User login
The Hospitalist Triage Role for Reducing Admission Delays: Impacts on Throughput, Quality, Interprofessional Practice, and Clinician Experience of Care
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).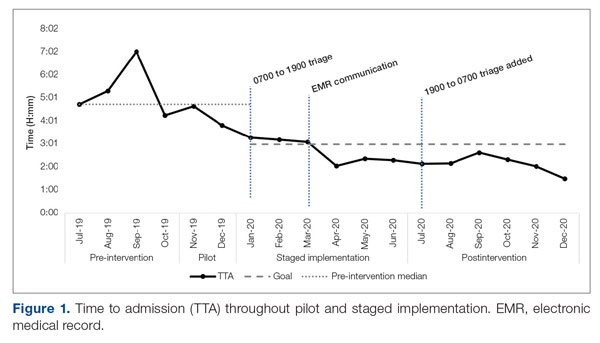
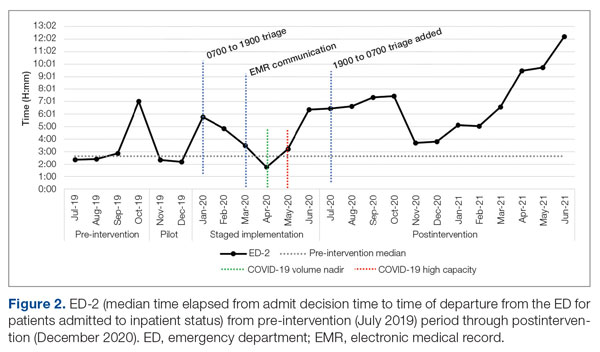
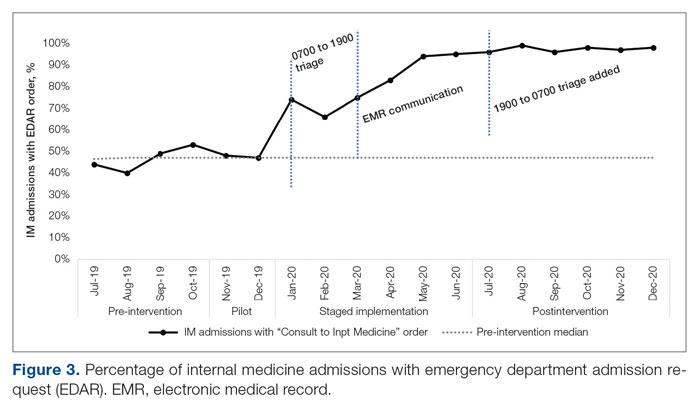
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).
There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.
Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions
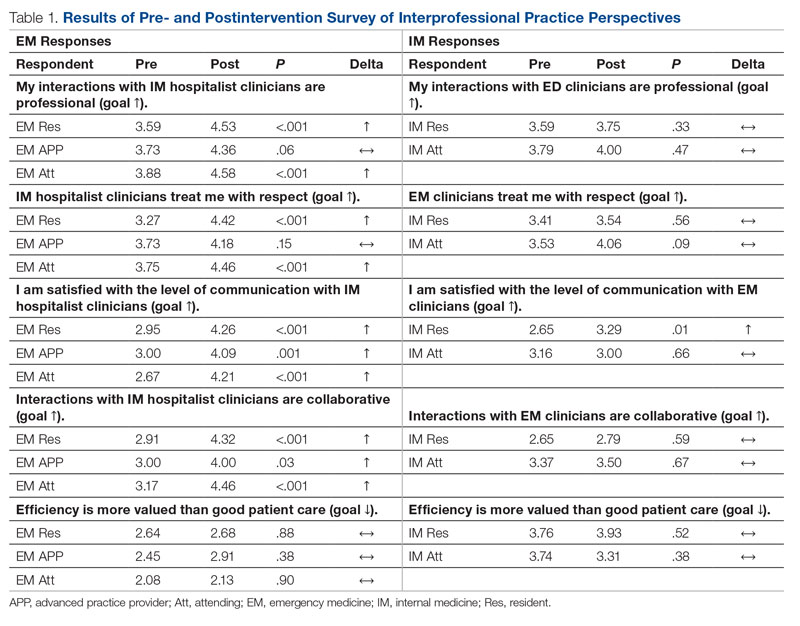
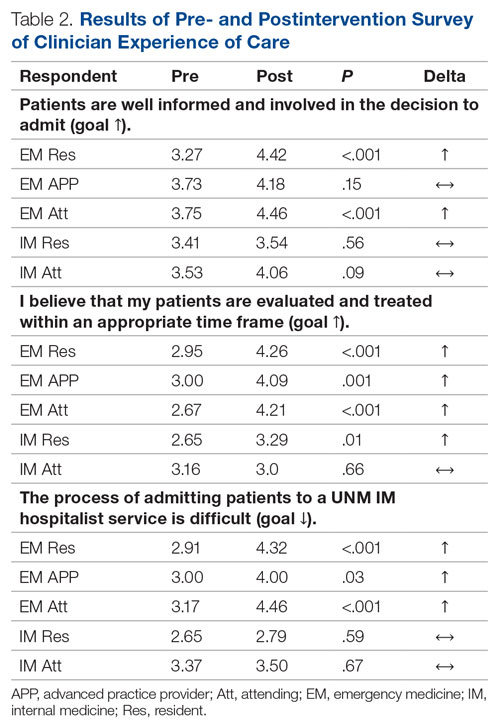
For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.
Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
From the Division of Hospital Medicine, University of New Mexico Hospital, Albuquerque (Drs. Bartlett, Pizanis, Angeli, Lacy, and Rogers), Department of Emergency Medicine, University of New Mexico Hospital, Albuquerque (Dr. Scott), and University of New Mexico School of Medicine, Albuquerque (Ms. Baca).
ABSTRACT
Background: Emergency department (ED) crowding is associated with deleterious consequences for patient care and throughput. Admission delays worsen ED crowding. Time to admission (TTA)—the time between an ED admission request and internal medicine (IM) admission orders—can be shortened through implementation of a triage hospitalist role. Limited research is available highlighting the impact of triage hospitalists on throughput, care quality, interprofessional practice, and clinician experience of care.
Methods: A triage hospitalist role was piloted and implemented. Run charts were interpreted using accepted rules for deriving statistically significant conclusions. Statistical analysis was applied to interprofessional practice and clinician experience-of-care survey results.
Results: Following implementation, TTA decreased from 5 hours 19 minutes to 2 hours 8 minutes. Emergency department crowding increased from baseline. The reduction in TTA was associated with decreased time from ED arrival to IM admission request, no change in critical care transfers during the initial 24 hours, and increased admissions to inpatient status. Additionally, decreased TTA was associated with no change in referring hospital transfer rates and no change in hospital medicine length of stay. Interprofessional practice attitudes improved among ED clinicians but not IM clinicians. Clinician experience-of-care results were mixed.
Conclusion: A triage hospitalist role is an effective approach for mitigating admission delays, with no evident adverse clinical consequences. A triage hospitalist alone was incapable of resolving ED crowding issues without a complementary focus on downstream bottlenecks.
Keywords: triage hospitalist, admission delay, quality improvement.
Excess time to admission (TTA), defined as the time between an emergency department (ED) admission request and internal medicine (IM) admission orders, contributes to ED crowding, which is associated with deleterious impacts on patient care and throughput. Prior research has correlated ED crowding with an increase in length of stay (LOS)1-3 and total inpatient cost,1 as well as increased inpatient mortality, higher left-without-being-seen rates,4 delays in clinically meaningful care,5,6 and poor patient and clinician satisfaction.6,7 While various solutions have been proposed to alleviate ED crowding,8 excess TTA is one aspect that IM can directly address.
Like many institutions, ours is challenged by ED crowding. Time to admission is a known bottleneck. Underlying factors that contribute to excess TTA include varied admission request volumes in relation to fixed admitting capacity; learner-focused admitting processes; and unreliable strategies for determining whether patients are eligible for ED observation, transfer to an alternative facility, or admission to an alternative primary service.
To address excess TTA, we piloted then implemented a triage hospitalist role, envisioned as responsible for evaluating ED admission requests to IM, making timely determinations of admission appropriateness, and distributing patients to admitting teams. This intervention was selected because of its strengths, including the ability to standardize admission processes, improve the proximity of clinical decision-makers to patient care to reduce delays, and decrease hierarchical imbalances experienced by trainees, and also because the institution expressed a willingness to mitigate its primary weakness (ie, ongoing financial support for sustainability) should it prove successful.
Previously, a triage hospitalist has been defined as “a physician who assesses patients for admission, actively supporting the transition of the patient from the outpatient to the inpatient setting.”9 Velásquez et al surveyed 10 academic medical centers and identified significant heterogeneity in the roles and responsibilities of a triage hospitalist.9 Limited research addresses the impact of this role on throughput. One report described the volume and source of requests evaluated by a triage hospitalist and the frequency with which the triage hospitalists’ assessment of admission appropriateness aligned with that of the referring clinicians.10 No prior research is available demonstrating the impact of this role on care quality, interprofessional practice, or clinician experience of care. This article is intended to address these gaps in the literature.
Methods
Setting
The University of New Mexico Hospital has 537 beds and is the only level-1 trauma and academic medical center in the state. On average, approximately 8000 patients register to be seen in the ED per month. Roughly 600 are admitted to IM per month. This study coincided with the COVID-19 pandemic, with low patient volumes in April 2020, overcapacity census starting in May 2020, and markedly high patient volumes in May/June 2020 and November/December 2020. All authors participated in project development, implementation, and analysis.
