User login
Post-Intensive Care Unit Psychiatric Comorbidity and Quality of Life
The prevalence of depression, anxiety, and posttraumatic stress disorder (PTSD) symptoms in intensive care unit (ICU) survivors ranges from 17% to 44%.1-4 Psychiatric comorbidity, the presence of 2 or more psychiatric disorders, is highly prevalent in survivors of acute respiratory distress syndrome and is associated with higher mortality in postsurgical ICU survivors.5-7 While long-term cognitive impairment in patients with ICU delirium has been associated with poor quality of life (QoL),1 the effects of psychiatric comorbidity on QoL among similar patients are not as well understood. In this study, we examined whether psychiatric comorbidity was associated with poorer QoL in survivors of ICU delirium.
METHODS
We examined subjects who participated in the Pharmacologic Management of Delirium (PMD) clinical trial. This trial examined the efficacy of a pharmacological intervention for patients who developed ICU delirium at a local tertiary-care academic hospital.8 Out of 62 patients who participated in the follow-up of the PMD study, 58 completed QoL interviews and validated psychiatric screens (Patient Health Questionnaire-9 [PHQ-9] for depression, the Generalized Anxiety Disorder-7 [GAD-7] questionnaire for anxiety, and the Post-Traumatic Stress Syndrome [PTSS-10] questionnaire for PTSD) at 3 months after hospital discharge. High psychiatric comorbidity was defined as having significant symptoms for all 3 conditions (depression: PHQ-9 score ≥ 10; anxiety: GAD-7 ≥ 10; and PTSD: PTSS-10 > 35). No psychiatric morbidity was defined as having no significant symptoms for all 3 conditions. Low to moderate (low-moderate) psychiatric morbidity was defined as having symptoms for 1 to 2 conditions.
Participants also completed 2 complementary QoL measures: the EuroQol 5 dimensions questionnaire 3-level (EQ-5D-3L) Index and the EuroQol 5 dimensions Visual Analog Scale (EQ-5D-VAS).9 The EQ-5D-3L Index asks participants to rate themselves as having (1) no problems, (2) some problems, or (3) extreme problems on the following 5 scales: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The scores are then indexed against the US population to create a continuous index scale ranging from −0.11 to 1.00.
Fisher’s exact tests were used to compare dichotomous outcomes. Analysis of variance (ANOVA) was used to compare continuous outcomes across the 3 psychiatric groups. Analysis of covariance (ANCOVA) was used to determine whether psychiatric comorbidity in survivors of ICU delirium was associated with QoL measures. Models were adjusted for the following covariates: age, gender, Charlson Comorbidity Index, discharged to home, prior history of depression, and prior history of anxiety. To assess the relationship of psychiatric comorbidity with QoL, we chose the 2 continuous QoL measures as the outcome. Because we were interested in the effect of psychiatric burden on QoL, we used ANCOVA with QoL as the dependent variable and psychiatric burden as an independent variable. Pairwise comparisons were then performed when overall differences were significant (P < 0.05). We performed 2 separate sensitivity analyses. The first analysis looked solely at the subgroup of patients from the medical intensive care unit. We also recalculated the EQ-5D-3L index excluding the anxiety/depression item.
RESULTS
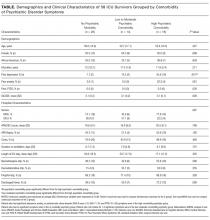
Nearly one-third of patients (18/58) had high psychiatric burden. The table looks at the demographic and clinical characteristics of patients with high psychiatric comorbidity versus those of low-moderate psychiatric comorbidity and those with no psychiatric morbidity. Patient groups did not differ significantly in terms of demographics. For clinical characteristics, patients with high psychiatric comorbidity were more likely than patients with low-moderate psychiatric comorbidity to have a prior history of depression (P < 0.05).
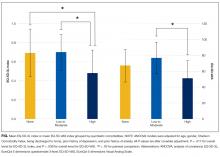
Patients with high psychiatric comorbidity were more likely to have a poorer QoL when compared with patients with low-moderate psychiatric comorbidity and to those with no morbidity as measured by a lower EQ-5D-3L Index (no, 0.69 ± 0.25; low-moderate, 0.70 ± 0.19; high, 0.48 ± 0.24; P = 0.006) and EQ-5D-VAS (no, 67.0 ± 20.7; low-moderate, 76.6 ± 20.0; high, 50.8 ± 22.4; P = 0.004). After adjusting for covariates, patients with high psychiatric comorbidity had a poorer QoL compared with those with no morbidity or low-moderate comorbidity on the EQ-5D-3L Index (P = 0.017 for overall differences), whereas patients who had high psychiatric comorbidity had a poorer QoL compared to those with low-moderate comorbidity on the EQ-5D-VAS (P = 0.039 for overall differences; Figure). Subgroup analysis of MICU patients yielded similar results. Patients with high psychiatric burden had significantly poorer QoL as measured by the EQ-5D-3L (unadjusted P = 0.044, adjusted P = 0.003) and the EQ-5D-VAS (unadjusted P = 0.007, adjusted P = 0.021). After excluding the anxiety/depression item from the EQ-5D-3L, we observed similar differences (no, 0.71 ± 0.24; low-moderate, 0.75 ± 0.15; high, 0.58 ± 0.22; unadjusted P = 0.062; adjusted P = 0.040).
DISCUSSION/CONCLUSION
Psychiatric comorbidities in ICU survivors are common and pose a significant clinical issue. Patients with multiple psychiatric comorbidities can be more complicated to identify from a diagnostic standpoint and often require more prolonged, intensive mental health treatment when compared with patients with a single psychiatric disorder.10,11 Our study showed that high psychiatric comorbidity in survivors of ICU delirium is associated with a decreased QoL compared with those with no psychiatric comorbidity or with low-moderate psychiatric comorbidity. This finding is consistent with previous studies in the general population that patients with multiple psychiatric comorbidities are associated with a poorer QoL compared with patients with a single psychiatric comorbidity.10,11
There is a pressing need to better characterize psychiatric comorbidities in ICU survivors because our current evidence suggests that the prevalence of psychiatric comorbidities of ICU survivors is substantially higher than that of the general population. We found that nearly one-third of survivors of ICU delirium had comorbid depression, anxiety, and PTSD symptoms at 3 months. This is consistent with the few other studies of ICU survivors, which showed a prevalence of psychiatric comorbidity of 25% to 33%.5,12 These rates are substantially higher than the prevalence in the general population of 6%.13
The high rate of psychiatric comorbidities may render it difficult to effectively treat the mental health symptoms in ICU survivors.14 Treating multiple psychiatric comorbidities may also be especially challenging in survivors of ICU delirium because they have a high prevalence of cognitive impairment. Mental health treatments for patients with psychiatric disorders and comorbid cognitive impairment are limited. Better characterization of psychiatric comorbidity in ICU survivors, particularly those with ICU delirium, is vital to the development of more effective, bundled treatments for this population with multiple comorbidities.
Standardized screenings of ICU survivors at a high risk for psychiatric disorders, such as survivors of ICU delirium, may help to identify patients with comorbid psychiatric disorder symptoms and have them referred to appropriate treatment earlier with the hope of improving their QoL sooner. Although opportunities to deliver integrated outpatient collaborative mental health and medical care for a subspecialty population are limited, one potential model of care would be to utilize a collaborative-care model in an ICU survivor clinic.15
Strengths of our study include the examination of psychiatric comorbidities in survivors of ICU delirium, who often have a poor QoL. A deeper understanding of psychiatric comorbidity and its relationship with QoL is needed to better understand how to deliver more effective treatments for these survivors. Limitations include the small sample size, a one-time measurement of psychiatric comorbidities at the 3-month follow-up based on screenings tools, and a lack of objective measures of physical functioning to determine the effects of psychiatric comorbidities on physical functioning. There may also have been differences in how patients with no psychiatric comorbidity responded to the EQ-5D-VAS as a result of premorbid differences (eg, they were healthier prior to their ICU stay and perceived their survivor status more negatively). This may explain why we did not see a statistically significant difference between no psychiatric comorbidity and high psychiatric comorbidity groups on the EQ-5D-VAS. Nevertheless, we did see a difference between the low-moderate psychiatric comorbidity group on EQ-5D-VAS and differences between the no comorbidity and low-moderate comorbidity groups versus the high comorbidity group on the EQ-5D-3L. Finally, data about psychiatric history and QoL prior to ICU hospitalization were limited. Therefore, truly determining incidence versus prevalence of post-ICU comorbidities and whether psychiatric symptoms and its effects on QoL were due to ICU hospitalization or to premorbid psychiatric symptoms is difficult.
Our study demonstrated that in survivors of ICU delirium, higher comorbidity of psychiatric symptoms was associated with poorer QoL. Future studies will need to confirm these findings. We will also need to identify potentially reversible risk factors for psychiatric comorbidity and poorer QoL and develop treatments to effectively target the mental health symptoms of survivors of ICU delirium.
Disclosure
Grant support: The PMD trial is funded through the National Institutes of Health grant R01AG054205-02. SW is supported by NIA 2P30AG010133. AP is supported by CMS 1 L1 CMS331444-02-00, Indiana CTSI, and NIA R01AG054205-02. SG is supported by NIA 2P30AG010133, NIA 5R01AG045350, and NIA R01AG054205-02. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00, NIA R01 AG030618-05A1 and NIA R01AG054205-02. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730. The funding agency had no role in the development of the study design, collection, analysis, interpretation of data, manuscript development, or the decision to submit the manuscript for publication. Conflicts of interest include MB, SG, and AP being funded by NIA R01AG054205-02 for the PMD study.
1. Jutte JE, Erb CT, Jackson JC. Physical, cognitive, and psychological disability following critical illness: what is the risk? Semin Respir Crit Care Med. 2015;36(6):943-958. PubMed
2. Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23-29. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43(3):642-653. PubMed
6. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric Symptoms in Acute Respiratory Distress Syndrome Survivors: A 1-Year National Multicenter Study. Crit Care Med. 2016;44(5):954-965. PubMed
7. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Influence of psychiatric comorbidity on surgical mortality. Arch Surg. 2010;145(10):947-953. PubMed
8. Campbell NL, Khan BA, Farber M, et al. Improving delirium care in the intensive care unit: the design of a pragmatic study. Trials. 2011;12:139. PubMed
9. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. PubMed
10. Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3(6):244–254. PubMed
11. Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression–PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6):711–718. PubMed
12. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813. PubMed
13. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. PubMed
14. Mehlhorn J, Freytag A, Schmidt K, et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42(5):1263-1271. PubMed
15. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115(3):24-31. PubMed
The prevalence of depression, anxiety, and posttraumatic stress disorder (PTSD) symptoms in intensive care unit (ICU) survivors ranges from 17% to 44%.1-4 Psychiatric comorbidity, the presence of 2 or more psychiatric disorders, is highly prevalent in survivors of acute respiratory distress syndrome and is associated with higher mortality in postsurgical ICU survivors.5-7 While long-term cognitive impairment in patients with ICU delirium has been associated with poor quality of life (QoL),1 the effects of psychiatric comorbidity on QoL among similar patients are not as well understood. In this study, we examined whether psychiatric comorbidity was associated with poorer QoL in survivors of ICU delirium.
METHODS
We examined subjects who participated in the Pharmacologic Management of Delirium (PMD) clinical trial. This trial examined the efficacy of a pharmacological intervention for patients who developed ICU delirium at a local tertiary-care academic hospital.8 Out of 62 patients who participated in the follow-up of the PMD study, 58 completed QoL interviews and validated psychiatric screens (Patient Health Questionnaire-9 [PHQ-9] for depression, the Generalized Anxiety Disorder-7 [GAD-7] questionnaire for anxiety, and the Post-Traumatic Stress Syndrome [PTSS-10] questionnaire for PTSD) at 3 months after hospital discharge. High psychiatric comorbidity was defined as having significant symptoms for all 3 conditions (depression: PHQ-9 score ≥ 10; anxiety: GAD-7 ≥ 10; and PTSD: PTSS-10 > 35). No psychiatric morbidity was defined as having no significant symptoms for all 3 conditions. Low to moderate (low-moderate) psychiatric morbidity was defined as having symptoms for 1 to 2 conditions.
Participants also completed 2 complementary QoL measures: the EuroQol 5 dimensions questionnaire 3-level (EQ-5D-3L) Index and the EuroQol 5 dimensions Visual Analog Scale (EQ-5D-VAS).9 The EQ-5D-3L Index asks participants to rate themselves as having (1) no problems, (2) some problems, or (3) extreme problems on the following 5 scales: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The scores are then indexed against the US population to create a continuous index scale ranging from −0.11 to 1.00.
Fisher’s exact tests were used to compare dichotomous outcomes. Analysis of variance (ANOVA) was used to compare continuous outcomes across the 3 psychiatric groups. Analysis of covariance (ANCOVA) was used to determine whether psychiatric comorbidity in survivors of ICU delirium was associated with QoL measures. Models were adjusted for the following covariates: age, gender, Charlson Comorbidity Index, discharged to home, prior history of depression, and prior history of anxiety. To assess the relationship of psychiatric comorbidity with QoL, we chose the 2 continuous QoL measures as the outcome. Because we were interested in the effect of psychiatric burden on QoL, we used ANCOVA with QoL as the dependent variable and psychiatric burden as an independent variable. Pairwise comparisons were then performed when overall differences were significant (P < 0.05). We performed 2 separate sensitivity analyses. The first analysis looked solely at the subgroup of patients from the medical intensive care unit. We also recalculated the EQ-5D-3L index excluding the anxiety/depression item.
RESULTS
Nearly one-third of patients (18/58) had high psychiatric burden. The table looks at the demographic and clinical characteristics of patients with high psychiatric comorbidity versus those of low-moderate psychiatric comorbidity and those with no psychiatric morbidity. Patient groups did not differ significantly in terms of demographics. For clinical characteristics, patients with high psychiatric comorbidity were more likely than patients with low-moderate psychiatric comorbidity to have a prior history of depression (P < 0.05).
Patients with high psychiatric comorbidity were more likely to have a poorer QoL when compared with patients with low-moderate psychiatric comorbidity and to those with no morbidity as measured by a lower EQ-5D-3L Index (no, 0.69 ± 0.25; low-moderate, 0.70 ± 0.19; high, 0.48 ± 0.24; P = 0.006) and EQ-5D-VAS (no, 67.0 ± 20.7; low-moderate, 76.6 ± 20.0; high, 50.8 ± 22.4; P = 0.004). After adjusting for covariates, patients with high psychiatric comorbidity had a poorer QoL compared with those with no morbidity or low-moderate comorbidity on the EQ-5D-3L Index (P = 0.017 for overall differences), whereas patients who had high psychiatric comorbidity had a poorer QoL compared to those with low-moderate comorbidity on the EQ-5D-VAS (P = 0.039 for overall differences; Figure). Subgroup analysis of MICU patients yielded similar results. Patients with high psychiatric burden had significantly poorer QoL as measured by the EQ-5D-3L (unadjusted P = 0.044, adjusted P = 0.003) and the EQ-5D-VAS (unadjusted P = 0.007, adjusted P = 0.021). After excluding the anxiety/depression item from the EQ-5D-3L, we observed similar differences (no, 0.71 ± 0.24; low-moderate, 0.75 ± 0.15; high, 0.58 ± 0.22; unadjusted P = 0.062; adjusted P = 0.040).
DISCUSSION/CONCLUSION
Psychiatric comorbidities in ICU survivors are common and pose a significant clinical issue. Patients with multiple psychiatric comorbidities can be more complicated to identify from a diagnostic standpoint and often require more prolonged, intensive mental health treatment when compared with patients with a single psychiatric disorder.10,11 Our study showed that high psychiatric comorbidity in survivors of ICU delirium is associated with a decreased QoL compared with those with no psychiatric comorbidity or with low-moderate psychiatric comorbidity. This finding is consistent with previous studies in the general population that patients with multiple psychiatric comorbidities are associated with a poorer QoL compared with patients with a single psychiatric comorbidity.10,11
There is a pressing need to better characterize psychiatric comorbidities in ICU survivors because our current evidence suggests that the prevalence of psychiatric comorbidities of ICU survivors is substantially higher than that of the general population. We found that nearly one-third of survivors of ICU delirium had comorbid depression, anxiety, and PTSD symptoms at 3 months. This is consistent with the few other studies of ICU survivors, which showed a prevalence of psychiatric comorbidity of 25% to 33%.5,12 These rates are substantially higher than the prevalence in the general population of 6%.13
The high rate of psychiatric comorbidities may render it difficult to effectively treat the mental health symptoms in ICU survivors.14 Treating multiple psychiatric comorbidities may also be especially challenging in survivors of ICU delirium because they have a high prevalence of cognitive impairment. Mental health treatments for patients with psychiatric disorders and comorbid cognitive impairment are limited. Better characterization of psychiatric comorbidity in ICU survivors, particularly those with ICU delirium, is vital to the development of more effective, bundled treatments for this population with multiple comorbidities.
Standardized screenings of ICU survivors at a high risk for psychiatric disorders, such as survivors of ICU delirium, may help to identify patients with comorbid psychiatric disorder symptoms and have them referred to appropriate treatment earlier with the hope of improving their QoL sooner. Although opportunities to deliver integrated outpatient collaborative mental health and medical care for a subspecialty population are limited, one potential model of care would be to utilize a collaborative-care model in an ICU survivor clinic.15
Strengths of our study include the examination of psychiatric comorbidities in survivors of ICU delirium, who often have a poor QoL. A deeper understanding of psychiatric comorbidity and its relationship with QoL is needed to better understand how to deliver more effective treatments for these survivors. Limitations include the small sample size, a one-time measurement of psychiatric comorbidities at the 3-month follow-up based on screenings tools, and a lack of objective measures of physical functioning to determine the effects of psychiatric comorbidities on physical functioning. There may also have been differences in how patients with no psychiatric comorbidity responded to the EQ-5D-VAS as a result of premorbid differences (eg, they were healthier prior to their ICU stay and perceived their survivor status more negatively). This may explain why we did not see a statistically significant difference between no psychiatric comorbidity and high psychiatric comorbidity groups on the EQ-5D-VAS. Nevertheless, we did see a difference between the low-moderate psychiatric comorbidity group on EQ-5D-VAS and differences between the no comorbidity and low-moderate comorbidity groups versus the high comorbidity group on the EQ-5D-3L. Finally, data about psychiatric history and QoL prior to ICU hospitalization were limited. Therefore, truly determining incidence versus prevalence of post-ICU comorbidities and whether psychiatric symptoms and its effects on QoL were due to ICU hospitalization or to premorbid psychiatric symptoms is difficult.
Our study demonstrated that in survivors of ICU delirium, higher comorbidity of psychiatric symptoms was associated with poorer QoL. Future studies will need to confirm these findings. We will also need to identify potentially reversible risk factors for psychiatric comorbidity and poorer QoL and develop treatments to effectively target the mental health symptoms of survivors of ICU delirium.
Disclosure
Grant support: The PMD trial is funded through the National Institutes of Health grant R01AG054205-02. SW is supported by NIA 2P30AG010133. AP is supported by CMS 1 L1 CMS331444-02-00, Indiana CTSI, and NIA R01AG054205-02. SG is supported by NIA 2P30AG010133, NIA 5R01AG045350, and NIA R01AG054205-02. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00, NIA R01 AG030618-05A1 and NIA R01AG054205-02. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730. The funding agency had no role in the development of the study design, collection, analysis, interpretation of data, manuscript development, or the decision to submit the manuscript for publication. Conflicts of interest include MB, SG, and AP being funded by NIA R01AG054205-02 for the PMD study.
The prevalence of depression, anxiety, and posttraumatic stress disorder (PTSD) symptoms in intensive care unit (ICU) survivors ranges from 17% to 44%.1-4 Psychiatric comorbidity, the presence of 2 or more psychiatric disorders, is highly prevalent in survivors of acute respiratory distress syndrome and is associated with higher mortality in postsurgical ICU survivors.5-7 While long-term cognitive impairment in patients with ICU delirium has been associated with poor quality of life (QoL),1 the effects of psychiatric comorbidity on QoL among similar patients are not as well understood. In this study, we examined whether psychiatric comorbidity was associated with poorer QoL in survivors of ICU delirium.
METHODS
We examined subjects who participated in the Pharmacologic Management of Delirium (PMD) clinical trial. This trial examined the efficacy of a pharmacological intervention for patients who developed ICU delirium at a local tertiary-care academic hospital.8 Out of 62 patients who participated in the follow-up of the PMD study, 58 completed QoL interviews and validated psychiatric screens (Patient Health Questionnaire-9 [PHQ-9] for depression, the Generalized Anxiety Disorder-7 [GAD-7] questionnaire for anxiety, and the Post-Traumatic Stress Syndrome [PTSS-10] questionnaire for PTSD) at 3 months after hospital discharge. High psychiatric comorbidity was defined as having significant symptoms for all 3 conditions (depression: PHQ-9 score ≥ 10; anxiety: GAD-7 ≥ 10; and PTSD: PTSS-10 > 35). No psychiatric morbidity was defined as having no significant symptoms for all 3 conditions. Low to moderate (low-moderate) psychiatric morbidity was defined as having symptoms for 1 to 2 conditions.
Participants also completed 2 complementary QoL measures: the EuroQol 5 dimensions questionnaire 3-level (EQ-5D-3L) Index and the EuroQol 5 dimensions Visual Analog Scale (EQ-5D-VAS).9 The EQ-5D-3L Index asks participants to rate themselves as having (1) no problems, (2) some problems, or (3) extreme problems on the following 5 scales: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The scores are then indexed against the US population to create a continuous index scale ranging from −0.11 to 1.00.
Fisher’s exact tests were used to compare dichotomous outcomes. Analysis of variance (ANOVA) was used to compare continuous outcomes across the 3 psychiatric groups. Analysis of covariance (ANCOVA) was used to determine whether psychiatric comorbidity in survivors of ICU delirium was associated with QoL measures. Models were adjusted for the following covariates: age, gender, Charlson Comorbidity Index, discharged to home, prior history of depression, and prior history of anxiety. To assess the relationship of psychiatric comorbidity with QoL, we chose the 2 continuous QoL measures as the outcome. Because we were interested in the effect of psychiatric burden on QoL, we used ANCOVA with QoL as the dependent variable and psychiatric burden as an independent variable. Pairwise comparisons were then performed when overall differences were significant (P < 0.05). We performed 2 separate sensitivity analyses. The first analysis looked solely at the subgroup of patients from the medical intensive care unit. We also recalculated the EQ-5D-3L index excluding the anxiety/depression item.
RESULTS
Nearly one-third of patients (18/58) had high psychiatric burden. The table looks at the demographic and clinical characteristics of patients with high psychiatric comorbidity versus those of low-moderate psychiatric comorbidity and those with no psychiatric morbidity. Patient groups did not differ significantly in terms of demographics. For clinical characteristics, patients with high psychiatric comorbidity were more likely than patients with low-moderate psychiatric comorbidity to have a prior history of depression (P < 0.05).
Patients with high psychiatric comorbidity were more likely to have a poorer QoL when compared with patients with low-moderate psychiatric comorbidity and to those with no morbidity as measured by a lower EQ-5D-3L Index (no, 0.69 ± 0.25; low-moderate, 0.70 ± 0.19; high, 0.48 ± 0.24; P = 0.006) and EQ-5D-VAS (no, 67.0 ± 20.7; low-moderate, 76.6 ± 20.0; high, 50.8 ± 22.4; P = 0.004). After adjusting for covariates, patients with high psychiatric comorbidity had a poorer QoL compared with those with no morbidity or low-moderate comorbidity on the EQ-5D-3L Index (P = 0.017 for overall differences), whereas patients who had high psychiatric comorbidity had a poorer QoL compared to those with low-moderate comorbidity on the EQ-5D-VAS (P = 0.039 for overall differences; Figure). Subgroup analysis of MICU patients yielded similar results. Patients with high psychiatric burden had significantly poorer QoL as measured by the EQ-5D-3L (unadjusted P = 0.044, adjusted P = 0.003) and the EQ-5D-VAS (unadjusted P = 0.007, adjusted P = 0.021). After excluding the anxiety/depression item from the EQ-5D-3L, we observed similar differences (no, 0.71 ± 0.24; low-moderate, 0.75 ± 0.15; high, 0.58 ± 0.22; unadjusted P = 0.062; adjusted P = 0.040).
DISCUSSION/CONCLUSION
Psychiatric comorbidities in ICU survivors are common and pose a significant clinical issue. Patients with multiple psychiatric comorbidities can be more complicated to identify from a diagnostic standpoint and often require more prolonged, intensive mental health treatment when compared with patients with a single psychiatric disorder.10,11 Our study showed that high psychiatric comorbidity in survivors of ICU delirium is associated with a decreased QoL compared with those with no psychiatric comorbidity or with low-moderate psychiatric comorbidity. This finding is consistent with previous studies in the general population that patients with multiple psychiatric comorbidities are associated with a poorer QoL compared with patients with a single psychiatric comorbidity.10,11
There is a pressing need to better characterize psychiatric comorbidities in ICU survivors because our current evidence suggests that the prevalence of psychiatric comorbidities of ICU survivors is substantially higher than that of the general population. We found that nearly one-third of survivors of ICU delirium had comorbid depression, anxiety, and PTSD symptoms at 3 months. This is consistent with the few other studies of ICU survivors, which showed a prevalence of psychiatric comorbidity of 25% to 33%.5,12 These rates are substantially higher than the prevalence in the general population of 6%.13
The high rate of psychiatric comorbidities may render it difficult to effectively treat the mental health symptoms in ICU survivors.14 Treating multiple psychiatric comorbidities may also be especially challenging in survivors of ICU delirium because they have a high prevalence of cognitive impairment. Mental health treatments for patients with psychiatric disorders and comorbid cognitive impairment are limited. Better characterization of psychiatric comorbidity in ICU survivors, particularly those with ICU delirium, is vital to the development of more effective, bundled treatments for this population with multiple comorbidities.
