User login
An Imposter Twice Over: A Case of IgG4-Related Disease
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
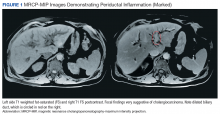
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
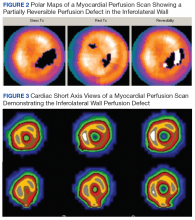
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated fibroinflammatory condition that involves multiple organs and appears as syndromes that were once thought to be unrelated. This disease leads to mass lesions, fibrosis, and subsequent organ failure if allowed to progress untreated.1 Involvement of gastrointestinal (GI) organs, salivary glands, lacrimal glands, lymph, prostate, pulmonary, and vascular system have all been reported.2 Elevated IgG4 serum levels are common, but about one-third of patients with biopsy-proven IgG4-RD do not manifest this characteristic.3,4
Diagnostic confirmation is with biopsy, and all patients with symptomatic, active IgG4-RD require treatment. Glucocorticoids are first-line treatment and are utilized for relapse of symptoms. In addition to glucocorticoids, steroid-sparing medications, including rituximab, azathioprine, mycophenolate mofetil, tacrolimus, and cyclophosphamide have all been used with successful remission.5,6 Here, the authors discuss a case of IgG4-RD that presented with intrahepatic biliary obstruction (mimicking cholangiocarcinoma) and subsequent development of coronary arteritis despite treatment.
Case Presentation
In June 2015, a 57-year-old Air Force veteran presented to Eglin AFB Hospital with pruritic jaundice and acute abdominal pain. He was found to have elevated bilirubin levels (total bilirubin 10 mg/dL [normal range 0.2-1.3 mg/dL], direct bilirubin 6.6 mg/dL [normal range 0.1-0.4 mg/dL]). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) also were moderately elevated (147 U/L and 337 U/L, respectively).
Prior to this presentation, the patient had been in his usual state of health. His past medical history was notable only for minimal change kidney disease (MCD). MCD is defined as effacement of the podocyte seen on electron microscopy, which allows the passage of large amounts of protein.
A cholangiogram showed abnormal filling into the left main intrahepatic duct and obvious obstruction at the bifurcation of the bile duct. A biliary drainage catheter was placed, and a repeat cholangiogram 2 days later showed involvement of both right and left intrahepatic ducts. The distal common bile duct appeared uninvolved as did the pancreas. Lymphadenopathy was noted at the liver hilum. Klatskin cholangiocarcinoma (type IIIB) was the presumed diagnosis. Based on these findings, tumor resection was performed 3 weeks later, including left hepatectomy, caudate lobe resection, complete bile duct resection, cholecystectomy, with reconstruction by Roux-en-Y intrahepaticojejunostomy. In addition, portal and hepatic artery lymph node dissection was completed.
Surgical specimens were sent for pathologic evaluation and were found negative for malignancy. Patchy areas of storiform fibrosis, obliterative phlebitis, and lymphoplasmacytic infiltrate were noted. IgG4 immunostain highlighted the presence of IgG4 positive plasma cells with a peak count of 145 IgG4 positive plasma cells/hpf. About 80% of the plasma cells were positive for IgG4. Unusually dense eosinophilic infiltrate with plasma cells and regions of dense fibrosis that strongly contributed to the masslike appearance on CT imaging also were noted. Final histology confirmed the diagnosis of IgG4-RD. Elevated levels of total IgG in the serum were observed without elevation in serum IgG4 (Table).
The patient was started on prednisone 40 mg and azathioprine 150 mg daily, with subsequent taper of prednisone over the next 6 months. After prednisone was discontinued, the patient reported new symptoms of lower extremity pain, neuropathy, and swelling of his face. Laboratory results were notable for elevated erythrocyte sedimentation rate. The patient was restarted on prednisone 40 mg daily. Azathioprine was replaced with a regimen of 4 doses every 6 months of IV rituximab 700 mg q week and mycophenolate mofetil (1,000 mg bid). After remission was induced, the patient was slowly weaned off prednisone again.
Following 6 months of successful discontinuation of prednisone and continued rituximab and mycophenolate mofetil therapy, the patient presented to the emergency department with new onset chest pain and shortness of breath. A CT angiography of the chest showed right upper and middle lobe infiltrate, and he was treated for community acquired pneumonia. Additionally, he was noted to have elevated troponin levels suggestive of myocardial infarction (MI). Initial troponin was 1.23 ng/mL (normal range < 0.015 ng/mL), which trended down over the next 18 hours. A bedside echocardiogram showed a normal left ventricular ejection fraction without wall motion abnormalities. Etiology for his acute MI was presumed to be demand ischemia from fixed atherosclerotic plaque. Further inpatient cardiac risk stratification was changed to the outpatient setting, and he was started on medical management for coronary artery disease with a beta blocker, a statin, and aspirin. He was discharged home on 10 mg prednisone daily, which was subsequently tapered over several weeks.
