User login
Safe Opioid Prescribing for Acute Noncancer Pain in Hospitalized Adults: A Systematic Review of Existing Guidelines
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
Pain is prevalent among hospitalized patients, occurring in 52%-71% of patients in cross-sectional surveys.1-3 Opioid administration is also common, with more than half of nonsurgical patients in United States (US) hospitals receiving at least one dose of opioid during hospitalization.4 Studies have also begun to define the degree to which hospital prescribing contributes to long-term use. Among opioid-naïve patients admitted to the hospital, 15%-25% fill an opioid prescription in the week after hospital discharge,5,6 43% of such patients fill another opioid prescription 90 days postdischarge,6 and 15% meet the criteria for long-term use at one year.7 With about 37 million discharges from US hospitals each year,8 these estimates suggest that hospitalization contributes to initiation of long-term opioid use in millions of adults each year.
Additionally, studies in the emergency department and hospital settings demonstrate large variations in prescribing of opioids between providers and hospitals.4,9 Variation unrelated to patient characteristics highlights areas of clinical uncertainty and the corresponding need for prescribing standards and guidance. To our knowledge, there are no existing guidelines on safe prescribing of opioids in hospitalized patients, aside from guidelines specifically focused on the perioperative, palliative care, or end-of-life settings.
Thus, in the context of the current opioid epidemic, the Society of Hospital Medicine (SHM) sought to develop a consensus statement to assist clinicians practicing medicine in the inpatient setting in safe prescribing of opioids for acute, noncancer pain on the medical services. We define “safe” prescribing as proposed by Aronson: “a process that recommends a medicine appropriate to the patient’s condition and minimizes the risk of undue harm from it.”10 To inform development of the consensus statement, SHM convened a working group to systematically review existing guidelines on the more general management of acute pain. This article describes the methods and results of our systematic review of existing guidelines for managing acute pain. The Consensus Statement derived from these existing guidelines, applied to the hospital setting, appears in a companion article.
METHODS
Steps in the systematic review process included: 1) searching for relevant guidelines, 2) applying exclusion criteria, 3) assessing the quality of the guidelines, and 4) synthesizing guideline recommendations to identify issues potentially relevant to medical inpatients with acute pain. Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=71846.
Data Sources and Search Terms
Guideline Inclusion/Exclusion Criteria
We defined guidelines as statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harm of alternative care options, consistent with the National Academies’ definition.11 To be eligible, guidelines had to be published in English and include recommendations on prescribing opioids for acute, noncancer pain. We excluded guidelines focused on chronic pain or palliative care, guidelines derived entirely from another guideline, and guidelines published before 2010, since such guidelines may contain outdated information.12 Because we were interested in general principles regarding safe use of opioids for managing acute pain, we excluded guidelines that focused exclusively on specific disease processes (eg, cancer, low-back pain, and sickle cell anemia). As we were specifically interested in the management of acute pain in the hospital setting, we also excluded guidelines that focused exclusively on specific nonhospital settings of care (eg, outpatient care clinics and nursing homes). We included guidelines related to care in the emergency department (ED) given the hospital-based location of care and the high degree of similarity in scope of practice and patient population, as most hospitalized adults are admitted through the ED. Finally, we excluded guidelines focusing on management in the intensive care setting (including the post-anesthesia care unit) given the inherent differences in patient population and management options between the intensive and nonintensive care areas of the hospital.
Guideline Quality Assessment
Guideline Synthesis and Analysis
We extracted recommendations from each guideline related to the following topics: 1) deciding when to use opioids, nonopioid medications, and nonmedication-based pain management modalities, 2) best practices in screening/monitoring/education prior to prescribing an opioid and/or during treatment, 3) opioid selection considerations, including selection of dose, duration, and route of administration, 4) strategies to minimize the risk of opioid-related adverse events, and 5) safe practices on discharge.
Role of the Funding Source
The Society of Hospital Medicine provided administrative and material support for the project, but had no role in the design or execution of the scientific evaluation.
RESULTS
Guideline Quality Assessment
See Table 1 for the AGREE II scaled domain scores, and Appendix Table 1 for the ratings on each individual item within a domain. The range of scaled scores for each of the AGREE II domains were as follows: Scope and purpose 52%-89%, stakeholder involvement 30%-81%, rigor of development 46%-81%, clarity of presentation 59%-72%, applicability 10%-57%, and editorial independence 42%-78%. Overall guideline assessment scores ranged from 4 to 5.33 on a scale from 1 to 7. Three of the guidelines (NICE, ACOEM, and WSAMDG)16,17,19 were recommended for use without modification by 2 out of 3 guideline appraisers, and one of the guidelines (ACEP)18 was recommended for use with modification by all 3 appraisers. The guideline by NICE19 was rated the highest both overall (5.33), and on 4 of the 6 AGREE II domains.
Although the guidelines each included a systematic review of the literature, the NICE19 and WSAMDG17 guidelines did not include the strength of recommendations or provide clear links between each recommendation and the underlying evidence base. When citations were present, we reviewed them to determine the type of data upon which the recommendations were based and included this information in Table 2. The majority of the recommendations in Table 2 are based on expert opinion alone, or other guidelines.
Guideline Synthesis and Analysis
Table 2 contains a synthesis of the recommendations related to each of our 5 prespecified content areas. Despite the generally low quality of the evidence supporting the recommendations, there were many areas of concordance across guidelines.
Deciding When to Use Opioids, Nonopioid Medications, and Nonmedication-Based Pain Management Modalities
Three out of 4 guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy,16-18 2 guidelines recommended treating mild to moderate pain with nonopioid medications, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs),16,17 and 2 guidelines recommended co-prescribing opioids with nonopioid analgesic medications to reduce total opioid requirements and improve pain control.16,17 Each of these recommendations was supported by at least one randomized controlled trial.