Standardized screenings of ICU survivors at a high risk for psychiatric disorders, such as survivors of ICU delirium, may help to identify patients with comorbid psychiatric disorder symptoms and have them referred to appropriate treatment earlier with the hope of improving their QoL sooner. Although opportunities to deliver integrated outpatient collaborative mental health and medical care for a subspecialty population are limited, one potential model of care would be to utilize a collaborative-care model in an ICU survivor clinic.15
Strengths of our study include the examination of psychiatric comorbidities in survivors of ICU delirium, who often have a poor QoL. A deeper understanding of psychiatric comorbidity and its relationship with QoL is needed to better understand how to deliver more effective treatments for these survivors. Limitations include the small sample size, a one-time measurement of psychiatric comorbidities at the 3-month follow-up based on screenings tools, and a lack of objective measures of physical functioning to determine the effects of psychiatric comorbidities on physical functioning. There may also have been differences in how patients with no psychiatric comorbidity responded to the EQ-5D-VAS as a result of premorbid differences (eg, they were healthier prior to their ICU stay and perceived their survivor status more negatively). This may explain why we did not see a statistically significant difference between no psychiatric comorbidity and high psychiatric comorbidity groups on the EQ-5D-VAS. Nevertheless, we did see a difference between the low-moderate psychiatric comorbidity group on EQ-5D-VAS and differences between the no comorbidity and low-moderate comorbidity groups versus the high comorbidity group on the EQ-5D-3L. Finally, data about psychiatric history and QoL prior to ICU hospitalization were limited. Therefore, truly determining incidence versus prevalence of post-ICU comorbidities and whether psychiatric symptoms and its effects on QoL were due to ICU hospitalization or to premorbid psychiatric symptoms is difficult.
Our study demonstrated that in survivors of ICU delirium, higher comorbidity of psychiatric symptoms was associated with poorer QoL. Future studies will need to confirm these findings. We will also need to identify potentially reversible risk factors for psychiatric comorbidity and poorer QoL and develop treatments to effectively target the mental health symptoms of survivors of ICU delirium.
Disclosure
Grant support: The PMD trial is funded through the National Institutes of Health grant R01AG054205-02. SW is supported by NIA 2P30AG010133. AP is supported by CMS 1 L1 CMS331444-02-00, Indiana CTSI, and NIA R01AG054205-02. SG is supported by NIA 2P30AG010133, NIA 5R01AG045350, and NIA R01AG054205-02. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00, NIA R01 AG030618-05A1 and NIA R01AG054205-02. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730. The funding agency had no role in the development of the study design, collection, analysis, interpretation of data, manuscript development, or the decision to submit the manuscript for publication. Conflicts of interest include MB, SG, and AP being funded by NIA R01AG054205-02 for the PMD study.
1. Jutte JE, Erb CT, Jackson JC. Physical, cognitive, and psychological disability following critical illness: what is the risk? Semin Respir Crit Care Med. 2015;36(6):943-958. PubMed
2. Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23-29. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43(3):642-653. PubMed
6. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric Symptoms in Acute Respiratory Distress Syndrome Survivors: A 1-Year National Multicenter Study. Crit Care Med. 2016;44(5):954-965. PubMed
7. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Influence of psychiatric comorbidity on surgical mortality. Arch Surg. 2010;145(10):947-953. PubMed
8. Campbell NL, Khan BA, Farber M, et al. Improving delirium care in the intensive care unit: the design of a pragmatic study. Trials. 2011;12:139. PubMed
9. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. PubMed
10. Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3(6):244–254. PubMed
11. Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression–PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6):711–718. PubMed
12. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813. PubMed
13. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. PubMed
14. Mehlhorn J, Freytag A, Schmidt K, et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42(5):1263-1271. PubMed
15. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115(3):24-31. PubMed
1. Jutte JE, Erb CT, Jackson JC. Physical, cognitive, and psychological disability following critical illness: what is the risk? Semin Respir Crit Care Med. 2015;36(6):943-958. PubMed
2. Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23-29. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Parker AM, Sricharoenchai T, Raparla S, Schneck KW, Bienvenu OJ, Needham DM. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121-1129. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43(3):642-653. PubMed
6. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric Symptoms in Acute Respiratory Distress Syndrome Survivors: A 1-Year National Multicenter Study. Crit Care Med. 2016;44(5):954-965. PubMed
7. Abrams TE, Vaughan-Sarrazin M, Rosenthal GE. Influence of psychiatric comorbidity on surgical mortality. Arch Surg. 2010;145(10):947-953. PubMed
8. Campbell NL, Khan BA, Farber M, et al. Improving delirium care in the intensive care unit: the design of a pragmatic study. Trials. 2011;12:139. PubMed
9. EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. PubMed
10. Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3(6):244–254. PubMed
11. Campbell DG, Felker BL, Liu CF, et al. Prevalence of depression–PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. 2007;22(6):711–718. PubMed
12. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813. PubMed
13. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. PubMed
14. Mehlhorn J, Freytag A, Schmidt K, et al. Rehabilitation interventions for postintensive care syndrome: a systematic review. Crit Care Med. 2014;42(5):1263-1271. PubMed
15. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115(3):24-31. PubMed
© 2017 Society of Hospital Medicine
Antidepressant Use and Depressive Symptoms in Intensive Care Unit Survivors
As the number of intensive care unit (ICU) survivors has steadily increased over the past few decades, there is growing awareness of the long-term physical, cognitive, and psychological impairments after ICU hospitalization, collectively known as post–intensive care syndrome (PICS).1 Systematic reviews based mostly on research studies suggest that the prevalence of depressive symptoms 2-12 months after ICU discharge is nearly 30%.2-5 Due to the scarcity of established models of care for ICU survivors, there is limited characterization of depressive symptoms and antidepressant regimens in this clinical population. The Critical Care Recovery Center (CCRC) at Eskenazi Hospital is one of the first ICU survivor clinics in the United States and targets a racially diverse, underserved population in the Indianapolis metropolitan area.6 In this study, we examined whether patients had depressive symptoms at their initial CCRC visit, and whether the risk factors for depressive symptoms differed if they were on an antidepressant at their initial CCRC visit.
METHODS
Referral criteria to the CCRC were 18 years or older, admitted to the Eskenazi ICU, were on mechanical ventilation or delirious for ≥48 hours (major risk factors for the development of PICS), and recommended for follow-up by a critical care physician. The exclusion criterion included was enrollment in hospice or palliative care services. Institutional review board approval was obtained to conduct retrospective analyses of de-identified clinical data. Medical history and medication lists were collected from patients, informal caregivers, and electronic medical records.
Two hundred thirty-three patients were seen in the CCRC from July 2011 to August 2016. Two hundred four patients rated symptoms of depression with either the Patient Health Questionnaire (PHQ-9; N = 99) or Geriatric Depression Scale (GDS-30; N = 105) at their initial visit to the CCRC prior to receiving any treatment at the CCRC. Twenty-nine patients who did not complete depression questionnaires were excluded from the analyses. Patients with PHQ-9 score ≥10 or GDS score ≥20 were categorized as having moderate to severe depressive symptoms.7,8
Electronic medical records were reviewed to determine whether patients were on an antidepressant at hospital admission, hospital discharge, and the initial CCRC visit prior to any treatment in the CCRC. Patients who were on a tricyclic antidepressant, selective serotonin reuptake inhibitor, selective serotonin-norepinephrine reuptake inhibitor, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), or norepinephrine and dopaminergic reuptake inhibitor (eg, bupropion) at any dose were designated as being on an antidepressant. Prescribers of antidepressants included primary care providers, clinical providers during their hospital stay, and various outpatient subspecialists other than those in the CCRC.
We then examined whether the risk factors for depressive symptoms differed if patients were on an antidepressant at their initial CCRC visit. We compared demographic and clinical characteristics between depressed and nondepressed patients not on an antidepressant. We repeated these analyses for those on an antidepressant. Dichotomous outcomes were compared using chi-square testing, and two-way Student t tests for continuous outcomes. Demographic and clinical variables with P < 0.1 were included as covariates in a logistic regression model for depressive symptoms separately for those not an antidepressant and those on an antidepressant. History of depression was not included as a covariate because it is highly collinear with post-ICU depression.
RESULTS
Two hundred four ICU survivors in this study reflected a racially diverse and underserved population (monthly income $745.3 ± $931.5). Although most had respiratory failure and/or delirium during their hospital stay, 94.1% (N = 160) mostly lived independently after discharge. Nearly one-third of patients (N = 69) were on at least 1 antidepressant at their initial CCRC visit. Of these 69 patients, 60.9% (N = 42) had an antidepressant prescription on hospital admission, and 60.9% (N = 42) had an antidepressant prescription on hospital discharge.
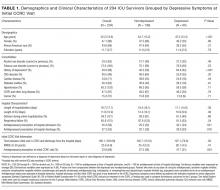
We first compared the demographic and clinical characteristics of patients with and without depressive symptoms at their initial CCRC visit. Patients with depressive symptoms were younger, less likely to have cardiac disease, more likely to have a history of depression, more likely to have been prescribed an antidepressant on hospital admission, more likely to be prescribed an antidepressant on hospital discharge, and more likely to be on an antidepressant at their initial CCRC visit (Table 1).
Patients with depressive symptoms on an antidepressant (n = 65) were younger and more likely to be African American (borderline significance; Supplementary Table 2). Multivariate logistic regression showed that both younger age (OR = 0.92 per year, P = 0.003) and African American race (OR = 4.3, P = 0.024) remained significantly associated with depressive symptoms (Table 2).
DISCUSSION
Our study demonstrated that about one-third of our ICU survivor clinical cohort had untreated or inadequately treated depressive symptoms at their CCRC initial visit. Many patients with depressive symptoms had a history of depression and/or antidepressant prescription on hospital admission. This suggests that pre-ICU depression is a major contributor to post-ICU depression. These findings are consistent with the results of a large retrospective analysis of Danish ICU survivors that found that patients were more likely to have premorbid psychiatric diagnoses, compared with the general population.9 Another ICU survivor research study that excluded patients who were on antidepressants prior to ICU hospitalization found that 49% of these patients were on an antidepressant after their ICU stay.10 Our much lower rate of patients on an antidepressant after their ICU stay may reflect the differences between patient populations, differences in healthcare systems, and differences in clinician prescribing practices.
Younger age was associated with a higher likelihood of depressive symptoms independent of antidepressant status. Findings about the relationship between age and post-ICU depression have varied. The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors group found that older age was associated with more depressive symptoms at 12 months postdischarge.11 On the other hand, a systematic review of post-ICU depression did not find any relationship between age and post-ICU depression.2,3 These differences may be due in part to demographic variations in cohorts.
Our logistic regression models suggest that there may also be different risk factors in patients who had untreated vs inadequately treated depressive symptoms. Patients who were not on an antidepressant at their initial CCRC visit were more likely to have a lower level of education. This is consistent with the Medical Expenditure Panel Surveys study, which showed that adults with less than a high school education were less likely to receive depression treatment.12 In patients who were on antidepressants at their initial CCRC visit, African Americans were more likely to have depressive symptoms. Possible reasons may include differences in receiving guideline-concordant antidepressant medication treatment, access to mental health subspecialty services, higher prevalence of treatment refractory depression, and differences in responses to antidepressant treatments.13,14
Strengths of our study include detailed characterization for a fairly large ICU survivor clinic population and a racially diverse cohort. To the best of our knowledge, our study is also the first to examine whether there may be different risk factors for depressive symptoms based on antidepressant status. Limitations include the lack of information about nonpharmacologic antidepressant treatment and the inability to assess whether noncompliance, insufficient dose, or insufficient time on antidepressants contributed to inadequate antidepressant treatment. Antidepressants may have also been prescribed for other purposes such as smoking cessation, neuropathic pain, and migraine headaches. However, because 72.4% of patients on antidepressants had a history of depression, it is likely that most of them were on antidepressants to treat depression.
Other limitations include potential biases in our clinical cohort. Over the last 5 years, the CCRC has provided care to more than 200 ICU survivors. With 1100 mechanically ventilated admissions per year, only 1.8% of survivors are seen. The referral criteria for the CCRC is a major source of selection bias, which likely overrepresents PICS. Because patients are seen in the CCRC about 3 months after hospital discharge, there is also informant censoring due to death. Physically sicker survivors in nursing home facilities were less likely to be included. Finally, the small cohort size may have resulted in an underpowered study.
Future studies will need to confirm our findings about the high prevalence of post-ICU depression and different responses to antidepressant medications by certain groups. Pre-ICU depression, lack of antidepressant treatment, and inadequate antidepressant treatment are major causes of post-ICU depression. Currently, the CCRC offers pharmacotherapy, problem-solving therapy, or referral to mental health specialists to treat patients with depressive symptoms. ICU survivor clinics, such as the CCRC, may become important settings that allow for increased access to depression treatment for those at higher risk for post-ICU depression as well as the testing of new antidepressant regimens for those with inadequately treated depression.
Acknowledgments
The authors thank Dr. Adil Sheikh for assistance with data entry and Cynthia Reynolds for her clinical services. Grant support: The Critical Care Recovery Center (CCRC) is supported by Eskenazi Health Services. SW is supported by NIA 2P30AG010133. SG is supported by NIA 2P30AG010133 and NIA 5R01AG045350. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00 and NIA R01 AG030618-05A1. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730.
Disclosure
There are no conflicts of interest. None of the above NIH grants supported the CCRC or this work.
1. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. PubMed
2. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796-809. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med 2016;44:954-965. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642-653. PubMed
6. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115:24-31. PubMed
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. PubMed
8. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. PubMed
9. Wuns ch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311:1133-1142. PubMed
10. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399-407. PubMed
11. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369-379. PubMed
12. Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482-1491. PubMed
13. González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67:37-46. PubMed
14. Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548-557. PubMed
As the number of intensive care unit (ICU) survivors has steadily increased over the past few decades, there is growing awareness of the long-term physical, cognitive, and psychological impairments after ICU hospitalization, collectively known as post–intensive care syndrome (PICS).1 Systematic reviews based mostly on research studies suggest that the prevalence of depressive symptoms 2-12 months after ICU discharge is nearly 30%.2-5 Due to the scarcity of established models of care for ICU survivors, there is limited characterization of depressive symptoms and antidepressant regimens in this clinical population. The Critical Care Recovery Center (CCRC) at Eskenazi Hospital is one of the first ICU survivor clinics in the United States and targets a racially diverse, underserved population in the Indianapolis metropolitan area.6 In this study, we examined whether patients had depressive symptoms at their initial CCRC visit, and whether the risk factors for depressive symptoms differed if they were on an antidepressant at their initial CCRC visit.
METHODS
Referral criteria to the CCRC were 18 years or older, admitted to the Eskenazi ICU, were on mechanical ventilation or delirious for ≥48 hours (major risk factors for the development of PICS), and recommended for follow-up by a critical care physician. The exclusion criterion included was enrollment in hospice or palliative care services. Institutional review board approval was obtained to conduct retrospective analyses of de-identified clinical data. Medical history and medication lists were collected from patients, informal caregivers, and electronic medical records.
Two hundred thirty-three patients were seen in the CCRC from July 2011 to August 2016. Two hundred four patients rated symptoms of depression with either the Patient Health Questionnaire (PHQ-9; N = 99) or Geriatric Depression Scale (GDS-30; N = 105) at their initial visit to the CCRC prior to receiving any treatment at the CCRC. Twenty-nine patients who did not complete depression questionnaires were excluded from the analyses. Patients with PHQ-9 score ≥10 or GDS score ≥20 were categorized as having moderate to severe depressive symptoms.7,8
Electronic medical records were reviewed to determine whether patients were on an antidepressant at hospital admission, hospital discharge, and the initial CCRC visit prior to any treatment in the CCRC. Patients who were on a tricyclic antidepressant, selective serotonin reuptake inhibitor, selective serotonin-norepinephrine reuptake inhibitor, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), or norepinephrine and dopaminergic reuptake inhibitor (eg, bupropion) at any dose were designated as being on an antidepressant. Prescribers of antidepressants included primary care providers, clinical providers during their hospital stay, and various outpatient subspecialists other than those in the CCRC.
We then examined whether the risk factors for depressive symptoms differed if patients were on an antidepressant at their initial CCRC visit. We compared demographic and clinical characteristics between depressed and nondepressed patients not on an antidepressant. We repeated these analyses for those on an antidepressant. Dichotomous outcomes were compared using chi-square testing, and two-way Student t tests for continuous outcomes. Demographic and clinical variables with P < 0.1 were included as covariates in a logistic regression model for depressive symptoms separately for those not an antidepressant and those on an antidepressant. History of depression was not included as a covariate because it is highly collinear with post-ICU depression.
RESULTS
Two hundred four ICU survivors in this study reflected a racially diverse and underserved population (monthly income $745.3 ± $931.5). Although most had respiratory failure and/or delirium during their hospital stay, 94.1% (N = 160) mostly lived independently after discharge. Nearly one-third of patients (N = 69) were on at least 1 antidepressant at their initial CCRC visit. Of these 69 patients, 60.9% (N = 42) had an antidepressant prescription on hospital admission, and 60.9% (N = 42) had an antidepressant prescription on hospital discharge.
We first compared the demographic and clinical characteristics of patients with and without depressive symptoms at their initial CCRC visit. Patients with depressive symptoms were younger, less likely to have cardiac disease, more likely to have a history of depression, more likely to have been prescribed an antidepressant on hospital admission, more likely to be prescribed an antidepressant on hospital discharge, and more likely to be on an antidepressant at their initial CCRC visit (Table 1).
Patients with depressive symptoms on an antidepressant (n = 65) were younger and more likely to be African American (borderline significance; Supplementary Table 2). Multivariate logistic regression showed that both younger age (OR = 0.92 per year, P = 0.003) and African American race (OR = 4.3, P = 0.024) remained significantly associated with depressive symptoms (Table 2).
DISCUSSION
Our study demonstrated that about one-third of our ICU survivor clinical cohort had untreated or inadequately treated depressive symptoms at their CCRC initial visit. Many patients with depressive symptoms had a history of depression and/or antidepressant prescription on hospital admission. This suggests that pre-ICU depression is a major contributor to post-ICU depression. These findings are consistent with the results of a large retrospective analysis of Danish ICU survivors that found that patients were more likely to have premorbid psychiatric diagnoses, compared with the general population.9 Another ICU survivor research study that excluded patients who were on antidepressants prior to ICU hospitalization found that 49% of these patients were on an antidepressant after their ICU stay.10 Our much lower rate of patients on an antidepressant after their ICU stay may reflect the differences between patient populations, differences in healthcare systems, and differences in clinician prescribing practices.
Younger age was associated with a higher likelihood of depressive symptoms independent of antidepressant status. Findings about the relationship between age and post-ICU depression have varied. The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors group found that older age was associated with more depressive symptoms at 12 months postdischarge.11 On the other hand, a systematic review of post-ICU depression did not find any relationship between age and post-ICU depression.2,3 These differences may be due in part to demographic variations in cohorts.
Our logistic regression models suggest that there may also be different risk factors in patients who had untreated vs inadequately treated depressive symptoms. Patients who were not on an antidepressant at their initial CCRC visit were more likely to have a lower level of education. This is consistent with the Medical Expenditure Panel Surveys study, which showed that adults with less than a high school education were less likely to receive depression treatment.12 In patients who were on antidepressants at their initial CCRC visit, African Americans were more likely to have depressive symptoms. Possible reasons may include differences in receiving guideline-concordant antidepressant medication treatment, access to mental health subspecialty services, higher prevalence of treatment refractory depression, and differences in responses to antidepressant treatments.13,14
Strengths of our study include detailed characterization for a fairly large ICU survivor clinic population and a racially diverse cohort. To the best of our knowledge, our study is also the first to examine whether there may be different risk factors for depressive symptoms based on antidepressant status. Limitations include the lack of information about nonpharmacologic antidepressant treatment and the inability to assess whether noncompliance, insufficient dose, or insufficient time on antidepressants contributed to inadequate antidepressant treatment. Antidepressants may have also been prescribed for other purposes such as smoking cessation, neuropathic pain, and migraine headaches. However, because 72.4% of patients on antidepressants had a history of depression, it is likely that most of them were on antidepressants to treat depression.
Other limitations include potential biases in our clinical cohort. Over the last 5 years, the CCRC has provided care to more than 200 ICU survivors. With 1100 mechanically ventilated admissions per year, only 1.8% of survivors are seen. The referral criteria for the CCRC is a major source of selection bias, which likely overrepresents PICS. Because patients are seen in the CCRC about 3 months after hospital discharge, there is also informant censoring due to death. Physically sicker survivors in nursing home facilities were less likely to be included. Finally, the small cohort size may have resulted in an underpowered study.
Future studies will need to confirm our findings about the high prevalence of post-ICU depression and different responses to antidepressant medications by certain groups. Pre-ICU depression, lack of antidepressant treatment, and inadequate antidepressant treatment are major causes of post-ICU depression. Currently, the CCRC offers pharmacotherapy, problem-solving therapy, or referral to mental health specialists to treat patients with depressive symptoms. ICU survivor clinics, such as the CCRC, may become important settings that allow for increased access to depression treatment for those at higher risk for post-ICU depression as well as the testing of new antidepressant regimens for those with inadequately treated depression.
Acknowledgments
The authors thank Dr. Adil Sheikh for assistance with data entry and Cynthia Reynolds for her clinical services. Grant support: The Critical Care Recovery Center (CCRC) is supported by Eskenazi Health Services. SW is supported by NIA 2P30AG010133. SG is supported by NIA 2P30AG010133 and NIA 5R01AG045350. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00 and NIA R01 AG030618-05A1. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730.
Disclosure
There are no conflicts of interest. None of the above NIH grants supported the CCRC or this work.
As the number of intensive care unit (ICU) survivors has steadily increased over the past few decades, there is growing awareness of the long-term physical, cognitive, and psychological impairments after ICU hospitalization, collectively known as post–intensive care syndrome (PICS).1 Systematic reviews based mostly on research studies suggest that the prevalence of depressive symptoms 2-12 months after ICU discharge is nearly 30%.2-5 Due to the scarcity of established models of care for ICU survivors, there is limited characterization of depressive symptoms and antidepressant regimens in this clinical population. The Critical Care Recovery Center (CCRC) at Eskenazi Hospital is one of the first ICU survivor clinics in the United States and targets a racially diverse, underserved population in the Indianapolis metropolitan area.6 In this study, we examined whether patients had depressive symptoms at their initial CCRC visit, and whether the risk factors for depressive symptoms differed if they were on an antidepressant at their initial CCRC visit.
METHODS
Referral criteria to the CCRC were 18 years or older, admitted to the Eskenazi ICU, were on mechanical ventilation or delirious for ≥48 hours (major risk factors for the development of PICS), and recommended for follow-up by a critical care physician. The exclusion criterion included was enrollment in hospice or palliative care services. Institutional review board approval was obtained to conduct retrospective analyses of de-identified clinical data. Medical history and medication lists were collected from patients, informal caregivers, and electronic medical records.
Two hundred thirty-three patients were seen in the CCRC from July 2011 to August 2016. Two hundred four patients rated symptoms of depression with either the Patient Health Questionnaire (PHQ-9; N = 99) or Geriatric Depression Scale (GDS-30; N = 105) at their initial visit to the CCRC prior to receiving any treatment at the CCRC. Twenty-nine patients who did not complete depression questionnaires were excluded from the analyses. Patients with PHQ-9 score ≥10 or GDS score ≥20 were categorized as having moderate to severe depressive symptoms.7,8
Electronic medical records were reviewed to determine whether patients were on an antidepressant at hospital admission, hospital discharge, and the initial CCRC visit prior to any treatment in the CCRC. Patients who were on a tricyclic antidepressant, selective serotonin reuptake inhibitor, selective serotonin-norepinephrine reuptake inhibitor, noradrenergic and specific serotonergic antidepressant (eg, mirtazapine), or norepinephrine and dopaminergic reuptake inhibitor (eg, bupropion) at any dose were designated as being on an antidepressant. Prescribers of antidepressants included primary care providers, clinical providers during their hospital stay, and various outpatient subspecialists other than those in the CCRC.
We then examined whether the risk factors for depressive symptoms differed if patients were on an antidepressant at their initial CCRC visit. We compared demographic and clinical characteristics between depressed and nondepressed patients not on an antidepressant. We repeated these analyses for those on an antidepressant. Dichotomous outcomes were compared using chi-square testing, and two-way Student t tests for continuous outcomes. Demographic and clinical variables with P < 0.1 were included as covariates in a logistic regression model for depressive symptoms separately for those not an antidepressant and those on an antidepressant. History of depression was not included as a covariate because it is highly collinear with post-ICU depression.
RESULTS
Two hundred four ICU survivors in this study reflected a racially diverse and underserved population (monthly income $745.3 ± $931.5). Although most had respiratory failure and/or delirium during their hospital stay, 94.1% (N = 160) mostly lived independently after discharge. Nearly one-third of patients (N = 69) were on at least 1 antidepressant at their initial CCRC visit. Of these 69 patients, 60.9% (N = 42) had an antidepressant prescription on hospital admission, and 60.9% (N = 42) had an antidepressant prescription on hospital discharge.
We first compared the demographic and clinical characteristics of patients with and without depressive symptoms at their initial CCRC visit. Patients with depressive symptoms were younger, less likely to have cardiac disease, more likely to have a history of depression, more likely to have been prescribed an antidepressant on hospital admission, more likely to be prescribed an antidepressant on hospital discharge, and more likely to be on an antidepressant at their initial CCRC visit (Table 1).
Patients with depressive symptoms on an antidepressant (n = 65) were younger and more likely to be African American (borderline significance; Supplementary Table 2). Multivariate logistic regression showed that both younger age (OR = 0.92 per year, P = 0.003) and African American race (OR = 4.3, P = 0.024) remained significantly associated with depressive symptoms (Table 2).
