User login
Case Studies in Toxicology: Double Take—Is Re-exposure Necessary to Explain Delayed Recurrent Opioid Toxicity?
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Case Studies in Toxicology: You Can’t See Dragonfly or Hear NBOMe, but They Can Still Hurt You
Case
A 24-year-old man was brought to the ED by emergency medical services (EMS) for altered mental status. The EMS crew reported they had picked up the patient at a nearby arts festival and concert series. A bystander at the event reported that the patient had taken something called “dragonfly.”
Initial assessment revealed the patient to be disoriented, with nonlinear thought patterns and an inability to follow commands. His vital signs were: blood pressure, 160/100 mm Hg; heart rate, 120 beats/minute; respiratory rate, 24 breaths/minute; and temperature, 102.2˚F. Oxygen saturation was 99% on room air. He was diaphoretic and agitated, and the nursing staff was concerned he would become aggressive and potentially violent. A quick Web search revealed that the agent the bystander mentioned was most likely Bromo-DragonFLY (BDF).
What is Bromo-DragonFLY?
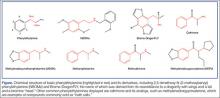
In the 1960s, an American chemist named Alexander Shulgin ushered in a new era of psychedelic drug use by establishing a simple synthesis of 3,4-methylenedioxy-methamphetamine (MDMA). Following this discovery, he suggested a therapist friend use the drug therapeutically.1 Shulgin then began a process of homologation (ie, creating novel compounds by slightly altering existing ones in an organized fashion) and developed systems for rating the drug experiences and naming the drugs in shorthand, both of which are still in use. The chemical structure common to nearly all of the drugs he studied is phenylethylamine. The Figure shows the structures of several phenylethylamine derivatives that were created by adding functional groups to the phenylethylamine backbone. Although the popularity of psychedelic drugs surged during this time period, 2,5-dimethoxy-N-(2-methoxybenzyl)phenylethylamine) (NBOMe), one of a number of newly popular psychedelics, only became available in 2003.
What is known about the pharmacology of Bromo-DragonFLY and NBOMe?
The major target of psychedelic drugs is the serotonin (5-HT2) receptor, specifically the central 5-HT2A subtype. Bromo-DragonFLY is a classic example of designer pharmacology in that the it was intended to potently exert its effect at this specific receptor site.
As its name suggests, BDF adds the “wings of the fly” to the phenylethylamine backbone furanyl rings at positions 2 and 5, and a halogen (bromine) at position 4. The furanyl ring impairs enzymatic clearance of the drug,2 resulting in a duration of action of up to 3 days.3 The addition of halogens increases drug potency, but the mechanism is not clear. The psychedelic agent NBOMe results from chemical additions of methoxy groups at position 2 and 5, and the halogen moiety (iodine in this case) at position 4 of the phenyl ring of the phenylethylamine structure.4
Through the work of Shulgin, some of his colleagues, and many disparate street chemists, a vast family of substituted phenylethylamines have been synthesized and used. Shulgin’s semiautobiographical book PiHKAL: A Chemical Love Story includes his laboratory notes for the synthesis and initial test-dose experience of 179 compounds1; this does not include research done by others or any work since its publication in 1995.
Notable popular drugs chemically similar to NBOMe and BDF are mescaline (found in peyote), cathinones (“bath salts”), and MDMA (found in ecstasy) (Figure). Naturally occurring (and more complex) compounds with similar effects include ayahuasca, a plant-derived beverage consisting of Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana from the Brazilian rainforest (see Emerg Med. 2014;46[12]:553-556); psilocybin (“magic mushrooms”); and lysergic acid diethylamide.
How are these drugs used and what are their clinical effects?
Most phenylethylamine compounds are well absorbed across the buccal mucosa, which is why BDF and NBOMe are commonly used in liquid form or on blotter paper. Dosing guides also exist for insufflation and claim equipotent dosing for this route.5 Regardless of delivery route, given the high potency, inadvertent exposures to these drugs should be expected.
Users simply seeking to hallucinate may not be aware of the significant risks associated with these potent serotonergic agents, which include both life- and limb-threatening effects.6 The high 5-HT2A potency results both in vasoconstriction and promotion of clot formation due to the presence of 5HT2A receptors on small blood vessels and platelets, respectively. Ergotism, historically called Saint Anthony’s fire, is an example of serotonergic vasoconstriction and hallucination.7 Chronic users of substituted amphetamines can develop necrotic ulcers in distal vascular beds such as the hands and feet; these ulcers may progress to amputation despite treatment attempts with vasodilators.
In addition to the vasoconstrictive properties, there are multiple reports of serotonin toxicity (serotonin syndrome) associated with use of these designer serotonergic amphetamines. This syndrome includes severe psychomotor agitation that can lead to personal injury, along with muscle rigidity, tremor, hyperthermia, rhabdomyolysis, and seizures.8
How are patients with phenylethylamine exposures managed?
Management of a patient with a substituted phenylethylamine exposure is similar to management of those with cocaine overdose. Attention to the life-threatening clinical effects of psychomotor agitation, hyperthermia, and seizures is paramount. Appropriate supportive care includes intravenous (IV) benzodiazepines to control agitation and muscle rigidity, replacement of lost volume with crystalloids, and active cooling measures. Failure of benzodiazepines (preferably in conjunction with continuous electroencephalogram monitoring) to control rigidity may lead to the need for propofol and/or result in paralysis. Similar to patients with cocaine intoxication, some may experience ischemic chest pain, and the usual protocol of sedation, nitroglycerin, morphine, and an antiplatelet drug is appropriate.
Identification of phenylethylamines typically requires specialized laboratory testing since most will not trigger a positive result on a standard urine immunoassay. Many specialized laboratories have test catalogs on their Web sites listing under the “stimulants panel” which drugs can be identified. However, none of these assays is likely truly comprehensive, and minor alterations or substitutions to the compounds result in new analogs that may not be in the reference laboratory’s identification library.
The patient was initially restrained and given 5 mg IV diazepam, which was followed by escalating doses every 5 minutes to a total of 35 mg for effect. He had a rectal temperature of 102.5˚F and was externally cooled after sedation. After 20 minutes, he had a generalized convulsion; an additional 10 mg of IV diazepam terminated the seizure, but he remained hyperthermic at 104˚F. The patient was intubated, placed on a propofol infusion, and admitted to the intensive care unit where his temperature was carefully monitored. The following day his temperature had normalized and he was weaned from the ventilator and discharged to the floor for monitoring. On hospital day 3, he was discharged in stable condition.
Mr Waldrop is a fourth-year medical student at the State University of New York, Upstate Medical University, Syracuse. Dr Nacca is a fellow in medical toxicology, department of emergency medicine, State University of New York, Upstate Medical University, Syracuse. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine, and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1995.
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving bromo-dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
- Gentry CL, Egleton RD, Gillespie T, et al. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20(10):1229-1238.
- Erowid. Bromo-Dragonfly Dosage. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_dose.shtml. Accessed January 14, 2015.
- Baumann MH, Ayestas MA Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192-1203.
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20-25.
- Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
Case
A 24-year-old man was brought to the ED by emergency medical services (EMS) for altered mental status. The EMS crew reported they had picked up the patient at a nearby arts festival and concert series. A bystander at the event reported that the patient had taken something called “dragonfly.”
Initial assessment revealed the patient to be disoriented, with nonlinear thought patterns and an inability to follow commands. His vital signs were: blood pressure, 160/100 mm Hg; heart rate, 120 beats/minute; respiratory rate, 24 breaths/minute; and temperature, 102.2˚F. Oxygen saturation was 99% on room air. He was diaphoretic and agitated, and the nursing staff was concerned he would become aggressive and potentially violent. A quick Web search revealed that the agent the bystander mentioned was most likely Bromo-DragonFLY (BDF).
What is Bromo-DragonFLY?
In the 1960s, an American chemist named Alexander Shulgin ushered in a new era of psychedelic drug use by establishing a simple synthesis of 3,4-methylenedioxy-methamphetamine (MDMA). Following this discovery, he suggested a therapist friend use the drug therapeutically.1 Shulgin then began a process of homologation (ie, creating novel compounds by slightly altering existing ones in an organized fashion) and developed systems for rating the drug experiences and naming the drugs in shorthand, both of which are still in use. The chemical structure common to nearly all of the drugs he studied is phenylethylamine. The Figure shows the structures of several phenylethylamine derivatives that were created by adding functional groups to the phenylethylamine backbone. Although the popularity of psychedelic drugs surged during this time period, 2,5-dimethoxy-N-(2-methoxybenzyl)phenylethylamine) (NBOMe), one of a number of newly popular psychedelics, only became available in 2003.
What is known about the pharmacology of Bromo-DragonFLY and NBOMe?
The major target of psychedelic drugs is the serotonin (5-HT2) receptor, specifically the central 5-HT2A subtype. Bromo-DragonFLY is a classic example of designer pharmacology in that the it was intended to potently exert its effect at this specific receptor site.
As its name suggests, BDF adds the “wings of the fly” to the phenylethylamine backbone furanyl rings at positions 2 and 5, and a halogen (bromine) at position 4. The furanyl ring impairs enzymatic clearance of the drug,2 resulting in a duration of action of up to 3 days.3 The addition of halogens increases drug potency, but the mechanism is not clear. The psychedelic agent NBOMe results from chemical additions of methoxy groups at position 2 and 5, and the halogen moiety (iodine in this case) at position 4 of the phenyl ring of the phenylethylamine structure.4
Through the work of Shulgin, some of his colleagues, and many disparate street chemists, a vast family of substituted phenylethylamines have been synthesized and used. Shulgin’s semiautobiographical book PiHKAL: A Chemical Love Story includes his laboratory notes for the synthesis and initial test-dose experience of 179 compounds1; this does not include research done by others or any work since its publication in 1995.
Notable popular drugs chemically similar to NBOMe and BDF are mescaline (found in peyote), cathinones (“bath salts”), and MDMA (found in ecstasy) (Figure). Naturally occurring (and more complex) compounds with similar effects include ayahuasca, a plant-derived beverage consisting of Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana from the Brazilian rainforest (see Emerg Med. 2014;46[12]:553-556); psilocybin (“magic mushrooms”); and lysergic acid diethylamide.