Best Practices in Screening/Monitoring/Education to Occur Prior to Prescribing an Opioid and/or During Treatment
Three guidelines recommended checking prescription drug monitoring programs (PDMPs), all based on expert consensus.16-18 Only the WSAMDG guideline offered guidance as to the optimal timing to check the PDMP in this setting, specifically recommending to check before prescribing opioids.17 Two guidelines also recommended helping patients set reasonable expectations about their recovery and educating patients about the risks/side effects of opioid therapy, all based on expert consensus or other guidelines.17,19
Opioid Selection Considerations, Including Selection of Dose, Duration, and Route of Administration
Three guidelines recommended using the lowest effective dose, supported by expert consensus and observational data in the outpatient setting demonstrating that overdose risk increases with opioid dose.16-18 Three guidelines recommended using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain based on expert consensus.16-18 Two guidelines recommended using as-needed rather than scheduled dosing of opioids based on expert recommendation.16, 17
Strategies to Minimize the Risk of Opioid-Related Adverse Events
Several strategies to minimize the risk of opioid-related adverse events were identified, but most were only recommended by a single guideline. Strategies recommended by more than one guideline included using a recognized opioid dose conversion guide when prescribing, reviewing, or changing opioid prescriptions (based on expert consensus);16,19 avoiding co-administration of parenteral and oral as-needed opioids, and if as-needed opioids from different routes are necessary, providing a clear indication for use of each (based on expert consensus and other guidelines);17,19 and avoiding/using caution when co-prescribing opioids with other central nervous system depressant medications16,17 (supported by observational studies demonstrating increased risk in the outpatient setting).
Safe Practices on Discharge
All 4 of the guidelines recommended prescribing a limited duration of opioids for the acute pain episode; however the maximum recommended duration varied widely from one week to 30 days.16-19 It is important to note that because these guidelines were not focused on hospitalization specifically, these maximum recommended durations of use reflect the entire acute pain episode (ie, not prescribing on discharge specifically). The guideline with the longest maximum recommended duration was from NICE, based in the United Kingdom, while the US-based guideline development groups uniformly recommended 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
DISCUSSION
This systematic review identified only 4 existing guidelines that included recommendations on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions, specific nonhospital settings, or the intensive care setting. Although 2 of the identified guidelines offered sparse recommendations specific to the hospital setting, we found no guidelines that focused exclusively on the period of hospitalization specifically outside of the perioperative period. Furthermore, the guideline recommendations were largely based on expert opinion. Although these factors limit the confidence with which the recommendations can be applied to the hospital setting, they nonetheless represent the best guidance currently available to standardize and improve the safety of prescribing opioids in the hospital setting.
This paucity of guidance specific to patients hospitalized in general, nonintensive care areas of the hospital is important because pain management in this setting differs in a number of ways from pain management in the ambulatory or intensive care unit settings (including the post-anesthesia care unit). First, there are differences in the monitoring strategies that are available in each of these settings (eg, variability in nurse-to-patient ratios, frequency of measuring vital signs, and availability of continuous pulse oximetry/capnography). Second, there are differences in available/feasible routes of medication administration depending on the setting of care. Finally, there are differences in the patients themselves, including severity of illness, baseline and expected functional status, pain severity, and ability to communicate.
Accordingly, to avoid substantial heterogeneity in recommendations obtained from this review, we chose to focus on guidelines most relevant to clinicians practicing medicine in nonintensive care areas of the hospital. This resulted in the exclusion of 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management and included use of advanced management procedures beyond the scope of practice for general internists,20,21 and one guideline that focused on management in the intensive care unit.22 Within the set of guidelines included in this review, we did include recommendations designated for the postoperative period that we felt were relevant to the care of hospitalized patients more generally. In fact, the ACOEM guideline, which includes postoperative recommendations, specifically noted that these recommendations are mostly comparable to those for treating acute pain more generally.16
In addition to the lack of guidance specific to the setting in which most hospitalists practice, most of the recommendations in the existing guidelines are based on expert consensus. Guidelines based on expert opinion typically carry a lower strength of recommendation, and, accordingly, should be applied with some caution and accompanied by diligent tracking of outcome metrics, as these recommendations are applied to local health systems. Recommendations may have unintended consequences that are not necessarily apparent at the outset, and the specific circumstances of each patient must be considered when deciding how best to apply recommendations. Additional research will be necessary to track the impact of the recommended prescribing practices on patient outcomes, particularly given that many states have already begun instituting regulations on safe opioid prescribing despite the limited nature of the evidence. Furthermore, although several studies have identified patient- and prescribing-related risk factors for opioid-related adverse events in surgical patient populations, given the differences in patient characteristics and prescribing patterns in these settings, research to understand the risk factors in hospitalized medical patients specifically is important to inform evidence-based, safe prescribing recommendations in this setting.
Despite the largely expert consensus-based nature of the recommendations, we found substantial overlap in the recommendations between the guidelines, spanning our prespecified topics of interest related to safe prescribing. Most guidelines recommended restricting opioid use to severe pain or pain that has not responded to nonopioid therapy, checking PDMPs, using the lowest effective dose, and using short-acting opioids and/or avoiding use of long-acting/extended-release opioids for acute pain. There was less consensus on risk mitigation strategies, where the majority of recommendations were endorsed by only 1 or 2 guidelines. Finally, all 4 guidelines recommended prescribing a limited duration of opioids for the acute pain episode, with US-based guidelines recommending 1 to 2 weeks as the maximum duration of opioid use, including the period of hospitalization.
