User login
Bortezomib may unlock resistance in WM with mutations
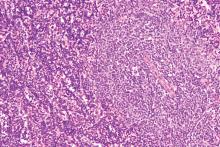
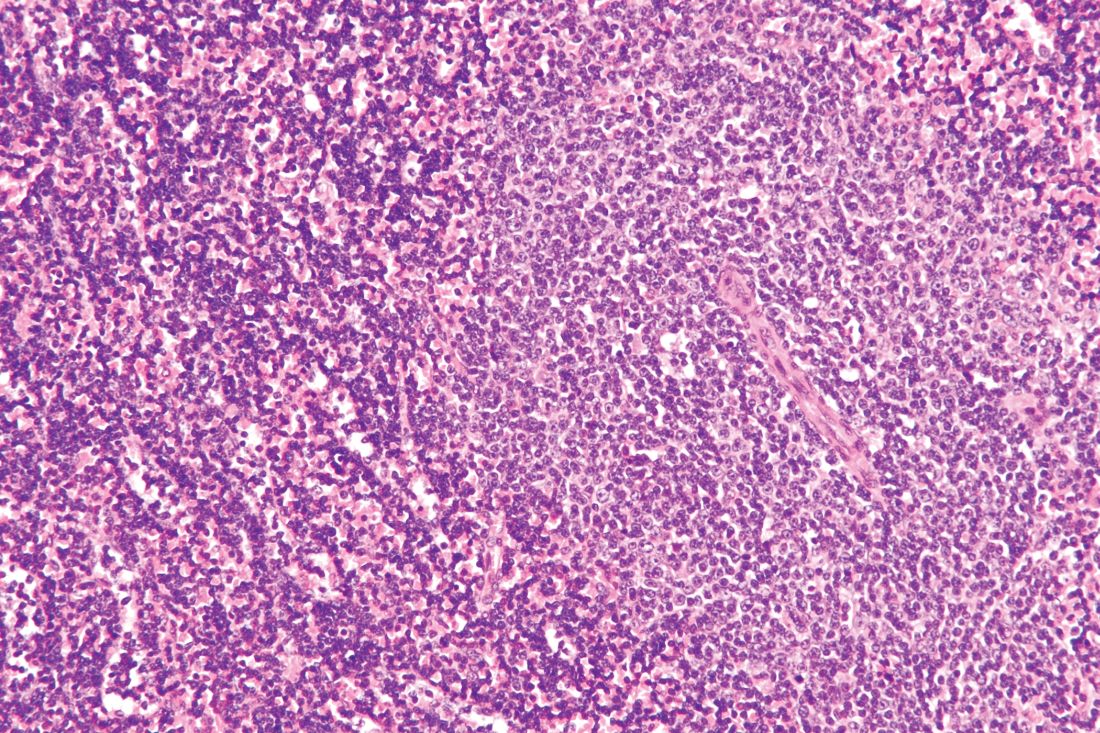
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
The use of bortezomib may help overcome treatment resistance in patients with Waldenström macroglobulinemia (WM) with CXCR4 mutations, according to new research.
Romanos Sklavenitis-Pistofidis, MD, of the Dana-Farber Cancer Institute in Boston, and his colleagues compared the effects of treatment with bortezomib/rituximab in patients with WM based on their CXCR4 mutation status. They found no significant difference in progression-free survival (Log-rank, P = .994) or overall survival (Log-rank, P = .407) when comparing patients who have CXCR4 mutations with those who have CXCR4 wild type.
“We report for the first time that a bortezomib-based combination is impervious to the impact of CXCR4 mutations in a cohort of patients with WM,” the researchers wrote in Blood. “Previously, we had shown this to be true in WM cell lines, whereby genetically engineering BCWM.1 and MWCL-1 to overexpress CXCR4 had no impact on bortezomib resistance.”
The researchers noted, however, that the mechanism at work may be different than that seen with bortezomib in other cancers.
“Different experiments have linked CXCR4 expression and bortezomib in a variety of ways in other hematological malignancies, including multiple myeloma. However, despite the complicated association in those cancer types, in WM there seems to be a consistently neutral effect of CXCR4 mutations on bortezomib resistance in both cell line and patient data,” they wrote.
The researchers recommended that the theory be tested in a prospective trial of bortezomib-based therapy in WM patients with CXCR4 mutations. Another question to be investigated, they pointed out, is the role of rituximab in the survival results seen in the current analysis.
The study included 63 patients with WM who were treated with bortezomib/rituximab either as upfront treatment or in the relapsed/refractory setting as part of a phase 2 trial.
Bortezomib was given by IV weekly at 1.6 mg/m2 for six cycles and rituximab was given at 375 mg/m2 during cycles one and four. Patients were taken off therapy after two cycles if they had progressive disease.
The researchers excluded 20 patients from the study because of a lack of material for genotyping. However, they noted that their clinical characteristics were not different from those patients who were included.
Out of 43 patients who were genotyped for CXCR4, 17 patients had a mutation. All patients who carried a CXCR4 mutation also had MYD88 L265P. Ten patients had frameshift mutations, one patient had a nonsense mutation, and six patients had missense mutations. The median follow-up of the analysis was 90.7 months.
The researchers repeated the analysis after excluding six patients with missense mutations and accounting for different treatment settings and found that survival remained unchanged.
