User login
Best timing for measuring orthostatic vital signs?
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old woman with a history of hypertension presents with a chief complaint of dizziness. You require an assessment of orthostatic vital signs to proceed. In your busy clinical practice, when should assessment take place to be most useful?
Orthostatic hypotension (OH) is defined as a postural reduction in systolic blood pressure (BP) of ≥ 20 mm Hg or diastolic BP of ≥ 10 mm Hg, measured within 3 minutes of rising from supine to standing. This definition is based on consensus guidelines from the American Academy of Neurology and the American Autonomic Society2 and has been upheld by European guidelines.3
The prevalence of OH is approximately 6% in the general population, with estimates ranging from 10% to 55% in older adults.4 Etiology is often multifactorial; causes may be neurogenic (mediated by autonomic failure as in Parkinson’s disease, multiple system atrophy, or diabetic neuropathy), non-neurogenic (related to medications or hypovolemia), or idiopathic.
It’s important to identify OH because of its associated increase in morbidities, such as an increased risk of falls (hazard ratio [HR] = 1.5),5 coronary heart disease (HR = 1.3), stroke (HR = 1.2), and all-cause mortality (HR = 1.4).6 Treatments include physical maneuvers (getting up slowly, leg crossing, and muscle clenching), increased salt and water intake, compression stockings, the addition of medications (such as fludrocortisone or midodrine), and the avoidance of other medications (such as benzodiazepines and diuretics).
The guideline-recommended 3-minute delay in assessment can be impractical in a busy clinical setting. Using data from the Atherosclerosis Risk in Communities (ARIC) study, investigators correlated the timing of measurements of postural change in BP with long-term adverse outcomes.1
STUDY SUMMARY
Early vs late OH assessment in middle-aged adults
The ARIC study is a longitudinal, prospective, cohort study of almost 16,000 adults followed since 1987. Juraschek et al1 assessed the optimal time to identify OH and its association with the adverse clinical outcomes of fall, fracture, syncope, motor vehicle crash, and mortality. The researchers sought to discover whether BP measurements determined immediately after standing predict adverse events as well as BP measurements taken closer to 3 minutes.
Study participants were between the ages of 45 and 64 years (mean 54 years), and 26% were black and 54% were female. They lived in 4 different US communities. The researchers excluded patients with missing OH assessments or other relevant cohort or historical data, leaving a cohort of 11,429 subjects.
Continue to: As part of their...
As part of their enrollment into the ARIC study, subjects had their BP measurements taken 2 to 5 times in the lying position (90% of participants had ≥ 4 measurements) and after standing (91% participants had ≥ 4 measurements) using a programmable automatic BP cuff. All 5 standing BP measurements (taken at a mean of 28, 53, 76, 100, and 116 seconds after standing) were measured for 7385 out of 11,429 (64.6%) participants. Subjects were asked if he or she “usually gets dizzy on standing up.”
Researchers determined the association between OH and postural change in systolic BP or postural change in diastolic BP with history of dizziness after standing. They also determined the incidence of falls, fracture, syncope, motor vehicle crash, and mortality via a review of hospitalizations and billing for Medicaid and Medicare services. Subjects were followed for a median of 23 years.
Results
Of the entire cohort, 1138 (10%) reported dizziness on standing. Only OH identified at the first BP measurement (mean 28 secs) was associated with a history of dizziness upon standing (odds ratio [OR] = 1.49; 95% confidence interval [CI], 1.18-1.89). Also, it was associated with the highest incidence of fracture, syncope, and death (18.9, 17, and 31.4 per 1000 person-years, respectively).
After adjusting for age, sex, and multiple other cardiovascular risk factors, the risk of falls was significantly associated with OH at BP measurements 1 to 4, but was most strongly associated with BP measurement 2 (taken at a mean of 53 secs after standing) (HR = 1.29; 95% CI, 1.12-1.49), which translates to 13.2 falls per 1000 patient-years. Fracture was associated with OH at measurements 1 (HR = 1.16; 95% CI, 1.01-1.34) and 2 (HR = 1.14; 95% CI, 1.01-1.29). Motor vehicle crashes were associated only with BP measurement 2 (HR = 1.43; 95% CI, 1.04-1.96). Finally, risk of syncope and risk of death were statistically associated with the presence of OH at all 5 BP measurements.
WHAT’S NEW
Earlier OH assessments are more informative than late ones
This study found OH identified within 1 minute of standing to be more clinically meaningful than OH identified after 1 minute. Also, the findings reinforce the relationship between OH and adverse events, including injury and overall mortality. Evaluation for OH performed only at 3 minutes may miss symptomatic OH.
Continue to: CAVEATS
CAVEATS
Could a healthy population skew the results?
The population in this study was relatively healthy, with a lower prevalence of diabetes and coronary artery disease than the general population. While there is no reason to expect detection of OH to differ in a population with more comorbidities, the possibility exists.
If OH is not identified in < 1 minute of standing, standard OH evaluation within 3 minutes after standing should be performed, as OH identified at any time point after standing is associated with adverse events and increased mortality.
