User login
Necrotizing pancreatitis: Diagnose, treat, consult
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
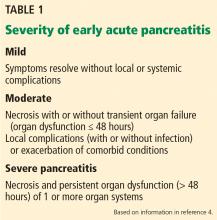
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
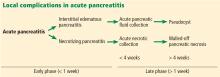
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
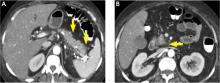
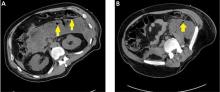
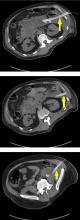
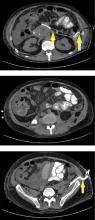
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
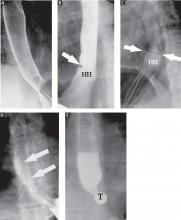
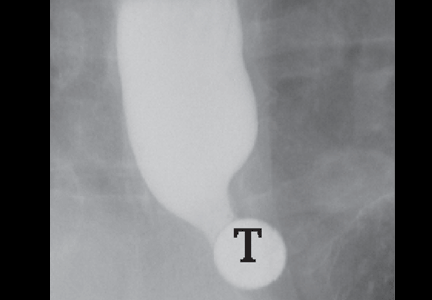
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
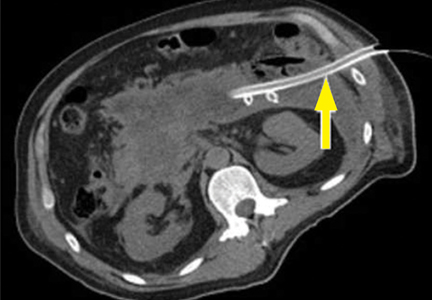
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
Acute pancreatitis accounted for more than 300,000 admissions and $2.6 billion in associated healthcare costs in the United States in 2012.1 First-line management is early aggressive fluid resuscitation and analgesics for pain control. Guidelines recommend estimating the clinical severity of each attack using a validated scoring system such as the Bedside Index of Severity in Acute Pancreatitis.2 Clinically severe pancreatitis is associated with necrosis.
Acute pancreatitis results from inappropriate activation of zymogens and subsequent autodigestion of the pancreas by its own enzymes. Though necrotizing pancreatitis is thought to be an ischemic complication, its pathogenesis is not completely understood. Necrosis increases the morbidity and mortality risk of acute pancreatitis because of its association with organ failure and infectious complications. As such, patients with necrotizing pancreatitis may need admission to the intensive care unit, nutritional support, antibiotics, and radiologic, endoscopic, or surgical interventions.
Here, we review current evidence regarding the diagnosis and management of necrotizing pancreatitis.
PROPER TERMINOLOGY HELPS COLLABORATION
Managing necrotizing pancreatitis requires the combined efforts of internists, gastroenterologists, radiologists, and surgeons. This collaboration is aided by proper terminology.
A classification system was devised in Atlanta, GA, in 1992 to facilitate communication and interdisciplinary collaboration.3 Severe pancreatitis was differentiated from mild by the presence of organ failure or the complications of pseudocyst, necrosis, or abscess.
The original Atlanta classification had several limitations. First, the terminology for fluid collections was ambiguous and frequently misused. Second, the assessment of clinical severity required either the Ranson score or the Acute Physiology and Chronic Health Evaluation II score, both of which are complex and have other limitations. Finally, advances in imaging and treatment have rendered the original Atlanta nomenclature obsolete.
In 2012, the Acute Pancreatitis Classification Working Group issued a revised Atlanta classification that modernized the terminology pertaining to natural history, severity, imaging features, and complications. It divides the natural course of acute pancreatitis into early and late phases.4
Early vs late phase
In the early phase, findings on computed tomography (CT) neither correlate with clinical severity nor alter clinical management.6 Thus, early imaging is not indicated unless there is diagnostic uncertainty, lack of response to appropriate treatment, or sudden deterioration.
Moderate pancreatitis describes patients with pancreatic necrosis with or without transient organ failure (organ dysfunction for ≤ 48 hours).
Severe pancreatitis is defined by pancreatic necrosis and persistent organ dysfunction.4 It may be accompanied by pancreatic and peripancreatic fluid collections; bacteremia and sepsis can occur in association with infection of necrotic collections.
Interstitial edematous pancreatitis vs necrotizing pancreatitis
The revised Atlanta classification maintains the original classification of acute pancreatitis into 2 main categories: interstitial edematous pancreatitis and necrotizing pancreatitis.
Necrotizing pancreatitis is further divided into 3 subtypes based on extent and location of necrosis:
- Parenchymal necrosis alone (5% of cases)
- Necrosis of peripancreatic fat alone (20%)
- Necrosis of both parenchyma and peripancreatic fat (75%).
Peripancreatic involvement is commonly found in the mesentery, peripancreatic and distant retroperitoneum, and lesser sac.
Of the three subtypes, peripancreatic necrosis has the best prognosis. However, all of the subtypes of necrotizing pancreatitis are associated with poorer outcomes than interstitial edematous pancreatitis.
Fluid collections
Acute pancreatic fluid collections contain exclusively nonsolid components without an inflammatory wall and are typically found in the peripancreatic fat. These collections often resolve without intervention as the patient recovers. If they persist beyond 4 weeks and develop a nonepithelialized, fibrous wall, they become pseudocysts. Intervention is generally not recommended for pseudocysts unless they are symptomatic.
ROLE OF IMAGING
Radiographic imaging is not usually necessary to diagnose acute pancreatitis. However, it can be a valuable tool to clarify an ambiguous presentation, determine severity, and identify complications.
The timing and appropriate type of imaging are integral to obtaining useful data. Any imaging obtained in acute pancreatitis to evaluate necrosis should be performed at least 3 to 5 days from the initial symptom onset; if imaging is obtained before 72 hours, necrosis cannot be confidently excluded.8
COMPUTED TOMOGRAPHY
CT is the imaging test of choice when evaluating acute pancreatitis. In addition, almost all percutaneous interventions are performed with CT guidance. The Balthazar score is the most well-known CT severity index. It is calculated based on the degree of inflammation, acute fluid collections, and parenchymal necrosis.9 However, a modified severity index incorporates extrapancreatic complications such as ascites and vascular compromise and was found to more strongly correlate with outcomes than the standard Balthazar score.10
Contrast-enhanced CT is performed in 2 phases:
The pancreatic parenchymal phase
The pancreatic parenchymal or late arterial phase is obtained approximately 40 to 45 seconds after the start of the contrast bolus. It is used to detect necrosis in the early phase of acute pancreatitis and to assess the peripancreatic arteries for pseudoaneurysms in the late phase of acute pancreatitis.11
Pancreatic necrosis appears as an area of decreased parenchymal enhancement, either well-defined or heterogeneous. The normal pancreatic parenchyma has a postcontrast enhancement pattern similar to that of the spleen. Parenchyma that does not enhance to the same degree is considered necrotic. The severity of necrosis is graded based on the percentage of the pancreas involved (< 30%, 30%–50%, or > 50%), and a higher percentage correlates with a worse outcome.12,13
Peripancreatic necrosis is harder to detect, as there is no method to assess fat enhancement as there is with pancreatic parenchymal enhancement. In general, radiologists assume that heterogeneous peripancreatic changes, including areas of fat, fluid, and soft tissue attenuation, are consistent with peripancreatic necrosis. After 7 to 10 days, if these changes become more homogeneous and confluent with a more mass-like process, peripancreatic necrosis can be more confidently identified.12,13
The portal venous phase
The later, portal venous phase of the scan is obtained approximately 70 seconds after the start of the contrast bolus. It is used to detect and characterize fluid collections and venous complications of the disease.