The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
SOURCE: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
FROM BLOOD
Key clinical point:
Major finding: Progression-free and overall survival were not significantly different between WM patients with CXCR4 mutations and those with CXCR4 wild type (log-rank, P = .994 and P = .407, respectively).
Study details: An analysis of 43 WM patients treated with bortezomib/rituximab and genotyped to determine CXCR4 mutation status.
Disclosures: The study was supported by the National Institutes of Health, the Leukemia and Lymphoma Society, and the International Waldenström Macroglobulinemia Foundation. One of the authors reported consulting and research funding from Takeda, which markets bortezomib, and other companies.
Source: Sklavenitis-Pistofidis R et al. Blood. 2018 Oct 26. doi: 10.1182/blood-2018-07-863241.
Study challenges LVEF assessment before DLBCL treatment
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Measuring left ventricular ejection fraction (LVEF) before administering doxorubicin-based chemotherapy doesn’t appear to add clinically meaningful information, according to an analysis of diffuse large B-cell lymphoma (DLBCL) patients.
Current guidelines recommend prescreening with either echocardiography or multiple-gated acquisition (MUGA) scan to identify asymptomatic left ventricular dysfunction before administering doxorubicin-containing chemotherapy, since doxorubicin is known for its cardiotoxicity.
But other studies have challenged the usefulness of routine LVEF screening in DLBCL patients.
In the current study, Deborah L. Enns, PhD, and her colleagues at Virginia Mason Medical Center in Seattle reviewed the medical records of 291 patients diagnosed with DLBCL between 2001 and 2013.
In total, 206 patients with normal LVEF and 8 patients with low LVEF received doxorubicin (P = .006). But while that association appears to support routine prescreening to inform clinical decision making, the link disappeared when the researchers factored out previous cardiac disease (P = .51).
“It is possible that previous [heart failure] may have played a larger role in shaping treatment decisions than did LVEF test results alone,” the researchers wrote. The report is in Mayo Clinic Proceedings: Innovations, Quality & Outcomes.
In addition, for patients who had their LVEF measured, the researchers found no difference in posttreatment incidence of heart failure based on whether patients received doxorubicin (7.0%) or did not (6.8%). The same was true for patients with LVEF values of less than 50% before treatment (13% vs. 14%).
The researchers noted that there are several reasons why LVEF prescreening may not be needed before treatment in DLBCL. For instance, DLBCL patients typically receive low cumulative doses of doxorubicin. Also, doxorubicin’s high efficacy in DLBCL should be balanced with the relatively low risk of death from heart failure due to doxorubicin treatment, at around 4% after 5 years for patients without preexisting cardiac conditions.
“We recommend that the policy of routinely performing prescreening LVEF measurements in all patients with DLBCL before administering anthracycline-based chemotherapy treatments be reevaluated,” the researchers wrote.
They reported having no competing interests.
SOURCE: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
FROM MAYO CLINIC PROCEEDINGS: INNOVATIONS, QUALITY & OUTCOMES
Key clinical point:
Major finding: Among diffuse large B-cell lymphoma (DLBCL) patients who had LVEF measured, the incidence of heart failure post treatment did not differ between patients who received doxorubicin and those who did not (P = 1.0).
Study details: A retrospective analysis of 291 patients diagnosed with DLBCL between 2001 and 2013.
Disclosures: The researchers reported having no competing interests.
Source: Enns DL et al. Mayo Clin Proc Innov Qual Outcomes. 2018 Aug 3;2(3):277-85.
Ibrutinib discontinuation harms survival in CLL
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Discontinuing ibrutinib therapy because of disease progression was associated with worse survival, according to a real-world study of ibrutinib dosing in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma patients.
Researchers at the University of Rochester Wilmot Cancer Institute in New York, who performed the single-center study, also found that optimal dosing early on in treatment has a significant impact on disease progression.
“Treating physicians need to be aware of these outcomes when initiating therapy on patients with high-risk CLL or lymphoma, as well as those with significant comorbidities or immune deficiencies,” AnnaLynn M. Williams. MS, and her colleagues reported in Clinical Lymphoma, Myeloma and Leukemia.
The researchers examined the impact of ibrutinib discontinuation and dose adherence on overall and progression-free survival in 170 patients with non-Hodgkin lymphoma and CLL treated with the drug at the Wilmot Cancer Institute between Jan. 1, 2014, and Dec. 1, 2016.
The study comprised 115 patients with CLL, 23 patients with Waldenstrom macroglobulinemia, 21 patients with mantle cell lymphoma, and 11 patients with other non-Hodgkin lymphomas. The median age of patients who started ibrutinib was 68 years, and the median treatment duration was 14.3 months. About a third of patients were taking ibrutinib as a first-line treatment.
Overall, 51 patients (30%) permanently discontinued ibrutinib during the study period, with more than half of the discontinuations stemming from adverse events or comorbidities. About 35% of the discontinuations were due to disease progression.
Median overall survival after discontinuation due to disease progression was 1.7 months. When patients discontinued for other reasons, median overall survival was not reached, compared with stopping for disease progression (P = .0008).
The researchers reported that among patients who discontinued for nonprogression reasons, 67% were alive after 1 year. Among CLL patients, 80% were alive after 1 year.