How are these drugs used and what are their clinical effects?
Most phenylethylamine compounds are well absorbed across the buccal mucosa, which is why BDF and NBOMe are commonly used in liquid form or on blotter paper. Dosing guides also exist for insufflation and claim equipotent dosing for this route.5 Regardless of delivery route, given the high potency, inadvertent exposures to these drugs should be expected.
Users simply seeking to hallucinate may not be aware of the significant risks associated with these potent serotonergic agents, which include both life- and limb-threatening effects.6 The high 5-HT2A potency results both in vasoconstriction and promotion of clot formation due to the presence of 5HT2A receptors on small blood vessels and platelets, respectively. Ergotism, historically called Saint Anthony’s fire, is an example of serotonergic vasoconstriction and hallucination.7 Chronic users of substituted amphetamines can develop necrotic ulcers in distal vascular beds such as the hands and feet; these ulcers may progress to amputation despite treatment attempts with vasodilators.
In addition to the vasoconstrictive properties, there are multiple reports of serotonin toxicity (serotonin syndrome) associated with use of these designer serotonergic amphetamines. This syndrome includes severe psychomotor agitation that can lead to personal injury, along with muscle rigidity, tremor, hyperthermia, rhabdomyolysis, and seizures.8
How are patients with phenylethylamine exposures managed?
Management of a patient with a substituted phenylethylamine exposure is similar to management of those with cocaine overdose. Attention to the life-threatening clinical effects of psychomotor agitation, hyperthermia, and seizures is paramount. Appropriate supportive care includes intravenous (IV) benzodiazepines to control agitation and muscle rigidity, replacement of lost volume with crystalloids, and active cooling measures. Failure of benzodiazepines (preferably in conjunction with continuous electroencephalogram monitoring) to control rigidity may lead to the need for propofol and/or result in paralysis. Similar to patients with cocaine intoxication, some may experience ischemic chest pain, and the usual protocol of sedation, nitroglycerin, morphine, and an antiplatelet drug is appropriate.
Identification of phenylethylamines typically requires specialized laboratory testing since most will not trigger a positive result on a standard urine immunoassay. Many specialized laboratories have test catalogs on their Web sites listing under the “stimulants panel” which drugs can be identified. However, none of these assays is likely truly comprehensive, and minor alterations or substitutions to the compounds result in new analogs that may not be in the reference laboratory’s identification library.
The patient was initially restrained and given 5 mg IV diazepam, which was followed by escalating doses every 5 minutes to a total of 35 mg for effect. He had a rectal temperature of 102.5˚F and was externally cooled after sedation. After 20 minutes, he had a generalized convulsion; an additional 10 mg of IV diazepam terminated the seizure, but he remained hyperthermic at 104˚F. The patient was intubated, placed on a propofol infusion, and admitted to the intensive care unit where his temperature was carefully monitored. The following day his temperature had normalized and he was weaned from the ventilator and discharged to the floor for monitoring. On hospital day 3, he was discharged in stable condition.
Mr Waldrop is a fourth-year medical student at the State University of New York, Upstate Medical University, Syracuse. Dr Nacca is a fellow in medical toxicology, department of emergency medicine, State University of New York, Upstate Medical University, Syracuse. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine, and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 24-year-old man was brought to the ED by emergency medical services (EMS) for altered mental status. The EMS crew reported they had picked up the patient at a nearby arts festival and concert series. A bystander at the event reported that the patient had taken something called “dragonfly.”
Initial assessment revealed the patient to be disoriented, with nonlinear thought patterns and an inability to follow commands. His vital signs were: blood pressure, 160/100 mm Hg; heart rate, 120 beats/minute; respiratory rate, 24 breaths/minute; and temperature, 102.2˚F. Oxygen saturation was 99% on room air. He was diaphoretic and agitated, and the nursing staff was concerned he would become aggressive and potentially violent. A quick Web search revealed that the agent the bystander mentioned was most likely Bromo-DragonFLY (BDF).
What is Bromo-DragonFLY?
In the 1960s, an American chemist named Alexander Shulgin ushered in a new era of psychedelic drug use by establishing a simple synthesis of 3,4-methylenedioxy-methamphetamine (MDMA). Following this discovery, he suggested a therapist friend use the drug therapeutically.1 Shulgin then began a process of homologation (ie, creating novel compounds by slightly altering existing ones in an organized fashion) and developed systems for rating the drug experiences and naming the drugs in shorthand, both of which are still in use. The chemical structure common to nearly all of the drugs he studied is phenylethylamine. The Figure shows the structures of several phenylethylamine derivatives that were created by adding functional groups to the phenylethylamine backbone. Although the popularity of psychedelic drugs surged during this time period, 2,5-dimethoxy-N-(2-methoxybenzyl)phenylethylamine) (NBOMe), one of a number of newly popular psychedelics, only became available in 2003.
What is known about the pharmacology of Bromo-DragonFLY and NBOMe?
The major target of psychedelic drugs is the serotonin (5-HT2) receptor, specifically the central 5-HT2A subtype. Bromo-DragonFLY is a classic example of designer pharmacology in that the it was intended to potently exert its effect at this specific receptor site.
As its name suggests, BDF adds the “wings of the fly” to the phenylethylamine backbone furanyl rings at positions 2 and 5, and a halogen (bromine) at position 4. The furanyl ring impairs enzymatic clearance of the drug,2 resulting in a duration of action of up to 3 days.3 The addition of halogens increases drug potency, but the mechanism is not clear. The psychedelic agent NBOMe results from chemical additions of methoxy groups at position 2 and 5, and the halogen moiety (iodine in this case) at position 4 of the phenyl ring of the phenylethylamine structure.4
Through the work of Shulgin, some of his colleagues, and many disparate street chemists, a vast family of substituted phenylethylamines have been synthesized and used. Shulgin’s semiautobiographical book PiHKAL: A Chemical Love Story includes his laboratory notes for the synthesis and initial test-dose experience of 179 compounds1; this does not include research done by others or any work since its publication in 1995.
Notable popular drugs chemically similar to NBOMe and BDF are mescaline (found in peyote), cathinones (“bath salts”), and MDMA (found in ecstasy) (Figure). Naturally occurring (and more complex) compounds with similar effects include ayahuasca, a plant-derived beverage consisting of Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana from the Brazilian rainforest (see Emerg Med. 2014;46[12]:553-556); psilocybin (“magic mushrooms”); and lysergic acid diethylamide.
How are these drugs used and what are their clinical effects?
Most phenylethylamine compounds are well absorbed across the buccal mucosa, which is why BDF and NBOMe are commonly used in liquid form or on blotter paper. Dosing guides also exist for insufflation and claim equipotent dosing for this route.5 Regardless of delivery route, given the high potency, inadvertent exposures to these drugs should be expected.
Users simply seeking to hallucinate may not be aware of the significant risks associated with these potent serotonergic agents, which include both life- and limb-threatening effects.6 The high 5-HT2A potency results both in vasoconstriction and promotion of clot formation due to the presence of 5HT2A receptors on small blood vessels and platelets, respectively. Ergotism, historically called Saint Anthony’s fire, is an example of serotonergic vasoconstriction and hallucination.7 Chronic users of substituted amphetamines can develop necrotic ulcers in distal vascular beds such as the hands and feet; these ulcers may progress to amputation despite treatment attempts with vasodilators.
In addition to the vasoconstrictive properties, there are multiple reports of serotonin toxicity (serotonin syndrome) associated with use of these designer serotonergic amphetamines. This syndrome includes severe psychomotor agitation that can lead to personal injury, along with muscle rigidity, tremor, hyperthermia, rhabdomyolysis, and seizures.8
How are patients with phenylethylamine exposures managed?
Management of a patient with a substituted phenylethylamine exposure is similar to management of those with cocaine overdose. Attention to the life-threatening clinical effects of psychomotor agitation, hyperthermia, and seizures is paramount. Appropriate supportive care includes intravenous (IV) benzodiazepines to control agitation and muscle rigidity, replacement of lost volume with crystalloids, and active cooling measures. Failure of benzodiazepines (preferably in conjunction with continuous electroencephalogram monitoring) to control rigidity may lead to the need for propofol and/or result in paralysis. Similar to patients with cocaine intoxication, some may experience ischemic chest pain, and the usual protocol of sedation, nitroglycerin, morphine, and an antiplatelet drug is appropriate.
Identification of phenylethylamines typically requires specialized laboratory testing since most will not trigger a positive result on a standard urine immunoassay. Many specialized laboratories have test catalogs on their Web sites listing under the “stimulants panel” which drugs can be identified. However, none of these assays is likely truly comprehensive, and minor alterations or substitutions to the compounds result in new analogs that may not be in the reference laboratory’s identification library.
The patient was initially restrained and given 5 mg IV diazepam, which was followed by escalating doses every 5 minutes to a total of 35 mg for effect. He had a rectal temperature of 102.5˚F and was externally cooled after sedation. After 20 minutes, he had a generalized convulsion; an additional 10 mg of IV diazepam terminated the seizure, but he remained hyperthermic at 104˚F. The patient was intubated, placed on a propofol infusion, and admitted to the intensive care unit where his temperature was carefully monitored. The following day his temperature had normalized and he was weaned from the ventilator and discharged to the floor for monitoring. On hospital day 3, he was discharged in stable condition.
Mr Waldrop is a fourth-year medical student at the State University of New York, Upstate Medical University, Syracuse. Dr Nacca is a fellow in medical toxicology, department of emergency medicine, State University of New York, Upstate Medical University, Syracuse. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine, and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1995.
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving bromo-dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
- Gentry CL, Egleton RD, Gillespie T, et al. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20(10):1229-1238.
- Erowid. Bromo-Dragonfly Dosage. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_dose.shtml. Accessed January 14, 2015.
- Baumann MH, Ayestas MA Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192-1203.
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20-25.
- Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1995.
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving bromo-dragonfly. Forensic Sci Int. 2009;183(1-3):91-96.
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila). 2011;49(8):705-719.
- Gentry CL, Egleton RD, Gillespie T, et al. The effect of halogenation on blood-brain barrier permeability of a novel peptide drug. Peptides. 1999;20(10):1229-1238.
