User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
No benefit in open massage over closed compressions in trauma cardiac arrest
SAN ANTONIO – Open-chest cardiac massage offers no benefit over closed-chest compressions in patients with traumatic cardiac arrest, according to a prospective observational study from the University of Maryland Shock Trauma Center in Baltimore.
The investigators compared 16 open-chest cardiac massage (OCCM) patients with 17 closed-chest compression (CCC) patients delivered directly to the level 1 trauma center in cardiac arrest. The open-massage group received closed compressions for a mean of 66 seconds before being converted to open massage for reasons that weren’t captured by the data.
End-tidal carbon dioxide (ETCO2) – the gold standard for determining the effectiveness of chest compressions and return of spontaneous circulation – was used as a surrogate for cardiac output and adequacy of resuscitation. Continuous high-resolution ETCO2 measurements were collected every 6 seconds in both groups.
When periods of OCCM were compared to equivalent periods of CCC, there were no differences in the initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
“Unless the patient has a thoracic injury that you need to get into the chest to fix, we didn’t see any benefit in opening the chest just to massage the heart. The data suggest that maybe we shouldn’t be so aggressive in doing open cardiac massage. There’s renewed interest in performing endovascular balloon occlusion techniques for the aorta to obtain hemorrhage control; if you do that and you do closed-chest compressions, it’s just as effective as opening up the chest and doing cardiac massage,” said investigator Dr. Matthew Bradley, a trauma surgeon at the Shock Trauma Center, at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Most of the patients were men, and there was a higher percentage of penetrating trauma in the OCCM group (81% versus 47%; P = .04).
The results were the same, however, in subgroup analyses limited to blunt and penetrating trauma.
All of the open massage patients died, but there were a few survivors in the CCC group. Dr. Bradley didn’t think the closed versus open approach was the reason for the survival difference.
Resuscitative endovascular balloon occlusion of the aorta patients were excluded from the trial to prevent confounding.
The investigators have no relevant disclosures.
SAN ANTONIO – Open-chest cardiac massage offers no benefit over closed-chest compressions in patients with traumatic cardiac arrest, according to a prospective observational study from the University of Maryland Shock Trauma Center in Baltimore.
The investigators compared 16 open-chest cardiac massage (OCCM) patients with 17 closed-chest compression (CCC) patients delivered directly to the level 1 trauma center in cardiac arrest. The open-massage group received closed compressions for a mean of 66 seconds before being converted to open massage for reasons that weren’t captured by the data.
End-tidal carbon dioxide (ETCO2) – the gold standard for determining the effectiveness of chest compressions and return of spontaneous circulation – was used as a surrogate for cardiac output and adequacy of resuscitation. Continuous high-resolution ETCO2 measurements were collected every 6 seconds in both groups.
When periods of OCCM were compared to equivalent periods of CCC, there were no differences in the initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
“Unless the patient has a thoracic injury that you need to get into the chest to fix, we didn’t see any benefit in opening the chest just to massage the heart. The data suggest that maybe we shouldn’t be so aggressive in doing open cardiac massage. There’s renewed interest in performing endovascular balloon occlusion techniques for the aorta to obtain hemorrhage control; if you do that and you do closed-chest compressions, it’s just as effective as opening up the chest and doing cardiac massage,” said investigator Dr. Matthew Bradley, a trauma surgeon at the Shock Trauma Center, at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Most of the patients were men, and there was a higher percentage of penetrating trauma in the OCCM group (81% versus 47%; P = .04).
The results were the same, however, in subgroup analyses limited to blunt and penetrating trauma.
All of the open massage patients died, but there were a few survivors in the CCC group. Dr. Bradley didn’t think the closed versus open approach was the reason for the survival difference.
Resuscitative endovascular balloon occlusion of the aorta patients were excluded from the trial to prevent confounding.
The investigators have no relevant disclosures.
SAN ANTONIO – Open-chest cardiac massage offers no benefit over closed-chest compressions in patients with traumatic cardiac arrest, according to a prospective observational study from the University of Maryland Shock Trauma Center in Baltimore.
The investigators compared 16 open-chest cardiac massage (OCCM) patients with 17 closed-chest compression (CCC) patients delivered directly to the level 1 trauma center in cardiac arrest. The open-massage group received closed compressions for a mean of 66 seconds before being converted to open massage for reasons that weren’t captured by the data.
End-tidal carbon dioxide (ETCO2) – the gold standard for determining the effectiveness of chest compressions and return of spontaneous circulation – was used as a surrogate for cardiac output and adequacy of resuscitation. Continuous high-resolution ETCO2 measurements were collected every 6 seconds in both groups.
When periods of OCCM were compared to equivalent periods of CCC, there were no differences in the initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
“Unless the patient has a thoracic injury that you need to get into the chest to fix, we didn’t see any benefit in opening the chest just to massage the heart. The data suggest that maybe we shouldn’t be so aggressive in doing open cardiac massage. There’s renewed interest in performing endovascular balloon occlusion techniques for the aorta to obtain hemorrhage control; if you do that and you do closed-chest compressions, it’s just as effective as opening up the chest and doing cardiac massage,” said investigator Dr. Matthew Bradley, a trauma surgeon at the Shock Trauma Center, at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Most of the patients were men, and there was a higher percentage of penetrating trauma in the OCCM group (81% versus 47%; P = .04).
The results were the same, however, in subgroup analyses limited to blunt and penetrating trauma.
All of the open massage patients died, but there were a few survivors in the CCC group. Dr. Bradley didn’t think the closed versus open approach was the reason for the survival difference.
Resuscitative endovascular balloon occlusion of the aorta patients were excluded from the trial to prevent confounding.
The investigators have no relevant disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point: There’s no benefit in opening the chest just to massage the heart.
Major finding: When periods of OCCM were compared to equivalent periods of CCC, there were no differences in initial, final, peak, or mean ETCO2 values, and there was no difference in return of spontaneous circulation (OCCM, 23.5% versus CCC, 38.9%; P = .53).
Data source: Prospective observational study in 33 patients
Disclosures: The investigators have no relevant disclosures.
Trauma hospitalists reduce mortality, readmissions
SAN ANTONIO – Embedding a hospitalist in the trauma service reduces mortality and readmissions, according to a review from Christiana Care Health System’s Level 1 trauma center in Newark, Del.
Investigators wanted to see how their trauma hospitalist program – launched in 2013 – was working, so they matched 469 patients who were comanaged by a hospitalist in 2014 to 938 patients who were not, based on age, injury severity score, and comorbidities.
“We were pleasantly surprised to see a dramatic reduction in mortality [2.9% to 0.4%] and 30-day trauma-related readmissions [2.3% to 0.6%]” when hospitalists were involved with care, said Dr. Mark Cipolle, chief of trauma surgery at the center. The findings were statistically significant.
More hospitalist patients were upgraded to the ICU [4.3% versus 2.1%], and hospitalist patients stayed in the hospital about a day and half longer. The increased ICU upgrades is probably from the extra vigilance trauma hospitalists bring to the team. As for length of stay, hospitalists probably “kept patients an extra day to tune up their diabetes, hypertension,” and other problems, and ensure they had good follow-up. “We strongly feel that many of these patients go home with their comorbidities better managed than when they came in,” Dr. Cipolle said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hospitalists also seemed to improve patient satisfaction on surveys.
Christiana currently employs about eight trauma hospitalists in Newark who rotate through week-long shifts. They’ve become so central to trauma care there that Dr. Cipolle suspects it would be difficult to find patients without hospitalist management for a new control group.
His hospitalists tend to work with older patients and manage comorbidities, especially insulin-dependent diabetes, heart failure, other significant heart disease, and chronic renal injury. Trauma surgeons, meanwhile, manage injury-related issues, such as additional diagnostics and pain control.
There can be some disagreements when two attendings care for the same patient, but, overall, “we get along pretty darn well,” Dr. Cipolle said.
Hospitalists in the study made no significant difference in the frequency of cardiology, nephrology, neurology, or endocrinology consultations. There was no difference in the development of venous thromboembolism, pneumonia, stroke, urinary tract infection, bacteremia, or alcohol withdrawal.
The patients were about 72 years old, on average, with a mean injury severity score of 10. Roughly 8% were recovering from a stroke, about 10% had heart failure, a quarter were diabetic, and three-quarters were hypertensive.
Dr. Cipolle said he has no relevant disclosures.
SAN ANTONIO – Embedding a hospitalist in the trauma service reduces mortality and readmissions, according to a review from Christiana Care Health System’s Level 1 trauma center in Newark, Del.
Investigators wanted to see how their trauma hospitalist program – launched in 2013 – was working, so they matched 469 patients who were comanaged by a hospitalist in 2014 to 938 patients who were not, based on age, injury severity score, and comorbidities.
