User login
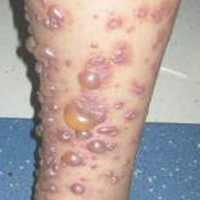
Erythematous Eruption on the Left Leg
The Diagnosis: Bullous Henoch-Schönlein Purpura
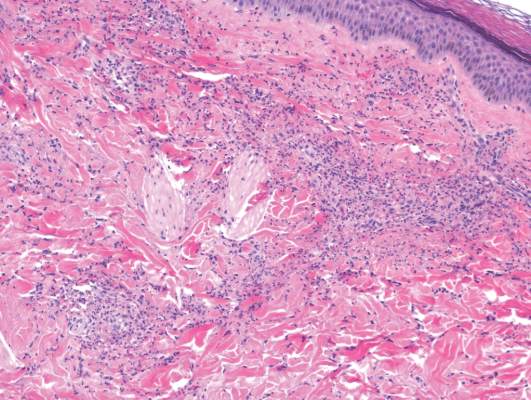
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).
Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).

Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).

Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.

A 12-year-old girl presented with an erythematous eruption that had started on the left leg approximately 1 week prior with subsequent spread to the abdomen and arms. She had associated knee pain, myalgia, abdominal pain, nausea, and nonbloody and nonbilious emesis. Her medical history was notable for methicillin-resistant Staphylococcus aureus abscesses, the most recent of which was treated with trimethoprim-sulfamethoxazole; treatment was completed 5 days before the onset of the rash. Family history was notable for her paternal aunt who died of systemic lupus erythematosus. Physical examination showed erythematous macules and purpuric papules with central vesiculation extending up the thighs and lower abdomen associated with edema of the lower extremities and pain after palpation. Tense bullae also were present.