User login
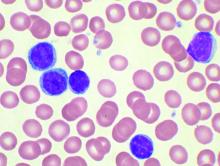
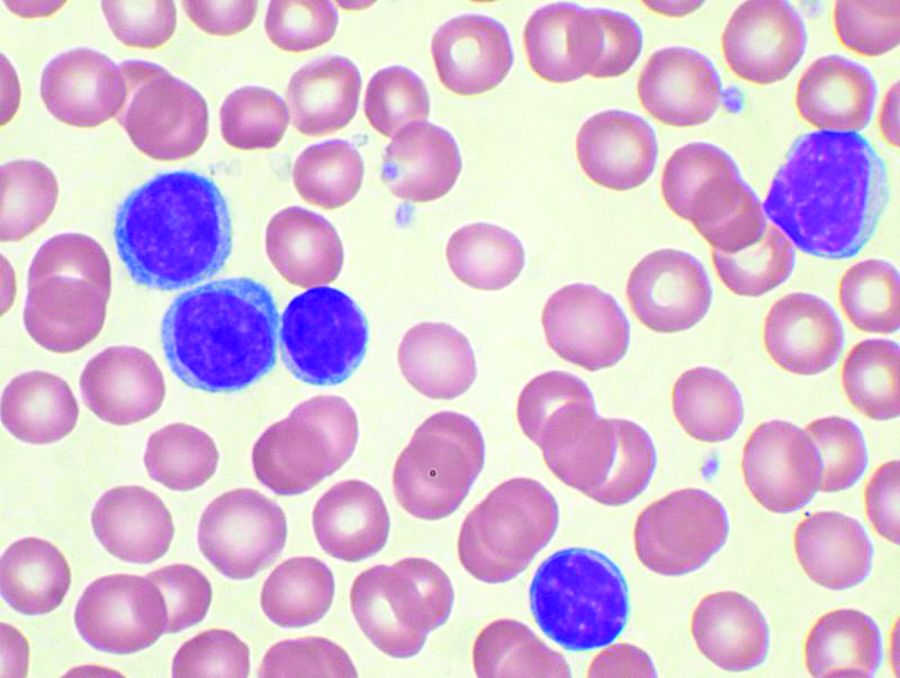
Short telomeres predict poorer response to chemo in CLL
A telomere-length analysis tool appears to identify reliably which chronic lymphocytic leukemia (CLL) patients will benefit from frontline chemotherapy, according to an analysis of 260 patients across two separate trials.
The analysis compared the use of high-throughput, single telomere–length analysis (HTSTELA) with other commonly used markers including beta-2 microglobulin, fluorescence-in-situ hybridization (FISH) cytogenetics, CD38 expression, ZAP70 expression, and IGHV mutation status. The researchers looked specifically at whether telomere length could predict response to frontline treatment with fludarabine, cyclophosphamide, rituximab (FCR)–based regimens.
“[T]elomere length is a powerful predictor of both [progression-free survival] and [overall survival] in patients treated with FCR-based therapies. In contrast, CD38 expression and beta-2 microglobulin expression were not predictive, and IGHV mutation status was only predictive of PFS (progression-free survival),” Kevin Norris, PhD, of Cardiff (Wales) University and his colleagues wrote in Leukemia.
Previous studies have shown that telomere-length analysis offers independent prognostic information in all stages of CLL. In the present study, the researchers used HTSTELA to analyze patient samples taken from two concurrent, phase 2 clinical trials of frontline FCR-based treatment – ARCTIC and ADMIRE.
The researchers divided the cohort based on a threshold of telomere dysfunction – the point at which the chromosome end-capping function is lost and there is genomic instability. Shorter telomeres are inside the fusogenic range (TL-IFR) and longer telomeres are outside fusogenic range (TL-OFR).
Patients with TL-IFR had significantly shorter PFS on FCR-based treatment (P less than .0001). They also had reduced overall survival (OS; P = .0002). In the same cohort of patients, IGHV mutation status was predictive of PFS (P = .0016), but it was not predictive for OS (P = .38), while CD38 and beta-2 microglobulin were not predictive of PFS or OS.
The researchers also looked at the value of telomere length in predicting outcomes among IGHV-mutated and -unmutated patients.
Patients with IGHV-mutated disease and TL-IFR had worse PFS and OS than did patients with TL-OFR. TL-IFR patients in this cohort were more likely to progress (hazard ratio, 4.35; P less than .0001) and more likely to die from their disease (HR, 3.81; P = .006).
“Although the number of IGHV-mutated patients with TL-IFR was relatively small (n = 16), our data suggests that telomere length can identify a subset of “bad risk” IGHV-mutated patients who do not respond well to FCR,” the researchers wrote.
Among IGHV unmutated patients, those with short telomeres had worse PFS (HR, 1.48; P = .08) and OS (HR, 2.18; P = .025) than did those with longer telomeres.
In multivariate modeling of all the potential markers, telomere length was the statistically significant dominant covariable for both PFS and OS.
The study was funded by a Bloodwise grant and the Wales Cancer Research Center. Dr. Norris and three coauthors reported that they are coinventors of patents relevant to the study and hold shares in a company set to provide telomere length testing.
SOURCE: Norris K et al. Leukemia. 2019 Jan 30. doi: 10.1038/s41375-019-0389-9.
A telomere-length analysis tool appears to identify reliably which chronic lymphocytic leukemia (CLL) patients will benefit from frontline chemotherapy, according to an analysis of 260 patients across two separate trials.
The analysis compared the use of high-throughput, single telomere–length analysis (HTSTELA) with other commonly used markers including beta-2 microglobulin, fluorescence-in-situ hybridization (FISH) cytogenetics, CD38 expression, ZAP70 expression, and IGHV mutation status. The researchers looked specifically at whether telomere length could predict response to frontline treatment with fludarabine, cyclophosphamide, rituximab (FCR)–based regimens.
“[T]elomere length is a powerful predictor of both [progression-free survival] and [overall survival] in patients treated with FCR-based therapies. In contrast, CD38 expression and beta-2 microglobulin expression were not predictive, and IGHV mutation status was only predictive of PFS (progression-free survival),” Kevin Norris, PhD, of Cardiff (Wales) University and his colleagues wrote in Leukemia.
Previous studies have shown that telomere-length analysis offers independent prognostic information in all stages of CLL. In the present study, the researchers used HTSTELA to analyze patient samples taken from two concurrent, phase 2 clinical trials of frontline FCR-based treatment – ARCTIC and ADMIRE.
The researchers divided the cohort based on a threshold of telomere dysfunction – the point at which the chromosome end-capping function is lost and there is genomic instability. Shorter telomeres are inside the fusogenic range (TL-IFR) and longer telomeres are outside fusogenic range (TL-OFR).
Patients with TL-IFR had significantly shorter PFS on FCR-based treatment (P less than .0001). They also had reduced overall survival (OS; P = .0002). In the same cohort of patients, IGHV mutation status was predictive of PFS (P = .0016), but it was not predictive for OS (P = .38), while CD38 and beta-2 microglobulin were not predictive of PFS or OS.
The researchers also looked at the value of telomere length in predicting outcomes among IGHV-mutated and -unmutated patients.
Patients with IGHV-mutated disease and TL-IFR had worse PFS and OS than did patients with TL-OFR. TL-IFR patients in this cohort were more likely to progress (hazard ratio, 4.35; P less than .0001) and more likely to die from their disease (HR, 3.81; P = .006).
“Although the number of IGHV-mutated patients with TL-IFR was relatively small (n = 16), our data suggests that telomere length can identify a subset of “bad risk” IGHV-mutated patients who do not respond well to FCR,” the researchers wrote.
Among IGHV unmutated patients, those with short telomeres had worse PFS (HR, 1.48; P = .08) and OS (HR, 2.18; P = .025) than did those with longer telomeres.
In multivariate modeling of all the potential markers, telomere length was the statistically significant dominant covariable for both PFS and OS.
The study was funded by a Bloodwise grant and the Wales Cancer Research Center. Dr. Norris and three coauthors reported that they are coinventors of patents relevant to the study and hold shares in a company set to provide telomere length testing.