- Erowid. Bromo-Dragonfly Dosage. http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly_dose.shtml. Accessed January 14, 2015.
- Baumann MH, Ayestas MA Jr, Partilla JS, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192-1203.
- Walterscheid JP, Phillips GT, Lopez AE, Gonsoulin ML, Chen HH, Sanchez LA. Pathological findings in 2 cases of fatal 25I-NBOMe toxicity. Am J Forensic Med Pathol. 2014;35(1):20-25.
- Wood DM, Looker JJ, Shaikh L, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5(4):226-229.
Case Studies in Toxicology: An Amazonian Herb Goes Mainstream
Case
A 23-year-old Hispanic woman with no past medical history is brought to the ED for the second time in one day. On her first presentation, which was for a fever and a headache, meningitis was excluded with normal laboratory tests that included a lumbar puncture. She was administered acetaminophen for fever and pain control, and was discharged with a diagnosis of viral illness. On this second visit, 10 hours after being discharged, she presented because her family noted convulsions that began 3 hours after taking an herbal headache remedy given to her by a naturopath.
The patient arrived to the ED with a persistent seizure that terminated following administration of 2 mg of lorazepam. Her initial vital signs were: blood pressure, 115/51 mm Hg; heart rate, 121 beats/minute; respiratory rate, 24 breaths/minute; temperature, 97.6oF. Oxygen (O2) saturation was 100% with 2 L of O2 administered via nasal cannula. Her neurological examination was significant for a depressed mental status, pupils that were 6 mm and minimally reactive, clonus, and hyperreflexia. Repeat laboratory evaluation found a leukocytosis of 22.0 x 103/µL, serum bicarbonate of 9 mEq/L, and an anion gap of 22 with a normal serum lactate.
What is the differential diagnosis of this patient?
The history of medicinal plant ingestion raises the possibility of a toxicologic etiology. However, because the patient took the “medication” to treat another disorder, a search for an alternate cause should be performed. The differential diagnosis of a toxin-induced seizure is broad and includes pharmaceuticals (eg, tramadol, antihistamines), which may be surreptitiously added to herbal medication to assure efficacy. Plants associated with seizures include those containing antimuscarinic tropane alkaloids such as Jimsonweed (though a rare side effect from this plant product) or the water hemlock (Cicuta maculata). Contaminants of the plant itself may include pesticides such as organophosphates.
Although unlikely in a 21 year old, withdrawal from benzodiazepines, ethanol, baclofen, or gamma hydroxybutyrate are other possible etiologies. In addition to pharmaceutical and plant-derived causes, carbon monoxide poisoning should be a consideration in any patient with headache and flu-like illness.
This patient also presented with a constellation of other findings that included hyperreflexia, clonus, tachycardia, and altered mental status. Together these signs are expected in patients with serotonin toxicity (also referred to as serotonin syndrome), neuroleptic malignant syndrome, exogenous thyrotoxicosis, and lithium poisoning.
Case Continuation
The naturopathic practitioner arrived at the ED concerned about the patient, informing the ED team that she had given the patient 2 ounces of ayahuasca tea.
What is ayahuasca? What is the mechanism by which it exerts toxic effects?
Ayahuasca is a plant-derived psychotropic beverage that is used for religious purposes by members of two Brazilian churches—Centro Espírita Beneficente União do Vegetal (UDV) and Santo Daime. The ayahuasca beverage consists of two pharmacologically active compounds that together, but not individually, are psychoactive. The desired active effects for church participants include hallucinations, and vomiting to bring about a “religious purge.”1
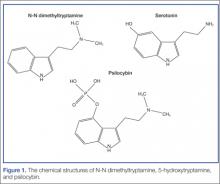
Ayahuasca is prepared by combining two plants indigenous to the Amazon Basin area: Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana. B caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydroharmine. These alkaloids act as reversible inhibitors of the monoamine oxidase A (MAO-A) enzyme. The bark and stems of B caapi are boiled along with either P viridis or D cabrerana, both of which contain the potent hallucinogen N-N dimethyltryptamine (DMT).2 Normally, DMT is not active orally because it is enzymatically metabolized by MAO-A. However, when taken in the presence of the B caapi-derived MAO-A–inhibiting harmine alkaloids, DMT reaches the systemic circulation and produces its clinical effects.3
What are the clinical findings of serotonin toxicity?
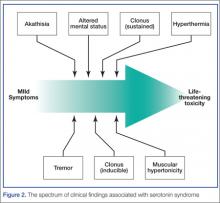
Serotonin toxicity is a collection of clinical findings that fall under three main categories: autonomic hyperactivity, altered mental status, and muscle rigidity.5 The autonomic findings may include tachycardia, hypertension, hyperthermia, shivering, diaphoresis, or mydriasis. Altered mental status ranges from mild agitation and hypervigilance to agitated delirium to obtundation. Other neurological findings may include tremor, myoclonus, hyperreflexia, or seizures. The onset of these signs is rapid, usually occurring within minutes after exposure to one or more serotonergic compounds. Although rare, severe serotonin toxicity may be associated with hypotension and shock, leading to death.4
The diagnosis of serotonin toxicity is based on the history and physical examination of the patient. Diagnostic criteria that have been suggested include the following: (1) a recent addition or increase in a known serotonergic agent; (2) absence of other possible etiologies; (3) no recent increase or addition of a neuroleptic agent (suggesting neuroleptic malignant syndrome); and/or (4) at least 3 of the following symptoms—mental status changes, myoclonus, agitation, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, fever5 (Figure 2).
How should this patient be managed?
The management of serotonin toxicity is primarily supportive with aggressive control of hyperthermia and autonomic instability. The precipitating xenobiotic agent should be immediately discontinued. In general, treatment with intravenous fluids, cooling measures, benzodiazepines, and a nonspecific 5-HT antagonist such as cyproheptadine should greatly improve the patient’s clinical status. Patients with severe toxicity may require induced paralysis and intubation.4 It is not clear in this case if the serotonin hyperactivation was due to the DMT (5-HT2A is associated with serotonin toxicity) or another serotonergic agent (eg, dextromethorphan from a cough and cold preparation) in combination with the MAO-inhibiting harmine alkaloids.
What is the availability of ayahuasca in the United States? How is it used in its nonherbal form?
...[Ayahuasca] is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches. Many clinicians are becoming increasingly familiar with this herbal preparation since the recreational use of ayahuasca is gaining popularity in the United States. Internet fora with information on how to safely use ayahuasca, such as avoiding aged cheeses, are becoming more prevalent.7 A recent article in the New York Times described an ayahuasca gathering in Brooklyn, New York, where participants use the herb in a communal fashion.8 This herbal product is also associated with the Hollywood social scene and has received celebrity endorsements.8
The National Survey on Drug Use and Health found that the number of people in the United States who have used DMT has gone up almost every year since 2006, from an estimated 688,000 in 2006 to 1,475,000 in 2012.9 When used alone (not as ayahuasca), DMT is almost exclusively insufflated as a nasal snuff, bypassing hepatic elimination. It has an onset of around 45 seconds and a duration of 5 to 10 minutes. Insufflating DMT was historically referred to as a “businessman’s trip” because users were able to have a brief hallucinogenic experience on a lunch break and recover rapidly to perform their normal work.10
International law declares that DMT is an illegal substance and its importation is banned. However, its use for religious purposes, as is allowed for mescaline found in peyote, remains controversial.7 The UDV brought suit in United States federal court to prevent interference with the church’s use of ayahuasca during religious ceremonies based on the Religious Freedom Restoration Act. This act states that the government should not cause substantial imposition on religious practices in the absence of a compelling government interest. The court sided with the UDV, finding that the government had not sufficiently proved the alleged health risks posed by ayahuasca and could not show a substantial risk that the drug would be abused recreationally.11 Thus it is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches.
Ayahuasca is not regulated by the US Food and Drug Administration. Many different types of preparations with different ingredients as well as different concentrations may exist, and clinical variability should be expected. Understanding that ayahuasca is capable of inhibiting MAO is important in order to avoid foods and medications, such as dextromethorphan, that may trigger adverse effects.
Case Conclusion
The patient’s hospital course was complicated by an additional seizure 12 hours after her initial presentation. By 36 hours she was back to her baseline mental status with a normal neurological examination.
Dr Fil is a senior fellow in medical toxicology at North Shore University Hospital, Manhasset, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34.
- Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and Disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4(7-8):610-616.
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73-83
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11);1112-1120.
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):6;705-713.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
- Erowid. Ayahuasca Vault. https://www.erowid.org/chemicals/ayahuasca/ayahuasca.shtml. Accessed November 25, 2014.
- Morris B. Ayahuasca: a strong cup of tea. New York Times. June 13, 2014. http://www.nytimes.com/2014/06/15/fashion/ayahuasca-a-strong-cup-of-tea.html. Accessed November 25, 2014.
- Quintanilla D. DMT: Hallucinogenic Drug Used in Shamanic Rituals Goes Mainstream. 10 Dec 2013. Available: http://www.opposingviews.com/i/health/dmt-hallucinogenic-drug-used-shamanic-rituals-goes-mainstream. Last accessed 11/14/14.
- Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin North Am. 2005;89(6):1259-1276.
- Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, 546 US 418 (2006). Available at http://scholar.google.com/scholar_case?case=7036734975431570669&hl=en&as_sdt=6&as_vis=1&oi=scholarr. Accessed November 25, 2014.
Case
A 23-year-old Hispanic woman with no past medical history is brought to the ED for the second time in one day. On her first presentation, which was for a fever and a headache, meningitis was excluded with normal laboratory tests that included a lumbar puncture. She was administered acetaminophen for fever and pain control, and was discharged with a diagnosis of viral illness. On this second visit, 10 hours after being discharged, she presented because her family noted convulsions that began 3 hours after taking an herbal headache remedy given to her by a naturopath.
