligohydramnios arises from prerenal, renal, and postrenal causes. The latter 2 groups reflect fetal kidney and urogenital abnormalities, which directly decrease fetal amniotic fluid production. Uteroplacental insufficiency is the most common cause of prerenal oligohydramnios, and the decreased amniotic fluid is a direct result of decreased fetal renal perfusion.14 Uteroplacental insufficiency may result in intrauterine growth restriction as the fetus shunts blood away from the growing torso and limbs and to vital organs such as the brain. Preeclampsia and postdate pregnancies both involve pathologic changes in the placenta that may result in uteroplacental insufficiency and oligohydramnios.
Oligohydramnios is difficult to assess
True oligohydramnios can be difficult to confirm due to the questionable accuracy of amniotic fluid measurement by ultrasound. There is controversy, for example, about whether (and how) to include pockets of amniotic fluid containing umbilical cord.15 The AFI was introduced in 19872 to replace the 2 cm “pocket technique” of fluid assessment, and studies continue to question to what extent the AFI reflects actual amniotic fluid volume.
AFI measurements may vary with the amount of pressure applied to the abdomen and with fetal position or movement.16
Serial measurements taken by the same ultrasound operator have been shown to differ from the true volume by 1 cm, or 10.8%; serial measurements taken by multiple operators have differed by as much as 2 cm, or 15.4%.17,18
O’Reilly-Green compared the diagnosis of oligohydramnios in 449 post-term patients with actual amniotic fluid volume measured at rupture of membranes.19 They found a positive predictive value of 50% for oligohydramnios at an AFI of 5 cm as the lower limit of normal. A study of 144 third trimester patients using the dye-dilution technique found that, to achieve 95% confidence for ruling out oligohydramnios, a cutoff AFI of 30 cm would need to be used, a value consistent with polyhydramnios.20
What is the association between oligohydramnios and poor fetal outcomes?
A number of studies over the past 15 years have shown an association between oligohydramnios and poor fetal outcomes. These were predominantly retrospective studies, which failed to control for the presence of factors known to be associated with oligohydramnios such as intrauterine growth restriction (IUGR) and urogenital malformations.
No studies have directly addressed whether labor induction improves outcomes. A meta-analysis of 18 studies examining outcomes of pregnancies with AFI <5 cm found an increased risk of cesarean delivery for fetal distress and low Apgar scores at 5 minutes. Most of these studies, however, had high-risk patients including IUGR (level of evidence [LOE]: 2).21
A recent study of high-risk patients failed to detect a difference in the incidence of nonreactive nonstress tests, meconium-stained amniotic fluid cesarean delivery for fetal distress, low Apgar scores, or infants with a cord pH of <7.10 when oligohydramnios (AFI <5.0 cm) was present (LOE: 1).2 The patients with oligohydramnios were all induced, while many of the other high-risk patients were expectantly managed. The study therefore provides no guidance on the safety of expectant management for patients with oligohydramnios. To eliminate the potential effect of induction versus expectant management the same authors performed a case-control study of 79 high-risk women with AFI <5 cm matched to 79 women with the same high-risk pregnancy complication who had an AFI >5 cm at the time of induction (LOE: 2).23 They failed to detect any significant differences in neonatal outcomes between the groups.
Studies of the “borderline” AFI (between 5 cm and 8 cm) may also demonstrate an association with adverse neonatal outcomes if researchers include fetuses with IUGR or malformations. In one retrospective case review of 214 women with AFI of 5 cm to 10 cm, the only statistically significant finding was an association with IUGR.3 The authors recommended antepartum surveillance twice a week for mothers with borderline AFI, but they did not comment on induction (LOE: 2). Correspondence regarding this study argued that this recommendation was not supported by the evidence and would lead to unnecessary antenatal testing.24
Studies of isolated oligohydramnios
Investigators have conducted studies (Table 1) excluding fetuses with intrauterine growth restriction or anomalies to try to determine if isolated oligohydramnios is associated with poorer outcomes.25-30
Rainford’s study of outcomes in exclusively term, low-risk patients failed to show significant outcome differences in Apgar scores, NICU admissions, or rates of cesarean delivery for non-reassuring fetal heart rate monitoring (LOE: 2).29 This study was limited due to its retrospective design. The authors comment that the relatively good outcomes in the oligohydramnios group may be due to the widespread practice of inducing such patients.
In a case-control study by Conway, 183 low-risk, term parturients with oligohydramnios were matched to 183 women of similar gestational age and parity who presented in spontaneous labor. The patients with isolated oligohydramnios were induced and showed an increased cesarean delivery rate. The increased rate of cesarean delivery was not due to nonreassuring fetal surveillance and was attributed to the induction process (LOE: 2).25
An analysis of woman diagnosed with isolated oligohydramnios (AFI <5) at any gestational age in the multicenter prospective RADIUS trial demonstrated similar perinatal outcomes and fetal growth compared with pregnancies with a normal amniotic fluid (LOE: 2).30
The only randomized clinical trial of labor induction vs expectant management for term isolated oligohydramnios showed similar outcomes in each group. But this study was small (n=61) and has only been published as an abstract.31
TABLE 1
Isolated oligohydramnios and perinatal outcomes
| Study | Design | Study number n vs controls | Patient-oriented outcomes | Comment | LOE | |
|---|---|---|---|---|---|---|
| Population | Significant findings | Non-significant findings | ||||
| Garmel19 | Prospective cohort | N=187 | Increased preterm birth (OR=3.23; 95% CI, 1.4–7.3) in oligohydramnios group | IUGR, asphyxia, death, NICU admit | Delivery recommended at 37 weeks | 2 |
| 17–37 week with subnormal EFW (>10%) | 65 AFI <8 cm vs 122 AFI >8 cm | |||||
| Conway18 | Prospective cohort | N=366 | Increased CS rate (OR=2.7 95% CI, 1.3–5.4) in oligohydramnios group | CS for fetal distress; all neonatal outcomes | Treatment group induced, controls spontaneous | 2 |
| Term, isolated oligohydramnios undergoing induction | 183 AFI <5 cm vs 183 AFI >5 cm | |||||
| Roberts21 | Prospective cohort | N=206 | Increased IUGR (OR=5.2; 95% CI, 1.6–22), induction (OR=34.4, 95% CI, 4–1425.5), NICU admit (OR=9.8; 95% CI, 1.3–432) | Fetal distress requiring CS | Used >5%ile to exclude IUGR. Included some high-risk pts (diabetes or hypertension) | 2 |
| 3rd trimester, isolated oligohydramnios | 103 AFI 3%ile (N=103) vs matched control | |||||
| Rainford22 | Retrospective cohort | N=232 | Induction rate for AFI <5 = 98% vs 51% AFI >5 P<.001; increased meconium staining in controls without oligohydramnios | NICU 2 admissions, 5-minute Apgar scores | 2 | |
| 37–41 week, low-risk. AFI within 4 days of delivery | AFI <5 (n=44) vs >5 (n=188) | |||||
| Zhang23 | Retrospective nested cohort | N=6657 | Malpresentation (RR=3.5, 95% CI 1.8–6.60) | Fetal growth, CS, low Apgar, overall neonatal morbidity | Benefit of routine ultrasound was the primary study outcome study endpoint | 2 |
| Term or near-term, low-risk | AFI <5 (n=86) vs >5 (n=6571) | |||||
| AFI, amniotic fluid index; CI, confidence interval; CS, cesarean section; EFW, estimated fetal weight; IUGR, intrauterine growth restriction; LOE, level of evidence; NICU, neonatal intensive care unit; OR, odds ratio; RR, relative risk. | ||||||
Effect of maternal hydration
Maternal hydration status and plasma osmolality have an affect on amniotic fluid volume (Table 2). Maternal hydration with oral water or intravenous hypotonic solutions has been shown to increase amniotic fluid volume.8,11-13 Oral hydration with hypotonic fluid has been demonstrated to increase fetal urine production in one observational study.32 Another observational study demonstrated increased amniotic fluid volume and uteroplacental perfusion without alteration of fetal urine production suggesting the possibility that transmembranous fluid shifts from the placenta to the amniotic cavity may be involved.12
Two small, randomized controlled trials (RCTs) demonstrated an increase in amniotic fluid volume in women with oligohydramnios after oral hydration.11,13 Doi demonstrated significant increases in AFI in women with oligohydramnios beyond 35 weeks when given oral hydration with free water (increase of 3.8 cm ± 1.9; P<. 001) or hypotonic intravenous solution (increase of 2.8 cm ± 1.9; P<.001) (LOE: 3).11 Interestingly, this study did not demonstrate an increase in amniotic fluid volume with intravenous hydration with isotonic fluid.
Kirkpatrick demonstrated a 30% increase in amniotic fluid compared with controls in women of unspecified gestational age with oligohydramnios given 2 liters of oral water 2 to 5 hours before repeat amniotic fluid index (LOE: 3).13
A randomized trial in women with normal amniotic fluid demonstrated a 16% increase in amniotic fluid index 4 to 6 hours after hydration with 2 liters of oral water, compared with an 8% decrease after fluid restriction during the same period.8
A recent study of daily oral hydration in women with amniotic fluid volume <10% percentile showed increased amniotic fluid volume at 1 week, suggesting long-term benefit, although the study lacked an appropriate control group (LOE: 3).33
There are no studies of clinical outcomes such as fetal heart rate decelerations during labor, or neonatal outcomes. A Cochrane systematic review concluded that maternal hydration appears to increase amniotic fluid and may be beneficial in management of oligohydramnios; however, it recommended controlled trials to assess clinical outcome benefits (LOE: 3).34
TABLE 2
Effect of hydration on amniotic fluid index
| Study | Design | Population | Intervention | Outcome | Comment | LOE |
|---|---|---|---|---|---|---|
| Kilpatrick32 | RCT | N=40, AFI 2.1–6.0; population of patients referred for antenatal testing | Treatment group drank 2 L water and repeat AFI same or next day | Increase of 1.5 ± 1.4 cm (P<.01) in treatment group | Gestational ages of subjects not stated | 3 |
| Kilpatrick37 | RCT | N=40, AFI 7–24 cm, gestational 28 weeks | Treatment group instructed to drink 2 L and restricted group 0.1 L water. AFI repeated in 4–6 h | Increase of 3.0 ± 2.4 cm (P<.0001) in treatment group; decrease of 1.5 ± 2.7cm in controls (P <.02) | Subjects had normal AFI at entry | 3 |
| Flack36 | Prospective cohort | N=20, 10 w/AFI <5 cm, 10 controls AFI >7, 3rd trimester | 2 L oral water over 2 h for treatment and control groups, repeat AFI at 2 h | Increase in 3.2 cm in AFI (95% CI, 1.1–5.3) in oligohydramnios group but not in normal AFI group | Improved uterine perfusion shown by increased uterine artery velocity only in oligohydramnios group | 3 |
| Doi35 | RCT | N=84, AFI <5, at least 35 wks; randomized three maternal hydration methods (2 L oral water, hypotonic saline IV, or isotonic saline IV) | Hydration with 2 L fluid and AFI repeated in 1 h compared with controls | Significant increases in AFI in oral water and hypotonic IV groups by 3.8 cm and 2.8 cm (P<.001) respectively | IV isotonic solutions did not increase amniotic fluid volume in study population | 3 |
| RCT, randomized controlled trial; AFI, amniotic fluid index; CI, confidence interval. | ||||||
Management recommendations
The AFI has low specificity and positive predictive value for oligohydramnios, and there is scant evidence that isolated term oligohydramnios causes adverse fetal outcomes. We recommend that an AFI under 5 cm should prompt additional antenatal testing rather than immediate induction in low-risk term pregnancies (SOR: B).
Though we acknowledge the lack of high-quality studies with patient-oriented outcomes to support observation and maternal hydration, we have developed a management strategy that does not require immediate induction of labor in women with uncomplicated term pregnancies.
The following recommendations apply to women having oligohydramnios as defined by amniotic fluid volume of less than 5 cm and gestational age between 37 and 41 weeks.
Initial assessment
- Assess for premature rupture of membranes with a thorough history and a sterile speculum exam
- Reassess dating as oligohydramnios in post-dates pregnancy (>41 weeks) is an indication for induction (SOR: C)35
- Perform a nonstress test to assess fetal wellbeing
- Assess for IUGR with an ultrasound for estimated fetal weight and for the ratio of head circumference (HC) to abdominal circumference (AC). A comparison with prior ultrasounds can aid in assessing interval growth. An estimated fetal weight below the 10%, an elevated HC/AC ratio, or poor interval growth would suggest IUGR
- Arrange for an ultrasound anatomic survey for fetal anomalies, if not done previously
- Determine if preeclampsia, chronic hypertension, diabetes, or other maternal conditions associated with uteroplacental insufficiency are present.
Action steps
With any positive findings in the initial evaluation, proceed to labor induction, as the patient does not have isolated, term oligohydramnios (SOR: C). If the initial assessment is unremarkable and the AFI is less than 5, consider hydration with oral water and repeating the AFI 2 to 6 hours later (SOR: B).
