User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
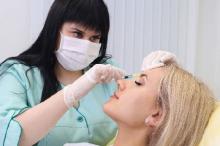
Expert advocates single pass CO2 laser resurfacing
NEWPORT BEACH, CALIF. – Facial CO2 laser resurfacing deserves a comeback, according to Victor Ross, MD, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego.
The practice fell out of favor in the late 1990s because of long recovery times, hypopigmentation, and the risk of keloid scars, among other issues. Physicians were just being too aggressive, doing multiple full-field passes in one session, he said at the Summit in Aesthetic Medicine, held by Global Academy for Medical Education.
It turns out that doing one pass very conservatively – with maybe a second pass around the mouth for deeper wrinkles – delivers a lot of the benefits with none of the downsides. It takes maybe 45 minutes, and “we get very nice results, I think better than fractional [laser] results,” Dr. Ross said. The wrinkle-smoothing effect may not be as potent or durable as the old-school approach, “but it’s a more natural look and [there’s] much faster recovery. Patients go pink instead of red,” and can wear makeup sooner. “You don’t get delayed hypopigmentation.”
The general trend in cosmetic dermatology is to do multiple procedures in one office visit instead of spacing them out over several appointments. For instance, lentigines on the hand could be targeted with intense pulsed light and then hand crepiness could be treated with a fractional laser. You “can get a lot done and a very nice result in one” session, but it makes sense to dial settings back maybe 15%-20% when different devices are used on the same area, he said.
I keep several lasers in one room” and sometimes “go back and forth, back and forth like a mad chef,” he said.
Companies are helping further the approach. One company, for instance, has added a nonablative fractional laser to its intense pulsed light platform. Others are combining ablative and nonablative fractional lasers. It’s all about “allowing you to have more flexibility in how you deliver energy,” Dr. Ross said.
Meanwhile, another newer kid on the block, the picosecond laser, has helped a bit with tattoo removal, but “we are still not really hitting a home run. There’s no doubt that picosecond lasers are more efficient than nanosecond lasers, but they’re maybe 20%-30% better,” he said. The problem is that most commercially available picosecond lasers have pulse durations on the order of 500-800 picoseconds, which is not optimal for the smallest pigment particles. If pulse durations are too short and energies too high, “you get this kind of white plasma in the skin, which doesn’t” help.
Industry hasn’t solved the problem yet; for now “I would give us a C-plus on tattoos,” he said.
Dr. Ross had some advice for dermatologists looking to outfit a new cosmetic practice. Right off the bat, “you will need something for red and brown spots, because you are going to see a lot of that.” An intense pulsed light device or a large-spot potassium titanyl phosphate (KTP) laser fills the bill, he said.
He added that he would have a device for resurfacing, and as the third device, “I would get maybe a Q-switch laser for tattoos,” he said.
So armed, a dermatologist could handle much of what’s likely to come through the door.
Dr. Ross works with a number of companies, including Lutronic, Cynosure, and Ellipse. Global Academy and this news organization are owned by the same company.
NEWPORT BEACH, CALIF. – Facial CO2 laser resurfacing deserves a comeback, according to Victor Ross, MD, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego.
The practice fell out of favor in the late 1990s because of long recovery times, hypopigmentation, and the risk of keloid scars, among other issues. Physicians were just being too aggressive, doing multiple full-field passes in one session, he said at the Summit in Aesthetic Medicine, held by Global Academy for Medical Education.
It turns out that doing one pass very conservatively – with maybe a second pass around the mouth for deeper wrinkles – delivers a lot of the benefits with none of the downsides. It takes maybe 45 minutes, and “we get very nice results, I think better than fractional [laser] results,” Dr. Ross said. The wrinkle-smoothing effect may not be as potent or durable as the old-school approach, “but it’s a more natural look and [there’s] much faster recovery. Patients go pink instead of red,” and can wear makeup sooner. “You don’t get delayed hypopigmentation.”
The general trend in cosmetic dermatology is to do multiple procedures in one office visit instead of spacing them out over several appointments. For instance, lentigines on the hand could be targeted with intense pulsed light and then hand crepiness could be treated with a fractional laser. You “can get a lot done and a very nice result in one” session, but it makes sense to dial settings back maybe 15%-20% when different devices are used on the same area, he said.
I keep several lasers in one room” and sometimes “go back and forth, back and forth like a mad chef,” he said.
Companies are helping further the approach. One company, for instance, has added a nonablative fractional laser to its intense pulsed light platform. Others are combining ablative and nonablative fractional lasers. It’s all about “allowing you to have more flexibility in how you deliver energy,” Dr. Ross said.
Meanwhile, another newer kid on the block, the picosecond laser, has helped a bit with tattoo removal, but “we are still not really hitting a home run. There’s no doubt that picosecond lasers are more efficient than nanosecond lasers, but they’re maybe 20%-30% better,” he said. The problem is that most commercially available picosecond lasers have pulse durations on the order of 500-800 picoseconds, which is not optimal for the smallest pigment particles. If pulse durations are too short and energies too high, “you get this kind of white plasma in the skin, which doesn’t” help.
Industry hasn’t solved the problem yet; for now “I would give us a C-plus on tattoos,” he said.
Dr. Ross had some advice for dermatologists looking to outfit a new cosmetic practice. Right off the bat, “you will need something for red and brown spots, because you are going to see a lot of that.” An intense pulsed light device or a large-spot potassium titanyl phosphate (KTP) laser fills the bill, he said.
He added that he would have a device for resurfacing, and as the third device, “I would get maybe a Q-switch laser for tattoos,” he said.
So armed, a dermatologist could handle much of what’s likely to come through the door.
Dr. Ross works with a number of companies, including Lutronic, Cynosure, and Ellipse. Global Academy and this news organization are owned by the same company.
NEWPORT BEACH, CALIF. – Facial CO2 laser resurfacing deserves a comeback, according to Victor Ross, MD, director of laser and cosmetic dermatology at the Scripps Clinic in San Diego.
The practice fell out of favor in the late 1990s because of long recovery times, hypopigmentation, and the risk of keloid scars, among other issues. Physicians were just being too aggressive, doing multiple full-field passes in one session, he said at the Summit in Aesthetic Medicine, held by Global Academy for Medical Education.
It turns out that doing one pass very conservatively – with maybe a second pass around the mouth for deeper wrinkles – delivers a lot of the benefits with none of the downsides. It takes maybe 45 minutes, and “we get very nice results, I think better than fractional [laser] results,” Dr. Ross said. The wrinkle-smoothing effect may not be as potent or durable as the old-school approach, “but it’s a more natural look and [there’s] much faster recovery. Patients go pink instead of red,” and can wear makeup sooner. “You don’t get delayed hypopigmentation.”
The general trend in cosmetic dermatology is to do multiple procedures in one office visit instead of spacing them out over several appointments. For instance, lentigines on the hand could be targeted with intense pulsed light and then hand crepiness could be treated with a fractional laser. You “can get a lot done and a very nice result in one” session, but it makes sense to dial settings back maybe 15%-20% when different devices are used on the same area, he said.
I keep several lasers in one room” and sometimes “go back and forth, back and forth like a mad chef,” he said.
Companies are helping further the approach. One company, for instance, has added a nonablative fractional laser to its intense pulsed light platform. Others are combining ablative and nonablative fractional lasers. It’s all about “allowing you to have more flexibility in how you deliver energy,” Dr. Ross said.
Meanwhile, another newer kid on the block, the picosecond laser, has helped a bit with tattoo removal, but “we are still not really hitting a home run. There’s no doubt that picosecond lasers are more efficient than nanosecond lasers, but they’re maybe 20%-30% better,” he said. The problem is that most commercially available picosecond lasers have pulse durations on the order of 500-800 picoseconds, which is not optimal for the smallest pigment particles. If pulse durations are too short and energies too high, “you get this kind of white plasma in the skin, which doesn’t” help.
Industry hasn’t solved the problem yet; for now “I would give us a C-plus on tattoos,” he said.
Dr. Ross had some advice for dermatologists looking to outfit a new cosmetic practice. Right off the bat, “you will need something for red and brown spots, because you are going to see a lot of that.” An intense pulsed light device or a large-spot potassium titanyl phosphate (KTP) laser fills the bill, he said.
He added that he would have a device for resurfacing, and as the third device, “I would get maybe a Q-switch laser for tattoos,” he said.
So armed, a dermatologist could handle much of what’s likely to come through the door.
Dr. Ross works with a number of companies, including Lutronic, Cynosure, and Ellipse. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM THE SUMMIT IN AESTHETIC MEDICINE
Endobariatrics: Coming to a clinic near you
SAN DIEGO – Device companies are working hard to bring obesity management to the endoscopy suite.
The field is called endobariatrics, and its goal is to fill the gap between surgery and pharmacotherapy. Drugs and lifestyle counseling don’t work too well, but a lot of people don’t want to go under the knife, so something is needed in the middle. Endobariatrics has the potential to be a boon for both obese patients and gastroenterology practices.
Several new investigational devices and approaches were showcased at the annual Digestive Disease Week; some “are beginning to approach the kind of results we see with surgical techniques,” said Steven Edmundowicz, MD, medical director of the University of Colorado Digestive Health Center, Aurora.
“We are seeing a tremendous amount of development in this space, but it’s early, and we have to be cautious,” he said. There have already been a few disappointments, including the EndoBarrier, a fluoropolymer liner anchored in the duodenal bulb and unfurled down the duodenum to block food absorption. A key U.S. trial was recently halted due to liver abscesses.
Dr. Edmundowicz reviewed the latest developments presented at DDW.
Self-assembling magnets for dual-path enteral anastomoses
The goal of the GI Windows system is to create a partial jejunoileal, side to side bypass without surgery. A 28-mm magnet ring is introduced to the ileum by colonoscopy, and a second ring to the jejunum by endoscopy. The rings snap together and tissue caught between them dies from pressure necrosis, leaving patients with a jejunoileal communication. Once food reaches that point, it either diverts through the anastomosis or continues past it down the digestive track. The magnets pass after the anastomosis forms in a week or so.
In a 6-month feasibility study from the Czech Republic, 10 obese patients lost 28.3% of their excess weight without diet restrictions. Those with diabetes had a mean hemoglobin A1c drop of 1.8%, and normalization of fasting blood glucose levels. The procedure took just over an hour and a half after the first five cases.
“I am very excited about [this]; I really want to see where the data are going,” Dr. Edmundowicz said.
Duodenal mucosal resurfacing
The idea of the Revita System (Fractyl) is to ablate “diabetic mucosa” in the duodenum so that normal mucosa can replace it. Saline is injected endoscopically under a portion of the duodenal mucosa to lift it off the muscularis; once isolated, the mucosa is destroyed – some in the audience thought “cooked” was a better word – by exposure to a hot water balloon catheter threaded into the lumen.
Thirty-nine overweight or obese type 2 diabetics had a 1.2% improvement at 6 months from a baseline hemoglobin A1c of 9.6% in a series from Santiago, Chile. Weight loss was modest in the trial; the system is being developed for type 2 diabetics.
There is some histologic support for the notion of a diabetic mucosa with both structural and hormonal aberrations, but it’s unclear if it’s a sign or cause of sickness. Even so, “the mucosa regenerates” and won’t be diabetic “for a while” after the procedure, said investigator Manoel Galvao Neto, MD, of the Gastro Obeso Center, São Paulo.
Gastric balloons
Inflating a balloon in the stomach to make people feel full isn’t new, but the notion of putting the balloon into a capsule that patients can swallow and inflating it through a tether is a more recent notion.
The Obalon (Obalon Therapeutics) is one such device. In an unblinded, sham-controlled trial with 336 obese patients, subjects who got the 250-mL, nitrogen-filled Obalons – most received three – lost about 3% more of their total body weight at 24 weeks than those who did not. Although swallowed, Obalon is removed endoscopically. Meanwhile, 34 obese patients who swallowed the 550-mL, fluid-filled Elipse balloon (Allurion) had a total body weight loss of 9.5% and mean excess weight loss of 37.2% at 4 months, by which time Elipse deflates on its own and passes without endoscopic retrieval.