Among 20 patients who had a dose adherence of less than 80% in the first 8 weeks, the researchers found worse progression-free survival (P = .002) and overall survival (P = .021). Among CLL patients only, progression-free survival was significantly worse (P = .043) but overall survival was not (P = .816).
The study also included five patients who reduced their ibrutinib dose in the first 8 weeks – down to 280 mg in two patients, 140 mg in two patients, and 420 mg in one patient. Again, the researchers observed worse progression-free survival (P = .004) and overall survival (P = .014), compared with patients who maintained their dosing level.
Interrupting ibrutinib dosing had an impact on survival but not as much as discontinuation. Among 10 patients who interrupted therapy for more than a week and then restarted, progression-free survival was worse, compared with those who stayed on treatment continuously (P = .047), but overall survival was not significantly worse (P = .577).
“This would suggest that the ideal treatment strategy would be to recommend initiation of therapy at standard dosing and interruption as needed as directed in the [Food and Drug Administration] label,” the researchers wrote.
The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
SOURCE: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
Key clinical point:
Major finding: Median overall survival after discontinuation of ibrutinib due to disease progression was 1.7 months.
Study details: A single-institution study of 170 patients with CLL or non-Hodgkin lymphoma who were taking ibrutinib.
Disclosures: The study was funded by the National Cancer Institute and the Cadregari Endowment Fund. The researchers reported having no conflicts of interest.
Source: Williams AM et al. Clin Lymphoma Myeloma Leuk. 2018 Oct 12. doi: 10.1016/j.clml.2018.10.005.
Ibrutinib plus obinutuzumab gets priority review in CLL/SLL
The Food and Drug Administration has granted priority review to an anti-CD20, chemotherapy-free combination – ibrutinib plus obinutuzumab – for the frontline treatment of chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL).
The agency will review the combination in previously untreated adults.
Ibrutinib (Imbruvica) is already approved as a single agent for adults with CLL/SLL for all lines of therapy and in combination with bendamustine and rituximab. Obinutuzumab (Gazyva) has been approved for patients with previously untreated CLL, in combination with chlorambucil.
The current application, which is sponsored by Janssen and Pharmacyclics, is based on results from the phase 3 iLLUMINATE trial. Preliminary results announced by Janssen and Pharmacyclics showed that ibrutinib plus obinutuzumab had statistically significant better progression-free survival, compared with chlorambucil plus obinutuzumab, as assessed by an independent review committee.
Complete results from the trial will be presented at an upcoming medical meeting, according to the sponsors.
The Food and Drug Administration has granted priority review to an anti-CD20, chemotherapy-free combination – ibrutinib plus obinutuzumab – for the frontline treatment of chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL).
The agency will review the combination in previously untreated adults.
Ibrutinib (Imbruvica) is already approved as a single agent for adults with CLL/SLL for all lines of therapy and in combination with bendamustine and rituximab. Obinutuzumab (Gazyva) has been approved for patients with previously untreated CLL, in combination with chlorambucil.
The current application, which is sponsored by Janssen and Pharmacyclics, is based on results from the phase 3 iLLUMINATE trial. Preliminary results announced by Janssen and Pharmacyclics showed that ibrutinib plus obinutuzumab had statistically significant better progression-free survival, compared with chlorambucil plus obinutuzumab, as assessed by an independent review committee.
Complete results from the trial will be presented at an upcoming medical meeting, according to the sponsors.
The Food and Drug Administration has granted priority review to an anti-CD20, chemotherapy-free combination – ibrutinib plus obinutuzumab – for the frontline treatment of chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL).
The agency will review the combination in previously untreated adults.
Ibrutinib (Imbruvica) is already approved as a single agent for adults with CLL/SLL for all lines of therapy and in combination with bendamustine and rituximab. Obinutuzumab (Gazyva) has been approved for patients with previously untreated CLL, in combination with chlorambucil.
The current application, which is sponsored by Janssen and Pharmacyclics, is based on results from the phase 3 iLLUMINATE trial. Preliminary results announced by Janssen and Pharmacyclics showed that ibrutinib plus obinutuzumab had statistically significant better progression-free survival, compared with chlorambucil plus obinutuzumab, as assessed by an independent review committee.
Complete results from the trial will be presented at an upcoming medical meeting, according to the sponsors.
Entospletinib falls short in relapsed/refractory DLBCL
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Entospletinib, a selective inhibitor of spleen tyrosine kinase (Syk), showed a dismal rate of progression-free survival and a high rate of adverse events in a cohort of previously treated patients with diffuse large B-cell lymphoma (DLBCL).
Entospletinib was evaluated in an open-label, single-agent, phase 2 trial (NCT01799889) with five relapsed/refractory patient cohorts: chronic lymphocytic leukemia (CLL), follicular lymphoma, other indolent non-Hodgkin lymphomas, mantle cell lymphoma, and DLBCL.
John M. Burke, MD, of Rocky Mountain Cancer Centers in Aurora, Colo., and his colleagues reported on the current analysis, which looked specifically at the 43 patients in the trial with previously treated DLBCL. Patients received at least one starting dose of 800 mg of entospletinib orally twice daily. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
In a previous report on the relapsed/refractory CLL cohort, the investigational agent demonstrated clinical activity with acceptable toxicity (Blood. 2015 Apr 9;125[15]:2336-43).
In the current report, the rate of progression-free survival (PFS) at 16 weeks was 3.6% and the median PFS was 1.5 months. None of the patients in the study achieved a complete or partial response to treatment, and just five patients had stable disease.