SOURCE: Norris K et al. Leukemia. 2019 Jan 30. doi: 10.1038/s41375-019-0389-9.
A telomere-length analysis tool appears to identify reliably which chronic lymphocytic leukemia (CLL) patients will benefit from frontline chemotherapy, according to an analysis of 260 patients across two separate trials.
The analysis compared the use of high-throughput, single telomere–length analysis (HTSTELA) with other commonly used markers including beta-2 microglobulin, fluorescence-in-situ hybridization (FISH) cytogenetics, CD38 expression, ZAP70 expression, and IGHV mutation status. The researchers looked specifically at whether telomere length could predict response to frontline treatment with fludarabine, cyclophosphamide, rituximab (FCR)–based regimens.
“[T]elomere length is a powerful predictor of both [progression-free survival] and [overall survival] in patients treated with FCR-based therapies. In contrast, CD38 expression and beta-2 microglobulin expression were not predictive, and IGHV mutation status was only predictive of PFS (progression-free survival),” Kevin Norris, PhD, of Cardiff (Wales) University and his colleagues wrote in Leukemia.
Previous studies have shown that telomere-length analysis offers independent prognostic information in all stages of CLL. In the present study, the researchers used HTSTELA to analyze patient samples taken from two concurrent, phase 2 clinical trials of frontline FCR-based treatment – ARCTIC and ADMIRE.
The researchers divided the cohort based on a threshold of telomere dysfunction – the point at which the chromosome end-capping function is lost and there is genomic instability. Shorter telomeres are inside the fusogenic range (TL-IFR) and longer telomeres are outside fusogenic range (TL-OFR).
Patients with TL-IFR had significantly shorter PFS on FCR-based treatment (P less than .0001). They also had reduced overall survival (OS; P = .0002). In the same cohort of patients, IGHV mutation status was predictive of PFS (P = .0016), but it was not predictive for OS (P = .38), while CD38 and beta-2 microglobulin were not predictive of PFS or OS.
The researchers also looked at the value of telomere length in predicting outcomes among IGHV-mutated and -unmutated patients.
Patients with IGHV-mutated disease and TL-IFR had worse PFS and OS than did patients with TL-OFR. TL-IFR patients in this cohort were more likely to progress (hazard ratio, 4.35; P less than .0001) and more likely to die from their disease (HR, 3.81; P = .006).
“Although the number of IGHV-mutated patients with TL-IFR was relatively small (n = 16), our data suggests that telomere length can identify a subset of “bad risk” IGHV-mutated patients who do not respond well to FCR,” the researchers wrote.
Among IGHV unmutated patients, those with short telomeres had worse PFS (HR, 1.48; P = .08) and OS (HR, 2.18; P = .025) than did those with longer telomeres.
In multivariate modeling of all the potential markers, telomere length was the statistically significant dominant covariable for both PFS and OS.
The study was funded by a Bloodwise grant and the Wales Cancer Research Center. Dr. Norris and three coauthors reported that they are coinventors of patents relevant to the study and hold shares in a company set to provide telomere length testing.
SOURCE: Norris K et al. Leukemia. 2019 Jan 30. doi: 10.1038/s41375-019-0389-9.
FROM LEUKEMIA
Bendamustine-rituximab shines in frontline treatment of MCL, iNHL
Frontline treatment with patients in the BRIGHT study.
The bendamustine-rituximab (BR) regimen had superior 5-year progression-free survival rates, event-free survival, and duration of response, compared with either rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine, and prednisone (R-CVP). The follow-up study did not find a significant difference in overall survival, however.
While the cumulative evidence from BRIGHT and other studies supports BR as a first-line treatment option for patients with indolent non-Hodgkin lymphoma (iNHL) and mantle cell lymphoma (MCL), the lack of an overall survival benefit indicates that the sequence of BR and R-CHOP or R-CVP “may not be critical,” Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute in Nashville, and his colleagues wrote in the Journal of Clinical Oncology.
“[The] choice of regimen for the initial treatment of iNHL may be driven more by patient preferences regarding the differences in toxicity profile,” the researchers wrote.
Initial results from the BRIGHT study found that BR was noninferior to R-CHOP/R-CVP in terms of complete response rate (P = .0225 for noninferiority). The present study includes outcomes data for at least 5 years after completion of the study treatment.
For the entire study, the median follow-up was 65.0 months for patients in the BR group and 64.1 months for patients in the R-CHOP/R-CVP group. Overall, the intention-to-treat population included 224 patients receiving BR and 223 patients receiving R-CHOP and R-CVP.
The median time to progression was not reached in either treatment group. The 5-year progression-free survival (PFS) rates were 65.5% in the BR group and 55.8% in the R-CHOP/R-CVP group. The difference between these rates was significant, with a hazard ratio of 0.61 (95% confidence interval, 0.45-0.85; P = .0025).
Similarly, event-free survival was better in the BR group versus the R-CHOP/R-CVP group (HR, 0.63; 95% CI, 0.46-0.84; P = .0020). Duration of response also favored the BR treatment regimen (HR, 0.66; 95% CI, 0.47-0.92; P = .0134).
The long-term follow-up showed no significant difference in overall survival, with an HR of 1.15 for BR versus R-CHOP/R-CVP (95% CI, 0.72-1.84; P = .5461). Overall, there were 40 deaths in the BR treatment group and 32 deaths in the R-CHOP/R-CVP group.
Whether patients received maintenance rituximab did not affect the overall survival between groups. Similarly, there was no difference in overall survival by lymphoma type.
“Benefit from BR treatment did not translate to prolonged [overall survival], possibly because of the subsequent lines of therapy, including the use of BR in patients in the R-CHOP/R-CVP group,” the researchers wrote.
In terms of safety, the follow-up data showed no significant difference in early non–disease-related mortality between the treatment groups. However, the BRIGHT study showed higher rates of secondary malignancies in the BR group, compared with R-CHOP/R-CVP. That finding was not seen in the Study Group of Indolent Lymphomas Non-Hodgkin Lymphoma (StiL NHL) 1 trial, and the authors could not provide an explanation for the increase in their research.
This study was supported by Teva Pharmaceuticals. Dr. Flinn reported receiving institutional research funding from Teva and receiving institutional research funding from or serving as a consultant to several other companies.
SOURCE: Flinn IW et al. J Clin Oncol. 2019 Feb 27. doi: 10.1200/JCO.18.00605.
Frontline treatment with patients in the BRIGHT study.
The bendamustine-rituximab (BR) regimen had superior 5-year progression-free survival rates, event-free survival, and duration of response, compared with either rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine, and prednisone (R-CVP). The follow-up study did not find a significant difference in overall survival, however.
While the cumulative evidence from BRIGHT and other studies supports BR as a first-line treatment option for patients with indolent non-Hodgkin lymphoma (iNHL) and mantle cell lymphoma (MCL), the lack of an overall survival benefit indicates that the sequence of BR and R-CHOP or R-CVP “may not be critical,” Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute in Nashville, and his colleagues wrote in the Journal of Clinical Oncology.
“[The] choice of regimen for the initial treatment of iNHL may be driven more by patient preferences regarding the differences in toxicity profile,” the researchers wrote.
Initial results from the BRIGHT study found that BR was noninferior to R-CHOP/R-CVP in terms of complete response rate (P = .0225 for noninferiority). The present study includes outcomes data for at least 5 years after completion of the study treatment.
For the entire study, the median follow-up was 65.0 months for patients in the BR group and 64.1 months for patients in the R-CHOP/R-CVP group. Overall, the intention-to-treat population included 224 patients receiving BR and 223 patients receiving R-CHOP and R-CVP.
The median time to progression was not reached in either treatment group. The 5-year progression-free survival (PFS) rates were 65.5% in the BR group and 55.8% in the R-CHOP/R-CVP group. The difference between these rates was significant, with a hazard ratio of 0.61 (95% confidence interval, 0.45-0.85; P = .0025).
