User login
Population vs Tailored Skin Cancer Screening: Which Is Best?
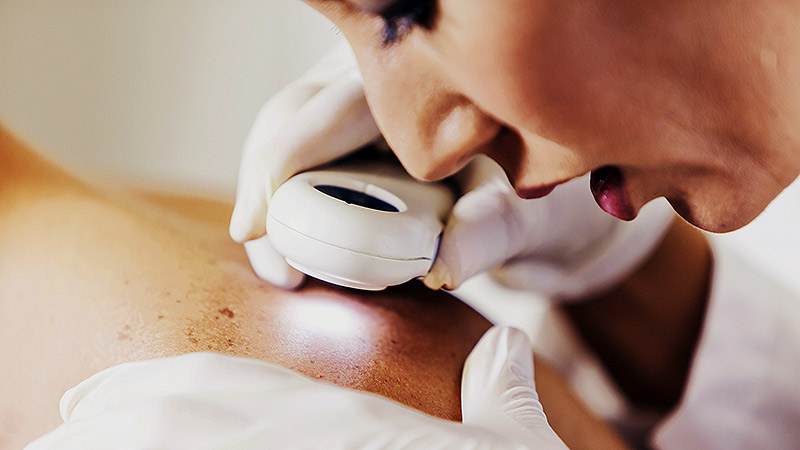
ATHENS, Greece — At the 11th World Congress of Melanoma and 21st EADO Congress 2025, experts presented divergent perspectives on the merits of population-wide skin cancer screening programs vs more targeted approaches. The debate highlighted concerns about healthcare resource allocation, overdiagnosis, and the true impact of mass skin cancer screening on mortality.
Arguing against widespread screening, particularly in low-to-medium incidence countries like Spain, was Susana Puig, MD, the head of Dermatology at Hospital Clínic de Barcelona, University of Barcelona, and a dermatologist at Barnaclínic+, Barcelona, Spain.
“It’s not efficient. We visit too many healthy individuals to detect melanoma,” she said. “We need to focus on treating patients, not checking healthy people without any risk.”
Championing for population-wide screening was Peter Mohr, MD, a dermatologist at the Clinic of Dermatology in Elbe Klinikum Buxtehude, Buxtehude, Germany, who noted a disproportionate focus on treatment rather than prevention. “The ultimate goal of screening,” he said, “is to prevent advanced disease and reduce melanoma-specific mortality.”
Avoid Population-Based Screening
Presenting data from Germany, Puig noted that population-based screening starting at any age requires examining more than 600 people and performing over 24 excisions to detect one melanoma. When setting screening to start at the age of 35 years, the number of people needed to screen to detect one melanoma decreased slightly to 559.
These findings highlight that population-based screening will include many people who don’t need it and can increase the potential for overdiagnosis, she argued.
Studies and guidelines from the United States align with Puig’s concern about broad-based screening likely leading to overdiagnosis. “The incidence of melanoma has risen sixfold in the past 40 years in the United States, while mortality has remained largely flat, an epidemiological signature consistent with overdiagnosis,” according to Adewole Adamson, MD, an assistant professor of internal medicine, in the Division of Dermatology at Dell Medical School at The University of Texas at Austin, Texas, who published findings to this effect in 2022.
“We cannot saturate the system with healthy people,” Puig said. Instead, “we need to use strategies to identify high-risk patients.” She proposed being more selective about who to screen by identifying those at higher risk of developing melanoma.
Identifying risk factors, such as the presence of atypical nevi and a personal or family history of melanoma, can help hone who is screened, she explained. Patients with a personal history of melanoma, in particular, face a higher risk of developing subsequent melanomas. Data show that patients with two or more primary melanomas had almost three times the risk of developing a subsequent one than those with one prior melanoma — 25.7% vs 8.6%. Puig also pointed out the significant correlation between age and melanoma risk, with people over 70 years exhibiting a 93-fold higher probability of diagnosis than those younger than 30 years.
Citing the German data, she noted that screening people 20 years and older with one risk factor reduced the number needed to screen by more than threefold — from more than 600 to 178.
Puig suggested dedicated surveillance programs for high-risk individuals alongside opportunistic screening during routine medical encounters.
“This would lead to a more efficient allocation of healthcare resources and better outcomes for those most vulnerable to melanoma,” Puig concluded.
Perform Population-Based Screening
In contrast, Mohr presented a defense of population-based skin cancer screening. Skin cancer is the most common cancer diagnosed in the United States and is prevalent worldwide, with more than 1.5 million new cases diagnosed globally in 2022.
Screening people and identifying the disease in its earliest stages is important, he said.
Mohr highlighted a recent study exploring biennial skin cancer screening in Germany and found that 4.2% of those screened had a skin cancer finding, but the number of interval melanomas was similar in both screened and unscreened populations.
However, a large retrospective cohort study from Germany involving about 1.4 million people showed a decrease in locoregional metastasis (from 13% to 4%), distant metastases (from 8% to 4%), and systemic treatments (from 21% to 11%) in screened vs unscreened people, as well as better overall survival rates in the screened population.
Mohr highlighted how Germany, in particular, is well-equipped for more broad-based, preventative screening.
Germany has had long-standing primary prevention programs, which have existed for about 24 years and involve extensive public awareness campaigns. Access to dermatologists is significantly better in Germany compared with the Netherlands, with an average waiting time for screening of around 6 weeks and only 1.2 weeks for suspicious lesions, compared with 14 weeks and 3.5 weeks, respectively, in the Netherlands. This access may make a broader screening strategy more feasible in a country like Germany.
However, Mohr did note that there are “no large, randomized trials to show us the value of skin cancer screening.”
A Role for Primary Care Physicians?
Although they disagreed about the utility of screening, both Puig and Mohr agreed on the important role primary care physicians play in improving early melanoma detection. “We cannot do it alone, and general practitioners are really fundamental,” Puig said.
Mohr said that continuous education for primary care physicians can dramatically improve their diagnostic skills. In Germany, an 8-hour training session significantly improved their ability to detect basal cell carcinoma and melanomas. However, he cautioned that this improved accuracy tended to wane within a year.
In Spain, Puig highlighted the successful implementation of teledermatology to support general practitioners. “We train them with dermoscopy, and we answer all teledermatology requests in 1 week, reducing in-person visits by 50%,” she explained. This approach allows general practitioners to assess potential skin cancer efficiently and streamline referrals.
Puig reported being on advisory boards for Almirall, Bristol Myers Squibb (BMS), ISDIN, La Roche-Posay, Leo Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi, and Sun Pharma. She conducts research and trials with AbbVie, Almirall, Amgen, BMS, Biofrontera, Canfield, Cantabria, Fotofinder, GSK, ISDIN, La Roche-Posay, Leo Pharma, MSD, MEDA, Novartis, Pfizer, Polychem, Sanofi, Roche, and Regeneron. She is involved with Athena Technology Solutions and Dermavision Solutions. Mohr reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATHENS, Greece — At the 11th World Congress of Melanoma and 21st EADO Congress 2025, experts presented divergent perspectives on the merits of population-wide skin cancer screening programs vs more targeted approaches. The debate highlighted concerns about healthcare resource allocation, overdiagnosis, and the true impact of mass skin cancer screening on mortality.
Arguing against widespread screening, particularly in low-to-medium incidence countries like Spain, was Susana Puig, MD, the head of Dermatology at Hospital Clínic de Barcelona, University of Barcelona, and a dermatologist at Barnaclínic+, Barcelona, Spain.
“It’s not efficient. We visit too many healthy individuals to detect melanoma,” she said. “We need to focus on treating patients, not checking healthy people without any risk.”
Championing for population-wide screening was Peter Mohr, MD, a dermatologist at the Clinic of Dermatology in Elbe Klinikum Buxtehude, Buxtehude, Germany, who noted a disproportionate focus on treatment rather than prevention. “The ultimate goal of screening,” he said, “is to prevent advanced disease and reduce melanoma-specific mortality.”
Avoid Population-Based Screening
Presenting data from Germany, Puig noted that population-based screening starting at any age requires examining more than 600 people and performing over 24 excisions to detect one melanoma. When setting screening to start at the age of 35 years, the number of people needed to screen to detect one melanoma decreased slightly to 559.
These findings highlight that population-based screening will include many people who don’t need it and can increase the potential for overdiagnosis, she argued.
Studies and guidelines from the United States align with Puig’s concern about broad-based screening likely leading to overdiagnosis. “The incidence of melanoma has risen sixfold in the past 40 years in the United States, while mortality has remained largely flat, an epidemiological signature consistent with overdiagnosis,” according to Adewole Adamson, MD, an assistant professor of internal medicine, in the Division of Dermatology at Dell Medical School at The University of Texas at Austin, Texas, who published findings to this effect in 2022.
“We cannot saturate the system with healthy people,” Puig said. Instead, “we need to use strategies to identify high-risk patients.” She proposed being more selective about who to screen by identifying those at higher risk of developing melanoma.
Identifying risk factors, such as the presence of atypical nevi and a personal or family history of melanoma, can help hone who is screened, she explained. Patients with a personal history of melanoma, in particular, face a higher risk of developing subsequent melanomas. Data show that patients with two or more primary melanomas had almost three times the risk of developing a subsequent one than those with one prior melanoma — 25.7% vs 8.6%. Puig also pointed out the significant correlation between age and melanoma risk, with people over 70 years exhibiting a 93-fold higher probability of diagnosis than those younger than 30 years.
Citing the German data, she noted that screening people 20 years and older with one risk factor reduced the number needed to screen by more than threefold — from more than 600 to 178.
Puig suggested dedicated surveillance programs for high-risk individuals alongside opportunistic screening during routine medical encounters.
“This would lead to a more efficient allocation of healthcare resources and better outcomes for those most vulnerable to melanoma,” Puig concluded.
Perform Population-Based Screening
In contrast, Mohr presented a defense of population-based skin cancer screening. Skin cancer is the most common cancer diagnosed in the United States and is prevalent worldwide, with more than 1.5 million new cases diagnosed globally in 2022.
Screening people and identifying the disease in its earliest stages is important, he said.
Mohr highlighted a recent study exploring biennial skin cancer screening in Germany and found that 4.2% of those screened had a skin cancer finding, but the number of interval melanomas was similar in both screened and unscreened populations.
However, a large retrospective cohort study from Germany involving about 1.4 million people showed a decrease in locoregional metastasis (from 13% to 4%), distant metastases (from 8% to 4%), and systemic treatments (from 21% to 11%) in screened vs unscreened people, as well as better overall survival rates in the screened population.
Mohr highlighted how Germany, in particular, is well-equipped for more broad-based, preventative screening.
Germany has had long-standing primary prevention programs, which have existed for about 24 years and involve extensive public awareness campaigns. Access to dermatologists is significantly better in Germany compared with the Netherlands, with an average waiting time for screening of around 6 weeks and only 1.2 weeks for suspicious lesions, compared with 14 weeks and 3.5 weeks, respectively, in the Netherlands. This access may make a broader screening strategy more feasible in a country like Germany.
However, Mohr did note that there are “no large, randomized trials to show us the value of skin cancer screening.”
A Role for Primary Care Physicians?
Although they disagreed about the utility of screening, both Puig and Mohr agreed on the important role primary care physicians play in improving early melanoma detection. “We cannot do it alone, and general practitioners are really fundamental,” Puig said.
Mohr said that continuous education for primary care physicians can dramatically improve their diagnostic skills. In Germany, an 8-hour training session significantly improved their ability to detect basal cell carcinoma and melanomas. However, he cautioned that this improved accuracy tended to wane within a year.
In Spain, Puig highlighted the successful implementation of teledermatology to support general practitioners. “We train them with dermoscopy, and we answer all teledermatology requests in 1 week, reducing in-person visits by 50%,” she explained. This approach allows general practitioners to assess potential skin cancer efficiently and streamline referrals.
Puig reported being on advisory boards for Almirall, Bristol Myers Squibb (BMS), ISDIN, La Roche-Posay, Leo Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi, and Sun Pharma. She conducts research and trials with AbbVie, Almirall, Amgen, BMS, Biofrontera, Canfield, Cantabria, Fotofinder, GSK, ISDIN, La Roche-Posay, Leo Pharma, MSD, MEDA, Novartis, Pfizer, Polychem, Sanofi, Roche, and Regeneron. She is involved with Athena Technology Solutions and Dermavision Solutions. Mohr reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATHENS, Greece — At the 11th World Congress of Melanoma and 21st EADO Congress 2025, experts presented divergent perspectives on the merits of population-wide skin cancer screening programs vs more targeted approaches. The debate highlighted concerns about healthcare resource allocation, overdiagnosis, and the true impact of mass skin cancer screening on mortality.
Arguing against widespread screening, particularly in low-to-medium incidence countries like Spain, was Susana Puig, MD, the head of Dermatology at Hospital Clínic de Barcelona, University of Barcelona, and a dermatologist at Barnaclínic+, Barcelona, Spain.
“It’s not efficient. We visit too many healthy individuals to detect melanoma,” she said. “We need to focus on treating patients, not checking healthy people without any risk.”
Championing for population-wide screening was Peter Mohr, MD, a dermatologist at the Clinic of Dermatology in Elbe Klinikum Buxtehude, Buxtehude, Germany, who noted a disproportionate focus on treatment rather than prevention. “The ultimate goal of screening,” he said, “is to prevent advanced disease and reduce melanoma-specific mortality.”
Avoid Population-Based Screening
Presenting data from Germany, Puig noted that population-based screening starting at any age requires examining more than 600 people and performing over 24 excisions to detect one melanoma. When setting screening to start at the age of 35 years, the number of people needed to screen to detect one melanoma decreased slightly to 559.
These findings highlight that population-based screening will include many people who don’t need it and can increase the potential for overdiagnosis, she argued.
Studies and guidelines from the United States align with Puig’s concern about broad-based screening likely leading to overdiagnosis. “The incidence of melanoma has risen sixfold in the past 40 years in the United States, while mortality has remained largely flat, an epidemiological signature consistent with overdiagnosis,” according to Adewole Adamson, MD, an assistant professor of internal medicine, in the Division of Dermatology at Dell Medical School at The University of Texas at Austin, Texas, who published findings to this effect in 2022.
“We cannot saturate the system with healthy people,” Puig said. Instead, “we need to use strategies to identify high-risk patients.” She proposed being more selective about who to screen by identifying those at higher risk of developing melanoma.
Identifying risk factors, such as the presence of atypical nevi and a personal or family history of melanoma, can help hone who is screened, she explained. Patients with a personal history of melanoma, in particular, face a higher risk of developing subsequent melanomas. Data show that patients with two or more primary melanomas had almost three times the risk of developing a subsequent one than those with one prior melanoma — 25.7% vs 8.6%. Puig also pointed out the significant correlation between age and melanoma risk, with people over 70 years exhibiting a 93-fold higher probability of diagnosis than those younger than 30 years.
Citing the German data, she noted that screening people 20 years and older with one risk factor reduced the number needed to screen by more than threefold — from more than 600 to 178.
Puig suggested dedicated surveillance programs for high-risk individuals alongside opportunistic screening during routine medical encounters.
“This would lead to a more efficient allocation of healthcare resources and better outcomes for those most vulnerable to melanoma,” Puig concluded.
Perform Population-Based Screening
In contrast, Mohr presented a defense of population-based skin cancer screening. Skin cancer is the most common cancer diagnosed in the United States and is prevalent worldwide, with more than 1.5 million new cases diagnosed globally in 2022.
Screening people and identifying the disease in its earliest stages is important, he said.
Mohr highlighted a recent study exploring biennial skin cancer screening in Germany and found that 4.2% of those screened had a skin cancer finding, but the number of interval melanomas was similar in both screened and unscreened populations.
However, a large retrospective cohort study from Germany involving about 1.4 million people showed a decrease in locoregional metastasis (from 13% to 4%), distant metastases (from 8% to 4%), and systemic treatments (from 21% to 11%) in screened vs unscreened people, as well as better overall survival rates in the screened population.
Mohr highlighted how Germany, in particular, is well-equipped for more broad-based, preventative screening.
Germany has had long-standing primary prevention programs, which have existed for about 24 years and involve extensive public awareness campaigns. Access to dermatologists is significantly better in Germany compared with the Netherlands, with an average waiting time for screening of around 6 weeks and only 1.2 weeks for suspicious lesions, compared with 14 weeks and 3.5 weeks, respectively, in the Netherlands. This access may make a broader screening strategy more feasible in a country like Germany.
However, Mohr did note that there are “no large, randomized trials to show us the value of skin cancer screening.”
A Role for Primary Care Physicians?
Although they disagreed about the utility of screening, both Puig and Mohr agreed on the important role primary care physicians play in improving early melanoma detection. “We cannot do it alone, and general practitioners are really fundamental,” Puig said.
Mohr said that continuous education for primary care physicians can dramatically improve their diagnostic skills. In Germany, an 8-hour training session significantly improved their ability to detect basal cell carcinoma and melanomas. However, he cautioned that this improved accuracy tended to wane within a year.
In Spain, Puig highlighted the successful implementation of teledermatology to support general practitioners. “We train them with dermoscopy, and we answer all teledermatology requests in 1 week, reducing in-person visits by 50%,” she explained. This approach allows general practitioners to assess potential skin cancer efficiently and streamline referrals.