DISCUSSION
Our study demonstrated that about one-third of our ICU survivor clinical cohort had untreated or inadequately treated depressive symptoms at their CCRC initial visit. Many patients with depressive symptoms had a history of depression and/or antidepressant prescription on hospital admission. This suggests that pre-ICU depression is a major contributor to post-ICU depression. These findings are consistent with the results of a large retrospective analysis of Danish ICU survivors that found that patients were more likely to have premorbid psychiatric diagnoses, compared with the general population.9 Another ICU survivor research study that excluded patients who were on antidepressants prior to ICU hospitalization found that 49% of these patients were on an antidepressant after their ICU stay.10 Our much lower rate of patients on an antidepressant after their ICU stay may reflect the differences between patient populations, differences in healthcare systems, and differences in clinician prescribing practices.
Younger age was associated with a higher likelihood of depressive symptoms independent of antidepressant status. Findings about the relationship between age and post-ICU depression have varied. The Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors group found that older age was associated with more depressive symptoms at 12 months postdischarge.11 On the other hand, a systematic review of post-ICU depression did not find any relationship between age and post-ICU depression.2,3 These differences may be due in part to demographic variations in cohorts.
Our logistic regression models suggest that there may also be different risk factors in patients who had untreated vs inadequately treated depressive symptoms. Patients who were not on an antidepressant at their initial CCRC visit were more likely to have a lower level of education. This is consistent with the Medical Expenditure Panel Surveys study, which showed that adults with less than a high school education were less likely to receive depression treatment.12 In patients who were on antidepressants at their initial CCRC visit, African Americans were more likely to have depressive symptoms. Possible reasons may include differences in receiving guideline-concordant antidepressant medication treatment, access to mental health subspecialty services, higher prevalence of treatment refractory depression, and differences in responses to antidepressant treatments.13,14
Strengths of our study include detailed characterization for a fairly large ICU survivor clinic population and a racially diverse cohort. To the best of our knowledge, our study is also the first to examine whether there may be different risk factors for depressive symptoms based on antidepressant status. Limitations include the lack of information about nonpharmacologic antidepressant treatment and the inability to assess whether noncompliance, insufficient dose, or insufficient time on antidepressants contributed to inadequate antidepressant treatment. Antidepressants may have also been prescribed for other purposes such as smoking cessation, neuropathic pain, and migraine headaches. However, because 72.4% of patients on antidepressants had a history of depression, it is likely that most of them were on antidepressants to treat depression.
Other limitations include potential biases in our clinical cohort. Over the last 5 years, the CCRC has provided care to more than 200 ICU survivors. With 1100 mechanically ventilated admissions per year, only 1.8% of survivors are seen. The referral criteria for the CCRC is a major source of selection bias, which likely overrepresents PICS. Because patients are seen in the CCRC about 3 months after hospital discharge, there is also informant censoring due to death. Physically sicker survivors in nursing home facilities were less likely to be included. Finally, the small cohort size may have resulted in an underpowered study.
Future studies will need to confirm our findings about the high prevalence of post-ICU depression and different responses to antidepressant medications by certain groups. Pre-ICU depression, lack of antidepressant treatment, and inadequate antidepressant treatment are major causes of post-ICU depression. Currently, the CCRC offers pharmacotherapy, problem-solving therapy, or referral to mental health specialists to treat patients with depressive symptoms. ICU survivor clinics, such as the CCRC, may become important settings that allow for increased access to depression treatment for those at higher risk for post-ICU depression as well as the testing of new antidepressant regimens for those with inadequately treated depression.
Acknowledgments
The authors thank Dr. Adil Sheikh for assistance with data entry and Cynthia Reynolds for her clinical services. Grant support: The Critical Care Recovery Center (CCRC) is supported by Eskenazi Health Services. SW is supported by NIA 2P30AG010133. SG is supported by NIA 2P30AG010133 and NIA 5R01AG045350. SK is supported by NHBLI 5T32HL091816-07. MB is supported by NIA R01 AG040220-05, AHRQ P30 HS024384-02, CMS 1 L1 CMS331444-02-00 and NIA R01 AG030618-05A1. BK is supported by NIA K23-AG043476 and NHLBI R01HL131730.
Disclosure
There are no conflicts of interest. None of the above NIH grants supported the CCRC or this work.
1. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. PubMed
2. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796-809. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med 2016;44:954-965. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642-653. PubMed
6. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115:24-31. PubMed
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. PubMed
8. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. PubMed
9. Wuns ch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311:1133-1142. PubMed
10. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399-407. PubMed
11. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369-379. PubMed
12. Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482-1491. PubMed
13. González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67:37-46. PubMed
14. Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548-557. PubMed
1. Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40:502-509. PubMed
2. Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796-809. PubMed
3. Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744-1753. PubMed
4. Huang M, Parker AM, Bienvenu OJ, et al. Psychiatric symptoms in acute respiratory distress syndrome survivors: A 1-year national multicenter study. Crit Care Med 2016;44:954-965. PubMed
5. Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, et al. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43:642-653. PubMed
6. Khan BA, Lasiter S, Boustani MA. CE: critical care recovery center: an innovative collaborative care model for ICU survivors. Am J Nurs. 2015;115:24-31. PubMed
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. PubMed
8. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17:37-49. PubMed
9. Wuns ch H, Christiansen CF, Johansen MB, et al. Psychiatric diagnoses and psychoactive medication use among nonsurgical critically ill patients receiving mechanical ventilation. JAMA. 2014;311:1133-1142. PubMed
10. Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399-407. PubMed
11. Jackson JC, Pandharipande PP, Girard TD, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369-379. PubMed
12. Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482-1491. PubMed
13. González HM, Vega WA, Williams DR, Tarraf W, West BT, Neighbors HW. Depression care in the United States: too little for too few. Arch Gen Psychiatry. 2010;67:37-46. PubMed
14. Bailey RK, Patel M, Barker NC, Ali S, Jabeen S. Major depressive disorder in the African American population. J Natl Med Assoc. 2011;103:548-557. PubMed
© 2017 Society of Hospital Medicine
Impact of Inpatient GCS on CI Patients
Under the Patient Protection and Affordable Care Act of 2010, commonly referred to as the Affordable Care Act, hospitals face up to a 3% penalty in Medicare reimbursements for patients readmitted within 30 days of initial discharge, and measures have been proposed for modifying payments to hospitals based on their performance on this metric.[1] Cognitive impairment (CI) is considered a major risk factor for poor postdischarge outcomes including mortality and hospital readmission.[2, 3] Hospitals are seeking strategies to reduce postdischarge mortality and rehospitalization among patients with and without CI.[4] Such strategies include use of transitional care coaches, patient and caregiver education, postdischarge follow‐up, and provision of geriatric consultative services (GCS) for the care of complex patients in the hospital setting.[5, 6, 7]
GCS utilize comprehensive geriatric assessments and multidisciplinary processes to recognize and modify risk factors that may lead to poor outcomes among hospitalized patients.[8, 9, 10, 11] Implementation of GCS models including Acute Care for Elders and, recently, the Mobile Acute Care of the Elderly services have shown many benefits among older patients including a reduction in the hospital length of stay and readmission rates.[12, 13] The benefits of such services among hospitalized elders suffering from CI, however, are not well established. The objective of this article was to evaluate the impact of GCS on the readmission and mortality rates of older adults with CI within 12 months of their hospitalization to an urban, public hospital. We hypothesized that GCS will reduce both 12‐month hospital readmissions and mortality rates among this vulnerable group of older adults.
METHODS
The study was approved by the Indiana University institutional review board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Setting
The study was conducted at Eskenazi hospital, Indianapolis, Indiana, a 340‐bed, university‐affiliated, public hospital with over 2300 admissions of patients aged 65 years or older every year.
Population
Four hundred fifteen hospitalized patients aged 65 years or older suffering from CI were enrolled into an original, randomized, controlled trial that evaluated the effect of a computerized decision support system on their quality and outcome of care between July 1, 2006 and May 30, 2008.[14] The computerized decision support included reminders for physicians to reduce the prescription of 18 anticholinergics, minimize physical restraints and Foley catheterization, and increase referral to the local GCS.[15] That previous trial neither showed an impact on quality of care nor health utilization among older patients, including mortality and hospital readmission rates. The current study uses the data from the clinical trial cohort to evaluate the effect of GCS on the 12‐month mortality and hospital readmission rates for hospitalized elders with CI (Figure 1).
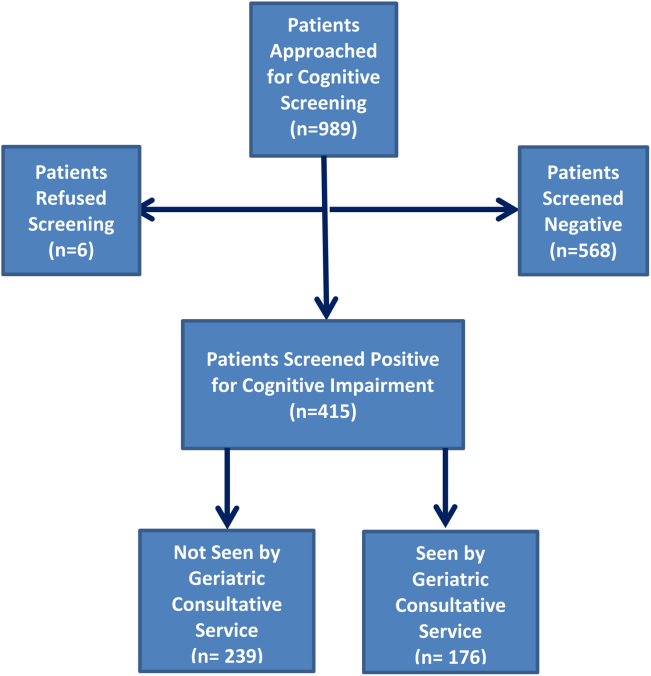
Inclusion and Exclusion Criteria
Individuals were eligible for enrollment if they were aged 65 years or older, hospitalized on a medical ward, able to speak English, and had evidence of CI within 48 hours of hospital admission. Individuals were excluded if they were previously enrolled, were aphasic, or unresponsive. The presence of CI was based on the Short Portable Mental Status Questionnaire (SPMSQ),[16] a brief 10‐item screening test with a sensitivity of 86% and specificity of 99% for dementia using a score of 7 or less (maximum possible score of 10).[16] The SPMSQ scoring process adjusts for participant educational and racial status, which was a benefit to its use given the urban setting of our hospital serving a large proportion of minority and low‐education patients. A physician‐trained research assistant administered the SPMSQ within 48 hours of hospital admission.
Geriatric Consultative Services
GCS is an interdisciplinary team of a geriatrician, a geriatric pharmacist, a case manager nurse, a social worker, a medical assistant, physical therapists, and a representative of the local Area Agency on Aging. There may be a geriatric fellow and/or medicine resident available to the team based on their rotation structure. Team‐based bedside rounds are performed on new consults only, but all patients are seen individually by the team clinicians. The team emphasizes prevention of functional decline and polypharmacy, recognition and treatment of geriatric syndromes including dementia and delirium, and early discharge/transition planning. Consensus recommendations are prepared and documented in the consult notes section of the electronic medical records. Recommendations deemed critical are discussed directly with the primary teams, but no orders are placed by the GCS team. The GCS team is available on all weekdays but not on weekends or major holidays.
Study Outcomes
For this secondary analysis, we used the Regenstrief Medical Record System (RMRS) to measure 2 outcomes: hospital readmission and mortality rates up to 1 year from discharge following index hospitalization, defined as the first admission in the original clinical trial. The RMRS is the primary instrument for processing data and monitoring patient and physician activity for the hospital.[17, 18] The RMRS is linked with a state‐wide health information exchange to capture data on hospitalization outside the hospital. The RMRS also contains death certificate information for all registered patients who die in or outside the Eskenazi hospital.
Other Data Collections
Delirium was assessed at screening and then every weekday using the Confusion Assessment Method (CAM) by a trained research assistant.[19] CAM evaluates 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders‐III‐Revision: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. Participant demographic characteristics, including age, sex, ethnicity, and years of education, were collected from the RMRS and from interviews performed at the time of cognitive screening. Information on length of hospital stay and discharge destination (eg, home vs facility, including skilled nursing and acute rehabilitation facilities) was also obtained from the RMRS. Charlson Comorbidity Index score was calculated using International Classification of Diseases, Ninth Revision codes gathered from 1 year before admission until the time of each participant's discharge from the hospital.[20] The Acute Physiology Score (APS) from the Acute Physiology and Chronic Health Evaluation (APACHE) III was derived from data available in the RMRS to measure the severity of illness.[21] Although the APACHE III was developed in the intensive care unit using data from the first 24 hours after admission, for our study we used the worst laboratory test value during the entire hospital stay to calculate the APS.[22]
Statistical Analysis
Baseline variables are presented as means and standard deviations for continuous variables, and percentages for binary categorical variables. Comparisons between patients receiving GCS and those who did not were performed using 2 tests for categorical variables and Kruskal‐Wallis test for continuous variables. Cox proportional hazard models were used to determine the association between receiving GCS and time to hospital readmission or mortality within 30 days or 1‐year postindex admission while adjusting for other covariates. For the models using time to readmission, patients without readmission were censored either at the endpoint (30 days or 1 year) or at time of death for those who died within the time frame in each model. Because GCS was not randomly assigned, we also conducted a propensity score analysis.[23] A logistic model for the probability of receiving GCS was conducted using patient demographic variables and information collected before and at the time of GCS. Stratified Cox proportional models using quintiles of predicted probability of receiving GCS were used in a propensity‐adjusted Cox model. All data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Between July 1, 2006 and May 30, 2008, 415 CI patients were enrolled in the original trial, with 176 receiving the GCS. As shown in Table 1, the GCS and non‐GCS groups differed significantly. The GCS group was older (79.2 years old, 8.1 standard deviation [SD] vs 75.8 years old, 7.8 SD; P0.001), scored lower on the SPMSQ (4.7, 2.7 SD vs 5.5, 2.7 SD; P=0.002), had fewer chronic conditions with a lower mean Charlson Comorbidity Index Score (2.1, 1.86 SD vs 2.8, 2.6 SD; P=0.023), but a higher percentage of delirium (48.9% vs 29.3%), a lower percentage of being discharged home (37.5% vs 56.1%), and a higher mean length of stay (6.4 days, 6.4 SD vs 5.6 days, 5.9 SD; P=0.004). They also had a lower malignancy rate (6.2% vs 14.6%; P=0.007) and a lower number of hospitalizations in the previous year (0.5 admissions, 0.9 SD vs 0.7 admissions, 1.1 SD; P=0.035). No differences were observed in regard to gender, ethnicity, history of myocardial infarctions, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, diabetes, and use of anticholinergic medicines.
| No GCS, n=239 | GCS, n=176 | P Value* | |
|---|---|---|---|
| |||
| Baseline characteristics | |||
| Mean age (SD) | 75.8 (7.8) | 79.2 (8.1) | <0.001 |
| % Female | 66.1 [n=158] | 68.2 [n=120] | 0.657 |
| % African American | 54.8 [n=131] | 63.6 [n=112] | 0.071 |
| Mean SPMSQ score (SD) | 5.5 (2.7) | 4.7 (2.7) | 0.002 |
| Admission diagnoses | |||
| MI | 15.5 [n=37] | 13.6 [n=24] | 0.675 |
| CHF | 38.1 [n=91] | 34.7 [n=61] | 0.475 |
| PVD | 7.1 [n=17] | 9.7 [n=17] | 0.370 |
| Cerebrovascular | 13.8 [n=33] | 19.3 [n=34] | 0.140 |
| COPD | 41.0 [n=98] | 33.0 [n=58] | 0.094 |
| Diabetes | 47.7 [n=114] | 40.9 [n=72] | 0.169 |
| Malignancy | 14.6 [n=35] | 6.2 [n=11] | 0.007 |
| Metastatic cancer | 8.8 [n=21] | 1.7 [n=3] | 0.002 |
| Mean Charlson Comorbidity (SD) | 2.8 (2.6) | 2.1 (1.8) | 0.023 |
| Mean APS (SD) | 24.5 (13.8) | 25.9 (13.5) | 0.231 |
| Definite ACB Use | 35.2 [n=84] | 27.8 [n=49] | 0.136 |
| Length of stay | 5.6 (5.9) | 6.4 (6.4) | 0.004 |
| % Any delirium | 29.3 [n=70] | 48.9 [n=156] | <0.001 |
| % Discharged home | 56.1 [n=134] | 37.5 [n=66] | <0.001 |
| No. of inpatient stays prior year | 0.7 (1.1) | 0.5 (0.9) | 0.035 |
| Follow‐up outcomes | |||
| % Readmission within 30 days | 15.1 [n=36] | 22.7 [n=40] | 0.054 |
| % Readmission within 1 year | 54.4 [n=130] | 56.3 [n=99] | 0.765 |
| % Death within 30 days | 4.2 [n=10] | 1.7 [n=3] | 0.253 |
| % Death within 1 year | 26.8 [n=64] | 23.9 [n=42] | 0.569 |
| % Readmission or death within 30 days | 18.0 [n=43] | 24.4 [n=43] | 0.113 |
| % Readmission or death within 1 year | 64.8 [n=155] | 63.1 [n=111] | 0.708 |
Table 2 describes the association of various factors with receiving GCS. Patients who were positive for delirium (odds ratio [OR]=1.65; 95% confidence interval=0.98‐2.77) and were older (OR=1.04; 95% confidence interval=1.01‐1.08) had a higher propensity to receive GCS, whereas, the presence of metastatic cancer resulted in a lower propensity (OR=0.15; 95% confidence interval=0.02‐1.16) of receiving GCS. The logistic model estimated area under the receiver operating characteristic curve was 0.707.
| Adjusted OR (95% CI) | P Value | |
|---|---|---|
| ||
| Age | 1.04 (1.011.08) | 0.006 |
| Female | 1.02 (0.641.63) | 0.942 |
| African American | 1.11 (0.711.72) | 0.657 |
| Short Portable Mental Status Questionnaire score | 1.00 (0.911.10) | 0.990 |
| Acute Physiology Score | 1.00 (0.981.02) | 0.769 |
| Charlson Comorbidity Score | 1.11 (0.841.46) | 0.471 |
| Length of hospital stay | 1.02 (0.981.07) | 0.299 |
| Definite anticholinergic use* | 0.74 (0.461.20) | 0.219 |
| Any delirium during hospital stay | 1.65 (0.982.77) | 0.061 |
| Diabetes mellitus | 0.72 (0.411.26) | 0.253 |
| Myocardial infarction | 0.83 (0.411.66) | 0.593 |
| Congestive heart failure | 0.83 (0.471.47) | 0.524 |
| Peripheral vascular disease | 1.39 (0.613.18) | 0.433 |
| Cerebrovascular disease | 1.30 (0.652.59) | 0.464 |
| Malignancy | 0.45 (0.171.21) | 0.113 |
| Metastatic cancer | 0.15 (0.021.16) | 0.069 |
| Chronic obstructive pulmonary disease | 0.91 (0.531.55) | 0.727 |
Table 3 provides results from the Cox models for receiving GCS on readmission and mortality outcomes adjusting for various sets of covariates and with the propensity score adjustment. Model 1 presents unadjusted hazard ratio (HR). Model 2 presents HRs adjusting for a common set of covariates that were significantly associated with at least 1 of the outcomes, whereas model 3 presents the results adjusting for all covariates. All 4 models yielded similar results. As evident from this table, propensity‐adjusted HR for 30‐day readmission was still significantly higher among patients receiving GCS (HR=1.75; 95% confidence interval=1.06‐2.88) but not at 1 year (HR=1.19; 95% confidence interval=0.89‐1.59). There was a trend for decreased mortality for the GCS group at 30 days (HR=0.35; 95% confidence interval=0.09‐1.35), but it disappeared at 1 year (HR=0.91; 95% confidence interval=0.59‐1.40). A composite outcome of readmissions and mortality did not show any difference between the GCS and no‐GCS groups.
| Outcome Variables | Model 1 | Model 2 | Model 3 | Propensity Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ||||||||
| Readmission within 30 days | 1.65 (1.05, 2.59) | 0.030 | 1.73 (1.08, 2.78) | 0.024 | 1.84 (1.133.00) | 0.015 | 1.75 (1.062.88) | 0.029 |
| Readmission within 1 year | 1.13 (0.87, 1.46) | 0.373 | 1.24 (0.94, 1.63) | 0.125 | 1.26 (0.941.68) | 0.117 | 1.19 (0.891.59) | 0.245 |
| Death within 30 days | 0.43 (0.12, 1.56) | 0.199 | 0.34 (0.09, 1.28) | 0.110 | 0.25 (0.061.02) | 0.053 | 0.35 (0.091.35) | 0.126 |
| Death within 1 year | 0.95 (0.65, 1.45) | 0.806 | 0.87 (0.58 1.31) | 0.506 | 0.93 (0.601.42) | 0.724 | 0.91 (0.591.40) | 0.669 |
| Readmission or Death within 30 days | 1.48 (0.97, 2.26) | 0.070 | 1.49 (0.96, 2.33) | 0.078 | 1.56 (0.982.47) | 0.061 | 1.55 (0.972.48) | 0.069 |
| Readmission or death within 1 year | 1.05 (0.82, 1.34) | 0.699 | 1.11 (0.86, 1.43) | 0.412 | 1.15 (0.881.50) | 0.318 | 1.08 (0.831.42) | 0.569 |
DISCUSSION
To our knowledge, this is the first study to analyze the impact of GCS on hospital readmission and mortality rates of CI patients. Our results did not show any short‐term or long‐term benefits of GCS for CI patients. Recent studies exploring cost benefits of the GCS have found trends toward lower readmission, but none focused on patients with CI.[6, 24, 25] It is important to note that our study did not use random allocation to assigning the patient into the GCS or control group, thus raising the possibility that patients who received GCS were sicker and were medically and socially more complex than those who did not receive the consult. Moreover, GCS consultation is preferentially sought for and completed for patients with CI and functional limitations, consistent with our finding that GCS patients more often have delirium and are less‐often discharged home.
The nature of the GCS team is another important consideration. Our GCS model did not include unit cohorting of patients, an important component of other proposed GCS models.[26] A recent meta‐analysis found that the GCS models without unit cohorting of patients did not have an impact on 1‐ or 12‐month readmission rates.[27] Low adherence to consultant recommendations (less than 33%) was thought to be a reason for such results. Importance of cohorting with regard to accomplishing recommendations by primary teams, importance of unit staff expertise in geriatric principles, and impact of a unit model on teamwork has also been highlighted by another review.[28] These findings lend to the hypothesis that unit cohorting and direct order placement by the GCS team may improve outcomes among CI patients, including a reduction in readmission rates.
Although readmissions rates were not statistically different between GCS and control groups at 1‐year postdischarge, 30‐day readmission rates were higher among the GCS group. Previous research among older heart failure patients found that a comprehensive transitional care intervention at the time of hospital discharge significantly shortened the time to readmission in the intervention group (P=0.026).[29] The factors identified by the study authors included enhanced supervision by the transitional healthcare teams along with improved awareness and education among treated patients that may have facilitated early recognition of clinical deterioration.[29] A recent study with intensive outpatient care that resulted in increased admissions among chronically ill adults provided a similar conclusion.[30]
GCS patients showed a trend toward decreased mortality as did patients enrolled in previous studies evaluating GCS models in the inpatient setting, as suggested by a recent review.[27] A caveat to note is that these trends favored ward‐styled GCS services as compared to our open GCS model,[27, 28] although the factors cited in these dedicated units affecting mortality included prompt attention to early rehabilitation, delirium management, and prevention of pressure ulcers and are also frequently implemented for patients in our GCS service model and therefore may have produced similar results.
Our neutral results in regard to the readmissions need to be interpreted with caution. First, this study was conducted in a hospital that supports expert geriatric and palliative care teams, both in the inpatient and the ambulatory settings, that provide consultative services and train medicine teams and hospital nursing staff. On the outpatient side, the presence of a robust geriatrics house‐calls program and the Geriatric Resources for Assessment and Care of Elders team results in above‐average care for the control group, and thus may also impact apparent outcomes.[31, 32] Second, 30‐day readmissions represent a complex outcome. Two recent reviews of hospital‐initiated interventions have shown that evidence regarding best strategies to decrease 30‐day readmissions is unclear.[33] Neither review included studies that targeted patients with CI only. The 2 programs that reduced 30‐day readmissions were multifaceted and included personnel who provide bridging between the hospital and the outpatient setting.[34] The GCS does include a focus on postdischarge resources, but does that on a case‐by‐case basis and no formal posthospital follow‐ups are provided. Moreover, the value of 30‐day readmission rates as a marker of quality, even though used by policymakers as an indicator of hospital quality, remains controversial.[35, 36] Broadening the outcomes of interest to include patient‐centered outcomes including satisfaction with care, that have shown to impact other health outcomes, may help improve understanding the benefits of GCS in hospitals.[37] Other comprehensive transitional care models that failed to show a benefit on 30‐day readmissions in older patients still resulted in higher satisfaction among patients.[38] Unfortunately, our evaluation did not include an assessment of patient satisfaction and quality of transitions.
Since the study period, GCS at our hospital now has incorporated a more robust focus on advance care planning (ACP) and execution of Physician Orders for Scope of Treatment that were legislated in the state in July 2013. The GCS team members are expert in carrying out complex ACP discussions and also partner with the inpatient palliative care team. It is quite possible that a study of more recent outcomes will yield more positive results for the selected outcomes. Thus, for future trials that aim to study the impact of GCS in the inpatient settings, it may be advisable to include important quality markers such as implementation of ACP and patient satisfaction along with the health utilization outcomes.