“We were pleasantly surprised to see a dramatic reduction in mortality [2.9% to 0.4%] and 30-day trauma-related readmissions [2.3% to 0.6%]” when hospitalists were involved with care, said Dr. Mark Cipolle, chief of trauma surgery at the center. The findings were statistically significant.
More hospitalist patients were upgraded to the ICU [4.3% versus 2.1%], and hospitalist patients stayed in the hospital about a day and half longer. The increased ICU upgrades is probably from the extra vigilance trauma hospitalists bring to the team. As for length of stay, hospitalists probably “kept patients an extra day to tune up their diabetes, hypertension,” and other problems, and ensure they had good follow-up. “We strongly feel that many of these patients go home with their comorbidities better managed than when they came in,” Dr. Cipolle said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hospitalists also seemed to improve patient satisfaction on surveys.
Christiana currently employs about eight trauma hospitalists in Newark who rotate through week-long shifts. They’ve become so central to trauma care there that Dr. Cipolle suspects it would be difficult to find patients without hospitalist management for a new control group.
His hospitalists tend to work with older patients and manage comorbidities, especially insulin-dependent diabetes, heart failure, other significant heart disease, and chronic renal injury. Trauma surgeons, meanwhile, manage injury-related issues, such as additional diagnostics and pain control.
There can be some disagreements when two attendings care for the same patient, but, overall, “we get along pretty darn well,” Dr. Cipolle said.
Hospitalists in the study made no significant difference in the frequency of cardiology, nephrology, neurology, or endocrinology consultations. There was no difference in the development of venous thromboembolism, pneumonia, stroke, urinary tract infection, bacteremia, or alcohol withdrawal.
The patients were about 72 years old, on average, with a mean injury severity score of 10. Roughly 8% were recovering from a stroke, about 10% had heart failure, a quarter were diabetic, and three-quarters were hypertensive.
Dr. Cipolle said he has no relevant disclosures.
SAN ANTONIO – Embedding a hospitalist in the trauma service reduces mortality and readmissions, according to a review from Christiana Care Health System’s Level 1 trauma center in Newark, Del.
Investigators wanted to see how their trauma hospitalist program – launched in 2013 – was working, so they matched 469 patients who were comanaged by a hospitalist in 2014 to 938 patients who were not, based on age, injury severity score, and comorbidities.
“We were pleasantly surprised to see a dramatic reduction in mortality [2.9% to 0.4%] and 30-day trauma-related readmissions [2.3% to 0.6%]” when hospitalists were involved with care, said Dr. Mark Cipolle, chief of trauma surgery at the center. The findings were statistically significant.
More hospitalist patients were upgraded to the ICU [4.3% versus 2.1%], and hospitalist patients stayed in the hospital about a day and half longer. The increased ICU upgrades is probably from the extra vigilance trauma hospitalists bring to the team. As for length of stay, hospitalists probably “kept patients an extra day to tune up their diabetes, hypertension,” and other problems, and ensure they had good follow-up. “We strongly feel that many of these patients go home with their comorbidities better managed than when they came in,” Dr. Cipolle said at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Hospitalists also seemed to improve patient satisfaction on surveys.
Christiana currently employs about eight trauma hospitalists in Newark who rotate through week-long shifts. They’ve become so central to trauma care there that Dr. Cipolle suspects it would be difficult to find patients without hospitalist management for a new control group.
His hospitalists tend to work with older patients and manage comorbidities, especially insulin-dependent diabetes, heart failure, other significant heart disease, and chronic renal injury. Trauma surgeons, meanwhile, manage injury-related issues, such as additional diagnostics and pain control.
There can be some disagreements when two attendings care for the same patient, but, overall, “we get along pretty darn well,” Dr. Cipolle said.
Hospitalists in the study made no significant difference in the frequency of cardiology, nephrology, neurology, or endocrinology consultations. There was no difference in the development of venous thromboembolism, pneumonia, stroke, urinary tract infection, bacteremia, or alcohol withdrawal.
The patients were about 72 years old, on average, with a mean injury severity score of 10. Roughly 8% were recovering from a stroke, about 10% had heart failure, a quarter were diabetic, and three-quarters were hypertensive.
Dr. Cipolle said he has no relevant disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point: The addition of a hospitalist to the trauma service resulted in statistically significant improvements in mortality and readmissions.
Major finding: When hospitalists were added to the trauma team, mortality fell from 2.9 to 0.4%, and 30-day readmissions fell from 2.3 to 0.6%.
Data source: Retrospective, single-center study of 1,407 trauma patients.
Disclosures: Dr. Cipolle said he has no relevant disclosures.
ACR: First U.S. clinical practice guidelines arise for Sjögren’s syndrome management
SAN FRANCISCO – Rituximab is, for now, the first-line option for Sjögren’s syndrome patients with systemic symptoms severe enough to require biologic therapy, according to new systemic treatment guidelines from the Sjögren’s Syndrome Foundation.
In general, tumor necrosis factor inhibitors are out because of the risk of lymphoma; Sjögren’s patients are already at risk for the disease.
The foundation has also released guidelines for the management of dry eyes and prevention of cavities in Sjögren’s. Thirteen more guidelines are in the works to tackle neurologic complications, lung disease, lymphoma, and other issues. The ocular guidelines have been published; the systemic and cavity ones will be soon.
“We are really excited about this. These are the first U.S. clinical practice guidelines for Sjögren’s. It will be a game changer,” said Katherine Hammitt, the Sjögren’s Syndrome Foundation’s vice president of medical and scientific affairs.
Topical fluoride should be used in all patients with dry mouth to prevent cavities, along with measures or medications to increase saliva. A comprehensive corneal exam is the first step for dry eyes to determine if they’re due to a lack of tears or abnormal oil secretion by eyelid glands, which causes tears to evaporate too soon. Treatment proceeds according to the findings.
The recommendations are based on a literature review and the consensus of about 100 Sjögren’s experts. The guidelines address what the experts considered to be the most pressing problems.
The foundation launched the efforts after fielding calls from patients reporting that doctors didn’t understand the disease; didn’t take it seriously; or said there was nothing that could be done. On the flip side, physicians were calling in about problem patients.
“So about 5 years ago, we launched an initiative to address the issues.” With current options, “to tell patients that they don’t need treatment or that nothing can be done is, in my view as a rheumatologist, malpractice,” said presenting author Dr. Frederick Vivino, chief of rheumatology at Penn Presbyterian Medical Center, a University of Pennsylvania teaching hospital in Philadelphia. He is also director of the Penn Sjögren’s Syndrome Center.
Rituximab (Rituxan) isn’t approved for Sjögren’s, but “clinicians are using it quite frequently because it’s really the only [biologic] out there” for the disease. “We’ve seen moderate improvement” of organ involvement, vasculitis, neuropathy, and other extraglandular problems, but “it hasn’t knocked our socks off. We need something better than rituximab” for the sickest patients, Ms. Hammitt said at the annual meeting of the American College of Rheumatology. The guidelines recommend hydroxychloroquine as the first step for inflammatory musculoskeletal pain, followed, as needed, by methotrexate, steroids, and other options. Azathioprine is a good option for recalcitrant musculoskeletal pain, as well as organ involvement. Meanwhile, aerobic exercise is important to help with fatigue.
A baseline corneal exam is “the most important recommendation” for eye patients, Dr. Vivino said.
A stepwise treatment algorithm based on the nature and severity of the problem comes next, and can included tear supplementation and stabilization; control of inflammation of the lacrimal glands and ocular surface; systemic therapy with secretagogues; tear preservation measures; and eyelid surgery. Salivary deficiency causes cavities in Sjögren’s. “If the patient has dry mouth, they need to be given topical fluoride. Either encourage them to ask their dentist, or, as I would do, just prescribe it, and they should use it on a regular basis. The group also felt that giving medications to stimulate saliva flow,” like pilocarpine or cevimeline, “would likely help prevent caries, as well,” Dr. Vivino said.
Sugar-free lozenges or chewing gum can also help with saliva flow. The group gave a weak recommendation for chlorhexidine varnishes, gels, and rinses, and a moderate one for nonfluoride remineralizing agents.
The work was supported by the Sjögren’s Syndrome Foundation, with no pharmaceutical industry funding. Some of the authors have financial ties to numerous pharmaceutical companies.
SAN FRANCISCO – Rituximab is, for now, the first-line option for Sjögren’s syndrome patients with systemic symptoms severe enough to require biologic therapy, according to new systemic treatment guidelines from the Sjögren’s Syndrome Foundation.
In general, tumor necrosis factor inhibitors are out because of the risk of lymphoma; Sjögren’s patients are already at risk for the disease.
The foundation has also released guidelines for the management of dry eyes and prevention of cavities in Sjögren’s. Thirteen more guidelines are in the works to tackle neurologic complications, lung disease, lymphoma, and other issues. The ocular guidelines have been published; the systemic and cavity ones will be soon.