The patient arrived to the ED with a persistent seizure that terminated following administration of 2 mg of lorazepam. Her initial vital signs were: blood pressure, 115/51 mm Hg; heart rate, 121 beats/minute; respiratory rate, 24 breaths/minute; temperature, 97.6oF. Oxygen (O2) saturation was 100% with 2 L of O2 administered via nasal cannula. Her neurological examination was significant for a depressed mental status, pupils that were 6 mm and minimally reactive, clonus, and hyperreflexia. Repeat laboratory evaluation found a leukocytosis of 22.0 x 103/µL, serum bicarbonate of 9 mEq/L, and an anion gap of 22 with a normal serum lactate.
What is the differential diagnosis of this patient?
The history of medicinal plant ingestion raises the possibility of a toxicologic etiology. However, because the patient took the “medication” to treat another disorder, a search for an alternate cause should be performed. The differential diagnosis of a toxin-induced seizure is broad and includes pharmaceuticals (eg, tramadol, antihistamines), which may be surreptitiously added to herbal medication to assure efficacy. Plants associated with seizures include those containing antimuscarinic tropane alkaloids such as Jimsonweed (though a rare side effect from this plant product) or the water hemlock (Cicuta maculata). Contaminants of the plant itself may include pesticides such as organophosphates.
Although unlikely in a 21 year old, withdrawal from benzodiazepines, ethanol, baclofen, or gamma hydroxybutyrate are other possible etiologies. In addition to pharmaceutical and plant-derived causes, carbon monoxide poisoning should be a consideration in any patient with headache and flu-like illness.
This patient also presented with a constellation of other findings that included hyperreflexia, clonus, tachycardia, and altered mental status. Together these signs are expected in patients with serotonin toxicity (also referred to as serotonin syndrome), neuroleptic malignant syndrome, exogenous thyrotoxicosis, and lithium poisoning.
Case Continuation
The naturopathic practitioner arrived at the ED concerned about the patient, informing the ED team that she had given the patient 2 ounces of ayahuasca tea.
What is ayahuasca? What is the mechanism by which it exerts toxic effects?
Ayahuasca is a plant-derived psychotropic beverage that is used for religious purposes by members of two Brazilian churches—Centro Espírita Beneficente União do Vegetal (UDV) and Santo Daime. The ayahuasca beverage consists of two pharmacologically active compounds that together, but not individually, are psychoactive. The desired active effects for church participants include hallucinations, and vomiting to bring about a “religious purge.”1
Ayahuasca is prepared by combining two plants indigenous to the Amazon Basin area: Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana. B caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydroharmine. These alkaloids act as reversible inhibitors of the monoamine oxidase A (MAO-A) enzyme. The bark and stems of B caapi are boiled along with either P viridis or D cabrerana, both of which contain the potent hallucinogen N-N dimethyltryptamine (DMT).2 Normally, DMT is not active orally because it is enzymatically metabolized by MAO-A. However, when taken in the presence of the B caapi-derived MAO-A–inhibiting harmine alkaloids, DMT reaches the systemic circulation and produces its clinical effects.3
What are the clinical findings of serotonin toxicity?
Serotonin toxicity is a collection of clinical findings that fall under three main categories: autonomic hyperactivity, altered mental status, and muscle rigidity.5 The autonomic findings may include tachycardia, hypertension, hyperthermia, shivering, diaphoresis, or mydriasis. Altered mental status ranges from mild agitation and hypervigilance to agitated delirium to obtundation. Other neurological findings may include tremor, myoclonus, hyperreflexia, or seizures. The onset of these signs is rapid, usually occurring within minutes after exposure to one or more serotonergic compounds. Although rare, severe serotonin toxicity may be associated with hypotension and shock, leading to death.4
The diagnosis of serotonin toxicity is based on the history and physical examination of the patient. Diagnostic criteria that have been suggested include the following: (1) a recent addition or increase in a known serotonergic agent; (2) absence of other possible etiologies; (3) no recent increase or addition of a neuroleptic agent (suggesting neuroleptic malignant syndrome); and/or (4) at least 3 of the following symptoms—mental status changes, myoclonus, agitation, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, fever5 (Figure 2).
How should this patient be managed?
The management of serotonin toxicity is primarily supportive with aggressive control of hyperthermia and autonomic instability. The precipitating xenobiotic agent should be immediately discontinued. In general, treatment with intravenous fluids, cooling measures, benzodiazepines, and a nonspecific 5-HT antagonist such as cyproheptadine should greatly improve the patient’s clinical status. Patients with severe toxicity may require induced paralysis and intubation.4 It is not clear in this case if the serotonin hyperactivation was due to the DMT (5-HT2A is associated with serotonin toxicity) or another serotonergic agent (eg, dextromethorphan from a cough and cold preparation) in combination with the MAO-inhibiting harmine alkaloids.
What is the availability of ayahuasca in the United States? How is it used in its nonherbal form?
...[Ayahuasca] is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches. Many clinicians are becoming increasingly familiar with this herbal preparation since the recreational use of ayahuasca is gaining popularity in the United States. Internet fora with information on how to safely use ayahuasca, such as avoiding aged cheeses, are becoming more prevalent.7 A recent article in the New York Times described an ayahuasca gathering in Brooklyn, New York, where participants use the herb in a communal fashion.8 This herbal product is also associated with the Hollywood social scene and has received celebrity endorsements.8
The National Survey on Drug Use and Health found that the number of people in the United States who have used DMT has gone up almost every year since 2006, from an estimated 688,000 in 2006 to 1,475,000 in 2012.9 When used alone (not as ayahuasca), DMT is almost exclusively insufflated as a nasal snuff, bypassing hepatic elimination. It has an onset of around 45 seconds and a duration of 5 to 10 minutes. Insufflating DMT was historically referred to as a “businessman’s trip” because users were able to have a brief hallucinogenic experience on a lunch break and recover rapidly to perform their normal work.10
International law declares that DMT is an illegal substance and its importation is banned. However, its use for religious purposes, as is allowed for mescaline found in peyote, remains controversial.7 The UDV brought suit in United States federal court to prevent interference with the church’s use of ayahuasca during religious ceremonies based on the Religious Freedom Restoration Act. This act states that the government should not cause substantial imposition on religious practices in the absence of a compelling government interest. The court sided with the UDV, finding that the government had not sufficiently proved the alleged health risks posed by ayahuasca and could not show a substantial risk that the drug would be abused recreationally.11 Thus it is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches.
Ayahuasca is not regulated by the US Food and Drug Administration. Many different types of preparations with different ingredients as well as different concentrations may exist, and clinical variability should be expected. Understanding that ayahuasca is capable of inhibiting MAO is important in order to avoid foods and medications, such as dextromethorphan, that may trigger adverse effects.
Case Conclusion
The patient’s hospital course was complicated by an additional seizure 12 hours after her initial presentation. By 36 hours she was back to her baseline mental status with a normal neurological examination.
Dr Fil is a senior fellow in medical toxicology at North Shore University Hospital, Manhasset, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 23-year-old Hispanic woman with no past medical history is brought to the ED for the second time in one day. On her first presentation, which was for a fever and a headache, meningitis was excluded with normal laboratory tests that included a lumbar puncture. She was administered acetaminophen for fever and pain control, and was discharged with a diagnosis of viral illness. On this second visit, 10 hours after being discharged, she presented because her family noted convulsions that began 3 hours after taking an herbal headache remedy given to her by a naturopath.
The patient arrived to the ED with a persistent seizure that terminated following administration of 2 mg of lorazepam. Her initial vital signs were: blood pressure, 115/51 mm Hg; heart rate, 121 beats/minute; respiratory rate, 24 breaths/minute; temperature, 97.6oF. Oxygen (O2) saturation was 100% with 2 L of O2 administered via nasal cannula. Her neurological examination was significant for a depressed mental status, pupils that were 6 mm and minimally reactive, clonus, and hyperreflexia. Repeat laboratory evaluation found a leukocytosis of 22.0 x 103/µL, serum bicarbonate of 9 mEq/L, and an anion gap of 22 with a normal serum lactate.
What is the differential diagnosis of this patient?
The history of medicinal plant ingestion raises the possibility of a toxicologic etiology. However, because the patient took the “medication” to treat another disorder, a search for an alternate cause should be performed. The differential diagnosis of a toxin-induced seizure is broad and includes pharmaceuticals (eg, tramadol, antihistamines), which may be surreptitiously added to herbal medication to assure efficacy. Plants associated with seizures include those containing antimuscarinic tropane alkaloids such as Jimsonweed (though a rare side effect from this plant product) or the water hemlock (Cicuta maculata). Contaminants of the plant itself may include pesticides such as organophosphates.
Although unlikely in a 21 year old, withdrawal from benzodiazepines, ethanol, baclofen, or gamma hydroxybutyrate are other possible etiologies. In addition to pharmaceutical and plant-derived causes, carbon monoxide poisoning should be a consideration in any patient with headache and flu-like illness.
This patient also presented with a constellation of other findings that included hyperreflexia, clonus, tachycardia, and altered mental status. Together these signs are expected in patients with serotonin toxicity (also referred to as serotonin syndrome), neuroleptic malignant syndrome, exogenous thyrotoxicosis, and lithium poisoning.
Case Continuation
The naturopathic practitioner arrived at the ED concerned about the patient, informing the ED team that she had given the patient 2 ounces of ayahuasca tea.
What is ayahuasca? What is the mechanism by which it exerts toxic effects?
Ayahuasca is a plant-derived psychotropic beverage that is used for religious purposes by members of two Brazilian churches—Centro Espírita Beneficente União do Vegetal (UDV) and Santo Daime. The ayahuasca beverage consists of two pharmacologically active compounds that together, but not individually, are psychoactive. The desired active effects for church participants include hallucinations, and vomiting to bring about a “religious purge.”1
Ayahuasca is prepared by combining two plants indigenous to the Amazon Basin area: Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana. B caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydroharmine. These alkaloids act as reversible inhibitors of the monoamine oxidase A (MAO-A) enzyme. The bark and stems of B caapi are boiled along with either P viridis or D cabrerana, both of which contain the potent hallucinogen N-N dimethyltryptamine (DMT).2 Normally, DMT is not active orally because it is enzymatically metabolized by MAO-A. However, when taken in the presence of the B caapi-derived MAO-A–inhibiting harmine alkaloids, DMT reaches the systemic circulation and produces its clinical effects.3
What are the clinical findings of serotonin toxicity?