All patients in the study eventually discontinued treatment and the median treatment duration was 1 month.
“The lack of activity of Syk inhibition in patients with relapsed DLBCL is in contrast to what would have been expected from preclinical data,” the investigators wrote. “Although it is unclear why entospletinib monotherapy lacked activity in the present study, it is possible that resistance to Syk inhibition played a role. Potential mechanisms of resistance of DLBCL to Syk inhibition include transcriptional upregulation of Syk mediated by FOXO1 and PTEN depletion.”
The investigators said that Syk inhibition in combination with BCL2 inhibitors could potentially overcome this resistance. Another approach, they suggested, would be to offer entospletinib in combination with Janus kinase (JAK) 1/3 inhibition.
“Based on results of the preclinical data, the efficacy of entospletinib in combination will be evaluated in future clinical trials,” the investigators wrote.
The rate of adverse events was high in the DLBCL cohort. Forty-two patients (98%) experienced an adverse event and nearly three-quarters experienced a grade 3 event. Overall, 30% of the grade 3 adverse events were related to treatment. More than 40% of patients interrupted treatment because of adverse events, and 19% discontinued. Four patients experienced an adverse event that led to death.
While the lack of clinical activity may have surprised investigators, the safety profile was in line with other patient cohorts in the phase 2 study. In the CLL and indolent non-Hodgkin lymphoma cohorts, the rates of treatment interruption were 45% and 54%, respectively.
The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
SOURCE: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The rate of progression-free survival at 16 weeks was 3.6% with a median PFS of 1.5 months.
Study details: An analysis of 43 relapsed/refractory DLBCL patients who received single-agent entospletinib.
Disclosures: The study was supported by Gilead Sciences. Dr. Burke reported relationships with Gilead and other companies.
Source: Burke JM et al. Clin Lymphoma Myeloma Leuk. 2018 Aug;18(8):e327-e331.
Frontline rituximab shows long-term success in indolent lymphoma
Advanced indolent lymphoma patients can be treated with a rituximab-containing regimen as first-line therapy and, in some cases, skip chemotherapy altogether, a study with 10 years of follow-up data suggests.
After a median of 10.6 years’ follow-up, almost three-quarters of patients (73%) in the study were alive, and 36% never required chemotherapy.
“This [overall survival] is at least as good as that observed in modern immunochemotherapy trials,” Sandra Lockmer, MD, of Karolinska University Hospital in Stockholm and her colleagues reported in the Journal of Clinical Oncology.
The study included 321 patients who were previously untreated and had been enrolled in two randomized clinical trials performed by the Nordic Lymphoma Group. The trials randomized patients to receive either rituximab monotherapy or rituximab combined with interferon alfa-2a. Neither trial used up-front chemotherapy.
Patients included in the follow-up analysis had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
The overall survival rate at 10 years after trial assignment was 75% and 66% after 15 years. Similarly, the lymphoma-specific survival rate was 81% at 10 years after trial assignment and 77% at 15 years, the researchers reported.
Overall, 117 patients did not require treatment with chemotherapy, but 24 patients were further treated with antibodies and/or radiation. Of the 93 patients who received no additional therapies after frontline treatment, 9 patients died from causes unrelated to their lymphoma.
Among the 237 patients who failed initial treatment, the median time to treatment failure was 1.5 years.
In terms of transformation to aggressive lymphoma, the rate was 2.4%/person-year overall. The cumulative risk of transformation was 20% at 10 years after trial assignment and 24% at 15 years.
The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
[email protected]
SOURCE: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
Advanced indolent lymphoma patients can be treated with a rituximab-containing regimen as first-line therapy and, in some cases, skip chemotherapy altogether, a study with 10 years of follow-up data suggests.
After a median of 10.6 years’ follow-up, almost three-quarters of patients (73%) in the study were alive, and 36% never required chemotherapy.
“This [overall survival] is at least as good as that observed in modern immunochemotherapy trials,” Sandra Lockmer, MD, of Karolinska University Hospital in Stockholm and her colleagues reported in the Journal of Clinical Oncology.
The study included 321 patients who were previously untreated and had been enrolled in two randomized clinical trials performed by the Nordic Lymphoma Group. The trials randomized patients to receive either rituximab monotherapy or rituximab combined with interferon alfa-2a. Neither trial used up-front chemotherapy.
Patients included in the follow-up analysis had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
The overall survival rate at 10 years after trial assignment was 75% and 66% after 15 years. Similarly, the lymphoma-specific survival rate was 81% at 10 years after trial assignment and 77% at 15 years, the researchers reported.
Overall, 117 patients did not require treatment with chemotherapy, but 24 patients were further treated with antibodies and/or radiation. Of the 93 patients who received no additional therapies after frontline treatment, 9 patients died from causes unrelated to their lymphoma.
Among the 237 patients who failed initial treatment, the median time to treatment failure was 1.5 years.
In terms of transformation to aggressive lymphoma, the rate was 2.4%/person-year overall. The cumulative risk of transformation was 20% at 10 years after trial assignment and 24% at 15 years.
The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
[email protected]
SOURCE: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
Advanced indolent lymphoma patients can be treated with a rituximab-containing regimen as first-line therapy and, in some cases, skip chemotherapy altogether, a study with 10 years of follow-up data suggests.