“This is a very promising approach. I am very excited about digested balloons,” said Dr. Edmundowicz, an investigator in the Obalon study.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty duplicates sleeve gastrectomy with stitches placed endoscopically to seal off the greater curvature of the stomach; functionally, patients are left with a narrow sleeve of a stomach. In a multicenter series presented at DDW, 242 patients had a mean total body weight loss of 19.8% at 18 months, with a low incidence of complications. “Weight loss appears to be continuing,” Dr. Edmundowicz said. Investigators used the Apollo OverStitch (Apollo Endosurgery) to place the sutures.
Aspiration therapy
With Food and Drug Administration approval on June 14, AspireAssist (Aspire Bariatrics) is probably the best known of the newer approaches. Patients drain a portion of their meals through an endoscopically placed percutaneous gastrostomy tube a half hour or so after eating. It takes 5-10 minutes. The agency is eager to keep it out of the hands of bulimics.
One-year data were reported at DDW; 111 obese AspireAssist subjects lost a mean of 37.2% of their excess weight versus 13% in 60 patients randomized to lifestyle counseling alone.
“It may not be aesthetically pleasing, but it certainly works. It’s a viable technology,” said Dr. Edmundowicz, who was an investigator.
The studies were funded by companies developing the devices and techniques. Dr. Edmundowicz has stock options, or is a consultant or researcher, Aspire, Obalon, GI Dynamics, Elira, and other firms.
SAN DIEGO – Device companies are working hard to bring obesity management to the endoscopy suite.
The field is called endobariatrics, and its goal is to fill the gap between surgery and pharmacotherapy. Drugs and lifestyle counseling don’t work too well, but a lot of people don’t want to go under the knife, so something is needed in the middle. Endobariatrics has the potential to be a boon for both obese patients and gastroenterology practices.
Several new investigational devices and approaches were showcased at the annual Digestive Disease Week; some “are beginning to approach the kind of results we see with surgical techniques,” said Steven Edmundowicz, MD, medical director of the University of Colorado Digestive Health Center, Aurora.
“We are seeing a tremendous amount of development in this space, but it’s early, and we have to be cautious,” he said. There have already been a few disappointments, including the EndoBarrier, a fluoropolymer liner anchored in the duodenal bulb and unfurled down the duodenum to block food absorption. A key U.S. trial was recently halted due to liver abscesses.
Dr. Edmundowicz reviewed the latest developments presented at DDW.
Self-assembling magnets for dual-path enteral anastomoses
The goal of the GI Windows system is to create a partial jejunoileal, side to side bypass without surgery. A 28-mm magnet ring is introduced to the ileum by colonoscopy, and a second ring to the jejunum by endoscopy. The rings snap together and tissue caught between them dies from pressure necrosis, leaving patients with a jejunoileal communication. Once food reaches that point, it either diverts through the anastomosis or continues past it down the digestive track. The magnets pass after the anastomosis forms in a week or so.
In a 6-month feasibility study from the Czech Republic, 10 obese patients lost 28.3% of their excess weight without diet restrictions. Those with diabetes had a mean hemoglobin A1c drop of 1.8%, and normalization of fasting blood glucose levels. The procedure took just over an hour and a half after the first five cases.
“I am very excited about [this]; I really want to see where the data are going,” Dr. Edmundowicz said.
Duodenal mucosal resurfacing
The idea of the Revita System (Fractyl) is to ablate “diabetic mucosa” in the duodenum so that normal mucosa can replace it. Saline is injected endoscopically under a portion of the duodenal mucosa to lift it off the muscularis; once isolated, the mucosa is destroyed – some in the audience thought “cooked” was a better word – by exposure to a hot water balloon catheter threaded into the lumen.
Thirty-nine overweight or obese type 2 diabetics had a 1.2% improvement at 6 months from a baseline hemoglobin A1c of 9.6% in a series from Santiago, Chile. Weight loss was modest in the trial; the system is being developed for type 2 diabetics.
There is some histologic support for the notion of a diabetic mucosa with both structural and hormonal aberrations, but it’s unclear if it’s a sign or cause of sickness. Even so, “the mucosa regenerates” and won’t be diabetic “for a while” after the procedure, said investigator Manoel Galvao Neto, MD, of the Gastro Obeso Center, São Paulo.
Gastric balloons
Inflating a balloon in the stomach to make people feel full isn’t new, but the notion of putting the balloon into a capsule that patients can swallow and inflating it through a tether is a more recent notion.
The Obalon (Obalon Therapeutics) is one such device. In an unblinded, sham-controlled trial with 336 obese patients, subjects who got the 250-mL, nitrogen-filled Obalons – most received three – lost about 3% more of their total body weight at 24 weeks than those who did not. Although swallowed, Obalon is removed endoscopically. Meanwhile, 34 obese patients who swallowed the 550-mL, fluid-filled Elipse balloon (Allurion) had a total body weight loss of 9.5% and mean excess weight loss of 37.2% at 4 months, by which time Elipse deflates on its own and passes without endoscopic retrieval.
“This is a very promising approach. I am very excited about digested balloons,” said Dr. Edmundowicz, an investigator in the Obalon study.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty duplicates sleeve gastrectomy with stitches placed endoscopically to seal off the greater curvature of the stomach; functionally, patients are left with a narrow sleeve of a stomach. In a multicenter series presented at DDW, 242 patients had a mean total body weight loss of 19.8% at 18 months, with a low incidence of complications. “Weight loss appears to be continuing,” Dr. Edmundowicz said. Investigators used the Apollo OverStitch (Apollo Endosurgery) to place the sutures.
Aspiration therapy
With Food and Drug Administration approval on June 14, AspireAssist (Aspire Bariatrics) is probably the best known of the newer approaches. Patients drain a portion of their meals through an endoscopically placed percutaneous gastrostomy tube a half hour or so after eating. It takes 5-10 minutes. The agency is eager to keep it out of the hands of bulimics.
One-year data were reported at DDW; 111 obese AspireAssist subjects lost a mean of 37.2% of their excess weight versus 13% in 60 patients randomized to lifestyle counseling alone.
“It may not be aesthetically pleasing, but it certainly works. It’s a viable technology,” said Dr. Edmundowicz, who was an investigator.
The studies were funded by companies developing the devices and techniques. Dr. Edmundowicz has stock options, or is a consultant or researcher, Aspire, Obalon, GI Dynamics, Elira, and other firms.
SAN DIEGO – Device companies are working hard to bring obesity management to the endoscopy suite.
The field is called endobariatrics, and its goal is to fill the gap between surgery and pharmacotherapy. Drugs and lifestyle counseling don’t work too well, but a lot of people don’t want to go under the knife, so something is needed in the middle. Endobariatrics has the potential to be a boon for both obese patients and gastroenterology practices.
Several new investigational devices and approaches were showcased at the annual Digestive Disease Week; some “are beginning to approach the kind of results we see with surgical techniques,” said Steven Edmundowicz, MD, medical director of the University of Colorado Digestive Health Center, Aurora.
“We are seeing a tremendous amount of development in this space, but it’s early, and we have to be cautious,” he said. There have already been a few disappointments, including the EndoBarrier, a fluoropolymer liner anchored in the duodenal bulb and unfurled down the duodenum to block food absorption. A key U.S. trial was recently halted due to liver abscesses.
Dr. Edmundowicz reviewed the latest developments presented at DDW.
Self-assembling magnets for dual-path enteral anastomoses
The goal of the GI Windows system is to create a partial jejunoileal, side to side bypass without surgery. A 28-mm magnet ring is introduced to the ileum by colonoscopy, and a second ring to the jejunum by endoscopy. The rings snap together and tissue caught between them dies from pressure necrosis, leaving patients with a jejunoileal communication. Once food reaches that point, it either diverts through the anastomosis or continues past it down the digestive track. The magnets pass after the anastomosis forms in a week or so.
In a 6-month feasibility study from the Czech Republic, 10 obese patients lost 28.3% of their excess weight without diet restrictions. Those with diabetes had a mean hemoglobin A1c drop of 1.8%, and normalization of fasting blood glucose levels. The procedure took just over an hour and a half after the first five cases.
“I am very excited about [this]; I really want to see where the data are going,” Dr. Edmundowicz said.
Duodenal mucosal resurfacing
The idea of the Revita System (Fractyl) is to ablate “diabetic mucosa” in the duodenum so that normal mucosa can replace it. Saline is injected endoscopically under a portion of the duodenal mucosa to lift it off the muscularis; once isolated, the mucosa is destroyed – some in the audience thought “cooked” was a better word – by exposure to a hot water balloon catheter threaded into the lumen.
Thirty-nine overweight or obese type 2 diabetics had a 1.2% improvement at 6 months from a baseline hemoglobin A1c of 9.6% in a series from Santiago, Chile. Weight loss was modest in the trial; the system is being developed for type 2 diabetics.
There is some histologic support for the notion of a diabetic mucosa with both structural and hormonal aberrations, but it’s unclear if it’s a sign or cause of sickness. Even so, “the mucosa regenerates” and won’t be diabetic “for a while” after the procedure, said investigator Manoel Galvao Neto, MD, of the Gastro Obeso Center, São Paulo.
Gastric balloons
Inflating a balloon in the stomach to make people feel full isn’t new, but the notion of putting the balloon into a capsule that patients can swallow and inflating it through a tether is a more recent notion.
The Obalon (Obalon Therapeutics) is one such device. In an unblinded, sham-controlled trial with 336 obese patients, subjects who got the 250-mL, nitrogen-filled Obalons – most received three – lost about 3% more of their total body weight at 24 weeks than those who did not. Although swallowed, Obalon is removed endoscopically. Meanwhile, 34 obese patients who swallowed the 550-mL, fluid-filled Elipse balloon (Allurion) had a total body weight loss of 9.5% and mean excess weight loss of 37.2% at 4 months, by which time Elipse deflates on its own and passes without endoscopic retrieval.
“This is a very promising approach. I am very excited about digested balloons,” said Dr. Edmundowicz, an investigator in the Obalon study.
Endoscopic sleeve gastroplasty
Endoscopic sleeve gastroplasty duplicates sleeve gastrectomy with stitches placed endoscopically to seal off the greater curvature of the stomach; functionally, patients are left with a narrow sleeve of a stomach. In a multicenter series presented at DDW, 242 patients had a mean total body weight loss of 19.8% at 18 months, with a low incidence of complications. “Weight loss appears to be continuing,” Dr. Edmundowicz said. Investigators used the Apollo OverStitch (Apollo Endosurgery) to place the sutures.
Aspiration therapy
With Food and Drug Administration approval on June 14, AspireAssist (Aspire Bariatrics) is probably the best known of the newer approaches. Patients drain a portion of their meals through an endoscopically placed percutaneous gastrostomy tube a half hour or so after eating. It takes 5-10 minutes. The agency is eager to keep it out of the hands of bulimics.
One-year data were reported at DDW; 111 obese AspireAssist subjects lost a mean of 37.2% of their excess weight versus 13% in 60 patients randomized to lifestyle counseling alone.
“It may not be aesthetically pleasing, but it certainly works. It’s a viable technology,” said Dr. Edmundowicz, who was an investigator.
The studies were funded by companies developing the devices and techniques. Dr. Edmundowicz has stock options, or is a consultant or researcher, Aspire, Obalon, GI Dynamics, Elira, and other firms.
AT DDW® 2016
Keep hyaluronidase in the office for filler complications
NEWPORT BEACH, CALIF. – If you inject fillers, Manhattan plastic surgeon Lawrence Bass, MD, recommends that you keep hyaluronidase handy.
It’s the go-to for intravascular injections and helpful for nodules and granulomas, the main filler worries, but some offices don’t stock it. “Complications are rare, but they can be devastating. You have to be prepared.”