Similarly, event-free survival was better in the BR group versus the R-CHOP/R-CVP group (HR, 0.63; 95% CI, 0.46-0.84; P = .0020). Duration of response also favored the BR treatment regimen (HR, 0.66; 95% CI, 0.47-0.92; P = .0134).
The long-term follow-up showed no significant difference in overall survival, with an HR of 1.15 for BR versus R-CHOP/R-CVP (95% CI, 0.72-1.84; P = .5461). Overall, there were 40 deaths in the BR treatment group and 32 deaths in the R-CHOP/R-CVP group.
Whether patients received maintenance rituximab did not affect the overall survival between groups. Similarly, there was no difference in overall survival by lymphoma type.
“Benefit from BR treatment did not translate to prolonged [overall survival], possibly because of the subsequent lines of therapy, including the use of BR in patients in the R-CHOP/R-CVP group,” the researchers wrote.
In terms of safety, the follow-up data showed no significant difference in early non–disease-related mortality between the treatment groups. However, the BRIGHT study showed higher rates of secondary malignancies in the BR group, compared with R-CHOP/R-CVP. That finding was not seen in the Study Group of Indolent Lymphomas Non-Hodgkin Lymphoma (StiL NHL) 1 trial, and the authors could not provide an explanation for the increase in their research.
This study was supported by Teva Pharmaceuticals. Dr. Flinn reported receiving institutional research funding from Teva and receiving institutional research funding from or serving as a consultant to several other companies.
SOURCE: Flinn IW et al. J Clin Oncol. 2019 Feb 27. doi: 10.1200/JCO.18.00605.
Frontline treatment with patients in the BRIGHT study.
The bendamustine-rituximab (BR) regimen had superior 5-year progression-free survival rates, event-free survival, and duration of response, compared with either rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine, and prednisone (R-CVP). The follow-up study did not find a significant difference in overall survival, however.
While the cumulative evidence from BRIGHT and other studies supports BR as a first-line treatment option for patients with indolent non-Hodgkin lymphoma (iNHL) and mantle cell lymphoma (MCL), the lack of an overall survival benefit indicates that the sequence of BR and R-CHOP or R-CVP “may not be critical,” Ian W. Flinn, MD, PhD, of Sarah Cannon Research Institute in Nashville, and his colleagues wrote in the Journal of Clinical Oncology.
“[The] choice of regimen for the initial treatment of iNHL may be driven more by patient preferences regarding the differences in toxicity profile,” the researchers wrote.
Initial results from the BRIGHT study found that BR was noninferior to R-CHOP/R-CVP in terms of complete response rate (P = .0225 for noninferiority). The present study includes outcomes data for at least 5 years after completion of the study treatment.
For the entire study, the median follow-up was 65.0 months for patients in the BR group and 64.1 months for patients in the R-CHOP/R-CVP group. Overall, the intention-to-treat population included 224 patients receiving BR and 223 patients receiving R-CHOP and R-CVP.
The median time to progression was not reached in either treatment group. The 5-year progression-free survival (PFS) rates were 65.5% in the BR group and 55.8% in the R-CHOP/R-CVP group. The difference between these rates was significant, with a hazard ratio of 0.61 (95% confidence interval, 0.45-0.85; P = .0025).
Similarly, event-free survival was better in the BR group versus the R-CHOP/R-CVP group (HR, 0.63; 95% CI, 0.46-0.84; P = .0020). Duration of response also favored the BR treatment regimen (HR, 0.66; 95% CI, 0.47-0.92; P = .0134).
The long-term follow-up showed no significant difference in overall survival, with an HR of 1.15 for BR versus R-CHOP/R-CVP (95% CI, 0.72-1.84; P = .5461). Overall, there were 40 deaths in the BR treatment group and 32 deaths in the R-CHOP/R-CVP group.
Whether patients received maintenance rituximab did not affect the overall survival between groups. Similarly, there was no difference in overall survival by lymphoma type.
“Benefit from BR treatment did not translate to prolonged [overall survival], possibly because of the subsequent lines of therapy, including the use of BR in patients in the R-CHOP/R-CVP group,” the researchers wrote.
In terms of safety, the follow-up data showed no significant difference in early non–disease-related mortality between the treatment groups. However, the BRIGHT study showed higher rates of secondary malignancies in the BR group, compared with R-CHOP/R-CVP. That finding was not seen in the Study Group of Indolent Lymphomas Non-Hodgkin Lymphoma (StiL NHL) 1 trial, and the authors could not provide an explanation for the increase in their research.
This study was supported by Teva Pharmaceuticals. Dr. Flinn reported receiving institutional research funding from Teva and receiving institutional research funding from or serving as a consultant to several other companies.
SOURCE: Flinn IW et al. J Clin Oncol. 2019 Feb 27. doi: 10.1200/JCO.18.00605.
FROM JOURNAL OF CLINICAL ONCOLOGY
Turoctocog alfa appears safe, effective in reducing bleeds
Turoctocog alfa, a recombinant Factor VIII molecule, was effective in preventing bleeding episodes and was well tolerated in more than 200 males with hemophilia A who were part of the Guardian 2 extension trial.
The open label, phase 3b study included patients who had completed the Guardian 1 or Guardian 3 phase 1 pharmacokinetics trials and who chose to continue prophylaxis with turoctocog alfa (Novoeight).
In total, 214 patients were enrolled and 213 were exposed to treatment. Prophylaxis was given as a standard dose level of 20-50 IU/kg once every second day or 20-60 IU/kg three times weekly. Less frequent prophylaxis was available at 40-60 IU/kg twice weekly or once every third day. For on-demand treatment, the recommended dose level was 20-50 IU/kg.
“The final results of guardian 2 are consistent with data from previous interim analyses,” Steven R. Lentz, MD, PhD, of the University of Iowa, Iowa City, and his colleagues wrote in Haemophilia. “Turoctocog alfa was well tolerated, with no unexpected safety signals and no development of FVIII inhibitors.”
Among the 207 patients on prophylaxis, 1,782 bleeds were reported. The overall median annualized bleeding rate (ABR) for this group was 1.37. More than 88% of these bleeds were stopped with one or two injections and the success rate for treatment of bleeds was more than 90%.
The median ABR dropped to zero, with a range of 0.00‐9.91, for the 27 patients who were on the less-frequent prophylaxis regimen. In this group, more than 75% of the 34 bleeds that occurred were stopped with one injection and the success rate for treatment of bleeds was about 94%.
“These data suggest a subset of patients might be well managed with less‐frequent dosing,” the researchers wrote.
For the 19 patients who received on-demand treatment, there were 391 bleeds reported. The overall median ABR was 30.44. Nearly 80% of bleeds were stopped with one injection and more than 15% with two injections. The treatment success rate in this group was more than 96%.
There were no Factor VIII inhibitors reported in the extension trial.
In total, there were 1,260 adverse events reported for 183 patients, corresponding to less than two adverse events per patients per years of exposure to the treatment.
The most common adverse event, seen in 14.6% of patients, was nasopharyngitis. Serious adverse events occurred in 18.3% of patients but all expect one event was deemed unlikely to be related to treatment. The one fatal event in the trial was deemed unlikely to be related to treatment.
The study was funded by Novo Nordisk. Dr. Lentz reported being a paid consultant to Novo Nordisk and receiving research funding from the company.
SOURCE: Lentz SR et al. Haemophilia. 2018 Nov;24(6):e391-4.
Turoctocog alfa, a recombinant Factor VIII molecule, was effective in preventing bleeding episodes and was well tolerated in more than 200 males with hemophilia A who were part of the Guardian 2 extension trial.
The open label, phase 3b study included patients who had completed the Guardian 1 or Guardian 3 phase 1 pharmacokinetics trials and who chose to continue prophylaxis with turoctocog alfa (Novoeight).
In total, 214 patients were enrolled and 213 were exposed to treatment. Prophylaxis was given as a standard dose level of 20-50 IU/kg once every second day or 20-60 IU/kg three times weekly. Less frequent prophylaxis was available at 40-60 IU/kg twice weekly or once every third day. For on-demand treatment, the recommended dose level was 20-50 IU/kg.
