User login
Tactics to prevent or slow progression of CKD in patients with diabetes
Chronic kidney disease (CKD) is a significant comorbidity of diabetes mellitus. The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as the presence of kidney damage or decreased kidney function for ≥ 3 months. CKD caused by diabetes is called diabetic kidney disease (DKD), which is 1 of 3 principal microvascular complications of diabetes. DKD can progress to end-stage renal disease (ESRD), requiring kidney replacement therapy, and is the leading cause of CKD and ESRD in the United States.1-3 Studies have also shown that, particularly in patients with diabetes, CKD considerably increases the risk of cardiovascular events, which often occur prior to ESRD.1,4
This article provides the latest recommendations for evaluating and managing DKD to help you prevent or slow its progression.
Defining and categorizing diabetic kidney disease
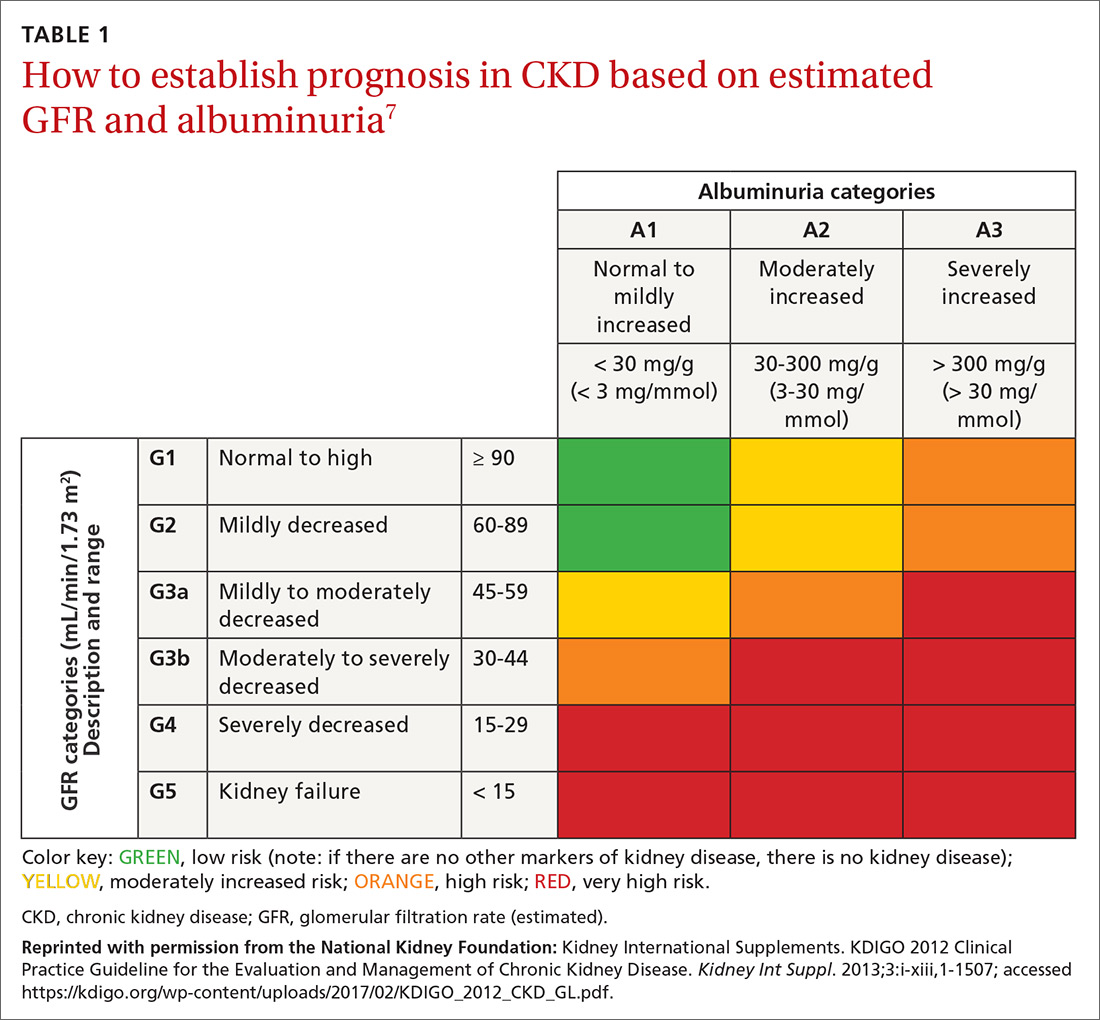
CKD is defined as persistently elevated excretion of urinary albumin (albuminuria) and decreased estimated glomerular filtration rate (eGFR), or as the presence of signs of progressive kidney damage.5,6 DKD, also known as diabetic nephropathy, is CKD attributed to long-term diabetes. A patient’s eGFR is the established basis for assignment to a stage (1, 2, 3a, 3b, 4, or 5) of CKD (TABLE 17) and, along with the category of albuminuria (A1, A2, or A3), can indicate prognosis.
Taking its toll in diabetes
As many as 40% of patients with diabetes develop DKD.8-10 Most studies of DKD have been conducted in patients with type 1 diabetes (T1D), because the time of clinical onset is typically known.
Type 1 diabetes. DKD usually occurs 10 to 15 years, or later, after the onset of diabetes.6 As many as 30% of people with T1D have albuminuria approximately 15 years after onset of diabetes; almost one-half of those develop DKD.5,11 After approximately 22.5 years without albuminuria, patients with T1D have approximately a 1% annual risk of DKD.12
Type 2 diabetes (T2D). DKD is often present at diagnosis, likely due to a delay in diagnosis and briefer clinical exposure, compared to T1D. Albuminuria has been reported in as many as 40% of patients with T2D approximately 10 years after onset of diabetes.12,13
Multiple risk factors with no standout “predictor”
Genetic susceptibility, ethnicity, glycemic control, smoking, blood pressure (BP), and the eGFR have been identified as risk factors for renal involvement in diabetes; obesity, oral contraceptives, and age can also contribute. Although each risk factor increases the risk of DKD, no single factor is adequately predictive. Moderately increased albuminuria, the earliest sign of DKD, is associated with progressive nephropathy.12
Continue to: How great is the risk?
How great is the risk? From disease onset to proteinuria and from proteinuria to ESRD, the risk of DKD in T1D and T2D is similar. With appropriate treatment, albuminuria can regress, and the risk of ESRD can be < 20% at 10 years in T1D.12 As in T1D, good glycemic control might result in regression of albuminuria in T2D.14
For unknown reasons, the degree of albuminuria can exist independent of the progression of DKD. Factors responsible for a progressive decline in eGFR in DKD without albuminuria are unknown.12,15
Patient evaluation with an eye toward comorbidities
A comprehensive initial medical evaluation for DKD includes a review of microvascular complications; visits to specialists; lifestyle and behavior patterns (eg, diet, sleep, substance use, and social support); and medication adherence, adverse drug effects, and alternative medicines. Although DKD is often a clinical diagnosis, it can be ruled in by persistent albuminuria or decreased eGFR, or both, in established diabetes or diabetic retinopathy when other causes are unlikely (see “Recommended DKD screening protocol,” below).
Screening for mental health conditions and barriers to self-management is also key.6
Comorbidities, of course, can complicate disease management in patients with diabetes.16-20 Providers and patients therefore need to be aware of potential diabetic comorbidities. For example, DKD and even moderately increased albuminuria significantly increase the risk of cardiovascular disease (CVD).12 Other possible comorbidities include (but are not limited to) nonalcoholic steatohepatitis, fracture, hearing impairment, cancer (eg, liver, pancreas, endometrium, colon, rectum, breast, and bladder), pancreatitis, hypogonadism, obstructive sleep apnea, periodontal disease, anxiety, depression, and eating disorders.6
Continue to: Recommended DKD screening protocol
Recommended DKD screening protocol
In all cases of T2D, in cases of T1D of ≥ 5 years’ duration, and in patients with diabetes and comorbid hypertension, perform annual screening for albuminuria, an elevated creatinine level, and a decline in eGFR.