Massage, aspiration, transdermal nitroglycerin, and other options might help when, for example, a patient’s lip turns purple after a nasolabial fold injection, but “really, it’s all about hyaluronidase,” whether the filler is hyaluronic acid or not. The enzyme digests hyaluronic acid – both the injected kind and what patients have naturally – and loosens and disperses other fillers.
A patient who says that the injection hurt more than usual is an indication that the vessel may have been injected, Dr. Bass said. In such cases, “you have to deal with it immediately,” and repeat dosing if skin color doesn’t improve within an hour, he noted. “If you manage patients correctly, you can usually settle it with little or no deformity.”
There are two hyaluronidase options in the United States: Vitrase, an ovine formulation, and Hylenex, a recombinant human formulation. It probably doesn’t matter which one is used, he said. Soft tissue vascular compromise generally requires 200 units, and retinal artery occlusion, 500 units or more. Restylane is the easiest filler to digest because it’s the least cross-linked; Juvederm is more difficult; and Belotero is the most difficult, Dr. Bass noted.
Nodules and granulomas from filler injections have a slower onset than vascular compromise. Infected nodules can appear months after injection, and granulomas – red or purplish hypersensitivity reactions with indistinct borders, often with all injection sites involved at once – can appear years afterwards, set off by the flu or some other inflammatory trigger, according to Miles Graivier, MD, a plastic surgeon in Roswell, Ga., who shared the podium with Dr. Bass at the meeting.
Dr. Graivier described his approach to granulomas as “very aggressive.” Patients with granulomas receive oral steroids plus intralesional triamcinolone/fluorouracil/lidocaine injections every 2 weeks or more frequently. Hyaluronidase is used to disperse the filler. Resistant patients also get oral allopurinol. Laser liquefaction can help with both granulomas and infected nodules, he added.
Retinal artery occlusion is probably the most feared filler complication. It’s most likely with glabellar or periorbital injections but possible with any facial injection. “It’s a real horror show when it happens. The bottom line is you must restore circulation within 60-90 minutes” with retrobulbar hyaluronidase injections “or patients will lose vision permanently,” Dr. Bass said. People might not feel comfortable with retrobulbar injections, “but when the alternative is blindness, maybe you want to give them a try,” he added.
A 25 gauge, 1.5 inch needle is introduced to the lateral limbus at the orbital rim. “You pass the needle along the orbital floor to the equator of the globe,” – about 13-14 mm – “angle it up to enter the muscular conus, and inject 500 units or more.” Injecting at the orbital floor is less risky, but probably less effective, he said.
Hard nodules, meanwhile, present early after injection and are usually a result of too much filler or poor technique, Dr. Graivier said. For hard nodules, “hyaluronidase is the number one treatment,” although cracking, massage, and saline injection might help.
Infected nodules look like abscesses, but usually grow nothing on culture. Hyaluronidase is also first line for infected nodules, along with drainage and broad spectrum oral antibiotics for at least 6 weeks, and, if they don’t work, intravenous antibiotics, he added.
Dr. Bass is an investigator and speaker for Cynosure; an investigator for Endo Pharmaceuticals and Neothetics, and an advisor to Merz. Dr. Graivier is an advisor for Merz, and an investigator for Merz, Galderma, Evolus, Ideal Implants, Exilis, and Cynosure. The Graivier Center for Plastic Surgery is a training center for Cynosure.
The Summit in Aesthetic Medicine is held by Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
NEWPORT BEACH, CALIF. – If you inject fillers, Manhattan plastic surgeon Lawrence Bass, MD, recommends that you keep hyaluronidase handy.
It’s the go-to for intravascular injections and helpful for nodules and granulomas, the main filler worries, but some offices don’t stock it. “Complications are rare, but they can be devastating. You have to be prepared.”
Massage, aspiration, transdermal nitroglycerin, and other options might help when, for example, a patient’s lip turns purple after a nasolabial fold injection, but “really, it’s all about hyaluronidase,” whether the filler is hyaluronic acid or not. The enzyme digests hyaluronic acid – both the injected kind and what patients have naturally – and loosens and disperses other fillers.
A patient who says that the injection hurt more than usual is an indication that the vessel may have been injected, Dr. Bass said. In such cases, “you have to deal with it immediately,” and repeat dosing if skin color doesn’t improve within an hour, he noted. “If you manage patients correctly, you can usually settle it with little or no deformity.”
There are two hyaluronidase options in the United States: Vitrase, an ovine formulation, and Hylenex, a recombinant human formulation. It probably doesn’t matter which one is used, he said. Soft tissue vascular compromise generally requires 200 units, and retinal artery occlusion, 500 units or more. Restylane is the easiest filler to digest because it’s the least cross-linked; Juvederm is more difficult; and Belotero is the most difficult, Dr. Bass noted.
Nodules and granulomas from filler injections have a slower onset than vascular compromise. Infected nodules can appear months after injection, and granulomas – red or purplish hypersensitivity reactions with indistinct borders, often with all injection sites involved at once – can appear years afterwards, set off by the flu or some other inflammatory trigger, according to Miles Graivier, MD, a plastic surgeon in Roswell, Ga., who shared the podium with Dr. Bass at the meeting.
Dr. Graivier described his approach to granulomas as “very aggressive.” Patients with granulomas receive oral steroids plus intralesional triamcinolone/fluorouracil/lidocaine injections every 2 weeks or more frequently. Hyaluronidase is used to disperse the filler. Resistant patients also get oral allopurinol. Laser liquefaction can help with both granulomas and infected nodules, he added.
Retinal artery occlusion is probably the most feared filler complication. It’s most likely with glabellar or periorbital injections but possible with any facial injection. “It’s a real horror show when it happens. The bottom line is you must restore circulation within 60-90 minutes” with retrobulbar hyaluronidase injections “or patients will lose vision permanently,” Dr. Bass said. People might not feel comfortable with retrobulbar injections, “but when the alternative is blindness, maybe you want to give them a try,” he added.
A 25 gauge, 1.5 inch needle is introduced to the lateral limbus at the orbital rim. “You pass the needle along the orbital floor to the equator of the globe,” – about 13-14 mm – “angle it up to enter the muscular conus, and inject 500 units or more.” Injecting at the orbital floor is less risky, but probably less effective, he said.
Hard nodules, meanwhile, present early after injection and are usually a result of too much filler or poor technique, Dr. Graivier said. For hard nodules, “hyaluronidase is the number one treatment,” although cracking, massage, and saline injection might help.
Infected nodules look like abscesses, but usually grow nothing on culture. Hyaluronidase is also first line for infected nodules, along with drainage and broad spectrum oral antibiotics for at least 6 weeks, and, if they don’t work, intravenous antibiotics, he added.
Dr. Bass is an investigator and speaker for Cynosure; an investigator for Endo Pharmaceuticals and Neothetics, and an advisor to Merz. Dr. Graivier is an advisor for Merz, and an investigator for Merz, Galderma, Evolus, Ideal Implants, Exilis, and Cynosure. The Graivier Center for Plastic Surgery is a training center for Cynosure.
The Summit in Aesthetic Medicine is held by Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
NEWPORT BEACH, CALIF. – If you inject fillers, Manhattan plastic surgeon Lawrence Bass, MD, recommends that you keep hyaluronidase handy.
It’s the go-to for intravascular injections and helpful for nodules and granulomas, the main filler worries, but some offices don’t stock it. “Complications are rare, but they can be devastating. You have to be prepared.”
Massage, aspiration, transdermal nitroglycerin, and other options might help when, for example, a patient’s lip turns purple after a nasolabial fold injection, but “really, it’s all about hyaluronidase,” whether the filler is hyaluronic acid or not. The enzyme digests hyaluronic acid – both the injected kind and what patients have naturally – and loosens and disperses other fillers.
A patient who says that the injection hurt more than usual is an indication that the vessel may have been injected, Dr. Bass said. In such cases, “you have to deal with it immediately,” and repeat dosing if skin color doesn’t improve within an hour, he noted. “If you manage patients correctly, you can usually settle it with little or no deformity.”
There are two hyaluronidase options in the United States: Vitrase, an ovine formulation, and Hylenex, a recombinant human formulation. It probably doesn’t matter which one is used, he said. Soft tissue vascular compromise generally requires 200 units, and retinal artery occlusion, 500 units or more. Restylane is the easiest filler to digest because it’s the least cross-linked; Juvederm is more difficult; and Belotero is the most difficult, Dr. Bass noted.
Nodules and granulomas from filler injections have a slower onset than vascular compromise. Infected nodules can appear months after injection, and granulomas – red or purplish hypersensitivity reactions with indistinct borders, often with all injection sites involved at once – can appear years afterwards, set off by the flu or some other inflammatory trigger, according to Miles Graivier, MD, a plastic surgeon in Roswell, Ga., who shared the podium with Dr. Bass at the meeting.
Dr. Graivier described his approach to granulomas as “very aggressive.” Patients with granulomas receive oral steroids plus intralesional triamcinolone/fluorouracil/lidocaine injections every 2 weeks or more frequently. Hyaluronidase is used to disperse the filler. Resistant patients also get oral allopurinol. Laser liquefaction can help with both granulomas and infected nodules, he added.
Retinal artery occlusion is probably the most feared filler complication. It’s most likely with glabellar or periorbital injections but possible with any facial injection. “It’s a real horror show when it happens. The bottom line is you must restore circulation within 60-90 minutes” with retrobulbar hyaluronidase injections “or patients will lose vision permanently,” Dr. Bass said. People might not feel comfortable with retrobulbar injections, “but when the alternative is blindness, maybe you want to give them a try,” he added.
A 25 gauge, 1.5 inch needle is introduced to the lateral limbus at the orbital rim. “You pass the needle along the orbital floor to the equator of the globe,” – about 13-14 mm – “angle it up to enter the muscular conus, and inject 500 units or more.” Injecting at the orbital floor is less risky, but probably less effective, he said.
Hard nodules, meanwhile, present early after injection and are usually a result of too much filler or poor technique, Dr. Graivier said. For hard nodules, “hyaluronidase is the number one treatment,” although cracking, massage, and saline injection might help.
Infected nodules look like abscesses, but usually grow nothing on culture. Hyaluronidase is also first line for infected nodules, along with drainage and broad spectrum oral antibiotics for at least 6 weeks, and, if they don’t work, intravenous antibiotics, he added.
Dr. Bass is an investigator and speaker for Cynosure; an investigator for Endo Pharmaceuticals and Neothetics, and an advisor to Merz. Dr. Graivier is an advisor for Merz, and an investigator for Merz, Galderma, Evolus, Ideal Implants, Exilis, and Cynosure. The Graivier Center for Plastic Surgery is a training center for Cynosure.
The Summit in Aesthetic Medicine is held by Global Academy for Medical Education. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM THE SUMMIT IN AESTHETIC MEDICINE
Newer St. Jude leads last as long as Medtronic Sprint Quattro
SAN FRANCISCO – St. Jude Medical’s Durata and Riata ST Optim defibrillator leads performed comparably to Medtronic’s Sprint Quattro out to 7 years in a Veterans Affairs analysis of almost 18,000 patients in the VA National Cardiac Device Surveillance Program.
The “highly satisfactory electrical survival” of the Optim leads, at least until year 5, should be of some reassurance to cardiologists, especially since the findings come from the VA, not a device company, investigator Seema Pursnani, MD, said.
The investigators combined Durata and Riata ST Optim leads together in their analysis, since they are similar; both are 7 Fr leads with St. Jude’s silicone/polyurethane Optim coating. After a mean follow-up of 3.4 years in 4,091 Durata patients and 351 Riata ST Optim patients, there were 26 electrical lead failures, which translated to 0.17% failures per device-year.
The investigators compared those results with Medtronic’s Sprint Quattro, which “is sort of a gold standard. It’s been around for quite a long time, and people have confidence in it,” said Dr. Pursnani. After a mean follow-up of 3.8 years in 13,254 patients, there were 57 failures, translating to 0.11% failures per device-year.
Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s. Although the difference was not statistically significant, “we need a little more follow-up to see why the curves are diverging at years 6 and 7,” said Dr. Pursnani, a cardiologist at the San Francisco Veterans Affairs Medical Center when the study was done, but now with the Kaiser Permanente San Leandro (Calif.) Medical Center.
There’s been lingering concern about St. Jude leads ever since the recall of earlier versions of Riata – with silicone-only insulation – in 2011 because of lead abrasion and subsequent safety problems. Optim was developed to address the issue.
“One of the most common modes of lead failure that we saw” with all three leads “was a rise in the pace-sense conductor impedance from the baseline impedance. Sometimes, there is nonphysiologic noise that can also be a sign of early failure,” she said.
There was no industry funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – St. Jude Medical’s Durata and Riata ST Optim defibrillator leads performed comparably to Medtronic’s Sprint Quattro out to 7 years in a Veterans Affairs analysis of almost 18,000 patients in the VA National Cardiac Device Surveillance Program.
The “highly satisfactory electrical survival” of the Optim leads, at least until year 5, should be of some reassurance to cardiologists, especially since the findings come from the VA, not a device company, investigator Seema Pursnani, MD, said.
The investigators combined Durata and Riata ST Optim leads together in their analysis, since they are similar; both are 7 Fr leads with St. Jude’s silicone/polyurethane Optim coating. After a mean follow-up of 3.4 years in 4,091 Durata patients and 351 Riata ST Optim patients, there were 26 electrical lead failures, which translated to 0.17% failures per device-year.
The investigators compared those results with Medtronic’s Sprint Quattro, which “is sort of a gold standard. It’s been around for quite a long time, and people have confidence in it,” said Dr. Pursnani. After a mean follow-up of 3.8 years in 13,254 patients, there were 57 failures, translating to 0.11% failures per device-year.
Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s. Although the difference was not statistically significant, “we need a little more follow-up to see why the curves are diverging at years 6 and 7,” said Dr. Pursnani, a cardiologist at the San Francisco Veterans Affairs Medical Center when the study was done, but now with the Kaiser Permanente San Leandro (Calif.) Medical Center.
There’s been lingering concern about St. Jude leads ever since the recall of earlier versions of Riata – with silicone-only insulation – in 2011 because of lead abrasion and subsequent safety problems. Optim was developed to address the issue.
“One of the most common modes of lead failure that we saw” with all three leads “was a rise in the pace-sense conductor impedance from the baseline impedance. Sometimes, there is nonphysiologic noise that can also be a sign of early failure,” she said.
There was no industry funding for the work, and the investigators have no disclosures.
SAN FRANCISCO – St. Jude Medical’s Durata and Riata ST Optim defibrillator leads performed comparably to Medtronic’s Sprint Quattro out to 7 years in a Veterans Affairs analysis of almost 18,000 patients in the VA National Cardiac Device Surveillance Program.
The “highly satisfactory electrical survival” of the Optim leads, at least until year 5, should be of some reassurance to cardiologists, especially since the findings come from the VA, not a device company, investigator Seema Pursnani, MD, said.
The investigators combined Durata and Riata ST Optim leads together in their analysis, since they are similar; both are 7 Fr leads with St. Jude’s silicone/polyurethane Optim coating. After a mean follow-up of 3.4 years in 4,091 Durata patients and 351 Riata ST Optim patients, there were 26 electrical lead failures, which translated to 0.17% failures per device-year.
The investigators compared those results with Medtronic’s Sprint Quattro, which “is sort of a gold standard. It’s been around for quite a long time, and people have confidence in it,” said Dr. Pursnani. After a mean follow-up of 3.8 years in 13,254 patients, there were 57 failures, translating to 0.11% failures per device-year.
Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s. Although the difference was not statistically significant, “we need a little more follow-up to see why the curves are diverging at years 6 and 7,” said Dr. Pursnani, a cardiologist at the San Francisco Veterans Affairs Medical Center when the study was done, but now with the Kaiser Permanente San Leandro (Calif.) Medical Center.
There’s been lingering concern about St. Jude leads ever since the recall of earlier versions of Riata – with silicone-only insulation – in 2011 because of lead abrasion and subsequent safety problems. Optim was developed to address the issue.
“One of the most common modes of lead failure that we saw” with all three leads “was a rise in the pace-sense conductor impedance from the baseline impedance. Sometimes, there is nonphysiologic noise that can also be a sign of early failure,” she said.
There was no industry funding for the work, and the investigators have no disclosures.
AT HEART RHYTHM 2016
Key clinical point: St. Jude may have solved the Riata lead problem.
Major finding: Seven-year lead survival was 97.7% with St. Jude’s products, and 98.9% with Medtronic’s; the difference was not statistically significant.
Data source: Veterans Affairs analysis of almost 18,000 patients
Disclosures: There was no industry funding for the work, and the investigators have no disclosures.
Endorectal balloons decrease prostate radiation’s rectal and bowel sequelae
SAN DIEGO – Endorectal balloons appeared to protect against rectal complications from prostate cancer radiation in a small randomized trial from Australia.
The balloons have been around for years, but the Australian study is likely the first randomized trial of long-term clinical outcomes. The balloons are inserted and inflated during radiation treatments, which in the study were daily for 6-7 weeks. They distend the rectum, reducing radiation exposure, and also stabilize the prostate. The investigators used the RectalPro balloon from Qlrad.
The 40 men were all undergoing image-guided radiation therapy; 20 got the balloon, 20 did not. At 2 years, four balloon patients (25%) had rectal bleeding, versus 10 (50%) of the controls (P less than .001). Balloon patients also had fewer bowel symptoms, less rectal hypersensitivity, and less reduction in internal anal sphincter thickness. Three (15%), versus six controls (30%), reported declines in physical function from their preradiation baseline, and one balloon patient (5%), versus three controls (15%) reported declines in role functioning (P less than .05 for both).
Rectal pain, however, was more common with the balloon at 2 years (six patients [30%] versus two [10%], P less than .001), and balloon patients had reduced anal squeeze pressures (P less than .05). They also reported a higher prevalence of urinary symptoms.
Further follow-up is needed to determine if benefits outweigh risks; if they do, then the balloon “will become standard procedure in our hospital,” said investigator Rochelle Botten, a research scientist in the department of radiation oncology at the Royal Adelaide Hospital.
Given the study’s small numbers, it’s unclear if urinary problems – for instance, incontinence, pain, frequency – are truly associated with the balloon, but it’s “something we’ve got to look at. We are going to study” the men “again at 3 years to see if they’ve resolved, and we’ll have more patients for 2-year follow-up. If we are pressing everything towards the bladder, it [could make] sense that the bladder is getting more radiation.
“We are hoping as the numbers increase, we’ll be able to look at how much of the bladder and how much of the rectum are actually irradiated, and if there’s a difference with the balloon,” she said at the annual Digestive Disease Week meeting.
Ms. Botten had no disclosures.
SAN DIEGO – Endorectal balloons appeared to protect against rectal complications from prostate cancer radiation in a small randomized trial from Australia.
The balloons have been around for years, but the Australian study is likely the first randomized trial of long-term clinical outcomes. The balloons are inserted and inflated during radiation treatments, which in the study were daily for 6-7 weeks. They distend the rectum, reducing radiation exposure, and also stabilize the prostate. The investigators used the RectalPro balloon from Qlrad.
The 40 men were all undergoing image-guided radiation therapy; 20 got the balloon, 20 did not. At 2 years, four balloon patients (25%) had rectal bleeding, versus 10 (50%) of the controls (P less than .001). Balloon patients also had fewer bowel symptoms, less rectal hypersensitivity, and less reduction in internal anal sphincter thickness. Three (15%), versus six controls (30%), reported declines in physical function from their preradiation baseline, and one balloon patient (5%), versus three controls (15%) reported declines in role functioning (P less than .05 for both).
Rectal pain, however, was more common with the balloon at 2 years (six patients [30%] versus two [10%], P less than .001), and balloon patients had reduced anal squeeze pressures (P less than .05). They also reported a higher prevalence of urinary symptoms.
Further follow-up is needed to determine if benefits outweigh risks; if they do, then the balloon “will become standard procedure in our hospital,” said investigator Rochelle Botten, a research scientist in the department of radiation oncology at the Royal Adelaide Hospital.
Given the study’s small numbers, it’s unclear if urinary problems – for instance, incontinence, pain, frequency – are truly associated with the balloon, but it’s “something we’ve got to look at. We are going to study” the men “again at 3 years to see if they’ve resolved, and we’ll have more patients for 2-year follow-up. If we are pressing everything towards the bladder, it [could make] sense that the bladder is getting more radiation.
“We are hoping as the numbers increase, we’ll be able to look at how much of the bladder and how much of the rectum are actually irradiated, and if there’s a difference with the balloon,” she said at the annual Digestive Disease Week meeting.
Ms. Botten had no disclosures.
SAN DIEGO – Endorectal balloons appeared to protect against rectal complications from prostate cancer radiation in a small randomized trial from Australia.
The balloons have been around for years, but the Australian study is likely the first randomized trial of long-term clinical outcomes. The balloons are inserted and inflated during radiation treatments, which in the study were daily for 6-7 weeks. They distend the rectum, reducing radiation exposure, and also stabilize the prostate. The investigators used the RectalPro balloon from Qlrad.
The 40 men were all undergoing image-guided radiation therapy; 20 got the balloon, 20 did not. At 2 years, four balloon patients (25%) had rectal bleeding, versus 10 (50%) of the controls (P less than .001). Balloon patients also had fewer bowel symptoms, less rectal hypersensitivity, and less reduction in internal anal sphincter thickness. Three (15%), versus six controls (30%), reported declines in physical function from their preradiation baseline, and one balloon patient (5%), versus three controls (15%) reported declines in role functioning (P less than .05 for both).
Rectal pain, however, was more common with the balloon at 2 years (six patients [30%] versus two [10%], P less than .001), and balloon patients had reduced anal squeeze pressures (P less than .05). They also reported a higher prevalence of urinary symptoms.
Further follow-up is needed to determine if benefits outweigh risks; if they do, then the balloon “will become standard procedure in our hospital,” said investigator Rochelle Botten, a research scientist in the department of radiation oncology at the Royal Adelaide Hospital.
Given the study’s small numbers, it’s unclear if urinary problems – for instance, incontinence, pain, frequency – are truly associated with the balloon, but it’s “something we’ve got to look at. We are going to study” the men “again at 3 years to see if they’ve resolved, and we’ll have more patients for 2-year follow-up. If we are pressing everything towards the bladder, it [could make] sense that the bladder is getting more radiation.
“We are hoping as the numbers increase, we’ll be able to look at how much of the bladder and how much of the rectum are actually irradiated, and if there’s a difference with the balloon,” she said at the annual Digestive Disease Week meeting.
Ms. Botten had no disclosures.
AT DDW 2016
Key clinical point: Endorectal balloons appeared to protect against rectal complications from prostate cancer radiation in a small randomized trial from Australia.
Major finding: At 2 years, four balloon patients (25%) had rectal bleeding, versus 10 (50%) of the controls (P less than 0.001).
Data source: Randomized trial of 40 men with prostate cancer.
Disclosures: The presenter had no disclosures.
ART less likely to work in women with UC
SAN DIEGO – Embryo transfers are less likely to result in live births when women with ulcerative colitis (UC) have fertility treatments, and the risk of preterm birth is higher, at least for twins, according to a 20-year nationwide cohort study from Denmark. On a brighter note, UC did not increase the risk of low birth weight or birth defects, and prior UC surgeries did not diminish success.
The take home conclusions from the study are that “women with ulcerative colitis may [want] to initiate ART [assisted reproductive technology] treatment” sooner than other women because “they cannot expect the same success per embryo transfer.” The greater risk of preterm birth means that “increased prenatal observation in UC pregnancies after ART should be considered,” but “women with UC should be reassured that surgery before ART treatment does not impact the chance of live birth per embryo transfer,” said senior investigator Dr. Sonia Friedman, a gastroenterologist at Brigham and Women’s Hospital in Boston.