“The final results of guardian 2 are consistent with data from previous interim analyses,” Steven R. Lentz, MD, PhD, of the University of Iowa, Iowa City, and his colleagues wrote in Haemophilia. “Turoctocog alfa was well tolerated, with no unexpected safety signals and no development of FVIII inhibitors.”
Among the 207 patients on prophylaxis, 1,782 bleeds were reported. The overall median annualized bleeding rate (ABR) for this group was 1.37. More than 88% of these bleeds were stopped with one or two injections and the success rate for treatment of bleeds was more than 90%.
The median ABR dropped to zero, with a range of 0.00‐9.91, for the 27 patients who were on the less-frequent prophylaxis regimen. In this group, more than 75% of the 34 bleeds that occurred were stopped with one injection and the success rate for treatment of bleeds was about 94%.
“These data suggest a subset of patients might be well managed with less‐frequent dosing,” the researchers wrote.
For the 19 patients who received on-demand treatment, there were 391 bleeds reported. The overall median ABR was 30.44. Nearly 80% of bleeds were stopped with one injection and more than 15% with two injections. The treatment success rate in this group was more than 96%.
There were no Factor VIII inhibitors reported in the extension trial.
In total, there were 1,260 adverse events reported for 183 patients, corresponding to less than two adverse events per patients per years of exposure to the treatment.
The most common adverse event, seen in 14.6% of patients, was nasopharyngitis. Serious adverse events occurred in 18.3% of patients but all expect one event was deemed unlikely to be related to treatment. The one fatal event in the trial was deemed unlikely to be related to treatment.
The study was funded by Novo Nordisk. Dr. Lentz reported being a paid consultant to Novo Nordisk and receiving research funding from the company.
SOURCE: Lentz SR et al. Haemophilia. 2018 Nov;24(6):e391-4.
Turoctocog alfa, a recombinant Factor VIII molecule, was effective in preventing bleeding episodes and was well tolerated in more than 200 males with hemophilia A who were part of the Guardian 2 extension trial.
The open label, phase 3b study included patients who had completed the Guardian 1 or Guardian 3 phase 1 pharmacokinetics trials and who chose to continue prophylaxis with turoctocog alfa (Novoeight).
In total, 214 patients were enrolled and 213 were exposed to treatment. Prophylaxis was given as a standard dose level of 20-50 IU/kg once every second day or 20-60 IU/kg three times weekly. Less frequent prophylaxis was available at 40-60 IU/kg twice weekly or once every third day. For on-demand treatment, the recommended dose level was 20-50 IU/kg.
“The final results of guardian 2 are consistent with data from previous interim analyses,” Steven R. Lentz, MD, PhD, of the University of Iowa, Iowa City, and his colleagues wrote in Haemophilia. “Turoctocog alfa was well tolerated, with no unexpected safety signals and no development of FVIII inhibitors.”
Among the 207 patients on prophylaxis, 1,782 bleeds were reported. The overall median annualized bleeding rate (ABR) for this group was 1.37. More than 88% of these bleeds were stopped with one or two injections and the success rate for treatment of bleeds was more than 90%.
The median ABR dropped to zero, with a range of 0.00‐9.91, for the 27 patients who were on the less-frequent prophylaxis regimen. In this group, more than 75% of the 34 bleeds that occurred were stopped with one injection and the success rate for treatment of bleeds was about 94%.
“These data suggest a subset of patients might be well managed with less‐frequent dosing,” the researchers wrote.
For the 19 patients who received on-demand treatment, there were 391 bleeds reported. The overall median ABR was 30.44. Nearly 80% of bleeds were stopped with one injection and more than 15% with two injections. The treatment success rate in this group was more than 96%.
There were no Factor VIII inhibitors reported in the extension trial.
In total, there were 1,260 adverse events reported for 183 patients, corresponding to less than two adverse events per patients per years of exposure to the treatment.
The most common adverse event, seen in 14.6% of patients, was nasopharyngitis. Serious adverse events occurred in 18.3% of patients but all expect one event was deemed unlikely to be related to treatment. The one fatal event in the trial was deemed unlikely to be related to treatment.
The study was funded by Novo Nordisk. Dr. Lentz reported being a paid consultant to Novo Nordisk and receiving research funding from the company.
SOURCE: Lentz SR et al. Haemophilia. 2018 Nov;24(6):e391-4.
FROM HAEMOPHILIA
Key clinical point:
Major finding: No Factor VIII inhibitors were reported. Among 207 patients who received prophylaxis, the median annualized bleeding rate was 1.37.
Study details: A phase 3b open label extension trial in which 213 males with hemophilia A were exposed to prophylaxis or on-demand treatment.
Disclosures: The study was funded by Novo Nordisk. Dr. Lentz reported being a paid consultant to Novo Nordisk and receiving research funding from the company.
Source: Lentz SR et al. Haemophilia. 2018 Nov;24(6):e391-e394.
Thrombin generation looks promising as a hemophilia biomarker
SAN DIEGO – Thrombin generation may edge out baseline factor activity as a biomarker for predicting bleeding severity among patients with mild and moderate hemophilia, according to a study of 81 patients with nonsevere hemophilia.
Both baseline factor activity and thrombin generation had a similar correlation with bleeding severity, but thrombin generation had a higher sensitivity when differentiating between bleeding severities, Fadi Nossair, MD, of Children’s Hospital of King’s Daughters in Norfolk, Va., reported in a poster at the annual meeting of the American Society of Hematology.
Nonsevere cases of hemophilia A and B account for about half of all hemophilia cases in which factor level does not consistently correlate with bleeding phenotype. That makes it difficult to determine prophylaxis or surgery and highlights the need for a predictive biomarker, the investigators noted.
In the study, 81 patients had their bleeding assessed using standardized, self-administered and investigator-administered questionnaires. Bleeding phenotypes were also collected from EMRs.
One-time venous blood samples were collected after a washout period, when applicable. Additionally, platelet poor plasma was obtained to measure thrombin generation, phospholipid-dependent factor Xa initiated clotting time, factor VIII and IX activities, and von Willebrand factor.
Nearly three-quarters of patients in the study had a low bleeding score.
Both baseline factor level and thrombin generation values obtained with a regular reagent (5 pM of tissue factor) demonstrated a significant correlation with bleeding score (P less than .05). Values obtained with other reagents and biomarkers did not show a significant correlation, according to the researchers.
However, a sensitivity and specificity analysis that helped the researchers narrow down the optimal cutoff values for differentiating between bleeding severities also found that thrombin generation had superior sensitivity, compared with baseline factor level. All thrombin generation values had a higher sensitivity to predict bleeding severity, compared with baseline factor level (57%-62% versus 29%).
“Long-term prospective studies should evaluate the utility of this approach in predicting bleeding severity in this population,” the researchers said.
The study was supported by grants from Novo Nordisk. Dr. Nossair reported financial disclosures related to Novo Nordisk.
SOURCE: Nossair F et al. ASH 2018, Poster 3788.
SAN DIEGO – Thrombin generation may edge out baseline factor activity as a biomarker for predicting bleeding severity among patients with mild and moderate hemophilia, according to a study of 81 patients with nonsevere hemophilia.
Both baseline factor activity and thrombin generation had a similar correlation with bleeding severity, but thrombin generation had a higher sensitivity when differentiating between bleeding severities, Fadi Nossair, MD, of Children’s Hospital of King’s Daughters in Norfolk, Va., reported in a poster at the annual meeting of the American Society of Hematology.
Nonsevere cases of hemophilia A and B account for about half of all hemophilia cases in which factor level does not consistently correlate with bleeding phenotype. That makes it difficult to determine prophylaxis or surgery and highlights the need for a predictive biomarker, the investigators noted.
In the study, 81 patients had their bleeding assessed using standardized, self-administered and investigator-administered questionnaires. Bleeding phenotypes were also collected from EMRs.
One-time venous blood samples were collected after a washout period, when applicable. Additionally, platelet poor plasma was obtained to measure thrombin generation, phospholipid-dependent factor Xa initiated clotting time, factor VIII and IX activities, and von Willebrand factor.
Nearly three-quarters of patients in the study had a low bleeding score.
