User login
Gabapentin Use in Acute Alcohol Withdrawal Management
The prevalence of alcohol dependence in the U.S. represents a significant public health concern. Alcohol use disorder (AUD) is estimated to affect 6.7% of Americans and is the fourth leading preventable cause of death.1 Men and women who have served in the military are at an even higher risk of excessive alcohol use. More than 20% of service members report binge drinking every week.2 This risk is further exacerbated in veterans who have experienced active combat or who have comorbid health conditions, such as posttraumatic stress disorder.3
Background
Individuals that regularly consume excessive amounts of alcohol can develop acute alcohol withdrawal syndrome (AWS) after abrupt discontinuation or significant reduction of alcohol intake. Patients admitted for acute alcohol withdrawal may experience complicated courses of treatment and extended lengths of hospitalization.4,5 Cessation from chronic alcohol intake elicits a pathophysiologic response from increased N-methyl-d-aspartate receptor activity and decreased γ-aminobutyric acid (GABA) receptor function.
Autonomic and psychomotor hyperactivity disturbances, such as anxiety, nausea, tremors, diaphoresis, and tachycardia, may occur as early as 6 to 8 hours after cessation of use. Within 48 to 72 hours of alcohol cessation, patients may be at an increased risk of experiencing tonic-clonic seizures, visual and auditory hallucinations, and delirium tremens (DTs), which may be accompanied by signs of extreme autonomic hyperactivity and agitation.6 Patients hospitalized within acute settings require frequent medical supervision for acute alcohol withdrawal, especially in patients at high risk for seizure or DTs because morbidity and mortality risk is increased.7
Benzodiazepines remain the standard of care for management of moderate-to-severe symptoms of AWS. Strong evidence supports the use of benzodiazepines to reduce withdrawal severity, incidence of delirium, and seizures in AWS by enhancing GABA activity.8 However, the adverse effect (AE) burden associated with benzodiazepines can be a major limitation throughout care. Benzodiazepines also may be limited in their use in select patient populations, such as in older adults or patients who present with hepatic dysfunction due to the risk of increased AEs or metabolite accumulation.6 A high dosing requirement of benzodiazepine for symptom management can lead to oversedation to the point of requiring intubation, increasing length of stay in the intensive care unit (ICU), and the risk of nosocomial infections.9
Anticonvulsants, such as carbamazepine, valproic acid, and gabapentin, have shown to be superior to placebo and equal in efficacy to benzodiazepines for symptom management in mild-to-moderate alcohol withdrawal in both inpatient and outpatient settings.6-8 However, these agents are not recommended as first-line monotherapy due to the limited number of randomized trials supporting their efficacy over benzodiazepines in preventing severe symptoms of withdrawal, such as seizures or delirium.10-12 Nonetheless, the mechanism of action of anticonvulsants may help raise seizure threshold in patients and provide a benzodiazepine-sparing effect by enhancing GABAergic activity and lowering neuronal excitability.13
Gabapentin makes an attractive agent for clinical use because of its anxiolytic and sedative properties that can be used to potentially target symptoms analogous with AWS when the use of benzodiazepines becomes a safety concern. Although similar in chemical structure, gabapentin is not metabolized to GABA and does not directly interact with the receptor. Gabapentin may increase GABA concentrations by direct synthesis of GABA and indirectly through interaction with voltage-gated calcium channels.13 In addition to its overall safety profile, gabapentin may be a viable adjuvant because emerging data may suggest a potential role in the management of acute alcohol withdrawal.12,14,15
Gabapentin for Alcohol Withdrawal at VAPORHCS
Although not currently included in the alcohol withdrawal protocol at Veterans Affairs Portland Health Care System (VAPORHCS), gabapentin has been added to the standard of care in select patients per the discretion of the attending physician. Anecdotal reports of patients experiencing milder symptoms and less benzodiazepine administration have facilitated use of gabapentin in alcohol withdrawal management at VAPORHCS. However, routine use of gabapentin is not consistent among all patients treated for acute alcohol withdrawal, and dosing schedules of gabapentin seem highly variable. Standard symptom management for acute alcohol withdrawal should be consistent for all affected individuals, using evidence-based medicine in order to achieve optimal outcomes and improve harm reduction.
The objective of this quality assurance/quality improvement (QA/QI) project was to assess the amount of lorazepam required for symptom management in acute alcohol withdrawal when gabapentin is used as an adjunct to treatment and to evaluate the impact on symptom management using the Clinical Institute Withdrawal Assessment for Alcohol scale, revised version (CIWA-Ar) in patients admitted to the ICU and general medicine wards for acute alcohol withdrawal at VAPORHCS.16 If a possible adjunct for the treatment of alcohol withdrawal has the potential to reduce benzodiazepine requirements and minimize AEs, a thorough evaluation of the treatment should be conducted before its practice is incorporated into the current standard of care.
Methods
The following QA/QI project was approved locally by the VAPORHCS associate chief of staff/Office of Research and Development and is considered to be nonresearch VHA operations activity and exempt from an institutional review board committee review. This project was a single-center, retrospective chart review of patients admitted to the ICU and general medicine wards at VAPORHCS with acute alcohol withdrawal. The CIWA-Ar protocol order sets between January 1, 2014 and December 31, 2015, were retrieved through the Computerized Patient Record System (CPRS) at VAPORHCS.