Puig reported being on advisory boards for Almirall, Bristol Myers Squibb (BMS), ISDIN, La Roche-Posay, Leo Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi, and Sun Pharma. She conducts research and trials with AbbVie, Almirall, Amgen, BMS, Biofrontera, Canfield, Cantabria, Fotofinder, GSK, ISDIN, La Roche-Posay, Leo Pharma, MSD, MEDA, Novartis, Pfizer, Polychem, Sanofi, Roche, and Regeneron. She is involved with Athena Technology Solutions and Dermavision Solutions. Mohr reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WCM-EADO 2025
Environmental and Socioeconomic Factors Fuel Respiratory Health Disparities in Rural and Urban Areas
In 2016, Brady Scott was in his parents’ home in Garrett, Kentucky, scrolling his Facebook feed when a post from a local newspaper caught his attention. “The article said that if you grew up in the region I grew up in, compared to the richer Central Kentucky region, the life expectancy differed by about 9 years,” he recalled.
The respiratory therapist, then a PhD student at Rush University, Chicago, was struck and began “Googling” to find out why this was the case. Initially, he thought diabetes, smoking, and economic distress — all prevalent problems in the area — were the culprits. However, he soon found that respiratory disease was particularly common in his region.
Now a professor and program director of the Respiratory Care Program at Rush University, Scott has spent several years trying to understand why people in certain regions experience respiratory illness at higher rates than in other places.
The Environment as a Determinant of Health
When Scott began his PhD, the prevalence of asthma in Southeast Kentucky, part of the Appalachian region, was already well-documented. He focused his research on uncontrolled asthma and the triggers that drove asthma exacerbations.
Housing quality emerged as an important factor. He found that exposure to mold, mildew, dust mites, pests, and rodents increased the risk for asthma and exacerbated existing cases. Lower-income families, more likely to live in poor-quality housing, were significantly affected, even in single-family homes.
Wanda Phipatanakul, MD, MS, director of the Division of Immunology Research Center at Boston Children’s Hospital and S. Jean Emans professor of Pediatrics at Harvard Medical School, Boston, has found similar results in urban environments. She said cockroach and mouse allergen exposure is disproportionately prevalent in urban, low-income neighborhoods. These exposures, closely tied to housing conditions, contribute to worse asthma and respiratory problems, particularly in children.
Scott and Phipatanakul agreed that the environment surrounding people’s homes can also exacerbate respiratory disease.
Rural areas present unique risks, such as agricultural activities that release pesticides and other particulates into the air, said Scott. In mountainous areas like Appalachia, mining operations are another significant contributor. For example, blasting mountains with dynamite creates large clouds of dust and pollutants that settle in valleys. Coal-hauling roads contribute to air quality issues, too. And houses near these roads may be exposed to increased levels of particulate matter, he said.
In the city, Phipatanakul has found that historical practices like redlining have systematically denied certain neighborhoods access to resources and investment, leaving a legacy of poor infrastructure, limited resources, and higher exposure to environmental risks. Today, these areas have more highways and fewer green spaces and are disproportionately linked with a higher incidence of respiratory illnesses.
The findings of both Scott and Phipatanakul underscore a critical bottom line: Health disparities are deeply influenced by environmental factors, which are themselves shaped by socioeconomic conditions and historical inequities. Poor housing quality, exposure to allergens, and proximity to environmental hazards disproportionately affect underserved and minority communities, whether in rural or urban settings.
The Role of Green Spaces in Improving Respiratory Health
Restoring and increasing tree cover and green spaces in urban areas can significantly improve respiratory health by addressing environmental challenges and reducing triggers for respiratory issues. Areas with greater greenness tend to have lower levels of pollutants and fewer environmental infestations, such as mice and cockroaches, explained Phipatanakul. Her research highlights that schools in greener areas have fewer airborne pollutants and particles than those in more urbanized, less green areas, which are usually in poorer suburbs.
Trees absorb pollutants such as particulate matter and sulfur dioxide through dry deposition and stomatal uptake, improving air quality. “The question is whether we can use trees as a public health tool, and this is being done in many cities,” said Alessandro Marcon, PhD, a professor of epidemiology and medical statistics at the University of Verona, Verona, Italy, while speaking at the European Respiratory Society conference held in Vienna last September.
A US analysis showed that existing natural vegetation, such as forests and grasslands, absorbs a large portion of emissions. By restoring land cover, pollution from harmful substances like sulfur dioxide and particulate matter could be reduced by about 30%. This approach is often more cost-effective than technological solutions for managing emissions.
Moreover, tree cover contributes to a healthier air microbiome. Research indicates that urban forest areas have lower pathogenic bacteria and fungi concentrations than nearby urban zones.
Another major advantage is the mitigation of the urban heat island effect. A study conducted in Paris found that municipalities with higher tree coverage experienced 20%-30% lower heat-related mortality than those with less greenery. Increasing tree coverage to 30% could reduce up to 40% of excess mortality associated with urban heat islands. Trees achieve this by providing shade and facilitating evapotranspiration, which cools the surrounding air.
Urban environments, unsurprisingly, often have higher levels of air pollution due to increased traffic and industrial activity. However, despite appearing greener, rural environments may harbor less obvious but significant sources of air pollution. “I live in an urban environment now, but I grew up in a rural environment,” Scott said. “Each has its own issues that affect air quality and health.”
Scott, Phipatanakul, and Marcon reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In 2016, Brady Scott was in his parents’ home in Garrett, Kentucky, scrolling his Facebook feed when a post from a local newspaper caught his attention. “The article said that if you grew up in the region I grew up in, compared to the richer Central Kentucky region, the life expectancy differed by about 9 years,” he recalled.
The respiratory therapist, then a PhD student at Rush University, Chicago, was struck and began “Googling” to find out why this was the case. Initially, he thought diabetes, smoking, and economic distress — all prevalent problems in the area — were the culprits. However, he soon found that respiratory disease was particularly common in his region.
Now a professor and program director of the Respiratory Care Program at Rush University, Scott has spent several years trying to understand why people in certain regions experience respiratory illness at higher rates than in other places.
The Environment as a Determinant of Health
When Scott began his PhD, the prevalence of asthma in Southeast Kentucky, part of the Appalachian region, was already well-documented. He focused his research on uncontrolled asthma and the triggers that drove asthma exacerbations.
Housing quality emerged as an important factor. He found that exposure to mold, mildew, dust mites, pests, and rodents increased the risk for asthma and exacerbated existing cases. Lower-income families, more likely to live in poor-quality housing, were significantly affected, even in single-family homes.
Wanda Phipatanakul, MD, MS, director of the Division of Immunology Research Center at Boston Children’s Hospital and S. Jean Emans professor of Pediatrics at Harvard Medical School, Boston, has found similar results in urban environments. She said cockroach and mouse allergen exposure is disproportionately prevalent in urban, low-income neighborhoods. These exposures, closely tied to housing conditions, contribute to worse asthma and respiratory problems, particularly in children.
Scott and Phipatanakul agreed that the environment surrounding people’s homes can also exacerbate respiratory disease.
Rural areas present unique risks, such as agricultural activities that release pesticides and other particulates into the air, said Scott. In mountainous areas like Appalachia, mining operations are another significant contributor. For example, blasting mountains with dynamite creates large clouds of dust and pollutants that settle in valleys. Coal-hauling roads contribute to air quality issues, too. And houses near these roads may be exposed to increased levels of particulate matter, he said.
In the city, Phipatanakul has found that historical practices like redlining have systematically denied certain neighborhoods access to resources and investment, leaving a legacy of poor infrastructure, limited resources, and higher exposure to environmental risks. Today, these areas have more highways and fewer green spaces and are disproportionately linked with a higher incidence of respiratory illnesses.
The findings of both Scott and Phipatanakul underscore a critical bottom line: Health disparities are deeply influenced by environmental factors, which are themselves shaped by socioeconomic conditions and historical inequities. Poor housing quality, exposure to allergens, and proximity to environmental hazards disproportionately affect underserved and minority communities, whether in rural or urban settings.
The Role of Green Spaces in Improving Respiratory Health
Restoring and increasing tree cover and green spaces in urban areas can significantly improve respiratory health by addressing environmental challenges and reducing triggers for respiratory issues. Areas with greater greenness tend to have lower levels of pollutants and fewer environmental infestations, such as mice and cockroaches, explained Phipatanakul. Her research highlights that schools in greener areas have fewer airborne pollutants and particles than those in more urbanized, less green areas, which are usually in poorer suburbs.
Trees absorb pollutants such as particulate matter and sulfur dioxide through dry deposition and stomatal uptake, improving air quality. “The question is whether we can use trees as a public health tool, and this is being done in many cities,” said Alessandro Marcon, PhD, a professor of epidemiology and medical statistics at the University of Verona, Verona, Italy, while speaking at the European Respiratory Society conference held in Vienna last September.
A US analysis showed that existing natural vegetation, such as forests and grasslands, absorbs a large portion of emissions. By restoring land cover, pollution from harmful substances like sulfur dioxide and particulate matter could be reduced by about 30%. This approach is often more cost-effective than technological solutions for managing emissions.
Moreover, tree cover contributes to a healthier air microbiome. Research indicates that urban forest areas have lower pathogenic bacteria and fungi concentrations than nearby urban zones.
Another major advantage is the mitigation of the urban heat island effect. A study conducted in Paris found that municipalities with higher tree coverage experienced 20%-30% lower heat-related mortality than those with less greenery. Increasing tree coverage to 30% could reduce up to 40% of excess mortality associated with urban heat islands. Trees achieve this by providing shade and facilitating evapotranspiration, which cools the surrounding air.
Urban environments, unsurprisingly, often have higher levels of air pollution due to increased traffic and industrial activity. However, despite appearing greener, rural environments may harbor less obvious but significant sources of air pollution. “I live in an urban environment now, but I grew up in a rural environment,” Scott said. “Each has its own issues that affect air quality and health.”
Scott, Phipatanakul, and Marcon reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In 2016, Brady Scott was in his parents’ home in Garrett, Kentucky, scrolling his Facebook feed when a post from a local newspaper caught his attention. “The article said that if you grew up in the region I grew up in, compared to the richer Central Kentucky region, the life expectancy differed by about 9 years,” he recalled.
The respiratory therapist, then a PhD student at Rush University, Chicago, was struck and began “Googling” to find out why this was the case. Initially, he thought diabetes, smoking, and economic distress — all prevalent problems in the area — were the culprits. However, he soon found that respiratory disease was particularly common in his region.
Now a professor and program director of the Respiratory Care Program at Rush University, Scott has spent several years trying to understand why people in certain regions experience respiratory illness at higher rates than in other places.
The Environment as a Determinant of Health
When Scott began his PhD, the prevalence of asthma in Southeast Kentucky, part of the Appalachian region, was already well-documented. He focused his research on uncontrolled asthma and the triggers that drove asthma exacerbations.
Housing quality emerged as an important factor. He found that exposure to mold, mildew, dust mites, pests, and rodents increased the risk for asthma and exacerbated existing cases. Lower-income families, more likely to live in poor-quality housing, were significantly affected, even in single-family homes.
Wanda Phipatanakul, MD, MS, director of the Division of Immunology Research Center at Boston Children’s Hospital and S. Jean Emans professor of Pediatrics at Harvard Medical School, Boston, has found similar results in urban environments. She said cockroach and mouse allergen exposure is disproportionately prevalent in urban, low-income neighborhoods. These exposures, closely tied to housing conditions, contribute to worse asthma and respiratory problems, particularly in children.
Scott and Phipatanakul agreed that the environment surrounding people’s homes can also exacerbate respiratory disease.
Rural areas present unique risks, such as agricultural activities that release pesticides and other particulates into the air, said Scott. In mountainous areas like Appalachia, mining operations are another significant contributor. For example, blasting mountains with dynamite creates large clouds of dust and pollutants that settle in valleys. Coal-hauling roads contribute to air quality issues, too. And houses near these roads may be exposed to increased levels of particulate matter, he said.
In the city, Phipatanakul has found that historical practices like redlining have systematically denied certain neighborhoods access to resources and investment, leaving a legacy of poor infrastructure, limited resources, and higher exposure to environmental risks. Today, these areas have more highways and fewer green spaces and are disproportionately linked with a higher incidence of respiratory illnesses.
The findings of both Scott and Phipatanakul underscore a critical bottom line: Health disparities are deeply influenced by environmental factors, which are themselves shaped by socioeconomic conditions and historical inequities. Poor housing quality, exposure to allergens, and proximity to environmental hazards disproportionately affect underserved and minority communities, whether in rural or urban settings.
The Role of Green Spaces in Improving Respiratory Health
Restoring and increasing tree cover and green spaces in urban areas can significantly improve respiratory health by addressing environmental challenges and reducing triggers for respiratory issues. Areas with greater greenness tend to have lower levels of pollutants and fewer environmental infestations, such as mice and cockroaches, explained Phipatanakul. Her research highlights that schools in greener areas have fewer airborne pollutants and particles than those in more urbanized, less green areas, which are usually in poorer suburbs.
Trees absorb pollutants such as particulate matter and sulfur dioxide through dry deposition and stomatal uptake, improving air quality. “The question is whether we can use trees as a public health tool, and this is being done in many cities,” said Alessandro Marcon, PhD, a professor of epidemiology and medical statistics at the University of Verona, Verona, Italy, while speaking at the European Respiratory Society conference held in Vienna last September.
A US analysis showed that existing natural vegetation, such as forests and grasslands, absorbs a large portion of emissions. By restoring land cover, pollution from harmful substances like sulfur dioxide and particulate matter could be reduced by about 30%. This approach is often more cost-effective than technological solutions for managing emissions.
Moreover, tree cover contributes to a healthier air microbiome. Research indicates that urban forest areas have lower pathogenic bacteria and fungi concentrations than nearby urban zones.
Another major advantage is the mitigation of the urban heat island effect. A study conducted in Paris found that municipalities with higher tree coverage experienced 20%-30% lower heat-related mortality than those with less greenery. Increasing tree coverage to 30% could reduce up to 40% of excess mortality associated with urban heat islands. Trees achieve this by providing shade and facilitating evapotranspiration, which cools the surrounding air.
Urban environments, unsurprisingly, often have higher levels of air pollution due to increased traffic and industrial activity. However, despite appearing greener, rural environments may harbor less obvious but significant sources of air pollution. “I live in an urban environment now, but I grew up in a rural environment,” Scott said. “Each has its own issues that affect air quality and health.”
Scott, Phipatanakul, and Marcon reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Environmental and Socioeconomic Factors Fuel Respiratory Health Disparities in Rural and Urban Areas
In 2016, Brady Scott was in his parents’ home in Garrett, Kentucky, scrolling his Facebook feed when a post from a local newspaper caught his attention. “The article said that if you grew up in the region I grew up in, compared to the richer Central Kentucky region, the life expectancy differed by about 9 years,” he recalled.
The respiratory therapist, then a PhD student at Rush University, Chicago, was struck and began “Googling” to find out why this was the case. Initially, he thought diabetes, smoking, and economic distress — all prevalent problems in the area — were the culprits. However, he soon found that respiratory disease was particularly common in his region.
Now a professor and program director of the Respiratory Care Program at Rush University, Scott has spent several years trying to understand why people in certain regions experience respiratory illness at higher rates than in other places.
The Environment as a Determinant of Health
When Scott began his PhD, the prevalence of asthma in Southeast Kentucky, part of the Appalachian region, was already well-documented. He focused his research on uncontrolled asthma and the triggers that drove asthma exacerbations.
Housing quality emerged as an important factor. He found that exposure to mold, mildew, dust mites, pests, and rodents increased the risk for asthma and exacerbated existing cases. Lower-income families, more likely to live in poor-quality housing, were significantly affected, even in single-family homes.
Wanda Phipatanakul, MD, MS, director of the Division of Immunology Research Center at Boston Children’s Hospital and S. Jean Emans professor of Pediatrics at Harvard Medical School, Boston, has found similar results in urban environments. She said cockroach and mouse allergen exposure is disproportionately prevalent in urban, low-income neighborhoods. These exposures, closely tied to housing conditions, contribute to worse asthma and respiratory problems, particularly in children.
Scott and Phipatanakul agreed that the environment surrounding people’s homes can also exacerbate respiratory disease.
Rural areas present unique risks, such as agricultural activities that release pesticides and other particulates into the air, said Scott. In mountainous areas like Appalachia, mining operations are another significant contributor. For example, blasting mountains with dynamite creates large clouds of dust and pollutants that settle in valleys. Coal-hauling roads contribute to air quality issues, too. And houses near these roads may be exposed to increased levels of particulate matter, he said.
In the city, Phipatanakul has found that historical practices like redlining have systematically denied certain neighborhoods access to resources and investment, leaving a legacy of poor infrastructure, limited resources, and higher exposure to environmental risks. Today, these areas have more highways and fewer green spaces and are disproportionately linked with a higher incidence of respiratory illnesses.
The findings of both Scott and Phipatanakul underscore a critical bottom line: Health disparities are deeply influenced by environmental factors, which are themselves shaped by socioeconomic conditions and historical inequities. Poor housing quality, exposure to allergens, and proximity to environmental hazards disproportionately affect underserved and minority communities, whether in rural or urban settings.
The Role of Green Spaces in Improving Respiratory Health
Restoring and increasing tree cover and green spaces in urban areas can significantly improve respiratory health by addressing environmental challenges and reducing triggers for respiratory issues. Areas with greater greenness tend to have lower levels of pollutants and fewer environmental infestations, such as mice and cockroaches, explained Phipatanakul. Her research highlights that schools in greener areas have fewer airborne pollutants and particles than those in more urbanized, less green areas, which are usually in poorer suburbs.
