User login
Assessment of IV Edaravone Use in the Management of Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
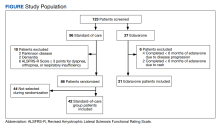
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
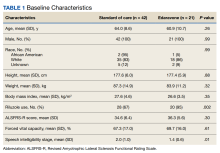
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
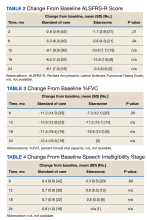
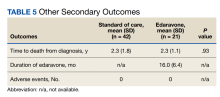
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disorder that results in progressive deterioration of motor neurons in the ventral horn of the spinal cord, which results in loss of voluntary muscle movements.1 Eventually, typical daily tasks become difficult to perform, and as the disease progresses, the ability to eat and breathe is impaired.2 Reports from 2015 show the annual incidence of ALS is 5 cases per 100,000 people, with the total number of cases reported at more than 16,000 in the United States.3 In clinical practice, disease progression is routinely assessed by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R). Typical decline is 1 point per month.4
Unfortunately, at this time, ALS care focuses on symptom management, including prevention of weight loss; implementation of communication strategies; and management of pain, constipation, excess secretions, cramping, and breathing. Despite copious research into treatment options, few exist. Riluzole is an oral medication administered twice daily and has been on the market since 1995.5-7 Efficacy was demonstrated in a study showing statistically significant survival at 12 months compared with controls (74% vs 58%, respectively; P = .014).6 Since its approval, riluzole has become part of standard-of-care ALS management.
In 2017, the US Food and Drug Administration (FDA) approved edaravone, an IV medication that was found to slow the progression of ALS in some patients.8-12 Oxidative stress caused by free radicals is hypothesized to increase the progression of ALS by motor neuron degradation.13 Edaravone works as a free radical and peroxynitrite scavenger and has been shown to eliminate lipid peroxides and hydroxyl radicals known to damage endothelial and neuronal cells.12
Given the mechanism of action of edaravone, it seemed to be a promising option to slow the progression of ALS. A 2019 systematic review analyzed 3 randomized studies with 367 patients and found a statistically significant difference in change in ALSFRS-R scores between patients treated with edaravone for 24 weeks compared with patients treated with the placebo (mean difference, 1.63; 95% CI, 0.26-3.00; P = .02).12 Secondary endpoints evaluated included percent forced vital capacity (%FVC), grip strength, and pinch strength: All showing no significant difference when comparing IV edaravone with placebo.
A 2022 postmarketing study of 324 patients with ALS evaluated the safety and efficacy of long-term edaravone treatment. IV edaravone therapy for > 24 weeks was well tolerated, although it was not associated with any disease-modifying benefit when comparing ALSFRS-R scores with patients not receiving edaravone over a median 13.9 months (ALSFRS-R points/month, -0.91 vs -0.85; P = .37).13 A third ALS treatment medication, sodium phenylbutyrate/taurursodiol was approved in 2022 but not available during our study period and not included here.14,15
Studies have shown an increased incidence of ALS in the veteran population. Veterans serving in the Gulf War were nearly twice as likely to develop ALS as those not serving in the Gulf.16 However, existing literature regarding the effectiveness of edaravone does not specifically examine the effect on this unique population. The objective of this study was to assess the effect of IV edaravone on ALS progression in veterans compared with veterans who received standard of care.
Methods
This study was conducted at a large, academic US Department of Veterans Affairs (VA) medical center. Patients with ALS are followed by a multidisciplinary clinic composed of a neurologist, pulmonologist, clinical pharmacist, social worker, speech therapist, physical therapist, occupational therapist, dietician, clinical psychologist, wheelchair clinic representative, and benefits representative. Patients are typically seen for a half-day appointment about every 3 months. During these visits, a comprehensive review of disease progression is performed. This review entails completion of the ALSFRS-R, physical examination, and pulmonary function testing. Speech intelligibility stage (SIS) is assessed by a speech therapist as well. SIS is scored from 1 (no detectable speech disorder) to 5 (no functional speech). All patients followed in this multidisciplinary ALS clinic receive standard-of-care treatment. This includes the discussion of treatment options that if appropriate are provided to help manage a wide range of complications associated with this disease (eg, pain, cramping, constipation, excessive secretions, weight loss, dysphagia). As a part of these personal discussions, treatment with riluzole is also offered as a standard-of-care pharmacologic option.