Patients with an alcohol withdrawal protocol order set who received gabapentin with or without lorazepam during hospitalization were identified for chart review. Patients were eligible for review if they were aged ≥ 18 years with a primary or secondary diagnosis of acute alcohol withdrawal and had a CIWA-Ar protocol order set placed during hospitalization. Patients must have been administered gabapentin, lorazepam, or both while the CIWA-Ar protocol was active. Patients with an active outpatient prescription for gabapentin or benzodiazepine filled within the previous 30 days, documented history of psychosis or epileptic seizure disorder, or other concomitant benzodiazepines or antiepileptics administered while on the CIWA-Ar protocol were excluded from the analysis.
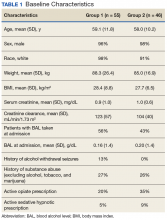
Baseline characteristics for patients eligible for review were collected and included age; sex; race, body mass index (BMI); estimated creatinine clearance (CrCl); toxicology screen at admission (if available), history of substance use disorder, AWS, or history of withdrawal seizures; and history of a sedative hypnotics (not including benzodiazepines) prescription within 30 days prior to admission.17
The primary endpoint was the total amount of lorazepam administered from the time of admission to the time of discontinuation of the alcohol withdrawal protocol. The dose, frequency, and amount of lorazepam and gabapentin administered daily were collected for each patient while on the CIWA-Ar protocol. Secondary endpoints included rate of the CIWA-Ar score reduction, time to protocol discontinuation, as well as incidence and onset of peak delirium scores during hospitalization. Cumulative CIWA-Ar scores over 24 hours were averaged per patient per day while on CIWA-Ar protocol. Peak CIWA-Ar scores per patient per day on the protocol also were collected. Time to protocol termination was determined by date of order for discontinuation or by date when scoring had ceased and protocol order was inadvertently continued. Peak Intensive Care Delirium Screening Checklist (ICDSC) scores were collected for patients admitted to the ICU.18 Day of peak ICDSC scores also were evaluated.
Statistical Analysis
The sample size for this analysis was determined by the number of patients identified who met the inclusion criteria and did not meet any of the exclusion criteria. Power was not calculated to estimate sample size needed to determine statistical significance. One hundred patients treated for alcohol withdrawal was established as the target sample size for this project. Descriptive statistics were performed to analyze patient baseline characteristics and primary and secondary objective data.
Results
A total of 1,611 CIWA-Ar protocol orders were identified between January 1, 2014 and December 31, 2015.
Primary Endpoint
The average amount of lorazepam administered for the total duration on CIWA-Ar protocol was 7.9 mg (median 6, ± 8.2) among
Secondary Endpoints
On average, the total number of days spent on CIWA-Ar protocol was 3.8 (median 4, ± 1.5) in group 1 compared with 4.1 (median 4, ± 1.6) in group 2. Rate of CIWA-Ar protocol discontinuation for patients in group 1 and group 2 is shown in Figure 3.
Discussion
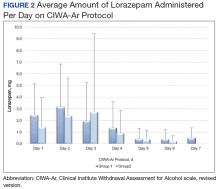
The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Patients who were started on gabapentin on the initiation of the alcohol withdrawal protocol received less lorazepam dosing compared with patients who received only lorazepam for symptom management for alcohol withdrawal. Except for day 3, average lorazepam dosage per day on the alcohol withdrawal protocol was lower in patients who were also taking gabapentin.
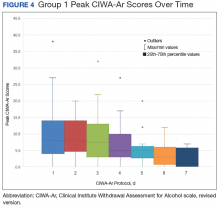
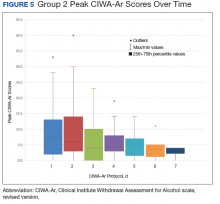
This trend also can be seen in the recorded peak CIWA-Ar scores per day as illustrated in Figures 4 and 5.
Limitations
Prior to evaluation, power analysis was not calculated to estimate an appropriate sample size necessary to determine statistical significance. Results from this evaluation are not definitive and are meant to be hypothesis generating for future analysis.
There were several limitations that were identified throughout this project. For this review, history and extent of patient’s prior alcohol use was not assessed. Therefore, the degree of symptom severity in which patients may have experienced during withdrawal may not have been adequately matched between groups. The inherent subjectivity of CIWA-Ar scoring was considered a limitation because scores were determined by clinical interpretation among various nursing staff. As this was a retrospective review, exact timing of medications administered as well as additional supportive care measures, such as ancillary medications for symptom management, were not accounted for and controlled between groups.
Patients presenting to the emergency department or from a facility outside of VAPORHCS for acute AWS may have had incomplete documentation of the onset of symptoms on presentation or of the medications administered prior to being admitted, which may have confounded initial CIWA-Ar scoring and total duration required to be on a withdrawal protocol. Some patients may have received benzodiazepines at initial presentation prior to gabapentin initiation and may have inaccurately reflected its efficacy potential to manage symptoms without the need for lorazepam.
There were 10 patients that were identified who received gabapentin on the alcohol withdrawal protocol and did not receive any lorazepam. This retrospective review could not be determined whether these patients did not require lorazepam because initiating gabapentin reduced severity or simply because their withdrawal symptoms were not severe enough to warrant the need for lorazepam, regardless of gabapentin use.
