User login
Lower Leg Hyperpigmentation in MYH9-Related Disorder
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
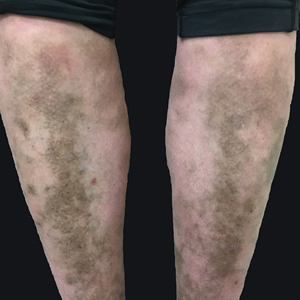
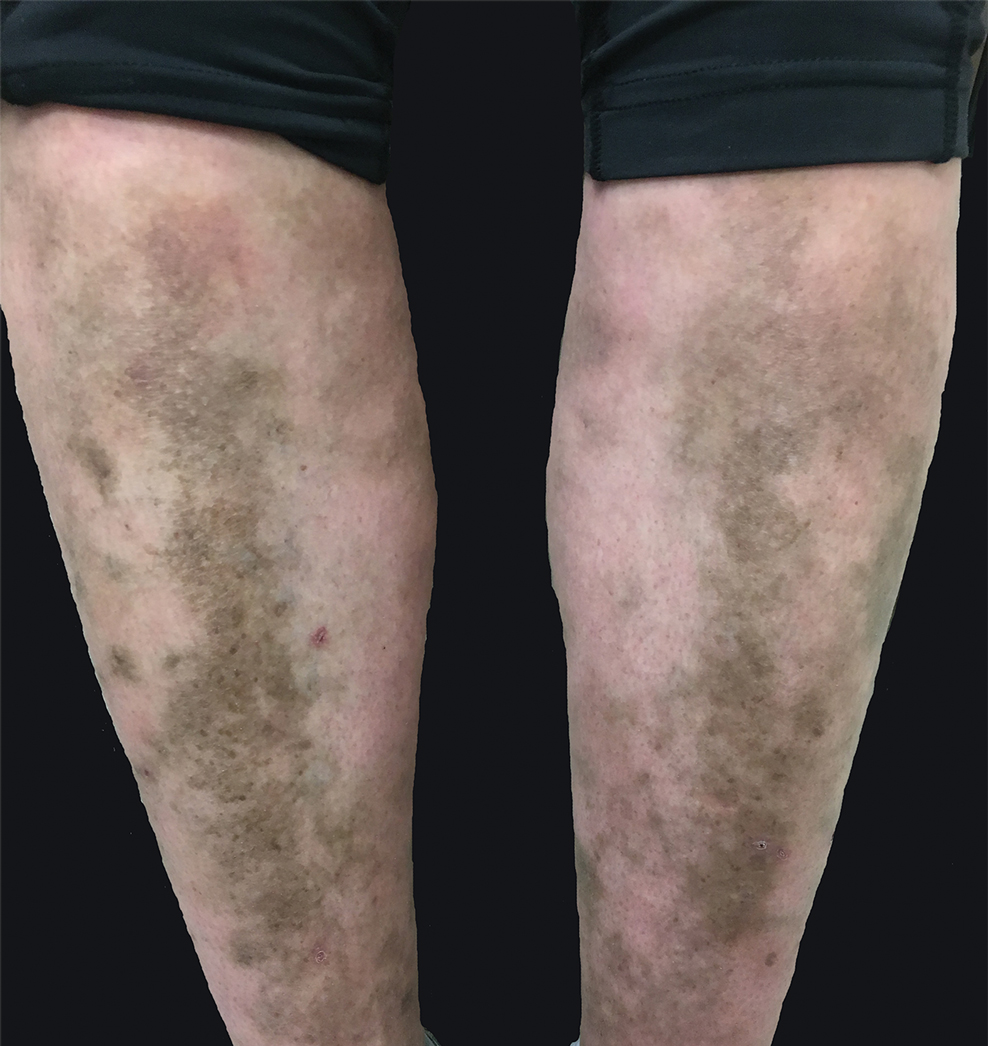
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.
Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.

Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.

Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
Practice Points
- MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.
- Leg hyperpigmentation can occur secondary to hemosiderin deposition from MYH9-related disorder.
- The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder.
- Lasers and intense pulsed light therapy are modalities to consider for the hyperpigmentation of MYH9- related disorder.