Preintervention IM Admission Process
When requesting IM admission, ED clinicians (resident, advanced practice provider [APP], or attending) contacted the IM triage person (typically an IM resident physician) by phone or in person. The IM triage person would then assess whether the patient needed critical care consultation (a unique and separate admission pathway), was eligible for ED observation or transfer to an outside hospital, or was clinically appropriate for IM subacute and floor admission. Pending admissions were evaluated in order of severity of illness or based on wait time if severity of illness was equal. Transfers from the intensive care unit (ICU) and referring hospitals were prioritized. Between 7:00
Triage Hospitalist Pilot
Key changes made during the pilot included scheduling an IM attending to serve as triage hospitalist for all IM admission requests from the ED between 7:00
Measures for Triage Hospitalist Pilot
Data collected included request type (new vs overflow from night) and patient details (name, medical record number). Two time points were recorded: when the EDAR order was entered and when admission orders were entered. Process indicators, including whether the EDAR order was entered and the final triage decision (eg, discharge, IM), were recorded. General feedback was requested at the end of each shift.
Phased Implementation of Triage Hospitalist Role
Triage hospitalist role implementation was approved following the pilot, with additional salary support funded by the institution. A new performance measure (time from admission request to admission order, self-identified goal < 3 hours) was approved by all parties.
In January 2020, the role was scheduled from 7:00
In March 2020, to create a single communication pathway while simultaneously hardwiring our measurement strategy, the EDAR order was modified such that it would automatically prompt a 1-way communication to the triage hospitalist using the institution’s secure messaging software. The message included patient name, medical record number, location, ED attending, reason for admission, and consultation priority, as well as 2 questions prompting ED clinicians to reflect on the most common reasons for the triage hospitalist to recommend against IM admission (eligible for admission to other primary service, transfer to alternative hospital).
In July 2020, the triage hospitalist role was scheduled 24 hours a day, 7 days a week, to meet an institutional request. The schedule was divided into a daytime 7:00
Measures for Triage Hospitalist Role
The primary outcome measure was TTA, defined as the time between EDAR (operationalized using EDAR order timestamp) and IM admission decision (operationalized using inpatient bed request order timestamp). Additional outcome measures included the Centers for Medicare & Medicaid Services Electronic Clinical Quality Measure ED-2 (eCQM ED-2), defined as the median time from admit decision to departure from the ED for patients admitted to inpatient status.
Process measures included time between patient arrival to the ED (operationalized using ED registration timestamp) and EDAR and percentage of IM admissions with an EDAR order. Balancing measures included time between bed request order (referred to as the IM admission order) and subsequent admission orders. While the IM admission order prompts an inpatient clinical encounter and inpatient bed assignment, subsequent admission orders are necessary for clinical care. Additional balancing measures included ICU transfer rate within the first 24 hours, referring facility transfer frequency to IM (an indicator of access for patients at outside hospitals), average hospital medicine LOS (operationalized using ED registration timestamp to discharge timestamp), and admission status (inpatient vs observation).
An anonymous preintervention (December 2019) and postintervention (August 2020) survey focusing on interprofessional practice and clinician experience of care was used to obtain feedback from ED and IM attendings, APPs, and trainees. Emergency department clinicians were asked questions pertaining to their IM colleagues and vice versa. A Likert 5-point scale was used to respond.
Data Analysis
The preintervention period was June 1, 2019, to October 31, 2019; the pilot period was November 1, 2019, to December 31, 2019; the staged implementation period was January 1, 2020, to June 30, 2020; and the postintervention period was July 1, 2020, to December 31, 2020. Run charts for outcome, process, and balancing measures were interpreted using rules for deriving statistically significant conclusions.11 Statistical analysis using a t test assuming unequal variances with P < . 05 to indicate statistical significance was applied to experience-of-care results. The study was approved by the Institutional Review Board.
Results
Triage Hospitalist Pilot Time Period
Seventy-four entries were recorded, 56 (75.7%) reflecting new admission requests. Average time between EDAR order and IM admission order was 40 minutes. The EDAR order was entered into the EMR without prompting in 22 (29.7%) cases. In 56 (75.7%) cases, the final triage decision was IM admission. Other dispositions included 3 discharges, 4 transfers, 3 alternative primary service admissions, 1 ED observation, and 7 triage deferrals pending additional workup or stabilization.
Feedback substantiated several benefits, including improved coordination among IM, ED, and consultant clinicians, as well as early admission of seriously ill patients. Feedback also confirmed several expected challenges, including evidence of communication lapses, difficulty with transfer coordinator integration, difficulty hardwiring elements of the verbal and bedside handoff, and perceived high cognitive load for the triage hospitalist. Several unexpected issues included whether ED APPs can request admission independently and how reconsultation is expected to occur if admission is initially deferred.
Triage Hospitalist Implementation Time Period
Time to admission decreased from a baseline pre-pilot average of 5 hours 19 minutes (median, 4 hours 45 minutes) to a postintervention average of 2 hours 8 minutes, with a statistically significant downward shift post intervention (Figure 1).
ED-2 increased from a baseline average of 3 hours 40 minutes (median, 2 hours 39 minutes), with a statistically significant upward shift starting in May 2020 (Figure 2). Time between patient arrival to the ED and EDAR order decreased from a baseline average of 8 hours 47 minutes (median, 8 hours 37 minutes) to a postintervention average of 5 hours 57 minutes, with a statistically significant downward shift post intervention. Percentage of IM admissions with an EDAR order increased from a baseline average of 47% (median, 47%) to 97%, with a statistically significant upward shift starting in January 2020 (Figure 3).

There was no change in observed average time between IM admission order and subsequent admission orders pre and post intervention (16 minutes vs 18 minutes). However, there was a statistically significant shift up to an average of 40 minutes from January through June 2020, which then resolved. The percentage of patients transferred to the ICU within 24 hours of admission to IM did not change (1.1% pre vs 1.4% post intervention). Frequency of patients transferred in from a referring facility also did not change (26/month vs 22/month). Average hospital medicine LOS did not change to a statistically significant degree (6.48 days vs 6.62 days). The percentage of inpatient admissions relative to short stays increased from a baseline of 74.0% (median, 73.6%) to a postintervention average of 82.4%, with a statistically significant shift upward starting March 2020.

Regarding interprofessional practice and clinician experience of care, 122 of 309 preintervention surveys (39.5% response rate) and 98 of 309 postintervention surveys (31.7% response rate) were completed. Pre- and postintervention responses were not linked.
Regarding interprofessional practice, EM residents and EM attendings experienced statistically significant improvements in all interprofessional practice domains (Table 1). Emergency medicine APPs experienced statistically significant improvements post intervention with “I am satisfied with the level of communication with IM hospitalist clinicians” and “Interactions

For clinician experience of care, EM residents (P < .001) and attendings (P < .001) experienced statistically significant improvements in “Patients are well informed and involved in the decision to admit,” whereas IM residents and attendings, as well as EM APPs, experienced nonstatistically significant improvements (Table 2). All groups except IM attendings experienced a statistically significant improvement (IM resident P = .011, EM resident P < .001, EM APP P = .001, EM attending P < .001) in “I believe that my patients are evaluated and treated within an appropriate time frame.” Internal medicine attendings felt that this indicator worsened to a nonstatistically significant degree. Post intervention, EM groups experienced a statistically significant worsening in “The process of admitting patients to a UNM IM hospitalist service is difficult,” while IM groups experienced a nonstatistically significant worsening.

Discussion
Implementation of the triage hospitalist role led to a significant reduction in average TTA, from 5 hours 19 minutes to 2 hours 8 minutes. Performance has been sustained at 1 hour 42 minutes on average over the past 6 months. The triage hospitalist was successful at reducing TTA because of their focus on evaluating new admission and transfer requests, deferring other admission responsibilities to on-call admitting teams. Early admission led to no increase in ICU transfers or hospitalist LOS. To ensure that earlier admission reflected improved timeliness of care and that new sources of delay were not being created, we measured the time between IM admission and subsequent admission orders. A statistically significant increase to 40 minutes from January through June 2020 was attributable to the hospitalist acclimating to their new role and the need to standardize workflow. This delay subsequently resolved. An additional benefit of the triage hospitalist was an increase in the proportion of inpatient admissions compared with short stays.
ED-2, an indicator of ED crowding, increased from 3 hours 40 minutes, with a statistically significant upward shift starting May 2020. Increasing ED-2 associated with the triage hospitalist role makes intuitive sense. Patients are admitted 2 hours 40 minutes earlier in their hospital course while downstream bottlenecks preventing patient movement to an inpatient bed remained unchanged. Unfortunately, the COVID-19 pandemic complicates interpretation of ED-2 because the measure reflects institutional capacity to match demand for inpatient beds. Fewer ED registrations and lower hospital medicine census (and resulting inpatient bed availability) in April 2020 during the first COVID-19 surge coincided with an ED-2 nadir of 1 hour 46 minutes. The statistically significant upward shift from May onward reflects ongoing and unprecedented patient volumes. It remains difficult to tease apart the presumed lesser contribution of the triage hospitalist role and presumed larger contribution of high patient volumes on ED-2 increases.
An important complementary change was linkage between the EDAR order and our secure messaging software, creating a single source of admission and transfer requests, prompting early ED clinician consideration of factors that could result in alternative disposition, and ensuring a sustainable data source for TTA. The order did not replace direct communication and included guidance for how triage hospitalists should connect with their ED colleagues. Percentage of IM admissions with the EDAR order increased to 97%. Fallouts are attributed to admissions from non-ED sources (eg, referring facility, endoscopy suite transfers). This communication strategy has been expanded as the primary mechanism of initiating consultation requests between IM and all consulting services.
This intervention was successful from the perspective of ED clinicians. Improvements can be attributed to the simplified admission process, timely patient assessment, a perception that patients are better informed of the decision to admit, and the ability to communicate with the triage hospitalist. Emergency medicine APPs may not have experienced similar improvements due to ongoing perceptions of a hierarchical imbalance. Unfortunately, the small but not statistically significant worsening perspective among ED clinicians that “efficiency is more valued than good patient care” and the statistically significant worsening perspective that “admitting patients to a UNM IM hospitalist service is difficult” may be due to the triage hospitalist responsibility for identifying the roughly 25% of patients who are safe for an alternative disposition.