After a median of 10.6 years’ follow-up, almost three-quarters of patients (73%) in the study were alive, and 36% never required chemotherapy.
“This [overall survival] is at least as good as that observed in modern immunochemotherapy trials,” Sandra Lockmer, MD, of Karolinska University Hospital in Stockholm and her colleagues reported in the Journal of Clinical Oncology.
The study included 321 patients who were previously untreated and had been enrolled in two randomized clinical trials performed by the Nordic Lymphoma Group. The trials randomized patients to receive either rituximab monotherapy or rituximab combined with interferon alfa-2a. Neither trial used up-front chemotherapy.
Patients included in the follow-up analysis had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
The overall survival rate at 10 years after trial assignment was 75% and 66% after 15 years. Similarly, the lymphoma-specific survival rate was 81% at 10 years after trial assignment and 77% at 15 years, the researchers reported.
Overall, 117 patients did not require treatment with chemotherapy, but 24 patients were further treated with antibodies and/or radiation. Of the 93 patients who received no additional therapies after frontline treatment, 9 patients died from causes unrelated to their lymphoma.
Among the 237 patients who failed initial treatment, the median time to treatment failure was 1.5 years.
In terms of transformation to aggressive lymphoma, the rate was 2.4%/person-year overall. The cumulative risk of transformation was 20% at 10 years after trial assignment and 24% at 15 years.
The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
[email protected]
SOURCE: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: After a median of 10.6 years’ follow up, 73% of patients were alive, and 36% did not require chemotherapy.
Study details: Ten-year follow-up data from two trials on 321 previously untreated patients who had follicular lymphoma, marginal zone lymphoma, small lymphocytic lymphoma, or indolent lymphoma not otherwise specified.
Disclosures: The study was funded in part by the Stockholm County Council and by the Nordic Lymphoma Group. The trials analyzed in the study were supported by Roche. Dr. Lockmer reported having no financial disclosures. Her coauthors reported relationships with Novartis, Gilead, Roche, and Takeda, among others.
Source: Lockmer S et al. J Clin Oncol. 2018 Oct 4:JCO1800262. doi: 10.1200/JCO.18.00262.
AITL responds to 5-azacytidine in small series
Older patients with refractory angioimmunoblastic T cell lymphoma (AITL) appear to respond well to treatment with 5-azacytidine, regardless of mutations.
Francois Lemonnier, MD, of Henri Mondor University Hospitals in Créteil, France, and his colleagues, reported on a retrospective series of 12 AITL patients who received 5-azacytidine for concomitant myeloid neoplasm or as compassionate therapy for relapsed or refractory AITL. The findings were published in Blood.
Patients were given 5-azacytidine subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days. The treatment was given every 28 days until progression or unacceptable toxicity for a median of 5.5 cycles. Along with 5-azacytidine, half of the patients received rituximab due to the presence of EBV replication or EBV B-blasts in the lymph node biopsy.
The patients were assessed via CT scan and responses were evaluated by investigators following the Cheson criteria.
This was a heavily pretreated patient population. The median age was 70 years and 11 of the patients had relapsed or refractory disease and had received a median of two lines of therapy. There was only one treatment-naive patient in the series.
Treatment with 5-azacytidine produced an overall response rate of 75%, with six patients achieving a complete response and three patients achieving a partial response. The median progression-free survival was 15 months and median overall survival was 21 months at a median follow-up of 27 months.
The researchers noted that some elderly patients with poor performance status achieved a sustained response after treatment with an acceptable tolerance.
Treatment was well tolerated overall. There were no treatment-related deaths and no patients developed neutropenia. Three patients required transfusion and another had grade 3 diarrhea.
The researchers also performed molecular studies using targeted deep sequencing. They detected TET2 mutations in all 12 patients, with seven patients having two mutations. Four patients had DNMT3A mutations, five patients had RHOA mutations, and four patients had p.G17V substitution. One patient had an IDH2R172 mutation.
Since all patients had a TET2 mutation, the researchers were unable to assess its impact on treatment response. However, they saw no association between the number of TET2 mutations and treatment response, or mutations in DNMT3A, IDH2, and RHOA and treatment response.
The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
SOURCE: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
Older patients with refractory angioimmunoblastic T cell lymphoma (AITL) appear to respond well to treatment with 5-azacytidine, regardless of mutations.
Francois Lemonnier, MD, of Henri Mondor University Hospitals in Créteil, France, and his colleagues, reported on a retrospective series of 12 AITL patients who received 5-azacytidine for concomitant myeloid neoplasm or as compassionate therapy for relapsed or refractory AITL. The findings were published in Blood.
Patients were given 5-azacytidine subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days. The treatment was given every 28 days until progression or unacceptable toxicity for a median of 5.5 cycles. Along with 5-azacytidine, half of the patients received rituximab due to the presence of EBV replication or EBV B-blasts in the lymph node biopsy.
The patients were assessed via CT scan and responses were evaluated by investigators following the Cheson criteria.
This was a heavily pretreated patient population. The median age was 70 years and 11 of the patients had relapsed or refractory disease and had received a median of two lines of therapy. There was only one treatment-naive patient in the series.
Treatment with 5-azacytidine produced an overall response rate of 75%, with six patients achieving a complete response and three patients achieving a partial response. The median progression-free survival was 15 months and median overall survival was 21 months at a median follow-up of 27 months.