Drawbacks of CT
A drawback of CT is the need for iodinated intravenous contrast media, which in severely ill patients may precipitate or worsen pre-existing acute kidney injury.
Further, several studies have shown that findings on CT rarely alter the management of patients in the early phase of acute pancreatitis and in fact may be an overuse of medical resources.14 Unless there are confounding clinical signs or symptoms, CT should be delayed for at least 72 hours.9,10,14,15
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) is not a first-line imaging test in this disease because it is not as available as CT and takes longer to perform—20 to 30 minutes. The patient must be evaluated for candidacy, as it is difficult for acutely ill patients to tolerate an examination that takes this long and requires them to hold their breath multiple times.
MRI is an appropriate alternative in patients who are pregnant or who have severe iodinated-contrast allergy. While contrast is necessary to detect pancreatic necrosis with CT, MRI can detect necrosis without the need for contrast in patients with acute kidney injury or severe chronic kidney disease. Also, MRI may be better in complicated cases requiring repeated imaging because it does not expose the patient to radiation.
On MRI, pancreatic necrosis appears as a heterogeneous area, owing to its liquid and solid components. Liquid components appear hyperintense, and solid components hypointense, on T2 fluid-weighted imaging. This ability to differentiate the components of a walled-off pancreatic necrosis can be useful in determining whether a collection requires drainage or debridement. MRI is also more sensitive for hemorrhagic complications, best seen on T1 fat-weighted images.12,16
Magnetic resonance cholangiopancreatography is an excellent method for ductal evaluation through heavily T2-weighted imaging. It is more sensitive than CT for detecting common bile duct stones and can also detect pancreatic duct strictures or extravasation into fluid collections.16
SUPPORTIVE MANAGEMENT OF EARLY NECROTIZING PANCREATITIS
In the early phase of necrotizing pancreatitis, management is supportive with the primary aim of preventing intravascular volume depletion. Aggressive fluid resuscitation in the first 48 to 72 hours, pain control, and bowel rest are the mainstays of supportive therapy. Intensive care may be necessary if organ failure and hemodynamic instability accompany necrotizing pancreatitis.
Prophylactic antibiotic and antifungal therapy to prevent infected necrosis has been controversial. Recent studies of its utility have not yielded supportive results, and the American College of Gastroenterology and the Infectious Diseases Society of America no longer recommend it.9,17 These medications should not be given unless concomitant cholangitis or extrapancreatic infection is clinically suspected.
Early enteral nutrition is recommended in patients in whom pancreatitis is predicted to be severe and in those not expected to resume oral intake within 5 to 7 days. Enteral nutrition most commonly involves bedside or endoscopic placement of a nasojejunal feeding tube and collaboration with a nutritionist to determine protein-caloric requirements.
Compared with enteral nutrition, total parenteral nutrition is associated with higher rates of infection, multiorgan dysfunction and failure, and death.18
MANAGING COMPLICATIONS OF PANCREATIC NECROSIS
Necrotizing pancreatitis is a defining complication of acute pancreatitis, and its presence alone indicates greater severity. However, superimposed complications may further worsen outcomes.
Infected pancreatic necrosis
Infection occurs in approximately 20% of patients with necrotizing pancreatitis and confers a mortality rate of 20% to 50%.19 Infected pancreatic necrosis occurs when gut organisms translocate into the nearby necrotic pancreatic and peripancreatic tissue. The most commonly identified organisms include Escherichia coli and Enterococcus species.20
This complication usually manifests 2 to 4 weeks after symptom onset; earlier onset is uncommon to rare. It should be considered when the systemic inflammatory response syndrome persists or recurs after 10 days to 2 weeks. Systemic inflammatory response syndrome is also common in sterile necrotizing pancreatitis and sometimes in interstitial pancreatitis, particularly during the first week. However, its sudden appearance or resurgence, high spiking fevers, or worsening organ failure in the later phase (2–4 weeks) of pancreatitis should heighten suspicion of infected pancreatic necrosis.
Imaging may also help diagnose infection, and the presence of gas within a collection or region of necrosis is highly specific. However, the presence of gas is not completely sensitive for infection, as it is seen in only 12% to 22% of infected cases.
Before minimally invasive techniques became available, the diagnosis of infected pancreatic necrosis was confirmed by percutaneous CT-guided aspiration of the necrotic mass or collection for Gram stain and culture.
Antibiotic therapy is indicated in confirmed or suspected cases of infected pancreatic necrosis. Antibiotics with gram-negative coverage and appropriate penetration such as carbapenems, metronidazole, fluoroquinolones, and selected cephalosporins are most commonly used. Meropenem is the antibiotic of choice at our institution.
CT-guided fine-needle aspiration is often done if suspected infected pancreatic necrosis fails to respond to empiric antibiotic therapy.
Debridement or drainage. Generally, the diagnosis or suspicion of infected pancreatic necrosis (suggestive signs are high fever, elevated white blood cell count, and sepsis) warrants an intervention to debride or drain infected pancreatic tissue and control sepsis.21
While source control is integral to the successful treatment of infected pancreatic necrosis, antibiotic therapy may provide a bridge to intervention for critically ill patients by suppressing bacteremia and subsequent sepsis. A 2013 meta-analysis found that 324 of 409 patients with suspected infected pancreatic necrosis were successfully stabilized with antibiotic treatment.21,22 The trend toward conservative management and promising outcomes with antibiotic therapy alone or with minimally invasive techniques has lessened the need for diagnostic CT-guided fine-needle aspiration.
Hemorrhage
Spontaneous hemorrhage into pancreatic necrosis is a rare but life-threatening complication. Because CT is almost always performed with contrast enhancement, this complication is rarely identified with imaging. The diagnosis is made by noting a drop in hemoglobin and hematocrit.
Hemorrhage into the retroperitoneum or the peritoneal cavity, or both, can occur when an inflammatory process erodes into a nearby artery. Luminal gastrointestinal bleeding can occur from gastric varices arising from splenic vein thrombosis and resulting left-sided portal hypertension, or from pseudoaneurysms. These can also bleed into the pancreatic duct (hemosuccus pancreaticus). Pseudoaneurysm is a later complication that occurs when an arterial wall (most commonly the splenic or gastroduodenal artery) is weakened by pancreatic enzymes.23
Prompt recognition of hemorrhagic events and consultation with an interventional radiologist or surgeon are required to prevent death.
Inflammation and abdominal compartment syndrome
Inflammation from necrotizing pancreatitis can cause further complications by blocking nearby structures. Reported complications include jaundice from biliary compression, hydronephrosis from ureteral compression, bowel obstruction, and gastric outlet obstruction.
Abdominal compartment syndrome is an increasingly recognized complication of acute pancreatitis. Abdominal pressure can rise due to a number of factors, including fluid collections, ascites, ileus, and overly aggressive fluid resuscitation.24 Elevated abdominal pressure is associated with complications such as decreased respiratory compliance, increased peak airway pressure, decreased cardiac preload, hypotension, mesenteric and intestinal ischemia, feeding intolerance, and lower-extremity ischemia and thrombosis.
Patients with necrotizing pancreatitis who have abdominal compartment syndrome have a mortality rate 5 times higher than patients without abdominal compartment syndrome.25
Abdominal pressures should be monitored using a bladder pressure sensor in critically ill or ventilated patients with acute pancreatitis. If the abdominal pressure rises above 20 mm Hg, medical and surgical interventions should be offered in a stepwise fashion to decrease it. Interventions include decompression by nasogastric and rectal tube, sedation or paralysis to relax abdominal wall tension, minimization of intravenous fluids, percutaneous drainage of ascites, and (rarely) surgical midline or subcostal laparotomy.