Internal medicine clinicians experienced no significant changes in attitudes. Underlying causes are likely multifactorial and a focus of ongoing work. Internal medicine residents experienced statistically significant improvements for “I am satisfied with the level of communication with EM clinicians” and nonstatistically significant improvements for the other 3 domains, likely because the intervention enabled them to focus on clinical care rather than the administrative tasks and decision-making complexities inherent to the IM admission process. Internal medicine attendings reported a nonstatistically significant worsening in “I am satisfied with the level of communication with EM clinicians,” which is possibly attributable to challenges connecting with ED attendings after being notified that a new admission is pending. Unfortunately, bedside handoff was not hardwired and is done sporadically. Independent of the data, we believe that the triage hospitalist role has facilitated closer ED-IM relationships by aligning clinical priorities, standardizing processes, improving communication, and reducing sources of hierarchical imbalance and conflict. We expected IM attendings and residents to experience some degree of resolution of the perception that “efficiency is more valued than good patient care” because of the addition of a dedicated triage role. Our data also suggest that IM attendings are less likely to agree that “patients are evaluated and treated within an appropriate time frame.” Both concerns may be linked to the triage hospitalist facing multiple admission and transfer sources with variable arrival rates and variable patient complexity, resulting in high cognitive load and the perception that individual tasks are not completed to the best of their abilities.
To our knowledge, this is the first study assessing the impact of the triage hospitalist role on throughput, clinical care quality, interprofessional practice, and clinician experience of care. In the cross-sectional survey of 10 academic medical centers, 8 had defined triage roles filled by IM attendings, while the remainder had IM attendings supervising trainees.9 A complete picture of the prevalence and varying approaches of triage hospitalists models is unknown. Howell et al12 reported on an approach that reduced admission delays without a resulting increase in mortality or LOS. Our approach differed in several ways, with greater involvement of the triage hospitalist in determining a final admission decision, incorporation of EMR communication, and presence of existing throughput challenges preventing patients from moving seamlessly to an inpatient unit.
Conclusion
We believe this effort was successful for several reasons, including adherence to quality improvement best practices, such as engagement of stakeholders early on, the use of data to inform decision-making, the application of technology to hardwire process, and alignment with institutional priorities. Spread of this intervention will be limited by the financial investment required to start and maintain a triage hospitalist role. A primary limitation of this study is the confounding effect of the COVID-19 pandemic on our analysis. Next steps include identification of clinicians wishing to specialize in triage and expanding triage to include non-IM primary services. Additional research to optimize the triage hospitalist experience of care, as well as to measure improvements in patient-centered outcomes, is necessary.
Corresponding author: Christopher Bartlett, MD, MPH; MSC10 5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected]
Disclosures: None reported.
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
1. Huang Q, Thind A, Dreyer JF, et al. The impact of delays to admission from the emergency department on inpatient outcomes. BMC Emerg Med. 2010;10:16. doi:10.1186/1471-227X-10-16
2. Liew D, Liew D, Kennedy MP. Emergency department length of stay independently predicts excess inpatient length of stay. Med J Aust. 2003;179:524-526. doi:10.5694/j.1326-5377.2003.tb05676.x
3. Richardson DB. The access-block effect: relationship between delay to reaching an inpatient bed and inpatient length of stay. Med J Aust. 2002;177:492-495. doi:10.5694/j.1326-5377.2002.tb04917.x
4. Polevoi SK, Quinn JV, Kramer KR. Factors associated with patients who leave without being seen. Acad Emerg Med. 2005;12:232-236. doi:10.1197/j.aem.2004.10.029
5. Bernstein SL, Aronsky D, Duseja R, et al. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med. 2009;16:1-10. doi:10.1111/j.1553-2712.2008.00295.x
6. Vieth TL, Rhodes KV. The effect of crowding on access and quality in an academic ED. Am J Emerg Med. 2006;24:787-794. doi:10.1016/j.ajem.2006.03.026
7. Rondeau KV, Francescutti LH. Emergency department overcrowding: the impact of resource scarcity on physician job satisfaction. J Healthc Manag. 2005;50:327-340; discussion 341-342.
8. Emergency Department Crowding: High Impact Solutions. American College of Emergency Physicians. Emergency Medicine Practice Committee. 2016. Accessed March 31, 2023. https://www.acep.org/globalassets/sites/acep/media/crowding/empc_crowding-ip_092016.pdf
9. Velásquez ST, Wang ES, White AW, et al. Hospitalists as triagists: description of the triagist role across academic medical centers. J Hosp Med. 2020;15:87-90. doi:10.12788/jhm.3327
10. Amick A, Bann M. Characterizing the role of the “triagist”: reasons for triage discordance and impact on disposition. J Gen Intern Med. 2021;36:2177-2179. doi:10.1007/s11606-020-05887-y
11. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning for variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51. doi:10.1136/bmjqs.2009.037895
12. Howell EE, Bessman ES, Rubin HR. Hospitalists and an innovative emergency department admission process. J Gen Intern Med. 2004;19:266-268. doi:10.1111/j.1525-1497.2004.30431.x
Becoming a high-value care physician
‘Culture shift’ comes from collective efforts
It’s Monday morning, and Mrs. Jones still has abdominal pain. Your ward team decides to order a CT. On chart review you notice she’s had three other abdominal CTs for the same indication this year. How did this happen? What should you do?
High-value care has been defined by the Institute of Medicine as “the best care for the patient, with the optimal result for the circumstances, delivered at the right price.”1 With an estimated $700 billion dollars – 30% of medical expenditures – spent on wasted care, there are rising calls for a transformational shift.2
You are now asked to consider not just everything you can do for a patient, but also the benefits, harms, and costs associated with those choices. But where to start? We recommend that trainees integrate these tips for high-value care into their routine practice.
1. Use evidence-based resources that highlight value
A great place to begin is the “Six Things Medical Students and Trainees Should Question,” originally published in Academic Medicine and created by Choosing Wisely Canada™. Recommendations range from avoiding tests or treatments that will not change a patient’s clinical course to holding off on ordering tests solely based on what you assume your preceptor will want (see the full list in Table 1).3
Other ways to avoid low-value care include following the United States Choosing Wisely™ campaign, which has collected more than 500 specialty society recommendations. Likewise, the American College of Radiology Appropriateness Criteria are designed to assist providers with ordering the appropriate imaging tests (for a more extensive list see Table 2).
2. Express your clinical reasoning
One driver of health care expenditures that is especially prevalent in academia is the pressure to demonstrate knowledge by recommending extensive testing. While these tests may rule out obscure diagnoses, they often do not change management.
You can still demonstrate a mastery of your patients’ care by expressing your thought process overtly. For instance, “I considered secondary causes of the patient’s severe hypertension but felt it was most reasonable to first treat her pain and restart her home medications before pursuing a larger work-up. If the patient’s blood pressure remains elevated and she is hypokalemic, we could consider testing for hyperaldosteronism.” If you explain why you think a diagnosis is less likely and order tests accordingly, others will be encouraged to consider value in their own medical decision making.
3. Hone your communication skills
One of the most cited reasons for providing unnecessary care is the time required to discuss treatment plans with patients – it’s much faster to just order the test than to explain why it isn’t needed. Research, however, shows that these cost conversations take 68 seconds on average.4 Costs of Care (see Table 2) has an excellent video series that highlights how effective communication allows for shared decision making, which promotes both patient engagement and helps avoid wasteful care.
Physicians’ first instincts are often defensive when a patient asks for care we perceive as unnecessary. However, exploring what the patient hopes to gain from said test or treatment frequently reveals concern for a specific, missed diagnosis or complication. Addressing this underlying fear, rather than defending your ordering patterns, can create improved rapport and may serve to provide more reassurance than a test ever could.5
As a physician-in-training, try to observe others having these conversations and take every opportunity to practice. By focusing on this key skill set, you will increase your comfort with in-depth discussions on the value of care.
4. Get involved in a project related to high-value care
While you are developing your own practice patterns, you may be inspired to tackle areas of overuse and underuse at a more systemwide level. If your hospital does not have a committee for high-value care, perhaps a quality improvement leader can support your ideas to launch a project or participate in an ongoing initiative. Physicians-in-training have been identified as crucial to these projects’ success – your frontline insight can highlight potential problems and the nuances of workflow that are key to effective solutions.6
5. Embrace lifelong learning and reflection
The process of becoming a physician and of practicing high-value care is not a sprint but a marathon. Multiple barriers to high-value care exist, and you may feel these pressures differently at various points in your career. These include malpractice concerns, addressing patient expectations, and the desire to take action “just to be safe.”6
Interestingly, fear of malpractice does not seem to dissipate in areas where tort reform has provided stronger provider protections.7 Practitioners may also inaccurately assume a patient’s desire for additional work-up or treatment.8 Furthermore, be aware of the role of “commission bias” by which a provider regrets not doing something that could have helped a previous patient. This regret can prove to be a stronger motivator than the potential harm related to unnecessary diagnostic tests or treatments.9
While these barriers cannot be removed easily, learners and providers can practice active reflection by examining their own fears, biases, and motivations before and after they order additional testing or treatment.
As a physician-in-training, you may feel that your decisions do not have a major impact on the health care system as a whole. However, the culture shift needed to “bend the cost curve” will come from the collective efforts of individuals like you. Practicing high-value care is not just a matter of ordering fewer tests – appropriate ordering of an expensive test that expedites a diagnosis may be more cost-effective and enhance the quality of care provided. Increasing your own awareness of both necessary and unnecessary practices is a major step toward realizing system change. Your efforts to resist and reform the medical culture that propagates low value care will encourage your colleagues to follow suit.
Dr. Lacy is assistant professor and associate clerkship director at the University of New Mexico, Albuquerque, as well as division director of high-value care for the division of hospital medicine. Dr. Goetz is assistant professor at Rush University Medical Center, Chicago. They met as 2015 Copello Fellows at the National Physician Alliance. Both have been involved in numerous high-value care initiatives, curricular development, and medical education at their respective institutions.