“We are really excited about this. These are the first U.S. clinical practice guidelines for Sjögren’s. It will be a game changer,” said Katherine Hammitt, the Sjögren’s Syndrome Foundation’s vice president of medical and scientific affairs.
Topical fluoride should be used in all patients with dry mouth to prevent cavities, along with measures or medications to increase saliva. A comprehensive corneal exam is the first step for dry eyes to determine if they’re due to a lack of tears or abnormal oil secretion by eyelid glands, which causes tears to evaporate too soon. Treatment proceeds according to the findings.
The recommendations are based on a literature review and the consensus of about 100 Sjögren’s experts. The guidelines address what the experts considered to be the most pressing problems.
The foundation launched the efforts after fielding calls from patients reporting that doctors didn’t understand the disease; didn’t take it seriously; or said there was nothing that could be done. On the flip side, physicians were calling in about problem patients.
“So about 5 years ago, we launched an initiative to address the issues.” With current options, “to tell patients that they don’t need treatment or that nothing can be done is, in my view as a rheumatologist, malpractice,” said presenting author Dr. Frederick Vivino, chief of rheumatology at Penn Presbyterian Medical Center, a University of Pennsylvania teaching hospital in Philadelphia. He is also director of the Penn Sjögren’s Syndrome Center.
Rituximab (Rituxan) isn’t approved for Sjögren’s, but “clinicians are using it quite frequently because it’s really the only [biologic] out there” for the disease. “We’ve seen moderate improvement” of organ involvement, vasculitis, neuropathy, and other extraglandular problems, but “it hasn’t knocked our socks off. We need something better than rituximab” for the sickest patients, Ms. Hammitt said at the annual meeting of the American College of Rheumatology. The guidelines recommend hydroxychloroquine as the first step for inflammatory musculoskeletal pain, followed, as needed, by methotrexate, steroids, and other options. Azathioprine is a good option for recalcitrant musculoskeletal pain, as well as organ involvement. Meanwhile, aerobic exercise is important to help with fatigue.
A baseline corneal exam is “the most important recommendation” for eye patients, Dr. Vivino said.
A stepwise treatment algorithm based on the nature and severity of the problem comes next, and can included tear supplementation and stabilization; control of inflammation of the lacrimal glands and ocular surface; systemic therapy with secretagogues; tear preservation measures; and eyelid surgery. Salivary deficiency causes cavities in Sjögren’s. “If the patient has dry mouth, they need to be given topical fluoride. Either encourage them to ask their dentist, or, as I would do, just prescribe it, and they should use it on a regular basis. The group also felt that giving medications to stimulate saliva flow,” like pilocarpine or cevimeline, “would likely help prevent caries, as well,” Dr. Vivino said.
Sugar-free lozenges or chewing gum can also help with saliva flow. The group gave a weak recommendation for chlorhexidine varnishes, gels, and rinses, and a moderate one for nonfluoride remineralizing agents.
The work was supported by the Sjögren’s Syndrome Foundation, with no pharmaceutical industry funding. Some of the authors have financial ties to numerous pharmaceutical companies.
SAN FRANCISCO – Rituximab is, for now, the first-line option for Sjögren’s syndrome patients with systemic symptoms severe enough to require biologic therapy, according to new systemic treatment guidelines from the Sjögren’s Syndrome Foundation.
In general, tumor necrosis factor inhibitors are out because of the risk of lymphoma; Sjögren’s patients are already at risk for the disease.
The foundation has also released guidelines for the management of dry eyes and prevention of cavities in Sjögren’s. Thirteen more guidelines are in the works to tackle neurologic complications, lung disease, lymphoma, and other issues. The ocular guidelines have been published; the systemic and cavity ones will be soon.
“We are really excited about this. These are the first U.S. clinical practice guidelines for Sjögren’s. It will be a game changer,” said Katherine Hammitt, the Sjögren’s Syndrome Foundation’s vice president of medical and scientific affairs.
Topical fluoride should be used in all patients with dry mouth to prevent cavities, along with measures or medications to increase saliva. A comprehensive corneal exam is the first step for dry eyes to determine if they’re due to a lack of tears or abnormal oil secretion by eyelid glands, which causes tears to evaporate too soon. Treatment proceeds according to the findings.
The recommendations are based on a literature review and the consensus of about 100 Sjögren’s experts. The guidelines address what the experts considered to be the most pressing problems.
The foundation launched the efforts after fielding calls from patients reporting that doctors didn’t understand the disease; didn’t take it seriously; or said there was nothing that could be done. On the flip side, physicians were calling in about problem patients.
“So about 5 years ago, we launched an initiative to address the issues.” With current options, “to tell patients that they don’t need treatment or that nothing can be done is, in my view as a rheumatologist, malpractice,” said presenting author Dr. Frederick Vivino, chief of rheumatology at Penn Presbyterian Medical Center, a University of Pennsylvania teaching hospital in Philadelphia. He is also director of the Penn Sjögren’s Syndrome Center.
Rituximab (Rituxan) isn’t approved for Sjögren’s, but “clinicians are using it quite frequently because it’s really the only [biologic] out there” for the disease. “We’ve seen moderate improvement” of organ involvement, vasculitis, neuropathy, and other extraglandular problems, but “it hasn’t knocked our socks off. We need something better than rituximab” for the sickest patients, Ms. Hammitt said at the annual meeting of the American College of Rheumatology. The guidelines recommend hydroxychloroquine as the first step for inflammatory musculoskeletal pain, followed, as needed, by methotrexate, steroids, and other options. Azathioprine is a good option for recalcitrant musculoskeletal pain, as well as organ involvement. Meanwhile, aerobic exercise is important to help with fatigue.
A baseline corneal exam is “the most important recommendation” for eye patients, Dr. Vivino said.
A stepwise treatment algorithm based on the nature and severity of the problem comes next, and can included tear supplementation and stabilization; control of inflammation of the lacrimal glands and ocular surface; systemic therapy with secretagogues; tear preservation measures; and eyelid surgery. Salivary deficiency causes cavities in Sjögren’s. “If the patient has dry mouth, they need to be given topical fluoride. Either encourage them to ask their dentist, or, as I would do, just prescribe it, and they should use it on a regular basis. The group also felt that giving medications to stimulate saliva flow,” like pilocarpine or cevimeline, “would likely help prevent caries, as well,” Dr. Vivino said.
Sugar-free lozenges or chewing gum can also help with saliva flow. The group gave a weak recommendation for chlorhexidine varnishes, gels, and rinses, and a moderate one for nonfluoride remineralizing agents.
The work was supported by the Sjögren’s Syndrome Foundation, with no pharmaceutical industry funding. Some of the authors have financial ties to numerous pharmaceutical companies.
AT THE ACR ANNUAL MEETING
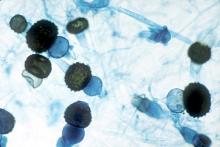
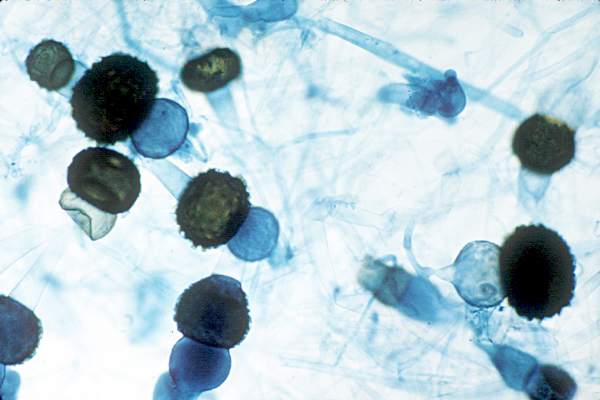
Hong Kong zygomycosis deaths pinned to dirty hospital laundry
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
Contaminated laundry led to an outbreak of cutaneous and pulmonary zygomycosis that killed three immunocompromised patients and sickened three others at Queen Mary Hospital in Hong Kong.
The contamination was traced to a contract laundry service that was, in short, a microbe Disneyland. It was hot and humid, with sealed windows, dim lights, and a thick layer of dust on just about everything. Washers weren’t hot enough to kill spores; washed items were packed while warm and moist; and dirty linens rich with organic material were transported with clean ones (Clin Infect Dis. 2015 Dec 13. doi:10.1093/cid/civ1006).
Of 195 environmental samples, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples. Freshly laundered items – including clothes and bedding – had bacteria counts of 1,028 colony forming units (CFU)/100 cm2, far exceeding the “hygienically clean” standard of 20 CFU/100 cm2 set by U.S. healthcare textile certification requirements.
Queen Mary didn’t regularly audit its linens for cleanliness and microbe counts. “Our findings [suggest] that such standards should be adopted to prevent similar outbreaks,” said the investigators, led by Dr. Vincent Cheng, an infection control officer at Queen Mary, one of Hong Kong’s largest hospitals and a teaching hospital for the University of Hong Kong.
It has since switched to a new laundry service.
