User login
Transcatheter mitral valve replacement: A frontier in cardiac intervention
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
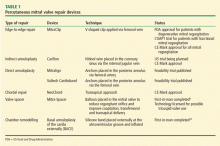
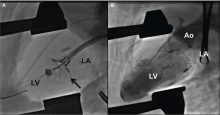
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
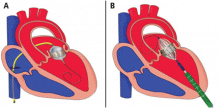
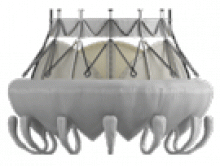
The NaviGate valve
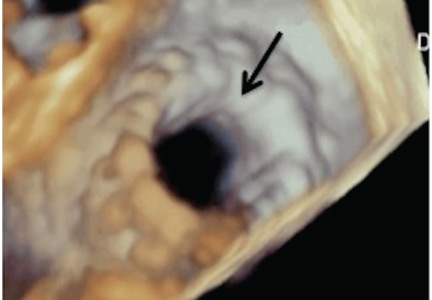
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.
The Intrepid valve
The Intrepid valve consists of an outer stent to provide fixation to the annulus and an inner stent that houses a bovine pericardial valve. The device is a self-expanding system that is delivered transapically.
In a series of 15 patients, 11 had functional mitral regurgitation (with an average left ventricular ejection fraction of 35%) and four had degenerative mitral regurgitation (with an average left ventricular ejection fraction of 57%).15 The device was successfully implanted in 14 patients, after which the average mitral valve gradient was 4 mm Hg. All patients but one were left with no regurgitation (the other patient had 1+ regurgitation).
A trial is currently under way in Europe.
The CardiAQ valve
The CardiAQ is constructed of bovine pericardium and can be delivered by the transseptal or transapical route.
Of 12 patients treated under compassionate use,16 two-thirds (eight patients) had functional mitral regurgitation. Two patients died during the procedure, three died of noncardiac complications within 30 days, and one more died of sepsis shortly after 30 days. This early experience demonstrates the importance of careful patient selection and postprocedural management in the feasibility assessment of these new technologies.
Patients are being enrolled in phase 1 trials.
The Tiara valve
The Tiara valve, a self-expanding prosthesis with bovine pericardial leaflets, is delivered by the transapical route.
Eleven patients underwent Tiara implantation as part of either a Canadian special access registry or an international feasibility trial. Their average Society of Thoracic Surgeons score (ie, their calculated risk of major morbidity or operative mortality) was 15.6%, and their average left ventricular ejection fraction was 29%. Only two patients had degenerative mitral regurgitation. Nine patients had uneventful procedures and demonstrated no residual mitral regurgitation and no left ventricular outflow tract obstruction. The procedure was converted to open surgery in two patients owing to valve malpositioning, and both of them died within 30 days. One patient in whom the procedure was successful suffered erosion of the septum and died on day 4.17
Patients are being enrolled in phase 1 trials.
DEGENERATIVE MITRAL STENOSIS
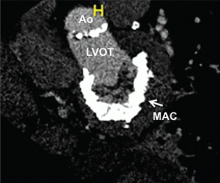
In patients with degenerative mitral stenosis, extensive mitral annular calcification may provide an adequate “frame” to hold a transcatheter valve prosthesis (Figure 3). Exploiting this feature, numerous investigators have successfully deployed prosthetic valves designed for TAVR in the calcified mitral annulus via the retrograde transapical and antegrade transseptal routes.
Guerrero and colleagues presented results from the first global registry of TMVR in mitral annular calcification at the 2016 EuroPCR Congress.18 Of 104 patients analyzed, almost all received an Edwards’ Sapien balloon-expandable valve (first-generation, Sapien XT, or Sapien 3); the others received Boston Scientific’s Lotus or Direct Flow Medical (Direct Flow Medical, Santa Clara, CA) valves. With an average age of 73 years and a high prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease, and prior cardiac surgery, the group presented extreme surgical risk, with an average Society of Thoracic Surgeons risk score of 14.4%. Slightly more than 40% of the patients underwent transapical implantation, slightly less than 40% underwent transfemoral or transseptal implantation, and just under 20% had a direct atrial approach.