Serotonin toxicity is a collection of clinical findings that fall under three main categories: autonomic hyperactivity, altered mental status, and muscle rigidity.5 The autonomic findings may include tachycardia, hypertension, hyperthermia, shivering, diaphoresis, or mydriasis. Altered mental status ranges from mild agitation and hypervigilance to agitated delirium to obtundation. Other neurological findings may include tremor, myoclonus, hyperreflexia, or seizures. The onset of these signs is rapid, usually occurring within minutes after exposure to one or more serotonergic compounds. Although rare, severe serotonin toxicity may be associated with hypotension and shock, leading to death.4
The diagnosis of serotonin toxicity is based on the history and physical examination of the patient. Diagnostic criteria that have been suggested include the following: (1) a recent addition or increase in a known serotonergic agent; (2) absence of other possible etiologies; (3) no recent increase or addition of a neuroleptic agent (suggesting neuroleptic malignant syndrome); and/or (4) at least 3 of the following symptoms—mental status changes, myoclonus, agitation, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, fever5 (Figure 2).
How should this patient be managed?
The management of serotonin toxicity is primarily supportive with aggressive control of hyperthermia and autonomic instability. The precipitating xenobiotic agent should be immediately discontinued. In general, treatment with intravenous fluids, cooling measures, benzodiazepines, and a nonspecific 5-HT antagonist such as cyproheptadine should greatly improve the patient’s clinical status. Patients with severe toxicity may require induced paralysis and intubation.4 It is not clear in this case if the serotonin hyperactivation was due to the DMT (5-HT2A is associated with serotonin toxicity) or another serotonergic agent (eg, dextromethorphan from a cough and cold preparation) in combination with the MAO-inhibiting harmine alkaloids.
What is the availability of ayahuasca in the United States? How is it used in its nonherbal form?
...[Ayahuasca] is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches. Many clinicians are becoming increasingly familiar with this herbal preparation since the recreational use of ayahuasca is gaining popularity in the United States. Internet fora with information on how to safely use ayahuasca, such as avoiding aged cheeses, are becoming more prevalent.7 A recent article in the New York Times described an ayahuasca gathering in Brooklyn, New York, where participants use the herb in a communal fashion.8 This herbal product is also associated with the Hollywood social scene and has received celebrity endorsements.8
The National Survey on Drug Use and Health found that the number of people in the United States who have used DMT has gone up almost every year since 2006, from an estimated 688,000 in 2006 to 1,475,000 in 2012.9 When used alone (not as ayahuasca), DMT is almost exclusively insufflated as a nasal snuff, bypassing hepatic elimination. It has an onset of around 45 seconds and a duration of 5 to 10 minutes. Insufflating DMT was historically referred to as a “businessman’s trip” because users were able to have a brief hallucinogenic experience on a lunch break and recover rapidly to perform their normal work.10
International law declares that DMT is an illegal substance and its importation is banned. However, its use for religious purposes, as is allowed for mescaline found in peyote, remains controversial.7 The UDV brought suit in United States federal court to prevent interference with the church’s use of ayahuasca during religious ceremonies based on the Religious Freedom Restoration Act. This act states that the government should not cause substantial imposition on religious practices in the absence of a compelling government interest. The court sided with the UDV, finding that the government had not sufficiently proved the alleged health risks posed by ayahuasca and could not show a substantial risk that the drug would be abused recreationally.11 Thus it is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches.
Ayahuasca is not regulated by the US Food and Drug Administration. Many different types of preparations with different ingredients as well as different concentrations may exist, and clinical variability should be expected. Understanding that ayahuasca is capable of inhibiting MAO is important in order to avoid foods and medications, such as dextromethorphan, that may trigger adverse effects.
Case Conclusion
The patient’s hospital course was complicated by an additional seizure 12 hours after her initial presentation. By 36 hours she was back to her baseline mental status with a normal neurological examination.
Dr Fil is a senior fellow in medical toxicology at North Shore University Hospital, Manhasset, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34.
- Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and Disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4(7-8):610-616.
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73-83
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11);1112-1120.
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):6;705-713.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
- Erowid. Ayahuasca Vault. https://www.erowid.org/chemicals/ayahuasca/ayahuasca.shtml. Accessed November 25, 2014.
- Morris B. Ayahuasca: a strong cup of tea. New York Times. June 13, 2014. http://www.nytimes.com/2014/06/15/fashion/ayahuasca-a-strong-cup-of-tea.html. Accessed November 25, 2014.
- Quintanilla D. DMT: Hallucinogenic Drug Used in Shamanic Rituals Goes Mainstream. 10 Dec 2013. Available: http://www.opposingviews.com/i/health/dmt-hallucinogenic-drug-used-shamanic-rituals-goes-mainstream. Last accessed 11/14/14.
- Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin North Am. 2005;89(6):1259-1276.
- Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, 546 US 418 (2006). Available at http://scholar.google.com/scholar_case?case=7036734975431570669&hl=en&as_sdt=6&as_vis=1&oi=scholarr. Accessed November 25, 2014.
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34.
- Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and Disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4(7-8):610-616.
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73-83
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11);1112-1120.
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):6;705-713.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
- Erowid. Ayahuasca Vault. https://www.erowid.org/chemicals/ayahuasca/ayahuasca.shtml. Accessed November 25, 2014.
- Morris B. Ayahuasca: a strong cup of tea. New York Times. June 13, 2014. http://www.nytimes.com/2014/06/15/fashion/ayahuasca-a-strong-cup-of-tea.html. Accessed November 25, 2014.
- Quintanilla D. DMT: Hallucinogenic Drug Used in Shamanic Rituals Goes Mainstream. 10 Dec 2013. Available: http://www.opposingviews.com/i/health/dmt-hallucinogenic-drug-used-shamanic-rituals-goes-mainstream. Last accessed 11/14/14.
- Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin North Am. 2005;89(6):1259-1276.
- Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, 546 US 418 (2006). Available at http://scholar.google.com/scholar_case?case=7036734975431570669&hl=en&as_sdt=6&as_vis=1&oi=scholarr. Accessed November 25, 2014.
Case Studies in Toxicology: Sippin’ on Some “Sizzurp”
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
Case Studies in Toxicology: A Common Procedure, an Uncommon Complication
Case
A 35-year-old woman underwent an elective hysteroscopic myomectomy to remove a symptomatic 2.7-cm uterine leiomyoma. The procedure was uncomplicated, and the patient awoke in the postanesthesia care unit (PACU) in good condition. Two hours later, however, she developed severe shortness of breath and required bilevel positive airway pressure ventilation. Her vital signs in the PACU were: blood pressure (BP), 110/70 mm Hg; heart rate, 90 beats/minute; respiratory rate, 12 breaths/minute; temperature, 98.4°F. Oxygen saturation was 94% on room air. She was diaphoretic and tachycardic on physical examination, but her pulmonary, abdominal, and gynecologic examinations were normal. During the examination, she complained of nausea, vomited, and then became increasingly lethargic and confused.
How can this patient’s clinical presentation be explained?
Uterine fibroids are the most common pelvic tumor in women.1 Hysteroscopic myomectomy is a minimally invasive surgical procedure commonly performed to resect submucosal fibroids. The procedure takes about 60 minutes, and is often performed on an outpatient basis under general anesthesia. During the procedure, an electrosurgery device called a resectoscope is inserted through the cervix. The uterine cavity is then distended with a large volume of irrigating solution. Maneuvering the resectoscope, the surgeon then shaves the protruding fibroid layer-by-layer until it aligns with the surrounding myometrium.
Surgical complications of hysteroscopic myomectomy may produce life-threatening effects. Excessive resection of the myometrium may increase blood loss, which can cause chest pain, shortness of breath, diaphoresis, lethargy, and confusion. Uterine perforation may produce hypotension, abdominal pain and distention, infection, and vaginal bleeding.
Venous Thromboembolism
Venous thromboembolism (VTE) is a common postoperative complication, with pulmonary embolism accounting for the most common preventable cause of hospital death in the United States.2 Gynecologic surgery, especially brief procedures, are associated with among the lowest rates of VTE, however, making this an unlikely explanation in this case.3 Additionally, VTE is not expected to produce the neurological findings observed in this patient.
Negative Pressure Pulmonary Edema
An uncommon but life-threatening complication for patients undergoing general anesthesia is negative pressure pulmonary edema, or “postextubation pulmonary edema,” which is estimated to occur in up to 1 in 1,000 procedures involving mechanical ventilation. During extubation, forced inspiration against a closed glottis causes intravascular fluid to be drawn into the interstitial space leading to pulmonary edema.4
Hyponatremia
An unusual but well described complication of endoscopic surgery is hyponatremia from systemic absorption of the irrigating fluid. Fluid overload may result in pulmonary edema, and dilutional hyponatremia may cause altered mental status or seizures.
Case Continuation
A chest X-ray performed after the patient became symptomatic revealed mild bilateral pulmonary edema. Her postoperative laboratory values were: sodium, 112 mEq/L; potassium, 3.3 mEq/L; chloride, 81 mEq/L; bicarbonate, 25 mEq/L; blood urea nitrogen, 18 mg/dL; creatinine, 0.6 mg/dL. Her ammonia level was 24 mmol/L (normal range, 11-35 mmol/L). An endotracheal tube was placed after her level of consciousness declined further. Her neurological examination revealed bilateral fixed and dilated pupils. An emergent computed tomography (CT) scan of the brain revealed severe generalized swelling of the brain.
What is the cause of this patient’s hyponatremia?
Monopolar electrosurgical devices such as the resectoscope cannot be used with electrolyte-containing irrigation fluids (eg, isotonic saline or lactated Ringer’s solution). Nonconductive, nonelectrolyte solutions such as glycine 1.5%, sorbitol 3%, or mannitol 5%, are the most common irrigating fluids employed to dilate the operating field and to wash away debris and blood.5
A dilutional hyponatremia can occur when the irrigating fluid is absorbed systemically. As it was first described following transurethral resection of the prostate procedures in the 1950s, the syndrome is referred to as “TURP” syndrome. Since then, several hundred life-threatening and even fatal cases of TURP syndrome have been reported.6 The syndrome occurs with other operations including transcervical resection of the endometrium (TCRE).5 The irrigating fluid is most frequently absorbed directly into the vascular system when a vein has been severed during the electrosurgery, particularly when the infusion pressure exceeds the venous pressure.6 Additionally, extravasation of the irrigating fluid into the intraperitoneal space can occur after instrument perforation of the uterine wall in TCRE, or via the fallopian tubes.6
What are the signs and symptoms of TURP syndrome?