Trees absorb pollutants such as particulate matter and sulfur dioxide through dry deposition and stomatal uptake, improving air quality. “The question is whether we can use trees as a public health tool, and this is being done in many cities,” said Alessandro Marcon, PhD, a professor of epidemiology and medical statistics at the University of Verona, Verona, Italy, while speaking at the European Respiratory Society conference held in Vienna last September.
A US analysis showed that existing natural vegetation, such as forests and grasslands, absorbs a large portion of emissions. By restoring land cover, pollution from harmful substances like sulfur dioxide and particulate matter could be reduced by about 30%. This approach is often more cost-effective than technological solutions for managing emissions.
Moreover, tree cover contributes to a healthier air microbiome. Research indicates that urban forest areas have lower pathogenic bacteria and fungi concentrations than nearby urban zones.
Another major advantage is the mitigation of the urban heat island effect. A study conducted in Paris found that municipalities with higher tree coverage experienced 20%-30% lower heat-related mortality than those with less greenery. Increasing tree coverage to 30% could reduce up to 40% of excess mortality associated with urban heat islands. Trees achieve this by providing shade and facilitating evapotranspiration, which cools the surrounding air.
Urban environments, unsurprisingly, often have higher levels of air pollution due to increased traffic and industrial activity. However, despite appearing greener, rural environments may harbor less obvious but significant sources of air pollution. “I live in an urban environment now, but I grew up in a rural environment,” Scott said. “Each has its own issues that affect air quality and health.”
Scott, Phipatanakul, and Marcon reported no relevant financial relationships.■
A version of this article first appeared on Medscape.com.
In 2016, Brady Scott was in his parents’ home in Garrett, Kentucky, scrolling his Facebook feed when a post from a local newspaper caught his attention. “The article said that if you grew up in the region I grew up in, compared to the richer Central Kentucky region, the life expectancy differed by about 9 years,” he recalled.
The respiratory therapist, then a PhD student at Rush University, Chicago, was struck and began “Googling” to find out why this was the case. Initially, he thought diabetes, smoking, and economic distress — all prevalent problems in the area — were the culprits. However, he soon found that respiratory disease was particularly common in his region.
Now a professor and program director of the Respiratory Care Program at Rush University, Scott has spent several years trying to understand why people in certain regions experience respiratory illness at higher rates than in other places.
The Environment as a Determinant of Health
When Scott began his PhD, the prevalence of asthma in Southeast Kentucky, part of the Appalachian region, was already well-documented. He focused his research on uncontrolled asthma and the triggers that drove asthma exacerbations.
Housing quality emerged as an important factor. He found that exposure to mold, mildew, dust mites, pests, and rodents increased the risk for asthma and exacerbated existing cases. Lower-income families, more likely to live in poor-quality housing, were significantly affected, even in single-family homes.
Wanda Phipatanakul, MD, MS, director of the Division of Immunology Research Center at Boston Children’s Hospital and S. Jean Emans professor of Pediatrics at Harvard Medical School, Boston, has found similar results in urban environments. She said cockroach and mouse allergen exposure is disproportionately prevalent in urban, low-income neighborhoods. These exposures, closely tied to housing conditions, contribute to worse asthma and respiratory problems, particularly in children.
Scott and Phipatanakul agreed that the environment surrounding people’s homes can also exacerbate respiratory disease.
Rural areas present unique risks, such as agricultural activities that release pesticides and other particulates into the air, said Scott. In mountainous areas like Appalachia, mining operations are another significant contributor. For example, blasting mountains with dynamite creates large clouds of dust and pollutants that settle in valleys. Coal-hauling roads contribute to air quality issues, too. And houses near these roads may be exposed to increased levels of particulate matter, he said.
In the city, Phipatanakul has found that historical practices like redlining have systematically denied certain neighborhoods access to resources and investment, leaving a legacy of poor infrastructure, limited resources, and higher exposure to environmental risks. Today, these areas have more highways and fewer green spaces and are disproportionately linked with a higher incidence of respiratory illnesses.
The findings of both Scott and Phipatanakul underscore a critical bottom line: Health disparities are deeply influenced by environmental factors, which are themselves shaped by socioeconomic conditions and historical inequities. Poor housing quality, exposure to allergens, and proximity to environmental hazards disproportionately affect underserved and minority communities, whether in rural or urban settings.
The Role of Green Spaces in Improving Respiratory Health
Restoring and increasing tree cover and green spaces in urban areas can significantly improve respiratory health by addressing environmental challenges and reducing triggers for respiratory issues. Areas with greater greenness tend to have lower levels of pollutants and fewer environmental infestations, such as mice and cockroaches, explained Phipatanakul. Her research highlights that schools in greener areas have fewer airborne pollutants and particles than those in more urbanized, less green areas, which are usually in poorer suburbs.
Trees absorb pollutants such as particulate matter and sulfur dioxide through dry deposition and stomatal uptake, improving air quality. “The question is whether we can use trees as a public health tool, and this is being done in many cities,” said Alessandro Marcon, PhD, a professor of epidemiology and medical statistics at the University of Verona, Verona, Italy, while speaking at the European Respiratory Society conference held in Vienna last September.
A US analysis showed that existing natural vegetation, such as forests and grasslands, absorbs a large portion of emissions. By restoring land cover, pollution from harmful substances like sulfur dioxide and particulate matter could be reduced by about 30%. This approach is often more cost-effective than technological solutions for managing emissions.
Moreover, tree cover contributes to a healthier air microbiome. Research indicates that urban forest areas have lower pathogenic bacteria and fungi concentrations than nearby urban zones.
Another major advantage is the mitigation of the urban heat island effect. A study conducted in Paris found that municipalities with higher tree coverage experienced 20%-30% lower heat-related mortality than those with less greenery. Increasing tree coverage to 30% could reduce up to 40% of excess mortality associated with urban heat islands. Trees achieve this by providing shade and facilitating evapotranspiration, which cools the surrounding air.
Urban environments, unsurprisingly, often have higher levels of air pollution due to increased traffic and industrial activity. However, despite appearing greener, rural environments may harbor less obvious but significant sources of air pollution. “I live in an urban environment now, but I grew up in a rural environment,” Scott said. “Each has its own issues that affect air quality and health.”
Scott, Phipatanakul, and Marcon reported no relevant financial relationships.■
A version of this article first appeared on Medscape.com.
In 2016, Brady Scott was in his parents’ home in Garrett, Kentucky, scrolling his Facebook feed when a post from a local newspaper caught his attention. “The article said that if you grew up in the region I grew up in, compared to the richer Central Kentucky region, the life expectancy differed by about 9 years,” he recalled.
The respiratory therapist, then a PhD student at Rush University, Chicago, was struck and began “Googling” to find out why this was the case. Initially, he thought diabetes, smoking, and economic distress — all prevalent problems in the area — were the culprits. However, he soon found that respiratory disease was particularly common in his region.
Now a professor and program director of the Respiratory Care Program at Rush University, Scott has spent several years trying to understand why people in certain regions experience respiratory illness at higher rates than in other places.
The Environment as a Determinant of Health
When Scott began his PhD, the prevalence of asthma in Southeast Kentucky, part of the Appalachian region, was already well-documented. He focused his research on uncontrolled asthma and the triggers that drove asthma exacerbations.
Housing quality emerged as an important factor. He found that exposure to mold, mildew, dust mites, pests, and rodents increased the risk for asthma and exacerbated existing cases. Lower-income families, more likely to live in poor-quality housing, were significantly affected, even in single-family homes.
Wanda Phipatanakul, MD, MS, director of the Division of Immunology Research Center at Boston Children’s Hospital and S. Jean Emans professor of Pediatrics at Harvard Medical School, Boston, has found similar results in urban environments. She said cockroach and mouse allergen exposure is disproportionately prevalent in urban, low-income neighborhoods. These exposures, closely tied to housing conditions, contribute to worse asthma and respiratory problems, particularly in children.
Scott and Phipatanakul agreed that the environment surrounding people’s homes can also exacerbate respiratory disease.
Rural areas present unique risks, such as agricultural activities that release pesticides and other particulates into the air, said Scott. In mountainous areas like Appalachia, mining operations are another significant contributor. For example, blasting mountains with dynamite creates large clouds of dust and pollutants that settle in valleys. Coal-hauling roads contribute to air quality issues, too. And houses near these roads may be exposed to increased levels of particulate matter, he said.
In the city, Phipatanakul has found that historical practices like redlining have systematically denied certain neighborhoods access to resources and investment, leaving a legacy of poor infrastructure, limited resources, and higher exposure to environmental risks. Today, these areas have more highways and fewer green spaces and are disproportionately linked with a higher incidence of respiratory illnesses.
The findings of both Scott and Phipatanakul underscore a critical bottom line: Health disparities are deeply influenced by environmental factors, which are themselves shaped by socioeconomic conditions and historical inequities. Poor housing quality, exposure to allergens, and proximity to environmental hazards disproportionately affect underserved and minority communities, whether in rural or urban settings.
The Role of Green Spaces in Improving Respiratory Health
Restoring and increasing tree cover and green spaces in urban areas can significantly improve respiratory health by addressing environmental challenges and reducing triggers for respiratory issues. Areas with greater greenness tend to have lower levels of pollutants and fewer environmental infestations, such as mice and cockroaches, explained Phipatanakul. Her research highlights that schools in greener areas have fewer airborne pollutants and particles than those in more urbanized, less green areas, which are usually in poorer suburbs.
Trees absorb pollutants such as particulate matter and sulfur dioxide through dry deposition and stomatal uptake, improving air quality. “The question is whether we can use trees as a public health tool, and this is being done in many cities,” said Alessandro Marcon, PhD, a professor of epidemiology and medical statistics at the University of Verona, Verona, Italy, while speaking at the European Respiratory Society conference held in Vienna last September.
A US analysis showed that existing natural vegetation, such as forests and grasslands, absorbs a large portion of emissions. By restoring land cover, pollution from harmful substances like sulfur dioxide and particulate matter could be reduced by about 30%. This approach is often more cost-effective than technological solutions for managing emissions.
Moreover, tree cover contributes to a healthier air microbiome. Research indicates that urban forest areas have lower pathogenic bacteria and fungi concentrations than nearby urban zones.
Another major advantage is the mitigation of the urban heat island effect. A study conducted in Paris found that municipalities with higher tree coverage experienced 20%-30% lower heat-related mortality than those with less greenery. Increasing tree coverage to 30% could reduce up to 40% of excess mortality associated with urban heat islands. Trees achieve this by providing shade and facilitating evapotranspiration, which cools the surrounding air.
Urban environments, unsurprisingly, often have higher levels of air pollution due to increased traffic and industrial activity. However, despite appearing greener, rural environments may harbor less obvious but significant sources of air pollution. “I live in an urban environment now, but I grew up in a rural environment,” Scott said. “Each has its own issues that affect air quality and health.”
Scott, Phipatanakul, and Marcon reported no relevant financial relationships.■
A version of this article first appeared on Medscape.com.
RSV Vaccines and Treatments Face Global Access Hurdles
Almost 70 years after the discovery of the respiratory syncytial virus (RSV), vaccines and preventive treatments are giving babies a chance to beat the potentially deadly childhood infection.
As doctors turn to monoclonal antibody therapies and governments plan vaccination programs, clinical researchers are asking whether these measures will reduce the spread of the virus. Will fewer babies die from RSV, and fewer children develop permanent wheezing?
Recent studies offer clues.
Fabio Midulla, an associate professor of pediatrics at Sapienza University of Rome in Rome, Italy, said that the pharmaceutical industry is poised to push governments to use vaccines and monoclonal antibodies for even more children. “Such a push might work,” he said at the European Respiratory Society (ERS) 2024 Congress, “given that several studies have already demonstrated that their use can improve outcomes for children who do become infected and reduce societal costs by reducing hospitalizations.”
But Mariëlle WH Pijnenburg, a pulmonary specialist at Erasmus University Rotterdam in the Netherlands, said at the Congress that greater rollout would require governments to force industry to lower prices. If treatments remain beyond the reach of lower-income countries — where the burden of RSV is the greatest — the death toll from this common childhood infection will remain stubbornly high, and the prospect of global elimination will remain forever out of reach, she said.
New Tools in the Fight Against RSV
Nirsevimab, a long-acting monoclonal antibody given to newborns to prevent severe infection, was approved by the European Medicines Agency (EMA) in October 2022 and the US Food and Drug Administration (FDA) in July 2023. And Abrysvo, a vaccine given to older adults and pregnant women to stop them from passing the virus to babies from birth through 6 months of age, was approved by the FDA and the EMA in 2023.
RSV is responsible for over 33 million lung infections in children younger than 5 years annually, with more than 4 million hospitalizations and nearly 200,000 deaths. According to the Centers for Disease Control and Prevention, every year, 2.1 million children younger than 5 years old visit a healthcare provider because of an RSV infection and between 58,000 and 80,000 children younger than 5 years old are hospitalized in the United States. The burden of severe RSV disease is also high among adults, with an estimated 123,000-193,000 hospitalizations, 24,400-34,900 ICU admissions, and 4680-8620 in-hospital deaths occurring annually among US adults.
Infection in infancy can lead to later complications, such as the development of wheezing, a condition that causes breathlessness and a feeling of tightening in the chest, and possibly also asthma.
Studies have shown that children and preterm infants infected with RSV who were given monoclonal antibodies experienced less post-infection wheezing, suggesting that RSV prophylaxis could prevent the development of wheezing bronchitis.
A study conducted in Galicia, Spain, showed that only 0.3% of infants who received prophylaxis with Nirsevimab were hospitalized for RSV-related lower respiratory tract infections. “This is very promising,” Yvonne Maldonado, MD, professor of pediatrics and epidemiology and population health at Stanford University in Stanford, California, told Medscape Medical News. “But this virus is ubiquitous. It’s found everywhere. It comes around every winter season. And immunity is not long-lasting.”
Older children who are not receiving monoclonal antibodies still experience RSV-related hospitalizations, suggesting the virus continues to circulate at high enough levels in the community. “The vaccine and monoclonal antibodies can reduce the risk of hospitalization and more severe disease in young kids, but they won’t eliminate the virus,” Maldonado said. “Right now, the goal is to prevent serious infection, not to prevent the spread of the virus completely.”
Expanding Access to RSV Prevention in Low-Income Countries
Currently, the RSV vaccine and monoclonal antibodies are only given in the United States, Europe, United Kingdom, and Canada to newborns, children at risk for severe disease, and pregnant women. However, Midulla said that pharmaceutical companies are pushing to broaden the rollout to a broader population within these countries. Yet, he said, over 99% of RSV infection–related deaths occur in the Global South.
No pharmaceutical company has sought approval in low-income countries such as those in Africa. “Unless they see there being a market in a country, they’re not going to go through the onerous process of getting [a vaccine] licensed,” Shabir Madhi, dean of the faculty of health sciences and a professor of vaccinology at the University of the Witwatersrand, Johannesburg, South Africa, told Medscape Medical News.
He highlighted that almost 50% of RSV-related deaths occur in African children younger than 5 years, despite these children comprising just one fifth of the global under-5 population. The high burden of RSV mortality in the Global South is mainly due to poor access to healthcare and supportive treatments, such as supplemental oxygen, which can help children recover from severe RSV infection.
Companies are unlikely to pursue regulatory approval and licensing in low- and middle-income countries until GAVI, the global vaccine alliance, commits to procuring and funding the vaccines for these regions. GAVI’s decision would provide the necessary market incentive for manufacturers to seek approval.
Madhi suggested that GAVI’s decision on RSV vaccine procurement is imminent, likely early next year, following the World Health Organization’s Strategic Advisory Group of Experts on Immunization recommendation to vaccinate all pregnant women with the RSV vaccine, regardless of whether they are in high-income or low-income countries.
Nevertheless, even if vaccines become available, many African countries may still struggle to afford them. Madhi said that these countries would likely depend on GAVI and organizations like UNICEF to procure the vaccines at affordable prices. “The unfortunate reality is that many countries — especially in Africa — still wouldn’t be able to afford it, even if the vaccine cost as little as $5,” said Madhi. “But that’s where they would have the greatest impact.”
Midulla, Pijnenburg reported no relevant financial relationships. Madhi’s research unit, the Vaccines and Infectious Disease Analytics Unit, was involved in the clinical trials for the Pfizer RSV vaccine, the GSK RSV vaccine (which was terminated), as well as the MEDLEY trial of palivizumab. All funding for these studies went to his institution, the University of the Witwatersrand. Maldonado was Stanford principal investigator for the Pfizer RSV vaccine.
A version of this article appeared on Medscape.com.
Almost 70 years after the discovery of the respiratory syncytial virus (RSV), vaccines and preventive treatments are giving babies a chance to beat the potentially deadly childhood infection.
As doctors turn to monoclonal antibody therapies and governments plan vaccination programs, clinical researchers are asking whether these measures will reduce the spread of the virus. Will fewer babies die from RSV, and fewer children develop permanent wheezing?
Recent studies offer clues.
Fabio Midulla, an associate professor of pediatrics at Sapienza University of Rome in Rome, Italy, said that the pharmaceutical industry is poised to push governments to use vaccines and monoclonal antibodies for even more children. “Such a push might work,” he said at the European Respiratory Society (ERS) 2024 Congress, “given that several studies have already demonstrated that their use can improve outcomes for children who do become infected and reduce societal costs by reducing hospitalizations.”