Although it’s known that UC diminishes fertility in general, it hasn’t been known until now how it affects assisted reproduction. The investigators turned to Denmark to find out because Denmark has one of the highest incidence rates of UC worldwide, especially among women and men 15-29 years old, and the country captures virtually every ART outcome in national databases.
The study included 1,360 embryo transfers in 432 women with UC and 149,094 in 52,661 women without UC from 1994 to 2013. Treatments included in vitro fertilization, intracytoplasmic sperm injection, and embryo transfers. Women in both groups were a median of 33 years old; women with UC had a median disease duration of 8 years.
The chance of live birth per embryo transfer was 22% less in women with UC (odds ratio, 0.78; 95% confidence interval, 0.67-0.91), after adjusting for age, infertility cause, body mass index (BMI), smoking, and other factors, including calendar year, to take into account changes in ART over the years.
The risk of preterm birth was five times higher when singletons and multiple births were considered together (adjusted OR, 5.29; 95% CI, 2.41-11.63), but not statistically elevated when singleton births were analyzed on their own (aOR, 1.80; 95% CI, 0.49-6.62).
About 35% of the women with UC had prior surgeries, most commonly total colon resections. There was no difference in odds of live birth per cycle versus women with UC and without surgery (OR, 0.97; 95% CI, 0.61-1.36) after adjusting for disease duration, comorbidities, year of treatment, and other factors.
The impact of UC is probably related to disease activity. “Even though gynecologists in Denmark tell us they really try to keep their patients in remission before ART, there is some disease activity,” Dr. Friedman said at the annual Digestive Disease Week.
Surgery for Crohn’s disease is known to reduce success in ART, so it was a bit surprising that it didn’t seem to do so in UC. Perhaps it’s because UC surgery is often curative, while Crohn’s operations often are not, so Crohn’s patients may be more likely to have continued disease activity during ART treatment, Dr. Friedman said.
The majority of women in both study groups were normal weight and didn’t smoke. Outside of UC, most were free of comorbidities. In the UC surgery group, about half the women had one procedure, and the rest two or three.
The work was funded by the Crohn’s and Colitis Foundation of America. Dr. Friedman has no disclosures.
SAN DIEGO – Embryo transfers are less likely to result in live births when women with ulcerative colitis (UC) have fertility treatments, and the risk of preterm birth is higher, at least for twins, according to a 20-year nationwide cohort study from Denmark. On a brighter note, UC did not increase the risk of low birth weight or birth defects, and prior UC surgeries did not diminish success.
The take home conclusions from the study are that “women with ulcerative colitis may [want] to initiate ART [assisted reproductive technology] treatment” sooner than other women because “they cannot expect the same success per embryo transfer.” The greater risk of preterm birth means that “increased prenatal observation in UC pregnancies after ART should be considered,” but “women with UC should be reassured that surgery before ART treatment does not impact the chance of live birth per embryo transfer,” said senior investigator Dr. Sonia Friedman, a gastroenterologist at Brigham and Women’s Hospital in Boston.
Although it’s known that UC diminishes fertility in general, it hasn’t been known until now how it affects assisted reproduction. The investigators turned to Denmark to find out because Denmark has one of the highest incidence rates of UC worldwide, especially among women and men 15-29 years old, and the country captures virtually every ART outcome in national databases.
The study included 1,360 embryo transfers in 432 women with UC and 149,094 in 52,661 women without UC from 1994 to 2013. Treatments included in vitro fertilization, intracytoplasmic sperm injection, and embryo transfers. Women in both groups were a median of 33 years old; women with UC had a median disease duration of 8 years.
The chance of live birth per embryo transfer was 22% less in women with UC (odds ratio, 0.78; 95% confidence interval, 0.67-0.91), after adjusting for age, infertility cause, body mass index (BMI), smoking, and other factors, including calendar year, to take into account changes in ART over the years.
The risk of preterm birth was five times higher when singletons and multiple births were considered together (adjusted OR, 5.29; 95% CI, 2.41-11.63), but not statistically elevated when singleton births were analyzed on their own (aOR, 1.80; 95% CI, 0.49-6.62).
About 35% of the women with UC had prior surgeries, most commonly total colon resections. There was no difference in odds of live birth per cycle versus women with UC and without surgery (OR, 0.97; 95% CI, 0.61-1.36) after adjusting for disease duration, comorbidities, year of treatment, and other factors.
The impact of UC is probably related to disease activity. “Even though gynecologists in Denmark tell us they really try to keep their patients in remission before ART, there is some disease activity,” Dr. Friedman said at the annual Digestive Disease Week.
Surgery for Crohn’s disease is known to reduce success in ART, so it was a bit surprising that it didn’t seem to do so in UC. Perhaps it’s because UC surgery is often curative, while Crohn’s operations often are not, so Crohn’s patients may be more likely to have continued disease activity during ART treatment, Dr. Friedman said.
The majority of women in both study groups were normal weight and didn’t smoke. Outside of UC, most were free of comorbidities. In the UC surgery group, about half the women had one procedure, and the rest two or three.
The work was funded by the Crohn’s and Colitis Foundation of America. Dr. Friedman has no disclosures.
SAN DIEGO – Embryo transfers are less likely to result in live births when women with ulcerative colitis (UC) have fertility treatments, and the risk of preterm birth is higher, at least for twins, according to a 20-year nationwide cohort study from Denmark. On a brighter note, UC did not increase the risk of low birth weight or birth defects, and prior UC surgeries did not diminish success.
The take home conclusions from the study are that “women with ulcerative colitis may [want] to initiate ART [assisted reproductive technology] treatment” sooner than other women because “they cannot expect the same success per embryo transfer.” The greater risk of preterm birth means that “increased prenatal observation in UC pregnancies after ART should be considered,” but “women with UC should be reassured that surgery before ART treatment does not impact the chance of live birth per embryo transfer,” said senior investigator Dr. Sonia Friedman, a gastroenterologist at Brigham and Women’s Hospital in Boston.
Although it’s known that UC diminishes fertility in general, it hasn’t been known until now how it affects assisted reproduction. The investigators turned to Denmark to find out because Denmark has one of the highest incidence rates of UC worldwide, especially among women and men 15-29 years old, and the country captures virtually every ART outcome in national databases.
The study included 1,360 embryo transfers in 432 women with UC and 149,094 in 52,661 women without UC from 1994 to 2013. Treatments included in vitro fertilization, intracytoplasmic sperm injection, and embryo transfers. Women in both groups were a median of 33 years old; women with UC had a median disease duration of 8 years.
The chance of live birth per embryo transfer was 22% less in women with UC (odds ratio, 0.78; 95% confidence interval, 0.67-0.91), after adjusting for age, infertility cause, body mass index (BMI), smoking, and other factors, including calendar year, to take into account changes in ART over the years.
The risk of preterm birth was five times higher when singletons and multiple births were considered together (adjusted OR, 5.29; 95% CI, 2.41-11.63), but not statistically elevated when singleton births were analyzed on their own (aOR, 1.80; 95% CI, 0.49-6.62).
About 35% of the women with UC had prior surgeries, most commonly total colon resections. There was no difference in odds of live birth per cycle versus women with UC and without surgery (OR, 0.97; 95% CI, 0.61-1.36) after adjusting for disease duration, comorbidities, year of treatment, and other factors.
The impact of UC is probably related to disease activity. “Even though gynecologists in Denmark tell us they really try to keep their patients in remission before ART, there is some disease activity,” Dr. Friedman said at the annual Digestive Disease Week.
Surgery for Crohn’s disease is known to reduce success in ART, so it was a bit surprising that it didn’t seem to do so in UC. Perhaps it’s because UC surgery is often curative, while Crohn’s operations often are not, so Crohn’s patients may be more likely to have continued disease activity during ART treatment, Dr. Friedman said.
The majority of women in both study groups were normal weight and didn’t smoke. Outside of UC, most were free of comorbidities. In the UC surgery group, about half the women had one procedure, and the rest two or three.
The work was funded by the Crohn’s and Colitis Foundation of America. Dr. Friedman has no disclosures.
AT DDW 2016
Key clinical point: Because they’ll probably need more treatment cycles, women with ulcerative colitis should turn to assisted reproductive technology sooner than most if they want to be moms.
Major finding: The chance of live birth per embryo transfer was 22% less in women with ulcerative colitis (OR, 0.78; 95% CI, 0.67-0.91).
Data source: 20-year nationwide cohort study from Denmark.
Disclosures: The work was funded by the Crohn’s and Colitis Foundation of America. The presenter has no disclosures.
Endoscopic, laparoscopic pseudocyst drainage comparable if necrotic debris minimal
SAN DIEGO – Endoscopic and laparoscopic drainage worked about equally well for pancreatic pseudocysts and walled off necrosis in a small randomized trial from India, the first to compare the two options.
Both are in common use, but until now it wasn’t clear if one was better than the other. The findings mean that “in general, one could do either; the choice of treatment depends [largely] on the expertise available. As an endoscopist, I prefer endoscopic drainage,” said gastroenterologist Pramod Garg, of the All India Institute of Medical Sciences, New Delhi.
Laparoscopic drainage was a technical success in 23 of the 30 patients (76.6%) randomized to it, six of whom (20%) had symptomatic pseudocysts larger than 6 cm for more than 6 weeks; the rest had walled off necrosis (WON) containing less than 30% necrotic debris. Five of the other patients were converted to open surgery, and two underwent percutaneous drainage. One of the 30 patients required endoscopic lavage and necrosectomy for secondary infection.
Endoscopic drainage, meanwhile, was technically successful in 22 of 30 patients (73.3%) with similar distributions of pseudocysts and WON. Most of the other patients needed subsequent endoscopic lavage and necrosectomy for secondary infection.
Clinical success – defined as resolution by week 4 – was 100% in the laparoscopic and 97% (29/30) in the endoscopic groups; one endoscopic patient had a splenic artery pseudoaneurysm that required further surgery. The differences in technical and clinical success rates were not statistically significant. There were no recurrences and no deaths in either group after an average follow-up of 22 months.
Although it seems okay to opt for either approach, “it’s very important for us to assess the amount of necrotic debris. If the amount is sizable, say 50% or more of the volume, one should hesitate before doing purely endoscopic drainage.” As seen in the study, “the chances of developing an infection are pretty high, especially if,” like the investigators, “you place only a plastic stent,” Dr. Garg said at the annual Digestive Disease Week.
Laparoscopic drainage would probably be better when there’s a lot of necrotic tissue, and certainly so if patients need their gallbladder removed, because it can be taken out at the same time. If endoscopy is still the choice, “you should be prepared to do repeat procedures for endoscopic lavage and necrosectomy. The chance of infection may be less if you use a metal stent with a wide diameter,” Dr. Garg said. Before tackling WON with endoscopy, he suggested getting input from a radiologist and surgeon.
Laparoscopic cystogastrostomy was done in the usual manner, with an endostapler to create a wide cystogastrostomy, necrotic debris suction, and concomitant cholecystectomies as needed.
Endoscopic drainage was performed under endosonographic guidance in the 13 patients without bulging cysts, and directly in the 17 patients whose cysts bulged. A balloon was used to dilate the cystogastrostomy tract to 12-15 mm, and a 10 F double pigtail plastic stent placed to keep it open.
Patients in both groups received perioperative antibiotics. The demographic, clinical, and laboratory parameters and etiology of acute pancreatitis were comparable between the two groups. Patients tended to be in their mid-30s, and about 75% in both groups were women. Over a third in each group had gallstone disease. The median hospital stay in both groups was about a week. Fever was more common following endoscopic drainage, probably because of the higher incidence of secondary infection.
Patients with complicated pseudocysts, coagulopathies, or organ failure were excluded from the investigation, as well as those otherwise unfit for surgery.
There was no industry funding for the work, and Dr. Garg had no disclosures.
SAN DIEGO – Endoscopic and laparoscopic drainage worked about equally well for pancreatic pseudocysts and walled off necrosis in a small randomized trial from India, the first to compare the two options.
