User login
Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
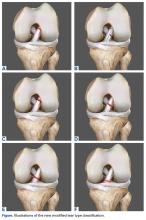
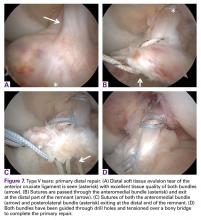
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
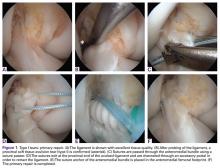
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
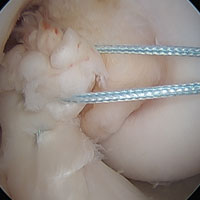
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
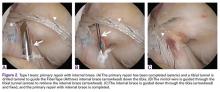
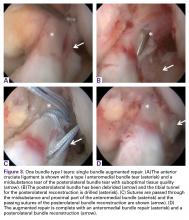
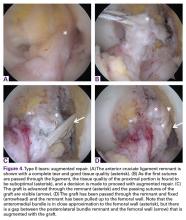
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
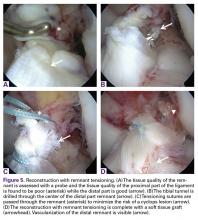
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
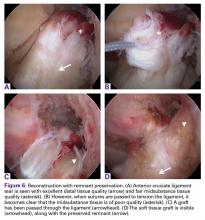
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
Preservation of the Anterior Cruciate Ligament: Surgical Techniques
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.