Persistent oligohydramnios at term, particularly with a ripe cervix, may lead you to consider labor induction. Continued expectant management of isolated term oligohydramnios with twice weekly fetal surveillance may also be a reasonable option due to the paucity of evidence that oligohydramnios is associated with an adverse outcome in this scenario (SOR: C). Normal results with umbilical artery Doppler flow studies have been used to decrease the need for induction in high-risk pregnancies with oligohydramnios, and this technique may eventually have a role in isolated term oligohydramnios.36
It is essential that patients receive counseling and give informed consent regarding the risks and benefits of observation or induction for isolated term oligohydramnios. The ease of induction based on parity and cervical ripeness should be considered.
A primiparous woman with an unfavorable cervix who strongly desires a spontaneous, vaginal birth could be told that, although there may be a small risk for her baby, no study has demonstrated any increased long-term morbidity or mortality associated with low fluid in her situation and that labor induction may double her chance of cesarean delivery.37,38 In such a situation, an acceptable approach for mother and clinician may be rehydration followed by a repeat AFI and close follow-up with testing for fetal well-being according to the algorithm (Figure). In a practical sense, rehydration with 2 liters of oral water for oligohydramnios may be done whether or not immediate induction is chosen, as this is a safe measure that has been shown to significantly increase AFI. Alternatively, the preferred approach for a multiparous woman with a ripe cervix by Bishop score may be labor induction.
As adverse fetal outcomes have not been demonstrated in women with isolated term oligohydramnios, there is no rationale for routinely inducing labor based on an isolated finding of a so called “borderline” amniotic fluid index in the 5-to-8 range. In this situation it is appropriate to perform the initial assessment described above and may be reasonable to repeat the amniotic fluid index in 3 to 4 days to determine if true oligohydramnios has developed.
FIGURE
Assessment of the pregnant woman with oligohydramnios at term
Acknowledgments
We appreciate the assistance of George Gilson MD, Lauren Plante MD, and William Rayburn MD in manuscript review.
Corresponding author
Lawrence Leeman, MD, MPH, University of New Mexico Depts of Family and Community Medicine, Obstetrics and Gynecology, 2400 Tucker NE, 3rd floor, Albuquerque, NM 87131. E-mail: [email protected].
1. Moore TR. Clinical assessment of amniotic fluid. Clin Obstet Gynecol 1997;40:303-313.
2. Phelan JP, Smith CV, Broussard P, Small M. Amniotic fluid volume assessment with the four-quadrant technique at 36–42weeks’ gestation. J Reprod Med 1987;32:540-542.
3. Banks EH. Perinatal risks associated with borderline amniotic fluid index. Am J Obstet Gynecol 1999;180:1461-1463.
4. Casey BM. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks gestation. Am J Obstet Gynecol 2000;182:909-912.
5. Hobbins JC. Oligohydramnios and post-term pregnancy. Ob/Gyn Clinical Alert December 2002;59-60.
6. Johnson DP, Davis NR, Brown AJ. Risk of cesarean delivery after induction at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol 2003;188:1565-1572.
7. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med 2001;345:3-8.
8. Kilpatrick SJ, Safford KL. Maternal hydration increases amniotic fluid index in women with normal amniotic fluid. Obstet Gynecol 1993;81:49-52.
9. Gilbert WM, Brace RA. Amniotic fluid volume and normal flows to and from the amniotic cavity. Seminars in Perinatology 1993;17:150-157.
10. Chandra PC, Schiavello HJ, Lewandowski MA. Effect of oral and intravenous hydration on oligohydramnios. J Reprod Med 2000;45:337-340.
11. Doi S, Osada H, Seki K, Sekiya S. Effect of maternal hydration on oligohydramnios: a comparison of three volume expansion methods. Obstet Gynecol 1998;92:525-529.
12. Flack NJ, Sepulveda W, Bower S, Fisk NM. Acute maternal hydration in third-trimester oligohydramnios: effects on amniotic fluid volume, uteroplacental perfusion, and fetal blood flow and urine output. Am J Obstet Gynecol 1995;173:1186-1191.
13. Kilpatrick SJ, Safford KL, Pomeroy T, Hoedt L, Scheerer L, Laros RK. Maternal hydration increases amniotic fluid index. Obstet Gynecol 1991;78:1098-1102.
14. Larmon JE, Ross BS. Clinical utility of amniotic fluid volume assessment. Obstet Gynecol Clin North Am 1998;25:639-661.
15. Hill LM. Oligohydramnios: sonographic diagnosis and clinical implications. Clin Obstet Gynecol 1997;40:314-327.
16. Flack NJ, Dore C, Southwell D, Kourtis P, Sepulveda W, Fisk NM. The influence of operator transducer pressure on ultrasonographic measurements of amniotic fluid volume. Am J Obstet Gynecol 1994;171:218-222.
17. Rutherford SE, Smith CV, Phelan JP, Kawakami K, Ahn MO. Four-quadrant assessment of amniotic fluid volume: interobserver and intraobserver variation. J Reprod Med 1987;32:587-589.
18. Bruner JP, Reed GW, Sarno AP, Harrington RA, Goodman MA. Intraobserver and interobserver variability of the amniotic fluid index. Am J Obstet Gynecol 1993;168:1309-1313.
19. O’Reilly-Green CP, Divon MY. Predictive value of amniotic fluid index for oligohydramnios in patients with prolonged pregnancies. J Matern Fetal Med 1996;5:218-226.
20. Chauhan SP, Magann EF, Morrison JC, Whitworth NS, Hendrix NW, Devoe LD. Ultrasonographic assessment of amniotic fluid does not reflect actual amniotic fluid volume. Am J Obstet Gynecol 1997;177:291-297.
21. Chauhan SP, Sanderson M, Hendrix NW, Magann EF, Devoe LD. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: a meta-analysis. Am J Obstet Gynecol 1999;181:1473-1478.
22. Magann EF. Antenatal testing among 1001 patients at high risk: the role of ultrasonographic estimate of amniotic fluid volume. Am J Obstet Gynecol 1999;180:1330-1336.
23. Magann EF. Does an amniotic fluid index of 5 cm necessitate delivery in high-risk pregnancies? A case-control study. Am J Obstet Gynecol 1999;180:1354-1359.
24. Nisell H, Ek S. Perinatal risks associated with borderline amniotic fluid index. Am J Obstet Gynecol. 2000;182:750-751.
25. Conway DL, et al. Isolated oligohydramnios in the term pregnancy: Is it a clinical entity. J Matern Fetal Med 1998;7:197-200.
26. Garmel SH, Chelmow D, Sha SJ, Roan JT, D’Alton ME. Oligohydramnios and the appropriately grown fetus. Am J Perinatol 1997;14:359-363.
27. Kreiser D, el-Sayed YY, Sorem KA, Chitkara U, Holbrook RH, Jr, Druzin ML. Decreased amniotic fluid index in low-risk pregnancy. J Reprod Med 2001;46:743-746.
28. Roberts D, Nwosu EC, Walkinshaw SA. The fetal outcome in pregnancies with isolated reduced amniotic fluid volume in the third trimester. J Perinat Med 1998;26:390-395.
29. Rainford M, Adair R, Scialli AR, Ghidini A, Spong CY. Amniotic fluid index in the uncomplicated term pregnancy. Prediction of outcome. J Reprod Med 2001;46:589-592.
30. Zhang J, Troendle J, Meikle S, Klebanoff MA, Rayburn WF. Isolated oligohydramnios is not associated with adverse perinatal outcomes. BJOG 2004;111:220-225.
31. Conway DL, Groth S, Adkins WB, Langer O. Management of isolated oligohyramnios in the term pregnancy: a randomized clinical trial. Am J Obstet Gynecol 2000;182:S21.-
32. Oosterhof H, Haak MC, Aarnoudse JG. Acute maternal rehydration increases the urine production rate in the near-term human fetus. Am J Obstet Gynecol 2000;183:226-229.
33. Fait G, Pauzner D, Gull I, Lessing JB, Jaffa AJ, Wolman I. Effect of 1 week or oral hydration on the amniotic fluid index. J Reprod Med 2003;48:187-190.
34. Hofmeyr GJ, Gulmezoglu AM. Maternal hydration for increasing amniotic fluid volume in oligohydramnios and normal amniotic fluid volute (Cochrane Review). In: The Cochrane Library. Issue 4, 2002. Oxford: Update Software.
35. Sherer DM, Langer O. Oligohydramnios: use and misuse in clinical management. Ultrasound Obstet Gynecol 2001;18:411-419.
36. Carroll BC, Bruner JP. Umbilical artery Doppler velocimetry in pregnancies complicated by oligohydramnios. J Reprod Med 2000;45:562-566.
37. Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol 1999;94:600-607.
38. Yeast JD, Jones A, Poskin M. Induction of labor and the relationship to cesarean delivery: A review of 7001 consecutive inductions. Am J Obstet Gynecol 1999;180:628-633.
1. Moore TR. Clinical assessment of amniotic fluid. Clin Obstet Gynecol 1997;40:303-313.
2. Phelan JP, Smith CV, Broussard P, Small M. Amniotic fluid volume assessment with the four-quadrant technique at 36–42weeks’ gestation. J Reprod Med 1987;32:540-542.
3. Banks EH. Perinatal risks associated with borderline amniotic fluid index. Am J Obstet Gynecol 1999;180:1461-1463.
4. Casey BM. Pregnancy outcomes after antepartum diagnosis of oligohydramnios at or beyond 34 weeks gestation. Am J Obstet Gynecol 2000;182:909-912.
5. Hobbins JC. Oligohydramnios and post-term pregnancy. Ob/Gyn Clinical Alert December 2002;59-60.
6. Johnson DP, Davis NR, Brown AJ. Risk of cesarean delivery after induction at term in nulliparous women with an unfavorable cervix. Am J Obstet Gynecol 2003;188:1565-1572.
7. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. N Engl J Med 2001;345:3-8.
8. Kilpatrick SJ, Safford KL. Maternal hydration increases amniotic fluid index in women with normal amniotic fluid. Obstet Gynecol 1993;81:49-52.
9. Gilbert WM, Brace RA. Amniotic fluid volume and normal flows to and from the amniotic cavity. Seminars in Perinatology 1993;17:150-157.
10. Chandra PC, Schiavello HJ, Lewandowski MA. Effect of oral and intravenous hydration on oligohydramnios. J Reprod Med 2000;45:337-340.
11. Doi S, Osada H, Seki K, Sekiya S. Effect of maternal hydration on oligohydramnios: a comparison of three volume expansion methods. Obstet Gynecol 1998;92:525-529.
12. Flack NJ, Sepulveda W, Bower S, Fisk NM. Acute maternal hydration in third-trimester oligohydramnios: effects on amniotic fluid volume, uteroplacental perfusion, and fetal blood flow and urine output. Am J Obstet Gynecol 1995;173:1186-1191.
13. Kilpatrick SJ, Safford KL, Pomeroy T, Hoedt L, Scheerer L, Laros RK. Maternal hydration increases amniotic fluid index. Obstet Gynecol 1991;78:1098-1102.
14. Larmon JE, Ross BS. Clinical utility of amniotic fluid volume assessment. Obstet Gynecol Clin North Am 1998;25:639-661.
15. Hill LM. Oligohydramnios: sonographic diagnosis and clinical implications. Clin Obstet Gynecol 1997;40:314-327.
16. Flack NJ, Dore C, Southwell D, Kourtis P, Sepulveda W, Fisk NM. The influence of operator transducer pressure on ultrasonographic measurements of amniotic fluid volume. Am J Obstet Gynecol 1994;171:218-222.
17. Rutherford SE, Smith CV, Phelan JP, Kawakami K, Ahn MO. Four-quadrant assessment of amniotic fluid volume: interobserver and intraobserver variation. J Reprod Med 1987;32:587-589.
18. Bruner JP, Reed GW, Sarno AP, Harrington RA, Goodman MA. Intraobserver and interobserver variability of the amniotic fluid index. Am J Obstet Gynecol 1993;168:1309-1313.
19. O’Reilly-Green CP, Divon MY. Predictive value of amniotic fluid index for oligohydramnios in patients with prolonged pregnancies. J Matern Fetal Med 1996;5:218-226.
20. Chauhan SP, Magann EF, Morrison JC, Whitworth NS, Hendrix NW, Devoe LD. Ultrasonographic assessment of amniotic fluid does not reflect actual amniotic fluid volume. Am J Obstet Gynecol 1997;177:291-297.
21. Chauhan SP, Sanderson M, Hendrix NW, Magann EF, Devoe LD. Perinatal outcome and amniotic fluid index in the antepartum and intrapartum periods: a meta-analysis. Am J Obstet Gynecol 1999;181:1473-1478.
22. Magann EF. Antenatal testing among 1001 patients at high risk: the role of ultrasonographic estimate of amniotic fluid volume. Am J Obstet Gynecol 1999;180:1330-1336.
23. Magann EF. Does an amniotic fluid index of 5 cm necessitate delivery in high-risk pregnancies? A case-control study. Am J Obstet Gynecol 1999;180:1354-1359.
24. Nisell H, Ek S. Perinatal risks associated with borderline amniotic fluid index. Am J Obstet Gynecol. 2000;182:750-751.
25. Conway DL, et al. Isolated oligohydramnios in the term pregnancy: Is it a clinical entity. J Matern Fetal Med 1998;7:197-200.
26. Garmel SH, Chelmow D, Sha SJ, Roan JT, D’Alton ME. Oligohydramnios and the appropriately grown fetus. Am J Perinatol 1997;14:359-363.