The researchers noted that some elderly patients with poor performance status achieved a sustained response after treatment with an acceptable tolerance.
Treatment was well tolerated overall. There were no treatment-related deaths and no patients developed neutropenia. Three patients required transfusion and another had grade 3 diarrhea.
The researchers also performed molecular studies using targeted deep sequencing. They detected TET2 mutations in all 12 patients, with seven patients having two mutations. Four patients had DNMT3A mutations, five patients had RHOA mutations, and four patients had p.G17V substitution. One patient had an IDH2R172 mutation.
Since all patients had a TET2 mutation, the researchers were unable to assess its impact on treatment response. However, they saw no association between the number of TET2 mutations and treatment response, or mutations in DNMT3A, IDH2, and RHOA and treatment response.
The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
SOURCE: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
Older patients with refractory angioimmunoblastic T cell lymphoma (AITL) appear to respond well to treatment with 5-azacytidine, regardless of mutations.
Francois Lemonnier, MD, of Henri Mondor University Hospitals in Créteil, France, and his colleagues, reported on a retrospective series of 12 AITL patients who received 5-azacytidine for concomitant myeloid neoplasm or as compassionate therapy for relapsed or refractory AITL. The findings were published in Blood.
Patients were given 5-azacytidine subcutaneously at a dose of 75 mg/m2 daily for 7 consecutive days. The treatment was given every 28 days until progression or unacceptable toxicity for a median of 5.5 cycles. Along with 5-azacytidine, half of the patients received rituximab due to the presence of EBV replication or EBV B-blasts in the lymph node biopsy.
The patients were assessed via CT scan and responses were evaluated by investigators following the Cheson criteria.
This was a heavily pretreated patient population. The median age was 70 years and 11 of the patients had relapsed or refractory disease and had received a median of two lines of therapy. There was only one treatment-naive patient in the series.
Treatment with 5-azacytidine produced an overall response rate of 75%, with six patients achieving a complete response and three patients achieving a partial response. The median progression-free survival was 15 months and median overall survival was 21 months at a median follow-up of 27 months.
The researchers noted that some elderly patients with poor performance status achieved a sustained response after treatment with an acceptable tolerance.
Treatment was well tolerated overall. There were no treatment-related deaths and no patients developed neutropenia. Three patients required transfusion and another had grade 3 diarrhea.
The researchers also performed molecular studies using targeted deep sequencing. They detected TET2 mutations in all 12 patients, with seven patients having two mutations. Four patients had DNMT3A mutations, five patients had RHOA mutations, and four patients had p.G17V substitution. One patient had an IDH2R172 mutation.
Since all patients had a TET2 mutation, the researchers were unable to assess its impact on treatment response. However, they saw no association between the number of TET2 mutations and treatment response, or mutations in DNMT3A, IDH2, and RHOA and treatment response.
The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
SOURCE: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
FROM BLOOD
Key clinical point:
Major finding: The overall response rate was 75% among the 12 patients, with 6 patients achieving complete response.
Study details: A retrospective case series of 12 patients with angioimmunoblastic T cell lymphoma.
Disclosures: The study was funded by a grant from the Leukemia & Lymphoma Society. Three of the coauthors received honoraria from Celgene.
Source: Lemonnier F et al. Blood. 2018 Oct 2. doi: 10.1182/blood-2018-04-840538.
Brentuximab vendotin plus CHP meets PFS endpoint in ECHELON-2
Takeda Pharmaceuticals and Seattle Genetics announced top-line results in the ECHELON-2 phase 3 trial of brentuximab vedotin plus CHP (cyclophosphamide, doxorubicin, prednisone) in the frontline treatment of CD-30 expressing peripheral T-cell lymphoma (PTCL).
The combination achieved statistically significant improvement in progression-free survival (PFS), compared with the control arm of standard chemotherapy alone using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The PFS was assessed by an Independent Review Facility (hazard ratio, 0.71; P = .0110).
The combination of brentuximab vedotin plus CHP also outperformed CHOP in overall survival, a secondary endpoint of the trial (hazard ratio, 0.66, P = .0244), according to the drug sponsors.
Full results of ECHELON-2 will be presented in December 2018 at the annual meeting of the American Society of Hematology, according to the announcement from Seattle Genetics and Takeda.
Takeda Pharmaceuticals and Seattle Genetics announced top-line results in the ECHELON-2 phase 3 trial of brentuximab vedotin plus CHP (cyclophosphamide, doxorubicin, prednisone) in the frontline treatment of CD-30 expressing peripheral T-cell lymphoma (PTCL).
The combination achieved statistically significant improvement in progression-free survival (PFS), compared with the control arm of standard chemotherapy alone using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The PFS was assessed by an Independent Review Facility (hazard ratio, 0.71; P = .0110).
The combination of brentuximab vedotin plus CHP also outperformed CHOP in overall survival, a secondary endpoint of the trial (hazard ratio, 0.66, P = .0244), according to the drug sponsors.
Full results of ECHELON-2 will be presented in December 2018 at the annual meeting of the American Society of Hematology, according to the announcement from Seattle Genetics and Takeda.
Takeda Pharmaceuticals and Seattle Genetics announced top-line results in the ECHELON-2 phase 3 trial of brentuximab vedotin plus CHP (cyclophosphamide, doxorubicin, prednisone) in the frontline treatment of CD-30 expressing peripheral T-cell lymphoma (PTCL).