Mild-to-moderate TURP syndrome occurs in 1% to 8% of TURP procedures performed. Fluid absorption is slightly more common during TCRE, and occurs more often during the resection of fibroids.6 The dilutional hyponatremia can result in brain edema, as well as pharmacological effects specific to the irrigant solutes.
Symptoms of TURP syndrome are primarily neurological, with nausea being the earliest sign of a mild syndrome. A “mini” mental-status test may show transient confusion with smaller absorption volumes.7 As the fluid absorption increases, the hyponatremia worsens, resulting in cerebral edema. This manifests as encephalopathy, which includes disorientation, twitching, and seizures. Hypotension is uncommon, since the fluid is being absorbed intravascularly.6 Shortness of breath, uneasiness, chest pain, and pulmonary edema may develop from systemic fluid overload. The intra-abdominal extravasation of fluid can result in abdominal pain. Other symptoms are specific to the irrigant.
Glycine
Glycine 1.5% is the most common irrigant solution used; as such, it produces the highest incidence of TURP syndrome.8 This solution is hypoosmotic (osmolality of 200 mosm/kg) compared with the normal serum (osmolality of 280 to 296 mosm/kg).5 In addition, glycine may cause visual disturbances.8 The metabolism of glycine produces ammonia, serine, and oxalate (Figure), and 10% of patients who absorb glycine show a marked hyperammonemia, further exacerbating the neurological effects.9,10
Sorbitol and mannitol
Sorbitol and mannitol irrigation fluids are used less frequently than glycine. Sorbitol 3% is metabolized to fructose and glucose, and has an osmolality of 165 mosm/kg.6 When absorbed systemically, sorbitol’s effects are similar to those of glycine, except that it does not induce visual symptoms. Mannitol 5% solution has the advantage of being isosmotic (275 mosm/kg). It is not metabolized and is excreted entirely in the urine. The excretion of mannitol creates an osmotic diuresis, thereby preventing hyponatremia from occurring.9Sorbitol and Mannitol
What are the treatment options for TURP Syndrome? Can it be prevented?
Patients with TURP syndrome in its mildest form can be asymptomatic, but severe cases can be life threatening or fatal. Unlike the treatment for hyponatremia caused by psychogenic polydipsia or the syndrome of inappropriate antidiuretic hormone, which calls for fluid restriction, plasma volume expansion is indicated in TURP syndrome, as hypovolemia and low-cardiac output develop as soon as irrigation is discontinued.
Hypertonic saline is indicated for neurological symptoms, or if the serum sodium concentration is <120mEq/L.6 Although furosemide has been used to treat acute pulmonary edema, no studies support its routine use in the treatment of fluid absorption,6 and its use may aggravate hyponatremia and hypovolemia.
Bipolar electrosurgical systems, unlike monopolar systems, permit the use of electrolyte solutions such as isotonic saline, thereby significantly reducing the risk of hyponatremia. For hysteroscopic procedures, the American College of Obstetricians and Gynecologists recommends the use of an automated fluid pump and monitoring system, thus minimizing the fluid pressure and halting or terminating the procedure before absorption thresholds are exceeded.11
Case Conclusion
The patient was immediately given a 1 mL/kg bolus of hypertonic saline. Two hours later, her serum sodium improved to 114 mEq/L and serum sodium concentration normalized over the next 24 hours. Her cardiovascular and neurological examinations worsened, however, and she required vasopressors. Her pupils remained fixed and dilated, and she lost her corneal and gag reflexes. A repeat CT of the brain showed persistent cerebral edema with signs of herniation, and she did not recover.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433-445.
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-455.
- McConkey PP. Postobstructive Pulmonary Oedema—a case series and review. Anaest Intensive Care. 2000;28(1):72-76.
- Charney AN, Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicological Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:249-264.
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96(1):8-20.
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26(1):1-5.
- Hahn RG. Glycine 1.5% for irrigation should be abandoned. Urol Int. 2013;91(3):249-255.
- Phillips DR, Milim SJ, Nathanson HG, Phillips RE, Haselkorn JS. Preventing hyponatremic encephalopathy: comparison of serum sodium and osmolality during operative hysteroscopy with 5.0% mannitol and 1.5% glycine distention media. J Am Assoc Gynecol Laparosc. 1997;4(5):567-576.
- Ghanem AN, Ward JP. Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol. 1990;66(1):71-78.
- American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106(2):439-442.
Case
A 35-year-old woman underwent an elective hysteroscopic myomectomy to remove a symptomatic 2.7-cm uterine leiomyoma. The procedure was uncomplicated, and the patient awoke in the postanesthesia care unit (PACU) in good condition. Two hours later, however, she developed severe shortness of breath and required bilevel positive airway pressure ventilation. Her vital signs in the PACU were: blood pressure (BP), 110/70 mm Hg; heart rate, 90 beats/minute; respiratory rate, 12 breaths/minute; temperature, 98.4°F. Oxygen saturation was 94% on room air. She was diaphoretic and tachycardic on physical examination, but her pulmonary, abdominal, and gynecologic examinations were normal. During the examination, she complained of nausea, vomited, and then became increasingly lethargic and confused.
How can this patient’s clinical presentation be explained?
Uterine fibroids are the most common pelvic tumor in women.1 Hysteroscopic myomectomy is a minimally invasive surgical procedure commonly performed to resect submucosal fibroids. The procedure takes about 60 minutes, and is often performed on an outpatient basis under general anesthesia. During the procedure, an electrosurgery device called a resectoscope is inserted through the cervix. The uterine cavity is then distended with a large volume of irrigating solution. Maneuvering the resectoscope, the surgeon then shaves the protruding fibroid layer-by-layer until it aligns with the surrounding myometrium.
Surgical complications of hysteroscopic myomectomy may produce life-threatening effects. Excessive resection of the myometrium may increase blood loss, which can cause chest pain, shortness of breath, diaphoresis, lethargy, and confusion. Uterine perforation may produce hypotension, abdominal pain and distention, infection, and vaginal bleeding.
Venous Thromboembolism
Venous thromboembolism (VTE) is a common postoperative complication, with pulmonary embolism accounting for the most common preventable cause of hospital death in the United States.2 Gynecologic surgery, especially brief procedures, are associated with among the lowest rates of VTE, however, making this an unlikely explanation in this case.3 Additionally, VTE is not expected to produce the neurological findings observed in this patient.
Negative Pressure Pulmonary Edema
An uncommon but life-threatening complication for patients undergoing general anesthesia is negative pressure pulmonary edema, or “postextubation pulmonary edema,” which is estimated to occur in up to 1 in 1,000 procedures involving mechanical ventilation. During extubation, forced inspiration against a closed glottis causes intravascular fluid to be drawn into the interstitial space leading to pulmonary edema.4
Hyponatremia
An unusual but well described complication of endoscopic surgery is hyponatremia from systemic absorption of the irrigating fluid. Fluid overload may result in pulmonary edema, and dilutional hyponatremia may cause altered mental status or seizures.
Case Continuation
A chest X-ray performed after the patient became symptomatic revealed mild bilateral pulmonary edema. Her postoperative laboratory values were: sodium, 112 mEq/L; potassium, 3.3 mEq/L; chloride, 81 mEq/L; bicarbonate, 25 mEq/L; blood urea nitrogen, 18 mg/dL; creatinine, 0.6 mg/dL. Her ammonia level was 24 mmol/L (normal range, 11-35 mmol/L). An endotracheal tube was placed after her level of consciousness declined further. Her neurological examination revealed bilateral fixed and dilated pupils. An emergent computed tomography (CT) scan of the brain revealed severe generalized swelling of the brain.
What is the cause of this patient’s hyponatremia?
Monopolar electrosurgical devices such as the resectoscope cannot be used with electrolyte-containing irrigation fluids (eg, isotonic saline or lactated Ringer’s solution). Nonconductive, nonelectrolyte solutions such as glycine 1.5%, sorbitol 3%, or mannitol 5%, are the most common irrigating fluids employed to dilate the operating field and to wash away debris and blood.5
A dilutional hyponatremia can occur when the irrigating fluid is absorbed systemically. As it was first described following transurethral resection of the prostate procedures in the 1950s, the syndrome is referred to as “TURP” syndrome. Since then, several hundred life-threatening and even fatal cases of TURP syndrome have been reported.6 The syndrome occurs with other operations including transcervical resection of the endometrium (TCRE).5 The irrigating fluid is most frequently absorbed directly into the vascular system when a vein has been severed during the electrosurgery, particularly when the infusion pressure exceeds the venous pressure.6 Additionally, extravasation of the irrigating fluid into the intraperitoneal space can occur after instrument perforation of the uterine wall in TCRE, or via the fallopian tubes.6
What are the signs and symptoms of TURP syndrome?
Mild-to-moderate TURP syndrome occurs in 1% to 8% of TURP procedures performed. Fluid absorption is slightly more common during TCRE, and occurs more often during the resection of fibroids.6 The dilutional hyponatremia can result in brain edema, as well as pharmacological effects specific to the irrigant solutes.
Symptoms of TURP syndrome are primarily neurological, with nausea being the earliest sign of a mild syndrome. A “mini” mental-status test may show transient confusion with smaller absorption volumes.7 As the fluid absorption increases, the hyponatremia worsens, resulting in cerebral edema. This manifests as encephalopathy, which includes disorientation, twitching, and seizures. Hypotension is uncommon, since the fluid is being absorbed intravascularly.6 Shortness of breath, uneasiness, chest pain, and pulmonary edema may develop from systemic fluid overload. The intra-abdominal extravasation of fluid can result in abdominal pain. Other symptoms are specific to the irrigant.