References
1. Committee on the Learning Health Care System in America, Institute of Medicine. “Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.” Edited by Smith M, Saunders R, Stuckhardt L, and McGinnis JM. (Washington: National Academies Press, 2013). http://www.ncbi.nlm.nih.gov/books/NBK207225/.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
3. Lakhani A et al. Choosing Wisely for Medical Education: Six things medical students and trainees should question. Acad Med. 2016 Oct;91(10):1374-8.
4. Hunter WG et al. Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res. 2016;16:108.
5. van Ravesteijn H et al. The reassuring value of diagnostic tests: a systematic review. Patient Educ Couns. 2012;86(1):3-8.
6. Moriates C, Wong BM. High-value care programmes from the bottom-up… and the top-down. BMJ Qual Saf. 2016;25(11):821-3.
7. Snyder Sulmasy L, Weinberger SE. Better care is the best defense: High-value clinical practice vs. defensive medicine. Cleve Clin J Med. 2014;81(8):464-7.
8. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients’ preferences matter. BMJ. 2012;345:e6572.
9. Scott IA. Cognitive challenges to minimising low value care. Intern Med J. 2017;47(9):1079-1083.
‘Culture shift’ comes from collective efforts
‘Culture shift’ comes from collective efforts
It’s Monday morning, and Mrs. Jones still has abdominal pain. Your ward team decides to order a CT. On chart review you notice she’s had three other abdominal CTs for the same indication this year. How did this happen? What should you do?
High-value care has been defined by the Institute of Medicine as “the best care for the patient, with the optimal result for the circumstances, delivered at the right price.”1 With an estimated $700 billion dollars – 30% of medical expenditures – spent on wasted care, there are rising calls for a transformational shift.2
You are now asked to consider not just everything you can do for a patient, but also the benefits, harms, and costs associated with those choices. But where to start? We recommend that trainees integrate these tips for high-value care into their routine practice.
1. Use evidence-based resources that highlight value
A great place to begin is the “Six Things Medical Students and Trainees Should Question,” originally published in Academic Medicine and created by Choosing Wisely Canada™. Recommendations range from avoiding tests or treatments that will not change a patient’s clinical course to holding off on ordering tests solely based on what you assume your preceptor will want (see the full list in Table 1).3
Other ways to avoid low-value care include following the United States Choosing Wisely™ campaign, which has collected more than 500 specialty society recommendations. Likewise, the American College of Radiology Appropriateness Criteria are designed to assist providers with ordering the appropriate imaging tests (for a more extensive list see Table 2).
2. Express your clinical reasoning
One driver of health care expenditures that is especially prevalent in academia is the pressure to demonstrate knowledge by recommending extensive testing. While these tests may rule out obscure diagnoses, they often do not change management.
You can still demonstrate a mastery of your patients’ care by expressing your thought process overtly. For instance, “I considered secondary causes of the patient’s severe hypertension but felt it was most reasonable to first treat her pain and restart her home medications before pursuing a larger work-up. If the patient’s blood pressure remains elevated and she is hypokalemic, we could consider testing for hyperaldosteronism.” If you explain why you think a diagnosis is less likely and order tests accordingly, others will be encouraged to consider value in their own medical decision making.
3. Hone your communication skills
One of the most cited reasons for providing unnecessary care is the time required to discuss treatment plans with patients – it’s much faster to just order the test than to explain why it isn’t needed. Research, however, shows that these cost conversations take 68 seconds on average.4 Costs of Care (see Table 2) has an excellent video series that highlights how effective communication allows for shared decision making, which promotes both patient engagement and helps avoid wasteful care.
Physicians’ first instincts are often defensive when a patient asks for care we perceive as unnecessary. However, exploring what the patient hopes to gain from said test or treatment frequently reveals concern for a specific, missed diagnosis or complication. Addressing this underlying fear, rather than defending your ordering patterns, can create improved rapport and may serve to provide more reassurance than a test ever could.5
As a physician-in-training, try to observe others having these conversations and take every opportunity to practice. By focusing on this key skill set, you will increase your comfort with in-depth discussions on the value of care.
4. Get involved in a project related to high-value care
While you are developing your own practice patterns, you may be inspired to tackle areas of overuse and underuse at a more systemwide level. If your hospital does not have a committee for high-value care, perhaps a quality improvement leader can support your ideas to launch a project or participate in an ongoing initiative. Physicians-in-training have been identified as crucial to these projects’ success – your frontline insight can highlight potential problems and the nuances of workflow that are key to effective solutions.6
5. Embrace lifelong learning and reflection
The process of becoming a physician and of practicing high-value care is not a sprint but a marathon. Multiple barriers to high-value care exist, and you may feel these pressures differently at various points in your career. These include malpractice concerns, addressing patient expectations, and the desire to take action “just to be safe.”6
Interestingly, fear of malpractice does not seem to dissipate in areas where tort reform has provided stronger provider protections.7 Practitioners may also inaccurately assume a patient’s desire for additional work-up or treatment.8 Furthermore, be aware of the role of “commission bias” by which a provider regrets not doing something that could have helped a previous patient. This regret can prove to be a stronger motivator than the potential harm related to unnecessary diagnostic tests or treatments.9
While these barriers cannot be removed easily, learners and providers can practice active reflection by examining their own fears, biases, and motivations before and after they order additional testing or treatment.
As a physician-in-training, you may feel that your decisions do not have a major impact on the health care system as a whole. However, the culture shift needed to “bend the cost curve” will come from the collective efforts of individuals like you. Practicing high-value care is not just a matter of ordering fewer tests – appropriate ordering of an expensive test that expedites a diagnosis may be more cost-effective and enhance the quality of care provided. Increasing your own awareness of both necessary and unnecessary practices is a major step toward realizing system change. Your efforts to resist and reform the medical culture that propagates low value care will encourage your colleagues to follow suit.
Dr. Lacy is assistant professor and associate clerkship director at the University of New Mexico, Albuquerque, as well as division director of high-value care for the division of hospital medicine. Dr. Goetz is assistant professor at Rush University Medical Center, Chicago. They met as 2015 Copello Fellows at the National Physician Alliance. Both have been involved in numerous high-value care initiatives, curricular development, and medical education at their respective institutions.
References
1. Committee on the Learning Health Care System in America, Institute of Medicine. “Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.” Edited by Smith M, Saunders R, Stuckhardt L, and McGinnis JM. (Washington: National Academies Press, 2013). http://www.ncbi.nlm.nih.gov/books/NBK207225/.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
3. Lakhani A et al. Choosing Wisely for Medical Education: Six things medical students and trainees should question. Acad Med. 2016 Oct;91(10):1374-8.
4. Hunter WG et al. Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res. 2016;16:108.
5. van Ravesteijn H et al. The reassuring value of diagnostic tests: a systematic review. Patient Educ Couns. 2012;86(1):3-8.
6. Moriates C, Wong BM. High-value care programmes from the bottom-up… and the top-down. BMJ Qual Saf. 2016;25(11):821-3.
7. Snyder Sulmasy L, Weinberger SE. Better care is the best defense: High-value clinical practice vs. defensive medicine. Cleve Clin J Med. 2014;81(8):464-7.
8. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients’ preferences matter. BMJ. 2012;345:e6572.
9. Scott IA. Cognitive challenges to minimising low value care. Intern Med J. 2017;47(9):1079-1083.
It’s Monday morning, and Mrs. Jones still has abdominal pain. Your ward team decides to order a CT. On chart review you notice she’s had three other abdominal CTs for the same indication this year. How did this happen? What should you do?
High-value care has been defined by the Institute of Medicine as “the best care for the patient, with the optimal result for the circumstances, delivered at the right price.”1 With an estimated $700 billion dollars – 30% of medical expenditures – spent on wasted care, there are rising calls for a transformational shift.2
You are now asked to consider not just everything you can do for a patient, but also the benefits, harms, and costs associated with those choices. But where to start? We recommend that trainees integrate these tips for high-value care into their routine practice.
1. Use evidence-based resources that highlight value
A great place to begin is the “Six Things Medical Students and Trainees Should Question,” originally published in Academic Medicine and created by Choosing Wisely Canada™. Recommendations range from avoiding tests or treatments that will not change a patient’s clinical course to holding off on ordering tests solely based on what you assume your preceptor will want (see the full list in Table 1).3
Other ways to avoid low-value care include following the United States Choosing Wisely™ campaign, which has collected more than 500 specialty society recommendations. Likewise, the American College of Radiology Appropriateness Criteria are designed to assist providers with ordering the appropriate imaging tests (for a more extensive list see Table 2).
2. Express your clinical reasoning
One driver of health care expenditures that is especially prevalent in academia is the pressure to demonstrate knowledge by recommending extensive testing. While these tests may rule out obscure diagnoses, they often do not change management.
You can still demonstrate a mastery of your patients’ care by expressing your thought process overtly. For instance, “I considered secondary causes of the patient’s severe hypertension but felt it was most reasonable to first treat her pain and restart her home medications before pursuing a larger work-up. If the patient’s blood pressure remains elevated and she is hypokalemic, we could consider testing for hyperaldosteronism.” If you explain why you think a diagnosis is less likely and order tests accordingly, others will be encouraged to consider value in their own medical decision making.
3. Hone your communication skills
One of the most cited reasons for providing unnecessary care is the time required to discuss treatment plans with patients – it’s much faster to just order the test than to explain why it isn’t needed. Research, however, shows that these cost conversations take 68 seconds on average.4 Costs of Care (see Table 2) has an excellent video series that highlights how effective communication allows for shared decision making, which promotes both patient engagement and helps avoid wasteful care.
Physicians’ first instincts are often defensive when a patient asks for care we perceive as unnecessary. However, exploring what the patient hopes to gain from said test or treatment frequently reveals concern for a specific, missed diagnosis or complication. Addressing this underlying fear, rather than defending your ordering patterns, can create improved rapport and may serve to provide more reassurance than a test ever could.5
As a physician-in-training, try to observe others having these conversations and take every opportunity to practice. By focusing on this key skill set, you will increase your comfort with in-depth discussions on the value of care.
