User login
Transcatheter mitral valve replacement: A frontier in cardiac intervention
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
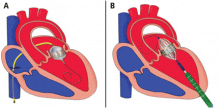
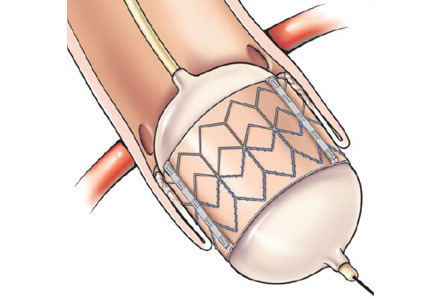
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
The NaviGate valve
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
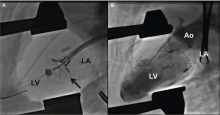
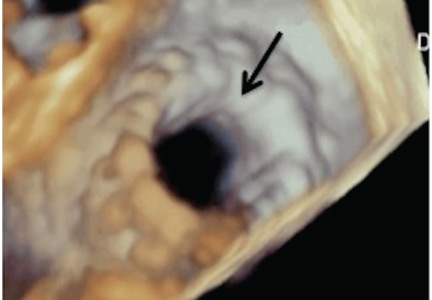
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.
The Intrepid valve
The Intrepid valve consists of an outer stent to provide fixation to the annulus and an inner stent that houses a bovine pericardial valve. The device is a self-expanding system that is delivered transapically.
In a series of 15 patients, 11 had functional mitral regurgitation (with an average left ventricular ejection fraction of 35%) and four had degenerative mitral regurgitation (with an average left ventricular ejection fraction of 57%).15 The device was successfully implanted in 14 patients, after which the average mitral valve gradient was 4 mm Hg. All patients but one were left with no regurgitation (the other patient had 1+ regurgitation).
A trial is currently under way in Europe.
The CardiAQ valve
The CardiAQ is constructed of bovine pericardium and can be delivered by the transseptal or transapical route.
Of 12 patients treated under compassionate use,16 two-thirds (eight patients) had functional mitral regurgitation. Two patients died during the procedure, three died of noncardiac complications within 30 days, and one more died of sepsis shortly after 30 days. This early experience demonstrates the importance of careful patient selection and postprocedural management in the feasibility assessment of these new technologies.
Patients are being enrolled in phase 1 trials.
The Tiara valve
The Tiara valve, a self-expanding prosthesis with bovine pericardial leaflets, is delivered by the transapical route.
Eleven patients underwent Tiara implantation as part of either a Canadian special access registry or an international feasibility trial. Their average Society of Thoracic Surgeons score (ie, their calculated risk of major morbidity or operative mortality) was 15.6%, and their average left ventricular ejection fraction was 29%. Only two patients had degenerative mitral regurgitation. Nine patients had uneventful procedures and demonstrated no residual mitral regurgitation and no left ventricular outflow tract obstruction. The procedure was converted to open surgery in two patients owing to valve malpositioning, and both of them died within 30 days. One patient in whom the procedure was successful suffered erosion of the septum and died on day 4.17
Patients are being enrolled in phase 1 trials.
DEGENERATIVE MITRAL STENOSIS
In patients with degenerative mitral stenosis, extensive mitral annular calcification may provide an adequate “frame” to hold a transcatheter valve prosthesis (Figure 3). Exploiting this feature, numerous investigators have successfully deployed prosthetic valves designed for TAVR in the calcified mitral annulus via the retrograde transapical and antegrade transseptal routes.
Guerrero and colleagues presented results from the first global registry of TMVR in mitral annular calcification at the 2016 EuroPCR Congress.18 Of 104 patients analyzed, almost all received an Edwards’ Sapien balloon-expandable valve (first-generation, Sapien XT, or Sapien 3); the others received Boston Scientific’s Lotus or Direct Flow Medical (Direct Flow Medical, Santa Clara, CA) valves. With an average age of 73 years and a high prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease, and prior cardiac surgery, the group presented extreme surgical risk, with an average Society of Thoracic Surgeons risk score of 14.4%. Slightly more than 40% of the patients underwent transapical implantation, slightly less than 40% underwent transfemoral or transseptal implantation, and just under 20% had a direct atrial approach.
The implantation was technically successful in 78 of 104 patients (75%); 13 patients (12.5%) required a second mitral valve to be placed, 11 patients (10.5%) had left ventricular outflow tract obstruction, four patients (4%) had valve embolization, and two patients (2%) had left ventricular perforation. At 30 days, 11 of 104 patients (10.6%) had died of cardiac causes and 15 patients (14.4%) had died of noncardiac causes. When divided roughly into three equal groups by chronological order, the last third of patients, compared with the first third of patients, enjoyed greater technical success (80%, n = 32/40 vs 62.5%, n = 20/32), better 30-day survival (85%, n = 34/40 vs 62.5%, n = 20/32), and no conversion to open surgery (0 vs 12.5%, n = 4/32), likely demonstrating both improved patient selection and lessons learned from shared experience. At 1 year, almost 90% of patients had New York Heart Association class I or II symptoms. Prior to the procedure, 91.5% had New York Heart Association class III or IV symptoms.
At present, TMVR in mitral annular calcification is not approved in the United States or elsewhere. However, multiple registries are currently enrolling patients or are in formative stages to push the frontier of the currently available technologies until better, dedicated devices are available for this group of patients.
BIOPROSTHETIC VALVE OR VALVE RING FAILURE
Implantation of a TAVR prosthetic inside a degenerated bioprosthetic mitral valve (valve-in-valve) and mitral valve ring (valve-in-ring) is generally limited to case series with short-term results using the Edwards Sapien series, Boston Scientific Lotus, Medtronic Melody (Medtronic, Minneapolis, MN), and Direct Flow Medical valves (Figure 4).19–23
The largest collective experience was presented in the Valve-in-Valve International Data (VIVID) registry, which included 349 patients who had mitral valve-in-valve placement and 88 patients who had mitral valve-in-ring procedures. Their average age was 74 and the mean Society of Thoracic Surgeons score was 12.9% in both groups.24 Of the 437 patients, 345 patients (78.9%) underwent transapical implantation, and 391 patients (89.5%) received a Sapien XT or Sapien 3 valve. In the valve-in-valve group, 41% of the patients had regurgitation, 25% had stenosis, and 34% had both. In the valve-in-ring group, 60% of the patients had regurgitation, 17% had stenosis, and 23% had both.
Valve placement was successful in most patients. The rate of stroke was low (2.9% with valve-in-valve placement, 1.1% with valve-in-ring placement), though the rate of moderate or greater residual mitral regurgitation was significantly higher in patients undergoing valve-in-ring procedures (14.8% vs 2.6%, P < .001), as was the rate of left ventricular outflow tract obstruction (8% vs 2.6%, P = .03). There was also a trend toward worse 30-day mortality in the valve-in-ring group (11.4% vs 7.7%, P = .15). As with aortic valve-in-valve procedures, small surgical mitral valves (≤ 25 mm) were associated with higher postprocedural gradients.
Eleid and colleagues25 published their experience with antegrade transseptal TMVR in 48 patients with an average Society of Thoracic Surgeons score of 13.2%, 33 of whom underwent valve-in-valve procedures and nine of whom underwent valve-in-ring procedures. (The other six patients underwent mitral valve implantation for severe mitral annular calcification.) In the valve-in-valve group, 31 patients successfully underwent implant procedures, but two patients died during the procedure from left ventricular perforation. Of the nine valve-in-ring patients, two had acute embolization of the valve and were converted to open surgery. Among the seven patients in whom implantation was successful, two developed significant left ventricular outflow tract obstruction; one was treated with surgical resection of the anterior mitral valve leaflet and the other was medically managed.
CONCLUSION
Transcatheter mitral valve replacement in regurgitant mitral valves, failing mitral valve bioprosthetics and rings, and calcified mitral annuli has been effectively conducted in a number of patients who had no surgical options due to prohibitive surgical risk. International registries and our experience have demonstrated that the valve-in-valve procedure using a TAVR prosthesis carries the greatest likelihood of success, given the rigid frame of the surgical bioprosthetic that allows stable valve deployment. While approved in Europe for this indication, use of these devices for this application in the United States is considered “off label” and is performed only in clinically extenuating circumstances. Implantation of TAVR prosthetics in patients with prior mitral ring repair or for native mitral stenosis also has been performed successfully, although left ventricular outflow tract obstruction is a significant risk in this early experience.
Devices designed specifically for TMVR are in their clinical infancy and have been implanted successfully in only small numbers of patients, most of whom had functional mitral regurgitation. Despite reasonable technical success, most of these trials have been plagued by high mortality rates at 30 days in large part due to the extreme risk of the patients in whom these procedures have been conducted. At present, enrollment in TMVR trials for patients with degenerative or functional mitral regurgitation is limited to those without a surgical option and who conform to very specific anatomic criteria.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63:185–186.
- DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010; 139:76–83; discussion 83–74.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28:1358–1365.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Sud K, Agarwal S, Parashar A, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016; 133:1594–1604.
- Svensson LG, Ye J, Piemonte TC, Kirker-Head C, Leon MB, Webb JG. Mitral valve regurgitation and left ventricular dysfunction treatment with an intravalvular spacer. J Card Surg 2015; 30:53–54.
- Raman J, Raghavan J, Chandrashekar P, Sugeng L. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011; 20:157–162.
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015; 66:1011–1019.
- Bapat V. FORTIS: design, clinical results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; Feburary 20–23, 2016; Washington, DC.
- Sorajja P. Tendyne: technology and clinical results update. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Navia J. Personal communication.
- Bapat V. Medtronic Intrepid transcatheter mitral valve replacement. Presented at EuroPCR 2015; May 19–22, 2015; Paris, France.
- Herrmann H. Cardiaq-Edwards TMVR. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Dvir D. Tiara: design, clincal results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitra valve disease with severe mitral annular calcification: results from the first global registry. JACC Cardiovasc Interv 2016; 9:1361–1371.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010; 76:608–615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010; 121:1848–1857.
- Cerillo AG, Chiaramonti F, Murzi M, et al. Transcatheter valve in valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv 2011; 78:987–995.
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012; 5:341–349.
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014; 147:210–219.
- Dvir D, on behalf of the VIVID Registry Investigators. Transcatheter mitral valve-in-valve and valve-in-ring implantations. Transcatheter Valve Therapies 2015.
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016; 9:1161–1174.
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
The NaviGate valve
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.
The Intrepid valve
The Intrepid valve consists of an outer stent to provide fixation to the annulus and an inner stent that houses a bovine pericardial valve. The device is a self-expanding system that is delivered transapically.