To confirm the diagnosis of DKD, at least 2 of 3 urine specimens must demonstrate an elevated urinary albumin:creatinine ratio (UACR) over a 3- to 6-month period.21 Apart from renal damage, exercise within 24 hours before specimen collection, infection, fever, congestive heart failure, hyperglycemia, menstruation, and hypertension can elevate the UACR.6
Levels of the UACR are established as follows22:
- Normal UACR is defined as < 30 milligrams of albumin per gram of creatinine (expressed as “mg/g”).
- Increased urinary albumin excretion is defined as ≥ 30 mg/g.
- Moderately increased albuminuria, a predictor of potential nephropathy, is the excretion of 30 to 300 mg/g.
- Severely increased albuminuria is excretion > 300 mg/g; it is often followed by a gradual decline in eGFR that, without treatment, eventually leads to ESRD.
The rate of decline in eGFR once albuminuria is severely increased is equivalent in T1D and T2D.12 Without intervention, the time from severely increased albuminuria to ESRD in T1D and T2D averages approximately 6 or 7 years.
Clinical features
DKD is typically a clinical diagnosis seen in patients with longstanding diabetes, albuminuria, retinopathy, or a reduced eGFR in the absence of another primary cause of kidney damage. In patients with T1D and DKD, signs of retinopathy and neuropathy are almost always present at diagnosis, unless a diagnosis is made early in the course of diabetes.12 Therefore, the presence of retinopathy suggests that diabetes is the likely cause of CKD.
Continue to: The presence of microvascular disease...
The presence of microvascular disease in patients with T2D and DKD is less predictable.12 In T2D patients who do not have retinopathy, consider causes of CKD other than DKD. Features suggesting that the cause of CKD is an underlying condition other than diabetes are rapidly increasing albuminuria or decreasing eGFR; urinary sediment comprising red blood cells or white blood cells; and nephrotic syndrome.6
As the prevalence of diabetes increases, it has become more common to diagnose DKD by eGFR without albuminuria—underscoring the importance of routine monitoring of eGFR in patients with diabetes.6
Sources of expert guidance. The Chronic Kidney Disease Epidemiology Collaboration equation23 is preferred for calculating eGFR from serum creatinine: An eGFR < 60 mL/min/1.73 m2 is considered abnormal.3,12 At these rates, the prevalence of complications related to CKD rises and screening for complications becomes necessary.
A more comprehensive classification of the stages of CKD, incorporating albuminuria and progression of CKD, has been recommended by Kidney Disease: Improving Global Outcomes (KDIGO).7 Because eGFR and excretion of albumin vary, abnormal test results need to be verified over time to stage the degree of CKD.3,12 Kidney damage often manifests as albuminuria, but also as hematuria, other types of abnormal urinary sediment, radiographic abnormalities, and other abnormal presentations.
Management
Nutritional factors
Excessive protein intake has been shown to increase albuminuria, worsen renal function, and increase CVD mortality in DKD.24-26 Therefore, daily dietary protein intake of 0.8 g/kg body weight is recommended for patients who are not on dialysis.3 Patients on dialysis might require higher protein intake to preserve muscle mass caused by protein-energy wasting, which is common in dialysis patients.6
Continue to: Low sodium intake
Low sodium intake in CKD patients has been shown to decrease BP and thus slow the progression of renal disease and lower the risk of CVD. The recommended dietary sodium intake in CKD patients is 1500-3000 mg/d.3
Low potassium intake. Hyperkalemia is a serious complication of CKD. A low-potassium diet is recommended in ESRD patients who have a potassium level > 5.5 mEq/L.6
Blood pressure
Preventing and treating hypertension is critical to slowing the progression of CKD and reducing cardiovascular risk. BP should be measured at every clinic visit. Aside from lifestyle changes, medication might be needed to reach target BP.
The American Diabetes Association recommends a BP goal of ≤ 140/90 mm Hg for hypertensive patients with diabetes, although they do state that a lower BP target (≤ 130/80 mm Hg) might be more appropriate for patients with DKD.27
The American College of Cardiology recommends that hypertensive patients with CKD have a BP target of ≤ 130/80 mm Hg.28
Continue to: ACE inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have renoprotective benefits. These agents are recommended as first-line medications for patients with diabetes, hypertension, and an eGFR < 60 mL/min/1.73 m2 and a UACR > 300 mg/g.29-31 Evidence also supports their use when the UACR is 30 to 299 mg/g.
Studies have shown that, in patients with DKD, ACE inhibitors and ARBs can slow the progression of renal disease.29,30,32 There is no difference between ACE inhibitors and ARBs in their effectiveness for preventing progression of DKD.6 There is no added benefit in combining an ACE inhibitor and an ARB33; notably, combination ACE inhibitor and ARB therapy can increase the risk of adverse events, such as hyperkalemia and acute kidney injury, especially in patients with DKD.33
There is no evidence for starting an ACE inhibitor or ARB to prevent CKD in patients with diabetes who are not hypertensive.5
ACE inhibitors and ARBs should be used with caution in women of childbearing age, who should use a reliable form of contraception if taking one of these drugs.
Diuretics. Thiazide-type and loop diuretics might potentiate the positive effects of ACE inhibitors and ARBs. KDOQI guidelines recommend that, in patients who require a second agent to control BP, a diuretic should be considered in combination with an ACE inhibitor or an ARB.20 A loop diuretic is preferred if the eGFR is < 30 mL/min/1.73 m2.
Continue to: Nondihydropyridine calcium-channel blockers
Nondihydropyridine calcium-channel blockers (CCBs), such as diltiazem and verapamil, have been shown to be more effective then dihydrophyridine CCBs, such as amlodipine and nifedipine, in slowing the progression of renal disease because of their antiproteinuric effects. However, the antiproteinuric effects of nondihydropyridine CCBs are not as strong as those of ACE inhibitors or ARBs, and these drugs do not appear to potentiate the effects of an ACE inhibitor or ARB when used in combination.20
Nondihydropyridine CCBs might be a reasonable alternative in patients who cannot tolerate an ACE inhibitor or an ARB.
Mineralocorticoid receptor antagonists in combination with an ACE inhibitor or ARB have been demonstrated to reduce albuminuria in short-term studies.34,35
Glycemic levels
Studies conducted in patients with T1D, and others in patients with T2D, have shown that tight glycemic control can delay the onset and slow the progression of albuminuria and a decline in the eGFR.10,36-39 The target glycated hemoglobin (A1C) should be < 7% to prevent or slow progression of DKD.40 However, patients with DKD have an increased risk of hypoglycemic events and increased mortality with more intensive glycemic control.40,41 Given those findings, some patients with DKD and significant comorbidities, ESRD, or limited life expectancy might need to have an A1C target set at 8%.6,42
Adjustments to antidiabetes medications in DKD
In patients with stages 3 to 5 DKD, several common antidiabetic medications might need to be adjusted or discontinued because they decrease creatinine clearance.