The implantation was technically successful in 78 of 104 patients (75%); 13 patients (12.5%) required a second mitral valve to be placed, 11 patients (10.5%) had left ventricular outflow tract obstruction, four patients (4%) had valve embolization, and two patients (2%) had left ventricular perforation. At 30 days, 11 of 104 patients (10.6%) had died of cardiac causes and 15 patients (14.4%) had died of noncardiac causes. When divided roughly into three equal groups by chronological order, the last third of patients, compared with the first third of patients, enjoyed greater technical success (80%, n = 32/40 vs 62.5%, n = 20/32), better 30-day survival (85%, n = 34/40 vs 62.5%, n = 20/32), and no conversion to open surgery (0 vs 12.5%, n = 4/32), likely demonstrating both improved patient selection and lessons learned from shared experience. At 1 year, almost 90% of patients had New York Heart Association class I or II symptoms. Prior to the procedure, 91.5% had New York Heart Association class III or IV symptoms.
At present, TMVR in mitral annular calcification is not approved in the United States or elsewhere. However, multiple registries are currently enrolling patients or are in formative stages to push the frontier of the currently available technologies until better, dedicated devices are available for this group of patients.
BIOPROSTHETIC VALVE OR VALVE RING FAILURE
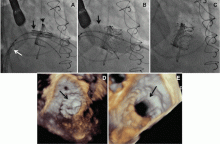
Implantation of a TAVR prosthetic inside a degenerated bioprosthetic mitral valve (valve-in-valve) and mitral valve ring (valve-in-ring) is generally limited to case series with short-term results using the Edwards Sapien series, Boston Scientific Lotus, Medtronic Melody (Medtronic, Minneapolis, MN), and Direct Flow Medical valves (Figure 4).19–23
The largest collective experience was presented in the Valve-in-Valve International Data (VIVID) registry, which included 349 patients who had mitral valve-in-valve placement and 88 patients who had mitral valve-in-ring procedures. Their average age was 74 and the mean Society of Thoracic Surgeons score was 12.9% in both groups.24 Of the 437 patients, 345 patients (78.9%) underwent transapical implantation, and 391 patients (89.5%) received a Sapien XT or Sapien 3 valve. In the valve-in-valve group, 41% of the patients had regurgitation, 25% had stenosis, and 34% had both. In the valve-in-ring group, 60% of the patients had regurgitation, 17% had stenosis, and 23% had both.
Valve placement was successful in most patients. The rate of stroke was low (2.9% with valve-in-valve placement, 1.1% with valve-in-ring placement), though the rate of moderate or greater residual mitral regurgitation was significantly higher in patients undergoing valve-in-ring procedures (14.8% vs 2.6%, P < .001), as was the rate of left ventricular outflow tract obstruction (8% vs 2.6%, P = .03). There was also a trend toward worse 30-day mortality in the valve-in-ring group (11.4% vs 7.7%, P = .15). As with aortic valve-in-valve procedures, small surgical mitral valves (≤ 25 mm) were associated with higher postprocedural gradients.
Eleid and colleagues25 published their experience with antegrade transseptal TMVR in 48 patients with an average Society of Thoracic Surgeons score of 13.2%, 33 of whom underwent valve-in-valve procedures and nine of whom underwent valve-in-ring procedures. (The other six patients underwent mitral valve implantation for severe mitral annular calcification.) In the valve-in-valve group, 31 patients successfully underwent implant procedures, but two patients died during the procedure from left ventricular perforation. Of the nine valve-in-ring patients, two had acute embolization of the valve and were converted to open surgery. Among the seven patients in whom implantation was successful, two developed significant left ventricular outflow tract obstruction; one was treated with surgical resection of the anterior mitral valve leaflet and the other was medically managed.
CONCLUSION
Transcatheter mitral valve replacement in regurgitant mitral valves, failing mitral valve bioprosthetics and rings, and calcified mitral annuli has been effectively conducted in a number of patients who had no surgical options due to prohibitive surgical risk. International registries and our experience have demonstrated that the valve-in-valve procedure using a TAVR prosthesis carries the greatest likelihood of success, given the rigid frame of the surgical bioprosthetic that allows stable valve deployment. While approved in Europe for this indication, use of these devices for this application in the United States is considered “off label” and is performed only in clinically extenuating circumstances. Implantation of TAVR prosthetics in patients with prior mitral ring repair or for native mitral stenosis also has been performed successfully, although left ventricular outflow tract obstruction is a significant risk in this early experience.