In follow-up, a Lexiscan myocardial perfusion imaging was conducted that demonstrated an inferolateral defect and associated wall motion abnormalities (Figures 2 and 3).
Discussion
IgG4-related disease has been found to be a systemic disorder. Typical characteristics include predominance in men aged > 50 years, elevated IgG4 levels, and findings on histology.1 It has been reported to involve many organs, including pancreas, liver, gallbladder, salivary glands, thyroid, and pleura of the lung.2,5
This case report begins with a presumptive diagnosis of cholangiocarcinoma, which was treated aggressively with extensive surgery. Several case reports of complex tumefactive lesions in the GI area (mostly pancreatic and biliary) have detailed IgG4-RD as both a risk factor for subsequent development of cholangiocarcinoma and as a separate entity of IgG4-related sclerosing cholangitis.7-9 It is hypothesized that the induction of IgG4- positive plasma cells has been intertwined with the development of cholangiocarcinoma. Differentiation between IgG4 reaction that is scattered around cancerous nests and IgG4 sclerosing cholangitis without malignancy is challenging. It has been documented that both elevated IgG4 levels and hilar hepatic lesions that resemble cholangiocarcinoma frequently accompany those cases of IgG4 sclerosing chlolangitis without pancreatic involvement.9 The histologic features of IgG4-RD need to be identified with multiple biopsies and cytology, and superficial biopsy from biliary mucosa cannot reliably exclude cholangiocarcinoma.
Lymphoplasmacytic aortitis and arteritis have been documented in IgG4-RD. In 2017, Barbu and colleagues described how one such case of coronary arteritis presented with typical angina and coronary catheterization revealing coronary artery stenosis.10 However, during coronary artery bypass surgery, the aorta and coronary vessels were noted to be abnormally stiff. A diffuse fibrotic tissue was identified to be causing the significant stenosis without evidence of atherosclerosis. Pathology showed typical findings of IgG4-RD, and there was a rapid response to immunosuppressive therapy. Involvement of coronary arteries has been described in a small number of cases at this time and is associated with progressive fibrotic changes resulting in an MI, aneurysms, and sudden cardiac death.2,10,11
IgG4-RD can be an extensively systemic disease. All presentations of fibrosis or vasculitis should be viewed with heightened suspicion in the future as being a facet of his IgG4-RD. Pleural involvement has been reported in 12% of cases presenting with systemic presentation, kidney involvement in 13%.2,12
Unfortunately, there is no standard laboratory parameter to date that is diagnostic for IgG4-RD. The gold standard remains confirmation of histologic findings with biopsy. According to an international consensus from 2015, 2 out of the 3 major findings need to be present: (1) dense lymphoplasmacytic infiltrate; (2) storiform fibrosis; and (3) obliterative phlebitis in veins and arteries.1,5 Most patients present with symptoms related to either tumefaction or fibrosis of an organ system.1 Peripheral eosinophilia and elevated serum IgE are often present in IgG4-RD.13 Although IgG4 values are elevated in 51% of biopsy-proven cases, flow cytometry of CD19lowCD38+CD20-CD27+ plasmablasts has been explored recently as a correlation with disease flare.3,14 These particular plasmablasts mark a stage between B cells and plasma cells and have been reported to have a sensitivity of 95% and a specificity of 82% in association with actual IgG4-RD.14 Furthermore, blood plasmablast concentrations decrease in response to glucocorticoid treatment, thereby providing a possible quantifiable value by which to measure success of IgG4 treatment.5,12
Treatment for this disease consists of immunosuppressive therapy. There is documentation of successful remission with rituximab and azathioprine, as well as methotrexate.1,5 Both 2015 consensus guidelines and a recent small single-center retrospective study support addition of second-line steroid sparing agents such as mycophenolate mofetil.5,6 For acute flairs, however, glucocorticoids with slow taper are usually utilized. In these cases, they should be tapered as soon as clinically feasible to avoid long-term adverse effects. Untreated IgG4-RD, even asymptomatic, has been shown to progress to fibrosis.5
Conclusion
IgG4-RD is a complicated disease process that requires a high index of suspicion to diagnose. In addition, for patients who are diagnosed with this condition, its ability to mimic other pathologic conditions should be taken into account with manifestation of any new illness. This case emphasizes the ability of this disease to localize in multiple organs over time and the need for lifetime surveillance in patients with IgG4-RD disease.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
1. Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189-199.
2. Brito-Zerón P, Ramos-Casals M, Bosch X, Stone JH. The clinical spectrum of IgG4-related disease. Autoimmun Rev. 2014;13(12):1203-1210.
3. Wallace ZS, Deshpande V, Mattoo H, et al. IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67(9):2466-2475.
4. Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-RD. Ann Rheum Dis. 2015;74(1):14-18.