The combination achieved statistically significant improvement in progression-free survival (PFS), compared with the control arm of standard chemotherapy alone using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The PFS was assessed by an Independent Review Facility (hazard ratio, 0.71; P = .0110).
The combination of brentuximab vedotin plus CHP also outperformed CHOP in overall survival, a secondary endpoint of the trial (hazard ratio, 0.66, P = .0244), according to the drug sponsors.
Full results of ECHELON-2 will be presented in December 2018 at the annual meeting of the American Society of Hematology, according to the announcement from Seattle Genetics and Takeda.
New mantle cell trials launching
At least four new trials in mantle cell lymphoma (MCL) are preparing to launch, according to records posted on Clinicaltrials.gov.
The range of new studies are occurring around the world and examine the association of lenalidomide with tumor flare reaction, maintenance ixazomib for newly diagnosed patients, the addition of bortezomib to ibrutinib in ibrutinib-released patients, and acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in untreated patients.
Acalabrutinib With Alternating Cycles of Bendamustine/Rituximab and Cytarabine/Rituximab for Untreated Mantle Cell Lymphoma (NCT03623373) is slated to start on Oct. 31, 2018, and be completed in 2025.
The trial, which is not yet recruiting, is estimated to enroll 15 participants. The phase 1 study will be aimed at evaluating the efficacy and safety of acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in MCL patients who are treatment naive. The primary outcome measure is stem cell mobilization success rate in patients treated with this regimen. The phase 1 study is preparation for a larger cooperative group trial that aims to achieve a standard induction regimen for MCL in transplant-eligible patients.
The study is sponsored by Washington University, St. Louis, in collaboration with Acerta Pharma.
Ibrutinib-relapsed MCL
Bortezomib in Combination With Ibrutinib in Ibrutinib Relapsed Mantle Cell Lymphoma (NCT03617484) is scheduled to start in September 2018 and be completed in 2021. It is estimated to enroll 35 patients but is not yet recruiting.
The phase 2, open-label study is aimed at evaluating the efficacy of the ibrutinib/bortezomib in MCL patients who relapsed on ibrutinib alone. The primary outcome is the overall response rate at 6 months based on the Lugano criteria.
The trial is sponsored by the University of Michigan, Ann Arbor.
Ixazomib Maintenance in Patients With Newly Diagnosed Mantle Cell Lymphoma (NCT03616782) launched in August 2018 and is expected to be completed in 2022.
The phase 2 trial is expected to enroll 98 patients but has not started recruitment yet. In the open-label, multicenter study, newly diagnosed MCL patients will receive induction chemotherapy and those who achieve at least a partial response will be eligible to move on to the experimental phase – maintenance ixazomib. At least 8 weeks after completing induction, patients can receive ixazomib orally on days 1, 8, and 15 for 4 weeks. Treatment repeats every 4 weeks for up to 2 years unless the disease progresses or there is unacceptable toxicity. The primary outcome measure is 2-year progression-free survival.
The trial is sponsored by Ho Sup Lee, MD, of Kosin University Gospel Hospital in Busan, South Korea, in collaboration with Takeda.
PASS MCL-005
The Noninterventional Study in Patients With Relapsed or Refractory Mantle Cell Lymphoma (R/R MCL) to Investigate the Association of Lenalidomide With Tumor Flare Reaction and High Tumor Burden, called PASS MCL-005 (NCT03647124) is slated to start at the end of September 2018 and wrap up in 2026.
The retrospective cohort study will rely on data collection from Nordic registries and national health databases. The researchers will analyze records from sites where lenalidomide treatment for relapsed/refractory MCL is reimbursed. The estimated enrollment is 560 participants.
The primary goal is to quantify and characterize the event of tumor flare reaction by tumor burden in relapsed/refractory MCL patients who were treated with lenalidomide in a real-world setting.
The European-based study is sponsored by Celgene.
At least four new trials in mantle cell lymphoma (MCL) are preparing to launch, according to records posted on Clinicaltrials.gov.
The range of new studies are occurring around the world and examine the association of lenalidomide with tumor flare reaction, maintenance ixazomib for newly diagnosed patients, the addition of bortezomib to ibrutinib in ibrutinib-released patients, and acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in untreated patients.
Acalabrutinib With Alternating Cycles of Bendamustine/Rituximab and Cytarabine/Rituximab for Untreated Mantle Cell Lymphoma (NCT03623373) is slated to start on Oct. 31, 2018, and be completed in 2025.
The trial, which is not yet recruiting, is estimated to enroll 15 participants. The phase 1 study will be aimed at evaluating the efficacy and safety of acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in MCL patients who are treatment naive. The primary outcome measure is stem cell mobilization success rate in patients treated with this regimen. The phase 1 study is preparation for a larger cooperative group trial that aims to achieve a standard induction regimen for MCL in transplant-eligible patients.
The study is sponsored by Washington University, St. Louis, in collaboration with Acerta Pharma.
Ibrutinib-relapsed MCL
Bortezomib in Combination With Ibrutinib in Ibrutinib Relapsed Mantle Cell Lymphoma (NCT03617484) is scheduled to start in September 2018 and be completed in 2021. It is estimated to enroll 35 patients but is not yet recruiting.