27. Kreiser D, el-Sayed YY, Sorem KA, Chitkara U, Holbrook RH, Jr, Druzin ML. Decreased amniotic fluid index in low-risk pregnancy. J Reprod Med 2001;46:743-746.
28. Roberts D, Nwosu EC, Walkinshaw SA. The fetal outcome in pregnancies with isolated reduced amniotic fluid volume in the third trimester. J Perinat Med 1998;26:390-395.
29. Rainford M, Adair R, Scialli AR, Ghidini A, Spong CY. Amniotic fluid index in the uncomplicated term pregnancy. Prediction of outcome. J Reprod Med 2001;46:589-592.
30. Zhang J, Troendle J, Meikle S, Klebanoff MA, Rayburn WF. Isolated oligohydramnios is not associated with adverse perinatal outcomes. BJOG 2004;111:220-225.
31. Conway DL, Groth S, Adkins WB, Langer O. Management of isolated oligohyramnios in the term pregnancy: a randomized clinical trial. Am J Obstet Gynecol 2000;182:S21.-
32. Oosterhof H, Haak MC, Aarnoudse JG. Acute maternal rehydration increases the urine production rate in the near-term human fetus. Am J Obstet Gynecol 2000;183:226-229.
33. Fait G, Pauzner D, Gull I, Lessing JB, Jaffa AJ, Wolman I. Effect of 1 week or oral hydration on the amniotic fluid index. J Reprod Med 2003;48:187-190.
34. Hofmeyr GJ, Gulmezoglu AM. Maternal hydration for increasing amniotic fluid volume in oligohydramnios and normal amniotic fluid volute (Cochrane Review). In: The Cochrane Library. Issue 4, 2002. Oxford: Update Software.
35. Sherer DM, Langer O. Oligohydramnios: use and misuse in clinical management. Ultrasound Obstet Gynecol 2001;18:411-419.
36. Carroll BC, Bruner JP. Umbilical artery Doppler velocimetry in pregnancies complicated by oligohydramnios. J Reprod Med 2000;45:562-566.
37. Seyb ST, Berka RJ, Socol ML, Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol 1999;94:600-607.
38. Yeast JD, Jones A, Poskin M. Induction of labor and the relationship to cesarean delivery: A review of 7001 consecutive inductions. Am J Obstet Gynecol 1999;180:628-633.
Do All Hospitals Need Cesarean Delivery Capability?
OBJECTIVES: We analyzed perinatal outcomes at a rural hospital without cesarean delivery capability.
STUDY DESIGN: This was a historical cohort outcomes study.
POPULATION: The study population included all pregnant women at 20 weeks or greater of gestational age (n = 1132) over a 5-year period in a predominantly Native American region of northwestern New Mexico.
OUTCOMES MEASURED: The outcomes studied included perinatal mortality, neonatal morbidity, obstetric emergencies, intrapartum and antepartum transfers, and cesarean delivery rate. We did a detailed case review of all obstetric emergencies and low-Apgar-score births at Zuni-Ramah Hospital and all cesarean deliveries for fetal distress at referral hospitals.
RESULTS: Of the 1132 women in the study population, 64.7% (n = 735) were able to give birth at the hospital without operative facilities; 25.6% (n = 290) were transferred before labor; and 9.5% (n = 107) were transferred during labor. The perinatal mortality rate of 11.4 per 1000 (95% confidence interval, 5.1-17.8) was similar to the nationwide rate of 12.8 per 1000 even though Zuni-Ramah has a high-risk obstetric population. No instances of major neonatal or maternal morbidity caused by lack of surgical facilities occurred. The cesarean delivery rate of 7.3% was significantly lower than the nationwide rate of 20.7% (P < .001). The incidence of neonates with low Apgar scores (0.54%) was significantly lower than the nationwide rate (1.4%). The incidence of neonates requiring resuscitation (3.4%) was comparable to the nationwide rate (2.9%).
CONCLUSIONS: The presence of a rural maternity care unit without surgical facilities can safely allow a high proportion of women to give birth closer to their communities. This study demonstrated a low level of perinatal risk. Most transfers were made for induction or augmentation of labor. Rural hospitals that do not have cesarean delivery capability but are part of an integrated perinatal system can safely offer obstetric services by using appropriate antepartum and intrapartum screening criteria for obstetric risk.
- Rural hospitals without cesarean delivery capability can safely offer obstetric care to selected patients as part of an integrated perinatal network.
- Measures of maternal and neonatal morbidity and mortality were at or below national averages despite a higher-risk population.
- Antepartum (25.6%) or intrapartum (9.5%) transfer to hospitals with surgical or tertiary-care facilities was required for 35% of pregnant women.
- The use of oxytocin induction or augmentation, if determined safe, may significantly lower the transfer rate from rural hospitals that lack cesarean delivery capability.
The availability of perinatal care in rural communities produces better pregnancy outcomes than do perinatal systems that require rural women to seek maternity care in distant urban areas.1-3 Unfortunately, rural maternity care has been affected by the loss of physicians who offer these services and by the closing of many rural hospitals’ maternity care units. Maintaining 24-hour operative obstetric capabilities is difficult in rural areas because they have an insufficient population base to support a physician trained in operative obstetrics. Another barrier is the lack of anesthesia services and operating room personnel.
The Guidelines for Perinatal Care developed by the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) state, “All hospitals offering labor and delivery services should be equipped to perform emergency cesarean delivery.” 4 Nevertheless, not all rural obstetric units can offer cesarean delivery and must transfer patients to a referral hospital for operative needs. Advisory panels in the United States and Canada have recommended similar models of rural perinatal care.5-8 A Canadian panel estimated that 125 Canadian hospitals offer obstetric care without surgical facilities.
Studies of rural hospitals in Canada, Australia, and the United Kingdom that lack continuous on-site cesarean capability are limited by the small number of deliveries.9-12 Most such studies are hospital based rather than population based and lack data on women who are transferred to outlying hospitals. The only population-based study that we identified found no evidence of adverse events caused by the lack of cesarean facilities; the sample size, however, was limited to 286 births.9
We studied all pregnancies occurring in a predominantly Native American region of New Mexico over a 5-year period to ascertain the safety of rural perinatal care based in a hospital without cesarean capability. Population-based and hospital-based outcomes are presented. This is the first study from a US community using this model of care.
Methods
We conducted an outcomes study using a historical cohort study design of all pregnancies beyond 20 weeks of gestation in the Zuni Pueblo and Ramah Navajo communities of northwestern New Mexico from 1992 to 1996. The perinatal services based at the Zuni-Ramah Indian Health Service (IHS) Hospital are the focus of this study. This 37-bed community hospital, staffed by family physicians and a part-time nurse-midwife, is part of an integrated perinatal system. The birthing unit has access to obstetrician-gynecologist (OBG) consultants at the Gallup Indian Medical Center (GIMC), 33 miles to the north, and perinatology and neonatology care in Albuquerque, 147 miles to the east. GIMC, the primary referral hospital and closest surgical facility, has an obstetric unit staffed by OBGs, family physicians, and nurse-mid-wives. Transportation time is 40 minutes by ground ambulance to GIMC or by fixed wing aircraft to Albuquerque.
The Zuni-Ramah Hospital limits intrapartum care to women designated as at low or moderate risk by criteria established by Zuni-Ramah family physicians and reviewed by GIMC OBGs. Criteria mandating transfer included prior cesarean, malpresentation, multiple gestation, intrauterine growth restriction, severe preeclampsia, placenta previa, significant vaginal bleeding, major fetal anomalies, anticipated preterm delivery (< 36 weeks), nonreassuring fetal heart tones (NRFHTs), and need for labor induction or augmentation with oxytocin. Women with gestational or type 2 diabetes who were well controlled could give birth at Zuni-Ramah unless they had end-organ damage or the fetus had known macrosomia. Physicians successfully completed the Advanced Life Support in Obstetrics (ALSO, ®American Academy of Family Physicians, 4th ed., 2000) course, attended weekly high-risk obstetric rounds, and performed quarterly reviews of obstetric complications. The family physicians performed vacuum-assisted deliveries, utilized amnioinfusion, and used continuous or intermittent fetal monitoring.
A review of the delivery and transfer records of the Zuni-Ramah Hospital and GIMC obstetric services revealed that there had been 1132 births of 1137 infants during the study period. The authors used a data collection form to review prenatal and newborn records from every birth. We reviewed intrapartum records for all births at the Zuni-Ramah and GIMC hospitals. We obtained discharge summaries from tertiary-care sites. We interviewed perinatal coordinators, public health nurses, and pediatric care providers to obtain information about patients who had received perinatal care outside of the IHS system.
The outcomes measured included perinatal mortality, neonatal morbidity, obstetric emergencies, intrapartum and antepartum transfers, and cesarean delivery rate. All obstetric emergencies originating at Zuni-Ramah Hospital were reviewed to determine whether the lack of surgical facilities had resulted in adverse outcomes. The physician’s notes were used to differentiate a NRFHT pattern requiring observation at a hospital with operative facilities from a truly worrisome pattern that required urgent intervention for fetal distress.
Births were defined as deliveries of infants at 20 weeks or more of estimated gestational age. We analyzed each birth in a multiple gestation individually. The population-based perinatal mortality rate was calculated from 20 weeks’ estimated gestational age to the 28th neonatal day. The Zuni-Ramah Hospital perinatal mortality rate was calculated by inclusion of all women delivered at Zuni-Ramah Hospital or transferred during labor. Approval for the study was obtained from the IHS Institutional Review Board and the Zuni Tribal Council.
Results
Study population
We identified 1137 births to 945 women between 1992 and 1996. Zuni and Navajo births were 66.9% and 30.8%, respectively; 30% of women were primiparous and 70%, multiparous. We found that 10.4% of births had occurred in women older than 35 years and 7.8% in women younger than 18 years. Regarding prenatal care, 3.9% of women had received none; 43.0% had established prenatal care in the first trimester; 40.4%, in the second trimester; and 12.8%, in the third trimester.
Delivery sites and maternal transfers
The majority of women (64.4%, n = 732) gave birth at the Zuni-Ramah Hospital (Figure) or at GIMC (29.6%, n = 337). A small number (2.2%, n = 25) gave birth at a private hospital with surgical facilities in Gallup. Albuquerque tertiary-care hospitals were the sites of 3.2% (n = 36) of deliveries. Primary indications for tertiary care were prematurity and fetal anomalies. Seven (0.6%) deliveries occurred at other sites, including home and ambulance.
The antepartum transfers (Table 1) were required primarily for pregnancy complications requiring labor induction. Preeclampsia, diabetes, nonreassuring antepartum testing, and post dates patients accounted for 56.8% of the 290 transfers. The 107 intrapartum transfers were made predominantly for labor induction or augmentation (64.5%, n = 69), a concerning fetal heart tracing (15.9%, n = 17), or fetal malpresentation diagnosed during labor (8.4%, n = 9).
FIGURE
PREGNANCIES AT ZUNI-RAMAH HOSPITAL
TABLE 1
ANTEPARTUM AND INTRAPARTUM TRANSFERS FROM ZUNI-RAMAH HOSPITAL
| Indication | Number of Transfers (%) |
|---|---|
| Antepartum Transfers* | |
| Preeclampsia | 83 (28.6) |
| Prior cesarean delivery | 55 (19.0) |
| Nonreassuring testing | 39 (13.4) |
| Preterm (includes PPROM) | 24 (8.3) |
| Diabetes | 22 (7.6) |
| Postdates | 21 (7.2) |
| Other | 18 (6.2) |
| Malpresentation | 16 (5.5) |
| Chronic HTN | 8 (2.8) |
| Macrosomia | 7 (2.4) |
| IUFD | 6 (2.1) |
| IUGR | 5 (1.7) |
| Anomalies | 4 (1.4) |
| Total | 290 (25.6% of population) |
| Intrapartum Transfers | |
| First-stage arrest of labor | 37 (34.6) |
| PROM without active labor | 32 (29.9) |
| Malpresentation | 9 (8.4) |
| Fetal distress | 5 (4.7) |
| Nonreassuring tracing | 12 (11.2) |
| Other | 12 (11.2) |
| Total | 107 (9.5% of population) |
| *Greater than 290 because of 18 patients with 2 reasons for antepartum transfer. | |
| HTN denotes hypertension; IUFD, intrauterine fetal demise; IUGR, intrauterine growth restriction; PROM, premature rupture of membranes; PPROM, preterm premature rupture of membranes. | |
Obstetric interventions
The total cesarean delivery rate (7.3%) was approximately one third the nationwide rate of 20.7% in 1996. The primary cesarean delivery rate (number of cesareans in women without prior cesarean divided by the number of women who have never had a cesarean) of 5.3% compares with a nationwide primary rate of 14.6%. The cesarean rate was 22.1% for antepartum transfers and 17.8% for intrapartum transfers. Operative vaginal delivery occurred in 5.4% of births, well below the nationwide rate of 9.4%. The induction rate of 13.8% is lower than the nationwide rate of 16.9%. The oxytocin augmentation rate of 7.7% is well below the nationwide rate of 16.9% in 1996.13 Parenteral narcotics were available at Zuni-Ramah; however, 81.4% of women elected to receive no labor analgesia. Epidural anesthesia was not available at Zuni-Ramah Hospital.