But Mariëlle WH Pijnenburg, a pulmonary specialist at Erasmus University Rotterdam in the Netherlands, said at the Congress that greater rollout would require governments to force industry to lower prices. If treatments remain beyond the reach of lower-income countries — where the burden of RSV is the greatest — the death toll from this common childhood infection will remain stubbornly high, and the prospect of global elimination will remain forever out of reach, she said.
New Tools in the Fight Against RSV
Nirsevimab, a long-acting monoclonal antibody given to newborns to prevent severe infection, was approved by the European Medicines Agency (EMA) in October 2022 and the US Food and Drug Administration (FDA) in July 2023. And Abrysvo, a vaccine given to older adults and pregnant women to stop them from passing the virus to babies from birth through 6 months of age, was approved by the FDA and the EMA in 2023.
RSV is responsible for over 33 million lung infections in children younger than 5 years annually, with more than 4 million hospitalizations and nearly 200,000 deaths. According to the Centers for Disease Control and Prevention, every year, 2.1 million children younger than 5 years old visit a healthcare provider because of an RSV infection and between 58,000 and 80,000 children younger than 5 years old are hospitalized in the United States. The burden of severe RSV disease is also high among adults, with an estimated 123,000-193,000 hospitalizations, 24,400-34,900 ICU admissions, and 4680-8620 in-hospital deaths occurring annually among US adults.
Infection in infancy can lead to later complications, such as the development of wheezing, a condition that causes breathlessness and a feeling of tightening in the chest, and possibly also asthma.
Studies have shown that children and preterm infants infected with RSV who were given monoclonal antibodies experienced less post-infection wheezing, suggesting that RSV prophylaxis could prevent the development of wheezing bronchitis.
A study conducted in Galicia, Spain, showed that only 0.3% of infants who received prophylaxis with Nirsevimab were hospitalized for RSV-related lower respiratory tract infections. “This is very promising,” Yvonne Maldonado, MD, professor of pediatrics and epidemiology and population health at Stanford University in Stanford, California, told Medscape Medical News. “But this virus is ubiquitous. It’s found everywhere. It comes around every winter season. And immunity is not long-lasting.”
Older children who are not receiving monoclonal antibodies still experience RSV-related hospitalizations, suggesting the virus continues to circulate at high enough levels in the community. “The vaccine and monoclonal antibodies can reduce the risk of hospitalization and more severe disease in young kids, but they won’t eliminate the virus,” Maldonado said. “Right now, the goal is to prevent serious infection, not to prevent the spread of the virus completely.”
Expanding Access to RSV Prevention in Low-Income Countries
Currently, the RSV vaccine and monoclonal antibodies are only given in the United States, Europe, United Kingdom, and Canada to newborns, children at risk for severe disease, and pregnant women. However, Midulla said that pharmaceutical companies are pushing to broaden the rollout to a broader population within these countries. Yet, he said, over 99% of RSV infection–related deaths occur in the Global South.
No pharmaceutical company has sought approval in low-income countries such as those in Africa. “Unless they see there being a market in a country, they’re not going to go through the onerous process of getting [a vaccine] licensed,” Shabir Madhi, dean of the faculty of health sciences and a professor of vaccinology at the University of the Witwatersrand, Johannesburg, South Africa, told Medscape Medical News.
He highlighted that almost 50% of RSV-related deaths occur in African children younger than 5 years, despite these children comprising just one fifth of the global under-5 population. The high burden of RSV mortality in the Global South is mainly due to poor access to healthcare and supportive treatments, such as supplemental oxygen, which can help children recover from severe RSV infection.
Companies are unlikely to pursue regulatory approval and licensing in low- and middle-income countries until GAVI, the global vaccine alliance, commits to procuring and funding the vaccines for these regions. GAVI’s decision would provide the necessary market incentive for manufacturers to seek approval.
Madhi suggested that GAVI’s decision on RSV vaccine procurement is imminent, likely early next year, following the World Health Organization’s Strategic Advisory Group of Experts on Immunization recommendation to vaccinate all pregnant women with the RSV vaccine, regardless of whether they are in high-income or low-income countries.
Nevertheless, even if vaccines become available, many African countries may still struggle to afford them. Madhi said that these countries would likely depend on GAVI and organizations like UNICEF to procure the vaccines at affordable prices. “The unfortunate reality is that many countries — especially in Africa — still wouldn’t be able to afford it, even if the vaccine cost as little as $5,” said Madhi. “But that’s where they would have the greatest impact.”
Midulla, Pijnenburg reported no relevant financial relationships. Madhi’s research unit, the Vaccines and Infectious Disease Analytics Unit, was involved in the clinical trials for the Pfizer RSV vaccine, the GSK RSV vaccine (which was terminated), as well as the MEDLEY trial of palivizumab. All funding for these studies went to his institution, the University of the Witwatersrand. Maldonado was Stanford principal investigator for the Pfizer RSV vaccine.
A version of this article appeared on Medscape.com.
Almost 70 years after the discovery of the respiratory syncytial virus (RSV), vaccines and preventive treatments are giving babies a chance to beat the potentially deadly childhood infection.
As doctors turn to monoclonal antibody therapies and governments plan vaccination programs, clinical researchers are asking whether these measures will reduce the spread of the virus. Will fewer babies die from RSV, and fewer children develop permanent wheezing?
Recent studies offer clues.
Fabio Midulla, an associate professor of pediatrics at Sapienza University of Rome in Rome, Italy, said that the pharmaceutical industry is poised to push governments to use vaccines and monoclonal antibodies for even more children. “Such a push might work,” he said at the European Respiratory Society (ERS) 2024 Congress, “given that several studies have already demonstrated that their use can improve outcomes for children who do become infected and reduce societal costs by reducing hospitalizations.”
But Mariëlle WH Pijnenburg, a pulmonary specialist at Erasmus University Rotterdam in the Netherlands, said at the Congress that greater rollout would require governments to force industry to lower prices. If treatments remain beyond the reach of lower-income countries — where the burden of RSV is the greatest — the death toll from this common childhood infection will remain stubbornly high, and the prospect of global elimination will remain forever out of reach, she said.
New Tools in the Fight Against RSV
Nirsevimab, a long-acting monoclonal antibody given to newborns to prevent severe infection, was approved by the European Medicines Agency (EMA) in October 2022 and the US Food and Drug Administration (FDA) in July 2023. And Abrysvo, a vaccine given to older adults and pregnant women to stop them from passing the virus to babies from birth through 6 months of age, was approved by the FDA and the EMA in 2023.
RSV is responsible for over 33 million lung infections in children younger than 5 years annually, with more than 4 million hospitalizations and nearly 200,000 deaths. According to the Centers for Disease Control and Prevention, every year, 2.1 million children younger than 5 years old visit a healthcare provider because of an RSV infection and between 58,000 and 80,000 children younger than 5 years old are hospitalized in the United States. The burden of severe RSV disease is also high among adults, with an estimated 123,000-193,000 hospitalizations, 24,400-34,900 ICU admissions, and 4680-8620 in-hospital deaths occurring annually among US adults.
Infection in infancy can lead to later complications, such as the development of wheezing, a condition that causes breathlessness and a feeling of tightening in the chest, and possibly also asthma.
Studies have shown that children and preterm infants infected with RSV who were given monoclonal antibodies experienced less post-infection wheezing, suggesting that RSV prophylaxis could prevent the development of wheezing bronchitis.
A study conducted in Galicia, Spain, showed that only 0.3% of infants who received prophylaxis with Nirsevimab were hospitalized for RSV-related lower respiratory tract infections. “This is very promising,” Yvonne Maldonado, MD, professor of pediatrics and epidemiology and population health at Stanford University in Stanford, California, told Medscape Medical News. “But this virus is ubiquitous. It’s found everywhere. It comes around every winter season. And immunity is not long-lasting.”
Older children who are not receiving monoclonal antibodies still experience RSV-related hospitalizations, suggesting the virus continues to circulate at high enough levels in the community. “The vaccine and monoclonal antibodies can reduce the risk of hospitalization and more severe disease in young kids, but they won’t eliminate the virus,” Maldonado said. “Right now, the goal is to prevent serious infection, not to prevent the spread of the virus completely.”
Expanding Access to RSV Prevention in Low-Income Countries
Currently, the RSV vaccine and monoclonal antibodies are only given in the United States, Europe, United Kingdom, and Canada to newborns, children at risk for severe disease, and pregnant women. However, Midulla said that pharmaceutical companies are pushing to broaden the rollout to a broader population within these countries. Yet, he said, over 99% of RSV infection–related deaths occur in the Global South.
No pharmaceutical company has sought approval in low-income countries such as those in Africa. “Unless they see there being a market in a country, they’re not going to go through the onerous process of getting [a vaccine] licensed,” Shabir Madhi, dean of the faculty of health sciences and a professor of vaccinology at the University of the Witwatersrand, Johannesburg, South Africa, told Medscape Medical News.
He highlighted that almost 50% of RSV-related deaths occur in African children younger than 5 years, despite these children comprising just one fifth of the global under-5 population. The high burden of RSV mortality in the Global South is mainly due to poor access to healthcare and supportive treatments, such as supplemental oxygen, which can help children recover from severe RSV infection.
Companies are unlikely to pursue regulatory approval and licensing in low- and middle-income countries until GAVI, the global vaccine alliance, commits to procuring and funding the vaccines for these regions. GAVI’s decision would provide the necessary market incentive for manufacturers to seek approval.
Madhi suggested that GAVI’s decision on RSV vaccine procurement is imminent, likely early next year, following the World Health Organization’s Strategic Advisory Group of Experts on Immunization recommendation to vaccinate all pregnant women with the RSV vaccine, regardless of whether they are in high-income or low-income countries.
Nevertheless, even if vaccines become available, many African countries may still struggle to afford them. Madhi said that these countries would likely depend on GAVI and organizations like UNICEF to procure the vaccines at affordable prices. “The unfortunate reality is that many countries — especially in Africa — still wouldn’t be able to afford it, even if the vaccine cost as little as $5,” said Madhi. “But that’s where they would have the greatest impact.”
Midulla, Pijnenburg reported no relevant financial relationships. Madhi’s research unit, the Vaccines and Infectious Disease Analytics Unit, was involved in the clinical trials for the Pfizer RSV vaccine, the GSK RSV vaccine (which was terminated), as well as the MEDLEY trial of palivizumab. All funding for these studies went to his institution, the University of the Witwatersrand. Maldonado was Stanford principal investigator for the Pfizer RSV vaccine.
A version of this article appeared on Medscape.com.
Is Oral XEN-D0501 the Next Obesity Drug Hype?
XEN-D0501, a transient receptor potential vanilloid 1 (TRPV1) antagonist, is gaining attention for its potential as an oral tablet to treat type 2 diabetes, obesity, and cardiovascular diseases. Dorte X. Gram, PhD, founder of Pila Pharma, a Swedish pharmaceutical company investigating XEN-D0501, first noticed the connection more than 20 years ago as a researcher at Novo Nordisk.
“In my very first experiments, I noticed that mice who would normally become diabetic didn’t get diabetes at all,” she said in an interview.
These surprising observations prompted Gram to investigate further the potential role of the TRPV1 receptor in regulating metabolism, leading her to file a patent and pursue the development of TRPV1 antagonists for obesity and related conditions.
The company has received enough attention from investors that it witnessed a triple-digit percentage gain on the Nasdaq First North stock exchange in 2024.
While XEN-D0501 shows promise, researchers urge caution, as the drug is still in early development. “There is simply no quality human data to say anything about the possibilities for this pathway,” said John B. Dixon, PhD, professor at Iverson Health Innovation Research Institute, Swinburne University of Technology, Melbourne, Australia.
What Is TRPV1 and How Do TRPV1 Modulators Work?
TRPV1 is a homotetrameric receptor with six transmembrane domains expressed primarily in sensory nerve fibers. It is responsible for detecting noxious signals, including heat and chemical irritants — particularly capsaicin, the active component of chili peppers.
TRPV1 mediates the sensation of burning pain, often associated with inflammation and heat exposure. It also helps detect and regulate body temperature and influences the release of inflammatory mediators. In the central nervous system, it affects synaptic function and plasticity.
Studies have shown that activating TRPV1 can help counter diet-induced obesity by increasing thermogenesis in brown adipose tissue and improving metabolic activity. TRPV1 agonists such as capsaicin have been shown to reduce weight gain in high-fat-diet‒induced obese mice, with clinical trials further supporting its potential for decreasing body weight in people with overweight.
For instance, a clinical trial showed that participants with obesity taking capsinoid supplementation for 12 weeks experienced a reduction in body weight compared with those who took a placebo.
While TRPV1 agonists have been more commonly studied for obesity management, most studies involving antagonists have focused on pain relief, inflammation, and conditions like erythromelalgia rather than weight loss.
However, some evidence suggests that TRPV1 antagonism may influence metabolism. For example, in one study, mice lacking TRPV1 were resistant to obesity, “but that is not sufficient [to come to any conclusion],” said Vincenzo Di Marzo, PhD, director of the Joint International Research Unit for the Chemical and Biomolecular Study of the Microbiome in Metabolic Health and Nutrition between the Consiglio Nazionale delle Ricerche, Italy, and Université Laval, Quebec City, Canada. He was not involved in the study.
Gram admits that the picture around the mechanism of action of TRPV1 modulators is unclear. “There is not a consensus in the literature about the effect of this receptor. Should it be agonized or should it be antagonized?” she said.
What Is XEN-D0501?
XEN-D0501 is a novel selective TRPV1 antagonist, which Pila Pharma is developing for treating erythromelalgia, a rare condition that causes burning pain, redness, and hotness in the skin, especially the feet. It has received orphan-drug status for this indication in the United States.
Initially, the company explored XEN-D0501 for treating overactive bladder, but the development of the drug for this condition has been discontinued. Now, attention has moved to investigating XEN-D0501 for its potential in treating type 2 diabetes, obesity, and cardiovascular disease.
Although phase 2a clinical trials showed that XEN-D0501 is generally well tolerated in healthy participants, it has been associated with several side effects, including hyperthermia and oral discomfort, thought to be due to TRPV1 antagonism at sensory nerve endings in the mouth, in addition to transient urinary retention and postvoiding residual volumes, indicating potential issues with bladder function.
Another phase 2a trial (PP-CT03) is planned to assess the maximum tolerable dose of XEN-D0501 in people with obesity and type 2 diabetes, focusing on safety and potential effects on body weight. Gram said that early data show these populations experience less hyperthermia than healthy participants. However, the mechanism behind it is still not understood. These studies also showed some positive effects on insulin sensitivity and a biomarker for heart failure.
“The company data provided so far for XEN-D0501 are promising but still too preliminary,” said Di Marzo.
The company is now planning a further 3-month-long dose-escalation study in people with obesity and diabetes. “If these studies show that the molecule is as efficacious and safe as we think it is, then it would make life a lot better for a lot of people because it is a tablet, not an injectable,” Gram said.
Also being explored by the company is the potential of the molecule for treating cardiovascular diseases, particularly abdominal aortic aneurysms, and as a potential nonopioid painkiller.
Dixon and Di Marzo disclosed no relevant financial relationships. Gram is founder and CSO at Pila Pharma.
A version of this article appeared on Medscape.com.
XEN-D0501, a transient receptor potential vanilloid 1 (TRPV1) antagonist, is gaining attention for its potential as an oral tablet to treat type 2 diabetes, obesity, and cardiovascular diseases. Dorte X. Gram, PhD, founder of Pila Pharma, a Swedish pharmaceutical company investigating XEN-D0501, first noticed the connection more than 20 years ago as a researcher at Novo Nordisk.
“In my very first experiments, I noticed that mice who would normally become diabetic didn’t get diabetes at all,” she said in an interview.
These surprising observations prompted Gram to investigate further the potential role of the TRPV1 receptor in regulating metabolism, leading her to file a patent and pursue the development of TRPV1 antagonists for obesity and related conditions.
The company has received enough attention from investors that it witnessed a triple-digit percentage gain on the Nasdaq First North stock exchange in 2024.
While XEN-D0501 shows promise, researchers urge caution, as the drug is still in early development. “There is simply no quality human data to say anything about the possibilities for this pathway,” said John B. Dixon, PhD, professor at Iverson Health Innovation Research Institute, Swinburne University of Technology, Melbourne, Australia.
What Is TRPV1 and How Do TRPV1 Modulators Work?
TRPV1 is a homotetrameric receptor with six transmembrane domains expressed primarily in sensory nerve fibers. It is responsible for detecting noxious signals, including heat and chemical irritants — particularly capsaicin, the active component of chili peppers.
TRPV1 mediates the sensation of burning pain, often associated with inflammation and heat exposure. It also helps detect and regulate body temperature and influences the release of inflammatory mediators. In the central nervous system, it affects synaptic function and plasticity.
Studies have shown that activating TRPV1 can help counter diet-induced obesity by increasing thermogenesis in brown adipose tissue and improving metabolic activity. TRPV1 agonists such as capsaicin have been shown to reduce weight gain in high-fat-diet‒induced obese mice, with clinical trials further supporting its potential for decreasing body weight in people with overweight.
For instance, a clinical trial showed that participants with obesity taking capsinoid supplementation for 12 weeks experienced a reduction in body weight compared with those who took a placebo.
While TRPV1 agonists have been more commonly studied for obesity management, most studies involving antagonists have focused on pain relief, inflammation, and conditions like erythromelalgia rather than weight loss.