Devices designed specifically for TMVR are in their clinical infancy and have been implanted successfully in only small numbers of patients, most of whom had functional mitral regurgitation. Despite reasonable technical success, most of these trials have been plagued by high mortality rates at 30 days in large part due to the extreme risk of the patients in whom these procedures have been conducted. At present, enrollment in TMVR trials for patients with degenerative or functional mitral regurgitation is limited to those without a surgical option and who conform to very specific anatomic criteria.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63:185–186.
- DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010; 139:76–83; discussion 83–74.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28:1358–1365.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Sud K, Agarwal S, Parashar A, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016; 133:1594–1604.
- Svensson LG, Ye J, Piemonte TC, Kirker-Head C, Leon MB, Webb JG. Mitral valve regurgitation and left ventricular dysfunction treatment with an intravalvular spacer. J Card Surg 2015; 30:53–54.
- Raman J, Raghavan J, Chandrashekar P, Sugeng L. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011; 20:157–162.
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015; 66:1011–1019.
- Bapat V. FORTIS: design, clinical results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; Feburary 20–23, 2016; Washington, DC.
- Sorajja P. Tendyne: technology and clinical results update. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Navia J. Personal communication.
- Bapat V. Medtronic Intrepid transcatheter mitral valve replacement. Presented at EuroPCR 2015; May 19–22, 2015; Paris, France.
- Herrmann H. Cardiaq-Edwards TMVR. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Dvir D. Tiara: design, clincal results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitra valve disease with severe mitral annular calcification: results from the first global registry. JACC Cardiovasc Interv 2016; 9:1361–1371.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010; 76:608–615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010; 121:1848–1857.
- Cerillo AG, Chiaramonti F, Murzi M, et al. Transcatheter valve in valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv 2011; 78:987–995.
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012; 5:341–349.
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014; 147:210–219.
- Dvir D, on behalf of the VIVID Registry Investigators. Transcatheter mitral valve-in-valve and valve-in-ring implantations. Transcatheter Valve Therapies 2015.
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016; 9:1161–1174.
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
The NaviGate valve
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.
The Intrepid valve
The Intrepid valve consists of an outer stent to provide fixation to the annulus and an inner stent that houses a bovine pericardial valve. The device is a self-expanding system that is delivered transapically.
In a series of 15 patients, 11 had functional mitral regurgitation (with an average left ventricular ejection fraction of 35%) and four had degenerative mitral regurgitation (with an average left ventricular ejection fraction of 57%).15 The device was successfully implanted in 14 patients, after which the average mitral valve gradient was 4 mm Hg. All patients but one were left with no regurgitation (the other patient had 1+ regurgitation).
A trial is currently under way in Europe.
The CardiAQ valve
The CardiAQ is constructed of bovine pericardium and can be delivered by the transseptal or transapical route.
Of 12 patients treated under compassionate use,16 two-thirds (eight patients) had functional mitral regurgitation. Two patients died during the procedure, three died of noncardiac complications within 30 days, and one more died of sepsis shortly after 30 days. This early experience demonstrates the importance of careful patient selection and postprocedural management in the feasibility assessment of these new technologies.
Patients are being enrolled in phase 1 trials.
The Tiara valve
The Tiara valve, a self-expanding prosthesis with bovine pericardial leaflets, is delivered by the transapical route.
Eleven patients underwent Tiara implantation as part of either a Canadian special access registry or an international feasibility trial. Their average Society of Thoracic Surgeons score (ie, their calculated risk of major morbidity or operative mortality) was 15.6%, and their average left ventricular ejection fraction was 29%. Only two patients had degenerative mitral regurgitation. Nine patients had uneventful procedures and demonstrated no residual mitral regurgitation and no left ventricular outflow tract obstruction. The procedure was converted to open surgery in two patients owing to valve malpositioning, and both of them died within 30 days. One patient in whom the procedure was successful suffered erosion of the septum and died on day 4.17
Patients are being enrolled in phase 1 trials.
DEGENERATIVE MITRAL STENOSIS
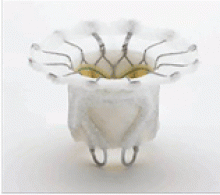
In patients with degenerative mitral stenosis, extensive mitral annular calcification may provide an adequate “frame” to hold a transcatheter valve prosthesis (Figure 3). Exploiting this feature, numerous investigators have successfully deployed prosthetic valves designed for TAVR in the calcified mitral annulus via the retrograde transapical and antegrade transseptal routes.