Limitations
As mentioned previously, it is possible that our risk adjustment was insufficient to account for all the medical and psychosocial differences among groups. For example, the overall anticholinergic impact of various medications such as antipsychotic medications and histamine‐2 blockers was assessed via the Anticholinergic Burden Scale on admission, but we did not have information on medication prescribing during the stay. We were further limited by lack of baseline functional status and socioeconomic details, both of which are related to 30‐day readmissions. For example, living alone, prior use of assist devices, and belonging to lower socioeconomic status are correlated with higher readmission rates.[39, 40] Patients with available social support may receive more intense supervision and may seek medical attention sooner. On the other hand, worsening health among CI patients without any approximate social support may be unnoticed for days. Absence of details of inpatient interventions may also have resulted in unmeasurable confounders that could have impacted our study outcomes. Finally, lack of information on the uptake of GCS recommendations by the primary teams is another limitation of this analysis. Future trials should include strategies to address these information gaps.
CONCLUSION
Our results comparing inpatient geriatrics consultative services with usual care in hospitalized elders having cognitive impairment failed to demonstrate an impact on readmissions and mortality. A clinical lesson learned, however, is that much work is still required to reduce readmission and mortality rates in this especially vulnerable patient population.
Disclosures
Disclosures: This work was supported by grants from a Geriatric Academic Career Award (K01HP20517) through Health Resources and Services Administration, R01AG034205 and K23‐AG043476 from the National Institute on Aging, and the John A. Hartford Foundation Center for Excellence in Geriatric Medicine. The sponsors had no role in the study design, evaluation, or manuscript development. The authors report no conflicts of interest.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , Cognitive impairment. Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34(8):579–585.
- , , , , , Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- , Understanding preventable hospital readmissions: masqueraders, markers, and true causal factors. J Hosp Med. 2011;6(2):51–53.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581.
- , ; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557.
- , , , Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139–2145.
- , , , et al. Screening of the risk of functional decline performed by an inpatient geriatric consultation team in a general hospital [in French]. Revue medicale de Bruxelles. 2013;34(6):462–468.
- , , , et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759.
- , , Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm. 2003;18(1):37–42, 47–39.
- , , , Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–996.
- , , , , , Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013;173(11):981–987.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567.
- , , , Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35(5):412–416.
- , , , et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253.
- , , , , , Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist. 1993;33(6):714–720.
- , , , , , Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963.
- , , , , , Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003.
- , , , et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. Jt Comm J Qual Improv. 1998;24(2):63–76.
- , , , et al. Developing a stroke unit using the acute care for elders intervention and model of care. J Am Geriatr Soc. 2003;51(11):1660–1667.
- , , , A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545–552.
- , , , , Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta‐analysis. BMC Med. 2013;11:48.
- , , , , Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , House calls for seniors: building and sustaining a model of care for homebound seniors. J Am Geriatr Soc. 2009;57(6):1103–1109.
- , , , et al. Geriatric care management for low‐income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633.
- , , , , Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs. 1999;14(1):44–54.
- Readmission to hospital: a measure of quality or outcome? Qual Saf Health Care. 2004;13(1):10–11.
- , , , , , Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff (Millwood). 2012;31(7):1623–1632.
- , , Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modeling approach. Patient Educ Couns. 2013;91(2):167–175.
- , , , The Care Transitions Innovation (C‐TraIn) for Socioeconomically Disadvantaged Adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460–1467.
- , , , Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62(3):489–494.
- , , , , , Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
Under the Patient Protection and Affordable Care Act of 2010, commonly referred to as the Affordable Care Act, hospitals face up to a 3% penalty in Medicare reimbursements for patients readmitted within 30 days of initial discharge, and measures have been proposed for modifying payments to hospitals based on their performance on this metric.[1] Cognitive impairment (CI) is considered a major risk factor for poor postdischarge outcomes including mortality and hospital readmission.[2, 3] Hospitals are seeking strategies to reduce postdischarge mortality and rehospitalization among patients with and without CI.[4] Such strategies include use of transitional care coaches, patient and caregiver education, postdischarge follow‐up, and provision of geriatric consultative services (GCS) for the care of complex patients in the hospital setting.[5, 6, 7]
GCS utilize comprehensive geriatric assessments and multidisciplinary processes to recognize and modify risk factors that may lead to poor outcomes among hospitalized patients.[8, 9, 10, 11] Implementation of GCS models including Acute Care for Elders and, recently, the Mobile Acute Care of the Elderly services have shown many benefits among older patients including a reduction in the hospital length of stay and readmission rates.[12, 13] The benefits of such services among hospitalized elders suffering from CI, however, are not well established. The objective of this article was to evaluate the impact of GCS on the readmission and mortality rates of older adults with CI within 12 months of their hospitalization to an urban, public hospital. We hypothesized that GCS will reduce both 12‐month hospital readmissions and mortality rates among this vulnerable group of older adults.
METHODS
The study was approved by the Indiana University institutional review board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Setting
The study was conducted at Eskenazi hospital, Indianapolis, Indiana, a 340‐bed, university‐affiliated, public hospital with over 2300 admissions of patients aged 65 years or older every year.
Population
Four hundred fifteen hospitalized patients aged 65 years or older suffering from CI were enrolled into an original, randomized, controlled trial that evaluated the effect of a computerized decision support system on their quality and outcome of care between July 1, 2006 and May 30, 2008.[14] The computerized decision support included reminders for physicians to reduce the prescription of 18 anticholinergics, minimize physical restraints and Foley catheterization, and increase referral to the local GCS.[15] That previous trial neither showed an impact on quality of care nor health utilization among older patients, including mortality and hospital readmission rates. The current study uses the data from the clinical trial cohort to evaluate the effect of GCS on the 12‐month mortality and hospital readmission rates for hospitalized elders with CI (Figure 1).

Inclusion and Exclusion Criteria
Individuals were eligible for enrollment if they were aged 65 years or older, hospitalized on a medical ward, able to speak English, and had evidence of CI within 48 hours of hospital admission. Individuals were excluded if they were previously enrolled, were aphasic, or unresponsive. The presence of CI was based on the Short Portable Mental Status Questionnaire (SPMSQ),[16] a brief 10‐item screening test with a sensitivity of 86% and specificity of 99% for dementia using a score of 7 or less (maximum possible score of 10).[16] The SPMSQ scoring process adjusts for participant educational and racial status, which was a benefit to its use given the urban setting of our hospital serving a large proportion of minority and low‐education patients. A physician‐trained research assistant administered the SPMSQ within 48 hours of hospital admission.
Geriatric Consultative Services
GCS is an interdisciplinary team of a geriatrician, a geriatric pharmacist, a case manager nurse, a social worker, a medical assistant, physical therapists, and a representative of the local Area Agency on Aging. There may be a geriatric fellow and/or medicine resident available to the team based on their rotation structure. Team‐based bedside rounds are performed on new consults only, but all patients are seen individually by the team clinicians. The team emphasizes prevention of functional decline and polypharmacy, recognition and treatment of geriatric syndromes including dementia and delirium, and early discharge/transition planning. Consensus recommendations are prepared and documented in the consult notes section of the electronic medical records. Recommendations deemed critical are discussed directly with the primary teams, but no orders are placed by the GCS team. The GCS team is available on all weekdays but not on weekends or major holidays.
Study Outcomes
For this secondary analysis, we used the Regenstrief Medical Record System (RMRS) to measure 2 outcomes: hospital readmission and mortality rates up to 1 year from discharge following index hospitalization, defined as the first admission in the original clinical trial. The RMRS is the primary instrument for processing data and monitoring patient and physician activity for the hospital.[17, 18] The RMRS is linked with a state‐wide health information exchange to capture data on hospitalization outside the hospital. The RMRS also contains death certificate information for all registered patients who die in or outside the Eskenazi hospital.
Other Data Collections
Delirium was assessed at screening and then every weekday using the Confusion Assessment Method (CAM) by a trained research assistant.[19] CAM evaluates 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders‐III‐Revision: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. Participant demographic characteristics, including age, sex, ethnicity, and years of education, were collected from the RMRS and from interviews performed at the time of cognitive screening. Information on length of hospital stay and discharge destination (eg, home vs facility, including skilled nursing and acute rehabilitation facilities) was also obtained from the RMRS. Charlson Comorbidity Index score was calculated using International Classification of Diseases, Ninth Revision codes gathered from 1 year before admission until the time of each participant's discharge from the hospital.[20] The Acute Physiology Score (APS) from the Acute Physiology and Chronic Health Evaluation (APACHE) III was derived from data available in the RMRS to measure the severity of illness.[21] Although the APACHE III was developed in the intensive care unit using data from the first 24 hours after admission, for our study we used the worst laboratory test value during the entire hospital stay to calculate the APS.[22]
Statistical Analysis
Baseline variables are presented as means and standard deviations for continuous variables, and percentages for binary categorical variables. Comparisons between patients receiving GCS and those who did not were performed using 2 tests for categorical variables and Kruskal‐Wallis test for continuous variables. Cox proportional hazard models were used to determine the association between receiving GCS and time to hospital readmission or mortality within 30 days or 1‐year postindex admission while adjusting for other covariates. For the models using time to readmission, patients without readmission were censored either at the endpoint (30 days or 1 year) or at time of death for those who died within the time frame in each model. Because GCS was not randomly assigned, we also conducted a propensity score analysis.[23] A logistic model for the probability of receiving GCS was conducted using patient demographic variables and information collected before and at the time of GCS. Stratified Cox proportional models using quintiles of predicted probability of receiving GCS were used in a propensity‐adjusted Cox model. All data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Between July 1, 2006 and May 30, 2008, 415 CI patients were enrolled in the original trial, with 176 receiving the GCS. As shown in Table 1, the GCS and non‐GCS groups differed significantly. The GCS group was older (79.2 years old, 8.1 standard deviation [SD] vs 75.8 years old, 7.8 SD; P0.001), scored lower on the SPMSQ (4.7, 2.7 SD vs 5.5, 2.7 SD; P=0.002), had fewer chronic conditions with a lower mean Charlson Comorbidity Index Score (2.1, 1.86 SD vs 2.8, 2.6 SD; P=0.023), but a higher percentage of delirium (48.9% vs 29.3%), a lower percentage of being discharged home (37.5% vs 56.1%), and a higher mean length of stay (6.4 days, 6.4 SD vs 5.6 days, 5.9 SD; P=0.004). They also had a lower malignancy rate (6.2% vs 14.6%; P=0.007) and a lower number of hospitalizations in the previous year (0.5 admissions, 0.9 SD vs 0.7 admissions, 1.1 SD; P=0.035). No differences were observed in regard to gender, ethnicity, history of myocardial infarctions, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, diabetes, and use of anticholinergic medicines.
| No GCS, n=239 | GCS, n=176 | P Value* | |
|---|---|---|---|
| |||
| Baseline characteristics | |||
| Mean age (SD) | 75.8 (7.8) | 79.2 (8.1) | <0.001 |
| % Female | 66.1 [n=158] | 68.2 [n=120] | 0.657 |
| % African American | 54.8 [n=131] | 63.6 [n=112] | 0.071 |
| Mean SPMSQ score (SD) | 5.5 (2.7) | 4.7 (2.7) | 0.002 |
| Admission diagnoses | |||
| MI | 15.5 [n=37] | 13.6 [n=24] | 0.675 |
| CHF | 38.1 [n=91] | 34.7 [n=61] | 0.475 |
| PVD | 7.1 [n=17] | 9.7 [n=17] | 0.370 |
| Cerebrovascular | 13.8 [n=33] | 19.3 [n=34] | 0.140 |
| COPD | 41.0 [n=98] | 33.0 [n=58] | 0.094 |
| Diabetes | 47.7 [n=114] | 40.9 [n=72] | 0.169 |
| Malignancy | 14.6 [n=35] | 6.2 [n=11] | 0.007 |
| Metastatic cancer | 8.8 [n=21] | 1.7 [n=3] | 0.002 |
| Mean Charlson Comorbidity (SD) | 2.8 (2.6) | 2.1 (1.8) | 0.023 |
| Mean APS (SD) | 24.5 (13.8) | 25.9 (13.5) | 0.231 |
| Definite ACB Use | 35.2 [n=84] | 27.8 [n=49] | 0.136 |
| Length of stay | 5.6 (5.9) | 6.4 (6.4) | 0.004 |
| % Any delirium | 29.3 [n=70] | 48.9 [n=156] | <0.001 |
| % Discharged home | 56.1 [n=134] | 37.5 [n=66] | <0.001 |
| No. of inpatient stays prior year | 0.7 (1.1) | 0.5 (0.9) | 0.035 |
| Follow‐up outcomes | |||
| % Readmission within 30 days | 15.1 [n=36] | 22.7 [n=40] | 0.054 |
| % Readmission within 1 year | 54.4 [n=130] | 56.3 [n=99] | 0.765 |
| % Death within 30 days | 4.2 [n=10] | 1.7 [n=3] | 0.253 |
| % Death within 1 year | 26.8 [n=64] | 23.9 [n=42] | 0.569 |
| % Readmission or death within 30 days | 18.0 [n=43] | 24.4 [n=43] | 0.113 |
| % Readmission or death within 1 year | 64.8 [n=155] | 63.1 [n=111] | 0.708 |
Table 2 describes the association of various factors with receiving GCS. Patients who were positive for delirium (odds ratio [OR]=1.65; 95% confidence interval=0.98‐2.77) and were older (OR=1.04; 95% confidence interval=1.01‐1.08) had a higher propensity to receive GCS, whereas, the presence of metastatic cancer resulted in a lower propensity (OR=0.15; 95% confidence interval=0.02‐1.16) of receiving GCS. The logistic model estimated area under the receiver operating characteristic curve was 0.707.
| Adjusted OR (95% CI) | P Value | |
|---|---|---|
| ||
| Age | 1.04 (1.011.08) | 0.006 |
| Female | 1.02 (0.641.63) | 0.942 |
| African American | 1.11 (0.711.72) | 0.657 |
| Short Portable Mental Status Questionnaire score | 1.00 (0.911.10) | 0.990 |
| Acute Physiology Score | 1.00 (0.981.02) | 0.769 |
| Charlson Comorbidity Score | 1.11 (0.841.46) | 0.471 |
| Length of hospital stay | 1.02 (0.981.07) | 0.299 |
| Definite anticholinergic use* | 0.74 (0.461.20) | 0.219 |
| Any delirium during hospital stay | 1.65 (0.982.77) | 0.061 |
| Diabetes mellitus | 0.72 (0.411.26) | 0.253 |
| Myocardial infarction | 0.83 (0.411.66) | 0.593 |
| Congestive heart failure | 0.83 (0.471.47) | 0.524 |
| Peripheral vascular disease | 1.39 (0.613.18) | 0.433 |
| Cerebrovascular disease | 1.30 (0.652.59) | 0.464 |
| Malignancy | 0.45 (0.171.21) | 0.113 |
| Metastatic cancer | 0.15 (0.021.16) | 0.069 |
| Chronic obstructive pulmonary disease | 0.91 (0.531.55) | 0.727 |
Table 3 provides results from the Cox models for receiving GCS on readmission and mortality outcomes adjusting for various sets of covariates and with the propensity score adjustment. Model 1 presents unadjusted hazard ratio (HR). Model 2 presents HRs adjusting for a common set of covariates that were significantly associated with at least 1 of the outcomes, whereas model 3 presents the results adjusting for all covariates. All 4 models yielded similar results. As evident from this table, propensity‐adjusted HR for 30‐day readmission was still significantly higher among patients receiving GCS (HR=1.75; 95% confidence interval=1.06‐2.88) but not at 1 year (HR=1.19; 95% confidence interval=0.89‐1.59). There was a trend for decreased mortality for the GCS group at 30 days (HR=0.35; 95% confidence interval=0.09‐1.35), but it disappeared at 1 year (HR=0.91; 95% confidence interval=0.59‐1.40). A composite outcome of readmissions and mortality did not show any difference between the GCS and no‐GCS groups.
| Outcome Variables | Model 1 | Model 2 | Model 3 | Propensity Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ||||||||
| Readmission within 30 days | 1.65 (1.05, 2.59) | 0.030 | 1.73 (1.08, 2.78) | 0.024 | 1.84 (1.133.00) | 0.015 | 1.75 (1.062.88) | 0.029 |
| Readmission within 1 year | 1.13 (0.87, 1.46) | 0.373 | 1.24 (0.94, 1.63) | 0.125 | 1.26 (0.941.68) | 0.117 | 1.19 (0.891.59) | 0.245 |
| Death within 30 days | 0.43 (0.12, 1.56) | 0.199 | 0.34 (0.09, 1.28) | 0.110 | 0.25 (0.061.02) | 0.053 | 0.35 (0.091.35) | 0.126 |
| Death within 1 year | 0.95 (0.65, 1.45) | 0.806 | 0.87 (0.58 1.31) | 0.506 | 0.93 (0.601.42) | 0.724 | 0.91 (0.591.40) | 0.669 |
| Readmission or Death within 30 days | 1.48 (0.97, 2.26) | 0.070 | 1.49 (0.96, 2.33) | 0.078 | 1.56 (0.982.47) | 0.061 | 1.55 (0.972.48) | 0.069 |
| Readmission or death within 1 year | 1.05 (0.82, 1.34) | 0.699 | 1.11 (0.86, 1.43) | 0.412 | 1.15 (0.881.50) | 0.318 | 1.08 (0.831.42) | 0.569 |
DISCUSSION
To our knowledge, this is the first study to analyze the impact of GCS on hospital readmission and mortality rates of CI patients. Our results did not show any short‐term or long‐term benefits of GCS for CI patients. Recent studies exploring cost benefits of the GCS have found trends toward lower readmission, but none focused on patients with CI.[6, 24, 25] It is important to note that our study did not use random allocation to assigning the patient into the GCS or control group, thus raising the possibility that patients who received GCS were sicker and were medically and socially more complex than those who did not receive the consult. Moreover, GCS consultation is preferentially sought for and completed for patients with CI and functional limitations, consistent with our finding that GCS patients more often have delirium and are less‐often discharged home.
The nature of the GCS team is another important consideration. Our GCS model did not include unit cohorting of patients, an important component of other proposed GCS models.[26] A recent meta‐analysis found that the GCS models without unit cohorting of patients did not have an impact on 1‐ or 12‐month readmission rates.[27] Low adherence to consultant recommendations (less than 33%) was thought to be a reason for such results. Importance of cohorting with regard to accomplishing recommendations by primary teams, importance of unit staff expertise in geriatric principles, and impact of a unit model on teamwork has also been highlighted by another review.[28] These findings lend to the hypothesis that unit cohorting and direct order placement by the GCS team may improve outcomes among CI patients, including a reduction in readmission rates.
Although readmissions rates were not statistically different between GCS and control groups at 1‐year postdischarge, 30‐day readmission rates were higher among the GCS group. Previous research among older heart failure patients found that a comprehensive transitional care intervention at the time of hospital discharge significantly shortened the time to readmission in the intervention group (P=0.026).[29] The factors identified by the study authors included enhanced supervision by the transitional healthcare teams along with improved awareness and education among treated patients that may have facilitated early recognition of clinical deterioration.[29] A recent study with intensive outpatient care that resulted in increased admissions among chronically ill adults provided a similar conclusion.[30]
GCS patients showed a trend toward decreased mortality as did patients enrolled in previous studies evaluating GCS models in the inpatient setting, as suggested by a recent review.[27] A caveat to note is that these trends favored ward‐styled GCS services as compared to our open GCS model,[27, 28] although the factors cited in these dedicated units affecting mortality included prompt attention to early rehabilitation, delirium management, and prevention of pressure ulcers and are also frequently implemented for patients in our GCS service model and therefore may have produced similar results.
Our neutral results in regard to the readmissions need to be interpreted with caution. First, this study was conducted in a hospital that supports expert geriatric and palliative care teams, both in the inpatient and the ambulatory settings, that provide consultative services and train medicine teams and hospital nursing staff. On the outpatient side, the presence of a robust geriatrics house‐calls program and the Geriatric Resources for Assessment and Care of Elders team results in above‐average care for the control group, and thus may also impact apparent outcomes.[31, 32] Second, 30‐day readmissions represent a complex outcome. Two recent reviews of hospital‐initiated interventions have shown that evidence regarding best strategies to decrease 30‐day readmissions is unclear.[33] Neither review included studies that targeted patients with CI only. The 2 programs that reduced 30‐day readmissions were multifaceted and included personnel who provide bridging between the hospital and the outpatient setting.[34] The GCS does include a focus on postdischarge resources, but does that on a case‐by‐case basis and no formal posthospital follow‐ups are provided. Moreover, the value of 30‐day readmission rates as a marker of quality, even though used by policymakers as an indicator of hospital quality, remains controversial.[35, 36] Broadening the outcomes of interest to include patient‐centered outcomes including satisfaction with care, that have shown to impact other health outcomes, may help improve understanding the benefits of GCS in hospitals.[37] Other comprehensive transitional care models that failed to show a benefit on 30‐day readmissions in older patients still resulted in higher satisfaction among patients.[38] Unfortunately, our evaluation did not include an assessment of patient satisfaction and quality of transitions.
Since the study period, GCS at our hospital now has incorporated a more robust focus on advance care planning (ACP) and execution of Physician Orders for Scope of Treatment that were legislated in the state in July 2013. The GCS team members are expert in carrying out complex ACP discussions and also partner with the inpatient palliative care team. It is quite possible that a study of more recent outcomes will yield more positive results for the selected outcomes. Thus, for future trials that aim to study the impact of GCS in the inpatient settings, it may be advisable to include important quality markers such as implementation of ACP and patient satisfaction along with the health utilization outcomes.
Limitations
As mentioned previously, it is possible that our risk adjustment was insufficient to account for all the medical and psychosocial differences among groups. For example, the overall anticholinergic impact of various medications such as antipsychotic medications and histamine‐2 blockers was assessed via the Anticholinergic Burden Scale on admission, but we did not have information on medication prescribing during the stay. We were further limited by lack of baseline functional status and socioeconomic details, both of which are related to 30‐day readmissions. For example, living alone, prior use of assist devices, and belonging to lower socioeconomic status are correlated with higher readmission rates.[39, 40] Patients with available social support may receive more intense supervision and may seek medical attention sooner. On the other hand, worsening health among CI patients without any approximate social support may be unnoticed for days. Absence of details of inpatient interventions may also have resulted in unmeasurable confounders that could have impacted our study outcomes. Finally, lack of information on the uptake of GCS recommendations by the primary teams is another limitation of this analysis. Future trials should include strategies to address these information gaps.
CONCLUSION
Our results comparing inpatient geriatrics consultative services with usual care in hospitalized elders having cognitive impairment failed to demonstrate an impact on readmissions and mortality. A clinical lesson learned, however, is that much work is still required to reduce readmission and mortality rates in this especially vulnerable patient population.
Disclosures
Disclosures: This work was supported by grants from a Geriatric Academic Career Award (K01HP20517) through Health Resources and Services Administration, R01AG034205 and K23‐AG043476 from the National Institute on Aging, and the John A. Hartford Foundation Center for Excellence in Geriatric Medicine. The sponsors had no role in the study design, evaluation, or manuscript development. The authors report no conflicts of interest.
Under the Patient Protection and Affordable Care Act of 2010, commonly referred to as the Affordable Care Act, hospitals face up to a 3% penalty in Medicare reimbursements for patients readmitted within 30 days of initial discharge, and measures have been proposed for modifying payments to hospitals based on their performance on this metric.[1] Cognitive impairment (CI) is considered a major risk factor for poor postdischarge outcomes including mortality and hospital readmission.[2, 3] Hospitals are seeking strategies to reduce postdischarge mortality and rehospitalization among patients with and without CI.[4] Such strategies include use of transitional care coaches, patient and caregiver education, postdischarge follow‐up, and provision of geriatric consultative services (GCS) for the care of complex patients in the hospital setting.[5, 6, 7]
GCS utilize comprehensive geriatric assessments and multidisciplinary processes to recognize and modify risk factors that may lead to poor outcomes among hospitalized patients.[8, 9, 10, 11] Implementation of GCS models including Acute Care for Elders and, recently, the Mobile Acute Care of the Elderly services have shown many benefits among older patients including a reduction in the hospital length of stay and readmission rates.[12, 13] The benefits of such services among hospitalized elders suffering from CI, however, are not well established. The objective of this article was to evaluate the impact of GCS on the readmission and mortality rates of older adults with CI within 12 months of their hospitalization to an urban, public hospital. We hypothesized that GCS will reduce both 12‐month hospital readmissions and mortality rates among this vulnerable group of older adults.
METHODS
The study was approved by the Indiana University institutional review board, and informed consent for identifiable chart review was obtained from subjects or their legally authorized representatives.
Setting
The study was conducted at Eskenazi hospital, Indianapolis, Indiana, a 340‐bed, university‐affiliated, public hospital with over 2300 admissions of patients aged 65 years or older every year.
Population
Four hundred fifteen hospitalized patients aged 65 years or older suffering from CI were enrolled into an original, randomized, controlled trial that evaluated the effect of a computerized decision support system on their quality and outcome of care between July 1, 2006 and May 30, 2008.[14] The computerized decision support included reminders for physicians to reduce the prescription of 18 anticholinergics, minimize physical restraints and Foley catheterization, and increase referral to the local GCS.[15] That previous trial neither showed an impact on quality of care nor health utilization among older patients, including mortality and hospital readmission rates. The current study uses the data from the clinical trial cohort to evaluate the effect of GCS on the 12‐month mortality and hospital readmission rates for hospitalized elders with CI (Figure 1).