There are limitations to our evaluation. As previously noted, in order to avoid substantial heterogeneity in management recommendations, we excluded 2 guidelines intended for anesthesiologists that focused exclusively on perioperative management,20,21 and one guideline focused on management in the intensive care unit.22 Accordingly, recommendations contained in this review may or may not be applicable to those settings, and readers interested in those settings specifically are directed to those guidelines. Additionally, we decided to exclude guidelines that focused on managing acute pain in specific conditions (eg, sickle cell disease and pancreatitis) because our goal was to identify generalizable principles of safe prescribing of opioids that apply regardless of clinical condition. Despite this goal, it is important to recognize that not all of the recommendations are generalizable to all types of pain; clinicians interested in management principles specific to certain disease states are encouraged to review disease-specific informational material. Finally, although we used rigorous, pre-defined search criteria and registered our protocol on PROSPERO, it is possible that our search strategy missed relevant guidelines.
In conclusion, we identified few guidelines on safe opioid prescribing practices for managing acute, noncancer pain, outside of the context of specific conditions or nonhospital settings, and no guidelines focused on acute pain management in general, nonintensive care areas of the hospital specifically. Nevertheless, the guidelines that we identified make consistent recommendations related to our prespecified topic areas of relevance to the hospital setting, although most recommendations are based exclusively on expert opinion. Our systematic review nonetheless provides guidance in an area where guidance has thus far been limited. Future research should investigate risk factors for opioid-related adverse events in hospitalized, nonsurgical patients, and the effectiveness of interventions designed to reduce their occurrence.
ACKNOWLEDGMENTS
Dr. Herzig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to acknowledge and thank Kevin Vuernick, Jenna Goldstein, Meghan Mallouk, and Chris Frost, MD, from SHM for their facilitation of this project and dedication to this purpose.
Disclosures: Dr. Herzig received compensation from the Society of Hospital Medicine for her editorial role at the Journal of Hospital Medicine (unrelated to the present work). Dr. Jena received consulting fees from Pfizer, Inc., Hill Rom Services, Inc., Bristol Myers Squibb, Novartis Pharmaceuticals, Vertex Pharmaceuticals, and Precision Health Economics (all unrelated to the present work). None of the other authors have any conflicts of interest to disclose.
Funding: The Society of Hospital Medicine (SHM) provided administrative assistance and material support, but had no role in or influence on the scientific conduct of the study. Dr. Herzig was funded by grant number K23AG042459 from the National Institute on Aging. Dr. Mosher was supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations and Office of Research and Development and Health Services Research and Development Service (HSR&D) through the Comprehensive Access and Delivery Research and Evaluation Center (CIN 13-412). None of the funding agencies had involvement in any aspect of the study, including design, conduct, or reporting of the study
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
1. Melotti RM, Samolsky-Dekel BG, Ricchi E, et al. Pain prevalence and predictors among inpatients in a major Italian teaching hospital. A baseline survey towards a pain free hospital. Eur J Pain. 2005;9(5):485-495. PubMed
2. Sawyer J, Haslam L, Robinson S, Daines P, Stilos K. Pain prevalence study in a large Canadian teaching hospital. Pain Manag Nurs. 2008;9(3):104-112. PubMed
3. Strohbuecker B, Mayer H, Evers GC, Sabatowski R. Pain prevalence in hospitalized patients in a German university teaching hospital. J Pain Symptom Manage. 2005;29(5):498-506. PubMed
4. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
5. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2015;31(5):478-485. PubMed
6. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to medicare neneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
7. Mosher HJ, Hofmeyer B, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. JHM. Accepted for Publication November 11, 2017. PubMed
8. Weiss AJ, Elixhauser A. Overview of hospital stays in the United States, 2012. HCUP Statistical Brief #180. 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf. Accessed June 29, 2015. PubMed
9. Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med. 2017;376(7):663-673. PubMed
10. Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62(6):629-632. PubMed
11. IOM (Institute of Medicine). 2011. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press.
12. Shekelle PG, Ortiz E, Rhodes S, et al. Validity of the agency for healthcare research and quality clinical practice guidelines: How quickly do guidelines become outdated? JAMA. 2001;286(12):1461-1467. PubMed
13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839-E842. PubMed
14. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 1: performance, usefulness and areas for improvement. CMAJ. 2010;182(10):1045-1052. PubMed
15. Brouwers MC, Kho ME, Browman GP, et al. Development of the AGREE II, part 2: Assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472-E478. PubMed
16. Hegmann KT, Weiss MS, Bowden K, et al. ACOEM practice guidelines: opioids for treatment of acute, subacute, chronic, and postoperative pain. J Occup Environ Med. 2014;56(12):e143-e159. PubMed
17. Washington State Agency Medical Directors’ Group. Interagency Guideline on Prescribing Opioids for Pain. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed December 5, 2017.
18. Cantrill SV, Brown MD, Carlisle RJ, et al. Clinical policy: critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499-525. PubMed
19. National Institute for Healthcare Excellence. Controlled drugs: Safe use and management. https://www.nice.org.uk/guidance/ng46/chapter/Recommendations. Accessed December 5, 2017.
20. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. PubMed
21. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology. 2013;118(2):291-307. PubMed
22. Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263-306. PubMed
© 2018 Society of Hospital Medicine
Screening for depression in hospitalized medical patients
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
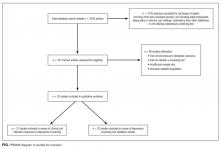
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.