The outbreak ran from June 2 to July 18, 2015, during Hong Kong’s hot and humid season, which didn’t help matters.
The six patients were 42-74 years old; one had interstitial lung disease and the rest were either cancer or transplant patients. Infection was due to the spore-forming mold Rhizopus microsporus. Two pulmonary and one cutaneous infection patient died.
Length of stay was the most significant risk factor for infection; the mean interval from admission to diagnosis was more than 2 months.
“Pulmonary zygomycosis due to contaminated hospital linens has never been reported.” Clinicians need to “maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,” the investigators said.
The U.S. recently had a cutaneous outbreak in Louisiana; hospital linens contaminated with Rhizopus species killed five immunocompromised children there in 2015.
“Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts;” previously reported sources include adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, adjacent building construction activity, and, as Queen Mary reported previously, contaminated allopurinol tablets.
Detecting the problem isn’t easy. None of the Replicate Organism Detection and Counting contact plates at Queen Mary recovered zygomycetes from the contaminated linen items. It took sponge swapping to find it; “without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked,” the investigators said.
Hong Kong government services helped support the work. The authors did not have any financial conflicts of interest.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Clinicians need to maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients,
Major finding: Of 195 environmental samples at the contaminated laundry, 119 (61%) were positive for Zygomycetes, as well as 100% of air samples.
Data source: Epidemiological study in Hong Kong.
Disclosures: Hong Kong government services helped support the work. The authors do not have any financial conflicts of interest.
ACR: Sulfasalazine reduces TNFi antibodies but more poorly than methotrexate
SAN FRANCISCO – Sulfasalazine prevents formation of antibodies against tumor necrosis factor inhibitors, but probably not as well as methotrexate, according to a European study of 140 axial spondyloarthritis patients.
“The effect of sulfasalazine on the development of antidrug antibodies has not been studied before. Our initial hypothesis was that methotrexate would [reduce] antibody formation” because it’s been shown to do that before, but that “sulfasalazine would not. This was a surprise to us,” said senior investigator Dr. Alejandro Balsa, chief of rheumatology at La Paz University Hospital in Madrid.
The findings suggest that sulfasalazine, like methotrexate, might “prevent immunogenicity and, hence ... secondary failure of” a tumor necrosis factor inhibitor (TNFi), he said at the annual meeting of the American College of Rheumatology.
Thirty-one patients (22%) were on infliximab (Remicade) and 109 (78%) were on adalimumab (Humira) in the year-long study, which was conducted in Madrid and Amsterdam.
Of the 90 patients on TNFi monotherapy, 33 (37%) developed TNFi antibodies, including 3 of 13 (23%) on infliximab monotherapy and 30 of 77 (39%) on adalimumab alone. The difference was not statistically significant.
Of the 50 patients on concomitant therapy, antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including three cases on infliximab and three on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
The trend toward better antibody protection with methotrexate was, again, not significant, probably because of the small numbers in the study.
Methotrexate and sulfasalazine are not routinely prescribed for axial spondyloarthritis; patients were on them in the study to help with peripheral manifestations. The drugs only prevent antibodies if started before a TNFi. “Once patients develop anti-[TNF] antibodies,” it’s too late, Dr. Balsa noted.
Despite the promising results, he said there’s not enough data at this point to recommend routine pretreatment with methotrexate or sulfasalazine to prevent TNFi antibodies.
As expected, antibodies diminished the clinical effect of a TNFi. Less than a quarter of antibody patients, versus more than a half free from antibodies, reached the investigators’ mark for low disease activity at 1 year: clinical improvement plus a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score below 4 and normal C-reactive protein (P = .03).
Also at 1 year, patients on monotherapy had an overall BASDAI improvement of about 1 point on the 10-point scale. Patients on methotrexate gained an additional point or so (P = .04), while those on sulfasalazine gained about a half point extra (P = .16).
“We only saw significant improvements in patients treated with methotrexate, probably because 100% cotreated with methotrexate had free” serum TNFi “at 1 year, as compared with only 82% cotreated with sulfasalazine,” Dr. Balsa said.
Oddly, the investigators detected free serum TNFi at 1 year in 78 (87%) monotherapy patients, which was more than in those cotreated with sulfasalazine; he didn’t address the finding.
The mean BASDAI at baseline was 6. Ankylosing spondylitis was the most common diagnosis in the study. More than half the subjects were men, the majority of patients were HLA-B27 positive, and their mean disease duration was 11 years.
Dr. Balsa’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
SAN FRANCISCO – Sulfasalazine prevents formation of antibodies against tumor necrosis factor inhibitors, but probably not as well as methotrexate, according to a European study of 140 axial spondyloarthritis patients.
“The effect of sulfasalazine on the development of antidrug antibodies has not been studied before. Our initial hypothesis was that methotrexate would [reduce] antibody formation” because it’s been shown to do that before, but that “sulfasalazine would not. This was a surprise to us,” said senior investigator Dr. Alejandro Balsa, chief of rheumatology at La Paz University Hospital in Madrid.
The findings suggest that sulfasalazine, like methotrexate, might “prevent immunogenicity and, hence ... secondary failure of” a tumor necrosis factor inhibitor (TNFi), he said at the annual meeting of the American College of Rheumatology.
Thirty-one patients (22%) were on infliximab (Remicade) and 109 (78%) were on adalimumab (Humira) in the year-long study, which was conducted in Madrid and Amsterdam.
Of the 90 patients on TNFi monotherapy, 33 (37%) developed TNFi antibodies, including 3 of 13 (23%) on infliximab monotherapy and 30 of 77 (39%) on adalimumab alone. The difference was not statistically significant.
Of the 50 patients on concomitant therapy, antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including three cases on infliximab and three on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
The trend toward better antibody protection with methotrexate was, again, not significant, probably because of the small numbers in the study.
Methotrexate and sulfasalazine are not routinely prescribed for axial spondyloarthritis; patients were on them in the study to help with peripheral manifestations. The drugs only prevent antibodies if started before a TNFi. “Once patients develop anti-[TNF] antibodies,” it’s too late, Dr. Balsa noted.
Despite the promising results, he said there’s not enough data at this point to recommend routine pretreatment with methotrexate or sulfasalazine to prevent TNFi antibodies.
As expected, antibodies diminished the clinical effect of a TNFi. Less than a quarter of antibody patients, versus more than a half free from antibodies, reached the investigators’ mark for low disease activity at 1 year: clinical improvement plus a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score below 4 and normal C-reactive protein (P = .03).
Also at 1 year, patients on monotherapy had an overall BASDAI improvement of about 1 point on the 10-point scale. Patients on methotrexate gained an additional point or so (P = .04), while those on sulfasalazine gained about a half point extra (P = .16).
“We only saw significant improvements in patients treated with methotrexate, probably because 100% cotreated with methotrexate had free” serum TNFi “at 1 year, as compared with only 82% cotreated with sulfasalazine,” Dr. Balsa said.
Oddly, the investigators detected free serum TNFi at 1 year in 78 (87%) monotherapy patients, which was more than in those cotreated with sulfasalazine; he didn’t address the finding.
The mean BASDAI at baseline was 6. Ankylosing spondylitis was the most common diagnosis in the study. More than half the subjects were men, the majority of patients were HLA-B27 positive, and their mean disease duration was 11 years.
Dr. Balsa’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
SAN FRANCISCO – Sulfasalazine prevents formation of antibodies against tumor necrosis factor inhibitors, but probably not as well as methotrexate, according to a European study of 140 axial spondyloarthritis patients.
“The effect of sulfasalazine on the development of antidrug antibodies has not been studied before. Our initial hypothesis was that methotrexate would [reduce] antibody formation” because it’s been shown to do that before, but that “sulfasalazine would not. This was a surprise to us,” said senior investigator Dr. Alejandro Balsa, chief of rheumatology at La Paz University Hospital in Madrid.
The findings suggest that sulfasalazine, like methotrexate, might “prevent immunogenicity and, hence ... secondary failure of” a tumor necrosis factor inhibitor (TNFi), he said at the annual meeting of the American College of Rheumatology.
Thirty-one patients (22%) were on infliximab (Remicade) and 109 (78%) were on adalimumab (Humira) in the year-long study, which was conducted in Madrid and Amsterdam.
Of the 90 patients on TNFi monotherapy, 33 (37%) developed TNFi antibodies, including 3 of 13 (23%) on infliximab monotherapy and 30 of 77 (39%) on adalimumab alone. The difference was not statistically significant.
Of the 50 patients on concomitant therapy, antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including three cases on infliximab and three on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
The trend toward better antibody protection with methotrexate was, again, not significant, probably because of the small numbers in the study.
Methotrexate and sulfasalazine are not routinely prescribed for axial spondyloarthritis; patients were on them in the study to help with peripheral manifestations. The drugs only prevent antibodies if started before a TNFi. “Once patients develop anti-[TNF] antibodies,” it’s too late, Dr. Balsa noted.