Both are in common use, but until now it wasn’t clear if one was better than the other. The findings mean that “in general, one could do either; the choice of treatment depends [largely] on the expertise available. As an endoscopist, I prefer endoscopic drainage,” said gastroenterologist Pramod Garg, of the All India Institute of Medical Sciences, New Delhi.
Laparoscopic drainage was a technical success in 23 of the 30 patients (76.6%) randomized to it, six of whom (20%) had symptomatic pseudocysts larger than 6 cm for more than 6 weeks; the rest had walled off necrosis (WON) containing less than 30% necrotic debris. Five of the other patients were converted to open surgery, and two underwent percutaneous drainage. One of the 30 patients required endoscopic lavage and necrosectomy for secondary infection.
Endoscopic drainage, meanwhile, was technically successful in 22 of 30 patients (73.3%) with similar distributions of pseudocysts and WON. Most of the other patients needed subsequent endoscopic lavage and necrosectomy for secondary infection.
Clinical success – defined as resolution by week 4 – was 100% in the laparoscopic and 97% (29/30) in the endoscopic groups; one endoscopic patient had a splenic artery pseudoaneurysm that required further surgery. The differences in technical and clinical success rates were not statistically significant. There were no recurrences and no deaths in either group after an average follow-up of 22 months.
Although it seems okay to opt for either approach, “it’s very important for us to assess the amount of necrotic debris. If the amount is sizable, say 50% or more of the volume, one should hesitate before doing purely endoscopic drainage.” As seen in the study, “the chances of developing an infection are pretty high, especially if,” like the investigators, “you place only a plastic stent,” Dr. Garg said at the annual Digestive Disease Week.
Laparoscopic drainage would probably be better when there’s a lot of necrotic tissue, and certainly so if patients need their gallbladder removed, because it can be taken out at the same time. If endoscopy is still the choice, “you should be prepared to do repeat procedures for endoscopic lavage and necrosectomy. The chance of infection may be less if you use a metal stent with a wide diameter,” Dr. Garg said. Before tackling WON with endoscopy, he suggested getting input from a radiologist and surgeon.
Laparoscopic cystogastrostomy was done in the usual manner, with an endostapler to create a wide cystogastrostomy, necrotic debris suction, and concomitant cholecystectomies as needed.
Endoscopic drainage was performed under endosonographic guidance in the 13 patients without bulging cysts, and directly in the 17 patients whose cysts bulged. A balloon was used to dilate the cystogastrostomy tract to 12-15 mm, and a 10 F double pigtail plastic stent placed to keep it open.
Patients in both groups received perioperative antibiotics. The demographic, clinical, and laboratory parameters and etiology of acute pancreatitis were comparable between the two groups. Patients tended to be in their mid-30s, and about 75% in both groups were women. Over a third in each group had gallstone disease. The median hospital stay in both groups was about a week. Fever was more common following endoscopic drainage, probably because of the higher incidence of secondary infection.
Patients with complicated pseudocysts, coagulopathies, or organ failure were excluded from the investigation, as well as those otherwise unfit for surgery.
There was no industry funding for the work, and Dr. Garg had no disclosures.
SAN DIEGO – Endoscopic and laparoscopic drainage worked about equally well for pancreatic pseudocysts and walled off necrosis in a small randomized trial from India, the first to compare the two options.
Both are in common use, but until now it wasn’t clear if one was better than the other. The findings mean that “in general, one could do either; the choice of treatment depends [largely] on the expertise available. As an endoscopist, I prefer endoscopic drainage,” said gastroenterologist Pramod Garg, of the All India Institute of Medical Sciences, New Delhi.
Laparoscopic drainage was a technical success in 23 of the 30 patients (76.6%) randomized to it, six of whom (20%) had symptomatic pseudocysts larger than 6 cm for more than 6 weeks; the rest had walled off necrosis (WON) containing less than 30% necrotic debris. Five of the other patients were converted to open surgery, and two underwent percutaneous drainage. One of the 30 patients required endoscopic lavage and necrosectomy for secondary infection.
Endoscopic drainage, meanwhile, was technically successful in 22 of 30 patients (73.3%) with similar distributions of pseudocysts and WON. Most of the other patients needed subsequent endoscopic lavage and necrosectomy for secondary infection.
Clinical success – defined as resolution by week 4 – was 100% in the laparoscopic and 97% (29/30) in the endoscopic groups; one endoscopic patient had a splenic artery pseudoaneurysm that required further surgery. The differences in technical and clinical success rates were not statistically significant. There were no recurrences and no deaths in either group after an average follow-up of 22 months.
Although it seems okay to opt for either approach, “it’s very important for us to assess the amount of necrotic debris. If the amount is sizable, say 50% or more of the volume, one should hesitate before doing purely endoscopic drainage.” As seen in the study, “the chances of developing an infection are pretty high, especially if,” like the investigators, “you place only a plastic stent,” Dr. Garg said at the annual Digestive Disease Week.
Laparoscopic drainage would probably be better when there’s a lot of necrotic tissue, and certainly so if patients need their gallbladder removed, because it can be taken out at the same time. If endoscopy is still the choice, “you should be prepared to do repeat procedures for endoscopic lavage and necrosectomy. The chance of infection may be less if you use a metal stent with a wide diameter,” Dr. Garg said. Before tackling WON with endoscopy, he suggested getting input from a radiologist and surgeon.
Laparoscopic cystogastrostomy was done in the usual manner, with an endostapler to create a wide cystogastrostomy, necrotic debris suction, and concomitant cholecystectomies as needed.
Endoscopic drainage was performed under endosonographic guidance in the 13 patients without bulging cysts, and directly in the 17 patients whose cysts bulged. A balloon was used to dilate the cystogastrostomy tract to 12-15 mm, and a 10 F double pigtail plastic stent placed to keep it open.
Patients in both groups received perioperative antibiotics. The demographic, clinical, and laboratory parameters and etiology of acute pancreatitis were comparable between the two groups. Patients tended to be in their mid-30s, and about 75% in both groups were women. Over a third in each group had gallstone disease. The median hospital stay in both groups was about a week. Fever was more common following endoscopic drainage, probably because of the higher incidence of secondary infection.
Patients with complicated pseudocysts, coagulopathies, or organ failure were excluded from the investigation, as well as those otherwise unfit for surgery.
There was no industry funding for the work, and Dr. Garg had no disclosures.
AT DDW 2016
Key clinical point: Choosing between endoscopic and laparoscopic drainage for pancreatic pseudocysts comes down to local expertise and the amount of necrotic tissue that needs to be removed.
Major finding: Clinical success – defined as resolution by week 4 – was 100% in the laparoscopic and 97% (29/30) in the endoscopic groups.
Data source: Randomized trial with 60 patients.
Disclosures: There was no industry funding for the work, and the presenter had no disclosures.
There’s still a place for HA fillers in fine facial lines
NEWPORT BEACH, CALIF. – Despite all the technical advances in aesthetic medicine, there’s still a place for treating fine facial lines with thin hyaluronic acid fillers, according to Dr. Mark G. Rubin, a dermatologist at the Lasky Skin Center in Beverly Hills, Calif.
“In the last couple of years, with all the volumizing products, a lot of people say they don’t treat wrinkles anymore. The idea is if you just put some volume in [a patient’s] nasojugal groove, you inflate the cheek, pull the skin tight, and the nasolabial fold will miraculously disappear. I don’t think that’s really true,” he said at the meeting held by Global Academy for Medical Education.
When patients don’t have good skin elasticity and tightness – and most older patients worried about fine lines don’t – volumizing will improve skin contour but not do much for atrophic lines.
That’s a good time to turn to fillers. “Basically, you are putting putty into a dent. Different wrinkles need different depths of material. We have fillers that are thin like sand, medium like pebbles, or thick like boulders.” Shallow lines need thinner material; deeper folds need volumizing boulders for lift. In some patients, “you need to layer them, boulders first then more superficial fillers to smooth out the surface,” Dr. Rubin said. “There are a lot of medium and deep fillers, but for really fine lines and superficial filling, there’s only Restylane Silk and Belotero.”
However, even superficial fillers need to be diluted sometimes with saline or lidocaine. “There isn’t a perfect filler; you need to create the one that works by changing its characteristics,” he said.
“With Restylane, if you dilute it too much, it turns into water, and you get no lift at all. You can dilute Juvederm down pretty well, but in some patients it still leaves ridges. You can dilute Belotero a lot without losing its ability to create lift, but I think in a lot of patients, Restylane Silk has a little better persistence than Belotero,” he said.
Staying in the dermis is important for superficial lines. “You need to come in at a very acute angle, and you have to see drug coming back at you through the pores. If you are not seeing that, you are definitely too deep. You also need to overcorrect, and see the area blanch a little bit,” he said.
Dr. Rubin warns patients that they might have what looks like a string of pearls under their skin after injection. “We massage [the beads] down with a Q-tip,” rolling it back and forth over the bulges, and they melt over time. A part of the reaction is histamine-related, so antihistamines like loratadine (Claritin) can help.
There can be more serious swelling as well, especially after perioral injections, and “it’s more common with Silk than with regular Restylane. Some patients have erythema that stays a long time, and some have a burning sensation. If it’s not horrific, you wait and see. If it’s still there in 10 days or 2 weeks, my inclination is to dissolve it out and switch drugs,” he said.
Dr. Rubin is a consultant for Merz, maker of Belotero, Radiesse, and other products. Global Academy and this news organization are owned by the same company.
NEWPORT BEACH, CALIF. – Despite all the technical advances in aesthetic medicine, there’s still a place for treating fine facial lines with thin hyaluronic acid fillers, according to Dr. Mark G. Rubin, a dermatologist at the Lasky Skin Center in Beverly Hills, Calif.
“In the last couple of years, with all the volumizing products, a lot of people say they don’t treat wrinkles anymore. The idea is if you just put some volume in [a patient’s] nasojugal groove, you inflate the cheek, pull the skin tight, and the nasolabial fold will miraculously disappear. I don’t think that’s really true,” he said at the meeting held by Global Academy for Medical Education.
When patients don’t have good skin elasticity and tightness – and most older patients worried about fine lines don’t – volumizing will improve skin contour but not do much for atrophic lines.
That’s a good time to turn to fillers. “Basically, you are putting putty into a dent. Different wrinkles need different depths of material. We have fillers that are thin like sand, medium like pebbles, or thick like boulders.” Shallow lines need thinner material; deeper folds need volumizing boulders for lift. In some patients, “you need to layer them, boulders first then more superficial fillers to smooth out the surface,” Dr. Rubin said. “There are a lot of medium and deep fillers, but for really fine lines and superficial filling, there’s only Restylane Silk and Belotero.”
However, even superficial fillers need to be diluted sometimes with saline or lidocaine. “There isn’t a perfect filler; you need to create the one that works by changing its characteristics,” he said.
“With Restylane, if you dilute it too much, it turns into water, and you get no lift at all. You can dilute Juvederm down pretty well, but in some patients it still leaves ridges. You can dilute Belotero a lot without losing its ability to create lift, but I think in a lot of patients, Restylane Silk has a little better persistence than Belotero,” he said.
Staying in the dermis is important for superficial lines. “You need to come in at a very acute angle, and you have to see drug coming back at you through the pores. If you are not seeing that, you are definitely too deep. You also need to overcorrect, and see the area blanch a little bit,” he said.
Dr. Rubin warns patients that they might have what looks like a string of pearls under their skin after injection. “We massage [the beads] down with a Q-tip,” rolling it back and forth over the bulges, and they melt over time. A part of the reaction is histamine-related, so antihistamines like loratadine (Claritin) can help.
There can be more serious swelling as well, especially after perioral injections, and “it’s more common with Silk than with regular Restylane. Some patients have erythema that stays a long time, and some have a burning sensation. If it’s not horrific, you wait and see. If it’s still there in 10 days or 2 weeks, my inclination is to dissolve it out and switch drugs,” he said.
Dr. Rubin is a consultant for Merz, maker of Belotero, Radiesse, and other products. Global Academy and this news organization are owned by the same company.