Perinatal mortality
The perinatal mortality rate for the population was 11.4 per 1000 births (95% CI, 5.1-17.8 by Poisson distribution), comparable to the 1991 nationwide peri-natal mortality rate of 12.8/1000.14 Nine of the 13 neonatal deaths were caused by intrauterine fetal demise before labor (Table 2). The Zuni-Ramah Hospital–based perinatal mortality rate of 1.2/1000 was comparable with the 1.3/1000 perinatal mortality rate for women in the National Birth Center study even though Zuni-Ramah Hospital accepts higher-risk patients.15
TABLE 2
PERINATAL MORTALITY IN ZUNI-RAMAH POPULATION
| Age (wk) | Weight (g) | Site | Cause |
|---|---|---|---|
| Intrauterine Fetal Death | |||
| 31 | 1410 | GIMC | Unexplained |
| 39 | 3130 | GIMC | Unexplained |
| 35 | 1540 | GIMC | Unexplained; IUGR |
| 35 | 1690 | GIMC | Unexplained; IUGR |
| 21 | 330 | GIMC | PPROM |
| 21 | 560 | GIMC | PPROM |
| 41 | 3040 | GIMC | Oligohydramnios and post dates. Two days prior, refused induction with amniotic fluid volume index of 3.8 |
| 28 | 1290 | Alb | Abruption |
| 32 | Unknown | Zuni | Necrotizing/calcifying encephalopathy (probable CMV) with severe IUGR |
| Early Neonatal Death (< 7 days) | |||
| 38 | 2805 | Alb | Osteogenesis imperfecta |
| 31 | Unknown | Alb | Potter’s syndrome |
| Late Neonatal Death (7 to 27 days) | |||
| 35 | 1590 | GIMC | Pulmonary interstitial emphysema caused by respiratory failure of unknown etiology/IUGR |
| 41 | 3220 | GIMC | Sepsis at 12 days; had been discharged home as healthy infant |
| Alb denotes Albuquerque tertiary-care hospital; CMV, cytomegalovirus; GIMC, Gallup Indian Medical Center; IUGR, intrauterine growth restriction; PPROM, preterm premature rupture of membranes. | |||
Neonatal morbidity
Measures of neonatal morbidity are summarized in Table 3. The frequency of 5-minute Apgar scores below 7, low birthweight, and prematurity compares favorably with 1996 US rates.13 The rate of assisted ventilation (intubation or bag-mask) for the entire population (4.6%, n = 52) is greater than the 1996 nationwide rate (2.9%), although the difference is of questionable clinical significance, since international studies have demonstrated a range for assisted ventilation of 1% to 10% of hospital births.16 Neonatal Intensive Care Unit (NICU) transfer occurred in 27 (2.4%) of deliveries from non-tertiary-care sites. Thirteen (1.8%) babies born at Zuni-Ramah were transferred to Albuquerque for NICU care because of respiratory distress (n = 10) or neonatal anomalies (n = 3). The 3 cases of low Apgar scores at Zuni-Ramah were attributed to pneumothorax, respiratory distress syndrome of prematurity, and sepsis with meconium aspiration.
TABLE 3
NEONATAL MORBIDITY IN ZUNI-RAMAH POPULATION, BASED ON LIVE BIRTHS
| Zuni-Ramah Hospital (N=732) | Zuni-Ramah Population (n = 1128) | 1996 US Population | |
|---|---|---|---|
| 5-minute Apgar score < 7 | 3 (0.41%), P = .023 | 6 (0.54%), P = .014 | 1.4% |
| Assisted ventilation | 19 (2.6%), P = 0.62 | 52 (4.6%), P < .001 | 2.9% |
| Birthweight < 2500 g | 14 (1.9%), P < .001 | 61 (5.4%), P < .001 | 11% |
| Preterm (37 weeks) | 22 (3.0%), P < .001 | 75 (6.7%), P = .36 | 7.4% |
| P values are based on comparison with the US population. US population figures for 1996 were extracted from Ventura SJ, Martin JA, Curtin SC, Mathews TJ. Report of Final Natality Statistics, 1996. Monthly vital statistics report; vol 46, no 11, supp. Hyattsville, Md: National Center for Health Statistics, 1998. | |||
Obstetric risk factors
The study population had a greater incidence of pregnancy-induced hypertension (14.5% vs 2.6% by 1996 ACOG criteria17), chronic hypertension (2.7% vs 0.7%15), and diabetes (9.2% vs 2.6%15) than the average US obstetric population. Gestational diabetes was diagnosed according to National Diabetes Data Group criteria:18 7.1% had gestational diabetes (class A1 and A2 ) and 2.1% had type 2 antepartum diabetes (classes B and C).
Outcomes of obstetric emergencies at zuni-ramah hospital
We reviewed all cases of placental abruption, uterine inversion, umbilical cord prolapse, and fetal distress at Zuni-Ramah Hospital to identify potentially preventable adverse outcomes caused by lack of operative facilities (Table W1). Umbilical cord prolapse and uterine inversion each occurred once and were appropriately managed, with excellent outcomes. In 3 of the 4 cases of placental abruption, there were clearly no adverse outcomes caused by lack of on-site operative facilities, as patients were expectantly managed upon arrival to the referral hospital (cases 3 and 4) or presented to Zuni-Ramah Hospital as an intrauterine demise (case 5).
The fourth patient with placental abruption (case 6) presented at Zuni-Ramah with vaginal bleeding, severe variable decelerations, and a 10-point drop from baseline hematocrit. She was scheduled to labor at GIMC because of a history of prior cesarean but presented to the Zuni-Ramah emergency room with vaginal bleeding. She was transferred to GIMC for an anticipated cesarean delivery; however, on arrival the patient rapidly progressed and gave birth to an infant vaginally with Apgar scores of 3 and 9. Her infant had a neonatal seizure and magnetic resonance imaging evidence of sagittal sinus thrombosis. The infant had a normal neurologic evaluation, developmental assessment, and electroencephalogram at 15 months.
We reviewed 5 cases of urgent transfer for fetal distress. These were differentiated from the 8 intrapartum transfers for NRFHTs based on the severity of fetal heart monitor tracings. Four of the 5 women who had been transferred for fetal distress gave birth to healthy infants vaginally more than 2 hours after arrival at the referral institution. One patient who was urgently transferred for repetitive late decelerations is discussed below.
Cesarean deliveries for fetal distress at referral hospitals
We reviewed all cases of cesarean deliveries for fetal distress (n = 10) at referral institutions to determine whether outcomes for any of the patients could potentially have been improved by having their cesarean deliveries earlier if operative facilities had been available at Zuni-Ramah Hospital or by being transferred earlier (Table W1). Seven of the 10 patients were transferred for preeclampsia, NRFHTs, or failure to progress. All had their cesareans for fetal distress many hours after arrival at the referral institution.
Two cases of cesarean delivery for fetal distress after transfer because of abruption were previously described. A patient presented in early labor with repetitive late decelerations and was urgently transferred to GIMC, where she underwent an immediate cesarean delivery. Her infant had Apgar scores of 1 and 7, an unremarkable neonatal course, and a normal 15-month developmental screen.
Discussion
Our outcomes demonstrate that with the use of appropriate screening criteria, childbirth can safely occur in institutions that lack surgical suites. The population-based perinatal mortality rate was similar to the nationwide rate. A review of obstetric emergencies and low Apgar scores among the 839 women laboring at Zuni-Ramah Hospital failed to identify adverse outcomes that might have been prevented if the hospital had had operative facilities. Cesarean rates were approximately one third the nationwide rate even though Zuni-Ramah patients had a higher prevalence of such risk factors as diabetes and preeclampsia.
Although they represented a high-risk obstetric population, 65% of women were able to give birth at Zuni-Ramah Hospital through use of the perinatal screening criteria. The 35% rate of transfer was caused largely by the need for oxytocin augmentation or induction. Only 21.6% of the women who were transferred for dysfunctional labor or premature rupture of membranes ultimately had a cesarean delivery. Oxytocin has not been permitted at Zuni-Ramah Hospital because of the ACOG guideline permitting oxytocin use only if “a physician capable of performing a cesarean delivery is readily available.”19 There are no studies addressing the safety of labor induction or augmentation without on-site cesarean capability.
Canadian guidelines for rural maternity care do not prohibit the use of prostaglandins or oxytocin at hospitals without operative facilities. A Consensus Conference on Obstetric Services in Rural or Remote Communities addressed the issue of labor induction or augmentation in hospitals without cesarean capability by stating, “If caring for a woman in labour is appropriate in the community, then caring for her during an augmented/induced labour is equally appropriate when there is support by trained local staff and resources.”20 We concur that use of oxytocin in rural hospital units without operative facilities should be considered under well-defined clinical guidelines or research protocols.
Limitations
Our study’s limitations include lack of long-term neonatal outcomes, small size of the Zuni-Ramah population, an almost exclusively Native American population, and lack of examiner blinding during record review. Transfer rates may be increased in populations with higher rates of cesarean delivery or epidural anesthesia use. Alternatively, the high incidence of preeclampsia, chronic hypertension, and diabetes in these communities may have resulted in a higher proportion of induction. Umbilical cord pro-lapse and significant placental abruption are routinely treated by urgent cesarean delivery; therefore, obstetric literature on outcomes without immediate operative intervention is limited.21,22 A larger study would be required to determine the potential increased neonatal morbidity or mortality resulting from delayed intervention.
Conclusions
The ACOG/AAP guideline requiring on-site surgical facilities and the ability to initiate a cesarean in 30 minutes is not based on evidence. Four small retrospective studies of emergency cesarean deliveries delayed for more than 30 minutes did not demonstrate adverse neonatal outcomes.23-26 In our study population, no adverse outcomes (none in 839 births) were determined to have been caused by a lack of surgical facilities. Despite these excellent outcomes, the possibility always exists for outcomes that can be prevented by doing a rapid emergent cesarean delivery. Women deciding to give birth in facilities without operative capabilities should receive information regarding the risks and benefits of delivering there and should have access to other facilities. Provider discretion and patient choice must be respected to ensure community support of these birthing units. Practitioners at the rural units must have assurance that any patients who require an urgent transfer will be readily accepted.
Rural communities, medical providers, and health care facilities need to consider the overall effect of maintaining local maternity care units, as the loss of rural maternity care can increase the risk of adverse perinatal outcomes.1-3 We concur with the Canadian panel that although maintenance of rural surgical and anesthesia capabilities is desirable, “good outcomes can be sustained within an integrated risk management system without local access to operative delivery.”8 Guidelines should be developed to permit rural hospitals without cesarean capability to provide maternity care as part of integrated perinatal systems with well-developed transport protocols and supportive referral institutions. Women living in rural areas should have the option to give birth near their homes in such units if they so desire.
Acknowledgments
The authors thank Robert Rhyne, MD, for editorial assistance in manuscript preparation and Betty Skipper, PhD, for statistical assistance. Current Zuni-Ramah Obstetric Guidelines are available at http://hsc.unm.edu/fcm/research/zuni.
1. Allen DT, Kamradt MS. Relationship of infant mortality to the availability of obstetric care in Indiana. J Fam Pract 1991;33:609-13.
2. Larimore WL, Davis A. Relation of infant mortality to the availability of maternity care in rural Florida. J Am Board Fam Pract 1995;8:392-9.
3. Nesbitt TS, Connell FA, Hart LG, Rosenblatt RA. Access to obstetric care in rural areas: effect on birth outcomes Am J Public Health. 1990;80:814-8.
4. American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 4th ed. Washington, DC: ACOG, 1997.
5. New York State Department of Health, Office of Rural Health. Report on the provision of birthing services in rural health networks. Albany, NY; 1994.
6. Nesbitt TS. Rural maternity care: new models of access. Birth. 1996;23:161-5.
7. Rosenthal TC, Holden DM, Woodward W. Primary care obstetrics in rural Western New York: a multi-center case review. NY State J Med 1990;90:537-40.
8. Iglesias S, Grzybowski S, Klein M, Gagne GP, Lalonde A. Joint position paper on rural maternity care. Society of Rural Physicians, Society of Obstetricians and Gynecologists of Canada, College of Family Physicians of Canada, 1998.
9. Grzybowski SCW, Cadesky AS, Hogg WE. Rural obstetrics: a 5-year prospective study of the outcomes of all pregnancies in a remote northern community. Can Med Assoc J 1991;144:987-94.
10. Black DB, Fyfe IM. The safety of obstetrics services in small communities in northern Ontario. Can Med Assoc J 1984;130:571-6.
11. Woollard LA, Hays RB. Rural obstetrics in NSW. Aust N Z J Obstet Gynaecol 1993;33:240-2.
12. McIlwain R, Smith S. Obstetrics in a small isolated community: the cesarean section dilemma. Can J Rural Med 2000;5:221-3.
13. Ventura SJ, Martin JA, Curtin SC, Mathews TJ. Report of Final Natality Statistics, 1996. Monthly vital statistics report; Vol 46 no 11, suppl. Hyattsville, Md: National Center for Health Statistics, 1998.
14. Hoyert DL. Perinatal mortality in the United States, 1985-91. National Center for Health Statistics. Vital Health Stat 20(26), 1995.
15. Rooks JP, Weatherby NL, Ernst EKM, Stapleton S, Rosen D, Rosenfield A. Outcomes of care in birth centers: the National Birth Center Study. N Engl J Med 1989;321:1804-11.
16. International guidelines for neonatal resuscitation: An excerpt from the guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2000;106(3):Available from: http://www.pediatrics.org/cgi/content/full/106/3/e29.
17. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. ACOG Technical Bulletin 219. Washington, DC: ACOG, 1996.
18. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57.
19. American College of Obstetricians and Gynecologists. Induction of labor. ACOG Practice Bulletin 10. Washington, DC: ACOG; 1999.
20. Torr E, ed, for the British Columbia Reproductive Care Program. Report on the findings of the Consensus Conference on Obstetric Services in Rural or Remote Communities. Can J Rural Med 2000;5:211-7.
21. Barrett J. Funic reduction for the management of umbilical cord prolapse. Am J Obstet Gynecol 1991;165:654-7.
22. Knab DR. Abruptio placentae: an assessment of the time and method of delivery. Obstet Gynecol 1978;52:625-9.
23. Chauhan SP, Roach H, Naef RW, Magann EF, Morrison JC, Martin JN. Cesarean section for suspected fetal distress: Does the decision-incision time make a difference? J Reprod Med 1997;42:347-52.
24. MacKenzie IZ, Cooke I. Prospective 12 month study of 30 minute decision to delivery intervals for “emergency” caesarean section. BMJ 2001;322:1334-5.
25. Schauberger CW, Rooney BL, Beguin EA, Schaper AM, Spindler J. Evaluating the thirty minute interval in emergency cesarean sections. J Am Coll Surg 1994;179:151-5.
26. Tuffnell DJ, Wilkinson K, Beresford N. Interval between decision and delivery by caesarean section: Are current standards achievable? BMJ 2001;322:1330-3.
OBJECTIVES: We analyzed perinatal outcomes at a rural hospital without cesarean delivery capability.
STUDY DESIGN: This was a historical cohort outcomes study.
POPULATION: The study population included all pregnant women at 20 weeks or greater of gestational age (n = 1132) over a 5-year period in a predominantly Native American region of northwestern New Mexico.
OUTCOMES MEASURED: The outcomes studied included perinatal mortality, neonatal morbidity, obstetric emergencies, intrapartum and antepartum transfers, and cesarean delivery rate. We did a detailed case review of all obstetric emergencies and low-Apgar-score births at Zuni-Ramah Hospital and all cesarean deliveries for fetal distress at referral hospitals.
RESULTS: Of the 1132 women in the study population, 64.7% (n = 735) were able to give birth at the hospital without operative facilities; 25.6% (n = 290) were transferred before labor; and 9.5% (n = 107) were transferred during labor. The perinatal mortality rate of 11.4 per 1000 (95% confidence interval, 5.1-17.8) was similar to the nationwide rate of 12.8 per 1000 even though Zuni-Ramah has a high-risk obstetric population. No instances of major neonatal or maternal morbidity caused by lack of surgical facilities occurred. The cesarean delivery rate of 7.3% was significantly lower than the nationwide rate of 20.7% (P < .001). The incidence of neonates with low Apgar scores (0.54%) was significantly lower than the nationwide rate (1.4%). The incidence of neonates requiring resuscitation (3.4%) was comparable to the nationwide rate (2.9%).
CONCLUSIONS: The presence of a rural maternity care unit without surgical facilities can safely allow a high proportion of women to give birth closer to their communities. This study demonstrated a low level of perinatal risk. Most transfers were made for induction or augmentation of labor. Rural hospitals that do not have cesarean delivery capability but are part of an integrated perinatal system can safely offer obstetric services by using appropriate antepartum and intrapartum screening criteria for obstetric risk.
- Rural hospitals without cesarean delivery capability can safely offer obstetric care to selected patients as part of an integrated perinatal network.
- Measures of maternal and neonatal morbidity and mortality were at or below national averages despite a higher-risk population.
- Antepartum (25.6%) or intrapartum (9.5%) transfer to hospitals with surgical or tertiary-care facilities was required for 35% of pregnant women.
- The use of oxytocin induction or augmentation, if determined safe, may significantly lower the transfer rate from rural hospitals that lack cesarean delivery capability.
The availability of perinatal care in rural communities produces better pregnancy outcomes than do perinatal systems that require rural women to seek maternity care in distant urban areas.1-3 Unfortunately, rural maternity care has been affected by the loss of physicians who offer these services and by the closing of many rural hospitals’ maternity care units. Maintaining 24-hour operative obstetric capabilities is difficult in rural areas because they have an insufficient population base to support a physician trained in operative obstetrics. Another barrier is the lack of anesthesia services and operating room personnel.
The Guidelines for Perinatal Care developed by the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) state, “All hospitals offering labor and delivery services should be equipped to perform emergency cesarean delivery.” 4 Nevertheless, not all rural obstetric units can offer cesarean delivery and must transfer patients to a referral hospital for operative needs. Advisory panels in the United States and Canada have recommended similar models of rural perinatal care.5-8 A Canadian panel estimated that 125 Canadian hospitals offer obstetric care without surgical facilities.
Studies of rural hospitals in Canada, Australia, and the United Kingdom that lack continuous on-site cesarean capability are limited by the small number of deliveries.9-12 Most such studies are hospital based rather than population based and lack data on women who are transferred to outlying hospitals. The only population-based study that we identified found no evidence of adverse events caused by the lack of cesarean facilities; the sample size, however, was limited to 286 births.9
We studied all pregnancies occurring in a predominantly Native American region of New Mexico over a 5-year period to ascertain the safety of rural perinatal care based in a hospital without cesarean capability. Population-based and hospital-based outcomes are presented. This is the first study from a US community using this model of care.
Methods
We conducted an outcomes study using a historical cohort study design of all pregnancies beyond 20 weeks of gestation in the Zuni Pueblo and Ramah Navajo communities of northwestern New Mexico from 1992 to 1996. The perinatal services based at the Zuni-Ramah Indian Health Service (IHS) Hospital are the focus of this study. This 37-bed community hospital, staffed by family physicians and a part-time nurse-midwife, is part of an integrated perinatal system. The birthing unit has access to obstetrician-gynecologist (OBG) consultants at the Gallup Indian Medical Center (GIMC), 33 miles to the north, and perinatology and neonatology care in Albuquerque, 147 miles to the east. GIMC, the primary referral hospital and closest surgical facility, has an obstetric unit staffed by OBGs, family physicians, and nurse-mid-wives. Transportation time is 40 minutes by ground ambulance to GIMC or by fixed wing aircraft to Albuquerque.
The Zuni-Ramah Hospital limits intrapartum care to women designated as at low or moderate risk by criteria established by Zuni-Ramah family physicians and reviewed by GIMC OBGs. Criteria mandating transfer included prior cesarean, malpresentation, multiple gestation, intrauterine growth restriction, severe preeclampsia, placenta previa, significant vaginal bleeding, major fetal anomalies, anticipated preterm delivery (< 36 weeks), nonreassuring fetal heart tones (NRFHTs), and need for labor induction or augmentation with oxytocin. Women with gestational or type 2 diabetes who were well controlled could give birth at Zuni-Ramah unless they had end-organ damage or the fetus had known macrosomia. Physicians successfully completed the Advanced Life Support in Obstetrics (ALSO, ®American Academy of Family Physicians, 4th ed., 2000) course, attended weekly high-risk obstetric rounds, and performed quarterly reviews of obstetric complications. The family physicians performed vacuum-assisted deliveries, utilized amnioinfusion, and used continuous or intermittent fetal monitoring.
A review of the delivery and transfer records of the Zuni-Ramah Hospital and GIMC obstetric services revealed that there had been 1132 births of 1137 infants during the study period. The authors used a data collection form to review prenatal and newborn records from every birth. We reviewed intrapartum records for all births at the Zuni-Ramah and GIMC hospitals. We obtained discharge summaries from tertiary-care sites. We interviewed perinatal coordinators, public health nurses, and pediatric care providers to obtain information about patients who had received perinatal care outside of the IHS system.
The outcomes measured included perinatal mortality, neonatal morbidity, obstetric emergencies, intrapartum and antepartum transfers, and cesarean delivery rate. All obstetric emergencies originating at Zuni-Ramah Hospital were reviewed to determine whether the lack of surgical facilities had resulted in adverse outcomes. The physician’s notes were used to differentiate a NRFHT pattern requiring observation at a hospital with operative facilities from a truly worrisome pattern that required urgent intervention for fetal distress.
Births were defined as deliveries of infants at 20 weeks or more of estimated gestational age. We analyzed each birth in a multiple gestation individually. The population-based perinatal mortality rate was calculated from 20 weeks’ estimated gestational age to the 28th neonatal day. The Zuni-Ramah Hospital perinatal mortality rate was calculated by inclusion of all women delivered at Zuni-Ramah Hospital or transferred during labor. Approval for the study was obtained from the IHS Institutional Review Board and the Zuni Tribal Council.
Results
Study population
We identified 1137 births to 945 women between 1992 and 1996. Zuni and Navajo births were 66.9% and 30.8%, respectively; 30% of women were primiparous and 70%, multiparous. We found that 10.4% of births had occurred in women older than 35 years and 7.8% in women younger than 18 years. Regarding prenatal care, 3.9% of women had received none; 43.0% had established prenatal care in the first trimester; 40.4%, in the second trimester; and 12.8%, in the third trimester.
Delivery sites and maternal transfers
The majority of women (64.4%, n = 732) gave birth at the Zuni-Ramah Hospital (Figure) or at GIMC (29.6%, n = 337). A small number (2.2%, n = 25) gave birth at a private hospital with surgical facilities in Gallup. Albuquerque tertiary-care hospitals were the sites of 3.2% (n = 36) of deliveries. Primary indications for tertiary care were prematurity and fetal anomalies. Seven (0.6%) deliveries occurred at other sites, including home and ambulance.
The antepartum transfers (Table 1) were required primarily for pregnancy complications requiring labor induction. Preeclampsia, diabetes, nonreassuring antepartum testing, and post dates patients accounted for 56.8% of the 290 transfers. The 107 intrapartum transfers were made predominantly for labor induction or augmentation (64.5%, n = 69), a concerning fetal heart tracing (15.9%, n = 17), or fetal malpresentation diagnosed during labor (8.4%, n = 9).
FIGURE
PREGNANCIES AT ZUNI-RAMAH HOSPITAL
TABLE 1
ANTEPARTUM AND INTRAPARTUM TRANSFERS FROM ZUNI-RAMAH HOSPITAL
| Indication | Number of Transfers (%) |
|---|---|
| Antepartum Transfers* | |
| Preeclampsia | 83 (28.6) |
| Prior cesarean delivery | 55 (19.0) |
| Nonreassuring testing | 39 (13.4) |
| Preterm (includes PPROM) | 24 (8.3) |
| Diabetes | 22 (7.6) |
| Postdates | 21 (7.2) |
| Other | 18 (6.2) |
| Malpresentation | 16 (5.5) |
| Chronic HTN | 8 (2.8) |
| Macrosomia | 7 (2.4) |
| IUFD | 6 (2.1) |
| IUGR | 5 (1.7) |
| Anomalies | 4 (1.4) |
| Total | 290 (25.6% of population) |
| Intrapartum Transfers | |
| First-stage arrest of labor | 37 (34.6) |
| PROM without active labor | 32 (29.9) |
| Malpresentation | 9 (8.4) |
| Fetal distress | 5 (4.7) |
| Nonreassuring tracing | 12 (11.2) |
| Other | 12 (11.2) |
| Total | 107 (9.5% of population) |
| *Greater than 290 because of 18 patients with 2 reasons for antepartum transfer. | |
| HTN denotes hypertension; IUFD, intrauterine fetal demise; IUGR, intrauterine growth restriction; PROM, premature rupture of membranes; PPROM, preterm premature rupture of membranes. | |
Obstetric interventions
The total cesarean delivery rate (7.3%) was approximately one third the nationwide rate of 20.7% in 1996. The primary cesarean delivery rate (number of cesareans in women without prior cesarean divided by the number of women who have never had a cesarean) of 5.3% compares with a nationwide primary rate of 14.6%. The cesarean rate was 22.1% for antepartum transfers and 17.8% for intrapartum transfers. Operative vaginal delivery occurred in 5.4% of births, well below the nationwide rate of 9.4%. The induction rate of 13.8% is lower than the nationwide rate of 16.9%. The oxytocin augmentation rate of 7.7% is well below the nationwide rate of 16.9% in 1996.13 Parenteral narcotics were available at Zuni-Ramah; however, 81.4% of women elected to receive no labor analgesia. Epidural anesthesia was not available at Zuni-Ramah Hospital.