Inclusion and Exclusion Criteria
Individuals were eligible for enrollment if they were aged 65 years or older, hospitalized on a medical ward, able to speak English, and had evidence of CI within 48 hours of hospital admission. Individuals were excluded if they were previously enrolled, were aphasic, or unresponsive. The presence of CI was based on the Short Portable Mental Status Questionnaire (SPMSQ),[16] a brief 10‐item screening test with a sensitivity of 86% and specificity of 99% for dementia using a score of 7 or less (maximum possible score of 10).[16] The SPMSQ scoring process adjusts for participant educational and racial status, which was a benefit to its use given the urban setting of our hospital serving a large proportion of minority and low‐education patients. A physician‐trained research assistant administered the SPMSQ within 48 hours of hospital admission.
Geriatric Consultative Services
GCS is an interdisciplinary team of a geriatrician, a geriatric pharmacist, a case manager nurse, a social worker, a medical assistant, physical therapists, and a representative of the local Area Agency on Aging. There may be a geriatric fellow and/or medicine resident available to the team based on their rotation structure. Team‐based bedside rounds are performed on new consults only, but all patients are seen individually by the team clinicians. The team emphasizes prevention of functional decline and polypharmacy, recognition and treatment of geriatric syndromes including dementia and delirium, and early discharge/transition planning. Consensus recommendations are prepared and documented in the consult notes section of the electronic medical records. Recommendations deemed critical are discussed directly with the primary teams, but no orders are placed by the GCS team. The GCS team is available on all weekdays but not on weekends or major holidays.
Study Outcomes
For this secondary analysis, we used the Regenstrief Medical Record System (RMRS) to measure 2 outcomes: hospital readmission and mortality rates up to 1 year from discharge following index hospitalization, defined as the first admission in the original clinical trial. The RMRS is the primary instrument for processing data and monitoring patient and physician activity for the hospital.[17, 18] The RMRS is linked with a state‐wide health information exchange to capture data on hospitalization outside the hospital. The RMRS also contains death certificate information for all registered patients who die in or outside the Eskenazi hospital.
Other Data Collections
Delirium was assessed at screening and then every weekday using the Confusion Assessment Method (CAM) by a trained research assistant.[19] CAM evaluates 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders‐III‐Revision: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. Participant demographic characteristics, including age, sex, ethnicity, and years of education, were collected from the RMRS and from interviews performed at the time of cognitive screening. Information on length of hospital stay and discharge destination (eg, home vs facility, including skilled nursing and acute rehabilitation facilities) was also obtained from the RMRS. Charlson Comorbidity Index score was calculated using International Classification of Diseases, Ninth Revision codes gathered from 1 year before admission until the time of each participant's discharge from the hospital.[20] The Acute Physiology Score (APS) from the Acute Physiology and Chronic Health Evaluation (APACHE) III was derived from data available in the RMRS to measure the severity of illness.[21] Although the APACHE III was developed in the intensive care unit using data from the first 24 hours after admission, for our study we used the worst laboratory test value during the entire hospital stay to calculate the APS.[22]
Statistical Analysis
Baseline variables are presented as means and standard deviations for continuous variables, and percentages for binary categorical variables. Comparisons between patients receiving GCS and those who did not were performed using 2 tests for categorical variables and Kruskal‐Wallis test for continuous variables. Cox proportional hazard models were used to determine the association between receiving GCS and time to hospital readmission or mortality within 30 days or 1‐year postindex admission while adjusting for other covariates. For the models using time to readmission, patients without readmission were censored either at the endpoint (30 days or 1 year) or at time of death for those who died within the time frame in each model. Because GCS was not randomly assigned, we also conducted a propensity score analysis.[23] A logistic model for the probability of receiving GCS was conducted using patient demographic variables and information collected before and at the time of GCS. Stratified Cox proportional models using quintiles of predicted probability of receiving GCS were used in a propensity‐adjusted Cox model. All data analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Between July 1, 2006 and May 30, 2008, 415 CI patients were enrolled in the original trial, with 176 receiving the GCS. As shown in Table 1, the GCS and non‐GCS groups differed significantly. The GCS group was older (79.2 years old, 8.1 standard deviation [SD] vs 75.8 years old, 7.8 SD; P0.001), scored lower on the SPMSQ (4.7, 2.7 SD vs 5.5, 2.7 SD; P=0.002), had fewer chronic conditions with a lower mean Charlson Comorbidity Index Score (2.1, 1.86 SD vs 2.8, 2.6 SD; P=0.023), but a higher percentage of delirium (48.9% vs 29.3%), a lower percentage of being discharged home (37.5% vs 56.1%), and a higher mean length of stay (6.4 days, 6.4 SD vs 5.6 days, 5.9 SD; P=0.004). They also had a lower malignancy rate (6.2% vs 14.6%; P=0.007) and a lower number of hospitalizations in the previous year (0.5 admissions, 0.9 SD vs 0.7 admissions, 1.1 SD; P=0.035). No differences were observed in regard to gender, ethnicity, history of myocardial infarctions, chronic obstructive pulmonary disease, cerebrovascular disease, peripheral vascular disease, diabetes, and use of anticholinergic medicines.
| No GCS, n=239 | GCS, n=176 | P Value* | |
|---|---|---|---|
| |||
| Baseline characteristics | |||
| Mean age (SD) | 75.8 (7.8) | 79.2 (8.1) | <0.001 |
| % Female | 66.1 [n=158] | 68.2 [n=120] | 0.657 |
| % African American | 54.8 [n=131] | 63.6 [n=112] | 0.071 |
| Mean SPMSQ score (SD) | 5.5 (2.7) | 4.7 (2.7) | 0.002 |
| Admission diagnoses | |||
| MI | 15.5 [n=37] | 13.6 [n=24] | 0.675 |
| CHF | 38.1 [n=91] | 34.7 [n=61] | 0.475 |
| PVD | 7.1 [n=17] | 9.7 [n=17] | 0.370 |
| Cerebrovascular | 13.8 [n=33] | 19.3 [n=34] | 0.140 |
| COPD | 41.0 [n=98] | 33.0 [n=58] | 0.094 |
| Diabetes | 47.7 [n=114] | 40.9 [n=72] | 0.169 |
| Malignancy | 14.6 [n=35] | 6.2 [n=11] | 0.007 |
| Metastatic cancer | 8.8 [n=21] | 1.7 [n=3] | 0.002 |
| Mean Charlson Comorbidity (SD) | 2.8 (2.6) | 2.1 (1.8) | 0.023 |
| Mean APS (SD) | 24.5 (13.8) | 25.9 (13.5) | 0.231 |
| Definite ACB Use | 35.2 [n=84] | 27.8 [n=49] | 0.136 |
| Length of stay | 5.6 (5.9) | 6.4 (6.4) | 0.004 |
| % Any delirium | 29.3 [n=70] | 48.9 [n=156] | <0.001 |
| % Discharged home | 56.1 [n=134] | 37.5 [n=66] | <0.001 |
| No. of inpatient stays prior year | 0.7 (1.1) | 0.5 (0.9) | 0.035 |
| Follow‐up outcomes | |||
| % Readmission within 30 days | 15.1 [n=36] | 22.7 [n=40] | 0.054 |
| % Readmission within 1 year | 54.4 [n=130] | 56.3 [n=99] | 0.765 |
| % Death within 30 days | 4.2 [n=10] | 1.7 [n=3] | 0.253 |
| % Death within 1 year | 26.8 [n=64] | 23.9 [n=42] | 0.569 |
| % Readmission or death within 30 days | 18.0 [n=43] | 24.4 [n=43] | 0.113 |
| % Readmission or death within 1 year | 64.8 [n=155] | 63.1 [n=111] | 0.708 |
Table 2 describes the association of various factors with receiving GCS. Patients who were positive for delirium (odds ratio [OR]=1.65; 95% confidence interval=0.98‐2.77) and were older (OR=1.04; 95% confidence interval=1.01‐1.08) had a higher propensity to receive GCS, whereas, the presence of metastatic cancer resulted in a lower propensity (OR=0.15; 95% confidence interval=0.02‐1.16) of receiving GCS. The logistic model estimated area under the receiver operating characteristic curve was 0.707.
| Adjusted OR (95% CI) | P Value | |
|---|---|---|
| ||
| Age | 1.04 (1.011.08) | 0.006 |
| Female | 1.02 (0.641.63) | 0.942 |
| African American | 1.11 (0.711.72) | 0.657 |
| Short Portable Mental Status Questionnaire score | 1.00 (0.911.10) | 0.990 |
| Acute Physiology Score | 1.00 (0.981.02) | 0.769 |
| Charlson Comorbidity Score | 1.11 (0.841.46) | 0.471 |
| Length of hospital stay | 1.02 (0.981.07) | 0.299 |
| Definite anticholinergic use* | 0.74 (0.461.20) | 0.219 |
| Any delirium during hospital stay | 1.65 (0.982.77) | 0.061 |
| Diabetes mellitus | 0.72 (0.411.26) | 0.253 |
| Myocardial infarction | 0.83 (0.411.66) | 0.593 |
| Congestive heart failure | 0.83 (0.471.47) | 0.524 |
| Peripheral vascular disease | 1.39 (0.613.18) | 0.433 |
| Cerebrovascular disease | 1.30 (0.652.59) | 0.464 |
| Malignancy | 0.45 (0.171.21) | 0.113 |
| Metastatic cancer | 0.15 (0.021.16) | 0.069 |
| Chronic obstructive pulmonary disease | 0.91 (0.531.55) | 0.727 |
Table 3 provides results from the Cox models for receiving GCS on readmission and mortality outcomes adjusting for various sets of covariates and with the propensity score adjustment. Model 1 presents unadjusted hazard ratio (HR). Model 2 presents HRs adjusting for a common set of covariates that were significantly associated with at least 1 of the outcomes, whereas model 3 presents the results adjusting for all covariates. All 4 models yielded similar results. As evident from this table, propensity‐adjusted HR for 30‐day readmission was still significantly higher among patients receiving GCS (HR=1.75; 95% confidence interval=1.06‐2.88) but not at 1 year (HR=1.19; 95% confidence interval=0.89‐1.59). There was a trend for decreased mortality for the GCS group at 30 days (HR=0.35; 95% confidence interval=0.09‐1.35), but it disappeared at 1 year (HR=0.91; 95% confidence interval=0.59‐1.40). A composite outcome of readmissions and mortality did not show any difference between the GCS and no‐GCS groups.
| Outcome Variables | Model 1 | Model 2 | Model 3 | Propensity Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ||||||||
| Readmission within 30 days | 1.65 (1.05, 2.59) | 0.030 | 1.73 (1.08, 2.78) | 0.024 | 1.84 (1.133.00) | 0.015 | 1.75 (1.062.88) | 0.029 |
| Readmission within 1 year | 1.13 (0.87, 1.46) | 0.373 | 1.24 (0.94, 1.63) | 0.125 | 1.26 (0.941.68) | 0.117 | 1.19 (0.891.59) | 0.245 |
| Death within 30 days | 0.43 (0.12, 1.56) | 0.199 | 0.34 (0.09, 1.28) | 0.110 | 0.25 (0.061.02) | 0.053 | 0.35 (0.091.35) | 0.126 |
| Death within 1 year | 0.95 (0.65, 1.45) | 0.806 | 0.87 (0.58 1.31) | 0.506 | 0.93 (0.601.42) | 0.724 | 0.91 (0.591.40) | 0.669 |
| Readmission or Death within 30 days | 1.48 (0.97, 2.26) | 0.070 | 1.49 (0.96, 2.33) | 0.078 | 1.56 (0.982.47) | 0.061 | 1.55 (0.972.48) | 0.069 |
| Readmission or death within 1 year | 1.05 (0.82, 1.34) | 0.699 | 1.11 (0.86, 1.43) | 0.412 | 1.15 (0.881.50) | 0.318 | 1.08 (0.831.42) | 0.569 |
DISCUSSION
To our knowledge, this is the first study to analyze the impact of GCS on hospital readmission and mortality rates of CI patients. Our results did not show any short‐term or long‐term benefits of GCS for CI patients. Recent studies exploring cost benefits of the GCS have found trends toward lower readmission, but none focused on patients with CI.[6, 24, 25] It is important to note that our study did not use random allocation to assigning the patient into the GCS or control group, thus raising the possibility that patients who received GCS were sicker and were medically and socially more complex than those who did not receive the consult. Moreover, GCS consultation is preferentially sought for and completed for patients with CI and functional limitations, consistent with our finding that GCS patients more often have delirium and are less‐often discharged home.
The nature of the GCS team is another important consideration. Our GCS model did not include unit cohorting of patients, an important component of other proposed GCS models.[26] A recent meta‐analysis found that the GCS models without unit cohorting of patients did not have an impact on 1‐ or 12‐month readmission rates.[27] Low adherence to consultant recommendations (less than 33%) was thought to be a reason for such results. Importance of cohorting with regard to accomplishing recommendations by primary teams, importance of unit staff expertise in geriatric principles, and impact of a unit model on teamwork has also been highlighted by another review.[28] These findings lend to the hypothesis that unit cohorting and direct order placement by the GCS team may improve outcomes among CI patients, including a reduction in readmission rates.
Although readmissions rates were not statistically different between GCS and control groups at 1‐year postdischarge, 30‐day readmission rates were higher among the GCS group. Previous research among older heart failure patients found that a comprehensive transitional care intervention at the time of hospital discharge significantly shortened the time to readmission in the intervention group (P=0.026).[29] The factors identified by the study authors included enhanced supervision by the transitional healthcare teams along with improved awareness and education among treated patients that may have facilitated early recognition of clinical deterioration.[29] A recent study with intensive outpatient care that resulted in increased admissions among chronically ill adults provided a similar conclusion.[30]
GCS patients showed a trend toward decreased mortality as did patients enrolled in previous studies evaluating GCS models in the inpatient setting, as suggested by a recent review.[27] A caveat to note is that these trends favored ward‐styled GCS services as compared to our open GCS model,[27, 28] although the factors cited in these dedicated units affecting mortality included prompt attention to early rehabilitation, delirium management, and prevention of pressure ulcers and are also frequently implemented for patients in our GCS service model and therefore may have produced similar results.
Our neutral results in regard to the readmissions need to be interpreted with caution. First, this study was conducted in a hospital that supports expert geriatric and palliative care teams, both in the inpatient and the ambulatory settings, that provide consultative services and train medicine teams and hospital nursing staff. On the outpatient side, the presence of a robust geriatrics house‐calls program and the Geriatric Resources for Assessment and Care of Elders team results in above‐average care for the control group, and thus may also impact apparent outcomes.[31, 32] Second, 30‐day readmissions represent a complex outcome. Two recent reviews of hospital‐initiated interventions have shown that evidence regarding best strategies to decrease 30‐day readmissions is unclear.[33] Neither review included studies that targeted patients with CI only. The 2 programs that reduced 30‐day readmissions were multifaceted and included personnel who provide bridging between the hospital and the outpatient setting.[34] The GCS does include a focus on postdischarge resources, but does that on a case‐by‐case basis and no formal posthospital follow‐ups are provided. Moreover, the value of 30‐day readmission rates as a marker of quality, even though used by policymakers as an indicator of hospital quality, remains controversial.[35, 36] Broadening the outcomes of interest to include patient‐centered outcomes including satisfaction with care, that have shown to impact other health outcomes, may help improve understanding the benefits of GCS in hospitals.[37] Other comprehensive transitional care models that failed to show a benefit on 30‐day readmissions in older patients still resulted in higher satisfaction among patients.[38] Unfortunately, our evaluation did not include an assessment of patient satisfaction and quality of transitions.
Since the study period, GCS at our hospital now has incorporated a more robust focus on advance care planning (ACP) and execution of Physician Orders for Scope of Treatment that were legislated in the state in July 2013. The GCS team members are expert in carrying out complex ACP discussions and also partner with the inpatient palliative care team. It is quite possible that a study of more recent outcomes will yield more positive results for the selected outcomes. Thus, for future trials that aim to study the impact of GCS in the inpatient settings, it may be advisable to include important quality markers such as implementation of ACP and patient satisfaction along with the health utilization outcomes.
Limitations
As mentioned previously, it is possible that our risk adjustment was insufficient to account for all the medical and psychosocial differences among groups. For example, the overall anticholinergic impact of various medications such as antipsychotic medications and histamine‐2 blockers was assessed via the Anticholinergic Burden Scale on admission, but we did not have information on medication prescribing during the stay. We were further limited by lack of baseline functional status and socioeconomic details, both of which are related to 30‐day readmissions. For example, living alone, prior use of assist devices, and belonging to lower socioeconomic status are correlated with higher readmission rates.[39, 40] Patients with available social support may receive more intense supervision and may seek medical attention sooner. On the other hand, worsening health among CI patients without any approximate social support may be unnoticed for days. Absence of details of inpatient interventions may also have resulted in unmeasurable confounders that could have impacted our study outcomes. Finally, lack of information on the uptake of GCS recommendations by the primary teams is another limitation of this analysis. Future trials should include strategies to address these information gaps.
CONCLUSION
Our results comparing inpatient geriatrics consultative services with usual care in hospitalized elders having cognitive impairment failed to demonstrate an impact on readmissions and mortality. A clinical lesson learned, however, is that much work is still required to reduce readmission and mortality rates in this especially vulnerable patient population.
Disclosures
Disclosures: This work was supported by grants from a Geriatric Academic Career Award (K01HP20517) through Health Resources and Services Administration, R01AG034205 and K23‐AG043476 from the National Institute on Aging, and the John A. Hartford Foundation Center for Excellence in Geriatric Medicine. The sponsors had no role in the study design, evaluation, or manuscript development. The authors report no conflicts of interest.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , Cognitive impairment. Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34(8):579–585.
- , , , , , Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- , Understanding preventable hospital readmissions: masqueraders, markers, and true causal factors. J Hosp Med. 2011;6(2):51–53.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581.
- , ; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557.
- , , , Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139–2145.
- , , , et al. Screening of the risk of functional decline performed by an inpatient geriatric consultation team in a general hospital [in French]. Revue medicale de Bruxelles. 2013;34(6):462–468.
- , , , et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759.
- , , Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm. 2003;18(1):37–42, 47–39.
- , , , Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–996.
- , , , , , Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013;173(11):981–987.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567.
- , , , Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35(5):412–416.
- , , , et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253.
- , , , , , Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist. 1993;33(6):714–720.
- , , , , , Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963.
- , , , , , Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003.
- , , , et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. Jt Comm J Qual Improv. 1998;24(2):63–76.
- , , , et al. Developing a stroke unit using the acute care for elders intervention and model of care. J Am Geriatr Soc. 2003;51(11):1660–1667.
- , , , A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545–552.
- , , , , Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta‐analysis. BMC Med. 2013;11:48.
- , , , , Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , House calls for seniors: building and sustaining a model of care for homebound seniors. J Am Geriatr Soc. 2009;57(6):1103–1109.
- , , , et al. Geriatric care management for low‐income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633.
- , , , , Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs. 1999;14(1):44–54.
- Readmission to hospital: a measure of quality or outcome? Qual Saf Health Care. 2004;13(1):10–11.
- , , , , , Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff (Millwood). 2012;31(7):1623–1632.
- , , Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modeling approach. Patient Educ Couns. 2013;91(2):167–175.
- , , , The Care Transitions Innovation (C‐TraIn) for Socioeconomically Disadvantaged Adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460–1467.
- , , , Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62(3):489–494.
- , , , , , Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , , , Cognitive impairment. Can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34(8):579–585.
- , , , , , Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279(15):1187–1193.
- , Understanding preventable hospital readmissions: masqueraders, markers, and true causal factors. J Hosp Med. 2011;6(2):51–53.
- , , , et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676.
- , , , et al. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581.
- , ; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557.
- , , , Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139–2145.
- , , , et al. Screening of the risk of functional decline performed by an inpatient geriatric consultation team in a general hospital [in French]. Revue medicale de Bruxelles. 2013;34(6):462–468.
- , , , et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759.
- , , Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm. 2003;18(1):37–42, 47–39.
- , , , Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990–996.
- , , , , , Effects of an acute care for elders unit on costs and 30‐day readmissions. JAMA Intern Med. 2013;173(11):981–987.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567.
- , , , Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly. J Am Geriatr Soc. 1987;35(5):412–416.
- , , , et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform. 1999;54(3):225–253.
- , , , , , Factors determining the decision to institutionalize dementing individuals: a prospective study. Gerontologist. 1993;33(6):714–720.
- , , , , , Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948.
- , , , , , Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322.
- , , , et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636.
- , , , et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc. 2013;61(11):1958–1963.
- , , , , , Propensity scores in intensive care and anaesthesiology literature: a systematic review. Intensive Care Med. 2010;36(12):1993–2003.
- , , , et al. Improving functional outcomes in older patients: lessons from an acute care for elders unit. Jt Comm J Qual Improv. 1998;24(2):63–76.
- , , , et al. Developing a stroke unit using the acute care for elders intervention and model of care. J Am Geriatr Soc. 2003;51(11):1660–1667.
- , , , A medical unit for the acute care of the elderly. J Am Geriatr Soc. 1994;42(5):545–552.
- , , , , Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta‐analysis. BMC Med. 2013;11:48.
- , , , , Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , , et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590.
- , , , et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157(1):1–10.
- , , , , House calls for seniors: building and sustaining a model of care for homebound seniors. J Am Geriatr Soc. 2009;57(6):1103–1109.
- , , , et al. Geriatric care management for low‐income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633.
- , , , , Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , The effects of a discharge planning and home follow‐up intervention on elders hospitalized with common medical and surgical cardiac conditions. J Cardiovasc Nurs. 1999;14(1):44–54.
- Readmission to hospital: a measure of quality or outcome? Qual Saf Health Care. 2004;13(1):10–11.
- , , , , , Unintended consequences of steps to cut readmissions and reform payment may threaten care of vulnerable older adults. Health Aff (Millwood). 2012;31(7):1623–1632.
- , , Analyzing the effects of shared decision‐making, empathy and team interaction on patient satisfaction and treatment acceptance in medical rehabilitation using a structural equation modeling approach. Patient Educ Couns. 2013;91(2):167–175.
- , , , The Care Transitions Innovation (C‐TraIn) for Socioeconomically Disadvantaged Adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460–1467.
- , , , Risk factors for early hospital readmission in low‐income elderly adults. J Am Geriatr Soc. 2014;62(3):489–494.
- , , , , , Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood). 2013;32(7):1196–1203.
© 2015 Society of Hospital Medicine
Impact of CI Among Hospitalized Elders
In 2001, approximately 12.6 million individuals age 65 and older were discharged from American hospitals with an average length of stay of 5.8 days1 and up to 66% of them suffered from cognitive impairment (CI).220 CI in hospitalized older adults includes a variety of disorders ranging from mild cognitive deficit, delirium, to full‐blown dementia. Dementia is a syndrome of decline in memory plus at least 1 other cognitive domain, such as language, visuospatial, or executive function sufficient to interfere with social or occupational functioning in an alert person.21 Delirium is a disturbance of consciousness with reduced ability to focus, sustain, or shift attention that occurs over a short period of time and tends to fluctuate over the course of the day.22 Mild CI without dementia is defined as the presence of a cognitive deficit in the absence of delirium that does not affect functional performance.23
Hospitalized older adults with CI are vulnerable to hospital complications, including delirium, physical restraints, urinary catheters, and tethers.2, 3, 2435 The management of their medical or surgical illnesses requires avoiding certain medications with anticholinergic activities that might worsen cognition.36 Furthermore, CI may delay diagnostic and therapeutic procedures, demand more time for informed consentrelated issues, and result in difficulty in adherence to medical recommendations.37, 38 The special needs of hospitalized older adults with delirium and dementia has been shown to increase demands on nursing staff, risk of postdischarge institutionalization, length of stay, and health care costs.310, 27, 3948 We wanted to look specifically at CI because it often goes undetected4951 and can have a great impact on the hospital course of elders.
Screening for CI among hospitalized older adults has been considered to have potential benefit in hospital care of older adults.52 Screening may lead to early detection by uncovering subtle symptoms not yet apparent to families or other caregivers who know the patient well but do not notice small declines or changes in day‐to‐day functioning. Early recognition of CI may lead to early treatment and subsequently may delay progression of cognitive decline and improve health outcomes. Screening may enhance physician prescribing practices and reduce exposure to harmful medications among these vulnerable patients. Finally, delirium is an important prognostic indicator, and screening patients could provide invaluable information toward the overall clinical picture. Despite all of this, the current literature does not provide sufficient information to support the use of routine screening on admission.220, 41, 5254 Most of the published studies were conducted among elders who stayed in the hospital for more than 48 hours, missing data on the crucial first 48 hours of the hospital course.220, 41, 5254 These studies did not evaluate the impact of unrecognized CI on the hospital course and the majority of these studies were not conducted in the urban and lower socioeconomic status populations of elders that are the most vulnerable to bad health outcomes.220, 41, 5254 Finally, few studies evaluated the impact of delirium superimposed on CI on the hospital course and mortality of elders.220, 41, 5254
With these details in mind, we wanted to explore the impact of CI recognition among patients age 65 years and older admitted to the medical services of an urban, public hospital in Indianapolis to determine the prevalence and the impact of recognized and unrecognized CI on the hospital course of these elders. Furthermore, we examined the role of delirium superimposed on these hospitalized elders with CI.
Patients and Methods
The study was approved by the Indiana University Purdue University at Indianapolis Institutional Review Board (IRB).
Study Setting and Population
The study was conducted on the inpatient general medicine service of Wishard Memorial Hospital (WMH). WMH is a 450‐bed, university‐affiliated, urban, public hospital that is staffed by Indiana University School of Medicine faculty and house staff. It serves a population of approximately 750,000 in Marion County.
Inclusion and Exclusion Criteria
Patients were enrolled in the study based on the following criteria: (1) at least 65 years of age; (2) hospitalized on a medical ward; (3) able to speak English; and (4) have CI at the time of hospital admission (see below). Patients were excluded if they had previously enrolled in the study, were enrolled in another clinical study at the time of admission, or were aphasic or unresponsive at the time of screening.