ROLE OF INTERVENTION
The treatment of necrotizing pancreatitis has changed rapidly, thanks to a growing experience with minimally invasive techniques.
Indications for intervention
Infected pancreatic necrosis is the primary indication for surgical, percutaneous, or endoscopic intervention.
In sterile necrosis, the threshold for intervention is less clear, and intervention is often reserved for patients who fail to clinically improve or who have intractable abdominal pain, gastric outlet obstruction, or fistulating disease.26
In asymptomatic cases, intervention is almost never indicated regardless of the location or size of the necrotic area.
In walled-off pancreatic necrosis, less-invasive and less-morbid interventions such as endoscopic or percutaneous drainage or video-assisted retroperitoneal debridement can be done.
Timing of intervention
In the past, delaying intervention was thought to increase the risk of death. However, multiple studies have found that outcomes are often worse if intervention is done early, likely due to the lack of a fully formed fibrous wall or demarcation of the necrotic area.27
If the patient remains clinically stable, it is best to delay intervention until at least 4 weeks after the index event to achieve optimal outcomes. Delay can often be achieved by antibiotic treatment to suppress bacteremia and endoscopic or percutaneous drainage of infected collections to control sepsis.
Open surgery
The gold-standard intervention for infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis is open necrosectomy. This involves exploratory laparotomy with blunt debridement of all visible necrotic pancreatic tissue.
Methods to facilitate later evacuation of residual infected fluid and debris vary widely. Multiple large-caliber drains can be placed to facilitate irrigation and drainage before closure of the abdominal fascia. As infected pancreatic necrosis carries the risk of contaminating the peritoneal cavity, the skin is often left open to heal by secondary intention. An interventional radiologist is frequently enlisted to place, exchange, or downsize drainage catheters.
Infected pancreatic necrosis or symptomatic sterile walled-off pancreatic necrosis often requires more than one operation to achieve satisfactory debridement.
The goals of open necrosectomy are to remove nonviable tissue and infection, preserve viable pancreatic tissue, eliminate fistulous connections, and minimize damage to local organs and vasculature.
Minimally invasive techniques
Video-assisted retroperitoneal debridement has been described as a hybrid between endoscopic and open retroperitoneal debridement.28 This technique requires first placing a percutaneous catheter into the necrotic area through the left flank to create a retroperitoneal tract. A 5-cm incision is made and the necrotic space is entered using the drain for guidance. Necrotic tissue is carefully debrided under direct vision using a combination of forceps, irrigation, and suction. A laparoscopic port can also be introduced into the incision when the procedure can no longer be continued under direct vision.29,30
Although not all patients are candidates for minimal-access surgery, it remains an evolving surgical option.
Endoscopic transmural debridement is another option for infected pancreatic necrosis and symptomatic walled-off pancreatic necrosis. Depending on the location of the necrotic area, an echoendoscope is passed to either the stomach or duodenum. Guided by endoscopic ultrasonography, a needle is passed into the collection, allowing subsequent fistula creation and stenting for internal drainage or debridement. In the past, this process required several steps, multiple devices, fluoroscopic guidance, and considerable time. But newer endoscopic lumen-apposing metal stents have been developed that can be placed in a single step without fluoroscopy. A slimmer endoscope can then be introduced into the necrotic cavity via the stent, and the necrotic debris can be debrided with endoscopic baskets, snares, forceps, and irrigation.9,31
Similar to surgical necrosectomy, satisfactory debridement is not often obtained with a single procedure; 2 to 5 endoscopic procedures may be needed to achieve resolution. However, the luminal approach in endoscopic necrosectomy avoids the significant morbidity of major abdominal surgery and the potential for pancreaticocutaneous fistulae that may occur with drains.
In a randomized trial comparing endoscopic necrosectomy vs surgical necrosectomy (video-assisted retroperitoneal debridement and exploratory laparotomy),32 endoscopic necrosectomy showed less inflammatory response than surgical necrosectomy and had a lower risk of new-onset organ failure, bleeding, fistula formation, and death.32
Selecting the best intervention for the individual patient
Given the multiple available techniques, selecting the best intervention for individual patients can be challenging. A team approach with input from a gastroenterologist, surgeon, and interventional radiologist is best when determining which technique would best suit each patient.
Surgical necrosectomy is still the treatment of choice for unstable patients with infected pancreatic necrosis or multiple, inaccessible collections, but current evidence suggests a different approach in stable infected pancreatic necrosis and symptomatic sterile walled-off pancreatic necrosis.
The Dutch Pancreatitis Group28 randomized 88 patients with infected pancreatic necrosis or symptomatic walled-off pancreatic necrosis to open necrosectomy or a minimally invasive “step-up” approach consisting of up to 2 percutaneous drainage or endoscopic debridement procedures before escalation to video-assisted retroperitoneal debridement. The step-up approach resulted in lower rates of morbidity and death than surgical necrosectomy as first-line treatment. Furthermore, some patients in the step-up group avoided the need for surgery entirely.30
SUMMING UP
Necrosis significantly increases rates of morbidity and mortality in acute pancreatitis. Hospitalists, general internists, and general surgeons are all on the front lines in identifying severe cases and consulting the appropriate specialists for optimal multidisciplinary care. Selective and appropriate timing of radiologic imaging is key, and a vital tool in the management of necrotizing pancreatitis.
While the primary indication for intervention is infected pancreatic necrosis, additional indications are symptomatic walled-off pancreatic necrosis secondary to intractable abdominal pain, bowel obstruction, and failure to thrive. As a result of improving technology and inpatient care, these patients may present with intractable symptoms in the outpatient setting rather than the inpatient setting. The onus is on the primary care physician to maintain a high level of suspicion and refer these patients to subspecialists as appropriate.
Open surgical necrosectomy remains an important approach for care of infected pancreatic necrosis or patients with intractable symptoms. A step-up approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic disease in the United States. Gastroenterology 2015; 149:1731–1741e3.
- Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol 2013; 108:1400–1416.
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, GA, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.
- Banks PA, Bollen TL, Dervenis C, et al; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62:102–111.
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–1652.
- Kadiyala V, Suleiman SL, McNabb-Baltar J, Wu BU, Banks PA, Singh VK. The Atlanta classification, revised Atlanta classification, and determinant-based classification of acute pancreatitis: which is best at stratifying outcomes? Pancreas 2016; 45:510–515.
- Singh VK, Bollen TL, Wu BU, et al. An assessment of the severity of interstitial pancreatitis. Clin Gastroenterol Hepatol 2011; 9:1098–1103.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002; 223:603–613.
- Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 2004; 183:1261–1265.
- Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: literature review. J Comput Assist Tomogr 2015; 39:7–12.
- Shyu JY, Sainani NI, Sahni VA, et al. Necrotizing pancreatitis: diagnosis, imaging, and intervention. Radiographics 2014; 34:1218–1239.
- Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012; 262:751–764.
- Morgan DE, Ragheb CM, Lockhart ME, Cary B, Fineberg NS, Berland LL. Acute pancreatitis: computed tomography utilization and radiation exposure are related to severity but not patient age. Clin Gastroenterol Hepatol 2010; 8:303–308.
- Vitellas KM, Paulson EK, Enns RA, Keogan MT, Pappas TN. Pancreatitis complicated by gland necrosis: evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr 1999; 23:898–905.
- Stimac D, Miletic D, Radic M, et al. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol 2007; 102:997–1004.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010; 11:79–109.
- Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23:336–345.
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820.
- Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev 2006; 4:CD002941.
- Baril NB, Ralls PW, Wren SM, et al. Does an infected peripancreatic fluid collection or abscess mandate operation? Ann Surg 2000; 231:361–367.
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology 2013; 144:333–340.e2.
- Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 2008; 31:957–970.