However, some evidence suggests that TRPV1 antagonism may influence metabolism. For example, in one study, mice lacking TRPV1 were resistant to obesity, “but that is not sufficient [to come to any conclusion],” said Vincenzo Di Marzo, PhD, director of the Joint International Research Unit for the Chemical and Biomolecular Study of the Microbiome in Metabolic Health and Nutrition between the Consiglio Nazionale delle Ricerche, Italy, and Université Laval, Quebec City, Canada. He was not involved in the study.
Gram admits that the picture around the mechanism of action of TRPV1 modulators is unclear. “There is not a consensus in the literature about the effect of this receptor. Should it be agonized or should it be antagonized?” she said.
What Is XEN-D0501?
XEN-D0501 is a novel selective TRPV1 antagonist, which Pila Pharma is developing for treating erythromelalgia, a rare condition that causes burning pain, redness, and hotness in the skin, especially the feet. It has received orphan-drug status for this indication in the United States.
Initially, the company explored XEN-D0501 for treating overactive bladder, but the development of the drug for this condition has been discontinued. Now, attention has moved to investigating XEN-D0501 for its potential in treating type 2 diabetes, obesity, and cardiovascular disease.
Although phase 2a clinical trials showed that XEN-D0501 is generally well tolerated in healthy participants, it has been associated with several side effects, including hyperthermia and oral discomfort, thought to be due to TRPV1 antagonism at sensory nerve endings in the mouth, in addition to transient urinary retention and postvoiding residual volumes, indicating potential issues with bladder function.
Another phase 2a trial (PP-CT03) is planned to assess the maximum tolerable dose of XEN-D0501 in people with obesity and type 2 diabetes, focusing on safety and potential effects on body weight. Gram said that early data show these populations experience less hyperthermia than healthy participants. However, the mechanism behind it is still not understood. These studies also showed some positive effects on insulin sensitivity and a biomarker for heart failure.
“The company data provided so far for XEN-D0501 are promising but still too preliminary,” said Di Marzo.
The company is now planning a further 3-month-long dose-escalation study in people with obesity and diabetes. “If these studies show that the molecule is as efficacious and safe as we think it is, then it would make life a lot better for a lot of people because it is a tablet, not an injectable,” Gram said.
Also being explored by the company is the potential of the molecule for treating cardiovascular diseases, particularly abdominal aortic aneurysms, and as a potential nonopioid painkiller.
Dixon and Di Marzo disclosed no relevant financial relationships. Gram is founder and CSO at Pila Pharma.
A version of this article appeared on Medscape.com.
XEN-D0501, a transient receptor potential vanilloid 1 (TRPV1) antagonist, is gaining attention for its potential as an oral tablet to treat type 2 diabetes, obesity, and cardiovascular diseases. Dorte X. Gram, PhD, founder of Pila Pharma, a Swedish pharmaceutical company investigating XEN-D0501, first noticed the connection more than 20 years ago as a researcher at Novo Nordisk.
“In my very first experiments, I noticed that mice who would normally become diabetic didn’t get diabetes at all,” she said in an interview.
These surprising observations prompted Gram to investigate further the potential role of the TRPV1 receptor in regulating metabolism, leading her to file a patent and pursue the development of TRPV1 antagonists for obesity and related conditions.
The company has received enough attention from investors that it witnessed a triple-digit percentage gain on the Nasdaq First North stock exchange in 2024.
While XEN-D0501 shows promise, researchers urge caution, as the drug is still in early development. “There is simply no quality human data to say anything about the possibilities for this pathway,” said John B. Dixon, PhD, professor at Iverson Health Innovation Research Institute, Swinburne University of Technology, Melbourne, Australia.
What Is TRPV1 and How Do TRPV1 Modulators Work?
TRPV1 is a homotetrameric receptor with six transmembrane domains expressed primarily in sensory nerve fibers. It is responsible for detecting noxious signals, including heat and chemical irritants — particularly capsaicin, the active component of chili peppers.
TRPV1 mediates the sensation of burning pain, often associated with inflammation and heat exposure. It also helps detect and regulate body temperature and influences the release of inflammatory mediators. In the central nervous system, it affects synaptic function and plasticity.
Studies have shown that activating TRPV1 can help counter diet-induced obesity by increasing thermogenesis in brown adipose tissue and improving metabolic activity. TRPV1 agonists such as capsaicin have been shown to reduce weight gain in high-fat-diet‒induced obese mice, with clinical trials further supporting its potential for decreasing body weight in people with overweight.
For instance, a clinical trial showed that participants with obesity taking capsinoid supplementation for 12 weeks experienced a reduction in body weight compared with those who took a placebo.
While TRPV1 agonists have been more commonly studied for obesity management, most studies involving antagonists have focused on pain relief, inflammation, and conditions like erythromelalgia rather than weight loss.
However, some evidence suggests that TRPV1 antagonism may influence metabolism. For example, in one study, mice lacking TRPV1 were resistant to obesity, “but that is not sufficient [to come to any conclusion],” said Vincenzo Di Marzo, PhD, director of the Joint International Research Unit for the Chemical and Biomolecular Study of the Microbiome in Metabolic Health and Nutrition between the Consiglio Nazionale delle Ricerche, Italy, and Université Laval, Quebec City, Canada. He was not involved in the study.
Gram admits that the picture around the mechanism of action of TRPV1 modulators is unclear. “There is not a consensus in the literature about the effect of this receptor. Should it be agonized or should it be antagonized?” she said.
What Is XEN-D0501?
XEN-D0501 is a novel selective TRPV1 antagonist, which Pila Pharma is developing for treating erythromelalgia, a rare condition that causes burning pain, redness, and hotness in the skin, especially the feet. It has received orphan-drug status for this indication in the United States.
Initially, the company explored XEN-D0501 for treating overactive bladder, but the development of the drug for this condition has been discontinued. Now, attention has moved to investigating XEN-D0501 for its potential in treating type 2 diabetes, obesity, and cardiovascular disease.
Although phase 2a clinical trials showed that XEN-D0501 is generally well tolerated in healthy participants, it has been associated with several side effects, including hyperthermia and oral discomfort, thought to be due to TRPV1 antagonism at sensory nerve endings in the mouth, in addition to transient urinary retention and postvoiding residual volumes, indicating potential issues with bladder function.
Another phase 2a trial (PP-CT03) is planned to assess the maximum tolerable dose of XEN-D0501 in people with obesity and type 2 diabetes, focusing on safety and potential effects on body weight. Gram said that early data show these populations experience less hyperthermia than healthy participants. However, the mechanism behind it is still not understood. These studies also showed some positive effects on insulin sensitivity and a biomarker for heart failure.
“The company data provided so far for XEN-D0501 are promising but still too preliminary,” said Di Marzo.
The company is now planning a further 3-month-long dose-escalation study in people with obesity and diabetes. “If these studies show that the molecule is as efficacious and safe as we think it is, then it would make life a lot better for a lot of people because it is a tablet, not an injectable,” Gram said.
Also being explored by the company is the potential of the molecule for treating cardiovascular diseases, particularly abdominal aortic aneurysms, and as a potential nonopioid painkiller.
Dixon and Di Marzo disclosed no relevant financial relationships. Gram is founder and CSO at Pila Pharma.
A version of this article appeared on Medscape.com.
Why Do People Struggle to Prioritize Their Long-Term Health?
Understanding how people make health-related decisions requires a deeper exploration of their motivations, beliefs, and circumstances, Christopher Dye, DPhil, professor of epidemiology at the University of Oxford in England, and former director of strategy at the World Health Organization, said in an interview. “In public health, we tend to prescribe solutions. But unless we understand how people really make choices about health and why they are less interested in prevention and happier to wait until they become ill, then we are not in the position to shift away from curative treatments to preventive treatments.”
Despite the well-documented benefits of preventive measures, many people fail to engage in proactive health behaviors. This can be attributed to psychological biases and socioeconomic factors that shape how people prioritize their health.
“The choices people make have some to do with facts, but they also have much to do with values and perception. We need to understand and take these perceptions and values seriously,” Dye said.
The Paradox of Prevention
People often recognize prevention as the right course of action but fail to act. “We know it’s the right thing to do, but we don’t do it,” Dye said.
He explained that, when considering potential future threats, we assess two key factors: The severity of the danger and the cost of addressing it. Action is more likely when the danger is significant and the cost of mitigation is low.
This dynamic can be broken down into three critical questions:
What is the nature of the hazard? Is the threat severe, like Ebola, which has a case fatality rate of around 50% in untreated cases, or relatively milder, like COVID-19, with a fatality rate of less than 1% but a much broader spread? The nastier the hazard, the more likely we are to take it seriously.
How likely is it to happen? Even a severe threat will not prompt much concern if its likelihood is perceived as low. Our willingness to act depends heavily on how probable people think the hazard is.
When is it likely to happen? A threat looming in the immediate future is more compelling than one projected weeks, months, or years away. This is because people tend to heavily discount the value of future risks.
When these factors — severity, likelihood, and immediacy — combine with low mitigation costs, the incentives for action align.
However, cost is not limited to financial expense. It encompasses effort, willpower, access to information, and personal inclination. Similarly, the perception of threat is shaped not just by hard data and epidemiology but also by subjective values and cultural interpretations.
“We place a high value on now rather than later,” Theresa Marteau, PhD, a psychologist and behavioral scientist and director of the Behaviour and Health Research Unit at the University of Cambridge in England, said in an interview. “Treatment is about fixing a problem that we have now, rather than trying to avoid a problem sometime in the future. We also place a high value on certainty: I’m ill today, and I want to avoid that, as opposed to putting resources on a possible disease that might or might not occur.”
Investing in the Future: A Privilege of Stability
People often undervalue future health risks because of temporal discounting, a cognitive bias where immediate rewards are prioritized over long-term benefits. This tendency makes it challenging to address health issues that may only manifest years later.
From a public health perspective, this creates challenges. Warning individuals that harmful behaviors, such as smoking, may lead to severe health problems in a decade often falls on deaf ears. People naturally focus on immediate concerns, particularly when grappling with present challenges. For those living in poverty or social instability, the urgency of daily survival frequently outweighs the perceived benefits of preventive health measures.
“A cigarette during the day is just one brief source of pleasure, a short-term escape from all the other stuff happening in their lives, and there’s more of that stuff happening to poorer people than there is to richer people,” Dye said.
He said that long-term thinking comes more naturally to those with stability and resources. People who are financially secure, have stable jobs, supportive families, and comfortable homes are better equipped to invest for the future and prioritize their health.
“People value their health regardless of their social and economic circumstances,” said Marteau. “But they might not have the resources to engage in behavior-changing activities.”
Bringing the Future to the Present
Effective interventions often involve a combination of “sticks” (deterrents) and “carrots” (rewards), Dye explained. Both approaches aim to bridge the gap between immediate actions and future benefits by making preventive behaviors more appealing in the short term. “We need to bring the future into the present,” he added.
Raising the cost of unhealthy behaviors has proven effective. For example, increasing the price of cigarettes leads to significant reductions in smoking rates. When smoking becomes less affordable, individuals are more likely to quit. Dye said that this approach works to a certain extent. At some point, the number of people quitting plateaus and those from low socioeconomic backgrounds are those more likely to continue to smoke.
Offering immediate rewards for preventive behaviors provides a powerful incentive. Things that give tangible benefits, like attending regular health checkups, receiving vaccinations, or joining fitness programs, can motivate individuals to engage in health-preserving activities. “The key is ensuring these benefits are timely and meaningful, as delayed rewards are less effective in overcoming the natural bias toward the present,” said Dye.
Healthcare providers are best placed to help people engage in preventive behavior by referring patients to the right services, such as programs to stop smoking, weight loss programs and medications, or mental health providers, Marteau said. “It’s not telling people to stop smoking or change their diet. It’s about signposting them to effective services that will help them change their behavior.”
Dye and Marteau reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Understanding how people make health-related decisions requires a deeper exploration of their motivations, beliefs, and circumstances, Christopher Dye, DPhil, professor of epidemiology at the University of Oxford in England, and former director of strategy at the World Health Organization, said in an interview. “In public health, we tend to prescribe solutions. But unless we understand how people really make choices about health and why they are less interested in prevention and happier to wait until they become ill, then we are not in the position to shift away from curative treatments to preventive treatments.”
Despite the well-documented benefits of preventive measures, many people fail to engage in proactive health behaviors. This can be attributed to psychological biases and socioeconomic factors that shape how people prioritize their health.
“The choices people make have some to do with facts, but they also have much to do with values and perception. We need to understand and take these perceptions and values seriously,” Dye said.
The Paradox of Prevention
People often recognize prevention as the right course of action but fail to act. “We know it’s the right thing to do, but we don’t do it,” Dye said.
He explained that, when considering potential future threats, we assess two key factors: The severity of the danger and the cost of addressing it. Action is more likely when the danger is significant and the cost of mitigation is low.
This dynamic can be broken down into three critical questions:
What is the nature of the hazard? Is the threat severe, like Ebola, which has a case fatality rate of around 50% in untreated cases, or relatively milder, like COVID-19, with a fatality rate of less than 1% but a much broader spread? The nastier the hazard, the more likely we are to take it seriously.
How likely is it to happen? Even a severe threat will not prompt much concern if its likelihood is perceived as low. Our willingness to act depends heavily on how probable people think the hazard is.
When is it likely to happen? A threat looming in the immediate future is more compelling than one projected weeks, months, or years away. This is because people tend to heavily discount the value of future risks.
When these factors — severity, likelihood, and immediacy — combine with low mitigation costs, the incentives for action align.
However, cost is not limited to financial expense. It encompasses effort, willpower, access to information, and personal inclination. Similarly, the perception of threat is shaped not just by hard data and epidemiology but also by subjective values and cultural interpretations.
“We place a high value on now rather than later,” Theresa Marteau, PhD, a psychologist and behavioral scientist and director of the Behaviour and Health Research Unit at the University of Cambridge in England, said in an interview. “Treatment is about fixing a problem that we have now, rather than trying to avoid a problem sometime in the future. We also place a high value on certainty: I’m ill today, and I want to avoid that, as opposed to putting resources on a possible disease that might or might not occur.”
Investing in the Future: A Privilege of Stability
People often undervalue future health risks because of temporal discounting, a cognitive bias where immediate rewards are prioritized over long-term benefits. This tendency makes it challenging to address health issues that may only manifest years later.
From a public health perspective, this creates challenges. Warning individuals that harmful behaviors, such as smoking, may lead to severe health problems in a decade often falls on deaf ears. People naturally focus on immediate concerns, particularly when grappling with present challenges. For those living in poverty or social instability, the urgency of daily survival frequently outweighs the perceived benefits of preventive health measures.
“A cigarette during the day is just one brief source of pleasure, a short-term escape from all the other stuff happening in their lives, and there’s more of that stuff happening to poorer people than there is to richer people,” Dye said.
He said that long-term thinking comes more naturally to those with stability and resources. People who are financially secure, have stable jobs, supportive families, and comfortable homes are better equipped to invest for the future and prioritize their health.
“People value their health regardless of their social and economic circumstances,” said Marteau. “But they might not have the resources to engage in behavior-changing activities.”
Bringing the Future to the Present
Effective interventions often involve a combination of “sticks” (deterrents) and “carrots” (rewards), Dye explained. Both approaches aim to bridge the gap between immediate actions and future benefits by making preventive behaviors more appealing in the short term. “We need to bring the future into the present,” he added.
Raising the cost of unhealthy behaviors has proven effective. For example, increasing the price of cigarettes leads to significant reductions in smoking rates. When smoking becomes less affordable, individuals are more likely to quit. Dye said that this approach works to a certain extent. At some point, the number of people quitting plateaus and those from low socioeconomic backgrounds are those more likely to continue to smoke.
Offering immediate rewards for preventive behaviors provides a powerful incentive. Things that give tangible benefits, like attending regular health checkups, receiving vaccinations, or joining fitness programs, can motivate individuals to engage in health-preserving activities. “The key is ensuring these benefits are timely and meaningful, as delayed rewards are less effective in overcoming the natural bias toward the present,” said Dye.
Healthcare providers are best placed to help people engage in preventive behavior by referring patients to the right services, such as programs to stop smoking, weight loss programs and medications, or mental health providers, Marteau said. “It’s not telling people to stop smoking or change their diet. It’s about signposting them to effective services that will help them change their behavior.”
Dye and Marteau reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Understanding how people make health-related decisions requires a deeper exploration of their motivations, beliefs, and circumstances, Christopher Dye, DPhil, professor of epidemiology at the University of Oxford in England, and former director of strategy at the World Health Organization, said in an interview. “In public health, we tend to prescribe solutions. But unless we understand how people really make choices about health and why they are less interested in prevention and happier to wait until they become ill, then we are not in the position to shift away from curative treatments to preventive treatments.”
Despite the well-documented benefits of preventive measures, many people fail to engage in proactive health behaviors. This can be attributed to psychological biases and socioeconomic factors that shape how people prioritize their health.
“The choices people make have some to do with facts, but they also have much to do with values and perception. We need to understand and take these perceptions and values seriously,” Dye said.
The Paradox of Prevention
People often recognize prevention as the right course of action but fail to act. “We know it’s the right thing to do, but we don’t do it,” Dye said.
He explained that, when considering potential future threats, we assess two key factors: The severity of the danger and the cost of addressing it. Action is more likely when the danger is significant and the cost of mitigation is low.
This dynamic can be broken down into three critical questions:
What is the nature of the hazard? Is the threat severe, like Ebola, which has a case fatality rate of around 50% in untreated cases, or relatively milder, like COVID-19, with a fatality rate of less than 1% but a much broader spread? The nastier the hazard, the more likely we are to take it seriously.