5. Khosroshahi A, Wallace ZS, Crowe JL, et al; Second International Symposium on IgG4-Related Disease. International consensus guidance statement on the management and treatment of IgG4-Related disease. Arthritis Rheumatol. 2015;67(7):1688-1699.
6. Gupta N, Mathew J, Mohan H, et al. Addition of second-line steroid sparing immunosuppressants like mycophenolate mofetil improves outcome of immunoglobulin G4-related disease (IgG4-RD): a series from a tertiary care teaching hospital in South India. Rheumatol Int. 2017;38(2):203-209.
7. Lin HP, Lin KT, Ho WC, Chen CB, Kuo, CY, Lin YC. IgG4-associated cholangitis mimicking cholangiocarcinoma-report of a case. J Intern Med Taiwan. 2013;24:137-141.
8. Douhara A, Mitoro A, Otani E, et al. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol. 2013;5(8):181-185.
9. Harada K, Nakanuma Y. Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol. 2014;2014:803876.
10. Barbu M, Lindström U, Nordborg C, Martinsson A, Dworeck C, Jeppsson A. Sclerosing aortic and coronary arteritis due to IgG4-related disease. Ann Thorac Surg. 2017;103(6):e487-e489.
11. Kim YJ, Park YS, Koo BS, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17(8):451-452.
12. Khosroshahi A, Digumarthy SR, Gibbons FK, Deshpande V. Case 34-2015: A 36-year-old woman with a lung mass, pleural effusion and hip pain. N Engl J Med. 2015;373(18):1762-1772.
13. Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):191-206.
14. Wallace ZS, Mattoo H, Carruthers M, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190-195.
Innovative Therapies for Severe Asthma
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
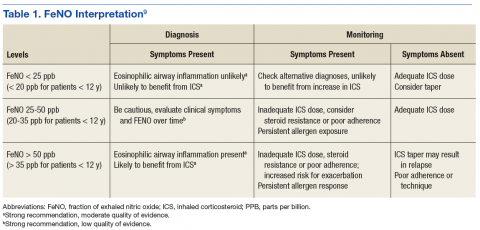
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
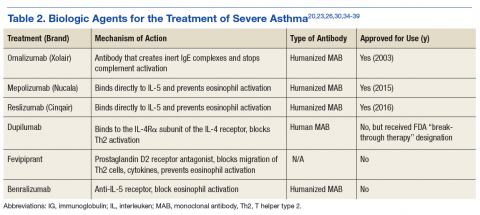
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
More than 39.5 million people in the U.S. have been diagnosed with asthma, and about 3,400 deaths occur annually due to asthma complications.1 Although the prevalence of atopy and asthma have increased over the past few decades in western countries, control and outcomes are improving.2 Use of asthma protocols and early recognition by the primary care provider (PCP) are among the main reasons for trends toward decreased hospitalization and fewer asthma-related deaths.3,4
In addition to the mainstay of treatments, including trigger avoidance, inhaled corticosteroids (ICS), and rescue bronchodilators, new therapies have been developed to supplement the treatment of severe persistent asthma, which constitutes about 5% to 10% of asthma cases. Severe asthma is defined as asthma that is unresponsive to baseline therapy.5
Three sets of guidelines and recommendations exist to provide structure to asthma treatment decision making. The Expert Panel Report-3 (EPR-3) was created by the National Education and Prevention Program (NAEPP) and was last published in 2007. The NAEPP favors a stepwise approach, based on asthma severity and age group.3 The International European Respiratory Society (ERS) and American Thoracic Society (ATS) task force report was updated in 2014.5 The Global Initiative for Asthma (GINA) report, updated in 2016, now includes several of the advances in asthma care for those patients refractory to standard treatments.
Asthma Therapies
In this review, the authors cover therapies for severe asthma that are becoming more important for PCPs to consider, including exhaled nitric oxide (NO) levels, the use of tiotropium for asthma, the applicability of biologic agents, the use of allergen immunotherapy, and the usefulness of roflumilast. This review also covers antileukotriene therapy, bronchial thermoplasty, and a discussion of long-acting beta-agonist (LABA) therapy.
Fractional Exhaled Nitric Oxide
Nitric oxide is present in the exhaled breath and is elevated in those with eosinophilic asthma.6 The role of NO in asthma pathology is complex, involving proinflammatory qualities that contribute to airway hyperresponsiveness (AHR) and as a weak mediator of smooth muscle relaxation. In exhaled air, NO correlates with up-regulation of NO synthase (NOS), which occurs with inflammation, therefore, quantifying airway inflammation.6-8
There has been some variability in the evidence supporting the use of fractional exhaled NO (FeNO) levels as a diagnostic tool. Some studies have suggested that FeNO is also elevated in other nonasthma conditions, such as eosinophilic bronchitis, atopy, and allergic rhinitis. Also, FeNO levels have been shown to be variably influenced by smoking, bronchoconstriction, and viral respiratory infections.9 However, FeNO levels > 50 ppb correlated most strongly with eosinophilic asthma and steroid responsiveness.9
Fractional exhaled NO tests now can be performed in the PCP office with NIOX VERO (Chicago, IL), a small, relatively inexpensive device. Although the 2016 GINA guidelines and the 2015 ERS/ATS guidelines do not offer specific recommendations for use and do not support withholding ICS based on FeNO test results, guidelines for FeNO use do exist. In 2011, ATS published a specific set of FeNO interpretive guidelines for office-based use.9 When performed in conjunction with standard testing, FeNO levels can provide valuable clinically relevant information, such as (1) detection of eosinophilic airway inflammation; (2) determining the likelihood of corticosteroid responsiveness; (3) monitoring of airway inflammation to determine the need for steroids; and (4) unmasking of otherwise unsuspected nonadherence to corticosteroid therapy (Table 1).