- De Waele JJ, Hoste E, Blot SI, Decruyenaere J, Colardyn F. Intra-abdominal hypertension in patients with severe acute pancreatitis. Crit Care 2005; 9:R452–R457.
- van Brunschot S, Schut AJ, Bouwense SA, et al; Dutch Pancreatitis Study Group. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas 2014; 43:665–674.
- Bugiantella W, Rondelli F, Boni M, et al. Necrotizing pancreatitis: a review of the interventions. Int J Surg 2016; 28(suppl 1):S163–S171.
- Besselink MG, Verwer TJ, Schoenmaeckers EJ, et al. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg 2007; 142:1194–1201.
- van Santvoort HC, Besselink MG, Horvath KD, et al; Dutch Acute Pancreatis Study Group. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007; 9:156–159.
- van Santvoort HC, Besselink MG, Bollen TL, Buskens E, van Ramshorst B, Gooszen HG; Dutch Acute Pancreatitis Study Group. Case-matched comparison of the retroperitoneal approach with laparotomy for necrotizing pancreatitis. World J Surg 2007; 31:1635–1642.
- van Santvoort HC, Besselink MG, Bakker OJ, et al; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010; 362:1491–1502.
- Thompson CC, Kumar N, Slattery J, et al. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016; 16:66–72.
- Bakker OJ, van Santvoort HC, van Brunschot S, et al; Dutch Pancreatitis Study Group. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012; 307:1053–1061.
KEY POINTS
- Selective and appropriate timing of radiologic imaging is vital in managing necrotizing pancreatitis. Protocols are valuable tools.
- While the primary indication for debridement and drainage in necrotizing pancreatitis is infection, other indications are symptomatic walled-off pancreatic necrosis, intractable abdominal pain, bowel obstruction, and failure to thrive.
- Open surgical necrosectomy remains an important treatment for infected pancreatic necrosis or intractable symptoms.
- A “step-up” approach starting with a minimally invasive procedure and escalating if the initial intervention is unsuccessful is gradually becoming the standard of care.
In reply: Barium esophagography
In Reply: We thank Dr. Keller for his kind remarks and feedback. However, we do not necessarily agree that the case presented was a bad example of a patient to be evaluated with a barium study. While a significant distal mucosal ring was identified on the study as the cause of the patient’s symptoms, this was not known before the examination. This patient could easily have had a subtle peptic stricture as the cause of the dysphagia. It is well known that subtle strictures can be missed with endoscopy. Further, if we knew that the patient had a significant distal mucosal ring before any testing, one could argue that all that was necessary was a dilation. When one knows, after the fact, what the cause of a patient’s symptoms are, one can always retrospectively determine which tests were necessary and which tests were not.
In our experience, we find that a well-performed barium study can identify many abnormalities that further direct a patient’s care. This examination, when performed correctly, provides both functional and anatomic information about the esophagus. We believe that too many patients undergo unnecessary endoscopic procedures and that endoscopy is not necessarily the initial examination in patients with dysphagia. As a result, the barium examination of the esophagus is underused. Furthermore, we view the barium examination and endoscopy as complementary examinations. We realize this is in many respects a philosophy. But Dr. Keller is also expressing a philosophy when he states, “I believe that patients with dysphagia and GERD are best served by initial endoscopy.” We, including most of our gastroenterologists and esophageal surgeons, believe that the barium examination is an important and often the best initial examination in patients with dysphagia.
In Reply: We thank Dr. Keller for his kind remarks and feedback. However, we do not necessarily agree that the case presented was a bad example of a patient to be evaluated with a barium study. While a significant distal mucosal ring was identified on the study as the cause of the patient’s symptoms, this was not known before the examination. This patient could easily have had a subtle peptic stricture as the cause of the dysphagia. It is well known that subtle strictures can be missed with endoscopy. Further, if we knew that the patient had a significant distal mucosal ring before any testing, one could argue that all that was necessary was a dilation. When one knows, after the fact, what the cause of a patient’s symptoms are, one can always retrospectively determine which tests were necessary and which tests were not.
In our experience, we find that a well-performed barium study can identify many abnormalities that further direct a patient’s care. This examination, when performed correctly, provides both functional and anatomic information about the esophagus. We believe that too many patients undergo unnecessary endoscopic procedures and that endoscopy is not necessarily the initial examination in patients with dysphagia. As a result, the barium examination of the esophagus is underused. Furthermore, we view the barium examination and endoscopy as complementary examinations. We realize this is in many respects a philosophy. But Dr. Keller is also expressing a philosophy when he states, “I believe that patients with dysphagia and GERD are best served by initial endoscopy.” We, including most of our gastroenterologists and esophageal surgeons, believe that the barium examination is an important and often the best initial examination in patients with dysphagia.
In Reply: We thank Dr. Keller for his kind remarks and feedback. However, we do not necessarily agree that the case presented was a bad example of a patient to be evaluated with a barium study. While a significant distal mucosal ring was identified on the study as the cause of the patient’s symptoms, this was not known before the examination. This patient could easily have had a subtle peptic stricture as the cause of the dysphagia. It is well known that subtle strictures can be missed with endoscopy. Further, if we knew that the patient had a significant distal mucosal ring before any testing, one could argue that all that was necessary was a dilation. When one knows, after the fact, what the cause of a patient’s symptoms are, one can always retrospectively determine which tests were necessary and which tests were not.
In our experience, we find that a well-performed barium study can identify many abnormalities that further direct a patient’s care. This examination, when performed correctly, provides both functional and anatomic information about the esophagus. We believe that too many patients undergo unnecessary endoscopic procedures and that endoscopy is not necessarily the initial examination in patients with dysphagia. As a result, the barium examination of the esophagus is underused. Furthermore, we view the barium examination and endoscopy as complementary examinations. We realize this is in many respects a philosophy. But Dr. Keller is also expressing a philosophy when he states, “I believe that patients with dysphagia and GERD are best served by initial endoscopy.” We, including most of our gastroenterologists and esophageal surgeons, believe that the barium examination is an important and often the best initial examination in patients with dysphagia.
Role of barium esophagography in evaluating dysphagia
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. The symptoms have occurred several times per year over the last 2 or 3 years and appear to be slowly worsening. She says she has no trouble swallowing liquids. She has a history of gastroesophageal reflux disease, for which she takes a proton pump inhibitor once a day. She says she has had no odynophagia, cough, regurgitation, or weight loss.
How should her symptoms best be evaluated?
DYSPHAGIA CAN BE OROPHARYNGEAL OR ESOPHAGEAL
Dysphagia is the subjective sensation of difficulty swallowing solids, liquids, or both. Symptoms can range from the inability to initiate a swallow to the sensation of esophageal obstruction. Other symptoms of esophageal disease may also be present, such as chest pain, heartburn, and regurgitation. There may also be nonesophageal symptoms related to the disease process causing the dysphagia.
Dysphagia can be separated into oropharyngeal and esophageal types.
Interestingly, many patients with symptoms of oropharyngeal dysphagia in fact have referred symptoms from primary esophageal dysphagia2; many patients with a distal mucosal ring describe a sense of something sticking in the cervical esophagus.
Esophageal dysphagia arises in the mid to distal esophagus or gastric cardia, and as a result, the symptoms are typically retrosternal.1 It can be caused by structural problems such as strictures, rings, webs, extrinsic compression, or a primary esophageal or gastroesophageal neoplasm, or by a primary motility abnormality such as achalasia (Table 1). Eosinophilic esophagitis is now a frequent cause of esophageal dysphagia, especially in white men.3
ESOPHAGOGRAPHY VS ENDOSCOPY IN EVALUATING DYSPHAGIA
Many gastroenterologists recommend endoscopy rather than barium esophagography as the initial examination in patients with dysphagia.4–8 Each test has certain advantages.