Study Design
This retrospective case-control study was conducted using electronic health record data to compare ALS progression in patients on IV edaravone therapy with standard of care. The Indiana University/Purdue University, Indianapolis Institutional Review Board and the VA Research and Development Committee approved the study. The control cohort received the standard of care. Patients in the case cohort received standard of care and edaravone 60 mg infusions daily for an initial cycle of 14 days on treatment, followed by 14 days off. All subsequent cycles were 10 of 14 days on treatment followed by 14 days off. The initial 2 doses were administered in the outpatient infusion clinic to monitor for a hypersensitivity reaction. Patients then had a peripherally inserted central catheter line placed and received doses on days 3 through 14 at home. A port was placed for subsequent cycles, which were also completed at home. Appropriateness of edaravone therapy was assessed by the neurologist at each follow-up appointment. Therapy was then discontinued if warranted based on disease progression or patient preference.
Study Population
Patients included were aged 18 to 75 years with diagnosed ALS. Patients with complications that might influence evaluation of medication efficacy (eg, Parkinson disease, schizophrenia, significant dementia, other major medical morbidity) were excluded. Patients were also excluded if they were on continuous bilevel positive airway pressure and/or had a total score of ≤ 3 points on ALSFRS-R items for dyspnea, orthopnea, or respiratory insufficiency. Due to our small sample size, patients were excluded if treatment was < 6 months, which is the gold standard of therapy duration established by clinical trials.9,11,12
The standard-of-care cohort included patients enrolled in the multidisciplinary clinic September 1, 2014 to August 31, 2017. These patients were compared in a 2:1 ratio with patients who received IV edaravone. The edaravone cohort included patients who initiated treatment with IV edaravone between September 1, 2017, and August 31, 2020. This date range prior to the approval of edaravone was chosen to compare patients at similar stages of disease progression and to have the largest sample size possible.
Data Collection
Data were obtained for eligible patients using the VA Computerized Patient Record System. Demographic data gathered for each patient included age, sex, weight, height, body mass index (BMI), race, and riluzole use.
The primary endpoint was the change in ALSFRS-R score after 6 months of IV edaravone compared with standard-of-care ALS management. Secondary outcomes included change in ALSFRS-R scores 3, 12, 18, and 24 months after therapy initiation, change in %FVC and SIS 3, 6, 12, 18, and 24 months after therapy initiation, duration of edaravone completed (months), time to death (months), and adverse events.
Statistical Analysis
Comparisons between the edaravone and control groups for differences in patient characteristics were made using χ2 and 2-sample t tests for categorical and continuous variables, respectively. Comparisons between the 2 groups for differences in study outcomes (ALSFRS-R scores, %FVC, SIS) at each time point were evaluated using 2-sample t tests. Adverse events and adverse drug reactions were compared between groups using χ2 tests. Statistical significance was set at 0.05.
We estimated that a sample size of 21 subjects in the edaravone (case) group and 42 in the standard-of-care (control) group would be needed to achieve 80% power to detect a difference of 6.5 between the 2 groups for the change in ALSFRS-R scores. This 80% power was calculated based on a 2-sample t test, and assuming a 2-sided 5% significance level and a within-group SD of 8.5.9 Statistical analysis was conducted using Microsoft Excel.
Results
Of the 96 patients, 10 met exclusion criteria. From the remaining 86, 42 were randomly selected for the standard-of-care group. A total of 27 patients seen in multidisciplinary ALS clinic between September 1, 2017, and August 31, 2020, received at least 1 dose of IV edaravone. Of the 27 edaravone patients, 6 were excluded for not completing a total of 6 months of edaravone. Two of the 6 excluded developed a rash, which resolved within 1 week after discontinuing edaravone. The other 4 discontinued edaravone before 6 months because of disease progression.
Baseline Characteristics
Efficacy
Discussion
This 24-month, case-control retrospective study assessed efficacy and safety of IV edaravone for the management of ALS. Although the landmark edaravone study showed slowed progression of ALS at 6 and 12 months, the effectiveness of edaravone outside the clinical trial setting has been less compelling.9-11,13 A later study showed no difference in change in ALSFRS-R score at 6 months compared with that of the placebo group.7 In our study, no statistically significant difference was found for change in ALSFRS-R scores at 6 months.