Gabapentin dosing was not standardized among patients, averaging from 100 mg to 3,600 mg per day. This wide variation in dose may have influenced the requirement of lorazepam needed for symptom management in patients receiving minimal doses or AEs experienced in patients who received large doses. Initiation and/or select dosing of gabapentin may have been dependent on the experience of the provider and familiarity with its use in alcohol withdrawal management. Interestingly, patients with a history of withdrawal seizures (13%) were identified only within the lorazepam-only group. This could suggest that patients with prior symptoms of severe alcohol withdrawal were selected to receive lorazepam-only at the discretion of the provider.
Existing literature investigating gabapentin utilization in alcohol withdrawal has demonstrated benefit for patients with mild-to-moderate symptoms in both inpatient and outpatient studies. However, supporting evidence is limited by the differences in design, methods, and comparators within each trial. Leung and colleagues identified 5 studies that utilized gabapentin as monotherapy or in combination with other agents in alcohol withdrawal.13 Three of these studies were performed within an inpatient setting, each differing in trial design, inclusion/exclusion criteria, intervention, and outcomes. Gabapentin dosing strategies were highly variable among studies. Collectively, the differences noted make it difficult to generalize that similar outcomes would result in other patient populations. The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Future projects could be designed to draw more specific conclusions.
Conclusion
On average, the required benzodiazepine dosage was lower with concomitant use of gabapentin in acute AWS management. The duration for patients on alcohol withdrawal protocol was not reduced with use of gabapentin. Between group (ie, history of withdrawal seizures, blood alcohol level) and among group (ie, gabapentin administration) differences prevent direct correlations to be drawn from this evaluation. Future reviews should include power analysis to establish an appropriate sample size to determine statistical significance among identified covariates. Further evaluation of the use of gabapentin for withdrawal management is warranted prior to incorporating its routine use in the current standard of care for patients experiencing acute AWS.
Acknowledgments
The authors thank Ryan Bickel, PharmD, BCCCP, Critical Care Clinical Pharmacist; Stephen M. Smith, PhD, Director of Medical Critical Care; Gordon Wong, PharmD, Clinical Applications Coordinator; and Eileen Wilbur, RPh, Research Pharmacy Supervisor.
1. Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109.
2. National Institute on Drug Abuse. Military. https://www.drugabuse.gov/related-topics/military. Updated April 2016. Accessed January 10, 2018.
3. Bohnert KM, Ilgen MA, Rosen CS, Desai RA, Austin K, Blow FC. The association between substance use disorders and mortality among a cohort of veterans with posttraumatic stress disorder: variation by age cohort and mortality type. Drug Alcohol Depend. 2013;128(1-2):98-103.
4. Foy A, Kay J, Taylor A. The course of alcohol withdrawal in a general hospital. QJM. 1997;90(4):253-261.
5. Carlson RW, Kumar NN, Wong-Mckinstry E, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28(4):549-585.
6. National Institute for Health and Care Excellence. Alcohol use disorders: diagnosis and clinical management of alcohol-related physical complications. https://www.nice.org.uk/guidance/cg100. Published June 2010. Updated April 2017. Accessed January 10, 2018.
7. Sarff MC, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med. 2010;38(suppl 9):494-501.
8. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf. Published December 2015. Accessed January 10, 2018.
9. Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724-730.
10. Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database Syst Rev. 2011(6):D008537.
11. Ntais C, Pakos E, Kyzas P, Ioannidis JP. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2005;20(3):CD005063.
12. Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol. 2010;45(2):143-145.
13. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. Role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906.
14. Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29:248-254.
15. Myrick H, Malcolm R, Randall PK, et al. A double blind trial of gabapentin vs lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
16. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
17 Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
18. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859-864.
The prevalence of alcohol dependence in the U.S. represents a significant public health concern. Alcohol use disorder (AUD) is estimated to affect 6.7% of Americans and is the fourth leading preventable cause of death.1 Men and women who have served in the military are at an even higher risk of excessive alcohol use. More than 20% of service members report binge drinking every week.2 This risk is further exacerbated in veterans who have experienced active combat or who have comorbid health conditions, such as posttraumatic stress disorder.3
Background
Individuals that regularly consume excessive amounts of alcohol can develop acute alcohol withdrawal syndrome (AWS) after abrupt discontinuation or significant reduction of alcohol intake. Patients admitted for acute alcohol withdrawal may experience complicated courses of treatment and extended lengths of hospitalization.4,5 Cessation from chronic alcohol intake elicits a pathophysiologic response from increased N-methyl-d-aspartate receptor activity and decreased γ-aminobutyric acid (GABA) receptor function.