4. Get involved in a project related to high-value care
While you are developing your own practice patterns, you may be inspired to tackle areas of overuse and underuse at a more systemwide level. If your hospital does not have a committee for high-value care, perhaps a quality improvement leader can support your ideas to launch a project or participate in an ongoing initiative. Physicians-in-training have been identified as crucial to these projects’ success – your frontline insight can highlight potential problems and the nuances of workflow that are key to effective solutions.6
5. Embrace lifelong learning and reflection
The process of becoming a physician and of practicing high-value care is not a sprint but a marathon. Multiple barriers to high-value care exist, and you may feel these pressures differently at various points in your career. These include malpractice concerns, addressing patient expectations, and the desire to take action “just to be safe.”6
Interestingly, fear of malpractice does not seem to dissipate in areas where tort reform has provided stronger provider protections.7 Practitioners may also inaccurately assume a patient’s desire for additional work-up or treatment.8 Furthermore, be aware of the role of “commission bias” by which a provider regrets not doing something that could have helped a previous patient. This regret can prove to be a stronger motivator than the potential harm related to unnecessary diagnostic tests or treatments.9
While these barriers cannot be removed easily, learners and providers can practice active reflection by examining their own fears, biases, and motivations before and after they order additional testing or treatment.
As a physician-in-training, you may feel that your decisions do not have a major impact on the health care system as a whole. However, the culture shift needed to “bend the cost curve” will come from the collective efforts of individuals like you. Practicing high-value care is not just a matter of ordering fewer tests – appropriate ordering of an expensive test that expedites a diagnosis may be more cost-effective and enhance the quality of care provided. Increasing your own awareness of both necessary and unnecessary practices is a major step toward realizing system change. Your efforts to resist and reform the medical culture that propagates low value care will encourage your colleagues to follow suit.
Dr. Lacy is assistant professor and associate clerkship director at the University of New Mexico, Albuquerque, as well as division director of high-value care for the division of hospital medicine. Dr. Goetz is assistant professor at Rush University Medical Center, Chicago. They met as 2015 Copello Fellows at the National Physician Alliance. Both have been involved in numerous high-value care initiatives, curricular development, and medical education at their respective institutions.
References
1. Committee on the Learning Health Care System in America, Institute of Medicine. “Best Care at Lower Cost: The Path to Continuously Learning Health Care in America.” Edited by Smith M, Saunders R, Stuckhardt L, and McGinnis JM. (Washington: National Academies Press, 2013). http://www.ncbi.nlm.nih.gov/books/NBK207225/.
2. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513-6.
3. Lakhani A et al. Choosing Wisely for Medical Education: Six things medical students and trainees should question. Acad Med. 2016 Oct;91(10):1374-8.
4. Hunter WG et al. Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res. 2016;16:108.
5. van Ravesteijn H et al. The reassuring value of diagnostic tests: a systematic review. Patient Educ Couns. 2012;86(1):3-8.
6. Moriates C, Wong BM. High-value care programmes from the bottom-up… and the top-down. BMJ Qual Saf. 2016;25(11):821-3.
7. Snyder Sulmasy L, Weinberger SE. Better care is the best defense: High-value clinical practice vs. defensive medicine. Cleve Clin J Med. 2014;81(8):464-7.
8. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: Patients’ preferences matter. BMJ. 2012;345:e6572.
9. Scott IA. Cognitive challenges to minimising low value care. Intern Med J. 2017;47(9):1079-1083.
Things We Do For No Reason: Prealbumin Testing to Diagnose Malnutrition in the Hospitalized Patient
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
© 2018 Society of Hospital Medicine
Does Preoperative Hypercapnia Predict Postoperative Complications in Patients with Obstructive Sleep Apnea?
Clinical question: Are patients with obstructive sleep apnea (OSA) and preoperative hypercapnia more likely to experience postoperative complications than those without?
Background: Obesity hypoventilation syndrome (OHS) is known to increase medical morbidity in patients with OSA, but its impact on postoperative outcome is unknown.
Study design: Retrospective cohort study.
Setting: Single tertiary-care center.
Synopsis: The study examined 1,800 patients with body mass index (BMI) ≥30 who underwent polysomnography, elective non-cardiac surgery (NCS), and had a blood gas performed. Of those, 194 patients were identified as having OSA with hypercapnia, and 325 were identified as having only OSA. Investigators found that the presence of hypercapnia in patients with OSA, whether from OHS, COPD, or another cause, was associated with worse postoperative outcomes. They found a statistically significant increase in postoperative respiratory failure (21% versus 2%), heart failure (8% versus 0%), tracheostomy (2% versus 1%), and ICU transfer (21% versus 6%). Mortality data did not reach significance.
The major limitation to the study is that hypercapnia is underrecognized in this patient population, and as a result, only patients who had a blood gas were included; many hypercapnic patients may have had elective NCS without receiving a blood gas and were thus excluded.
Bottom line: Consider performing a preoperative blood gas in patients with OSA undergoing elective NCS to help with postoperative complication risk assessment.
Citation: Kaw R, Bhateja P, Paz y Mar H, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest. 2016;149(1):84-91 doi:10.1378/chest.14-3216.
Clinical question: Are patients with obstructive sleep apnea (OSA) and preoperative hypercapnia more likely to experience postoperative complications than those without?
Background: Obesity hypoventilation syndrome (OHS) is known to increase medical morbidity in patients with OSA, but its impact on postoperative outcome is unknown.
Study design: Retrospective cohort study.
Setting: Single tertiary-care center.
Synopsis: The study examined 1,800 patients with body mass index (BMI) ≥30 who underwent polysomnography, elective non-cardiac surgery (NCS), and had a blood gas performed. Of those, 194 patients were identified as having OSA with hypercapnia, and 325 were identified as having only OSA. Investigators found that the presence of hypercapnia in patients with OSA, whether from OHS, COPD, or another cause, was associated with worse postoperative outcomes. They found a statistically significant increase in postoperative respiratory failure (21% versus 2%), heart failure (8% versus 0%), tracheostomy (2% versus 1%), and ICU transfer (21% versus 6%). Mortality data did not reach significance.
The major limitation to the study is that hypercapnia is underrecognized in this patient population, and as a result, only patients who had a blood gas were included; many hypercapnic patients may have had elective NCS without receiving a blood gas and were thus excluded.
Bottom line: Consider performing a preoperative blood gas in patients with OSA undergoing elective NCS to help with postoperative complication risk assessment.
Citation: Kaw R, Bhateja P, Paz y Mar H, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest. 2016;149(1):84-91 doi:10.1378/chest.14-3216.
Clinical question: Are patients with obstructive sleep apnea (OSA) and preoperative hypercapnia more likely to experience postoperative complications than those without?
Background: Obesity hypoventilation syndrome (OHS) is known to increase medical morbidity in patients with OSA, but its impact on postoperative outcome is unknown.
Study design: Retrospective cohort study.
Setting: Single tertiary-care center.
Synopsis: The study examined 1,800 patients with body mass index (BMI) ≥30 who underwent polysomnography, elective non-cardiac surgery (NCS), and had a blood gas performed. Of those, 194 patients were identified as having OSA with hypercapnia, and 325 were identified as having only OSA. Investigators found that the presence of hypercapnia in patients with OSA, whether from OHS, COPD, or another cause, was associated with worse postoperative outcomes. They found a statistically significant increase in postoperative respiratory failure (21% versus 2%), heart failure (8% versus 0%), tracheostomy (2% versus 1%), and ICU transfer (21% versus 6%). Mortality data did not reach significance.
The major limitation to the study is that hypercapnia is underrecognized in this patient population, and as a result, only patients who had a blood gas were included; many hypercapnic patients may have had elective NCS without receiving a blood gas and were thus excluded.
Bottom line: Consider performing a preoperative blood gas in patients with OSA undergoing elective NCS to help with postoperative complication risk assessment.
Citation: Kaw R, Bhateja P, Paz y Mar H, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest. 2016;149(1):84-91 doi:10.1378/chest.14-3216.
Rapid Immunoassays for Heparin-Induced Thrombocytopenia Offer Fast Screening Possibilities
Clinical question: How useful are rapid immunoassays (RIs) compared to other tests for heparin-induced thrombocytopenia (HIT)?
Background: HIT is a clinicopathologic diagnosis, which traditionally requires clinical criteria and laboratory confirmation through initial testing with enzyme-linked immunosorbent assay (ELISA) and “gold standard” testing with washed platelet functional assays when available. There are an increasing number of RIs available, which have lab turnaround times of less than one hour. Their clinical utility is not well understood.
Study design: Meta-analysis.
Setting: Twenty-three studies.
Synopsis: The authors found 23 articles to include for review. These studies included 5,637 unique patients and included heterogeneous (medical, surgical, non-ICU) populations. These articles examined six different rapid immunoassays, which have been developed in recent years. All RIs examined had excellent negative predictive values (NPVs) ranging from 0.99 to 1.00, though positive predictive values (PPVs) had much wider variation (0.42–0.71). The greatest limitation in this meta-analysis is that 17 of the studies were marked as “high risk of bias” because they did not compare the RIs to the “gold standard” assay.
Bottom line: RIs for the diagnosis of HIT have very high NPVs and may be usefully incorporated into the diagnostic algorithm for HIT, but they cannot take the place of “gold standard” washed platelet functional assays.
Citation: Sun L, Gimotty PA, Lakshmanan S, Cuker A. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis [published online ahead of print January 14, 2016]. Thromb Haemost. doi:10.1160/TH15-06-0523.
Clinical question: How useful are rapid immunoassays (RIs) compared to other tests for heparin-induced thrombocytopenia (HIT)?
Background: HIT is a clinicopathologic diagnosis, which traditionally requires clinical criteria and laboratory confirmation through initial testing with enzyme-linked immunosorbent assay (ELISA) and “gold standard” testing with washed platelet functional assays when available. There are an increasing number of RIs available, which have lab turnaround times of less than one hour. Their clinical utility is not well understood.