In a series of 15 patients, 11 had functional mitral regurgitation (with an average left ventricular ejection fraction of 35%) and four had degenerative mitral regurgitation (with an average left ventricular ejection fraction of 57%).15 The device was successfully implanted in 14 patients, after which the average mitral valve gradient was 4 mm Hg. All patients but one were left with no regurgitation (the other patient had 1+ regurgitation).
A trial is currently under way in Europe.
The CardiAQ valve
The CardiAQ is constructed of bovine pericardium and can be delivered by the transseptal or transapical route.
Of 12 patients treated under compassionate use,16 two-thirds (eight patients) had functional mitral regurgitation. Two patients died during the procedure, three died of noncardiac complications within 30 days, and one more died of sepsis shortly after 30 days. This early experience demonstrates the importance of careful patient selection and postprocedural management in the feasibility assessment of these new technologies.
Patients are being enrolled in phase 1 trials.
The Tiara valve
The Tiara valve, a self-expanding prosthesis with bovine pericardial leaflets, is delivered by the transapical route.
Eleven patients underwent Tiara implantation as part of either a Canadian special access registry or an international feasibility trial. Their average Society of Thoracic Surgeons score (ie, their calculated risk of major morbidity or operative mortality) was 15.6%, and their average left ventricular ejection fraction was 29%. Only two patients had degenerative mitral regurgitation. Nine patients had uneventful procedures and demonstrated no residual mitral regurgitation and no left ventricular outflow tract obstruction. The procedure was converted to open surgery in two patients owing to valve malpositioning, and both of them died within 30 days. One patient in whom the procedure was successful suffered erosion of the septum and died on day 4.17
Patients are being enrolled in phase 1 trials.
DEGENERATIVE MITRAL STENOSIS
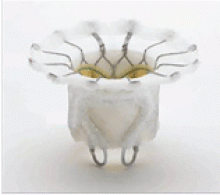
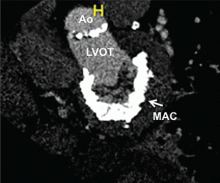
In patients with degenerative mitral stenosis, extensive mitral annular calcification may provide an adequate “frame” to hold a transcatheter valve prosthesis (Figure 3). Exploiting this feature, numerous investigators have successfully deployed prosthetic valves designed for TAVR in the calcified mitral annulus via the retrograde transapical and antegrade transseptal routes.
Guerrero and colleagues presented results from the first global registry of TMVR in mitral annular calcification at the 2016 EuroPCR Congress.18 Of 104 patients analyzed, almost all received an Edwards’ Sapien balloon-expandable valve (first-generation, Sapien XT, or Sapien 3); the others received Boston Scientific’s Lotus or Direct Flow Medical (Direct Flow Medical, Santa Clara, CA) valves. With an average age of 73 years and a high prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease, and prior cardiac surgery, the group presented extreme surgical risk, with an average Society of Thoracic Surgeons risk score of 14.4%. Slightly more than 40% of the patients underwent transapical implantation, slightly less than 40% underwent transfemoral or transseptal implantation, and just under 20% had a direct atrial approach.
The implantation was technically successful in 78 of 104 patients (75%); 13 patients (12.5%) required a second mitral valve to be placed, 11 patients (10.5%) had left ventricular outflow tract obstruction, four patients (4%) had valve embolization, and two patients (2%) had left ventricular perforation. At 30 days, 11 of 104 patients (10.6%) had died of cardiac causes and 15 patients (14.4%) had died of noncardiac causes. When divided roughly into three equal groups by chronological order, the last third of patients, compared with the first third of patients, enjoyed greater technical success (80%, n = 32/40 vs 62.5%, n = 20/32), better 30-day survival (85%, n = 34/40 vs 62.5%, n = 20/32), and no conversion to open surgery (0 vs 12.5%, n = 4/32), likely demonstrating both improved patient selection and lessons learned from shared experience. At 1 year, almost 90% of patients had New York Heart Association class I or II symptoms. Prior to the procedure, 91.5% had New York Heart Association class III or IV symptoms.
At present, TMVR in mitral annular calcification is not approved in the United States or elsewhere. However, multiple registries are currently enrolling patients or are in formative stages to push the frontier of the currently available technologies until better, dedicated devices are available for this group of patients.
BIOPROSTHETIC VALVE OR VALVE RING FAILURE
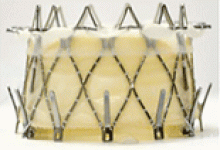
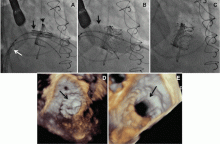
Implantation of a TAVR prosthetic inside a degenerated bioprosthetic mitral valve (valve-in-valve) and mitral valve ring (valve-in-ring) is generally limited to case series with short-term results using the Edwards Sapien series, Boston Scientific Lotus, Medtronic Melody (Medtronic, Minneapolis, MN), and Direct Flow Medical valves (Figure 4).19–23
The largest collective experience was presented in the Valve-in-Valve International Data (VIVID) registry, which included 349 patients who had mitral valve-in-valve placement and 88 patients who had mitral valve-in-ring procedures. Their average age was 74 and the mean Society of Thoracic Surgeons score was 12.9% in both groups.24 Of the 437 patients, 345 patients (78.9%) underwent transapical implantation, and 391 patients (89.5%) received a Sapien XT or Sapien 3 valve. In the valve-in-valve group, 41% of the patients had regurgitation, 25% had stenosis, and 34% had both. In the valve-in-ring group, 60% of the patients had regurgitation, 17% had stenosis, and 23% had both.
Valve placement was successful in most patients. The rate of stroke was low (2.9% with valve-in-valve placement, 1.1% with valve-in-ring placement), though the rate of moderate or greater residual mitral regurgitation was significantly higher in patients undergoing valve-in-ring procedures (14.8% vs 2.6%, P < .001), as was the rate of left ventricular outflow tract obstruction (8% vs 2.6%, P = .03). There was also a trend toward worse 30-day mortality in the valve-in-ring group (11.4% vs 7.7%, P = .15). As with aortic valve-in-valve procedures, small surgical mitral valves (≤ 25 mm) were associated with higher postprocedural gradients.
Eleid and colleagues25 published their experience with antegrade transseptal TMVR in 48 patients with an average Society of Thoracic Surgeons score of 13.2%, 33 of whom underwent valve-in-valve procedures and nine of whom underwent valve-in-ring procedures. (The other six patients underwent mitral valve implantation for severe mitral annular calcification.) In the valve-in-valve group, 31 patients successfully underwent implant procedures, but two patients died during the procedure from left ventricular perforation. Of the nine valve-in-ring patients, two had acute embolization of the valve and were converted to open surgery. Among the seven patients in whom implantation was successful, two developed significant left ventricular outflow tract obstruction; one was treated with surgical resection of the anterior mitral valve leaflet and the other was medically managed.
CONCLUSION
Transcatheter mitral valve replacement in regurgitant mitral valves, failing mitral valve bioprosthetics and rings, and calcified mitral annuli has been effectively conducted in a number of patients who had no surgical options due to prohibitive surgical risk. International registries and our experience have demonstrated that the valve-in-valve procedure using a TAVR prosthesis carries the greatest likelihood of success, given the rigid frame of the surgical bioprosthetic that allows stable valve deployment. While approved in Europe for this indication, use of these devices for this application in the United States is considered “off label” and is performed only in clinically extenuating circumstances. Implantation of TAVR prosthetics in patients with prior mitral ring repair or for native mitral stenosis also has been performed successfully, although left ventricular outflow tract obstruction is a significant risk in this early experience.
Devices designed specifically for TMVR are in their clinical infancy and have been implanted successfully in only small numbers of patients, most of whom had functional mitral regurgitation. Despite reasonable technical success, most of these trials have been plagued by high mortality rates at 30 days in large part due to the extreme risk of the patients in whom these procedures have been conducted. At present, enrollment in TMVR trials for patients with degenerative or functional mitral regurgitation is limited to those without a surgical option and who conform to very specific anatomic criteria.
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
The NaviGate valve
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.
The Intrepid valve
The Intrepid valve consists of an outer stent to provide fixation to the annulus and an inner stent that houses a bovine pericardial valve. The device is a self-expanding system that is delivered transapically.
In a series of 15 patients, 11 had functional mitral regurgitation (with an average left ventricular ejection fraction of 35%) and four had degenerative mitral regurgitation (with an average left ventricular ejection fraction of 57%).15 The device was successfully implanted in 14 patients, after which the average mitral valve gradient was 4 mm Hg. All patients but one were left with no regurgitation (the other patient had 1+ regurgitation).
A trial is currently under way in Europe.
The CardiAQ valve
The CardiAQ is constructed of bovine pericardium and can be delivered by the transseptal or transapical route.
Of 12 patients treated under compassionate use,16 two-thirds (eight patients) had functional mitral regurgitation. Two patients died during the procedure, three died of noncardiac complications within 30 days, and one more died of sepsis shortly after 30 days. This early experience demonstrates the importance of careful patient selection and postprocedural management in the feasibility assessment of these new technologies.
Patients are being enrolled in phase 1 trials.
The Tiara valve
The Tiara valve, a self-expanding prosthesis with bovine pericardial leaflets, is delivered by the transapical route.
Eleven patients underwent Tiara implantation as part of either a Canadian special access registry or an international feasibility trial. Their average Society of Thoracic Surgeons score (ie, their calculated risk of major morbidity or operative mortality) was 15.6%, and their average left ventricular ejection fraction was 29%. Only two patients had degenerative mitral regurgitation. Nine patients had uneventful procedures and demonstrated no residual mitral regurgitation and no left ventricular outflow tract obstruction. The procedure was converted to open surgery in two patients owing to valve malpositioning, and both of them died within 30 days. One patient in whom the procedure was successful suffered erosion of the septum and died on day 4.17
Patients are being enrolled in phase 1 trials.
DEGENERATIVE MITRAL STENOSIS
In patients with degenerative mitral stenosis, extensive mitral annular calcification may provide an adequate “frame” to hold a transcatheter valve prosthesis (Figure 3). Exploiting this feature, numerous investigators have successfully deployed prosthetic valves designed for TAVR in the calcified mitral annulus via the retrograde transapical and antegrade transseptal routes.
Guerrero and colleagues presented results from the first global registry of TMVR in mitral annular calcification at the 2016 EuroPCR Congress.18 Of 104 patients analyzed, almost all received an Edwards’ Sapien balloon-expandable valve (first-generation, Sapien XT, or Sapien 3); the others received Boston Scientific’s Lotus or Direct Flow Medical (Direct Flow Medical, Santa Clara, CA) valves. With an average age of 73 years and a high prevalence of comorbidities such as diabetes, chronic obstructive pulmonary disease, atrial fibrillation, chronic kidney disease, and prior cardiac surgery, the group presented extreme surgical risk, with an average Society of Thoracic Surgeons risk score of 14.4%. Slightly more than 40% of the patients underwent transapical implantation, slightly less than 40% underwent transfemoral or transseptal implantation, and just under 20% had a direct atrial approach.