Perinatal mortality
The perinatal mortality rate for the population was 11.4 per 1000 births (95% CI, 5.1-17.8 by Poisson distribution), comparable to the 1991 nationwide peri-natal mortality rate of 12.8/1000.14 Nine of the 13 neonatal deaths were caused by intrauterine fetal demise before labor (Table 2). The Zuni-Ramah Hospital–based perinatal mortality rate of 1.2/1000 was comparable with the 1.3/1000 perinatal mortality rate for women in the National Birth Center study even though Zuni-Ramah Hospital accepts higher-risk patients.15
TABLE 2
PERINATAL MORTALITY IN ZUNI-RAMAH POPULATION
| Age (wk) | Weight (g) | Site | Cause |
|---|---|---|---|
| Intrauterine Fetal Death | |||
| 31 | 1410 | GIMC | Unexplained |
| 39 | 3130 | GIMC | Unexplained |
| 35 | 1540 | GIMC | Unexplained; IUGR |
| 35 | 1690 | GIMC | Unexplained; IUGR |
| 21 | 330 | GIMC | PPROM |
| 21 | 560 | GIMC | PPROM |
| 41 | 3040 | GIMC | Oligohydramnios and post dates. Two days prior, refused induction with amniotic fluid volume index of 3.8 |
| 28 | 1290 | Alb | Abruption |
| 32 | Unknown | Zuni | Necrotizing/calcifying encephalopathy (probable CMV) with severe IUGR |
| Early Neonatal Death (< 7 days) | |||
| 38 | 2805 | Alb | Osteogenesis imperfecta |
| 31 | Unknown | Alb | Potter’s syndrome |
| Late Neonatal Death (7 to 27 days) | |||
| 35 | 1590 | GIMC | Pulmonary interstitial emphysema caused by respiratory failure of unknown etiology/IUGR |
| 41 | 3220 | GIMC | Sepsis at 12 days; had been discharged home as healthy infant |
| Alb denotes Albuquerque tertiary-care hospital; CMV, cytomegalovirus; GIMC, Gallup Indian Medical Center; IUGR, intrauterine growth restriction; PPROM, preterm premature rupture of membranes. | |||
Neonatal morbidity
Measures of neonatal morbidity are summarized in Table 3. The frequency of 5-minute Apgar scores below 7, low birthweight, and prematurity compares favorably with 1996 US rates.13 The rate of assisted ventilation (intubation or bag-mask) for the entire population (4.6%, n = 52) is greater than the 1996 nationwide rate (2.9%), although the difference is of questionable clinical significance, since international studies have demonstrated a range for assisted ventilation of 1% to 10% of hospital births.16 Neonatal Intensive Care Unit (NICU) transfer occurred in 27 (2.4%) of deliveries from non-tertiary-care sites. Thirteen (1.8%) babies born at Zuni-Ramah were transferred to Albuquerque for NICU care because of respiratory distress (n = 10) or neonatal anomalies (n = 3). The 3 cases of low Apgar scores at Zuni-Ramah were attributed to pneumothorax, respiratory distress syndrome of prematurity, and sepsis with meconium aspiration.
TABLE 3
NEONATAL MORBIDITY IN ZUNI-RAMAH POPULATION, BASED ON LIVE BIRTHS
| Zuni-Ramah Hospital (N=732) | Zuni-Ramah Population (n = 1128) | 1996 US Population | |
|---|---|---|---|
| 5-minute Apgar score < 7 | 3 (0.41%), P = .023 | 6 (0.54%), P = .014 | 1.4% |
| Assisted ventilation | 19 (2.6%), P = 0.62 | 52 (4.6%), P < .001 | 2.9% |
| Birthweight < 2500 g | 14 (1.9%), P < .001 | 61 (5.4%), P < .001 | 11% |
| Preterm (37 weeks) | 22 (3.0%), P < .001 | 75 (6.7%), P = .36 | 7.4% |
| P values are based on comparison with the US population. US population figures for 1996 were extracted from Ventura SJ, Martin JA, Curtin SC, Mathews TJ. Report of Final Natality Statistics, 1996. Monthly vital statistics report; vol 46, no 11, supp. Hyattsville, Md: National Center for Health Statistics, 1998. | |||
Obstetric risk factors
The study population had a greater incidence of pregnancy-induced hypertension (14.5% vs 2.6% by 1996 ACOG criteria17), chronic hypertension (2.7% vs 0.7%15), and diabetes (9.2% vs 2.6%15) than the average US obstetric population. Gestational diabetes was diagnosed according to National Diabetes Data Group criteria:18 7.1% had gestational diabetes (class A1 and A2 ) and 2.1% had type 2 antepartum diabetes (classes B and C).
Outcomes of obstetric emergencies at zuni-ramah hospital
We reviewed all cases of placental abruption, uterine inversion, umbilical cord prolapse, and fetal distress at Zuni-Ramah Hospital to identify potentially preventable adverse outcomes caused by lack of operative facilities (Table W1). Umbilical cord prolapse and uterine inversion each occurred once and were appropriately managed, with excellent outcomes. In 3 of the 4 cases of placental abruption, there were clearly no adverse outcomes caused by lack of on-site operative facilities, as patients were expectantly managed upon arrival to the referral hospital (cases 3 and 4) or presented to Zuni-Ramah Hospital as an intrauterine demise (case 5).
The fourth patient with placental abruption (case 6) presented at Zuni-Ramah with vaginal bleeding, severe variable decelerations, and a 10-point drop from baseline hematocrit. She was scheduled to labor at GIMC because of a history of prior cesarean but presented to the Zuni-Ramah emergency room with vaginal bleeding. She was transferred to GIMC for an anticipated cesarean delivery; however, on arrival the patient rapidly progressed and gave birth to an infant vaginally with Apgar scores of 3 and 9. Her infant had a neonatal seizure and magnetic resonance imaging evidence of sagittal sinus thrombosis. The infant had a normal neurologic evaluation, developmental assessment, and electroencephalogram at 15 months.
We reviewed 5 cases of urgent transfer for fetal distress. These were differentiated from the 8 intrapartum transfers for NRFHTs based on the severity of fetal heart monitor tracings. Four of the 5 women who had been transferred for fetal distress gave birth to healthy infants vaginally more than 2 hours after arrival at the referral institution. One patient who was urgently transferred for repetitive late decelerations is discussed below.
Cesarean deliveries for fetal distress at referral hospitals
We reviewed all cases of cesarean deliveries for fetal distress (n = 10) at referral institutions to determine whether outcomes for any of the patients could potentially have been improved by having their cesarean deliveries earlier if operative facilities had been available at Zuni-Ramah Hospital or by being transferred earlier (Table W1). Seven of the 10 patients were transferred for preeclampsia, NRFHTs, or failure to progress. All had their cesareans for fetal distress many hours after arrival at the referral institution.
Two cases of cesarean delivery for fetal distress after transfer because of abruption were previously described. A patient presented in early labor with repetitive late decelerations and was urgently transferred to GIMC, where she underwent an immediate cesarean delivery. Her infant had Apgar scores of 1 and 7, an unremarkable neonatal course, and a normal 15-month developmental screen.
Discussion
Our outcomes demonstrate that with the use of appropriate screening criteria, childbirth can safely occur in institutions that lack surgical suites. The population-based perinatal mortality rate was similar to the nationwide rate. A review of obstetric emergencies and low Apgar scores among the 839 women laboring at Zuni-Ramah Hospital failed to identify adverse outcomes that might have been prevented if the hospital had had operative facilities. Cesarean rates were approximately one third the nationwide rate even though Zuni-Ramah patients had a higher prevalence of such risk factors as diabetes and preeclampsia.
Although they represented a high-risk obstetric population, 65% of women were able to give birth at Zuni-Ramah Hospital through use of the perinatal screening criteria. The 35% rate of transfer was caused largely by the need for oxytocin augmentation or induction. Only 21.6% of the women who were transferred for dysfunctional labor or premature rupture of membranes ultimately had a cesarean delivery. Oxytocin has not been permitted at Zuni-Ramah Hospital because of the ACOG guideline permitting oxytocin use only if “a physician capable of performing a cesarean delivery is readily available.”19 There are no studies addressing the safety of labor induction or augmentation without on-site cesarean capability.
Canadian guidelines for rural maternity care do not prohibit the use of prostaglandins or oxytocin at hospitals without operative facilities. A Consensus Conference on Obstetric Services in Rural or Remote Communities addressed the issue of labor induction or augmentation in hospitals without cesarean capability by stating, “If caring for a woman in labour is appropriate in the community, then caring for her during an augmented/induced labour is equally appropriate when there is support by trained local staff and resources.”20 We concur that use of oxytocin in rural hospital units without operative facilities should be considered under well-defined clinical guidelines or research protocols.
Limitations
Our study’s limitations include lack of long-term neonatal outcomes, small size of the Zuni-Ramah population, an almost exclusively Native American population, and lack of examiner blinding during record review. Transfer rates may be increased in populations with higher rates of cesarean delivery or epidural anesthesia use. Alternatively, the high incidence of preeclampsia, chronic hypertension, and diabetes in these communities may have resulted in a higher proportion of induction. Umbilical cord pro-lapse and significant placental abruption are routinely treated by urgent cesarean delivery; therefore, obstetric literature on outcomes without immediate operative intervention is limited.21,22 A larger study would be required to determine the potential increased neonatal morbidity or mortality resulting from delayed intervention.
Conclusions
The ACOG/AAP guideline requiring on-site surgical facilities and the ability to initiate a cesarean in 30 minutes is not based on evidence. Four small retrospective studies of emergency cesarean deliveries delayed for more than 30 minutes did not demonstrate adverse neonatal outcomes.23-26 In our study population, no adverse outcomes (none in 839 births) were determined to have been caused by a lack of surgical facilities. Despite these excellent outcomes, the possibility always exists for outcomes that can be prevented by doing a rapid emergent cesarean delivery. Women deciding to give birth in facilities without operative capabilities should receive information regarding the risks and benefits of delivering there and should have access to other facilities. Provider discretion and patient choice must be respected to ensure community support of these birthing units. Practitioners at the rural units must have assurance that any patients who require an urgent transfer will be readily accepted.
Rural communities, medical providers, and health care facilities need to consider the overall effect of maintaining local maternity care units, as the loss of rural maternity care can increase the risk of adverse perinatal outcomes.1-3 We concur with the Canadian panel that although maintenance of rural surgical and anesthesia capabilities is desirable, “good outcomes can be sustained within an integrated risk management system without local access to operative delivery.”8 Guidelines should be developed to permit rural hospitals without cesarean capability to provide maternity care as part of integrated perinatal systems with well-developed transport protocols and supportive referral institutions. Women living in rural areas should have the option to give birth near their homes in such units if they so desire.
Acknowledgments
The authors thank Robert Rhyne, MD, for editorial assistance in manuscript preparation and Betty Skipper, PhD, for statistical assistance. Current Zuni-Ramah Obstetric Guidelines are available at http://hsc.unm.edu/fcm/research/zuni.
OBJECTIVES: We analyzed perinatal outcomes at a rural hospital without cesarean delivery capability.
STUDY DESIGN: This was a historical cohort outcomes study.
POPULATION: The study population included all pregnant women at 20 weeks or greater of gestational age (n = 1132) over a 5-year period in a predominantly Native American region of northwestern New Mexico.
OUTCOMES MEASURED: The outcomes studied included perinatal mortality, neonatal morbidity, obstetric emergencies, intrapartum and antepartum transfers, and cesarean delivery rate. We did a detailed case review of all obstetric emergencies and low-Apgar-score births at Zuni-Ramah Hospital and all cesarean deliveries for fetal distress at referral hospitals.
RESULTS: Of the 1132 women in the study population, 64.7% (n = 735) were able to give birth at the hospital without operative facilities; 25.6% (n = 290) were transferred before labor; and 9.5% (n = 107) were transferred during labor. The perinatal mortality rate of 11.4 per 1000 (95% confidence interval, 5.1-17.8) was similar to the nationwide rate of 12.8 per 1000 even though Zuni-Ramah has a high-risk obstetric population. No instances of major neonatal or maternal morbidity caused by lack of surgical facilities occurred. The cesarean delivery rate of 7.3% was significantly lower than the nationwide rate of 20.7% (P < .001). The incidence of neonates with low Apgar scores (0.54%) was significantly lower than the nationwide rate (1.4%). The incidence of neonates requiring resuscitation (3.4%) was comparable to the nationwide rate (2.9%).
CONCLUSIONS: The presence of a rural maternity care unit without surgical facilities can safely allow a high proportion of women to give birth closer to their communities. This study demonstrated a low level of perinatal risk. Most transfers were made for induction or augmentation of labor. Rural hospitals that do not have cesarean delivery capability but are part of an integrated perinatal system can safely offer obstetric services by using appropriate antepartum and intrapartum screening criteria for obstetric risk.
- Rural hospitals without cesarean delivery capability can safely offer obstetric care to selected patients as part of an integrated perinatal network.
- Measures of maternal and neonatal morbidity and mortality were at or below national averages despite a higher-risk population.
- Antepartum (25.6%) or intrapartum (9.5%) transfer to hospitals with surgical or tertiary-care facilities was required for 35% of pregnant women.
- The use of oxytocin induction or augmentation, if determined safe, may significantly lower the transfer rate from rural hospitals that lack cesarean delivery capability.
The availability of perinatal care in rural communities produces better pregnancy outcomes than do perinatal systems that require rural women to seek maternity care in distant urban areas.1-3 Unfortunately, rural maternity care has been affected by the loss of physicians who offer these services and by the closing of many rural hospitals’ maternity care units. Maintaining 24-hour operative obstetric capabilities is difficult in rural areas because they have an insufficient population base to support a physician trained in operative obstetrics. Another barrier is the lack of anesthesia services and operating room personnel.
The Guidelines for Perinatal Care developed by the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) state, “All hospitals offering labor and delivery services should be equipped to perform emergency cesarean delivery.” 4 Nevertheless, not all rural obstetric units can offer cesarean delivery and must transfer patients to a referral hospital for operative needs. Advisory panels in the United States and Canada have recommended similar models of rural perinatal care.5-8 A Canadian panel estimated that 125 Canadian hospitals offer obstetric care without surgical facilities.