Both baseline factor level and thrombin generation values obtained with a regular reagent (5 pM of tissue factor) demonstrated a significant correlation with bleeding score (P less than .05). Values obtained with other reagents and biomarkers did not show a significant correlation, according to the researchers.
However, a sensitivity and specificity analysis that helped the researchers narrow down the optimal cutoff values for differentiating between bleeding severities also found that thrombin generation had superior sensitivity, compared with baseline factor level. All thrombin generation values had a higher sensitivity to predict bleeding severity, compared with baseline factor level (57%-62% versus 29%).
“Long-term prospective studies should evaluate the utility of this approach in predicting bleeding severity in this population,” the researchers said.
The study was supported by grants from Novo Nordisk. Dr. Nossair reported financial disclosures related to Novo Nordisk.
SOURCE: Nossair F et al. ASH 2018, Poster 3788.
SAN DIEGO – Thrombin generation may edge out baseline factor activity as a biomarker for predicting bleeding severity among patients with mild and moderate hemophilia, according to a study of 81 patients with nonsevere hemophilia.
Both baseline factor activity and thrombin generation had a similar correlation with bleeding severity, but thrombin generation had a higher sensitivity when differentiating between bleeding severities, Fadi Nossair, MD, of Children’s Hospital of King’s Daughters in Norfolk, Va., reported in a poster at the annual meeting of the American Society of Hematology.
Nonsevere cases of hemophilia A and B account for about half of all hemophilia cases in which factor level does not consistently correlate with bleeding phenotype. That makes it difficult to determine prophylaxis or surgery and highlights the need for a predictive biomarker, the investigators noted.
In the study, 81 patients had their bleeding assessed using standardized, self-administered and investigator-administered questionnaires. Bleeding phenotypes were also collected from EMRs.
One-time venous blood samples were collected after a washout period, when applicable. Additionally, platelet poor plasma was obtained to measure thrombin generation, phospholipid-dependent factor Xa initiated clotting time, factor VIII and IX activities, and von Willebrand factor.
Nearly three-quarters of patients in the study had a low bleeding score.
Both baseline factor level and thrombin generation values obtained with a regular reagent (5 pM of tissue factor) demonstrated a significant correlation with bleeding score (P less than .05). Values obtained with other reagents and biomarkers did not show a significant correlation, according to the researchers.
However, a sensitivity and specificity analysis that helped the researchers narrow down the optimal cutoff values for differentiating between bleeding severities also found that thrombin generation had superior sensitivity, compared with baseline factor level. All thrombin generation values had a higher sensitivity to predict bleeding severity, compared with baseline factor level (57%-62% versus 29%).
“Long-term prospective studies should evaluate the utility of this approach in predicting bleeding severity in this population,” the researchers said.
The study was supported by grants from Novo Nordisk. Dr. Nossair reported financial disclosures related to Novo Nordisk.
SOURCE: Nossair F et al. ASH 2018, Poster 3788.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Compared with baseline factor level, all thrombin generation values had a higher sensitivity to predict bleeding severity (57%-62% versus 29%).
Study details: The study included 81 patients with mild or moderate hemophilia A or B and compared biomarkers for differentiating between bleeding phenotype severities.
Disclosures: The study was supported by grants from Novo Nordisk. Dr. Nossair reported financial disclosures related to Novo Nordisk.
Source: Nossair F et al. ASH 2018, Poster 3788.
Study identifies four patient subgroups in hemophilia A
SAN DIEGO – A small study of infants with severe hemophilia A revealed that there are four distinct patient subgroups based on their unique antibody signatures.
Bagirath Gangadharan, PhD, and his colleagues reported the results of HIPS, the Hemophilia Inhibitor Previously Untreated Patient Study, in a poster at the annual meeting of the American Society of Hematology.
The findings included data on 23 infants who had a baseline Factor VIII coagulant activity of less than 0.01 IU/mL at enrollment in the study. All patients received a single type of recombinant FVIII replacement therapy (Advate by Baxalta) with the dose, frequency, and regimen at the discretion of the treating physician.
Patients with prior exposure to clotting factor concentrates or blood products, another clinically significant chronic disease, and those participating in another investigational drug study were excluded.
The researchers discovered that patients fell into four distinct subgroups based on their antibody characteristics after 50 days’ exposure to FVIII infusions:
- Subgroup one, which included seven patients, had no detectable FVIII-specific antibodies and no FVIII inhibitors.
- Subgroup two – also made up of seven patients – had FVIII-specific IgG1, no detectable IgG class–switched antibodies, and no FVIII inhibitors.
- The third subgroup included just two patients. These patients had FVIII-specific IgG1, no detectable IgG class–switched antibodies, but low-titer FVIII inhibitors.
- The final subgroup, which had seven patients, had FVIII-specific IgG1, high-affinity IgG class–switched antibodies (IgG3, IgG4, and IgG2), and FVIII inhibitors.
The researchers concluded that high-affinity FVIII-specific class-switched antibodies precede clinical FVIII inhibitor detection, adding to the evidence of their “potential role as suitable predictive biomarkers for inhibitor development.”
The study is funded by Baxalta, a part of Shire. Dr. Gangadharan is employed by Shire and other authors reported financial disclosures related to the study sponsor.
SOURCE: Gangadharan B et al. ASH 2018, Poster 3774.
SAN DIEGO – A small study of infants with severe hemophilia A revealed that there are four distinct patient subgroups based on their unique antibody signatures.
Bagirath Gangadharan, PhD, and his colleagues reported the results of HIPS, the Hemophilia Inhibitor Previously Untreated Patient Study, in a poster at the annual meeting of the American Society of Hematology.
The findings included data on 23 infants who had a baseline Factor VIII coagulant activity of less than 0.01 IU/mL at enrollment in the study. All patients received a single type of recombinant FVIII replacement therapy (Advate by Baxalta) with the dose, frequency, and regimen at the discretion of the treating physician.
Patients with prior exposure to clotting factor concentrates or blood products, another clinically significant chronic disease, and those participating in another investigational drug study were excluded.
The researchers discovered that patients fell into four distinct subgroups based on their antibody characteristics after 50 days’ exposure to FVIII infusions:
- Subgroup one, which included seven patients, had no detectable FVIII-specific antibodies and no FVIII inhibitors.
- Subgroup two – also made up of seven patients – had FVIII-specific IgG1, no detectable IgG class–switched antibodies, and no FVIII inhibitors.
- The third subgroup included just two patients. These patients had FVIII-specific IgG1, no detectable IgG class–switched antibodies, but low-titer FVIII inhibitors.
- The final subgroup, which had seven patients, had FVIII-specific IgG1, high-affinity IgG class–switched antibodies (IgG3, IgG4, and IgG2), and FVIII inhibitors.
The researchers concluded that high-affinity FVIII-specific class-switched antibodies precede clinical FVIII inhibitor detection, adding to the evidence of their “potential role as suitable predictive biomarkers for inhibitor development.”
The study is funded by Baxalta, a part of Shire. Dr. Gangadharan is employed by Shire and other authors reported financial disclosures related to the study sponsor.
SOURCE: Gangadharan B et al. ASH 2018, Poster 3774.
SAN DIEGO – A small study of infants with severe hemophilia A revealed that there are four distinct patient subgroups based on their unique antibody signatures.
Bagirath Gangadharan, PhD, and his colleagues reported the results of HIPS, the Hemophilia Inhibitor Previously Untreated Patient Study, in a poster at the annual meeting of the American Society of Hematology.
The findings included data on 23 infants who had a baseline Factor VIII coagulant activity of less than 0.01 IU/mL at enrollment in the study. All patients received a single type of recombinant FVIII replacement therapy (Advate by Baxalta) with the dose, frequency, and regimen at the discretion of the treating physician.
Patients with prior exposure to clotting factor concentrates or blood products, another clinically significant chronic disease, and those participating in another investigational drug study were excluded.
The researchers discovered that patients fell into four distinct subgroups based on their antibody characteristics after 50 days’ exposure to FVIII infusions:
- Subgroup one, which included seven patients, had no detectable FVIII-specific antibodies and no FVIII inhibitors.