The implantation was technically successful in 78 of 104 patients (75%); 13 patients (12.5%) required a second mitral valve to be placed, 11 patients (10.5%) had left ventricular outflow tract obstruction, four patients (4%) had valve embolization, and two patients (2%) had left ventricular perforation. At 30 days, 11 of 104 patients (10.6%) had died of cardiac causes and 15 patients (14.4%) had died of noncardiac causes. When divided roughly into three equal groups by chronological order, the last third of patients, compared with the first third of patients, enjoyed greater technical success (80%, n = 32/40 vs 62.5%, n = 20/32), better 30-day survival (85%, n = 34/40 vs 62.5%, n = 20/32), and no conversion to open surgery (0 vs 12.5%, n = 4/32), likely demonstrating both improved patient selection and lessons learned from shared experience. At 1 year, almost 90% of patients had New York Heart Association class I or II symptoms. Prior to the procedure, 91.5% had New York Heart Association class III or IV symptoms.
At present, TMVR in mitral annular calcification is not approved in the United States or elsewhere. However, multiple registries are currently enrolling patients or are in formative stages to push the frontier of the currently available technologies until better, dedicated devices are available for this group of patients.
BIOPROSTHETIC VALVE OR VALVE RING FAILURE
Implantation of a TAVR prosthetic inside a degenerated bioprosthetic mitral valve (valve-in-valve) and mitral valve ring (valve-in-ring) is generally limited to case series with short-term results using the Edwards Sapien series, Boston Scientific Lotus, Medtronic Melody (Medtronic, Minneapolis, MN), and Direct Flow Medical valves (Figure 4).19–23
The largest collective experience was presented in the Valve-in-Valve International Data (VIVID) registry, which included 349 patients who had mitral valve-in-valve placement and 88 patients who had mitral valve-in-ring procedures. Their average age was 74 and the mean Society of Thoracic Surgeons score was 12.9% in both groups.24 Of the 437 patients, 345 patients (78.9%) underwent transapical implantation, and 391 patients (89.5%) received a Sapien XT or Sapien 3 valve. In the valve-in-valve group, 41% of the patients had regurgitation, 25% had stenosis, and 34% had both. In the valve-in-ring group, 60% of the patients had regurgitation, 17% had stenosis, and 23% had both.
Valve placement was successful in most patients. The rate of stroke was low (2.9% with valve-in-valve placement, 1.1% with valve-in-ring placement), though the rate of moderate or greater residual mitral regurgitation was significantly higher in patients undergoing valve-in-ring procedures (14.8% vs 2.6%, P < .001), as was the rate of left ventricular outflow tract obstruction (8% vs 2.6%, P = .03). There was also a trend toward worse 30-day mortality in the valve-in-ring group (11.4% vs 7.7%, P = .15). As with aortic valve-in-valve procedures, small surgical mitral valves (≤ 25 mm) were associated with higher postprocedural gradients.
Eleid and colleagues25 published their experience with antegrade transseptal TMVR in 48 patients with an average Society of Thoracic Surgeons score of 13.2%, 33 of whom underwent valve-in-valve procedures and nine of whom underwent valve-in-ring procedures. (The other six patients underwent mitral valve implantation for severe mitral annular calcification.) In the valve-in-valve group, 31 patients successfully underwent implant procedures, but two patients died during the procedure from left ventricular perforation. Of the nine valve-in-ring patients, two had acute embolization of the valve and were converted to open surgery. Among the seven patients in whom implantation was successful, two developed significant left ventricular outflow tract obstruction; one was treated with surgical resection of the anterior mitral valve leaflet and the other was medically managed.
CONCLUSION
Transcatheter mitral valve replacement in regurgitant mitral valves, failing mitral valve bioprosthetics and rings, and calcified mitral annuli has been effectively conducted in a number of patients who had no surgical options due to prohibitive surgical risk. International registries and our experience have demonstrated that the valve-in-valve procedure using a TAVR prosthesis carries the greatest likelihood of success, given the rigid frame of the surgical bioprosthetic that allows stable valve deployment. While approved in Europe for this indication, use of these devices for this application in the United States is considered “off label” and is performed only in clinically extenuating circumstances. Implantation of TAVR prosthetics in patients with prior mitral ring repair or for native mitral stenosis also has been performed successfully, although left ventricular outflow tract obstruction is a significant risk in this early experience.
Devices designed specifically for TMVR are in their clinical infancy and have been implanted successfully in only small numbers of patients, most of whom had functional mitral regurgitation. Despite reasonable technical success, most of these trials have been plagued by high mortality rates at 30 days in large part due to the extreme risk of the patients in whom these procedures have been conducted. At present, enrollment in TMVR trials for patients with degenerative or functional mitral regurgitation is limited to those without a surgical option and who conform to very specific anatomic criteria.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63:185–186.
- DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010; 139:76–83; discussion 83–74.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28:1358–1365.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Sud K, Agarwal S, Parashar A, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016; 133:1594–1604.
- Svensson LG, Ye J, Piemonte TC, Kirker-Head C, Leon MB, Webb JG. Mitral valve regurgitation and left ventricular dysfunction treatment with an intravalvular spacer. J Card Surg 2015; 30:53–54.
- Raman J, Raghavan J, Chandrashekar P, Sugeng L. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011; 20:157–162.
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015; 66:1011–1019.
- Bapat V. FORTIS: design, clinical results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; Feburary 20–23, 2016; Washington, DC.
- Sorajja P. Tendyne: technology and clinical results update. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Navia J. Personal communication.
- Bapat V. Medtronic Intrepid transcatheter mitral valve replacement. Presented at EuroPCR 2015; May 19–22, 2015; Paris, France.
- Herrmann H. Cardiaq-Edwards TMVR. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Dvir D. Tiara: design, clincal results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitra valve disease with severe mitral annular calcification: results from the first global registry. JACC Cardiovasc Interv 2016; 9:1361–1371.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010; 76:608–615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010; 121:1848–1857.
- Cerillo AG, Chiaramonti F, Murzi M, et al. Transcatheter valve in valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv 2011; 78:987–995.
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012; 5:341–349.
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014; 147:210–219.
- Dvir D, on behalf of the VIVID Registry Investigators. Transcatheter mitral valve-in-valve and valve-in-ring implantations. Transcatheter Valve Therapies 2015.
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016; 9:1161–1174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Goel SS, Bajaj N, Aggarwal B, et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63:185–186.
- DiBardino DJ, ElBardissi AW, McClure RS, Razo-Vasquez OA, Kelly NE, Cohn LH. Four decades of experience with mitral valve repair: analysis of differential indications, technical evolution, and long-term outcome. J Thorac Cardiovasc Surg 2010; 139:76–83; discussion 83–74.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007; 28:1358–1365.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Sud K, Agarwal S, Parashar A, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016; 133:1594–1604.
- Svensson LG, Ye J, Piemonte TC, Kirker-Head C, Leon MB, Webb JG. Mitral valve regurgitation and left ventricular dysfunction treatment with an intravalvular spacer. J Card Surg 2015; 30:53–54.
- Raman J, Raghavan J, Chandrashekar P, Sugeng L. Can we repair the mitral valve from outside the heart? A novel extra-cardiac approach to functional mitral regurgitation. Heart Lung Circ 2011; 20:157–162.
- Abdul-Jawad Altisent O, Dumont E, Dagenais F, et al. Initial experience of transcatheter mitral valve replacement with a novel transcatheter mitral valve: procedural and 6-month follow-up results. J Am Coll Cardiol 2015; 66:1011–1019.
- Bapat V. FORTIS: design, clinical results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; Feburary 20–23, 2016; Washington, DC.
- Sorajja P. Tendyne: technology and clinical results update. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Navia J. Personal communication.
- Bapat V. Medtronic Intrepid transcatheter mitral valve replacement. Presented at EuroPCR 2015; May 19–22, 2015; Paris, France.
- Herrmann H. Cardiaq-Edwards TMVR. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Dvir D. Tiara: design, clincal results, and next steps. Presented at CRT (Cardiovascular Research Technologies) 16; February 20–23, 2016; Washington, DC.
- Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitra valve disease with severe mitral annular calcification: results from the first global registry. JACC Cardiovasc Interv 2016; 9:1361–1371.
- Seiffert M, Franzen O, Conradi L, et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv 2010; 76:608–615.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010; 121:1848–1857.
- Cerillo AG, Chiaramonti F, Murzi M, et al. Transcatheter valve in valve implantation for failed mitral and tricuspid bioprosthesis. Catheter Cardiovasc Interv 2011; 78:987–995.
- Seiffert M, Conradi L, Baldus S, et al. Transcatheter mitral valve-in-valve implantation in patients with degenerated bioprostheses. JACC Cardiovasc Interv 2012; 5:341–349.
- Wilbring M, Alexiou K, Tugtekin SM, et al. Pushing the limits—further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg 2014; 147:210–219.
- Dvir D, on behalf of the VIVID Registry Investigators. Transcatheter mitral valve-in-valve and valve-in-ring implantations. Transcatheter Valve Therapies 2015.
- Eleid MF, Cabalka AK, Williams MR, et al. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. JACC Cardiovasc Interv 2016; 9:1161–1174.
KEY POINTS
- Most TMVR procedures are performed by either a retrograde transapical approach or an antegrade transseptal approach.
- In the small number of patients who have undergone TMVR for native mitral valve regurgitation to date, mortality rates at 30 days have been high, reflecting the seriousness of illness in these patients.
- At present, none of the new devices for TMVR in patients with native mitral valve regurgitation are approved for general use, although some of them are being tested in phase 1 clinical trials that are enrolling patients.
- Valves made for TAVR have been used for TMVR in patients with degenerative mitral stenosis or failure of mitral bioprostheses; however, these are off-label uses of these devices.
Transcatheter aortic valve replacement: History and current indications
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
Transcatheter aortic valve replacement (TAVR) has established itself as an effective way of treating high-risk patients with severe aortic valve stenosis. With new generations of existing valves and newer alternative devices, the procedure promises to become increasingly safer. The field is evolving rapidly and it will be important for interventional cardiologists and cardiac surgeons alike to stay abreast of developments. This article reviews the history of this promising procedure and examines its use in current practice.