How likely is it to happen? Even a severe threat will not prompt much concern if its likelihood is perceived as low. Our willingness to act depends heavily on how probable people think the hazard is.
When is it likely to happen? A threat looming in the immediate future is more compelling than one projected weeks, months, or years away. This is because people tend to heavily discount the value of future risks.
When these factors — severity, likelihood, and immediacy — combine with low mitigation costs, the incentives for action align.
However, cost is not limited to financial expense. It encompasses effort, willpower, access to information, and personal inclination. Similarly, the perception of threat is shaped not just by hard data and epidemiology but also by subjective values and cultural interpretations.
“We place a high value on now rather than later,” Theresa Marteau, PhD, a psychologist and behavioral scientist and director of the Behaviour and Health Research Unit at the University of Cambridge in England, said in an interview. “Treatment is about fixing a problem that we have now, rather than trying to avoid a problem sometime in the future. We also place a high value on certainty: I’m ill today, and I want to avoid that, as opposed to putting resources on a possible disease that might or might not occur.”
Investing in the Future: A Privilege of Stability
People often undervalue future health risks because of temporal discounting, a cognitive bias where immediate rewards are prioritized over long-term benefits. This tendency makes it challenging to address health issues that may only manifest years later.
From a public health perspective, this creates challenges. Warning individuals that harmful behaviors, such as smoking, may lead to severe health problems in a decade often falls on deaf ears. People naturally focus on immediate concerns, particularly when grappling with present challenges. For those living in poverty or social instability, the urgency of daily survival frequently outweighs the perceived benefits of preventive health measures.
“A cigarette during the day is just one brief source of pleasure, a short-term escape from all the other stuff happening in their lives, and there’s more of that stuff happening to poorer people than there is to richer people,” Dye said.
He said that long-term thinking comes more naturally to those with stability and resources. People who are financially secure, have stable jobs, supportive families, and comfortable homes are better equipped to invest for the future and prioritize their health.
“People value their health regardless of their social and economic circumstances,” said Marteau. “But they might not have the resources to engage in behavior-changing activities.”
Bringing the Future to the Present
Effective interventions often involve a combination of “sticks” (deterrents) and “carrots” (rewards), Dye explained. Both approaches aim to bridge the gap between immediate actions and future benefits by making preventive behaviors more appealing in the short term. “We need to bring the future into the present,” he added.
Raising the cost of unhealthy behaviors has proven effective. For example, increasing the price of cigarettes leads to significant reductions in smoking rates. When smoking becomes less affordable, individuals are more likely to quit. Dye said that this approach works to a certain extent. At some point, the number of people quitting plateaus and those from low socioeconomic backgrounds are those more likely to continue to smoke.
Offering immediate rewards for preventive behaviors provides a powerful incentive. Things that give tangible benefits, like attending regular health checkups, receiving vaccinations, or joining fitness programs, can motivate individuals to engage in health-preserving activities. “The key is ensuring these benefits are timely and meaningful, as delayed rewards are less effective in overcoming the natural bias toward the present,” said Dye.
Healthcare providers are best placed to help people engage in preventive behavior by referring patients to the right services, such as programs to stop smoking, weight loss programs and medications, or mental health providers, Marteau said. “It’s not telling people to stop smoking or change their diet. It’s about signposting them to effective services that will help them change their behavior.”
Dye and Marteau reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Artificial Intelligence Helps Diagnose Lung Disease in Infants and Outperforms Trainee Doctors
VIENNA — Artificial Intelligence (AI) can assist doctors in assessing and diagnosing respiratory illnesses in infants and children, according to two new studies presented at the European Respiratory Society (ERS) 2024 Congress.
Researchers can train artificial neural networks (ANNs) to detect lung disease in premature babies by analyzing their breathing patterns while they sleep. “Our noninvasive test is less distressing for the baby and their parents, meaning they can access treatment more quickly, and may also be relevant for their long-term prognosis,” said Edgar Delgado-Eckert, PhD, adjunct professor in the Department of Biomedical Engineering at The University of Basel, Switzerland, and a research group leader at the University Children’s Hospital, Switzerland.
Manjith Narayanan, MD, a consultant in pediatric pulmonology at the Royal Hospital for Children and Young People, Edinburgh, and honorary senior clinical lecturer at The University of Edinburgh, United Kingdom, said chatbots such as ChatGPT, Bard, and Bing can perform as well as or better than trainee doctors when assessing children with respiratory issues. He said chatbots could triage patients more quickly and ease pressure on health services.
Chatbots Show Promise in Triage of Pediatric Respiratory Illnesses
Researchers at The University of Edinburgh provided 10 trainee doctors with less than 4 months of clinical experience in pediatrics with clinical scenarios that covered topics such as cystic fibrosis, asthma, sleep-disordered breathing, breathlessness, chest infections, or no obvious diagnosis.
The trainee doctors had 1 hour to use the internet, although they were not allowed to use chatbots to solve each scenario with a descriptive answer.
Each scenario was also presented to the three large language models (LLMs): OpenAI’s ChatGPT, Google’s Bard, and Microsoft’s Bing.
Six pediatric respiratory experts assessed all responses, scoring correctness, comprehensiveness, usefulness, plausibility, and coherence on a scale of 0-9. They were also asked to say whether they thought a human or a chatbot generated each response.
ChatGPT scored an average of 7 out of 9 overall and was believed to be more human-like than responses from the other chatbots. Bard scored an average of 6 out of 9 and was more “coherent” than trainee doctors, but in other respects, it was no better or worse than trainee doctors. Bing and trainee doctors scored an average of 4 out of 9.
“Our study is the first, to our knowledge, to test LLMs against trainee doctors in situations that reflect real-life clinical practice,” Narayanan said. “We did this by allowing the trainee doctors to have full access to resources available on the internet, as they would in real life. This moves the focus away from testing memory, where LLMs have a clear advantage.”
Narayanan said that these models could help nurses, trainee doctors, and primary care physicians triage patients quickly and assist medical professionals in their studies by summarizing their thought processes. “The key word, though, is “assist.” They cannot replace conventional medical training yet,” he told Medscape Medical News.
The researchers found no obvious hallucinations — seemingly made-up information — with any of the three LLMs. Still, Narayanan said, “We need to be aware of this possibility and build mitigations.”
Hilary Pinnock, ERS education council chair and professor of primary care respiratory medicine at The University of Edinburgh who was not involved in the research, said seeing how widely available AI tools can provide solutions to complex cases of respiratory illness in children is exciting and worrying at the same time. “It certainly points the way to a brave new world of AI-supported care.”
“However, before we start to use AI in routine clinical practice, we need to be confident that it will not create errors either through ‘hallucinating’ fake information or because it has been trained on data that does not equitably represent the population we serve,” she said.
AI Predicts Lung Disease in Premature Babies
Identifying bronchopulmonary dysplasia (BPD) in premature babies remains a challenge. Lung function tests usually require blowing out on request, which is a task babies cannot perform. Current techniques require sophisticated equipment to measure an infant’s lung ventilation characteristics, so doctors usually diagnose BPD by the presence of its leading causes, prematurity and the need for respiratory support.
Researchers at the University of Basel in Switzerland trained an ANN model to predict BPD in premature babies.
The team studied a group of 139 full-term and 190 premature infants who had been assessed for BPD, recording their breathing for 10 minutes while they slept. For each baby, 100 consecutive regular breaths, carefully inspected to exclude sighs or other artifacts, were used to train, validate, and test an ANN called a Long Short-Term Memory model (LSTM), which is particularly effective at classifying sequential data such as tidal breathing.
Researchers used 60% of the data to teach the network how to recognize BPD, 20% to validate the model, and then fed the remaining 20% of the data to the model to see if it could correctly identify those babies with BPD.
The LSTM model classified a series of flow values in the unseen test data set as belonging to a patient diagnosed with BPD or not with 96% accuracy.
“Until recently, this need for large amounts of data has hindered efforts to create accurate models for lung disease in infants because it is so difficult to assess their lung function,” Delgado-Eckert said. “Our research delivers, for the first time, a comprehensive way of analyzing infants’ breathing and allows us to detect which babies have BPD as early as 1 month of corrected age.”
The study presented by Delgado-Eckert received funding from the Swiss National Science Foundation. Narayanan and Pinnock reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — Artificial Intelligence (AI) can assist doctors in assessing and diagnosing respiratory illnesses in infants and children, according to two new studies presented at the European Respiratory Society (ERS) 2024 Congress.
Researchers can train artificial neural networks (ANNs) to detect lung disease in premature babies by analyzing their breathing patterns while they sleep. “Our noninvasive test is less distressing for the baby and their parents, meaning they can access treatment more quickly, and may also be relevant for their long-term prognosis,” said Edgar Delgado-Eckert, PhD, adjunct professor in the Department of Biomedical Engineering at The University of Basel, Switzerland, and a research group leader at the University Children’s Hospital, Switzerland.
Manjith Narayanan, MD, a consultant in pediatric pulmonology at the Royal Hospital for Children and Young People, Edinburgh, and honorary senior clinical lecturer at The University of Edinburgh, United Kingdom, said chatbots such as ChatGPT, Bard, and Bing can perform as well as or better than trainee doctors when assessing children with respiratory issues. He said chatbots could triage patients more quickly and ease pressure on health services.
Chatbots Show Promise in Triage of Pediatric Respiratory Illnesses
Researchers at The University of Edinburgh provided 10 trainee doctors with less than 4 months of clinical experience in pediatrics with clinical scenarios that covered topics such as cystic fibrosis, asthma, sleep-disordered breathing, breathlessness, chest infections, or no obvious diagnosis.
The trainee doctors had 1 hour to use the internet, although they were not allowed to use chatbots to solve each scenario with a descriptive answer.
Each scenario was also presented to the three large language models (LLMs): OpenAI’s ChatGPT, Google’s Bard, and Microsoft’s Bing.
Six pediatric respiratory experts assessed all responses, scoring correctness, comprehensiveness, usefulness, plausibility, and coherence on a scale of 0-9. They were also asked to say whether they thought a human or a chatbot generated each response.
ChatGPT scored an average of 7 out of 9 overall and was believed to be more human-like than responses from the other chatbots. Bard scored an average of 6 out of 9 and was more “coherent” than trainee doctors, but in other respects, it was no better or worse than trainee doctors. Bing and trainee doctors scored an average of 4 out of 9.
“Our study is the first, to our knowledge, to test LLMs against trainee doctors in situations that reflect real-life clinical practice,” Narayanan said. “We did this by allowing the trainee doctors to have full access to resources available on the internet, as they would in real life. This moves the focus away from testing memory, where LLMs have a clear advantage.”
Narayanan said that these models could help nurses, trainee doctors, and primary care physicians triage patients quickly and assist medical professionals in their studies by summarizing their thought processes. “The key word, though, is “assist.” They cannot replace conventional medical training yet,” he told Medscape Medical News.
The researchers found no obvious hallucinations — seemingly made-up information — with any of the three LLMs. Still, Narayanan said, “We need to be aware of this possibility and build mitigations.”
Hilary Pinnock, ERS education council chair and professor of primary care respiratory medicine at The University of Edinburgh who was not involved in the research, said seeing how widely available AI tools can provide solutions to complex cases of respiratory illness in children is exciting and worrying at the same time. “It certainly points the way to a brave new world of AI-supported care.”
“However, before we start to use AI in routine clinical practice, we need to be confident that it will not create errors either through ‘hallucinating’ fake information or because it has been trained on data that does not equitably represent the population we serve,” she said.
AI Predicts Lung Disease in Premature Babies
Identifying bronchopulmonary dysplasia (BPD) in premature babies remains a challenge. Lung function tests usually require blowing out on request, which is a task babies cannot perform. Current techniques require sophisticated equipment to measure an infant’s lung ventilation characteristics, so doctors usually diagnose BPD by the presence of its leading causes, prematurity and the need for respiratory support.
Researchers at the University of Basel in Switzerland trained an ANN model to predict BPD in premature babies.
The team studied a group of 139 full-term and 190 premature infants who had been assessed for BPD, recording their breathing for 10 minutes while they slept. For each baby, 100 consecutive regular breaths, carefully inspected to exclude sighs or other artifacts, were used to train, validate, and test an ANN called a Long Short-Term Memory model (LSTM), which is particularly effective at classifying sequential data such as tidal breathing.
Researchers used 60% of the data to teach the network how to recognize BPD, 20% to validate the model, and then fed the remaining 20% of the data to the model to see if it could correctly identify those babies with BPD.
The LSTM model classified a series of flow values in the unseen test data set as belonging to a patient diagnosed with BPD or not with 96% accuracy.
“Until recently, this need for large amounts of data has hindered efforts to create accurate models for lung disease in infants because it is so difficult to assess their lung function,” Delgado-Eckert said. “Our research delivers, for the first time, a comprehensive way of analyzing infants’ breathing and allows us to detect which babies have BPD as early as 1 month of corrected age.”
The study presented by Delgado-Eckert received funding from the Swiss National Science Foundation. Narayanan and Pinnock reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — Artificial Intelligence (AI) can assist doctors in assessing and diagnosing respiratory illnesses in infants and children, according to two new studies presented at the European Respiratory Society (ERS) 2024 Congress.
Researchers can train artificial neural networks (ANNs) to detect lung disease in premature babies by analyzing their breathing patterns while they sleep. “Our noninvasive test is less distressing for the baby and their parents, meaning they can access treatment more quickly, and may also be relevant for their long-term prognosis,” said Edgar Delgado-Eckert, PhD, adjunct professor in the Department of Biomedical Engineering at The University of Basel, Switzerland, and a research group leader at the University Children’s Hospital, Switzerland.
Manjith Narayanan, MD, a consultant in pediatric pulmonology at the Royal Hospital for Children and Young People, Edinburgh, and honorary senior clinical lecturer at The University of Edinburgh, United Kingdom, said chatbots such as ChatGPT, Bard, and Bing can perform as well as or better than trainee doctors when assessing children with respiratory issues. He said chatbots could triage patients more quickly and ease pressure on health services.
Chatbots Show Promise in Triage of Pediatric Respiratory Illnesses
Researchers at The University of Edinburgh provided 10 trainee doctors with less than 4 months of clinical experience in pediatrics with clinical scenarios that covered topics such as cystic fibrosis, asthma, sleep-disordered breathing, breathlessness, chest infections, or no obvious diagnosis.
The trainee doctors had 1 hour to use the internet, although they were not allowed to use chatbots to solve each scenario with a descriptive answer.
Each scenario was also presented to the three large language models (LLMs): OpenAI’s ChatGPT, Google’s Bard, and Microsoft’s Bing.
Six pediatric respiratory experts assessed all responses, scoring correctness, comprehensiveness, usefulness, plausibility, and coherence on a scale of 0-9. They were also asked to say whether they thought a human or a chatbot generated each response.
ChatGPT scored an average of 7 out of 9 overall and was believed to be more human-like than responses from the other chatbots. Bard scored an average of 6 out of 9 and was more “coherent” than trainee doctors, but in other respects, it was no better or worse than trainee doctors. Bing and trainee doctors scored an average of 4 out of 9.
“Our study is the first, to our knowledge, to test LLMs against trainee doctors in situations that reflect real-life clinical practice,” Narayanan said. “We did this by allowing the trainee doctors to have full access to resources available on the internet, as they would in real life. This moves the focus away from testing memory, where LLMs have a clear advantage.”
Narayanan said that these models could help nurses, trainee doctors, and primary care physicians triage patients quickly and assist medical professionals in their studies by summarizing their thought processes. “The key word, though, is “assist.” They cannot replace conventional medical training yet,” he told Medscape Medical News.
The researchers found no obvious hallucinations — seemingly made-up information — with any of the three LLMs. Still, Narayanan said, “We need to be aware of this possibility and build mitigations.”
Hilary Pinnock, ERS education council chair and professor of primary care respiratory medicine at The University of Edinburgh who was not involved in the research, said seeing how widely available AI tools can provide solutions to complex cases of respiratory illness in children is exciting and worrying at the same time. “It certainly points the way to a brave new world of AI-supported care.”
“However, before we start to use AI in routine clinical practice, we need to be confident that it will not create errors either through ‘hallucinating’ fake information or because it has been trained on data that does not equitably represent the population we serve,” she said.
AI Predicts Lung Disease in Premature Babies
Identifying bronchopulmonary dysplasia (BPD) in premature babies remains a challenge. Lung function tests usually require blowing out on request, which is a task babies cannot perform. Current techniques require sophisticated equipment to measure an infant’s lung ventilation characteristics, so doctors usually diagnose BPD by the presence of its leading causes, prematurity and the need for respiratory support.
Researchers at the University of Basel in Switzerland trained an ANN model to predict BPD in premature babies.
The team studied a group of 139 full-term and 190 premature infants who had been assessed for BPD, recording their breathing for 10 minutes while they slept. For each baby, 100 consecutive regular breaths, carefully inspected to exclude sighs or other artifacts, were used to train, validate, and test an ANN called a Long Short-Term Memory model (LSTM), which is particularly effective at classifying sequential data such as tidal breathing.
Researchers used 60% of the data to teach the network how to recognize BPD, 20% to validate the model, and then fed the remaining 20% of the data to the model to see if it could correctly identify those babies with BPD.
The LSTM model classified a series of flow values in the unseen test data set as belonging to a patient diagnosed with BPD or not with 96% accuracy.