Despite the promising results, he said there’s not enough data at this point to recommend routine pretreatment with methotrexate or sulfasalazine to prevent TNFi antibodies.
As expected, antibodies diminished the clinical effect of a TNFi. Less than a quarter of antibody patients, versus more than a half free from antibodies, reached the investigators’ mark for low disease activity at 1 year: clinical improvement plus a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score below 4 and normal C-reactive protein (P = .03).
Also at 1 year, patients on monotherapy had an overall BASDAI improvement of about 1 point on the 10-point scale. Patients on methotrexate gained an additional point or so (P = .04), while those on sulfasalazine gained about a half point extra (P = .16).
“We only saw significant improvements in patients treated with methotrexate, probably because 100% cotreated with methotrexate had free” serum TNFi “at 1 year, as compared with only 82% cotreated with sulfasalazine,” Dr. Balsa said.
Oddly, the investigators detected free serum TNFi at 1 year in 78 (87%) monotherapy patients, which was more than in those cotreated with sulfasalazine; he didn’t address the finding.
The mean BASDAI at baseline was 6. Ankylosing spondylitis was the most common diagnosis in the study. More than half the subjects were men, the majority of patients were HLA-B27 positive, and their mean disease duration was 11 years.
Dr. Balsa’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
AT THE ACR ANNUAL MEETING
Key clinical point: Pretreatment with sulfasalazine seems to prevent TNFi antibodies, but not as well as methotrexate.
Major finding: Antibodies against anti-TNF agents occurred in 6 of 35 on sulfasalazine (17%), including 3 cases on infliximab and 3 on adalimumab. There was just one antibody case in 15 patients on methotrexate (7%); the patient was on adalimumab.
Data source: European study of 140 axial spondyloarthritis patients
Disclosures: The senior investigator’s institution receives research funding from Pfizer, UCB, and Roche, and he receives speakers fees from those companies plus AbbVie, Merck, and Bristol-Myers Squibb. Other investigators disclosed financial ties to those or other companies.
WDC: Breastfeeding linked to reduced diabetes risk in mom and child
VANCOUVER – Breastfeeding was associated with a reduced risk of type 2 diabetes in an analysis of 250,392 children born to 180,107 women in the Canadian province of Manitoba.
Data were culled from hospital records, which captured the initiation of breastfeeding in the hospital, but not the subsequent duration. First-nation women have higher rates of diabetes than do others in Manitoba, so the investigators assessed the benefits of their breastfeeding separately. Initiating breastfeeding in the hospital was associated with a 23% reduced risk of diabetes among non–first nation mothers (HR 0.768, 95% CI: 0.719-0.820, P less than .0001) and a 14% reduced risk of diabetes among first nation mothers (HR 0.859, 95% CI 0.799-0.9230, P less than .0001), investigators at the University of Manitoba in Winnipeg reported at the World Diabetes Congress.
Initiating breastfeeding in the hospital also protected children against type 2 diabetes during up to 24 years of follow-up (HR 0.821, CI 0.686-0.983, P = .0317). Just a small percentage of children – 0.2% among the breastfed, and 0.4% among those not breastfed – developed type 2 diabetes during follow-up.
After the researchers controlled for a range of potential confounders, the findings were independent of gestational diabetes, gestational hypertension, family income, and location of residence, age of mother at birth, parity, and birth weight of offspring.
“We believe this is the first study to provide evidence that breastfeeding initiation has a significant impact on diabetes in both mothers and children. We recommend enhanced education on breastfeeding initiation and duration,” said Dr. Gary Shen, professor of endocrinology and metabolism at the university.
The women were in their mid-20s, on average. About 56% of first-nation and 83% of non–first-nation women started breast feeding in the hospital. Gestational diabetes, children born small for gestational age, low income, and rural living were more common among women who did not breastfeed.
The prevalence of diabetes and obesity is up to three times higher in first nation people than in the general population of Manitoba, according to Dr. Shen.
He and his colleagues have launched an education website for moms-to-be called “Moms in Motion.” They had no conflicts of interest to disclose.
VANCOUVER – Breastfeeding was associated with a reduced risk of type 2 diabetes in an analysis of 250,392 children born to 180,107 women in the Canadian province of Manitoba.
Data were culled from hospital records, which captured the initiation of breastfeeding in the hospital, but not the subsequent duration. First-nation women have higher rates of diabetes than do others in Manitoba, so the investigators assessed the benefits of their breastfeeding separately. Initiating breastfeeding in the hospital was associated with a 23% reduced risk of diabetes among non–first nation mothers (HR 0.768, 95% CI: 0.719-0.820, P less than .0001) and a 14% reduced risk of diabetes among first nation mothers (HR 0.859, 95% CI 0.799-0.9230, P less than .0001), investigators at the University of Manitoba in Winnipeg reported at the World Diabetes Congress.
Initiating breastfeeding in the hospital also protected children against type 2 diabetes during up to 24 years of follow-up (HR 0.821, CI 0.686-0.983, P = .0317). Just a small percentage of children – 0.2% among the breastfed, and 0.4% among those not breastfed – developed type 2 diabetes during follow-up.
After the researchers controlled for a range of potential confounders, the findings were independent of gestational diabetes, gestational hypertension, family income, and location of residence, age of mother at birth, parity, and birth weight of offspring.
“We believe this is the first study to provide evidence that breastfeeding initiation has a significant impact on diabetes in both mothers and children. We recommend enhanced education on breastfeeding initiation and duration,” said Dr. Gary Shen, professor of endocrinology and metabolism at the university.
The women were in their mid-20s, on average. About 56% of first-nation and 83% of non–first-nation women started breast feeding in the hospital. Gestational diabetes, children born small for gestational age, low income, and rural living were more common among women who did not breastfeed.
The prevalence of diabetes and obesity is up to three times higher in first nation people than in the general population of Manitoba, according to Dr. Shen.
He and his colleagues have launched an education website for moms-to-be called “Moms in Motion.” They had no conflicts of interest to disclose.
VANCOUVER – Breastfeeding was associated with a reduced risk of type 2 diabetes in an analysis of 250,392 children born to 180,107 women in the Canadian province of Manitoba.
Data were culled from hospital records, which captured the initiation of breastfeeding in the hospital, but not the subsequent duration. First-nation women have higher rates of diabetes than do others in Manitoba, so the investigators assessed the benefits of their breastfeeding separately. Initiating breastfeeding in the hospital was associated with a 23% reduced risk of diabetes among non–first nation mothers (HR 0.768, 95% CI: 0.719-0.820, P less than .0001) and a 14% reduced risk of diabetes among first nation mothers (HR 0.859, 95% CI 0.799-0.9230, P less than .0001), investigators at the University of Manitoba in Winnipeg reported at the World Diabetes Congress.
Initiating breastfeeding in the hospital also protected children against type 2 diabetes during up to 24 years of follow-up (HR 0.821, CI 0.686-0.983, P = .0317). Just a small percentage of children – 0.2% among the breastfed, and 0.4% among those not breastfed – developed type 2 diabetes during follow-up.
After the researchers controlled for a range of potential confounders, the findings were independent of gestational diabetes, gestational hypertension, family income, and location of residence, age of mother at birth, parity, and birth weight of offspring.
“We believe this is the first study to provide evidence that breastfeeding initiation has a significant impact on diabetes in both mothers and children. We recommend enhanced education on breastfeeding initiation and duration,” said Dr. Gary Shen, professor of endocrinology and metabolism at the university.
The women were in their mid-20s, on average. About 56% of first-nation and 83% of non–first-nation women started breast feeding in the hospital. Gestational diabetes, children born small for gestational age, low income, and rural living were more common among women who did not breastfeed.
The prevalence of diabetes and obesity is up to three times higher in first nation people than in the general population of Manitoba, according to Dr. Shen.
He and his colleagues have launched an education website for moms-to-be called “Moms in Motion.” They had no conflicts of interest to disclose.
AT THE WORLD DIABETES CONGRESS
Key clinical point: Initiating breastfeeding in the hospital was linked with lower type 2 diabetes risk.
Major finding: Initiating breastfeeding in the hospital was associated with a 23% reduced risk of diabetes among non–first nation mothers and a 14% reduced risk of diabetes among first nation mothers in the Canadian province of Manitoba.
Data source: Review of 250,392 children born to 180,107 women.
Disclosures: The investigators had no conflicts of interest.
ACR: The pain of inflammatory disease goes beyond the physical
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
SAN FRANCISCO – The most common fear of rheumatoid arthritis (RA) and axial spondyloarthritis (axSpA) patients is that their future suffering will be as bad as their past suffering, according to a French survey of 474 patients.
Overall, 182 of the 303 RA patients (60%) and 122 of the 171 axSpA patients (71%) in the study ranked that fear as at least a 7 on a 10-point scale, and it remained a serious and common concern even among the roughly half of patients who were in remission.