Tiotropium as an Adjunct Treatment
Tiotropium is a long-acting inhaled anticholinergic. A sentinel 1984 study by Gross and Skorodin demonstrated that parasympathetic activity is the dominant reversible component in patients with chronic obstructive pulmonary disease (COPD), including emphysema.10 In addition, all achievable bronchodilation was obtained with an inhaled anticholinergic compared with that of separate or simultaneous administration of adrenergics. Sympathetic neural pathways are sparse in human lungs and have their endings on the cells of the cholinergic postganglionic fibers, because sympathetic terminals on airway smooth muscle cells are rare or nonexistent.11 Therefore, sympathetic modulation or activation of beta cells could change the parasympathetic tone.11
The FDA approved the addition of tiotropium for treating asthma in September 2015 for patients aged ≥ 12 years. The use of tiotropium is supported by both the ERS/ASTS and GINA 2016 guidelines. The recommended and approved dose of tiotropium for asthma is 2.5 µg daily (the recommended dose for COPD treatment is 5 µg).12 A recent phase 3 study compared 2.5 µg vs 5 µg dosing with ICS but no LABA in adolescents, noting significant improvement with the 2.5 µg dose.13 Adding tiotropium to ICS + LABA in patients with severe symptomatic asthma has been associated with positive results in initial studies by Kersjens and colleagues.14 Even as early as 2010, the use of tiotropium was shown to produce statistically significant improvement in morning peak expiratory flow (PEF), with a mean difference of 25.8 L/min (n = 210, P < .001).15
Tiotropium also has been shown to provide a sustained reduction in lung hyperinflation for those with COPD, thus providing an improvement in exertional dyspnea and exercise tolerance. On day 42 of a randomized, double-blinded, placebo-controlled, parallel-group study of 187 patients, vital capacity and inspiratory capacity were noted to be increased with decreases in residual volume and functional residual capacity. Exercise endurance times increased by 105 ± 40 sec (21%).16 This effect has not been studied yet in a population of patients with asthma; however, the same principles may hold true.
Biologic Agents
Recent asthma research has been focused on disrupting the inflammatory cascade. Both GINA and ERS/ATS divide asthma into allergic vs nonallergic endotypes. Allergic asthma usually is manifested by sputum eosinophilia and high serum eosinophil counts, whereas other endotypes include aspirin-sensitive and exercise-induced asthmas that present with a neutrophilic predominance. Nonallergic asthma is more severe typically and has been linked to steroid resistance.17 Many differentphenotypes have been identified, but the main categories include eosinophilic, neutrophilic, mixed, and paucigranulocytic.18
Mast cells, bronchial epithelium, and macrophages are involved in asthma progression. Targeting the cytokines produced by these pathways can be achieved through direct and indirect modulation. Interleukin (IL)-13 is central to development of AHR, and its effect is mediated through binding to its receptor and IL-4 receptor α complexes.19 Patients with severe asthma with an eosinophilic phenotype can benefit with the use of the new biologics, which decrease the amount of eosinophilia in lung tissue by blocking specific receptors for IL-5.
Omalizumab
Omalizumab, an anti-immunoglobulin E (IgE) antibody, has been shown to be helpful in treating patients with allergic asthma. Omalizumab is a 95% humanized IgE monoclonal antibody (MAB) that binds to the IgE molecule at the fc region and prevents IgE from binding to cell-surface receptors. In a humanized MAB, only the hypervariable regions are from mouse origin vs the newer completely human MABs. Omalizumab forms small, biologically inert IgE+ anti-IgE complexes that cannot activate the complement cascade. The serum free IgE level is decreased.20 Approved in 2003 for those aged ≤ 12 years, its use is restricted to patients with severe asthma, allergic sensitization (positive allergen skin testing), and an elevated serum IgE level (30-700 IU/mL). It is administered subcutaneously every 2 to 4 weeks, based on body weight and serum IgE levels.