HISTORICAL PERSPECTIVE
In 1980, Danish researcher H. R. Anderson reported developing and testing a balloon-expandable valve in animals.1 The technology was eventually acquired and further developed by Edwards Life Sciences (Irvine, California).
Alain Cribier started early work in humans in 2002 in France.2 He used a transfemoral arterial access to approach the aortic valve transseptally, but this procedure was associated with high rates of mortality and stroke.3 At the same time, in the United States, animal studies were being carried out by Lars G. Svensson, Todd Dewey, and Michael Mack to develop a transapical method of implantation,4,5 while John Webb and colleagues were also developing a transapical aortic valve implantation technique,6,7 and later went on to develop a retrograde transfemoral technique. This latter technique became feasible once Edwards developed a catheter that could be flexed to get around the aortic arch and across the aortic valve.
As the Edwards balloon-expandable valve (Sapien) was being developed, a nitinol-based self-expandable valve system was introduced by Medtronic: the CoreValve. Following feasibility studies,5,8 the safety and efficacy of these valves were established thorough the Placement of Aortic Transcatheter Valves (PARTNER) trial and the US Core Valve Pivotal Trial. These valves are currently approved by the US Food and Drug Administration (FDA) for patients for whom conventional surgery would pose an extreme or high risk.9–11
CLINICAL TRIALS OF TAVR
The two landmark prospective randomized trials of TAVR were the PARTNER trial and CoreValve Pivotal Trial.
The PARTNER trial consisted of two parts: PARTNER A, which compared the Sapien balloon-expandable transcatheter valve with surgical aortic valve replacement in patients at high surgical risk (Society of Thoracic Surgeons [STS] score > 10%), and PARTNER B, which compared TAVR with medical therapy in patients who could not undergo surgery (combined risk of serious morbidity or death of 50% or more, and two surgeons agreeing that the patient was inoperable).
Similarly, the CoreValve Pivotal Trial compared the self-expandable transcatheter valve with conventional medical and surgical treatment.
TAVR is comparable to surgery in outcomes, with caveats
In the PARTNER A trial, mortality rates were similar between patients who underwent Sapien TAVR and those who underwent surgical valve replacement at 30 days (3.4% and 6.5%, P = .07), 1 year (24.2% and 26.8%), and 2 years (33.9% and 35.0%). The patients in this group were randomized to either Sapien TAVR or surgery (Table 1).10,12
The combined rate of stroke and transient ischemic attack was higher in the patients assigned to TAVR at 30 days (5.5% with TAVR vs 2.4% with surgery, P = .04) and at 1 year (8.3% with TAVR vs 4.3% with surgery, P = .04). The difference was of small significance at 2 years (11.2% vs 6.5%, P = .05). At 30 days, the rate of major vascular complications was higher with TAVR (11.0% vs 3.2%), while surgery was associated with more frequent major bleeding episodes (19.5% vs 9.3%) and new-onset atrial fibrillation (16.0% vs 8.6%). The rate of new pacemaker requirement at 30 days was similar between the TAVR and surgical groups (3.8% vs 3.6%). Moderate or severe paravalvular aortic regurgitation was more common after TAVR at 30 days, 1 year, and 2 years. This aortic insufficiency was associated with increased late mortality.10,12
In the US CoreValve High Risk Study, no difference was found in the 30-day mortality rate in patients at high surgical risk randomized to CoreValve TAVR or surgery (3.3% and 4.5%) (Table 1). Surprisingly, the 1-year mortality rate was lower in the TAVR group than in the surgical group (14.1% vs 18.9%, respectively), a finding sustained at 2 years in data presented at the American College of Cardiology conference in March 2015.13–16
TAVR is superior to medical management, but the risk of stroke is higher
In the PARTNER B trial, inoperable patients were randomly assigned to undergo TAVR with a Sapien valve or medical management. TAVR resulted in lower mortality rates at 1 year (30.7% vs 50.7%) and 2 years (43.4% vs 68.0%) compared with medical management (Table 1).17 Of note, medical management included balloon valvuloplasty. The rate of the composite end point of death or repeat hospitalization was also lower with TAVR compared with medical therapy (44.1% vs 71.6%, respectively, at 1 year and 56.7% and 87.9%, respectively, at 2 years).17 The TAVR group had a higher stroke rate than the medical therapy group at 30 days (11.2% vs 5.5%, respectively) and at 2 years (13.8% vs 5.5%).17 Survival improved with TAVR in patients with an STS score of less than 15% but not in those with an STS score of 15% or higher.9
The very favorable results from the PARTNER trial rendered a randomized trial comparing self-expanding (CoreValve) TAVR and medical therapy unethical. Instead, a prospective single-arm study, the CoreValve Extreme Risk US Pivotal Trial, was used to compare the 12-month rate of death or major stroke with CoreValve TAVR vs a prespecified estimate of this rate with medical therapy.14 In about 500 patients who had a CoreValve attempt, the rate of all-cause mortality or major stroke at 1 year was significantly lower than the prespecified expected rate (26% vs 43%), reinforcing the results from the PARTNER Trial.14
Five-year outcomes
The 5-year PARTNER clinical and valve performance outcomes were published recently18 and continued to demonstrate equivalent outcomes for high-risk patients who underwent surgical aortic valve replacement or TAVR; there were no significant differences in all-cause mortality, cardiovascular mortality, stroke, or need for readmission to the hospital. The functional outcomes were similar as well, and no differences were demonstrated between surgical and TAVR valve performance.
Of note, moderate or severe aortic regurgitation occurred in 14% of patients in the TAVR group compared with 1% in the surgical aortic valve replacement group (P < .0001). This was associated with increased 5-year risk of death in the TAVR group (72.4% in those with moderate or severe aortic regurgitation vs 56.6% in those with mild aortic regurgitation or less; P = .003).
If the available randomized data are combined with observational reports, overall mortality and stroke rates are comparable between surgical aortic valve replacement and balloon-expandable or self-expandable TAVR in high-risk surgical candidates. Vascular complications, aortic regurgitation and permanent pacemaker insertion occur more frequently after TAVR, while major bleeding is more likely to occur after surgery.19 As newer generations of valves are developed, it is expected that aortic regurgitation and pacemaker rates will decrease over time. Indeed, trial data presented at the American College of Cardiology meeting in March 2015 for the third-generation Sapien valve (Sapien S3) showed only a 3.0% to 4.2% rate of significant paravalvular leak.
Contemporary valve comparison data
The valve used in the original PARTNER data was the first-generation Sapien valve. Since then, the second generation of this valve, the Sapien XT, has been introduced and is the model currently used in the United States (with the third-generation valve mentioned above, the Sapien S3, still available only through clinical trials). Thus, the two contemporary valves available for commercial use in the United States are the Edwards Sapien XT and Medtronic CoreValve. There are limited data comparing these valves head-to-head, but one recent trial attempted to do just that.
The Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve vs Edwards Sapien XT (CHOICE) trial compared the Edwards Sapien XT and CoreValve devices. Two hundred and forty-one patients were randomized. The primary end point of this trial was “device success” (a composite end point of four components: successful vascular access and deployment of the device with retrieval of the delivery system, correct position of the device, intended performance of the valve without moderate or severe insufficiency, and only one valve implanted in the correct anatomical location).
In this trial, the balloon-expandable Sapien XT valve showed a significantly higher device success rate than the self-expanding CoreValve, due to a significantly lower rate of aortic regurgitation (4.1% vs 18.3%, P < .001) and the less frequent need for implantation of more than one valve (0.8% vs 5.8%, P = .03). Placement of a permanent pacemaker was considerably less frequent in the balloon-expandable valve group (17.3% vs 37.6%, P = .001).20
PREOPERATIVE CONSIDERATIONS AND EVALUATION CRITERIA
Currently, TAVR is indicated for patients with symptomatic severe native aortic valve stenosis who are deemed at high risk or inoperable by a heart team including interventional cardiologists and cardiac surgeons. The CoreValve was also recently approved for valve-in-valve insertion in high-risk or inoperable patients with a prosthetic aortic valve in place.
The STS risk score is a reasonable preliminary risk assessment tool and is applicable to most patients being evaluated for aortic valve replacement. The STS risk score represents the percentage risk of unfavorable outcomes based on certain clinical variables. A calculator is available at riskcalc.sts.org. Patients considered at high risk are those with an STS operative risk score of 8% or higher or a postoperative 30-day risk of death of 15% or higher.
It is important to remember, though, that the STS score does not account for certain severe surgical risk factors. These include the presence of a "porcelain aorta" (heavy circumferential calcification of the ascending aorta precluding cross-clamping), history of mediastinal radiation, “hostile chest” (kyphoscoliosis, other deformities, previous coronary artery bypass grafting with adhesion of internal mammary artery to the back of sternum), severely compromised respiratory function (forced expiratory volume in 1 second < 1 L or < 40% predicted, diffusing capacity for carbon monoxide < 30%), severe pulmonary hypertension, severe liver disease (Model for End-stage Liver Disease score 8–20), severe dementia, severe cerebrovascular disease, and frailty.
With regard to this last risk factor, frailty is not simply old age but rather a measurable characteristic akin to weakness or disability. Several tests exist to measure frailty, including the “eyeball test” (the physician’s subjective assessment), Mini-Mental State Examination, gait speed/15-foot walk test, hand grip strength, serum albumin, and assessment of activities of daily living. Formal frailty testing is recommended during the course of a TAVR workup.
Risk assessment and patient suitability for TAVR is ultimately determined by the combined judgment of the heart valve team using both the STS score and consideration of these other factors.
Implantation approaches
Today, TAVR could be performed by several approaches: transfemoral arterial, transapical, transaortic via partial sternotomy or right anterior thoracotomy,21,22 transcarotid,23–25 and transaxillary or subclavian.26,27 Less commonly, transfemoral-venous routes have been performed utilizing either transseptal28 or caval-aortic puncture.29
The transfemoral approach is used most commonly by most institutions, including Cleveland Clinic. It allows for a completely percutaneous insertion and, in select cases, without endotracheal intubation and general anesthesia (Figure 1).
In patients with difficult femoral access due to severe calcification, extreme tortuosity, or small diameter, alternative access routes become a consideration. In this situation, at our institution, we favor the transaortic approach in patients who have not undergone cardiac surgery in the past, while the transapical approach is used in patients who had previous cardiac surgery. With the transapical approach, we have found the outcomes similar to those of transfemoral TAVR after propensity matching.30,31 Although there is a learning curve,32 transapical TAVR can be performed with very limited mortality and morbidity. In a recent series at Cleveland Clinic, the mortality rate with the transapical approach was 1.2%, renal failure occurred in 4.7%, and a pacemaker was placed in 5.9% of patients; there were no strokes.33 This approach can be utilized for simultaneous additional procedures like transcatheter mitral valve reimplantation and percutaneous coronary interventions.34–36
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704– 708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case descrip- tion. Circulation 2002; 106:3006–3008.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698– 703.