Guerrero and colleagues presented results from the first global registry of TMVR in mitral annular calcification at the 2016 EuroPCR Congress.18 Of 104 patients analyzed, almost all received an Edwards’ Sapien balloon-expandable valve (first-generation, Sapien XT, or Sapien 3); the others received Boston Scientific’s Lotus or Direct Flow Medical (Direct Flow Medical, Santa Clara, CA) valves. With an average age of 73 years and a high prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease, and prior cardiac surgery, the group presented extreme surgical risk, with an average Society of Thoracic Surgeons risk score of 14.4%. Slightly more than 40% of the patients underwent transapical implantation, slightly less than 40% underwent transfemoral or transseptal implantation, and just under 20% had a direct atrial approach.
The implantation was technically successful in 78 of 104 patients (75%); 13 patients (12.5%) required a second mitral valve to be placed, 11 patients (10.5%) had left ventricular outflow tract obstruction, four patients (4%) had valve embolization, and two patients (2%) had left ventricular perforation. At 30 days, 11 of 104 patients (10.6%) had died of cardiac causes and 15 patients (14.4%) had died of noncardiac causes. When divided roughly into three equal groups by chronological order, the last third of patients, compared with the first third of patients, enjoyed greater technical success (80%, n = 32/40 vs 62.5%, n = 20/32), better 30-day survival (85%, n = 34/40 vs 62.5%, n = 20/32), and no conversion to open surgery (0 vs 12.5%, n = 4/32), likely demonstrating both improved patient selection and lessons learned from shared experience. At 1 year, almost 90% of patients had New York Heart Association class I or II symptoms. Prior to the procedure, 91.5% had New York Heart Association class III or IV symptoms.
At present, TMVR in mitral annular calcification is not approved in the United States or elsewhere. However, multiple registries are currently enrolling patients or are in formative stages to push the frontier of the currently available technologies until better, dedicated devices are available for this group of patients.
BIOPROSTHETIC VALVE OR VALVE RING FAILURE
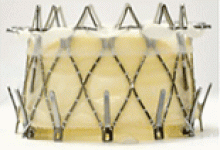
Implantation of a TAVR prosthetic inside a degenerated bioprosthetic mitral valve (valve-in-valve) and mitral valve ring (valve-in-ring) is generally limited to case series with short-term results using the Edwards Sapien series, Boston Scientific Lotus, Medtronic Melody (Medtronic, Minneapolis, MN), and Direct Flow Medical valves (Figure 4).19–23
The largest collective experience was presented in the Valve-in-Valve International Data (VIVID) registry, which included 349 patients who had mitral valve-in-valve placement and 88 patients who had mitral valve-in-ring procedures. Their average age was 74 and the mean Society of Thoracic Surgeons score was 12.9% in both groups.24 Of the 437 patients, 345 patients (78.9%) underwent transapical implantation, and 391 patients (89.5%) received a Sapien XT or Sapien 3 valve. In the valve-in-valve group, 41% of the patients had regurgitation, 25% had stenosis, and 34% had both. In the valve-in-ring group, 60% of the patients had regurgitation, 17% had stenosis, and 23% had both.
Valve placement was successful in most patients. The rate of stroke was low (2.9% with valve-in-valve placement, 1.1% with valve-in-ring placement), though the rate of moderate or greater residual mitral regurgitation was significantly higher in patients undergoing valve-in-ring procedures (14.8% vs 2.6%, P < .001), as was the rate of left ventricular outflow tract obstruction (8% vs 2.6%, P = .03). There was also a trend toward worse 30-day mortality in the valve-in-ring group (11.4% vs 7.7%, P = .15). As with aortic valve-in-valve procedures, small surgical mitral valves (≤ 25 mm) were associated with higher postprocedural gradients.
Eleid and colleagues25 published their experience with antegrade transseptal TMVR in 48 patients with an average Society of Thoracic Surgeons score of 13.2%, 33 of whom underwent valve-in-valve procedures and nine of whom underwent valve-in-ring procedures. (The other six patients underwent mitral valve implantation for severe mitral annular calcification.) In the valve-in-valve group, 31 patients successfully underwent implant procedures, but two patients died during the procedure from left ventricular perforation. Of the nine valve-in-ring patients, two had acute embolization of the valve and were converted to open surgery. Among the seven patients in whom implantation was successful, two developed significant left ventricular outflow tract obstruction; one was treated with surgical resection of the anterior mitral valve leaflet and the other was medically managed.