Cognitive Screening
CI was determined by the Short Portable Mental Status Questionnaire (SPMSQ),55, 56 chosen for its accuracy56 and the fact that it is entirely verbal in administration. In most cases, patients were followed and reassessed daily. Patients having 2 or more errors, indicating a score of 8 or less on the SPMSQ after adjusting for race and education were considered to have cognitive impairment. The SPMSQ is a brief 10‐item screening test with a sensitivity of 86% and specificity 99.0% for dementia among medical inpatients.56 At the time of cognitive screening, delirium was assessed by using the Confusion Assessment Method (CAM).22 This was also done daily in most cases. The CAM22 is a structured instrument that evaluates the 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III‐R: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. The CAM score is determined by examining the patient, investigating the chart and interviewing the nurse and/or a family member for: (1) acute and fluctuating changes in mental status, (2) inattention, (3) disorganized or incoherent thinking, and (4) altered level of consciousness. A CAM score is considered to be positive if the patient displays both (1) and (2) with at least one of (3) or (4). The CAM diagnosis of delirium was validated against the clinical judgment of a psychiatrist and found to have a sensitivity of 97% and a specificity of 92%.22 A research assistant (RA) was trained for a period of 9 months by a physician as a rater to interview the patient and administer both the SPMSQ and the CAM at the time of admission and then every weekday. When feasible, the RA administered both the SPMSQ and the CAM within the first few hours of hospitalization, and then followed up with our patients each day. More than 70% of our initial cognitive screening occurred in the first 48 hours of hospital admission, and was repeated on a daily basis. In addition to cognitive assessment, the RA reported the presence or absence of Foley catheterization, physical restraints, and tethers during the cognitive assessment. Agreement was obtained from the general internal medicine group practice physicians both to participate in the study and to request screening for CI as part of the recognized admission standard of care among their hospitalized patients aged 65 years and older. The study coordinator was notified of all admissions for patients aged 65 or older by the hospital intranet e‐mail and paging system. Admission notifications were sent by page and e‐mail on an hourly basis from Monday through Friday, 8:00 AM through 5:00 PM. Those admissions occurring between the hours of 5:00 PM and 8:00 AM were sent during the next normal batch notification. Pages and e‐mails for admissions occurring on Saturday and Sunday were sent on Monday morning at 8:00 AM.
Regenstrief Medical Record System at WMH
The computerized Regenstrief Medical Record System (RMRS) is the primary instrument for processing data and monitoring patient and physician activity for Wishard Health System.57, 58 The RMRS is a modular system, composed of Registration and Scheduling, Laboratory, and Pharmacy database modules. The Registration and Scheduling module is used to make all outpatient appointments for the office practices associated with Wishard Health System. The Laboratory module handles all data for all inpatient and outpatient laboratories. This module also produces all laboratory reports and data used for billing. In addition to laboratory data, this module stores coded results and full‐text interpretations of all imaging studies and special procedures. The Pharmacy module contains information on medication orders captured by the computerized physician order enter (CPOE). The Database module stores all the above data by date in a fully‐coded form. Thus, these data are readily retrievable for individual patients by healthcare providers using online terminals. Data for large numbers of patients are retrievable using a locally developed English‐like language called CARE. Patients can be identified either by a certain restriction list (eg, the list of subjects in a study) or by clinical criteria. The RMRS also maintains a number of other databases including diagnoses, vital signs, results of laboratory tests and diagnostic tests, full‐text discharge summaries, preventive health maneuvers, and detailed information on all inpatient and outpatient charges. It contains death certificate information from the Indiana State Board of Health for all registered patients who die in, or outside of, Indiana. Therefore, the RMRS collects and monitors a broad array of physician and patient activity, practice patterns, utilization, diagnostic test finding, and offers a wonderful array of outcome measures.
Other Data Collections
Patient demographics such as age, gender, race, and education level were determined by the RMRS and by information obtained during the time of cognitive screening. Length of hospital stay and 30‐day posthospitalization mortality were obtained from the RMRS. Comorbidity level was measured by reviewing the RMRS and determining each patient's Charlson comorbidity index total score.59, 60 This score was determined using International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD‐9) codes gathered from 1 year prior to admission until the patient was discharged from the hospital. Anticholinergic medications were determined by using the Anticholinergic Cognitive Burden Scale,61 an expert‐based practical index. The scale was developed based on a review of all published studies from 1996 to 2007 that measured the anticholinergic activities of a drug and its association with cognitive function in older adults. The list of drugs reviewed was presented to an expert interdisciplinary panel that included geriatricians, geriatric pharmacists, geriatric psychiatrists, general physicians, geriatric nurses, and aging brain researchers. The panel categorized each medication into a possible or definite anticholinergic category based on the severity of its cognitive anticholinergic effects.61 A patient who received at least 1 order of a possible or definite anticholinergic during their hospitalization was considered to be an anticholinergic user. Prior recognition of CI was determined by searching the RMRS for any ICD‐9 code (see Appendix) indicative of dementia, Alzheimer disease, or delirium reported at hospital admission, discharge, or during an 1‐year period prior to hospitalization for every patient enrolled in the study. Those patients with documented ICD‐9 codes were felt recognized as having some form of cognitive impairment. Those who had a positive screen but no prior documentation according to ICD‐9 coding, were said to have unrecognized CI.
Analysis
Descriptive statistics were calculated, including percentages for binary categorical variables, and means and standard deviations for continuous variables. Comparisons between groups were based upon Fisher's Exact Tests for binary categorical variables and t tests for continuous variables. When controlling for covariates such as age, gender, race, Charlson comorbidity index, and SPMSQ at screening, group comparisons were made by using logistic regression for binary categorical variables and multiple regression for continuous variables. Since the distributions of length of stay and Charlson comorbidity index were skewed, all statistical tests comparing them across groups were actually performed on their log‐transformed values.
Results
The Prevalence and Recognition of CI
Table 1 describes the demographic characteristic of our study population, which is a reflection of the public and urban nature of our target hospital. Our study assessed the cognitive status of 997 older adults usually (>70% of the time) within 48 hours of their admission to the medical ward of this urban hospital between July of 2006 and March 2008 (see Table 1) and found that 43% of these elders had evidence of CI as determined by a SPMSQ score of 8 points or less. However, 61% of the 424 cognitively impaired elders were not documented or recognized by the electronic medical record system to have cognitive deficit.
| Variable | n | %/Mean (SD) |
|---|---|---|
| ||
| Age (years), mean (SD) | 997 | 74.8 (7.5) |
| Age 85 (%) | 997 | 12.6 |
| Female (%) | 997 | 67.8 |
| African American (%) | 997 | 59.4 |
| Education (years), mean (SD) | 910 | 10.3 (2.8) |
| Education <12 years (%) | 910 | 59.1 |
| Screened within 48 hours of admission (%) | 997 | 73.2 |
| SPMSQ score at screening, mean (SD) | 997 | 7.7 (2.8) |
| Cognitive impairment based on the SPMSQ score 8 (%) | 997 | 42.5 |
The Impact of Unrecognized CI on the Hospital Course
As expected, hospitalized elders with documented CI were older (mean age 79.1 years vs. 76.1 years; P < 0.001) and had worse cognitive function upon screening than those with unrecognized CI (mean SPMSQ 3.4 points vs. 6.3; P < 0.001). Furthermore, CI recognition was influenced by the elders' race and comorbidity (Table 2); a higher percentage of elders with documented CI were African American (69% vs. 54%; P = 0.003) and had less comorbidity (mean Charlson index 1.9 vs. 2.3; P = 0.03). After adjusting for age, gender, race, comorbidity, and cognitive function at screening, our study found no differences between elders with previously recognized CI and those with unrecognized CI in regard to the length of hospital stay (6.7 days vs. 7.5 days; P = 0.59), 30‐day posthospital mortality (4.8% vs. 6.6%; P > 0.2), home discharge (32% vs. 45%; P > 0.7), hospital readmission (19.2% vs.18.8%; P > 0.6), delirium incidence (27% vs. 21%; P > 0.9), and physical restraints (1.8% vs. 1.5%; P > 0.4). We also found that elders with undocumented CI were not more likely to receive definite anticholinergics (33.2% vs. 32.7%; P > 0.9).
| CI Documented | CI Undocumented | P Value | P Value* | |
|---|---|---|---|---|
| ||||
| n (%) | 165 (39) | 259 (61) | n/a | |
| Age, mean (SD) | 79.1 (7.9) | 76.1 (8.0) | <0.001 | |
| Female (%) | 68.5 | 64.5 | 0.40 | |
| African American (%) | 68.5 | 53.7 | <0.01 | |
| SPMSQ at screen, mean (SD) | 3.4 (2.7) | 6.3 (2.1) | <0.001 | |
| Charlson comorbidity index, mean (SD) | 1.9 (1.9) | 2.3 (2.1) | 0.03 | |
| Length of hospital stay, mean (SD) | 6.7 (5.1) | 7.5 (7.1) | 0.49 | 0.59 |
| Survived at 30 days postdischarge (%) | 95.2 | 93.4 | 0.53 | 0.25 |
| Discharged home (%) | 31.5 | 45.2 | 0.01 | 0.74 |
| Readmission within 30 days after discharge home (%) | 19.2 | 18.8 | 0.99 | 0.66 |
| Incidence of delirium (%) | 26.7 | 20.6 | 0.52 | 0.99 |
| Observed with Foley catheter (%) | 43.6 | 27.4 | <0.001 | 0.61 |
| Observed with physical restraint (%) | 1.8 | 1.5 | 0.99 | 0.31 |
| Observed with tethers (%) | 81.8 | 73.8 | 0.06 | 0.58 |
| With at least 1 Ach (%) | 83.6 | 90.7 | 0.03 | 0.22 |
| Possible Ach (%) | 81.2 | 88.4 | 0.05 | 0.31 |
| Definite Ach (%) | 32.7 | 33.2 | 0.99 | 0.64 |
The Impact of Delirium on the Hospital Course of Elders with CI
Among the 424 hospitalized elders with CI, 163 (38%) had delirium at least once during their hospital course and 24% had delirium on the day of hospital discharge. In comparison to elders who had CI but not delirium during their hospitalization (Table 3), those with at least 1 day of delirium had a higher 30‐day posthospitalization mortality risk (8.6% vs. 4.2%; P = 0.09), stayed in the hospital 3.3 additional days (9.2 days vs. 5.9 days; P < 0.001), were less likely to be discharged home (25% vs. 49%; P < 0.001), were more likely to receive a Foley catheterization (52% vs. 23%; P < 0.001), more likely to be physically restrained (4% vs. 0%; P < 0.01), and more likely to receive tethers during their care (89% vs. 69%; P < 0.001). There was no statistically significant difference between the 2 groups in terms of 30‐day hospital readmission rates or in their use of definite anticholinergics (Table 3).
| Delirium+* | Delirium | P value | |
|---|---|---|---|
| |||
| n (%) | 163 (38) | 261 (62) | n/a |
| Age, mean (SD) | 78.4 (8.5) | 76.5 (7.8) | 0.02 |
| Female (%) | 60.1 | 69.7 | 0.05 |
| African American (%) | 64.4 | 56.3 | 0.10 |
| Charlson comorbidity index, mean (SD) | 1.8 (1.9) | 2.3 (2.1) | 0.01 |
| Length of hospital stay, mean (SD) | 9.2 (7.9) | 5.9 (4.9) | <0.001 |
| Survived at 30‐day postdischarge (%) | 91.4 | 95.8 | 0.09 |
| Discharged home (%) | 24.5 | 49.4 | <0.001 |
| Readmission within 30 days after discharge home (%) | 22.5 | 17.8 | 0.50 |
| Observed with Foley catheter (%) | 51.5 | 22.6 | <0.001 |
| Observed with physical restraint (%) | 4.3 | 0.0 | <0.01 |
| Observed with tethers (%) | 89.0 | 69.4 | <0.001 |
| With at least 1 anticholinergic (%) | 83.4 | 90.8 | 0.03 |
| Possible anticholinergic (%) | 80.4 | 88.9 | 0.02 |
| Definite anticholinergic (%) | 36.8 | 30.7 | 0.20 |
Discussion
Our study found that in an urban, public hospital, acute or preexisting CI affects more than one‐third of hospitalized elders admitted to general medical services. Unfortunately, our hospital system does not currently recognize the majority of these vulnerable patients. Our study also found that delirium affects more than one‐third of hospitalized elders with CI during their hospital course. Delirium complicates hospital care by prolonging length of stay and decreasing the probability of surviving and getting discharged home. It leads to high use of Foley catheterization, physical restraints, and tethers.
The high prevalence of CI with and without delirium in our cohort is within the rates reported previously in the literature. It is estimated that the prevalence of CI in hospitalized older adults ranges from 14% to 66%, depending on the method used to measure cognition, the definition of CI, and the type of hospital ward (surgical, medical, and geriatric units).220 One particular study that used a similar cognitive assessment method reported higher prevalence rates for both CI and delirium.11 The study randomly evaluated a sample of 201 patients age 65 and over who were hospitalized for a medical illness and found that 56% of the cohort suffered from CI and among those with CI, 47% had delirium.11 The difference between this finding and our study is most likely due to our sampling technique; more than 70% of our cognitive screening occurred in the first 48 hours of hospital admission whereas the Australian study, in similar enrollment criteria to all of the published studies in this area, excluded patients who were discharged within 48 hours of admission. We believe, however, that by including the first 48 hours of admission in our design, our study provides a more generalizable reflection of the actual acute care experience.
The impact of delirium on the course of hospital care found in our study supports some of the findings from previous studies conducted in the past 2 decades.5, 6, 11 Despite 2 decades of clinical research, delirium continues to increase mortality, hospital stays, and posthospital institutionalization.
We were surprised to find that patients suffering from delirium continue to receive at least 1 definite anticholinergic medication. Such medications are considered inappropriate among patients with any form of cognitive impairment.36, 62 Although the impact of anticholinergic medications on hospitalized outcomes is less well‐described, their use has been suspected to negatively impact long‐term outcomes of cognitive impairment.61, 63 Our study found no difference in the use of anticholinergic medications between those with CI who experienced delirium and those who did not; however, the total burden of anticholinergic medication was not assessed in a quantitative manner. It is still unknown if certain anticholinergic medications or a cumulative effect of anticholinergic medications may impact cognitive or health‐related outcomes in a vulnerable older population with CI.
Although our study reported for the first time in a systematic way the rate of undocumented CI among hospitalized elders found to have CI on admission, we found no impact of such underrecognition on the length of hospital stay, mortality, discharge location, and delirium occurrence. Although the use of anticholinergic medications is not recommended for patients with any form of CI, our results indicate that a significant number of patients with cognitive impairment continue to receive inappropriate medications. CI recognition in the elderly was not shown to have a statistically significant affect on length of stay, cost, or mortality.
Our study has some limitations. First of all, we did not determine the underlying types of CI such as Alzheimer disease, vascular dementia, mild cognitive impairment, or reversible etiology other than delirium. Such a categorization requires posthospital assessment, which was not included in our study design. Second, our delirium incidence rate and delirium impact on hospital outcomes might be very conservative and may underestimate its true prevalence and correlation due to our data collection methods. Despite the fluctuating nature of delirium, our study was not designed to assess the presence of delirium every shift and tried to assess cognitive function on a daily basis throughout the patient's hospitalization. Therefore, the severity and duration of delirium could not be accurately assessed. Our reported rates of use of Foley catheterization, physical restraints, and tethers are also very conservative and we could not determine the appropriateness of these procedures. Our study was conducted in 1 public hospital in an urban city with a higher percentage of African Americans. Thus, our sample is not a true representative sample. However, studies with significant representation of minority groups are not common in the research literature, especially in CI research; we hope to fulfill some of the gaps in the literature regarding the most vulnerable older American population. Finally, we were limited in our use of ICD‐9 coding to determine if patients had previously been recognized by other providers as having CI. ICD‐9 coding, while useful, is not perfect in identifying all if a patient's medical problems. Use of coding to determine whether a patient had been recognized as impaired also does not allow us to determine when the diagnosis was made.
In conclusion, our study evaluated cognitive impairment in hospitalized elders and found that in our cohort of 997 patients, 43% were cognitively impaired on admission. Of those with CI, 61% were not documented or recognized as impaired. We found no statistically significant difference between those with documented CI and those with undocumented CI in terms of length of stay, mortality, home discharge, readmission rates, incidence of delirium, or potential to receive anticholinergics or restraints. Among those with CI, 38% had delirium. Those with delirium experienced increased length of stay, decreased discharge to home, and increased use of Foley catheters and restraints.
- ,.National hospital discharge survey: annual summary, 1994.Vital Health Stat 13.1997;(128):i–v;1–50.
- .The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients.Am J Med.1994;97:278–288.
- ,,.Dementia in medical wards.J Clin Epidemiol.1988;41:123–126.
- ,,,,,.Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging.Am J Psychiatry.2000;157:708–714.
- ,,, et al.Randomized, placebo‐controlled, double‐blind clinical trial of sertraline in the treatment of depression complicating Alzheimer's disease: initial results from the Depression in Alzheimer's Disease study.Am J Psychiatry.2000;157:1686–1689.
- ,,.Dementia in elderly persons in a general hospital.Am J Psychiatry.2000;157:704–707.
- ,,,,.A prospective study of the impact of psychiatric comorbidity on length of hospital stays of elderly medical‐surgical inpatients.Psychosomatics.1998;39:273–280.
- ,.Psychiatric comorbidity and length of stay in the general hospital. A critical review of outcome studies.Psychosomatics.1994;35:233–252.
- ,,,.Dementia among medical inpatients. Evaluation of 2000 consecutive admissions.Arch Intern Med.1986;146:1923–1926.
- ,,,.The consequences of non‐cognitive symptoms of dementia in medical hospital departments.Int J Psychiatry Med.2003;33:257–271.
- ,,.Cognitive impairment in medical inpatients. I: Screening for dementia—is history better than mental state?Age Ageing.1997;26:31–35.
- ,,, et al.Acute confusional states in elderly patients treated for femoral neck fracture.J Am Geriatr Soc.1988;36:525–530.
- ,.A prospective study of elderly general surgical patients: II. Post‐operative complications.Age Ageing.1989;18:316–326.
- ,,,.Dementia and depression in elderly medical inpatients.Int Psychogeriatr.2000;12:67–75.
- ,,.[Psychiatric disorders in elderly general hospital patients: incidence and long‐term prognosis].Nervenarzt.1993;64:53–61. [German]
- ,.Delirium and dementia in acute medical admissions of elderly patients in Iceland.Acta Psychiatr Scand.1993;87:123–127.
- ,,,,,.[Psychiatric morbidity in elderly patients admitted to a general hospital. A day‐prevalence study].Med Clin (Barc).1991;97:206–210. [Spanish]
- ,,,,,.Detection of psychiatric disorders in elderly medical inpatients.Age Ageing.1994;23:307–311.
- ,,,.Cognitive impairment, emotional disorder and length of stay of elderly patients in a district general hospital.Br J Med Psychol.1987;60(Pt 2):133–139.
- ,,.An investigation of the components of best nursing practice in the care of acutely ill hospitalized older patients with coincidental dementia: a multi‐method design.J Adv Nurs1999;30:1127–1136.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,, et al.Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging.Neurology.2001;57:1655–1662.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care.Am J Med.1999;106:565–573.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Iatrogenic causes of falls in hospitalised elderly patients: a case‐control study.Postgrad Med J.2002;78:487–489.
- ,,.A prospective study of delirium in hospitalized elderly.JAMA.1990;263:1097–1101.
- ,.The prognostic significance of delirium in older hospital patients.J Am Geriatr Soc.1997;45:174–178.
- ,.Prognosis of delirium in elderly hospital patients.CMAJ.1993;149:41–46.
- ,,,,.The detection of psychiatric morbidity and its effects on outcome in acute elderly medical admissions.Int J Ger Psych1991;6:861–866.
- ,,.Adverse consequences of hospitalization in the elderly.Soc Sci Med.1982;16:1033–1038.
- ,,, et al.Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I.N Engl J Med.1991;324:370–376.
- ,,,.Delirium in elderly patients: an overview of the state of the science.J Gerontol Nurs.2001;27:12–20.
- ,,,,.A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics.Ann Intern Med.1993;119:474–481.
- ,.Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability.JAMA.1996;275:852–857.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- ,,, et al.A clinical prediction rule for delirium after elective noncardiac surgery.JAMA.1994;271:134–139.
- ,,, et al.The relationship of postoperative delirium with psychoactive medications.JAMA.1994;272:1518–1522.
- ,,, et al.How do delirium and dementia increase length of stay of elderly general medical inpatients?Psychosomatics.2004;45:235–242.
- ,,,,,.The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use.J Am Geriatr Soc.2004;52:187–194.
- ,,, et al.Risk factors for delirium in hospitalized elderly.JAMA.1992;267:827–831.
- ,,,,.Psychological comorbidity and length of stay in the general hospital.Am J Psychiatry.1991;148:324–329.
- ,,, et al.Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients.Arch Intern Med.1992;152:334–340.
- ,,.What happens to medical patients with psychiatric disorder?J Psychosom Res1988;32:541–549.
- ,,,,.[Dementia syndromes and length of stay of elderly patients in internal medicine].Ann Med Interne (Paris).1997;148:424–426. [French]
- ,,, et al.[Hospital discharge planning and length of hospital stay in elderly patients admitted through the emergency department].Rev Epidemiol Sante Publique.1995;43:337–347. [French]
- ,,,,.The effect of dementia on acute care in a geriatric medical unit.Int Psychogeriatr.1992;4:231–239.
- ,,,.Cognitive impairment. Can it predict the course of hospitalized patients?J Am Geriatr Soc.1986;34:579–585.
- ,,,,;US Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2003;138(11):927–937.
- ,,,.Cognitive impairment in the elderly medically ill: how often is it missed?Int J Geriatr Psychiatry.1993;8:929–937.
- .Recognition of cognitive impairment in elderly medical in‐patients.J R Soc Med.1995;88:183–184.
- ,.Quality indicators for dementia in vulnerable community‐dwelling and hospitalized elders.Ann Intern Med.2001;135:668–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,.Prevalence of psychotic symptoms in delirium.Psychosomatics.2000;41:519–522.
- .A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients.J Am Geriatr Soc.1975;23:433–441.
- ,,,.Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly.J Am Geriatr Soc.1987;35:412–416.
- ,,, et al.The Regenstrief Medical Record System: a quarter century experience.Int J Med Inform.1999;54:225–253.
- ,,,,,.Factors determining the decision to institutionalize dementing individuals: a prospective study.Gerontologist.1993;33:714–720.
- ,,,,,.Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients.JAMA.1986;255:1316–1322.
- ,,,,,.Assessing illness severity: does clinical judgment work?J Chronic Dis.1986;39:439–452.
- ,,,,.Impact of anticholinergics on the aging brain: a review and practical application.Aging Health.2008;4(3):311–320.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, eds.Primary Care Geriatrics: A Case‐Based Approach.5th ed.Philadelphia, PA:Mosby Elsevier;2007:210–218.
- ,,, et al.The association between cognition and histamine‐2 receptor antagonists in African Americans.J Am Geriatr Soc.2007;55(8):1248–1253.
In 2001, approximately 12.6 million individuals age 65 and older were discharged from American hospitals with an average length of stay of 5.8 days1 and up to 66% of them suffered from cognitive impairment (CI).220 CI in hospitalized older adults includes a variety of disorders ranging from mild cognitive deficit, delirium, to full‐blown dementia. Dementia is a syndrome of decline in memory plus at least 1 other cognitive domain, such as language, visuospatial, or executive function sufficient to interfere with social or occupational functioning in an alert person.21 Delirium is a disturbance of consciousness with reduced ability to focus, sustain, or shift attention that occurs over a short period of time and tends to fluctuate over the course of the day.22 Mild CI without dementia is defined as the presence of a cognitive deficit in the absence of delirium that does not affect functional performance.23
Hospitalized older adults with CI are vulnerable to hospital complications, including delirium, physical restraints, urinary catheters, and tethers.2, 3, 2435 The management of their medical or surgical illnesses requires avoiding certain medications with anticholinergic activities that might worsen cognition.36 Furthermore, CI may delay diagnostic and therapeutic procedures, demand more time for informed consentrelated issues, and result in difficulty in adherence to medical recommendations.37, 38 The special needs of hospitalized older adults with delirium and dementia has been shown to increase demands on nursing staff, risk of postdischarge institutionalization, length of stay, and health care costs.310, 27, 3948 We wanted to look specifically at CI because it often goes undetected4951 and can have a great impact on the hospital course of elders.
Screening for CI among hospitalized older adults has been considered to have potential benefit in hospital care of older adults.52 Screening may lead to early detection by uncovering subtle symptoms not yet apparent to families or other caregivers who know the patient well but do not notice small declines or changes in day‐to‐day functioning. Early recognition of CI may lead to early treatment and subsequently may delay progression of cognitive decline and improve health outcomes. Screening may enhance physician prescribing practices and reduce exposure to harmful medications among these vulnerable patients. Finally, delirium is an important prognostic indicator, and screening patients could provide invaluable information toward the overall clinical picture. Despite all of this, the current literature does not provide sufficient information to support the use of routine screening on admission.220, 41, 5254 Most of the published studies were conducted among elders who stayed in the hospital for more than 48 hours, missing data on the crucial first 48 hours of the hospital course.220, 41, 5254 These studies did not evaluate the impact of unrecognized CI on the hospital course and the majority of these studies were not conducted in the urban and lower socioeconomic status populations of elders that are the most vulnerable to bad health outcomes.220, 41, 5254 Finally, few studies evaluated the impact of delirium superimposed on CI on the hospital course and mortality of elders.220, 41, 5254
With these details in mind, we wanted to explore the impact of CI recognition among patients age 65 years and older admitted to the medical services of an urban, public hospital in Indianapolis to determine the prevalence and the impact of recognized and unrecognized CI on the hospital course of these elders. Furthermore, we examined the role of delirium superimposed on these hospitalized elders with CI.