- Subgroup two – also made up of seven patients – had FVIII-specific IgG1, no detectable IgG class–switched antibodies, and no FVIII inhibitors.
- The third subgroup included just two patients. These patients had FVIII-specific IgG1, no detectable IgG class–switched antibodies, but low-titer FVIII inhibitors.
- The final subgroup, which had seven patients, had FVIII-specific IgG1, high-affinity IgG class–switched antibodies (IgG3, IgG4, and IgG2), and FVIII inhibitors.
The researchers concluded that high-affinity FVIII-specific class-switched antibodies precede clinical FVIII inhibitor detection, adding to the evidence of their “potential role as suitable predictive biomarkers for inhibitor development.”
The study is funded by Baxalta, a part of Shire. Dr. Gangadharan is employed by Shire and other authors reported financial disclosures related to the study sponsor.
SOURCE: Gangadharan B et al. ASH 2018, Poster 3774.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Two of the subgroups had Factor VIII inhibitors and two of the subgroups did not.
Study details: The study included 23 infants with previously untreated severe hemophilia A who received 50 days’ exposure to FVIII infusions.
Disclosures: The study is funded by Baxalta, a part of Shire. Dr. Gangadharan is employed by Shire and other authors reported financial disclosures related to the study sponsor.
Source: Gangadharan B et al. ASH 2018, Poster 3774.
Myeloma expert named as endowed cancer chair at Emory
Sagar Lonial, MD, an expert in the biology and treatment of multiple myeloma, was recently named as the Anne and Bernard Gray Family Chair in Cancer at Winship Cancer Institute of Emory University, Atlanta.
The endowment was created in honor of Mrs. Gray’s sister, Karen Ammons Howell, who died of breast cancer. Dr. Lonial, who joined Emory more than 20 years ago, is also the chief medical officer for Winship Cancer Institute and the chairman of the department of hematology and medical oncology. He is currently leading a global genome sequencing study for patients with newly diagnosed myeloma.
The Moffitt Cancer Center, Tampa, is taking a step toward precision medicine. They recently created the position of artificial intelligence officer. J. Ross Mitchell, PhD, is taking on the new role and is tasked with using computer science – and, specifically, artificial intelligence applications – to improve efficiency and quality of care at the center. Dr. Mitchell previously worked at the Mayo Clinic in Scottsdale, Ariz., leading medical imaging information projects, including the application of machine learning in brain tumor imaging.
Oren Cahlon, MD, a radiation oncologist at Memorial Sloan Kettering Cancer Center, New York, has been tapped as associate deputy physician in chief for the institution’s Regional Care Network. This puts Dr. Cahlon, an expert in proton therapy, in a new role overseeing outpatient clinical programs across seven locations in New York and New Jersey. Dr. Cahlon will continue in his position as vice chair of the department of radiation oncology, in which he has served since 2016.
John Barrett, MD, is the new editor in chief of the British Journal of Haematology. He begins his term in January 2019. Dr. Barrett, who is a clinical professor of medicine at George Washington University Cancer Center in Washington takes over from Finbarr E. Cotter, MBBS, PhD, who had served in the role since 2006.
The cancer community is mourning the passage of Bertrand Coiffier, MD, PhD, who died in January 2019 at the age of 71. The well-known lymphoma researcher led the team that first described the use of the R-CHOP chemotherapy regimen for the treatment of diffuse large B-cell lymphoma in 2002. Dr. Coiffier also was a founding member of the Groupe d’Etude des Lymphomes de l’Adulte (GELA), which began in Europe in 1984 to advance basic and clinical research on lymphoma in adults, and later became LYSA (Lymphoma Study Association).
Sagar Lonial, MD, an expert in the biology and treatment of multiple myeloma, was recently named as the Anne and Bernard Gray Family Chair in Cancer at Winship Cancer Institute of Emory University, Atlanta.
The endowment was created in honor of Mrs. Gray’s sister, Karen Ammons Howell, who died of breast cancer. Dr. Lonial, who joined Emory more than 20 years ago, is also the chief medical officer for Winship Cancer Institute and the chairman of the department of hematology and medical oncology. He is currently leading a global genome sequencing study for patients with newly diagnosed myeloma.
The Moffitt Cancer Center, Tampa, is taking a step toward precision medicine. They recently created the position of artificial intelligence officer. J. Ross Mitchell, PhD, is taking on the new role and is tasked with using computer science – and, specifically, artificial intelligence applications – to improve efficiency and quality of care at the center. Dr. Mitchell previously worked at the Mayo Clinic in Scottsdale, Ariz., leading medical imaging information projects, including the application of machine learning in brain tumor imaging.
Oren Cahlon, MD, a radiation oncologist at Memorial Sloan Kettering Cancer Center, New York, has been tapped as associate deputy physician in chief for the institution’s Regional Care Network. This puts Dr. Cahlon, an expert in proton therapy, in a new role overseeing outpatient clinical programs across seven locations in New York and New Jersey. Dr. Cahlon will continue in his position as vice chair of the department of radiation oncology, in which he has served since 2016.
John Barrett, MD, is the new editor in chief of the British Journal of Haematology. He begins his term in January 2019. Dr. Barrett, who is a clinical professor of medicine at George Washington University Cancer Center in Washington takes over from Finbarr E. Cotter, MBBS, PhD, who had served in the role since 2006.
The cancer community is mourning the passage of Bertrand Coiffier, MD, PhD, who died in January 2019 at the age of 71. The well-known lymphoma researcher led the team that first described the use of the R-CHOP chemotherapy regimen for the treatment of diffuse large B-cell lymphoma in 2002. Dr. Coiffier also was a founding member of the Groupe d’Etude des Lymphomes de l’Adulte (GELA), which began in Europe in 1984 to advance basic and clinical research on lymphoma in adults, and later became LYSA (Lymphoma Study Association).
Sagar Lonial, MD, an expert in the biology and treatment of multiple myeloma, was recently named as the Anne and Bernard Gray Family Chair in Cancer at Winship Cancer Institute of Emory University, Atlanta.
The endowment was created in honor of Mrs. Gray’s sister, Karen Ammons Howell, who died of breast cancer. Dr. Lonial, who joined Emory more than 20 years ago, is also the chief medical officer for Winship Cancer Institute and the chairman of the department of hematology and medical oncology. He is currently leading a global genome sequencing study for patients with newly diagnosed myeloma.
The Moffitt Cancer Center, Tampa, is taking a step toward precision medicine. They recently created the position of artificial intelligence officer. J. Ross Mitchell, PhD, is taking on the new role and is tasked with using computer science – and, specifically, artificial intelligence applications – to improve efficiency and quality of care at the center. Dr. Mitchell previously worked at the Mayo Clinic in Scottsdale, Ariz., leading medical imaging information projects, including the application of machine learning in brain tumor imaging.
Oren Cahlon, MD, a radiation oncologist at Memorial Sloan Kettering Cancer Center, New York, has been tapped as associate deputy physician in chief for the institution’s Regional Care Network. This puts Dr. Cahlon, an expert in proton therapy, in a new role overseeing outpatient clinical programs across seven locations in New York and New Jersey. Dr. Cahlon will continue in his position as vice chair of the department of radiation oncology, in which he has served since 2016.
John Barrett, MD, is the new editor in chief of the British Journal of Haematology. He begins his term in January 2019. Dr. Barrett, who is a clinical professor of medicine at George Washington University Cancer Center in Washington takes over from Finbarr E. Cotter, MBBS, PhD, who had served in the role since 2006.
The cancer community is mourning the passage of Bertrand Coiffier, MD, PhD, who died in January 2019 at the age of 71. The well-known lymphoma researcher led the team that first described the use of the R-CHOP chemotherapy regimen for the treatment of diffuse large B-cell lymphoma in 2002. Dr. Coiffier also was a founding member of the Groupe d’Etude des Lymphomes de l’Adulte (GELA), which began in Europe in 1984 to advance basic and clinical research on lymphoma in adults, and later became LYSA (Lymphoma Study Association).