Studies of rural hospitals in Canada, Australia, and the United Kingdom that lack continuous on-site cesarean capability are limited by the small number of deliveries.9-12 Most such studies are hospital based rather than population based and lack data on women who are transferred to outlying hospitals. The only population-based study that we identified found no evidence of adverse events caused by the lack of cesarean facilities; the sample size, however, was limited to 286 births.9
We studied all pregnancies occurring in a predominantly Native American region of New Mexico over a 5-year period to ascertain the safety of rural perinatal care based in a hospital without cesarean capability. Population-based and hospital-based outcomes are presented. This is the first study from a US community using this model of care.
Methods
We conducted an outcomes study using a historical cohort study design of all pregnancies beyond 20 weeks of gestation in the Zuni Pueblo and Ramah Navajo communities of northwestern New Mexico from 1992 to 1996. The perinatal services based at the Zuni-Ramah Indian Health Service (IHS) Hospital are the focus of this study. This 37-bed community hospital, staffed by family physicians and a part-time nurse-midwife, is part of an integrated perinatal system. The birthing unit has access to obstetrician-gynecologist (OBG) consultants at the Gallup Indian Medical Center (GIMC), 33 miles to the north, and perinatology and neonatology care in Albuquerque, 147 miles to the east. GIMC, the primary referral hospital and closest surgical facility, has an obstetric unit staffed by OBGs, family physicians, and nurse-mid-wives. Transportation time is 40 minutes by ground ambulance to GIMC or by fixed wing aircraft to Albuquerque.
The Zuni-Ramah Hospital limits intrapartum care to women designated as at low or moderate risk by criteria established by Zuni-Ramah family physicians and reviewed by GIMC OBGs. Criteria mandating transfer included prior cesarean, malpresentation, multiple gestation, intrauterine growth restriction, severe preeclampsia, placenta previa, significant vaginal bleeding, major fetal anomalies, anticipated preterm delivery (< 36 weeks), nonreassuring fetal heart tones (NRFHTs), and need for labor induction or augmentation with oxytocin. Women with gestational or type 2 diabetes who were well controlled could give birth at Zuni-Ramah unless they had end-organ damage or the fetus had known macrosomia. Physicians successfully completed the Advanced Life Support in Obstetrics (ALSO, ®American Academy of Family Physicians, 4th ed., 2000) course, attended weekly high-risk obstetric rounds, and performed quarterly reviews of obstetric complications. The family physicians performed vacuum-assisted deliveries, utilized amnioinfusion, and used continuous or intermittent fetal monitoring.
A review of the delivery and transfer records of the Zuni-Ramah Hospital and GIMC obstetric services revealed that there had been 1132 births of 1137 infants during the study period. The authors used a data collection form to review prenatal and newborn records from every birth. We reviewed intrapartum records for all births at the Zuni-Ramah and GIMC hospitals. We obtained discharge summaries from tertiary-care sites. We interviewed perinatal coordinators, public health nurses, and pediatric care providers to obtain information about patients who had received perinatal care outside of the IHS system.
The outcomes measured included perinatal mortality, neonatal morbidity, obstetric emergencies, intrapartum and antepartum transfers, and cesarean delivery rate. All obstetric emergencies originating at Zuni-Ramah Hospital were reviewed to determine whether the lack of surgical facilities had resulted in adverse outcomes. The physician’s notes were used to differentiate a NRFHT pattern requiring observation at a hospital with operative facilities from a truly worrisome pattern that required urgent intervention for fetal distress.
Births were defined as deliveries of infants at 20 weeks or more of estimated gestational age. We analyzed each birth in a multiple gestation individually. The population-based perinatal mortality rate was calculated from 20 weeks’ estimated gestational age to the 28th neonatal day. The Zuni-Ramah Hospital perinatal mortality rate was calculated by inclusion of all women delivered at Zuni-Ramah Hospital or transferred during labor. Approval for the study was obtained from the IHS Institutional Review Board and the Zuni Tribal Council.
Results
Study population
We identified 1137 births to 945 women between 1992 and 1996. Zuni and Navajo births were 66.9% and 30.8%, respectively; 30% of women were primiparous and 70%, multiparous. We found that 10.4% of births had occurred in women older than 35 years and 7.8% in women younger than 18 years. Regarding prenatal care, 3.9% of women had received none; 43.0% had established prenatal care in the first trimester; 40.4%, in the second trimester; and 12.8%, in the third trimester.
Delivery sites and maternal transfers
The majority of women (64.4%, n = 732) gave birth at the Zuni-Ramah Hospital (Figure) or at GIMC (29.6%, n = 337). A small number (2.2%, n = 25) gave birth at a private hospital with surgical facilities in Gallup. Albuquerque tertiary-care hospitals were the sites of 3.2% (n = 36) of deliveries. Primary indications for tertiary care were prematurity and fetal anomalies. Seven (0.6%) deliveries occurred at other sites, including home and ambulance.
The antepartum transfers (Table 1) were required primarily for pregnancy complications requiring labor induction. Preeclampsia, diabetes, nonreassuring antepartum testing, and post dates patients accounted for 56.8% of the 290 transfers. The 107 intrapartum transfers were made predominantly for labor induction or augmentation (64.5%, n = 69), a concerning fetal heart tracing (15.9%, n = 17), or fetal malpresentation diagnosed during labor (8.4%, n = 9).
FIGURE
PREGNANCIES AT ZUNI-RAMAH HOSPITAL
TABLE 1
ANTEPARTUM AND INTRAPARTUM TRANSFERS FROM ZUNI-RAMAH HOSPITAL
| Indication | Number of Transfers (%) |
|---|---|
| Antepartum Transfers* | |
| Preeclampsia | 83 (28.6) |
| Prior cesarean delivery | 55 (19.0) |
| Nonreassuring testing | 39 (13.4) |
| Preterm (includes PPROM) | 24 (8.3) |
| Diabetes | 22 (7.6) |
| Postdates | 21 (7.2) |
| Other | 18 (6.2) |
| Malpresentation | 16 (5.5) |
| Chronic HTN | 8 (2.8) |
| Macrosomia | 7 (2.4) |
| IUFD | 6 (2.1) |
| IUGR | 5 (1.7) |
| Anomalies | 4 (1.4) |
| Total | 290 (25.6% of population) |
| Intrapartum Transfers | |
| First-stage arrest of labor | 37 (34.6) |
| PROM without active labor | 32 (29.9) |
| Malpresentation | 9 (8.4) |
| Fetal distress | 5 (4.7) |
| Nonreassuring tracing | 12 (11.2) |
| Other | 12 (11.2) |
| Total | 107 (9.5% of population) |
| *Greater than 290 because of 18 patients with 2 reasons for antepartum transfer. | |
| HTN denotes hypertension; IUFD, intrauterine fetal demise; IUGR, intrauterine growth restriction; PROM, premature rupture of membranes; PPROM, preterm premature rupture of membranes. | |
Obstetric interventions
The total cesarean delivery rate (7.3%) was approximately one third the nationwide rate of 20.7% in 1996. The primary cesarean delivery rate (number of cesareans in women without prior cesarean divided by the number of women who have never had a cesarean) of 5.3% compares with a nationwide primary rate of 14.6%. The cesarean rate was 22.1% for antepartum transfers and 17.8% for intrapartum transfers. Operative vaginal delivery occurred in 5.4% of births, well below the nationwide rate of 9.4%. The induction rate of 13.8% is lower than the nationwide rate of 16.9%. The oxytocin augmentation rate of 7.7% is well below the nationwide rate of 16.9% in 1996.13 Parenteral narcotics were available at Zuni-Ramah; however, 81.4% of women elected to receive no labor analgesia. Epidural anesthesia was not available at Zuni-Ramah Hospital.
Perinatal mortality
The perinatal mortality rate for the population was 11.4 per 1000 births (95% CI, 5.1-17.8 by Poisson distribution), comparable to the 1991 nationwide peri-natal mortality rate of 12.8/1000.14 Nine of the 13 neonatal deaths were caused by intrauterine fetal demise before labor (Table 2). The Zuni-Ramah Hospital–based perinatal mortality rate of 1.2/1000 was comparable with the 1.3/1000 perinatal mortality rate for women in the National Birth Center study even though Zuni-Ramah Hospital accepts higher-risk patients.15
TABLE 2
PERINATAL MORTALITY IN ZUNI-RAMAH POPULATION
| Age (wk) | Weight (g) | Site | Cause |
|---|---|---|---|
| Intrauterine Fetal Death | |||
| 31 | 1410 | GIMC | Unexplained |
| 39 | 3130 | GIMC | Unexplained |
| 35 | 1540 | GIMC | Unexplained; IUGR |
| 35 | 1690 | GIMC | Unexplained; IUGR |
| 21 | 330 | GIMC | PPROM |
| 21 | 560 | GIMC | PPROM |
| 41 | 3040 | GIMC | Oligohydramnios and post dates. Two days prior, refused induction with amniotic fluid volume index of 3.8 |
| 28 | 1290 | Alb | Abruption |
| 32 | Unknown | Zuni | Necrotizing/calcifying encephalopathy (probable CMV) with severe IUGR |
| Early Neonatal Death (< 7 days) | |||
| 38 | 2805 | Alb | Osteogenesis imperfecta |
| 31 | Unknown | Alb | Potter’s syndrome |
| Late Neonatal Death (7 to 27 days) | |||
| 35 | 1590 | GIMC | Pulmonary interstitial emphysema caused by respiratory failure of unknown etiology/IUGR |
| 41 | 3220 | GIMC | Sepsis at 12 days; had been discharged home as healthy infant |
| Alb denotes Albuquerque tertiary-care hospital; CMV, cytomegalovirus; GIMC, Gallup Indian Medical Center; IUGR, intrauterine growth restriction; PPROM, preterm premature rupture of membranes. | |||
Neonatal morbidity
Measures of neonatal morbidity are summarized in Table 3. The frequency of 5-minute Apgar scores below 7, low birthweight, and prematurity compares favorably with 1996 US rates.13 The rate of assisted ventilation (intubation or bag-mask) for the entire population (4.6%, n = 52) is greater than the 1996 nationwide rate (2.9%), although the difference is of questionable clinical significance, since international studies have demonstrated a range for assisted ventilation of 1% to 10% of hospital births.16 Neonatal Intensive Care Unit (NICU) transfer occurred in 27 (2.4%) of deliveries from non-tertiary-care sites. Thirteen (1.8%) babies born at Zuni-Ramah were transferred to Albuquerque for NICU care because of respiratory distress (n = 10) or neonatal anomalies (n = 3). The 3 cases of low Apgar scores at Zuni-Ramah were attributed to pneumothorax, respiratory distress syndrome of prematurity, and sepsis with meconium aspiration.
TABLE 3
NEONATAL MORBIDITY IN ZUNI-RAMAH POPULATION, BASED ON LIVE BIRTHS
| Zuni-Ramah Hospital (N=732) | Zuni-Ramah Population (n = 1128) | 1996 US Population | |
|---|---|---|---|
| 5-minute Apgar score < 7 | 3 (0.41%), P = .023 | 6 (0.54%), P = .014 | 1.4% |
| Assisted ventilation | 19 (2.6%), P = 0.62 | 52 (4.6%), P < .001 | 2.9% |
| Birthweight < 2500 g | 14 (1.9%), P < .001 | 61 (5.4%), P < .001 | 11% |
| Preterm (37 weeks) | 22 (3.0%), P < .001 | 75 (6.7%), P = .36 | 7.4% |
| P values are based on comparison with the US population. US population figures for 1996 were extracted from Ventura SJ, Martin JA, Curtin SC, Mathews TJ. Report of Final Natality Statistics, 1996. Monthly vital statistics report; vol 46, no 11, supp. Hyattsville, Md: National Center for Health Statistics, 1998. | |||
Obstetric risk factors
The study population had a greater incidence of pregnancy-induced hypertension (14.5% vs 2.6% by 1996 ACOG criteria17), chronic hypertension (2.7% vs 0.7%15), and diabetes (9.2% vs 2.6%15) than the average US obstetric population. Gestational diabetes was diagnosed according to National Diabetes Data Group criteria:18 7.1% had gestational diabetes (class A1 and A2 ) and 2.1% had type 2 antepartum diabetes (classes B and C).
Outcomes of obstetric emergencies at zuni-ramah hospital
We reviewed all cases of placental abruption, uterine inversion, umbilical cord prolapse, and fetal distress at Zuni-Ramah Hospital to identify potentially preventable adverse outcomes caused by lack of operative facilities (Table W1). Umbilical cord prolapse and uterine inversion each occurred once and were appropriately managed, with excellent outcomes. In 3 of the 4 cases of placental abruption, there were clearly no adverse outcomes caused by lack of on-site operative facilities, as patients were expectantly managed upon arrival to the referral hospital (cases 3 and 4) or presented to Zuni-Ramah Hospital as an intrauterine demise (case 5).
The fourth patient with placental abruption (case 6) presented at Zuni-Ramah with vaginal bleeding, severe variable decelerations, and a 10-point drop from baseline hematocrit. She was scheduled to labor at GIMC because of a history of prior cesarean but presented to the Zuni-Ramah emergency room with vaginal bleeding. She was transferred to GIMC for an anticipated cesarean delivery; however, on arrival the patient rapidly progressed and gave birth to an infant vaginally with Apgar scores of 3 and 9. Her infant had a neonatal seizure and magnetic resonance imaging evidence of sagittal sinus thrombosis. The infant had a normal neurologic evaluation, developmental assessment, and electroencephalogram at 15 months.