Patients and Methods
The study was approved by the Indiana University Purdue University at Indianapolis Institutional Review Board (IRB).
Study Setting and Population
The study was conducted on the inpatient general medicine service of Wishard Memorial Hospital (WMH). WMH is a 450‐bed, university‐affiliated, urban, public hospital that is staffed by Indiana University School of Medicine faculty and house staff. It serves a population of approximately 750,000 in Marion County.
Inclusion and Exclusion Criteria
Patients were enrolled in the study based on the following criteria: (1) at least 65 years of age; (2) hospitalized on a medical ward; (3) able to speak English; and (4) have CI at the time of hospital admission (see below). Patients were excluded if they had previously enrolled in the study, were enrolled in another clinical study at the time of admission, or were aphasic or unresponsive at the time of screening.
Cognitive Screening
CI was determined by the Short Portable Mental Status Questionnaire (SPMSQ),55, 56 chosen for its accuracy56 and the fact that it is entirely verbal in administration. In most cases, patients were followed and reassessed daily. Patients having 2 or more errors, indicating a score of 8 or less on the SPMSQ after adjusting for race and education were considered to have cognitive impairment. The SPMSQ is a brief 10‐item screening test with a sensitivity of 86% and specificity 99.0% for dementia among medical inpatients.56 At the time of cognitive screening, delirium was assessed by using the Confusion Assessment Method (CAM).22 This was also done daily in most cases. The CAM22 is a structured instrument that evaluates the 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III‐R: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. The CAM score is determined by examining the patient, investigating the chart and interviewing the nurse and/or a family member for: (1) acute and fluctuating changes in mental status, (2) inattention, (3) disorganized or incoherent thinking, and (4) altered level of consciousness. A CAM score is considered to be positive if the patient displays both (1) and (2) with at least one of (3) or (4). The CAM diagnosis of delirium was validated against the clinical judgment of a psychiatrist and found to have a sensitivity of 97% and a specificity of 92%.22 A research assistant (RA) was trained for a period of 9 months by a physician as a rater to interview the patient and administer both the SPMSQ and the CAM at the time of admission and then every weekday. When feasible, the RA administered both the SPMSQ and the CAM within the first few hours of hospitalization, and then followed up with our patients each day. More than 70% of our initial cognitive screening occurred in the first 48 hours of hospital admission, and was repeated on a daily basis. In addition to cognitive assessment, the RA reported the presence or absence of Foley catheterization, physical restraints, and tethers during the cognitive assessment. Agreement was obtained from the general internal medicine group practice physicians both to participate in the study and to request screening for CI as part of the recognized admission standard of care among their hospitalized patients aged 65 years and older. The study coordinator was notified of all admissions for patients aged 65 or older by the hospital intranet e‐mail and paging system. Admission notifications were sent by page and e‐mail on an hourly basis from Monday through Friday, 8:00 AM through 5:00 PM. Those admissions occurring between the hours of 5:00 PM and 8:00 AM were sent during the next normal batch notification. Pages and e‐mails for admissions occurring on Saturday and Sunday were sent on Monday morning at 8:00 AM.
Regenstrief Medical Record System at WMH
The computerized Regenstrief Medical Record System (RMRS) is the primary instrument for processing data and monitoring patient and physician activity for Wishard Health System.57, 58 The RMRS is a modular system, composed of Registration and Scheduling, Laboratory, and Pharmacy database modules. The Registration and Scheduling module is used to make all outpatient appointments for the office practices associated with Wishard Health System. The Laboratory module handles all data for all inpatient and outpatient laboratories. This module also produces all laboratory reports and data used for billing. In addition to laboratory data, this module stores coded results and full‐text interpretations of all imaging studies and special procedures. The Pharmacy module contains information on medication orders captured by the computerized physician order enter (CPOE). The Database module stores all the above data by date in a fully‐coded form. Thus, these data are readily retrievable for individual patients by healthcare providers using online terminals. Data for large numbers of patients are retrievable using a locally developed English‐like language called CARE. Patients can be identified either by a certain restriction list (eg, the list of subjects in a study) or by clinical criteria. The RMRS also maintains a number of other databases including diagnoses, vital signs, results of laboratory tests and diagnostic tests, full‐text discharge summaries, preventive health maneuvers, and detailed information on all inpatient and outpatient charges. It contains death certificate information from the Indiana State Board of Health for all registered patients who die in, or outside of, Indiana. Therefore, the RMRS collects and monitors a broad array of physician and patient activity, practice patterns, utilization, diagnostic test finding, and offers a wonderful array of outcome measures.
Other Data Collections
Patient demographics such as age, gender, race, and education level were determined by the RMRS and by information obtained during the time of cognitive screening. Length of hospital stay and 30‐day posthospitalization mortality were obtained from the RMRS. Comorbidity level was measured by reviewing the RMRS and determining each patient's Charlson comorbidity index total score.59, 60 This score was determined using International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD‐9) codes gathered from 1 year prior to admission until the patient was discharged from the hospital. Anticholinergic medications were determined by using the Anticholinergic Cognitive Burden Scale,61 an expert‐based practical index. The scale was developed based on a review of all published studies from 1996 to 2007 that measured the anticholinergic activities of a drug and its association with cognitive function in older adults. The list of drugs reviewed was presented to an expert interdisciplinary panel that included geriatricians, geriatric pharmacists, geriatric psychiatrists, general physicians, geriatric nurses, and aging brain researchers. The panel categorized each medication into a possible or definite anticholinergic category based on the severity of its cognitive anticholinergic effects.61 A patient who received at least 1 order of a possible or definite anticholinergic during their hospitalization was considered to be an anticholinergic user. Prior recognition of CI was determined by searching the RMRS for any ICD‐9 code (see Appendix) indicative of dementia, Alzheimer disease, or delirium reported at hospital admission, discharge, or during an 1‐year period prior to hospitalization for every patient enrolled in the study. Those patients with documented ICD‐9 codes were felt recognized as having some form of cognitive impairment. Those who had a positive screen but no prior documentation according to ICD‐9 coding, were said to have unrecognized CI.
Analysis
Descriptive statistics were calculated, including percentages for binary categorical variables, and means and standard deviations for continuous variables. Comparisons between groups were based upon Fisher's Exact Tests for binary categorical variables and t tests for continuous variables. When controlling for covariates such as age, gender, race, Charlson comorbidity index, and SPMSQ at screening, group comparisons were made by using logistic regression for binary categorical variables and multiple regression for continuous variables. Since the distributions of length of stay and Charlson comorbidity index were skewed, all statistical tests comparing them across groups were actually performed on their log‐transformed values.
Results
The Prevalence and Recognition of CI
Table 1 describes the demographic characteristic of our study population, which is a reflection of the public and urban nature of our target hospital. Our study assessed the cognitive status of 997 older adults usually (>70% of the time) within 48 hours of their admission to the medical ward of this urban hospital between July of 2006 and March 2008 (see Table 1) and found that 43% of these elders had evidence of CI as determined by a SPMSQ score of 8 points or less. However, 61% of the 424 cognitively impaired elders were not documented or recognized by the electronic medical record system to have cognitive deficit.
| Variable | n | %/Mean (SD) |
|---|---|---|
| ||
| Age (years), mean (SD) | 997 | 74.8 (7.5) |
| Age 85 (%) | 997 | 12.6 |
| Female (%) | 997 | 67.8 |
| African American (%) | 997 | 59.4 |
| Education (years), mean (SD) | 910 | 10.3 (2.8) |
| Education <12 years (%) | 910 | 59.1 |
| Screened within 48 hours of admission (%) | 997 | 73.2 |
| SPMSQ score at screening, mean (SD) | 997 | 7.7 (2.8) |
| Cognitive impairment based on the SPMSQ score 8 (%) | 997 | 42.5 |
The Impact of Unrecognized CI on the Hospital Course
As expected, hospitalized elders with documented CI were older (mean age 79.1 years vs. 76.1 years; P < 0.001) and had worse cognitive function upon screening than those with unrecognized CI (mean SPMSQ 3.4 points vs. 6.3; P < 0.001). Furthermore, CI recognition was influenced by the elders' race and comorbidity (Table 2); a higher percentage of elders with documented CI were African American (69% vs. 54%; P = 0.003) and had less comorbidity (mean Charlson index 1.9 vs. 2.3; P = 0.03). After adjusting for age, gender, race, comorbidity, and cognitive function at screening, our study found no differences between elders with previously recognized CI and those with unrecognized CI in regard to the length of hospital stay (6.7 days vs. 7.5 days; P = 0.59), 30‐day posthospital mortality (4.8% vs. 6.6%; P > 0.2), home discharge (32% vs. 45%; P > 0.7), hospital readmission (19.2% vs.18.8%; P > 0.6), delirium incidence (27% vs. 21%; P > 0.9), and physical restraints (1.8% vs. 1.5%; P > 0.4). We also found that elders with undocumented CI were not more likely to receive definite anticholinergics (33.2% vs. 32.7%; P > 0.9).
| CI Documented | CI Undocumented | P Value | P Value* | |
|---|---|---|---|---|
| ||||
| n (%) | 165 (39) | 259 (61) | n/a | |
| Age, mean (SD) | 79.1 (7.9) | 76.1 (8.0) | <0.001 | |
| Female (%) | 68.5 | 64.5 | 0.40 | |
| African American (%) | 68.5 | 53.7 | <0.01 | |
| SPMSQ at screen, mean (SD) | 3.4 (2.7) | 6.3 (2.1) | <0.001 | |
| Charlson comorbidity index, mean (SD) | 1.9 (1.9) | 2.3 (2.1) | 0.03 | |
| Length of hospital stay, mean (SD) | 6.7 (5.1) | 7.5 (7.1) | 0.49 | 0.59 |
| Survived at 30 days postdischarge (%) | 95.2 | 93.4 | 0.53 | 0.25 |
| Discharged home (%) | 31.5 | 45.2 | 0.01 | 0.74 |
| Readmission within 30 days after discharge home (%) | 19.2 | 18.8 | 0.99 | 0.66 |
| Incidence of delirium (%) | 26.7 | 20.6 | 0.52 | 0.99 |
| Observed with Foley catheter (%) | 43.6 | 27.4 | <0.001 | 0.61 |
| Observed with physical restraint (%) | 1.8 | 1.5 | 0.99 | 0.31 |
| Observed with tethers (%) | 81.8 | 73.8 | 0.06 | 0.58 |
| With at least 1 Ach (%) | 83.6 | 90.7 | 0.03 | 0.22 |
| Possible Ach (%) | 81.2 | 88.4 | 0.05 | 0.31 |
| Definite Ach (%) | 32.7 | 33.2 | 0.99 | 0.64 |
The Impact of Delirium on the Hospital Course of Elders with CI
Among the 424 hospitalized elders with CI, 163 (38%) had delirium at least once during their hospital course and 24% had delirium on the day of hospital discharge. In comparison to elders who had CI but not delirium during their hospitalization (Table 3), those with at least 1 day of delirium had a higher 30‐day posthospitalization mortality risk (8.6% vs. 4.2%; P = 0.09), stayed in the hospital 3.3 additional days (9.2 days vs. 5.9 days; P < 0.001), were less likely to be discharged home (25% vs. 49%; P < 0.001), were more likely to receive a Foley catheterization (52% vs. 23%; P < 0.001), more likely to be physically restrained (4% vs. 0%; P < 0.01), and more likely to receive tethers during their care (89% vs. 69%; P < 0.001). There was no statistically significant difference between the 2 groups in terms of 30‐day hospital readmission rates or in their use of definite anticholinergics (Table 3).
| Delirium+* | Delirium | P value | |
|---|---|---|---|
| |||
| n (%) | 163 (38) | 261 (62) | n/a |
| Age, mean (SD) | 78.4 (8.5) | 76.5 (7.8) | 0.02 |
| Female (%) | 60.1 | 69.7 | 0.05 |
| African American (%) | 64.4 | 56.3 | 0.10 |
| Charlson comorbidity index, mean (SD) | 1.8 (1.9) | 2.3 (2.1) | 0.01 |
| Length of hospital stay, mean (SD) | 9.2 (7.9) | 5.9 (4.9) | <0.001 |
| Survived at 30‐day postdischarge (%) | 91.4 | 95.8 | 0.09 |
| Discharged home (%) | 24.5 | 49.4 | <0.001 |
| Readmission within 30 days after discharge home (%) | 22.5 | 17.8 | 0.50 |
| Observed with Foley catheter (%) | 51.5 | 22.6 | <0.001 |
| Observed with physical restraint (%) | 4.3 | 0.0 | <0.01 |
| Observed with tethers (%) | 89.0 | 69.4 | <0.001 |
| With at least 1 anticholinergic (%) | 83.4 | 90.8 | 0.03 |
| Possible anticholinergic (%) | 80.4 | 88.9 | 0.02 |
| Definite anticholinergic (%) | 36.8 | 30.7 | 0.20 |
Discussion
Our study found that in an urban, public hospital, acute or preexisting CI affects more than one‐third of hospitalized elders admitted to general medical services. Unfortunately, our hospital system does not currently recognize the majority of these vulnerable patients. Our study also found that delirium affects more than one‐third of hospitalized elders with CI during their hospital course. Delirium complicates hospital care by prolonging length of stay and decreasing the probability of surviving and getting discharged home. It leads to high use of Foley catheterization, physical restraints, and tethers.
The high prevalence of CI with and without delirium in our cohort is within the rates reported previously in the literature. It is estimated that the prevalence of CI in hospitalized older adults ranges from 14% to 66%, depending on the method used to measure cognition, the definition of CI, and the type of hospital ward (surgical, medical, and geriatric units).220 One particular study that used a similar cognitive assessment method reported higher prevalence rates for both CI and delirium.11 The study randomly evaluated a sample of 201 patients age 65 and over who were hospitalized for a medical illness and found that 56% of the cohort suffered from CI and among those with CI, 47% had delirium.11 The difference between this finding and our study is most likely due to our sampling technique; more than 70% of our cognitive screening occurred in the first 48 hours of hospital admission whereas the Australian study, in similar enrollment criteria to all of the published studies in this area, excluded patients who were discharged within 48 hours of admission. We believe, however, that by including the first 48 hours of admission in our design, our study provides a more generalizable reflection of the actual acute care experience.
The impact of delirium on the course of hospital care found in our study supports some of the findings from previous studies conducted in the past 2 decades.5, 6, 11 Despite 2 decades of clinical research, delirium continues to increase mortality, hospital stays, and posthospital institutionalization.
We were surprised to find that patients suffering from delirium continue to receive at least 1 definite anticholinergic medication. Such medications are considered inappropriate among patients with any form of cognitive impairment.36, 62 Although the impact of anticholinergic medications on hospitalized outcomes is less well‐described, their use has been suspected to negatively impact long‐term outcomes of cognitive impairment.61, 63 Our study found no difference in the use of anticholinergic medications between those with CI who experienced delirium and those who did not; however, the total burden of anticholinergic medication was not assessed in a quantitative manner. It is still unknown if certain anticholinergic medications or a cumulative effect of anticholinergic medications may impact cognitive or health‐related outcomes in a vulnerable older population with CI.
Although our study reported for the first time in a systematic way the rate of undocumented CI among hospitalized elders found to have CI on admission, we found no impact of such underrecognition on the length of hospital stay, mortality, discharge location, and delirium occurrence. Although the use of anticholinergic medications is not recommended for patients with any form of CI, our results indicate that a significant number of patients with cognitive impairment continue to receive inappropriate medications. CI recognition in the elderly was not shown to have a statistically significant affect on length of stay, cost, or mortality.
Our study has some limitations. First of all, we did not determine the underlying types of CI such as Alzheimer disease, vascular dementia, mild cognitive impairment, or reversible etiology other than delirium. Such a categorization requires posthospital assessment, which was not included in our study design. Second, our delirium incidence rate and delirium impact on hospital outcomes might be very conservative and may underestimate its true prevalence and correlation due to our data collection methods. Despite the fluctuating nature of delirium, our study was not designed to assess the presence of delirium every shift and tried to assess cognitive function on a daily basis throughout the patient's hospitalization. Therefore, the severity and duration of delirium could not be accurately assessed. Our reported rates of use of Foley catheterization, physical restraints, and tethers are also very conservative and we could not determine the appropriateness of these procedures. Our study was conducted in 1 public hospital in an urban city with a higher percentage of African Americans. Thus, our sample is not a true representative sample. However, studies with significant representation of minority groups are not common in the research literature, especially in CI research; we hope to fulfill some of the gaps in the literature regarding the most vulnerable older American population. Finally, we were limited in our use of ICD‐9 coding to determine if patients had previously been recognized by other providers as having CI. ICD‐9 coding, while useful, is not perfect in identifying all if a patient's medical problems. Use of coding to determine whether a patient had been recognized as impaired also does not allow us to determine when the diagnosis was made.
In conclusion, our study evaluated cognitive impairment in hospitalized elders and found that in our cohort of 997 patients, 43% were cognitively impaired on admission. Of those with CI, 61% were not documented or recognized as impaired. We found no statistically significant difference between those with documented CI and those with undocumented CI in terms of length of stay, mortality, home discharge, readmission rates, incidence of delirium, or potential to receive anticholinergics or restraints. Among those with CI, 38% had delirium. Those with delirium experienced increased length of stay, decreased discharge to home, and increased use of Foley catheters and restraints.
In 2001, approximately 12.6 million individuals age 65 and older were discharged from American hospitals with an average length of stay of 5.8 days1 and up to 66% of them suffered from cognitive impairment (CI).220 CI in hospitalized older adults includes a variety of disorders ranging from mild cognitive deficit, delirium, to full‐blown dementia. Dementia is a syndrome of decline in memory plus at least 1 other cognitive domain, such as language, visuospatial, or executive function sufficient to interfere with social or occupational functioning in an alert person.21 Delirium is a disturbance of consciousness with reduced ability to focus, sustain, or shift attention that occurs over a short period of time and tends to fluctuate over the course of the day.22 Mild CI without dementia is defined as the presence of a cognitive deficit in the absence of delirium that does not affect functional performance.23
Hospitalized older adults with CI are vulnerable to hospital complications, including delirium, physical restraints, urinary catheters, and tethers.2, 3, 2435 The management of their medical or surgical illnesses requires avoiding certain medications with anticholinergic activities that might worsen cognition.36 Furthermore, CI may delay diagnostic and therapeutic procedures, demand more time for informed consentrelated issues, and result in difficulty in adherence to medical recommendations.37, 38 The special needs of hospitalized older adults with delirium and dementia has been shown to increase demands on nursing staff, risk of postdischarge institutionalization, length of stay, and health care costs.310, 27, 3948 We wanted to look specifically at CI because it often goes undetected4951 and can have a great impact on the hospital course of elders.
Screening for CI among hospitalized older adults has been considered to have potential benefit in hospital care of older adults.52 Screening may lead to early detection by uncovering subtle symptoms not yet apparent to families or other caregivers who know the patient well but do not notice small declines or changes in day‐to‐day functioning. Early recognition of CI may lead to early treatment and subsequently may delay progression of cognitive decline and improve health outcomes. Screening may enhance physician prescribing practices and reduce exposure to harmful medications among these vulnerable patients. Finally, delirium is an important prognostic indicator, and screening patients could provide invaluable information toward the overall clinical picture. Despite all of this, the current literature does not provide sufficient information to support the use of routine screening on admission.220, 41, 5254 Most of the published studies were conducted among elders who stayed in the hospital for more than 48 hours, missing data on the crucial first 48 hours of the hospital course.220, 41, 5254 These studies did not evaluate the impact of unrecognized CI on the hospital course and the majority of these studies were not conducted in the urban and lower socioeconomic status populations of elders that are the most vulnerable to bad health outcomes.220, 41, 5254 Finally, few studies evaluated the impact of delirium superimposed on CI on the hospital course and mortality of elders.220, 41, 5254
With these details in mind, we wanted to explore the impact of CI recognition among patients age 65 years and older admitted to the medical services of an urban, public hospital in Indianapolis to determine the prevalence and the impact of recognized and unrecognized CI on the hospital course of these elders. Furthermore, we examined the role of delirium superimposed on these hospitalized elders with CI.
Patients and Methods
The study was approved by the Indiana University Purdue University at Indianapolis Institutional Review Board (IRB).
Study Setting and Population
The study was conducted on the inpatient general medicine service of Wishard Memorial Hospital (WMH). WMH is a 450‐bed, university‐affiliated, urban, public hospital that is staffed by Indiana University School of Medicine faculty and house staff. It serves a population of approximately 750,000 in Marion County.
Inclusion and Exclusion Criteria
Patients were enrolled in the study based on the following criteria: (1) at least 65 years of age; (2) hospitalized on a medical ward; (3) able to speak English; and (4) have CI at the time of hospital admission (see below). Patients were excluded if they had previously enrolled in the study, were enrolled in another clinical study at the time of admission, or were aphasic or unresponsive at the time of screening.
Cognitive Screening
CI was determined by the Short Portable Mental Status Questionnaire (SPMSQ),55, 56 chosen for its accuracy56 and the fact that it is entirely verbal in administration. In most cases, patients were followed and reassessed daily. Patients having 2 or more errors, indicating a score of 8 or less on the SPMSQ after adjusting for race and education were considered to have cognitive impairment. The SPMSQ is a brief 10‐item screening test with a sensitivity of 86% and specificity 99.0% for dementia among medical inpatients.56 At the time of cognitive screening, delirium was assessed by using the Confusion Assessment Method (CAM).22 This was also done daily in most cases. The CAM22 is a structured instrument that evaluates the 10 symptoms of delirium specified in the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III‐R: acute onset, fluctuating course, inattention, disorganized thinking, altered level of consciousness, disorientation, memory impairment, perceptual disturbances, psychomotor agitation or retardation, and sleep/wake disturbance. The CAM score is determined by examining the patient, investigating the chart and interviewing the nurse and/or a family member for: (1) acute and fluctuating changes in mental status, (2) inattention, (3) disorganized or incoherent thinking, and (4) altered level of consciousness. A CAM score is considered to be positive if the patient displays both (1) and (2) with at least one of (3) or (4). The CAM diagnosis of delirium was validated against the clinical judgment of a psychiatrist and found to have a sensitivity of 97% and a specificity of 92%.22 A research assistant (RA) was trained for a period of 9 months by a physician as a rater to interview the patient and administer both the SPMSQ and the CAM at the time of admission and then every weekday. When feasible, the RA administered both the SPMSQ and the CAM within the first few hours of hospitalization, and then followed up with our patients each day. More than 70% of our initial cognitive screening occurred in the first 48 hours of hospital admission, and was repeated on a daily basis. In addition to cognitive assessment, the RA reported the presence or absence of Foley catheterization, physical restraints, and tethers during the cognitive assessment. Agreement was obtained from the general internal medicine group practice physicians both to participate in the study and to request screening for CI as part of the recognized admission standard of care among their hospitalized patients aged 65 years and older. The study coordinator was notified of all admissions for patients aged 65 or older by the hospital intranet e‐mail and paging system. Admission notifications were sent by page and e‐mail on an hourly basis from Monday through Friday, 8:00 AM through 5:00 PM. Those admissions occurring between the hours of 5:00 PM and 8:00 AM were sent during the next normal batch notification. Pages and e‐mails for admissions occurring on Saturday and Sunday were sent on Monday morning at 8:00 AM.
Regenstrief Medical Record System at WMH
The computerized Regenstrief Medical Record System (RMRS) is the primary instrument for processing data and monitoring patient and physician activity for Wishard Health System.57, 58 The RMRS is a modular system, composed of Registration and Scheduling, Laboratory, and Pharmacy database modules. The Registration and Scheduling module is used to make all outpatient appointments for the office practices associated with Wishard Health System. The Laboratory module handles all data for all inpatient and outpatient laboratories. This module also produces all laboratory reports and data used for billing. In addition to laboratory data, this module stores coded results and full‐text interpretations of all imaging studies and special procedures. The Pharmacy module contains information on medication orders captured by the computerized physician order enter (CPOE). The Database module stores all the above data by date in a fully‐coded form. Thus, these data are readily retrievable for individual patients by healthcare providers using online terminals. Data for large numbers of patients are retrievable using a locally developed English‐like language called CARE. Patients can be identified either by a certain restriction list (eg, the list of subjects in a study) or by clinical criteria. The RMRS also maintains a number of other databases including diagnoses, vital signs, results of laboratory tests and diagnostic tests, full‐text discharge summaries, preventive health maneuvers, and detailed information on all inpatient and outpatient charges. It contains death certificate information from the Indiana State Board of Health for all registered patients who die in, or outside of, Indiana. Therefore, the RMRS collects and monitors a broad array of physician and patient activity, practice patterns, utilization, diagnostic test finding, and offers a wonderful array of outcome measures.
Other Data Collections
Patient demographics such as age, gender, race, and education level were determined by the RMRS and by information obtained during the time of cognitive screening. Length of hospital stay and 30‐day posthospitalization mortality were obtained from the RMRS. Comorbidity level was measured by reviewing the RMRS and determining each patient's Charlson comorbidity index total score.59, 60 This score was determined using International Statistical Classification of Diseases and Related Health Problems, 9th edition (ICD‐9) codes gathered from 1 year prior to admission until the patient was discharged from the hospital. Anticholinergic medications were determined by using the Anticholinergic Cognitive Burden Scale,61 an expert‐based practical index. The scale was developed based on a review of all published studies from 1996 to 2007 that measured the anticholinergic activities of a drug and its association with cognitive function in older adults. The list of drugs reviewed was presented to an expert interdisciplinary panel that included geriatricians, geriatric pharmacists, geriatric psychiatrists, general physicians, geriatric nurses, and aging brain researchers. The panel categorized each medication into a possible or definite anticholinergic category based on the severity of its cognitive anticholinergic effects.61 A patient who received at least 1 order of a possible or definite anticholinergic during their hospitalization was considered to be an anticholinergic user. Prior recognition of CI was determined by searching the RMRS for any ICD‐9 code (see Appendix) indicative of dementia, Alzheimer disease, or delirium reported at hospital admission, discharge, or during an 1‐year period prior to hospitalization for every patient enrolled in the study. Those patients with documented ICD‐9 codes were felt recognized as having some form of cognitive impairment. Those who had a positive screen but no prior documentation according to ICD‐9 coding, were said to have unrecognized CI.