Continue to: First-generation sulfonylureas
First-generation sulfonylureas should be avoided in DKD. Glipizide and gliclazide are preferred among second-generation sulfonylureas because they do not increase the risk of hypoglycemia in DKD patients, although patients taking these medications still require close monitoring of their blood glucose level.20
Metformin. In 2016, recommendations changed for the use of metformin in patients with DKD: The eGFR, not the serum creatinine level, should guide treatment.43 Metformin can be used safely in patients with (1) an eGFR of < 60 mL/min/1.73 m2 and (2) an eGFR of 30 mL/min/1.73 m2 with close monitoring. Metformin should not be initiated if the eGFR is < 45 mL/min/1.73 m2.43
Antidiabetes medications with direct effect on the kidney
Several antidiabetes medications have a direct effect on the kidney apart from their effect on the blood glucose level.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to reduce albuminuria and slow the decrease of eGFR independent of glycemic control. In addition, SGLT2 inhibitors have also been shown to have cardiovascular benefits in patients with DKD.44,45
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to delay and decrease the progression of DKD.46-48 Also, similar to what is seen with SGLT2 inhibitors, GLP-1 agonists have demonstrable cardiovascular benefit in patients with DKD.46,48
Continue to: Dyslipidemia and DKD
Dyslipidemia and DKD
Because the risk of CVD is increased in patients with DKD, addressing other modifiable risk factors, including dyslipidemia, is recommended in these patients. Patients with diabetes and stages 1 to 4 DKD should be treated with a high-intensity statin or a combination of a statin and ezetimibe.49,50
If a patient is taking a statin and starting dialysis, it’s important to discuss with him or her whether to continue the statin, based on perceived benefits and risks. It is not recommended that statins be initiated in patients on dialysis unless there is a specific cardiovascular indication for doing so. Risk reduction with a statin has been shown to be significantly less in dialysis patients than in patients who are not being treated with dialysis.49
Complications of CKD
Anemia is a common complication of CKD. KDIGO recommends measuring the hemoglobin concentration annually in DKD stage 3 patients without anemia; at least every 6 months in stage 4 patients; and at least every 3 months in stage 5. DKD patients with anemia should have additional laboratory testing: the absolute reticulocyte count, serum ferritin, serum transferrin saturation, vitamin B12, and folate.51
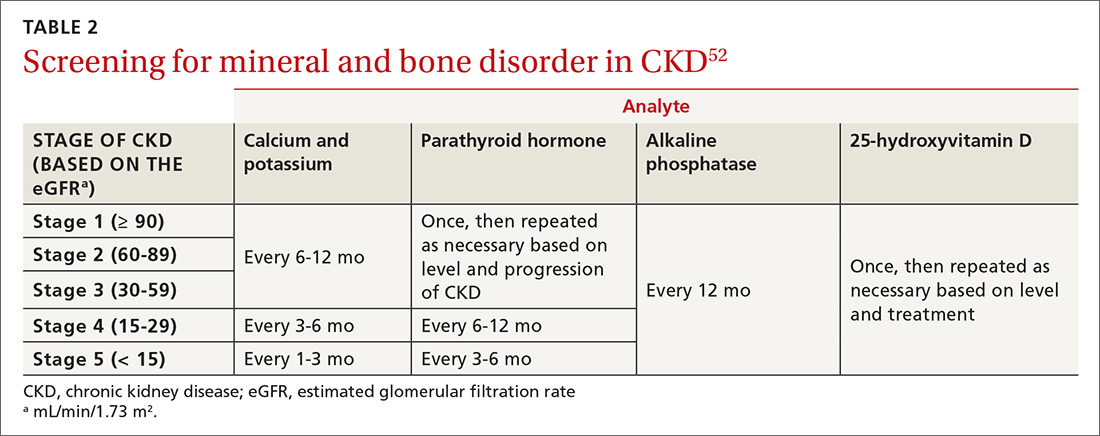
Mineral and bone disorder should be screened for in patients with DKD. TABLE 252 outlines when clinical laboratory tests should be ordered to assess for mineral bone disease.
When to refer to a nephrologist
Refer patients with stage 4 or 5 CKD (eGFR, ≤ 30 mL/min/1.73 m2) to a nephrologist for discussion of kidney replacement therapy.6 Patients with stage 3a CKD and severely increased albuminuria or with stage 3b CKD and moderately or severely increased albuminuria should also be referred to a nephrologist for intervention to delay disease progression.
Continue to: Identifying the need for early referral...
Identifying the need for early referral to a nephrologist has been shown to reduce the cost, and improve the quality, of care.53 Other indications for earlier referral include uncertainty about the etiology of renal disease, persistent or severe albuminuria, persistent hematuria, a rapid decline in eGFR, and acute kidney injury. Additionally, referral at an earlier stage of DKD might be needed to assist with complications associated with DKD, such as anemia, secondary hyperparathyroidism, mineral and bone disorder, resistant hypertension, fluid overload, and electrolyte disturbances.6
ACKNOWLEDGEMENT
The authors thank Colleen Colbert, PhD, and Iqbal Ahmad, PhD, for their review and critique of the manuscript of this article. They also thank Christopher Babiuch, MD, for his guidance in the preparation of the manuscript.
CORRESPONDENCE
Faraz Ahmad, MD, MPH, Care Point East Family Medicine, 543 Taylor Avenue, 2nd floor, Columbus, OH 43203; faraz. [email protected].
1. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83:1373-1381.
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7.
3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533.
4. Fox CS, Matsushita K, Woodward M, et al; . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673.
5. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124.
6. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159. Accessed January 5, 2021. https://care.diabetesjournals.org/content/41/Supplement_1
7. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. Accessed January 5, 2021. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
8. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-610.
9. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539.
10. de Boer IH; DCCT/EDIC Research Group. Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:24-30.
11. Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S3-S21.
12. Mottl AK, Tuttle KR. Diabetic kidney disease: Pathogenesis and epidemiology. UpToDate. Updated August 19, 2019. Accessed January 5, 2021. www.uptodate.com/contents/diabetic-kidney-disease-pathogenesis-and-epidemiology
13. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 2 diabetes mellitus. UpToDate. Updated November 3, 2020. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-2-diabetes-mellitus
14. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428.
15. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7-A8.
16. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277-284.
17. de Boer IH, Gao X, Cleary PA, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: The DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969-1977.
18. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941-1949.
19. Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patient with insulin dependent diabetes? BMJ. 1993;306:1722-1725.
20. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12-154.
21. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus. UpToDate. Updated December 3, 2019. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-1-diabetes-mellitus
22. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17-26.
23. Levey AS, Stevens LA, Schmid CH, et al; , A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
24. Wrone EM, Carnethon MR, Palaniappan L, et al; . Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580-587.
25. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467.
26. Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876-883.
27. de Boer, IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284.
28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
29. Brenner BM, Cooper ME, de Zeeuw D, et al; Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
30. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462.
31. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355;253-259.
32. Lewis EJ, Hunsicker LG, Clarke WR, et al; . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
33. Fried LF, Emanuele N, Zhang JH, et al; . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
34. Bakris GL, Agarwal R, Chan JC, et al; . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884-894.
35. Filippatos G, Anker SD, M, et al. Randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114.
36. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.N Engl J Med. 2008;358:2560-2572.
37. Ismail-Beigi F, Craven T, Banerji MA, et al; . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
38. Zoungas S, Chalmers J, Neal B, et al; . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392-1406.
39. Zoungas S, Arima H, Gerstein HC, et al; . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
40. Miller ME, Bonds DE, Gerstein HC, et al; . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340;b5444.
41. Papademetriou V, Lovato L, Doumas M, et al; . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659.
42. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886.
43. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J. 2017;10:301-304.
44. Wanner C, Inzucchi SE, Lachin JM, et al; Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
45. Neal B, Perkovic V, Mahaffey KW, et al; . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
46. Marso SP, Daniels GH, Brown-Frandsen K, et al; . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
47. Mann JFE, DD, Brown-Frandsen K, et al; . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
48. Marso SP, Bain SC, Consoli A, et al; . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
49. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309.
50. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143.
51. National Kidney Foundation KDOQI. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335. Accessed January 5, 2021. www.sciencedirect.com/journal/kidney-international-supplements/vol/2/issue/4
52. National Kidney Foundation KDOQI. Evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). 2010. Accessed January 5, 2021. www.kidney.org/sites/default/files/02-10-390B_LBA_KDOQI_BoneGuide.pdf
53. Smart MA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
Chronic kidney disease (CKD) is a significant comorbidity of diabetes mellitus. The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as the presence of kidney damage or decreased kidney function for ≥ 3 months. CKD caused by diabetes is called diabetic kidney disease (DKD), which is 1 of 3 principal microvascular complications of diabetes. DKD can progress to end-stage renal disease (ESRD), requiring kidney replacement therapy, and is the leading cause of CKD and ESRD in the United States.1-3 Studies have also shown that, particularly in patients with diabetes, CKD considerably increases the risk of cardiovascular events, which often occur prior to ESRD.1,4
This article provides the latest recommendations for evaluating and managing DKD to help you prevent or slow its progression.
Defining and categorizing diabetic kidney disease
CKD is defined as persistently elevated excretion of urinary albumin (albuminuria) and decreased estimated glomerular filtration rate (eGFR), or as the presence of signs of progressive kidney damage.5,6 DKD, also known as diabetic nephropathy, is CKD attributed to long-term diabetes. A patient’s eGFR is the established basis for assignment to a stage (1, 2, 3a, 3b, 4, or 5) of CKD (TABLE 17) and, along with the category of albuminuria (A1, A2, or A3), can indicate prognosis.

Taking its toll in diabetes
As many as 40% of patients with diabetes develop DKD.8-10 Most studies of DKD have been conducted in patients with type 1 diabetes (T1D), because the time of clinical onset is typically known.
Type 1 diabetes. DKD usually occurs 10 to 15 years, or later, after the onset of diabetes.6 As many as 30% of people with T1D have albuminuria approximately 15 years after onset of diabetes; almost one-half of those develop DKD.5,11 After approximately 22.5 years without albuminuria, patients with T1D have approximately a 1% annual risk of DKD.12
Type 2 diabetes (T2D). DKD is often present at diagnosis, likely due to a delay in diagnosis and briefer clinical exposure, compared to T1D. Albuminuria has been reported in as many as 40% of patients with T2D approximately 10 years after onset of diabetes.12,13
Multiple risk factors with no standout “predictor”
Genetic susceptibility, ethnicity, glycemic control, smoking, blood pressure (BP), and the eGFR have been identified as risk factors for renal involvement in diabetes; obesity, oral contraceptives, and age can also contribute. Although each risk factor increases the risk of DKD, no single factor is adequately predictive. Moderately increased albuminuria, the earliest sign of DKD, is associated with progressive nephropathy.12
Continue to: How great is the risk?
How great is the risk? From disease onset to proteinuria and from proteinuria to ESRD, the risk of DKD in T1D and T2D is similar. With appropriate treatment, albuminuria can regress, and the risk of ESRD can be < 20% at 10 years in T1D.12 As in T1D, good glycemic control might result in regression of albuminuria in T2D.14
For unknown reasons, the degree of albuminuria can exist independent of the progression of DKD. Factors responsible for a progressive decline in eGFR in DKD without albuminuria are unknown.12,15
Patient evaluation with an eye toward comorbidities
A comprehensive initial medical evaluation for DKD includes a review of microvascular complications; visits to specialists; lifestyle and behavior patterns (eg, diet, sleep, substance use, and social support); and medication adherence, adverse drug effects, and alternative medicines. Although DKD is often a clinical diagnosis, it can be ruled in by persistent albuminuria or decreased eGFR, or both, in established diabetes or diabetic retinopathy when other causes are unlikely (see “Recommended DKD screening protocol,” below).
Screening for mental health conditions and barriers to self-management is also key.6
Comorbidities, of course, can complicate disease management in patients with diabetes.16-20 Providers and patients therefore need to be aware of potential diabetic comorbidities. For example, DKD and even moderately increased albuminuria significantly increase the risk of cardiovascular disease (CVD).12 Other possible comorbidities include (but are not limited to) nonalcoholic steatohepatitis, fracture, hearing impairment, cancer (eg, liver, pancreas, endometrium, colon, rectum, breast, and bladder), pancreatitis, hypogonadism, obstructive sleep apnea, periodontal disease, anxiety, depression, and eating disorders.6
Continue to: Recommended DKD screening protocol
Recommended DKD screening protocol
In all cases of T2D, in cases of T1D of ≥ 5 years’ duration, and in patients with diabetes and comorbid hypertension, perform annual screening for albuminuria, an elevated creatinine level, and a decline in eGFR.
To confirm the diagnosis of DKD, at least 2 of 3 urine specimens must demonstrate an elevated urinary albumin:creatinine ratio (UACR) over a 3- to 6-month period.21 Apart from renal damage, exercise within 24 hours before specimen collection, infection, fever, congestive heart failure, hyperglycemia, menstruation, and hypertension can elevate the UACR.6
Levels of the UACR are established as follows22:
- Normal UACR is defined as < 30 milligrams of albumin per gram of creatinine (expressed as “mg/g”).
- Increased urinary albumin excretion is defined as ≥ 30 mg/g.
- Moderately increased albuminuria, a predictor of potential nephropathy, is the excretion of 30 to 300 mg/g.
- Severely increased albuminuria is excretion > 300 mg/g; it is often followed by a gradual decline in eGFR that, without treatment, eventually leads to ESRD.
The rate of decline in eGFR once albuminuria is severely increased is equivalent in T1D and T2D.12 Without intervention, the time from severely increased albuminuria to ESRD in T1D and T2D averages approximately 6 or 7 years.
Clinical features
DKD is typically a clinical diagnosis seen in patients with longstanding diabetes, albuminuria, retinopathy, or a reduced eGFR in the absence of another primary cause of kidney damage. In patients with T1D and DKD, signs of retinopathy and neuropathy are almost always present at diagnosis, unless a diagnosis is made early in the course of diabetes.12 Therefore, the presence of retinopathy suggests that diabetes is the likely cause of CKD.
Continue to: The presence of microvascular disease...
The presence of microvascular disease in patients with T2D and DKD is less predictable.12 In T2D patients who do not have retinopathy, consider causes of CKD other than DKD. Features suggesting that the cause of CKD is an underlying condition other than diabetes are rapidly increasing albuminuria or decreasing eGFR; urinary sediment comprising red blood cells or white blood cells; and nephrotic syndrome.6
As the prevalence of diabetes increases, it has become more common to diagnose DKD by eGFR without albuminuria—underscoring the importance of routine monitoring of eGFR in patients with diabetes.6
Sources of expert guidance. The Chronic Kidney Disease Epidemiology Collaboration equation23 is preferred for calculating eGFR from serum creatinine: An eGFR < 60 mL/min/1.73 m2 is considered abnormal.3,12 At these rates, the prevalence of complications related to CKD rises and screening for complications becomes necessary.
A more comprehensive classification of the stages of CKD, incorporating albuminuria and progression of CKD, has been recommended by Kidney Disease: Improving Global Outcomes (KDIGO).7 Because eGFR and excretion of albumin vary, abnormal test results need to be verified over time to stage the degree of CKD.3,12 Kidney damage often manifests as albuminuria, but also as hematuria, other types of abnormal urinary sediment, radiographic abnormalities, and other abnormal presentations.