CONCLUSION
Transcatheter mitral valve replacement in regurgitant mitral valves, failing mitral valve bioprosthetics and rings, and calcified mitral annuli has been effectively conducted in a number of patients who had no surgical options due to prohibitive surgical risk. International registries and our experience have demonstrated that the valve-in-valve procedure using a TAVR prosthesis carries the greatest likelihood of success, given the rigid frame of the surgical bioprosthetic that allows stable valve deployment. While approved in Europe for this indication, use of these devices for this application in the United States is considered “off label” and is performed only in clinically extenuating circumstances. Implantation of TAVR prosthetics in patients with prior mitral ring repair or for native mitral stenosis also has been performed successfully, although left ventricular outflow tract obstruction is a significant risk in this early experience.
Devices designed specifically for TMVR are in their clinical infancy and have been implanted successfully in only small numbers of patients, most of whom had functional mitral regurgitation. Despite reasonable technical success, most of these trials have been plagued by high mortality rates at 30 days in large part due to the extreme risk of the patients in whom these procedures have been conducted. At present, enrollment in TMVR trials for patients with degenerative or functional mitral regurgitation is limited to those without a surgical option and who conform to very specific anatomic criteria.
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
The NaviGate valve
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.
The Intrepid valve
The Intrepid valve consists of an outer stent to provide fixation to the annulus and an inner stent that houses a bovine pericardial valve. The device is a self-expanding system that is delivered transapically.
In a series of 15 patients, 11 had functional mitral regurgitation (with an average left ventricular ejection fraction of 35%) and four had degenerative mitral regurgitation (with an average left ventricular ejection fraction of 57%).15 The device was successfully implanted in 14 patients, after which the average mitral valve gradient was 4 mm Hg. All patients but one were left with no regurgitation (the other patient had 1+ regurgitation).
A trial is currently under way in Europe.
The CardiAQ valve
The CardiAQ is constructed of bovine pericardium and can be delivered by the transseptal or transapical route.
Of 12 patients treated under compassionate use,16 two-thirds (eight patients) had functional mitral regurgitation. Two patients died during the procedure, three died of noncardiac complications within 30 days, and one more died of sepsis shortly after 30 days. This early experience demonstrates the importance of careful patient selection and postprocedural management in the feasibility assessment of these new technologies.
Patients are being enrolled in phase 1 trials.
The Tiara valve
The Tiara valve, a self-expanding prosthesis with bovine pericardial leaflets, is delivered by the transapical route.
Eleven patients underwent Tiara implantation as part of either a Canadian special access registry or an international feasibility trial. Their average Society of Thoracic Surgeons score (ie, their calculated risk of major morbidity or operative mortality) was 15.6%, and their average left ventricular ejection fraction was 29%. Only two patients had degenerative mitral regurgitation. Nine patients had uneventful procedures and demonstrated no residual mitral regurgitation and no left ventricular outflow tract obstruction. The procedure was converted to open surgery in two patients owing to valve malpositioning, and both of them died within 30 days. One patient in whom the procedure was successful suffered erosion of the septum and died on day 4.17
Patients are being enrolled in phase 1 trials.
DEGENERATIVE MITRAL STENOSIS
In patients with degenerative mitral stenosis, extensive mitral annular calcification may provide an adequate “frame” to hold a transcatheter valve prosthesis (Figure 3). Exploiting this feature, numerous investigators have successfully deployed prosthetic valves designed for TAVR in the calcified mitral annulus via the retrograde transapical and antegrade transseptal routes.
Guerrero and colleagues presented results from the first global registry of TMVR in mitral annular calcification at the 2016 EuroPCR Congress.18 Of 104 patients analyzed, almost all received an Edwards’ Sapien balloon-expandable valve (first-generation, Sapien XT, or Sapien 3); the others received Boston Scientific’s Lotus or Direct Flow Medical (Direct Flow Medical, Santa Clara, CA) valves. With an average age of 73 years and a high prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease, and prior cardiac surgery, the group presented extreme surgical risk, with an average Society of Thoracic Surgeons risk score of 14.4%. Slightly more than 40% of the patients underwent transapical implantation, slightly less than 40% underwent transfemoral or transseptal implantation, and just under 20% had a direct atrial approach.