Study design: Meta-analysis.
Setting: Twenty-three studies.
Synopsis: The authors found 23 articles to include for review. These studies included 5,637 unique patients and included heterogeneous (medical, surgical, non-ICU) populations. These articles examined six different rapid immunoassays, which have been developed in recent years. All RIs examined had excellent negative predictive values (NPVs) ranging from 0.99 to 1.00, though positive predictive values (PPVs) had much wider variation (0.42–0.71). The greatest limitation in this meta-analysis is that 17 of the studies were marked as “high risk of bias” because they did not compare the RIs to the “gold standard” assay.
Bottom line: RIs for the diagnosis of HIT have very high NPVs and may be usefully incorporated into the diagnostic algorithm for HIT, but they cannot take the place of “gold standard” washed platelet functional assays.
Citation: Sun L, Gimotty PA, Lakshmanan S, Cuker A. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis [published online ahead of print January 14, 2016]. Thromb Haemost. doi:10.1160/TH15-06-0523.
Clinical question: How useful are rapid immunoassays (RIs) compared to other tests for heparin-induced thrombocytopenia (HIT)?
Background: HIT is a clinicopathologic diagnosis, which traditionally requires clinical criteria and laboratory confirmation through initial testing with enzyme-linked immunosorbent assay (ELISA) and “gold standard” testing with washed platelet functional assays when available. There are an increasing number of RIs available, which have lab turnaround times of less than one hour. Their clinical utility is not well understood.
Study design: Meta-analysis.
Setting: Twenty-three studies.
Synopsis: The authors found 23 articles to include for review. These studies included 5,637 unique patients and included heterogeneous (medical, surgical, non-ICU) populations. These articles examined six different rapid immunoassays, which have been developed in recent years. All RIs examined had excellent negative predictive values (NPVs) ranging from 0.99 to 1.00, though positive predictive values (PPVs) had much wider variation (0.42–0.71). The greatest limitation in this meta-analysis is that 17 of the studies were marked as “high risk of bias” because they did not compare the RIs to the “gold standard” assay.
Bottom line: RIs for the diagnosis of HIT have very high NPVs and may be usefully incorporated into the diagnostic algorithm for HIT, but they cannot take the place of “gold standard” washed platelet functional assays.
Citation: Sun L, Gimotty PA, Lakshmanan S, Cuker A. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis [published online ahead of print January 14, 2016]. Thromb Haemost. doi:10.1160/TH15-06-0523.
Impact of Delayed Discharge Summary Completion on Hospital Readmission
Clinical question: Is a delay in completion of hospital discharge summary associated with hospital readmissions?
Background: Inpatient discharge summaries serve as a communication tool to future care providers. Previous studies have shown mixed impact on the timeliness of discharge summaries on hospital readmissions.
Study design: Retrospective cohort study.
Setting: Adult medical patients at Johns Hopkins University Hospital, Baltimore.
Synopsis: Study authors examined the time between hospital discharge and discharge summary completion on 87,994 hospitalizations to assess whether a delay increased the odds of hospital readmission. In those hospitalizations, 14,248 patients (16.2%) were readmitted within 30 days of discharge. There was a statistically significant adjusted odds ratio of 1.09 (P=0.001) for readmission associated with discharge summaries completed more than three days after discharge.
The main advantage of the study is that the investigators reviewed a large number of hospitalizations. The major limitation is that deaths or admissions to other hospitals within 30 days of discharge were not measured.
Bottom line: Completing a discharge summary within three days of discharge may decrease the risk of 30-day readmission.
Citation: Hoyer EH, Odonkor CA, Bhatia SN, Leung C, Deutschendorf A, Brotman DJ. Association between days to complete inpatient discharge summaries with all-payer hospital readmissions in Maryland [published online ahead of print February 23, 2016]. J Hosp Med. doi:10.1002/jhm.2556
Short Take
Effectiveness of Rapid Response Teams
A meta-analysis of 30 eligible studies evaluating the impact of rapid response teams (RRTs) from 2000 to 2016 found that RRTs are effective at reducing both in-hospital cardiac arrest and hospital mortality.
Citation: Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in-hospital cardiopulmonary arrest and mortality: a systematic review and meta-analysis [published online ahead of print Febraury 1, 2016]. J Hosp Med. doi:10.1002/jhm.2554.
Clinical question: Is a delay in completion of hospital discharge summary associated with hospital readmissions?
Background: Inpatient discharge summaries serve as a communication tool to future care providers. Previous studies have shown mixed impact on the timeliness of discharge summaries on hospital readmissions.
Study design: Retrospective cohort study.
Setting: Adult medical patients at Johns Hopkins University Hospital, Baltimore.
Synopsis: Study authors examined the time between hospital discharge and discharge summary completion on 87,994 hospitalizations to assess whether a delay increased the odds of hospital readmission. In those hospitalizations, 14,248 patients (16.2%) were readmitted within 30 days of discharge. There was a statistically significant adjusted odds ratio of 1.09 (P=0.001) for readmission associated with discharge summaries completed more than three days after discharge.
The main advantage of the study is that the investigators reviewed a large number of hospitalizations. The major limitation is that deaths or admissions to other hospitals within 30 days of discharge were not measured.
Bottom line: Completing a discharge summary within three days of discharge may decrease the risk of 30-day readmission.
Citation: Hoyer EH, Odonkor CA, Bhatia SN, Leung C, Deutschendorf A, Brotman DJ. Association between days to complete inpatient discharge summaries with all-payer hospital readmissions in Maryland [published online ahead of print February 23, 2016]. J Hosp Med. doi:10.1002/jhm.2556
Short Take
Effectiveness of Rapid Response Teams
A meta-analysis of 30 eligible studies evaluating the impact of rapid response teams (RRTs) from 2000 to 2016 found that RRTs are effective at reducing both in-hospital cardiac arrest and hospital mortality.
Citation: Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in-hospital cardiopulmonary arrest and mortality: a systematic review and meta-analysis [published online ahead of print Febraury 1, 2016]. J Hosp Med. doi:10.1002/jhm.2554.
Clinical question: Is a delay in completion of hospital discharge summary associated with hospital readmissions?
Background: Inpatient discharge summaries serve as a communication tool to future care providers. Previous studies have shown mixed impact on the timeliness of discharge summaries on hospital readmissions.
Study design: Retrospective cohort study.
Setting: Adult medical patients at Johns Hopkins University Hospital, Baltimore.
Synopsis: Study authors examined the time between hospital discharge and discharge summary completion on 87,994 hospitalizations to assess whether a delay increased the odds of hospital readmission. In those hospitalizations, 14,248 patients (16.2%) were readmitted within 30 days of discharge. There was a statistically significant adjusted odds ratio of 1.09 (P=0.001) for readmission associated with discharge summaries completed more than three days after discharge.
The main advantage of the study is that the investigators reviewed a large number of hospitalizations. The major limitation is that deaths or admissions to other hospitals within 30 days of discharge were not measured.
Bottom line: Completing a discharge summary within three days of discharge may decrease the risk of 30-day readmission.
Citation: Hoyer EH, Odonkor CA, Bhatia SN, Leung C, Deutschendorf A, Brotman DJ. Association between days to complete inpatient discharge summaries with all-payer hospital readmissions in Maryland [published online ahead of print February 23, 2016]. J Hosp Med. doi:10.1002/jhm.2556
Short Take
Effectiveness of Rapid Response Teams
A meta-analysis of 30 eligible studies evaluating the impact of rapid response teams (RRTs) from 2000 to 2016 found that RRTs are effective at reducing both in-hospital cardiac arrest and hospital mortality.
Citation: Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in-hospital cardiopulmonary arrest and mortality: a systematic review and meta-analysis [published online ahead of print Febraury 1, 2016]. J Hosp Med. doi:10.1002/jhm.2554.
Effects of Assigning Medical Teams to Nursing Units on Patient Care
Clinical question: Does assigning a single medical team to a nursing unit (regionalizing) improve communication and prevent adverse events?
Background: Many factors impact communication in healthcare delivery. Failures in communication are a known source of adverse events in hospital care. Previous studies of the impact of regionalized care (assigning medical physician teams to nursing units) on communication and outcomes have had mixed results.
Study design: Pre-post intervention cohort analysis.
Setting: Brigham and Women’s Hospital, Boston.
Synopsis: Three medical teams were assigned to 15-bed nursing units with structured multidisciplinary meeting times for one year. Assessments of concordance of care plan and adverse event detection (with a focus on adverse drug events and poor glycemic control) were performed before and after this assignment. Regionalization of care in the study site improved recognition of care team members (0.56 versus 0.86; P<0.001), discussion of care plan (0.73 versus 0.88; P<0.001), and agreement on estimated discharge date (0.56 versus 0.68; P<0.003). However, it did not significantly improve nurse and physician concordance of the plan or reduce the odds of preventable adverse events.
This study may not have captured an impact on more subtle adverse events or other aspects of interprofessional relationships that enhance patient care.
Bottom line: Regionalization effectively promotes communication but may not lead to patient safety improvements.
Citation: Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services [published online ahead of print February 24, 2016]. J Hosp Med. doi:10.1002/jhm.2566.
Clinical question: Does assigning a single medical team to a nursing unit (regionalizing) improve communication and prevent adverse events?
Background: Many factors impact communication in healthcare delivery. Failures in communication are a known source of adverse events in hospital care. Previous studies of the impact of regionalized care (assigning medical physician teams to nursing units) on communication and outcomes have had mixed results.
Study design: Pre-post intervention cohort analysis.
Setting: Brigham and Women’s Hospital, Boston.
Synopsis: Three medical teams were assigned to 15-bed nursing units with structured multidisciplinary meeting times for one year. Assessments of concordance of care plan and adverse event detection (with a focus on adverse drug events and poor glycemic control) were performed before and after this assignment. Regionalization of care in the study site improved recognition of care team members (0.56 versus 0.86; P<0.001), discussion of care plan (0.73 versus 0.88; P<0.001), and agreement on estimated discharge date (0.56 versus 0.68; P<0.003). However, it did not significantly improve nurse and physician concordance of the plan or reduce the odds of preventable adverse events.