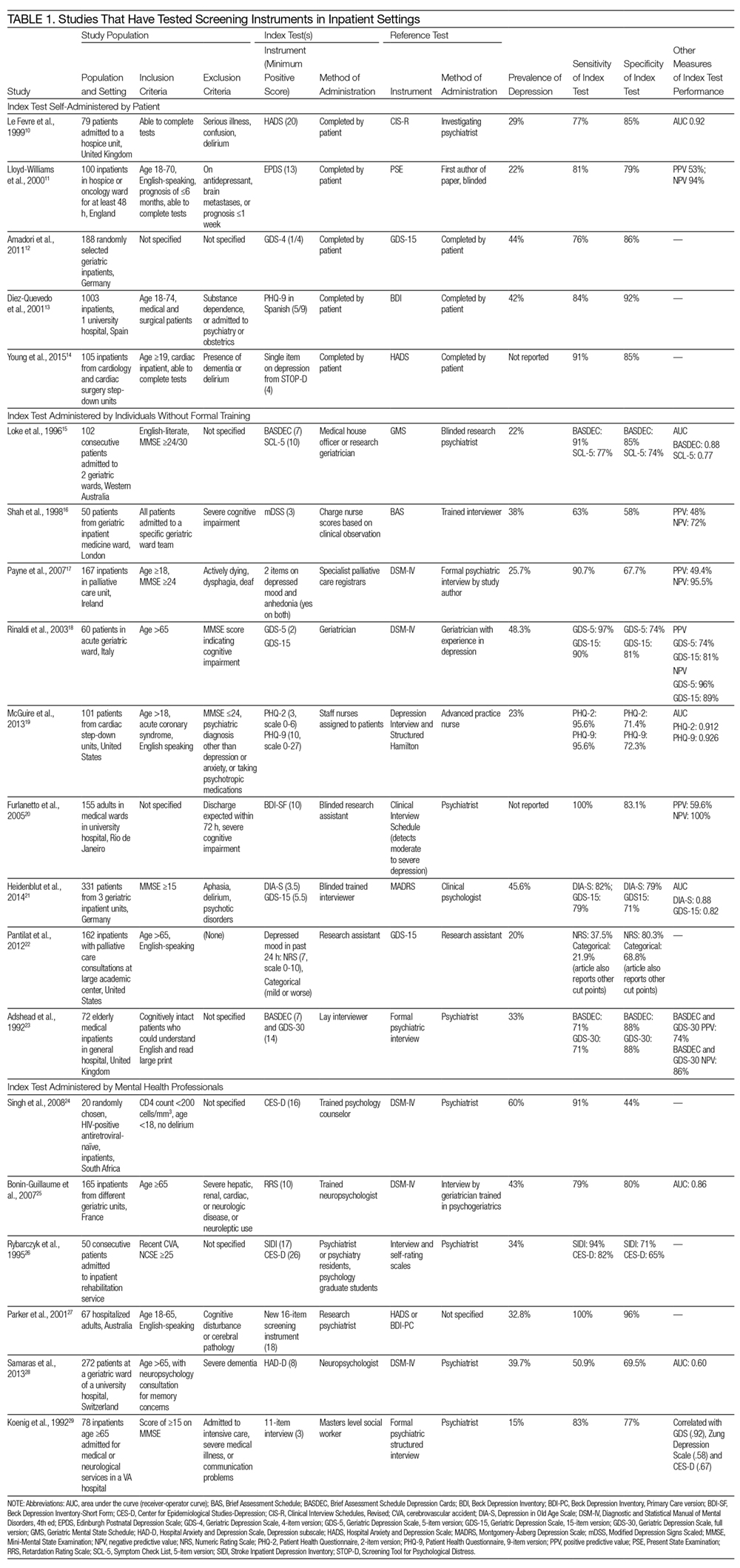
The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.
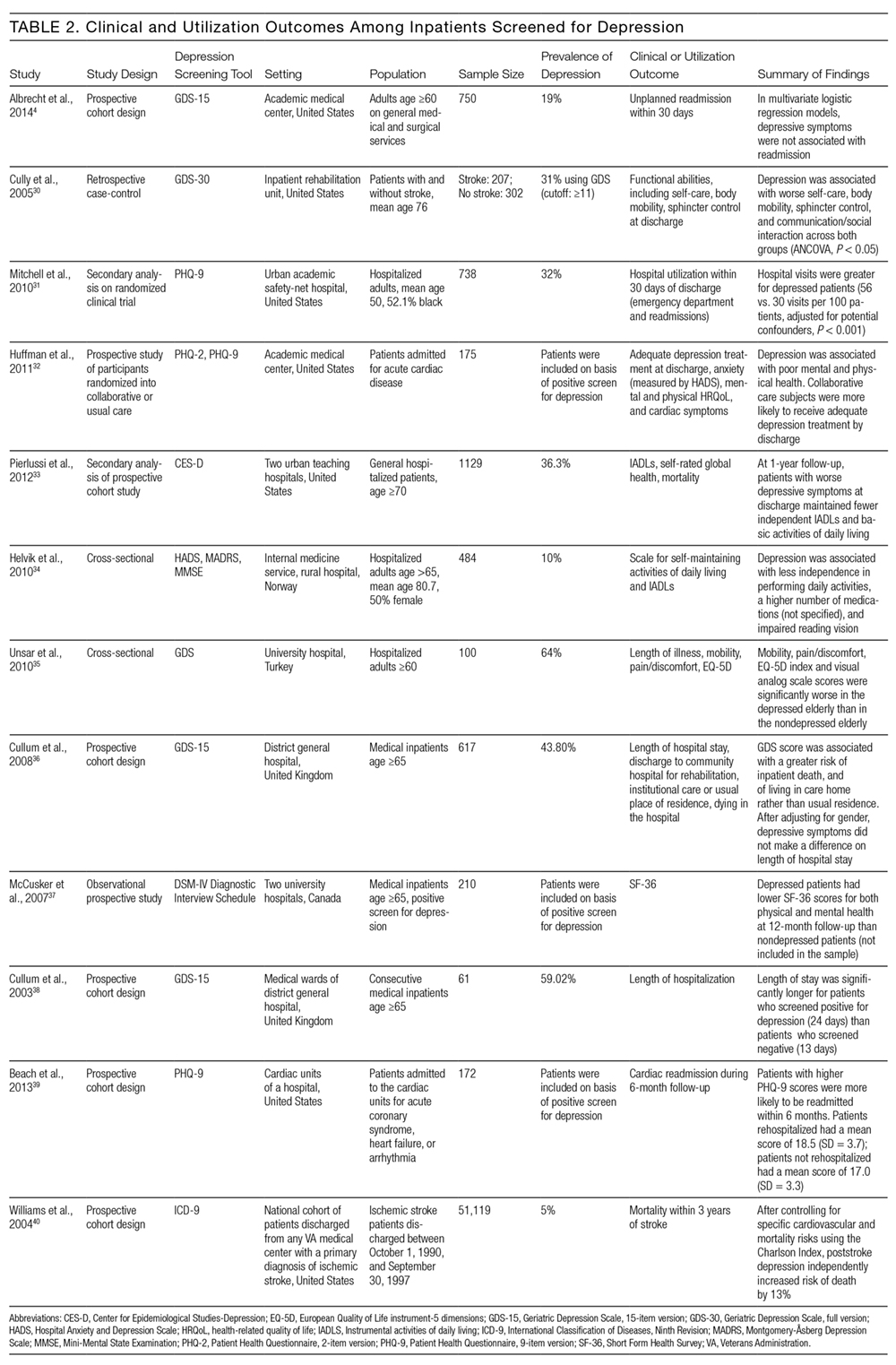
Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.