For those with baseline eosinophil counts > 300 µL, addition of omalizumab most likely has been shown to improve quality of life (QOL) and reduce exacerbations, the use of rescue medications, ICS dosages, and ED visits.21-26 The most dangerous adverse effect (AE) was found to be an anaphylaxis rate of 0.09%, most frequently occurring in the first 2 hours after the first dose. Therefore, the patient must be monitored for 2 hours after the first dose and 30 minutes after subsequent doses. Epinephrine injection also should be prescribed. Although a 5-year prospective cohort study and retrospective pooled analysis of more than 10,000 patients did not support any relationship with malignancy.27,28 A higher incidence of cardiovascular and cerebrovascular AEs has been observed, and the FDA issued a safety announcement regarding this finding.29
Both ERS/ATS and GINA 2016 recommended that a therapeutic trial of omalizumab should be performed in all patients with severe confirmed IgE dependent allergic asthma.4,5 If there is no response in 4 months, it is unlikely that further administration would be beneficial.
Mepolizumab
Interleukin-5 is a key cytokine in the eosinophil life pathway. There are receptors for IL-5 on eosinophils, basophils, and β cells.30 Mepolizumab is an anti-IL-5 antibody for those with refractory eosinophilic asthma and a history of continued exacerbations. It has beneficial effects in the management of persistent airways eosinophilia among corticosteroid-resistant subjects. In a 2009 study, rates of exacerbations at 50 weeks were significantly lower than with placebo (2.0 vs 3.4 mean exacerbations per subject, 95% confidence interval [CI], 0.32-0.92; P = .002) as were eosinophil counts in blood and sputum (P < .001 and P = .002 respectively.31 A 2014 randomized, double-blind trial by Ortega and colleagues demonstrated reduction in rate of asthma exacerbations (primary outcome) to 47% (95% CI, 29-61) among patients receiving IV dosing and 53% (95% CI, 37-65) in the oral mepolizumab group (P < .001 for both groups, n = 576).32
In addition, there is significant data to show that even if the patient did not respond to omalizumab, he or she might still respond to mepolizumab. Data were collected from 2 randomized, double-blind, placebo-controlled studies with rate of exacerbation and percentage reduction in oral corticosteroid dose as the primary outcomes. In one of the studies (n = 576), the subjects were noted to have prior omalizumab use but still decreased exacerbation rate by 57%.33
Reslizumab
Reslizumab also is an FDA-approved anti-IL-5 antibody. It binds directly to IL-5 and prevents it from binding to eosinophils.34 For adults with severe eosinophilic asthma and refractory exacerbations, the goal of reslizumab therapy is to reduce eosinophil maturation, recruitment, and activation. Reslizumab is delivered in a weight-based IV dose (3 mg/kg) every 4 weeks. The FDA has issued a boxed warning for a 0.3% anaphylaxis rate.35 The most common AEs are elevated creatinine kinase, musculoskeletal pain, and oropharyngeal pain. Use of reslizumab resulted in greater reduction in sputum eosinophils, improvements in airway function, and a trend toward greater asthma control compared with that of placebo.34
Other Biologic Therapies
Many biologics are being developed as medical researchers continue to understand more of the mechanisms and pathways that contribute to allergic disease (Table 2). Dupilumab is an IL-4 inhibitor designated as a “breakthrough therapy” in 2014 by the FDA. This biologic blocks the downstream signaling events induced by IL-4 and IL-13 by binding to a subunit of the IL-4 receptor in the complexes. It has been found beneficial for those with high blood eosinophil counts and moderate-to-severe asthma and decreased asthma exacerbations when LABA and ICS were withdrawn.36,37
Fevipiprant is a prostaglandin D2 inhibitor that blocks T-helper type 2 (Th2) cell migration and subsequent bronchoconstriction and cytokine effects with decreased IL-4, IL-5, and IL-13. Although sputum eosinophil percentage was noted to be decreased in a study involving 61 patients randomized to treatment for 12 weeks, asthma QOL questionnaires and prebronchodilator spirometry did not change.38,39
Benralizumab is an anti-IL-5 receptor antibody that has been more effective in reduction of airway and blood eosinophils levels compared with that of mepolizumab (undetectable vs 52% reduction), within 24 hours of IV dosing. In contrast, the anti-IL-5 antibodies take about 4 weeks to decrease eosinophil levels in blood and sputum.34 There have been no documented AEs aside from nasopharyngitis and injection site reactions and no safety concerns to date. It is currently undergoing phase 3 trials.40
Immunotherapies
Allergen immunotherapy is recommended for mild-to-moderate asthma. A 2010 Cochrane Review found that subcutaneous immunotherapy compared with placebo demonstrated improvements in bronchial hyperresponsiveness and decreased medication use.41 Expert Panel Report-3 guidelines recommend consideration of immunotherapy for mild-to-moderate asthma.5 While ERS/ATS guidelines for severe asthma do not address allergen immunotherapy, GINA guidelines incorporate it as Evidence A for treating modifiable risk factors to reduce exacerbations, although the efficacy is limited.6
Roflumilast
Roflumilast is a selective PDE4 inhibitor that has shown an anti-inflammatory effect in COPD. Studies evaluating the reversibility and prevention of airway remodeling showed good promise in mouse models.42 Data from 8 placebo-controlled, double-blind, phase 1, 2, and 3 studies conducted at 14 sites in Europe, North America, and South Africa from 1997 to 2005 showed reduced sputum eosinophil and neutrophil counts, consistent with findings during COPD treatment. However, forced expiratory volume in one second (FEV1) and PEF values were unchanged, suggesting that there was no acute bronchodilatory effect with roflumilast therapy.43 Roflumilast is not addressed in the 2016 GINA guidelines and at this time does not have a role in the treatment of severe asthma.