Management
Nutritional factors
Excessive protein intake has been shown to increase albuminuria, worsen renal function, and increase CVD mortality in DKD.24-26 Therefore, daily dietary protein intake of 0.8 g/kg body weight is recommended for patients who are not on dialysis.3 Patients on dialysis might require higher protein intake to preserve muscle mass caused by protein-energy wasting, which is common in dialysis patients.6
Continue to: Low sodium intake
Low sodium intake in CKD patients has been shown to decrease BP and thus slow the progression of renal disease and lower the risk of CVD. The recommended dietary sodium intake in CKD patients is 1500-3000 mg/d.3
Low potassium intake. Hyperkalemia is a serious complication of CKD. A low-potassium diet is recommended in ESRD patients who have a potassium level > 5.5 mEq/L.6
Blood pressure
Preventing and treating hypertension is critical to slowing the progression of CKD and reducing cardiovascular risk. BP should be measured at every clinic visit. Aside from lifestyle changes, medication might be needed to reach target BP.
The American Diabetes Association recommends a BP goal of ≤ 140/90 mm Hg for hypertensive patients with diabetes, although they do state that a lower BP target (≤ 130/80 mm Hg) might be more appropriate for patients with DKD.27
The American College of Cardiology recommends that hypertensive patients with CKD have a BP target of ≤ 130/80 mm Hg.28
Continue to: ACE inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have renoprotective benefits. These agents are recommended as first-line medications for patients with diabetes, hypertension, and an eGFR < 60 mL/min/1.73 m2 and a UACR > 300 mg/g.29-31 Evidence also supports their use when the UACR is 30 to 299 mg/g.
Studies have shown that, in patients with DKD, ACE inhibitors and ARBs can slow the progression of renal disease.29,30,32 There is no difference between ACE inhibitors and ARBs in their effectiveness for preventing progression of DKD.6 There is no added benefit in combining an ACE inhibitor and an ARB33; notably, combination ACE inhibitor and ARB therapy can increase the risk of adverse events, such as hyperkalemia and acute kidney injury, especially in patients with DKD.33
There is no evidence for starting an ACE inhibitor or ARB to prevent CKD in patients with diabetes who are not hypertensive.5
ACE inhibitors and ARBs should be used with caution in women of childbearing age, who should use a reliable form of contraception if taking one of these drugs.
Diuretics. Thiazide-type and loop diuretics might potentiate the positive effects of ACE inhibitors and ARBs. KDOQI guidelines recommend that, in patients who require a second agent to control BP, a diuretic should be considered in combination with an ACE inhibitor or an ARB.20 A loop diuretic is preferred if the eGFR is < 30 mL/min/1.73 m2.
Continue to: Nondihydropyridine calcium-channel blockers
Nondihydropyridine calcium-channel blockers (CCBs), such as diltiazem and verapamil, have been shown to be more effective then dihydrophyridine CCBs, such as amlodipine and nifedipine, in slowing the progression of renal disease because of their antiproteinuric effects. However, the antiproteinuric effects of nondihydropyridine CCBs are not as strong as those of ACE inhibitors or ARBs, and these drugs do not appear to potentiate the effects of an ACE inhibitor or ARB when used in combination.20
Nondihydropyridine CCBs might be a reasonable alternative in patients who cannot tolerate an ACE inhibitor or an ARB.
Mineralocorticoid receptor antagonists in combination with an ACE inhibitor or ARB have been demonstrated to reduce albuminuria in short-term studies.34,35
Glycemic levels
Studies conducted in patients with T1D, and others in patients with T2D, have shown that tight glycemic control can delay the onset and slow the progression of albuminuria and a decline in the eGFR.10,36-39 The target glycated hemoglobin (A1C) should be < 7% to prevent or slow progression of DKD.40 However, patients with DKD have an increased risk of hypoglycemic events and increased mortality with more intensive glycemic control.40,41 Given those findings, some patients with DKD and significant comorbidities, ESRD, or limited life expectancy might need to have an A1C target set at 8%.6,42
Adjustments to antidiabetes medications in DKD
In patients with stages 3 to 5 DKD, several common antidiabetic medications might need to be adjusted or discontinued because they decrease creatinine clearance.
Continue to: First-generation sulfonylureas
First-generation sulfonylureas should be avoided in DKD. Glipizide and gliclazide are preferred among second-generation sulfonylureas because they do not increase the risk of hypoglycemia in DKD patients, although patients taking these medications still require close monitoring of their blood glucose level.20
Metformin. In 2016, recommendations changed for the use of metformin in patients with DKD: The eGFR, not the serum creatinine level, should guide treatment.43 Metformin can be used safely in patients with (1) an eGFR of < 60 mL/min/1.73 m2 and (2) an eGFR of 30 mL/min/1.73 m2 with close monitoring. Metformin should not be initiated if the eGFR is < 45 mL/min/1.73 m2.43
Antidiabetes medications with direct effect on the kidney
Several antidiabetes medications have a direct effect on the kidney apart from their effect on the blood glucose level.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to reduce albuminuria and slow the decrease of eGFR independent of glycemic control. In addition, SGLT2 inhibitors have also been shown to have cardiovascular benefits in patients with DKD.44,45
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to delay and decrease the progression of DKD.46-48 Also, similar to what is seen with SGLT2 inhibitors, GLP-1 agonists have demonstrable cardiovascular benefit in patients with DKD.46,48
Continue to: Dyslipidemia and DKD
Dyslipidemia and DKD
Because the risk of CVD is increased in patients with DKD, addressing other modifiable risk factors, including dyslipidemia, is recommended in these patients. Patients with diabetes and stages 1 to 4 DKD should be treated with a high-intensity statin or a combination of a statin and ezetimibe.49,50
If a patient is taking a statin and starting dialysis, it’s important to discuss with him or her whether to continue the statin, based on perceived benefits and risks. It is not recommended that statins be initiated in patients on dialysis unless there is a specific cardiovascular indication for doing so. Risk reduction with a statin has been shown to be significantly less in dialysis patients than in patients who are not being treated with dialysis.49
Complications of CKD
Anemia is a common complication of CKD. KDIGO recommends measuring the hemoglobin concentration annually in DKD stage 3 patients without anemia; at least every 6 months in stage 4 patients; and at least every 3 months in stage 5. DKD patients with anemia should have additional laboratory testing: the absolute reticulocyte count, serum ferritin, serum transferrin saturation, vitamin B12, and folate.51
Mineral and bone disorder should be screened for in patients with DKD. TABLE 252 outlines when clinical laboratory tests should be ordered to assess for mineral bone disease.

When to refer to a nephrologist
Refer patients with stage 4 or 5 CKD (eGFR, ≤ 30 mL/min/1.73 m2) to a nephrologist for discussion of kidney replacement therapy.6 Patients with stage 3a CKD and severely increased albuminuria or with stage 3b CKD and moderately or severely increased albuminuria should also be referred to a nephrologist for intervention to delay disease progression.
Continue to: Identifying the need for early referral...
Identifying the need for early referral to a nephrologist has been shown to reduce the cost, and improve the quality, of care.53 Other indications for earlier referral include uncertainty about the etiology of renal disease, persistent or severe albuminuria, persistent hematuria, a rapid decline in eGFR, and acute kidney injury. Additionally, referral at an earlier stage of DKD might be needed to assist with complications associated with DKD, such as anemia, secondary hyperparathyroidism, mineral and bone disorder, resistant hypertension, fluid overload, and electrolyte disturbances.6
ACKNOWLEDGEMENT
The authors thank Colleen Colbert, PhD, and Iqbal Ahmad, PhD, for their review and critique of the manuscript of this article. They also thank Christopher Babiuch, MD, for his guidance in the preparation of the manuscript.