Majorities in both groups were highly concerned about deformity, wheel chair dependence, burdening loved ones, losing autonomy, and disease spread to other joints. Less common fears, but still ranked at least a 7 by about one-third to well over half of patients, were more frequent flares, inability to care for others, losing friends, loss of treatment effectiveness, fear of treatment side effects, and not finding help if independence is lost.
In general, axSpA was perceived as the more frightening disease, with patients more likely than those with RA to give fears presented on the survey a score of 7 or higher; axSpA patients also were more likely to fear the impact of disease on pregnancy and work, and more worried about passing disease onto their children. Fears about joints seizing up, bone and spine fusion, and increased flare activity were far more prevalent in the axSpA group.
The findings are from a test run of a new questionnaire being developed in France to capture the psychological burden of chronic inflammatory disease. The idea is to make patients’ fears and convictions explicit so that providers know what they are and can help patients cope.
“We’ve had this idea for a long time. Patients have fears and beliefs that” are difficult to express, and they get in the way of effective office communication. The questionnaire might break down the walls, so “patients know their doctors understand and are concerned” about their overall well-being, said senior investigator Dr. Francis Berenbaum, chief of rheumatology at Saint Antoine Hospital in Paris.
It’s hoped that the efforts will improve trust and dialogue between patients and doctors and lead to better treatment adherence and follow-up, more effective counseling, and perhaps even new patient-related outcomes for clinical trials, he said at the annual meeting of the American College of Rheumatology.
To create the survey, the team conducted semi-structured patient interviews at rheumatology practices across France. They whittled the responses down to identify 23 common fears and 19 disease-related beliefs in RA and axSpA. The resulting 44-item survey – there are two additional items about pregnancy and work-related concerns – asks patients to rate each one on a scale of 1-10. The team hopes to have data soon to show whether or not the efforts improve outcomes.
Common beliefs in both groups were that fatigue, over-exertion, stress, and weather changes trigger flares, but that moderate physical activity reduces them.
Almost half of RA patients, versus about a quarter of axSpA patients, believed that their disease was triggered by an emotional shock or stressful event, and small minorities in both groups attributed their disease to pollution, vaccines, or passive or active smoking. About 70% of patients in both groups were on biologics, and about one-third in each were very worried that their treatments would cause cancer.
Some “disease perceptions may not be accurate” and “provide a basis for discussion … to dispel misconceptions, align treatment expectations, and provide reassurance,” the investigators noted,
The RA patients were 60 years old on average, and about three-quarters were women. The median disease duration was 10 years, and mean Disease Activity Score (DAS28) was 2.7; axSpA patients were a mean age of 47 years, 43% were women, and the median disease duration was 12 years. Their mean Bath Ankylosing Spondylitis Disease Activity Index score was 3.2.
Foundation Arthritis Jacques Courtin and UCB Pharma funded the work. Dr. Berenbaum has no relevant disclosures. Two investigators are UCB employees.
AT THE ACR ANNUAL MEETING
Key clinical point: Ask patients what they are worried about; you might put them at ease by addressing their misconceptions.
Major finding: Overall, 60% of RA patients and 71% of axSpA patients were fearful that their future suffering would be as bad as their past suffering.
Data source: A French survey of 474 patients.
Disclosures: Foundation Arthritis Jacques Courtin and UCB Pharma funded the study. The senior investigator has no relevant disclosures. Two investigators are UCB employees.
ACR: VEBs predict sudden cardiac death in systemic sclerosis
SAN FRANCISCO – In systemic sclerosis, more than 1,190 ventricular ectopic beats per 24 hours of Holter monitoring was 100% sensitive and 83% specific for sudden cardiac death or defibrillator implantation within a year in a prospective cohort study from the Catholic University of the Sacred Heart in Rome.
Twenty-four hour Holter monitoring needs to be “part of the routine evaluation in SSc [systemic sclerosis] with suspicious cardiac involvement” so that patients get implantable cardioverter defibrillators (ICDs) in time, said investigator Dr. Giacomo De Luca, a rheumatologist at the university.
Arrhythmias secondary to myocardial fibrosis are killers in SSc and sometimes strike patients with mild heart symptoms such as dyspnea that are easily mistaken for lung involvement, and patients who fall outside of traditional ICD indications such as ejection fractions below 35%. Because of that, “better risk stratification is desperately needed” to catch patients early “and prevent sudden cardiac death [SCD],” Dr. De Luca said at the annual meeting of the American College of Rheumatology.
Holter monitoring isn’t a part of routine SSc workup, but Dr. De Luca and his team think it might solve the arrhythmia problem. Ventricular ectopic beats (VEBs) over 1,190 per 24 hours have an “excellent positive predictive value. [They are a] warning biomarker of major arrhythmias,” he said.
The conclusions come from 100 SSc patients with new-onset cardiac symptoms, generally palpitations and mild dyspnea, but also some with chest pain or heart failure signs. The subjects wore Holter monitors for 24 hours and then were followed for a mean of 2 years.
At baseline, 56 patients had Holter abnormalities. VEBs were the most common, present in 24 patients at a “strikingly high” mean of 2,046/24 hours. The number of VEBs correlated with cardiac troponin T (cTnT) levels and inversely correlated with left ventricular ejection fractions.
The team also found 19 patients with supraventricular ectopic beats, 14 with episodes of supraventricular paroxysmal tachycardia, and 11 with runs of nonsustained ventricular tachycardia, but those problems didn’t prove to be predictors of SCD.
During follow-up, seven patients met the study’s combined primary endpoint of SCD or ICD implantation. Five died and two had implants at a mean of 8.5 months. The age range was 32-77 years, and median baseline ejection fraction was 40%, but ejection fractions ranged widely and were normal in some, indicating that they don’t reliably predict SCD in SSc patients. None of the seven subjects had pulmonary arterial hypertension; it was ruled out by right heart catheterization.
Compared with the overall cohort, the seven patients who met the primary endpoint had lower ejection fractions and higher numbers of VEBs – all were above 1,000/24 hours at baseline – plus higher levels of cTnT and NT-proBNP [N-terminal prohormone brain natriuretic peptide].
The 1,190 VEBs/day cutoff emerged on a receiver operating characteristic (ROC) analysis that proved robust (AUROC = 0.94, P less than .0001); cTnT above 0.014 ng/mL and right bundle branch block were independent predictors of VEBs above the cutoff, but prolonged baseline QT intervals had no prognostic value.
The investigators had no disclosures.

|
Dr. Francesco Boin |
Cardiac involvement in scleroderma is an emerging topic. People focus on the right side of the heart, but when we really look at what patients die from, it’s arrhythmias; the buildup of cardiac scarring leads to arrhythmias that kill our patients.
This is an important study because it raises awareness that we need to do a better job at looking for early signs of heart involvement, but we need bigger, multicenter studies to have confidence in the cutoff value found in this study.
Dr. Francesco Boin is the director of the scleroderma clinic at the University of California, San Francisco. He moderated the study presentation, and has no relevant disclosures.

|
Dr. Francesco Boin |
Cardiac involvement in scleroderma is an emerging topic. People focus on the right side of the heart, but when we really look at what patients die from, it’s arrhythmias; the buildup of cardiac scarring leads to arrhythmias that kill our patients.
This is an important study because it raises awareness that we need to do a better job at looking for early signs of heart involvement, but we need bigger, multicenter studies to have confidence in the cutoff value found in this study.
Dr. Francesco Boin is the director of the scleroderma clinic at the University of California, San Francisco. He moderated the study presentation, and has no relevant disclosures.

|
Dr. Francesco Boin |
Cardiac involvement in scleroderma is an emerging topic. People focus on the right side of the heart, but when we really look at what patients die from, it’s arrhythmias; the buildup of cardiac scarring leads to arrhythmias that kill our patients.
This is an important study because it raises awareness that we need to do a better job at looking for early signs of heart involvement, but we need bigger, multicenter studies to have confidence in the cutoff value found in this study.
Dr. Francesco Boin is the director of the scleroderma clinic at the University of California, San Francisco. He moderated the study presentation, and has no relevant disclosures.
SAN FRANCISCO – In systemic sclerosis, more than 1,190 ventricular ectopic beats per 24 hours of Holter monitoring was 100% sensitive and 83% specific for sudden cardiac death or defibrillator implantation within a year in a prospective cohort study from the Catholic University of the Sacred Heart in Rome.
Twenty-four hour Holter monitoring needs to be “part of the routine evaluation in SSc [systemic sclerosis] with suspicious cardiac involvement” so that patients get implantable cardioverter defibrillators (ICDs) in time, said investigator Dr. Giacomo De Luca, a rheumatologist at the university.