Autonomic and psychomotor hyperactivity disturbances, such as anxiety, nausea, tremors, diaphoresis, and tachycardia, may occur as early as 6 to 8 hours after cessation of use. Within 48 to 72 hours of alcohol cessation, patients may be at an increased risk of experiencing tonic-clonic seizures, visual and auditory hallucinations, and delirium tremens (DTs), which may be accompanied by signs of extreme autonomic hyperactivity and agitation.6 Patients hospitalized within acute settings require frequent medical supervision for acute alcohol withdrawal, especially in patients at high risk for seizure or DTs because morbidity and mortality risk is increased.7
Benzodiazepines remain the standard of care for management of moderate-to-severe symptoms of AWS. Strong evidence supports the use of benzodiazepines to reduce withdrawal severity, incidence of delirium, and seizures in AWS by enhancing GABA activity.8 However, the adverse effect (AE) burden associated with benzodiazepines can be a major limitation throughout care. Benzodiazepines also may be limited in their use in select patient populations, such as in older adults or patients who present with hepatic dysfunction due to the risk of increased AEs or metabolite accumulation.6 A high dosing requirement of benzodiazepine for symptom management can lead to oversedation to the point of requiring intubation, increasing length of stay in the intensive care unit (ICU), and the risk of nosocomial infections.9
Anticonvulsants, such as carbamazepine, valproic acid, and gabapentin, have shown to be superior to placebo and equal in efficacy to benzodiazepines for symptom management in mild-to-moderate alcohol withdrawal in both inpatient and outpatient settings.6-8 However, these agents are not recommended as first-line monotherapy due to the limited number of randomized trials supporting their efficacy over benzodiazepines in preventing severe symptoms of withdrawal, such as seizures or delirium.10-12 Nonetheless, the mechanism of action of anticonvulsants may help raise seizure threshold in patients and provide a benzodiazepine-sparing effect by enhancing GABAergic activity and lowering neuronal excitability.13
Gabapentin makes an attractive agent for clinical use because of its anxiolytic and sedative properties that can be used to potentially target symptoms analogous with AWS when the use of benzodiazepines becomes a safety concern. Although similar in chemical structure, gabapentin is not metabolized to GABA and does not directly interact with the receptor. Gabapentin may increase GABA concentrations by direct synthesis of GABA and indirectly through interaction with voltage-gated calcium channels.13 In addition to its overall safety profile, gabapentin may be a viable adjuvant because emerging data may suggest a potential role in the management of acute alcohol withdrawal.12,14,15
Gabapentin for Alcohol Withdrawal at VAPORHCS
Although not currently included in the alcohol withdrawal protocol at Veterans Affairs Portland Health Care System (VAPORHCS), gabapentin has been added to the standard of care in select patients per the discretion of the attending physician. Anecdotal reports of patients experiencing milder symptoms and less benzodiazepine administration have facilitated use of gabapentin in alcohol withdrawal management at VAPORHCS. However, routine use of gabapentin is not consistent among all patients treated for acute alcohol withdrawal, and dosing schedules of gabapentin seem highly variable. Standard symptom management for acute alcohol withdrawal should be consistent for all affected individuals, using evidence-based medicine in order to achieve optimal outcomes and improve harm reduction.
The objective of this quality assurance/quality improvement (QA/QI) project was to assess the amount of lorazepam required for symptom management in acute alcohol withdrawal when gabapentin is used as an adjunct to treatment and to evaluate the impact on symptom management using the Clinical Institute Withdrawal Assessment for Alcohol scale, revised version (CIWA-Ar) in patients admitted to the ICU and general medicine wards for acute alcohol withdrawal at VAPORHCS.16 If a possible adjunct for the treatment of alcohol withdrawal has the potential to reduce benzodiazepine requirements and minimize AEs, a thorough evaluation of the treatment should be conducted before its practice is incorporated into the current standard of care.
Methods
The following QA/QI project was approved locally by the VAPORHCS associate chief of staff/Office of Research and Development and is considered to be nonresearch VHA operations activity and exempt from an institutional review board committee review. This project was a single-center, retrospective chart review of patients admitted to the ICU and general medicine wards at VAPORHCS with acute alcohol withdrawal. The CIWA-Ar protocol order sets between January 1, 2014 and December 31, 2015, were retrieved through the Computerized Patient Record System (CPRS) at VAPORHCS.
Patients with an alcohol withdrawal protocol order set who received gabapentin with or without lorazepam during hospitalization were identified for chart review. Patients were eligible for review if they were aged ≥ 18 years with a primary or secondary diagnosis of acute alcohol withdrawal and had a CIWA-Ar protocol order set placed during hospitalization. Patients must have been administered gabapentin, lorazepam, or both while the CIWA-Ar protocol was active. Patients with an active outpatient prescription for gabapentin or benzodiazepine filled within the previous 30 days, documented history of psychosis or epileptic seizure disorder, or other concomitant benzodiazepines or antiepileptics administered while on the CIWA-Ar protocol were excluded from the analysis.
Baseline characteristics for patients eligible for review were collected and included age; sex; race, body mass index (BMI); estimated creatinine clearance (CrCl); toxicology screen at admission (if available), history of substance use disorder, AWS, or history of withdrawal seizures; and history of a sedative hypnotics (not including benzodiazepines) prescription within 30 days prior to admission.17
The primary endpoint was the total amount of lorazepam administered from the time of admission to the time of discontinuation of the alcohol withdrawal protocol. The dose, frequency, and amount of lorazepam and gabapentin administered daily were collected for each patient while on the CIWA-Ar protocol. Secondary endpoints included rate of the CIWA-Ar score reduction, time to protocol discontinuation, as well as incidence and onset of peak delirium scores during hospitalization. Cumulative CIWA-Ar scores over 24 hours were averaged per patient per day while on CIWA-Ar protocol. Peak CIWA-Ar scores per patient per day on the protocol also were collected. Time to protocol termination was determined by date of order for discontinuation or by date when scoring had ceased and protocol order was inadvertently continued. Peak Intensive Care Delirium Screening Checklist (ICDSC) scores were collected for patients admitted to the ICU.18 Day of peak ICDSC scores also were evaluated.