Advantages of endoscopy. Endoscopy is superior to esophagography in detecting milder grades of esophagitis. Further, interventions can be performed endoscopically (eg, dilation, biopsy, attachment of a wireless pH testing probe) that cannot be done during a radiographic procedure, and endoscopy does not expose the patient to radiation.
Advantages of esophagography. Endoscopy cannot detect evidence of gastroesophageal reflux disease unless mucosal injury is present. In dysphagia, the radiologic findings correlate well with endoscopic findings, including the detection of esophageal malignancy and moderate to severe esophagitis. Further, motility disorders can be detected with barium esophagography but not with endoscopy.9,10
Subtle abnormalities, especially rings and strictures, may be missed by narrow-diameter (9.8–10 mm) modern upper-endoscopic equipment. Further, esophagography is noninvasive, costs less, and may be more convenient (it does not require sedation and a chaperone for the patient after sedation). This examination also provides dynamic evaluation of the complex process of swallowing. Causes of dysphagia external to the esophagus can also be determined.
In view of the respective advantages and disadvantages of the two methods, we believe that in most instances barium esophagography should be the initial examination,1,9,11–15 and at our institution most patients presenting with dysphagia undergo barium esophagography before they undergo other examinations.14
OBTAIN A HISTORY BEFORE ORDERING ESOPHAGOGRAPHY
Before a barium examination of the esophagus is done, a focused medical history should be obtained, as it can guide the further workup as well as the esophageal study itself.
An attempt should be made to determine whether the dysphagia is oropharyngeal or if it is esophageal, as the former is generally best initially evaluated by a speech and language pathologist. Generally, the physician who orders the test judges whether the patient has oropharyngeal or esophageal dysphagia. Often, both an oropharyngeal examination, performed by a speech and language pathologist, and an esophageal examination, performed by a radiologist, are ordered.
Rapidly progressive symptoms, especially if accompanied by weight loss, should make one suspect cancer. Chronic symptoms usually point to gastroesophageal reflux disease or a motility disorder such as achalasia. Liquid dysphagia almost always means the patient has a motility disorder such as achalasia.
In view of the possibility of eosinophilic esophagitis, one should ask about food or seasonal allergies, especially in young patients with intermittent difficulty swallowing solids.3
BARIUM ESOPHAGOGRAPHY HAS EIGHT SEPARATE PHASES
Barium esophagography is tailored to the patient with dysphagia on the basis of his or her history. The standard examination is divided into eight separate phases (see below).14 Each phase addresses a specific question or questions concerning the structure and function of the esophagus.
At our institution, the first phase of the examination is determined by the presenting symptoms. If the patient has liquid dysphagia, we start with a timed barium swallow to assess esophageal emptying. If the patient does not have liquid dysphagia, we start with an air-contrast mucosal examination.
The patient must be cooperative and mobile to complete all phases of the examination.
Timed barium swallow to measure esophageal emptying
The timed barium swallow is an objective measure of esophageal emptying.16–18 This technique is essential in the initial evaluation of a patient with liquid dysphagia, a symptom common in patients with severe dysmotility, usually achalasia.
We use this examination in our patients with suspected or confirmed achalasia and to follow up patients who have been treated with pneumatic dilatation, botulinum toxin injection, and Heller myotomy.17,18 In addition, this timed test is an objective measure of emptying in patients who have undergone intervention but whose symptoms have not subjectively improved, and can suggest that further intervention may be required.
Air-contrast or mucosal phase
Although this phase is not as sensitive as endoscopy, it can detect masses, mucosal erosions, ulcers, and—most importantly in our experience—fixed hernias. Patients with a fixed hernia have a foreshortened esophagus, which is important to know about before repairing the hernia. Many esophageal surgeons believe that a foreshortened esophagus precludes a standard Nissen fundoplication and necessitates an esophageal lengthening procedure (ie, Collis gastroplasty with a Nissen fundoplication).14
Motility phase
The third phase examines esophageal motility. With the patient in a semiprone position, low-density barium is given in single swallows, separated by 25 to 30 seconds. The images are recorded on digital media to allow one to review them frame by frame.
The findings on this phase correlate well with those of manometry.19 This portion of the examination also uses impedance monitoring to assess bolus transfer, an aspect not evaluated by manometry.20,21 Impedance monitoring detects changes in resistance to current flow and correlates well with esophagraphic findings regarding bolus transfer.
While many patients with dysphagia also undergo esophageal manometry, the findings from this phase of the esophagographic examination may be the first indication of an esophageal motility disorder. In fact, this portion of the examination shows the distinct advantage of esophagography over endoscopy as the initial test in patients with dysphagia, as endoscopy may not identify patients with achalasia, especially early on.4
Single-contrast (full-column) phase to detect strictures, rings
The fourth phase of the esophagographic evaluation is the distended, single-contrast examination (Figure 2B). This is performed in the semiprone position with the patient rapidly drinking thin barium. It is done to detect esophageal strictures, rings, and contour abnormalities caused by extrinsic processes. Subtle abnormalities shown by this technique, including benign strictures and rings, are often not visualized with endoscopy.
Mucosal relief phase
The fifth phase is performed with a collapsed esophagus immediately after the distended, single-contrast phase, where spot films are taken of the barium-coated, collapsed esophagus (Figure 2C). This phase is used to evaluate thickened mucosal folds, a common finding in moderate to severe reflux esophagitis.
Reflux evaluation
Provocative maneuvers are used in the sixth phase to elicit gastroesophageal reflux (Figure 2D). With the patient supine, he or she is asked to roll side to side, do a Valsalva maneuver, and do a straight-leg raise. The patient then sips water in the supine position to assess for reflux (the water siphon test). If reflux is seen, the cause, the height of the reflux, and the duration of reflux retention are recorded.
Solid-bolus phase to assess strictures
In the seventh phase, the patient swallows a 13-mm barium tablet (Figure 2E). This allows one to assess the significance of a ring or stricture and to assess if dysphagia symptoms recur as a result of tablet obstruction. Subtle strictures that were not detected during the prior phases can also be detected using a tablet. If obstruction or impaired passage occurs, the site of obstruction and the presence or absence of symptoms are recorded.
Modified esophagography to assess the oropharynx
The final or eighth phase of barium esophagography is called “modified barium esophagography” or the modified barium swallow. However, it may be the first phase of the examination performed or the only portion of the examination performed, or it may not be performed at all.
Modified barium esophagography is used to define the anatomy of the oropharynx and to assess its function in swallowing.12 It may also guide rehabilitation strategies aimed at eliminating a patient’s swallowing symptoms.
Most patients referred for this test have sustained damage to the central nervous system or structures of the oropharynx, such as stroke or radiation therapy for laryngeal cancer. Many have difficulty in starting to swallow, aspirate when they try to swallow, or both.
The final esophagographic report should document the findings of each phase of the examination (Table 2).
WHAT HAPPENED TO OUR PATIENT?
Our patient underwent barium esophagography (Figure 2). A distal mucosal ring that transiently obstructed a 13-mm tablet was found. The patient underwent endoscopy and the ring was dilated. No biopsies were necessary.
- Levine MS, Rubesin SE. Radiologic investigation of dysphagia. AJR Am J Roentgenol 1990; 154:1157–1163.
- Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998; 171:1361–1365.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Spechler SJ. American Gastroenterological Association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology 1999; 117:229–233.
- American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52:831–837.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Standards of Practice Committee. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007; 66:219–224.
- Halpert RD, Feczko PJ, Spickler EM, Ackerman LV. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985; 157:599–602.
- Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am 1994; 32:1147–1166.
- Ekberg O, Pokieser P. Radiologic evaluation of the dysphagic patient. Eur Radiol 1997; 7:1285–1295.
- Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg 1997; 116:335–338.
- Baker ME, Rice TW. Radiologic evaluation of the esophagus: methods and value in motility disorders and GERD. Semin Thorac Cardiovasc Surg 2001; 13:201–225.
- Baker ME, Einstein DM, Herts BR, et al. Gastroesophageal reflux disease: integrating the barium esophagram before and after antire-flux surgery. Radiology 2007; 243:329–339.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol 2008; 6:11–25.
- deOliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997; 169:473–479.
- Kostic SV, Rice TW, Baker ME, et al. Time barium esophagram: a simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 2000; 120:935–943.
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50:765–770.
- Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 1990; 98:626–632.
- Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, video-esophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005;G1000–G1006.
- Imam H, Baker M, Shay S. Simultaneous barium esophagram, impedance monitoring and manometry in patients with dysphagia due to a tight fundoplication [abstract]. Gastroenterology 2004; 126:A-639.
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. The symptoms have occurred several times per year over the last 2 or 3 years and appear to be slowly worsening. She says she has no trouble swallowing liquids. She has a history of gastroesophageal reflux disease, for which she takes a proton pump inhibitor once a day. She says she has had no odynophagia, cough, regurgitation, or weight loss.
How should her symptoms best be evaluated?
DYSPHAGIA CAN BE OROPHARYNGEAL OR ESOPHAGEAL
Dysphagia is the subjective sensation of difficulty swallowing solids, liquids, or both. Symptoms can range from the inability to initiate a swallow to the sensation of esophageal obstruction. Other symptoms of esophageal disease may also be present, such as chest pain, heartburn, and regurgitation. There may also be nonesophageal symptoms related to the disease process causing the dysphagia.
Dysphagia can be separated into oropharyngeal and esophageal types.
Interestingly, many patients with symptoms of oropharyngeal dysphagia in fact have referred symptoms from primary esophageal dysphagia2; many patients with a distal mucosal ring describe a sense of something sticking in the cervical esophagus.
Esophageal dysphagia arises in the mid to distal esophagus or gastric cardia, and as a result, the symptoms are typically retrosternal.1 It can be caused by structural problems such as strictures, rings, webs, extrinsic compression, or a primary esophageal or gastroesophageal neoplasm, or by a primary motility abnormality such as achalasia (Table 1). Eosinophilic esophagitis is now a frequent cause of esophageal dysphagia, especially in white men.3
ESOPHAGOGRAPHY VS ENDOSCOPY IN EVALUATING DYSPHAGIA
Many gastroenterologists recommend endoscopy rather than barium esophagography as the initial examination in patients with dysphagia.4–8 Each test has certain advantages.
Advantages of endoscopy. Endoscopy is superior to esophagography in detecting milder grades of esophagitis. Further, interventions can be performed endoscopically (eg, dilation, biopsy, attachment of a wireless pH testing probe) that cannot be done during a radiographic procedure, and endoscopy does not expose the patient to radiation.
Advantages of esophagography. Endoscopy cannot detect evidence of gastroesophageal reflux disease unless mucosal injury is present. In dysphagia, the radiologic findings correlate well with endoscopic findings, including the detection of esophageal malignancy and moderate to severe esophagitis. Further, motility disorders can be detected with barium esophagography but not with endoscopy.9,10
Subtle abnormalities, especially rings and strictures, may be missed by narrow-diameter (9.8–10 mm) modern upper-endoscopic equipment. Further, esophagography is noninvasive, costs less, and may be more convenient (it does not require sedation and a chaperone for the patient after sedation). This examination also provides dynamic evaluation of the complex process of swallowing. Causes of dysphagia external to the esophagus can also be determined.
In view of the respective advantages and disadvantages of the two methods, we believe that in most instances barium esophagography should be the initial examination,1,9,11–15 and at our institution most patients presenting with dysphagia undergo barium esophagography before they undergo other examinations.14
OBTAIN A HISTORY BEFORE ORDERING ESOPHAGOGRAPHY
Before a barium examination of the esophagus is done, a focused medical history should be obtained, as it can guide the further workup as well as the esophageal study itself.
An attempt should be made to determine whether the dysphagia is oropharyngeal or if it is esophageal, as the former is generally best initially evaluated by a speech and language pathologist. Generally, the physician who orders the test judges whether the patient has oropharyngeal or esophageal dysphagia. Often, both an oropharyngeal examination, performed by a speech and language pathologist, and an esophageal examination, performed by a radiologist, are ordered.
Rapidly progressive symptoms, especially if accompanied by weight loss, should make one suspect cancer. Chronic symptoms usually point to gastroesophageal reflux disease or a motility disorder such as achalasia. Liquid dysphagia almost always means the patient has a motility disorder such as achalasia.
In view of the possibility of eosinophilic esophagitis, one should ask about food or seasonal allergies, especially in young patients with intermittent difficulty swallowing solids.3
BARIUM ESOPHAGOGRAPHY HAS EIGHT SEPARATE PHASES
Barium esophagography is tailored to the patient with dysphagia on the basis of his or her history. The standard examination is divided into eight separate phases (see below).14 Each phase addresses a specific question or questions concerning the structure and function of the esophagus.
At our institution, the first phase of the examination is determined by the presenting symptoms. If the patient has liquid dysphagia, we start with a timed barium swallow to assess esophageal emptying. If the patient does not have liquid dysphagia, we start with an air-contrast mucosal examination.
The patient must be cooperative and mobile to complete all phases of the examination.
Timed barium swallow to measure esophageal emptying
The timed barium swallow is an objective measure of esophageal emptying.16–18 This technique is essential in the initial evaluation of a patient with liquid dysphagia, a symptom common in patients with severe dysmotility, usually achalasia.
We use this examination in our patients with suspected or confirmed achalasia and to follow up patients who have been treated with pneumatic dilatation, botulinum toxin injection, and Heller myotomy.17,18 In addition, this timed test is an objective measure of emptying in patients who have undergone intervention but whose symptoms have not subjectively improved, and can suggest that further intervention may be required.
Air-contrast or mucosal phase
Although this phase is not as sensitive as endoscopy, it can detect masses, mucosal erosions, ulcers, and—most importantly in our experience—fixed hernias. Patients with a fixed hernia have a foreshortened esophagus, which is important to know about before repairing the hernia. Many esophageal surgeons believe that a foreshortened esophagus precludes a standard Nissen fundoplication and necessitates an esophageal lengthening procedure (ie, Collis gastroplasty with a Nissen fundoplication).14
Motility phase
The third phase examines esophageal motility. With the patient in a semiprone position, low-density barium is given in single swallows, separated by 25 to 30 seconds. The images are recorded on digital media to allow one to review them frame by frame.
The findings on this phase correlate well with those of manometry.19 This portion of the examination also uses impedance monitoring to assess bolus transfer, an aspect not evaluated by manometry.20,21 Impedance monitoring detects changes in resistance to current flow and correlates well with esophagraphic findings regarding bolus transfer.
While many patients with dysphagia also undergo esophageal manometry, the findings from this phase of the esophagographic examination may be the first indication of an esophageal motility disorder. In fact, this portion of the examination shows the distinct advantage of esophagography over endoscopy as the initial test in patients with dysphagia, as endoscopy may not identify patients with achalasia, especially early on.4
Single-contrast (full-column) phase to detect strictures, rings
The fourth phase of the esophagographic evaluation is the distended, single-contrast examination (Figure 2B). This is performed in the semiprone position with the patient rapidly drinking thin barium. It is done to detect esophageal strictures, rings, and contour abnormalities caused by extrinsic processes. Subtle abnormalities shown by this technique, including benign strictures and rings, are often not visualized with endoscopy.