CORRESPONDENCE
Faraz Ahmad, MD, MPH, Care Point East Family Medicine, 543 Taylor Avenue, 2nd floor, Columbus, OH 43203; faraz. [email protected].
Chronic kidney disease (CKD) is a significant comorbidity of diabetes mellitus. The Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation defines CKD as the presence of kidney damage or decreased kidney function for ≥ 3 months. CKD caused by diabetes is called diabetic kidney disease (DKD), which is 1 of 3 principal microvascular complications of diabetes. DKD can progress to end-stage renal disease (ESRD), requiring kidney replacement therapy, and is the leading cause of CKD and ESRD in the United States.1-3 Studies have also shown that, particularly in patients with diabetes, CKD considerably increases the risk of cardiovascular events, which often occur prior to ESRD.1,4
This article provides the latest recommendations for evaluating and managing DKD to help you prevent or slow its progression.
Defining and categorizing diabetic kidney disease
CKD is defined as persistently elevated excretion of urinary albumin (albuminuria) and decreased estimated glomerular filtration rate (eGFR), or as the presence of signs of progressive kidney damage.5,6 DKD, also known as diabetic nephropathy, is CKD attributed to long-term diabetes. A patient’s eGFR is the established basis for assignment to a stage (1, 2, 3a, 3b, 4, or 5) of CKD (TABLE 17) and, along with the category of albuminuria (A1, A2, or A3), can indicate prognosis.

Taking its toll in diabetes
As many as 40% of patients with diabetes develop DKD.8-10 Most studies of DKD have been conducted in patients with type 1 diabetes (T1D), because the time of clinical onset is typically known.
Type 1 diabetes. DKD usually occurs 10 to 15 years, or later, after the onset of diabetes.6 As many as 30% of people with T1D have albuminuria approximately 15 years after onset of diabetes; almost one-half of those develop DKD.5,11 After approximately 22.5 years without albuminuria, patients with T1D have approximately a 1% annual risk of DKD.12
Type 2 diabetes (T2D). DKD is often present at diagnosis, likely due to a delay in diagnosis and briefer clinical exposure, compared to T1D. Albuminuria has been reported in as many as 40% of patients with T2D approximately 10 years after onset of diabetes.12,13
Multiple risk factors with no standout “predictor”
Genetic susceptibility, ethnicity, glycemic control, smoking, blood pressure (BP), and the eGFR have been identified as risk factors for renal involvement in diabetes; obesity, oral contraceptives, and age can also contribute. Although each risk factor increases the risk of DKD, no single factor is adequately predictive. Moderately increased albuminuria, the earliest sign of DKD, is associated with progressive nephropathy.12
Continue to: How great is the risk?
How great is the risk? From disease onset to proteinuria and from proteinuria to ESRD, the risk of DKD in T1D and T2D is similar. With appropriate treatment, albuminuria can regress, and the risk of ESRD can be < 20% at 10 years in T1D.12 As in T1D, good glycemic control might result in regression of albuminuria in T2D.14
For unknown reasons, the degree of albuminuria can exist independent of the progression of DKD. Factors responsible for a progressive decline in eGFR in DKD without albuminuria are unknown.12,15
Patient evaluation with an eye toward comorbidities
A comprehensive initial medical evaluation for DKD includes a review of microvascular complications; visits to specialists; lifestyle and behavior patterns (eg, diet, sleep, substance use, and social support); and medication adherence, adverse drug effects, and alternative medicines. Although DKD is often a clinical diagnosis, it can be ruled in by persistent albuminuria or decreased eGFR, or both, in established diabetes or diabetic retinopathy when other causes are unlikely (see “Recommended DKD screening protocol,” below).
Screening for mental health conditions and barriers to self-management is also key.6
Comorbidities, of course, can complicate disease management in patients with diabetes.16-20 Providers and patients therefore need to be aware of potential diabetic comorbidities. For example, DKD and even moderately increased albuminuria significantly increase the risk of cardiovascular disease (CVD).12 Other possible comorbidities include (but are not limited to) nonalcoholic steatohepatitis, fracture, hearing impairment, cancer (eg, liver, pancreas, endometrium, colon, rectum, breast, and bladder), pancreatitis, hypogonadism, obstructive sleep apnea, periodontal disease, anxiety, depression, and eating disorders.6
Continue to: Recommended DKD screening protocol
Recommended DKD screening protocol
In all cases of T2D, in cases of T1D of ≥ 5 years’ duration, and in patients with diabetes and comorbid hypertension, perform annual screening for albuminuria, an elevated creatinine level, and a decline in eGFR.
To confirm the diagnosis of DKD, at least 2 of 3 urine specimens must demonstrate an elevated urinary albumin:creatinine ratio (UACR) over a 3- to 6-month period.21 Apart from renal damage, exercise within 24 hours before specimen collection, infection, fever, congestive heart failure, hyperglycemia, menstruation, and hypertension can elevate the UACR.6
Levels of the UACR are established as follows22:
- Normal UACR is defined as < 30 milligrams of albumin per gram of creatinine (expressed as “mg/g”).
- Increased urinary albumin excretion is defined as ≥ 30 mg/g.
- Moderately increased albuminuria, a predictor of potential nephropathy, is the excretion of 30 to 300 mg/g.
- Severely increased albuminuria is excretion > 300 mg/g; it is often followed by a gradual decline in eGFR that, without treatment, eventually leads to ESRD.
The rate of decline in eGFR once albuminuria is severely increased is equivalent in T1D and T2D.12 Without intervention, the time from severely increased albuminuria to ESRD in T1D and T2D averages approximately 6 or 7 years.
Clinical features
DKD is typically a clinical diagnosis seen in patients with longstanding diabetes, albuminuria, retinopathy, or a reduced eGFR in the absence of another primary cause of kidney damage. In patients with T1D and DKD, signs of retinopathy and neuropathy are almost always present at diagnosis, unless a diagnosis is made early in the course of diabetes.12 Therefore, the presence of retinopathy suggests that diabetes is the likely cause of CKD.
Continue to: The presence of microvascular disease...
The presence of microvascular disease in patients with T2D and DKD is less predictable.12 In T2D patients who do not have retinopathy, consider causes of CKD other than DKD. Features suggesting that the cause of CKD is an underlying condition other than diabetes are rapidly increasing albuminuria or decreasing eGFR; urinary sediment comprising red blood cells or white blood cells; and nephrotic syndrome.6
As the prevalence of diabetes increases, it has become more common to diagnose DKD by eGFR without albuminuria—underscoring the importance of routine monitoring of eGFR in patients with diabetes.6
Sources of expert guidance. The Chronic Kidney Disease Epidemiology Collaboration equation23 is preferred for calculating eGFR from serum creatinine: An eGFR < 60 mL/min/1.73 m2 is considered abnormal.3,12 At these rates, the prevalence of complications related to CKD rises and screening for complications becomes necessary.
A more comprehensive classification of the stages of CKD, incorporating albuminuria and progression of CKD, has been recommended by Kidney Disease: Improving Global Outcomes (KDIGO).7 Because eGFR and excretion of albumin vary, abnormal test results need to be verified over time to stage the degree of CKD.3,12 Kidney damage often manifests as albuminuria, but also as hematuria, other types of abnormal urinary sediment, radiographic abnormalities, and other abnormal presentations.