This study may not have captured an impact on more subtle adverse events or other aspects of interprofessional relationships that enhance patient care.
Bottom line: Regionalization effectively promotes communication but may not lead to patient safety improvements.
Citation: Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services [published online ahead of print February 24, 2016]. J Hosp Med. doi:10.1002/jhm.2566.
Clinical question: Does assigning a single medical team to a nursing unit (regionalizing) improve communication and prevent adverse events?
Background: Many factors impact communication in healthcare delivery. Failures in communication are a known source of adverse events in hospital care. Previous studies of the impact of regionalized care (assigning medical physician teams to nursing units) on communication and outcomes have had mixed results.
Study design: Pre-post intervention cohort analysis.
Setting: Brigham and Women’s Hospital, Boston.
Synopsis: Three medical teams were assigned to 15-bed nursing units with structured multidisciplinary meeting times for one year. Assessments of concordance of care plan and adverse event detection (with a focus on adverse drug events and poor glycemic control) were performed before and after this assignment. Regionalization of care in the study site improved recognition of care team members (0.56 versus 0.86; P<0.001), discussion of care plan (0.73 versus 0.88; P<0.001), and agreement on estimated discharge date (0.56 versus 0.68; P<0.003). However, it did not significantly improve nurse and physician concordance of the plan or reduce the odds of preventable adverse events.
This study may not have captured an impact on more subtle adverse events or other aspects of interprofessional relationships that enhance patient care.
Bottom line: Regionalization effectively promotes communication but may not lead to patient safety improvements.
Citation: Mueller SK, Schnipper JL, Giannelli K, Roy CL, Boxer R. Impact of regionalized care on concordance of plan and preventable adverse events on general medicine services [published online ahead of print February 24, 2016]. J Hosp Med. doi:10.1002/jhm.2566.
Troponin Leak Portends Poorer Outcomes in Congestive Heart Disease Hospitalizations
Clinical question: What is the association between detectable cardiac troponin (cTn) levels and outcomes in persons hospitalized with acute decompensated heart failure (ADHF)?
Background: There are millions of ADHF hospitalizations per year, and all-cause mortality and readmission rates are high. Efforts to better risk-stratify such patients have included measuring cTn levels and determining risk of increased length of stay, hospital readmission, and mortality.
Study design: Systematic review and meta-analysis.
Setting: Twenty-six observational cohort studies.
Synopsis: Compared with an undetectable cTn, detectable or elevated cTn levels were associated with greater length of stay (odds ratio [OR], 1.05; 95% CI, 1.01¬–1.10) and greater in-hospital death (OR, 2.57; 95% CI, 2.27–2.91). ADHF patients with detectable or elevated cTn were also at increased risk for mortality and composite of mortality and readmission over the short, intermediate, and long term. Reviewers eventually considered the overall association of a detectable or elevated troponin with mortality and readmission as moderate (relative association measure >2.0).
Meanwhile, few studies in this analysis showed a continuous and graded relationship between cTn levels and clinical outcomes.
Limitations of the review include arbitrarily stratifying groups by the level of cTn from assays whose lower limit of detection vary. The authors also admit the various associations are likely affected by several confounders for which they could not adjust because individual participant data were unavailable.
Finally, while acknowledging patients with chronic stable heart failure often have baseline elevated cTn levels, accounting for this in the analysis was limited.
Bottom line: A detectable or elevated level of cTn during ADHF hospitalization leads to worse outcomes both during and after discharge.
Citation: Yousufuddin M, Abdalrhim AD, Wang Z, Murad MH. Cardiac troponin in patients hospitalized with acute decompensated heart failure: a systematic review and meta-analysis [published online ahead of print February 18, 2016]. J Hosp Med. doi:10.1002/jhm.2558.
Clinical question: What is the association between detectable cardiac troponin (cTn) levels and outcomes in persons hospitalized with acute decompensated heart failure (ADHF)?
Background: There are millions of ADHF hospitalizations per year, and all-cause mortality and readmission rates are high. Efforts to better risk-stratify such patients have included measuring cTn levels and determining risk of increased length of stay, hospital readmission, and mortality.
Study design: Systematic review and meta-analysis.
Setting: Twenty-six observational cohort studies.
Synopsis: Compared with an undetectable cTn, detectable or elevated cTn levels were associated with greater length of stay (odds ratio [OR], 1.05; 95% CI, 1.01¬–1.10) and greater in-hospital death (OR, 2.57; 95% CI, 2.27–2.91). ADHF patients with detectable or elevated cTn were also at increased risk for mortality and composite of mortality and readmission over the short, intermediate, and long term. Reviewers eventually considered the overall association of a detectable or elevated troponin with mortality and readmission as moderate (relative association measure >2.0).
Meanwhile, few studies in this analysis showed a continuous and graded relationship between cTn levels and clinical outcomes.
Limitations of the review include arbitrarily stratifying groups by the level of cTn from assays whose lower limit of detection vary. The authors also admit the various associations are likely affected by several confounders for which they could not adjust because individual participant data were unavailable.
Finally, while acknowledging patients with chronic stable heart failure often have baseline elevated cTn levels, accounting for this in the analysis was limited.
Bottom line: A detectable or elevated level of cTn during ADHF hospitalization leads to worse outcomes both during and after discharge.
Citation: Yousufuddin M, Abdalrhim AD, Wang Z, Murad MH. Cardiac troponin in patients hospitalized with acute decompensated heart failure: a systematic review and meta-analysis [published online ahead of print February 18, 2016]. J Hosp Med. doi:10.1002/jhm.2558.
Clinical question: What is the association between detectable cardiac troponin (cTn) levels and outcomes in persons hospitalized with acute decompensated heart failure (ADHF)?
Background: There are millions of ADHF hospitalizations per year, and all-cause mortality and readmission rates are high. Efforts to better risk-stratify such patients have included measuring cTn levels and determining risk of increased length of stay, hospital readmission, and mortality.
Study design: Systematic review and meta-analysis.
Setting: Twenty-six observational cohort studies.
Synopsis: Compared with an undetectable cTn, detectable or elevated cTn levels were associated with greater length of stay (odds ratio [OR], 1.05; 95% CI, 1.01¬–1.10) and greater in-hospital death (OR, 2.57; 95% CI, 2.27–2.91). ADHF patients with detectable or elevated cTn were also at increased risk for mortality and composite of mortality and readmission over the short, intermediate, and long term. Reviewers eventually considered the overall association of a detectable or elevated troponin with mortality and readmission as moderate (relative association measure >2.0).
Meanwhile, few studies in this analysis showed a continuous and graded relationship between cTn levels and clinical outcomes.
Limitations of the review include arbitrarily stratifying groups by the level of cTn from assays whose lower limit of detection vary. The authors also admit the various associations are likely affected by several confounders for which they could not adjust because individual participant data were unavailable.
Finally, while acknowledging patients with chronic stable heart failure often have baseline elevated cTn levels, accounting for this in the analysis was limited.
Bottom line: A detectable or elevated level of cTn during ADHF hospitalization leads to worse outcomes both during and after discharge.
Citation: Yousufuddin M, Abdalrhim AD, Wang Z, Murad MH. Cardiac troponin in patients hospitalized with acute decompensated heart failure: a systematic review and meta-analysis [published online ahead of print February 18, 2016]. J Hosp Med. doi:10.1002/jhm.2558.
New Guidelines for Cardiovascular Imaging in Chest Pain
Clinical question: Which cardiovascular imaging modalities can augment triage of ED patients with chest pain?
Background: Because absolute event rates for patients with chest pain and normal initial ECG findings are not low enough to drive discharge triage decisions, and findings that patients with acute myocardial infarction (AMI) are inadvertently discharged because of less-sensitive troponin assays, there is great interest in what imaging modalities can facilitate safer triages.
Study design: Clinical guideline.
Setting: Meta-analysis of studies in multiple clinical settings.
Synopsis: This guideline adopted two pathways: an early assessment pathway, which considers imaging without the need for serial biomarker analysis, and an observational pathway, which involves serial biomarker testing.
For the early assessment pathway, when ECG and/or biomarker analysis is unequivocally positive for ischemia, all rest-imaging modalities are rarely appropriate. When the initial troponin level is equivocal, both rest single-photon emission computed tomography (SPECT) and coronary CT angiography (CCTA) are appropriate, though rest echocardiography and rest cardiovascular magnetic resonance (CMR) may be alternatives. Resting imaging may also be appropriate when chest pain resolves prior to evaluation and/or initial ECG plus troponin is non-ischemic/normal.
In the observational pathway, for patients with ECG changes and/or serial troponins unequivocally positive for AMI, only cardiac catheterization is recommended. When serial ECGs/troponins are borderline, stress-test modalities and CCTA are appropriate. When serial ECGs/ troponins are negative, outpatient testing may be appropriate.
Bottom line: Experts recommend cardiac catheterization as the imaging modality of choice for patients with an unequivocal AMI diagnosis. When ECG and/or biomarkers are equivocal or negative, outpatient evaluation may be appropriate.
Citation: Rybicki FJ, Udelson JE, Peacock WF, et al. Appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J Am Coll Radiol. 2016;(2):e1-e29. doi:10.1016/j.jacr.2015.07.007.
Short Take
Family Reflections on End-of-Life Cancer Care
In this multicenter, prospective, observational study, family members of patients with advanced-stage cancer who received aggressive care at end of life were less likely to report the overall quality of end-of-life care as “excellent” or “very good.”
Citation: Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
Clinical question: Which cardiovascular imaging modalities can augment triage of ED patients with chest pain?
Background: Because absolute event rates for patients with chest pain and normal initial ECG findings are not low enough to drive discharge triage decisions, and findings that patients with acute myocardial infarction (AMI) are inadvertently discharged because of less-sensitive troponin assays, there is great interest in what imaging modalities can facilitate safer triages.
