User login
Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
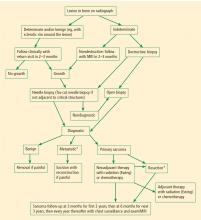
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
Prior to the 1970s, bone sarcomas were routinely treated with amputation, yet most patients still died from metastatic disease.1 The advent of the use of chemotherapy for bone sarcomas in the 1970s was shown to increase long-term survival,2–5 contributing in part to tremendous subsequent advances in the treatment of the most common bone sarcomas—osteosarcoma and Ewing sarcoma. Today, long-term disease-free survival rates of about 60% to 80% are observed for patients with Ewing sarcoma or osteosarcoma with no metastasis at presentation.6,7 In addition to the chemotherapy advances, modular metallic prosthetic limb reconstruction systems are now readily available, eliminating the need to wait for custom reconstructive hardware. Moreover, these systems can be used in combination with large bone allografts or vascularized bone flaps.
The majority of patients with bone sarcomas require multimodal treatment, primarily with surgery and chemotherapy. Patients with chondrosarcomas are the primary exception, as chondrosarcomas are generally treated with resection alone. Thus, management of most patients with bone sarcomas requires a multidisciplinary team that includes orthopedic, medical, and radiation oncologists as well as plastic and reconstructive surgeons, physical therapy specialists, pathologists, and radiologists with expertise in bone tumors.
Despite this broad need for multimodal therapy, surgical resection is fundamental to the management of virtually all bone sarcomas and is the primary focus of this article. The roles of chemotherapy and radiation therapy for bone sarcomas are detailed in the final two articles in this supplement.
INITIAL EVALUATION OF SUSPICIOUS BONE MASSES
History and physical examination
As noted in the preceding article in this supplement, most bone sarcomas (particularly osteosarcomas and Ewing sarcomas) occur in pediatric patients and young adults and develop in the extremities (especially the distal femur) or pelvis.
In terms of history, most patients with a bone sarcoma will report pain, but pain is not a good indicator of malignancy, as some patients with no pain or an improvement in pain have sarcomas while many patients with pain do not have malignancies.1
The other most common finding in patients with a bone sarcoma is an enlarging mass. The presence of a mass, as well as its location, depth, size, and overlying skin quality, can be determined on physical examination. An accurate neurovascular exam should be performed as well, although damage to neurovascular structures is a late finding in sarcoma patients.
Imaging
Radiographs are important in any patient with prolonged unexplained bone pain and will almost always reveal an aggressive lesion in the patient with a bone sarcoma. Lengthy delays in the diagnosis of a bone sarcoma are nearly always explained by failure to obtain a radiograph.
Magnetic resonance imaging (MRI). Questions about whether a radiograph of a lesion is determinate or not are best resolved by MRI, which is the primary imaging method for evaluating bone lesions, their exact location, and their proximity to neurovascular structures. While “determinate” and “indeterminate” are most precisely used to refer to imaging studies of a lesion, these terms are often used in clinical parlance to refer to the lesions themselves. As such, “determinate lesions” by imaging are those that can be accurately judged malignant or benign with a high level of certainty. Determinate benign inactive lesions such as enchondromas and osteochondromas, if asymptomatic and without severe bony destruction, do not require a bone biopsy. “Indeterminate lesions” by imaging are those whose imaging findings are not clearly consistent with a single diagnosis, and nearly all of these lesions require a biopsy.
In general, any patient with a bone mass with indeterminate imaging results should be referred to an orthopedic oncologist.
Staging
When imaging findings are highly suggestive of bone sarcoma, efforts should be made to delineate how far the tumor extends and whether systemic disease is present. Bone sarcomas can metastasize to other bones, but their most common site for metastasis is the lung.
MRI of the lesion without gadolinium is indicated, and the entire bone is imaged to determine the extent of the external mass outside the bone and to look for medullary extension and skip lesions (eg, smaller foci of sarcoma occurring in the same bone or on the opposing side of a joint). The precision offered by MRI has dramatically increased surgeons’ ability to achieve negative margins during resection.
Radiography or computed tomography of the chest is required to accurately assess the lungs for metastasis. A nuclear medicine technetium scan can be obtained to look for other similar bone lesions (metachronous lesions) or metastatic bony disease.
Laboratory tests are not helpful in the staging of bone sarcomas.
BIOPSY
Biopsy is the gold standard for diagnosis of bone sarcoma (Figure 1). The primary biopsy methods used are needle or open biopsy techniques, and Tru-cut needles or core bone biopsy needles are increasingly used. If the core needle biopsy is diagnostically inconclusive, an open biopsy can promptly be performed. Biopsies yielding specimens that are too small can result in inconclusive pathology reports. Regardless of the biopsy technique, hemostasis is of paramount importance, and patients are generally advised to not use the affected limb for at least several days after the procedure to reduce the risk of a cancer cell–laden hematoma.
If a needle biopsy is performed, 2 to 10 minutes of gentle pressure is applied to the site. In an open biopsy, electrocauterization is used extensively. Aggressive hemostasis is achieved, and if a drain is placed it should be in proximity to the incision site itself so that the drain site will be resected with the specimen at the time of definitive resection. Open biopsies are performed in the operating room with regional or general anesthesia. Incisions are made longitudinally and never transversely.