Statistical Analysis
The sample size for this analysis was determined by the number of patients identified who met the inclusion criteria and did not meet any of the exclusion criteria. Power was not calculated to estimate sample size needed to determine statistical significance. One hundred patients treated for alcohol withdrawal was established as the target sample size for this project. Descriptive statistics were performed to analyze patient baseline characteristics and primary and secondary objective data.
Results
A total of 1,611 CIWA-Ar protocol orders were identified between January 1, 2014 and December 31, 2015.
Primary Endpoint
The average amount of lorazepam administered for the total duration on CIWA-Ar protocol was 7.9 mg (median 6, ± 8.2) among
Secondary Endpoints
On average, the total number of days spent on CIWA-Ar protocol was 3.8 (median 4, ± 1.5) in group 1 compared with 4.1 (median 4, ± 1.6) in group 2. Rate of CIWA-Ar protocol discontinuation for patients in group 1 and group 2 is shown in Figure 3.
Discussion
The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Patients who were started on gabapentin on the initiation of the alcohol withdrawal protocol received less lorazepam dosing compared with patients who received only lorazepam for symptom management for alcohol withdrawal. Except for day 3, average lorazepam dosage per day on the alcohol withdrawal protocol was lower in patients who were also taking gabapentin.
This trend also can be seen in the recorded peak CIWA-Ar scores per day as illustrated in Figures 4 and 5.
Limitations
Prior to evaluation, power analysis was not calculated to estimate an appropriate sample size necessary to determine statistical significance. Results from this evaluation are not definitive and are meant to be hypothesis generating for future analysis.
There were several limitations that were identified throughout this project. For this review, history and extent of patient’s prior alcohol use was not assessed. Therefore, the degree of symptom severity in which patients may have experienced during withdrawal may not have been adequately matched between groups. The inherent subjectivity of CIWA-Ar scoring was considered a limitation because scores were determined by clinical interpretation among various nursing staff. As this was a retrospective review, exact timing of medications administered as well as additional supportive care measures, such as ancillary medications for symptom management, were not accounted for and controlled between groups.
Patients presenting to the emergency department or from a facility outside of VAPORHCS for acute AWS may have had incomplete documentation of the onset of symptoms on presentation or of the medications administered prior to being admitted, which may have confounded initial CIWA-Ar scoring and total duration required to be on a withdrawal protocol. Some patients may have received benzodiazepines at initial presentation prior to gabapentin initiation and may have inaccurately reflected its efficacy potential to manage symptoms without the need for lorazepam.
There were 10 patients that were identified who received gabapentin on the alcohol withdrawal protocol and did not receive any lorazepam. This retrospective review could not be determined whether these patients did not require lorazepam because initiating gabapentin reduced severity or simply because their withdrawal symptoms were not severe enough to warrant the need for lorazepam, regardless of gabapentin use.
Gabapentin dosing was not standardized among patients, averaging from 100 mg to 3,600 mg per day. This wide variation in dose may have influenced the requirement of lorazepam needed for symptom management in patients receiving minimal doses or AEs experienced in patients who received large doses. Initiation and/or select dosing of gabapentin may have been dependent on the experience of the provider and familiarity with its use in alcohol withdrawal management. Interestingly, patients with a history of withdrawal seizures (13%) were identified only within the lorazepam-only group. This could suggest that patients with prior symptoms of severe alcohol withdrawal were selected to receive lorazepam-only at the discretion of the provider.
Existing literature investigating gabapentin utilization in alcohol withdrawal has demonstrated benefit for patients with mild-to-moderate symptoms in both inpatient and outpatient studies. However, supporting evidence is limited by the differences in design, methods, and comparators within each trial. Leung and colleagues identified 5 studies that utilized gabapentin as monotherapy or in combination with other agents in alcohol withdrawal.13 Three of these studies were performed within an inpatient setting, each differing in trial design, inclusion/exclusion criteria, intervention, and outcomes. Gabapentin dosing strategies were highly variable among studies. Collectively, the differences noted make it difficult to generalize that similar outcomes would result in other patient populations. The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Future projects could be designed to draw more specific conclusions.
Conclusion
On average, the required benzodiazepine dosage was lower with concomitant use of gabapentin in acute AWS management. The duration for patients on alcohol withdrawal protocol was not reduced with use of gabapentin. Between group (ie, history of withdrawal seizures, blood alcohol level) and among group (ie, gabapentin administration) differences prevent direct correlations to be drawn from this evaluation. Future reviews should include power analysis to establish an appropriate sample size to determine statistical significance among identified covariates. Further evaluation of the use of gabapentin for withdrawal management is warranted prior to incorporating its routine use in the current standard of care for patients experiencing acute AWS.
Acknowledgments
The authors thank Ryan Bickel, PharmD, BCCCP, Critical Care Clinical Pharmacist; Stephen M. Smith, PhD, Director of Medical Critical Care; Gordon Wong, PharmD, Clinical Applications Coordinator; and Eileen Wilbur, RPh, Research Pharmacy Supervisor.