The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.

Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
In our current healthcare system, pressure to provide cost- and time-efficient care is immense. Inpatient care often focuses on assessing the patient’s presenting illness or injury and treating that condition in a manner that gets the patient on their feet and out of the hospital quickly. Because depression is not an indication for hospitalization so long as active suicidality is absent, inpatient physicians may view it as a problem best managed in the outpatient setting. Yet both psychosocial and physical factors associated with depression put patients at risk for rehospitalization.1 Furthermore, hospitalization represents an unrecognized opportunity to optimize both mental and physical health outcomes.2
Indeed, poor physical and mental health often occur together. Depressed inpatients have poorer outcomes, increased length of stay, and greater vulnerability to hospital readmission.3,4 Among elderly hospitalized patients, depression is particularly common, especially in those with poor physical health, alcoholism,5 hip fracture, and stroke.6 Yet little is known about how often depression goes unrecognized, undiagnosed, and, therefore, untreated.
The US Preventive Services Task Force (USPSTF) recommends screening for depression in the general adult population, including pregnant and postpartum women, and further suggests that screening should be implemented “with adequate systems in place to ensure accurate diagnosis, effective treatment, and appropriate follow-up.”2 The USPSTF guidelines do not distinguish between inpatient and outpatient settings. However, the preponderance of evidence for screening comes from outpatient care settings, and little is known about screening among inpatient populations.7
This study had 2 objectives. First, we sought to examine the performance of depression screening tools in inpatient settings. If depression screening were to become routine in hospital settings, screening tools would need to be sensitive and specific as well as brief and suitable for self-administration by patients or for administration by nurses, resident physicians, or hospitalists. It is also important to consider administration by mental health professionals, who may be best trained to administer such tests. We, therefore, examined 3 types of studies: (1) studies that tested a self-administered screening instrument, (2) studies that tested screening by individuals without formal training, and (3) studies that compared screening tools administered by mental health professionals. Second, we sought to describe associations between depression and clinical or utilization outcomes among hospitalized patients.
METHODS
We adhered to recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement,8,9 including designing the analysis before performing the review. However, we did not post a protocol in an online registry, formally assess study quality, or perform a meta-analysis.
Data Sources and Searches
We searched PsycINFO and PubMed databases for articles published between 1990 and 2016 (as of July 31, 2016). In PubMed, 2 search term strings were used to capture studies of depression screening tools in inpatient settings. The first used the advanced search option to exclude studies related to primary care settings or children and adolescents, and the second used MeSH terms to ensure that a wide variety of studies were included. Specific search terms are included in the Appendix. A similar search was conducted in the PsycINFO database and these search terms are also included in the Appendix.
Study Selection
Articles were eligible if they were published in English in peer-reviewed journals, included at least 20 adults hospitalized for nonpsychiatric reasons, and described the use of at least 1 measure of depression. The studies must have either tested the validity of a depression screening tool or examined the association between depression screening and clinical or utilization outcomes. Two investigators reviewed each title, abstract, and full-text article to determine eligibility, then reached a consensus on which studies to include in this review.
Data Extraction
Two investigators reviewed each full-text article to extract information related to study design, population, and outcomes regarding screening tool analysis or clinical results. From articles that assessed the performance of depression screening tools, we extracted information related to the nature and application of the index test, the nature and application of the reference test, the prevalence of depression, and the sensitivity and specificity of the index test compared with the reference test. For articles that focused on the association between depression screening and clinical or utilization outcomes, the data on relevant clinical outcomes included symptom severity, quality of life, and daily functioning, whereas the data on utilization outcomes included length of stay, readmission, and the cost of care.
RESULTS
Altogether, the search identified 3226 records. After eliminating duplicates and abstracts not suitable for inclusion (Figure), 101 articles underwent full-text review and 32 were found to be eligible. Of these, 12 focused on the association between depression and clinical or utilization outcomes, while 20 assessed the performance of depression screening tools.
Depression Screening Tools
Table 1 describes the index and reference instruments as well as methods of administration, the prevalence of depression, and the sensitivity and specificity of the index instruments relative to the reference instruments. Across the 20 studies, the prevalence of depression ranged from 15% to 60%, with a median of 34%.10–29 This finding may reflect different methods of screening or variation among diverse hospitalized populations. Many of the studies excluded patients with cognitive impairment or communication barriers.

The included studies tested a wide range of unique instruments, and compared them with diverse reference standards. Five studies examined instruments that were self-administered by patients10–14; 9 studies assessed instruments administered by nurses, physicians, or research staff members without formal psychiatric training15–23; and 6 studies evaluated instruments administered by mental health professionals.24–29 Four studies compared different instruments that were administered in the same manner (eg, both self-administered by patients).12–14,22 In the remaining studies, both instruments and methods of administration differed between the index and reference conditions.