Antileukotrienes
After the activation of mast cells and eosinophils, leukotrienes are generated by 5-lipoxygenase from arachidonic acid and create bronchoconstriction, vasodilation, increased mucus production, increased recruitment of eosinophils, and decreased ciliary motility. Some studies have encouraged addingleukotriene receptor blockers (both montelukast and zafirlukast) to ICS therapy44,45 and to therapy for patients with aspirin-intolerant asthma or allergic asthma.46,47 However, other studies have shown them to be of limited benefit.48,49 A recent Cochrane Reviewof 18 randomized-controlled trials with 7,208 adults and children compared ICS + leukotriene receptor antagonist (LTRA) vs ICS + LABA.50 The ICS + LABA resulted in greater improvements in lung function, symptoms score, and rates of exacerbations.50
Most recommendations recognize the limitations of antileukotriene medications and agree that they are an adjunct rather than primary therapy. The GINA 2016 guidelines support the use of LTRAs in mild asthma, stating that although LTRAs are less effective than ICS (Evidence A), they may be appropriate for initial controller treatment for some patients who are unable or unwilling to use ICS or for patients with concurrent allergic rhinitis (Evidence B).51,52
Zileuton is a different type of antileukotriene. It inhibits leukotrienes B4, C4, D4, and E4 by inhibition of 5-lipoxygenase, interfering with leukotriene formation. It is approved for patients aged ≥ 12 years and is more expensive than montelukast or zafirlukast. Most studies supporting its use were conducted in patients with mild-to-moderate asthma on β-agonist therapy only. A 1988 study showed that zileuton therapy improved FEV1, reduced nasal symptoms, and decreased bronchial responsiveness to inhaled aspirin and histamine.53 All but 1 study patient were on ICS or oral corticosteroids. Zileuton was noted to be effective for patients with aspirin-intolerant asthma.
Some earlier studies reported that a small number of subjects had an increase in transaminases that resolved when they discontinued the medication. Therefore, it is recommended to check baseline laboratory results every 2 to 3 months.54,55 Neither GINA nor ERS/ATS guidelines address the use of zileuton.
Bronchial Thermoplasty
With asthma there is marked hypertrophy and hyperplasia that occurs in the airway smooth muscle. The airway of the patient with asthma also is lined with cells that promote inflammation. Thermal energy is used to perform controlled destruction of the inflammatory lining and pathologic hyperplasia. Three sequential bronchoscopies are performed 3 weeks apart to treat the right lower lobe, left lower lobe, and bilateral upper lobes. The right middle lobe is not treated due to its smaller diameter. Each bronchoscopy takes about 30 to 60 minutes. Patients are given perioperative steroids.56
Three large phase 3 clinical trials have evaluated the efficacy of bronchial thermoplasty (BT). The AIR (Asthma Intervention Research) trial in 2007 was a randomized controlled study of 112 patients with moderate or severe asthma that showed improved exacerbation rates, symptom-free days and QOL scores (1.3 ± 1.0 vs 0.6 ± 1.1; P = .003), but no difference in prebronchodilator FEV1 or AHR.57 There was a significant reduction in the rate of mild exacerbations and increase in morning PEF rates.57 Findings at 5 years showed improved AHR but no difference in frequency of need for oral corticosteroids and frequency of hospital or emergency department (ED) visits.58
The Research in Severe Asthma (RISA) clinical trial was a randomized controlled study (n = 32, 15 randomly assigned to BT) that showed improved prebronchodilator FEV1 in patients with severe, symptomatic asthma and baseline FEV1 of 62% to 66% with half the patients requiring oral corticosteroids (percentage predicted; 14.9 ± 17.4 vs -0.9 ± 22.3; P =.04).57 Quality of life scores were also significantly improved. At 5 years (14 BT patients were followed), the frequency of hospitalizations and ED visits decreased.59
The 2010 AIR2 study was a randomized, double blind, sham-controlled study (n = 288) developed to address the limitations of the 2 previous studies. It excluded the severest asthma cases, and its blinded nature was created with sham bronchoscopy to eliminate possible placebo effect. The study questionnaires showed improved QOL overall (79% vs 64%); however, there was a definite placebo effect noted.60 Decreased frequency of severe exacerbations as well as ED visits and days lost from work or school also were documented as secondary endpoints. At 5 years, decreased frequency of severe exacerbations and ED visits continued in the control group (85% consented to follow-up).61 Importantly, despite the placebo effect in QOL scores, there were no improvements in exacerbation rates or hospitalizations in those receiving sham bronchoscopy at the 1-year mark.61
Although more longitudinal studies need to be planned, including evaluation of those with the most severe asthma, there seems to be a sustained improvement in patients. Those who have received BT generally are found to have reduced airway smooth muscle with lower concentration of key inflammatory cytokines on follow-up bronchoscopy. However, variability in response has been documented.56 There has been no documented deterioration in pulmonary function with BT, and no significant structural abnormalities have been seen on high-resolution computed tomography.56,58 Both GINA 2016 and ERS/ATS support the use of BT in the context of adults with severe asthma, calling for more long-term studies to address delayed benefits and safety.