Glycine
Glycine 1.5% is the most common irrigant solution used; as such, it produces the highest incidence of TURP syndrome.8 This solution is hypoosmotic (osmolality of 200 mosm/kg) compared with the normal serum (osmolality of 280 to 296 mosm/kg).5 In addition, glycine may cause visual disturbances.8 The metabolism of glycine produces ammonia, serine, and oxalate (Figure), and 10% of patients who absorb glycine show a marked hyperammonemia, further exacerbating the neurological effects.9,10
Sorbitol and mannitol
Sorbitol and mannitol irrigation fluids are used less frequently than glycine. Sorbitol 3% is metabolized to fructose and glucose, and has an osmolality of 165 mosm/kg.6 When absorbed systemically, sorbitol’s effects are similar to those of glycine, except that it does not induce visual symptoms. Mannitol 5% solution has the advantage of being isosmotic (275 mosm/kg). It is not metabolized and is excreted entirely in the urine. The excretion of mannitol creates an osmotic diuresis, thereby preventing hyponatremia from occurring.9Sorbitol and Mannitol
What are the treatment options for TURP Syndrome? Can it be prevented?
Patients with TURP syndrome in its mildest form can be asymptomatic, but severe cases can be life threatening or fatal. Unlike the treatment for hyponatremia caused by psychogenic polydipsia or the syndrome of inappropriate antidiuretic hormone, which calls for fluid restriction, plasma volume expansion is indicated in TURP syndrome, as hypovolemia and low-cardiac output develop as soon as irrigation is discontinued.
Hypertonic saline is indicated for neurological symptoms, or if the serum sodium concentration is <120mEq/L.6 Although furosemide has been used to treat acute pulmonary edema, no studies support its routine use in the treatment of fluid absorption,6 and its use may aggravate hyponatremia and hypovolemia.
Bipolar electrosurgical systems, unlike monopolar systems, permit the use of electrolyte solutions such as isotonic saline, thereby significantly reducing the risk of hyponatremia. For hysteroscopic procedures, the American College of Obstetricians and Gynecologists recommends the use of an automated fluid pump and monitoring system, thus minimizing the fluid pressure and halting or terminating the procedure before absorption thresholds are exceeded.11
Case Conclusion
The patient was immediately given a 1 mL/kg bolus of hypertonic saline. Two hours later, her serum sodium improved to 114 mEq/L and serum sodium concentration normalized over the next 24 hours. Her cardiovascular and neurological examinations worsened, however, and she required vasopressors. Her pupils remained fixed and dilated, and she lost her corneal and gag reflexes. A repeat CT of the brain showed persistent cerebral edema with signs of herniation, and she did not recover.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 35-year-old woman underwent an elective hysteroscopic myomectomy to remove a symptomatic 2.7-cm uterine leiomyoma. The procedure was uncomplicated, and the patient awoke in the postanesthesia care unit (PACU) in good condition. Two hours later, however, she developed severe shortness of breath and required bilevel positive airway pressure ventilation. Her vital signs in the PACU were: blood pressure (BP), 110/70 mm Hg; heart rate, 90 beats/minute; respiratory rate, 12 breaths/minute; temperature, 98.4°F. Oxygen saturation was 94% on room air. She was diaphoretic and tachycardic on physical examination, but her pulmonary, abdominal, and gynecologic examinations were normal. During the examination, she complained of nausea, vomited, and then became increasingly lethargic and confused.
How can this patient’s clinical presentation be explained?
Uterine fibroids are the most common pelvic tumor in women.1 Hysteroscopic myomectomy is a minimally invasive surgical procedure commonly performed to resect submucosal fibroids. The procedure takes about 60 minutes, and is often performed on an outpatient basis under general anesthesia. During the procedure, an electrosurgery device called a resectoscope is inserted through the cervix. The uterine cavity is then distended with a large volume of irrigating solution. Maneuvering the resectoscope, the surgeon then shaves the protruding fibroid layer-by-layer until it aligns with the surrounding myometrium.
Surgical complications of hysteroscopic myomectomy may produce life-threatening effects. Excessive resection of the myometrium may increase blood loss, which can cause chest pain, shortness of breath, diaphoresis, lethargy, and confusion. Uterine perforation may produce hypotension, abdominal pain and distention, infection, and vaginal bleeding.
Venous Thromboembolism
Venous thromboembolism (VTE) is a common postoperative complication, with pulmonary embolism accounting for the most common preventable cause of hospital death in the United States.2 Gynecologic surgery, especially brief procedures, are associated with among the lowest rates of VTE, however, making this an unlikely explanation in this case.3 Additionally, VTE is not expected to produce the neurological findings observed in this patient.
Negative Pressure Pulmonary Edema
An uncommon but life-threatening complication for patients undergoing general anesthesia is negative pressure pulmonary edema, or “postextubation pulmonary edema,” which is estimated to occur in up to 1 in 1,000 procedures involving mechanical ventilation. During extubation, forced inspiration against a closed glottis causes intravascular fluid to be drawn into the interstitial space leading to pulmonary edema.4
Hyponatremia
An unusual but well described complication of endoscopic surgery is hyponatremia from systemic absorption of the irrigating fluid. Fluid overload may result in pulmonary edema, and dilutional hyponatremia may cause altered mental status or seizures.
Case Continuation
A chest X-ray performed after the patient became symptomatic revealed mild bilateral pulmonary edema. Her postoperative laboratory values were: sodium, 112 mEq/L; potassium, 3.3 mEq/L; chloride, 81 mEq/L; bicarbonate, 25 mEq/L; blood urea nitrogen, 18 mg/dL; creatinine, 0.6 mg/dL. Her ammonia level was 24 mmol/L (normal range, 11-35 mmol/L). An endotracheal tube was placed after her level of consciousness declined further. Her neurological examination revealed bilateral fixed and dilated pupils. An emergent computed tomography (CT) scan of the brain revealed severe generalized swelling of the brain.
What is the cause of this patient’s hyponatremia?
Monopolar electrosurgical devices such as the resectoscope cannot be used with electrolyte-containing irrigation fluids (eg, isotonic saline or lactated Ringer’s solution). Nonconductive, nonelectrolyte solutions such as glycine 1.5%, sorbitol 3%, or mannitol 5%, are the most common irrigating fluids employed to dilate the operating field and to wash away debris and blood.5
A dilutional hyponatremia can occur when the irrigating fluid is absorbed systemically. As it was first described following transurethral resection of the prostate procedures in the 1950s, the syndrome is referred to as “TURP” syndrome. Since then, several hundred life-threatening and even fatal cases of TURP syndrome have been reported.6 The syndrome occurs with other operations including transcervical resection of the endometrium (TCRE).5 The irrigating fluid is most frequently absorbed directly into the vascular system when a vein has been severed during the electrosurgery, particularly when the infusion pressure exceeds the venous pressure.6 Additionally, extravasation of the irrigating fluid into the intraperitoneal space can occur after instrument perforation of the uterine wall in TCRE, or via the fallopian tubes.6
What are the signs and symptoms of TURP syndrome?
Mild-to-moderate TURP syndrome occurs in 1% to 8% of TURP procedures performed. Fluid absorption is slightly more common during TCRE, and occurs more often during the resection of fibroids.6 The dilutional hyponatremia can result in brain edema, as well as pharmacological effects specific to the irrigant solutes.
Symptoms of TURP syndrome are primarily neurological, with nausea being the earliest sign of a mild syndrome. A “mini” mental-status test may show transient confusion with smaller absorption volumes.7 As the fluid absorption increases, the hyponatremia worsens, resulting in cerebral edema. This manifests as encephalopathy, which includes disorientation, twitching, and seizures. Hypotension is uncommon, since the fluid is being absorbed intravascularly.6 Shortness of breath, uneasiness, chest pain, and pulmonary edema may develop from systemic fluid overload. The intra-abdominal extravasation of fluid can result in abdominal pain. Other symptoms are specific to the irrigant.
Glycine
Glycine 1.5% is the most common irrigant solution used; as such, it produces the highest incidence of TURP syndrome.8 This solution is hypoosmotic (osmolality of 200 mosm/kg) compared with the normal serum (osmolality of 280 to 296 mosm/kg).5 In addition, glycine may cause visual disturbances.8 The metabolism of glycine produces ammonia, serine, and oxalate (Figure), and 10% of patients who absorb glycine show a marked hyperammonemia, further exacerbating the neurological effects.9,10
Sorbitol and mannitol
Sorbitol and mannitol irrigation fluids are used less frequently than glycine. Sorbitol 3% is metabolized to fructose and glucose, and has an osmolality of 165 mosm/kg.6 When absorbed systemically, sorbitol’s effects are similar to those of glycine, except that it does not induce visual symptoms. Mannitol 5% solution has the advantage of being isosmotic (275 mosm/kg). It is not metabolized and is excreted entirely in the urine. The excretion of mannitol creates an osmotic diuresis, thereby preventing hyponatremia from occurring.9Sorbitol and Mannitol
What are the treatment options for TURP Syndrome? Can it be prevented?
Patients with TURP syndrome in its mildest form can be asymptomatic, but severe cases can be life threatening or fatal. Unlike the treatment for hyponatremia caused by psychogenic polydipsia or the syndrome of inappropriate antidiuretic hormone, which calls for fluid restriction, plasma volume expansion is indicated in TURP syndrome, as hypovolemia and low-cardiac output develop as soon as irrigation is discontinued.
Hypertonic saline is indicated for neurological symptoms, or if the serum sodium concentration is <120mEq/L.6 Although furosemide has been used to treat acute pulmonary edema, no studies support its routine use in the treatment of fluid absorption,6 and its use may aggravate hyponatremia and hypovolemia.
Bipolar electrosurgical systems, unlike monopolar systems, permit the use of electrolyte solutions such as isotonic saline, thereby significantly reducing the risk of hyponatremia. For hysteroscopic procedures, the American College of Obstetricians and Gynecologists recommends the use of an automated fluid pump and monitoring system, thus minimizing the fluid pressure and halting or terminating the procedure before absorption thresholds are exceeded.11
Case Conclusion
The patient was immediately given a 1 mL/kg bolus of hypertonic saline. Two hours later, her serum sodium improved to 114 mEq/L and serum sodium concentration normalized over the next 24 hours. Her cardiovascular and neurological examinations worsened, however, and she required vasopressors. Her pupils remained fixed and dilated, and she lost her corneal and gag reflexes. A repeat CT of the brain showed persistent cerebral edema with signs of herniation, and she did not recover.