“Until recently, this need for large amounts of data has hindered efforts to create accurate models for lung disease in infants because it is so difficult to assess their lung function,” Delgado-Eckert said. “Our research delivers, for the first time, a comprehensive way of analyzing infants’ breathing and allows us to detect which babies have BPD as early as 1 month of corrected age.”
The study presented by Delgado-Eckert received funding from the Swiss National Science Foundation. Narayanan and Pinnock reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ERS 2024
Is Wildfire Smoke More Toxic Than General Air Pollution?
Wildfire-related air pollution in Europe kills more than non-wildfire air pollution. As climate change exacerbates the frequency and violence of wildfires, researchers are studying the health implications of mitigation methods such as prescribed fires.
Presenting at the annual congress of the European Respiratory Society (ERS), Cathryn Tonne, PhD, an environmental epidemiologist at the Instituto de Salud Global de Barcelona, Spain, said wildfire-related PM2.5 is more toxic than general PM2.5, leading to significantly higher mortality rates.
Prescribed, controlled fires have been employed worldwide to reduce the chance of uncontrolled, catastrophic fires. However, researchers wonder whether the techniques reduce the overall fire-related PM2.5 or add up to it. “Prescribed fire increases ecosystem resilience and can reduce the risk of catastrophic wildfire,” said Jason Sacks, MPH, an epidemiologist in the Center for Public Health and Environmental Assessment in the Office of Research and Development at the Environmental Protection Agency (EPA), at the congress. “But it also leads to poorer air quality and health impacts, and we still don’t know what this means at a regional scale.”
Wildfire Pollution Kills More Than Other Air Pollution
Researchers at the Instituto de Salud Global de Barcelona used a large dataset of daily mortality data from 32 European countries collected through the EARLY-ADAPT project. They utilized the SILAM model to derive daily average concentrations of wildfire-related PM2.5, non-fire PM2.5, and total PM2.5 levels. They also employed GEOSTAT population grids at a 1-km resolution to calculate the attributable number of deaths across different regions, specifically focusing on data from 2006, 2011, and 2018.
The data analysis indicated that the relative risk per unit of PM2.5 is substantially larger for wildfire-related PM2.5, compared with non-fire PM2.5. “We essentially assume that wildfire smoke PM2.5 has the same toxicity as total PM2.5, but it’s increasingly clear that’s likely not the case,” Dr. Tonne said, presenting the study.
When employing exposure-response functions (ERFs) specific to wildfire smoke, researchers found that the attributable deaths from all causes of wildfire PM2.5 were approximately 10 times larger than those calculated using total PM2.5 exposure estimates. Dr. Tonne explained that this stark difference highlights the critical need for tailored ERFs that accurately reflect the unique health risks posed by wildfire smoke.
“Respiratory mortality usually has the strongest relative risks, and we’re seeing that in this study as well,” Dr. Tonne said. “Wildfire smoke seems to operate through quite immediate mechanisms, likely through inflammation and oxidative stress.”
One significant challenge of the study was the lack of uniform spatial resolution across all countries involved in the analysis. This inconsistency may affect how accurately mortality estimates can be attributed to specific PM2.5 sources. Additionally, the study had limited statistical power for generating age- and sex-specific mortality estimates, which could obscure important demographic differences in vulnerability to wildfire smoke exposure. The analysis was also constrained to data available only up to 2020, thereby excluding critical wildfire events from subsequent years, such as those in 2022 and 2023, which may have further elucidated the health impacts of wildfire smoke in Europe.
Fires Prescription
Prescribed fires or controlled burns are intentional fires set by land managers under carefully managed conditions.
Historically, many forested areas have been subjected to fire suppression practices, which allow combustible materials like dry leaves, twigs, and shrubs to accumulate over time. This buildup leads to a higher likelihood of severe, uncontrollable wildfires. Prescribed fires can reduce these fuel loads and improve the health and resilience of ecosystems.
They release fewer pollutants and emissions than the large-scale, unmanageable wildfires they help prevent because they happen at lower temperatures. But they still introduce pollutants in the air that can negatively affect nearby communities’ health.
People with preexisting respiratory conditions, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly vulnerable to smoke, which can trigger health issues like breathing difficulties, coughing, and eye irritation. The cumulative impact of increased burns raises concerns about long-term air quality, especially in densely populated areas. “We need to understand if we’re actually tipping the scale to having less wildfire smoke or just increasing the total amount of smoke.”
Mitigation strategies include accurately picking the right timing and weather conditions to determine when and where to conduct controlled burns and effective and timely communication to inform local communities about upcoming burns, the potential for smoke exposure, and how to protect themselves.
There is a growing need to improve public messaging around prescribed fires, Mr. Sacks said, because often the message communicated is oversimplified, such as “there will be smoke, but don’t worry. But that’s not the message we want to convey, especially for people with asthma or COPD.”
Instead, he said public health agencies should provide clearer, science-based guidance on the risks for smoke exposure and practical steps people can take to reduce their risk.
What Can Doctors Do?
Chris Carlsten, MD, director of the Centre for Lung Health and professor and head of the Respiratory Medicine Division at the University of British Columbia, Vancouver, Canada, told this news organization that determining whether an exacerbation of a respiratory condition is caused by fire exposure or other factors, such as viral infections, is complex because both can trigger similar responses and may complement each other. “It’s very difficult for any individual to know whether, when they’re having an exacerbation of asthma or COPD, that’s due to the fire,” he said. Fire smoke also increases infection risks, further complicating diagnosis.
Dr. Carlsten suggested that physicians could recommend preventative use of inhalers for at-risk patients when wildfires occur rather than waiting for symptoms to worsen. “That is a really interesting idea that could be practical.” Still, he advises caution, stressing that patients should consult their providers because not all may react well to increased inhaler use.
He also highlighted a significant shift in the healthcare landscape, noting that traditionally, the focus has been on the cardiovascular impacts of pollution, particularly traffic-related pollution. However, as wildfire smoke becomes a growing issue, the focus is shifting back to respiratory problems, with profound implications for healthcare resources, budgets, and drug approvals based on the burden of respiratory disease. “Fire smoke is becoming more of a problem. This swing back to respiratory has huge implications for healthcare systems and respiratory disease burden.”
Mr. Sacks and Dr. Carlsten reported no relevant financial relationships. The study presented by Dr. Tonne received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101057131.
A version of this article first appeared on Medscape.com.
Wildfire-related air pollution in Europe kills more than non-wildfire air pollution. As climate change exacerbates the frequency and violence of wildfires, researchers are studying the health implications of mitigation methods such as prescribed fires.
Presenting at the annual congress of the European Respiratory Society (ERS), Cathryn Tonne, PhD, an environmental epidemiologist at the Instituto de Salud Global de Barcelona, Spain, said wildfire-related PM2.5 is more toxic than general PM2.5, leading to significantly higher mortality rates.
Prescribed, controlled fires have been employed worldwide to reduce the chance of uncontrolled, catastrophic fires. However, researchers wonder whether the techniques reduce the overall fire-related PM2.5 or add up to it. “Prescribed fire increases ecosystem resilience and can reduce the risk of catastrophic wildfire,” said Jason Sacks, MPH, an epidemiologist in the Center for Public Health and Environmental Assessment in the Office of Research and Development at the Environmental Protection Agency (EPA), at the congress. “But it also leads to poorer air quality and health impacts, and we still don’t know what this means at a regional scale.”
Wildfire Pollution Kills More Than Other Air Pollution
Researchers at the Instituto de Salud Global de Barcelona used a large dataset of daily mortality data from 32 European countries collected through the EARLY-ADAPT project. They utilized the SILAM model to derive daily average concentrations of wildfire-related PM2.5, non-fire PM2.5, and total PM2.5 levels. They also employed GEOSTAT population grids at a 1-km resolution to calculate the attributable number of deaths across different regions, specifically focusing on data from 2006, 2011, and 2018.
The data analysis indicated that the relative risk per unit of PM2.5 is substantially larger for wildfire-related PM2.5, compared with non-fire PM2.5. “We essentially assume that wildfire smoke PM2.5 has the same toxicity as total PM2.5, but it’s increasingly clear that’s likely not the case,” Dr. Tonne said, presenting the study.
When employing exposure-response functions (ERFs) specific to wildfire smoke, researchers found that the attributable deaths from all causes of wildfire PM2.5 were approximately 10 times larger than those calculated using total PM2.5 exposure estimates. Dr. Tonne explained that this stark difference highlights the critical need for tailored ERFs that accurately reflect the unique health risks posed by wildfire smoke.
“Respiratory mortality usually has the strongest relative risks, and we’re seeing that in this study as well,” Dr. Tonne said. “Wildfire smoke seems to operate through quite immediate mechanisms, likely through inflammation and oxidative stress.”
One significant challenge of the study was the lack of uniform spatial resolution across all countries involved in the analysis. This inconsistency may affect how accurately mortality estimates can be attributed to specific PM2.5 sources. Additionally, the study had limited statistical power for generating age- and sex-specific mortality estimates, which could obscure important demographic differences in vulnerability to wildfire smoke exposure. The analysis was also constrained to data available only up to 2020, thereby excluding critical wildfire events from subsequent years, such as those in 2022 and 2023, which may have further elucidated the health impacts of wildfire smoke in Europe.
Fires Prescription
Prescribed fires or controlled burns are intentional fires set by land managers under carefully managed conditions.
Historically, many forested areas have been subjected to fire suppression practices, which allow combustible materials like dry leaves, twigs, and shrubs to accumulate over time. This buildup leads to a higher likelihood of severe, uncontrollable wildfires. Prescribed fires can reduce these fuel loads and improve the health and resilience of ecosystems.
They release fewer pollutants and emissions than the large-scale, unmanageable wildfires they help prevent because they happen at lower temperatures. But they still introduce pollutants in the air that can negatively affect nearby communities’ health.
People with preexisting respiratory conditions, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly vulnerable to smoke, which can trigger health issues like breathing difficulties, coughing, and eye irritation. The cumulative impact of increased burns raises concerns about long-term air quality, especially in densely populated areas. “We need to understand if we’re actually tipping the scale to having less wildfire smoke or just increasing the total amount of smoke.”
Mitigation strategies include accurately picking the right timing and weather conditions to determine when and where to conduct controlled burns and effective and timely communication to inform local communities about upcoming burns, the potential for smoke exposure, and how to protect themselves.
There is a growing need to improve public messaging around prescribed fires, Mr. Sacks said, because often the message communicated is oversimplified, such as “there will be smoke, but don’t worry. But that’s not the message we want to convey, especially for people with asthma or COPD.”
Instead, he said public health agencies should provide clearer, science-based guidance on the risks for smoke exposure and practical steps people can take to reduce their risk.
What Can Doctors Do?
Chris Carlsten, MD, director of the Centre for Lung Health and professor and head of the Respiratory Medicine Division at the University of British Columbia, Vancouver, Canada, told this news organization that determining whether an exacerbation of a respiratory condition is caused by fire exposure or other factors, such as viral infections, is complex because both can trigger similar responses and may complement each other. “It’s very difficult for any individual to know whether, when they’re having an exacerbation of asthma or COPD, that’s due to the fire,” he said. Fire smoke also increases infection risks, further complicating diagnosis.
Dr. Carlsten suggested that physicians could recommend preventative use of inhalers for at-risk patients when wildfires occur rather than waiting for symptoms to worsen. “That is a really interesting idea that could be practical.” Still, he advises caution, stressing that patients should consult their providers because not all may react well to increased inhaler use.
He also highlighted a significant shift in the healthcare landscape, noting that traditionally, the focus has been on the cardiovascular impacts of pollution, particularly traffic-related pollution. However, as wildfire smoke becomes a growing issue, the focus is shifting back to respiratory problems, with profound implications for healthcare resources, budgets, and drug approvals based on the burden of respiratory disease. “Fire smoke is becoming more of a problem. This swing back to respiratory has huge implications for healthcare systems and respiratory disease burden.”
Mr. Sacks and Dr. Carlsten reported no relevant financial relationships. The study presented by Dr. Tonne received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101057131.
A version of this article first appeared on Medscape.com.
Wildfire-related air pollution in Europe kills more than non-wildfire air pollution. As climate change exacerbates the frequency and violence of wildfires, researchers are studying the health implications of mitigation methods such as prescribed fires.
Presenting at the annual congress of the European Respiratory Society (ERS), Cathryn Tonne, PhD, an environmental epidemiologist at the Instituto de Salud Global de Barcelona, Spain, said wildfire-related PM2.5 is more toxic than general PM2.5, leading to significantly higher mortality rates.
Prescribed, controlled fires have been employed worldwide to reduce the chance of uncontrolled, catastrophic fires. However, researchers wonder whether the techniques reduce the overall fire-related PM2.5 or add up to it. “Prescribed fire increases ecosystem resilience and can reduce the risk of catastrophic wildfire,” said Jason Sacks, MPH, an epidemiologist in the Center for Public Health and Environmental Assessment in the Office of Research and Development at the Environmental Protection Agency (EPA), at the congress. “But it also leads to poorer air quality and health impacts, and we still don’t know what this means at a regional scale.”
Wildfire Pollution Kills More Than Other Air Pollution
Researchers at the Instituto de Salud Global de Barcelona used a large dataset of daily mortality data from 32 European countries collected through the EARLY-ADAPT project. They utilized the SILAM model to derive daily average concentrations of wildfire-related PM2.5, non-fire PM2.5, and total PM2.5 levels. They also employed GEOSTAT population grids at a 1-km resolution to calculate the attributable number of deaths across different regions, specifically focusing on data from 2006, 2011, and 2018.
The data analysis indicated that the relative risk per unit of PM2.5 is substantially larger for wildfire-related PM2.5, compared with non-fire PM2.5. “We essentially assume that wildfire smoke PM2.5 has the same toxicity as total PM2.5, but it’s increasingly clear that’s likely not the case,” Dr. Tonne said, presenting the study.
When employing exposure-response functions (ERFs) specific to wildfire smoke, researchers found that the attributable deaths from all causes of wildfire PM2.5 were approximately 10 times larger than those calculated using total PM2.5 exposure estimates. Dr. Tonne explained that this stark difference highlights the critical need for tailored ERFs that accurately reflect the unique health risks posed by wildfire smoke.
“Respiratory mortality usually has the strongest relative risks, and we’re seeing that in this study as well,” Dr. Tonne said. “Wildfire smoke seems to operate through quite immediate mechanisms, likely through inflammation and oxidative stress.”
One significant challenge of the study was the lack of uniform spatial resolution across all countries involved in the analysis. This inconsistency may affect how accurately mortality estimates can be attributed to specific PM2.5 sources. Additionally, the study had limited statistical power for generating age- and sex-specific mortality estimates, which could obscure important demographic differences in vulnerability to wildfire smoke exposure. The analysis was also constrained to data available only up to 2020, thereby excluding critical wildfire events from subsequent years, such as those in 2022 and 2023, which may have further elucidated the health impacts of wildfire smoke in Europe.
Fires Prescription
Prescribed fires or controlled burns are intentional fires set by land managers under carefully managed conditions.
Historically, many forested areas have been subjected to fire suppression practices, which allow combustible materials like dry leaves, twigs, and shrubs to accumulate over time. This buildup leads to a higher likelihood of severe, uncontrollable wildfires. Prescribed fires can reduce these fuel loads and improve the health and resilience of ecosystems.
They release fewer pollutants and emissions than the large-scale, unmanageable wildfires they help prevent because they happen at lower temperatures. But they still introduce pollutants in the air that can negatively affect nearby communities’ health.
People with preexisting respiratory conditions, such as asthma or chronic obstructive pulmonary disease (COPD), are particularly vulnerable to smoke, which can trigger health issues like breathing difficulties, coughing, and eye irritation. The cumulative impact of increased burns raises concerns about long-term air quality, especially in densely populated areas. “We need to understand if we’re actually tipping the scale to having less wildfire smoke or just increasing the total amount of smoke.”
Mitigation strategies include accurately picking the right timing and weather conditions to determine when and where to conduct controlled burns and effective and timely communication to inform local communities about upcoming burns, the potential for smoke exposure, and how to protect themselves.
There is a growing need to improve public messaging around prescribed fires, Mr. Sacks said, because often the message communicated is oversimplified, such as “there will be smoke, but don’t worry. But that’s not the message we want to convey, especially for people with asthma or COPD.”
Instead, he said public health agencies should provide clearer, science-based guidance on the risks for smoke exposure and practical steps people can take to reduce their risk.
What Can Doctors Do?
Chris Carlsten, MD, director of the Centre for Lung Health and professor and head of the Respiratory Medicine Division at the University of British Columbia, Vancouver, Canada, told this news organization that determining whether an exacerbation of a respiratory condition is caused by fire exposure or other factors, such as viral infections, is complex because both can trigger similar responses and may complement each other. “It’s very difficult for any individual to know whether, when they’re having an exacerbation of asthma or COPD, that’s due to the fire,” he said. Fire smoke also increases infection risks, further complicating diagnosis.
Dr. Carlsten suggested that physicians could recommend preventative use of inhalers for at-risk patients when wildfires occur rather than waiting for symptoms to worsen. “That is a really interesting idea that could be practical.” Still, he advises caution, stressing that patients should consult their providers because not all may react well to increased inhaler use.
He also highlighted a significant shift in the healthcare landscape, noting that traditionally, the focus has been on the cardiovascular impacts of pollution, particularly traffic-related pollution. However, as wildfire smoke becomes a growing issue, the focus is shifting back to respiratory problems, with profound implications for healthcare resources, budgets, and drug approvals based on the burden of respiratory disease. “Fire smoke is becoming more of a problem. This swing back to respiratory has huge implications for healthcare systems and respiratory disease burden.”