Ideally, the biopsy should be performed or supervised by a physician experienced with limb salvage for bone sarcomas. Otherwise there is risk for an inappropriate biopsy tract or approach, misinterpretation of the radiographic studies, misinterpretation of the pathology, or biopsy complications. These errors may lead to undertreatment or even unnecessary amputation.8,9
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.
AMPUTATION
When curative surgery is possible and limb-salvaging resection is unlikely to obtain a negative margin or a functionally viable extremity, amputations are still performed. For example, amputation is recommended in a patient with a high-grade calcaneal (heel bone) sarcoma with a large soft-tissue mass. However, amputation is not the usual approach for most bone sarcomas today and it is not benign in outcome. Notably, phantom limb pain and stump pain have been reported after amputation in the typically sensate tumor patient.
Meticulous hemostasis is necessary in all amputations, and myodesis, or direct suturing of muscle to the distal end of the bone, is important for soft-tissue coverage over the distal stump. In general, a fish-mouth incision is used for the upper extremity and thigh, and a posterior flap is used, when possible, below the knee. However, the choice of technique depends on factors such as the presence or absence of a biopsy incision and the location of tumor soft-tissue mass, so local tissue rearrangement or flaps may need to be used for stable coverage or closure.
For all amputation patients, early involvement of an acute pain specialist reduces the incidence of phantom limb pain.
SURVEILLANCE AND FOLLOW-UP
Post-therapy follow-up of patients with bone sarcomas is critical. Even among patients who receive appropriate surgery with negative margins there is a recurrence rate of approximately 9% (personal communication from Dr. Dempsey Springfield), and previously undetectable metastatic disease may become detectable in the postoperative period. In general, patients are followed at 3-month intervals for the first 2 years, at 6-month intervals for the next 3 years, and at yearly intervals thereafter. Follow-up evaluations must include examination of the the involved extremity and imaging of the chest, with radiography or computed tomography, to assess for metastasis.
Rehabilitation is specific to the site of resection and the reconstruction. In general, range of motion is important around the knee, whereas in patients with resection and reconstruction involving the shoulder, hip, or pelvis, it is more important that the affected muscles be given time to heal (6–12 weeks) before aggressive rehabilitation is begun.
Many patients limp postoperatively, particularly in the initial period, and the degree of limp depends primarily on the amount of muscle and the bony insertion sites that are resected with the tumor. Improvements in function are common over time, even at several years after surgery.
FUTURE DIRECTIONS
Despite the advances in bone sarcoma outcomes in recent decades, sarcomas of the pelvis continue to carry a worse prognosis than those of the extremities and thus represent an opportunity for improvement. Among the improvements hoped for is an ability to accomplish partial pelvic resections—eg, of the wing, ischium, or ramus—without need for reconstruction for these smaller localized tumors. Options include amputation (hemipelvectomy) with loss of leg; internal hemipelvectomy (where the pelvis is resected but the leg is left attached without reconstruction of the defect); or resection of the pelvic/acetabular area but with reconstruction using pelvic allografts/total hip composites or large metallic prostheses.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.
- Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998.
- Cortes EP, Holland JF, Wang JJ, et al. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974; 291:998–1000.
- Goorin AM, Abelson HT, Frei E III. Osteosarcoma: fifteen years later. N Engl J Med 1985; 313:1637–1643.
- Goorin AM, Frei E, Abelson HT. Adjuvant chemotherapy for osteosarcoma: a decade of experience. Surg Clin North Am 1981; 61:1379–1389.
- Jaffe N, Goorin A, Link M, et al. High-dose methotrexate in osteogenic sarcoma adjuvant chemotherapy and limb salvage results. Cancer Treat Rep 1981; 65(suppl 1):99–106.
- Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 2008; 19:814–820.
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival: a report from the Children’s Oncology Group. J Clin Oncol 2008; 26:633–638.
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78:656–663.
- Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982; 64:1121–1127.
- Blakely ML, Spurbeck WW, Pappo AS, et al. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 1999; 34:672–675.
- Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol 1997; 66:81–87.
- Gupta GR, Yasko AW, Lewis VO, et al. Risk of local recurrence after deltoid-sparing resection for osteosarcoma of the proximal humerus. Cancer 2009; 115:3767–3773.
- Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 2008; 90:203–208.
- Bauer HC, Brosjö O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities: 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66:283–288.
- Graham-Pole J. Ewing sarcoma: treatment with high dose radiation and adjuvant chemotherapy. Med Pediatr Oncol 1979; 7:1–8.
- Merchant TE, Kushner BH, Sheldon JM, LaQuaglia M, Healey JH. Effect of low-dose radiation therapy when combined with surgical resection for Ewing sarcoma. Med Pediatr Oncol 1999; 33:65–70.
- Rosito P, Mancini AF, Rondelli R, et al. Italian Cooperative Study for the treatment of children and young adults with localized Ewing sarcoma of bone: a preliminary report of 6 years of experience. Cancer 1999; 86:421–428.
- Goldsby R, Burke C, Nagarajan R, et al. Second solid malignancies among children, adolescents, and young adults diagnosed with malignant bone tumors after 1976: follow-up of a Children’s Oncology Group cohort. Cancer 2008; 113:2597–2604.