Arrhythmias secondary to myocardial fibrosis are killers in SSc and sometimes strike patients with mild heart symptoms such as dyspnea that are easily mistaken for lung involvement, and patients who fall outside of traditional ICD indications such as ejection fractions below 35%. Because of that, “better risk stratification is desperately needed” to catch patients early “and prevent sudden cardiac death [SCD],” Dr. De Luca said at the annual meeting of the American College of Rheumatology.
Holter monitoring isn’t a part of routine SSc workup, but Dr. De Luca and his team think it might solve the arrhythmia problem. Ventricular ectopic beats (VEBs) over 1,190 per 24 hours have an “excellent positive predictive value. [They are a] warning biomarker of major arrhythmias,” he said.
The conclusions come from 100 SSc patients with new-onset cardiac symptoms, generally palpitations and mild dyspnea, but also some with chest pain or heart failure signs. The subjects wore Holter monitors for 24 hours and then were followed for a mean of 2 years.
At baseline, 56 patients had Holter abnormalities. VEBs were the most common, present in 24 patients at a “strikingly high” mean of 2,046/24 hours. The number of VEBs correlated with cardiac troponin T (cTnT) levels and inversely correlated with left ventricular ejection fractions.
The team also found 19 patients with supraventricular ectopic beats, 14 with episodes of supraventricular paroxysmal tachycardia, and 11 with runs of nonsustained ventricular tachycardia, but those problems didn’t prove to be predictors of SCD.
During follow-up, seven patients met the study’s combined primary endpoint of SCD or ICD implantation. Five died and two had implants at a mean of 8.5 months. The age range was 32-77 years, and median baseline ejection fraction was 40%, but ejection fractions ranged widely and were normal in some, indicating that they don’t reliably predict SCD in SSc patients. None of the seven subjects had pulmonary arterial hypertension; it was ruled out by right heart catheterization.
Compared with the overall cohort, the seven patients who met the primary endpoint had lower ejection fractions and higher numbers of VEBs – all were above 1,000/24 hours at baseline – plus higher levels of cTnT and NT-proBNP [N-terminal prohormone brain natriuretic peptide].
The 1,190 VEBs/day cutoff emerged on a receiver operating characteristic (ROC) analysis that proved robust (AUROC = 0.94, P less than .0001); cTnT above 0.014 ng/mL and right bundle branch block were independent predictors of VEBs above the cutoff, but prolonged baseline QT intervals had no prognostic value.
The investigators had no disclosures.
SAN FRANCISCO – In systemic sclerosis, more than 1,190 ventricular ectopic beats per 24 hours of Holter monitoring was 100% sensitive and 83% specific for sudden cardiac death or defibrillator implantation within a year in a prospective cohort study from the Catholic University of the Sacred Heart in Rome.
Twenty-four hour Holter monitoring needs to be “part of the routine evaluation in SSc [systemic sclerosis] with suspicious cardiac involvement” so that patients get implantable cardioverter defibrillators (ICDs) in time, said investigator Dr. Giacomo De Luca, a rheumatologist at the university.
Arrhythmias secondary to myocardial fibrosis are killers in SSc and sometimes strike patients with mild heart symptoms such as dyspnea that are easily mistaken for lung involvement, and patients who fall outside of traditional ICD indications such as ejection fractions below 35%. Because of that, “better risk stratification is desperately needed” to catch patients early “and prevent sudden cardiac death [SCD],” Dr. De Luca said at the annual meeting of the American College of Rheumatology.
Holter monitoring isn’t a part of routine SSc workup, but Dr. De Luca and his team think it might solve the arrhythmia problem. Ventricular ectopic beats (VEBs) over 1,190 per 24 hours have an “excellent positive predictive value. [They are a] warning biomarker of major arrhythmias,” he said.
The conclusions come from 100 SSc patients with new-onset cardiac symptoms, generally palpitations and mild dyspnea, but also some with chest pain or heart failure signs. The subjects wore Holter monitors for 24 hours and then were followed for a mean of 2 years.
At baseline, 56 patients had Holter abnormalities. VEBs were the most common, present in 24 patients at a “strikingly high” mean of 2,046/24 hours. The number of VEBs correlated with cardiac troponin T (cTnT) levels and inversely correlated with left ventricular ejection fractions.
The team also found 19 patients with supraventricular ectopic beats, 14 with episodes of supraventricular paroxysmal tachycardia, and 11 with runs of nonsustained ventricular tachycardia, but those problems didn’t prove to be predictors of SCD.
During follow-up, seven patients met the study’s combined primary endpoint of SCD or ICD implantation. Five died and two had implants at a mean of 8.5 months. The age range was 32-77 years, and median baseline ejection fraction was 40%, but ejection fractions ranged widely and were normal in some, indicating that they don’t reliably predict SCD in SSc patients. None of the seven subjects had pulmonary arterial hypertension; it was ruled out by right heart catheterization.
Compared with the overall cohort, the seven patients who met the primary endpoint had lower ejection fractions and higher numbers of VEBs – all were above 1,000/24 hours at baseline – plus higher levels of cTnT and NT-proBNP [N-terminal prohormone brain natriuretic peptide].
The 1,190 VEBs/day cutoff emerged on a receiver operating characteristic (ROC) analysis that proved robust (AUROC = 0.94, P less than .0001); cTnT above 0.014 ng/mL and right bundle branch block were independent predictors of VEBs above the cutoff, but prolonged baseline QT intervals had no prognostic value.
The investigators had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Even equivocal cardiac signs should get a Holter monitor in systemic sclerosis.
Major finding: In systemic sclerosis, more than 1,190 ventricular ectopic beats per 24 hours of Holter monitoring was 100% sensitive and 83% specific for sudden cardiac death or defibrillator implantation within a year.
Data source: Prospective cohort of 100 patients.
Disclosures: The investigators had no disclosures.
ACR: Etanercept during pregnancy doubles the odds of major malformations
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
SAN FRANCISCO – Etanercept during pregnancy more than doubled the risk of major congenital malformations in a study by the Organization of Teratology Information Specialists.
The group keeps a prospective registry on exposures to biologics during pregnancy. It is finishing up its adalimumab (Humira) investigation and hasn’t found much to worry about, and continues to gather data on abatacept (Orencia), tocilizumab (Actemra), tofacitinib (Xeljanz), apremilast (Otezla), and certolizumab pegol (Cimzia).
Etanercept (Enbrel), however, seems to be a different story; major malformations turned up in the group’s recently completed investigation. Even so, Organization of Teratology Information Specialists (OTIS) investigator Dr. Christina D. Chambers, Ph.D., of the University of California, San Diego, was careful to note at the annual meeting of the American College of Rheumatology that “etanercept is not meeting the criteria for causality. There’s no pattern” in major defects and “no biological plausibility” because the drug doesn’t seem to cross the placenta when the fetus is most vulnerable to adverse outcomes.
“It is difficult to draw the conclusion that this drug is causing harm. With true teratogens, you tend to see reduced birth weights and an increased risk of spontaneous abortion, which is not the case with etanercept. We are seeing only this one finding that kind of stands alone, and everything else looks pretty good,” she said.
The etanercept study investigated pregnancy outcomes in 370 women exposed to the drug while pregnant, mostly women with rheumatoid arthritis, but also women with psoriasis and ankylosing spondylitis. Their outcomes were compared with 164 pregnant women with the same diseases but no etanercept exposure – the disease control group – and 296 healthy pregnant women.
Women in all three groups were about 33 years old on average, and about 80% were white. The women were enrolled toward the end of their first trimester. Disease severity, comorbidities, and use of vitamins, alcohol, and tobacco were similar between etanercept and disease control women. About 40% of the etanercept and disease control women, but just one in the healthy pregnancy group, were exposed to systemic corticosteroids while pregnant.
There were 33 major structural defects in children born to women taking etanercept versus 7 in the disease control group. That translated to a more than doubling of risk with etanercept (odds ratio, 2.37; 95% confidence interval, 1.02-5.52), and a more than doubling of risk versus the 10 major structural defects in children born to healthy control women (OR, 2.91; 95% CI, 1.37-6.76).
A subanalysis excluded chromosomal anomalies, but “did not [change] our conclusions,” Dr. Chambers said.
Major structural defects generally refer to problems that need a surgical fix, including spina bifida, atrial septal defects, cleft palates, hypospadias, polydactyly, and craniosynostosis.
Minor defects that don’t need surgery, like a missing earlobe, occurred in six children exposed to etanercept and showed two different patterns that involved “three specific minor malformations” not seen in either of the control groups, Dr. Chambers said. She did not elaborate on what those patterns were, but noted that the parents usually had them, too, “suggesting a genetic component as opposed to a drug effect.”
Children in the three study groups had no statistically significant differences in 1-year malignancy rates, serious infections, and hospitalizations, even when exposed to etanercept in the third trimester.
Children exposed to etanercept, however, were more likely to be born preterm and more likely to be small for gestational age in weight, length, and head circumference. They were also more likely than disease control children to screen positive for developmental issues at 1 year, but none of those differences were statistically significant.