Eight studies tested brief instruments with 5 or fewer items, most of which exhibited good sensitivity (range 38%–91%) and specificity (range 68%–86%) relative to longer instruments.12,14–19,22 In 2 of these studies, instruments were self-administered. In 1 case, a single self-administered item from the STOP-D instrument (“Over the past 2 weeks, how much have you been bothered by feeling sad, down, or uninterested in life?”) performed nearly as well as the 14-item Hospital Anxiety and Depression Scale.14 In the other 6 studies testing brief instruments, the instruments were administered by individuals without formal training.15–19,22 In 1 such study, geriatricians asking 2 questions about depressed mood and anhedonia performed well compared with a formal psychiatric interview.17
Four studies tested variations of the Geriatric Depression Scale (GDS).12,18,21,23 In 3 of these studies, abbreviated versions of the GDS exhibited relatively high sensitivity and specificity.12,18,21 However, a study comparing the 15-item GDS (GDS-15) with the GDS-4 found that GDS-15 correctly classified 10% more patients with suspected depression.12 Two studies examined variations of the Patient Health Questionnaire (PHQ). One study found that both the PHQ-2 and PHQ-9 obtained by staff nurses performed well relative to a comprehensive assessment by a trained advanced practice nurse.13,19
When reported, positive predictive value, negative predictive value, and area under the receiver-operator curve were generally high.
Depression and Clinical or Utilization Outcomes
Of the 12 studies that reported either clinical or utilization outcomes for depression screening in an inpatient setting,4,30–40 3 measured rates of rehospitalization.4,31,39 The other 9 studies tested for associations between symptoms of depression and either health or treatment outcomes. Table 2 provides a more detailed description of the study designs and results.

Other studies found that depression was associated with reduced functional abilities such as mobility and self-care,30,32–34 and increased hospital readmission31 as well as physical and mental health deficits.37 Interestingly, although 1 study did not find that depression and hospital readmission were closely linked (frequency at 19%), it found that comorbid illness and previous hospitalizations predicted readmission.4
We also evaluated the associations between depression diagnosed in the inpatient studies and 2 types of outcomes. The first type includes clinical outcomes including symptom severity, quality of life, and daily functioning. Most studies we identified assessed clinical outcomes, and all detected an association between depression and worse clinical outcomes. The second type includes healthcare utilization, which can be measured with the patients’ length of hospital stay, readmission and cost of care. In 1 such study, Mitchell aet al.31 reported a 54% increase in readmission within 30 days of discharge among patients who screened positive for depression.31 Additionally, Cully et al.30 found that depression may impinge on the recovery process of acute rehabilitation patients.
DISCUSSION
The purpose of this study was to describe the feasibility and performance of depression screening tools in inpatient medical settings, as well as associations between depression diagnosed in the inpatient setting and clinical and utilization outcomes. The median rate at which depression was detected among inpatients was 33%, ranging from 5% to 60%. Studies from several individual hospitals indicated that depression can be associated with higher healthcare utilization, including return to the hospital after discharge, as well as worse clinical outcomes. To detect undiagnosed depression among inpatients, screening appears feasible. Depression screening instruments generally exhibited good sensitivity and specificity relative to comprehensive clinical evaluations by mental health professionals. Furthermore, several self-administered and brief instruments had good performance. Prior authors have reported that screening for depression among inpatients may not be particularly burdensome to patients or staff members.41
The studies we reviewed used diverse screening instruments. Further research is needed to determine which tools are preferable in which patient populations, and to confirm that brief instruments are adequate for screening. The GDS is widely used, and many patients hospitalized in the United States fall into the geriatric group. The PHQ has been validated for self-administration and is widely used among outpatients42; it may be more suitable for younger populations. We found that several abbreviated versions of these and other screening instruments have exhibited good sensitivity and specificity among inpatients. However, many of the studies excluded patients with cognitive impairment or communication barriers. For individuals with auditory impairment, the Brief Assessment Schedule Depression Cards (BASDEC) might be an option. Used in 2 studies, the BASDEC involves showing patients a deck of 19 easy-to-read cards. The time required to administer the BASDEC is modest.15,23 Sets of smiley face diagrams might also be suitable for some patients with communication barriers or cognitive impairment. An ineligible study among stroke survivors found that selecting a sad face had a sensitivity of 76% and specificity of 77% relative to a formal diagnostic evaluation for depression.43
In considering the instruments that may be most suitable for inpatients, the role of somatic symptoms is also important because these can overlap between depression and the medical conditions that lead to hospitalization.44–46 Prior investigators found, for example, that 47% of Beck Depression Inventory (BDI) scores were attributable to somatic symptoms among patients hospitalized after myocardial infarction, whereas 37% of BDI scores were attributable to somatic symptoms among depressed outpatients.47 Future research is needed to determine the significance of somatic symptoms among inpatients, including whether they should be considered during screening, add prognostic value, or warrant specific treatment. In addition, although positive and negative predictive values were generally high among the screening instruments we evaluated, confirming the diagnosis of depression with a thorough clinical assessment is likely to be necessary.44,45
Despite the high prevalence of depression, associations with suboptimal outcomes, and the good performance of screening tools to date, screening for depression in the inpatient setting has received little attention. Prior authors have questioned whether hospital-based screening is an efficient and effective way to detect depression, and have raised valid concerns regarding false-positive diagnoses and unnecessary treatment, as well as a lack of randomized controlled trials.7,48,49 Whereas some studies suggest that depression is associated with greater healthcare utilization,3,4 little information exists regarding whether screening during hospitalization and treating previously undiagnosed depression improves clinical outcomes or reduces healthcare utilization.