The prevalence of alcohol dependence in the U.S. represents a significant public health concern. Alcohol use disorder (AUD) is estimated to affect 6.7% of Americans and is the fourth leading preventable cause of death.1 Men and women who have served in the military are at an even higher risk of excessive alcohol use. More than 20% of service members report binge drinking every week.2 This risk is further exacerbated in veterans who have experienced active combat or who have comorbid health conditions, such as posttraumatic stress disorder.3
Background
Individuals that regularly consume excessive amounts of alcohol can develop acute alcohol withdrawal syndrome (AWS) after abrupt discontinuation or significant reduction of alcohol intake. Patients admitted for acute alcohol withdrawal may experience complicated courses of treatment and extended lengths of hospitalization.4,5 Cessation from chronic alcohol intake elicits a pathophysiologic response from increased N-methyl-d-aspartate receptor activity and decreased γ-aminobutyric acid (GABA) receptor function.
Autonomic and psychomotor hyperactivity disturbances, such as anxiety, nausea, tremors, diaphoresis, and tachycardia, may occur as early as 6 to 8 hours after cessation of use. Within 48 to 72 hours of alcohol cessation, patients may be at an increased risk of experiencing tonic-clonic seizures, visual and auditory hallucinations, and delirium tremens (DTs), which may be accompanied by signs of extreme autonomic hyperactivity and agitation.6 Patients hospitalized within acute settings require frequent medical supervision for acute alcohol withdrawal, especially in patients at high risk for seizure or DTs because morbidity and mortality risk is increased.7
Benzodiazepines remain the standard of care for management of moderate-to-severe symptoms of AWS. Strong evidence supports the use of benzodiazepines to reduce withdrawal severity, incidence of delirium, and seizures in AWS by enhancing GABA activity.8 However, the adverse effect (AE) burden associated with benzodiazepines can be a major limitation throughout care. Benzodiazepines also may be limited in their use in select patient populations, such as in older adults or patients who present with hepatic dysfunction due to the risk of increased AEs or metabolite accumulation.6 A high dosing requirement of benzodiazepine for symptom management can lead to oversedation to the point of requiring intubation, increasing length of stay in the intensive care unit (ICU), and the risk of nosocomial infections.9
Anticonvulsants, such as carbamazepine, valproic acid, and gabapentin, have shown to be superior to placebo and equal in efficacy to benzodiazepines for symptom management in mild-to-moderate alcohol withdrawal in both inpatient and outpatient settings.6-8 However, these agents are not recommended as first-line monotherapy due to the limited number of randomized trials supporting their efficacy over benzodiazepines in preventing severe symptoms of withdrawal, such as seizures or delirium.10-12 Nonetheless, the mechanism of action of anticonvulsants may help raise seizure threshold in patients and provide a benzodiazepine-sparing effect by enhancing GABAergic activity and lowering neuronal excitability.13
Gabapentin makes an attractive agent for clinical use because of its anxiolytic and sedative properties that can be used to potentially target symptoms analogous with AWS when the use of benzodiazepines becomes a safety concern. Although similar in chemical structure, gabapentin is not metabolized to GABA and does not directly interact with the receptor. Gabapentin may increase GABA concentrations by direct synthesis of GABA and indirectly through interaction with voltage-gated calcium channels.13 In addition to its overall safety profile, gabapentin may be a viable adjuvant because emerging data may suggest a potential role in the management of acute alcohol withdrawal.12,14,15
Gabapentin for Alcohol Withdrawal at VAPORHCS
Although not currently included in the alcohol withdrawal protocol at Veterans Affairs Portland Health Care System (VAPORHCS), gabapentin has been added to the standard of care in select patients per the discretion of the attending physician. Anecdotal reports of patients experiencing milder symptoms and less benzodiazepine administration have facilitated use of gabapentin in alcohol withdrawal management at VAPORHCS. However, routine use of gabapentin is not consistent among all patients treated for acute alcohol withdrawal, and dosing schedules of gabapentin seem highly variable. Standard symptom management for acute alcohol withdrawal should be consistent for all affected individuals, using evidence-based medicine in order to achieve optimal outcomes and improve harm reduction.
The objective of this quality assurance/quality improvement (QA/QI) project was to assess the amount of lorazepam required for symptom management in acute alcohol withdrawal when gabapentin is used as an adjunct to treatment and to evaluate the impact on symptom management using the Clinical Institute Withdrawal Assessment for Alcohol scale, revised version (CIWA-Ar) in patients admitted to the ICU and general medicine wards for acute alcohol withdrawal at VAPORHCS.16 If a possible adjunct for the treatment of alcohol withdrawal has the potential to reduce benzodiazepine requirements and minimize AEs, a thorough evaluation of the treatment should be conducted before its practice is incorporated into the current standard of care.
Methods
The following QA/QI project was approved locally by the VAPORHCS associate chief of staff/Office of Research and Development and is considered to be nonresearch VHA operations activity and exempt from an institutional review board committee review. This project was a single-center, retrospective chart review of patients admitted to the ICU and general medicine wards at VAPORHCS with acute alcohol withdrawal. The CIWA-Ar protocol order sets between January 1, 2014 and December 31, 2015, were retrieved through the Computerized Patient Record System (CPRS) at VAPORHCS.