We reviewed 5 cases of urgent transfer for fetal distress. These were differentiated from the 8 intrapartum transfers for NRFHTs based on the severity of fetal heart monitor tracings. Four of the 5 women who had been transferred for fetal distress gave birth to healthy infants vaginally more than 2 hours after arrival at the referral institution. One patient who was urgently transferred for repetitive late decelerations is discussed below.
Cesarean deliveries for fetal distress at referral hospitals
We reviewed all cases of cesarean deliveries for fetal distress (n = 10) at referral institutions to determine whether outcomes for any of the patients could potentially have been improved by having their cesarean deliveries earlier if operative facilities had been available at Zuni-Ramah Hospital or by being transferred earlier (Table W1). Seven of the 10 patients were transferred for preeclampsia, NRFHTs, or failure to progress. All had their cesareans for fetal distress many hours after arrival at the referral institution.
Two cases of cesarean delivery for fetal distress after transfer because of abruption were previously described. A patient presented in early labor with repetitive late decelerations and was urgently transferred to GIMC, where she underwent an immediate cesarean delivery. Her infant had Apgar scores of 1 and 7, an unremarkable neonatal course, and a normal 15-month developmental screen.
Discussion
Our outcomes demonstrate that with the use of appropriate screening criteria, childbirth can safely occur in institutions that lack surgical suites. The population-based perinatal mortality rate was similar to the nationwide rate. A review of obstetric emergencies and low Apgar scores among the 839 women laboring at Zuni-Ramah Hospital failed to identify adverse outcomes that might have been prevented if the hospital had had operative facilities. Cesarean rates were approximately one third the nationwide rate even though Zuni-Ramah patients had a higher prevalence of such risk factors as diabetes and preeclampsia.
Although they represented a high-risk obstetric population, 65% of women were able to give birth at Zuni-Ramah Hospital through use of the perinatal screening criteria. The 35% rate of transfer was caused largely by the need for oxytocin augmentation or induction. Only 21.6% of the women who were transferred for dysfunctional labor or premature rupture of membranes ultimately had a cesarean delivery. Oxytocin has not been permitted at Zuni-Ramah Hospital because of the ACOG guideline permitting oxytocin use only if “a physician capable of performing a cesarean delivery is readily available.”19 There are no studies addressing the safety of labor induction or augmentation without on-site cesarean capability.
Canadian guidelines for rural maternity care do not prohibit the use of prostaglandins or oxytocin at hospitals without operative facilities. A Consensus Conference on Obstetric Services in Rural or Remote Communities addressed the issue of labor induction or augmentation in hospitals without cesarean capability by stating, “If caring for a woman in labour is appropriate in the community, then caring for her during an augmented/induced labour is equally appropriate when there is support by trained local staff and resources.”20 We concur that use of oxytocin in rural hospital units without operative facilities should be considered under well-defined clinical guidelines or research protocols.
Limitations
Our study’s limitations include lack of long-term neonatal outcomes, small size of the Zuni-Ramah population, an almost exclusively Native American population, and lack of examiner blinding during record review. Transfer rates may be increased in populations with higher rates of cesarean delivery or epidural anesthesia use. Alternatively, the high incidence of preeclampsia, chronic hypertension, and diabetes in these communities may have resulted in a higher proportion of induction. Umbilical cord pro-lapse and significant placental abruption are routinely treated by urgent cesarean delivery; therefore, obstetric literature on outcomes without immediate operative intervention is limited.21,22 A larger study would be required to determine the potential increased neonatal morbidity or mortality resulting from delayed intervention.
Conclusions
The ACOG/AAP guideline requiring on-site surgical facilities and the ability to initiate a cesarean in 30 minutes is not based on evidence. Four small retrospective studies of emergency cesarean deliveries delayed for more than 30 minutes did not demonstrate adverse neonatal outcomes.23-26 In our study population, no adverse outcomes (none in 839 births) were determined to have been caused by a lack of surgical facilities. Despite these excellent outcomes, the possibility always exists for outcomes that can be prevented by doing a rapid emergent cesarean delivery. Women deciding to give birth in facilities without operative capabilities should receive information regarding the risks and benefits of delivering there and should have access to other facilities. Provider discretion and patient choice must be respected to ensure community support of these birthing units. Practitioners at the rural units must have assurance that any patients who require an urgent transfer will be readily accepted.
Rural communities, medical providers, and health care facilities need to consider the overall effect of maintaining local maternity care units, as the loss of rural maternity care can increase the risk of adverse perinatal outcomes.1-3 We concur with the Canadian panel that although maintenance of rural surgical and anesthesia capabilities is desirable, “good outcomes can be sustained within an integrated risk management system without local access to operative delivery.”8 Guidelines should be developed to permit rural hospitals without cesarean capability to provide maternity care as part of integrated perinatal systems with well-developed transport protocols and supportive referral institutions. Women living in rural areas should have the option to give birth near their homes in such units if they so desire.
Acknowledgments
The authors thank Robert Rhyne, MD, for editorial assistance in manuscript preparation and Betty Skipper, PhD, for statistical assistance. Current Zuni-Ramah Obstetric Guidelines are available at http://hsc.unm.edu/fcm/research/zuni.
1. Allen DT, Kamradt MS. Relationship of infant mortality to the availability of obstetric care in Indiana. J Fam Pract 1991;33:609-13.
2. Larimore WL, Davis A. Relation of infant mortality to the availability of maternity care in rural Florida. J Am Board Fam Pract 1995;8:392-9.
3. Nesbitt TS, Connell FA, Hart LG, Rosenblatt RA. Access to obstetric care in rural areas: effect on birth outcomes Am J Public Health. 1990;80:814-8.
4. American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 4th ed. Washington, DC: ACOG, 1997.
5. New York State Department of Health, Office of Rural Health. Report on the provision of birthing services in rural health networks. Albany, NY; 1994.
6. Nesbitt TS. Rural maternity care: new models of access. Birth. 1996;23:161-5.
7. Rosenthal TC, Holden DM, Woodward W. Primary care obstetrics in rural Western New York: a multi-center case review. NY State J Med 1990;90:537-40.
8. Iglesias S, Grzybowski S, Klein M, Gagne GP, Lalonde A. Joint position paper on rural maternity care. Society of Rural Physicians, Society of Obstetricians and Gynecologists of Canada, College of Family Physicians of Canada, 1998.
9. Grzybowski SCW, Cadesky AS, Hogg WE. Rural obstetrics: a 5-year prospective study of the outcomes of all pregnancies in a remote northern community. Can Med Assoc J 1991;144:987-94.
10. Black DB, Fyfe IM. The safety of obstetrics services in small communities in northern Ontario. Can Med Assoc J 1984;130:571-6.
11. Woollard LA, Hays RB. Rural obstetrics in NSW. Aust N Z J Obstet Gynaecol 1993;33:240-2.
12. McIlwain R, Smith S. Obstetrics in a small isolated community: the cesarean section dilemma. Can J Rural Med 2000;5:221-3.
13. Ventura SJ, Martin JA, Curtin SC, Mathews TJ. Report of Final Natality Statistics, 1996. Monthly vital statistics report; Vol 46 no 11, suppl. Hyattsville, Md: National Center for Health Statistics, 1998.
14. Hoyert DL. Perinatal mortality in the United States, 1985-91. National Center for Health Statistics. Vital Health Stat 20(26), 1995.
15. Rooks JP, Weatherby NL, Ernst EKM, Stapleton S, Rosen D, Rosenfield A. Outcomes of care in birth centers: the National Birth Center Study. N Engl J Med 1989;321:1804-11.
16. International guidelines for neonatal resuscitation: An excerpt from the guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2000;106(3):Available from: http://www.pediatrics.org/cgi/content/full/106/3/e29.
17. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. ACOG Technical Bulletin 219. Washington, DC: ACOG, 1996.
18. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57.
19. American College of Obstetricians and Gynecologists. Induction of labor. ACOG Practice Bulletin 10. Washington, DC: ACOG; 1999.
20. Torr E, ed, for the British Columbia Reproductive Care Program. Report on the findings of the Consensus Conference on Obstetric Services in Rural or Remote Communities. Can J Rural Med 2000;5:211-7.
21. Barrett J. Funic reduction for the management of umbilical cord prolapse. Am J Obstet Gynecol 1991;165:654-7.
22. Knab DR. Abruptio placentae: an assessment of the time and method of delivery. Obstet Gynecol 1978;52:625-9.
23. Chauhan SP, Roach H, Naef RW, Magann EF, Morrison JC, Martin JN. Cesarean section for suspected fetal distress: Does the decision-incision time make a difference? J Reprod Med 1997;42:347-52.
24. MacKenzie IZ, Cooke I. Prospective 12 month study of 30 minute decision to delivery intervals for “emergency” caesarean section. BMJ 2001;322:1334-5.
25. Schauberger CW, Rooney BL, Beguin EA, Schaper AM, Spindler J. Evaluating the thirty minute interval in emergency cesarean sections. J Am Coll Surg 1994;179:151-5.
26. Tuffnell DJ, Wilkinson K, Beresford N. Interval between decision and delivery by caesarean section: Are current standards achievable? BMJ 2001;322:1330-3.
1. Allen DT, Kamradt MS. Relationship of infant mortality to the availability of obstetric care in Indiana. J Fam Pract 1991;33:609-13.
2. Larimore WL, Davis A. Relation of infant mortality to the availability of maternity care in rural Florida. J Am Board Fam Pract 1995;8:392-9.
3. Nesbitt TS, Connell FA, Hart LG, Rosenblatt RA. Access to obstetric care in rural areas: effect on birth outcomes Am J Public Health. 1990;80:814-8.
4. American Academy of Pediatrics and American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 4th ed. Washington, DC: ACOG, 1997.
5. New York State Department of Health, Office of Rural Health. Report on the provision of birthing services in rural health networks. Albany, NY; 1994.
6. Nesbitt TS. Rural maternity care: new models of access. Birth. 1996;23:161-5.
7. Rosenthal TC, Holden DM, Woodward W. Primary care obstetrics in rural Western New York: a multi-center case review. NY State J Med 1990;90:537-40.
8. Iglesias S, Grzybowski S, Klein M, Gagne GP, Lalonde A. Joint position paper on rural maternity care. Society of Rural Physicians, Society of Obstetricians and Gynecologists of Canada, College of Family Physicians of Canada, 1998.
9. Grzybowski SCW, Cadesky AS, Hogg WE. Rural obstetrics: a 5-year prospective study of the outcomes of all pregnancies in a remote northern community. Can Med Assoc J 1991;144:987-94.
10. Black DB, Fyfe IM. The safety of obstetrics services in small communities in northern Ontario. Can Med Assoc J 1984;130:571-6.
11. Woollard LA, Hays RB. Rural obstetrics in NSW. Aust N Z J Obstet Gynaecol 1993;33:240-2.
12. McIlwain R, Smith S. Obstetrics in a small isolated community: the cesarean section dilemma. Can J Rural Med 2000;5:221-3.
13. Ventura SJ, Martin JA, Curtin SC, Mathews TJ. Report of Final Natality Statistics, 1996. Monthly vital statistics report; Vol 46 no 11, suppl. Hyattsville, Md: National Center for Health Statistics, 1998.
14. Hoyert DL. Perinatal mortality in the United States, 1985-91. National Center for Health Statistics. Vital Health Stat 20(26), 1995.
15. Rooks JP, Weatherby NL, Ernst EKM, Stapleton S, Rosen D, Rosenfield A. Outcomes of care in birth centers: the National Birth Center Study. N Engl J Med 1989;321:1804-11.
16. International guidelines for neonatal resuscitation: An excerpt from the guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2000;106(3):Available from: http://www.pediatrics.org/cgi/content/full/106/3/e29.
17. American College of Obstetricians and Gynecologists. Hypertension in pregnancy. ACOG Technical Bulletin 219. Washington, DC: ACOG, 1996.
18. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57.
19. American College of Obstetricians and Gynecologists. Induction of labor. ACOG Practice Bulletin 10. Washington, DC: ACOG; 1999.
20. Torr E, ed, for the British Columbia Reproductive Care Program. Report on the findings of the Consensus Conference on Obstetric Services in Rural or Remote Communities. Can J Rural Med 2000;5:211-7.
21. Barrett J. Funic reduction for the management of umbilical cord prolapse. Am J Obstet Gynecol 1991;165:654-7.
22. Knab DR. Abruptio placentae: an assessment of the time and method of delivery. Obstet Gynecol 1978;52:625-9.
23. Chauhan SP, Roach H, Naef RW, Magann EF, Morrison JC, Martin JN. Cesarean section for suspected fetal distress: Does the decision-incision time make a difference? J Reprod Med 1997;42:347-52.
24. MacKenzie IZ, Cooke I. Prospective 12 month study of 30 minute decision to delivery intervals for “emergency” caesarean section. BMJ 2001;322:1334-5.
25. Schauberger CW, Rooney BL, Beguin EA, Schaper AM, Spindler J. Evaluating the thirty minute interval in emergency cesarean sections. J Am Coll Surg 1994;179:151-5.
26. Tuffnell DJ, Wilkinson K, Beresford N. Interval between decision and delivery by caesarean section: Are current standards achievable? BMJ 2001;322:1330-3.