RNA expression may hold key to hemophilia A severity
A decrease in the expression of two long noncoding RNAs in the factor VIII gene may explain the development of severe hemophilia, according to an analysis recently published in Hematology.
Researchers at the Iranian Comprehensive Hemophilia Care Center in Tehran identified two long noncoding RNAs for investigation – NONHSAT139219.2 and NONHSAT139215.2. They collected 5 mL of venous blood from 50 males with severe hemophilia A and 50 healthy male donors and analyzed the RNA expression.
The mean of the transcript levels of two long noncoding RNAs was significantly lower in the hemophilia A samples, compared with the normal samples – 5.52 for controls versus 1.25 for hemophilia A for NONHSAT139215 and 4.86 for controls versus 2.14 for hemophilia A samples for NONHSAT139219 (P less than .05).
Low expression levels of long noncoding RNAs in severe hemophilia A cases may be linked with the reduction of factor VIII levels, according to the investigators. “It is possible that the transcription of [long noncoding] RNAs leads to gene silencing or activation.”
This provides a potential biomarker with application in both prognosis and therapeutics; long noncoding RNA functional analysis should be performed in future studies, they wrote.
There was no outside funding for the study, and no potential conflict of interest was reported by the authors.
SOURCE: Niloofar N et al. Hematology. 2019;24(1):255-62.
A decrease in the expression of two long noncoding RNAs in the factor VIII gene may explain the development of severe hemophilia, according to an analysis recently published in Hematology.
Researchers at the Iranian Comprehensive Hemophilia Care Center in Tehran identified two long noncoding RNAs for investigation – NONHSAT139219.2 and NONHSAT139215.2. They collected 5 mL of venous blood from 50 males with severe hemophilia A and 50 healthy male donors and analyzed the RNA expression.
The mean of the transcript levels of two long noncoding RNAs was significantly lower in the hemophilia A samples, compared with the normal samples – 5.52 for controls versus 1.25 for hemophilia A for NONHSAT139215 and 4.86 for controls versus 2.14 for hemophilia A samples for NONHSAT139219 (P less than .05).
Low expression levels of long noncoding RNAs in severe hemophilia A cases may be linked with the reduction of factor VIII levels, according to the investigators. “It is possible that the transcription of [long noncoding] RNAs leads to gene silencing or activation.”
This provides a potential biomarker with application in both prognosis and therapeutics; long noncoding RNA functional analysis should be performed in future studies, they wrote.
There was no outside funding for the study, and no potential conflict of interest was reported by the authors.
SOURCE: Niloofar N et al. Hematology. 2019;24(1):255-62.
A decrease in the expression of two long noncoding RNAs in the factor VIII gene may explain the development of severe hemophilia, according to an analysis recently published in Hematology.
Researchers at the Iranian Comprehensive Hemophilia Care Center in Tehran identified two long noncoding RNAs for investigation – NONHSAT139219.2 and NONHSAT139215.2. They collected 5 mL of venous blood from 50 males with severe hemophilia A and 50 healthy male donors and analyzed the RNA expression.
The mean of the transcript levels of two long noncoding RNAs was significantly lower in the hemophilia A samples, compared with the normal samples – 5.52 for controls versus 1.25 for hemophilia A for NONHSAT139215 and 4.86 for controls versus 2.14 for hemophilia A samples for NONHSAT139219 (P less than .05).
Low expression levels of long noncoding RNAs in severe hemophilia A cases may be linked with the reduction of factor VIII levels, according to the investigators. “It is possible that the transcription of [long noncoding] RNAs leads to gene silencing or activation.”
This provides a potential biomarker with application in both prognosis and therapeutics; long noncoding RNA functional analysis should be performed in future studies, they wrote.
There was no outside funding for the study, and no potential conflict of interest was reported by the authors.
SOURCE: Niloofar N et al. Hematology. 2019;24(1):255-62.
FROM HEMATOLOGY
Key clinical point:
Major finding: Transcript levels of two long noncoding RNAs were significantly lower in severe hemophilia A patients, compared with healthy controls (P less than .05). Study details: An analysis of RNA expression levels in 50 patients with severe hemophilia A and 50 healthy controls at a single center in Iran.
Disclosures: There was no outside funding for the study, and no potential conflict of interest information was reported by the authors.
Source: Niloofar N et al. Hematology. 2019;24(1):255-62.
Study supports need for less toxic therapies in FL
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Despite improvements in the treatment for follicular lymphoma, including the introduction of anti-CD20 therapies like rituximab, the leading cause of death remains lymphoma, according to a recent analysis.
Researchers led by Clementine Sarkozy, MD, of the University of Lyon (France), analyzed the cause of death for 1,654 follicular lymphoma patients across one French and one U.S. cohort. The French cohort enrolled patients between 2001 and 2013 and the U.S. cohort enrolled patients between 2002 and 2012.
Among the 734 patients in the French cohort, there were 113 deaths after a median 89 months follow-up. Similarly, following a median follow-up of 84 months, there were 170 deaths among the 920 U.S. patients. The 10-year overall survival was similar in the two cohorts at 79.8% among the French patients and 76.6% among the U.S. patients, the researchers reported in the Journal of Clinical Oncology.
Cause of death information was available for 283 patients across the two cohorts. In 140 patients (56.5%), the cause of death was lymphoma; more than half of those cases occurred in patients whose disease had transformed at some point. That puts the cumulative risk of mortality from lymphoma at 10.3% at 10 years, according to the researchers.
The researchers also noted that the Follicular Lymphoma International Prognostic Index score was strongly linked to lymphoma-related mortality but not to nonlymphoma causes of death.
Another 42 patients (17%) died from treatment-related causes, mainly infection. About 13% of the cohort died from other cancers and another 13% died from other causes.
“Deaths related to treatment seem to also be a significant burden and new, less-toxic treatment options need to be investigated,” the researchers wrote.
The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
SOURCE: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: The cumulative risk of mortality from lymphoma was 10.3% at 10 years for patients with follicular lymphoma.
Study details: A pooled cohort study of 1,654 patients with follicular lymphoma in the United States and France.
Disclosures: The study was supported by the National Institutes of Health. Dr. Sarkozy reported financial relationships with Genentech, Celgene, and Takeda.
Source: Sarkozy C et al. J Clin Oncol. 2019 Jan 10;37(2):144-52.
Baselga moves to industry; MD Anderson mourns
Jose Baselga, MD, PhD, the former chief medical officer at Memorial Sloan Kettering Cancer Center in New York, made headlines recently by accepting a position with the drugmaker AstraZeneca.
Dr. Baselga had stepped down from his position at Memorial Sloan Kettering in September 2018 after a ProPublica investigation revealed that he had failed to disclose industry funding in dozens of instances when publishing in top medical journals. In his new role, Dr. Baselga, who acknowledged the missing disclosures but said they were unintentional, will head up the research and development unit for oncology, AstraZeneca said in a press release.
The American Society of Clinical Oncology (ASCO) has elected new leadership. Lori J. Pierce, MD, a radiation oncologist and leader in breast cancer research, is the newly elected president-elect. She will serve in that role beginning June 2019 and will end her 1-year term as president of ASCO in June 2020. Dr. Pierce is a professor and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, and the director of the Michigan Radiation Oncology Quality Consortium.
Three physicians were also elected to the ASCO board of directors. Michael A. Thompson, MD, PhD, of Advocate Aurora Health in Milwaukee, was elected to the community oncologist seat. Elizabeth A. Mittendorf, MD, PhD, of Brigham and Women’s Hospital and the Dana-Farber Cancer Institute in Boston, was elected to the surgical oncologist seat. Ethan Basch, MD, of the University of North Carolina, was elected to the undesignated specialty seat. They will all begin 4-year terms on the board of directors starting in June 2019.
A radiation oncologist is moving up the ranks at the Centers for Medicare & Medicaid Services. Anand Shah, MD, has been named senior medical advisor for innovation at the Center for Medicare & Medicaid Innovation (CMMI), part of CMS. He had previously served as the CMMI’s chief medical officer. Dr. Shah is getting congratulations from ASCO and the American Society of Radiation Oncology (ASTRO), both of whom have a lot to say about CMMI policies such as attempts to revive the Competitive Acquisition Program for Part B drugs and alternative payment models for oncology.