Dr Nguyen is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433-445.
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-455.
- McConkey PP. Postobstructive Pulmonary Oedema—a case series and review. Anaest Intensive Care. 2000;28(1):72-76.
- Charney AN, Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicological Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:249-264.
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96(1):8-20.
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26(1):1-5.
- Hahn RG. Glycine 1.5% for irrigation should be abandoned. Urol Int. 2013;91(3):249-255.
- Phillips DR, Milim SJ, Nathanson HG, Phillips RE, Haselkorn JS. Preventing hyponatremic encephalopathy: comparison of serum sodium and osmolality during operative hysteroscopy with 5.0% mannitol and 1.5% glycine distention media. J Am Assoc Gynecol Laparosc. 1997;4(5):567-576.
- Ghanem AN, Ward JP. Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol. 1990;66(1):71-78.
- American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106(2):439-442.
- Buttram VC Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433-445.
- Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163(14):1711-1717.
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90(3):446-455.
- McConkey PP. Postobstructive Pulmonary Oedema—a case series and review. Anaest Intensive Care. 2000;28(1):72-76.
- Charney AN, Hoffman RS. Fluid, Electrolyte, and Acid-Base Principles. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicological Emergencies. 9th ed. New York, NY: McGraw Hill; 2010:249-264.
- Hahn RG. Fluid absorption in endoscopic surgery. Br J Anaesth. 2006;96(1):8-20.
- Nilsson A, Hahn RG. Mental status after transurethral resection of the prostate. Eur Urol. 1994;26(1):1-5.
- Hahn RG. Glycine 1.5% for irrigation should be abandoned. Urol Int. 2013;91(3):249-255.
- Phillips DR, Milim SJ, Nathanson HG, Phillips RE, Haselkorn JS. Preventing hyponatremic encephalopathy: comparison of serum sodium and osmolality during operative hysteroscopy with 5.0% mannitol and 1.5% glycine distention media. J Am Assoc Gynecol Laparosc. 1997;4(5):567-576.
- Ghanem AN, Ward JP. Osmotic and metabolic sequelae of volumetric overload in relation to the TUR syndrome. Br J Urol. 1990;66(1):71-78.
- American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106(2):439-442.
Case Studies in Toxicology: Hot as a Hare and Red as a Beet
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
Case Studies in Toxicology: A Patchwork of Problems in Parkinson Patients
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 76-year-old man with Parkinson disease (PD) and hypertension presented to the ED with acute onset of severe tremulousness, blurred vision, salivation, lacrimation, diffuse muscle aches, and extremity weakness. His initial vital signs were: blood pressure, 175/74 mm Hg; heart rate, 62 beats/minute; respiratory rate, 16 breaths/minute; temperature, 37°C (98.6°F). Oxygen saturation was 100% on room air. On physical examination, the patient had excessive lacrimation and salivation, a coarse resting tremor, and 2/5 strength in both the upper and lower extremities. The remainder of the examination, including abdominal and pulmonary systems, was unremarkable compared with baseline findings.
How does the pathophysiology of PD explain how treatments are targeted?
What medications are used to treat PD? What are some associated complications?
Dopamine Precursors and Agonists
(L-dopa) can be combined with the L-amino acid decarboxylase inhibitor carbidopa to prevent peripheral metabolism by this enzyme and thereby increase brain concentrations of DA following metabolism by DA decarboxylase in the central nervous system (CNS).1 Dopamine agonists, including bromocriptine, ropinirole, and pramipexole, do not depend on endogenous conversion to DA and have substantially longer durations of action, limiting the dose-related fluctuations in motor function common in some PD patients taking L-dopa.1 For these reasons, DA agonists have often replaced L-dopa as initial treatment, especially in younger patients. Catechol-O-methyltransferase inhibitors (tolcapone, entacapone) prevent peripheral breakdown of DA, allowing a higher fraction to reach the CNS.
With respect to side effects, all of the dopaminergic medications can cause nausea, hallucinations, confusion, and orthostatic hypotension.
Anticholinergic Drugs
Although the precise mechanism by which anticholinergic drugs improve PD is not fully understood, agents such as trihexyphenidyl, benztropine mesylate, and diphenhydramine hydrochloride were prescribed even before the discovery of L-dopa and continue to be used today.1 Adverse effects are a function of the antimuscarinic (anticholinergic) properties of the drugs and may include mydriasis and blurred vision, dry flushed skin, tachycardia, hyperthermia, constipation, urinary retention, and altered mental status.
Amantadine
In addition to the anticholinergics, amantadine is also used to treat PD. This antiviral agent alters DA release in the brain, produces anticholinergic effects, and blocks N-methyl-D-aspartate glutamate receptors.1 Common adverse drug effects include anticholinergic signs as well as nausea, vomiting, dizziness, lethargy, and sleep disturbance, all of which are usually mild and reversible.
Case Continuation
A review of the patient’s medication history revealed he has been taking L-dopa/carbidopa. In addition to L-dopa/carbidopa, he was recently prescribed transdermal rivastigmine patches (13.3 mg/24 h). At bedtime the evening prior to presentation, the patient applied more than 20 rivastigmine patches. Approximately 5 hours later, he awoke with the previously described findings whereupon his wife removed the patches and brought him to the ED.
What is rivastigmine and what is its role in PD
Rivastigmine is a carbamate-type cholinesterase inhibitor (CEI) indicated for the treatment of mild-to-moderate dementia associated with PD and Alzheimer disease.2 Tacrine, a medicinal noncarbamate CEI, is also prescribed for this use.2 Both drugs increase ACh concentrations in relevant brain regions and foster the formation of new memory.
Cholinesterase inhibitors are mechanistically analogous to the insecticidal carbamates (eg, aldicarb) and the organophosphates (OPs) (eg, malathion). They inhibit the metabolism of ACh by acetylcholinesterase (AChE) in the various cholinergic synapses, increasing the intrasynaptic concentration of ACh.
Additional AChEs include physostigmine, a carbamate commonly used in the ED to treat anticholinergic toxicity. Physostigmine raises the local synaptic concentration of ACh to compete for the muscarinic ACh receptor with drugs such as diphenhydramine or atropine. Other CEIs (eg, neostigmine, pyridostigmine, edrophonium) are used to raise intrasynaptic ACh concentrations and overcome antibody blockade of nicotinic ACh receptors at the neuromuscular junction in patients with myasthenia gravis.
What is the toxidrome associated with carbamate overdose
Carbamate toxicity, as manifested by the cholinergic toxidrome, largely resembles OP toxicity but with an important difference: Both OPs and carbamates function by binding to and inhibiting AChE; however, the carbamate-AChE bond undergoes spontaneous hydrolysis, thereby reactivating the enzyme. Consequently, the clinical effects of carbamate toxicity, though potentially severe, are self-limited and usually only last 24 hours or less.4
How should this patient be managed?
The general approach to a patient with medical carbamate toxicity is similar to that of a patient with OP poisoning. Dermal exposure, as is the case with this patient, should prompt skin decontamination to minimize ongoing exposure. Patch removal is necessary but is not sufficient to prevent ongoing absorption, since a depot of medication typically forms in the dermal tissue. In the presence of significant or life-threatening muscarinic effects (eg, bronchorrhea, bronchospasm, seizure), an antimuscarinic agent such as atropine is indicated. Various dosing schemes of atropine exist; at our institution, we recommend an initial dose of 1 to 3 mg intravenously (IV), with escalating doses every 5 minutes until reversal of bronchorrhea and bronchospasm occur.4 This is followed by initiation of an atropine infusion at a rate of 10% to 20% of the total loading dose per hour (to a maximum of 2 mg/h).4
Pralidoxime (2-PAM) and other oximes, accelerate the reactivation of carbamate-inhibited AChE and have effects at both the nicotinic and muscarinic synapses. Reactivation results in the enhanced metabolism of intrasynaptic ACh and decreased clinical cholinergic effects. Since atropine is only effective at muscarinic receptors, oximes were administered in this case to reverse neuromuscular weakness.
Although early administration of 2-PAM is indicated in the setting of significant OP poisoning (due to irreversible inhibition of AChE), its use for medical carbamate toxicity is controversial. Early animal studies of carbamate toxicity suggested that treatment with oximes worsened outcomes; however, this has not been demonstrated in more recent studies.5,6 Therefore, although 2-PAM may be beneficial in treating cases of clinically significant carbamate poisoning (which can be prolonged and severe), these benefits should be weighed against the potential risks.
Case Conclusion
Upon arrival to the ED, the patient’s skin was cleansed thoroughly. As he did not exhibit muscarinic findings of bradycardia, bronchoconstriction, or bronchorrhea, atropine was not indicated. He was treated conservatively with IV fluid hydration and admitted to the medicine floor. Since he continued to exhibit profound extremity weakness with no improvement 12 hours from the onset of symptoms, pralidoxime 1 g IV was administered over a 30-minute period. Shortly thereafter, patient’s motor strength improved from 2/5 to 4/5 in both upper and lower extremities. No complications were noted, and the patient‘s weakness and tremulousness continued to resolve. He was transferred to a skilled nursing facility on hospital day 6.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
- Standaert DG, Roberson ED. Treatment of central nervous system degenerative disorders. In: Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman’s The Pharmacologic Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011:609-628
- Rösler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633-638.
- Exelon Patch [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
- Eddleston M, Clark RF. Insecticides: organic phosphorus compounds and carbamates. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011:1450-1466.
- Natoff IL, Reiff B. Effect of oximes on the acute toxicity of anticholinesterase carbamates. Toxicol Appl Pharmacol. 1973;25(4):569-575.
- Mercurio-Zappala M, Hack JB, Salvador A, Hoffman RS. Pralidoxime in carbaryl poisoning: an animal model. Hum Exp Toxicol. 2007;26(2)125-129.
Tiny Bubbles: Or, the Dangers of Cleaning Fruit
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
Case Studies in Toxicology: Tiny Bubbles (Or, the Dangers of Cleaning Your Fruit)
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.