Analysis
Descriptive statistics were calculated, including percentages for binary categorical variables, and means and standard deviations for continuous variables. Comparisons between groups were based upon Fisher's Exact Tests for binary categorical variables and t tests for continuous variables. When controlling for covariates such as age, gender, race, Charlson comorbidity index, and SPMSQ at screening, group comparisons were made by using logistic regression for binary categorical variables and multiple regression for continuous variables. Since the distributions of length of stay and Charlson comorbidity index were skewed, all statistical tests comparing them across groups were actually performed on their log‐transformed values.
Results
The Prevalence and Recognition of CI
Table 1 describes the demographic characteristic of our study population, which is a reflection of the public and urban nature of our target hospital. Our study assessed the cognitive status of 997 older adults usually (>70% of the time) within 48 hours of their admission to the medical ward of this urban hospital between July of 2006 and March 2008 (see Table 1) and found that 43% of these elders had evidence of CI as determined by a SPMSQ score of 8 points or less. However, 61% of the 424 cognitively impaired elders were not documented or recognized by the electronic medical record system to have cognitive deficit.
| Variable | n | %/Mean (SD) |
|---|---|---|
| ||
| Age (years), mean (SD) | 997 | 74.8 (7.5) |
| Age 85 (%) | 997 | 12.6 |
| Female (%) | 997 | 67.8 |
| African American (%) | 997 | 59.4 |
| Education (years), mean (SD) | 910 | 10.3 (2.8) |
| Education <12 years (%) | 910 | 59.1 |
| Screened within 48 hours of admission (%) | 997 | 73.2 |
| SPMSQ score at screening, mean (SD) | 997 | 7.7 (2.8) |
| Cognitive impairment based on the SPMSQ score 8 (%) | 997 | 42.5 |
The Impact of Unrecognized CI on the Hospital Course
As expected, hospitalized elders with documented CI were older (mean age 79.1 years vs. 76.1 years; P < 0.001) and had worse cognitive function upon screening than those with unrecognized CI (mean SPMSQ 3.4 points vs. 6.3; P < 0.001). Furthermore, CI recognition was influenced by the elders' race and comorbidity (Table 2); a higher percentage of elders with documented CI were African American (69% vs. 54%; P = 0.003) and had less comorbidity (mean Charlson index 1.9 vs. 2.3; P = 0.03). After adjusting for age, gender, race, comorbidity, and cognitive function at screening, our study found no differences between elders with previously recognized CI and those with unrecognized CI in regard to the length of hospital stay (6.7 days vs. 7.5 days; P = 0.59), 30‐day posthospital mortality (4.8% vs. 6.6%; P > 0.2), home discharge (32% vs. 45%; P > 0.7), hospital readmission (19.2% vs.18.8%; P > 0.6), delirium incidence (27% vs. 21%; P > 0.9), and physical restraints (1.8% vs. 1.5%; P > 0.4). We also found that elders with undocumented CI were not more likely to receive definite anticholinergics (33.2% vs. 32.7%; P > 0.9).
| CI Documented | CI Undocumented | P Value | P Value* | |
|---|---|---|---|---|
| ||||
| n (%) | 165 (39) | 259 (61) | n/a | |
| Age, mean (SD) | 79.1 (7.9) | 76.1 (8.0) | <0.001 | |
| Female (%) | 68.5 | 64.5 | 0.40 | |
| African American (%) | 68.5 | 53.7 | <0.01 | |
| SPMSQ at screen, mean (SD) | 3.4 (2.7) | 6.3 (2.1) | <0.001 | |
| Charlson comorbidity index, mean (SD) | 1.9 (1.9) | 2.3 (2.1) | 0.03 | |
| Length of hospital stay, mean (SD) | 6.7 (5.1) | 7.5 (7.1) | 0.49 | 0.59 |
| Survived at 30 days postdischarge (%) | 95.2 | 93.4 | 0.53 | 0.25 |
| Discharged home (%) | 31.5 | 45.2 | 0.01 | 0.74 |
| Readmission within 30 days after discharge home (%) | 19.2 | 18.8 | 0.99 | 0.66 |
| Incidence of delirium (%) | 26.7 | 20.6 | 0.52 | 0.99 |
| Observed with Foley catheter (%) | 43.6 | 27.4 | <0.001 | 0.61 |
| Observed with physical restraint (%) | 1.8 | 1.5 | 0.99 | 0.31 |
| Observed with tethers (%) | 81.8 | 73.8 | 0.06 | 0.58 |
| With at least 1 Ach (%) | 83.6 | 90.7 | 0.03 | 0.22 |
| Possible Ach (%) | 81.2 | 88.4 | 0.05 | 0.31 |
| Definite Ach (%) | 32.7 | 33.2 | 0.99 | 0.64 |
The Impact of Delirium on the Hospital Course of Elders with CI
Among the 424 hospitalized elders with CI, 163 (38%) had delirium at least once during their hospital course and 24% had delirium on the day of hospital discharge. In comparison to elders who had CI but not delirium during their hospitalization (Table 3), those with at least 1 day of delirium had a higher 30‐day posthospitalization mortality risk (8.6% vs. 4.2%; P = 0.09), stayed in the hospital 3.3 additional days (9.2 days vs. 5.9 days; P < 0.001), were less likely to be discharged home (25% vs. 49%; P < 0.001), were more likely to receive a Foley catheterization (52% vs. 23%; P < 0.001), more likely to be physically restrained (4% vs. 0%; P < 0.01), and more likely to receive tethers during their care (89% vs. 69%; P < 0.001). There was no statistically significant difference between the 2 groups in terms of 30‐day hospital readmission rates or in their use of definite anticholinergics (Table 3).
| Delirium+* | Delirium | P value | |
|---|---|---|---|
| |||
| n (%) | 163 (38) | 261 (62) | n/a |
| Age, mean (SD) | 78.4 (8.5) | 76.5 (7.8) | 0.02 |
| Female (%) | 60.1 | 69.7 | 0.05 |
| African American (%) | 64.4 | 56.3 | 0.10 |
| Charlson comorbidity index, mean (SD) | 1.8 (1.9) | 2.3 (2.1) | 0.01 |
| Length of hospital stay, mean (SD) | 9.2 (7.9) | 5.9 (4.9) | <0.001 |
| Survived at 30‐day postdischarge (%) | 91.4 | 95.8 | 0.09 |
| Discharged home (%) | 24.5 | 49.4 | <0.001 |
| Readmission within 30 days after discharge home (%) | 22.5 | 17.8 | 0.50 |
| Observed with Foley catheter (%) | 51.5 | 22.6 | <0.001 |
| Observed with physical restraint (%) | 4.3 | 0.0 | <0.01 |
| Observed with tethers (%) | 89.0 | 69.4 | <0.001 |
| With at least 1 anticholinergic (%) | 83.4 | 90.8 | 0.03 |
| Possible anticholinergic (%) | 80.4 | 88.9 | 0.02 |
| Definite anticholinergic (%) | 36.8 | 30.7 | 0.20 |
Discussion
Our study found that in an urban, public hospital, acute or preexisting CI affects more than one‐third of hospitalized elders admitted to general medical services. Unfortunately, our hospital system does not currently recognize the majority of these vulnerable patients. Our study also found that delirium affects more than one‐third of hospitalized elders with CI during their hospital course. Delirium complicates hospital care by prolonging length of stay and decreasing the probability of surviving and getting discharged home. It leads to high use of Foley catheterization, physical restraints, and tethers.
The high prevalence of CI with and without delirium in our cohort is within the rates reported previously in the literature. It is estimated that the prevalence of CI in hospitalized older adults ranges from 14% to 66%, depending on the method used to measure cognition, the definition of CI, and the type of hospital ward (surgical, medical, and geriatric units).220 One particular study that used a similar cognitive assessment method reported higher prevalence rates for both CI and delirium.11 The study randomly evaluated a sample of 201 patients age 65 and over who were hospitalized for a medical illness and found that 56% of the cohort suffered from CI and among those with CI, 47% had delirium.11 The difference between this finding and our study is most likely due to our sampling technique; more than 70% of our cognitive screening occurred in the first 48 hours of hospital admission whereas the Australian study, in similar enrollment criteria to all of the published studies in this area, excluded patients who were discharged within 48 hours of admission. We believe, however, that by including the first 48 hours of admission in our design, our study provides a more generalizable reflection of the actual acute care experience.
The impact of delirium on the course of hospital care found in our study supports some of the findings from previous studies conducted in the past 2 decades.5, 6, 11 Despite 2 decades of clinical research, delirium continues to increase mortality, hospital stays, and posthospital institutionalization.
We were surprised to find that patients suffering from delirium continue to receive at least 1 definite anticholinergic medication. Such medications are considered inappropriate among patients with any form of cognitive impairment.36, 62 Although the impact of anticholinergic medications on hospitalized outcomes is less well‐described, their use has been suspected to negatively impact long‐term outcomes of cognitive impairment.61, 63 Our study found no difference in the use of anticholinergic medications between those with CI who experienced delirium and those who did not; however, the total burden of anticholinergic medication was not assessed in a quantitative manner. It is still unknown if certain anticholinergic medications or a cumulative effect of anticholinergic medications may impact cognitive or health‐related outcomes in a vulnerable older population with CI.
Although our study reported for the first time in a systematic way the rate of undocumented CI among hospitalized elders found to have CI on admission, we found no impact of such underrecognition on the length of hospital stay, mortality, discharge location, and delirium occurrence. Although the use of anticholinergic medications is not recommended for patients with any form of CI, our results indicate that a significant number of patients with cognitive impairment continue to receive inappropriate medications. CI recognition in the elderly was not shown to have a statistically significant affect on length of stay, cost, or mortality.
Our study has some limitations. First of all, we did not determine the underlying types of CI such as Alzheimer disease, vascular dementia, mild cognitive impairment, or reversible etiology other than delirium. Such a categorization requires posthospital assessment, which was not included in our study design. Second, our delirium incidence rate and delirium impact on hospital outcomes might be very conservative and may underestimate its true prevalence and correlation due to our data collection methods. Despite the fluctuating nature of delirium, our study was not designed to assess the presence of delirium every shift and tried to assess cognitive function on a daily basis throughout the patient's hospitalization. Therefore, the severity and duration of delirium could not be accurately assessed. Our reported rates of use of Foley catheterization, physical restraints, and tethers are also very conservative and we could not determine the appropriateness of these procedures. Our study was conducted in 1 public hospital in an urban city with a higher percentage of African Americans. Thus, our sample is not a true representative sample. However, studies with significant representation of minority groups are not common in the research literature, especially in CI research; we hope to fulfill some of the gaps in the literature regarding the most vulnerable older American population. Finally, we were limited in our use of ICD‐9 coding to determine if patients had previously been recognized by other providers as having CI. ICD‐9 coding, while useful, is not perfect in identifying all if a patient's medical problems. Use of coding to determine whether a patient had been recognized as impaired also does not allow us to determine when the diagnosis was made.
In conclusion, our study evaluated cognitive impairment in hospitalized elders and found that in our cohort of 997 patients, 43% were cognitively impaired on admission. Of those with CI, 61% were not documented or recognized as impaired. We found no statistically significant difference between those with documented CI and those with undocumented CI in terms of length of stay, mortality, home discharge, readmission rates, incidence of delirium, or potential to receive anticholinergics or restraints. Among those with CI, 38% had delirium. Those with delirium experienced increased length of stay, decreased discharge to home, and increased use of Foley catheters and restraints.
- ,.National hospital discharge survey: annual summary, 1994.Vital Health Stat 13.1997;(128):i–v;1–50.
- .The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients.Am J Med.1994;97:278–288.
- ,,.Dementia in medical wards.J Clin Epidemiol.1988;41:123–126.
- ,,,,,.Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging.Am J Psychiatry.2000;157:708–714.
- ,,, et al.Randomized, placebo‐controlled, double‐blind clinical trial of sertraline in the treatment of depression complicating Alzheimer's disease: initial results from the Depression in Alzheimer's Disease study.Am J Psychiatry.2000;157:1686–1689.
- ,,.Dementia in elderly persons in a general hospital.Am J Psychiatry.2000;157:704–707.
- ,,,,.A prospective study of the impact of psychiatric comorbidity on length of hospital stays of elderly medical‐surgical inpatients.Psychosomatics.1998;39:273–280.
- ,.Psychiatric comorbidity and length of stay in the general hospital. A critical review of outcome studies.Psychosomatics.1994;35:233–252.
- ,,,.Dementia among medical inpatients. Evaluation of 2000 consecutive admissions.Arch Intern Med.1986;146:1923–1926.
- ,,,.The consequences of non‐cognitive symptoms of dementia in medical hospital departments.Int J Psychiatry Med.2003;33:257–271.
- ,,.Cognitive impairment in medical inpatients. I: Screening for dementia—is history better than mental state?Age Ageing.1997;26:31–35.
- ,,, et al.Acute confusional states in elderly patients treated for femoral neck fracture.J Am Geriatr Soc.1988;36:525–530.
- ,.A prospective study of elderly general surgical patients: II. Post‐operative complications.Age Ageing.1989;18:316–326.
- ,,,.Dementia and depression in elderly medical inpatients.Int Psychogeriatr.2000;12:67–75.
- ,,.[Psychiatric disorders in elderly general hospital patients: incidence and long‐term prognosis].Nervenarzt.1993;64:53–61. [German]
- ,.Delirium and dementia in acute medical admissions of elderly patients in Iceland.Acta Psychiatr Scand.1993;87:123–127.
- ,,,,,.[Psychiatric morbidity in elderly patients admitted to a general hospital. A day‐prevalence study].Med Clin (Barc).1991;97:206–210. [Spanish]
- ,,,,,.Detection of psychiatric disorders in elderly medical inpatients.Age Ageing.1994;23:307–311.
- ,,,.Cognitive impairment, emotional disorder and length of stay of elderly patients in a district general hospital.Br J Med Psychol.1987;60(Pt 2):133–139.
- ,,.An investigation of the components of best nursing practice in the care of acutely ill hospitalized older patients with coincidental dementia: a multi‐method design.J Adv Nurs1999;30:1127–1136.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,, et al.Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging.Neurology.2001;57:1655–1662.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care.Am J Med.1999;106:565–573.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Iatrogenic causes of falls in hospitalised elderly patients: a case‐control study.Postgrad Med J.2002;78:487–489.
- ,,.A prospective study of delirium in hospitalized elderly.JAMA.1990;263:1097–1101.
- ,.The prognostic significance of delirium in older hospital patients.J Am Geriatr Soc.1997;45:174–178.
- ,.Prognosis of delirium in elderly hospital patients.CMAJ.1993;149:41–46.
- ,,,,.The detection of psychiatric morbidity and its effects on outcome in acute elderly medical admissions.Int J Ger Psych1991;6:861–866.
- ,,.Adverse consequences of hospitalization in the elderly.Soc Sci Med.1982;16:1033–1038.
- ,,, et al.Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I.N Engl J Med.1991;324:370–376.
- ,,,.Delirium in elderly patients: an overview of the state of the science.J Gerontol Nurs.2001;27:12–20.
- ,,,,.A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics.Ann Intern Med.1993;119:474–481.
- ,.Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability.JAMA.1996;275:852–857.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- ,,, et al.A clinical prediction rule for delirium after elective noncardiac surgery.JAMA.1994;271:134–139.
- ,,, et al.The relationship of postoperative delirium with psychoactive medications.JAMA.1994;272:1518–1522.
- ,,, et al.How do delirium and dementia increase length of stay of elderly general medical inpatients?Psychosomatics.2004;45:235–242.
- ,,,,,.The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use.J Am Geriatr Soc.2004;52:187–194.
- ,,, et al.Risk factors for delirium in hospitalized elderly.JAMA.1992;267:827–831.
- ,,,,.Psychological comorbidity and length of stay in the general hospital.Am J Psychiatry.1991;148:324–329.
- ,,, et al.Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients.Arch Intern Med.1992;152:334–340.
- ,,.What happens to medical patients with psychiatric disorder?J Psychosom Res1988;32:541–549.
- ,,,,.[Dementia syndromes and length of stay of elderly patients in internal medicine].Ann Med Interne (Paris).1997;148:424–426. [French]
- ,,, et al.[Hospital discharge planning and length of hospital stay in elderly patients admitted through the emergency department].Rev Epidemiol Sante Publique.1995;43:337–347. [French]
- ,,,,.The effect of dementia on acute care in a geriatric medical unit.Int Psychogeriatr.1992;4:231–239.
- ,,,.Cognitive impairment. Can it predict the course of hospitalized patients?J Am Geriatr Soc.1986;34:579–585.
- ,,,,;US Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2003;138(11):927–937.
- ,,,.Cognitive impairment in the elderly medically ill: how often is it missed?Int J Geriatr Psychiatry.1993;8:929–937.
- .Recognition of cognitive impairment in elderly medical in‐patients.J R Soc Med.1995;88:183–184.
- ,.Quality indicators for dementia in vulnerable community‐dwelling and hospitalized elders.Ann Intern Med.2001;135:668–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,.Prevalence of psychotic symptoms in delirium.Psychosomatics.2000;41:519–522.
- .A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients.J Am Geriatr Soc.1975;23:433–441.
- ,,,.Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly.J Am Geriatr Soc.1987;35:412–416.
- ,,, et al.The Regenstrief Medical Record System: a quarter century experience.Int J Med Inform.1999;54:225–253.
- ,,,,,.Factors determining the decision to institutionalize dementing individuals: a prospective study.Gerontologist.1993;33:714–720.
- ,,,,,.Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients.JAMA.1986;255:1316–1322.
- ,,,,,.Assessing illness severity: does clinical judgment work?J Chronic Dis.1986;39:439–452.
- ,,,,.Impact of anticholinergics on the aging brain: a review and practical application.Aging Health.2008;4(3):311–320.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, eds.Primary Care Geriatrics: A Case‐Based Approach.5th ed.Philadelphia, PA:Mosby Elsevier;2007:210–218.
- ,,, et al.The association between cognition and histamine‐2 receptor antagonists in African Americans.J Am Geriatr Soc.2007;55(8):1248–1253.
- ,.National hospital discharge survey: annual summary, 1994.Vital Health Stat 13.1997;(128):i–v;1–50.
- .The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients.Am J Med.1994;97:278–288.
- ,,.Dementia in medical wards.J Clin Epidemiol.1988;41:123–126.
- ,,,,,.Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging.Am J Psychiatry.2000;157:708–714.
- ,,, et al.Randomized, placebo‐controlled, double‐blind clinical trial of sertraline in the treatment of depression complicating Alzheimer's disease: initial results from the Depression in Alzheimer's Disease study.Am J Psychiatry.2000;157:1686–1689.
- ,,.Dementia in elderly persons in a general hospital.Am J Psychiatry.2000;157:704–707.
- ,,,,.A prospective study of the impact of psychiatric comorbidity on length of hospital stays of elderly medical‐surgical inpatients.Psychosomatics.1998;39:273–280.
- ,.Psychiatric comorbidity and length of stay in the general hospital. A critical review of outcome studies.Psychosomatics.1994;35:233–252.
- ,,,.Dementia among medical inpatients. Evaluation of 2000 consecutive admissions.Arch Intern Med.1986;146:1923–1926.
- ,,,.The consequences of non‐cognitive symptoms of dementia in medical hospital departments.Int J Psychiatry Med.2003;33:257–271.
- ,,.Cognitive impairment in medical inpatients. I: Screening for dementia—is history better than mental state?Age Ageing.1997;26:31–35.
- ,,, et al.Acute confusional states in elderly patients treated for femoral neck fracture.J Am Geriatr Soc.1988;36:525–530.
- ,.A prospective study of elderly general surgical patients: II. Post‐operative complications.Age Ageing.1989;18:316–326.
- ,,,.Dementia and depression in elderly medical inpatients.Int Psychogeriatr.2000;12:67–75.
- ,,.[Psychiatric disorders in elderly general hospital patients: incidence and long‐term prognosis].Nervenarzt.1993;64:53–61. [German]
- ,.Delirium and dementia in acute medical admissions of elderly patients in Iceland.Acta Psychiatr Scand.1993;87:123–127.
- ,,,,,.[Psychiatric morbidity in elderly patients admitted to a general hospital. A day‐prevalence study].Med Clin (Barc).1991;97:206–210. [Spanish]
- ,,,,,.Detection of psychiatric disorders in elderly medical inpatients.Age Ageing.1994;23:307–311.
- ,,,.Cognitive impairment, emotional disorder and length of stay of elderly patients in a district general hospital.Br J Med Psychol.1987;60(Pt 2):133–139.
- ,,.An investigation of the components of best nursing practice in the care of acutely ill hospitalized older patients with coincidental dementia: a multi‐method design.J Adv Nurs1999;30:1127–1136.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed.Washington, DC:American Psychiatric Association;1994.
- ,,,,,.Clarifying confusion: the confusion assessment method. A new method for detection of delirium.Ann Intern Med.1990;113:941–948.
- ,,, et al.Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging.Neurology.2001;57:1655–1662.
- ,,.Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care.Am J Med.1999;106:565–573.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,.Iatrogenic causes of falls in hospitalised elderly patients: a case‐control study.Postgrad Med J.2002;78:487–489.
- ,,.A prospective study of delirium in hospitalized elderly.JAMA.1990;263:1097–1101.
- ,.The prognostic significance of delirium in older hospital patients.J Am Geriatr Soc.1997;45:174–178.
- ,.Prognosis of delirium in elderly hospital patients.CMAJ.1993;149:41–46.
- ,,,,.The detection of psychiatric morbidity and its effects on outcome in acute elderly medical admissions.Int J Ger Psych1991;6:861–866.
- ,,.Adverse consequences of hospitalization in the elderly.Soc Sci Med.1982;16:1033–1038.
- ,,, et al.Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I.N Engl J Med.1991;324:370–376.
- ,,,.Delirium in elderly patients: an overview of the state of the science.J Gerontol Nurs.2001;27:12–20.
- ,,,,.A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics.Ann Intern Med.1993;119:474–481.
- ,.Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability.JAMA.1996;275:852–857.
- ,,,,,.Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts.Arch Intern Med.2003;163:2716–2724.
- ,,, et al.A clinical prediction rule for delirium after elective noncardiac surgery.JAMA.1994;271:134–139.
- ,,, et al.The relationship of postoperative delirium with psychoactive medications.JAMA.1994;272:1518–1522.
- ,,, et al.How do delirium and dementia increase length of stay of elderly general medical inpatients?Psychosomatics.2004;45:235–242.
- ,,,,,.The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use.J Am Geriatr Soc.2004;52:187–194.
- ,,, et al.Risk factors for delirium in hospitalized elderly.JAMA.1992;267:827–831.
- ,,,,.Psychological comorbidity and length of stay in the general hospital.Am J Psychiatry.1991;148:324–329.
- ,,, et al.Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients.Arch Intern Med.1992;152:334–340.
- ,,.What happens to medical patients with psychiatric disorder?J Psychosom Res1988;32:541–549.
- ,,,,.[Dementia syndromes and length of stay of elderly patients in internal medicine].Ann Med Interne (Paris).1997;148:424–426. [French]
- ,,, et al.[Hospital discharge planning and length of hospital stay in elderly patients admitted through the emergency department].Rev Epidemiol Sante Publique.1995;43:337–347. [French]
- ,,,,.The effect of dementia on acute care in a geriatric medical unit.Int Psychogeriatr.1992;4:231–239.
- ,,,.Cognitive impairment. Can it predict the course of hospitalized patients?J Am Geriatr Soc.1986;34:579–585.
- ,,,,;US Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force.Ann Intern Med.2003;138(11):927–937.
- ,,,.Cognitive impairment in the elderly medically ill: how often is it missed?Int J Geriatr Psychiatry.1993;8:929–937.
- .Recognition of cognitive impairment in elderly medical in‐patients.J R Soc Med.1995;88:183–184.
- ,.Quality indicators for dementia in vulnerable community‐dwelling and hospitalized elders.Ann Intern Med.2001;135:668–676.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49:516–522.
- ,.Prevalence of psychotic symptoms in delirium.Psychosomatics.2000;41:519–522.
- .A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients.J Am Geriatr Soc.1975;23:433–441.
- ,,,.Short Portable Mental Status Questionnaire as a screening test for dementia and delirium among the elderly.J Am Geriatr Soc.1987;35:412–416.
- ,,, et al.The Regenstrief Medical Record System: a quarter century experience.Int J Med Inform.1999;54:225–253.
- ,,,,,.Factors determining the decision to institutionalize dementing individuals: a prospective study.Gerontologist.1993;33:714–720.
- ,,,,,.Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients.JAMA.1986;255:1316–1322.
- ,,,,,.Assessing illness severity: does clinical judgment work?J Chronic Dis.1986;39:439–452.
- ,,,,.Impact of anticholinergics on the aging brain: a review and practical application.Aging Health.2008;4(3):311–320.
- ,.Delirium in hospitalized older adults. In: Ham R, Sloane P, Warshaw G, eds.Primary Care Geriatrics: A Case‐Based Approach.5th ed.Philadelphia, PA:Mosby Elsevier;2007:210–218.
- ,,, et al.The association between cognition and histamine‐2 receptor antagonists in African Americans.J Am Geriatr Soc.2007;55(8):1248–1253.
Copyright © 2010 Society of Hospital Medicine