Julian Schink, MD, has been appointed chief medical officer at Cancer Treatment Centers of America (CTCA). Dr. Schink, a gynecologic oncologist, joined CTCA in 2017 as chief of gynecologic oncology. Before that, he had worked at the University of Wisconsin–Madison and the Northwestern University, Chicago.
In sad news, John Mendelsohn, MD, the president emeritus of the University of Texas MD Anderson Cancer Center, died on Jan. 7 at the age of 82 years. He had been diagnosed with glioblastoma 15 months earlier. Before working in medical leadership, Dr. Mendelsohn had worked with his colleagues at the University of California, San Diego, on research to block epidermal growth factor receptors. That work led to the development of the drug cetuximab, which was approved by the Food and Drug Administration to treat advanced colorectal cancer and later head and neck cancer.
The MD Anderson community also mourned the passing of Waun Ki Hong, MD, a physician-scientist who led the institution’s division of cancer medicine during 2001-2014. He died at age 76 years. Dr. Hong, who was also a past president of the American Association for Cancer Research, was well known for advancing the fields of targeted therapy and chemoprevention.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Jose Baselga, MD, PhD, the former chief medical officer at Memorial Sloan Kettering Cancer Center in New York, made headlines recently by accepting a position with the drugmaker AstraZeneca.
Dr. Baselga had stepped down from his position at Memorial Sloan Kettering in September 2018 after a ProPublica investigation revealed that he had failed to disclose industry funding in dozens of instances when publishing in top medical journals. In his new role, Dr. Baselga, who acknowledged the missing disclosures but said they were unintentional, will head up the research and development unit for oncology, AstraZeneca said in a press release.
The American Society of Clinical Oncology (ASCO) has elected new leadership. Lori J. Pierce, MD, a radiation oncologist and leader in breast cancer research, is the newly elected president-elect. She will serve in that role beginning June 2019 and will end her 1-year term as president of ASCO in June 2020. Dr. Pierce is a professor and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, and the director of the Michigan Radiation Oncology Quality Consortium.
Three physicians were also elected to the ASCO board of directors. Michael A. Thompson, MD, PhD, of Advocate Aurora Health in Milwaukee, was elected to the community oncologist seat. Elizabeth A. Mittendorf, MD, PhD, of Brigham and Women’s Hospital and the Dana-Farber Cancer Institute in Boston, was elected to the surgical oncologist seat. Ethan Basch, MD, of the University of North Carolina, was elected to the undesignated specialty seat. They will all begin 4-year terms on the board of directors starting in June 2019.
A radiation oncologist is moving up the ranks at the Centers for Medicare & Medicaid Services. Anand Shah, MD, has been named senior medical advisor for innovation at the Center for Medicare & Medicaid Innovation (CMMI), part of CMS. He had previously served as the CMMI’s chief medical officer. Dr. Shah is getting congratulations from ASCO and the American Society of Radiation Oncology (ASTRO), both of whom have a lot to say about CMMI policies such as attempts to revive the Competitive Acquisition Program for Part B drugs and alternative payment models for oncology.
Julian Schink, MD, has been appointed chief medical officer at Cancer Treatment Centers of America (CTCA). Dr. Schink, a gynecologic oncologist, joined CTCA in 2017 as chief of gynecologic oncology. Before that, he had worked at the University of Wisconsin–Madison and the Northwestern University, Chicago.
In sad news, John Mendelsohn, MD, the president emeritus of the University of Texas MD Anderson Cancer Center, died on Jan. 7 at the age of 82 years. He had been diagnosed with glioblastoma 15 months earlier. Before working in medical leadership, Dr. Mendelsohn had worked with his colleagues at the University of California, San Diego, on research to block epidermal growth factor receptors. That work led to the development of the drug cetuximab, which was approved by the Food and Drug Administration to treat advanced colorectal cancer and later head and neck cancer.
The MD Anderson community also mourned the passing of Waun Ki Hong, MD, a physician-scientist who led the institution’s division of cancer medicine during 2001-2014. He died at age 76 years. Dr. Hong, who was also a past president of the American Association for Cancer Research, was well known for advancing the fields of targeted therapy and chemoprevention.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Jose Baselga, MD, PhD, the former chief medical officer at Memorial Sloan Kettering Cancer Center in New York, made headlines recently by accepting a position with the drugmaker AstraZeneca.
Dr. Baselga had stepped down from his position at Memorial Sloan Kettering in September 2018 after a ProPublica investigation revealed that he had failed to disclose industry funding in dozens of instances when publishing in top medical journals. In his new role, Dr. Baselga, who acknowledged the missing disclosures but said they were unintentional, will head up the research and development unit for oncology, AstraZeneca said in a press release.
The American Society of Clinical Oncology (ASCO) has elected new leadership. Lori J. Pierce, MD, a radiation oncologist and leader in breast cancer research, is the newly elected president-elect. She will serve in that role beginning June 2019 and will end her 1-year term as president of ASCO in June 2020. Dr. Pierce is a professor and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, and the director of the Michigan Radiation Oncology Quality Consortium.
Three physicians were also elected to the ASCO board of directors. Michael A. Thompson, MD, PhD, of Advocate Aurora Health in Milwaukee, was elected to the community oncologist seat. Elizabeth A. Mittendorf, MD, PhD, of Brigham and Women’s Hospital and the Dana-Farber Cancer Institute in Boston, was elected to the surgical oncologist seat. Ethan Basch, MD, of the University of North Carolina, was elected to the undesignated specialty seat. They will all begin 4-year terms on the board of directors starting in June 2019.
A radiation oncologist is moving up the ranks at the Centers for Medicare & Medicaid Services. Anand Shah, MD, has been named senior medical advisor for innovation at the Center for Medicare & Medicaid Innovation (CMMI), part of CMS. He had previously served as the CMMI’s chief medical officer. Dr. Shah is getting congratulations from ASCO and the American Society of Radiation Oncology (ASTRO), both of whom have a lot to say about CMMI policies such as attempts to revive the Competitive Acquisition Program for Part B drugs and alternative payment models for oncology.
Julian Schink, MD, has been appointed chief medical officer at Cancer Treatment Centers of America (CTCA). Dr. Schink, a gynecologic oncologist, joined CTCA in 2017 as chief of gynecologic oncology. Before that, he had worked at the University of Wisconsin–Madison and the Northwestern University, Chicago.
In sad news, John Mendelsohn, MD, the president emeritus of the University of Texas MD Anderson Cancer Center, died on Jan. 7 at the age of 82 years. He had been diagnosed with glioblastoma 15 months earlier. Before working in medical leadership, Dr. Mendelsohn had worked with his colleagues at the University of California, San Diego, on research to block epidermal growth factor receptors. That work led to the development of the drug cetuximab, which was approved by the Food and Drug Administration to treat advanced colorectal cancer and later head and neck cancer.
The MD Anderson community also mourned the passing of Waun Ki Hong, MD, a physician-scientist who led the institution’s division of cancer medicine during 2001-2014. He died at age 76 years. Dr. Hong, who was also a past president of the American Association for Cancer Research, was well known for advancing the fields of targeted therapy and chemoprevention.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Sickle cell infusion gains FDA breakthrough designation
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.
The in patients with sickle cell disease of all genotypes.
The designation allows the treatment to be reviewed on an expedited schedule.
Crizanlizumab, marketed by Novartis, is a humanized anti–P-selectin monoclonal antibody that has been shown to inhibit interactions between endothelial cells, platelets, red blood cells, sickled red blood cells, and leukocytes.
In the phase 2 SUSTAIN trial, crizanlizumab reduced the median annual rate of vasoocclusive crises that resulted in health care visits by about 45%, compared with placebo (1.63 vs. 2.98; P = .010). The drug also increased the percentage of patients who did not experience any vasoocclusive crises, compared with placebo (35.8% vs. 16.9%; P = .010).
The rates of treatment-emergent and serious adverse events was similar in the drug and placebo arms of the trial.