- Dewey TM, Walther T, Doss M, et al. Transapical aortic valve implantation: an animal feasibility study. Ann Thorac Surg 2006; 82:110–116.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of trans- catheter insertion of a stented aortic valve by the left ventricular apex. Ann Thorac Surg 2008; 86:46–54.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve im- plantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Webb JG, Pasupati S, Hyumphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Leon MB, Kodali S, Williams M, et al. Transcatheter aortic valve replacement in patients with critical aortic stenosis: rationale, device descriptions, early clinical experiences, and perspectives. Semin Thorac Cardiovasc Surg 2006; 18:165–174.
- Leon MB, Smith CR, Mack M, et al; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo sur- gery. N Engl J Med 2010; 363:1597–1607.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Adams DH, Popma JJ, Reardon MJ, et al; U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790–1798.
- Kodali SK, Williams MR, Smith CR, et al; PARTNER Trial Investigators. Two- year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012; 366:1686–1695.
- Reardon M, et al. A randomized comparison of self-expanding
- Popma JJ, Adams DH, Reardon MJ, et al; CoreValve United States Clinical In- vestigators. Transcatheter aortic valve replacement using a self-expanding biopros- thesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol 2014; 63:1972–1981.
- Adams DH, Popma JJ, Reardon MJ. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967–968.
- Kaul S. Transcatheter aortic-valve replacement with a self-expanding prosthesis (letter). N Engl J Med 2014; 371:967.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcathether aortic-valve re- placement for inoperable severe aortic stenosis. N Engl J Med 2012; 366: 1696–704.
- Mack MJ, Leon MB, Smith CR, et al; PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve re- placement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of trans- catheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013; 2:10–23.
- Abdel-Wahab M, Mehilli J, Frerker C, et al; CHOICE investigators. Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcath- eter aortic valve replacement: the CHOICE randomized clinical trial. JAMA 2014; 311:1503–1514.
- Okuyama K, Jilaihawi H, Mirocha J, et al. Alternative access for balloon-ex- pandable transcatheter aortic valve replacement: comparison of the transaortic approach using right anterior thoracotomy to partial J-sternotomy. J Thorac Car- diovasc Surg 2014; 149:789–797.
- Lardizabal JA, O’Neill BP, Desai HV, et al. The transaortic approach for transcath- eter aortic valve replacement: initial clinical experience in the United States. J Am Coll Cardiol 2013; 61:2341–2345.
- Thourani VH, Gunter RL, Neravetla S, et al. Use of transaortic, transapical, and transcarotid transcatheter aortic valve replacement in inoperable patients. Ann Thorac Surg 2013; 96:1349–1357.
- Azmoun A, Amabile N, Ramadan R, et al. Transcatheter aortic valve implantation through carotid artery access under local anaesthesia. Eur J Cardiothorac Surg 2014; 46: 693–698.
- Rajagopal R, More RS, Roberts DH. Transcatheter aortic valve implantation through a transcarotid approach under local anesthesia. Catheter Cardiovasc In- terv 2014; 84:903–907.
- Fraccaro C, Napodano M, Tarantini G, et al. Expanding the eligibility for trans- catheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system. JACC Cardiovasc Interv 2009; 2:828–333.
- Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv 2010; 3:359–366.
- Cohen MG, Singh V, Martinez CA, et al. Transseptal antegrade transcatheter aor- tic valve replacement for patients with no other access approach—a contemporary experience. Catheter Cardiovasc Interv 2013; 82:987–993.
- Greenbaum AB, O’Neill WW, Paone G, et al. Caval-aortic access to allow trans- catheter aortic valve replacement in otherwise ineligible patients: initial human experience. J Am Coll Cardiol 2014; 63:2795–2804.
- D’Onofrio A, Salizzoni S, Agrifoglio M, et al. Medium term outcomes of trans- apical aortic valve implantation: results from the Italian Registry of Trans-Apical Aortic Valve Implantation. Ann Thorac Surg 2013; 96:830–835.
- Johansson M, Nozohoor S, Kimblad PO, Harnek J, Olivecrona GK, Sjögren J. Transapical versus transfemoral aortic valve implantation: a comparison of survival and safety. Ann Thorac Surg 2011; 91:57–63.
- Kempfert J, Rastan A, Holzhey D, et al. Transapical aortic valve implantation: analysis of risk factors and learning experience in 299 patients. Circulation 2011; 124(suppl):S124–S129.
- Aguirre J, Waskowski R, Poddar K, et al. Transcatheter aortic valve replacement: experience with the transapical approach, alternate access sites, and concomitant cardiac repairs. J Thorac Cardiovasc Surg 2014; 148:1417–1422.
- Al Kindi AH, Salhab KF, Roselli EE, Kapadia S, Tuzcu EM, Svensson LG. Alternative access options for transcatheter aortic valve replacement in patients with no conventional access and chest pathology. J Thorac Cardiovasc Surg 2014; 147:644–651.
- Salhab KF, Al Kindi AH, Lane JH, et al. Concomitant percutaneous coronary intervention and transcatheter aortic valve replacement: safe and feasible replace- ment alternative approaches in high-risk patients with severe aortic stenosis and coronary artery disease. J Card Surg 2013; 28:481–483.
- Al Kindi AH, Salhab KF, Kapadia S, et al. Simultaneous transapical transcatheter aortic and mitral valve replacement in a high-risk patient with a previous mitral bioprosthesis. J Thorac Cardiovasc Surg 2012; 144:e90–e91.
KEY POINTS
- In randomized trials, transcatheter aortic valve replacement (TAVR) has produced results that are comparable to surgical aortic valve replacement in high-risk patients. TAVR is superior to medical management in patients who cannot undergo surgery, although it is associated with higher rates of stroke.
- Risk assessment and suitability for TAVR is determined by a heart team composed of interventional cardiologists and cardiac surgeons. Society of Thoracic Surgeons Score and a number of other criteria mentioned below are considered during this process.
- The transfemoral arterial approach is the most common approach used by most institutions, but other approaches such as transaortic, transapical, transaxillary, and transcarotid are utilized if suitable in patients who have difficult femoral access.
Challenges in the management of aortic stenosis
The classic case of aortic stenosis is in an otherwise healthy middle-aged patient with symptomatic severe disease who is referred to a cardiac surgeon for surgical aortic valve replacement. Unfortunately, physicians who manage valvular heart disease do not encounter this straightforward scenario on a regular basis. Rather, patients come with comorbidities such as advanced age, pulmonary disease, renal dysfunction, coronary artery disease, and significant left ventricular dysfunction. They also come with severe aortic stenosis without symptoms.
In this issue of the Cleveland Clinic Journal of Medicine, Sawaya and colleagues1 review the management of aortic stenosis, focusing on clinically challenging scenarios such as low-flow, low-gradient aortic stenosis, low-gradient severe aortic stenosis with a normal ejection fraction, aortic stenosis in elderly patients, moderate aortic stenosis in patients undergoing other cardiac surgery, and transcatheter aortic valve replacement, according to the guidelines from the American College of Cardiology and American Heart Association.2
In addition to the situations covered in their review, a few other complicated situations in patients with severe aortic stenosis also merit discussion. We discuss these below.
ASYMPTOMATIC SEVERE AORTIC STENOSIS AND A NORMAL EJECTION FRACTION
Patients with aortic stenosis may be unaware of their decline in functional capacity, since the illness is gradually progressive. In these patients, exercise testing is often done, as it can uncover limitations and determine the need for aortic valve replacement. Another group of patients with asymptomatic severe aortic stenosis who need aortic valve replacement are those whose ejection fraction is less than 50%.
However, many patients with asymptomatic aortic stenosis pass the stress test with flying colors—no symptoms, no blood pressure changes, no arrhythmias—and have a normal ejection fraction. Managing these patients can be more complicated.
Lancellotti et al3 described a group of patients with asymptomatic severe aortic stenosis, a normal ejection fraction, an aortic valve area smaller than 1 cm2, and normal results on exercise testing. Rates of the primary end point (cardiovascular death or need for aortic valve replacement due to symptoms or left ventricular dysfunction) were assessed in subsets of patients grouped on the basis of two variables:
- Left ventricular stroke volume index (flow)—either normal or low (< 35 mL/m2) and
- Mean gradient—either high or low (< 40 mm Hg).
The prevalence rates and 2-year event rates (which were substantial) were as follows:
- Normal flow, high gradient—51% of patients; event rate 56%
- Normal flow, low gradient—31% of patients; event rate 17%
- Low flow, high gradient—10% of patients; event rate 70%
- Low flow, low gradient—7% of patients; event rate 73%.
Mihaljevic et al4 at our institution found that left ventricular hypertrophy at the time of surgery for aortic stenosis may have lasting negative consequences. In an observational study of 3,049 patients who underwent aortic valve replacement, severe left ventricular hypertrophy preceded symptoms in 17%. Additionally, the survival rate at 10 years in the group whose left ventricular mass was greater than 185 g/m2 was 45% at 10 years, compared with 65% in patients whose left ventricular mass was less than 100 g/m2. Left ventricular hypertrophy may, therefore, eventually become another factor that we use in defining the appropriateness of surgery in patients with severe but asymptomatic aortic stenosis.
Comment. Not all patients who have severe aortic stenosis, no symptoms, and a “normal” ejection fraction are the same. Our view of what constitutes appropriate left ventricular function in aortic stenosis has changed and now encompasses diastolic filling values, myocardial velocity, and patterns of hypertrophy in addition to ejection fraction. Surgery is already considered reasonable for patients with asymptomatic but “extremely severe” aortic stenosis (aortic valve area < 0.6 cm2, jet velocity > 5 m/sec, mean gradient > 60 mm Hg), and it may improve long-term survival.2,5
However, closer inspection of left ventricular mechanics may also identify another group of patients whose prognosis is worse than in the rest. It is possible that a more thorough evaluation of left ventricular mechanics, including strain imaging, will provide a more elegant way to risk-stratify patients and help clinicians decide when to intervene in this difficult group of patients.6
While these factors are not yet a part of the diagnostic algorithm, the work by Lancellotti et al3 and Mihaljevic et al4 sheds light on the idea that evaluation of advanced echocardiographic variables may provide clinical insights into whether patients should undergo aortic valve replacement.
PCI FOR CONCOMITANT SEVERE CORONARY ARTERY DISEASE
The risk factors for aortic stenosis are similar to those for coronary artery disease, and many patients with moderate or severe aortic stenosis also have significant coronary disease. These patients are traditionally referred for combined surgical aortic valve replacement and coronary artery bypass grafting.