LABA Inhalers
A multicenter, double-blind, 26-week study of 11,693 patients randomized to ICS + LABA (budesonide/formoterol) vs ICS (budesonide) alone has shown no increased AEs in either arm. The study found that treatment with budesonide/formoterol was associated with lower risk of asthma exacerbations than using budesonide alone (16.5%; P = .002).62
The safety of adding a LABA to fluticasone also has been evaluated recently. A 2016 study of almost 12,000 patients (aged > 12 years) compared fluticasone proprionate alone vs fluticasone with salmeterol.63 There were no asthma-related deaths, but 2 patients in the fluticasone-only group were intubated with asthma complications. The risk of a severe asthma exacerbation seemed to be lower in the combination group (8% vs 10%; P < .001).63
A 2014 Cochrane Review supported the view that LABAs in adults seem to be safe when used concurrently with an ICS with a A-level recommendation, based on consistent good-quality, patient-oriented evidence.64 Multiple organizations have issued guidelines to this effect in the past, but previous results of studies showed that asthma deaths and a small increase in nonfatal serious AEs were noted in those using LABA monotherapy alone.64
NAEPP (EPR-3) and ERS/ATS recommend stepwise increases in the dose of ICS in combination with a LABA. The GINA guidelines recommend controller therapy to include combination IHS and LABA but with the consideration of higher doses of ICS than are routinely recommended for general use.
Inhaler and Inhaler Combinations
Many different inhalers of ICS alone and ICS/LABA combinations exist on the market today. There are differences in delivery that affect patient preference but these differences have not been found to improve delivery. Small particle ICS therapy could possibly correlate with improved delivery to the small airways.65 There are 3 preparations of inhaled steroids that fit in to this group, including beclomethasone, ciclesonide, and flunisolide. Other inhaled steroid formulations include budesonide, fluticasone propionate, fluticasone furoate, and mometasone.
Combination therapy (ICS + LABA) inhalers also are widely available. They include budesonide/formoterol, fluticasone proprionate/salmeterol, mometasone/formoterol, and the newer fluticasone furoate/vilanterol, a once-daily combination approved for those aged ≥ 18 years.
Conclusion
The treatment of severe asthma has progressed from simple manipulation of avoidance, bronchodilators, and corticosteroids to include many other treatments that have improved QOL for patients with refractory asthma. Although many of these options are delivered in coordination with an allergy and pulmonary specialist, it is important for the PCP to have a good knowledge base and awareness of additional treatments that are currently available.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.
1. Centers for Disease Control and Prevention. Asthma facts: CDC’s national asthma control program grantees. https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Published July 2013. Accessed November 9, 2017.
2. Wilson DH, Adams RJ, Tucker G, Appleton S, Taylor AW, Ruffin RE. Trends in asthma prevalence and population changes in South Australia, 1990-2003. Med J Aust. 2006;184(5):226-229.
3. National Asthma Education Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(suppl 5):S94-S138.
4. Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 update. http://ginasthma.org/wp-content/up loads/2016/04/wms-GINA-2016-main-report-final.pdf. Accessed November 9, 2017.
5. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-373.
6. Reid DW, Johns DP, Feltis B, Ward C, Walters EH. Exhaled nitric oxide continues to reflect airway hyperresponsiveness and disease activity in inhaled corticosteroid-treated adult asthmatic patients. Respirology. 2003;8(4):479-486.
7. De Sanctis GT, MacLean JA, Hamada K, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189(10):1621-1630.
8. Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182.
9. Dweik RA, Boggs PB, Erzurum SC, et al; American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
10. Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311(7):421-425.
11. Gelb AF, Nadel JA. Affirmation of the adoration of the vagi and role of tiotropium in asthmatic patients. J Allergy Clin Immunol. 2016;138(4):1011-1013.
12. Chin SJ, Durmowicz AG, Chowdhury BA. Tiotropium respimat is effective for the treatment of asthma at a dose lower than that for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(2):173-179.
13. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441-450.e8.
14. Kerstjens HA, Casale TB, Bleeker ER, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respir Med. 2015;3(5):367-376.
15. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715-1726.
16. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnea, and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832-840.
17. Lötvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355-360.
18. Wenzel SE. Phenotypes in asthma: useful guides for therapy, distinct biological processes, or both? Am J Respir Crit Care Med. 2004;170(6):579-580.
19. Wang E, Hoyte FC. Traditional therapies for severe asthma. Immunol Allergy Clin North Am. 2016;36(3):581-608.
20. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354(25):2689-2695.
21. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378-1386.
22. Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309-316.
23. Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573-582.
24. Holgate ST, Djukanovic´ R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408-416.
25. Finn A, Gross G, van Bavel J, et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol. 2003;111(2):278-284.
26. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184-190.
27. Long A, Rahmaoui A, Rothman KJ, et al. Incidence of malignancy in patients with moderate-to-severe asthma treated with or without omalizumab. J Allergy Clin Immunol. 2014;134(3):560-567.e4.
28. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs /DrugSafety/ucm414911.htm. Updated February 10, 2016. Accessed November 9, 2017.
30. Tan HT, Sugita K, Akdis CA. Novel biologicals for the treatment of allergic diseases and asthma. Curr Allergy Asthma Rep. 2016;16(10):70.
31. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973-984.
32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
33. Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71(9):1335-1344.
34. Castro M, Mathur S, Hargreave F, et al; Res-5-0010 Study Group. Resilizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184(10):1125-1132.
35. Cinqair [package insert]. Frazier, PA: Teva Respiratory; 2016.
36. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455-2466.
37. Chung KF. Dupilumab: a potential new treatment for severe asthma. Lancet. 2016;388(10039):3-4.
38. Gonem S, Berair R, Singapuri A, et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med. 2016;4(9):699-707.
39. Erpenbeck VJ, Popov TA, Miller D, et al. The oral CRTh2 antagonist QAWO39 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther. 2016;39:54-63.
40. Tan LD, Bratt JM, Godor D, Louie S, Kenyon NJ. Benralizumab: a unique IL-5 inhibitor for severe asthma. J Asthma Allergy. 2016;9:71-81.
41. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
42. Kim SW, Kim JH, Park CK, et al. Effect of roflumilast on airway remodeling in a murine model of chronic asthma. Clin Exp Allergy. 2016;46(5):754-763.
43. Bardin P, Kanniess F, Gauvreau G, Bredenbröker D, Rabe KF. Roflumilast for asthma: efficacy findings in mechanism of action studies. Pulm Pharmacol Ther. 2015;(suppl 35):S4-S10.
44. Virchow JC Jr, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Resp Crit Care Med. 2000;162(2, pt 1):558-585.
45. Price DB, Hernandez D, Magyar P, et al; Clinical Outcomes with Montelukast as a Partner Agent to Corticosteroid Therapy (COMPACT) International Study Group. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax. 2003;58(3):211-216.
46. Dahlén SE, Malmström K, Nizankowska E, et al. Improvement of aspirin intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165(1):9-14.
47. Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006; 61(6):737-742.
48. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001;357(9273):2007-2011.
49. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta 2 agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014;(1):CD003137.
50. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
51. Philip G, Nayak AS, Berger WE, et al. The effect of montelukast on rhinitis symptoms in patients with asthma and seasonal allergic rhinitis. Curr Med Res Opin. 2004;20(10):1549-1558.
52. Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of topical budesonide and oral montelukast in seasonal allergic rhinitis and asthma. Clin Exp Allergy. 2001;31(4):616-624.
53. Dahlén B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157(4, pt 1):1187-1194.
54. Israel E, Cohn J, Dubé L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zilueton Clinical Trial Group. JAMA. 1996;275(12):931-936.
55. Nelson H, Kemp J, Berger W, et al. Efficacy of zileuton controlled-release tablets administered twice daily in the treatment of moderate persistent asthma: a 3-month randomized controlled study. Ann Allergy Asthma Immunol. 2007;99(2):178-184.
56. Laxmanan B, Egressy K, Murgu SD, White SR, Hogarth DK. Advances in bronchial thermoplasty. Chest. 2016;150(3):694-704.
57. Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327-1337.
58. Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011:11:8.
59. Pavord, ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185-1191.
60. Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116-124.
61. Wechsler ME, Laviolette M, Rubin AS, et al; Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295-1302.
62. Peters SP, Bleecker ER, Canonica GW, et al. Serious asthma events with budesonide plus formoterol vs budesonide alone. N Engl J Med. 2016;375(9):850-860.
63. Stempel DA, Raphiou IH, Kral KM, et al; AUSTRI Investigators. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374(19):1822-1830.
64. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844.
65. Finkas LK, Martin R. Role of small airways in asthma. Immunol Allergy Clin North Am. 2016;36(3):473-482.