Our study was unique given we evaluated a veteran population. The link between the military and ALS is largely unknown, although studies have shown increased incidence of ALS in people with a military history compared with that of the general population.16-18 Our study was also unique because it was single-centered in design and allowed for outcome assessments, including ALSFRS-R scores, SIS, and %FVC measurements, to all be conducted by the same practitioner to limit variability. Unfortunately, our sample size resulted in a cohort that was underpowered at 12, 18, and 24 months. In addition, there was a lack of data on chart review for SIS and %FVC measurements at 24 months. As ALS progresses toward end stage, SIS and %FVC measurements can become difficult and burdensome on the patient to obtain, and the ALS multidisciplinary team may decide not to gather these data points as ALS progresses. As a result, change in SIS and %FVC measurements were unable to be reported due to lack of gathering this information at the 24-month mark in the edaravone group. Due to the cost and administration burden associated with edaravone, it is important that assessment of disease progression is performed regularly to assess benefit and appropriateness of continued therapy. The oral formulation of edaravone was approved in 2022, shortly after the completion of data collection for this study.19,20 Although our study did not analyze oral edaravone, the administration burden of treatment would be reduced with the oral formulation, and we hypothesize there will be increased patient interest in ALS management with oral vs IV edaravone. Evaluation of long-term treatment for efficacy and safety beyond 24 months has not been evaluated. Future studies should continue to evaluate edaravone use in a larger veteran population.
Limitations
One limitation for our study alluded to earlier in the discussion was sample size. Although this study met power at the 6-month mark, it was limited by the number of patients who received more than 6 months of edaravone (n = 21). As a result, statistical analyses between treatment groups were underpowered at 12, 18, and 24 months. Our study had 80% power to detect a difference of 6.5 between the groups for the change in ALSFRS-R scores. Previous studies detected a statistically significant difference in ALSFRS-R scores, with a difference in ALSFRS-R scores of 2.49 between groups.8 Future studies should evaluate a larger sample size of patients who are prescribed edaravone.
Another limitation was that the edaravone and standard-of-care group data were gathered from different time periods. Two different time frames were selected to increase sample size by gathering data over a longer period and to account for patients who may have qualified for IV edaravone but could not receive it as it was not yet available on the market. There were no known changes to the standard of care between the time periods that would affect results. As noted previously, the standard-of-care group had fewer patients taking riluzole compared with the edaravone group, which may have confounded our results. We concluded patients opting for edaravone were more likely to trial riluzole, taken by mouth twice daily, before starting edaravone, a once-daily IV infusion.
Conclusions
No difference in the rate of ALS progression was noted between patients who received IV edaravone vs standard of care at 6 months. In addition, no difference was noted in other objective measures of disease progression, including %FVC, SIS, and time to death. As a result, the decision to initiate and continue edaravone therapy should be made on an individualized basis according to a prescriber’s clinical judgment and a patient’s goals. Edaravone therapy should be discontinued when disease progression occurs or when medication administration becomes a burden.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at Veteran Health Indiana.
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952
1. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. doi:10.1016/S0140-6736(10)61156-7
2. Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344(22):1688-1700. doi:0.1056/NEJM200105313442207
3. Mehta P, Kaye W, Raymond J, et al. Prevalence of amyotrophic lateral sclerosis–United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(46):1285-1289. doi:10.15585/mmwr.mm6746a1
4. Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178-180. doi:10.3109/17482960903093710
5. Rilutek. Package insert. Covis Pharmaceuticals; 1995.
6. Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585-591. doi:10.1056/NEJM199403033300901
7. Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425-1431. doi:10.1016/s0140-6736(96)91680-3
8. Radicava. Package insert. MT Pharma America Inc; 2017.
9. Abe K, Itoyama Y, Sobue G, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7-8):610-617. doi:10.3109/21678421.2014.959024
10. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505-512. doi:10.1016/S1474-4422(17)30115-1
11. Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(suppl 1):40-48. doi:10.1080/21678421.2017.1361441
12. Luo L, Song Z, Li X, et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis–a systematic review and meta-analysis. Neurol Sci. 2019;40(2):235-241. doi:10.1007/s10072-018-3653-2
13. Witzel S, Maier A, Steinbach R, et al; German Motor Neuron Disease Network (MND-NET). Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121-130. doi:10.1001/jamaneurol.2021.4893
14. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919-930. doi:10.1056/NEJMoa1916945
15. Relyvrio. Package insert. Amylyx Pharmaceuticals Inc; 2022.
16. McKay KA, Smith KA, Smertinaite L, Fang F, Ingre C, Taube F. Military service and related risk factors for amyotrophic lateral sclerosis. Acta Neurol Scand. 2021;143(1):39-50. doi:10.1111/ane.13345
17. Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y. How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr. 2018;62(1):20-38. doi:10.3164/jcbn.17-62
18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742-749. doi:10.1212/01.wnl.0000069922.32557.ca
19. Radicava ORS. Package insert. Mitsubishi Tanabe Pharma America Inc; 2022.
20. Shimizu H, Nishimura Y, Shiide Y, et al. Bioequivalence study of oral suspension and intravenous formulation of edaravone in healthy adult subjects. Clin Pharmacol Drug Dev. 2021;10(10):1188-1197. doi:10.1002/cpdd.952