Patients with an alcohol withdrawal protocol order set who received gabapentin with or without lorazepam during hospitalization were identified for chart review. Patients were eligible for review if they were aged ≥ 18 years with a primary or secondary diagnosis of acute alcohol withdrawal and had a CIWA-Ar protocol order set placed during hospitalization. Patients must have been administered gabapentin, lorazepam, or both while the CIWA-Ar protocol was active. Patients with an active outpatient prescription for gabapentin or benzodiazepine filled within the previous 30 days, documented history of psychosis or epileptic seizure disorder, or other concomitant benzodiazepines or antiepileptics administered while on the CIWA-Ar protocol were excluded from the analysis.
Baseline characteristics for patients eligible for review were collected and included age; sex; race, body mass index (BMI); estimated creatinine clearance (CrCl); toxicology screen at admission (if available), history of substance use disorder, AWS, or history of withdrawal seizures; and history of a sedative hypnotics (not including benzodiazepines) prescription within 30 days prior to admission.17
The primary endpoint was the total amount of lorazepam administered from the time of admission to the time of discontinuation of the alcohol withdrawal protocol. The dose, frequency, and amount of lorazepam and gabapentin administered daily were collected for each patient while on the CIWA-Ar protocol. Secondary endpoints included rate of the CIWA-Ar score reduction, time to protocol discontinuation, as well as incidence and onset of peak delirium scores during hospitalization. Cumulative CIWA-Ar scores over 24 hours were averaged per patient per day while on CIWA-Ar protocol. Peak CIWA-Ar scores per patient per day on the protocol also were collected. Time to protocol termination was determined by date of order for discontinuation or by date when scoring had ceased and protocol order was inadvertently continued. Peak Intensive Care Delirium Screening Checklist (ICDSC) scores were collected for patients admitted to the ICU.18 Day of peak ICDSC scores also were evaluated.
Statistical Analysis
The sample size for this analysis was determined by the number of patients identified who met the inclusion criteria and did not meet any of the exclusion criteria. Power was not calculated to estimate sample size needed to determine statistical significance. One hundred patients treated for alcohol withdrawal was established as the target sample size for this project. Descriptive statistics were performed to analyze patient baseline characteristics and primary and secondary objective data.
Results
A total of 1,611 CIWA-Ar protocol orders were identified between January 1, 2014 and December 31, 2015.
Primary Endpoint
The average amount of lorazepam administered for the total duration on CIWA-Ar protocol was 7.9 mg (median 6, ± 8.2) among
Secondary Endpoints
On average, the total number of days spent on CIWA-Ar protocol was 3.8 (median 4, ± 1.5) in group 1 compared with 4.1 (median 4, ± 1.6) in group 2. Rate of CIWA-Ar protocol discontinuation for patients in group 1 and group 2 is shown in Figure 3.
Discussion
The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Patients who were started on gabapentin on the initiation of the alcohol withdrawal protocol received less lorazepam dosing compared with patients who received only lorazepam for symptom management for alcohol withdrawal. Except for day 3, average lorazepam dosage per day on the alcohol withdrawal protocol was lower in patients who were also taking gabapentin.
This trend also can be seen in the recorded peak CIWA-Ar scores per day as illustrated in Figures 4 and 5.
Limitations
Prior to evaluation, power analysis was not calculated to estimate an appropriate sample size necessary to determine statistical significance. Results from this evaluation are not definitive and are meant to be hypothesis generating for future analysis.
There were several limitations that were identified throughout this project. For this review, history and extent of patient’s prior alcohol use was not assessed. Therefore, the degree of symptom severity in which patients may have experienced during withdrawal may not have been adequately matched between groups. The inherent subjectivity of CIWA-Ar scoring was considered a limitation because scores were determined by clinical interpretation among various nursing staff. As this was a retrospective review, exact timing of medications administered as well as additional supportive care measures, such as ancillary medications for symptom management, were not accounted for and controlled between groups.
Patients presenting to the emergency department or from a facility outside of VAPORHCS for acute AWS may have had incomplete documentation of the onset of symptoms on presentation or of the medications administered prior to being admitted, which may have confounded initial CIWA-Ar scoring and total duration required to be on a withdrawal protocol. Some patients may have received benzodiazepines at initial presentation prior to gabapentin initiation and may have inaccurately reflected its efficacy potential to manage symptoms without the need for lorazepam.
There were 10 patients that were identified who received gabapentin on the alcohol withdrawal protocol and did not receive any lorazepam. This retrospective review could not be determined whether these patients did not require lorazepam because initiating gabapentin reduced severity or simply because their withdrawal symptoms were not severe enough to warrant the need for lorazepam, regardless of gabapentin use.
Gabapentin dosing was not standardized among patients, averaging from 100 mg to 3,600 mg per day. This wide variation in dose may have influenced the requirement of lorazepam needed for symptom management in patients receiving minimal doses or AEs experienced in patients who received large doses. Initiation and/or select dosing of gabapentin may have been dependent on the experience of the provider and familiarity with its use in alcohol withdrawal management. Interestingly, patients with a history of withdrawal seizures (13%) were identified only within the lorazepam-only group. This could suggest that patients with prior symptoms of severe alcohol withdrawal were selected to receive lorazepam-only at the discretion of the provider.