Dr. Chambers disclosed funding from 14 companies, including Amgen, the maker of etanercept, and Janssen, Pfizer, Roche, Sanofi/Genzyme, GlaxoSmithKline, and AbbVie, the maker of adalimumab.
AT THE ACR ANNUAL MEETING
Key clinical point: Although etanercept exposure was associated with more than twofold higher odds of major structural defects, there was no pattern to the defects and no biological plausibility to etanercept causing the defects.
Major finding: There were 33 major structural defects in children born to etanercept women versus 7 in a disease comparison group, translating to a more than doubling of risk with etanercept (OR, 2.37; 95% CI, 1.02-5.52).
Data source: Prospective investigation of 830 pregnant women.
Disclosures: The presenting investigator disclosed funding from 14 companies, including Amgen, the maker of etanercept, and AbbVie, the maker of adalimumab.
ACR: Fewer GCA relapses with add-on abatacept
SAN FRANCISCO – The addition of abatacept (Orencia) to standard prednisone regimens seemed to reduce the risk of relapse in a study by the Vasculitis Clinical Research Consortiumof 49 patients with giant cell arteritis.
The patients were initially treated with 40-60 mg of prednisone daily on a standard taper plus abatacept 10 mg/kg IV on days 1, 15, and 29, and week 8. The 41 patients who were in remission at week 12 were then randomized to continued abatacept or placebo IV once monthly. Prednisone was stopped at week 28.
By the time the study concluded a year after the last patient was randomized, 10 abatacept patients had relapsed and 10 had stayed in remission, while 14 placebo patients had relapsed and 7 had stayed in remission. Relapse-free survival at 12 months was calculated to be 48% in the abatacept group and 31% in the placebo group (P = .049). A longer median duration of remission was seen with abatacept, as well (9.9 months versus 3.9 months with placebo).
“Abatacept prolonged the rate of relapse-free survival and was associated with a favorable safety profile.” These are “very encouraging results supporting abatacept” for giant cell arteritis (GCA), lead investigator Dr. Carol Langford, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, said at the annual meeting of the American College of Rheumatology.
There were 129 adverse events in 35 patients, including 33 infections and three malignancies; 23 adverse events in 15 patients were deemed serious.
Even so, “there was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection,” and “no evidence of enhanced toxicity during the first 3 months [when] patients received abatacept and high-dose prednisone,” the investigators said.
While steroids remain the standard of care, “positive signals for new drugs in GCA will [give us] the opportunity to refine their use,” perhaps, for example, by allowing for a lower steroid dose up front, said Dr. Peter Merkel, chief of rheumatology at the University of Pennsylvania in Philadelphia.
Patients in the abatacept arm were 64 years old on average, while placebo patients were about 72 years old. All of the placebo patients and 80% of the abatacept patients were women, and most of the subjects were white. Median disease duration was a few months in both groups.
There were no statistically significant differences in GCA characteristics between the groups at time of enrollment. Vascular pain, headaches, and tongue and jaw pain were common in both. One patient in both arms had ischemic retinopathy.
Abatacept counters T cell activation, which has been implicated in the pathophysiology of GCA. Promising results for tocilizumab (Actemra) in GCA also were presented at the annual meeting.
The National Institutes of Health funded the study. Bristol-Myers Squibb provided the abatacept. Nearly all of the investigators, including Dr. Merkel and Dr. Langford, disclosed relationships with the company.
SAN FRANCISCO – The addition of abatacept (Orencia) to standard prednisone regimens seemed to reduce the risk of relapse in a study by the Vasculitis Clinical Research Consortiumof 49 patients with giant cell arteritis.
The patients were initially treated with 40-60 mg of prednisone daily on a standard taper plus abatacept 10 mg/kg IV on days 1, 15, and 29, and week 8. The 41 patients who were in remission at week 12 were then randomized to continued abatacept or placebo IV once monthly. Prednisone was stopped at week 28.
By the time the study concluded a year after the last patient was randomized, 10 abatacept patients had relapsed and 10 had stayed in remission, while 14 placebo patients had relapsed and 7 had stayed in remission. Relapse-free survival at 12 months was calculated to be 48% in the abatacept group and 31% in the placebo group (P = .049). A longer median duration of remission was seen with abatacept, as well (9.9 months versus 3.9 months with placebo).
“Abatacept prolonged the rate of relapse-free survival and was associated with a favorable safety profile.” These are “very encouraging results supporting abatacept” for giant cell arteritis (GCA), lead investigator Dr. Carol Langford, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, said at the annual meeting of the American College of Rheumatology.
There were 129 adverse events in 35 patients, including 33 infections and three malignancies; 23 adverse events in 15 patients were deemed serious.
Even so, “there was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection,” and “no evidence of enhanced toxicity during the first 3 months [when] patients received abatacept and high-dose prednisone,” the investigators said.
While steroids remain the standard of care, “positive signals for new drugs in GCA will [give us] the opportunity to refine their use,” perhaps, for example, by allowing for a lower steroid dose up front, said Dr. Peter Merkel, chief of rheumatology at the University of Pennsylvania in Philadelphia.
Patients in the abatacept arm were 64 years old on average, while placebo patients were about 72 years old. All of the placebo patients and 80% of the abatacept patients were women, and most of the subjects were white. Median disease duration was a few months in both groups.
There were no statistically significant differences in GCA characteristics between the groups at time of enrollment. Vascular pain, headaches, and tongue and jaw pain were common in both. One patient in both arms had ischemic retinopathy.
Abatacept counters T cell activation, which has been implicated in the pathophysiology of GCA. Promising results for tocilizumab (Actemra) in GCA also were presented at the annual meeting.
The National Institutes of Health funded the study. Bristol-Myers Squibb provided the abatacept. Nearly all of the investigators, including Dr. Merkel and Dr. Langford, disclosed relationships with the company.
SAN FRANCISCO – The addition of abatacept (Orencia) to standard prednisone regimens seemed to reduce the risk of relapse in a study by the Vasculitis Clinical Research Consortiumof 49 patients with giant cell arteritis.
The patients were initially treated with 40-60 mg of prednisone daily on a standard taper plus abatacept 10 mg/kg IV on days 1, 15, and 29, and week 8. The 41 patients who were in remission at week 12 were then randomized to continued abatacept or placebo IV once monthly. Prednisone was stopped at week 28.
By the time the study concluded a year after the last patient was randomized, 10 abatacept patients had relapsed and 10 had stayed in remission, while 14 placebo patients had relapsed and 7 had stayed in remission. Relapse-free survival at 12 months was calculated to be 48% in the abatacept group and 31% in the placebo group (P = .049). A longer median duration of remission was seen with abatacept, as well (9.9 months versus 3.9 months with placebo).
“Abatacept prolonged the rate of relapse-free survival and was associated with a favorable safety profile.” These are “very encouraging results supporting abatacept” for giant cell arteritis (GCA), lead investigator Dr. Carol Langford, director of the Center for Vasculitis Care and Research at the Cleveland Clinic, said at the annual meeting of the American College of Rheumatology.
There were 129 adverse events in 35 patients, including 33 infections and three malignancies; 23 adverse events in 15 patients were deemed serious.
Even so, “there was no difference in the frequency or severity of adverse events between treatment arms, including the rate of infection,” and “no evidence of enhanced toxicity during the first 3 months [when] patients received abatacept and high-dose prednisone,” the investigators said.
While steroids remain the standard of care, “positive signals for new drugs in GCA will [give us] the opportunity to refine their use,” perhaps, for example, by allowing for a lower steroid dose up front, said Dr. Peter Merkel, chief of rheumatology at the University of Pennsylvania in Philadelphia.
Patients in the abatacept arm were 64 years old on average, while placebo patients were about 72 years old. All of the placebo patients and 80% of the abatacept patients were women, and most of the subjects were white. Median disease duration was a few months in both groups.
There were no statistically significant differences in GCA characteristics between the groups at time of enrollment. Vascular pain, headaches, and tongue and jaw pain were common in both. One patient in both arms had ischemic retinopathy.
Abatacept counters T cell activation, which has been implicated in the pathophysiology of GCA. Promising results for tocilizumab (Actemra) in GCA also were presented at the annual meeting.
The National Institutes of Health funded the study. Bristol-Myers Squibb provided the abatacept. Nearly all of the investigators, including Dr. Merkel and Dr. Langford, disclosed relationships with the company.
AT THE ACR ANNUAL MEETING
Key clinical point: Abatacept appears to be a useful adjunct for giant cell arteritis.
Major finding: Relapse-free survival at 12 months was 48% in the abatacept group and 31% in the placebo group (P = .049).
Data source: Randomized, placebo-controlled study of 49 patients with giant cell arteritis.
Disclosures: The National Institutes of Health funded the study. Nearly all of the investigators disclosed relationships with Bristol-Myers Squibb, which provided the abatacept.