Mucosal relief phase
The fifth phase is performed with a collapsed esophagus immediately after the distended, single-contrast phase, where spot films are taken of the barium-coated, collapsed esophagus (Figure 2C). This phase is used to evaluate thickened mucosal folds, a common finding in moderate to severe reflux esophagitis.
Reflux evaluation
Provocative maneuvers are used in the sixth phase to elicit gastroesophageal reflux (Figure 2D). With the patient supine, he or she is asked to roll side to side, do a Valsalva maneuver, and do a straight-leg raise. The patient then sips water in the supine position to assess for reflux (the water siphon test). If reflux is seen, the cause, the height of the reflux, and the duration of reflux retention are recorded.
Solid-bolus phase to assess strictures
In the seventh phase, the patient swallows a 13-mm barium tablet (Figure 2E). This allows one to assess the significance of a ring or stricture and to assess if dysphagia symptoms recur as a result of tablet obstruction. Subtle strictures that were not detected during the prior phases can also be detected using a tablet. If obstruction or impaired passage occurs, the site of obstruction and the presence or absence of symptoms are recorded.
Modified esophagography to assess the oropharynx
The final or eighth phase of barium esophagography is called “modified barium esophagography” or the modified barium swallow. However, it may be the first phase of the examination performed or the only portion of the examination performed, or it may not be performed at all.
Modified barium esophagography is used to define the anatomy of the oropharynx and to assess its function in swallowing.12 It may also guide rehabilitation strategies aimed at eliminating a patient’s swallowing symptoms.
Most patients referred for this test have sustained damage to the central nervous system or structures of the oropharynx, such as stroke or radiation therapy for laryngeal cancer. Many have difficulty in starting to swallow, aspirate when they try to swallow, or both.
The final esophagographic report should document the findings of each phase of the examination (Table 2).
WHAT HAPPENED TO OUR PATIENT?
Our patient underwent barium esophagography (Figure 2). A distal mucosal ring that transiently obstructed a 13-mm tablet was found. The patient underwent endoscopy and the ring was dilated. No biopsies were necessary.
A 55-year-old woman presents with an intermittent sensation of food getting stuck in her mid to lower chest. The symptoms have occurred several times per year over the last 2 or 3 years and appear to be slowly worsening. She says she has no trouble swallowing liquids. She has a history of gastroesophageal reflux disease, for which she takes a proton pump inhibitor once a day. She says she has had no odynophagia, cough, regurgitation, or weight loss.
How should her symptoms best be evaluated?
DYSPHAGIA CAN BE OROPHARYNGEAL OR ESOPHAGEAL
Dysphagia is the subjective sensation of difficulty swallowing solids, liquids, or both. Symptoms can range from the inability to initiate a swallow to the sensation of esophageal obstruction. Other symptoms of esophageal disease may also be present, such as chest pain, heartburn, and regurgitation. There may also be nonesophageal symptoms related to the disease process causing the dysphagia.
Dysphagia can be separated into oropharyngeal and esophageal types.
Interestingly, many patients with symptoms of oropharyngeal dysphagia in fact have referred symptoms from primary esophageal dysphagia2; many patients with a distal mucosal ring describe a sense of something sticking in the cervical esophagus.
Esophageal dysphagia arises in the mid to distal esophagus or gastric cardia, and as a result, the symptoms are typically retrosternal.1 It can be caused by structural problems such as strictures, rings, webs, extrinsic compression, or a primary esophageal or gastroesophageal neoplasm, or by a primary motility abnormality such as achalasia (Table 1). Eosinophilic esophagitis is now a frequent cause of esophageal dysphagia, especially in white men.3
ESOPHAGOGRAPHY VS ENDOSCOPY IN EVALUATING DYSPHAGIA
Many gastroenterologists recommend endoscopy rather than barium esophagography as the initial examination in patients with dysphagia.4–8 Each test has certain advantages.
Advantages of endoscopy. Endoscopy is superior to esophagography in detecting milder grades of esophagitis. Further, interventions can be performed endoscopically (eg, dilation, biopsy, attachment of a wireless pH testing probe) that cannot be done during a radiographic procedure, and endoscopy does not expose the patient to radiation.
Advantages of esophagography. Endoscopy cannot detect evidence of gastroesophageal reflux disease unless mucosal injury is present. In dysphagia, the radiologic findings correlate well with endoscopic findings, including the detection of esophageal malignancy and moderate to severe esophagitis. Further, motility disorders can be detected with barium esophagography but not with endoscopy.9,10
Subtle abnormalities, especially rings and strictures, may be missed by narrow-diameter (9.8–10 mm) modern upper-endoscopic equipment. Further, esophagography is noninvasive, costs less, and may be more convenient (it does not require sedation and a chaperone for the patient after sedation). This examination also provides dynamic evaluation of the complex process of swallowing. Causes of dysphagia external to the esophagus can also be determined.
In view of the respective advantages and disadvantages of the two methods, we believe that in most instances barium esophagography should be the initial examination,1,9,11–15 and at our institution most patients presenting with dysphagia undergo barium esophagography before they undergo other examinations.14
OBTAIN A HISTORY BEFORE ORDERING ESOPHAGOGRAPHY
Before a barium examination of the esophagus is done, a focused medical history should be obtained, as it can guide the further workup as well as the esophageal study itself.
An attempt should be made to determine whether the dysphagia is oropharyngeal or if it is esophageal, as the former is generally best initially evaluated by a speech and language pathologist. Generally, the physician who orders the test judges whether the patient has oropharyngeal or esophageal dysphagia. Often, both an oropharyngeal examination, performed by a speech and language pathologist, and an esophageal examination, performed by a radiologist, are ordered.
Rapidly progressive symptoms, especially if accompanied by weight loss, should make one suspect cancer. Chronic symptoms usually point to gastroesophageal reflux disease or a motility disorder such as achalasia. Liquid dysphagia almost always means the patient has a motility disorder such as achalasia.
In view of the possibility of eosinophilic esophagitis, one should ask about food or seasonal allergies, especially in young patients with intermittent difficulty swallowing solids.3
BARIUM ESOPHAGOGRAPHY HAS EIGHT SEPARATE PHASES
Barium esophagography is tailored to the patient with dysphagia on the basis of his or her history. The standard examination is divided into eight separate phases (see below).14 Each phase addresses a specific question or questions concerning the structure and function of the esophagus.
At our institution, the first phase of the examination is determined by the presenting symptoms. If the patient has liquid dysphagia, we start with a timed barium swallow to assess esophageal emptying. If the patient does not have liquid dysphagia, we start with an air-contrast mucosal examination.
The patient must be cooperative and mobile to complete all phases of the examination.
Timed barium swallow to measure esophageal emptying
The timed barium swallow is an objective measure of esophageal emptying.16–18 This technique is essential in the initial evaluation of a patient with liquid dysphagia, a symptom common in patients with severe dysmotility, usually achalasia.
We use this examination in our patients with suspected or confirmed achalasia and to follow up patients who have been treated with pneumatic dilatation, botulinum toxin injection, and Heller myotomy.17,18 In addition, this timed test is an objective measure of emptying in patients who have undergone intervention but whose symptoms have not subjectively improved, and can suggest that further intervention may be required.
Air-contrast or mucosal phase
Although this phase is not as sensitive as endoscopy, it can detect masses, mucosal erosions, ulcers, and—most importantly in our experience—fixed hernias. Patients with a fixed hernia have a foreshortened esophagus, which is important to know about before repairing the hernia. Many esophageal surgeons believe that a foreshortened esophagus precludes a standard Nissen fundoplication and necessitates an esophageal lengthening procedure (ie, Collis gastroplasty with a Nissen fundoplication).14
Motility phase
The third phase examines esophageal motility. With the patient in a semiprone position, low-density barium is given in single swallows, separated by 25 to 30 seconds. The images are recorded on digital media to allow one to review them frame by frame.