This study did not address the effects of medical intervention for OH on injury or mortality. Also, whether OH is the direct cause of the adverse outcomes or a marker for other disease is unknown.
CHALLENGES TO IMPLEMENTATION
A change to protocols and guidelines
Although none were noted, any change in practice requires updating clinical protocols and guidelines, which can take time.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
1. Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Internal Med. 2017;177:1316-1323.
2. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470.
3. Lahrmann H, Cortelli P, Hilz M, et al. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930-936.
4. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69-72.
5. Rutan GH, Hermanson B, Bild DE, et al. Orthostatic hypotension in older adults: the Cardiovascular Health Study. Hypertension. 1992;19(6 Pt 1):508-519.
6. Xin W, Lin Z, Mi S. Orthostatic hypotension and mortality risk: a meta-analysis of cohort studies. Heart. 2014;100:406-413.
PRACTICE CHANGER
Measure orthostatic vital signs within 1 minute of standing to most accurately correlate dizziness with long-term adverse outcomes. 1
STRENGTH OF RECOMMENDATION
B: Based on a single, high-quality, prospective cohort study with patient-oriented outcomes and good follow-up.
Juraschek SP, Daya N, Rawlings AM, et al. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316-1323.
A Better Approach to the Diagnosis of PE
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.

Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).

Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
A better approach to the diagnosis of PE
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
Penny E is a 48-year-old woman with a history of asthma who presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. Pulmonary embolism (PE) is not your most likely diagnosis, but it is included in the differential, so you order a D-dimer concentration and it returns at 700 ng/mL. Should you order computed tomography pulmonary angiography (CTPA) to evaluate for PE?
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2 people/1000 population and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
The use of a diagnostic algorithm that includes the Wells’ criteria and a
Further, it is common for a
Three items of the original Wells’ criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 After excluding 151 patients who met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA), investigators managed 3465 study patients according to the YEARS algorithm. This algorithm called for obtaining a
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a
Continue to: Eighteen of the 2964 patients...
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%; 95% CI, 0.36-0.96), with 6 patients (0.20%; 95% CI, 0.07-0.44) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43% (95% CI, 0.17-0.88), which is similar to the 0.34% (0.036-0.96) reported in a previous meta-analysis of the Wells’ rule algorithm.13 Overall, fatal PE occurred in 0.3% (95% CI, 0.12-0.78) of patients in the YEARS cohort vs 0.6% (0.4-1.1) in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells’ algorithm, for an absolute difference of 13% (95% CI, 10-15) and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% (95% CI, 12-16) and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference; 95% CI, 12-16) when compared with using the Wells’ rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria does not consider an age-adjusted
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38:S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and D-dimer testing to rule out pulmonary embolism. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including D-dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating D-dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
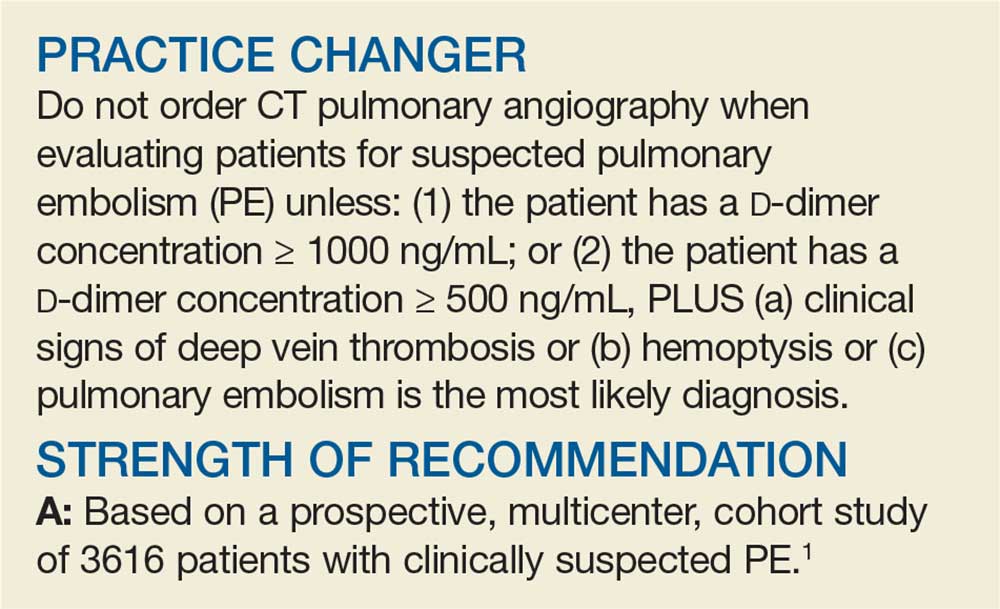
PRACTICE CHANGER
Do not order computed tomography pulmonary angiography when evaluating patients for suspected pulmonary embolism unless: (1) the patient has a
STRENGTH OF RECOMMENDATION
A: Based on a prospective, multicenter, cohort study of 3616 patients with clinically suspected pulmonary embolism.1
van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.