Existing literature investigating gabapentin utilization in alcohol withdrawal has demonstrated benefit for patients with mild-to-moderate symptoms in both inpatient and outpatient studies. However, supporting evidence is limited by the differences in design, methods, and comparators within each trial. Leung and colleagues identified 5 studies that utilized gabapentin as monotherapy or in combination with other agents in alcohol withdrawal.13 Three of these studies were performed within an inpatient setting, each differing in trial design, inclusion/exclusion criteria, intervention, and outcomes. Gabapentin dosing strategies were highly variable among studies. Collectively, the differences noted make it difficult to generalize that similar outcomes would result in other patient populations. The purpose of this project was to evaluate gabapentin use at VAPORHCS for alcohol withdrawal and evaluate the impact on symptom management. Future projects could be designed to draw more specific conclusions.
Conclusion
On average, the required benzodiazepine dosage was lower with concomitant use of gabapentin in acute AWS management. The duration for patients on alcohol withdrawal protocol was not reduced with use of gabapentin. Between group (ie, history of withdrawal seizures, blood alcohol level) and among group (ie, gabapentin administration) differences prevent direct correlations to be drawn from this evaluation. Future reviews should include power analysis to establish an appropriate sample size to determine statistical significance among identified covariates. Further evaluation of the use of gabapentin for withdrawal management is warranted prior to incorporating its routine use in the current standard of care for patients experiencing acute AWS.
Acknowledgments
The authors thank Ryan Bickel, PharmD, BCCCP, Critical Care Clinical Pharmacist; Stephen M. Smith, PhD, Director of Medical Critical Care; Gordon Wong, PharmD, Clinical Applications Coordinator; and Eileen Wilbur, RPh, Research Pharmacy Supervisor.
1. Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109.
2. National Institute on Drug Abuse. Military. https://www.drugabuse.gov/related-topics/military. Updated April 2016. Accessed January 10, 2018.
3. Bohnert KM, Ilgen MA, Rosen CS, Desai RA, Austin K, Blow FC. The association between substance use disorders and mortality among a cohort of veterans with posttraumatic stress disorder: variation by age cohort and mortality type. Drug Alcohol Depend. 2013;128(1-2):98-103.
4. Foy A, Kay J, Taylor A. The course of alcohol withdrawal in a general hospital. QJM. 1997;90(4):253-261.
5. Carlson RW, Kumar NN, Wong-Mckinstry E, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28(4):549-585.
6. National Institute for Health and Care Excellence. Alcohol use disorders: diagnosis and clinical management of alcohol-related physical complications. https://www.nice.org.uk/guidance/cg100. Published June 2010. Updated April 2017. Accessed January 10, 2018.
7. Sarff MC, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med. 2010;38(suppl 9):494-501.
8. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf. Published December 2015. Accessed January 10, 2018.
9. Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724-730.
10. Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database Syst Rev. 2011(6):D008537.
11. Ntais C, Pakos E, Kyzas P, Ioannidis JP. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2005;20(3):CD005063.
12. Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol. 2010;45(2):143-145.
13. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. Role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906.
14. Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29:248-254.
15. Myrick H, Malcolm R, Randall PK, et al. A double blind trial of gabapentin vs lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
16. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
17 Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
18. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859-864.
1. Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109.
2. National Institute on Drug Abuse. Military. https://www.drugabuse.gov/related-topics/military. Updated April 2016. Accessed January 10, 2018.
3. Bohnert KM, Ilgen MA, Rosen CS, Desai RA, Austin K, Blow FC. The association between substance use disorders and mortality among a cohort of veterans with posttraumatic stress disorder: variation by age cohort and mortality type. Drug Alcohol Depend. 2013;128(1-2):98-103.
4. Foy A, Kay J, Taylor A. The course of alcohol withdrawal in a general hospital. QJM. 1997;90(4):253-261.
5. Carlson RW, Kumar NN, Wong-Mckinstry E, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28(4):549-585.
6. National Institute for Health and Care Excellence. Alcohol use disorders: diagnosis and clinical management of alcohol-related physical complications. https://www.nice.org.uk/guidance/cg100. Published June 2010. Updated April 2017. Accessed January 10, 2018.
7. Sarff MC, Gold JA. Alcohol withdrawal syndromes in the intensive care unit. Crit Care Med. 2010;38(suppl 9):494-501.
8. U.S. Department of Veteran Affairs, U.S. Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPGRevised22216.pdf. Published December 2015. Accessed January 10, 2018.
9. Gold JA, Rimal B, Nolan A, Nelson LS. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724-730.
10. Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the alcohol withdrawal syndrome. Cochrane Database Syst Rev. 2011(6):D008537.
11. Ntais C, Pakos E, Kyzas P, Ioannidis JP. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2005;20(3):CD005063.
12. Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol. 2010;45(2):143-145.
13. Leung JG, Hall-Flavin D, Nelson S, Schmidt KA, Schak KM. Role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906.
14. Johnson BA, Swift RM, Addolorato G, Ciraulo DA, Myrick H. Safety and efficacy of GABAergic medications for treating alcoholism. Alcohol Clin Exp Res. 2005;29:248-254.
15. Myrick H, Malcolm R, Randall PK, et al. A double blind trial of gabapentin vs lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
16. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
17 Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
18. Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859-864.