Patients who have the combination of both diseases have a worse prognosis, and adding coronary artery bypass grafting to surgical aortic valve replacement increases the perioperative mortality rate.7
With advances in transcatheter aortic valve replacement, attention has turned to managing concomitant coronary artery disease percutaneously as well. Until recently, however, there were few data on the safety of percutaneous coronary intervention (PCI) in patients with severe aortic stenosis.
Goel et al8 analyzed the outcomes of 254 patients with severe aortic stenosis who underwent PCI at our institution, compared with a propensity-matched group of 508 patients without aortic stenosis undergoing PCI. Overall, the 30-day mortality rate did not differ significantly between the two groups (4.3% vs 4.7%, P = .20), nor did the rate of complications such as contrast nephropathy, periprocedural myocardial infarction, and hemodynamic compromise during the procedure. In subgroup analysis, patients who had severe aortic stenosis and ejection fractions of 30% or less had a significantly higher risk of death than those with ejection fractions greater than 30% (15.4% vs 1.2%, P < .001).
Comment. This information is important, since many patients with severe aortic stenosis also have coronary artery disease. Certainly, for patients with significant coronary artery disease and severe aortic stenosis who cannot undergo surgery, the findings are especially encouraging with respect to the safety of PCI.
The findings also suggest that in patients for whom transcatheter aortic valve replacement can be performed in a timely fashion, a completely percutaneous approach to treating aortic stenosis and coronary artery disease may be reasonable. This hypothesis must be further investigated, but the preliminary data are encouraging.
TRANSCATHETER AORTIC VALVE REPLACEMENT IN LOWER-RISK PATIENTS
The PARTNER (Placement of Aortic Transcatheter Valves) trial showed that transcatheter aortic valve replacement was superior to medical therapy alone for patients who cannot undergo surgery, and not inferior to surgical aortic valve replacement for patients at high surgical risk, ie, a Society of Thoracic Surgeons (STS) mortality risk score greater than 10%.9
Given these encouraging results, the PARTNER II trial is now randomizing patients who are at moderate surgical risk (STS score > 4%) to surgical vs transcatheter aortic valve replacement.
Since transcatheter aortic valve replacement has been performed in Europe under the Conformité Européenne (CE) marking since 2007, diffusion of the procedure there has occurred in a more rapid fashion than in the United States. As a result, a number of patients with low or moderate surgical risk have undergone this procedure.
Lange et al10 summarized their experience at a single center in Munich, Germany, with an eye toward patient selection and surgical risk. Between 2007 and 2010, 420 patients underwent transcatheter aortic valve replacement. When the authors divided the cases into quartiles according to the sequence in which they were seen, they found a statistically significant decline in the STS score over time, from 7.1% in the earliest quartile to 4.8% in the latest quartile (P < .001), indicating the procedure was diffusing into lower-risk groups. With respect to outcome, the 6-month mortality rate declined from 23.5% to 12.4%; this was likely due to a combination of patient-related factors (more patients at lower risk over time), device advances, and greater operator experience. Also of note, only 70% of patients in the latest quartile were intubated for the procedure.
Comment. Diffusion of transcatheter aortic valve replacement in the United States is following a thoughtful path, with patients being assessed by “heart teams” of clinical cardiologists, interventional cardiologists, imaging cardiologists, and cardiac surgeons, and with strict criteria for site approval to perform commercial placement of the Edwards Sapien valve. In keeping with this controlled process, future randomized studies (such as PARTNER II) of transcatheter aortic valve replacement in lower-risk patients will be necessary before this procedure can be widely applied to this patient group. The results are, therefore, eagerly anticipated, but preliminary experience from Europe is encouraging.
BALLOON AORTIC VALVULOPLASTY IS SEEING A RESURGENCE
In large part due to rising interest in managing aortic stenosis and to the anticipated diffusion of transcatheter aortic valve replacement, balloon aortic valvuloplasty has seen a resurgence in recent years.
This procedure can be considered in a number of situations. In patients with severe aortic stenosis who are hemodynamically unstable and for whom urgent aortic valve replacement is not feasible, balloon valvuloplasty may serve as a “bridge” to valve replacement. Similarly, we have seen significant functional improvement in patients after balloon aortic valvuloplasty, so that some who initially were unable to undergo aortic valve replacement have improved to a point that either transcatheter or surgical replacement could be performed safely. In patients who need urgent noncardiac surgery, balloon valvuloplasty may be considered as a temporizing measure in the hope of reducing the risks of perioperative hemodynamic changes associated with anesthesia.
Many patients with severe aortic stenosis have comorbidities such as chronic obstructive pulmonary disease or liver or kidney disease that make it difficult to discern the degree to which aortic stenosis contributes to their symptoms. In such cases, the balloon procedure may provide a therapeutic answer; improvement of symptoms points to aortic stenosis as the driver of symptoms and may push for a more definitive valve replacement option.
Finally, in patients with no option for either transcatheter or surgical aortic valve replacement, balloon aortic valvuloplasty may be considered as a palliative measure.
The benefit of this procedure is only temporary, and restenosis generally occurs within 6 months. Therefore, its value as a stand-alone procedure is limited, and the overall survival rate is significantly improved only when it is used as a bridge to valve replacement.
It should be noted that balloon aortic valvuloplasty carries significant risk. The 30-day mortality rate may be as high as 10%, usually due to either aortic regurgitation (as a complication of the procedure) or persistent heart failure. Other complications occur in up to 15% of cases and include stroke, peripheral vascular complications (due to the size of the devices used and concomitant incidence of peripheral arterial disease), coronary occlusion, need for permanent pacemaker implantation, cardiac tamponade, and cardiac arrest. In patients who require a repeat procedure, it entails similar risks and outcomes as the first procedure.
Comment. Balloon aortic valvuloplasty holds an important place in the treatment of patients with severe aortic stenosis. In our experience, it is most often performed to bridge severely symptomatic patients to transcatheter or surgical aortic valve replacement, or to better understand the contribution of aortic stenosis to functional limitation in patients with multiple comorbidities. It has tremendous potential to alleviate symptoms and provide an opportunity for functional improvement, in turn allowing definitive treatment with aortic valve replacement and improved quality and quantity of life in patients with severe aortic stenosis.
MANAGING SEVERE STENOSIS IS FULFILLING, BUT CHALLENGING
Managing patients with severe aortic stenosis is very fulfilling but at the same time can be extraordinarily challenging. It requires a patient-by-patient analysis of clinical, echocardiographic, and hemodynamic data. In some cases, the relationship between aortic stenosis and current symptoms or future outcomes may be in doubt, and provocative testing or balloon aortic valvuloplasty may be necessary to provide further direction. A meticulous assessment, requiring the expertise of clinicians, imagers, interventionalists, and surgeons is often necessary to deliver optimal care to this group of patients.
- Sawaya F, Stewart J, Babaliaros V. Aortic stenosis: who should undergo surgery, transcatheter valve replacement? Cleve Clin J Med 2012; 79:487–497.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661
- Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012; 59:235–243.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1278; discussion 1278–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Ozkan A, Kapadia S, Tuzcu M, Marwick TH. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 2011; 8:494–501.
- Nowicki ER, Birkmeyer NJ, Weintraub RW, et al; Northern New England Cardiovascular Disease Study Group and the Center for Evaluative Clinical Sciences, Dartmouth Medical School. Multivariable prediction of in-hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg 2004; 77:1966–1977.
- Goel SS, Agarwal S, Tuzcu EM, et al. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation 2012; 125:1005–1013.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Lange R, Bleiziffer S, Mazzitelli D, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol 2012; 59:280–287.
The classic case of aortic stenosis is in an otherwise healthy middle-aged patient with symptomatic severe disease who is referred to a cardiac surgeon for surgical aortic valve replacement. Unfortunately, physicians who manage valvular heart disease do not encounter this straightforward scenario on a regular basis. Rather, patients come with comorbidities such as advanced age, pulmonary disease, renal dysfunction, coronary artery disease, and significant left ventricular dysfunction. They also come with severe aortic stenosis without symptoms.
In this issue of the Cleveland Clinic Journal of Medicine, Sawaya and colleagues1 review the management of aortic stenosis, focusing on clinically challenging scenarios such as low-flow, low-gradient aortic stenosis, low-gradient severe aortic stenosis with a normal ejection fraction, aortic stenosis in elderly patients, moderate aortic stenosis in patients undergoing other cardiac surgery, and transcatheter aortic valve replacement, according to the guidelines from the American College of Cardiology and American Heart Association.2
In addition to the situations covered in their review, a few other complicated situations in patients with severe aortic stenosis also merit discussion. We discuss these below.
ASYMPTOMATIC SEVERE AORTIC STENOSIS AND A NORMAL EJECTION FRACTION
Patients with aortic stenosis may be unaware of their decline in functional capacity, since the illness is gradually progressive. In these patients, exercise testing is often done, as it can uncover limitations and determine the need for aortic valve replacement. Another group of patients with asymptomatic severe aortic stenosis who need aortic valve replacement are those whose ejection fraction is less than 50%.
However, many patients with asymptomatic aortic stenosis pass the stress test with flying colors—no symptoms, no blood pressure changes, no arrhythmias—and have a normal ejection fraction. Managing these patients can be more complicated.
Lancellotti et al3 described a group of patients with asymptomatic severe aortic stenosis, a normal ejection fraction, an aortic valve area smaller than 1 cm2, and normal results on exercise testing. Rates of the primary end point (cardiovascular death or need for aortic valve replacement due to symptoms or left ventricular dysfunction) were assessed in subsets of patients grouped on the basis of two variables:
- Left ventricular stroke volume index (flow)—either normal or low (< 35 mL/m2) and
- Mean gradient—either high or low (< 40 mm Hg).
The prevalence rates and 2-year event rates (which were substantial) were as follows:
- Normal flow, high gradient—51% of patients; event rate 56%
- Normal flow, low gradient—31% of patients; event rate 17%
- Low flow, high gradient—10% of patients; event rate 70%
- Low flow, low gradient—7% of patients; event rate 73%.
Mihaljevic et al4 at our institution found that left ventricular hypertrophy at the time of surgery for aortic stenosis may have lasting negative consequences. In an observational study of 3,049 patients who underwent aortic valve replacement, severe left ventricular hypertrophy preceded symptoms in 17%. Additionally, the survival rate at 10 years in the group whose left ventricular mass was greater than 185 g/m2 was 45% at 10 years, compared with 65% in patients whose left ventricular mass was less than 100 g/m2. Left ventricular hypertrophy may, therefore, eventually become another factor that we use in defining the appropriateness of surgery in patients with severe but asymptomatic aortic stenosis.