The implantation was technically successful in 78 of 104 patients (75%); 13 patients (12.5%) required a second mitral valve to be placed, 11 patients (10.5%) had left ventricular outflow tract obstruction, four patients (4%) had valve embolization, and two patients (2%) had left ventricular perforation. At 30 days, 11 of 104 patients (10.6%) had died of cardiac causes and 15 patients (14.4%) had died of noncardiac causes. When divided roughly into three equal groups by chronological order, the last third of patients, compared with the first third of patients, enjoyed greater technical success (80%, n = 32/40 vs 62.5%, n = 20/32), better 30-day survival (85%, n = 34/40 vs 62.5%, n = 20/32), and no conversion to open surgery (0 vs 12.5%, n = 4/32), likely demonstrating both improved patient selection and lessons learned from shared experience. At 1 year, almost 90% of patients had New York Heart Association class I or II symptoms. Prior to the procedure, 91.5% had New York Heart Association class III or IV symptoms.
At present, TMVR in mitral annular calcification is not approved in the United States or elsewhere. However, multiple registries are currently enrolling patients or are in formative stages to push the frontier of the currently available technologies until better, dedicated devices are available for this group of patients.
BIOPROSTHETIC VALVE OR VALVE RING FAILURE
Implantation of a TAVR prosthetic inside a degenerated bioprosthetic mitral valve (valve-in-valve) and mitral valve ring (valve-in-ring) is generally limited to case series with short-term results using the Edwards Sapien series, Boston Scientific Lotus, Medtronic Melody (Medtronic, Minneapolis, MN), and Direct Flow Medical valves (Figure 4).19–23
The largest collective experience was presented in the Valve-in-Valve International Data (VIVID) registry, which included 349 patients who had mitral valve-in-valve placement and 88 patients who had mitral valve-in-ring procedures. Their average age was 74 and the mean Society of Thoracic Surgeons score was 12.9% in both groups.24 Of the 437 patients, 345 patients (78.9%) underwent transapical implantation, and 391 patients (89.5%) received a Sapien XT or Sapien 3 valve. In the valve-in-valve group, 41% of the patients had regurgitation, 25% had stenosis, and 34% had both. In the valve-in-ring group, 60% of the patients had regurgitation, 17% had stenosis, and 23% had both.
Valve placement was successful in most patients. The rate of stroke was low (2.9% with valve-in-valve placement, 1.1% with valve-in-ring placement), though the rate of moderate or greater residual mitral regurgitation was significantly higher in patients undergoing valve-in-ring procedures (14.8% vs 2.6%, P < .001), as was the rate of left ventricular outflow tract obstruction (8% vs 2.6%, P = .03). There was also a trend toward worse 30-day mortality in the valve-in-ring group (11.4% vs 7.7%, P = .15). As with aortic valve-in-valve procedures, small surgical mitral valves (≤ 25 mm) were associated with higher postprocedural gradients.
Eleid and colleagues25 published their experience with antegrade transseptal TMVR in 48 patients with an average Society of Thoracic Surgeons score of 13.2%, 33 of whom underwent valve-in-valve procedures and nine of whom underwent valve-in-ring procedures. (The other six patients underwent mitral valve implantation for severe mitral annular calcification.) In the valve-in-valve group, 31 patients successfully underwent implant procedures, but two patients died during the procedure from left ventricular perforation. Of the nine valve-in-ring patients, two had acute embolization of the valve and were converted to open surgery. Among the seven patients in whom implantation was successful, two developed significant left ventricular outflow tract obstruction; one was treated with surgical resection of the anterior mitral valve leaflet and the other was medically managed.
CONCLUSION
Transcatheter mitral valve replacement in regurgitant mitral valves, failing mitral valve bioprosthetics and rings, and calcified mitral annuli has been effectively conducted in a number of patients who had no surgical options due to prohibitive surgical risk. International registries and our experience have demonstrated that the valve-in-valve procedure using a TAVR prosthesis carries the greatest likelihood of success, given the rigid frame of the surgical bioprosthetic that allows stable valve deployment. While approved in Europe for this indication, use of these devices for this application in the United States is considered “off label” and is performed only in clinically extenuating circumstances. Implantation of TAVR prosthetics in patients with prior mitral ring repair or for native mitral stenosis also has been performed successfully, although left ventricular outflow tract obstruction is a significant risk in this early experience.