NEWPORT BEACH, CALIF. – Despite all the technical advances in aesthetic medicine, there’s still a place for treating fine facial lines with thin hyaluronic acid fillers, according to Dr. Mark G. Rubin, a dermatologist at the Lasky Skin Center in Beverly Hills, Calif.
“In the last couple of years, with all the volumizing products, a lot of people say they don’t treat wrinkles anymore. The idea is if you just put some volume in [a patient’s] nasojugal groove, you inflate the cheek, pull the skin tight, and the nasolabial fold will miraculously disappear. I don’t think that’s really true,” he said at the meeting held by Global Academy for Medical Education.
When patients don’t have good skin elasticity and tightness – and most older patients worried about fine lines don’t – volumizing will improve skin contour but not do much for atrophic lines.
That’s a good time to turn to fillers. “Basically, you are putting putty into a dent. Different wrinkles need different depths of material. We have fillers that are thin like sand, medium like pebbles, or thick like boulders.” Shallow lines need thinner material; deeper folds need volumizing boulders for lift. In some patients, “you need to layer them, boulders first then more superficial fillers to smooth out the surface,” Dr. Rubin said. “There are a lot of medium and deep fillers, but for really fine lines and superficial filling, there’s only Restylane Silk and Belotero.”
However, even superficial fillers need to be diluted sometimes with saline or lidocaine. “There isn’t a perfect filler; you need to create the one that works by changing its characteristics,” he said.
“With Restylane, if you dilute it too much, it turns into water, and you get no lift at all. You can dilute Juvederm down pretty well, but in some patients it still leaves ridges. You can dilute Belotero a lot without losing its ability to create lift, but I think in a lot of patients, Restylane Silk has a little better persistence than Belotero,” he said.
Staying in the dermis is important for superficial lines. “You need to come in at a very acute angle, and you have to see drug coming back at you through the pores. If you are not seeing that, you are definitely too deep. You also need to overcorrect, and see the area blanch a little bit,” he said.
Dr. Rubin warns patients that they might have what looks like a string of pearls under their skin after injection. “We massage [the beads] down with a Q-tip,” rolling it back and forth over the bulges, and they melt over time. A part of the reaction is histamine-related, so antihistamines like loratadine (Claritin) can help.
There can be more serious swelling as well, especially after perioral injections, and “it’s more common with Silk than with regular Restylane. Some patients have erythema that stays a long time, and some have a burning sensation. If it’s not horrific, you wait and see. If it’s still there in 10 days or 2 weeks, my inclination is to dissolve it out and switch drugs,” he said.
Dr. Rubin is a consultant for Merz, maker of Belotero, Radiesse, and other products. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM THE SUMMIT IN AESTHETIC MEDICINE
European experience: Biosimilar infliximab works as well as Remicade for IBD
SAN DIEGO – Biosimilar infliximab (Inflectra) was as safe and effective as the original version (Remicade) in an Italian observational cohort study of 547 inflammatory bowel disease (IBD) patients, the largest to date for the biosimilar.
“We can save money with similar efficacy and safety,” said Dr. Gionata Fiorino, a gastroenterologist at the Humanitas Institute in Milan. The findings should reassure physicians in the United States following recent Food and Drug Administration approval of Inflectra.
In the Italian study, 311 patients were new to anti–tumor necrosis factor (TNF) blockers, 139 were previously exposed to anti-TNF therapy, and 97 were switched directly from Remicade.
A total of 64 patients had adverse events, 38 (59%) of which were infusion reactions. Infusion reactions occurred in 9 new patients (3%), 7 switched patents (7%), and 21 patients (15%) starting the biosimilar after an anti-TNF drug holiday. Infusion reactions were most likely in patients starting biosimilar infliximab after a break from Remicade; 29% of those patients had infusion reactions, versus 11% exposed to other anti-TNFs.
“When you look at the historical literature, infusion reactions were also reported mainly after a drug holiday” from Remicade, Dr. Fiorino said at the annual Digestive Disease Week meeting.
There were no statistically significant between-group differences in other adverse events, which were mostly dermatitis. Adverse events led to discontinuation in six new patients (2%), two switched patients (2%), and seven drug-holiday patients (5%); again, the differences were not statistically significant.
The study is ongoing, with a median follow-up so far of just 4.5 months. Because of that, the investigators estimated efficacy using a time-to-treat analysis for censored observations. They calculated that more than 90% of patients in all three arms would have a clinical remission at 12 weeks, and about 90% at 24 weeks.
At least for now, the data “do not show any significant issues in terms of safety. Infusion reaction rates in patients previously exposed to anti-TNFs are in line with the literature, especially for those exposed previously to the originator infliximab. The efficacy profile [also] seems to be in line with the originator,” said Dr. Fiorino, who noted that his hospital has saved about $200,000 using biosimilar infliximab over the past year.
There were no patients who remained on Remicade to compare with those who switched; that was the major limitation of the study, and it was because of payers forcing the switch in Italy. Even so, “the infusion reaction rates in those previously exposed to infliximab is lower than in some previous reports” of Remicade, he said.
“In America, doctors are not ready to use” biosimilar infliximab because there are no good head-to-head trials against Remicade, and it’s unclear if there ever will be. But meanwhile, “we use it all over Europe. It’s just as good, and about 30%-50% cheaper than Remicade,” Dr. John Kaimakliotis, a gastroenterologist in Cyprus, said after the presentation. “I’ve been using it in my practice for the last 3 years and checking antibodies. There’s basically no difference; it’s just a lot cheaper.”
About 57% of the patients in the Italian study had Crohn’s disease, and the rest had ulcerative colitis. About 42% of the patients were women. Subjects were an average of 32 years old at diagnosis and had a mean disease duration of about 8 years. The study will continue through 2016; serum is being collected for antibody analysis.
There was no industry funding for the work. Dr. Kaimakliotis has no disclosures. Dr. Fiorino is a consultant for numerous companies, including Janssen, a Remicade marketer.

|
Dr. Brian Bressler |
With regards to starting biosimilar infliximab in anti-TNF–naive patients with IBD, there have been a lot of cohort studies throughout the world showing outcomes similar to the originator. I don’t think that’s disputed much anymore. The fundamental question we are dealing with right now is whether it’s safe to switch patients who are on Remicade. This study didn’t really tell us that [since] there is no comparison group of people who stayed on Remicade.
Dr. Brian Bressler is a gastroenterologist at the University of British Columbia, Vancouver. He moderated Dr. Fiorini’s presentation. Dr. Bressler has ties to numerous pharmaceutical companies. He is a consultant for Janssen, which markets Remicade.

|
Dr. Brian Bressler |
With regards to starting biosimilar infliximab in anti-TNF–naive patients with IBD, there have been a lot of cohort studies throughout the world showing outcomes similar to the originator. I don’t think that’s disputed much anymore. The fundamental question we are dealing with right now is whether it’s safe to switch patients who are on Remicade. This study didn’t really tell us that [since] there is no comparison group of people who stayed on Remicade.
Dr. Brian Bressler is a gastroenterologist at the University of British Columbia, Vancouver. He moderated Dr. Fiorini’s presentation. Dr. Bressler has ties to numerous pharmaceutical companies. He is a consultant for Janssen, which markets Remicade.

|
Dr. Brian Bressler |
With regards to starting biosimilar infliximab in anti-TNF–naive patients with IBD, there have been a lot of cohort studies throughout the world showing outcomes similar to the originator. I don’t think that’s disputed much anymore. The fundamental question we are dealing with right now is whether it’s safe to switch patients who are on Remicade. This study didn’t really tell us that [since] there is no comparison group of people who stayed on Remicade.
Dr. Brian Bressler is a gastroenterologist at the University of British Columbia, Vancouver. He moderated Dr. Fiorini’s presentation. Dr. Bressler has ties to numerous pharmaceutical companies. He is a consultant for Janssen, which markets Remicade.
SAN DIEGO – Biosimilar infliximab (Inflectra) was as safe and effective as the original version (Remicade) in an Italian observational cohort study of 547 inflammatory bowel disease (IBD) patients, the largest to date for the biosimilar.
“We can save money with similar efficacy and safety,” said Dr. Gionata Fiorino, a gastroenterologist at the Humanitas Institute in Milan. The findings should reassure physicians in the United States following recent Food and Drug Administration approval of Inflectra.
In the Italian study, 311 patients were new to anti–tumor necrosis factor (TNF) blockers, 139 were previously exposed to anti-TNF therapy, and 97 were switched directly from Remicade.
A total of 64 patients had adverse events, 38 (59%) of which were infusion reactions. Infusion reactions occurred in 9 new patients (3%), 7 switched patents (7%), and 21 patients (15%) starting the biosimilar after an anti-TNF drug holiday. Infusion reactions were most likely in patients starting biosimilar infliximab after a break from Remicade; 29% of those patients had infusion reactions, versus 11% exposed to other anti-TNFs.
“When you look at the historical literature, infusion reactions were also reported mainly after a drug holiday” from Remicade, Dr. Fiorino said at the annual Digestive Disease Week meeting.
There were no statistically significant between-group differences in other adverse events, which were mostly dermatitis. Adverse events led to discontinuation in six new patients (2%), two switched patients (2%), and seven drug-holiday patients (5%); again, the differences were not statistically significant.
The study is ongoing, with a median follow-up so far of just 4.5 months. Because of that, the investigators estimated efficacy using a time-to-treat analysis for censored observations. They calculated that more than 90% of patients in all three arms would have a clinical remission at 12 weeks, and about 90% at 24 weeks.
At least for now, the data “do not show any significant issues in terms of safety. Infusion reaction rates in patients previously exposed to anti-TNFs are in line with the literature, especially for those exposed previously to the originator infliximab. The efficacy profile [also] seems to be in line with the originator,” said Dr. Fiorino, who noted that his hospital has saved about $200,000 using biosimilar infliximab over the past year.
There were no patients who remained on Remicade to compare with those who switched; that was the major limitation of the study, and it was because of payers forcing the switch in Italy. Even so, “the infusion reaction rates in those previously exposed to infliximab is lower than in some previous reports” of Remicade, he said.
“In America, doctors are not ready to use” biosimilar infliximab because there are no good head-to-head trials against Remicade, and it’s unclear if there ever will be. But meanwhile, “we use it all over Europe. It’s just as good, and about 30%-50% cheaper than Remicade,” Dr. John Kaimakliotis, a gastroenterologist in Cyprus, said after the presentation. “I’ve been using it in my practice for the last 3 years and checking antibodies. There’s basically no difference; it’s just a lot cheaper.”
About 57% of the patients in the Italian study had Crohn’s disease, and the rest had ulcerative colitis. About 42% of the patients were women. Subjects were an average of 32 years old at diagnosis and had a mean disease duration of about 8 years. The study will continue through 2016; serum is being collected for antibody analysis.
There was no industry funding for the work. Dr. Kaimakliotis has no disclosures. Dr. Fiorino is a consultant for numerous companies, including Janssen, a Remicade marketer.
SAN DIEGO – Biosimilar infliximab (Inflectra) was as safe and effective as the original version (Remicade) in an Italian observational cohort study of 547 inflammatory bowel disease (IBD) patients, the largest to date for the biosimilar.
“We can save money with similar efficacy and safety,” said Dr. Gionata Fiorino, a gastroenterologist at the Humanitas Institute in Milan. The findings should reassure physicians in the United States following recent Food and Drug Administration approval of Inflectra.
In the Italian study, 311 patients were new to anti–tumor necrosis factor (TNF) blockers, 139 were previously exposed to anti-TNF therapy, and 97 were switched directly from Remicade.
A total of 64 patients had adverse events, 38 (59%) of which were infusion reactions. Infusion reactions occurred in 9 new patients (3%), 7 switched patents (7%), and 21 patients (15%) starting the biosimilar after an anti-TNF drug holiday. Infusion reactions were most likely in patients starting biosimilar infliximab after a break from Remicade; 29% of those patients had infusion reactions, versus 11% exposed to other anti-TNFs.