Comment. Not all patients who have severe aortic stenosis, no symptoms, and a “normal” ejection fraction are the same. Our view of what constitutes appropriate left ventricular function in aortic stenosis has changed and now encompasses diastolic filling values, myocardial velocity, and patterns of hypertrophy in addition to ejection fraction. Surgery is already considered reasonable for patients with asymptomatic but “extremely severe” aortic stenosis (aortic valve area < 0.6 cm2, jet velocity > 5 m/sec, mean gradient > 60 mm Hg), and it may improve long-term survival.2,5
However, closer inspection of left ventricular mechanics may also identify another group of patients whose prognosis is worse than in the rest. It is possible that a more thorough evaluation of left ventricular mechanics, including strain imaging, will provide a more elegant way to risk-stratify patients and help clinicians decide when to intervene in this difficult group of patients.6
While these factors are not yet a part of the diagnostic algorithm, the work by Lancellotti et al3 and Mihaljevic et al4 sheds light on the idea that evaluation of advanced echocardiographic variables may provide clinical insights into whether patients should undergo aortic valve replacement.
PCI FOR CONCOMITANT SEVERE CORONARY ARTERY DISEASE
The risk factors for aortic stenosis are similar to those for coronary artery disease, and many patients with moderate or severe aortic stenosis also have significant coronary disease. These patients are traditionally referred for combined surgical aortic valve replacement and coronary artery bypass grafting.
Patients who have the combination of both diseases have a worse prognosis, and adding coronary artery bypass grafting to surgical aortic valve replacement increases the perioperative mortality rate.7
With advances in transcatheter aortic valve replacement, attention has turned to managing concomitant coronary artery disease percutaneously as well. Until recently, however, there were few data on the safety of percutaneous coronary intervention (PCI) in patients with severe aortic stenosis.
Goel et al8 analyzed the outcomes of 254 patients with severe aortic stenosis who underwent PCI at our institution, compared with a propensity-matched group of 508 patients without aortic stenosis undergoing PCI. Overall, the 30-day mortality rate did not differ significantly between the two groups (4.3% vs 4.7%, P = .20), nor did the rate of complications such as contrast nephropathy, periprocedural myocardial infarction, and hemodynamic compromise during the procedure. In subgroup analysis, patients who had severe aortic stenosis and ejection fractions of 30% or less had a significantly higher risk of death than those with ejection fractions greater than 30% (15.4% vs 1.2%, P < .001).
Comment. This information is important, since many patients with severe aortic stenosis also have coronary artery disease. Certainly, for patients with significant coronary artery disease and severe aortic stenosis who cannot undergo surgery, the findings are especially encouraging with respect to the safety of PCI.
The findings also suggest that in patients for whom transcatheter aortic valve replacement can be performed in a timely fashion, a completely percutaneous approach to treating aortic stenosis and coronary artery disease may be reasonable. This hypothesis must be further investigated, but the preliminary data are encouraging.
TRANSCATHETER AORTIC VALVE REPLACEMENT IN LOWER-RISK PATIENTS
The PARTNER (Placement of Aortic Transcatheter Valves) trial showed that transcatheter aortic valve replacement was superior to medical therapy alone for patients who cannot undergo surgery, and not inferior to surgical aortic valve replacement for patients at high surgical risk, ie, a Society of Thoracic Surgeons (STS) mortality risk score greater than 10%.9
Given these encouraging results, the PARTNER II trial is now randomizing patients who are at moderate surgical risk (STS score > 4%) to surgical vs transcatheter aortic valve replacement.
Since transcatheter aortic valve replacement has been performed in Europe under the Conformité Européenne (CE) marking since 2007, diffusion of the procedure there has occurred in a more rapid fashion than in the United States. As a result, a number of patients with low or moderate surgical risk have undergone this procedure.
Lange et al10 summarized their experience at a single center in Munich, Germany, with an eye toward patient selection and surgical risk. Between 2007 and 2010, 420 patients underwent transcatheter aortic valve replacement. When the authors divided the cases into quartiles according to the sequence in which they were seen, they found a statistically significant decline in the STS score over time, from 7.1% in the earliest quartile to 4.8% in the latest quartile (P < .001), indicating the procedure was diffusing into lower-risk groups. With respect to outcome, the 6-month mortality rate declined from 23.5% to 12.4%; this was likely due to a combination of patient-related factors (more patients at lower risk over time), device advances, and greater operator experience. Also of note, only 70% of patients in the latest quartile were intubated for the procedure.
Comment. Diffusion of transcatheter aortic valve replacement in the United States is following a thoughtful path, with patients being assessed by “heart teams” of clinical cardiologists, interventional cardiologists, imaging cardiologists, and cardiac surgeons, and with strict criteria for site approval to perform commercial placement of the Edwards Sapien valve. In keeping with this controlled process, future randomized studies (such as PARTNER II) of transcatheter aortic valve replacement in lower-risk patients will be necessary before this procedure can be widely applied to this patient group. The results are, therefore, eagerly anticipated, but preliminary experience from Europe is encouraging.
BALLOON AORTIC VALVULOPLASTY IS SEEING A RESURGENCE
In large part due to rising interest in managing aortic stenosis and to the anticipated diffusion of transcatheter aortic valve replacement, balloon aortic valvuloplasty has seen a resurgence in recent years.
This procedure can be considered in a number of situations. In patients with severe aortic stenosis who are hemodynamically unstable and for whom urgent aortic valve replacement is not feasible, balloon valvuloplasty may serve as a “bridge” to valve replacement. Similarly, we have seen significant functional improvement in patients after balloon aortic valvuloplasty, so that some who initially were unable to undergo aortic valve replacement have improved to a point that either transcatheter or surgical replacement could be performed safely. In patients who need urgent noncardiac surgery, balloon valvuloplasty may be considered as a temporizing measure in the hope of reducing the risks of perioperative hemodynamic changes associated with anesthesia.
Many patients with severe aortic stenosis have comorbidities such as chronic obstructive pulmonary disease or liver or kidney disease that make it difficult to discern the degree to which aortic stenosis contributes to their symptoms. In such cases, the balloon procedure may provide a therapeutic answer; improvement of symptoms points to aortic stenosis as the driver of symptoms and may push for a more definitive valve replacement option.
Finally, in patients with no option for either transcatheter or surgical aortic valve replacement, balloon aortic valvuloplasty may be considered as a palliative measure.
The benefit of this procedure is only temporary, and restenosis generally occurs within 6 months. Therefore, its value as a stand-alone procedure is limited, and the overall survival rate is significantly improved only when it is used as a bridge to valve replacement.
It should be noted that balloon aortic valvuloplasty carries significant risk. The 30-day mortality rate may be as high as 10%, usually due to either aortic regurgitation (as a complication of the procedure) or persistent heart failure. Other complications occur in up to 15% of cases and include stroke, peripheral vascular complications (due to the size of the devices used and concomitant incidence of peripheral arterial disease), coronary occlusion, need for permanent pacemaker implantation, cardiac tamponade, and cardiac arrest. In patients who require a repeat procedure, it entails similar risks and outcomes as the first procedure.
Comment. Balloon aortic valvuloplasty holds an important place in the treatment of patients with severe aortic stenosis. In our experience, it is most often performed to bridge severely symptomatic patients to transcatheter or surgical aortic valve replacement, or to better understand the contribution of aortic stenosis to functional limitation in patients with multiple comorbidities. It has tremendous potential to alleviate symptoms and provide an opportunity for functional improvement, in turn allowing definitive treatment with aortic valve replacement and improved quality and quantity of life in patients with severe aortic stenosis.
MANAGING SEVERE STENOSIS IS FULFILLING, BUT CHALLENGING
Managing patients with severe aortic stenosis is very fulfilling but at the same time can be extraordinarily challenging. It requires a patient-by-patient analysis of clinical, echocardiographic, and hemodynamic data. In some cases, the relationship between aortic stenosis and current symptoms or future outcomes may be in doubt, and provocative testing or balloon aortic valvuloplasty may be necessary to provide further direction. A meticulous assessment, requiring the expertise of clinicians, imagers, interventionalists, and surgeons is often necessary to deliver optimal care to this group of patients.
The classic case of aortic stenosis is in an otherwise healthy middle-aged patient with symptomatic severe disease who is referred to a cardiac surgeon for surgical aortic valve replacement. Unfortunately, physicians who manage valvular heart disease do not encounter this straightforward scenario on a regular basis. Rather, patients come with comorbidities such as advanced age, pulmonary disease, renal dysfunction, coronary artery disease, and significant left ventricular dysfunction. They also come with severe aortic stenosis without symptoms.
In this issue of the Cleveland Clinic Journal of Medicine, Sawaya and colleagues1 review the management of aortic stenosis, focusing on clinically challenging scenarios such as low-flow, low-gradient aortic stenosis, low-gradient severe aortic stenosis with a normal ejection fraction, aortic stenosis in elderly patients, moderate aortic stenosis in patients undergoing other cardiac surgery, and transcatheter aortic valve replacement, according to the guidelines from the American College of Cardiology and American Heart Association.2
In addition to the situations covered in their review, a few other complicated situations in patients with severe aortic stenosis also merit discussion. We discuss these below.
ASYMPTOMATIC SEVERE AORTIC STENOSIS AND A NORMAL EJECTION FRACTION
Patients with aortic stenosis may be unaware of their decline in functional capacity, since the illness is gradually progressive. In these patients, exercise testing is often done, as it can uncover limitations and determine the need for aortic valve replacement. Another group of patients with asymptomatic severe aortic stenosis who need aortic valve replacement are those whose ejection fraction is less than 50%.
However, many patients with asymptomatic aortic stenosis pass the stress test with flying colors—no symptoms, no blood pressure changes, no arrhythmias—and have a normal ejection fraction. Managing these patients can be more complicated.
Lancellotti et al3 described a group of patients with asymptomatic severe aortic stenosis, a normal ejection fraction, an aortic valve area smaller than 1 cm2, and normal results on exercise testing. Rates of the primary end point (cardiovascular death or need for aortic valve replacement due to symptoms or left ventricular dysfunction) were assessed in subsets of patients grouped on the basis of two variables:
- Left ventricular stroke volume index (flow)—either normal or low (< 35 mL/m2) and
- Mean gradient—either high or low (< 40 mm Hg).
The prevalence rates and 2-year event rates (which were substantial) were as follows:
- Normal flow, high gradient—51% of patients; event rate 56%
- Normal flow, low gradient—31% of patients; event rate 17%
- Low flow, high gradient—10% of patients; event rate 70%
- Low flow, low gradient—7% of patients; event rate 73%.