Devices designed specifically for TMVR are in their clinical infancy and have been implanted successfully in only small numbers of patients, most of whom had functional mitral regurgitation. Despite reasonable technical success, most of these trials have been plagued by high mortality rates at 30 days in large part due to the extreme risk of the patients in whom these procedures have been conducted. At present, enrollment in TMVR trials for patients with degenerative or functional mitral regurgitation is limited to those without a surgical option and who conform to very specific anatomic criteria.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63:185–186.
- DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010; 139:76–83; discussion 83–74.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28:1358–1365.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Sud K, Agarwal S, Parashar A, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016; 133:1594–1604.
- Svensson LG, Ye J, Piemonte TC, Kirker-Head C, Leon MB, Webb JG. Mitral valve regurgitation and left ventricular dysfunction treatment with an intravalvular spacer. J Card Surg 2015; 30:53–54.
- Raman J, Raghavan J, Chandrashekar P, Sugeng L. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011; 20:157–162.
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015; 66:1011–1019.
- Bapat V. FORTIS: design, clinical results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; Feburary 20–23, 2016; Washington, DC.
- Sorajja P. Tendyne: technology and clinical results update. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Navia J. Personal communication.
- Bapat V. Medtronic Intrepid transcatheter mitral valve replacement. Presented at EuroPCR 2015; May 19–22, 2015; Paris, France.
- Herrmann H. Cardiaq-Edwards TMVR. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Dvir D. Tiara: design, clincal results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitra valve disease with severe mitral annular calcification: results from the first global registry. JACC Cardiovasc Interv 2016; 9:1361–1371.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010; 76:608–615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010; 121:1848–1857.
- Cerillo AG, Chiaramonti F, Murzi M, et al. Transcatheter valve in valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv 2011; 78:987–995.
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012; 5:341–349.
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014; 147:210–219.
- Dvir D, on behalf of the VIVID Registry Investigators. Transcatheter mitral valve-in-valve and valve-in-ring implantations. Transcatheter Valve Therapies 2015.
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016; 9:1161–1174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63:185–186.
- DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010; 139:76–83; discussion 83–74.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28:1358–1365.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Sud K, Agarwal S, Parashar A, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016; 133:1594–1604.
- Svensson LG, Ye J, Piemonte TC, Kirker-Head C, Leon MB, Webb JG. Mitral valve regurgitation and left ventricular dysfunction treatment with an intravalvular spacer. J Card Surg 2015; 30:53–54.
- Raman J, Raghavan J, Chandrashekar P, Sugeng L. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011; 20:157–162.
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015; 66:1011–1019.
- Bapat V. FORTIS: design, clinical results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; Feburary 20–23, 2016; Washington, DC.
- Sorajja P. Tendyne: technology and clinical results update. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Navia J. Personal communication.
- Bapat V. Medtronic Intrepid transcatheter mitral valve replacement. Presented at EuroPCR 2015; May 19–22, 2015; Paris, France.
- Herrmann H. Cardiaq-Edwards TMVR. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Dvir D. Tiara: design, clincal results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitra valve disease with severe mitral annular calcification: results from the first global registry. JACC Cardiovasc Interv 2016; 9:1361–1371.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010; 76:608–615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010; 121:1848–1857.
- Cerillo AG, Chiaramonti F, Murzi M, et al. Transcatheter valve in valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv 2011; 78:987–995.
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012; 5:341–349.
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014; 147:210–219.
- Dvir D, on behalf of the VIVID Registry Investigators. Transcatheter mitral valve-in-valve and valve-in-ring implantations. Transcatheter Valve Therapies 2015.
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016; 9:1161–1174.
KEY POINTS
- Most TMVR procedures are performed by either a retrograde transapical approach or an antegrade transseptal approach.
- In the small number of patients who have undergone TMVR for native mitral valve regurgitation to date, mortality rates at 30 days have been high, reflecting the seriousness of illness in these patients.
- At present, none of the new devices for TMVR in patients with native mitral valve regurgitation are approved for general use, although some of them are being tested in phase 1 clinical trials that are enrolling patients.
- Valves made for TAVR have been used for TMVR in patients with degenerative mitral stenosis or failure of mitral bioprostheses; however, these are off-label uses of these devices.