“When you look at the historical literature, infusion reactions were also reported mainly after a drug holiday” from Remicade, Dr. Fiorino said at the annual Digestive Disease Week meeting.
There were no statistically significant between-group differences in other adverse events, which were mostly dermatitis. Adverse events led to discontinuation in six new patients (2%), two switched patients (2%), and seven drug-holiday patients (5%); again, the differences were not statistically significant.
The study is ongoing, with a median follow-up so far of just 4.5 months. Because of that, the investigators estimated efficacy using a time-to-treat analysis for censored observations. They calculated that more than 90% of patients in all three arms would have a clinical remission at 12 weeks, and about 90% at 24 weeks.
At least for now, the data “do not show any significant issues in terms of safety. Infusion reaction rates in patients previously exposed to anti-TNFs are in line with the literature, especially for those exposed previously to the originator infliximab. The efficacy profile [also] seems to be in line with the originator,” said Dr. Fiorino, who noted that his hospital has saved about $200,000 using biosimilar infliximab over the past year.
There were no patients who remained on Remicade to compare with those who switched; that was the major limitation of the study, and it was because of payers forcing the switch in Italy. Even so, “the infusion reaction rates in those previously exposed to infliximab is lower than in some previous reports” of Remicade, he said.
“In America, doctors are not ready to use” biosimilar infliximab because there are no good head-to-head trials against Remicade, and it’s unclear if there ever will be. But meanwhile, “we use it all over Europe. It’s just as good, and about 30%-50% cheaper than Remicade,” Dr. John Kaimakliotis, a gastroenterologist in Cyprus, said after the presentation. “I’ve been using it in my practice for the last 3 years and checking antibodies. There’s basically no difference; it’s just a lot cheaper.”
About 57% of the patients in the Italian study had Crohn’s disease, and the rest had ulcerative colitis. About 42% of the patients were women. Subjects were an average of 32 years old at diagnosis and had a mean disease duration of about 8 years. The study will continue through 2016; serum is being collected for antibody analysis.
There was no industry funding for the work. Dr. Kaimakliotis has no disclosures. Dr. Fiorino is a consultant for numerous companies, including Janssen, a Remicade marketer.
AT DDW 2016
Key clinical point: Biosimilar infliximab (Inflectra) was as safe and effective as Remicade in the largest inflammatory bowel disease observational cohort study to date,
Major finding: Twelve percent of infliximab biosimilar patients had adverse events, most of which were infusion reactions after restarting Remicade following a drug holiday. The rate was the same as in historical Remicade controls.
Data source: Italian observational cohort study of 547 inflammatory bowel disease patients.
Disclosures: There was no industry funding for the work. The presenter is a consultant for numerous companies, including Janssen, which markets Remicade.
Phentermine-topiramate tops competition for long-term weight loss
SAN DIEGO – Phentermine-topiramate was the most effective long-term weight loss drug, based on findings from a network meta-analysis from the University of California, San Diego.
The investigators pooled data from 28 studies of phentermine-topiramate (Qsymia) and four other drugs: orlistat (Xenical, Alli), lorcaserin (Belviq), naltrexone-bupropion (Contrave), and liraglutide (Victoza, Saxenda). The most commonly prescribed weight loss drug in the United States – generic phentermine – was not included because it’s not indicated for long-term use.
“It’s good to have a problem of plenty,” said Dr. Siddharth Singh, but it also makes it hard to figure out which drug to prescribe, especially given the lack of head-to-head studies. He said he hopes the results will help. However, the 30%-45% attrition rate across the studies means that the results have to be interpreted cautiously, said Dr. Singh, who is in the division of gastroenterology in the department of internal medicine, UCSD.
Most of the studies were company-funded phase III trials of weight loss drugs vs. placebo; the trials all were at least 1 year long and included more than 29,000 overweight or obese adults. The analysis was limited to patients on the maximum recommended dose of each drug.
All the drugs were more effective than placebo, but phentermine-topiramate was the most effective, with about 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%, with an average weight loss above placebo of 9 kg. Phentermine-topiramate patients were 10 times more likely than patients on placebo to hit the 5% mark at 1 year; liraglutide patients were 5.5 times more likely to do so; naltrexone-bupropion patients were 4 times more likely; lorcaserin patients, 3.1 times more likely; and orlistat patients, 2.7 times more likely to hit the 5% mark. The spread was similar for weight loss of 10% or more at 1 year vs. placebo.
Phentermine-topiramate’s tolerability profile was acceptable, with 10% of patients quitting the drug by 1 year because of adverse events. The best tolerated was lorcaserin, with a discontinuation rate of 6%, and the least tolerated was liraglutide, with a 13% discontinuation rate. Patients were more likely to quit active drug than placebo in all the studies.
Of course, there are other considerations, especially comorbidities; liraglutide might be the best choice in diabetics, for instance, and patients with psychiatric issues might want to avoid naltrexone-bupropion and lorcaserin, Dr. Singh said.
Subjects were a median of 46 years old, and 74% were women. The median body mass index was 36 kg/m2. All the patients had lifestyle and nutrition counseling along with their study medications.
The investigators next plan to compare the drugs’ safety and efficacy in real world settings.
There was no industry funding for the work, and Dr. Singh had no relevant financial disclosures.

|
Dr. John Morton |
The findings do not correlate with the way weight loss drugs are prescribed. The drug that’s being prescribed the most – outside of generic phentermine – is Contrave (naltrexone-bupropion), but the one that seemed to have the most effect was Qsymia (phentermine-topiramate), which is not being prescribed as often. Qsymia is probably underprescribed, perhaps as a result of its high price tag. Cost is a big factor for these drugs.
Dr. John Morton is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He moderated Dr. Singh’s presentation, and had no disclosures.

|
Dr. John Morton |
The findings do not correlate with the way weight loss drugs are prescribed. The drug that’s being prescribed the most – outside of generic phentermine – is Contrave (naltrexone-bupropion), but the one that seemed to have the most effect was Qsymia (phentermine-topiramate), which is not being prescribed as often. Qsymia is probably underprescribed, perhaps as a result of its high price tag. Cost is a big factor for these drugs.
Dr. John Morton is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He moderated Dr. Singh’s presentation, and had no disclosures.

|
Dr. John Morton |
The findings do not correlate with the way weight loss drugs are prescribed. The drug that’s being prescribed the most – outside of generic phentermine – is Contrave (naltrexone-bupropion), but the one that seemed to have the most effect was Qsymia (phentermine-topiramate), which is not being prescribed as often. Qsymia is probably underprescribed, perhaps as a result of its high price tag. Cost is a big factor for these drugs.
Dr. John Morton is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He moderated Dr. Singh’s presentation, and had no disclosures.
SAN DIEGO – Phentermine-topiramate was the most effective long-term weight loss drug, based on findings from a network meta-analysis from the University of California, San Diego.
The investigators pooled data from 28 studies of phentermine-topiramate (Qsymia) and four other drugs: orlistat (Xenical, Alli), lorcaserin (Belviq), naltrexone-bupropion (Contrave), and liraglutide (Victoza, Saxenda). The most commonly prescribed weight loss drug in the United States – generic phentermine – was not included because it’s not indicated for long-term use.
“It’s good to have a problem of plenty,” said Dr. Siddharth Singh, but it also makes it hard to figure out which drug to prescribe, especially given the lack of head-to-head studies. He said he hopes the results will help. However, the 30%-45% attrition rate across the studies means that the results have to be interpreted cautiously, said Dr. Singh, who is in the division of gastroenterology in the department of internal medicine, UCSD.
Most of the studies were company-funded phase III trials of weight loss drugs vs. placebo; the trials all were at least 1 year long and included more than 29,000 overweight or obese adults. The analysis was limited to patients on the maximum recommended dose of each drug.
All the drugs were more effective than placebo, but phentermine-topiramate was the most effective, with about 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%, with an average weight loss above placebo of 9 kg. Phentermine-topiramate patients were 10 times more likely than patients on placebo to hit the 5% mark at 1 year; liraglutide patients were 5.5 times more likely to do so; naltrexone-bupropion patients were 4 times more likely; lorcaserin patients, 3.1 times more likely; and orlistat patients, 2.7 times more likely to hit the 5% mark. The spread was similar for weight loss of 10% or more at 1 year vs. placebo.
Phentermine-topiramate’s tolerability profile was acceptable, with 10% of patients quitting the drug by 1 year because of adverse events. The best tolerated was lorcaserin, with a discontinuation rate of 6%, and the least tolerated was liraglutide, with a 13% discontinuation rate. Patients were more likely to quit active drug than placebo in all the studies.
Of course, there are other considerations, especially comorbidities; liraglutide might be the best choice in diabetics, for instance, and patients with psychiatric issues might want to avoid naltrexone-bupropion and lorcaserin, Dr. Singh said.
Subjects were a median of 46 years old, and 74% were women. The median body mass index was 36 kg/m2. All the patients had lifestyle and nutrition counseling along with their study medications.
The investigators next plan to compare the drugs’ safety and efficacy in real world settings.
There was no industry funding for the work, and Dr. Singh had no relevant financial disclosures.
SAN DIEGO – Phentermine-topiramate was the most effective long-term weight loss drug, based on findings from a network meta-analysis from the University of California, San Diego.
The investigators pooled data from 28 studies of phentermine-topiramate (Qsymia) and four other drugs: orlistat (Xenical, Alli), lorcaserin (Belviq), naltrexone-bupropion (Contrave), and liraglutide (Victoza, Saxenda). The most commonly prescribed weight loss drug in the United States – generic phentermine – was not included because it’s not indicated for long-term use.
“It’s good to have a problem of plenty,” said Dr. Siddharth Singh, but it also makes it hard to figure out which drug to prescribe, especially given the lack of head-to-head studies. He said he hopes the results will help. However, the 30%-45% attrition rate across the studies means that the results have to be interpreted cautiously, said Dr. Singh, who is in the division of gastroenterology in the department of internal medicine, UCSD.
Most of the studies were company-funded phase III trials of weight loss drugs vs. placebo; the trials all were at least 1 year long and included more than 29,000 overweight or obese adults. The analysis was limited to patients on the maximum recommended dose of each drug.
All the drugs were more effective than placebo, but phentermine-topiramate was the most effective, with about 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%, with an average weight loss above placebo of 9 kg. Phentermine-topiramate patients were 10 times more likely than patients on placebo to hit the 5% mark at 1 year; liraglutide patients were 5.5 times more likely to do so; naltrexone-bupropion patients were 4 times more likely; lorcaserin patients, 3.1 times more likely; and orlistat patients, 2.7 times more likely to hit the 5% mark. The spread was similar for weight loss of 10% or more at 1 year vs. placebo.
Phentermine-topiramate’s tolerability profile was acceptable, with 10% of patients quitting the drug by 1 year because of adverse events. The best tolerated was lorcaserin, with a discontinuation rate of 6%, and the least tolerated was liraglutide, with a 13% discontinuation rate. Patients were more likely to quit active drug than placebo in all the studies.
Of course, there are other considerations, especially comorbidities; liraglutide might be the best choice in diabetics, for instance, and patients with psychiatric issues might want to avoid naltrexone-bupropion and lorcaserin, Dr. Singh said.
Subjects were a median of 46 years old, and 74% were women. The median body mass index was 36 kg/m2. All the patients had lifestyle and nutrition counseling along with their study medications.
The investigators next plan to compare the drugs’ safety and efficacy in real world settings.
There was no industry funding for the work, and Dr. Singh had no relevant financial disclosures.
AT DDW® 2016
Key clinical point: Phentermine-topiramate was the most effective long-term weight loss drug in a network meta-analysis from the University of California, San Diego.
Major finding: All the drugs were better than placebo, but phentermine-topiramate was the most effective, with 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%.
Data source: Twenty-eight clinical trials involving more than 29,000 overweight or obese adults
Disclosures: There was no industry funding, and the presenter didn’t have any relevant financial disclosures.