Several important questions remain. What is the pathophysiology of depressed mood during hospitalization? How often does depressed mood during hospitalization reflect longstanding undiagnosed depression, longstanding undertreated depression, an acute stress disorder, or a normal if unpleasant short-term reaction to the stress of acute illnesses? Do the manifestations and effects of depressed mood differ among these situations? What is the prognosis of depressed mood occurring during hospitalization, and how many patients continue to have depression after recovery from acute illness; what factors affect prognosis? In a small sample of hospitalized patients, nearly 50% of those who had been depressed at intake remained depressed 1 month after discharge.50 Given that most antidepressant medications have to be taken for several weeks before effects can be detected, what, if any, approach to treatment should be taken? More research is needed on the effectiveness and cost-effectiveness of diagnosing and treating depression in the inpatient setting.
This work has several limitations. We found relatively few studies meeting eligibility criteria, particularly studies assessing clinical and utilization outcomes among depressed inpatients. Among the screening tools that were studied in the hospital setting, the highly diverse instruments and modes of administration precluded a quantitative synthesis such as meta-analysis. Prior meta-analyses on specific screening tools have focused on outpatient populations.51–53 Furthermore, we did not evaluate study quality or risk of bias.
In conclusion, screening for depression in the inpatient setting via patient self-assessment or assessment by hospital staff appears feasible. Several brief screening tools are available that have good sensitivity and specificity relative to diagnoses made by mental health professionals. Limited evidence suggests that screening tools for depression may be ready to integrate into inpatient care.41 Yet, although depression appears to be common and associated with worse clinical outcomes and higher healthcare utilization, more research is needed on the benefits, risks, and potential costs of adding depression screening in the inpatient healthcare setting.
Disclosures
The authors report no conflicts of interest.
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
1. Kahn KL, Keeler EB, Sherwood MJ, et al. Comparing outcomes of care before and after implementation of the DRG-based prospective payment system. JAMA. 1990;264(15):1984-1988. PubMed
2. U.S. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(4):380-387. PubMed
3. Dennis M, Kadri A, Coffey J. Depression in older people in the general hospital: a systematic review of screening instruments. Age Ageing. 2012;41(2):148-154. PubMed
4. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Depressive symptoms and hospital readmission in older adults. J Am Geriatr Soc. 2014;62(3):495-499. PubMed
5. Grant BF, Hasin DS, Harford TC. Screening for major depression among alcoholics: an application of receiver operating characteristic analysis. Drug Alcohol Depend. 1989;23(2):123-131. PubMed
6. Lieberman D, Galinsky D, Fried V, et al. Geriatric Depression Screening Scale (GDS) in patients hospitalized for physical rehabilitation. Int J Geriatr Psychiatry. 1999;14(7):549-555. PubMed
7. Canadian Task Force on Preventive Health Care. Recommendations on screening for depression in adults. CMAJ. 2013;185(9):775-782.
8. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed
9. Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013-1020. PubMed
10. Le Fevre P, Devereux J, Smith S, Lawrie SM, Cornbleet M. Screening for psychiatric illness in the palliative care inpatient setting: a comparison between the Hospital Anxiety and Depression Scale and the General Health Questionnaire-12. Palliat Med. 1999;13(5):399-407. PubMed
11. Lloyd-Williams M, Friedman T, Rudd N. Criterion validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in patients with advanced metastatic cancer. J Pain Symptom Manag. 2000;20(4):259-265. PubMed
12. Amadori K, Herrmann E, Püllen RK. Comparison of the 15-item Geriatric Depression Scale (GDS-15) and the GDS-4 during screening for depression in an in-patient geriatric patient group. J Am Geriatr Soc. 2011;59(1):171-172. PubMed
13. Diez-Quevedo C, Rangil T, Sanchez-Planell L, Kroenke K, Spitzer RL. Validation and utility of the Patient Health Questionnaire in diagnosing mental disorders in 1003 general hospital Spanish inpatients. Psychosom Med. 2001;63(4):679-686. PubMed
14. Young Q-R, Nguyen M, Roth S, Broadberry A, Mackay MH. Single-item measures for depression and anxiety: validation of the screening tool for psychological distress in an inpatient cardiology setting. Eur J Cardiovasc Nursing. 2015;14(6):544-551. PubMed
15. Loke B, Nicklason F, Burvill P. Screening for depression: clinical validation of geriatricians’ diagnosis, the Brief Assessment Schedule Depression Cards and the 5-item version of the Symptom Check List among non-demented geriatric inpatients. Int J Geriatr Psychiatry. 1996;11(5):461-465.
16. Shah A, Karasu M, De T. Nursing staff and screening for depression among acutely ill geriatric inpatients: a pilot study. Aging Ment Health. 1998;2(1):71-74.
17. Payne A, Barry S, Creedon B, et al. Sensitivity and specificity of a two-question screening tool for depression in a specialist palliative care unit. Palliat Med. 2007;21(3):193-198. PubMed
18. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. PubMed
19. McGuire AW, Eastwood J, Macabasco-O’Connell A, Hays RD, Doering LV. Depression screening: utility of the Patient Health Questionnaire in patients with acute coronary syndrome. Am J Crit Care. 2013;22(1):12-19. PubMed
20. Furlanetto LM, Mendlowicz MV, Bueno JR. The validity of the Beck Depression Inventory-Short Form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. J Affect Disord. 2005;86(1):87-91. PubMed
21. Heidenblut S, Zank S. Screening for depression with the Depression in Old Age Scale (DIA-S) and the Geriatric Depression Scale (GDS15): diagnostic accuracy in a geriatric inpatient setting. GeroPsych (Bern). 2014;27(1):41. PubMed
22. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. An assessment of the screening performance of a single-item measure of depression from the Edmonton Symptom Assessment Scale among chronically ill hospitalized patients. J Pain Symptom Manage. 2012;43(5):866-873. PubMed
23. Adshead F, Cody DD, Pitt B. BASDEC: a novel screening instrument for depression in elderly medical inpatients. BMJ. 1992;305(6850):397. PubMed
24. Singh D, Sunpath H, John S, Eastham L, Gouden R. The utility of a rapid screening tool for depression and HIV dementia amongst patients with low CD4 counts – a preliminary report. Afr J Psychiatry (Johannesbg). 2008;11(4):282-286. PubMed
25. Bonin-Guillaume S, Sautel L, Demattei C, Jouve E, Blin O. Validation of the Retardation Rating Scale for detecting in geriatric inpatients. Int J Geriatr Psychiatry. 2007;22(1):68-76. PubMed
26. Rybarczyk B, Winemiller DR, Lazarus LW, Haut A, Hartman C. Validation of a depression screening measure for stroke inpatients. Am J Geriatr Psychiatry. 1996;4(2):131-139.