The findings on this phase correlate well with those of manometry.19 This portion of the examination also uses impedance monitoring to assess bolus transfer, an aspect not evaluated by manometry.20,21 Impedance monitoring detects changes in resistance to current flow and correlates well with esophagraphic findings regarding bolus transfer.
While many patients with dysphagia also undergo esophageal manometry, the findings from this phase of the esophagographic examination may be the first indication of an esophageal motility disorder. In fact, this portion of the examination shows the distinct advantage of esophagography over endoscopy as the initial test in patients with dysphagia, as endoscopy may not identify patients with achalasia, especially early on.4
Single-contrast (full-column) phase to detect strictures, rings
The fourth phase of the esophagographic evaluation is the distended, single-contrast examination (Figure 2B). This is performed in the semiprone position with the patient rapidly drinking thin barium. It is done to detect esophageal strictures, rings, and contour abnormalities caused by extrinsic processes. Subtle abnormalities shown by this technique, including benign strictures and rings, are often not visualized with endoscopy.
Mucosal relief phase
The fifth phase is performed with a collapsed esophagus immediately after the distended, single-contrast phase, where spot films are taken of the barium-coated, collapsed esophagus (Figure 2C). This phase is used to evaluate thickened mucosal folds, a common finding in moderate to severe reflux esophagitis.
Reflux evaluation
Provocative maneuvers are used in the sixth phase to elicit gastroesophageal reflux (Figure 2D). With the patient supine, he or she is asked to roll side to side, do a Valsalva maneuver, and do a straight-leg raise. The patient then sips water in the supine position to assess for reflux (the water siphon test). If reflux is seen, the cause, the height of the reflux, and the duration of reflux retention are recorded.
Solid-bolus phase to assess strictures
In the seventh phase, the patient swallows a 13-mm barium tablet (Figure 2E). This allows one to assess the significance of a ring or stricture and to assess if dysphagia symptoms recur as a result of tablet obstruction. Subtle strictures that were not detected during the prior phases can also be detected using a tablet. If obstruction or impaired passage occurs, the site of obstruction and the presence or absence of symptoms are recorded.
Modified esophagography to assess the oropharynx
The final or eighth phase of barium esophagography is called “modified barium esophagography” or the modified barium swallow. However, it may be the first phase of the examination performed or the only portion of the examination performed, or it may not be performed at all.
Modified barium esophagography is used to define the anatomy of the oropharynx and to assess its function in swallowing.12 It may also guide rehabilitation strategies aimed at eliminating a patient’s swallowing symptoms.
Most patients referred for this test have sustained damage to the central nervous system or structures of the oropharynx, such as stroke or radiation therapy for laryngeal cancer. Many have difficulty in starting to swallow, aspirate when they try to swallow, or both.
The final esophagographic report should document the findings of each phase of the examination (Table 2).
WHAT HAPPENED TO OUR PATIENT?
Our patient underwent barium esophagography (Figure 2). A distal mucosal ring that transiently obstructed a 13-mm tablet was found. The patient underwent endoscopy and the ring was dilated. No biopsies were necessary.
- Levine MS, Rubesin SE. Radiologic investigation of dysphagia. AJR Am J Roentgenol 1990; 154:1157–1163.
- Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998; 171:1361–1365.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Spechler SJ. American Gastroenterological Association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology 1999; 117:229–233.
- American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52:831–837.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Standards of Practice Committee. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007; 66:219–224.
- Halpert RD, Feczko PJ, Spickler EM, Ackerman LV. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985; 157:599–602.
- Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am 1994; 32:1147–1166.
- Ekberg O, Pokieser P. Radiologic evaluation of the dysphagic patient. Eur Radiol 1997; 7:1285–1295.
- Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg 1997; 116:335–338.
- Baker ME, Rice TW. Radiologic evaluation of the esophagus: methods and value in motility disorders and GERD. Semin Thorac Cardiovasc Surg 2001; 13:201–225.
- Baker ME, Einstein DM, Herts BR, et al. Gastroesophageal reflux disease: integrating the barium esophagram before and after antire-flux surgery. Radiology 2007; 243:329–339.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol 2008; 6:11–25.
- deOliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997; 169:473–479.
- Kostic SV, Rice TW, Baker ME, et al. Time barium esophagram: a simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 2000; 120:935–943.
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50:765–770.
- Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 1990; 98:626–632.
- Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, video-esophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005;G1000–G1006.
- Imam H, Baker M, Shay S. Simultaneous barium esophagram, impedance monitoring and manometry in patients with dysphagia due to a tight fundoplication [abstract]. Gastroenterology 2004; 126:A-639.
- Levine MS, Rubesin SE. Radiologic investigation of dysphagia. AJR Am J Roentgenol 1990; 154:1157–1163.
- Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol 1998; 171:1361–1365.
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007; 133:1342–1363.
- Spechler SJ. American Gastroenterological Association medical position statement on treatment of patients with dysphagia caused by benign disorders of the distal esophagus. Gastroenterology 1999; 117:229–233.
- American Society for Gastrointestinal Endoscopy. Appropriate use of gastrointestinal endoscopy. Gastrointest Endosc 2000; 52:831–837.
- Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol 2002; 97:2733–2737.
- Varadarajulu S, Eloubeidi MA, Patel RS, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc 2005; 61:804–808.
- Standards of Practice Committee. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007; 66:219–224.
- Halpert RD, Feczko PJ, Spickler EM, Ackerman LV. Radiological assessment of dysphagia with endoscopic correlation. Radiology 1985; 157:599–602.
- Ott DJ. Gastroesophageal reflux disease. Radiol Clin North Am 1994; 32:1147–1166.
- Ekberg O, Pokieser P. Radiologic evaluation of the dysphagic patient. Eur Radiol 1997; 7:1285–1295.
- Logemann JA. Role of the modified barium swallow in management of patients with dysphagia. Otolaryngol Head Neck Surg 1997; 116:335–338.
- Baker ME, Rice TW. Radiologic evaluation of the esophagus: methods and value in motility disorders and GERD. Semin Thorac Cardiovasc Surg 2001; 13:201–225.
- Baker ME, Einstein DM, Herts BR, et al. Gastroesophageal reflux disease: integrating the barium esophagram before and after antire-flux surgery. Radiology 2007; 243:329–339.
- Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol 2008; 6:11–25.
- deOliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. AJR Am J Roentgenol 1997; 169:473–479.
- Kostic SV, Rice TW, Baker ME, et al. Time barium esophagram: a simple physiologic assessment for achalasia. J Thorac Cardiovasc Surg 2000; 120:935–943.
- Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50:765–770.
- Hewson EG, Ott DJ, Dalton CB, Chen YM, Wu WC, Richter JE. Manometry and radiology. Complementary studies in the assessment of esophageal motility disorders. Gastroenterology 1990; 98:626–632.
- Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, video-esophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol 2005;G1000–G1006.
- Imam H, Baker M, Shay S. Simultaneous barium esophagram, impedance monitoring and manometry in patients with dysphagia due to a tight fundoplication [abstract]. Gastroenterology 2004; 126:A-639.
KEY POINTS
- Dysphagia can be due to problems in the oropharynx and cervical esophagus or in the distal esophagus.
- Radiologic evaluation of dysphagia has distinct advantages over endoscopy, including its ability to diagnose both structural changes and motility disorders.
- A barium evaluation can include a modified barium-swallowing study to evaluate the oropharynx, barium esophagography to evaluate the esophagus, and a timed study to evaluate esophageal emptying.
- Often, the true cause of dysphagia is best approached with a combination of radiographic and endoscopic studies.