The phase 2, open-label study is aimed at evaluating the efficacy of the ibrutinib/bortezomib in MCL patients who relapsed on ibrutinib alone. The primary outcome is the overall response rate at 6 months based on the Lugano criteria.
The trial is sponsored by the University of Michigan, Ann Arbor.
Ixazomib Maintenance in Patients With Newly Diagnosed Mantle Cell Lymphoma (NCT03616782) launched in August 2018 and is expected to be completed in 2022.
The phase 2 trial is expected to enroll 98 patients but has not started recruitment yet. In the open-label, multicenter study, newly diagnosed MCL patients will receive induction chemotherapy and those who achieve at least a partial response will be eligible to move on to the experimental phase – maintenance ixazomib. At least 8 weeks after completing induction, patients can receive ixazomib orally on days 1, 8, and 15 for 4 weeks. Treatment repeats every 4 weeks for up to 2 years unless the disease progresses or there is unacceptable toxicity. The primary outcome measure is 2-year progression-free survival.
The trial is sponsored by Ho Sup Lee, MD, of Kosin University Gospel Hospital in Busan, South Korea, in collaboration with Takeda.
PASS MCL-005
The Noninterventional Study in Patients With Relapsed or Refractory Mantle Cell Lymphoma (R/R MCL) to Investigate the Association of Lenalidomide With Tumor Flare Reaction and High Tumor Burden, called PASS MCL-005 (NCT03647124) is slated to start at the end of September 2018 and wrap up in 2026.
The retrospective cohort study will rely on data collection from Nordic registries and national health databases. The researchers will analyze records from sites where lenalidomide treatment for relapsed/refractory MCL is reimbursed. The estimated enrollment is 560 participants.
The primary goal is to quantify and characterize the event of tumor flare reaction by tumor burden in relapsed/refractory MCL patients who were treated with lenalidomide in a real-world setting.
The European-based study is sponsored by Celgene.
At least four new trials in mantle cell lymphoma (MCL) are preparing to launch, according to records posted on Clinicaltrials.gov.
The range of new studies are occurring around the world and examine the association of lenalidomide with tumor flare reaction, maintenance ixazomib for newly diagnosed patients, the addition of bortezomib to ibrutinib in ibrutinib-released patients, and acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in untreated patients.
Acalabrutinib With Alternating Cycles of Bendamustine/Rituximab and Cytarabine/Rituximab for Untreated Mantle Cell Lymphoma (NCT03623373) is slated to start on Oct. 31, 2018, and be completed in 2025.
The trial, which is not yet recruiting, is estimated to enroll 15 participants. The phase 1 study will be aimed at evaluating the efficacy and safety of acalabrutinib plus bendamustine and rituximab and cytarabine and rituximab in MCL patients who are treatment naive. The primary outcome measure is stem cell mobilization success rate in patients treated with this regimen. The phase 1 study is preparation for a larger cooperative group trial that aims to achieve a standard induction regimen for MCL in transplant-eligible patients.
The study is sponsored by Washington University, St. Louis, in collaboration with Acerta Pharma.
Ibrutinib-relapsed MCL
Bortezomib in Combination With Ibrutinib in Ibrutinib Relapsed Mantle Cell Lymphoma (NCT03617484) is scheduled to start in September 2018 and be completed in 2021. It is estimated to enroll 35 patients but is not yet recruiting.
The phase 2, open-label study is aimed at evaluating the efficacy of the ibrutinib/bortezomib in MCL patients who relapsed on ibrutinib alone. The primary outcome is the overall response rate at 6 months based on the Lugano criteria.
The trial is sponsored by the University of Michigan, Ann Arbor.
Ixazomib Maintenance in Patients With Newly Diagnosed Mantle Cell Lymphoma (NCT03616782) launched in August 2018 and is expected to be completed in 2022.
The phase 2 trial is expected to enroll 98 patients but has not started recruitment yet. In the open-label, multicenter study, newly diagnosed MCL patients will receive induction chemotherapy and those who achieve at least a partial response will be eligible to move on to the experimental phase – maintenance ixazomib. At least 8 weeks after completing induction, patients can receive ixazomib orally on days 1, 8, and 15 for 4 weeks. Treatment repeats every 4 weeks for up to 2 years unless the disease progresses or there is unacceptable toxicity. The primary outcome measure is 2-year progression-free survival.
The trial is sponsored by Ho Sup Lee, MD, of Kosin University Gospel Hospital in Busan, South Korea, in collaboration with Takeda.
PASS MCL-005
The Noninterventional Study in Patients With Relapsed or Refractory Mantle Cell Lymphoma (R/R MCL) to Investigate the Association of Lenalidomide With Tumor Flare Reaction and High Tumor Burden, called PASS MCL-005 (NCT03647124) is slated to start at the end of September 2018 and wrap up in 2026.
The retrospective cohort study will rely on data collection from Nordic registries and national health databases. The researchers will analyze records from sites where lenalidomide treatment for relapsed/refractory MCL is reimbursed. The estimated enrollment is 560 participants.
The primary goal is to quantify and characterize the event of tumor flare reaction by tumor burden in relapsed/refractory MCL patients who were treated with lenalidomide in a real-world setting.
The European-based study is sponsored by Celgene.
SUMMARY FROM CLINICALTRIALS.GOV
New BTK inhibitor under review in China
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.