Mr. Sacks and Dr. Carlsten reported no relevant financial relationships. The study presented by Dr. Tonne received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101057131.
A version of this article first appeared on Medscape.com.
FROM ERS 2024
How Doctors Can Overcome Vaccine Hesitancy Through Empathy, Storytelling, and Patient-Centered Communication
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When Kimberly Fisher, MD, was a junior doctor, she got fired up when patients showed hesitancy about vaccines. She responded by providing numbers, data, and facts that proved vaccines were safe and effective in preventing life-threatening diseases. But she soon realized that regurgitating scientific evidence wasn’t a winning strategy. “I’ve made the mistake of launching into a let me tell you all the things that I know that you don’t know kind of lecture,” Dr. Fisher, now an associate professor of medicine at UMass Chan Medical School, Worcester, Massachusetts, a pulmonary physician, and a researcher interested in patient-provider communication, told this news organization. “Through experience and research, I have learned that when you do that, they stop listening.”
She said when patients give reasons for not getting vaccinated that are factually wrong and rooted in misinformation, the most common reaction is to correct that information and not let it stand. “That is important; it just can’t be the first thing you do,” she said.
Diane Arnaout, MD, a pediatrician at Cook Children’s Pediatrics in Fort Worth, Texas, said listening to some patients explaining why vaccine injections are poisonous or a conspiracy can be exhausting and frustrating, but she agrees that presenting scientific facts alone won’t change people’s minds. “Even in my worst days, I take the time to stop talking for a moment and let the parents talk about what concerns them because if you just get mad and put a wall up, then that trust is gone, possibly forever, not just about vaccines.”
The Default Option
Since the start of the COVID-19 pandemic, Dr. Fisher has dedicated much of her time researching vaccine hesitancy. One of the most “fascinating and unexpected” findings of her work was that people are more likely to get vaccinated if a healthcare provider recommends that they get vaccinated in a “presumptive style,” which means that the provider uses language that presupposes that the person’s going to get vaccinated. “Rather than asking whether they wanted to get the vaccine conveying that the option of not getting it is just as valid, you make vaccination the default option,” she suggested.
The strategy wins many undecided, but it might not work on the most reluctant. “The presumptive recommendation is very directive, and if that works, great, but if it doesn’t, you need to shift to almost the opposite strategy, showing empathy and understanding about the person’s reasons for not wanting to be vaccinated,” Dr. Fisher said.
Find One Thing to Agree On
During a focus group on COVID-19 vaccine hesitancy that Dr. Fisher conducted in December 2021, most physicians expressed frustration that some patients remained resistant despite their best efforts. However, one participant shared an approach she found effective with even the most hesitant patients. The physician would listen carefully and express understanding, and even if what the patient said wasn’t accurate, she would find a kernel of truth to agree with and align herself with the patient. By doing this, she made patients feel like they were a team.
The example she gave was if a patient said, “I don’t know. I’ve heard different things and don’t feel comfortable taking the vaccine,” she might respond with something like, “I think it’s great that you’re thinking critically about this before making a decision. I was the same way — I wanted to fully understand the data before getting vaccinated. I also wouldn’t want to take something if I thought it wasn’t safe. It’s good that you’re being thorough.” Acknowledging their careful thought process, the physician helped patients feel seen and understood only after she introduced additional information to guide them toward understanding why the vaccine might be beneficial.
Focus on the Disease
Dr. Arnaout’s frustration grows when at the end of an appointment some parents object to vaccines with irrational and misguided concerns. “You’ve trusted me with everything else we’ve discussed today — whether it’s a diaper rash or an ear infection — so why wouldn’t you trust me on this? Sometimes it feels almost offensive — why trust my medical expertise on everything else but not vaccines?” she said.
The answer, she believes, is that vaccines are preventive, and when the threat of disease feels distant, it’s hard to see the necessity of a painful shot for your healthy child. “But if your baby were dying from meningitis, the needles we use to deliver life-saving medications in the hospital would feel absolutely necessary. It’s hard as a parent to inflict pain for something you’ve never personally seen.”
Dr. Arnaout thinks it is important to bring the focus on the disease the vaccine prevents. “Let’s talk about measles — how if a baby in my waiting room has measles and coughs, the virus can stay suspended in the air for 2 hours, and 100% of unvaccinated people in that room will get measles.”
She said sharing personal stories can also help physicians connect with their patients. “I talk to parents every day about their vaccine concerns, and I’ve found that if I take the time to explain why we vaccinate, they start to understand. I also tell them, ‘I vaccinated my children for everything on time and give them the flu shot every year. Why would I offer your child something I wouldn’t give my own?’ That personal decision, made without hesitation, resonates with parents.”
Wired for Stories
Medical professionals have a professional necessity to think and speak with precision. Their training is based on analyzing studies and data, and they develop a specialized vocabulary to describe their findings accurately.
But the human brain is naturally inclined to process and make sense of information through the structure and narrative of stories. We instinctively organize reality into a “shape of a story” rather than just isolated facts, explained Ben Riggs, senior communications specialist at Kettering Health, Dayton, Ohio, a nonfiction writing coach and author. Storytelling also taps into the emotional, rather than just the rational, parts of the brain. This emotional connection helps make the information more memorable and impactful for the listener.
Mr. Riggs said that moving from this world of precision and accuracy to one that also requires effective communication with those who haven’t had that same training is much like learning a new language. “If they can’t speak in a way that non-scientists understand, it’s like the old saying: If a tree falls in the woods and no one hears it, does it make a sound?”
Metaphors can help doctors translate scientific facts into language that meets people where they are, allowing patients to make informed decisions about their health. They can help physicians transform abstract concepts into vivid, tangible mental images that are easier for people to understand and relate to, Mr. Riggs explained. “We are predominantly concrete thinkers. Metaphors can create concrete scenes and do much of the heavy lifting when communicating complex ideas.”
“It’s important to align yourself with the other person by showing that you care, that you’re truly listening, and understand their perspective,” concluded Dr. Fisher. “Acknowledge their point of view and emphasize that they have autonomy in the decision-making process. This can open people up to hearing your perspective. You also need to know when to let go don’t cause a rift in the relationship.”
Dr. Fisher, Dr. Arnaout, and Mr. Riggs reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monitor Asthma Patients on Biologics for Remission, Potential EGPA Symptoms During Steroid Tapering
VIENNA — , according to pulmonary experts presenting at the European Respiratory Society (ERS) 2024 International Congress.
Biologics have revolutionized the treatment of severe asthma, significantly improving patient outcomes. However, the focus has recently shifted toward achieving more comprehensive disease control. Remission, already a well-established goal in conditions like rheumatoid arthritis and inflammatory bowel disease, is now being explored in patients with asthma receiving biologics.
Peter Howarth, medical director at Global Medical, Specialty Medicine, GSK, in Brentford, England, said that new clinical remission criteria in asthma may be overly rigid and of little use. He said that more attainable limits must be created. Meanwhile, clinicians should collect clinical data more thoroughly.
In parallel, studies have also raised questions about the role of biologics in the emergence of EGPA.
Defining Clinical Remission in Asthma
Last year, a working group, including members from the American Thoracic Society and the American College and Academy of Allergy, Asthma, and Immunology, proposed new guidelines to define clinical remission in asthma. These guidelines extended beyond the typical outcomes of no severe exacerbations, no maintenance oral corticosteroid use, good asthma control, and stable lung function. The additional recommendations included no missed work or school due to asthma, limited use of rescue medication (no more than once a month), and reduced inhaled corticosteroid use to low or medium doses.
To explore the feasibility of achieving these clinical remission outcomes, GSK partnered with the Mayo Clinic for a retrospective analysis of the medical records of 700 patients with asthma undergoing various biologic therapies. The study revealed that essential data for determining clinical remission, such as asthma control and exacerbation records, were inconsistently documented. While some data were recorded, such as maintenance corticosteroid use in 50%-60% of cases, other key measures, like asthma control, were recorded in less than a quarter of the patients.
GSK researchers analyzed available data and found that around 30% of patients on any biologic therapy met three components of remission. Mepolizumab performed better than other corticosteroids, with over 40% of those receiving the drug meeting these criteria. However, when stricter definitions were applied, such as requiring four or more remission components, fewer patients achieved remission — less than 10% for four components, with no patients meeting the full seven-point criteria proposed by the working group.
An ongoing ERS Task Force is now exploring what clinical remission outcomes are practical to achieve, as the current definitions may be too aspirational, said Mr. Howarth. “It’s a matter of defying what is practical to achieve because if you can’t achieve it, then it won’t be valuable.”
He also pointed out that biologics are often used for the most severe cases of asthma after other treatments have failed. Evidence suggests that introducing biologics earlier in the disease, before chronic damage occurs, may result in better patient outcomes.
Biologics and EGPA
In a retrospective study, clinical details of 27 patients with adult-onset asthma from 28 countries, all on biologic therapy, were analyzed. The study, a multicounty collaboration, was led by ERS Severe Heterogeneous Asthma Research Collaboration, Patient-centred (SHARP), and aimed to understand the role of biologics in the emergence of EGPA.
The most significant finding presented at the ERS 2024 International Congress was that EGPA was not associated with maintenance corticosteroids; instead, it often emerged when corticosteroid doses were reduced or tapered off. “This might suggest that steroid withdrawal may unmask the underlying disease,” said Hitasha Rupani, MD, a consultant respiratory physician at the University Hospital Southampton, in Southampton, England. Importantly, the rate at which steroids were tapered did not influence the onset of EGPA, indicating that the tapering process, rather than its speed, may be the critical factor. However, due to the small sample size, this remains a hypothesis, Dr. Rupani explained.
The study also found that when clinicians had a clinical suspicion of EGPA before starting biologic therapy, the diagnosis was made earlier than in cases without such suspicion. Dr. Rupani concluded that this underscores the importance of clinical vigilance and the need to monitor patients closely for EGPA symptoms, especially during corticosteroid tapering.
The study was funded by GSK. Mr. Howarth is an employee at GSK. Dr. Rupani reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — , according to pulmonary experts presenting at the European Respiratory Society (ERS) 2024 International Congress.
Biologics have revolutionized the treatment of severe asthma, significantly improving patient outcomes. However, the focus has recently shifted toward achieving more comprehensive disease control. Remission, already a well-established goal in conditions like rheumatoid arthritis and inflammatory bowel disease, is now being explored in patients with asthma receiving biologics.
Peter Howarth, medical director at Global Medical, Specialty Medicine, GSK, in Brentford, England, said that new clinical remission criteria in asthma may be overly rigid and of little use. He said that more attainable limits must be created. Meanwhile, clinicians should collect clinical data more thoroughly.
In parallel, studies have also raised questions about the role of biologics in the emergence of EGPA.
Defining Clinical Remission in Asthma
Last year, a working group, including members from the American Thoracic Society and the American College and Academy of Allergy, Asthma, and Immunology, proposed new guidelines to define clinical remission in asthma. These guidelines extended beyond the typical outcomes of no severe exacerbations, no maintenance oral corticosteroid use, good asthma control, and stable lung function. The additional recommendations included no missed work or school due to asthma, limited use of rescue medication (no more than once a month), and reduced inhaled corticosteroid use to low or medium doses.
To explore the feasibility of achieving these clinical remission outcomes, GSK partnered with the Mayo Clinic for a retrospective analysis of the medical records of 700 patients with asthma undergoing various biologic therapies. The study revealed that essential data for determining clinical remission, such as asthma control and exacerbation records, were inconsistently documented. While some data were recorded, such as maintenance corticosteroid use in 50%-60% of cases, other key measures, like asthma control, were recorded in less than a quarter of the patients.
GSK researchers analyzed available data and found that around 30% of patients on any biologic therapy met three components of remission. Mepolizumab performed better than other corticosteroids, with over 40% of those receiving the drug meeting these criteria. However, when stricter definitions were applied, such as requiring four or more remission components, fewer patients achieved remission — less than 10% for four components, with no patients meeting the full seven-point criteria proposed by the working group.
An ongoing ERS Task Force is now exploring what clinical remission outcomes are practical to achieve, as the current definitions may be too aspirational, said Mr. Howarth. “It’s a matter of defying what is practical to achieve because if you can’t achieve it, then it won’t be valuable.”
He also pointed out that biologics are often used for the most severe cases of asthma after other treatments have failed. Evidence suggests that introducing biologics earlier in the disease, before chronic damage occurs, may result in better patient outcomes.
Biologics and EGPA
In a retrospective study, clinical details of 27 patients with adult-onset asthma from 28 countries, all on biologic therapy, were analyzed. The study, a multicounty collaboration, was led by ERS Severe Heterogeneous Asthma Research Collaboration, Patient-centred (SHARP), and aimed to understand the role of biologics in the emergence of EGPA.
The most significant finding presented at the ERS 2024 International Congress was that EGPA was not associated with maintenance corticosteroids; instead, it often emerged when corticosteroid doses were reduced or tapered off. “This might suggest that steroid withdrawal may unmask the underlying disease,” said Hitasha Rupani, MD, a consultant respiratory physician at the University Hospital Southampton, in Southampton, England. Importantly, the rate at which steroids were tapered did not influence the onset of EGPA, indicating that the tapering process, rather than its speed, may be the critical factor. However, due to the small sample size, this remains a hypothesis, Dr. Rupani explained.
The study also found that when clinicians had a clinical suspicion of EGPA before starting biologic therapy, the diagnosis was made earlier than in cases without such suspicion. Dr. Rupani concluded that this underscores the importance of clinical vigilance and the need to monitor patients closely for EGPA symptoms, especially during corticosteroid tapering.
The study was funded by GSK. Mr. Howarth is an employee at GSK. Dr. Rupani reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — , according to pulmonary experts presenting at the European Respiratory Society (ERS) 2024 International Congress.
Biologics have revolutionized the treatment of severe asthma, significantly improving patient outcomes. However, the focus has recently shifted toward achieving more comprehensive disease control. Remission, already a well-established goal in conditions like rheumatoid arthritis and inflammatory bowel disease, is now being explored in patients with asthma receiving biologics.
Peter Howarth, medical director at Global Medical, Specialty Medicine, GSK, in Brentford, England, said that new clinical remission criteria in asthma may be overly rigid and of little use. He said that more attainable limits must be created. Meanwhile, clinicians should collect clinical data more thoroughly.
In parallel, studies have also raised questions about the role of biologics in the emergence of EGPA.
Defining Clinical Remission in Asthma
Last year, a working group, including members from the American Thoracic Society and the American College and Academy of Allergy, Asthma, and Immunology, proposed new guidelines to define clinical remission in asthma. These guidelines extended beyond the typical outcomes of no severe exacerbations, no maintenance oral corticosteroid use, good asthma control, and stable lung function. The additional recommendations included no missed work or school due to asthma, limited use of rescue medication (no more than once a month), and reduced inhaled corticosteroid use to low or medium doses.
To explore the feasibility of achieving these clinical remission outcomes, GSK partnered with the Mayo Clinic for a retrospective analysis of the medical records of 700 patients with asthma undergoing various biologic therapies. The study revealed that essential data for determining clinical remission, such as asthma control and exacerbation records, were inconsistently documented. While some data were recorded, such as maintenance corticosteroid use in 50%-60% of cases, other key measures, like asthma control, were recorded in less than a quarter of the patients.
GSK researchers analyzed available data and found that around 30% of patients on any biologic therapy met three components of remission. Mepolizumab performed better than other corticosteroids, with over 40% of those receiving the drug meeting these criteria. However, when stricter definitions were applied, such as requiring four or more remission components, fewer patients achieved remission — less than 10% for four components, with no patients meeting the full seven-point criteria proposed by the working group.
An ongoing ERS Task Force is now exploring what clinical remission outcomes are practical to achieve, as the current definitions may be too aspirational, said Mr. Howarth. “It’s a matter of defying what is practical to achieve because if you can’t achieve it, then it won’t be valuable.”
He also pointed out that biologics are often used for the most severe cases of asthma after other treatments have failed. Evidence suggests that introducing biologics earlier in the disease, before chronic damage occurs, may result in better patient outcomes.
Biologics and EGPA
In a retrospective study, clinical details of 27 patients with adult-onset asthma from 28 countries, all on biologic therapy, were analyzed. The study, a multicounty collaboration, was led by ERS Severe Heterogeneous Asthma Research Collaboration, Patient-centred (SHARP), and aimed to understand the role of biologics in the emergence of EGPA.
The most significant finding presented at the ERS 2024 International Congress was that EGPA was not associated with maintenance corticosteroids; instead, it often emerged when corticosteroid doses were reduced or tapered off. “This might suggest that steroid withdrawal may unmask the underlying disease,” said Hitasha Rupani, MD, a consultant respiratory physician at the University Hospital Southampton, in Southampton, England. Importantly, the rate at which steroids were tapered did not influence the onset of EGPA, indicating that the tapering process, rather than its speed, may be the critical factor. However, due to the small sample size, this remains a hypothesis, Dr. Rupani explained.
The study also found that when clinicians had a clinical suspicion of EGPA before starting biologic therapy, the diagnosis was made earlier than in cases without such suspicion. Dr. Rupani concluded that this underscores the importance of clinical vigilance and the need to monitor patients closely for EGPA symptoms, especially during corticosteroid tapering.
The study was funded by GSK. Mr. Howarth is an employee at GSK. Dr. Rupani reports no relevant financial relationships.
A version of this article appeared on Medscape.com.