Mihaljevic et al4 at our institution found that left ventricular hypertrophy at the time of surgery for aortic stenosis may have lasting negative consequences. In an observational study of 3,049 patients who underwent aortic valve replacement, severe left ventricular hypertrophy preceded symptoms in 17%. Additionally, the survival rate at 10 years in the group whose left ventricular mass was greater than 185 g/m2 was 45% at 10 years, compared with 65% in patients whose left ventricular mass was less than 100 g/m2. Left ventricular hypertrophy may, therefore, eventually become another factor that we use in defining the appropriateness of surgery in patients with severe but asymptomatic aortic stenosis.
Comment. Not all patients who have severe aortic stenosis, no symptoms, and a “normal” ejection fraction are the same. Our view of what constitutes appropriate left ventricular function in aortic stenosis has changed and now encompasses diastolic filling values, myocardial velocity, and patterns of hypertrophy in addition to ejection fraction. Surgery is already considered reasonable for patients with asymptomatic but “extremely severe” aortic stenosis (aortic valve area < 0.6 cm2, jet velocity > 5 m/sec, mean gradient > 60 mm Hg), and it may improve long-term survival.2,5
However, closer inspection of left ventricular mechanics may also identify another group of patients whose prognosis is worse than in the rest. It is possible that a more thorough evaluation of left ventricular mechanics, including strain imaging, will provide a more elegant way to risk-stratify patients and help clinicians decide when to intervene in this difficult group of patients.6
While these factors are not yet a part of the diagnostic algorithm, the work by Lancellotti et al3 and Mihaljevic et al4 sheds light on the idea that evaluation of advanced echocardiographic variables may provide clinical insights into whether patients should undergo aortic valve replacement.
PCI FOR CONCOMITANT SEVERE CORONARY ARTERY DISEASE
The risk factors for aortic stenosis are similar to those for coronary artery disease, and many patients with moderate or severe aortic stenosis also have significant coronary disease. These patients are traditionally referred for combined surgical aortic valve replacement and coronary artery bypass grafting.
Patients who have the combination of both diseases have a worse prognosis, and adding coronary artery bypass grafting to surgical aortic valve replacement increases the perioperative mortality rate.7
With advances in transcatheter aortic valve replacement, attention has turned to managing concomitant coronary artery disease percutaneously as well. Until recently, however, there were few data on the safety of percutaneous coronary intervention (PCI) in patients with severe aortic stenosis.
Goel et al8 analyzed the outcomes of 254 patients with severe aortic stenosis who underwent PCI at our institution, compared with a propensity-matched group of 508 patients without aortic stenosis undergoing PCI. Overall, the 30-day mortality rate did not differ significantly between the two groups (4.3% vs 4.7%, P = .20), nor did the rate of complications such as contrast nephropathy, periprocedural myocardial infarction, and hemodynamic compromise during the procedure. In subgroup analysis, patients who had severe aortic stenosis and ejection fractions of 30% or less had a significantly higher risk of death than those with ejection fractions greater than 30% (15.4% vs 1.2%, P < .001).
Comment. This information is important, since many patients with severe aortic stenosis also have coronary artery disease. Certainly, for patients with significant coronary artery disease and severe aortic stenosis who cannot undergo surgery, the findings are especially encouraging with respect to the safety of PCI.
The findings also suggest that in patients for whom transcatheter aortic valve replacement can be performed in a timely fashion, a completely percutaneous approach to treating aortic stenosis and coronary artery disease may be reasonable. This hypothesis must be further investigated, but the preliminary data are encouraging.
TRANSCATHETER AORTIC VALVE REPLACEMENT IN LOWER-RISK PATIENTS
The PARTNER (Placement of Aortic Transcatheter Valves) trial showed that transcatheter aortic valve replacement was superior to medical therapy alone for patients who cannot undergo surgery, and not inferior to surgical aortic valve replacement for patients at high surgical risk, ie, a Society of Thoracic Surgeons (STS) mortality risk score greater than 10%.9
Given these encouraging results, the PARTNER II trial is now randomizing patients who are at moderate surgical risk (STS score > 4%) to surgical vs transcatheter aortic valve replacement.
Since transcatheter aortic valve replacement has been performed in Europe under the Conformité Européenne (CE) marking since 2007, diffusion of the procedure there has occurred in a more rapid fashion than in the United States. As a result, a number of patients with low or moderate surgical risk have undergone this procedure.
Lange et al10 summarized their experience at a single center in Munich, Germany, with an eye toward patient selection and surgical risk. Between 2007 and 2010, 420 patients underwent transcatheter aortic valve replacement. When the authors divided the cases into quartiles according to the sequence in which they were seen, they found a statistically significant decline in the STS score over time, from 7.1% in the earliest quartile to 4.8% in the latest quartile (P < .001), indicating the procedure was diffusing into lower-risk groups. With respect to outcome, the 6-month mortality rate declined from 23.5% to 12.4%; this was likely due to a combination of patient-related factors (more patients at lower risk over time), device advances, and greater operator experience. Also of note, only 70% of patients in the latest quartile were intubated for the procedure.
Comment. Diffusion of transcatheter aortic valve replacement in the United States is following a thoughtful path, with patients being assessed by “heart teams” of clinical cardiologists, interventional cardiologists, imaging cardiologists, and cardiac surgeons, and with strict criteria for site approval to perform commercial placement of the Edwards Sapien valve. In keeping with this controlled process, future randomized studies (such as PARTNER II) of transcatheter aortic valve replacement in lower-risk patients will be necessary before this procedure can be widely applied to this patient group. The results are, therefore, eagerly anticipated, but preliminary experience from Europe is encouraging.
BALLOON AORTIC VALVULOPLASTY IS SEEING A RESURGENCE
In large part due to rising interest in managing aortic stenosis and to the anticipated diffusion of transcatheter aortic valve replacement, balloon aortic valvuloplasty has seen a resurgence in recent years.
This procedure can be considered in a number of situations. In patients with severe aortic stenosis who are hemodynamically unstable and for whom urgent aortic valve replacement is not feasible, balloon valvuloplasty may serve as a “bridge” to valve replacement. Similarly, we have seen significant functional improvement in patients after balloon aortic valvuloplasty, so that some who initially were unable to undergo aortic valve replacement have improved to a point that either transcatheter or surgical replacement could be performed safely. In patients who need urgent noncardiac surgery, balloon valvuloplasty may be considered as a temporizing measure in the hope of reducing the risks of perioperative hemodynamic changes associated with anesthesia.
Many patients with severe aortic stenosis have comorbidities such as chronic obstructive pulmonary disease or liver or kidney disease that make it difficult to discern the degree to which aortic stenosis contributes to their symptoms. In such cases, the balloon procedure may provide a therapeutic answer; improvement of symptoms points to aortic stenosis as the driver of symptoms and may push for a more definitive valve replacement option.
Finally, in patients with no option for either transcatheter or surgical aortic valve replacement, balloon aortic valvuloplasty may be considered as a palliative measure.
The benefit of this procedure is only temporary, and restenosis generally occurs within 6 months. Therefore, its value as a stand-alone procedure is limited, and the overall survival rate is significantly improved only when it is used as a bridge to valve replacement.
It should be noted that balloon aortic valvuloplasty carries significant risk. The 30-day mortality rate may be as high as 10%, usually due to either aortic regurgitation (as a complication of the procedure) or persistent heart failure. Other complications occur in up to 15% of cases and include stroke, peripheral vascular complications (due to the size of the devices used and concomitant incidence of peripheral arterial disease), coronary occlusion, need for permanent pacemaker implantation, cardiac tamponade, and cardiac arrest. In patients who require a repeat procedure, it entails similar risks and outcomes as the first procedure.
Comment. Balloon aortic valvuloplasty holds an important place in the treatment of patients with severe aortic stenosis. In our experience, it is most often performed to bridge severely symptomatic patients to transcatheter or surgical aortic valve replacement, or to better understand the contribution of aortic stenosis to functional limitation in patients with multiple comorbidities. It has tremendous potential to alleviate symptoms and provide an opportunity for functional improvement, in turn allowing definitive treatment with aortic valve replacement and improved quality and quantity of life in patients with severe aortic stenosis.
MANAGING SEVERE STENOSIS IS FULFILLING, BUT CHALLENGING
Managing patients with severe aortic stenosis is very fulfilling but at the same time can be extraordinarily challenging. It requires a patient-by-patient analysis of clinical, echocardiographic, and hemodynamic data. In some cases, the relationship between aortic stenosis and current symptoms or future outcomes may be in doubt, and provocative testing or balloon aortic valvuloplasty may be necessary to provide further direction. A meticulous assessment, requiring the expertise of clinicians, imagers, interventionalists, and surgeons is often necessary to deliver optimal care to this group of patients.
- Sawaya F, Stewart J, Babaliaros V. Aortic stenosis: who should undergo surgery, transcatheter valve replacement? Cleve Clin J Med 2012; 79:487–497.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661
- Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012; 59:235–243.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1278; discussion 1278–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Ozkan A, Kapadia S, Tuzcu M, Marwick TH. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 2011; 8:494–501.
- Nowicki ER, Birkmeyer NJ, Weintraub RW, et al; Northern New England Cardiovascular Disease Study Group and the Center for Evaluative Clinical Sciences, Dartmouth Medical School. Multivariable prediction of in-hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg 2004; 77:1966–1977.
- Goel SS, Agarwal S, Tuzcu EM, et al. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation 2012; 125:1005–1013.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Lange R, Bleiziffer S, Mazzitelli D, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol 2012; 59:280–287.
- Sawaya F, Stewart J, Babaliaros V. Aortic stenosis: who should undergo surgery, transcatheter valve replacement? Cleve Clin J Med 2012; 79:487–497.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661
- Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012; 59:235–243.
- Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg 2008; 135:1270–1278; discussion 1278–1279.
- Kang DH, Park SJ, Rim JH, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010; 121:1502–1509.
- Ozkan A, Kapadia S, Tuzcu M, Marwick TH. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol 2011; 8:494–501.
- Nowicki ER, Birkmeyer NJ, Weintraub RW, et al; Northern New England Cardiovascular Disease Study Group and the Center for Evaluative Clinical Sciences, Dartmouth Medical School. Multivariable prediction of in-hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg 2004; 77:1966–1977.
- Goel SS, Agarwal S, Tuzcu EM, et al. Percutaneous coronary intervention in patients with severe aortic stenosis: implications for transcatheter aortic valve replacement. Circulation 2012; 125:1005–1013.
- Smith CR, Leon MB, Mack MJ, et al; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Lange R, Bleiziffer S, Mazzitelli D, et al. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol 2012; 59:280–287.