Management
Nutritional factors
Excessive protein intake has been shown to increase albuminuria, worsen renal function, and increase CVD mortality in DKD.24-26 Therefore, daily dietary protein intake of 0.8 g/kg body weight is recommended for patients who are not on dialysis.3 Patients on dialysis might require higher protein intake to preserve muscle mass caused by protein-energy wasting, which is common in dialysis patients.6
Continue to: Low sodium intake
Low sodium intake in CKD patients has been shown to decrease BP and thus slow the progression of renal disease and lower the risk of CVD. The recommended dietary sodium intake in CKD patients is 1500-3000 mg/d.3
Low potassium intake. Hyperkalemia is a serious complication of CKD. A low-potassium diet is recommended in ESRD patients who have a potassium level > 5.5 mEq/L.6
Blood pressure
Preventing and treating hypertension is critical to slowing the progression of CKD and reducing cardiovascular risk. BP should be measured at every clinic visit. Aside from lifestyle changes, medication might be needed to reach target BP.
The American Diabetes Association recommends a BP goal of ≤ 140/90 mm Hg for hypertensive patients with diabetes, although they do state that a lower BP target (≤ 130/80 mm Hg) might be more appropriate for patients with DKD.27
The American College of Cardiology recommends that hypertensive patients with CKD have a BP target of ≤ 130/80 mm Hg.28
Continue to: ACE inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) have renoprotective benefits. These agents are recommended as first-line medications for patients with diabetes, hypertension, and an eGFR < 60 mL/min/1.73 m2 and a UACR > 300 mg/g.29-31 Evidence also supports their use when the UACR is 30 to 299 mg/g.
Studies have shown that, in patients with DKD, ACE inhibitors and ARBs can slow the progression of renal disease.29,30,32 There is no difference between ACE inhibitors and ARBs in their effectiveness for preventing progression of DKD.6 There is no added benefit in combining an ACE inhibitor and an ARB33; notably, combination ACE inhibitor and ARB therapy can increase the risk of adverse events, such as hyperkalemia and acute kidney injury, especially in patients with DKD.33
There is no evidence for starting an ACE inhibitor or ARB to prevent CKD in patients with diabetes who are not hypertensive.5
ACE inhibitors and ARBs should be used with caution in women of childbearing age, who should use a reliable form of contraception if taking one of these drugs.
Diuretics. Thiazide-type and loop diuretics might potentiate the positive effects of ACE inhibitors and ARBs. KDOQI guidelines recommend that, in patients who require a second agent to control BP, a diuretic should be considered in combination with an ACE inhibitor or an ARB.20 A loop diuretic is preferred if the eGFR is < 30 mL/min/1.73 m2.
Continue to: Nondihydropyridine calcium-channel blockers
Nondihydropyridine calcium-channel blockers (CCBs), such as diltiazem and verapamil, have been shown to be more effective then dihydrophyridine CCBs, such as amlodipine and nifedipine, in slowing the progression of renal disease because of their antiproteinuric effects. However, the antiproteinuric effects of nondihydropyridine CCBs are not as strong as those of ACE inhibitors or ARBs, and these drugs do not appear to potentiate the effects of an ACE inhibitor or ARB when used in combination.20
Nondihydropyridine CCBs might be a reasonable alternative in patients who cannot tolerate an ACE inhibitor or an ARB.
Mineralocorticoid receptor antagonists in combination with an ACE inhibitor or ARB have been demonstrated to reduce albuminuria in short-term studies.34,35
Glycemic levels
Studies conducted in patients with T1D, and others in patients with T2D, have shown that tight glycemic control can delay the onset and slow the progression of albuminuria and a decline in the eGFR.10,36-39 The target glycated hemoglobin (A1C) should be < 7% to prevent or slow progression of DKD.40 However, patients with DKD have an increased risk of hypoglycemic events and increased mortality with more intensive glycemic control.40,41 Given those findings, some patients with DKD and significant comorbidities, ESRD, or limited life expectancy might need to have an A1C target set at 8%.6,42
Adjustments to antidiabetes medications in DKD
In patients with stages 3 to 5 DKD, several common antidiabetic medications might need to be adjusted or discontinued because they decrease creatinine clearance.
Continue to: First-generation sulfonylureas
First-generation sulfonylureas should be avoided in DKD. Glipizide and gliclazide are preferred among second-generation sulfonylureas because they do not increase the risk of hypoglycemia in DKD patients, although patients taking these medications still require close monitoring of their blood glucose level.20
Metformin. In 2016, recommendations changed for the use of metformin in patients with DKD: The eGFR, not the serum creatinine level, should guide treatment.43 Metformin can be used safely in patients with (1) an eGFR of < 60 mL/min/1.73 m2 and (2) an eGFR of 30 mL/min/1.73 m2 with close monitoring. Metformin should not be initiated if the eGFR is < 45 mL/min/1.73 m2.43
Antidiabetes medications with direct effect on the kidney
Several antidiabetes medications have a direct effect on the kidney apart from their effect on the blood glucose level.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to reduce albuminuria and slow the decrease of eGFR independent of glycemic control. In addition, SGLT2 inhibitors have also been shown to have cardiovascular benefits in patients with DKD.44,45
Glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to delay and decrease the progression of DKD.46-48 Also, similar to what is seen with SGLT2 inhibitors, GLP-1 agonists have demonstrable cardiovascular benefit in patients with DKD.46,48
Continue to: Dyslipidemia and DKD
Dyslipidemia and DKD
Because the risk of CVD is increased in patients with DKD, addressing other modifiable risk factors, including dyslipidemia, is recommended in these patients. Patients with diabetes and stages 1 to 4 DKD should be treated with a high-intensity statin or a combination of a statin and ezetimibe.49,50
If a patient is taking a statin and starting dialysis, it’s important to discuss with him or her whether to continue the statin, based on perceived benefits and risks. It is not recommended that statins be initiated in patients on dialysis unless there is a specific cardiovascular indication for doing so. Risk reduction with a statin has been shown to be significantly less in dialysis patients than in patients who are not being treated with dialysis.49
Complications of CKD
Anemia is a common complication of CKD. KDIGO recommends measuring the hemoglobin concentration annually in DKD stage 3 patients without anemia; at least every 6 months in stage 4 patients; and at least every 3 months in stage 5. DKD patients with anemia should have additional laboratory testing: the absolute reticulocyte count, serum ferritin, serum transferrin saturation, vitamin B12, and folate.51
Mineral and bone disorder should be screened for in patients with DKD. TABLE 252 outlines when clinical laboratory tests should be ordered to assess for mineral bone disease.

When to refer to a nephrologist
Refer patients with stage 4 or 5 CKD (eGFR, ≤ 30 mL/min/1.73 m2) to a nephrologist for discussion of kidney replacement therapy.6 Patients with stage 3a CKD and severely increased albuminuria or with stage 3b CKD and moderately or severely increased albuminuria should also be referred to a nephrologist for intervention to delay disease progression.
Continue to: Identifying the need for early referral...
Identifying the need for early referral to a nephrologist has been shown to reduce the cost, and improve the quality, of care.53 Other indications for earlier referral include uncertainty about the etiology of renal disease, persistent or severe albuminuria, persistent hematuria, a rapid decline in eGFR, and acute kidney injury. Additionally, referral at an earlier stage of DKD might be needed to assist with complications associated with DKD, such as anemia, secondary hyperparathyroidism, mineral and bone disorder, resistant hypertension, fluid overload, and electrolyte disturbances.6
ACKNOWLEDGEMENT
The authors thank Colleen Colbert, PhD, and Iqbal Ahmad, PhD, for their review and critique of the manuscript of this article. They also thank Christopher Babiuch, MD, for his guidance in the preparation of the manuscript.
CORRESPONDENCE
Faraz Ahmad, MD, MPH, Care Point East Family Medicine, 543 Taylor Avenue, 2nd floor, Columbus, OH 43203; faraz. [email protected].
1. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83:1373-1381.
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7.
3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533.
4. Fox CS, Matsushita K, Woodward M, et al; . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673.
5. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124.
6. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159. Accessed January 5, 2021. https://care.diabetesjournals.org/content/41/Supplement_1
7. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. Accessed January 5, 2021. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
8. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-610.
9. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539.
10. de Boer IH; DCCT/EDIC Research Group. Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:24-30.
11. Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S3-S21.
12. Mottl AK, Tuttle KR. Diabetic kidney disease: Pathogenesis and epidemiology. UpToDate. Updated August 19, 2019. Accessed January 5, 2021. www.uptodate.com/contents/diabetic-kidney-disease-pathogenesis-and-epidemiology
13. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 2 diabetes mellitus. UpToDate. Updated November 3, 2020. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-2-diabetes-mellitus
14. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428.
15. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7-A8.
16. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277-284.
17. de Boer IH, Gao X, Cleary PA, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: The DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969-1977.
18. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941-1949.
19. Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patient with insulin dependent diabetes? BMJ. 1993;306:1722-1725.
20. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12-154.
21. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus. UpToDate. Updated December 3, 2019. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-1-diabetes-mellitus
22. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17-26.
23. Levey AS, Stevens LA, Schmid CH, et al; , A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
24. Wrone EM, Carnethon MR, Palaniappan L, et al; . Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580-587.
25. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467.
26. Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876-883.
27. de Boer, IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284.
28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
29. Brenner BM, Cooper ME, de Zeeuw D, et al; Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
30. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462.
31. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355;253-259.
32. Lewis EJ, Hunsicker LG, Clarke WR, et al; . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
33. Fried LF, Emanuele N, Zhang JH, et al; . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
34. Bakris GL, Agarwal R, Chan JC, et al; . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884-894.
35. Filippatos G, Anker SD, M, et al. Randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114.
36. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.N Engl J Med. 2008;358:2560-2572.
37. Ismail-Beigi F, Craven T, Banerji MA, et al; . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
38. Zoungas S, Chalmers J, Neal B, et al; . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392-1406.
39. Zoungas S, Arima H, Gerstein HC, et al; . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
40. Miller ME, Bonds DE, Gerstein HC, et al; . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340;b5444.
41. Papademetriou V, Lovato L, Doumas M, et al; . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659.
42. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886.
43. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J. 2017;10:301-304.
44. Wanner C, Inzucchi SE, Lachin JM, et al; Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
45. Neal B, Perkovic V, Mahaffey KW, et al; . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
46. Marso SP, Daniels GH, Brown-Frandsen K, et al; . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
47. Mann JFE, DD, Brown-Frandsen K, et al; . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
48. Marso SP, Bain SC, Consoli A, et al; . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
49. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309.
50. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143.
51. National Kidney Foundation KDOQI. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335. Accessed January 5, 2021. www.sciencedirect.com/journal/kidney-international-supplements/vol/2/issue/4
52. National Kidney Foundation KDOQI. Evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). 2010. Accessed January 5, 2021. www.kidney.org/sites/default/files/02-10-390B_LBA_KDOQI_BoneGuide.pdf
53. Smart MA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
1. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83:1373-1381.
2. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7.
3. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533.
4. Fox CS, Matsushita K, Woodward M, et al; . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673.
5. Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116-1124.
6. American Diabetes Association. Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159. Accessed January 5, 2021. https://care.diabetesjournals.org/content/41/Supplement_1
7. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150. Accessed January 5, 2021. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
8. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602-610.
9. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532-2539.
10. de Boer IH; DCCT/EDIC Research Group. Kidney disease and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2014;37:24-30.
11. Stanton RC. Clinical challenges in diagnosis and management of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S3-S21.
12. Mottl AK, Tuttle KR. Diabetic kidney disease: Pathogenesis and epidemiology. UpToDate. Updated August 19, 2019. Accessed January 5, 2021. www.uptodate.com/contents/diabetic-kidney-disease-pathogenesis-and-epidemiology
13. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 2 diabetes mellitus. UpToDate. Updated November 3, 2020. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-2-diabetes-mellitus
14. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6:e005428.
15. Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7-A8.
16. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277-284.
17. de Boer IH, Gao X, Cleary PA, et al; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group. Albuminuria changes and cardiovascular and renal outcomes in type 1 diabetes: The DCCT/EDIC study. Clin J Am Soc Nephrol. 2016;11:1969-1977.
18. Sumida K, Molnar MZ, Potukuchi PK, et al. Changes in albuminuria and subsequent risk of incident kidney disease. Clin J Am Soc Nephrol. 2017;12:1941-1949.
19. Borch-Johnsen K, Wenzel H, Viberti GC, et al. Is screening and intervention for microalbuminuria worthwhile in patient with insulin dependent diabetes? BMJ. 1993;306:1722-1725.
20. KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12-154.
21. Bakris GL. Moderately increased albuminuria (microalbuminuria) in type 1 diabetes mellitus. UpToDate. Updated December 3, 2019. Accessed January 5, 2021. https://www.uptodate.com/contents/moderately-increased-albuminuria-microalbuminuria-in-type-1-diabetes-mellitus
22. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of chronic kidney disease: rationale and benefits. Clin Biochem Rev. 2016;37:17-26.
23. Levey AS, Stevens LA, Schmid CH, et al; , A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612.
24. Wrone EM, Carnethon MR, Palaniappan L, et al; . Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:580-587.
25. Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138:460-467.
26. Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876-883.
27. de Boer, IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273-1284.
28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
29. Brenner BM, Cooper ME, de Zeeuw D, et al; Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
30. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456-1462.
31. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355;253-259.
32. Lewis EJ, Hunsicker LG, Clarke WR, et al; . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
33. Fried LF, Emanuele N, Zhang JH, et al; . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892-1903.
34. Bakris GL, Agarwal R, Chan JC, et al; . Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884-894.
35. Filippatos G, Anker SD, M, et al. Randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105-2114.
36. The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes.N Engl J Med. 2008;358:2560-2572.
37. Ismail-Beigi F, Craven T, Banerji MA, et al; . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430.
38. Zoungas S, Chalmers J, Neal B, et al; . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392-1406.
39. Zoungas S, Arima H, Gerstein HC, et al; . Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431-437.
40. Miller ME, Bonds DE, Gerstein HC, et al; . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340;b5444.
41. Papademetriou V, Lovato L, Doumas M, et al; . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649-659.
42. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850-886.
43. Imam TH. Changes in metformin use in chronic kidney disease. Clin Kidney J. 2017;10:301-304.
44. Wanner C, Inzucchi SE, Lachin JM, et al; Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334.
45. Neal B, Perkovic V, Mahaffey KW, et al; . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
46. Marso SP, Daniels GH, Brown-Frandsen K, et al; . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
47. Mann JFE, DD, Brown-Frandsen K, et al; . Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
48. Marso SP, Bain SC, Consoli A, et al; . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
49. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303-1309.
50. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143.
51. National Kidney Foundation KDOQI. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335. Accessed January 5, 2021. www.sciencedirect.com/journal/kidney-international-supplements/vol/2/issue/4
52. National Kidney Foundation KDOQI. Evaluation and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). 2010. Accessed January 5, 2021. www.kidney.org/sites/default/files/02-10-390B_LBA_KDOQI_BoneGuide.pdf
53. Smart MA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
PRACTICE RECOMMENDATIONS
› Screen patients with diabetes annually for diabetic kidney disease with measurement of urinary albumin and the estimated glomerular filtration rate. B
› Optimize blood glucose and blood pressure control in patients with diabetes to prevent or delay progression to diabetic kidney disease. A
› Treat hypertensive patients with diabetes and stages 1 to 4 chronic kidney disease with an angiotensin-converting enzyme inhibitor or angiotensin II-receptor blocker as a first-line antihypertensive, absent contraindications. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series