27. Parker G, Hilton T, Hadzi-Pavlovic D, Bains J. Screening for depression in the medically ill: the suggested utility of a cognitive-based approach. Aust N Z J Psychiatry. 2001;35(4):474-480. PubMed
28. Samaras N, Herrmann FR, Samaras D, et al. The Hospital Anxiety and Depression Scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr. 2013;25(1):82-87. PubMed
29. Koenig HG, Cohen HJ, Blazer DG, Meador KG, Westlund R. A brief depression scale for use in the medically ill. Int J Psychiatry Med. 1992;22(2):183-195. PubMed
30. Cully JA, Gfeller JD, Heise RA, Ross MJ, Teal CR, Kunik ME. Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch Phys Med Rehabil. 2005;86(12):2256-2260. PubMed
31. Mitchell SE, Paasche-Orlow MK, Forsythe SR, et al. Post-discharge hospital utilization among adult medical inpatients with depressive symptoms. J Hosp Med. 2010;5(7):378-384. PubMed
32. Huffman JC, Mastromauro CA, Sowden GL, Wittmann C, Rodman R, Januzzi JL. A collaborative care depression management program for cardiac inpatients: depression characteristics and in-hospital outcomes. Psychosomatics. 2011;52(1):26-3. 2007;22(11):1596-1602.J Gen Intern Med PubMed
53. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. 2010;126(3):335-348.J Affect Disord. PubMed
52. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. 2010;69(4):371-378.J Psychosom Res. PubMed
51. Brennan C, Worrall-Davis A, McMillan D, Gilbody S, House A. The Hospital Anxiety and Depression Scale: a diagnostic meta-analysis of case-finding ability. . 1992;22(3):281-289.Int J Psychiatry Med PubMed
50. Pomerantz AS, de-Nesnera A, West AN. Resolution of depressive symptoms in medical inpatients after discharge. 2014;12(1):13.BMC Med PubMed
49. Thombs BD, Ziegelstein RC, Roseman M, Kloda LA, Ioannidis JPA. There are no randomized controlled trials that support the United States Preventive Services Task Force guideline on screening for depression in primary care: a systematic review. 2013;1(4):E159-E167.CMAJ Open PubMed
48. Keshavarz H, Fitzpatrick-Lewis D, Streiner DL, et al. Screening for depression: a systematic review and meta-analysis. 2012;73(3):157-162.J Psychosom Res. PubMed
47. Delisle VC, Beck AT, Ziegelstein RC, Thombs BD. Symptoms of heart disease or its treatment may increase Beck Depression Inventory Scores in hospitalized post-myocardial infarction patients. 2014;23(9):1079.Psychooncology PubMed
46. Palmer SC. Study provides little insight into routine screening for depression.
2005;20(3):289.Int J Geriatr Psychiatry. PubMed
45. Baldwin RC. Validation of short screening tests for depression, response to Seymour [letter to the editor]. 2005;20(3):289.Int J Geriatr Psychiatry.
44. Seymour J. Validation of short screening tests for depression: comment on Goring et al. (2004) [letter to the editor]. 2008;45(7):1081-1089.Int J Nurs Stud. PubMed
43. Lee ACK, Tang SW, Yu GKK, Cheung RTF. The smiley as a simple screening tool for depression after stroke: a preliminary study. 1999;282(18):1737-1744.JAMA. PubMed
42. Spitzer RL, Kroenke K, Williams JW. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. . 2011;14(3):275-279.J Palliat Med PubMed
41. Rao S, Ferris FD, Irwin SA. Ease of screening for depression and delirium in patients enrolled in inpatient hospice care. . 2004;161(6):1090-1095.Am J Psychiatry PubMed
40. Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. 2013;75(5):409-413.J Psychosom Res. PubMed
39. Beach SR, Januzzi JL, Mastromauro CA, et al. Patient Health Questionnaire-9 score and adverse cardiac outcomes in patients hospitalized for acute cardiac disease. 2003;18(4):358-359.Int J Geriatr Psychiatry PubMed
38. Cullum S, Nandhra H, Darley J, Todd C. Screening for depression in older people on medical wards: which cut-point should we use? 2007;29(4):340-348.Gen Hosp Psychiatry. PubMed
37. McCusker J, Cole M, Ciampi A, Latimer E, Windholz S, Belzile E. Major depression in older medical inpatients predicts poor physical and mental health status over 12 months. 2008;37(6):690-695.Age Ageing PubMed
36. Cullum S, Metcalfe C, Todd C, Brayne C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial.
. 2010;50(1):6-10.Arch Gerontol Geriatr PubMed
35. Unsar S, Sut N. Depression and health status in elderly hospitalized patients with chronic illness. 150-159.:2010;25(2)Int J Geriatr Psychiatry. PubMed
34. Helvik A-S, Skancke RH, Selbæk G. Screening for depression in elderly medical inpatients from rural area of Norway: prevalence and associated factors. . 2012;60(12):2254-2262.J Am Geriatr Soc PubMed
33. Pierlussi E, Mehta KM, Kirby KA, et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. PubMed
© 2017 Society of Hospital Medicine