Study design: Clinical guideline.
Setting: Meta-analysis of studies in multiple clinical settings.
Synopsis: This guideline adopted two pathways: an early assessment pathway, which considers imaging without the need for serial biomarker analysis, and an observational pathway, which involves serial biomarker testing.
For the early assessment pathway, when ECG and/or biomarker analysis is unequivocally positive for ischemia, all rest-imaging modalities are rarely appropriate. When the initial troponin level is equivocal, both rest single-photon emission computed tomography (SPECT) and coronary CT angiography (CCTA) are appropriate, though rest echocardiography and rest cardiovascular magnetic resonance (CMR) may be alternatives. Resting imaging may also be appropriate when chest pain resolves prior to evaluation and/or initial ECG plus troponin is non-ischemic/normal.
In the observational pathway, for patients with ECG changes and/or serial troponins unequivocally positive for AMI, only cardiac catheterization is recommended. When serial ECGs/troponins are borderline, stress-test modalities and CCTA are appropriate. When serial ECGs/ troponins are negative, outpatient testing may be appropriate.
Bottom line: Experts recommend cardiac catheterization as the imaging modality of choice for patients with an unequivocal AMI diagnosis. When ECG and/or biomarkers are equivocal or negative, outpatient evaluation may be appropriate.
Citation: Rybicki FJ, Udelson JE, Peacock WF, et al. Appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J Am Coll Radiol. 2016;(2):e1-e29. doi:10.1016/j.jacr.2015.07.007.
Short Take
Family Reflections on End-of-Life Cancer Care
In this multicenter, prospective, observational study, family members of patients with advanced-stage cancer who received aggressive care at end of life were less likely to report the overall quality of end-of-life care as “excellent” or “very good.”
Citation: Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
Clinical question: Which cardiovascular imaging modalities can augment triage of ED patients with chest pain?
Background: Because absolute event rates for patients with chest pain and normal initial ECG findings are not low enough to drive discharge triage decisions, and findings that patients with acute myocardial infarction (AMI) are inadvertently discharged because of less-sensitive troponin assays, there is great interest in what imaging modalities can facilitate safer triages.
Study design: Clinical guideline.
Setting: Meta-analysis of studies in multiple clinical settings.
Synopsis: This guideline adopted two pathways: an early assessment pathway, which considers imaging without the need for serial biomarker analysis, and an observational pathway, which involves serial biomarker testing.
For the early assessment pathway, when ECG and/or biomarker analysis is unequivocally positive for ischemia, all rest-imaging modalities are rarely appropriate. When the initial troponin level is equivocal, both rest single-photon emission computed tomography (SPECT) and coronary CT angiography (CCTA) are appropriate, though rest echocardiography and rest cardiovascular magnetic resonance (CMR) may be alternatives. Resting imaging may also be appropriate when chest pain resolves prior to evaluation and/or initial ECG plus troponin is non-ischemic/normal.
In the observational pathway, for patients with ECG changes and/or serial troponins unequivocally positive for AMI, only cardiac catheterization is recommended. When serial ECGs/troponins are borderline, stress-test modalities and CCTA are appropriate. When serial ECGs/ troponins are negative, outpatient testing may be appropriate.
Bottom line: Experts recommend cardiac catheterization as the imaging modality of choice for patients with an unequivocal AMI diagnosis. When ECG and/or biomarkers are equivocal or negative, outpatient evaluation may be appropriate.
Citation: Rybicki FJ, Udelson JE, Peacock WF, et al. Appropriate utilization of cardiovascular imaging in emergency department patients with chest pain: a joint document of the American College of Radiology Appropriateness Criteria Committee and the American College of Cardiology Appropriate Use Criteria Task Force. J Am Coll Radiol. 2016;(2):e1-e29. doi:10.1016/j.jacr.2015.07.007.
Short Take
Family Reflections on End-of-Life Cancer Care
In this multicenter, prospective, observational study, family members of patients with advanced-stage cancer who received aggressive care at end of life were less likely to report the overall quality of end-of-life care as “excellent” or “very good.”
Citation: Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
Hospitalist Quality Improvement Initiative Reduces Inpatient Laboratory Costs
Clinical question: Will a multifaceted quality improvement initiative targeted at hospitalists reduce inpatient laboratory costs?
Background: Routine inpatient laboratory testing is a well-recognized area of healthcare waste and was highlighted by the American Board of Internal Medicine Choosing Wisely campaign as a practice that should be questioned. Multifaceted quality improvement interventions, especially those that incorporate interventions beyond education, are more successful at achieving sustainable change.
Study design: Retrospective, controlled, interrupted time series study.
Setting: University of Utah, academic general internal medicine hospitalist service.
Synopsis: The intervention group, a teaching hospitalist service, received targeted education, cost feedback comparing individual provider performance, and divisional financial incentives. Additionally, a standardized rounding checklist was implemented and completed by rotating medical students. The control group included all non-hospitalist services. Approximately 20% of the 31,896 encounters measured in pre-intervention and post-intervention periods took place in the intervention group. Lab cost per day was reduced from $138 to $123 in the intervention group (P<0.001), while cost per day was non-significantly increased in the control group from $130 to $132 (P=0.37). Limitations of this study include the fact that the University of Utah already prioritizes high-value care and utilizes a local tool to provide individual cost and ordering feedback to providers as well as the financial incentives. Additionally, the use of medical students to implement the rounding checklist may not be feasible in many practice settings.
Bottom line: An approach of targeted education, direct provider feedback, consistent use of a rounding checklist, and financial incentives may decrease lab utilization.
Citation: Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs [published online ahead of print February 4, 2016]. J Hosp Med. doi:10.1002/jhm.2552.
Short Take
aVL ST-Depression Differentiates Inferior Stemi from Pericarditis
This retrospective analysis showed that any aVL ST-depression helps to distinguish inferior myocardial infarctions from pericarditis.
Citation: Bischof JE, Worrall C, Thompson P, Marti D, Smith SW. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am J Emerg Med. 2016;34(2):149-154.
Clinical question: Will a multifaceted quality improvement initiative targeted at hospitalists reduce inpatient laboratory costs?
Background: Routine inpatient laboratory testing is a well-recognized area of healthcare waste and was highlighted by the American Board of Internal Medicine Choosing Wisely campaign as a practice that should be questioned. Multifaceted quality improvement interventions, especially those that incorporate interventions beyond education, are more successful at achieving sustainable change.
Study design: Retrospective, controlled, interrupted time series study.
Setting: University of Utah, academic general internal medicine hospitalist service.
Synopsis: The intervention group, a teaching hospitalist service, received targeted education, cost feedback comparing individual provider performance, and divisional financial incentives. Additionally, a standardized rounding checklist was implemented and completed by rotating medical students. The control group included all non-hospitalist services. Approximately 20% of the 31,896 encounters measured in pre-intervention and post-intervention periods took place in the intervention group. Lab cost per day was reduced from $138 to $123 in the intervention group (P<0.001), while cost per day was non-significantly increased in the control group from $130 to $132 (P=0.37). Limitations of this study include the fact that the University of Utah already prioritizes high-value care and utilizes a local tool to provide individual cost and ordering feedback to providers as well as the financial incentives. Additionally, the use of medical students to implement the rounding checklist may not be feasible in many practice settings.
Bottom line: An approach of targeted education, direct provider feedback, consistent use of a rounding checklist, and financial incentives may decrease lab utilization.
Citation: Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs [published online ahead of print February 4, 2016]. J Hosp Med. doi:10.1002/jhm.2552.
Short Take
aVL ST-Depression Differentiates Inferior Stemi from Pericarditis
This retrospective analysis showed that any aVL ST-depression helps to distinguish inferior myocardial infarctions from pericarditis.
Citation: Bischof JE, Worrall C, Thompson P, Marti D, Smith SW. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am J Emerg Med. 2016;34(2):149-154.
Clinical question: Will a multifaceted quality improvement initiative targeted at hospitalists reduce inpatient laboratory costs?
Background: Routine inpatient laboratory testing is a well-recognized area of healthcare waste and was highlighted by the American Board of Internal Medicine Choosing Wisely campaign as a practice that should be questioned. Multifaceted quality improvement interventions, especially those that incorporate interventions beyond education, are more successful at achieving sustainable change.
Study design: Retrospective, controlled, interrupted time series study.
Setting: University of Utah, academic general internal medicine hospitalist service.
Synopsis: The intervention group, a teaching hospitalist service, received targeted education, cost feedback comparing individual provider performance, and divisional financial incentives. Additionally, a standardized rounding checklist was implemented and completed by rotating medical students. The control group included all non-hospitalist services. Approximately 20% of the 31,896 encounters measured in pre-intervention and post-intervention periods took place in the intervention group. Lab cost per day was reduced from $138 to $123 in the intervention group (P<0.001), while cost per day was non-significantly increased in the control group from $130 to $132 (P=0.37). Limitations of this study include the fact that the University of Utah already prioritizes high-value care and utilizes a local tool to provide individual cost and ordering feedback to providers as well as the financial incentives. Additionally, the use of medical students to implement the rounding checklist may not be feasible in many practice settings.
Bottom line: An approach of targeted education, direct provider feedback, consistent use of a rounding checklist, and financial incentives may decrease lab utilization.
Citation: Yarbrough PM, Kukhareva PV, Horton D, Edholm K, Kawamoto K. Multifaceted intervention including education, rounding checklist implementation, cost feedback, and financial incentives reduces inpatient laboratory costs [published online ahead of print February 4, 2016]. J Hosp Med. doi:10.1002/jhm.2552.
Short Take
aVL ST-Depression Differentiates Inferior Stemi from Pericarditis
This retrospective analysis showed that any aVL ST-depression helps to distinguish inferior myocardial infarctions from pericarditis.
Citation: Bischof JE, Worrall C, Thompson P, Marti D, Smith SW. ST depression in lead aVL differentiates inferior ST-elevation myocardial infarction from pericarditis. Am J Emerg Med. 2016;34(2):149-154.